Abstract
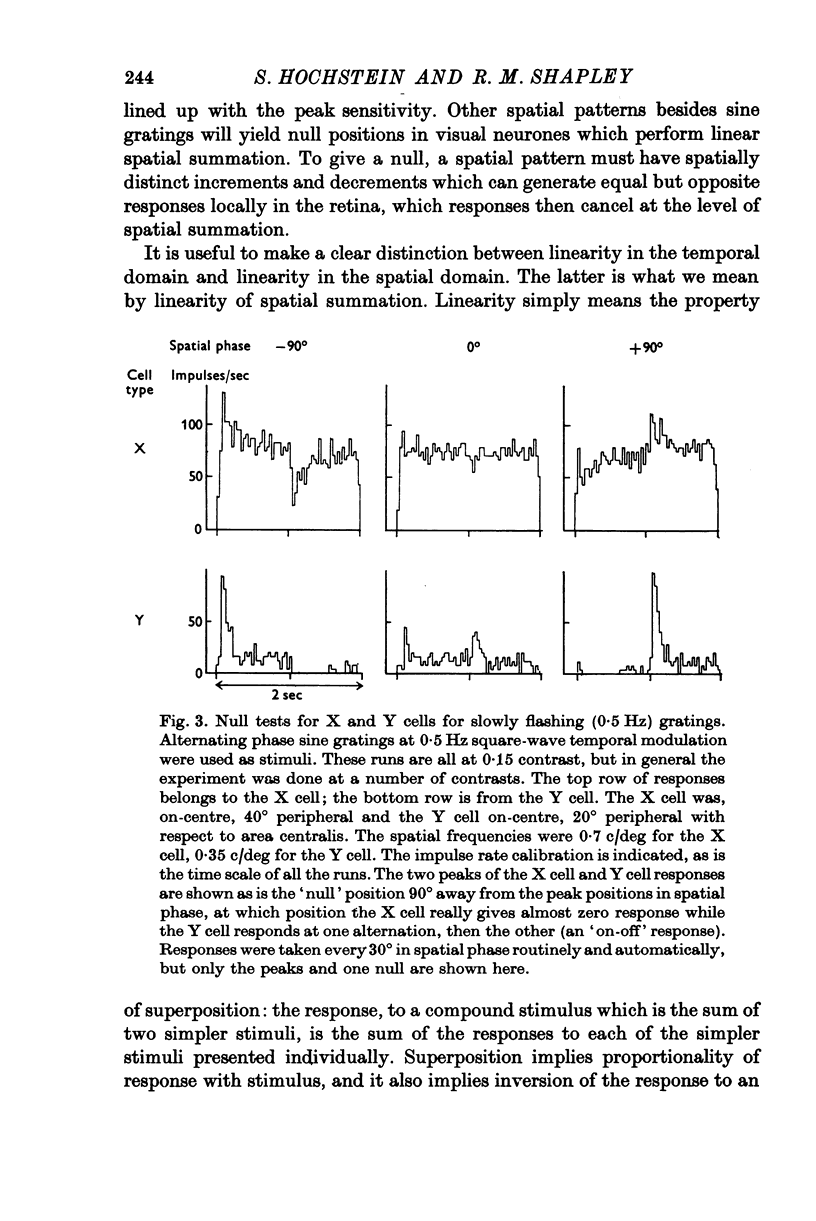
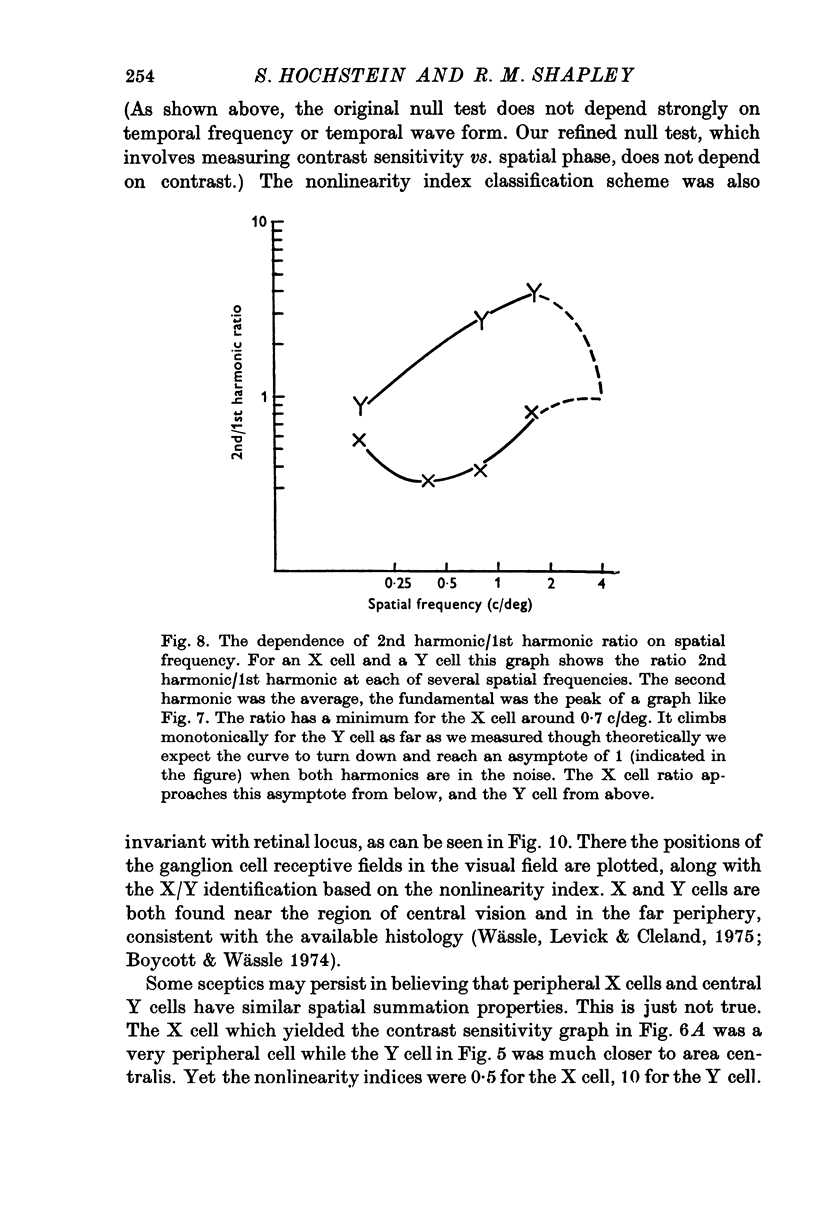
The classification of cat retinal ganglion cells as X or Y on the basis of linearity or nonlinearity of spatial summation has been confirmed and extended. Recordings were taken from optic tract fibres of anaesthetized, paralysed cats. 2. When an alternating phase sine wave grating was used as a stimulus, X cells had null positions and Y cells responded at all positions of the grating. 3. These results did not depend on the temporal wave form or the temporal frequency of pattern alternation over a wide range. 4. At high spatial frequencies for the particular cell, a Y cell gave abig 'on-off' response, or frequency doubling, at all positions of the grating, while an X cell did not. 5. The use of contrast sensitivity versus spatial phase also served to differentiate the two cell types. With an alternating sine grating stimulus X cells had a sinusoidal dependence on spatial phase, while each Y cell's sensitivity depended in a complicated manner on spatial phase. 6. Sensitivity versus spatial phase for different Fourier components of the neural response also separated the two classes of cells. Significant second harmonic distortion was present in Y cells. The second harmonic component was spatial phase insensitive, and became dominant at high spatial frequencies. 7. The maximum of the 2nd/1st harmonic ratio was taken as an index of nonlinearity. X cells always had a nonlinearity index less than 1 while in Y cells this index always exceeded 1. 8. Response to spots, diffuse light and drifting gratings were compared to the nonlinearity index as a basis for classifying cells. The nonlinearity index was most reliable because it was least dependent on retinal eccentricity.
Full text
PDF

























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boycott B. B., Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol. 1974 Jul;240(2):397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Adaptation and dynamics of cat retinal ganglion cells. J Physiol. 1973 Sep;233(2):271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Flux, not retinal illumination, is what cat retinal ganglion cells really care about. J Physiol. 1973 Sep;233(2):311–326. doi: 10.1113/jphysiol.1973.sp010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald R., Chase R. An improved method for plotting retinal landmarks and focusing the eyes. Vision Res. 1971 Jan;11(1):95–96. doi: 10.1016/0042-6989(71)90207-0. [DOI] [PubMed] [Google Scholar]
- Fukada Y. Receptive field organization of cat optic nerve fibers with special reference to conduction velocity. Vision Res. 1971 Mar;11(3):209–226. doi: 10.1016/0042-6989(71)90186-6. [DOI] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K. P., Stone J., Sherman S. M. Relay of receptive-field properties in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1972 Jul;35(4):518–531. doi: 10.1152/jn.1972.35.4.518. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Receptive field organization of 'sustained' and 'transient' retinal ganglion cells which subserve different function roles. J Physiol. 1972 Dec;227(3):769–800. doi: 10.1113/jphysiol.1972.sp010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakiela H. G., Enroth-Cugell C. Adaptation and dynamics in X-cells and Y-cells of the cat retina. Exp Brain Res. 1976 Feb 26;24(4):335–342. doi: 10.1007/BF00235001. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Form and function of cat retinal ganglion cells. Nature. 1975 Apr 24;254(5502):659–662. doi: 10.1038/254659a0. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res. 1973 Jul;13(7):1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Pettigrew J. D., Bishop P. O., Nikara T. Residual eye movements in receptive-field studies of paralyzed cats. Vision Res. 1967 Jan;7(1):107–110. doi: 10.1016/0042-6989(67)90031-4. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res. 1965 Dec;5(11):583–601. doi: 10.1016/0042-6989(65)90033-7. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Shapley R., Hochstein S. Visual spatial summation in two classes of geniculate cells. Nature. 1975 Jul 31;256(5516):411–413. doi: 10.1038/256411a0. [DOI] [PubMed] [Google Scholar]
- Shkolnik-Yarros E. G. Neurons of the cat's retina. Vision Res. 1971 Jan;11(1):7–26. doi: 10.1016/0042-6989(71)90202-1. [DOI] [PubMed] [Google Scholar]
- Stone J., Hoffman K. P. Conduction velocity as a parameter in the organisation of the afferent relay in the cat's lateral geniculate nucleus. Brain Res. 1971 Sep 24;32(2):454–459. doi: 10.1016/0006-8993(71)90339-8. [DOI] [PubMed] [Google Scholar]
- Wässle H., Levick W. R., Cleland B. G. The distribution of the alpha type of ganglion cells in the cat's retina. J Comp Neurol. 1975 Feb 1;159(3):419–438. doi: 10.1002/cne.901590308. [DOI] [PubMed] [Google Scholar]
- Yoon M. Influence of adaptation level on response pattern and sensitivity of ganglion cells in the cat's retina. J Physiol. 1972 Feb;221(1):93–104. doi: 10.1113/jphysiol.1972.sp009741. [DOI] [PMC free article] [PubMed] [Google Scholar]


