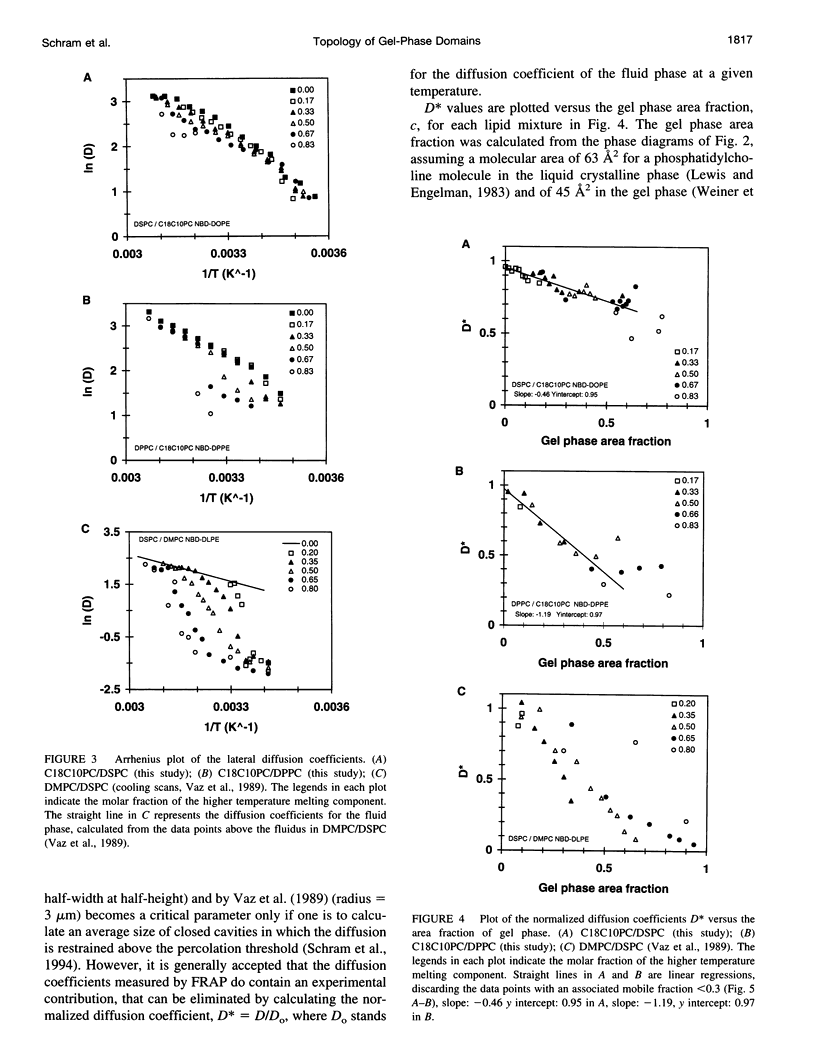
Abstract
The influence of the lipid mixing properties on the lateral organization in a two-component, two-phase phosphatidylcholine bilayer was investigated using both an experimental (fluorescence recovery after photobleaching (FRAP)) and a simulated (Monte Carlo) approach. With the FRAP technique, we have examined binary mixtures of 1-stearoyl-2-capryl-phosphatidylcholine/1,2-distearoyl-phosphat idylcholine (C18C10PC/DSPC), and 1-stearoyl-2-capryl-phosphatidylcholine/1,2-dipalmitoyl-phospha tid ylcholine (C18C10PC/DPPC). Comparison with the 1,2-dimyristoyl-phosphatidylcholine/1,2-distearoyl-phosphatidylcholine (DMPC/DSPC) previously investigated by FRAP by Vaz and co-workers (Biophys. J., 1989, 56:869-876) shows that the gel phase domains become more effective in restricting the diffusion coefficient when the ideality of the mixture increases (i.e., in the order C18C10PC/DSPC-->C18C10PC/DPPC-->DMPC/DSPC). However, an increased lipid miscibility is accompanied by an increasing compositional dependence: the higher the proportion of the high-temperature melting component, the less efficient the gel phase is in compartmentalizing the diffusion plane, a trend that is best accounted for by a variation of the gel phase domain shape rather than size. Computer-simulated fluorescence recoveries obtained in a matrix obstructed with obstacle aggregates of various fractal dimension demonstrate that: 1) for a given obstacle size and area fraction, the relative diffusion coefficient increases linearly with the obstacle fractal dimension and 2) aggregates with a lower fractal dimension are more efficient in compartmentalizing the diffusion plane. Comparison of the simulated with the experimental mobile fractions strongly suggests that the fractal dimension of the gel phase domains increases with the proportion of high-temperature melting component in DMPC/DSPC and (slightly) in C18C10PC/DPPC.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney J. R., Scalettar B. A., Owicki J. C. Self diffusion of interacting membrane proteins. Biophys J. 1989 May;55(5):817–833. doi: 10.1016/S0006-3495(89)82882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida P. F., Vaz W. L., Thompson T. E. Lateral diffusion and percolation in two-phase, two-component lipid bilayers. Topology of the solid-phase domains in-plane and across the lipid bilayer. Biochemistry. 1992 Aug 11;31(31):7198–7210. doi: 10.1021/bi00146a024. [DOI] [PubMed] [Google Scholar]
- Bultmann T., Vaz W. L., Melo E. C., Sisk R. B., Thompson T. E. Fluid-phase connectivity and translational diffusion in a eutectic, two-component, two-phase phosphatidylcholine bilayer. Biochemistry. 1991 Jun 4;30(22):5573–5579. doi: 10.1021/bi00236a033. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Flores J., Petersen W. P. A milling crowd model for local and long-range obstructed lateral diffusion. Mobility of excimeric probes in the membrane of intact erythrocytes. Biophys J. 1986 May;49(5):987–1001. doi: 10.1016/S0006-3495(86)83727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flörsheimer M., Möhwald H. Development of equilibrium domain shapes in phospholipid monolayers. Chem Phys Lipids. 1989 Mar;49(4):231–241. doi: 10.1016/0009-3084(89)90071-6. [DOI] [PubMed] [Google Scholar]
- Halladay H. N., Stark R. E., Ali S., Bittman R. Magic-angle spinning NMR studies of molecular organization in multibilayers formed by 1-octadecanoyl-2-decanoyl-sn-glycero-3-phosphocholine. Biophys J. 1990 Dec;58(6):1449–1461. doi: 10.1016/S0006-3495(90)82490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S. W., Mason J. T., Huang C. Acyl chain interdigitation in saturated mixed-chain phosphatidylcholine bilayer dispersions. Biochemistry. 1984 Nov 6;23(23):5570–5577. doi: 10.1021/bi00318a029. [DOI] [PubMed] [Google Scholar]
- Jørgensen K., Mouritsen O. G. Phase separation dynamics and lateral organization of two-component lipid membranes. Biophys J. 1995 Sep;69(3):942–954. doi: 10.1016/S0006-3495(95)79968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen K., Sperotto M. M., Mouritsen O. G., Ipsen J. H., Zuckermann M. J. Phase equilibria and local structure in binary lipid bilayers. Biochim Biophys Acta. 1993 Oct 10;1152(1):135–145. doi: 10.1016/0005-2736(93)90240-z. [DOI] [PubMed] [Google Scholar]
- Knoll W., Ibel K., Sackmann E. Small-angle neutron scattering study of lipid phase diagrams by the contrast variation method. Biochemistry. 1981 Oct 27;20(22):6379–6383. doi: 10.1021/bi00525a015. [DOI] [PubMed] [Google Scholar]
- Koppel D. E. Fluorescence redistribution after photobleaching. A new multipoint analysis of membrane translational dynamics. Biophys J. 1979 Nov;28(2):281–291. doi: 10.1016/S0006-3495(79)85176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983 May 15;166(2):211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- Lopez A., Dupou L., Altibelli A., Trotard J., Tocanne J. F. Fluorescence recovery after photobleaching (FRAP) experiments under conditions of uniform disk illumination. Critical comparison of analytical solutions, and a new mathematical method for calculation of diffusion coefficient D. Biophys J. 1988 Jun;53(6):963–970. doi: 10.1016/S0006-3495(88)83177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. T. Mixing behavior of symmetric chain length and mixed chain length phosphatidylcholines in two-component multilamellar bilayers: evidence for gel and liquid-crystalline phase immiscibility. Biochemistry. 1988 Jun 14;27(12):4421–4429. doi: 10.1021/bi00412a032. [DOI] [PubMed] [Google Scholar]
- Mason J. T. Properties of phosphatidylcholine bilayers as revealed by mixed-acyl phospholipid fluorescent probes containing n-(9-anthroyloxy) fatty acids. Biochim Biophys Acta. 1994 Aug 24;1194(1):99–108. doi: 10.1016/0005-2736(94)90207-0. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A., Ellington J. C., Jr, Porter N. A. New structural model for mixed-chain phosphatidylcholine bilayers. Biochemistry. 1984 Aug 28;23(18):4038–4044. doi: 10.1021/bi00313a005. [DOI] [PubMed] [Google Scholar]
- Piknová B., Marsh D., Thompson T. E. Fluorescence-quenching study of percolation and compartmentalization in two-phase lipid bilayers. Biophys J. 1996 Aug;71(2):892–897. doi: 10.1016/S0006-3495(96)79291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram M. B., Marsh D., Thompson T. E. Determination of fluid and gel domain sizes in two-component, two-phase lipid bilayers. An electron spin resonance spin label study. Biophys J. 1992 Aug;63(2):340–349. doi: 10.1016/S0006-3495(92)81619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion and aggregation. A Monte Carlo study. Biophys J. 1992 Jan;61(1):119–128. doi: 10.1016/S0006-3495(92)81821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Distance dependence of the diffusion coefficient. Biophys J. 1989 Sep;56(3):615–622. doi: 10.1016/S0006-3495(89)82708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram V., Thompson T. E. Interdigitation does not affect translational diffusion of lipids in liquid crystalline bilayers. Biophys J. 1995 Dec;69(6):2517–2520. doi: 10.1016/S0006-3495(95)80122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram V., Tocanne J. F., Lopez A. Influence of obstacles on lipid lateral diffusion: computer simulation of FRAP experiments and application to proteoliposomes and biomembranes. Eur Biophys J. 1994;23(5):337–348. doi: 10.1007/BF00188657. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Slater J. L., Huang C. H. Interdigitated bilayer membranes. Prog Lipid Res. 1988;27(4):325–359. doi: 10.1016/0163-7827(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Clegg R. M., Hallmann D. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 1985 Jan 29;24(3):781–786. doi: 10.1021/bi00324a037. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Melo E. C., Thompson T. E. Fluid phase connectivity in an isomorphous, two-component, two-phase phosphatidylcholine bilayer. Biophys J. 1990 Jul;58(1):273–275. doi: 10.1016/S0006-3495(90)82373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Melo E. C., Thompson T. E. Translational diffusion and fluid domain connectivity in a two-component, two-phase phospholipid bilayer. Biophys J. 1989 Nov;56(5):869–876. doi: 10.1016/S0006-3495(89)82733-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M. C., Suter R. M., Nagle J. F. Structure of the fully hydrated gel phase of dipalmitoylphosphatidylcholine. Biophys J. 1989 Feb;55(2):315–325. doi: 10.1016/S0006-3495(89)82807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. E., Maynard V. M., McKinnon C. A., Melchior D. L. Lipid domains in the ram sperm plasma membrane demonstrated by differential scanning calorimetry. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6893–6896. doi: 10.1073/pnas.87.17.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dreele P. H. Estimation of lateral species separation from phase transitions in nonideal two-dimensional lipid mixtures. Biochemistry. 1978 Sep 19;17(19):3939–3943. doi: 10.1021/bi00612a009. [DOI] [PubMed] [Google Scholar]


