Key Points
Question
Is it safe to omit sentinel lymph node biopsy in patients with small breast cancer (BC) and a negative preoperative axillary ultrasonography result?
Findings
In this randomized clinical trial that included 1463 women with small node-negative BC, patients who did not undergo axillary surgery had noninferior 5-year distant disease–free survival compared with those who underwent sentinel lymph node biopsy.
Meaning
These findings suggest that patients with BC of a diameter equal to or smaller than 2 cm and a negative result on preoperative axillary lymph node ultrasonography can be safely spared any axillary surgery whenever the lack of pathological information does not affect the postoperative treatment plan.
Abstract
Importance
Sentinel lymph node biopsy (SLNB) is the standard of care for axillary node staging of patients with early breast cancer (BC), but its necessity can be questioned since surgery for examination of axillary nodes is not performed with curative intent.
Objective
To determine whether the omission of axillary surgery is noninferior to SLNB in patients with small BC and a negative result on preoperative axillary lymph node ultrasonography.
Design, Setting, and Participants
The SOUND (Sentinel Node vs Observation After Axillary Ultra-Sound) trial was a prospective noninferiority phase 3 randomized clinical trial conducted in Italy, Switzerland, Spain, and Chile. A total of 1463 women of any age with BC up to 2 cm and a negative preoperative axillary ultrasonography result were enrolled and randomized between February 6, 2012, and June 30, 2017. Of those, 1405 were included in the intention-to-treat analysis. Data were analyzed from October 10, 2022, to January 13, 2023.
Intervention
Eligible patients were randomized on a 1:1 ratio to receive SLNB (SLNB group) or no axillary surgery (no axillary surgery group).
Main Outcomes and Measures
The primary end point of the study was distant disease–free survival (DDFS) at 5 years, analyzed as intention to treat. Secondary end points were the cumulative incidence of distant recurrences, the cumulative incidence of axillary recurrences, DFS, overall survival (OS), and the adjuvant treatment recommendations.
Results
Among 1405 women (median [IQR] age, 60 [52-68] years) included in the intention-to-treat analysis, 708 were randomized to the SLNB group, and 697 were randomized to the no axillary surgery group. Overall, the median (IQR) tumor size was 1.1 (0.8-1.5) cm, and 1234 patients (87.8%) had estrogen receptor–positive ERBB2 (formerly HER2 or HER2/neu), nonoverexpressing BC. In the SLNB group, 97 patients (13.7%) had positive axillary nodes. The median (IQR) follow-up for disease assessment was 5.7 (5.0-6.8) years in the SLNB group and 5.7 (5.0-6.6) years in the no axillary surgery group. Five-year distant DDFS was 97.7% in the SLNB group and 98.0% in the no axillary surgery group (log-rank P = .67; hazard ratio, 0.84; 90% CI, 0.45-1.54; noninferiority P = .02). A total of 12 (1.7%) locoregional relapses, 13 (1.8%) distant metastases, and 21 (3.0%) deaths were observed in the SLNB group, and 11 (1.6%) locoregional relapses, 14 (2.0%) distant metastases, and 18 (2.6%) deaths were observed in the no axillary surgery group.
Conclusions and Relevance
In this randomized clinical trial, omission of axillary surgery was noninferior to SLNB in patients with small BC and a negative result on ultrasonography of the axillary lymph nodes. These results suggest that patients with these features can be safely spared any axillary surgery whenever the lack of pathological information does not affect the postoperative treatment plan.
Trial Registration
ClinicalTrials.gov Identifier: NCT02167490
This randomized clinical trial assesses whether the omission of sentinel node biopsy is noninferior to sentinel lymph node biopsy in female patients with small breast cancer and negative results on preoperative ultrasonography of axillary lymph nodes.
Introduction
Sentinel lymph node biopsy (SLNB) is the standard of care for axillary node staging in patients with early breast cancer (BC). The application of this technique represented a milestone in surgical de-escalation, providing the same outcome as axillary lymph node dissection (ALND).1,2 The results of the American College of Surgeons Oncology Group Z0011 (ACOSOG Z0011) randomized clinical trial3 showed that there is no advantage in performing ALND compared with not performing ALND, even when up to 2 sentinel nodes are positive, for patients receiving breast-conserving surgery, adjuvant radiotherapy, and medical treatment. These data were supported by other randomized clinical trials4,5 and became the basis for a new standard of axillary care.6,7 In the past, SLNB was conceived as a reliable means to distinguish between patients with negative nodes who can be spared the morbidity associated with a complete ALND and those with nodal involvement who might benefit from a more extensive surgical procedure. But the absence of advantages from ALND revealed in the ACOSOG Z0111 trial3 raised 2 questions: first, whether it is really necessary to perform surgical staging of axillary lymph nodes, and second, whether imaging might replace surgery for reliable staging of axillary lymph nodes. The SOUND (Sentinel Node vs Observation After Axillary Ultra-Sound) trial was launched in February 2012 with the aim of evaluating the oncological safety of omitting axillary surgery in patients with BC of a diameter equal to or smaller than 2 cm and a negative result on preoperative axillary lymph node ultrasonography.
Methods
The SOUND trial was a prospective multicenter noninferiority phase 3 randomized clinical trial conducted in 18 hospitals in Italy, Spain, Switzerland, and Chile (eTable in Supplement 2). Participants were recruited and enrolled from February 6, 2012, to June 30, 2017. Data were analyzed between October 10, 2022, and January 13, 2023. The Trial Protocol and Statistical Analysis Plan for this trial are provided in Supplement 1. Of note, the SOUND trial was unintentionally registered late with ClinicalTrials.gov on June 18, 2014, after 492 patients had already been randomized. No interval analysis was conducted, and no data were examined before clinical trial registration. The trial was conducted in accordance with the amended Declaration of Helsinki,8 and the protocol was approved by the institutional review board at each participating center. All patients provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.
Study Population
Eligible patients were women of any age with invasive BC up to 2 cm in diameter, lack of involvement of axillary nodes at clinical evaluation, and a plan to undergo a breast-conserving surgery and radiotherapy. All patients were required to have preoperative axillary ultrasonography showing no lymph node involvement at imaging. In the case of a doubtful finding on ultrasonography concerning an isolated lymph node, fine-needle aspiration was performed to rule out the presence of nodal metastases by cytological examination. Axillary lymph nodes with micrometastases or macrometastases were defined as positive. Exclusion criteria were the preoperative presence of multiple doubtful or suspicious lymph nodes, extensive multifocality or multicentricity, bilateral BC, diagnosis of synchronous distant metastases, previous cancer, ongoing pregnancy or lactation, and obstacles to obtaining informed consent or undergoing regular follow-up.
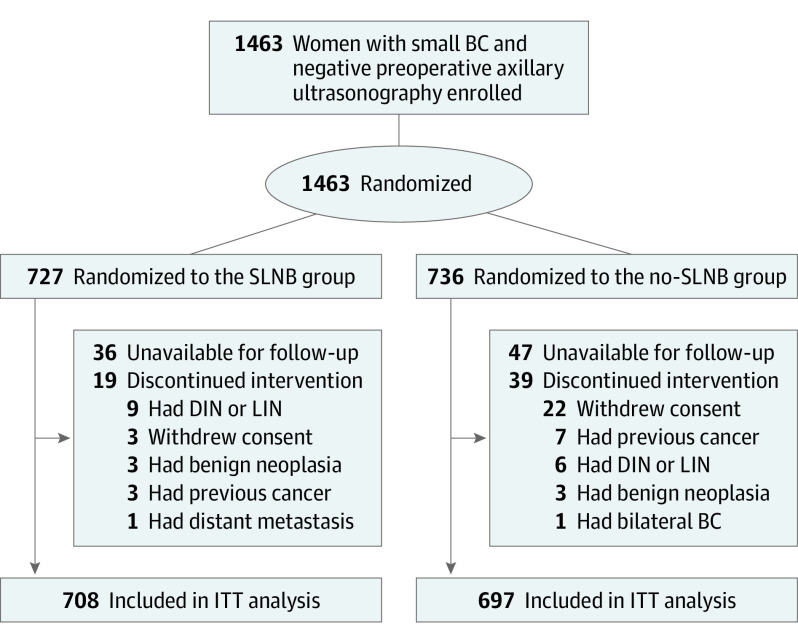
For the sample size calculation, the 5-year distant disease–free survival (DDFS) in the group randomized to receive no axillary surgery (no axillary surgery group) was assumed to be 96.5%. We calculated a target sample size of 1560 participants (780 per group) to test whether the no axillary surgery group experienced outcomes that were no worse than those of the group randomized to receive SLNB (SLNB group) given a margin of noninferiority for the 5-year DDFS of 2.5%. Between February 6, 2012, and June 30, 2017, 1463 women were recruited and enrolled in the study (93.8% of the planned sample size). The steering committee decided to close the accrual period early because the enrollment became too slow after most of the participating hospitals adopted the ACOSOG Z0011 criteria. Most of the enrolled patients (n = 1406) had negative ultrasonographic results; 57 patients who had a single doubtful node on ultrasonography were randomized after undergoing fine-needle aspiration cytological examination with negative results. Overall, 727 patients were randomized to the SLNB group and 736 to the no axillary surgery group. In the SLNB group, 19 patients discontinued intervention; in the no axillary surgery group, 39 patients discontinued intervention (Figure 1). The remaining 1405 women (708 in the SLNB group and 697 in the no axillary surgery group) were included in the intention-to-treat analysis.
Figure 1. Flow Diagram.
BC indicates breast cancer; DIN, ductal intraepithelial neoplasia; ITT, intention to treat; LIN, lobular intraepithelial neoplasia; and SLNB, sentinel lymph node biopsy.
Randomization and Treatment
The study design is summarized in Figure 1 and has been previously described.9,10,11 Eligible patients were randomly assigned in a 1:1 ratio to undergo SLNB or no axillary surgery. Of note, in the SLNB group, patients with metastases in the sentinel lymph node had to undergo ALND. Randomization was performed online using computer-generated allocation without stratification.
Trial End Points
The protocol-specified primary end point of the study was DDFS. Secondary end points were the cumulative incidence of distant recurrences, the cumulative incidence of axillary recurrences, DFS, overall survival (OS), and the adjuvant treatment recommendations.
Statistical Analysis
To describe the study population, we used frequencies, percentages, medians, and IQRs. Differences in the distribution of categorical variables were assessed using the χ2 test.
We compared women in the SLNB group with those in the no axillary surgery group using an intention-to-treat approach. The primary end point was DDFS, with distant metastases and deaths from all causes as the events of interest.12 Ipsilateral BC recurrences, axillary recurrences, contralateral BC, and nonbreast primary tumors were treated as censoring events. The secondary end points were DFS and OS. In the DFS analysis, all available events (ie, ipsilateral BC recurrences, axillary recurrences, distant metastases, contralateral BC, nonbreast primary tumors, and deaths from all causes) were events of interest. In the OS analysis, deaths from all causes were events of interest. In case of no events, observations were censored at last disease assessment for DDFS and DFS, while observations were censored at last vital status assessment for OS. Survival curves were estimated using the nonparametric Kaplan-Meier method. Differences in survival were assessed by means of the log-rank test. As additional secondary end points, we calculated the cumulative incidence of distant metastases and the cumulative incidence of axillary recurrences in a competing risk framework; events that were not distant metastases or axillary recurrences were treated as competing events. Differences in the cumulative incidence between groups were evaluated by means of the Gray test. For the analysis of DDFS, DFS, and cumulative incidence, only the data from the first event were used. All tests were 2-sided.
Based on a margin of noninferiority for the 5-year DDFS of 2.5%, the statistical power was set at 80%, and the 1-sided type 1 error was set at 5%. Noninferiority was shown if the upper limit of the 2-sided 90% CI for the hazard ratio for no axillary surgery vs SLNB, calculated at 5 years (ie, right-censoring follow-up at 5 years), was less than 1.74. The corresponding 1-sided P value for noninferiority was reported. The significance threshold was 1-sided P < .05. Analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
Results
Among 1405 women included in the intention-to-treat analysis, the median (IQR) age was 60 (52-68) years, the median (IQR) tumor size was 1.1 (0.8-1.5) cm, and 1234 patients (87.8%) had estrogen receptor (ER)–positive ERBB2 (formerly HER2 or HER2/neu), nonoverexpressing BC. Baseline characteristics were similar between treatment groups (Table 1). In the SLNB group (n = 708), 97 patients (13.7%) had positive axillary nodes (36 [5.1%] with micrometastases and 61 [8.6%] with macrometastases), and 4 (0.6%) had 4 or more positive lymph nodes.
Table 1. Baseline Patient and Tumor Characteristics.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| SLNB (n = 708) | No axillary surgery (n = 697) | |
| Age at surgery, y | ||
| <40 | 10 (1.4) | 10 (1.4) |
| 40-49 | 114 (16.1) | 128 (18.4) |
| 50-64 | 324 (45.8) | 298 (42.8) |
| ≥65 | 260 (36.7) | 261 (37.4) |
| Median (IQR) | 60 (52-68) | 60 (51-68) |
| Menopausal statusa | ||
| Premenopausal | 145 (20.6) | 154 (22.3) |
| Perimenopausal or postmenopausal | 558 (79.4) | 538 (77.7) |
| Histotype | ||
| Ductal | 551 (77.8) | 543 (77.9) |
| Lobular | 61 (8.6) | 59 (8.5) |
| Tubular | 27 (3.8) | 33 (4.7) |
| Other | 69 (9.7) | 62 (8.9) |
| Pathological tumor size | ||
| pT1mic or pT1a | 71 (10.0) | 61 (8.8) |
| pT1b | 251 (35.5) | 240 (34.4) |
| pT1c | 355 (50.1) | 361 (51.8) |
| pT2 | 31 (4.4) | 35 (5.0) |
| Median (IQR), cm | 1.1 (0.8-1.5) | 1.1 (0.8-1.5) |
| No. of positive SLNs | ||
| 0 | 599 (84.6) | 12 (1.7) |
| 1 | 83 (11.7) | 10 (1.4) |
| ≥2 | 14 (2.0) | 0 |
| SLNB not performed | 12 (1.7) | 675 (96.8) |
| No. of positive LNs | ||
| 0 | 599 (84.6) | 12 (1.7) |
| 1-3 | 93 (13.1) | 9 (1.3) |
| 4-9 | 2 (0.3) | 1 (0.1) |
| ≥10 | 2 (0.3) | 0 |
| No information | 12 (1.7) | 675 (96.8) |
| Pathological node status | ||
| pNx | 12 (1.7) | 675 (96.8) |
| pN0 | 584 (82.5) | 12 (1.7) |
| pN0(i+) | 15 (2.1) | 0 |
| pN1mi | 36 (5.1) | 4 (0.6) |
| pN1 | 57 (8.1) | 5 (0.7) |
| pN2 | 4 (0.6) | 1 (0.1) |
| Gradeb | ||
| 1 | 194 (27.7) | 204 (29.9) |
| 2 | 377 (53.8) | 356 (52.2) |
| 3 | 130 (18.5) | 122 (17.9) |
| ER status | ||
| 0 | 56 (7.9) | 44 (6.3) |
| >0 | 652 (92.1) | 653 (93.7) |
| PgR status | ||
| 0 | 108 (15.3) | 95 (13.6) |
| >0 | 600 (84.7) | 602 (86.4) |
| Ki-67 indexc | ||
| <20 | 455 (64.4) | 439 (63.2) |
| ≥20 | 252 (35.6) | 256 (36.8) |
| Median (IQR) | 15 (10-23) | 15 (10-24) |
| ERBB2 overexpression | ||
| Not overexpressed | 660 (93.2) | 650 (93.3) |
| Overexpressed | 48 (6.8) | 47 (6.7) |
| Surrogate subtype | ||
| Luminal ERBB2-negative | 617 (87.1) | 617 (88.5) |
| ERBB2-enriched | 48 (6.8) | 47 (6.7) |
| Triple-negative | 43 (6.1) | 33 (4.7) |
Abbreviations: ER, estrogen receptor; LN, lymph node; PgR, progesterone receptor; SLN, sentinel lymph node; SLNB, sentinel lymph node biopsy.
Frequencies do not sum to total due to missing data. Percentages were based on 703 patients in the SLNB group and 692 patients in the no axillary surgery group.
Frequencies do not sum to total due to missing data. Percentages were based on 701 patients in the SLNB group and 682 patients in the no axillary surgery group.
Frequencies do not sum to total due to missing data. Percentages were based on 707 patients in the SLNB group and 695 patients in the no axillary surgery group.
The recommended adjuvant systemic therapy and radiotherapy were similar in the 2 groups (Table 2). In the SLNB group, 652 patients (92.1%) had ER-positive BC; of those, 638 (97.9%) received hormone therapy. In the no axillary surgery group (n = 697), 653 patients (93.7%) had ER-positive BC; of those, 646 (98.9%) received hormone therapy. In the SLNB group, 48 patients (6.8%) had ERBB2-overexpressing BC; of those, 45 (93.8%) received trastuzumab. In the no axillary surgery group, 47 patients (6.7%) had ERBB2-overexpressing BC; of those, 46 (97.9%) received trastuzumab. Overall, 142 women (20.1%) in the SLNB group and 122 women (17.5%) in the no axillary surgery group received chemotherapy, while 694 women (98.0%) in the SLNB group and 680 women (97.6%) in the no axillary surgery group received radiotherapy. In total, 76 patients (10.7%) in the SLNB group and 75 patients (10.8%) in the no axillary surgery group received partial breast radiotherapy (intraoperative electron radiotherapy [ELIOT], 21 Gy). A total of 24 patients (3.4%) in the SLNB group and 39 patients (5.6%) in the no axillary surgery group received an intraoperative boost of ELIOT (12 Gy) followed by a hypofractionated course of whole-breast radiotherapy (37.05 Gy in 13 fractions). In total, 593 patients (83.8%) in the SLNB group and 565 patients (81.1%) in the no axillary surgery group received whole-breast radiotherapy with conventional fractionation delivered over 3 to 5 weeks according to the standard of care applied in the different institutions.
Table 2. Final Surgical Treatment and Recommended Adjuvant Therapy.
| Treatment | Patients, No. (%) | P value | |
|---|---|---|---|
| SLNB (n = 708) | No axillary surgery (n = 697) | ||
| Surgery | |||
| Breast-conserving | 12 (1.7) | 675 (96.8) | NA |
| Breast-conserving and SLNB | 646 (91.2) | 13 (1.9) | |
| Breast-conserving, SLNB, and AD | 45 (6.4) | 5 (0.7) | |
| Mastectomy and SLNB | 5 (0.7) | 4 (0.6) | |
| Hormone therapy | |||
| No | 66 (9.3) | 49 (7.0) | .12 |
| Yes | 642 (90.7) | 648 (93.0) | |
| Hormone therapy in ER-positive casesa | |||
| No | 14 (2.1) | 7 (1.1) | .12 |
| Yes | 638 (97.9) | 646 (98.9) | |
| Chemotherapy | |||
| No | 566 (79.9) | 575 (82.5) | .22 |
| Yes | 142 (20.1) | 122 (17.5) | |
| Hormone therapy and chemotherapy | |||
| Neither hormone therapy nor chemotherapy | 17 (2.4) | 11 (1.6) | .35 |
| Hormone therapy without chemotherapy | 549 (77.5) | 564 (80.9) | |
| Chemotherapy without hormone therapy | 49 (6.9) | 38 (5.5) | |
| Both hormone therapy and chemotherapy | 93 (13.1) | 84 (12.1) | |
| Radiotherapy | |||
| No | 14 (2.0) | 17 (2.4) | .56 |
| Yes | 694 (98.0) | 680 (97.6) | |
| Trastuzumab | |||
| No | 661 (93.4) | 651 (93.4) | .98 |
| Yes | 47 (6.6) | 46 (6.6) | |
| Trastuzumab in overexpressed ERBB2-positive casesb | |||
| No | 3 (6.2) | 1 (2.1) | .62 |
| Yes | 45 (93.8) | 46 (97.9) | |
Abbreviations: AD, axillary dissection; ER, estrogen receptor; NA, not applicable; SLNB, sentinel lymph node biopsy.
Percentages were based on 652 patients in the SLNB group and 653 patients in the no axillary surgery group.
Percentages were based on 48 patients in the SLNB group and 47 patients in the no axillary surgery group.
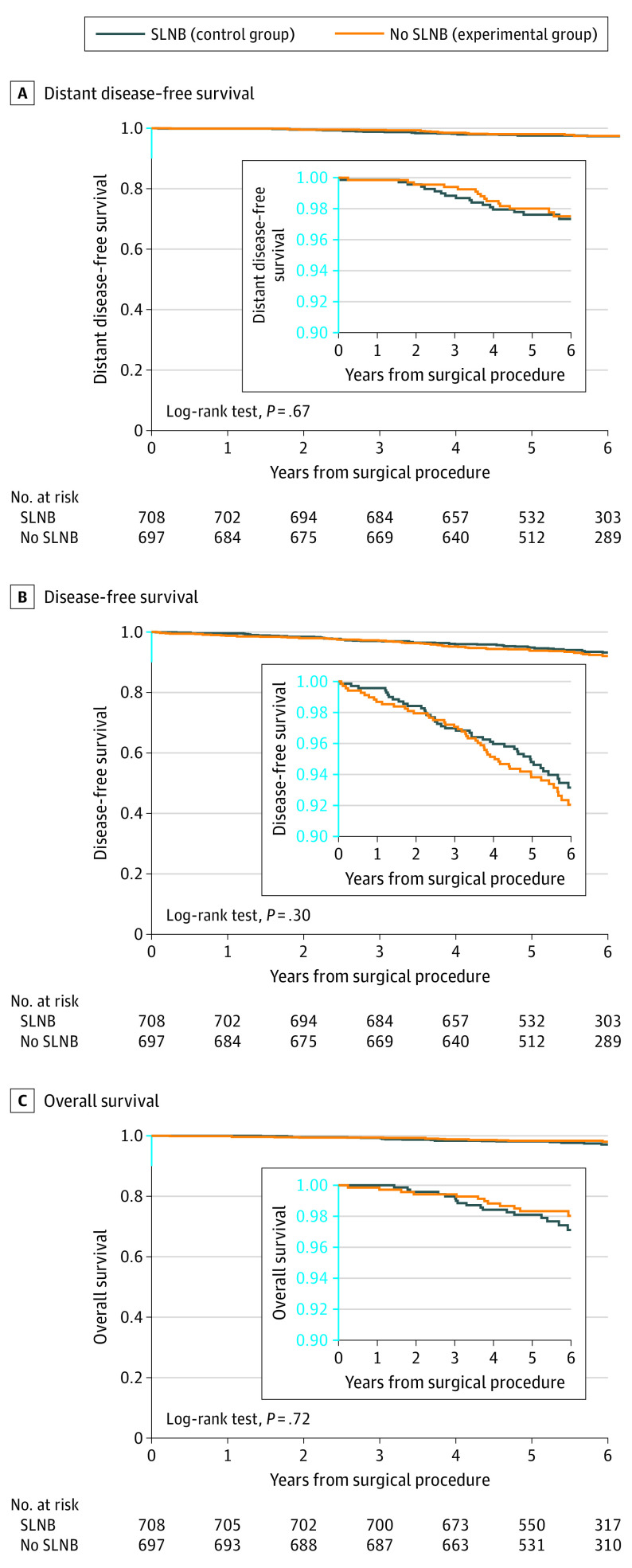
The median (IQR) follow-up for disease assessment was 5.7 (5.0-6.8) years in the SLNB group and 5.7 (5.0-6.6) years in the no axillary surgery group. The median (IQR) follow-up for vital status assessment was 5.8 (5.0-6.9) years in the SLNB group and 5.8 (5.0-6.8) years in the no axillary surgery group. During the follow-up period, 12 (1.7%) locoregional relapses, 13 (1.8%) distant metastases, and 21 (3.0%) deaths occurred in the SLNB group, while 11 (1.6%) locoregional relapses, 14 (2.0%) distant metastases, and 18 (2.6%) deaths occurred in the no axillary surgery group (Table 3). The 5-year DDFS was 97.7% in the SLNB group and 98.0% in the no axillary surgery group (log-rank P = .67) (Figure 2A). When evaluating the primary hypothesis of noninferiority for 5-year DDFS, we found that the omission of axillary surgery was noninferior to SLNB (hazard ratio, 0.84; 90% CI, 0.45-1.54; noninferiority P = .02). The 5-year DFS was 94.7% in the SLNB group and 93.9% in the no axillary surgery group (log-rank P = .30) (Figure 2B). The 5-year OS was 98.2% in the SLNB group and 98.4% in the no axillary surgery group (log-rank P = .72) (Figure 2C).
Table 3. Summary of First Events, Deaths, and Follow-Up Time.
| Outcome | Events, No. (%) | |
|---|---|---|
| SLNB (n = 708) | No axillary surgery (n = 697) | |
| First events | ||
| Ipsilateral breast recurrence | 7 (1.0) | 6 (0.9) |
| Axillary recurrence | 3 (0.4) | 5 (0.7) |
| Ipsilateral breast and axillary recurrence | 2 (0.3) | 0 |
| Distant metastasis | 13 (1.8) | 14 (2.0) |
| Contralateral breast cancer | 5 (0.7) | 7 (1.0) |
| Nonbreast primary tumors | 17 (2.4) | 22 (3.2) |
| Death from breast cancer | 0 | 0 |
| Death from cause other than breast cancer | 5 (0.7) | 6 (0.9) |
| Death from unknown cause | 1 (0.1) | 1 (0.1) |
| Follow-up, median (IQR), y | 5.7 (5.0-6.8) | 5.7 (5.0-6.6) |
| All deaths, cause | ||
| Breast cancer | 7 (1.0) | 4 (0.6) |
| Cause other than breast cancer | 10 (1.4) | 12 (1.7) |
| Unknown cause | 4 (0.6) | 2 (0.3) |
| Follow-up, median (IQR), y | 5.8 (5.0-6.9) | 5.8 (5.0-6.8) |
Abbreviation: SLNB, sentinel lymph node biopsy.
Figure 2. Kaplan-Meier Estimates of Distant Disease–Free Survival, Disease-Free Survival, and Overall Survival.
SLNB indicates sentinel lymph node biopsy.
The 5-year cumulative incidence of distant metastases was 2.3% in the SLNB group and 1.9% in the no axillary surgery group (Gray P = .69) (eFigure 1 in Supplement 2). The 5-year cumulative incidence of axillary recurrences was 0.4% in both groups (Gray P = .91) (eFigure 2 in Supplement 2).
Discussion
In the SOUND trial, the omission of axillary surgery was noninferior to surgical staging performed by SLNB when evaluating DDFS at 5 years in patients with BC up to 2 cm and a negative result on preoperative ultrasonography of axillary lymph nodes. Of note, in the no axillary surgery group, the cumulative incidence of lymph node recurrences in the axilla was very low (0.4% at 5 years), despite a 13.7% rate of nodal involvement in the SLNB group.
Other prospective randomized clinical trials13,14,15 conducted in the pre-SLNB era have shown that ALND did not improve outcomes compared with no surgery in the axilla, confirming that removal of axillary lymph nodes had no therapeutic effect by itself and was performed mainly as a staging procedure. Thus, a relevant research aim addressed as a secondary end point was to evaluate possible differences in terms of adjuvant treatment recommendations. Data from the current trial indicated that adjuvant treatments were not significantly different in the 2 study groups, regardless of whether the pathological information from SLNB was available. These data confirm the increasing pattern of guiding adjuvant treatment mostly through the use of biological parameters rather than clinicopathological variables.16,17,18
The data in this trial were consistent with guidelines from the Choosing Wisely campaign of the Society of Surgical Oncology,19 which recommends omitting SLNB in patients older than 70 years with small ER-positive ERBB2-negative BC when the adjuvant treatment plan is clear and does not include the addition of chemotherapy to endocrine treatment. However, the information provided by nodal status is currently not being completely ignored when selecting postoperative treatment for younger patients, and the absence of the pathological information acquired from SLNB might still create challenges in the management of ER-positive ERBB2-negative BC. Even with the wide availability of genomic testing, chemotherapy can be prescribed or at least considered in addition to endocrine treatments for women with endocrine-responsive disease and axillary lymph node involvement, especially for premenopausal patients. In the Rx-PONDER (A Clinical Trial Rx for Positive Node, Endocrine Responsive Breast Cancer) trial,20 the advantage of adding cytotoxic agents could not be ruled out in patients younger than 50 years with nodal metastases, even when the recurrence score was low or intermediate. In addition, the duration of adjuvant endocrine therapy can be adapted according to the risk estimate and usually prolonged after 5 years in patients with node-positive disease.21,22,23 On the other hand, the absence of pathological nodal involvement might allow de-escalation of the hormonal treatment, both in terms of drug choice (tamoxifen vs aromatase inhibitors or ovarian suppression vs no ovarian suppression) and duration, especially in the case of adverse effects that substantially affect patient quality of life. Furthermore, in the subset of patients with ERBB2-positive disease undergoing upfront surgery, information on nodal status is relevant to properly tailor adjuvant treatment, which in node-negative disease might be restricted to paclitaxel and trastuzumab.24 In addition, in patients with small triple-negative BC undergoing upfront surgery, pathological staging of nodal status might be relevant to modulate the postoperative treatment plan. Moreover, nodal radiation fields are frequently adapted for women with nodal involvement as a complement to breast radiotherapy after breast conservation. In contrast, some patients 65 years and older with node-negative disease might even be spared from undergoing any radiotherapy with a limited number of locoregional events and no detrimental effect on OS.25,26
We were also interested in evaluating the capacity of ultrasonography to detect nodal involvement to understand whether imaging might eventually replace surgery for reliable staging.9 It is well known that ultrasonography of axillary nodes has several limitations in detecting lymph node involvement, with a sensitivity ranging from 24% to 94%.27 However, in the current study, the use of ultrasonography was able to rule out the presence of relevant nodal burden, which might not have been identified with clinical evaluation alone. In the SLNB group, the presence of micro- and macrometastases was limited (13.7%) and much lower than the rate reported in previous trials,1,2 likely due to the screening effect of the negative preoperative axillary ultrasonography result required to enter the trial. Given the limited number of patients with macrometastases, the very low number of patients with extensive nodal involvement (0.6% with 4 or more positive nodes) in the axillary surgery group, and the extremely low cumulative incidence of axillary lymph node recurrence in the no axillary surgery group (0.4% at 5 years), the performance of ultrasonography can be considered clinically meaningful. Despite the need for further research to improve imaging methods, the multi-institutional nature of our study supported the wide reproducibility of ultrasonography as a simple and inexpensive method that can be routinely applied in the preoperative workup of all patients with BC.
The results of this trial support the safety of omitting axillary surgery in older postmenopausal women with ER-positive ERBB2-negative BC who met the SOUND eligibility criteria. This subset of women represents approximately 25% of the whole population of women with BC.28,29 Considering that an estimated 2.3 million women are diagnosed with BC every year, approximately 500 000 patients might be able to take advantage of the total omission of axillary surgery, which has been shown to improve arm function in the early postoperative period.11,30,31 Data from the SOUND trial should be considered in the multidisciplinary decision-making process of the individual patient to identify those who might be able to omit SLNB without affecting the postoperative treatment plan. Moreover, the incorporation of these data in future guidelines might lead to a substantial decrease in health care costs due to the reduced involvement of human resources and savings in terms of materials and time.
Limitations
This study has several limitations. The inclusion criteria of the study led to enrollment of patients who could be considered to be at low risk of recurrence in the short term. Thus, we cannot exclude the possibility that differences in outcome might appear over a longer follow-up period because the curve of event onset is expected to occur later in patients with ER-positive ERBB2-negative BC than in patients with triple-negative or ERBB2-positive disease.32,33 Therefore, we have planned to continue the follow-up with a formal analysis after 10 years. We also highlight that the analysis of adjuvant treatments was not the primary end point and that sample size calculations were not performed for this purpose. Therefore, this trial might be underpowered to detect small differences in the details of medical or radiotherapy treatment recommendations.
The SOUND trial was designed in 2011, immediately after publication of the ACOSOG Z0011 trial34 and as its natural continuation. At that time, ALND was the standard of care in the presence of sentinel lymph node metastases because data from the ACOSOG Z0011 trial had not yet been incorporated into guidelines. Because the objective of the SOUND trial was to evaluate the oncological safety of omitting axillary surgery, we decided to compare the experimental group with the most standardized and radical approach comprising ALND in the presence of nodal macrometastases. It was only between 2016 and 2017 that most of the participating centers embraced the ACOSOG Z0011 approach, slowing down the recruitment pace of our enrollment. Furthermore, all available options of radiotherapy were allowed, even partial breast radiotherapy. Of note, 114 patients (16.3%) randomized to the no axillary surgery group received ELIOT as a full dose or an intraoperative boost.
We also highlight the unintentional late registration of the SOUND trial in ClinicalTrials.gov even though we specify that no data were examined and no interval analysis was conducted before the trial was registered. Late registration occurred without any intention to bias the reporting, which is confirmed by the fact that study design, inclusion criteria, exclusion criteria, end points, and sample size were published in a peer-reviewed journal9 immediately after the study started. Another article10 was later published reporting the number of patients included in the SOUND trial up to that time, which provided a forecast on the conclusion of patient enrollment.
Conclusions
This randomized clinical trial found that omission of axillary surgery was noninferior to SLNB in women with small BC and negative results on ultrasonography of the axillary lymph nodes. These results suggest that patients with these features can be safely spared any axillary surgery when the lack of pathological information does not affect the postoperative treatment plan.
Trial Protocol and Statistical Analysis Plan
eFigure 1. Cumulative Incidence of Distant Metastases
eFigure 2. Cumulative Incidence of Axillary Recurrences
eTable. Randomized and Included Participants by Participating Institutions
Nonauthor Collaborators. The SOUND Trial Group
Data Sharing Statement
References
- 1.Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349(9069):1864-1867. doi: 10.1016/S0140-6736(97)01004-0 [DOI] [PubMed] [Google Scholar]
- 2.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927-933. doi: 10.1016/S1470-2045(10)70207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918-926. doi: 10.1001/jama.2017.11470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartels SAL, Donker M, Poncet C, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-year results of the randomized controlled EORTC 10981-22023 AMAROS trial. J Clin Oncol. 2023;41(12):2159-2165. doi: 10.1200/JCO.22.01565 [DOI] [PubMed] [Google Scholar]
- 5.Galimberti V, Cole BF, Viale G, et al. ; International Breast Cancer Study Group Trial 23-01 . Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(10):1385-1393. doi: 10.1016/S1470-2045(18)30380-2 [DOI] [PubMed] [Google Scholar]
- 6.Brackstone M, Baldassarre FG, Perera FE, et al. Management of the axilla in early-stage breast cancer: Ontario Health (Cancer Care Ontario) and ASCO guideline. J Clin Oncol. 2021;39(27):3056-3082. doi: 10.1200/JCO.21.00934 [DOI] [PubMed] [Google Scholar]
- 7.Gradishar WJ, Moran MS, Abraham J, et al. NCCN guidelines: breast cancer. Version 4.2023. National Comprehensive Cancer Network. March 3, 2023. Accessed March 8, 2023. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419
- 8.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 9.Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? a new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel Node vs Observation After Axillary Ultrasonography). Breast. 2012;21(5):678-681. doi: 10.1016/j.breast.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 10.Gentilini O, Veronesi U. Staging the axilla in early breast cancer: will imaging replace surgery? JAMA Oncol. 2015;1(8):1031-1032. doi: 10.1001/jamaoncol.2015.2337 [DOI] [PubMed] [Google Scholar]
- 11.Gentilini O, Botteri E, Dadda P, et al. Physical function of the upper limb after breast cancer surgery: results from the SOUND (Sentinel Node vs. Observation After Axillary Ultra-Sound) trial. Eur J Surg Oncol. 2016;42(5):685-689. doi: 10.1016/j.ejso.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 12.Gourgou-Bourgade S, Cameron D, Poortmans P, et al. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-Event Endpoints in Cancer trials). Ann Oncol. 2015;26(5):873-879. doi: 10.1093/annonc/mdv106 [DOI] [PubMed] [Google Scholar]
- 13.Rudenstam CM, Zahrieh D, Forbes JF, et al. ; International Breast Cancer Study Group . Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of International Breast Cancer Study Group Trial 10-93. J Clin Oncol. 2006;24(3):337-344. doi: 10.1200/JCO.2005.01.5784 [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five–year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347(8):567-575. doi: 10.1056/NEJMoa020128 [DOI] [PubMed] [Google Scholar]
- 15.Agresti R, Martelli G, Sandri M, et al. Axillary lymph node dissection versus no dissection in patients with T1N0 breast cancer: a randomized clinical trial (INT09/98). Cancer. 2014;120(6):885-893. doi: 10.1002/cncr.28499 [DOI] [PubMed] [Google Scholar]
- 16.Burstein HJ, Curigliano G, Thürlimann B, et al. ; Panelists of the St Gallen Consensus Conference . Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32(10):1216-1235. doi: 10.1016/j.annonc.2021.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolberg-Liedtke C, Lüftner D, Brucker SY, et al. ; Gallen International Consensus Conference . Practice-changing perspectives regarding systemic therapy in early breast cancer: opinions of German experts regarding the 17th St. Gallen International Consensus Conference. Breast Care (Basel). 2022;17(3):336-345. doi: 10.1159/000517501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ; Panel Members . Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22(8):1736-1747. doi: 10.1093/annonc/mdr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ABIM Foundation. Choosing Wisely: five things physicians and patients should question. American Board of Internal Medicine. April 24, 2014. Accessed March 9, 2023. https://www.aabb.org/docs/default-source/default-document-library/resources/choosing-wisely-five-things-physicians-and-patients-should-question.pdf
- 20.Kalinsky K, Barlow WE, Gralow JR, et al. 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021;385(25):2336-2347. doi: 10.1056/NEJMoa2108873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375(3):209-219. doi: 10.1056/NEJMoa1604700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies C, Pan H, Godwin J, et al. ; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group . Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor–positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805-816. doi: 10.1016/S0140-6736(12)61963-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gnant M, Fitzal F, Rinnerthaler G, et al. ; Austrian Breast and Colorectal Cancer Study Group . Duration of adjuvant aromatase-inhibitor therapy in postmenopausal breast cancer. N Engl J Med. 2021;385(5):395-405. doi: 10.1056/NEJMoa2104162 [DOI] [PubMed] [Google Scholar]
- 24.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134-141. doi: 10.1056/NEJMoa1406281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382-2387. doi: 10.1200/JCO.2012.45.2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunkler IH, Williams LJ, Jack WJL, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in early breast cancer. N Engl J Med. 2023;388(7):585-594. doi: 10.1056/NEJMoa2207586 [DOI] [PubMed] [Google Scholar]
- 27.Le Boulc’h M, Gilhodes J, Steinmeyer Z, Molière S, Mathelin C. Pretherapeutic imaging for axillary staging in breast cancer: a systematic review and meta-analysis of ultrasonography, MRI and FDG PET. J Clin Med. 2021;10(7):1543. doi: 10.3390/jcm10071543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyld L, Reed MWR. The need for targeted research into breast cancer in the elderly. Br J Surg. 2003;90(4):388-399. doi: 10.1002/bjs.4124 [DOI] [PubMed] [Google Scholar]
- 29.Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst. 2000;92(7):550-556. doi: 10.1093/jnci/92.7.550 [DOI] [PubMed] [Google Scholar]
- 30.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 31.Reimer T, Stachs A, Veselinovic K, et al. ; INSEMA Investigators . Patient-reported outcomes for the Intergroup Sentinel Mamma study (INSEMA): a randomised trial with persistent impact of axillary surgery on arm and breast symptoms in patients with early breast cancer. EClinicalMedicine. 2022;55:101756. doi: 10.1016/j.eclinm.2022.101756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan H, Gray R, Braybrooke J, et al. ; EBCTCG . 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836-1846. doi: 10.1056/NEJMoa1701830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group trials I to V. J Clin Oncol. 2016;34(9):927-935. doi: 10.1200/JCO.2015.62.3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569-575. doi: 10.1001/jama.2011.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eFigure 1. Cumulative Incidence of Distant Metastases
eFigure 2. Cumulative Incidence of Axillary Recurrences
eTable. Randomized and Included Participants by Participating Institutions
Nonauthor Collaborators. The SOUND Trial Group
Data Sharing Statement