Abstract
The rapid development of therapies for severe and rare genetic conditions underlines the need to incorporate first-tier genetic testing into newborn screening (NBS) programs. A workflow was developed to screen newborns for 165 treatable pediatric disorders by deep sequencing of regions of interest in 405 genes. The prospective observational BabyDetect pilot project was launched in September 2022 in a maternity ward of a public hospital in the Liege area, Belgium. In this ongoing observational study, 4,260 families have been informed of the project, and 3,847 consented to participate. To date, 71 disease cases have been identified, 30 of which were not detected by conventional NBS. Glucose-6-phosphate dehydrogenase deficiency was the most frequent disorder detected, with 44 positive individuals. Of the remaining 27 cases, 17 were recessive disorders. We also identified one false-positive case in a newborn in whom two variants in the AGXT gene were identified, which were subsequently shown to be located on the maternal allele. Nine heterozygous variants were identified in genes associated with dominant conditions. Results from the BabyDetect project demonstrate the importance of integrating biochemical and genomic methods in NBS programs. Challenges must be addressed in variant interpretation within a presymptomatic population and in result reporting and diagnostic confirmation.
Subject terms: Paediatrics, Preventive medicine, Population screening
The BabyDetect project offered expanded newborn genomic screening covering more than 400 genes to 4,260 families, leading to 71 clinical diagnoses.
Main
Every year, thousands of children are born with rare genetic diseases that may lead to death or lifelong disability1. Newborn screening (NBS) has been used for decades to identify treatable conditions before the onset of the first symptoms to allow timely interventions that can prevent or minimize long-term health effects. Traditionally, NBS involves collecting a few drops of blood immediately after birth and analyzing this sample by biochemical methods to detect the presence of specific biomarkers. The inclusion of new conditions into an NBS program is driven by criteria formulated by Wilson and Jungner2 in 1968. The criteria include the existence of an effective treatment and a reliable and cost-effective analytical method.
Recent technological advances have led to the identification of the genetic causes of several diseases, and the rate of introduction of new therapies for rare diseases has remarkably increased in the past decade3. Spinal muscular atrophy and severe combined immunodeficiency are examples of diseases for which new treatments are now available. Importantly, these treatments are most effective if initiated before symptoms appear4–6. The US Food and Drug Administration estimates that by 2025, there will be 10–20 new cell and gene therapy approvals per year7, and it is expected that early or presymptomatic administration of treatments will be correlated with higher life expectancy, avoidance of severe disabilities and fewer complications.
The rarity and lack of medical awareness of rare genetic diseases often lead to a long diagnostic journey, as biomarkers that can be detected by biochemical assays have not been identified for many rare disorders. This has prompted a growing interest in expanding NBS by integrating genomic technologies8–12. In September 2022, we launched the BabyDetect project (ClinicalTrials.gov identifier: NCT05687474; www.babydetect.com) to explore the feasibility and acceptability of a population-based, first-tier genomic NBS using targeted next-generation sequencing (tNGS). We report here the results of the first 18 months of this ongoing observational study, which was conducted in a single maternity ward in southern Belgium.
Results
Screened population and samples
From September 2022 to the end of April 2024, the families of 4,260 neonates were informed of the BabyDetect trial. A total of 3,847 neonates were enrolled, corresponding to a 90% acceptance rate. Most (53%) of the parents who opted not to enroll their baby in the study did not disclose a reason for their refusal. Among those who were more forthcoming, the primary rationale was that they deemed the test unnecessary considering that the family and siblings were healthy, the pregnancy had proceeded smoothly or the child appeared to be in good health13. The characteristics of the newborns enrolled in the study are presented in Table 1. Of the 3,847 samples analyzed, 84 (2.2%) were retested because of technical issues. The main reasons for testing failures were sample cross-contamination (n = 16), sequencing workstation failure (n = 48) and poor library quality (n = 20).
Table 1.
Characteristics of the newborn population
| Characteristics | n | (%) |
|---|---|---|
| Sex | ||
| Male | 1,957 | 50.9 |
| Female | 1,890 | 49.1 |
| Birth weight (g) | ||
| <2,500 | 478 | 12.4 |
| 2,500–4,000 | 3,155 | 82.0 |
| >4,000 | 214 | 5.6 |
| Gestational age (weeks) | ||
| <37 | 497 | 12.9 |
| 37–38 | 964 | 25.1 |
| 39–40 | 2,092 | 54.4 |
| >40 | 294 | 7.6 |
Variant filtering and review
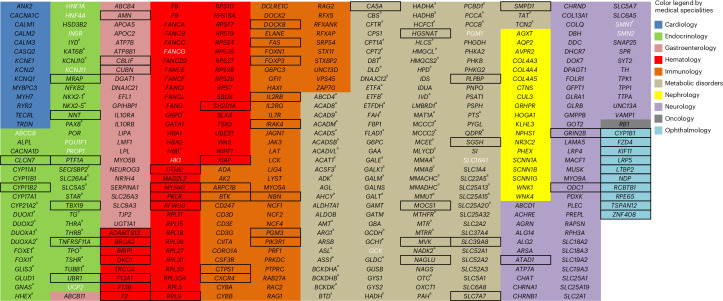
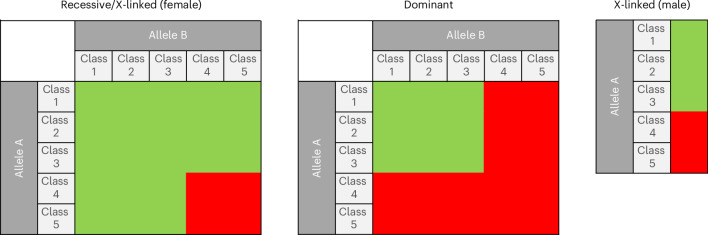
The list of genes included in BabyDetect target panel is shown in Fig. 1. Zygosity criteria for variant reporting are outlined in Fig. 2. Between 4,000 and 11,000 variants were inferred for each neonate. A dedicated classification tree on the Alissa Interpret platform was used to automatically process variants. The sorting algorithm consisted of a sequence of filters and output bins with optional labels and scores, incorporated into a decision tree topology. The tree allowed us to systematically triage and classify variants. Benign and likely benign variants and variants of unknown significance (VUS) were discarded by the tree, and pathogenic or likely pathogenic genome variants were flagged for manual review before reporting. To comply with the requirement for actionable screening, we report only variants with genotypes known to be associated with a disease. Figure 3 summarizes the applied filtering criteria.
Fig. 1. List of genes included in the BabyDetect target panel.
Boxed genes were added to version 2.0 of the panel, whereas genes in white font were removed from version 1.0. Genes marked with a superscript letter (a) are associated with a disorder covered by our conventional NBS.
Fig. 2. Zygosity criteria, based on Mendelian inheritance, for pathogenic (class 5) or likely pathogenic (class 4) variant reporting.
Classes 1, 2 and 3 are benign, likely benign and VUS, respectively. Green indicates negative results. Red indicates samples reviewed manually for the accuracy of variant annotations.
Fig. 3. Variant filtering criteria.
P, pathogenic; LP, likely pathogenic.
After filtering, flagged samples were manually reviewed to validate variant classification as pathogenic or likely pathogenic and to rule out any potential conflicting interpretations before reporting. This variant review included the American College of Medical Genetics and Genomics interpretation using the Franklin14,15 and VarSome16 tools, an extended literature review, and correlation with biochemical results when available. Samples were considered negative if no consensus on the variant was found among the ClinVar, VarSome and Franklin databases.
Positive screening cases
In this ongoing observational study, 3,847 neonates have been tested thus far. After variant filtering, 1% of screened samples required manual review, of which 71 were identified as positive cases for a pathogenic or likely pathogenic variant. Among those neonates, no issues related to discrepancies between phenotypic and genetic sex were observed. Of the positive cases, 44 neonates were identified to have glucose-6-phosphate dehydrogenase (G6PD) deficiency. The positive cases are summarized in Table 2. Nine heterozygous variants were detected in genes associated with conditions that can be inherited in a dominant manner: familial exudative vitreoretinopathy in one neonate, maturity-onset diabetes of the young 13 in one neonate, cardiomyopathy due to a mutation in MYBPC3 in two neonates and cardiomyopathy due to a mutation in MYH7 in five neonates. Eighteen neonates were identified to have recessive disorders, including two with glycogen storage disease 1b/c, one with Shwachman–Diamond syndrome, two with hemophilia A, two with hemophilia B, five with cystic fibrosis, one with phenylketonuria, two with partial biotinidase deficiency, one with short-chain acyl-CoA dehydrogenase deficiency, one with carnitine palmitoyltransferase 2 (CPT2) deficiency and one with two class 5 variants in the AGXT gene.
Table 2.
Positive cases of diseases detected in the BabyDetect study
| Case no. | Disorder | Sex | Genotype | Conventional NBS | Conventional NBS result (if available) | Confirmatory result, follow-up | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | G6PD deficiency | Female | G6PD:c.[292G>A;466A>G]–c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 2 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 3 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 4 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 5 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 6 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 7 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 8 | G6PD deficiency | Male | G6PD:c.1450C>T | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 9 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 10 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 11 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 12 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 13 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 14 | G6PD deficiency | Male | G6PD:c.494A>C | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 15 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 16 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 17 | G6PD deficiency | Female | G6PD:c.[292G>A;466A>G]–c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 18 | G6PD deficiency | Female | G6PD:c.[292G>A;466A>G]–c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 19 | G6PD deficiency | Male | G6PD:c.653C>T | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 20 | G6PD deficiency | Male | G6PD:c.653C>T | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 21 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 22 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 23 | G6PD deficiency | Female | G6PD:c.653C>T–c.1093G>A | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 24 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 25 | G6PD deficiency | Male | G6PD:c.494A>C | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 26 | G6PD deficiency | Male | G6PD:c.653C>T | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 27 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 28 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 29 | G6PD deficiency | Male | G6PD:c.1058T>C–c.466A>G | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 30 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 31 | G6PD deficiency | Female | G6PD:c.[292G>A;466A>G]–c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 32 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 33 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 34 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Positive | G6PD activity < 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 35 | Biotinidase deficiency | Female | BTD:c.1270G>C–c.1308A>C | Positive | Biotinidase activity = 31.5% (RR > 47%) | Confirmed by NGS and decreased biotinidase activity in serum, under conventional NBS follow-up | Standard of care: biotin administration |
| 36 | Biotinidase deficiency | Male | BTD:c.535G>A–c.1270G>C | Positive | Biotinidase activity = 30.5% (RR > 47%) | Confirmed by NGS and decreased biotinidase activity in serum, under conventional NBS follow-up | Standard of care: biotin administration |
| 37 | Cystic fibrosis | Male | CFTR:c.1521_1523delCTT–c.1521_1523delCTT | Positive | Positive IRT + CFTR analysis | Under conventional NBS follow-up | Standard of carea |
| 38 | Cystic fibrosis | Male | CFTR:c.1521_1523delCTT–c.1521_1523delCTT | Positive | Positive CFTR analysis | Under conventional NBS follow-up | Standard of carea |
| 39 | Cystic fibrosis | Female | CFTR:c.1521_1523delCTT–c.1521_1523delCTT | Positive | Positive IRT + CFTR analysis | Under conventional NBS follow-up | Standard of carea |
| 40 | Cystic fibrosis | Female | CFTR:c.1521_1523delCTT–c.2657+5G>A | Positive | Positive IRT + CFTR analysis | Under conventional NBS follow-up | Standard of carea |
| 41 | Mild phenylketonuria | Male | PAH:c.1222C>T–c.688G>A | Positive | Phenylalanine = 164 µmol l−1 (RR < 120 μmol l−1) | Confirmed by NGS, under conventional NBS follow-up | Standard of care: restrictive diet |
| 42 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Negative | G6PD activity > 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 43 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Negative | G6PD activity > 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 44 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Negative | G6PD activity > 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 45 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Negative | G6PD activity > 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 46 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Negative | G6PD activity > 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 47 | G6PD deficiency | Female | G6PD:c.[292G>A;466A>G]–c.[292G>A;466A>G] | Negative | G6PD activity > 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 48 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Negative | G6PD activity > 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 49 | G6PD deficiency | Male | G6PD:c.934G>C | Negative | G6PD activity > 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 50 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Negative | G6PD activity > 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 51 | G6PD deficiency | Male | G6PD:c.[292G>A;466A>G] | Negative | G6PD activity > 30% (RR ≥ 30%) | Reduced G6PD activity measured in red blood cells, under conventional NBS follow-up | Preventive measures |
| 52 | Short-chain acyl-CoA dehydrogenase deficiency | Female | ACADS:c.596C>T–c.1147C>T | Negative | C4-carnitine = 0.06 µmol l−1 (RR < 0.08 μmol l−1) | Pending | |
| 53 | Cystic fibrosis | Male | CFTR:c.1865G>A–c.1865G>A | Negative | IRT = 31 µg l−1 (RR < 59.8 μg l−1) | Neonate not referred, see the text | / |
| 54 | CPT2 deficiency | Male | CPT2:c.1339C>T–c.1436A>T | Negative | Long-chain acylcarnitines within normal values | CPT2 activity: 2.6 nmol min−1 per mg protein (RR = 9–23 nmol min−1 per mg protein) | Standard of care initiated at 5 months of ageb |
| 55 | Hyperoxaluria 1 | Female | AGXT:c.33dupC–c.332G>A | / | / | Not confirmed—false-positive BabyDetect result | Not applicable |
| 56 | Hemophilia A | Male | F8:c.396A>C | / | / | Factor VIII: 48% (RR > 50%) | Preventive care in preoperative settings |
| 57 | Hemophilia A | Male | F8:c.6089G>A | / | / | Factor VIII: 21% (RR > 50%) | Preventive care in preoperative settings |
| 58 | Hemophilia B | Male | F9:c.1345C>T | / | / | Lost to follow-up | / |
| 59 | Hemophilia B | Male | F9:c.1024A>G | / | / | Pending | |
| 60 | Familial exudative vitreoretinopathy | Female | FZD4:c.313A>G | / | / | Fundus of the eye examination planned at 9 months of age | Surveillance |
| 61 | Maturity-onset diabetes of the young 13 | Male | KCNJ11:c.902G>A | / | / | Confirmed by Sanger sequencing, positive familial history | Surveillance |
| 62 | Cardiomyopathy, hypertrophic, 4 | Male | MYBPC3:c.3407_3409delACT | / | / | Pending | |
| 63 | Cardiomyopathy, hypertrophic, 4 | Female | MYBPC3:c.2618C>T | / | / | Pending | |
| 64 | Cardiomyopathy, dilated 1S | Female | MYH7:c.4498C>T | / | / | Confirmed by Sanger sequencing, positive familial history | Surveillance |
| 65 | Cardiomyopathy, dilated 1S | Male | MYH7:c.1750G>A | / | / | Confirmed by Sanger sequencing | Surveillance |
| 66 | Cardiomyopathy, dilated 1S | Male | MYH7:c.4498C>T | / | / | Pending | |
| 67 | Cardiomyopathy, dilated 1S | Male | MYH7:c.2572C>T | / | / | Pending | |
| 68 | Cardiomyopathy, dilated 1S | Male | MYH7:c.1370T>C | / | / | Pending | |
| 69 | Shwachman–Diamond syndrome | Male | SBDS:c.258+2T>C–c.258+2T>C | / | / | Pending | |
| 70 | Glycogen storage disease 1b/c | Female | SLC37A4:c.1015G>T–c.1015G>T | / | / | Increased 1,5-anhydroglucitol | Standard of care: restrictive diet; empagliflozin initiated at 8 months of age |
| 71 | Glycogen storage disease 1b/c | Female | SLC37A4:c.1015G>T–c.1015G>T | / | / | Increased 1,5-anhydroglucitol | Standard of care: restrictive diet; empagliflozin initiated at 8 months of age |
In the fifth and sixth columns, a slash indicates that the disease is not included in conventional NBS. RR, reference range; IRT, immunoreactive trypsin.
aNovel therapies for cystic fibrosis are available in Belgium only to patients aged 2 years and older.
bInitiation of care was subject to the availability of biochemical results, which were obtained after several months. The baby received standard of care at 5 months of age.
We also recorded one false-negative case in a neonate who was referred for cholestasis, jaundice and skin ichthyosis. As part of the diagnostic evaluation, whole-exome sequencing (WES) analysis identified a nonsense c.1030C>T (p.Arg344*) homozygous variant in the TJP2 gene that was reported as pathogenic given the clinical context. This alteration was detected in the BabyDetect sequencing data, but this variant was not present in our curated variant list and in ClinVar and, to our knowledge, had not been described previously. Consequently, the variant was not flagged for manual review by our sorting tree and was not reported by our workflow. This variant has now been added to our managed variant database.
Seventeen cases were flagged by the filtering tree but were not subsequently reported. These cases included 16 newborns with the benign homozygous Duarte variant c.940A>G (p.Asn314Asp) in the GALT gene. All had galactose concentrations within normal limits based on routine NBS, and the BabyDetect results were thus not reported. One neonate was also identified to have the c.1397C>G (p.Ser466*) and c.3209G>A (p.Arg1070Gln) variants in the cystic fibrosis transmembrane conductance regulator gene (CFTR). A comprehensive literature review revealed that these variants are frequently reported in a complex cis-segregating allele17,18. The level of immunoreactive trypsin in this neonate was also far below the cutoff for reporting. Thus, we decided not to report these variants to the attending pediatrician.
Follow-up of positive screening cases
Of the 71 positive cases reported by BabyDetect, 41 cases were identified through conventional NBS (Fig. 4). Among the 30 cases not identified by standard NBS, 10 were G6PD deficiency cases. Measurement of G6PD activity in whole blood confirmed mild deficiencies in all these babies. Patients with G6PD deficiency do not require interventional care unless they experience a hemolytic crisis. However, preventive measures have been taken for the 44 newborns identified to have G6PD deficiency by providing the parents with a list of drugs, chemicals and foods likely to trigger oxidative stress and whose consumption should, therefore, be avoided. These babies are followed up by community pediatricians.
Fig. 4. BabyDetect screening and diagnostic flowchart.
The Goldcard is a dedicated golden filter paper card from LaCAR MDx. The BabyDetect timeline represents the theoretical schedule for BabyDetect result availability.
In one newborn suspected of having CPT2 deficiency, the condition was also not detected by biochemical NBS. The two variants identified in the CPT2 gene (c.1339C>T and c.1436A>T) were suggestive of a myopathic form of this deficiency. The result was reported to the pediatrician, and the baby was referred for further metabolic testing. The diagnosis was confirmed by measuring CPT2 activity in cultured cells from the patient. The neonate had a CPT2 activity of 2.6 nmol min−1 per mg protein, notably lower than the reference range of 9–22.6 nmol min−1 per mg protein. The deficiency was also confirmed by acylcarnitine profiling performed on a plasma specimen, which showed a moderate increase in long-chain acylcarnitines compared to the reference range. Conventional NBS analysis is known to have poor sensitivity for CPT2 screening19. This neonate was hospitalized for rhabdomyolysis attacks and myoglobinuria. The availability of BabyDetect results allowed for rapid and appropriate care.
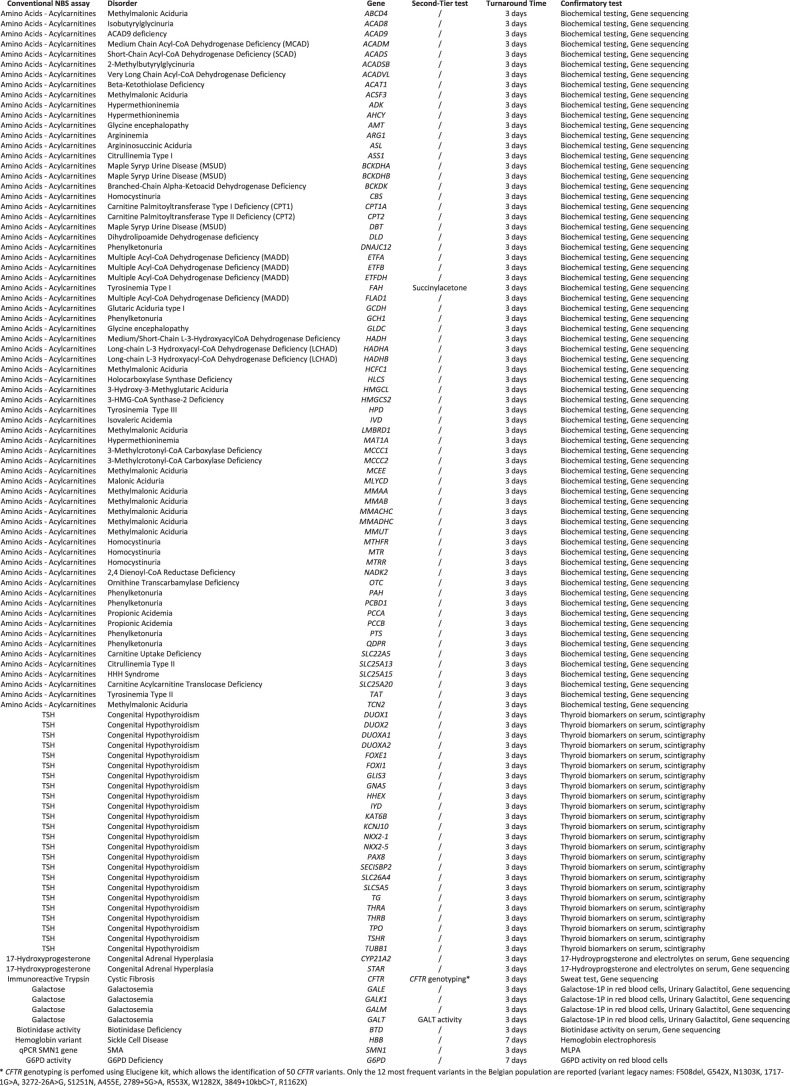
One neonate with a homozygous CFTR:c.1865G>A variant, known to be prevalent in African populations and on Reunion Island and associated with a broad spectrum of cystic fibrosis-related phenotypes20, was also identified. Conventional NBS tests for cystic fibrosis use a two-tier protocol: the first-tier assay measures immunoreactive trypsin, and the second-tier assay involves CFTR genotyping. Reporting of the CFTR genotyping results is restricted to the 12 most frequent variants found in the Belgian population (Extended Data Table 1). As the CFTR:c.1865G>A variant is not one of these variants and because the immunoreactive trypsin level in the patient was far below the positivity threshold, the clinical expert who evaluated the BabyDetect results did not recall the baby for further evaluation.
Extended Data Table 1.
Conventional NBS
Two neonates were identified to have hemizygous variants in the F8 gene. Factor VIII activity measured in fresh plasma samples confirmed mild (activity: 48%) and moderate (activity: 21%) hemophilia A in these neonates. These patients would benefit from the preventive use of desmopressin in preoperative settings to reduce the risk of bleeding complications. The two neonates with glycogen storage disease b/c were twin sisters. After the diagnosis was confirmed, they were immediately placed on a restricted diet. Treatment with empagliflozin was initiated at 8 months of age to prevent neutropenia. One case of MYH7-related cardiomyopathy with the heterozygous variant MYH7:c.4498C>T was noteworthy. During the confirmatory evaluation, a familial investigation revealed that the father exhibited signs of undiagnosed cardiac hypertrophy. None of these patients were treated with innovative therapies.
The neonate carrying two class 5 variants in the AGXT gene (c.33dupC and c.332G>A) was demonstrated to be a false-positive case. Segregation analysis of parental DNA showed that the father carries neither variant and the mother carries both mutations. The mother showed no symptoms of hyperoxaluria.
Turnaround time
The average turnaround time for the BabyDetect screening was calculated as the average of the intervals between the consent date and the variant interpretation date. Goldcards were processed in batches of 96 samples. As around 50 neonates were enrolled per week, analyses were run every 2 weeks. We observed a notable improvement in our average turnaround time over the 18 months of the study to date. The average turnaround time for the first 300 samples analyzed was 64 days (s.d. 33 days), whereas that for the last 300 samples was 51 days (s.d. 10 days). When a conventional NBS comparator was not available, reanalysis took an average of 3 weeks.
Cost
The cost per sample of screening for 165 diseases in the context of this study was 365 euros, which was entirely covered by study funds. This cost does not include material depreciation, overhead, license for secondary analysis (as bioinformatics was conducted on the Humanomics program developed in-house) or follow-up of positive cases.
Discussion
Through the prospective BabyDetect pilot project, we demonstrated the feasibility of a genomic NBS approach at a midscale level. The rapid development of innovative therapies for severe genetic conditions, which cannot be diagnosed by current NBS assays, underlines the need to incorporate genetic testing into NBS. Accordingly, several large prospective studies of genomic NBS have been launched across North America, Europe and Australia to assess the acceptability and feasibility of a first-tier genomic NBS approach12,21. To identify affected babies, these pilot trials have implemented tNGS, WES or whole-genome sequencing (WGS) approaches, and the genes queried vary widely between programs21,22.
At 18 months after the BabyDetect project was launched in one maternity ward, the acceptance rate of the test (>90%) by families attests to the strong buy-in to genomic NBS by the southern Belgian population. The proportion of positive cases identified (1.8%; 0.8% not identified by conventional NBS) must be understood in the context of the broad gene list covered by the panel and by the inclusion of G6PD. G6PD deficiency was by far the most frequent disorder detected, with 44 positive individuals (38 male and 6 female neonates). Thirty-five were diagnosed with moderate deficiencies associated with the common A haplotype (G6PD:c.[292G>A;466A>G]); the residual G6PD activity in the identified neonates was between 10% and 60% of the reference levels. Conventional NBS programs usually do not screen for G6PD deficiency, but the G6PD gene has been included in most genomic NBS trials21. Genomic NBS has the potential to diagnose cases not identified by conventional screening, such as the myopathic form of CPT2 deficiency as illustrated in the identification of an infant during our pilot.
The BabyDetect trial is an ongoing prospective observational study. As part of the diagnosis of positive cases, DNA extracted from an independently collected, fresh sample is analyzed by an external laboratory. For certain cases, confirmatory analysis results are still pending, with some delayed by several months. This accounts for the absence of confirmatory and follow-up information for some newborns. We acknowledge this missing information as a limitation of this ongoing study and note that this highlights the challenges inherent in genomic NBS programs.
For the BabyDetect study, a relatively conservative approach in variant reporting is used. Our gene panel is designed to capture only exons and intron–exon boundaries. Consequently, pathogenic variants located within introns, promoters or untranslated regions are not detected by our approach. Additionally, the methodology is not designed to identify certain genetic alterations, such as copy number variations, large deletions, mosaicism or other complex structural abnormalities (for example, translocations, inversions or intricate genomic rearrangements), which further limits its diagnostic accuracy. We also report only pathogenic and likely pathogenic variants that have a consensual curation in several databases, disregarding VUS. Following variant filtering, 1% of screened samples required manual review. The workload generated by this manual review stems from the limitations of our variant filtering tool in assigning X-linked disorders, as well as the conflicting interpretations of certain variants found in the ClinVar database. Whether or not to report VUS is a subject of debate. In the presymptomatic context of population-based NBS, reporting VUS could overload the variant review process, result in an unmanageable recall rate, increase anxiety and mistrust among the screened populations, and require the allocation of substantial resources for confirmatory testing. The risk of false-negative results is illustrated by a patient with a nonsense homozygous variant in the TJP2 gene, not reported by BabyDetect, in whom the disease was subsequently diagnosed following symptom onset. As genomic NBS becomes more widely used, new pathogenic variants will be detected, particularly in populations traditionally underrepresented in genetic databases. Continuously populating our curated variant list with newly validated disease-causing variants will improve sensitivity and negative predictive value, enhancing the identification of disorders not covered by conventional NBS. Data sharing between genomic NBS programs and careful documentation of false-negative cases are crucial for this process.
False-positive screening results occurred due to cis-located double-heterozygous mutations. Variants with a cis configuration are not uncommon in the general population18,23. Unless parental blood is collected simultaneously with the collection of the baby’s blood, biallelic localization of variants cannot be confirmed without contacting the parents. We do not collect blood samples from parents at the time of blood sample collection in neonates owing to logistical hurdles (for example, the need for informed consent and sample storage and tracking), because of the potential of this requirement to decrease the consent rate and because samples are useful only if blood can be collected from both parents. Addressing this limitation will require future genomic NBS programs to incorporate second-tier tests to ascertain the allelic status of variants (for example, using long-read sequencing technologies).
The biochemical data available through coordination with the conventional NBS program also notably assisted in variant assessment. For example, the correlation of BabyDetect results with biomarker concentrations, such as galactose for the homozygous Duarte variant and immunoreactive trypsin for complex CFTR alleles, prevented 17 cases from being reported unnecessarily.
The recall rate of 1 in 54 newborns reported here is challenging to manage in a population-based NBS context. The identification of 33 cases of mild G6PD deficiency raises the question of whether reporting such genotypes is warranted, as 10 of these 33 cases were not identified through conventional screening. Within the BabyDetect framework, members of the expert panel and clinical geneticists convene biannually to review and refine the gene panel. This process involves assessing the inclusion of genes linked to newly approved treatments and addressing the challenges of reporting variants with poorly defined penetrance. As an example, the panel chose to revise the reporting of variants in MYH7, MYBPC3 and KCNJ11 after the identification of five, two and one babies, respectively. The natural history of MYH7- and MYBPC3-related cardiomyopathies, which have variable ages of onset, and the phenotypic heterogeneity among members of the same family24,25 are inconsistent with our disease selection criteria. Consequently, the reporting criteria for MYH7 and MYBPC3 variants were revised to restrict reporting to instances in which two variants are identified (either homozygous or possibly compound heterozygous). We removed the KCNJ11 gene from our panel. These examples highlight that the current variant reporting process, which primarily relies on the mode of inheritance, does not take into account crucial information regarding the penetrance of each variant and the age of symptom onset of each disease within populations. Enhancing the characterization of variants will require support from large-scale, long-term, multigenerational studies.
The identification of disorders with a dominant mode of inheritance raises additional questions. For instance, the detection of a heterozygous MYH7 variant in a neonate subsequently resulted in the diagnosis of cardiomyopathy in the father. Except in cases of de novo mutations, dominant phenotypes are generally expected to be known within affected families. Moreover, as a public health effort, NBS should not aim to identify mild phenotypes.
The sensitivity and positive predictive value of genomic NBS are challenging to calculate reliably. Although sequencing results are generally accurate, the age of symptom onset and the penetrance of many variants are not well defined, making it difficult to determine whether or not to report positive screening results and making it almost impossible to identify false-negative cases. To partially address this limitation, we have amended our consent form to ask parents to agree to be recontacted when their child reaches 1 year of age; this will allow us to collect information on the child’s development. Additionally, we will collect information on the treatment and follow-up of positive cases from the physicians.
Despite promising results, tNGS and WES have several shortcomings, including poor coverage, diversity of captured regions, challenges in variant calling and filtering, lack of consensus on the interpretation of many variants, and the absence of information on whether variants are located cis or trans. Although WES allows a greater number of diseases to be screened than does tNGS, WES has lower sensitivity and specificity than conventional NBS as a primary screening method for inborn errors of metabolism8,26. Implementation of large-scale pilot programs and intergenerational population follow-up are necessary to enhance the accuracy of genomic NBS. The development of guidelines for clinical practice will depend on furthering our understanding of how genomic sequences correlate with pathology. Dominant, epistatic, epigenetic and oligogenic mechanisms or other processes that remain unexplained may cause false-negative results. The parents and medical teams need to be aware of this limitation.
BabyDetect sample preparation is currently performed manually. Managing around 2,000 samples a year requires highly skilled staff to minimize errors. Extending coverage to screen several thousand babies a year, which would be the case if genomic NBS were extended to all of Belgium, would not be feasible without workflow automation. We made notable strides in reducing our average turnaround time over the 18 months evaluated here, with negative results now available in 51 days, on average, after parental consent is obtained. The improvement in the turnaround time was due to the optimization of the entire process, from double-checking consents before submission to the NBS laboratory to automating DNA extraction with QIAsymphony and accelerating variant inference through our in-house bioinformatics pipeline. Manual assessment of variants is a time-consuming process that adversely affects turnaround time. The number of variants reviewed was limited by precurated lists of pathogenic and likely pathogenic variants, which allowed for higher degrees of automation of the reporting workflow12. Finally, with around 50 newborns enrolled each week, analyses were conducted on a biweekly basis. Expanding the project to include a larger population would increase the analysis throughput, thereby notably reducing the turnaround time. Future optimization of methods and processes could also decrease turnaround time, as demonstrated in NBS for spinal muscular atrophy5.
Managing the substantial volume of data generated by genomic NBS demands scalable, resilient solutions ensuring data encryption, access control and deidentification to safeguard privacy, adhering to GDPR (General Data Protection Regulation) or HIPAA (Health Insurance Portability and Accountability Act of 1996) regulations. Given that sequencing samples from 3,847 babies by tNGS resulted in 3.5 terabytes of data and raw data must be securely stored for 5 years, expanding the project to hundreds of thousands of babies will inevitably lead to data storage issues.
The cost per sample (365 euros) is notably higher than the 42 euros dedicated by the southern Belgium government to screen for 19 diseases through conventional NBS. However, when considered on a disease basis, the screening of 405 genes is in the same price range as conventional NBS. We expect that technology development and increased volume will considerably decrease these costs over the next several years.
A disadvantage of tNGS is its limited flexibility. For instance, adding 61 new genes to the second version of our panel required a 4-month validation process. To increase the adaptability of the BabyDetect framework, and given that the cost of WES is now comparable to that of tNGS, we plan to transition to WES technology. This shift will eliminate the need for revalidating the entire panel with each new gene inclusion and will streamline the process overall. Looking ahead, we are also considering WGS, as global WGS costs (that is, sequencing, analysis and data storage) are expected to decrease over time. WGS has numerous advantages over WES, including reduced hands-on time for sample preparation and more consistent coverage allowing for easier interpretation of copy number variations or tandem repeat expansions27.
Although genomic NBS has considerable potential, its practical implementation is undeniably complex. Biochemical and genomic strategies are expected to complement each other in future NBS programs. However, healthcare systems must prepare to handle the increased demand for genetic counseling and follow-up care that will result from the implementation of genomic NBS. Pilot programs such as BabyDetect will help identify and solve clinical, economic, societal, legal and ethical issues that must be addressed before the broad implementation of genomic NBS.
Methods
Ethics
The project was approved by the CHU Liege ethics committee (no. 2021/239) and was conducted in accordance with the Declaration of Helsinki. Patients or guardians provided voluntary informed consent to participate in the study, free of coercion or coercive circumstances.
Population
Newborn recruitment was carried out over 18 months in one maternity ward at the public hospital of CHR Citadelle in Liege, Belgium, one of the largest in our area, with approximately 2,500 births annually. From September 2022 to the end of April 2024, a total of 4,260 babies were born at CHR Citadelle. Patients of this hospital reflect the general population of the Liege area, which is highly diverse in terms of ethnic origin and socioeconomic status. The proportion of non-Belgian inhabitants in Liege is 20.38% (versus 10.97% in southern Belgium), and the median income per household is 21,589 euros (14.3% below the southern Belgium average; https://walstat.iweps.be). Consanguinity in this population is uncommon but occasionally observed in some ethnic groups. Information on the sex of the neonates (male or female) was collected to support the interpretation of sex-chromosome sequencing data. This phenotypic sex was provided by the referring pediatrician. Information on gender was not relevant as the study population comprised newborns.
We previously reported the overall study setup and consent process13. Briefly, all parents are informed about the project before delivery. Flyers, posters and audiovisual content with information and links to the study website are available in the waiting rooms of the maternity ward and those of gynecologists who support the project. After birth and before sample collection, good clinical practice-certified data managers and trained students collect digital informed consent from parents on a dedicated and secured website. This consent confirms that data could be used for further medical consults and research purposes. Enrollment in the trial is free of charge. For consented babies, a few drops of blood are collected on the Goldcard, a dedicated golden filter paper card (LaCAR MDx), on the first days after birth. Then, testing is performed in our region’s conventional NBS reference laboratory.
Selection of genes
The general inclusion criteria for genes incorporated into the initial test panel and the version of the panel implemented after 1 year were as follows: notable consequences for life expectancy or severe disability associated with an untreated disease, disease onset before 5 years of age, strong genotype–phenotype correlation, the existence of a disease-modifying treatment accessible to the diagnosed patients and notable benefit of prompt treatment. Genes with mutations that underlie diseases currently screened in our biochemical panel were also included even if they did not match these criteria (for example, G6PD deficiency). Approval by pediatricians who specialize in the treatment of the disease was mandatory. Screening for a given disease would have been discontinued if a disease-modifying treatment became unavailable (for example, withdrawn from the market or no longer reimbursed, which did not occur) or if there were operational issues that precluded accurate testing. Discussions were held periodically with experts to review the list of disorders and genes in the tNGS panel. We first used a panel targeting 359 genes, including 104 genes coding for disorders currently screened by conventional NBS (Extended Data Table 1) and 255 additional genes coding for defects not amenable to biochemical screening. The panel was reviewed after 1 year of testing; 61 genes were added and 15 were removed, leaving a total of 405 genes. These genes are associated with 165 treatable severe pediatric disorders. The full list of genes included is presented in Fig. 1.
Gene panel-based sequencing
DNA was initially extracted manually from three 3.2-mm dried blood spots using the QIAamp DNA Investigator kit (Qiagen). Currently, DNA is extracted using the QIAsymphony instrument (Qiagen). Target enrichment is performed using Twist Bioscience preparation reagents. Captured regions cover only the coding regions and intron–exon boundaries (~50 base pairs from the intronic borders) of selected genes. Deep intronic variants, promoter and untranslated regions, and homopolymeric regions are not sequenced. With target panel version 2.0, approximately 1.5 Mb are sequenced.
Libraries are sequenced on the NovaSeq 6000 or NextSeq 550 platform (Illumina) with an average read depth coverage of 200×. Sequence alignment onto the GRCh37 (hg19) human reference genome, data quality control and variant inference are performed on the Humanomics (12 September 2024) bioinformatics pipeline developed in the Genetics Department of the CHU Liege following the GATK (Genome Analysis Toolkit) best practices pipeline28,29. Briefly, all paired-end reads are mapped to the reference genome, and optical and PCR duplicates are removed. Identification of small nucleotide variants, insertions and deletions, and quality control evaluation are performed with HaplotypeCaller. All values of quality control metrics are stored in a local database for traceability. Raw sequencing data and results are stored in a hospital-grade storage facility that follows the standard policies for redundancy, data integrity and availability, and network security. Computation is performed on the hospital-hosted high-performance computing infrastructure. The Humanomics tool allows identifying single-nucleotide polymorphisms and indels located within exons or at the intron–exon boundary (~50 base pairs of flanking regions). The pipeline does not call copy number variations, large deletions, mosaicism or other structural abnormalities (for example, translocations and inversions).
Variant reporting
Variant annotation, prioritization, classification and interpretation are performed using Alissa Interpret v.5.4.2 (Agilent Technologies), which is a secure variant assessment cloud platform also intended for variant storage. Phenotype-driven interpretation of variants using Human Phenotype Ontology codes is not useful for neonates. Therefore, variant annotation is performed using an internally curated list of genomic variations and the ClinVar database30. According to the American College of Medical Genetics and Genomics classification of variants31, we report only class 4 (likely pathogenic) and class 5 (pathogenic) variants. Benign, likely benign and VUS are disregarded. Additionally, variants not documented in ClinVar or our curated list are not reported. Variants reported in ClinVar are subsequently reviewed with particular caution using the Franklin14,15 and VarSome16 platforms, which have an advanced artificial intelligence-driven engine designed to prioritize and interpret variant data. In genes associated with autosomal recessive disorders, the identification of two pathogenic or likely pathogenic variants is necessary to report a positive result for the corresponding disease. For autosomal dominant diseases, the identification of one pathogenic or likely pathogenic variant is considered a positive result. For genes located on the X chromosome, hemizygous identification of pathogenic or likely pathogenic variants in male neonates and homozygous or possible compound heterozygous identification of pathogenic or likely pathogenic variants in female neonates are considered positive results (Fig. 2).
Screening process
Goldcard specimens are registered in the laboratory information system used for the conventional NBS program under the same patient entry, enabling genotype–phenotype correlation assessment for genes covered by both the conventional NBS program and BabyDetect. For disorders not covered by conventional NBS, first-tier positive samples are reanalyzed (from DNA extraction to variant interpretation) to rule out errors in specimen assignment to a particular individual. For disorders included in our conventional NBS program, BabyDetect sequencing data are matched to biochemical results to confirm the result.
Parents were informed that, as is the case for conventional NBS in southern Belgium, no negative reports will be issued, and the test should be considered negative in the absence of a report within 3 months. When BabyDetect identified a disease also identified by conventional NBS, no further action was taken through the BabyDetect program as the baby was managed per standard of care. Positive tests not identified by conventional NBS were communicated by the laboratory to the neonate’s pediatrician, the referent specialists of the identified disorder and referent geneticists. Parents were contacted to plan a consultation as soon as possible. At this consultation, a fresh blood sample was collected from the neonate, and blood samples were collected from the parents for segregation analysis. Independent confirmatory testing was performed by Sanger sequencing, tNGS or biochemical assays depending on the disorder (Fig. 4). After the confirmation of a positive screening result, appropriate care was initiated, and parents were recommended to seek genetic counseling.
Outcomes
The study outcomes focused on assessing the acceptability and feasibility of genomic NBS within the studied population. The proportion of parents who provided consent for the proposed screening was meticulously recorded in relation to the total number of mothers approached. The clinical performance of the screening process was rigorously evaluated, with particular attention to the rate of positive findings. Estimates of false-positive and false-negative results were derived through close collaboration with physicians managing the associated conditions. Furthermore, the turnaround time of the screening process was carefully monitored to ensure the timely delivery of results.
Reporting
We report the study results following the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines32.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41591-024-03465-x.
Supplementary information
Acknowledgements
This work was supported by private and public grants (Sanofi (SGZ-2019-12843), Orchard Therapeutics, Takeda, Léon Frédéricq Foundation) and by a donation from LaCAR MDx. V.B. was funded by a Région Wallonne grant (WALGEMED project 1710180). The funders of this study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
We thank J. Wyatt for the language editing.
Extended data
Author contributions
F.B. contributed to conceptualization, data curation, formal analysis, investigation, methodology, resources, software, supervision, validation, and the writing, review and editing of the paper. K.H. contributed to data curation, formal analysis, investigation, methodology, software, validation, and the writing, review and editing of the paper. F.P. contributed to methodology, validation (gene panel curation), and the writing, review and editing of the paper. F.M. contributed to formal analysis, resources and software. M.M. contributed to formal analysis and resources. V.J. contributed to data curation, formal analysis, investigation, validation, and the writing, review and editing of the paper. D.M. contributed to funding acquisition and project administration. N.B. contributed resources. V.B. contributed resources and to the writing, review and editing of the paper. A.J. contributed to methodology, validation (gene panel curation), and the writing, review and editing of the paper. J.H. contributed to methodology, validation (gene panel curation), and the writing, review and editing of the paper. S.B. contributed to methodology, validation (gene panel curation), and the writing, review and editing of the paper. V.D. contributed to methodology, validation (confirmation of screening results), and the writing, review and editing of the paper. L.H. contributed to software development (consolidated the bioinformatics pipeline and sequencing results analysis) and the writing, review and editing of the paper. L.P. contributed to software development (consolidated the bioinformatics pipeline and sequencing results analysis) and the writing, review and editing of the paper. T.D. contributed to funding acquisition, methodology, project administration, resources, and the writing, review and editing of the paper. L.S. contributed to conceptualization, funding acquisition, investigation, project administration, resources, supervision, and the writing, review and editing of the paper. The BabyDetect Expert Panel consists of a group of medical specialists who contributed to the selection of genes and are in charge of managing the care of confirmed cases. All authors approved the paper and agreed to be accountable for the accuracy of the reported findings.
Peer review
Peer review information
Nature Medicine thanks Roberto Giugliani and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Anna Maria Ranzoni, in collaboration with the Nature Medicine team.
Data availability
In accordance with the informed consent agreements, the raw sequencing data can be stored for each patient for a period of 10 years. Metadata files are retained with no time limit. The raw sequencing data and metadata files generated in the study cannot be made publicly available because of ethical and data protection constraints. Deidentified data that support the results reported in this article will be made available to suitably qualified researchers through any requests that comply with ethical standards to the corresponding author (F.B., f.boemer@chuliege.be). Data must be requested between 1 and 12 months after the paper has been published, and the proposed use of the data must be approved by an independent review committee identified for this purpose by mutual agreement. Requests will be forwarded by the corresponding author to the identified ethics review committee. Upon acceptance by that committee, deidentified data will be provided by the corresponding author to the applicants through a secured web platform within 2 months. The minimum dataset required to run our code and reproduce results is available via Zenodo at 10.5281/zenodo.13935241 (ref. 29).
Code availability
The Humanomics pipeline used in this article is publicly distributed under GNU Affero General Public License version 3 (https://gitlab.uliege.be/bif-chu/humanomics). The version used for analyses described here (12 September 2024) is available as an official release on the GitLab repository. For traceability and reproducibility concerns, a Zenodo record is provided (10.5281/zenodo.13935241)29.
Competing interests
F.B. has consulted for LaCAR MDx and has presented lectures for Novartis and Sanofi. T.D. has presented lectures for Biogen, Roche and Novartis. L.S. is a member of scientific advisory boards or has consulted for Biogen, Novartis, Roche, Illumina, Sanofi, Scholar Rock, LaCAR MDx and BioHaven. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: François Boemer, Kristine Hovhannesyan.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
François Boemer, Email: f.boemer@chuliege.be.
BabyDetect Expert Panel:
Serpil Alkan, Christophe Barrea, Paulina Bartoszek, Emeline Bequet, Hedwige Boboli, Romain Bruninx, Laure Collard, Aurore Daron, François-Guillaume Debray, Marie-Françoise Dresse, Jean-Marie Dubru, Iulia Ebetiuc, Benoit Florkin, Caroline Jacquemart, Céline Kempeneers, Nadège Hennuy, Marie-Christine Lebrethon, Marie Leonard, Patricia Leroy, Angélique Lhomme, Jacques Lombet, Sabine Michotte, Ariane Milet, Anne-Simone Parent, Vincent Rigo, Marie-Christine Seghaye, and Sandrine Vaessen
Extended data
is available for this paper at 10.1038/s41591-024-03465-x.
Supplementary information
The online version contains supplementary material available at 10.1038/s41591-024-03465-x.
References
- 1.Gonzaludo, N., Belmont, J. W., Gainullin, V. G. & Taft, R. J. Estimating the burden and economic impact of pediatric genetic disease. Genet. Med.21, 1781–1789 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson, J. M. G. & Jungner, G. Principles and Practice of Screening for Disease (World Health Organization, 1968).
- 3.Subbiah, V. The next generation of evidence-based medicine. Nat. Med.29, 49–58 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Brown, L. et al. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening. Blood117, 3243–3246 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Boemer, F. et al. Three years pilot of spinal muscular atrophy newborn screening turned into official program in Southern Belgium. Sci. Rep.11, 19922 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dangouloff, T. et al. Financial cost and quality of life of patients with spinal muscular atrophy identified by symptoms or newborn screening. Dev. Med. Child Neurol.65, 67–77 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb, S. Statement from FDA Commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.D., Director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies. FDAwww.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics (2020).
- 8.Boemer, F. et al. A next-generation newborn screening pilot study: NGS on dried blood spots detects causal mutations in patients with inherited metabolic diseases. Sci. Rep.7, 17641 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey, D. B. Jr et al. Early Check: translational science at the intersection of public health and newborn screening. BMC Pediatr.19, 238 (2019). [DOI] [PMC free article] [PubMed]
- 10.Kingsmore, S. F. et al. A genome sequencing system for universal newborn screening, diagnosis, and precision medicine for severe genetic diseases. Am. J. Hum. Genet.109, 1605–1619 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold, N. B. et al. Perspectives of rare disease experts on newborn genome sequencing. JAMA Netw. Open6, e2312231 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stark, Z. & Scott, R. H. Genomic newborn screening for rare diseases. Nat. Rev. Genet.24, 755–766 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Dangouloff, T. et al. Feasibility and acceptability of a newborn screening program using targeted next-generation sequencing in one maternity hospital in southern Belgium. Children11, 926 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da S Rodrigues, E. et al. Variant-level matching for diagnosis and discovery: challenges and opportunities. Hum. Mutat.43, 782–790 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franklin by Genoox (Genoox, accessed 21 July 2024); franklin.genoox.com/
- 16.Kopanos, C. et al. VarSome: the human genomic variant search engine. Bioinformatics35, 1978–1980 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krasnov, K. V. et al. Localization studies of rare missense mutations in cystic fibrosis transmembrane conductance regulator (CFTR) facilitate interpretation of genotype–phenotype relationships. Hum. Mutat.29, 1364–1372 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanov, M. et al. Targeted sequencing reveals complex, phenotype-correlated genotypes in cystic fibrosis. BMC Med. Genomics11, 13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yazıcı, H. et al. Experience with carnitine palmitoyltransferase II deficiency: diagnostic challenges in the myopathic form. J. Pediatr. Endocrinol. Metab.37, 33–41 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Marion, H. et al. The p.Gly622Asp (G622D) mutation, frequently found in Reunion Island and in black populations, is associated with a wide spectrum of CF and CFTR-RD phenotypes. J. Cyst. Fibros.14, 305–309 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Minten, T. et al. Data-driven prioritization of genetic disorders for global genomic newborn screening programs. Preprint at medRxiv10.1101/2024.03.24.24304797 (2024).
- 22.Downie, L. et al. Gene selection for genomic newborn screening: moving toward consensus? Genet. Med.26, 101077 (2024). [DOI] [PubMed] [Google Scholar]
- 23.Chen, T. et al. Genomic sequencing as a first-tier screening test and outcomes of newborn screening. JAMA Netw. Open6, e2331162 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Frutos, F. et al. Natural history of MYH7-related dilated cardiomyopathy. J. Am. Coll. Cardiol.80, 1447–1461 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Field, E. et al. Cardiac myosin binding protein-C variants in paediatric-onset hypertrophic cardiomyopathy: natural history and clinical outcomes. J. Med. Genet.59, 768–775 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adhikari, A. N. et al. The role of exome sequencing in newborn screening for inborn errors of metabolism. Nat. Med.26, 1392–1397 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wojcik, M. H. et al. Genome sequencing for diagnosing rare diseases. N. Engl. J. Med.390, 1985–1997 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Auwera, G. A. et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinformatics43, 11.10.1–11.10.33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charloteaux, B. et al. Humanomics. Zenodo10.5281/zenodo.13935241 (2024).
- 30.Landrum, M. J. et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res.46, D1062–D1067 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med.17, 405–424 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet370, 1453–1457 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In accordance with the informed consent agreements, the raw sequencing data can be stored for each patient for a period of 10 years. Metadata files are retained with no time limit. The raw sequencing data and metadata files generated in the study cannot be made publicly available because of ethical and data protection constraints. Deidentified data that support the results reported in this article will be made available to suitably qualified researchers through any requests that comply with ethical standards to the corresponding author (F.B., f.boemer@chuliege.be). Data must be requested between 1 and 12 months after the paper has been published, and the proposed use of the data must be approved by an independent review committee identified for this purpose by mutual agreement. Requests will be forwarded by the corresponding author to the identified ethics review committee. Upon acceptance by that committee, deidentified data will be provided by the corresponding author to the applicants through a secured web platform within 2 months. The minimum dataset required to run our code and reproduce results is available via Zenodo at 10.5281/zenodo.13935241 (ref. 29).
The Humanomics pipeline used in this article is publicly distributed under GNU Affero General Public License version 3 (https://gitlab.uliege.be/bif-chu/humanomics). The version used for analyses described here (12 September 2024) is available as an official release on the GitLab repository. For traceability and reproducibility concerns, a Zenodo record is provided (10.5281/zenodo.13935241)29.