Abstract
The aim of this study was to investigate the effect of arterial hypertension (AH) and of obstructive sleep apnea (OSA) on cognitive course in the neurocognitive disorder (NCD) cohort RIFADE which enrolled patients with NCD due to Alzheimer’s disease (AD), vascular NCD (vNCD), and mixed NCD (AD + vNCD = mNCD). Multiple risk factors (RF), including AH and OSA, that contribute to the development of various kinds of dementia have been identified in previous studies. Studies that observed AH lacked investigation of long-term effects and did not isolate it from other RF. Studies involving OSA as a risk factor did not include participants with all stages of NCD. 126 subjects were screened for AH and OSA. Repeated cognitive measurements were performed with the DemTect as primary outcome and the clock drawing test as secondary outcome measure. 90 patients had AH (71.4%) and 40 patients had OSA (31.7%). RF-status had a significant effect on cognitive outcome in models with RF as single factors (AH p = 0.027, OSA p < 0.001), a 2-factor analysis with AH × OSA (AH as main factor p = 0.027) as well as a model including the 3 factors AH × OSA × diagnosis (p = 0.038). Similarly, a 3-factor model was significant for the clock-drawing test, whereas single factor-models remained insignificant. AH and OSA appear to be risk factors in common NCD and cognitive decline can be mitigated by treatment of these RF.
Subject terms: Alzheimer's disease, Hypertension, Dementia, Sleep disorders
Introduction
There is increasing evidence that certain risk factors play a role in the two most common types of dementia, i.e. Alzheimer’s disease (AD) and vascular dementia (VaD). Shown primarily by observational studies, it turned out, that vascular risk factors, hypacusis, environmental conditions and lifestyle behaviors could account for the development of up to 40% of dementias1.
This is of particular interest for Alzheimer’s disease (AD), where successful treatments improving or even stabilizing cognitive outcomes for time periods of years have so far been lacking. However, correction or elimination of a suspected pathological factor does not necessarily lead to successful treatment, which has been revealed by numerous attempts to address cellular pathology. Immunotherapies against amyloid or tau pathology have so far failed to show cognitive improvement or stabilization in the long term in AD2–6.
As a first important example of such factors contributing to the pathology of neurocognitive disorders (NCD), arterial hypertension (AH) is presented and analyzed in this publication. Being one of the most relevant factors in medicine, AH is clearly proven as a risk factor for Alzheimer´s disease and vascular dementia7–9. In addition, there is initial evidence, that correcting elevated blood pressure has positive effects on cognitive outcome10–13. However, this has not been shown in the long term and for AH as a single factor, i.e. in an isolated analysis without complexing and merging with other factors14–16. In addition, there are few studies addressing the question of whether treatment of AH in patients with a pre-existing NCD could have favorable effects. Wharton et al.17 found that participants affected by AH were less likely to convert from MCI to AD when treated with antihypertensive medications. Similar effects were found in participants with already existing AD, usage of antihypertensive medication was associated with a slower rate of cognitive decline compared to AD individuals that had never been treated with this kind of medication18.
Another suspected risk factor for dementia is obstructive sleep apnea (OSA). The estimated prevalence of OSA ranges from 9 to 38 percent in the general population. In elderly males, it is estimated at 90 percent19. Similarly, a greatly increased odds ratio for developing OSA has been reported with increasing age20.
Obstructive sleep apnea is characterized by the collapse of upper airways through relaxed throat musculature causing intermittent hypoxia and sleep fragmentation21. For the sleep disturbance to be considered OSA, the breathing cessation must persist for a duration of at least 10 s and occur more than 5 times per hour of sleep. Alternatively, airflow must show a reduction at least five times per hour, including a drop in oxygen or a rise in carbon dioxide22. The results of disrupted sleep and hypoxia are excessive daytime sleepiness (EDS)23, fatigue, depression24, and cognitive complaints25,26. Cognitive domains usually impaired in OSA are working memory, vigilance/attention27 and executive functioning19,21,28,29.
There are several lines of evidence showing OSA as a putative risk factor for neurocognitive disorders including AD. First, the hippocampus as a major target of pathologies leading to dementia, shows high vulnerability to hypoxic events such as those found in OSA30. Continuous positive airway pressure (CPAP), the gold standard in the treatment of OSA31, seems to have a beneficial influence on mild cognitive impairment (MCI). Improved attention, psychomotor speed and everyday functioning, and reduced EDS have been shown after one year of CPAP32. Treating OSA may slow disease progression in MCI patients and even show short-term improvements on cognitive scales33. Untreated OSA causes arterial hypertension and is often associated with other vascular risk factors, increasing the risk of cardiac or cerebrovascular diseases potentially leading to dementia34–36. A long-term follow-up analysis showed a partial remission of cognitive deficits, a reduction of EDS and depressive symptoms37,38.
Neuroimaging studies indicated not only functional brain alterations in OSA39 but also recovery after CPAP-treatment in brain regions which were affected by hypoxic damage40 i.e. the hippocampus41,42, the frontal gyri43, and the default mode network44,45.
Studies including patients already suffering from mild cognitive impairment (MCI) and Alzheimer’s disease showed a benefit for treatment of OSA delaying dementia onset or slowing disease progression32,37,46–48. These studies, however, did not investigate the effect of treatment in patient groups including all stages of pre-existing NCD.
Finally, a meta-analysis indicated an epidemiological argument for OSA as a risk factor for AD showing a five-fold increased prevalence of OSA in patients with AD compared to cognitive healthy individuals49.
The cohort RIFADE (RIsk FActors of DEmentia) presented here is a single-center cohort with neurocognitive disorder patients enrolled in the lower Rhine area of Germany. This cohort is in detail described in a prior publication50. The current study aims to investigate the isolated effects of the risk factors AH and OSA in all stages of NCD.
Methods
Study population
The present analysis used the clinical data of the German neurocognitive disorder (NCD) cohort RIFADE (n = 126), which is a retrospective single-center study focusing on the role of risk factors in NCD50. Patients of RIFADE entered the study with the diagnoses NCD due to Alzheimer`s disease (AD-NCD), NCD of vascular type (vascular-NCD) or a combination of both diseases (mixed-NCD). Few patients did not fulfill the criteria of one of these disorders, denoted as neurocognitive disorder of unclear etiology (unspecified-NCD). Important exclusion criteria were the presence of severe Parkinson’s disease, frontotemporal degeneration, Lewy-body-disease, and being resident of a nursing home.
Informed consent was obtained from all subjects. The RIFADE cohort is registered on GermanCTR.de with identifier DRKS00027217. It complies with the Declaration of Helsinki and Good Clinical Practice Guidelines and has been approved by The Ethics Committee at the Faculty of Medicine of Heinrich-Heine-University Düsseldorf.
Assessments
Obstructive sleep apnea
The Epworth sleepiness scale was applied to each patient at the first visit. If scores were suspicious for obstructive sleep apnea (score ≥ 10), patients were referred for a polygraphy. In case of an apnea–hypopnea index (AHI) ≥ 5 a polysomnography (PSG) was performed. If PSG indicated a diagnosis of obstructive sleep apnea, an AHI of 5/h was considered as cut-off for the need of treatment according to the criteria of the International Classification of Sleep Disorders51. Stages of OSA were classified for severity as mild grade (AHI 5–15), moderate grade (AHI 15–29) and severe grade (AHI ≥ 30).
In cases showing OSA with the need for treatment, continuous positive airway pressure ventilation during sleep (CPAP) was initiated and patients were followed according to local clinical practice. Adherence to treatment by CPAP was defined as a mean use ≥ 4 h per night for > 5 nights per week with a residual AHI < 5/h.
In the cases where CPAP was not tolerated or not possible, another treatment was initiated including the following treatment options: (1) a mandibular advancement device, (2) a positional therapy.
Obstructive sleep apnea (OSA) was considered present in case of the above criteria were met. OSA was considered corrected in case of CPAP or alternative treatment according to the above criteria.
Arterial hypertension
Arterial hypertension (AH) was considered present in case of (1) a pre-existing medication with an antihypertensive drug and/or (2) a mean value of blood pressure (BP) > 140/90 mm Hg in at least 10 successive measurements during 5 days and/or (3) an anamnesis indicating existing arterial hypertension.
Arterial hypertension was considered corrected in case of (1) regular intake of at least 1 antihypertensive drug and/or (2) a mean value BP < 140/90 mm Hg in at least 10 successive measurements during 5 days.
Primary outcome
As primary outcome variable the DemTect score was assessed at each visit in the patients cohort. Times between visits followed a general scheme of 3, 6, and 12 months after baseline visit followed by yearly visits. According to a natural setting, this scheme varied due to adherence and clinical acuity. The DemTect represents a common cognitive test, which is validated to categorize and predict outcome in NCD52. Repeated measurements of DemTect scores were used as absolute scores as well as the change in scores of two neighboured measurements according to the formula: DemTect change = DemTect score at the current measurement minus DemTect score at the previous measurement.
Since the DemTect uses an age-dependent algorithm for the calculation of normalized scores from raw values with a cut-off at the age of 60, there may be comparability problems with repeated measurements in subjects who pass the 60-year limit during observation. Therefore, in these patients the scores derived from the algorithm for subjects aged < 60 (DemTect score < 60) and ≥ 60 years (DemTect score ≥ 60) were averaged at each visit due to the formula: (DemTect score < 60 + DemTect score ≥ 60)/2 in order to achieve age-independent scores for calculation of the DemTect change53.
In order to stratify data for the initial stages of the longitudinal cognitive course, initial DemTect scores were referred to groups with initial scores of 13–18 (stage 1), 9–12 (stage 2) and < 9 (stage 3), aiming to achieve a staging similar to that of subjective cognitive disorder (stage 1) mild cognitive impairment (stage 2), and dementia (stage 3). For this staging, the original age-dependent scores of the DemTect were used.
To achieve a measure for the final cognitive outcome in addition to repeated measurements of the primary outcome measure, stages at baseline and stages at the final visit were evaluated for favorable and unfavorable outcomes due to the algorithm: (A) favorable outcome was assessed, if patients started (1) in stage 1 or 2 and remained in the same stage, (2) started in stage 2 and improved to stage 1, (3) started in stage 3 and improved to stage 1 or 2. (B) Unfavorable outcome was assigned to all other cognitive courses, which were different from A.
As a secondary outcome, Shulman’s clock-drawing test was performed54. This brief screening test relying on visuo-constructive abilities has proven to reliably discriminate between patients suffering from Alzheimer’s disease, mild cognitive impairment and healthy individuals55,56. Testing intervals were the same as for the DemTect (3, 6, 12 months and yearly follow up visits).
Evaluation of risk factors
To study the influence of risk factors on the cognitive outcome, neurocognitive time periods (NCT) between 2 successive measurements of the primary outcome variable were established. Since the primary outcome variable was recorded as repeated measures, each patient exhibits at least 1 NCT. The risk factors AH and OSA were evaluated for each NCT regarding (1) presence status, (2) correction status, according to the above-mentioned criteria. If the time of correction of a risk factor exceeded 50% of NCT, the correction status for this factor in this NCT was assigned as "corrected", otherwise the correction status was recorded as "uncorrected". For more details see the previous publication on RIFADE50.
Both types of risk factor status, presence and correction status, were integrated into a three-fold status, i.e. the status "absent" (A), if the risk factor is not present, "treated" (T+), if the factor is considered corrected due to the criteria given above, "untreated" (T−), if the factor is not considered corrected.
Statistics
The relationships between DemTect score/DemTect change and the status of AH and OSA were investigated by mixed effects repeated measurement models. For this purpose, the risk factors AH and OSA as well as the combination thereof were taken as a fixed effect and extra variability in repeated measurements originating from individual patients was taken as a random effect. Different models according to possible combinations of risk factors were calculated and corrected for the parameters age, DemTect at baseline, education and time since inclusion or time to measurement (NCT). In addition, a model with diagnosis as a further factor was performed. According to the analysis performed for the DemTect as the primary outcome all four models/linear mixed models were calculated for the clock-drawing test/secondary outcome as well.
χ2-tests are calculated to examine the influence of treated risk factors on favourable cognitive outcome. T-tests are used to compare the observation time of the treated and untreated groups with respect to the risk factors.
Analysis was performed with SPSS 26. N = 126 patients were observed.
Ethical considerations
This study was designed and conducted according to the Declaration of Helsinki. The study protocol was approved by the Ethical Committee of the Heinrich Heine University Düsseldorf. The study was performed under the laws of General Data Protection Regulation (GDPR) and the Code of Good Conduct.
Results
Demographic and clinical data
All patients of the RIFADE cohort were included in this study (n = 126). According to the above mentioned criteria, 90 patients had arterial hypertension (AH) in this cohort (71.4%) and 40 (31.7%) appeared to have obstructive sleep apnea (OSA). Numbers of combinations of AH and OSA are shown in the flow chart (Fig. 1). Seventy nine of the AH patients had permanently corrected hypertension (87.8%) and 13 of the OSA patients had permanent OSA treatment (33.3%). 11 of the 13 permanently treated patients with OSA received CPAP treatment, one patient used a mandibular advancement device, one patient performed position therapy. Four patients with OSA received treatment non-permanently, i.e. not in all NCTs.
Figure 1.
Flow chart patient inclusion. AH, arterial hypertension; OSA, obstructive sleep apnea; Treated(+), risk factor is permanently treated during patient observation; Untreated(−), risk factor is not treated in at least 1 neurocognitive time period (NCT) of patient observation.
Patients with AH were older, more often male and had a lower score in the DemTect at baseline compared to those without AH. No differences were present in the level of education and comorbidity with OSA between these groups.
Patients with OSA were more often male compared to gender distribution across groups. Patients in the treated group were higher educated, younger, had a higher score in the DemTect baseline, and had a lower proportion of vascular NCD as compared to the untreated group. T-Tests revealed only significant differences for the covariate age since the AH+ (p = 0.0001) and OSA− (p = 0.0002) groups were older than their counterparts. Demographic and clinical data are shown in Tables 1 and 2.
Table 1.
Demographic data of patients with and without arterial hypertension.
| Demography | Missings | Total (n = 126, 100%) |
AH− (n = 36, 28.6%) |
AH+ (n = 90, 71.4%) |
T-test/χ2-test | p-value |
|---|---|---|---|---|---|---|
| Mean age (yrs, SD) | 0 | 70.60 ± 10.61 | 65.31 ± 11.38 | 72.71 ± 9.49 | t(124) = − 3.730 | 0.0001 |
| Female sex (n, %) | 0 | 66 (52.4%) | 22 (61.1%) | 44 (49%) | χ2(1) = 1.540 | 0.215 |
| Education (yrs, SD) | 0 | 9.25 ± 2.53 | 9.25 ± 2.1 | 9.26 ± 2.69 | t(124) = − 0.020 | 0.492 |
| DemTect baseline | 0 | 12.64 ± 4.24 | 13.40 ± 4.12 | 12.33 ± 4.27 | t(124) = 1.283 | 0.201 |
| OSA present (n, %) | 0 | 40 (31.7%) | 11 (30.6%) | 29 (32.2%) | χ2(1) = 0.033 | 0.856 |
| Diagnosis (n) | 0 | 80/28/7 | 22/7/2 | 58/21/5 | χ2(3) = 1.756 | 0.624 |
AD Alzheimer’s disease, AH arterial hypertension, Diagnosis patients with the neurocognitive disorder (NCD) of mixed/vascular/Alzheimer type (unspecified omitted), n number of subjects, SD standard deviation, +/− with/without AH, yrs years.
Table 2.
Demographic data of OSA patients.
| (a) Obstructive sleep apnea patients divided by Apnea–Hypopnea Indexa | |||||
|---|---|---|---|---|---|
| Demography | Missings | Total (n = 40, 100%) | OSA mild (n = 11, 27.5%) | OSA moderate (n = 14, 35%) | OSA severe (n = 15, 37.5%) |
| Mean AHI | 0 | 25.34 ± 14.96 | 9.6 ± 3.69 | 20.45 ± 4.07 | 40.97 ± 11.11 |
| Female sex (n, %) | 0 | 11 (27.5%) | 5 (45%) | 1 (7.1%) | 5 (33%) |
| Education (yrs, SD) | 0 | 10.25 ± 3.24 | 9.25 ± 2.1 | 9.26 ± 2.69 | 9.26 ± 2.69 |
| Mean age (yrs, SD) | 0 | 68.54 ± 11.10 | 72.92 ± 8.65 | 65.17 ± 11.05 | 68.47 ± 11.62 |
| DemTect baseline | 0 | 13.1 ± 3.79 | 14.21 ± 3.32 | 12.67 ± 4.53 | 12.28 ± 2.80 |
| BMI | 11 | 27.27 ± 3.97 | 26.76 ± 4.45 | 26.96 ± 3.28 | 28.83 ± 3.72 |
| Diagnosis (n) | 0 | 20/11/1 | 6/2/0 | 4/6/0 | 10/3/1 |
| AH present (n, %) | 0 | 29 (72.5%) | 9 (82%) | 9 (64.3%) | 11 (73.3%) |
| (b) Obstructive sleep apnea patients divided by treatment statusb | |||||
|---|---|---|---|---|---|
| Demography | OSA+ (n = 13) | OSA− (n = 27) | T-test/χ2-test | p-value | |
| Mean AHI | 29.175 ± 14.59 | 23.82 ± 14.9 | t(38) = 1.071 | 0.290 | |
| Female sex (n, %) | 4(31%) | 6(22%) | χ2(1) = 0.342 | 0.559 | |
| Education (yrs, SD) | 11.15 ± 3.30 | 9.81 ± 3.12 | t(38) = 1.249 | 0.219 | |
| Mean age (yrs, SD) | 60.12 ± 11.63 | 72.59 ± 8.17 | t(38) = − 3.929 | 0.0002 | |
| DemTect baseline | 14.31 ± 4.16 | 12.52 ± 3.46 | t(38) = 1.343 | 0.160 | |
| BMI | 27.57 ± 3.46 (2m) | 27.08 ± 4.27 (9m) | t(27) = 0.321 | 0.751 | |
| AH present | 9(69%) | 20(74%) | χ2(1) = 0.103 | 0.748 | |
| Diagnosis | 7/1/1 | 13/9/0 | χ2(1) = 5.027 | 0.151 | |
aAHI Apnea–Hypopnea Index, BMI body mass index, Diagnosis see Table 1, moderate AHI 15–29.9, n number of subjects, OSA obstructive sleep apnea, SD standard deviation, severe AHI ≥ 30, mild AHI 5–14.9, yrs years.
bBMI Body Mass Index, Diagnosis see Table 1, m missings, OSA- untreated OSA patients who were either CPAP incompliant (n = 6), or received CPAP therapy for < 50% of total NCT (n = 4), or did not receive any OSA therapy according to medical records (n = 17), OSA + treated OSA patients, yrs years.
Mixed model analysis
In order to analyze the relationships between DemTect score/DemTect change and AH- and OSA-status, 4 models were run as mixed linear models. In addition to the analysis of the single factors AH (model 1) and OSA (model 2), the combination of both factors (model 3) and a model additionally including diagnosis as factor (model 4) was calculated. Each model was corrected for the parameters age, time since inclusion (for DemTect score) or time to measurement (NCT) for DemTect change, DemTect score at baseline and education. For analyses which included diagnosis as a factor, patients with NCD-unspecified were excluded from the analysis. Results are shown in Tables 3 and 4 and Figs. 2, 3, 4, 5, 6 and 7. Further details can be found Supplementary Tables 1–5 .
Table 3.
Mixed linear model analysis of repeated DemTect scores as dependent variable with (a) fixed effects type III, and (b) mean values for risk factors in different states.
| (1a) Model 1: AH as single factorA | ||||||
|---|---|---|---|---|---|---|
| Source | Numerator-df | Denominator-df | F | p | ||
| Constant term | 1 | 140.649 | 29.918 | 0.000 | ||
| AH | 2 | 212.023 | 1.583 | 0.208 | ||
| Age (yrs) | 1 | 160.070 | 15.972 | 0.000 | ||
| Education | 1 | 120.031 | 0.208 | 0.649 | ||
| Time | 1 | 356.930 | 24.515 | 0.000 | ||
| DemTect baseline | 1 | 119.459 | 123.074 | 0.000 | ||
| (1b) Model 1: AH as single factorB | ||||||
|---|---|---|---|---|---|---|
| AH | M | SE | Df | Confidence interval 95% | ||
| Lower bound | Upper bound | |||||
| Absent | 12.026a | 0.484 | 131.536 | 11.067 | 12.984 | |
| Treated | 11.820a | 0.296 | 133.749 | 11.235 | 12.406 | |
| Untreated | 10.337a | 0.877 | 358.256 | 8.611 | 12.062 | |
| (2) Model 2: OSA as single factorC | ||||||
|---|---|---|---|---|---|---|
| Source | Numerator-df | Denominator-df | F | p | ||
| Constant term | 1 | 127.529 | 26.073 | 0.000 | ||
| OSA | 2 | 205.267 | 8.507 | 0.000 | ||
| Age (yrs) | 1 | 149.177 | 7.405 | 0.007 | ||
| Education | 1 | 113.613 | 1.252 | 0.265 | ||
| Time | 1 | 352.392 | 37.170 | 0.000 | ||
| DemTect baseline | 1 | 111.640 | 115.519 | 0.000 | ||
| (3a) Model 3: AH × OSAD | ||||||
|---|---|---|---|---|---|---|
| Source | Numerator-df | Denominator-df | F | p | ||
| Constant term | 1 | 134.739 | 27.847 | 0.000 | ||
| AH | 2 | 180.897 | 2.735 | 0.068 | ||
| OSA | 2 | 256.012 | 1.475 | 0.231 | ||
| AH × OSA | 4 | 256.666 | 1.689 | 0.153 | ||
| Age (yrs) | 1 | 153.478 | 10.631 | 0.001 | ||
| Education | 1 | 115.844 | 0.953 | 0.331 | ||
| Time | 1 | 354.551 | 37.306 | 0.000 | ||
| DemTect baseline | 1 | 114.613 | 122.135 | 0.000 | ||
| (3b) Model 3: AH × OSAE | ||||||
|---|---|---|---|---|---|---|
| OSA | AH | M | SE | Df | Confidence interval 95% | |
| Lower bound | Upper bound | |||||
| Absent | Absent | 11.222a | 0.573 | 144.825 | 10.089 | 12.354 |
| Treated | 11.553a | 0.363 | 140.246 | 10.834 | 12.271 | |
| Untreated | 9.974a | 1.213 | 365.969 | 7.589 | 12.359 | |
| Treated | Absent | 15.489a | 0.931 | 206.883 | 13.654 | 17.325 |
| Treated | 13.237a | 0.988 | 150.161 | 11.286 | 15.188 | |
| Untreated | 9.328a | 2.269 | 173.977 | 4.850 | 13.806 | |
| Untreated | Absent | 11.562a | 0.951 | 244.244 | 9.690 | 13.435 |
| Treated | 11.855a | 0.579 | 132.295 | 10.710 | 12.999 | |
| Untreated | 11.391a | 1.439 | 365.788 | 8.562 | 14.221 | |
AAH arterial hypertension in the states absent, treated, untreated, DemTect baseline DemTect score in initial testing, Denominator df = denominator degrees of freedom, Education Education in the categories low/intermediate/high, Numerator df numerator degrees of freedom, Time time since inclusion.
BNote: aCovariates in the model were calculated using: Age 72.932, Education 1.45, Time since inclusion 973.1284, DemTect score 11.993. Absent risk factor (RF) is not present, treated RF is present and treated, untreated RF is present and untreated, AH arterial hypertension in the states absent, treated, untreated, SE Standard error.
COSA obstructive sleep apnea in the states absent, treated, untreated. For further abbreviations see Table 3(1a) and (1b).
DFor abbreviations see Table 3(1a) and (1b).
ENote: aCovariates in the model were calculated using: Age 72.932, Education 1.45, Time since inclusion 973.1284, DemTect score baseline 11.993. For other abbreviations see Table 3(1a) and (1b).
Table 4.
Mixed linear model analysis of DemTect change as dependent variable with (a) fixed effects type III and (b) mean values for risk factors in different states.
| (1) Model 1: AH as single factorA | |||||||
|---|---|---|---|---|---|---|---|
| Source | Numerator-df | Denominator-df | F | p | |||
| Constant term | 1 | 366 | 12.748 | 0.000 | |||
| AH | 2 | 366 | 3.642 | 0.027 | |||
| Age (yrs) | 1 | 366 | 10.589 | 0.001 | |||
| Education | 1 | 366 | 0.258 | 0.612 | |||
| NCT | 1 | 366 | 16.582 | 0.000 | |||
| DemTect start | 1 | 366 | 5.487 | 0.020 | |||
| (2a) Model 2: OSA as single factorB | |||||||
|---|---|---|---|---|---|---|---|
| Source | Numerator-df | Denominator-df | F | p | |||
| Constant term | 1 | 366 | 12.041 | 0.001 | |||
| OSA | 2 | 366 | 1.287 | 0.277 | |||
| Age (yrs) | 1 | 366 | 6.094 | 0.014 | |||
| Education | 1 | 366 | 0.355 | 0.551 | |||
| NCT | 1 | 366 | 19.017 | 0.000 | |||
| DemTect start | 1 | 366 | 5.049 | 0.025 | |||
| (2b) Model 2: OSA as single factorC | |||||||
|---|---|---|---|---|---|---|---|
| OSA | M | SE | Df | Confidence interval 95% | |||
| Lower bound | Upper bound | ||||||
| Absent | − 0.147d | 0.201 | 366 | − 0.542 | 0.248 | ||
| Treated | 0.409d | 0.478 | 366 | − 0.531 | 1.348 | ||
| Untreated | − 0.480d | 0.286 | 366 | − 1.042 | 0.082 | ||
| (3) Model 3: AH × OSAD | |||||||
|---|---|---|---|---|---|---|---|
| Source | Numerator-df | Denominator-df | F | p | |||
| Constant term | 1 | 366 | 12.194 | 0.001 | |||
| AH | 2 | 366 | 3.638 | 0.027 | |||
| OSA | 2 | 366 | 0.158 | 0.854 | |||
| AH × OSA | 4 | 366 | 0.550 | 0.699 | |||
| Age (yrs) | 1 | 366 | 8.598 | 0.004 | |||
| Education | 1 | 366 | 0.263 | 0.608 | |||
| NCT | 1 | 366 | 17.211 | 0.000 | |||
| DemTect start | 1 | 366 | 5.776 | 0.017 | |||
| (4a) Model 4: AH × OSA × diagnosisE | |||||||
|---|---|---|---|---|---|---|---|
| Source | Numerator-df | Denominator-df | F | p | |||
| Constant term | 1 | 366 | 15.154 | 0.000 | |||
| Diagnosis | 3 | 366 | 1.221 | 0.302 | |||
| AH | 2 | 366 | 2.964 | 0.053 | |||
| OSA | 2 | 366 | 1.514 | 0.221 | |||
| AH × OSA | 4 | 366 | 0.308 | 0.873 | |||
| Diagnosis × AH | 4 | 366 | 0.400 | 0.809 | |||
| Diagnosis × OSA | 6 | 366 | 1.588 | 0.149 | |||
| Diagnosis × AH × OSA | 2 | 366 | 2.076 | 0.127 | |||
| Age (yrs) | 1 | 366 | 9.503 | 0.002 | |||
| Education | 1 | 366 | 0.023 | 0.879 | |||
| NCT | 1 | 366 | 20.260 | 0.000 | |||
| DemTect start | 1 | 366 | 8.654 | 0.003 | |||
| (4b) Model 4: AH × OSA × diagnosisF | |||||||
|---|---|---|---|---|---|---|---|
| OSA | AH | Diagnosis | M | SE | Df | Confidence interval 95% | |
| Lower bound | Upper bound | ||||||
| Absent | Absent | AD | − 0.443d | 1.405 | 366 | − 3.207 | 2.320 |
| MIXED | − 0.430d | 0.422 | 366 | − 1.260 | 0.400 | ||
| VASC | 1.252d | 1.089 | 366 | − 0.890 | 3.394 | ||
| Treated | AD | − 0.023d | 0.985 | 366 | − 1.959 | 1.913 | |
| MIXED | − 0.117d | 0.264 | 366 | − 0.637 | 0.402 | ||
| VASC | 0.948d | 0.636 | 366 | − 0.303 | 2.198 | ||
| Untreated | AD | c,d | |||||
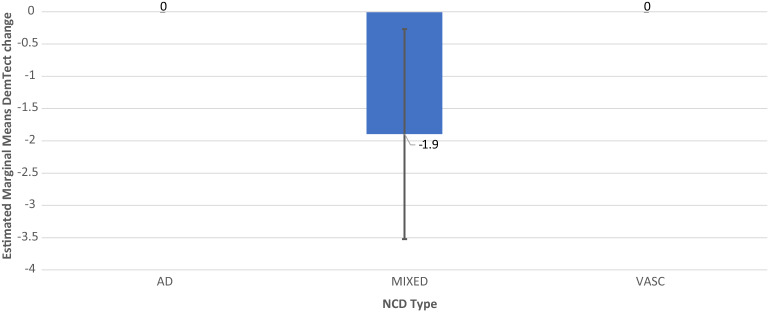
| MIXED | − 2.202d | 1.256 | 366 | − 4.672 | 0.268 | ||
| VASC | c,d | ||||||
| Present | Absent | AD | c,d | ||||
| MIXED | 0.379d | 0.861 | 366 | − 1.313 | 2.071 | ||
| VASC | − 0.070d | 0.917 | 366 | − 1.874 | 1.733 | ||
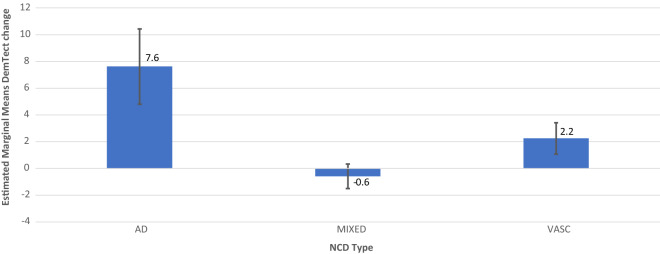
| Treated | AD | 7.622d | 2.810 | 366 | 2.095 | 13.148 | |
| MIXED | − 0.589d | 0.913 | 366 | − 2.385 | 1.207 | ||
| VASC | 2.244d | 1.175 | 366 | − 0.067 | 4.554 | ||
| Untreated | AD | c,d | |||||
| MIXED | c,d | ||||||
| VASC | c,d | ||||||
| Untreated | Absent | AD | c,d | ||||
| MIXED | − 1.366d | 1.035 | 366 | − 3.402 | 0.670 | ||
| VASC | 0.827d | 0.994 | 366 | − 1.128 | 2.781 | ||
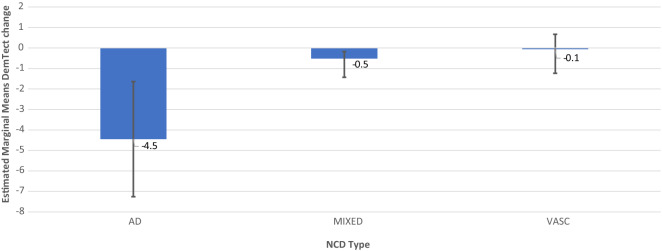
| Treated | AD | − 4.446d | 2.809 | 366 | − 9.970 | 1.078 | |
| MIXED | − 0.514d | 0.344 | 366 | − 1.191 | 0.163 | ||
| VASC | − 0.058d | 0.729 | 366 | − 1.492 | 1.376 | ||
| Untreated | AD | c,d | |||||
| MIXED | − 1.895d | 1.626 | 366 | − 5.093 | 1.302 | ||
| VASC | c,d | ||||||
ADemTect start DemTect score at begin of NCT, NCT time between repeated measurements of the DemTect. For other abbreviations see Table 3(1a) and (1b).
BFor abbreviations see Tables 3(1a) and (1b) and 4(1).
CFor abbreviations and notes see Tables 3(1a) and (1b) and 4(1).
DFor abbreviations see Tables 3(1a) and (1b) and 4(1).
EFor abbreviations see Tables 3(1a) and (1b), 4(1) and Supplementary Tables 2, 3.
FFor abbreviations and notes see Tables 3(1a) and (1b), 4(1) and Supplementary Tables 2, 3.
Figure 2.
Model 2: OSA as Single Factor, including M and SE.
Figure 3.
Model 4 AH × OSA × Diagnosis for AH+OSA− including M and SE.
Figure 4.
Model 4 AH × OSA × Diagnosis for AH+OSA+ DemTect score, including M and SE.
Figure 5.
Model 4 AH × OSA × Diagnosis for AH−OSA− including M and SE.
Figure 6.
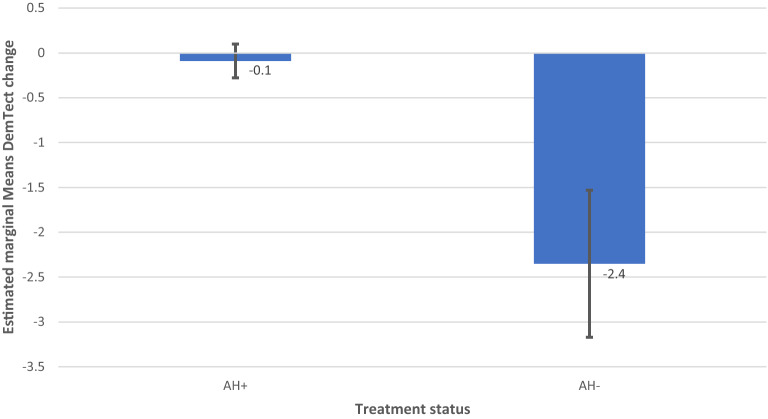
Model 1 AH as Single Factor DemTect change including M and SE.
Figure 7.
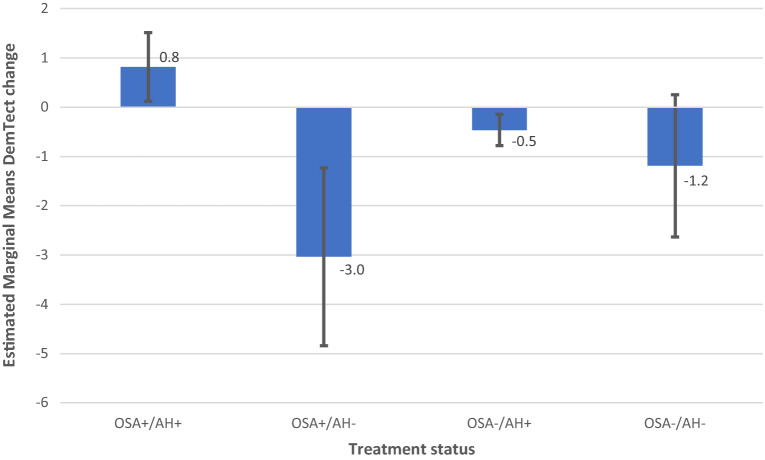
Model 3 AH × OSA DemTect change including M and SE.
Model 1 revealed a non-significant effect of AH on DemTect scores (F (2, 212.023) = 1.583 p = 0.208) and a significant effect of AH on DemTect change (F (2, 366) = 3.642, p = 0.027).
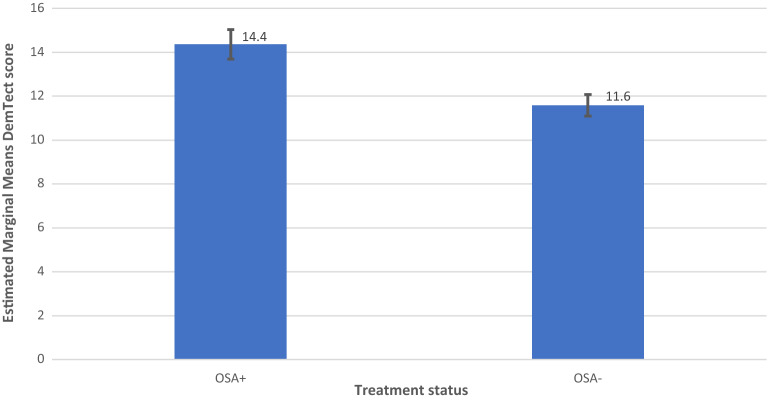
Model 2 showed a significant effect of OSA on DemTect scores (F (2, 205.267) = 8.507, p = 0.0003) and a non-significant effect of OSA on DemTect change (F (2, 366) = 1.287, p = 0.277).
In model 3, 2-factorial analysis with the predictors OSA and AH revealed a non-significant effect of AH (F (2, 180.897) = 2.735, p = 0.068) and of OSA and OSA x OH (F (2, 256.012) = 1.475, p = 0.231) and (F (4, 256.666) = 1.689, p = 0.153) on DemTect scores. The same analysis resulted in a significant effect of AH (F (2, 366) = 3.638, p = 0.027) and a non-significant effect of OSA (F (2, 366) = 0.158, p = 0.854) and AH × OSA (F (4, 366) = 0.55, p = 0.699) on DemTect change.
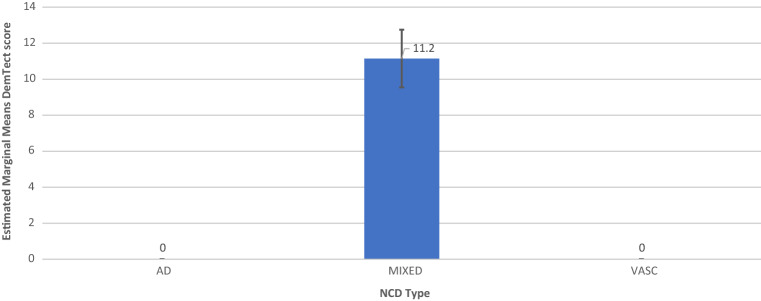
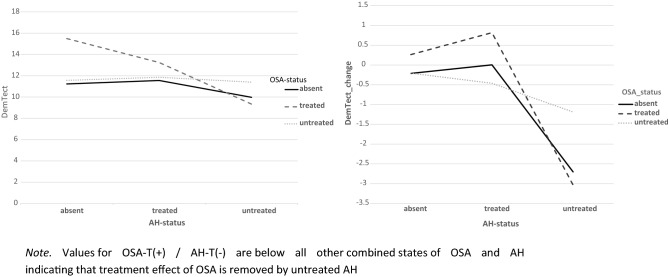
A synopsis of mean DemTect scores as revealed in model 3 for different states of OSA and AH is shown in Fig. 8.
Figure 8.
Synopsis of mean DemTect scores (l) and mean DemTect change (r) derived from model 3 for different states of OSA and AH. Note. Values for OSA-T(+)/AH-T(−) are below all other combined states of OSA and AH indicating that treatment effect of OSA is removed by untreated AH.
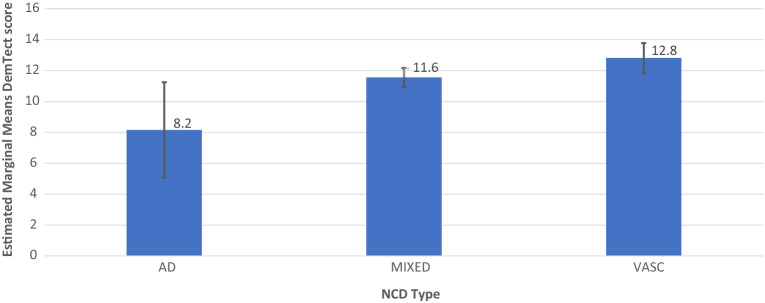
Model 4 showed a significant interaction effect of all 3 factors AH × OSA × diagnosis on DemTect scores (F (2, 202.324) = 3.329, p = 0.038) and a significant main effect of OSA on DemTect scores (F (2, 262.129) = 6.141, p = 0.002) and a non-significant main effect of AH on DemTect change (F (2, 366) = 2.964, p = 0.053). Diagnosis revealed a non-significant effect on DemTect scores (F (2, 123.538) = 2.941, p = 0.057). All other analyses in this model proved non-significant for single or combined factors on both outcome variables.
In order to analyze the effects of different states of risk factors AH and OSA in diagnostic groups, two separate models were calculated with AH and diagnosis (model 5) and OSA and diagnosis (model 6) as factors and DemTect change as the dependent variable, including the same parameters as covariates as in the above-reported models. Whereas model 5 showed no significant effect on DemTect change, neither for AH as a single factor, nor for the combination AH x diagnosis, model 6 revealed a significant effect of OSA (F(2, 357) = 5.16, p = 0.006) and a non-significant effect of OSA x diagnosis (F(4,357) = 2.20, p = 0.068). Effects of diagnosis in different states of risk factors could be evaluated by estimated marginal means to give an impression of diagnosis-related patterns (see Table 5 and Supplementary Table 6).
Table 5.
Estimated marginal means of DemTect change as dependent variable in (a) AH × diagnosis, and (b) OSA × diagnosis.
| (a) Diagnosis × AHA | ||||||
|---|---|---|---|---|---|---|
| Diagnosis | AH | M | SE | Df | Confidence interval 95% | |
| Lower bound | Upper bound | |||||
| AD | Absent | − 0.356e | 1.438 | 357 | − 3.185 | 2.472 |
| Treated | 0.338e | 0.901 | 357 | − 1.433 | 2.110 | |
| Untreated | c, e | |||||
| MIXED | Absent | − 0.391e | 0.377 | 357 | − 1.131 | 0.350 |
| Treated | − 0.281e | 0.207 | 357 | − 0.689 | 0.127 | |
| Untreated | − 2.046e | 1.017 | 357 | − 4.047 | − 0.046 | |
| VASC | Absent | 0.525e | 0.596 | 357 | − 0.647 | 1.697 |
| Treated | 0.694e | 0.458 | 357 | − 0.208 | 1.595 | |
| Untreated | c, e | |||||
| (b) Diagnosis × OSAB | ||||||
|---|---|---|---|---|---|---|
| Diagnosis | OSA | M | SE | Df | Confidence interval 95% | |
| Lower bound | Upper bound | |||||
| AD | Absent | − 0.134e | 0.821 | 357 | − 1.747 | 1.480 |
| Treated | 7.429e | 2.843 | 357 | 1.838 | 13.021 | |
| Untreated | − 4.618e | 2.842 | 357 | − 10.208 | 0.971 | |
| MIXED | Absent | − 0.287e | 0.218 | 357 | − 0.715 | 0.141 |
| Treated | 0.045e | 0.642 | 357 | − 1.218 | 1.308 | |
| Untreated | − 0.627e | 0.321 | 357 | − 1.259 | 0.004 | |
| VASC | Absent | 0.959e | 0.563 | 357 | − 0.148 | 2.066 |
| Treated | 0.820e | 0.733 | 357 | − 0.622 | 2.262 | |
| Untreated | 0.220e | 0.595 | 357 | − 0.950 | 1.391 | |
ANote: eCovariates in the model were calculated using: Age 73.505, Education 1.44, Time between measurements/neurocognitive time (NCT) 415.3361, DemTect score baseline 11.903. For other abbreviations see Tables 3(1a) and (1b) and Supplementary Tables 2, 3.
BFor other abbreviations see Tables 3(1a) and (1b), 5(a) and Supplementary Tables 2, 3.
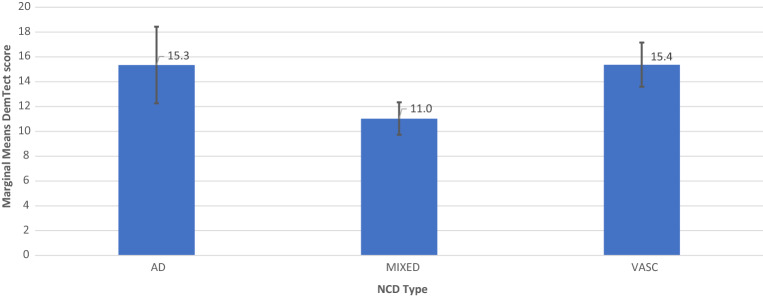
In all diagnostic groups, i.e. AD-NCD, vascular-NCD, and mixed-NCD, DemTect change is best, if OSA is treated and DemTect change is better in treated compared to untreated groups with regard to OSA or AH. Even if the untreated condition is not observed, as is the case with AH in AD-NCD and vascular-NCD, DemTect change is better in the treated state compared to the absent state. However, whereas in AD-NCD and vascular-NCD the combination of treated OSA and treated hypertension achieves the best results, in mixed-NCD this is the case in the combination with absent AH. On the other hand, worst outcomes are obtained in combinations with untreated OSA in AD-NCD and vascular-NCD, but in mixed-NCD this appears for the combination of absent OSA and untreated AH (see Figs. 8, 9, 10, 11 and Supplementary Table 6).
Figure 9.
Model 6 AH × OSA Diagnosis OSA+ AH+ DemTect change including M and SE.
Figure 10.
Model 6 AH x OSA Diagnosis OSA− AH− DemTect change including M and SE.
Figure 11.
Model 6 AH x OSA Diagnosis OSA− AH+ DemTect change including M and SE.
Model 4 showed a significant interaction effect of all 3 factors AH × OSA × diagnosis on clock-drawing test scores (F (2, 210.670) = 3.432, p = 0.034) whereas OSA and AH as single factors remained insignificant. Time (F (1, 329) = 43.1197, p = 0.000) and clock-drawing test results baseline (F (1, 116.259) = 100.588, p = 0.000) were found to be significant covariates in the model.
Ancillary analysis
In order to analyze risk factors on a patient level, subjects were divided into permanently corrected hypertension (n = 79) and non-permanently corrected hypertension (n = 11). Whereas 50 of the permanently corrected patients had a favorable cognitive outcome, this was only the case in 3 of the non-permanently corrected patients. A significant association could be found between this assignment and the development of the clinical stage (favorable or unfavorable) from baseline to last observation (Fisher-test: χ2 = 5.17, p = 0.045). There was no significant difference between the mean observation time in both groups (t(124) = − 0.145, p = 0.885).
Similarly, patients with OSA were divided into permanently corrected OSA (n = 13) and non-permanently corrected OSA (n = 27). Here, no association could be found between corrected and non-corrected OSA with regard to favorable vs non-favorable outcome (Fisher-test: χ2 = 3.25, df = 1, p = 0.178). There was also no significant difference between the mean observation time in both groups (t(38) = 0.412, p = 0.682).
An analysis of diagnostic attributions in the non-corrected, i.e. the untreated group of OSA patients with regard to outcome revealed, that 9 of 10 untreated OSA patients with vascular-NCD, but only 8 of 17 untreated OSA patients with mixed-NCD showed a favorable cognitive outcome (Fisher-test:χ2 = 4.98, df = 1, p = 0.042).
To obtain data regarding hypertension in the elderly, patients with treated hypertension aged ≥ 80 years were compared to those with treated AH aged < 80 years. When covaried for DemTect baseline, these comparisons did not reveal a significant difference in outcomes, neither for DemTect scores (F(1, 75.489), p = 0.273) nor for DemTect change (F(1,255), p = 0.508).
Discussion
The major objective of this study was to evaluate the effect of treatment for arterial hypertension (AH) and obstructive sleep apnea (OSA) on cognitive decline in elderly patients from a cohort with all stages of common neurocognitive disorder. Our major findings are the following. AH and OSA as assessed for the states absent, treated and untreated predict cognitive course as analyzed by DemTect scores or their change and sufficient treatment of each factor results in a positive effect on the cognitive course. When both factors are investigated together, OSA turns out as a significant factor and a non-significant interaction of OSA with AH is revealed. The 3 factors AH, OSA and diagnosis predict cognitive outcome as assessed by DemTect scores.
For the clock-drawing test diagnosis, AH and OSA status revealed a significant interaction effect.
The harmful interplay/additive effect of the investigated risk factors and underlying pathology may worsen cognitive outcome/visuo-constructive skills as measured by the clock-drawing test. Since AH and OSA as single risk factors were not sufficient to predict cognitive outcome this test might be less sensitive than the DemTect.
In a COPD cohort worse DemTect scores were linked to oxygen saturation parameters confirming its sensitivity for detecting cognitive changes due to hypoxic states which are also characteristic for OSA53.
Arterial hypertension
Hypertension reveals an effect on the cognitive outcome as presented by the change in DemTect scores. Here AH reveals a significant effect in both the single factor analysis as well as in the 2-factor statistical approach with AH and OSA as independent variables. Significance disappears for AH when diagnosis as a factor is included in the model. Detailed analyzes of estimated marginal means show an effect of hypertension in all diagnostic groups with a more profound effect in mixed than in vascular NCD (Table 5). Since none of the patients in the vascular group had uncorrected hypertension, this missing data may explain the loss of significance in the analysis including diagnosis as a factor, where a level of p = 0.053 is obtained for AH as a factor in this model.
The results showing an effect of blood pressure (BP) on cognitive outcome are in line with reports of high BP resulting in faster cognitive decline as compared to borderline BP or normal BP in subjects without pre-existing NCD11. As shown by the current results, it should be noted that treatment of blood pressure reduces cognitive decline also in individuals with NCD. There are only few reports on the comparison of treated vs untreated hypertension in subjects with NCD. A Brazilian study analyzed the effects of the antihypertensive calcium channel antagonist (CCA) nimodipine as a co-medication in mixed dementia showing no effect of nimodipine on psychomotor speed or quality of life. Although using an RCT design, this study gives no information about the treatment status of hypertension given by other antihypertensive agents and their effect on cognitive outcomes57. A study on AD patients responding to cholinesterase inhibitors demonstrated an independent effect of antihypertensive treatment in this subgroup over 40 weeks58. A further study on the CCA nilvadipine demonstrated significant effects on cognitive measures in AD patients over 6 weeks59. In a French cohort, AD patients treated with renin-angiotensin system (RAS) acting medications had slower long-term cognitive decline than those without such medication17. Whereas hypertension status in this study appears sure in RAS-users, the compared group of non-users might include patients without hypertension.
In sum, most of the few studies on the treatment of hypertension in subjects with NCD reveal a favorable effect on cognitive outcome up to a time period of less than 1 year. For the first time, the current study demonstrates favorable results of BP correction in NCD patients in the long term.
In the current study, patients with hypertension aged over 80 years had no different cognitive outcome of antihypertensive treatment than those younger than 80 years. This is in conflict with reports of a failing or even worsening cognitive effect of lowering BP in the elderly60–63, contrasting with positive results in middle-aged subjects64. Possible reasons for the positive results of lowering BP in older patients in RIFADE could be the following: (1) In RIFADE the coupling of the cognitive outcome as assessed by repeated measurements in neurocognitive time periods (NCT) with the concomitant status of treated or untreated hypertension in each NCT allows for evaluation of medium-term effects of treatment and not only of long-term effects on cognition. The analysis of favorable outcome in the long-term in the current study also shows an effect of lowering BP in older individuals, indicating that the positive results in mixed linear models may not be solely due to medium-term effects. Thus, positive results might be obtained due to methodological reasons by analysis of different time windows. (2) RIFADE investigates subjects with preexisting NCD. Positive results are in line with reports showing an increased risk for dementia with high BP in subjects with MCI65. (3) No subjects over 90 years old were included in RIFADE. Therefore, no data are available in this study about nonagenarians or centenarians. Moreover, it is to consider that with preceding historical times and improving treatments for cardiovascular diseases, lifespan expectations might be extended and biological aging retarded. Therefore, the octogenarians of the 1990s years for example might be comparable to the nonagenarians of the 2010s years, which may further complicate comparisons of the current data with previous reports. (4) Reports of a raising systolic BP in subjects with NCD up to the age of 80 years with decreasing values only in those over 80 years66 support the importance of this observation in RIFADE and suggest that when BP is elevated in elderly patients, should also be lowered in these patients. (5) For individuals older than 90 years, other pathogenetic profiles may exist and other guidelines for BP regulation might be needed.
There are several pathophysiological assumptions about how hypertension might affect cognition. A central role is seen in mitochondrial dysfunction resulting into dysregulation of cellular redox condition and reduced cell survival67. Since tau phosphorylation is involved in mitochondrial functioning, it is conceivable, that hypertension, as a classical vascular risk factor, could also be linked to Alzheimer’s pathology8,9. This is also supported by recent reports of greater amyloid deposition in the presence of hypertension in middle-aged subjects68. A broader and unifying concept of how elevated BP affects cognition brings the link between vasculature and neural function to the fore in terms of the neurovascular unit69–75. Further research is needed to clarify the association between elevated BP and neurodegeneration on a molecular level.
It is conceivable that the regulation of AH could be improved above the level achieved in RIFADE. Among others, 2 strategies appear promising. First, frequent use of home blood pressure measurement devices including applications for documentation of BP-values could provide a more detailed picture of the individual BP history and allow for a tighter circadian regulation of BP to normative values. This would be of particular importance for common cases of labile hypertension with high BP variability.
Second, national strategies to reduce sodium in nutrients should be intensified and prospectively evaluated due to methodologically incoherent results in former reports76–82. Moreover, educational campaigns should be enforced to increase awareness of the deleterious effects of increased sodium intake, in particular with regard to hypertension and cardiovascular disease, including hazardous effects apart from hypertension83. Finally, identifying and treating OSA will improve the effectiveness of treatment in hypertension and lead to a higher chance of favorable cognitive outcomes84.
It can be concluded that BP should be measured more frequently in subjects with cognitive complaints, not only to diagnose hypertension but also to monitor its correction status in order to avoid negative cognitive effects.
Obstructive sleep apnea
OSA shows a significant effect on DemTect score as a single factor and in the 3-factorial analysis including AH and diagnosis as a factor. Results are in line with a small study on patients with moderate dementia, in which sufficient use of CPAP during a median time of 13.3 years led to a reduced amount of deterioration in global cognition measures and in improved executive functioning as compared to non-users of CPAP37. In a great study on the ADNI cohort, based on self-reported sleep apnea or obstructive sleep apnea, CPAP users with varying duration of CPAP use showed a delayed age at onset of MCI or Alzheimer’s dementia as compared to non-users of CPAP47. A French study on patients with mild to moderate AD found even stable or improved cognitive courses in 9 of 14 CPAP-treated OSA patients46. In MCI-patients, an increased psychomotor speed was observed in CPAP-users31. Recently Liguori et al.48 compared CPAP-adherent patients with non-adherent CPAP-users in a small cohort of patients with MCI or AD observing a smaller cognitive decline in the adherent group.
In sum, the current study is in accordance with findings of positive cognitive effects of OSA treatment in patients with NCD and is the first report on treated and untreated OSA patients in all stages of pre-existing neurocognitive disorder, showing favorable cognitive effects of treatment on the long-term in a substantial proportion of OSA patients.
Similar to the above-mentioned link between Alzheimer’s pathology and AH, there are also reports on associations of OSA with amyloid pathology43,85 contributing to the understanding of the pathophysiological consequences of OSA linked to hypoxia-induced dysfunctions86. Another aspect in this context is sleep fragmentation as an OSA-related factor resulting into cognitive decline21.
Whereas an exciting high proportion of patients with AH in this cohort received treatment (88%), only one-third of OSA patients got therapy, predominantly by CPAP, the gold standard of OSA treatment. This indicates a high need for intensified efforts in NCD patients with identified OSA to assess, whether CPAP treatment is applicable. On the other hand, it should be noted that a substantial amount of patients with untreated OSA had a favorable cognitive outcome. It is conceivable that a subgroup of OSA patients might have adapted to apnea-related hypoxia and other pathological events associated with OSA. Given the high proportion of patients, who do not tolerate CPAP treatment87–89, a search for biomarkers is required to estimate cognitive outcome in untreated OSA and the urgency of treatment at an individual level. In this context, it should be considered that recent reports point to a lack of erythrocytosis in OSA, which would be expected as an effect of chronic hypoxia90. This indicates, that markers of the hematopoietic system may not be suitable as such predictive biomarkers. Apart from these considerations, multidisciplinary efforts should be undertaken to reduce the prevalence of obesity as a main causal factor of OSA, which simultaneously helps to address a couple of other risk factors, e.g. hypertension.
The glymphatic system as link of the risk factors OSA and AH
The term glymphatic is a neologism of ‘glio-lymphatic system’ and was coined by Iliff and Nedergaard91. In mice aquaporin-4 (AQP-4), water channels were found to be expressed at the endfeet of astrocytes. Usually, these endfeet enclose vasculature, arteries as well as veins, allowing an exchange of cerebrospinal (CSF) and interstitial (ISF) fluid thereby promoting the clearance of waste products from brain parenchyma (for review see92,93). This process is facilitated during sleep since interstitial space widens by about 60%94. CSF influx into the glymphatic system is dependent on arterial pulsation driven by respiratory and cardiac cycles. ISF efflux occurs along big sinuses and veins and finally drains into lymphatic vessels on the dura mater terminating the cervical lymph nodes95,96.
Two of the above expounded glymphatic features are particularly vulnerable to disturbances. Firstly, the CSF influx depends on arterial pulsation/cardiac cycle. In arterial hypertonus/AH there is a remodelling of the vascular wall leading to stiffness and reduced elasticity/pulsation. Impediments of pulsation in turn lead to decreased clearance of waste products from brain parenchyma/interstitial space97. The formation of ß amyloid plaques in Alzheimer’s disease has been shown to be promoted in AH. However longitudinal analysis of Danish patient registers revealed that high blood brain barrier permeable ß-blockers (Propranolol and Carvedilol) reduced the risk of Alzheimer’s disease compared to low permeable ß-blockers98. Treating AH may therefore be a preventive strategy to avoid later Alzheimer’s disease. This notion is supported by a recent meta-analysis of five randomized controlled trials showing reduced odds for getting Alzheimer’s disease in patients with blood pressure lowering medication99.
Secondly, sleep is facilitating the clearance of waste products from brain parenchyma. One night of sleep deprivation in healthy adults was shown to increase ß amyloid levels in a PET study100. Similarly, clearance of contrast agent gadobutrol was impaired in individuals with 24 h of sleep deprivation101. New diffusion tensor imaging studies using analysis along perivascular space (APLS) as measure of glymphatic functioning link both AH102 and OSA103 to glymphatic impairment. A recent study employing dynamic contrast enhanced MRI in OSA patients revealed enlarged perivascular spaces, lateral ventricles and disturbed glymphatic flow as well as cognitive deficits measured by MMST and MoCA104. After 1 month of CPAP glymphatic function of OSA patients improved, thus emphasizing the need of treating OSA as a risk factor for dementia/NCD.
Arterial hypertension and obstructive sleep apnea
Following a concept of an integrated approach in the treatment of vascular risk factors, with a focus on endothelial dysfunction as a common final path resulting in malfunction of the neuro-vascular unit105, it seems obvious to consider hypertension and OSA together in the treatment of NCD. This is supported by the result in the current study, that patients who had treatment for both factors (AH × OSA) appear to have the best estimated marginal means for DemTect change (Table 4). The 2-factor analysis with AH and OSA as independent variables, however, revealed a non-significant result for both outcome variables. This could be mainly due to two reasons. First, the level of close adjustments of BP to normative values in RIFADE could be not as high as it could have been achievable. Moreover, the limited statistical power of RIFADE may have prevented significant results.
On the other hand, a synoptic view on cognitive outcome measures in OSA, which depend on the status of hypertension reveals interesting results (Fig. 2). DemTect change is best, if OSA and AH are treated. Untreated AH results in the worst cognitive outcome in OSA among all OSA states. In other words, an untreated hypertension status appears to reduce or even remove the effect of treatment in OSA. However, due to the small case numbers in this study, such results should be treated with caution and be verified in greater, well-balanced cohorts.
If the diagnosis is considered in analysis of the risk factors, a diagnosis-related pattern appears with regard to cognitive outcome (Supplementary Table 6). In all diagnostic groups, i.e. AD-NCD, vascular-NCD and mixed-NCD, the outcome is best, if OSA is treated and the outcome is better in treated compared to untreated groups with regard to OSA or AH. However, whereas in AD-NCD and vascular-NCD the combination of treated OSA and treated hypertension achieves the best results, in mixed-NCD this is the case in the combination with absent AH. On the other hand, the worst outcomes are obtained in combinations with untreated OSA in AD-NCD and vascular-NCD, in mixed-NCD this appears for the combination of absent OSA and untreated AH. Although caution is recommended to generalize those results due to small case numbers, those data point to a special relevance of hypertension in mixed-NCD. It is conceivable that the combination of Alzheimer’s and vascular pathology makes patients with mixed-NCD particularly susceptible to deleterious effects of untreated hypertension promoting cognitive decline.
Compared to other diagnostic groups, AD-NCD patients revealed the best cognitive outcome in the treated OSA condition averaged over all conditions of AH (treated, untreated, absent) (Table 5). Otherwise, in untreated OSA, AD-NCD showed the worst outcome compared to other diagnostic groups, when averaged over all AH conditions. This may indicate an essential effect of OSA in AD, which is in line with recent reports on favorable cognitive effects of OSA treatment in Alzheimer´s48.
For the group with vascular-NCD it can be stated, that cognitive improvements were observed in all conditions except for the untreated OSA combination with treated AH (Supplementary Table 6). However, it has to be considered, that the untreated condition for OSA and AH was not represented in this diagnostic group. Nevertheless, vascular-NCD reveals to be the most favorable condition with regard to the cognitive outcome as compared to AD-NCD and mixed-NCD (Table 5).
Another interesting observation was given by the analysis of diagnostic attributions in the group of untreated OSA patients. Namely, 90% of the patients with vascular-NCD and untreated OSA showed a favorable final cognitive outcome whereas this was only the case in 48% of the untreated OSA patients with mixed-NCD. This is in accordance with the above-mentioned approach, that in vascular patients treatment concepts should consider risk factors in an integrated view rather than treating each factor isolated. On the other hand, treatment of OSA appears all the more important, when AD pathology comes into play in form of mixed-NCD.
As mentioned elsewhere50, RIFADE has several limitations. One of them is the small number of enrolled patients. This disadvantage is partially compensated for by repeated measurements, which allow the analysis of both short-term and long-term effects of predictors such as risk factors. While it has been widely assumed in research for decades, that vascular risk factors, for example, only have an effect on the long term, the current data show that factors like hypertension may also exert cognotropic effects over shorter periods of time, from months to a few years.
As the main conclusion, it is stated that treatment of two risk factors contributed to favorable effects on cognitive outcomes in patients suffering from both most common neurocognitive disorders. Patients with AD- or vascular- / mixed-NCD should be screened for hypertension and obstructive sleep apnea and treated for these factors as far as possible. Efforts by health organizations such as the Berlin manifesto are helpful in putting this into action106.
Supplementary Information
Acknowledgements
The authors would like to thank all investigators from the study center, who contributed in patient recruitment and data collection/capture: Ulrike Winterscheidt, Ute Plath, Susanne Weber, Marion Mulder-Walter and Sükrya Özden.
Author contributions
B.B. and M.K. wrote the paper, took part in consensus conferences on diagnostic attributions, revised the manuscript and contributed substantially to the final version of the text, L.B. was involved in clinical practice/patient screening, M.J. performed the statistical analysis and revised the manuscript, M.Z. revised the manuscript and T.L. revised the manuscript and contributed to sample collection.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to patient confidentiality but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-33701-2.
References
- 1.Livingston G, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song C, et al. Immunotherapy for Alzheimer’s disease: Targeting β-amyloid and beyond. Transl. Neurodegener. 2022;11(1):18. doi: 10.1186/s40035-022-00292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo JJ, Wallace W, Kusiak JW. A tough trek in the development of an anti-amyloid therapy for Alzheimer’s disease: Do we see hope in the distance? J. Neurol. Sci. 2022;438:120294. doi: 10.1016/j.jns.2022.120294. [DOI] [PubMed] [Google Scholar]
- 4.Lacorte E, et al. Safety and efficacy of monoclonal antibodies for alzheimer’s disease: A systematic review and meta-analysis of published and unpublished clinical trials. J. Alzheimer. Dis. 2022;87(1):101–129. doi: 10.3233/JAD-220046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bittar A, Bhatt N, Kayed R. Advances and considerations in AD tau-targeted immunotherapy. Neurobiol. Dis. 2020;134:104707. doi: 10.1016/j.nbd.2019.104707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandusky-Beltran LA, Sigurdsson EM. Tau immunotherapies: Lessons learned, current status and future considerations. Neuropharmacology. 2020;175:108104. doi: 10.1016/j.neuropharm.2020.108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pase MP, Beiser A, Enserro D, Xanthakis V, Aparicio H, Satizabal CL, Himali JJ, Kase CS, Vasan RS, DeCarli C, Seshadri S. Association of ideal cardiovascular health with vascular brain injury and incident dementia. Stroke. 2016;47(5):1201–1206. doi: 10.1161/STROKEAHA.115.012608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, Csiszar A. Hypertension-induced cognitive impairment: From pathophysiology to public health. Nat. Rev. Nephrol. 2021;17(10):639–654. doi: 10.1038/s41581-021-00430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension. Circ. Res. 2019;124(7):1025–1044. doi: 10.1161/CIRCRESAHA.118.313260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun X, et al. Systolic blood pressure and cognition in the elderly: The northern manhattan study. J. Alzheimer. Dis. 2021;82(2):689–699. doi: 10.3233/jad-210252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, Hua R, Yang Z, Zhong B, Yan L, Xie W. Different hypertension thresholds and cognitive decline: A pooled analysis of three ageing cohorts. BMC Med. 2021;19(1):287. doi: 10.1186/s12916-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Menezes ST, Giatti L, Brant LCC, Griep RH, Schmidt MI, Duncan BB, Suemoto CK, Ribeiro ALP, Barreto SM. Hypertension, prehypertension, and hypertension control: Association with decline in cognitive performance in the ELSA-Brasil Cohort. Hypertens. (Dallas Tex: 1979) 2021;77(2):672–681. doi: 10.1161/HYPERTENSIONAHA.120.16080. [DOI] [PubMed] [Google Scholar]
- 13.Mahinrad S, Sorond FA, Gorelick PB. Hypertension and cognitive dysfunction: A review of mechanisms, life-course observational studies and clinical trial results. Rev. Cardiovasc. Med. 2021;22(4):1429–1449. doi: 10.31083/j.rcm2204148. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Perdomo S, Billinger S, Beddhu S, Burns J, Gronseth G. Treatment of hypertension reduces cognitive decline in older adults: A systematic review and meta-analysis. BMJ Open. 2020;10(11):e038971. doi: 10.1136/bmjopen-2020-038971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp SI, Aarsland D, Day S, Sønnesyn H, Ballard C. Hypertension is a potential risk factor for vascular dementia: Systematic review. Int. J. Geriatr. Psychiatry. 2011;26(7):661–669. doi: 10.1002/gps.2572. [DOI] [PubMed] [Google Scholar]
- 16.Bermejo-Pareja F, Benito-León J, Louis ED, Trincado R, Carro E, Villarejo A, de La-Cámara AG. Risk of incident dementia in drug-untreated arterial hypertension: A population-based study. J. Alzheim. Dis. 2010;22(3):949–958. doi: 10.3233/JAD-2010-101110. [DOI] [PubMed] [Google Scholar]
- 17.Wharton W, et al. Modulation of renin-angiotensin system may slow conversion from mild cognitive impairment to alzheimer’s disease. J. Am. Geriatr. Soc. 2015;63(9):1749–1756. doi: 10.1111/jgs.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soto ME, van Kan GA, Nourhashemi F, Gillette-Guyonnet S, Cesari M, Cantet C, Rolland Y, Vellas B. Angiotensin-converting enzyme inhibitors and Alzheimer’s disease progression in older adults: Results from the Réseau sur la Maladie d’s Alzheimer Français cohort. J. Am. Geriatr. Soc. 2013;61(9):1482–1488. doi: 10.1111/jgs.12415. [DOI] [PubMed] [Google Scholar]
- 19.Linssen B, Bergman E, Klarenbeek P, Hoff E. Prevalence of obstructive sleep apnea at an outpatient memory clinic. Health Sci. Rep. 2021;4(1):e228. doi: 10.1002/hsr2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LRA. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Mullins AE, Kam K, Parekh A, Bubu OM, Osorio RS, Varga AW. Obstructive Sleep apnea and its treatment in aging: Effects on alzheimer’s disease biomarkers, cognition, brain structure and neurophysiology. Neurobiol. Dis. 2020;145:105054. doi: 10.1016/j.nbd.2020.105054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavigne GJ, Herrero Babiloni A, Beetz G, Dal Fabbro C, Sutherland K, Huynh N, Cistulli PA. Critical issues in dental and medical management of obstructive sleep apnea. J. Dent. Res. 2020;99(1):26–35. doi: 10.1177/0022034519885644. [DOI] [PubMed] [Google Scholar]
- 23.Rosenzweig I, Glasser M, Crum WR, Kempton MJ, Milosevic M, McMillan A, Leschziner GD, Kumari V, Goadsby P, Simonds AK, Williams SCR, Morrell MJ. Changes in neurocognitive architecture in patients with obstructive sleep apnea treated with continuous positive airway pressure. EBioMedicine. 2016;7:221–229. doi: 10.1016/j.ebiom.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Ad B, Lavie P. Effect of sleep apnea on cognition and mood. Int. Rev. Psychiatry (Abingdon, Engl.) 2005;17(4):277–282. doi: 10.1080/09540260500104508. [DOI] [PubMed] [Google Scholar]
- 25.Gilley RR. The role of sleep in cognitive function: The value of a good night’s rest. Clin. EEG Neurosci. 2022 doi: 10.1177/15500594221090067. [DOI] [PubMed] [Google Scholar]
- 26.Macchitella L, Romano DL, Marinelli CV, Toraldo DM, Arigliani M, de Benedetto M, Angelelli P. Neuropsychological and socio-cognitive deficits in patients with obstructive sleep apnea. J. Clin. Exp. Neuropsychol. 2021;43(5):514–533. doi: 10.1080/13803395.2021.1944609. [DOI] [PubMed] [Google Scholar]
- 27.Angelelli P, Macchitella L, Toraldo DM, Abbate E, Marinelli CV, Arigliani M, de Benedetto M. The neuropsychological profile of attention deficits of patients with obstructive sleep apnea: An update on the daytime attentional impairment. Brain Sci. 2020;10:6. doi: 10.3390/brainsci10060325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: A meta-analysis. Sleep. 2013;36(9):1297–1305. doi: 10.5665/sleep.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olaithe M, Bucks RS, Hillman DR, Eastwood PR. Cognitive deficits in obstructive sleep apnea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med. Rev. 2018;38:39–49. doi: 10.1016/j.smrv.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Owen JE, BenediktsdÓttir B, Gislason T, Robinson SR. Neuropathological investigation of cell layer thickness and myelination in the hippocampus of people with obstructive sleep apnea. Sleep. 2019;42:1. doi: 10.1093/sleep/zsy199. [DOI] [PubMed] [Google Scholar]
- 31.Seda G, Matwiyoff G, Parrish JS. Effects of obstructive sleep apnea and CPAP on cognitive function. Curr. Neurol. Neurosci. Rep. 2021;21(7):32. doi: 10.1007/s11910-021-01123-0. [DOI] [PubMed] [Google Scholar]
- 32.Richards KC, Gooneratne N, Dicicco B, Hanlon A, Moelter S, Onen F, Wang Y, Sawyer A, Weaver T, Lozano A, Carter P, Johnson J. Cpap adherence may slow 1-year cognitive decline in older adults with mild cognitive impairment and apnea. J. Am. Geriatr. Soc. 2019;67(3):558–564. doi: 10.1111/jgs.15758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Cheng C, Moelter S, Fuentecilla JL, Kincheloe K, Lozano AJ, Carter P, Gooneratne N, Richards KC. One year of continuous positive airway pressure adherence improves cognition in older adults with mild apnea and mild cognitive impairment. Nurs. Res. 2020;69(2):157–164. doi: 10.1097/NNR.0000000000000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 35.Hou H, Zhao Y, Yu W, Dong H, Xue X, Ding J, Xing W, Wang W. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J. Glob. Health. 2018;8(1):10405. doi: 10.7189/jogh.08.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tveit RL, Lehmann S, Bjorvatn B. Prevalence of several somatic diseases depends on the presence and severity of obstructive sleep apnea. PLoS ONE. 2018;13(2):e0192671. doi: 10.1371/journal.pone.0192671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooke JR, Ayalon L, Palmer BW, Loredo JS, Corey-Bloom J, Natarajan L, Liu L, Ancoli-Israel S. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: A preliminary study. J. Clin. Sleep Med. 2009;5(4):305–309. doi: 10.5664/jcsm.27538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dzierzewski JM, Wallace DM, Wohlgemuth WK. Adherence to continuous positive airway pressure in existing users: Self-efficacy enhances the association between continuous positive airway pressure and adherence. J. Clin. Sleep Med. 2016;12(2):169–176. doi: 10.5664/jcsm.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan W, Liu X, Ping L, Jin S, Yu H, Dong M, Xu F, Li N, Li Y, Xu Y, Ji Z, Cheng Y, Xu X, Zhou C. Distinct functional brain abnormalities in insomnia disorder and obstructive sleep apnea. Eur. Arch. Psychiatry Clin. Neurosci. 2022 doi: 10.1007/s00406-022-01485-7. [DOI] [PubMed] [Google Scholar]
- 40.Salsone M, et al. Microstructural changes in normal-appearing white matter in male sleep apnea patients are reversible after treatment: A pilot study. J. Neurosci. Res. 2021;99(10):2646–2656. doi: 10.1002/jnr.24858. [DOI] [PubMed] [Google Scholar]
- 41.Kim H, Joo E, Suh S, Kim J-H, Kim ST, Hong SB. Effects of long-term treatment on brain volume in patients with obstructive sleep apnea syndrome. Hum. Brain Mapp. 2016;37(1):395–409. doi: 10.1002/hbm.23038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torelli F, Moscufo N, Garreffa G, Placidi F, Romigi A, Zannino S, Bozzali M, Fasano F, Giulietti G, Djonlagic I, Malhotra A, Marciani MG, Guttmann CRG. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54(2):787–793. doi: 10.1016/j.neuroimage.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandes M, Mari L, Chiaravalloti A, Paoli B, Nuccetelli M, Izzi F, Giambrone MP, Camedda R, Bernardini S, Schillaci O, Mercuri NB, Placidi F, Liguori C. 18f-FDG PET, cognitive functioning, and CSF biomarkers in patients with obstructive sleep apnoea before and after continuous positive airway pressure treatment. J. Neurol. 2022;269(10):5356–5367. doi: 10.1007/s00415-022-11182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalmases M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: A randomized pilot study. Chest. 2015;148(5):1214–1223. doi: 10.1378/chest.15-0171. [DOI] [PubMed] [Google Scholar]
- 45.Chang Y-T, Chen Y-C, Chen Y-L, Hsu S-W, Yang F-Y, Lee C-C, Hsu P-Y, Lin M-C. Functional connectivity in default mode network correlates with severity of hypoxemia in obstructive sleep apnea. Brain Behav. 2020;10(12):e01889. doi: 10.1002/brb3.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Troussière A-C, Charley CM, Salleron J, Richard F, Delbeuck X, Derambure P, Pasquier F, Bombois S. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2014;85(12):1405–1408. doi: 10.1136/jnnp-2013-307544. [DOI] [PubMed] [Google Scholar]
- 47.Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu S-E, Lim J, Wohlleber ME, Ducca EL, Koushyk V, Glodzik L, Mosconi L, Ayappa I, Rapoport DM, de Leon MJ. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964–1971. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liguori C, Cremascoli R, Maestri M, Fernandes M, Izzi F, Tognoni G, Scarpina F, Siciliano G, Mercuri NB, Priano L, Bonanni E, Placidi F. Obstructive sleep apnea syndrome and Alzheimer's disease pathology: May continuous positive airway pressure treatment delay cognitive deterioration? Sleep Breath. 2021;25(4):2135–2139. doi: 10.1007/s11325-021-02320-4. [DOI] [PubMed] [Google Scholar]
- 49.Emamian F, Khazaie H, Tahmasian M, Leschziner GD, Morrell MJ, Hsiung G-YR, Rosenzweig I, Sepehry AA. The association between obstructive sleep apnea and alzheimer’s disease: A meta-analysis perspective. Front. Aging Neurosci. 2016;8:78. doi: 10.3389/fnagi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baumann B, Lipka T, Jänner M, Kujovic M. The Neurocognitive Disorder Cohort RIFADE: Aims, methods, first results showing cognitive improvement in a subgroup. Eur. Arch. Psychiatry Clin. Neurosci. 2022 doi: 10.1007/s00406-022-01516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sateia MJ. International classification of sleep disorders-third edition: Highlights and modifications. Chest. 2014;146(5):1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 52.Aycicek GS, Çalıskan H, Ozsurekci C, Unsal P, Kessler J, Kalbe E, Esme M, Dogrul RT, Balcı C, Seven U, Karabulut E, Halil M, Cankurtaran M, Yavuz BB. A reliable tool for assessing MCI and dementia: Validation study of DemTect for Turkish population. Am. J. Alzheim. Dis. Other Demen. 2020;35:1533317520949805. doi: 10.1177/1533317520949805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Siemens SM, Perneczky R, Vogelmeier CF, Behr J, Kauffmann-Guerrero D, Alter P, Trudzinski FC, Bals R, Grohé C, Söhler S, Waschki B, Lutter JI, Welte T, Jörres RA, Kahnert K. The association of cognitive functioning as measured by the DemTect with functional and clinical characteristics of COPD: Results from the COSYCONET cohort. Respir. Res. 2019;20(1):257. doi: 10.1186/s12931-019-1217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shulman KI. Clock-drawing: Is it the ideal cognitive screening test? Int. J. Geriatr. Psychiatry. 2000;15(6):548–561. doi: 10.1002/1099-1166(200006)15:6<548::aid-gps242>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 55.Amodeo S, Mainland BJ, Herrmann N, Shulman KI. The times they are a-changin': Clock drawing and prediction of dementia. J. Geriatr. Psychiatry Neurol. 2015;28(2):145–155. doi: 10.1177/0891988714554709. [DOI] [PubMed] [Google Scholar]
- 56.Park J, Jeong E, Seomun G. The clock drawing test: A systematic review and meta-analysis of diagnostic accuracy. J. Adv. Nurs. 2018;74(12):2742–2754. doi: 10.1111/jan.13810. [DOI] [PubMed] [Google Scholar]
- 57.Caramelli P, et al. Effects of galantamine and galantamine combined with nimodipine on cognitive speed and quality of life in mixed dementia: A 24-week, randomized, placebo-controlled exploratory trial (the REMIX study) Arq. Neuropsiquiatr. 2014;72(6):411–417. doi: 10.1590/0004-282X20140055. [DOI] [PubMed] [Google Scholar]
- 58.Rozzini L, Vicini Chilovi B, Bellelli G, Bertoletti E, Trabucchi M, Padovani A. Effects of cholinesterase inhibitors appear greater in patients on established antihypertensive therapy. Int. J. Geriatr. Psychiatry. 2005;20(6):547–551. doi: 10.1002/gps.1312. [DOI] [PubMed] [Google Scholar]
- 59.Kennelly S, Abdullah L, Kenny RA, Mathura V, Luis CA, Mouzon B, Crawford F, Mullan M, Lawlor B. Apolipoprotein E genotype-specific short-term cognitive benefits of treatment with the antihypertensive nilvadipine in Alzheimer’s patients—an open-label trial. Int. J. Geriatr. Psychiatry. 2012;27(4):415–422. doi: 10.1002/gps.2735. [DOI] [PubMed] [Google Scholar]
- 60.Hebert LE, Scherr PA, Bennett DA, Bienias JL, Wilson RS, Morris MC, Evans DA. Blood pressure and late-life cognitive function change: A biracial longitudinal population study. Neurology. 2004;62(11):2021–2024. doi: 10.1212/01.WNL.0000129258.93137.4B. [DOI] [PubMed] [Google Scholar]
- 61.Di-Carlo A, Baldereschi M, Amaducci L, Maggi S, Grigoletto F, Scarlato G, Inzitari D. Cognitive impairment without dementia in older people: Prevalence, vascular risk factors, impact on disability. The Italian Longitudinal Study on Aging. J. Am. Geriatr. Soc. 2000;48(7):775–782. doi: 10.1111/j.1532-5415.2000.tb04752.x. [DOI] [PubMed] [Google Scholar]
- 62.Scherr PA, Hebert LE, Smith LA, Evans DA. Relation of blood pressure to cognitive function in the elderly. Am. J. Epidemiol. 1991;134(11):1303–1315. doi: 10.1093/oxfordjournals.aje.a116033. [DOI] [PubMed] [Google Scholar]
- 63.Kabayama M, et al. The association of blood pressure with physical frailty and cognitive function in community-dwelling septuagenarians, octogenarians, and nonagenarians: The SONIC study. Hypertens. Res. 2020;43(12):1421–1429. doi: 10.1038/s41440-020-0499-9. [DOI] [PubMed] [Google Scholar]
- 64.Rouch L, Cestac P, Hanon O, Ruidavets J-B, Ehlinger V, Gentil C, Cool C, Helmer C, Dartigues J-F, Bouhanick B, Chamontin B, Sallerin B, Vellas B, Marquié J-C, Esquirol Y, Andrieu S. Blood pressure and cognitive performances in middle-aged adults: The Aging, Health and Work longitudinal study. J. Hypertens. 2019;37(6):1244–1253. doi: 10.1097/HJH.0000000000002013. [DOI] [PubMed] [Google Scholar]
- 65.Gaussoin SA, Pajewski NM, Chelune G, Cleveland ML, Crowe MG, Launer LJ, Lerner AJ, Martindale-Adams J, Nichols LO, Ogrocki PK, Sachs BC, Sink KM, Supiano MA, Wadley VG, Wilson VM, Wright CB, Williamson JD, Reboussin DM, Rapp SR. Effect of intensive blood pressure control on subtypes of mild cognitive impairment and risk of progression from SPRINT study. J. Am. Geriatr. Soc. 2022;70(5):1384–1393. doi: 10.1111/jgs.17583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hestad K, Engedal K, Horndalsveen P, Strand BH. Blood pressure in different dementia disorders, mild cognitive impairment, and subjective cognitive decline. Front. Aging Neurosci. 2020;12:257. doi: 10.3389/fnagi.2020.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rahimi R, Nikfar S, Sadeghi M, Abdollahi M, Moghaddam RH, Farzaei MH. Effect of antihypertensive drugs on cognition and behavioral symptoms of patients with alzheimer’s disease: A meta-analysis. Curr. Pharm. Biotechnol. 2021;22(11):1511–1519. doi: 10.2174/1386207323666201211101720. [DOI] [PubMed] [Google Scholar]
- 68.Gottesman RF, Schneider ALC, Zhou Y, Coresh J, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, Wagenknecht LE, Wong DF, Mosley TH. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317(14):1443–1450. doi: 10.1001/jama.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kazama K, et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ. Res. 2004;95(10):1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 70.Kazama K, Wang G, Frys K, Anrather JJ, Iadecola C. Angiotensin II attenuates functional hyperemia in the mouse somatosensory cortex. Am. J. Physiol. Heart Circul. Physiol. 2003;285(5):H1890–H1899. doi: 10.1152/ajpheart.00464.2003. [DOI] [PubMed] [Google Scholar]
- 71.Girouard H, Park L, Anrather JJ, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2007;27(2):303–309. doi: 10.1161/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- 72.Capone C, Anrather JJ, Milner TA, Iadecola C. Estrous cycle-dependent neurovascular dysfunction induced by angiotensin II in the mouse neocortex. Hypertens. (Dallas, Tex: 1979) 2009;54(2):302–307. doi: 10.1161/HYPERTENSIONAHA.109.133249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Capone C, Faraco G, Anrather JJ, Zhou P, Iadecola C. Cyclooxygenase 1-derived prostaglandin E2 and EP1 receptors are required for the cerebrovascular dysfunction induced by angiotensin II. Hypertens. (Dallas, Tex: 1979) 2010;55(4):911–917. doi: 10.1161/HYPERTENSIONAHA.109.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Capone C, Faraco G, Park L, Cao X, Davisson RL, Iadecola C. The cerebrovascular dysfunction induced by slow pressor doses of angiotensin II precedes the development of hypertension. Am. J. Physiol. Heart Circul. Physiol. 2011;300(1):H397–407. doi: 10.1152/ajpheart.00679.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faraco G, et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J. Clin. Investig. 2016;126(12):4674–4689. doi: 10.1172/jci86950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohan D, et al. Link between dietary sodium intake, cognitive function, and dementia risk in middle-aged and older adults: A systematic review. J. Alzheim. Dis. 2020;76(4):1347–1373. doi: 10.3233/JAD-191339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang NX, et al. The World Hypertension League Science of Salt: A regularly updated systematic review of salt and health outcomes studies (Sept 2019 to Dec 2020) J. Hum. Hypertens. 2022 doi: 10.1038/s41371-022-00710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tekle DY, et al. Monitoring and implementation of salt reduction initiatives in Africa: A systematic review. J. Clin. Hypertens. (Greenwich Conn.) 2020;22(8):1355–1370. doi: 10.1111/jch.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cappuccio FP, Beer M, Strazzullo P. Population dietary salt reduction and the risk of cardiovascular disease. A scientific statement from the European Salt Action Network. Nutr. Metabol. Cardiovasc. Dis. 2018;29(2):107–114. doi: 10.1016/j.numecd.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 80.He FJ, Tan M, Ma YY, MacGregor GA. Salt reduction to prevent hypertension and cardiovascular disease: Jacc state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(6):632–647. doi: 10.1016/j.jacc.2019.11.055. [DOI] [PubMed] [Google Scholar]
- 81.Burt HE, Brown MK, He FJ, MacGregor GA. Salt: The forgotten foe in UK public health policy. BMJ (Clin. Res. Ed.) 2022;377:e070686. doi: 10.1136/bmj-2022-070686. [DOI] [PubMed] [Google Scholar]
- 82.Tsuchihashi T. Dietary salt intake in Japan—past, present, and future. Hypertens. Res. 2022;45(5):748–757. doi: 10.1038/s41440-022-00888-2. [DOI] [PubMed] [Google Scholar]
- 83.Faraco G, et al. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature. 2019;574(7780):686–690. doi: 10.1038/s41586-019-1688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kapa S, Sert-Kuniyoshi FH, Somers VK. Sleep apnea and hypertension: Interactions and implications for management. Hypertension (Dallas, Tex: 1979) 2008;51(3):605–608. doi: 10.1161/HYPERTENSIONAHA.106.076190. [DOI] [PubMed] [Google Scholar]
- 85.Bhuniya S, Goyal M, Chowdhury N, Mishra P. Intermittent hypoxia and sleep disruption in obstructive sleep apnea increase serum tau and amyloid-beta levels. J. Sleep Res. 2022;2022:e13566. doi: 10.1111/jsr.13566. [DOI] [PubMed] [Google Scholar]
- 86.Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams SCR, Morrell MJ. Sleep apnoea and the brain: A complex relationship. Lancet Respir. Med. 2015;3(5):404–414. doi: 10.1016/S2213-2600(15)00090-9. [DOI] [PubMed] [Google Scholar]
- 87.Tsuyumu M, Tsurumoto T, Iimura J, Nakajima T, Kojima H. Ten-year adherence to continuous positive airway pressure treatment in patients with moderate-to-severe obstructive sleep apnea. Sleep Breath. 2020;24(4):1565–1571. doi: 10.1007/s11325-020-02033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palm A, Midgren B, Theorell-Haglöw J, Ekström M, Ljunggren M, Janson C, Lindberg E. Factors influencing adherence to continuous positive airway pressure treatment in obstructive sleep apnea and mortality associated with treatment failure—a national registry-based cohort study. Sleep Med. 2018;51:85–91. doi: 10.1016/j.sleep.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 89.Koehler J, Hildebrandt O, Cassel W, Conradt R, Mayr P, Alter P, Viniol C. Therapieadhärenz 3 Monate nach Einleitung einer nichtinvasiven CPAP-Therapie bei 1078 Patienten mit obstruktiver Schlafapnoe (OSA) [adherence to CPAP three months after starting therapy in 1078 Patients with obstructive sleep apnea (OSA)] Pneumol. (Stuttgart, Germany) 2022;76(4):251–259. doi: 10.1055/a-1666-5369. [DOI] [PubMed] [Google Scholar]
- 90.Gangaraju R, Sundar KM, Song J, Prchal JT. Polycythemia is rarely caused by obstructive sleep apnea. Blood. 2016;128(22):2444. doi: 10.1182/blood.V128.22.2444.2444. [DOI] [Google Scholar]
- 91.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012;4(147):147. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Benveniste H, Nedergaard M. Cerebral small vessel disease: A glymphopathy? Curr. Opin. Neurobiol. 2022;72:15–21. doi: 10.1016/j.conb.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 93.Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science (N. Y.) 2020;370(6512):50–56. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science (NY, N. Y.) 2013;342(6156):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015;212(7):991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mortensen KN, Sanggaard S, Mestre H, Lee H, Kostrikov S, Xavier ALR, Gjedde A, Benveniste H, Nedergaard M. Impaired glymphatic transport in spontaneously hypertensive rats. J. Neurosci. 2019;39(32):6365–6377. doi: 10.1523/JNEUROSCI.1974-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beaman EE, Bonde AN, Larsen SMU, Ozenne B, Lohela TJ, Nedergaard M, Gíslason GH, Knudsen GM, Holst SC. Blood-brain barrier permeable β-blockers linked to lower risk of Alzheimer's disease in hypertension. Brain. 2023;146(3):1141–1151. doi: 10.1093/brain/awac076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peters R, et al. Blood pressure lowering and prevention of dementia: An individual patient data meta-analysis. Eur. Heart J. 2022;43(48):4980–4990. doi: 10.1093/eurheartj/ehac584. [DOI] [PubMed] [Google Scholar]
- 100.Shokri-Kojori E, Wang G-J, Wiers CE, Demiral SB, Guo M, Kim SW, Lindgren E, Ramirez V, Zehra A, Freeman C, Miller G, Manza P, Srivastava T, de Santi S, Tomasi D, Benveniste H, Volkow ND. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. 2018;115(17):4483–4488. doi: 10.1073/pnas.1721694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eide PK, Vinje V, Pripp AH, Mardal K-A, Ringstad G. Sleep deprivation impairs molecular clearance from the human brain. Brain. 2021;144(3):863–874. doi: 10.1093/brain/awaa443. [DOI] [PubMed] [Google Scholar]
- 102.Kikuta J, Kamagata K, Takabayashi K, Taoka T, Yokota H, Andica C, Wada A, Someya Y, Tamura Y, Kawamori R, Watada H, Naganawa S, Aoki S. An investigation of water diffusivity changes along the perivascular space in elderly subjects with hypertension. AJNR Am. J. Neuroradiol. 2022;43(1):48–55. doi: 10.3174/ajnr.A7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roy B, Nunez A, Aysola RS, Kang DW, Vacas S, Kumar R. Impaired glymphatic system actions in obstructive sleep apnea adults. Front. Neurosci. 2022;16:884234. doi: 10.3389/fnins.2022.884234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J, Tian Y, Qin C, Meng L, Feng R, Xu S, Zhai Y, Liang D, Zhang R, Tian H, Liu H, Chen Y, Fu Y, Chen P, Zhu Q, Teng J, Wang X. Impaired glymphatic drainage underlying obstructive sleep apnea is associated with cognitive dysfunction. J. Neurol. 2023;270(4):2204–2216. doi: 10.1007/s00415-022-11530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iadecola C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hachinski V, et al. Preventing dementia by preventing stroke: The Berlin Manifesto. Alzheimer. Dementia J. Alzheim. Assoc. 2019;15(7):961–984. doi: 10.1016/j.jalz.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to patient confidentiality but are available from the corresponding author on reasonable request.