Abstract
A 62-kDa cell surface antigen (M9) of Mycoplasma gallisepticum PG31 that mediates antibody-induced agglutination of the organism was purified and subjected to N-terminal amino-acid sequencing. A 999-bp region of the cDNA encoding the M9 protein was generated by reverse transcription-PCR, and its nucleotide sequence was determined. PCR primers based on this sequence were used to screen a genomic DNA library of PG31. A full-length M9 protein-encoding gene was isolated and sequenced, revealing 96% nucleotide identity with the pMGA1.1 gene of M. gallisepticum S6. Sequence analyses of the M9 gene and flanking open reading frames that encode other pMGA family members suggest that a tandemly repeated GAA sequence may influence pMGA gene expression.
A major plasma membrane protein, pMGA, of Mycoplasma gallisepticum S6 has been identified as a cell adhesin (hemagglutinin) molecule (14, 15). Recent studies indicate that the genetic determinants that code for the hemagglutinin are organized into a large family of genes but that only one of these genes is predominately expressed in any given strain (3, 9, 15, 16). We have previously described a monoclonal antibody (MAb), G9, that reacts with an epitope of the M9 protein of M. gallisepticum PG31, resulting in agglutination of the organism (17). In the present study, the amino acid and nucleotide sequences of the purified M9 protein and its gene were determined. Sequence comparisons between the M9 gene of PG31 and the pMGA hemagglutinin gene revealed considerable homology, demonstrating that the M9 protein is a member of the pMGA multigene family. Thus, M9 is the second member of this large gene family which has been shown to be expressed as a surface protein in M. gallisepticum.
Purification of M9 protein.
M. gallisepticum PG31 (ATCC 19610) was cultured in modified Frey (8) broth supplemented with 10% swine serum as described previously (11). Cells from 50 ml of culture were harvested by centrifugation (8,000 × g, 20 min) and solubilized in 1.0 ml of lysis buffer (25 mM Tris-HCl, 0.25% [wt/vol] sodium deoxycholate, 1.0% Nonidet P-40, 0.05% Tween 20, 0.15 M NaCl [pH 8.1] [4°C]). Affinity purification of the M9 antigen was monitored by immunoblot analysis. Proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (7.5% polyacrylamide) followed by electrophoretic transfer onto a nitrocellulose membrane (pore size, 0.2 μm; Schleicher & Schuell, Inc., Keene, N.H.) and reaction with MAb G9 (in ascites fluid at a dilution of 1:400) as described previously (17). The M9 antigen in cell lysates was detected as a single protein band (apparent Mr of 62,000) (Fig. 1A and B). To purify M9, cell lysates (1.0 ml) were centrifuged at 10,000 × g for 5 min at 4°C to remove insoluble debris, ascites fluid containing 160 μg of MAb G9 was added, and the mixture was incubated at 4°C for 1 h. A secondary, affinity-purified, rabbit anti-mouse immunoglobulin G antibody (0.67 mg; Jackson ImmunoResearch Laboratories, West Grove, Pa.) was added to 0.34 ml of protein A–Sepharose CL-4B (Pharmacia, Piscataway, N.J.) in 3.0 ml of lysis buffer (10% [vol/vol] suspension) and gently mixed at 4°C for 1 h. After the beads were washed (twice, in 10 volumes of lysis buffer) and resuspended in 3 volumes of lysis buffer, 1.3 ml of the final suspension (25% [vol/vol]) was added to the solubilized M. gallisepticum and ascites fluid and incubated at 4°C for 1 h with gentle agitation. The Sepharose-bound immune complexes were harvested by centrifugation at 3,000 × g for 20 s at 4°C and washed three times in lysis buffer (4 ml). For elution, the immune complexes were suspended in Laemmli sample buffer (0.5 ml) (12) and heated at 95°C for 1 min. Elution was repeated once, and the pooled eluate containing the M9 antigen protein was further fractionated by preparative SDS-PAGE (7.5% polyacrylamide). The M9 protein band was located following rapid Coomassie blue staining and destaining of two gel strips excised from each edge of the gel. The M9 protein band was excised from the unstained portion of the gel, and the gel slice was placed in a 50-ml polyallomer tube and macerated with a Teflon pestle. The crushed gel was transferred to a 50-ml conical centrifuge tube and suspended in 6 volumes (typically about 8 ml) of extraction buffer (25 mM Tris, 190 mM glycine, 0.1% SDS, 2 mM dithiothreitol [pH 8.3]). Protein was extracted by vigorous shaking for 1 h at room temperature, followed by collection of the supernatant after centrifugation (4,000 × g, 1 min). Extraction was completed by rinsing the gel pellet three times with extraction buffer (3 volumes). The extracted protein (∼20 ml) was concentrated to ∼50 μl by sequential use of Centriprep-30 and Centricon-30 concentrators (Amicon, Inc., Beverly, Mass.). About 6 μg of purified M9 protein was typically obtained from 50 ml of M. gallisepticum culture.
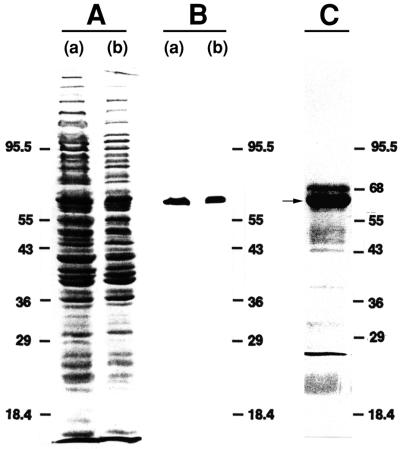
FIG. 1.
Separation and analysis of M9 by SDS-PAGE and electroblotting. (A) SDS-PAGE-resolved, Ponceau S-stained blot of whole-cell lysate of M. gallisepticum PG31 (a) and the supernatant retrieved after centrifugation of this whole-cell lysate (b). (B) The blot from panel A was destained and probed with MAb G9. (C) The immunoaffinity- and gel-purified protein sample was concentrated, subjected to SDS-PAGE, blotted onto a PVDF membrane, and stained with Ponceau S. One of four identical lanes is shown, each of which contained 10 μg of M9 protein. The M9 protein bands (indicated by the arrow) were excised for sequencing. (A small amount of albumin originating from the ascitic fluid appears as a band above the M9 protein band.) Molecular size markers (in kilodaltons; Diversified Biotech, Boston, Mass.) are for, from top to bottom, phosphorylase b, bovine serum albumin (68 kDa, panel C only), glutamate dehydrogenase, ovalbumin, lactate dehydrogenase, carbonic anhydrase, and lactoglobulin.
M9 protein sequence determination.
Purified M9 protein (∼40 μg) was subjected to SDS-PAGE followed by electrophoretic transfer to a polyvinylidene difluoride (PVDF) membrane (Fig. 1C). After we located the PVDF-immobilized M9 protein bands by staining the membrane with Ponceau S (1), the bands were excised and submitted to the Harvard Microchemistry Facility (Cambridge, Mass.), where the N-terminal sequence of a portion of the sample was determined directly by automated Edman degradation. Comparison of this sequence with the deduced amino acid sequences in the GenBank database indicated that the M9 protein was distinct but that it showed homology with a large multigene family in M. gallisepticum S6 designated pMGA (16). The probable presence of numerous family members in the M. gallisepticum PG31 genome (by analogy to the S6 strain) complicated our strategy for cloning the M9 gene.
To identify internal peptides with sequences unique to M9 protein, portions of the PVDF-immobilized M9 protein sample were digested in situ with endoproteinase Lys-C or trypsin and analyzed by capillary reverse-phase liquid chromatography coupled with an electrospray ionization quadrupole ion trap mass spectrometer (Finnigan LQC) (6, 22) at the Harvard Microchemistry Facility. Individual peptide masses and fragmentation patterns (i.e., tandem mass spectra) were then compared with those of predicted peptides from the pMGA gene family by using the SEQUEST algorithm (6, 22). This analysis revealed M9’s significant sequence homology with pMGA1.1 and pMGA1.2 (GenBank accession no. L28423 and L28424 [16]), confirming the results from N-terminal sequence analysis. For example, M9 tryptic peptides with masses and fragmentation patterns and, therefore, sequences corresponding to 25 of 51 predicted peptides from pMGA1.1 and pMGA1.2 were identified. Additionally, we identified at least nine M9 peptides which contained one or more sequence differences from the predicted sequences of peptides from pMGA1.1 and pMGA1.2. This information was used to select candidates for further chemical microsequence analysis, by focusing primarily on those with unique sequences (i.e., unique masses and tandem mass spectra) not predicted for peptides from pMGA1.1 and pMGA1.2. Eight additional peptides from M9 were chemically sequenced, including four which contained differences with corresponding regions in pMGA1.1 and pMGA1.2; the other four M9 peptides were identical in sequence to predicted peptides from the two homologous proteins of strain S6. Overall, unambiguous sequence information for 129 amino acid residues (including the N terminus), representing 20% of the entire M9 protein, was obtained by chemical microsequencing.
RT-PCR products.
Because of the large number of members of the pMGA family, reverse transcription-PCR (RT-PCR) was the method of choice for identifying the particular gene encoding the M9 protein produced in strain PG31. Based on the protein microsequence information, degenerate oligonucleotides were designed (Table 1; Fig. 2) as primers for RT-PCR amplification of M9 mRNA. Primers were synthesized by Research Genetics, Inc. (Huntsville, Ala.). From a 40-ml log-phase culture of M. gallisepticum PG31, 221 μg of total RNA was isolated with a ToTally RNA kit (Ambion, Inc., Austin, Tex.) according to instructions provided. Prior to cDNA synthesis, aliquots of total RNA (1 μg) were treated with 2 U of RNase-free DNase I (Ambion) in a 16-μl volume for reaction at 37°C for 2 h and DNase was denatured at 75°C for 20 min. cDNA was synthesized by addition of 20 U of avian myeloblastosis virus reverse transcriptase (Boehringer Mannheim, Indianapolis, Ind.), deoxynucleoside triphosphates (1 mM each), and either primer B4 (0.5 μM) or primer R2 (1 μM) in a 20-μl volume for reaction at 55°C for 1 h, followed by reaction at 65°C for 10 min. Following denaturation at 95°C for 1 min, second-strand synthesis and amplification were accomplished with a modified “touchdown” PCR protocol (4) consisting of denaturation (20 s) at 95°C for each cycle, annealing (90 s) starting at 66°C but with the temperature decreasing 2°C after every two cycles until it reached 52°C (14 cycles, total) and then 26 cycles at 52°C, extension (30 s) at 72°C for each cycle, and final extension (7 min) at 72°C. PCR amplification was performed with a GeneAmp model 9600 thermal cycler and GeneAmp kit (Perkin-Elmer Cetus, Norwalk, Conn.). Primary PCR mixtures (50 μl) contained 1.5 mM MgCl2, deoxynucleoside triphosphates (0.2 mM each), 1.25 U of Taq polymerase, primer pairs (L7 and B4 or L12 and R2, at the concentrations indicated in Table 1), and 1 μl of the RT product as the template. PCRs with nested primer pairs (L9 and R4, L9 and R8, or L13 and R9) for secondary amplifications were performed as described above with 1 μl of samples from primary PCR mixtures as templates.
TABLE 1.
Sequences of primers for RT-PCR and library screeninga
| Primer | Final concn in PCR mixture (μM) | Sequence (5′–3′) | Source |
|---|---|---|---|
| Sense primers | |||
| L7 | 0.5 | ATGAAYGGNGGNGAYACNAA | N terminus |
| L9 | 0.5 | GGNGAYGGNCARGGNATGATG | N terminus |
| L12 | 1.0 | AATTAGCTGCTGCAAGAATGGG | L9 and R8 |
| L13 | 1.0 | CCTTTGATGAACAACATGCCG | L9 and R8 |
| F2 | 1.0 | AAAAACACAGGACACTCTAACAAAAGC | L9 and R8 |
| Antisense primers | |||
| R4b | 1.0 | ACYAAYTCNGCRTGYTGYTCRTCRAA | PK266 |
| 2.0 | ACNAGYTCNGCRTGYTGYTCRTCRAA | ||
| R8 | 0.5 | GTNGCNGCCATCATNACNAC | PT75-2 |
| B4 | 0.5 | GGYTGNACCATNGTYTGNCC | PT108 |
| R9b | 0.5 | CCRAAYTTYAARTTNGCYAANG | PK253 |
| 1.0 | CCRAAYTTYAARTTNGCNAGNG | ||
| 1.0 | CCRAAYTTNAGRTTNGCYAANG | ||
| 2.0 | CCRAAYTTNAGRTTNGCNAGNG | ||
| R2 | 1.0 | ARRTACATRTTNCCDATCATNGG | PT75-1 |
| B2 | 1.0 | CAGTAACAGTTCCAGTTATATCAACGC | L13 and R9 |
Sequences of degenerate oligonucleotides were derived from amino acid sequences of the indicated peptides, except for underlined residues, which were derived from amino acids conserved in all previously described pMGA family members (total of five) (16). Sequences of nondegenerate oligonucleotides were derived from nucleic acid sequences of RT-PCR products obtained with the indicated primer pairs.
To reduce both the degree of degeneracy and the number of mismatches, primers containing anticodons for leucine were synthesized separately with either NAG or YAA and then combined in PCR mixtures to yield approximately equimolar mixtures of all individual oligonucleotides.
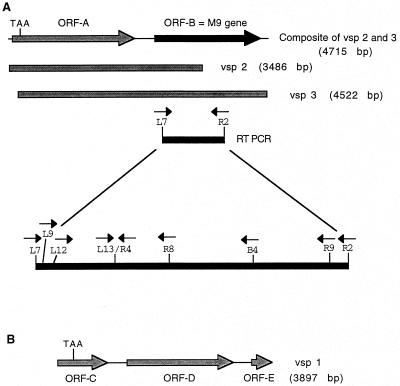
FIG. 2.
Schematic diagrams of M. gallisepticum DNA fragments containing the M9 gene. (A) Composite diagram (top line) of the chromosomal region containing the M9 gene (ORF-B) based on sequence analysis of the clones VSP 2 and VSP 3 (middle shaded lines) and locations of transcribed sequences identified by RT-PCR (bottom filled line). The locations of primer binding sites used for RT-PCR analysis are shown with arrows indicating directionality. (B) Schematic diagram of the insert in clone VSP 1 showing the locations of orf-C, -D, and -E. Positions of TAA stop codons in orf-A and -C are indicated.
RT-PCR of PG31 mRNA with primers L7 and B4 yielded no product that was detectable on ethidium bromide-stained agarose gels. However, nested PCR with the product of the initial RT-PCR as the template and the internal primer pairs L9 and R4 and L9 and R8 yielded specific DNA fragments of 284- and 479-bp lengths, respectively. PCR products were directly sequenced, without cloning, with the fmol DNA Sequencing System (Promega Corp., Madison, Wis.) according to instructions provided. From the nucleotide sequences of these PCR products, the specific (nondegenerate) sense primers L12 and L13 were designed. RT-PCR with the primer pair L12 and R2 also did not produce any detectable product on agarose gels, but nested PCR with primer pair L13 and R9 yielded a DNA fragment of 806 bp. By combining the sequences from these three nested PCR products, the nucleotide sequence of a 999-bp region comprising approximately 50% of the entire M9 gene was obtained (Fig. 2). Comparison of the deduced amino acid sequence of the 999-bp region with the M9 peptide sequences (Fig. 3) confirmed the identities of the RT-PCR products as M9-derived sequences.
FIG. 3.
Nucleotide sequence of the M9 gene and predicted amino acid sequence. Double-underlined amino acids agree with sequence data obtained from peptide fragments. Underlined nucleotides indicate positions of oligonucleotide primers (indicated in parentheses) used for RT-PCR and library screening. Arrows mark the sequence determined from RT-PCR product analysis as well as from the genomic clones. An asterisk denotes the stop codon.
Nucleotide sequence of the M9 gene and predicted protein.
A genomic library of PG31 DNA in the lambda ZAP II expression vector (Stratagene, La Jolla, Calif.) was constructed. PG31 DNA was isolated as described by Voelker et al. (20) and mechanically sheared by repeated passage through a 27-gauge hypodermic needle. The resulting DNA fragments (3 to 5 kb) were ligated into the ZAP II vector as described previously (10), except that EcoRI-BstXI adapters (Invitrogen) instead of EcoRI linkers were used. The ligation mixture was packaged to produce viable phage particles with Gigapack III Gold in vitro packaging extract (Stratagene). Packaged phage particles representing the genomic library were amplified on lawns of Escherichia coli XL1-Blue MRF′ on Luria-Bertani plates.
Isolation of individual clones from the genomic DNA library was accomplished as described previously by sequential plating of the library at decreasing plaque densities and PCR analysis of phage extracted from plates at each step (2). Primers F2 and B2 (Table 1 and Fig. 3), which were designed to amplify a 557-bp region of the M9 gene and which exhibited maximum divergence from pMGA1.1 and pMGA1.2, were used for PCR screening of phage stocks (5 μl/reaction) with the touchdown program described above for RT-PCR. Each positive individual phage stock was subjected to an additional round of plaque purification and PCR screening (1 μl/reaction) to ensure isolation of a clonal phage population. The pBluescript SK(−) phagemids containing the cloned DNA inserts were excised from the phage according to the protocol provided by the manufacturer (Stratagene) and purified with a Plasmid Mini Kit (Qiagen, Inc., Chatsworth, Calif.). DNA sequencing of both strands of the plasmid DNA templates was performed via automated sequencing with an Applied Biosystems (Foster City, Calif.) 377 Prism sequencer at the Iowa State University DNA Sequencing and Synthesis Facility, Ames.
Three genomic clones (VSPs 1, 2, and 3) were isolated. The complete nucleotide sequences of the inserts in VSP 3 (4,522 bp) and VSP 2 (3,486 bp) were determined and found to contain a region exhibiting nucleotide identity to the M9 sequence generated by RT-PCR. Alignment of the DNA sequence of VSP 3 with that of VSP 2 revealed a large overlapping region of nucleotide identity, indicating that these two clones contain inserts from the same region of the M. gallisepticum genome. The composite nucleotide sequence (4,715 bp) obtained for VSPs 2 and 3 is shown schematically in Fig. 2A.
One of the open reading frames (orfs) in VSP 3 (orf-B) apparently is the M9 gene. orf-B starts with a GTG initiation codon, ends with a TAG stop codon, and encodes a predicted polypeptide of 645 amino acid residues with a molecular mass of 69.8 kDa (Fig. 3). orf-B contains a 999-bp region identical to the nucleotide sequence obtained by RT-PCR analysis of M9. Also, the deduced amino acid sequence of the orf-B protein was identical to M9 peptide sequences obtained by both chemical microsequencing and mass spectrometry, except for a single amino acid difference in one peptide (PT75-2). (This difference involves a low-confidence assignment of an alanine residue during chemical sequencing; since the mass spectrometry analysis confirmed the DNA sequencing results, the discrepancy is considered to be of no consequence.) Amino acid and DNA sequence data, therefore, strongly suggest that orf-B encodes the M9 protein.
The nucleotide sequence of the M9 gene (orf-B) is extremely similar (>96% identity) to those of the previously described pMGA1.1 and pMGA1.2 genes (16). The predicted amino acid sequences of the M9 and pMGA1.1 proteins are aligned in Fig. 4, illustrating that M9 is clearly a member of the pMGA family. As with other members of the pMGA family, the amino-terminal region of the predicted M9 protein begins with a hydrophobic sequence that is not present in the N-terminal sequence of the M9 protein. This putative signal peptide ends with a typical consensus sequence for mycoplasma lipoproteins (Ala, Ala, Ser, and Cys) which is presumably recognized by the signal peptidase II enzyme that hydrolyzes the peptide bond between the serine and cysteine residues and catalyzes the acylation reaction (21).
FIG. 4.
Alignment of the M9 protein’s predicted amino acid sequence with those of the products of related ORFs from strain PG31 and with that of the pMGA1.1 protein of strain S6. Alignment was performed with the CLUSTAL W (19) program of the MacVector software package. Regions of dark shading and light boxed areas indicate amino acid identity and similarity, respectively. Amino acids that are different are unshaded. Gaps indicated by dashes were added to obtain the best alignment.
Clones VSP 1 to 3 contain other M9-like genes.
In addition to the putative M9 gene, clones VSP 2 and 3 contain a second orf designated orf-A (Fig. 2A). orf-A contains 2,202 bp and would encode a protein showing 38% amino acid sequence identity with the M9 protein and 41% amino acid sequence identity with pMGA1.4 (16). However, orf-A contains an internal TAA stop codon at nucleotide positions 139 to 141. Therefore, orf-A encodes a truncated gene product of only 46 amino acids.
The nucleotide sequence of the 3,897-bp insert in VSP 1 was determined and found to contain predicted genes designated orf-C (nucleotide positions 1 to 903), orf-D (positions 1254 to 3188), and orf-E (positions 3528 to 3896) (Fig. 2B). orf-D is a full-length member of the pMGA family bearing 95% nucleotide sequence identity to the M9 gene, and the deduced amino acid sequence of the orf-D gene product has 88% identity with the sequence of the M9 protein. The partial genes orf-C and orf-E flanking orf-D (Fig. 2B) are potentially two additional members of the M9 gene family. The nucleotide sequence of orf-C revealed the presence of an internal TAA stop codon at nucleotide positions 286 to 288. Thus, if the 5′ end of orf-C, which has not yet been cloned, is like that of other members of the M9-pMGA family, orf-C expression would result in a truncated product of about 534 amino acids. orf-E contains the 5′ end of another M9-pMGA family member that appears to be more closely related to pMGA1.3 and pMGA1.5 than to M9 and pMGA1.1.
Analysis of the intergenic regions upstream of orf-B, -D, and -E show that all three regions have similar structures. Each region has a different number of tandem GAA repeats, similar to what has been described for the pMGA multigene family (3). The transcription start site has been previously determined for pMGA1.1 (9) as a specific G nucleotide that is conserved in the intergenic regions upstream of orf-B, -D, and -E, suggesting that all members of the M9-pMGA family use this nucleotide as the transcription start site. However, the postulated transcriptional promoter consensus sequences (−10 and −35 regions) differ considerably among the various genes of the M9-pMGA family.
A dendrogram comparing the pMGA protein family from S6 with the M9 protein family from PG31 was generated by the MegAlign program of the DNASTAR (Madison, Wis.) software package. Based on this analysis, the M9 protein is very closely related to the pMGA1.1 and pMGA1.2 proteins and the orf-D gene product is also closely related to pMGA1.1, pMGA1.2, and M9. However, other proteins such as the orf-A gene product are more closely related to pMGA1.3, pMGA1.4, and pMGA1.5. These data indicate that polymorphisms exhibited by members of these families are not necessarily strain specific. However, the divergence of individual family members is such that strains likely possess unique protein sequences.
M9 and pMGA gene expression.
The amino acid sequence of the purified M9 protein is consistent with a single polypeptide and not with the other members of the M9-pMGA family described thus far. Therefore, either the G9 antibody is specific for the M9 protein and does not react with other members of the family (e.g., the gene products of orf-A, -C, -D, and -E) or the other proteins of this family are not synthesized in PG31 under the growth conditions that were used in this study. An unresolved issue is the mechanism that regulates expression of the M9 and pMGA genes. A notable similarity in the intergenic regions of members of the M9-pMGA family is the fact that they have a different number of tandem GAA repeats located upstream of the putative promoter. Both the pMGA1.1 gene expressed in the S6 strain and the M9 gene expressed in the PG31 strain have exactly 12 GAA repeats. None of the other genes of the M9-pMGA family for which nucleotide sequence data are available have 12 repeats; some have fewer than 12 repeats (from 7 to 11) and others have more than 12 (from 14 to 16). The number of GAA repeats associated with a particular member of the M9-pMGA family is expected to sometimes vary, either expand or contract, because of DNA replication errors referred to as slipped-strand mispairing (13). A speculative possibility is that exactly 12 GAA repeats are required for expression of genes in this family, but the mechanism by which the number of repeats might regulate gene expression is unknown. The GAA repeat region is homopyrimidine on one DNA strand and homopurine on the other, and under the right conditions it should form a triple helix structure referred to as H-DNA (7). H-DNA formation leaves the fourth DNA strand unpaired and susceptible to single-strand-specific nucleases. Regions of H-DNA may be hot spots for DNA recombination (18), but recombination between members of the M9-pMGA gene family has not yet been reported. The formation of H-DNA in vitro is superhelix induced and pH dependent (5). Environmental factors may regulate the ability of GAA repeats in M. gallisepticum to form H-DNA, and such regulation may affect M9 and pMGA gene expression.
Nucleotide sequence accession numbers.
The nucleotide sequence for VSP 1 has been deposited in the GenBank database under accession no. AF053978. The combined sequences of VSP 2 and VSP 3 have been assigned accession no. AF032890.
Acknowledgments
We gratefully acknowledge Bill Lane and his colleagues at the Harvard Microchemistry Facility for their skillful protein sequencing effort, as well as for the additional effort and time spent for tandem mass spectrometry and SEQUEST analyses.
This work was supported by the National Research Initiative Competitive Grants Program (grant 93-37204-9113), U.S. Department of Agriculture, and by the College of Veterinary Medicine (grant ALAV 304), Auburn University.
REFERENCES
- 1.Aebersold R. Internal amino acid sequence analysis of proteins after in situ protease digestion on nitrocellulose. In: Matsudaira P, editor. A practical guide to protein and peptide purification for microsequencing. 2nd ed. San Diego, Calif: Academic Press, Inc.; 1993. pp. 102–124. [Google Scholar]
- 2.Amaravadi L, King M W. A rapid and efficient, nonradioactive method for screening recombinant DNA libraries. BioTechniques. 1994;16:98–103. [PubMed] [Google Scholar]
- 3.Baseggio N, Glew M D, Markham P F, Whithear K G, Browning G F. Size and genomic location of the pMGA multigene family of Mycoplasma gallisepticum. Microbiology. 1996;142:1429–1435. doi: 10.1099/13500872-142-6-1429. [DOI] [PubMed] [Google Scholar]
- 4.Don R H, Cox P T, Wainwright B J, Baker K, Mattick J S. Touchdown PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991;19:4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dybvig K, Clark C D, Aliperti G, Schlesinger M J. A chicken repetitive DNA sequence that is highly sensitive to single-strand specific endonucleases. Nucleic Acids Res. 1983;11:8495–8508. doi: 10.1093/nar/11.23.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eng J K, McCormack A L, Yates J R., III An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 7.Frank-Kamenetskii M D. Triplex DNA structures. Annu Rev Biochem. 1995;64:65–95. doi: 10.1146/annurev.bi.64.070195.000433. [DOI] [PubMed] [Google Scholar]
- 8.Frey M L, Hanson R P, Anderson D P. A medium for isolation of avian mycoplasmas. Am J Vet Res. 1968;29:2163–2171. [PubMed] [Google Scholar]
- 9.Glew M D, Markham P F, Browning G F, Walker I D. Expression studies on four members of the pMGA multigene family in Mycoplasma gallisepticum S6. Microbiology. 1995;141:3005–3014. doi: 10.1099/13500872-141-11-3005. [DOI] [PubMed] [Google Scholar]
- 10.Huynh T V, Young R A, Davis R W. Constructing and screening cDNA libraries in λgt 10 and λgt 11. In: Glover D M, editor. DNA cloning: a practical approach. Vol. 1. Oxford, United Kingdom: IRL Press; 1985. pp. 49–78. [Google Scholar]
- 11.Hwang Y S, Panangala V S, Rossi C R, Giambrone J J, Lauerman L H. Monoclonal antibodies that recognize specific antigens of Mycoplasma gallisepticum and Mycoplasma synoviae. Avian Dis. 1989;33:42–52. [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Levinson G, Gutman G A. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987;4:203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- 14.Markham P F, Glew M D, Brandon M R, Walker I D, Whithear K G. Characterization of a major hemagglutinin protein from Mycoplasma gallisepticum. Infect Immun. 1992;60:3885–3891. doi: 10.1128/iai.60.9.3885-3891.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markham P F, Glew M D, Whithear K G, Walker I D. Molecular cloning of a member of the gene family that encodes pMGA, a hemagglutinin of Mycoplasma gallisepticum. Infect Immun. 1993;61:903–909. doi: 10.1128/iai.61.3.903-909.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markham P F, Glew M D, Sykes J E, Bowden T R, Pollock T D, Browning G F, Whithear K G, Walker I D. The organisation of the multigene family which encodes the major cell surface protein, pMGA, of Mycoplasma gallisepticum. FEBS Lett. 1994;352:347–352. doi: 10.1016/0014-5793(94)00991-0. [DOI] [PubMed] [Google Scholar]
- 17.Panangala V S, Morsy M A, Gresham M M, Toivio-Kinnucan M. Antigenic variation of Mycoplasma gallisepticum, as detected by use of monoclonal antibodies. Am J Vet Res. 1992;53:1139–1144. [PubMed] [Google Scholar]
- 18.Rooney S M, Moore P D. Antiparallel, intramolecular triplex DNA stimulates homologous recombination in human cells. Proc Natl Acad Sci USA. 1995;92:2141–2144. doi: 10.1073/pnas.92.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voelker L L, Weaver K E, Ehle L J, Washburn L R. Association of lysogenic bacteriophage MAV1 with virulence in Mycoplasma arthritidis. Infect Immun. 1995;63:4016–4023. doi: 10.1128/iai.63.10.4016-4023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H C, Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- 22.Yates J R, III, McCormack A L, Eng J. Mining genomes with MS. Anal Chem. 1996;68:534A–540A. doi: 10.1021/ac962050l. [DOI] [PubMed] [Google Scholar]