Abstract
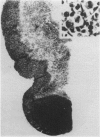
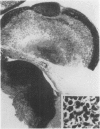
The deuterated benzaldehyde derivative zilascorb(2H), 5,6-O-benzylidene-d-L-ascorbic acid, was administered once daily by i.v. injection in nude mice with grafted tumours of a human malignant melanoma (E.E.) and ovarian carcinoma (OVCAR-3) origins. Like benzaldehyde, zilascorb(2H) has been shown to induce protein synthesis inhibition at otherwise non-toxic doses in cells grown in vitro, and acts reversibly in the sense that protein synthesis returns to normal shortly after removal of the drug. The present data indicate that daily injections with zilascorb(2H) induce a tumour volume growth inhibitory effect in both tumour xenografts studied. Furthermore, from histological examinations of each single tumour it was found that tumours of drug-treated animals, although smaller than those of placebo-treated (i.e. control) animals, had, on average, a higher necrotic fraction than control tumours. Thus, it is concluded that zilascorb(2H) induces tumour necrotisation and not just inhibition of the rate of tumour cell production. Continued measurement of tumour volume after ended treatment with zilascorb(2H) indicated that surviving tumour cells resumed their normal growth rate immediately. The reversibility of the effect induced by this compound, earlier observed in vitro only, is therefore here confirmed to be valid also in two different tumour xenografts in vivo. The present data accords well with the assumption that protein synthesis inhibition is the primary cellular effect of zilascorb(2H) in vivo. We therefore conclude that zilascorb(2H)-induced cancer cell lethality in tumour xenografts probably comes as a secondary consequence of prolonged protein synthesis inhibition.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balázová E., Koza I. Therapy of P388 leukemia with benzaldehyde. Neoplasma. 1988;35(6):725–728. [PubMed] [Google Scholar]
- Boyland E. Experiments on the chemotherapy of cancer: Further experiments with aldehydes and their derivatives. Biochem J. 1940 Sep;34(8-9):1196–1201. doi: 10.1042/bj0341196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett T. H., Valeriote F. A., Baker L. H. Is the P388 murine tumor no longer adequate as a drug discovery model? Invest New Drugs. 1987;5(1):3–20. doi: 10.1007/BF00217664. [DOI] [PubMed] [Google Scholar]
- Dornish J. M., Larsen R. O., Schwarze P. E., Børretzen B., Pettersen E. O. In vivo and in vitro pharmacokinetics of 4,6-benzylidene-D-glucose (BG) in rats. Invest New Drugs. 1990 May;8(2):149–157. doi: 10.1007/BF00177250. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Young R. C., McKoy W. M., Grotzinger K. R., Green J. A., Chu E. W., Whang-Peng J., Rogan A. M., Green W. R., Ozols R. F. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983 Nov;43(11):5379–5389. [PubMed] [Google Scholar]
- Kochi M., Isono N., Niwayama M., Shirakabe K. Antitumor activity of a benzaldehyde derivative. Cancer Treat Rep. 1985 May;69(5):533–537. [PubMed] [Google Scholar]
- Kochi M., Takeuchi S., Mizutani T., Mochizuki K., Matsumoto Y., Saito Y. Antitumor activity of benzaldehyde. Cancer Treat Rep. 1980 Jan;64(1):21–23. [PubMed] [Google Scholar]
- Osato S. Chemotherapy of human carcinoma with citronellal and citral and their action on carcinoma tissue in its histological aspects up to healing. Tohoku J Exp Med. 1965 Jul 25;86(2):102–147. doi: 10.1620/tjem.86.102. [DOI] [PubMed] [Google Scholar]
- Pettersen E. O., Dornish J. M., Rønning O. W. 4,6-Benzylidene-D-glucose, a benzaldehyde derivative that inhibits protein synthesis but not mitosis of NHIK 3025 cells. Cancer Res. 1985 May;45(5):2085–2091. [PubMed] [Google Scholar]
- Pettersen E. O., Larsen R. O., Boerretzen B., Dornish J. M., Oftebro R. Effect on protein synthesis and cell survival of the benzaldehyde derivatives sodium benzylidene ascorbate (SBA) and the deuterated compound zilascorb(2H). Anticancer Res. 1991 May-Jun;11(3):1077–1081. [PubMed] [Google Scholar]
- Pettersen E. O., Larsen R. O., Børretzen B., Dornish J. M., Oftebro R. Increased effect of benzaldehyde by exchanging the hydrogen in the formyl group with deuterium. Anticancer Res. 1991 Jan-Feb;11(1):369–373. [PubMed] [Google Scholar]
- Pettersen E. O., Nome O., Rønning O. W., Oftebro R. Effects of benzaldehyde on survival and cell-cycle kinetics of human cells cultivated in vitro. Eur J Cancer Clin Oncol. 1983 Apr;19(4):507–514. doi: 10.1016/0277-5379(83)90114-1. [DOI] [PubMed] [Google Scholar]
- Pettersen E. O., Schwarze P. E., Dornish J. M., Nesland J. M. Antitumour effect of benzylidene-glucose (BG) in rats with chemically induced hepatocellular carcinoma. Anticancer Res. 1986 Mar-Apr;6(2):147–152. [PubMed] [Google Scholar]
- Rofstad E. K., Brustad T., Johannessen J. V., Mossige J. Effect of cobalt-60 gamma rays and DTIC (5-(3,3 dimethyl-1-triazeno)-imidazole-r-carboxamide) on human malignant melanomas grown in athymic nude mice. Br J Radiol. 1977 May;50(593):314–320. doi: 10.1259/0007-1285-50-593-314. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K. Growth and vascular structure of human melanoma xenografts. Cell Tissue Kinet. 1984 Jan;17(1):91–101. doi: 10.1111/j.1365-2184.1984.tb00571.x. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K., Lindmo T., Brustad T. Effect of single dose irradiation on the proliferation kinetics in a human malignant melanoma in athymic nude mice. Acta Radiol Oncol. 1980;19(4):261–269. doi: 10.3109/02841868009130163. [DOI] [PubMed] [Google Scholar]
- Rønning O. W., Lindmo T., Pettersen E. O., Seglen P. O. The role of protein accumulation in the cell cycle control of human NHIK 3025 cells. J Cell Physiol. 1981 Dec;109(3):411–418. doi: 10.1002/jcp.1041090306. [DOI] [PubMed] [Google Scholar]
- Rønning O. W., Lindmo T. Progress through G1 and S in relation to net protein accumulation in human NHIK 3025 cells. Exp Cell Res. 1983 Mar;144(1):171–179. doi: 10.1016/0014-4827(83)90451-2. [DOI] [PubMed] [Google Scholar]
- Rønning O. W., Pettersen E. O. Doubling of cell mass is not necessary in order to achieve cell division in cultured human cells. Exp Cell Res. 1984 Nov;155(1):267–272. doi: 10.1016/0014-4827(84)90788-2. [DOI] [PubMed] [Google Scholar]
- Sakagami H., Asano K., Fukuchi K., Gomi K., Ota H., Kazama K., Tanuma S., Kochi M. Induction of tumor degeneration by sodium benzylideneascorbate. Anticancer Res. 1991 Jul-Aug;11(4):1533–1538. [PubMed] [Google Scholar]
- Taetle R., Howell S. B. Preclinical re-evaluation of benzaldehyde as a chemotherapeutic agent. Cancer Treat Rep. 1983 Jun;67(6):561–566. [PubMed] [Google Scholar]
- Tanum G., Tveit K. M., Høst H., Pettersen E. O. Benzylidene-glucose: no effect after all? Am J Clin Oncol. 1990 Apr;13(2):161–163. [PubMed] [Google Scholar]
- Tatsumura T., Tsujimoto M., Koyama S., Furuno T., Komori Y., Sato H., Yamamoto K., Kitagawa M., Kagamimori S. 4, 6-0-benzylidene-D-glucopyranose (BG) in the treatment of solid malignant tumours, an extended phase I study. Br J Cancer. 1990 Sep;62(3):436–439. doi: 10.1038/bjc.1990.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington R. C. A novel approach for improving the efficacy of experimental cancer chemotherapy using combinations of anticancer drugs and L-histidinol. Anticancer Res. 1986 May-Jun;6(3 Pt B):451–464. [PubMed] [Google Scholar]