Abstract
Background
The effects of restrictive versus liberal transfusion strategies in critically ill patients with traumatic brain injury (TBI) and anemia, particularly in adult patients with moderate to severe TBI, remain inconclusive. Therefore, this systematic review and meta-analysis aim to evaluate the comparative impact of restrictive and liberal red blood cell transfusion strategies among critically ill adult patients with moderate to severe TBI.
Methods
We conducted a search of PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials from their inception through October 20, 2024, to identify randomized controlled trials that compared restrictive (transfusions at a hemoglobin level of ≤ 7 g/dL) and liberal (transfusions at a hemoglobin level of ≤ 9–10 g/dL) transfusion strategies in adult patients with TBI. The primary outcome was mortality, with secondary outcomes including an unfavorable neurological outcome at six months, as determined by the Glasgow Outcome Scale (GOS < 4; or Glasgow Outcome Scale-Extended [GOSE] < 6), and the number of units of packed red blood cells (pRBCs) transfused.
Results
Five randomized controlled trials involving 1,528 patients were included in the analysis. The results showed that restrictive transfusion, compared to liberal transfusion, had no impact on mortality (RR 1.00, 95% CI 0.80 to 1.24, I2 = 0%) or unfavorable neurological outcome at 6 months (RR 1.06, 95% CI 0.94 to 1.20, I2 = 47%). Restrictive transfusion was associated with a reduction in the number of units of pRBCs transfused (MD -2.62, 95% CI -3.33 to -1.90, I2 = 63%).
Conclusion
In patients with TBI, a restrictive transfusion strategy did not reduce the risk of mortality or unfavorable neurological outcome compared with a liberal transfusion strategy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-024-01411-1.
Keywords: Traumatic brain injury, Transfusion, Meta-analysis
Introduction
Anemia is a common complication in critically ill patients with traumatic brain injury (TBI), and it is associated with unfavorable neurological outcomes and elevated mortality rates in patients with TBI requiring intensive care unit admission [1, 2]. Red blood cell (RBC) transfusions are frequently used to treat anemia, yet their use in this population can increase the risk of infections and transfusion-related acute lung injury [2–4]. Clinical guidelines and reviews comparing the outcomes of liberal and restrictive transfusion strategies suggest that a restrictive approach is safe in most settings [5, 6]. However, these guidelines caution that current evidence is insufficient to guide transfusion practices in patients with TBI, as restrictive strategies may not meet the heightened metabolic demands of the cerebral parenchyma, potentially affecting its oxygen delivery capacity and neuronal functionality [5, 6].
Previous meta-analysis on TBI has explored the impacts of transfusions under either restrictive or liberal strategies have yielded inconclusive results, as they were primarily based on observational studies, which may introduce bias [7]. Recently, the emergence of two large-scale randomized controlled trials (RCTs) has provided substantial additional data, enhancing the overall sample size significantly [8, 9]. Now, we’re conducting a systematic review and meta-analysis of RCTs to compare the efficacy of two transfusion thresholds in patients with TBI.
Methods
Protocol and guidance
This systematic review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. Moreover, prior to initiating the study, we prospectively registered the research protocol at PROSPERO with the registration number CRD42024574035. This step was implemented to enhance transparency and adherence to established standards in evidence-based research.
Search methods and selection criteria
Studies were included if they (1) enrolled patients with moderate to severe TBI; (2) compared restrictive transfusion strategies with liberal transfusion strategies (restrictive transfusion strategies were defined as transfusions at a hemoglobin level of ≤ 7 g/dl, while liberal transfusion strategies were defined as transfusions at a hemoglobin level of ≤ 9–10 g/dl); (3) were RCTs; and (4) reported at least one of the predefined outcome measures of interest, such as mortality, unfavorable outcome.
Outcomes
The primary outcome was mortality at 6 months. Secondary outcomes included an unfavorable neurological outcome at 6 months, assessed using the Glasgow Outcome Scale (GOS < 4) or the Glasgow Outcome Scale-Extended (GOSE < 6) [11], and the total number of packed red blood cell units transfused. The number of pRBC units transfused was considered to capture the differences in blood product utilization and potential transfusion-related risks between restrictive and liberal transfusion strategies.
Information sources and search strategy
We searched PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials from inception until October 20, 2024. The search equation includes free terms and MeH terms incorporating these keywords: “traumatic brain injury,” “anemia,” “blood transfusion,” “erythrocyte transfusion,” and “blood component transfusion.” There were no limitations based on language. Table S1 in the Data Supplement outlines the comprehensive search terms employed in each database searched.
Study inclusion
Two reviewers (XY and SZ) independently evaluated all titles and abstracts identified through the systematic search. To ensure accuracy, a third reviewer (YZ) checked for any discrepancies in the collected data. Any disagreements were resolved through discussion or adjudicated by a third reviewer (YZ).
Data extraction
Two reviewers (XY and SZ) independently extracted data on the characteristics of the included trials, including the study author, publication year, study methodology, research aim, hemoglobin threshold (in grams per deciliter), patient demographics, and presentation attributes such as age and gender. To ensure accuracy, a third reviewer (YZ) reviewed the extracted data for any errors. Disagreements between reviewers were resolved through discussion.
Assessment of the risk of bias
Two reviewers (XY and SZ) independently assessed the risk of bias in the trials using the Cochrane Risk of Bias-2 tool [12] across five domains. Each domain in all trials was assigned a study-level score indicating the level of bias risk: low, high, or unclear. Disagreements between reviewers were resolved through discussion. If consensus could not be reached, a final judgment was provided by a third author (YZ).
Confidence of evidence
Two authors (XY and SZ) independently assessed the quality of evidence for primary and secondary outcomes using the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) [13]. The quality of evidence was categorized as high, moderate, low, or very low based on multiple factors including the evaluation of study design, risk of bias, inconsistency, imprecision, and indirectness of the included trials.
Data analysis
We performed the statistical analysis utilizing Review Manager (version 5.4, The Cochrane Collaboration). For binary results, we computed the relative risk (RR) with 95% confidence intervals (CI). To assess continuous results, we calculated the mean difference (MD) with a 95% CI. Study heterogeneity was assessed using Cochrane’s Q and Higgins’ I2 statistics, with Q statistic values at a 10% level of significance (p < 0.1) and I2 values > 50% indicating significant heterogeneity. To ensure the validity of the findings, we utilized random-effect models for all outcomes. We considered a predefined two-sided p-value < 0.05 as statistically significant. Forest diagrams were constructed to illustrate the magnitude of effects and combined estimations. All statistical analyses were conducted using two-tailed tests, with a significance threshold of p < 0.05 indicating a statistically significant association. In this systematic review and meta-analysis, we did not pool individual patient data.
Publication bias
If a meta-analysis included 10 or more studies, we would generate a funnel plot and perform quantitative assessments utilizing the Egger’s [14] and Begg’s tests [15] to comprehensively assess any potential biases associated with small study effects.
Subgroup analysis
We conducted subgroup analysis on the primary outcome based on follow-up time, hemoglobin at randomization, and mean age.
Sensitivity analysis
We performed a sensitivity analysis utilizing the following approaches: (1) utilizing a fixed-effect model; (2) excluding trials with a weight less than 10%; (3) excluding each trial individually.
Results
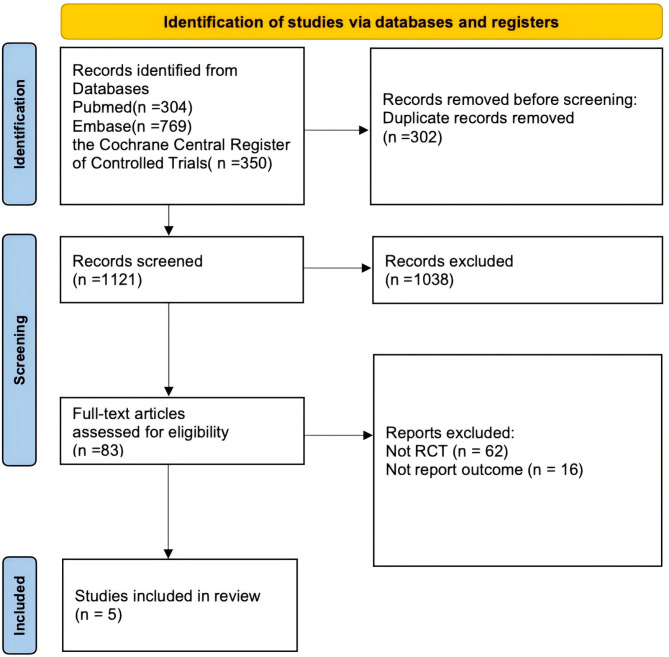
Our search strategy initially identified 1423 records. After removing duplicates, we screened a total of 1121 unique records. We then systematically evaluated the titles and abstracts of these records, followed by an in-depth assessment of the full texts of potentially eligible studies. Ultimately, we identified five trials [8, 9, 16–18] that satisfied the inclusion criteria for this systematic review (Fig. 1).
Fig. 1.
Search strategy and final included and excluded studies
Our systematic review and meta-analysis included five RCTs [8, 9, 16–18], with four [8, 16–18] focusing on TBI and one [9] studying acute brain injury that included TBI. The trials were published between 2006 and 2024, and the sample size ranged from 44 to 742 patients (Table 1).
Table 1.
Characteristics of studies included in the meta-analysis
| Study | Mean age, years | Patients, N | Female, no. (%) | Transfused Threshold, g/dl | Glasgow Coma Scale: median (IQR) | Injury Severity Score: mean ± SD or median (IQR) |
Hemoglobin at Randomization, g/dl: mean ± SD or median (IQR) |
Units of Packed Red Blood Cells Transfused: mean ± SD |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liberal group | Restrictive group |
Liberal group | Restrictive group |
Liberal group | Restrictive group |
Liberal group | Restrictive group |
Liberal group | Restrictive group |
||||
| McIntyre et al., 2006[17] | 41 | 67 | 26 | ≤ 10 | ≤ 7 | 7 (5–9) | 8 (5–10) | 31 ± 13 | 30 ± 14 | NR | NR | 4.6 ± 2.5 | 1.4 ± 2.2 |
| Robertson et al., 2014[18] | 32 | 200 | 14 | ≤ 10 | ≤ 7 | NR | NR | 29 (25–35) | 29 (25–38) | 14.3 ± 2.0 | 14.3 ± 2.0 | 7.1 ± 5.3 | 4.7 ± 5.3 |
| Gobatto et al., 2019[16] | 35 | 44 | 9 | ≤ 9 | ≤ 7 | 5 (3–7) | 5 (3–7) | 28 ± 9 | 31 ± 9 | 7.9 ± 0.6 | 8.2 ± 1.0 | 3.1 ± 1.6 | 1.5 ± 1.7 |
| Turgeon et al., 2024[8] | 49 | 742 | 27 | ≤ 10 | ≤ 7 | 4 (1–5) * | 4 (1–5) * | 30 ± 11 | 32 ± 11 | 9.1 ± 0.8 | 9.1 ± 0.8 | 3.4 ± 2.2 | 0.4 ± 0.7 |
| Taccone et al.,2024[9] | NR | 475 | NR | ≤ 9 | ≤ 7 | NR | NR | NR | NR | NR | NR | NR | NR |
GCS: Glasgow Coma Scale, SD: Standard Deviation, IQR: Inter-Quartile Range, NR: not reported, * Glasgow Coma Scale motor score
Figure S1 in the Data Supplement presents the Risk-of-bias assessments. One trial had low risk of bias [16], while four trials had some concerns [9, 17–19] (Figure S1 in the Data Supplement). The quality of evidence for the mortality was moderate, while the unfavorable neurological outcome and the units of packed red blood cells transfused were low, as evaluated by GRADE (Table 2).
Table 2.
Summary of findings and strength of evidence
| Outcome | NO. of patients (trials) |
Relative Effect | Absolute effect estimates (per 1000) | Quality of the evidence | |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Mortality | 1045 (4) | RR = 1.00; 95% CI (0.80 to 1.24) | 0 (47 to 56) | Moderate a | |
| Secondary outcomes | |||||
| Unfavorable neurological outcome at 6 months | 1422 (4) | RR = 1.06; 95% CI (0.94 to 1.20) | 54 (69 to 169) | Low a, c | |
| Units of packed red blood cells transfused | 1047 (4) | MD = -2.62; 95% CI (-3.33 to -1.90) | 2.62 (1.9 to 3.33) | Low a, b | |
CI: confidence interval; RR: risk ratio
a We downgraded our assessment of the evidence one level for imprecision due to the limited number of patients
b We downgraded our assessment of the evidence one level for inconsistency due to the serious I2 value of 63%
c We downgraded our assessment of the evidence one level for inconsistency due to the moderate I2 value of 47%. This inconsistency was further highlighted by the sensitivity analysis, which showed a significant reduction in heterogeneity and a change in the result when one particular study was excluded
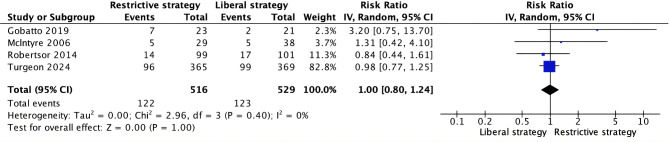
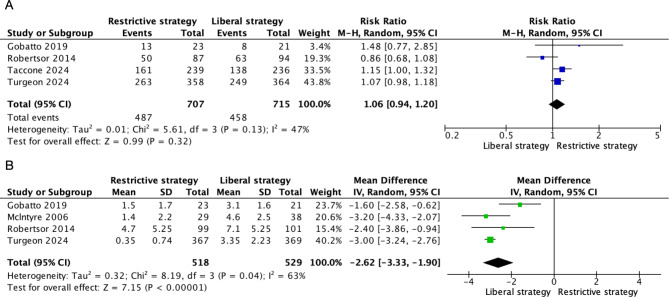
Four trials [16–19] reported on mortality, with no significant difference observed between restrictive and liberal transfusion thresholds (RR 0.80, 95% CI 0.80 to 1.24, I² = 0%) (Fig. 2). Four trials [9, 16, 18, 19] reported on the GOS or GOSE, with no significant difference in unfavorable neurological outcome at six months between the two transfusion thresholds (RR 1.06, 95% CI 0.94 to 1.20, I² = 47%) (Fig. 3A). Additionally, four trials [16–19] reported on the number of units of pRBCs transfused, with restrictive transfusion thresholds resulting in significantly fewer units being transfused (MD -2.62, 95% CI -3.33 to -1.90, I² = 63%) (Fig. 3B).
Fig. 2.
Forest plots comparing: mortality with restrictive and liberal transfusion thresholds in patients with traumatic brain injury. M–H: Mantel–Haenszel, CI: Confidence interval, df: Degrees of freedom
Fig. 3.
Forest plots comparing: (A) Unfavorable neurological outcome at six months (B) Units of packed red blood cells transfused. M–H: Mantel–Haenszel, CI: Confidence interval, df: Degrees of freedom
The robustness of mortality findings was confirmed through sensitivity analyses. Our sensitivity analyses of mortality revealed no significant differences in results, regardless of whether we employed a fixed-effect model, excluded trials with a weight of less than 10%, or excluded each trial individually (Table S2 in the Data Supplement). Conversely, when we excluded the trial by Robertson et al. (2014) [18], the effect on unfavorable neurological outcome became significant (Table S3 in the Data Supplement).
The results of the subgroups for mortality in patients with TBI indicated that none of the following factors significantly contributed to the source of heterogeneity: mean age (< 40 vs. >40, P for interaction = 0.61), follow-up time (two months vs. six months, P for interaction = 0.68), and mean hemoglobin at randomization (< 10 vs. >10, P for interaction = 0.42) (Figure S2 in the Data Supplement).
Discussion
This systematic review and meta-analysis, which included five RCTs involving a total of 1528 participants, revealed that a restrictive transfusion strategy, compared to a liberal strategy, did not lead to a reduction in mortality or an unfavorable neurological outcome.
A previous meta-analysis [7] also examined this topic. Similar to our study, it similarly concluded that a restrictive transfusion strategy did not reduce mortality (odds ratio 0.94, 95% CI 0.60 to 1.48). However, that analysis included both trials and comparative observational studies, incorporating only three relatively small RCTs involving a total of 311 patients. In contrast, our comparative analysis was strictly focused on RCTs, including two newer large RCTs available in the literature, involving a total of 1528 patients. The data from these extensive studies served to strengthen the conclusions and enhance the precision of the treatment effects associated with a restrictive transfusion strategy.
Another meta-analysis of RCTs [20] encompassed a heterogeneous patient cohort with diverse neurocritical conditions (RR 1.42, 95% CI 0.42 to 4.78), including TBI, intracerebral hemorrhage, and subarachnoid hemorrhage. This broad scope has posed challenges in deriving precise implications for patients specifically with TBI. In contrast, our study exclusively investigated TBI patients, enabling us to deliver more tailored and concentrated outcomes for this distinct patient group. This focused methodology elevated the relevance and practicality of our conclusions for individuals with TBI.
The management of TBI patients involves a delicate balance between ensuring adequate blood flow and oxygenation to the brain, while minimizing the risks associated with blood transfusions. The debate between liberal and restrictive transfusion strategies in TBI patients is a critical aspect of this management, with each approach having its own set of advantages and disadvantages. Liberal transfusion, by setting a higher hemoglobin transfusion threshold (e.g., Hemoglobin (Hb) ≤ 10 g/dL), aims to maintain a sufficient Hb level to ensure adequate oxygen supply to the brain, potentially mitigating secondary brain injury due to anemia and improving neurological outcomes [21–23]. However, this approach may increase the risk of transfusion-related adverse events and complications, such as acute respiratory distress syndrome [24], transfusion-related acute lung injury [25], and infections, as well as consume more medical resources. On the other hand, restrictive transfusion, which adopts a lower Hb transfusion threshold (e.g., Hb ≤ 7 g/dL), seeks to reduce unnecessary transfusions and thus minimize transfusion-related risks and conserve medical resources. While this strategy has demonstrated advantages in mitigating transfusion-related hazards, it may also lead to inadequate blood supply to the brain, potentially impacting patient outcomes due to cerebral hypoxia [5, 26].
Hb concentration, while commonly used to guide transfusion decisions, reflects a measure of concentration in the blood rather than the actual volume of red blood cells. This limitation becomes critical when considering that TBI patients, especially those with intracranial hypertension, may require a more nuanced assessment of oxygen delivery to the brain [27]. Given that Hb concentration alone may not fully capture the adequacy of brain oxygen supply, alternative indicators such as jugular venous oxygen saturation and brain tissue oxygen monitoring can provide invaluable insights. These parameters can help inform transfusion decisions in situations where maintaining adequate brain oxygenation amidst elevated intracranial pressure is paramount [28–30]. A comprehensive approach that incorporates these alternative indicators, alongside considerations for the differences in intracranial pressure between moderate and severe TBI patients, is essential for optimizing treatment strategies and potentially improving patient outcomes while mitigating the risk of secondary brain injury. Future studies exploring optimal transfusion thresholds in TBI patients should consider incorporating these additional metrics to further refine and personalize transfusion strategies, ensuring that oxygen delivery to the brain is adequate and tailored to individual patient needs.
There are several limitations that need to be considered. First, the inconsistency in inclusion criteria, particularly regarding the definition of TBI, introduces variability into the research landscape. Second, variations in follow-up time among studies and characteristics of the patient population contribute to further heterogeneity. Variation in these parameters impart heterogeneity to a systematic review and meta-analysis of pooled data. To mitigate the impact of these disparities, we conducted stratified subgroup analyses. However, certain subgroup analyses, such as the severity of TBI could not be performed due to a lack of individual patient data from the component trials. Third, our primary outcome is primarily shaped by the HEMOTION trial [19]. While this enhances the statistical power of our analysis, it also means that our conclusions may be disproportionately affected by the characteristics and results of this particular study. Fourth, our primary outcome includes two very small RCTs which could potentially introduce bias. Consequently, we conducted a sensitivity analysis to assess the impact of these smaller studies on our findings. The results of these sensitivity analyses remain consistent with our findings, indicating that our conclusions are not significantly influenced by these smaller RCTs. Fifth, the limited number of RCTs included provided insufficient power to detect potential publication bias. Nevertheless, the likelihood of publication bias is minimal as most trials yielded neutral results in the primary outcome.
Additionally, our systematic review and meta-analysis did not demonstrate significant differences in mortality or unfavorable neurological outcome between restrictive and liberal transfusion strategies, this finding aligns with the results of the HEMOTION trial [19]. While HEMOTION was designed to detect a 10% absolute difference, the observed 5% difference suggests a non-significant trend favoring the liberal strategy, potentially due to inadequate power to detect smaller effect sizes, given the sample size [8, 31]. This underscores the complexity and uncertainty associated with the application of transfusion strategies in clinical practice. Therefore, while current evidence does not definitively support the superiority of one strategy over the other, these findings highlight the need for future research. Large-scale studies are warranted to investigate whether specific transfusion thresholds can provide meaningful clinical benefits for patients with TBI, further elucidating how to optimize transfusion strategies to improve patient outcomes.
Conclusions
This systematic review and meta-analysis indicated that a restrictive transfusion strategy did not reduce the risk of mortality or unfavorable neurological outcome compared with a liberal transfusion strategy in patients with TBI. Nevertheless, current evidence does not definitively support the superiority of one strategy over the other. Further large-scale studies are warranted to determine whether specific transfusion thresholds provide clinically meaningful benefits in this patient population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the use of GPT-based AI for language refinement in the manuscript preparation.
Abbreviations
- TBI
Traumatic brain injury
- RBC
Red blood cell
- Hb
Hemoglobin
- GOS
Glasgow outcome scale
- GOSE
Glasgow outcome scale-extended
- GCS
Glasgow coma scale
- pRBCs
Packed red blood cells
- RCT
Randomized controlled trial
- GRADE
Grading of recommendation, assessment, development and evaluation
- PRISMA
Preferred reporting items for systematic reviews and Meta-analysis
- CI
Confidence intervals
- RR
Relative risk
- OR
Odds ratio
- MD
Mean difference
Author contributions
Study concept: YZ. Design: All authors. Acquisition, analysis, or interpretation of data: XY, SZ, JW, CC, PW, SF, YL, JY, JH, QY, XL, KL, ZX and YR. Statistical analysis: XY and SZ. Drafting of the manuscript: XY, SZ, JW and CC. Critical revision of the manuscript for important intellectual content: All authors.
Funding
This work is supported by National Natural Science Foundation of China (82271364), the innovation team project of Affiliated Hospital of Clinical Medicine College of Chengdu University (CDFYCX202203), and the project of Sichuan Science and Technology Bureau (22ZDYF0798), the 1·3·5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (21HXFH046), the project of health commission of Sichuan province (2019HR50), Experimental Teaching Research and Reform Project of Chengdu University (cdsyjg2022022), National Entrepreneurship Training Program for College Students of Chengdu University (202411079002 S), Chengdu Science and Technology Project (2024-YF05-00871-SN), and Science and Technology Research Program of Chongging Municipal Education Commission (KJQN202200452).
Data availability
All relevant data are within the manuscript and its Supporting Information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiang Yuan, Sen Zhang, Cheng Chen, and Jun Wan contributed equally to this work.
References
- 1.Guglielmi A, Graziano F, Bogossian EG, et al. Haemoglobin values, transfusion practices, and long-term outcomes in critically ill patients with traumatic brain injury: a secondary analysis of CENTER-TBI. Crit Care. 2024;28(1):199. [DOI] [PMC free article] [PubMed]
- 2.Lelubre C, Bouzat P, Crippa IA, et al. Anemia management after acute brain injury. Crit Care. 2016;20(1):152. [DOI] [PMC free article] [PubMed]
- 3.Van Beek JG, Mushkudiani NA, Steyerberg EW, et al. Prognostic value of admission laboratory parameters in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):315–28. [DOI] [PubMed] [Google Scholar]
- 4.Warner MA, O’Keeffe T, Bhavsar P, et al. Transfusions and long-term functional outcomes in traumatic brain injury. J Neurosurg. 2010;113(3):539 – 46. [DOI] [PubMed]
- 5.Carson JL, Stanworth SJ, Dennis JA, et al. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst Rev. 2021;12(12):Cd002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson JL, Stanworth SJ, Guyatt G, et al. Red blood cell transfusion: 2023 AABB International guidelines. JAMA. 2023;330(19):1892–902. [DOI] [PubMed] [Google Scholar]
- 7.Montgomery EY, Barrie U, Kenfack YJ, et al. Transfusion guidelines in traumatic Brain Injury: a systematic review and Meta-analysis of the currently available evidence. Neurotrauma Rep. 2022;3(1):554–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turgeon AF, Fergusson DA, Clayton L, et al. Liberal or Restrictive Transfusion Strategy in Patients with Traumatic Brain Injury. N Engl J Med. 2024. [DOI] [PubMed]
- 9.Taccone FS, Rynkowski Bittencourt C, Møller K, et al. Restrictive vs Liberal Transfusion Strategy in Patients With Acute Brain Injury: The TRAIN Randomized Clinical Trial. Jama. 2024. [DOI] [PMC free article] [PubMed]
- 10.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021;372:n71. [DOI] [PMC free article] [PubMed]
- 11.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet1975. p. 480-4. [DOI] [PubMed]
- 12.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. 2019;366:l4898. [DOI] [PubMed]
- 13.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629 – 34. [DOI] [PMC free article] [PubMed]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088 – 101. [PubMed]
- 16.Gobatto ALN, Link MA, Solla DJ, et al. Transfusion requirements after head trauma: a randomized feasibility controlled trial. Crit Care. 2019;23(1):89. [DOI] [PMC free article] [PubMed]
- 17.McIntyre LA, Fergusson DA, Hutchison JS, et al. Effect of a liberal versus restrictive transfusion strategy on mortality in patients with moderate to severe head injury. Neurocrit Care. 2006;5(1):4–9. [DOI] [PubMed]
- 18.Robertson CS, Hannay HJ, Yamal JM, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA. 2014;312(1):36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turgeon AF, Fergusson DA, Clayton L, et al. Liberal or Restrictive Transfusion Strategy in patients with traumatic brain Injury. N Engl J Med. 2024;391(8):722–35. [DOI] [PubMed] [Google Scholar]
- 20.Yu Y, Fu Y, Li W, et al. Red blood cell transfusion in neurocritical patients: a systematic review and meta-analysis. BMC Anesthesiol. 2024;24(1):106. [DOI] [PMC free article] [PubMed]
- 21.Smith MJ, Stiefel MF, Magge S, et al. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med. 2005;33(5):1104–8. [DOI] [PubMed] [Google Scholar]
- 22.Zygun DA, Nortje J, Hutchinson PJ, et al. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Crit Care Med. 2009;37(3):1074-8. [DOI] [PubMed]
- 23.Leal-Noval SR, Rincón-Ferrari MD, Marin-Niebla A, et al. Transfusion of erythrocyte concentrates produces a variable increment on cerebral oxygenation in patients with severe traumatic brain injury: a preliminary study. Intensive Care Med. 2006;32(11):1733-40. [DOI] [PubMed]
- 24.Zilberberg MD, Carter C, Lefebvre P, et al. Red blood cell transfusions and the risk of acute respiratory distress syndrome among the critically ill: a cohort study. Crit Care. 2007;11(3):R63. [DOI] [PMC free article] [PubMed]
- 25.Yu Y, Lian Z. Update on transfusion-related acute lung injury: an overview of its pathogenesis and management. Front Immunol. 2023;14:1175387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carson JL, Hill S, Carless P, et al. Transfusion triggers: a systematic review of the literature. Transfus Med Rev. 2002;16(3):187–99. [DOI] [PubMed] [Google Scholar]
- 27.Johnston IH, Johnston JA, Jennett B. Intracranial-pressure changes following head injury. Lancet. 1970;2(7670):433–6. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C, Zhou L, Zhang K, et al. Brain tissue oxygen pressure combined with intracranial pressure monitoring may improve clinical outcomes for patients with severe traumatic brain injury: a systemic review and meta-analysis. PeerJ. 2024;12:e18086. [DOI] [PMC free article] [PubMed]
- 29.Vigué B, Ract C, Benayed M, et al. Early SjvO2 monitoring in patients with severe brain trauma. Intensive Care Med. 1999;25(5):445–51. [DOI] [PubMed] [Google Scholar]
- 30.Chesnut R, Aguilera S, Buki A, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International severe traumatic brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020;46(5):919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turgeon AF, Fergusson DA, Clayton L, et al. Haemoglobin transfusion threshold in traumatic brain injury optimisation (HEMOTION): a multicentre, randomised, clinical trial protocol. BMJ Open. 2022;12(10):e067117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.