Abstract
Treatment of cultured tracheal smooth-muscle cells (TSM) with phorbol 12-myristate 13-acetate (PMA) (100 nM) or bradykinin (100 nM) elicited enhanced basal and guanosine 5'-[beta gamma-imido]-triphosphate-stimulated adenylate cyclase activities in subsequently isolated membranes. Combined stimulation of cells was non-additive, indicating that both agents activate adenylate cyclase via similar routes. Both PMA (100 nM) and bradykinin (100 nM) allowed the alpha subunit of Gs to act as a more favourable substrate for its cholera-toxin-catalysed ADP-ribosylation in vitro. PMA was without effect on intracellular cyclic AMP in control cells. However, constitutive activation of Gs by treatment in vivo with cholera toxin (0.5 ng/ml, 18 h) sensitized the cells to PMA stimulation, resulting in a concentration-dependent increase in intracellular cyclic AMP accumulation (EC50 = 7.3 +/- 2.5 nM, n = 5). Bradykinin also elicited a concentration-dependent increase in intracellular cyclic AMP (EC50 = 63.3 +/- 14.5 nM, n = 3). Constitutive activation of Gs resulted in an increased maximal response (10-fold) and potency (EC50 = 6.17 +/- 1.6 nM, n = 3) to bradykinin. This response was not affected by the B2-receptor antagonist, NPC567 [which selectively blocks bradykinin-stimulated phospholipase C (PLC), with minor activity against phospholipase D (PLD) activity]. Des-Arg9-bradykinin (a B1-receptor agonist) was without activity. These results suggest that the receptor sub-type capable of activating PLD may also be stimulatory for cyclic AMP accumulation. Furthermore, pre-treatment of the cells with butan-l-ol (0.3%, v/v), which traps phosphatidate derived from PLD reactions, blocked the bradykinin-stimulated increase in intracellular cyclic AMP. These studies suggest that there may be a causal link between PLD-derived phosphatidate and the positive modulation of adenylate cyclase activity. In support of this, the concentration-dependence for bradykinin-stimulated adenylate cyclase activity was identical with that of bradykinin-stimulated phospholipase D activity (EC50 = 5 nM). Bradykinin, but not PMA, was also capable of eliciting the inhibition of cyclic AMP phosphodiesterase activity in TSM cells (EC50 > 100 nM) via an unidentified mechanism. These studies indicate that cross-regulation between the cyclic AMP pathway and phospholipid-derived second messengers in TSM cells does not occur as a consequence of PLC-catalysed PtdIns(4,5)P2 hydrolysis, but may involve, in part, PLD-catalysed phosphatidylcholine hydrolysis.
Full text
PDF


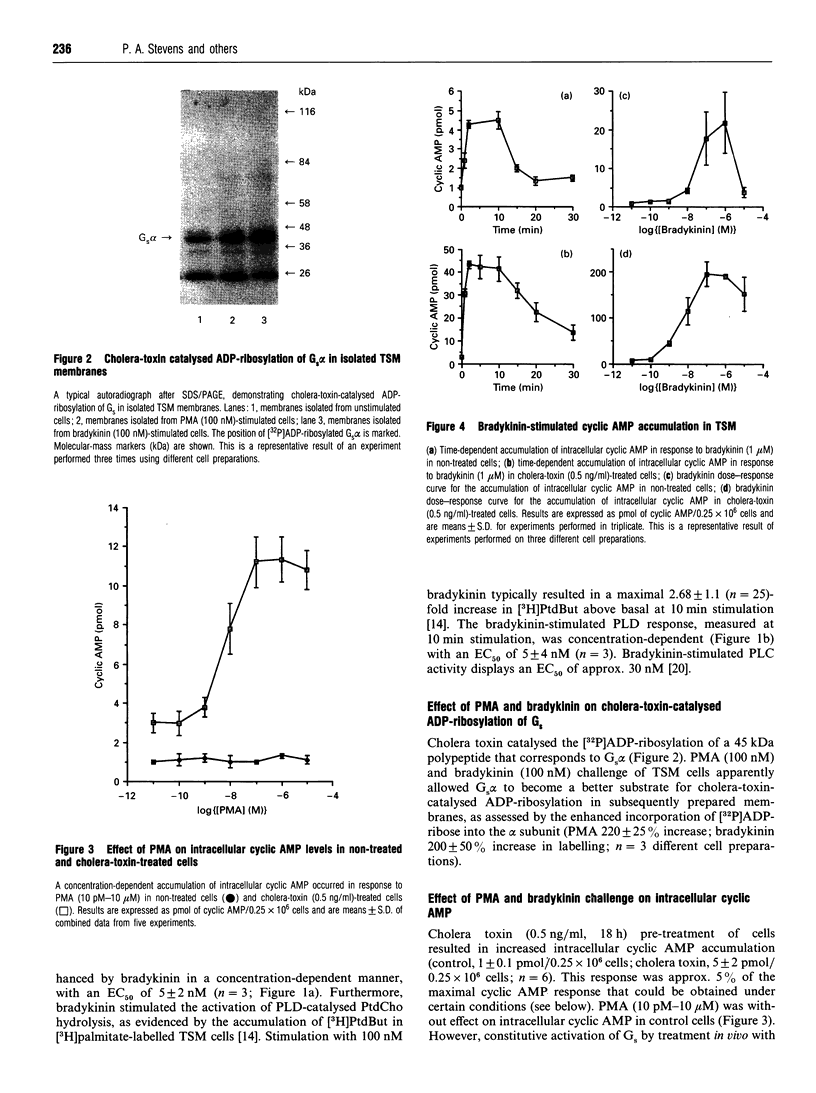



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bocckino S. B., Wilson P. B., Exton J. H. Phosphatidate-dependent protein phosphorylation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6210–6213. doi: 10.1073/pnas.88.14.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushfield M., Griffiths S. L., Murphy G. J., Pyne N. J., Knowler J. T., Milligan G., Parker P. J., Mollner S., Houslay M. D. Diabetes-induced alterations in the expression, functioning and phosphorylation state of the inhibitory guanine nucleotide regulatory protein Gi-2 in hepatocytes. Biochem J. 1990 Oct 15;271(2):365–372. doi: 10.1042/bj2710365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushfield M., Pyne N. J., Houslay M. D. Changes in the phosphorylation state of the inhibitory guanine-nucleotide-binding protein Gi-2 in hepatocytes from lean (Fa/Fa) and obese (fa/fa) Zucker rats. Eur J Biochem. 1990 Sep 11;192(2):537–542. doi: 10.1111/j.1432-1033.1990.tb19258.x. [DOI] [PubMed] [Google Scholar]
- Cronin M. J., Summers S. T., Sortino M. A., Hewlett E. L. Protein kinase C enhances growth hormone releasing factor (1-40)-stimulated cyclic AMP levels in anterior pituitary. Actions of somatostatin and pertussis toxin. J Biol Chem. 1986 Oct 25;261(30):13932–13935. [PubMed] [Google Scholar]
- Dobson P. R., Brown B. L., Michelangeli V. P., Short A. D., Moseley J. M., Russell R. G., Martin T. J. Interactive regulation of signalling pathways in bone cells: possible modulation of PGE2-stimulated adenylyl cyclase activity by protein kinase C. Biochim Biophys Acta. 1990 May 2;1052(2):323–326. doi: 10.1016/0167-4889(90)90228-6. [DOI] [PubMed] [Google Scholar]
- Farmer S. G., Ensor J. E., Burch R. M. Evidence that cultured airway smooth muscle cells contain bradykinin B2 and B3 receptors. Am J Respir Cell Mol Biol. 1991 Mar;4(3):273–277. doi: 10.1165/ajrcmb/4.3.273. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Pitcher J. A., Luttrell D. K., Linder M. E., Kurose H., Parsons S. J., Caron M. G., Lefkowitz R. J. Tyrosine phosphorylation of G protein alpha subunits by pp60c-src. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5720–5724. doi: 10.1073/pnas.89.13.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Whetton A. D., Wong S., Martin B. R., Houslay M. D. Insulin inhibits the cholera-toxin-catalysed ribosylation of a Mr-25000 protein in rat liver plasma membranes. Biochem J. 1985 Jun 15;228(3):593–603. doi: 10.1042/bj2280593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D. 'Crosstalk': a pivotal role for protein kinase C in modulating relationships between signal transduction pathways. Eur J Biochem. 1991 Jan 1;195(1):9–27. doi: 10.1111/j.1432-1033.1991.tb15671.x. [DOI] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Marsh K. A., Hill S. J. Bradykinin B2 receptor-mediated phosphoinositide hydrolysis in bovine cultured tracheal smooth muscle cells. Br J Pharmacol. 1992 Oct;107(2):443–447. doi: 10.1111/j.1476-5381.1992.tb12765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H., Exton J. H. Purification and characterization of the zeta isoform of protein kinase C from bovine kidney. J Biol Chem. 1992 Aug 15;267(23):16347–16354. [PubMed] [Google Scholar]
- O'Brien R. M., Houslay M. D., Milligan G., Siddle K. The insulin receptor tyrosyl kinase phosphorylates holomeric forms of the guanine nucleotide regulatory proteins Gi and Go. FEBS Lett. 1987 Feb 23;212(2):281–288. doi: 10.1016/0014-5793(87)81361-3. [DOI] [PubMed] [Google Scholar]
- Pyne N. J., Freissmuth M., Palmer S. Phosphorylation of the spliced variant forms of the recombinant stimulatory guanine-nucleotide-binding regulatory protein (Gs alpha) by protein kinase C. Biochem J. 1992 Jul 1;285(Pt 1):333–338. doi: 10.1042/bj2850333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne N. J., Freissmuth M., Pyne S. Phosphorylation of the recombinant spliced variants of the alpha-sub-unit of the stimulatory guanine-nucleotide binding regulatory protein (Gs) by the catalytic sub-unit of protein kinase A. Biochem Biophys Res Commun. 1992 Jul 31;186(2):1081–1086. doi: 10.1016/0006-291x(92)90857-h. [DOI] [PubMed] [Google Scholar]
- Pyne N. J., Murphy G. J., Milligan G., Houslay M. D. Treatment of intact hepatocytes with either the phorbol ester TPA or glucagon elicits the phosphorylation and functional inactivation of the inhibitory guanine nucleotide regulatory protein Gi. FEBS Lett. 1989 Jan 16;243(1):77–82. doi: 10.1016/0014-5793(89)81221-9. [DOI] [PubMed] [Google Scholar]
- Pyne S., Pyne N. J. Bradykinin stimulates phospholipase D in primary cultures of guinea-pig tracheal smooth muscle. Biochem Pharmacol. 1993 Feb 9;45(3):593–603. doi: 10.1016/0006-2952(93)90132-g. [DOI] [PubMed] [Google Scholar]
- Pyne S., Pyne N. J. Differential effects of B2 receptor antagonists upon bradykinin-stimulated phospholipase C and D in guinea-pig cultured tracheal smooth muscle. Br J Pharmacol. 1993 Sep;110(1):477–481. doi: 10.1111/j.1476-5381.1993.tb13835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Cyclic AMP-dependent protein kinase from bovine heart muscle. Methods Enzymol. 1974;38:308–315. doi: 10.1016/0076-6879(74)38047-0. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Simmoteit R., Schulzki H. D., Palm D., Mollner S., Pfeuffer T. Chemical and functional analysis of components of adenylyl cyclase from human platelets treated with phorbolesters. FEBS Lett. 1991 Jul 8;285(1):99–103. doi: 10.1016/0014-5793(91)80734-k. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Yoshimasa T., Sibley D. R., Bouvier M., Lefkowitz R. J., Caron M. G. Cross-talk between cellular signalling pathways suggested by phorbol-ester-induced adenylate cyclase phosphorylation. Nature. 1987 May 7;327(6117):67–70. doi: 10.1038/327067a0. [DOI] [PubMed] [Google Scholar]



