Abstract
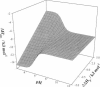
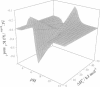
A theoretical development in the evaluation of proton linkage in protein binding reactions by isothermal titration calorimetry (ITC) is presented. For a system in which binding is linked to protonation of an ionizable group on a protein, we show that by performing experiments as a function of pH in buffers with varying ionization enthalpy, one can determine the pK(a)'s of the group responsible for the proton linkage in the free and the liganded states, the protonation enthalpy for this group in these states, as well as the intrinsic energetics for ligand binding (delta H(o), delta S(o), and delta C(p)). Determination of intrinsic energetics in this fashion allows for comparison with energetics calculated empirically from structural information. It is shown that in addition to variation of the ligand binding constant with pH, the observed binding enthalpy and heat capacity change can undergo extreme deviations from their intrinsic values, depending upon pH and buffer conditions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. L. Temperature dependence of the hydrophobic interaction in protein folding. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8069–8072. doi: 10.1073/pnas.83.21.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat T. N., Bentley G. A., Boulot G., Greene M. I., Tello D., Dall'Acqua W., Souchon H., Schwarz F. P., Mariuzza R. A., Poljak R. J. Bound water molecules and conformational stabilization help mediate an antigen-antibody association. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1089–1093. doi: 10.1073/pnas.91.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly P. R., Aldape R. A., Bruzzese F. J., Chambers S. P., Fitzgibbon M. J., Fleming M. A., Itoh S., Livingston D. J., Navia M. A., Thomson J. A. Enthalpy of hydrogen bond formation in a protein-ligand binding reaction. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1964–1968. doi: 10.1073/pnas.91.5.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly P. R., Thomson J. A. Heat capacity changes and hydrophobic interactions in the binding of FK506 and rapamycin to the FK506 binding protein. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4781–4785. doi: 10.1073/pnas.89.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly P. R., Varadarajan R., Sturtevant J. M., Richards F. M. Thermodynamics of protein-peptide interactions in the ribonuclease S system studied by titration calorimetry. Biochemistry. 1990 Jun 26;29(25):6108–6114. doi: 10.1021/bi00477a031. [DOI] [PubMed] [Google Scholar]
- Dill K. A. The meaning of hydrophobicity. Science. 1990 Oct 12;250(4978):297–298. doi: 10.1126/science.2218535. [DOI] [PubMed] [Google Scholar]
- Doyle M. L., Louie G., Dal Monte P. R., Sokoloski T. D. Tight binding affinities determined from thermodynamic linkage to protons by titration calorimetry. Methods Enzymol. 1995;259:183–194. doi: 10.1016/0076-6879(95)59044-7. [DOI] [PubMed] [Google Scholar]
- Eftink M. R., Anusiem A. C., Biltonen R. L. Enthalpy-entropy compensation and heat capacity changes for protein-ligand interactions: general thermodynamic models and data for the binding of nucleotides to ribonuclease A. Biochemistry. 1983 Aug 2;22(16):3884–3896. doi: 10.1021/bi00285a025. [DOI] [PubMed] [Google Scholar]
- Gill S. J., Wadsö I. An equation of state describing hydrophobic interactions. Proc Natl Acad Sci U S A. 1976 Sep;73(9):2955–2958. doi: 10.1073/pnas.73.9.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez J., Freire E. Thermodynamic mapping of the inhibitor site of the aspartic protease endothiapepsin. J Mol Biol. 1995 Sep 22;252(3):337–350. doi: 10.1006/jmbi.1995.0501. [DOI] [PubMed] [Google Scholar]
- Kresheck G. C., Vitello L. B., Erman J. E. Calorimetric studies on the interaction of horse ferricytochrome c and yeast cytochrome c peroxidase. Biochemistry. 1995 Jul 4;34(26):8398–8405. doi: 10.1021/bi00026a022. [DOI] [PubMed] [Google Scholar]
- Makhatadze G. I., Privalov P. L. Heat capacity of proteins. I. Partial molar heat capacity of individual amino acid residues in aqueous solution: hydration effect. J Mol Biol. 1990 May 20;213(2):375–384. doi: 10.1016/S0022-2836(05)80197-4. [DOI] [PubMed] [Google Scholar]
- Murphy K. P., Freire E., Paterson Y. Configurational effects in antibody-antigen interactions studied by microcalorimetry. Proteins. 1995 Feb;21(2):83–90. doi: 10.1002/prot.340210202. [DOI] [PubMed] [Google Scholar]
- Murphy K. P., Privalov P. L., Gill S. J. Common features of protein unfolding and dissolution of hydrophobic compounds. Science. 1990 Feb 2;247(4942):559–561. doi: 10.1126/science.2300815. [DOI] [PubMed] [Google Scholar]
- Murphy K. P., Xie D., Garcia K. C., Amzel L. M., Freire E. Structural energetics of peptide recognition: angiotensin II/antibody binding. Proteins. 1993 Feb;15(2):113–120. doi: 10.1002/prot.340150203. [DOI] [PubMed] [Google Scholar]
- Spolar R. S., Record M. T., Jr Coupling of local folding to site-specific binding of proteins to DNA. Science. 1994 Feb 11;263(5148):777–784. doi: 10.1126/science.8303294. [DOI] [PubMed] [Google Scholar]
- Sturtevant J. M. Heat capacity and entropy changes in processes involving proteins. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2236–2240. doi: 10.1073/pnas.74.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Fukada H. Calorimetric studies of the binding of Streptomyces subtilisin inhibitor to subtilisin of Bacillus subtilis strain N'. Biochemistry. 1985 Jan 15;24(2):297–300. doi: 10.1021/bi00323a009. [DOI] [PubMed] [Google Scholar]
- Wiseman T., Williston S., Brandts J. F., Lin L. N. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem. 1989 May 15;179(1):131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]
- Ysern X., Fields B. A., Bhat T. N., Goldbaum F. A., Dall'Acqua W., Schwarz F. P., Poljak R. J., Mariuzza R. A. Solvent rearrangement in an antigen-antibody interface introduced by site-directed mutagenesis of the antibody combining site. J Mol Biol. 1994 May 13;238(4):496–500. doi: 10.1006/jmbi.1994.1309. [DOI] [PubMed] [Google Scholar]
- Yung B. Y., Trowbridge C. G. A calorimetric comparison of trypsin and its anhydro modification in complex formation with Kunitz soybean inhibitor. J Biol Chem. 1980 Oct 25;255(20):9724–9730. [PubMed] [Google Scholar]