Abstract
OBJECTIVE—To determine the material properties of the subchondral bone plate in patients with osteoarthritis or osteoporosis. METHODS—Femoral heads were obtained after surgical removal from age and sex matched groups of patients with either osteoporosis (OP), after a fractured neck of femur, or osteoarthritis (OA) and compared with a normal group. The mechanical stiffness, density, and composition of the subchondral bone plate from sites selected to represent areas of heavy, intermittent, and light loading were measured. RESULTS—Overall, OP bone was the least stiff and dense, followed by OA bone; normal bone was stiffer and more dense (p < 0.05). Though OP bone contained less mineral, the organic and water contents were increased in proportion suggesting no change in the relative amount of organic matrix. OA bone was also hypomineralised (p < 0.05) but had different organic and water fractions suggesting a defect in the matrix. Site variation of most properties was small, though across all the groups the superior region was significantly stiffer than the inferior. CONCLUSION—This study shows that subchondral bone plate is less stiff than normal in both OP and OA and so cannot, by itself, explain the preserving of the overlying cartilage in OP while aiding its destruction in OA. However, the subchondral bone plate is only one part of the bony structure of the femoral head and changes in the cancellous bone need to be considered. The generalised changes in bone composition found in patients with OA support the hypothesis that the disease could involve the bone in the primary pathogenesis.
Full Text
The Full Text of this article is available as a PDF (174.9 KB).
Figure 1 .
Schematic diagram of the sites on the human femoral head from which samples of subchondral bone were removed.
Figure 2 .
Median values of the stiffness of the subchondral bone plate determined from all sites over the femoral head of patients undergoing a hip replacement for osteoarthritis (OA), or osteoporosis (OP) compared with normal controls. The extent of the shaded bars shows the 25% and 75% confidence limits, the error bars show the 5% and 95% limits. (* p<0.05).
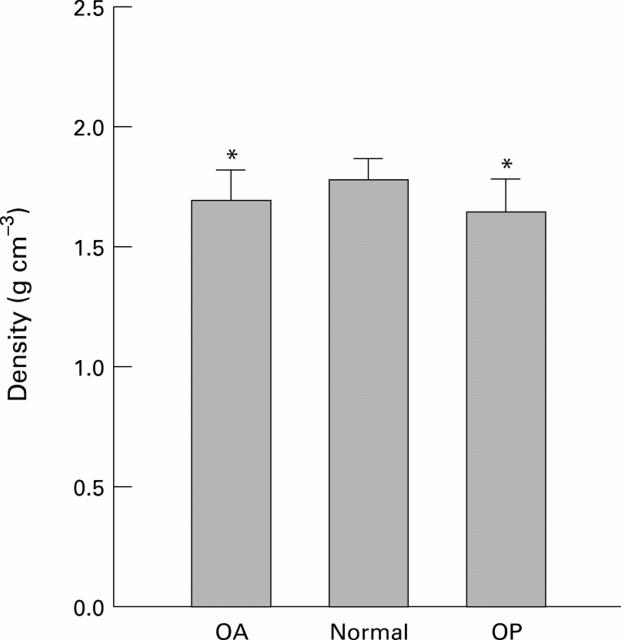
Figure 3 .
Density of the subchondral bone plate from the femoral heads of patients with OA or OP compared with normal controls. Both OA and OP groups are significantly reduced from normal and OP is slightly but significantly less than OA. (* p<0.05).
Figure 4 .
Composition of normal, OA, and OP subchondral bone plate from human femoral heads expressed as a fraction of the wet mass of the sample. Both OP and OA differed from normal but in different ways. (* p<0.05).
Figure 5 .
Composition of normal, OA, and OP subchondral bone plate expressed as mass per unit volume of tissue showing the changes seen in figure 4 in a different way. Both the OA and OP bone contain less mineral than normal (p < 0.05), though they have the same organic content. The increased water content in the OA bone matrix (p < 0.05 compared with normal) suggests that matrix changes occurring in the OA bone are different to those in OP bone.
Figure 6 .
Linear regression relations between stiffness and density of the subchondral bone plate. There is no difference between the gradients of the regression lines over the density range found in this study. See text for regression equations.
Figure 7 .

Site variation of stiffness of the subchondral bone plate from human femoral heads. (A) Stiffnesses are not normally distributed and the median values are shown. Shaded bars represent 25% and 75% and the errors bars the 5% and 95% confidence intervals. (B) Stiffness of each site expressed as a fractional change compared with the equivalent site from the normal group.
Figure 8 .

(A) Site variation of density of the subchondral bone plate from human femoral heads. (B) Density of each site expressed as a fractional change compared with the equivalent site from the normal group.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir G., Pirie C. J., Rashad S., Revell P. A. Remodelling of subchondral bone in osteoarthritis: a histomorphometric study. J Clin Pathol. 1992 Nov;45(11):990–992. doi: 10.1136/jcp.45.11.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong S. J., Read R. A., Price R. Topographical variation within the articular cartilage and subchondral bone of the normal ovine knee joint: a histological approach. Osteoarthritis Cartilage. 1995 Mar;3(1):25–33. doi: 10.1016/s1063-4584(05)80035-4. [DOI] [PubMed] [Google Scholar]
- Benske J., Schünke M., Tillmann B. Subchondral bone formation in arthrosis. Polychrome labeling studies in mice. Acta Orthop Scand. 1988 Oct;59(5):536–541. doi: 10.3109/17453678809148779. [DOI] [PubMed] [Google Scholar]
- Björkström S., Goldie I. F. Hardness of the subchondral bone of the patella in the normal state, in chondromalacia, and in osteoarthrosis. Acta Orthop Scand. 1982 Jun;53(3):451–462. doi: 10.3109/17453678208992240. [DOI] [PubMed] [Google Scholar]
- Brown T. D., Shaw D. T. In vitro contact stress distributions in the natural human hip. J Biomech. 1983;16(6):373–384. doi: 10.1016/0021-9290(83)90071-4. [DOI] [PubMed] [Google Scholar]
- Byers P. D., Contepomi C. A., Farkas T. A. A post mortem study of the hip joint. Including the prevalence of the features of the right side. Ann Rheum Dis. 1970 Jan;29(1):15–31. doi: 10.1136/ard.29.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C., Cook P. L., Osmond C., Fisher L., Cawley M. I. Osteoarthritis of the hip and osteoporosis of the proximal femur. Ann Rheum Dis. 1991 Aug;50(8):540–542. doi: 10.1136/ard.50.8.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedrick D. K., Goldstein S. A., Brandt K. D., O'Connor B. L., Goulet R. W., Albrecht M. A longitudinal study of subchondral plate and trabecular bone in cruciate-deficient dogs with osteoarthritis followed up for 54 months. Arthritis Rheum. 1993 Oct;36(10):1460–1467. doi: 10.1002/art.1780361019. [DOI] [PubMed] [Google Scholar]
- Dequeker J., Johnell O. Osteoarthritis protects against femoral neck fracture: the MEDOS study experience. Bone. 1993;14 (Suppl 1):S51–S56. doi: 10.1016/8756-3282(93)90350-j. [DOI] [PubMed] [Google Scholar]
- Grynpas M. D., Alpert B., Katz I., Lieberman I., Pritzker K. P. Subchondral bone in osteoarthritis. Calcif Tissue Int. 1991 Jul;49(1):20–26. doi: 10.1007/BF02555898. [DOI] [PubMed] [Google Scholar]
- Havdrup T., Hulth A., Telhag H. The subchondral bone in osteoarthritis and rheumatoid arthritis of the knee. A histological and microradiographical study. Acta Orthop Scand. 1976 Jun;47(3):345–350. doi: 10.3109/17453677608992003. [DOI] [PubMed] [Google Scholar]
- Hodge W. A., Fijan R. S., Carlson K. L., Burgess R. G., Harris W. H., Mann R. W. Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci U S A. 1986 May;83(9):2879–2883. doi: 10.1073/pnas.83.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S. B. Ultrasonic method for measuring elastic coefficients of bone and results on fresh and dried bovine bones. IEEE Trans Biomed Eng. 1970 Apr;17(2):101–105. doi: 10.1109/tbme.1970.4502706. [DOI] [PubMed] [Google Scholar]
- Lees S., Ahern J. M., Leonard M. Parameters influencing the sonic velocity in compact calcified tissues of various species. J Acoust Soc Am. 1983 Jul;74(1):28–33. doi: 10.1121/1.389723. [DOI] [PubMed] [Google Scholar]
- Lees S., Heeley J. D., Cleary P. F. A study of some properties of a sample of bovine cortical bone using ultrasound. Calcif Tissue Int. 1979 Nov 26;29(2):107–117. doi: 10.1007/BF02408065. [DOI] [PubMed] [Google Scholar]
- Lereim P., Goldie I., Dahlberg E. Hardness of the subchondral bone of the tibial condyles in the normal state and in osteoarthritis and rheumatoid arthritis. Acta Orthop Scand. 1974;45(4):614–627. doi: 10.3109/17453677408989184. [DOI] [PubMed] [Google Scholar]
- Oettmeier R., Arokoski J., Roth A. J., Helminen H. J., Tammi M., Abendroth K. Quantitative study of articular cartilage and subchondral bone remodeling in the knee joint of dogs after strenuous running training. J Bone Miner Res. 1992 Dec;7 (Suppl 2):S419–S424. doi: 10.1002/jbmr.5650071410. [DOI] [PubMed] [Google Scholar]
- Pogrund H., Rutenberg M., Makin M., Robin G., Menczel J., Steinberg R. Osteoarthritis of the hip joint and osteoporosis: a radiological study in a random population sample in Jerusalem. Clin Orthop Relat Res. 1982 Apr;(164):130–135. [PubMed] [Google Scholar]
- Radin E. L., Paul I. L. Does cartilage compliance reduce skeletal impact loads? The relative force-attenuating properties of articular cartilage, synovial fluid, periarticular soft tissues and bone. Arthritis Rheum. 1970 Mar-Apr;13(2):139–144. doi: 10.1002/art.1780130206. [DOI] [PubMed] [Google Scholar]
- Radin E. L., Rose R. M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986 Dec;(213):34–40. [PubMed] [Google Scholar]
- Simon S. R., Radin E. L., Paul I. L., Rose R. M. The response of joints to impact loading. II. In vivo behavior of subchondral bone. J Biomech. 1972 May;5(3):267–272. doi: 10.1016/0021-9290(72)90042-5. [DOI] [PubMed] [Google Scholar]
- Solomon L., Schnitzler C. M., Browett J. P. Osteoarthritis of the hip: the patient behind the disease. Ann Rheum Dis. 1982 Apr;41(2):118–125. doi: 10.1136/ard.41.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli M. B., Evans J. A. Dependence of the velocity and attenuation of ultrasound in bone on the mineral content. Phys Med Biol. 1991 Nov;36(11):1529–1537. doi: 10.1088/0031-9155/36/11/012. [DOI] [PubMed] [Google Scholar]
- Turner C. H., Eich M. Ultrasonic velocity as a predictor of strength in bovine cancellous bone. Calcif Tissue Int. 1991 Aug;49(2):116–119. doi: 10.1007/BF02565132. [DOI] [PubMed] [Google Scholar]
- Yoon H. S., Katz J. L. Ultrasonic wave propagation in human cortical bone--II. Measurements of elastic properties and microhardness. J Biomech. 1976;9(7):459–464. doi: 10.1016/0021-9290(76)90089-0. [DOI] [PubMed] [Google Scholar]