Abstract
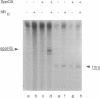
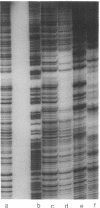
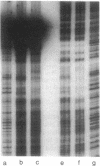
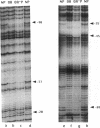
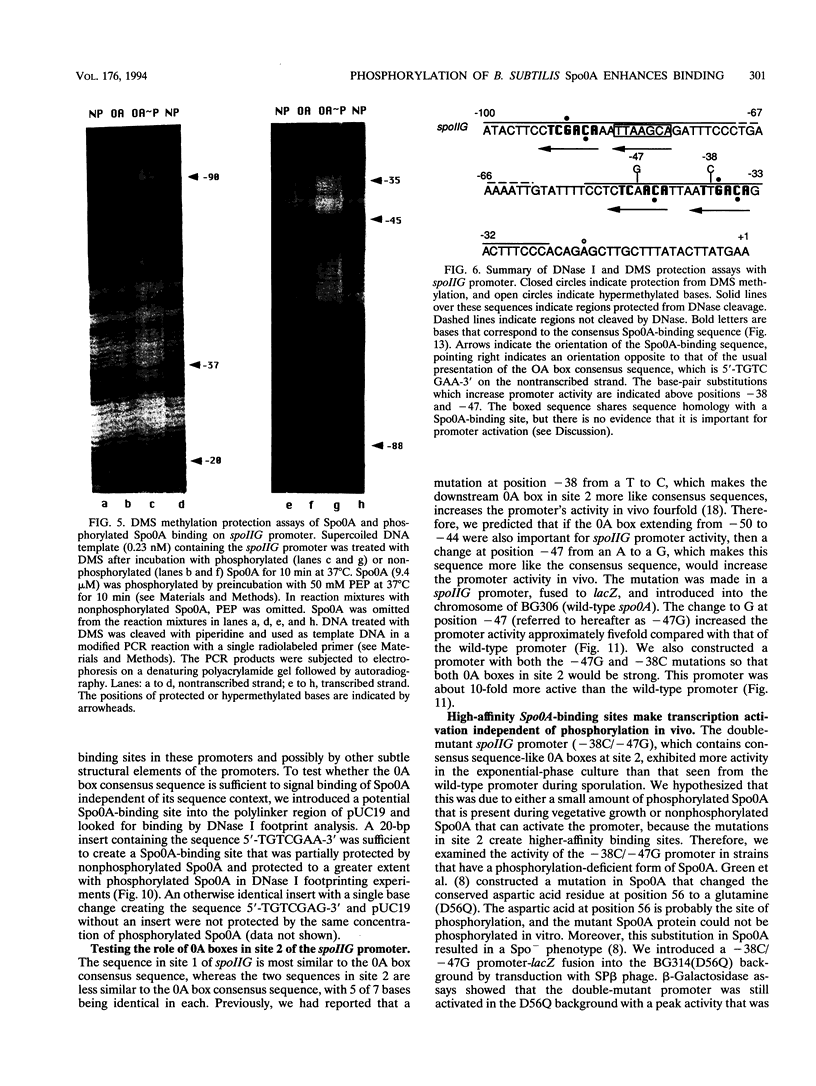
Activation of the spoIIG promoter at the onset of sporulation in Bacillus subtilis requires the regulatory protein, Spo0A, which binds to two sites in the promoter, sites 1 and 2. Phosphorylation of Spo0A is essential for the initiation of sporulation. Therefore, we examined the role of Spo0A phosphorylation in spoIIG promoter activation. Phosphorylation of Spo0A stimulated transcription from the spoIIG promoter in vitro. In DNAse I footprinting experiments with the spoIIG promoter, we found that phosphorylation of Spo0A increased its affinity for site 2 more than for site 1, which is the site to which nonphosphorylated Spo0A binds most avidly. This result could not be explained by increased cooperativity between Spo0A bound at sites 1 and 2 because the increased affinity for site 2 by phosphorylated Spo0A was also observed with a deletion derivative of the spoIIG promoter containing only site 2. We have located Spo0A-binding sequences in the spoIIG promoter by DMS protection assays and mutational analysis, and found that site 1 contains one higher-affinity binding sequence whereas site 2 contains two weaker-binding sites. Two substitutions in site 2 of the spoIIG promoter that change the sequence to be more like an optimal Spo0A-binding site were found to increase promoter activity. Moreover, phosphorylation of Spo0A was not required in vivo for activation of the spoIIG promoter containing these strong binding sites. The results suggest that the primary role for phosphorylation of Spo0A is to increase its affinity for specific sites rather than to activate an activity of Spo0A that acts on RNA polymerase at promoters.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird T. H., Grimsley J. K., Hoch J. A., Spiegelman G. B. Phosphorylation of Spo0A activates its stimulation of in vitro transcription from the Bacillus subtilis spoIIG operon. Mol Microbiol. 1993 Aug;9(4):741–749. doi: 10.1111/j.1365-2958.1993.tb01734.x. [DOI] [PubMed] [Google Scholar]
- Burbulys D., Trach K. A., Hoch J. A. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991 Feb 8;64(3):545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- Carter H. L., 3rd, Moran C. P., Jr New RNA polymerase sigma factor under spo0 control in Bacillus subtilis. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9438–9442. doi: 10.1073/pnas.83.24.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowing D. W., Mecsas J., Record M. T., Jr, Gross C. A. Intermediates in the formation of the open complex by RNA polymerase holoenzyme containing the sigma factor sigma 32 at the groE promoter. J Mol Biol. 1989 Dec 5;210(3):521–530. doi: 10.1016/0022-2836(89)90128-9. [DOI] [PubMed] [Google Scholar]
- Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993 Mar;57(1):1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F. A., Nguyen A., Lang D., Hoch J. A. Construction and properties of an integrable plasmid for Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1513–1515. doi: 10.1128/jb.154.3.1513-1515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. D., Olmedo G., Youngman P. A genetic analysis of Spo0A structure and function. Res Microbiol. 1991 Sep-Oct;142(7-8):825–830. doi: 10.1016/0923-2508(91)90061-e. [DOI] [PubMed] [Google Scholar]
- Inoue T., Cech T. R. Secondary structure of the circular form of the Tetrahymena rRNA intervening sequence: a technique for RNA structure analysis using chemical probes and reverse transcriptase. Proc Natl Acad Sci U S A. 1985 Feb;82(3):648–652. doi: 10.1073/pnas.82.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K., Rudner D. Z., Siranosian K. J., Grossman A. D. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev. 1993 Feb;7(2):283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- Kenney T. J., Kirchman P. A., Moran C. P., Jr Gene encoding sigma E is transcribed from a sigma A-like promoter in Bacillus subtilis. J Bacteriol. 1988 Jul;170(7):3058–3064. doi: 10.1128/jb.170.7.3058-3064.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney T. J., Moran C. P., Jr Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3329–3339. doi: 10.1128/jb.169.7.3329-3339.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleary W. R., Stock J. B., Ninfa A. J. Is acetyl phosphate a global signal in Escherichia coli? J Bacteriol. 1993 May;175(10):2793–2798. doi: 10.1128/jb.175.10.2793-2798.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo G., Ninfa E. G., Stock J., Youngman P. Novel mutations that alter the regulation of sporulation in Bacillus subtilis. Evidence that phosphorylation of regulatory protein SpoOA controls the initiation of sporulation. J Mol Biol. 1990 Oct 5;215(3):359–372. doi: 10.1016/s0022-2836(05)80357-2. [DOI] [PubMed] [Google Scholar]
- Popham D. L., Szeto D., Keener J., Kustu S. Function of a bacterial activator protein that binds to transcriptional enhancers. Science. 1989 Feb 3;243(4891):629–635. doi: 10.1126/science.2563595. [DOI] [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. Footprinting protein-DNA complexes in vivo. Methods Enzymol. 1991;208:146–168. doi: 10.1016/0076-6879(91)08012-7. [DOI] [PubMed] [Google Scholar]
- Satola S. W., Baldus J. M., Moran C. P., Jr Binding of Spo0A stimulates spoIIG promoter activity in Bacillus subtilis. J Bacteriol. 1992 Mar;174(5):1448–1453. doi: 10.1128/jb.174.5.1448-1453.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satola S., Kirchman P. A., Moran C. P., Jr Spo0A binds to a promoter used by sigma A RNA polymerase during sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4533–4537. doi: 10.1073/pnas.88.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schickor P., Metzger W., Werel W., Lederer H., Heumann H. Topography of intermediates in transcription initiation of E.coli. EMBO J. 1990 Jul;9(7):2215–2220. doi: 10.1002/j.1460-2075.1990.tb07391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch M. A., Trach K. A., Day J., Hoch J. A. Spo0A activates and represses its own synthesis by binding at its dual promoters. Biochimie. 1992 Jul-Aug;74(7-8):619–626. doi: 10.1016/0300-9084(92)90133-y. [DOI] [PubMed] [Google Scholar]
- Strauch M. A., Wu J. J., Jonas R. H., Hoch J. A. A positive feedback loop controls transcription of the spoOF gene, a component of the sporulation phosphorelay in Bacillus subtilis. Mol Microbiol. 1993 Mar;7(6):967–974. doi: 10.1111/j.1365-2958.1993.tb01188.x. [DOI] [PubMed] [Google Scholar]
- Strauch M., Webb V., Spiegelman G., Hoch J. A. The SpoOA protein of Bacillus subtilis is a repressor of the abrB gene. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1801–1805. doi: 10.1073/pnas.87.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trach K., Burbulys D., Strauch M., Wu J. J., Dhillon N., Jonas R., Hanstein C., Kallio P., Perego M., Bird T. Control of the initiation of sporulation in Bacillus subtilis by a phosphorelay. Res Microbiol. 1991 Sep-Oct;142(7-8):815–823. doi: 10.1016/0923-2508(91)90060-n. [DOI] [PubMed] [Google Scholar]
- Wu J. J., Howard M. G., Piggot P. J. Regulation of transcription of the Bacillus subtilis spoIIA locus. J Bacteriol. 1989 Feb;171(2):692–698. doi: 10.1128/jb.171.2.692-698.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S., Yoshikawa H., Kawamura F., Takahashi H., Yamamoto T., Kobayashi Y., Saito H. The effect of spo0 mutations on the expression of spo0A- and spo0F-lacZ fusions. Mol Gen Genet. 1986 Oct;205(1):28–33. doi: 10.1007/BF02428029. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- York K., Kenney T. J., Satola S., Moran C. P., Jr, Poth H., Youngman P. Spo0A controls the sigma A-dependent activation of Bacillus subtilis sporulation-specific transcription unit spoIIE. J Bacteriol. 1992 Apr;174(8):2648–2658. doi: 10.1128/jb.174.8.2648-2658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]