Abstract
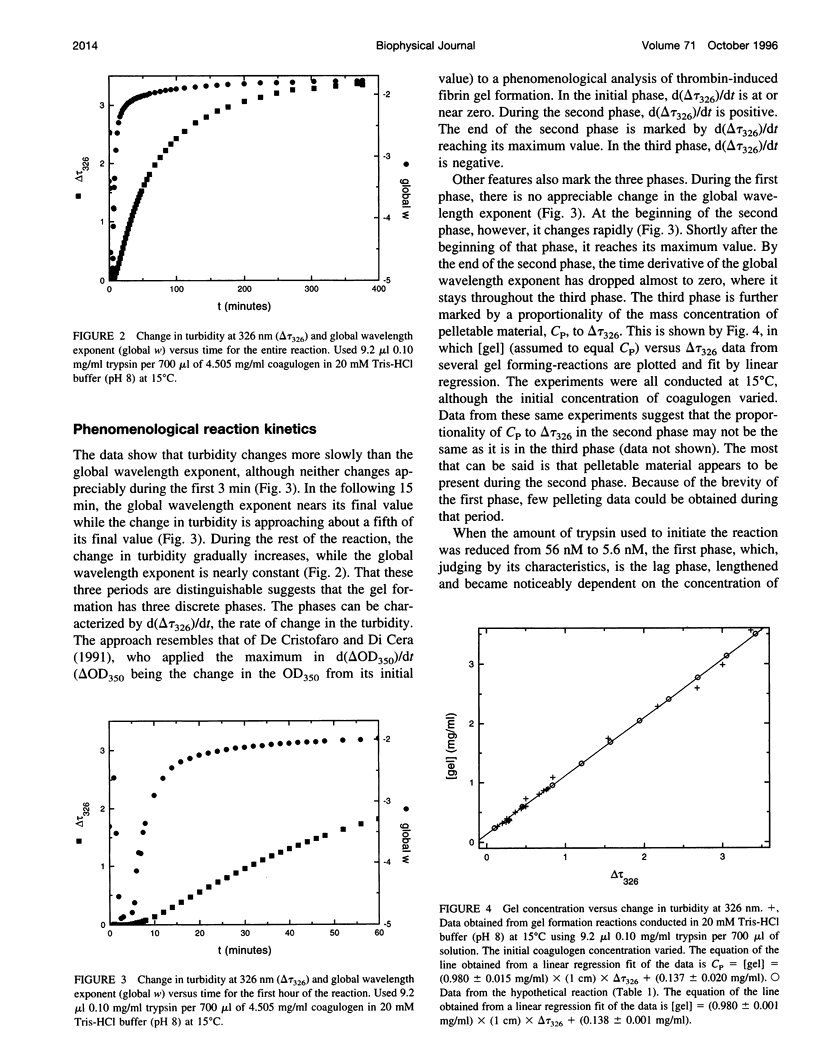
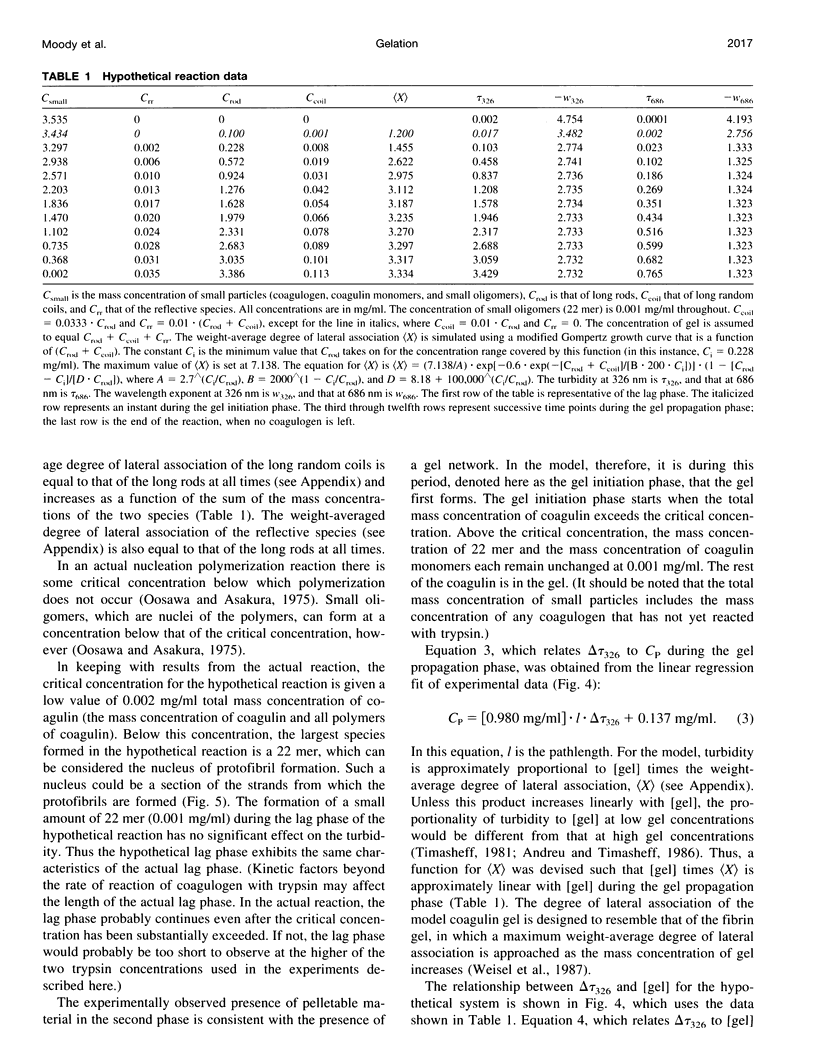
The turbidity during trypsin-induced coagulin gel formation was studied over a range of wavelengths. The range of wavelengths used (686-326 nm) also made it possible to investigate the dependence of turbidity on wavelength (the wavelength exponent). Using the results from that work, and structural information on coagulin and the coagulin gel from other studies, a model gel-forming system was designed that consists of species for which the turbidity can be calculated relatively simply. These species include small particles (small in all dimensions relative to the wavelength of incident light); long rods and long random coils (particles that are large in just one dimension relative to the wavelength of incident light); and reflective regions (aggregated material that is large in more than one dimension relative to the wavelength of incident light). The turbidimetric characteristics of the real coagulin gel-forming system are compared with those of the model system.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu J. M., Timasheff S. N. The measurement of cooperative protein self-assembly by turbidity and other techniques. Methods Enzymol. 1986;130:47–59. doi: 10.1016/0076-6879(86)30007-7. [DOI] [PubMed] [Google Scholar]
- BANG F. B. A bacterial disease of Limulus polyphemus. Bull Johns Hopkins Hosp. 1956 May;98(5):325–351. [PubMed] [Google Scholar]
- Berkowitz S. A., Day L. A. Turbidity measurements in an analytical ultracentrifuge. Determinations of mass per length for filamentous viruses fd, Xf, and Pf3. Biochemistry. 1980 Jun 10;19(12):2696–2702. doi: 10.1021/bi00553a025. [DOI] [PubMed] [Google Scholar]
- CASASSA E. F., EISENBERG H. THERMODYNAMIC ANALYSIS OF MULTICOMPONENT SOLUTIONS. Adv Protein Chem. 1964;19:287–395. doi: 10.1016/s0065-3233(08)60191-6. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Franklin R. M., Day L. A. Molecular weights, dispersion of refractive index increments, and dimensions from transmittance spectrophotometry. Bacteriophages R17, T7, and PM2, and tobacco mosaic virus. Biochemistry. 1974 Aug 27;13(18):3763–3773. doi: 10.1021/bi00715a023. [DOI] [PubMed] [Google Scholar]
- Cheng S. M., Suzuki A., Zon G., Liu T. Y. Characterization of a complementary deoxyribonucleic acid for the coagulogen of Limulus polyphemus. Biochim Biophys Acta. 1986 Oct 16;868(1):1–8. doi: 10.1016/0167-4781(86)90079-5. [DOI] [PubMed] [Google Scholar]
- Chou R. G., Stromer M. H., Robson R. M., Huiatt T. W. Determination of the critical concentration required for desmin assembly. Biochem J. 1990 Nov 15;272(1):139–145. doi: 10.1042/bj2720139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cristofaro R., Di Cera E. Phenomenological analysis of the clotting curve. J Protein Chem. 1991 Oct;10(5):455–468. doi: 10.1007/BF01025473. [DOI] [PubMed] [Google Scholar]
- Donovan M. A., Laue T. M. A novel trypsin inhibitor from the hemolymph of the horseshoe crab Limulus polyphemus. J Biol Chem. 1991 Feb 5;266(4):2121–2125. [PubMed] [Google Scholar]
- GEIDUSCHEK E. P., HOLTZER A. Application of light scattering to biological systems: deoxyribonucleic acid and the muscle proteins. Adv Biol Med Phys. 1958;6:431–551. doi: 10.1016/b978-1-4832-3112-9.50014-1. [DOI] [PubMed] [Google Scholar]
- Gaffin S. L. The clotting of the lysed white cells of Limulus induced by endotoxin--I: Preparation and characterization of clot-forming proteins. Biorheology. 1976 Nov;13(5):273–280. doi: 10.3233/bir-1976-13501. [DOI] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Hantgan R. R., Hermans J. Assembly of fibrin. A light scattering study. J Biol Chem. 1979 Nov 25;254(22):11272–11281. [PubMed] [Google Scholar]
- Hantgan R., McDonagh J., Hermans J. Fibrin assembly. Ann N Y Acad Sci. 1983 Jun 27;408:344–366. doi: 10.1111/j.1749-6632.1983.tb23256.x. [DOI] [PubMed] [Google Scholar]
- Holme R., Solum N. O. Electron microscopy of the gel protein formed by clotting of Limulus polyphemus hemocyte extracts. J Ultrastruct Res. 1973 Sep;44(5):329–338. doi: 10.1016/s0022-5320(73)90001-4. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Borisy G. G. Thermodynamic analysis of microtubule self-assembly in vitro. J Mol Biol. 1979 Sep 15;133(2):199–216. doi: 10.1016/0022-2836(79)90530-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Seid R. C., Jr, Tai J. Y., Liang S. M., Sakmar T. P., Robbins J. B. Studies on Limulus lysate coagulating system. Prog Clin Biol Res. 1979;29:147–158. [PubMed] [Google Scholar]
- Miyata T., Hiranaga M., Umezu M., Iwanaga S. Amino acid sequence of the coagulogen from Limulus polyphemus hemocytes. J Biol Chem. 1984 Jul 25;259(14):8924–8933. [PubMed] [Google Scholar]
- Nakamura S., Iwanaga S., Harada T., Niwa M. A clottable protein (coagulogen) from amoebocyte lysate of Japanese horseshoe crab (Tachypleus tridentatus). Its isolation and biochemical properties. J Biochem. 1976 Nov;80(5):1011–1021. doi: 10.1093/oxfordjournals.jbchem.a131357. [DOI] [PubMed] [Google Scholar]
- Shishikura F., Nakamura S., Takahashi K., Sekiguchi K. Coagulogens from four living species of horseshoe crabs (Limulidae): comparison of their biochemical and immunochemical properties. J Biochem. 1983 Oct;94(4):1279–1287. doi: 10.1093/oxfordjournals.jbchem.a134473. [DOI] [PubMed] [Google Scholar]
- Solum N. O. Some characteristics of the clottable protein of limulus polyphemus blood cells. Thromb Diath Haemorrh. 1970 Feb 28;23(1):170–181. [PubMed] [Google Scholar]
- Tai J. Y., Seid R. C., Jr, Huhn R. D., Liu T. Y. Studies on Limulus amoebocyte lysate II. Purification of the coagulogen and the mechanism of clotting. J Biol Chem. 1977 Jul 25;252(14):4773–4776. [PubMed] [Google Scholar]
- Takagi T., Hokama Y., Morita T., Iwanaga S., Nakamura S., Niwa M. Amino acid sequence studies on horseshoe crab (Tachypleus tridentatus) coagulogen and the mechanism of gel formation. Prog Clin Biol Res. 1979;29:169–184. [PubMed] [Google Scholar]
- Weisel J. W., Nagaswami C., Makowski L. Twisting of fibrin fibers limits their radial growth. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8991–8995. doi: 10.1073/pnas.84.24.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]


