Abstract
Objective
To compare conventional treatment (heparin and warfarin) of iliofemoral venous thrombosis with multimodality treatment (lysis and stenting).
Summary Background Data
Several studies have reported on conventional therapy for iliofemoral venous thrombosis with disappointing results. However, more recent studies have reported better results with multimodality treatment.
Methods
Fifty-one consecutive patients with extensive iliofemoral venous thrombosis were treated during a 10-year period. If there were no contraindications, patients were given the option to choose between conventional therapy (group 1) and multimodality therapy (group 2). The multimodality treatment strategy included catheter-directed lysis followed by percutaneous transluminal balloon angioplasty (PTA) and stenting for residual iliac stenoses. All patients underwent routine venous duplex imaging at 30 days, 3 months, 6 months, and every 6 months thereafter.
Results
There were 33 patients in group 1 and 18 patients in group 2. Demographic and clinical characteristics were comparable for both groups. Initial lysis was achieved in 16 of 18 patients (89%) in group 2. Ten of 18 patients in group 2 had residual stenosis after lysis (8 primary and 2 secondary to malignancy), and they were treated with PTA/stenting with an initial success rate of 90%. Two patients in group 1 (6%) had a symptomatic pulmonary embolism (none in group 2). At 30 days, venous patency and symptom resolution were achieved in 1 of 33 patients (3%) in group 1 versus 15 of 18 (83%) in group 2. Kaplan-Meier analysis showed primary iliofemoral venous patency rates at 1, 3, and 5 years of 24%, 18%, and 18% and 83%, 69%, and 69% for groups 1 and 2, respectively. Long-term symptom resolution was achieved in 10 of 33 patients (30%) in group 1 versus 14 of 18 (78%) in group 2. Kaplan-Meier life table analysis showed similar survival rates at 1, 3, and 5 years of 100%, 93%, and 85% for group 1 and 100%, 93%, and 81% for group 2.
Conclusions
Lysis/stenting treatment was more effective than conventional treatment in patients with iliofemoral vein thrombosis.
Conventional therapy for iliofemoral deep vein thrombosis (DVT) is systemic heparinization followed by oral anticoagulants (warfarin). However, many studies have reported disappointing results with systemic heparinization. Conventional therapy has not been associated with rapid resolution of symptoms or recanalization of long venous occlusions and has been associated with long-term disability secondary to chronic venous insufficiencies. 1 Only 6% of patients with acute proximal DVT show complete lysis of the thrombus within 10 days. 2 Akesson 3 showed that approximately 95% of patients with iliofemoral DVT treated with anticoagulation alone had severely compromised muscle pump function and valvular competency at 5 years of follow-up, despite improvement in venous outflow.
Until the late 1970s, interventional treatment for iliofemoral venous thrombosis was confined to thrombectomy. Preliminary reports 4 on femoral venous thrombectomies were encouraging, but long-term follow-up studies failed to show objective benefits. 5,6 Recent studies have shown, however, that surgical thrombectomy may provide long-term iliac venous patency rates of approximately 80% when combined with the creation of an arteriovenous fistula. 7–9 However, for many patients, thrombectomy is incomplete, with early occurrence of rethrombosis. As a result, venous thrombectomy has not been accepted as the standard treatment for these patients. Beginning with the study by Elliot et al 10 comparing systemic streptokinase with heparin, a new modality was ushered into the forefront. Systemic lytic therapy has been shown to quadruple the lysis of venous thrombi, but it does not decrease the incidence of pulmonary embolism and the death rate when compared with heparin. 8 However, some studies have shown a decrease in the incidence of postthrombotic syndrome when streptokinase was compared with heparin. 9,10 Because of the disappointing results of systemic lytic therapy, others have adapted an aggressive regional approach for treatment of patients with iliofemoral DVT, including catheter-directed lytic therapy. 11–14 Transcatheter regional lytic therapy allows a higher concentration of the lytic agency to be introduced directly into the clot, resulting in a more rapid dissolution of the thrombus and reduced hemorrhagic complications compared than with systemic lysis. 15,16 This study compares catheter-directed thrombolysis combined with angioplasty and stenting with conventional anticoagulation in treating patients with iliofemoral DVT.
METHODS
Patient Population
Fifty-one consecutive patients with extensive iliofemoral venous thrombosis were treated during a 10-year period (January 1, 1990, through December 30, 1999). If there were no contraindications to lytic therapy, patients were given the option to choose between conventional therapy (heparin and warfarin, group 1) and multimodality therapy (lytic therapy with or without percutaneous transluminal angioplasty [PTA] and stenting, group 2).
Patients were excluded from lytic therapy if they had contraindications to contrast media or thrombolytic agents or contraindications to anticoagulation: bleeding disorders, active internal bleeding, recent gastrointestinal bleeding or recent cerebrovascular accident, recent major surgery (<10 days), recent serious trauma, severe hypertension, pregnancy or recent delivery, or metastatic malignancy with central nervous system involvement. In addition, lytic therapy was not offered to patients with an onset of iliofemoral venous thrombosis of more than 14 days. Patients with a history of DVT or chronic DVT were also excluded. Informed consent was obtained from all patients after the risks and benefits of treatment were fully explained.
All patients were symptomatic at the time of presentation, and the diagnosis was confirmed by venous duplex imaging/iliofemoral phlebography. Venous duplex imaging was used to evaluate the infrainguinal deep venous system. Phlebography was performed to assess the proximal extent of the thrombus, and routine contralateral iliocavography was used to evaluate the contralateral iliofemoral venous system and the inferior vena cava. All patients underwent routine venous duplex imaging at 30 days, 3 months, 6 months, and every 6 months thereafter. Six patients underwent pretreatment or posttreatment ventilation/perfusion lung scanning, and only two were positive for pulmonary emboli. Routine posttreatment ventilation/perfusion scanning was not performed. Every effort was made to identify the cause for the acute venous thrombosis.
Treatment Options
In group 1, conventional treatment consisted of intravenous heparin followed by sodium warfarin (Coumadin). A loading dose of 5,000 to 10,000 units heparin followed by 1,000 to 2,000 units per hour was given by continuous intravenous infusion. The dose was adjusted to achieve an activated partial thromboplastin time (aPTT) of twice the control value. Heparin was given for 5 to 7 days. Warfarin was initiated within 48 to 72 hours and was continued for 6 months for all patients, except for those with pulmonary emboli, in whom it was continued for 9 to 12 months, or patients with a hypercoagulable state, where it was given indefinitely. Patients in whom recurrent DVT developed were also given warfarin indefinitely. The warfarin dose was adjusted to achieve a prothrombin time of 1.5 to 2 times control or an international normalized ratio (INR) of 2 to 3. All patients were treated with limb elevation and moist heat during their initial admission and maintained on prescription gradient compression stockings.
In group 2, lytic therapy was achieved by placing a catheter in the contralateral femoral vein, the right internal jugular vein, or the ipsilateral popliteal vein for direct intraclot infusion. An attempt was made to cross the thrombosed vein with a 0.035-inch guidewire. Once the guidewire was across the thrombus, multiple side-hole catheters were advanced into the thrombus to assure maximum delivery of the lytic agent. Urokinase (Abbokinase; Abbott Laboratories, North Chicago, IL) was given as a loading dose of 4,500 units per kilogram followed by infusion of 4,500 units per kilogram per hour for 24 to 48 hours. Heparin was administered concomitantly with the lytic therapy and continued until therapeutic anticoagulation with warfarin was accomplished. Fibrinogen and aPTT levels were obtained before and every 12 hours after thrombolytic therapy was started. The fibrinogen level was maintained at more than 100 mg/dL and the desired aPTT was approximately twice the control. If the fibrinogen level decreased to less than 100 mg/dL, the lytic agent was stopped for 6 to 12 hours and additional heparin was given. Lytic infusion was resumed when the fibrinogen level increased to more than 100 mg/dL. Other laboratory parameters obtained included hematocrit levels, prothrombin time, and INR. Venous duplex imaging and repeat phlebography through the infusion catheters were performed at 12- and 24-hour intervals, and therapy was continued until maximum lysis was achieved.
More recently (the last 12 months of the study), recombinant tissue-type plasminogen activator (rtPA) was the lytic agent of choice, replacing urokinase, which was infused as a bolus of 4 to 8 mg, with a continuous infusion of 2 to 4 mg per hour.
We did not routinely place caval filters before lytic therapy during this study.
After lytic therapy, further intervention (PTA/stenting) was performed if there was an underlying venous stenosis of 50% or more. Stent placement was done with balloon-expandable Palmaz stents (Johnson & Johnson Interventional Systems, Warren, NJ) or self-expanding stainless-steel wall stents (Schneider, Plymouth, MN; currently Boston Scientific). All stents were placed above the inguinal ligament. All stented patients were given warfarin indefinitely (INR 2–3).
Clinical Outcome and Definitions
Every effort was made to comply with the Reporting Standards on Venous Disease as reported by the Ad Hoc Committee of the Society for Vascular Surgery/International Society of Cardiovascular Surgeons. 17 Long-term symptom resolution was analyzed according to CEAP classifications. 17 The CEAP clinical class severity score was calculated according to the consensus statement on classification and grading of chronic venous disease in the lower limbs by members of the executive committee (ad hoc committee) of the American Venous Forum. 18 Lysis was considered complete if there was less than 5% residual thrombus. In long-term follow-up, venous patency was defined as an open vein with less than 30% residual stenosis. Veins that were occluded or had residual stenosis of 30% or more (with venous reflux), as shown by color duplex ultrasonography, were considered a failure.
Major complications included bleeding or hematoma necessitating a transfusion, pulmonary embolism, neurologic event, and death. Minor complications included minor bleeding or hematoma, fever, nausea and vomiting.
Statistical Methods
Life table methods were used to calculate the cumulative primary patency rates from the time of intervention, uninterrupted by thrombosis, by using duplex ultrasound results obtained at various follow-up intervals. The generalized Wilcoxon and the log-rank tests were used to determine whether there were significant differences between survival curves and patency in the subgroups. The Fisher exact test for comparison of proportions of categoric variables was used to test statistical significance.
RESULTS
There were 33 patients in group 1, including 5 with contraindications to lytic therapy, 3 who refused lysis, and 5 in whom the catheter could not be positioned in the thrombus. Eighteen patients (group 2) received multimodality therapy. The mean follow-up was 63 and 51 months for groups 1 and 2, respectively. The demographic and clinical characteristics were comparable for both groups. As noted in Table 1, the risk factors were comparable for both groups. The mean duration of the lower extremity symptoms was also comparable for both groups: 3.8 days for group 1 and 3.6 days for group 2 (range 1–11 for the whole series). The age range was 23 to 74 years (mean 49 in group 1, 46 in group 2). There were 19 (58%) women in group 1 and 11 (61%) in group 2.
Table 1. RISK FACTORS

*P = .7538.
The primary venous access site for lytic therapy was the right internal jugular vein in eight patients (44%), the contralateral femoral vein in four patients (22%), and the ipsilateral popliteal vein in six patients (33%). Initial lysis was achieved in 16 of 18 (89%) patients in group 2. Ten of the 18 patients (9 on the left side) in this group had residual stenoses after lysis (8 primary, 2 secondary to malignancies); they were treated with PTA/stenting with an initial success rate of 90% (9/10). Four patients in group 2 had disease secondary to malignancy (three on the right side, one on the left); in two of these, lysis failed, and two had partial lysis with residual stenoses, both of whom were treated with PTA/stenting (one on the right side, one on the left). Fourteen others had disease secondary to nonmalignant causes (12 on the left side, 2 on the right). The two on the right side underwent successful lysis with no residual stenoses; 8 of the 12 patients on the left side had residual stenoses, and all of these underwent PTA/stenting.
Overall, treatment failed in five patients in group 2 in the long term: two early failures (unsuccessful lysis, both secondary to malignancy), one thrombosis partially lysed but could not be opened by PTA/stenting (secondary to malignancy), one thrombosis successfully lysed but failed later (secondary to malignancy); in the remaining patient, treatment failed because of secondary recurrent DVT. In contrast, only 1 patient of 33 in group 1 had venous patency at 30 days and 7 others at 6 months. Two of these eight patients had recurrent DVT later with a venous occlusion; both were treated with anticoagulation.
Table 2 summarizes the clinical outcome and the type of therapy. As noted, the 30-day and 6-month venous patency rates were significantly better in group 2 than in group 1 (83% vs. 3% and 83% vs. 24%, respectively, P < .0001). Long-term symptom resolution was also better in group 2 (78% vs. 30%, P = .0015).
Table 2. CLINICAL OUTCOME

Major bleeding complications were noted in two patients in each group (P = NS). These included one retroperitoneal hematoma and one venous bleed at the venous insertion site in group 2, and one gastrointestinal bleed and one retroperitoneal hematoma in group 1. There were no immediate deaths in either group. Three of the 18 patients in group 2 (17%) had minor bleeding complications (venous insertion site), in contrast to 3 of the 33 patients in group 1 (9%). None of the patients in group 2, in contrast to two patients in group 1 (6%), had symptomatic pulmonary emboli (P = .534). No other complications were noted.
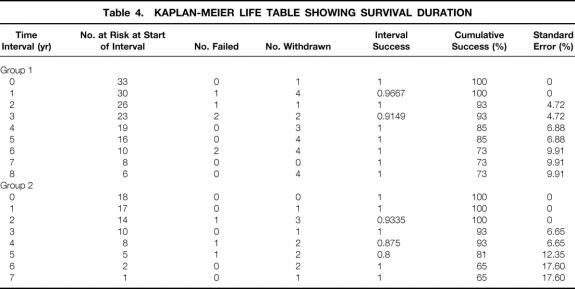
Kaplan-Meier life table analyses showed that the primary iliofemoral venous patency rates at 1, 3, and 5 years were 24%, 18%, and 18% in group 1 versus 83%, 69%, and 69% in group 2 (P < .01;Table 3, Fig. 1). The survival rates at 1, 3, and 5 years were similar for both groups: 100%, 93%, and 85% for group 1 and 100%, 93%, and 81% for group 2 (P = .740;Table 4, Fig. 2). Overall, there were six late deaths in group 1 (five secondary to malignancy and one secondary to myocardial infarction) and three deaths in group 2 (two secondary to malignancies and one secondary to myocardial infarction).
Table 3. KAPLAN-MEIER LIFE TABLE SHOWING TIME TO ACHIEVING PATENCY
FU, follow-up.
Figure 1. Kaplan-Meier curves for primary iliofemoral venous patency.
Table 4. KAPLAN-MEIER LIFE TABLE SHOWING SURVIVAL DURATION
Figure 2. Kaplan-Meier survival curves.
Long-term symptom resolution (0–2 CEAP classes) was achieved in 10 of 33 (30%) group 1 patients (clinical severity score range of 0–1; this included six patients with a patent iliofemoral venous system and four patients because of the development of extensive collaterals) versus 14 of 18 (78%) group 2 patients (clinical severity score range of 0–1; 13 patients with a patent iliofemoral venous system and one with collaterals, P = .0015). Seven other patients in group 1 with CEAP 5 to 6 classes (three C5S and four C6S with a clinical severity score range of 7–18) were treated with femoral vein crossover femoral vein bypass grafting (for iliac occlusion); five had satisfactory results. The remaining 16 patients in group 1 (two C3A, three C3S, four C4A, and seven C4S with a clinical severity score range of 1–10) were treated conservatively. Of the four remaining in group 2, two (one C5S and one C6S with a clinical severity score of 7 and 18) had femorofemoral vein bypass grafts with satisfactory results and two (one C3A and one C4S with a clinical severity score of 1 and 6) were treated conservatively.
DISCUSSION
The goal of treatment of acute DVT should be prevention of early and late complications of venous thrombosis—that is, prevention of pulmonary embolism and restoration of blood flow through a thrombosed vein with preservation of venous valve function. Achieving these goals minimizes deaths and complications in pulmonary embolism and diminishes the sequelae of postthrombotic syndrome. Most patients with iliofemoral DVT have a substantial rate of acute complications and severe postthrombotic sequelae, 1,3,14,15,19 which are associated with the higher ambulatory venous pressure that occurs in patients who have both venous obstruction and valvular incompetance. 15,19–21
With conventional anticoagulation therapy, recanalization of the occluded veins depends solely on the effectiveness of the patient’s fibrinolytic system. Unfortunately, in most patients, anticoagulation cannot resolve iliofemoral venous thrombosis, and only 6% of patients with acute proximal DVT show complete lysis of the thrombus within 10 days. 2 In addition, anticoagulation alone in patients with iliofemoral DVT resulted in severely compromised muscle pump function and valvular competency in approximately 95% of patients at 5 years of follow-up, despite improvement in venous outflow. 3
Thrombolysis is an attractive treatment alternative to standard anticoagulation because it has the potential to lyse the thrombus rapidly and may preserve valve function, which may prevent postthrombotic syndrome. This clinical condition is heralded by intractable edema, leg pain, hyperpigmentation, and cutaneous ulceration. Studies have shown that the risk for symptoms of pain and edema ranges from 30% to 67%. Skin complications, which consist of pigmentation and ulceration, are considered the most severe manifestations of the syndrome. These complications have been found at follow-up to occur in 2% to 28% of patients diagnosed with DVT. 8–10,19
Several studies have shown that valvular function can be preserved after physiologic lysis of DVT, especially when it occurs over a short period. 16,22 Several randomized studies have concluded that thrombolytic agents, even when administered systemically, are superior to standard anticoagulation therapy in achieving early thrombolysis. In a pooled analysis of the results from 13 randomized studies, Comerota and Aldridge 22 found that only 4% of patients treated with heparin had substantial or complete lysis, compared with 45% of patients randomly assigned to receive systemic streptokinase therapy. Similarly, Goldhaber et al, 23 in a review of pooled data from six randomized trials, found that systemic thrombolysis was 3.7 times more effective than heparin in producing some degree of lysis. The experience of others with systemic thrombolysis for iliofemoral venous thrombosis has been somewhat disappointing. 15,24 Because of the presence of venous collaterals that develop in venous occlusion, it is not usually possible to deliver the lytic agent directly to the thrombus when systemic infusion is used. Transcatheter infusion of a lytic agent can deliver high concentrations directly into the thrombus while minimizing the potential for a systemic fibrinolytic effect. 15,25
A theoretical consideration with catheter-directed lytic therapy is dislodgement of significant thrombotic material, which may lead to a pulmonary embolism. In 1994, Semba and Dake 14 reported one of the first series of patients treated for venous thrombosis with catheter-directed thrombolysis. In their study, which consisted of 21 patients who underwent catheter-directed thrombolysis, there were no clinically detectable pulmonary emboli. Other studies likewise failed to show symptomatic pulmonary embolism. 15
In our practice, we do not routinely insert caval filters before lytic therapy unless there is an indication for one. In our study of 18 patients who underwent thrombolysis, there were no episodes of pulmonary embolism. In contrast, two patients who were treated with conventional anticoagulation had a pulmonary embolism. Because of the sample size, this disparity was not found to be statistically significant (P = .5341). The Venous Registry, a multicenter trial of 473 patients treated with catheter-directed urokinase, reported a 1% incidence rate of pulmonary embolism. 16 These results were comparable to data extrapolated by Grossman and McPherson 20 : in their review of 15 studies published concerning catheter-directed thrombolysis, they found only 2 cases (0.9%) of symptomatic pulmonary emboli in 214 patients who underwent thrombolytic therapy but did not have prophylactic placement of an inferior vena cava filter. In addition, their review of 12 studies of conventional heparin treatment for DVT of the lower extremity revealed an incidence of nonfatal symptomatic emboli of 7.9%.
One of the parameters that this study compared was iliofemoral venous patency. Of the 33 patients treated with conventional anticoagulation, only 1 (3%) was found to be patent at 30 days. By comparison, 83% (15/18) of the patients in the lysis/stenting group were found to be patent (P < .0001). Further evaluation by venous duplex imaging at 6 months revealed an improved patency rate of 24% (8/33) at 6 months in the group treated with conventional therapy, but this patency stayed at 24%, 18%, and 18% at 1, 3, and 5 years. This improvement was not unexpected: previous studies have shown that recanalization with this form of therapy can occur. 22 However, the patency rates at 1, 3, and 5 years for group 2 were superior at 83%, 69%, and 69%, respectively (P < .01).
The end point of comparison between our two groups was the long-term symptom resolution. In group 1, 30% of patients reported resolution of symptoms in contrast to 78% for group 2.
Other studies using lytic therapy have documented similar sustained patency rates. Semba and Dake 14 reported a 92% continued patency rate for treated iliofemoral veins at 3 months. Mewissen’s report 16 on data collected from the Venous Registry showed a primary patency rate of 65% at 6 months. Patency rates were also more likely to be sustained in patients with iliofemoral DVT and in those treated with adjunctive stenting. 14,16
Our superior results in group 2 patients (lysis/PTA/stenting) can at least partially be explained by the use of adjunctive procedures. Angioplasty/stenting was performed in 10 of 18 patients who showed residual stenosis after lysis. In a recent study by Grossman and McPherson 20 on the safety and efficacy of catheter-directed thrombolysis for iliofemoral venous thrombosis, the data from 15 published studies were reviewed; angioplasty was performed in approximately 91 of 263 patients and stents were placed in 83 patients. Most of the authors in these studies preferred the wall stent device (Schneider, Minneapolis, MN), but Palmaz stents (Johnson & Johnson, Cordis, Warren, NJ) or Gianturco stents (Cook, Bloomington, IN) were also used. When angioplasty alone was performed, it was most often done to macerate any residual clot after lytic therapy. Stent placement was usually indicated for patients with venous wall elastic recoil or residual luminal stenosis. Most of these stents were placed in the common or external iliac veins. Generally, wall stent endoprostheses seem to have a considerable advantage over other stent models in this location because they are flexible and allow nontraumatic manipulation within curved vessel segments.
Several routes of local lytic therapy are generally available: the transjugular vein approach, ipsilateral femoral vein puncture, contralateral femoral vein puncture with passage of an infusion catheter over the caval bifurcation, ipsilateral popliteal vein puncture for transcatheter therapy of an iliofemoral thrombus, and infusion into the foot vein, with Ace-type elastic bandages or cuffs used to direct the drug into the ipsilateral deep veins. In general, infusion into the foot vein is minimally effective, and it is difficult for both patients and the nursing staff to maintain a catheter in the foot vein for 2 to 3 days. Ipsilateral popliteal vein puncture is most useful for the thrombus that extends from the inferior vena cava into the superficial femoral vein, and when the transjugular approach is difficult because of venous valves, or there is resistance in passing the guidewire through an organized thrombus. Ipsilateral femoral or contralateral femoral vein approaches are easier but may induce femoral vein thrombosis if it is not present. The jugular approach, however, spares the femoral vein from catheter-related trauma, especially when PTA or stenting is being done through larger sheaths. Thrombosis of the jugular vein is less likely to lead to significant complications in these circumstances. As indicated in our results, the jugular vein approach was used in a significant number of our patients (44%), the ipsilateral popliteal vein in 33%, and the contralateral femoral vein in 22%. At present, an ultrasound-guided popliteal vein puncture is the recommended access to the thrombosed deep venous system. 16
CONCLUSIONS
The results of this study show that catheter-directed thrombolysis, with the addition of angioplasty/stenting for residual stenosis, is more effective than conventional anticoagulation in the treatment of iliofemoral venous thrombosis. This treatment modality produces significantly improved long-term patency rates and resolution of symptoms.
Discussion
Dr. Ralph G. Depalma (Washington, DC): President Aust, members, and guests. It was a great pleasure to hear this paper and to read the manuscript. I can tell that Dr. AbuRhama has been thinking about this problem for about 10 years, probably related to his work in the axillary vein.
I have had experience with this technique, or at least in our clinic, and I have observed serious complications of lysis for venous thrombosis, two related to CNS hemorrhage and two related to retroperitoneal hemorrhage. So it is not something you want to go home and undertake lightly. On the other hand, the results in terms of patency have been truly remarkable in the author’s hands. I have several questions.
How have the authors adjusted the rtPA dosage in heparin since your kinase has been withdrawn? We have had lots of problems with dosing rtPA. It is tricky and there is more of a tendency toward bleeding.
Based on this extensive experience, what wall stent do you recommend for the iliac veins? Have you come to a choice, or is it just a generic choice?
I really commend their nonuse of caval filters. It shows a great deal of restraint, in my opinion. But were you not tempted, in the group 1 patients where there were two pulmonary emboli, to treat these patients with filters?
And, finally, has any patient in either group really developed venous ulceration or class 5 disease over the period of study?
Thank you very much for sharing this information.
Dr. J. Alex Haller, Jr. (Baltimore, Maryland): Dr. Aust, Dr. Townsend, ladies and gentlemen. First I would like to compliment the authors also on an excellent study and on focusing again on a common problem, namely deep venous thrombosis.
Some of you may think, as I stand up here, that certainly he is having a “senior moment,” because this is not a pediatric surgery problem in any sense of the word. Very rarely seen in children. But a few of you may remember that at one time I was a real surgery in Louisville, Kentucky, and had a great interest in this problem of deep venous thrombosis and continued that interest for a few years also in Baltimore.
I thought it might be helpful, if I may have that first discussion slide, since the authors did not have time to show examples of just what they are talking about, to remind the audience of what is involved in removal of these clots from the deep venous system. Because what we have proposed in Louisville and had an extensive experience at that time was a new operation in which there was open removal of the clot in the deep femoral system, as you see here diagrammatically, in which the patient with deep venous thrombosis was approached through the groin and was an incision in the thrombosed vessel. It was possible, with pressure above and below, to extrude this clot in the operating room. This immediately relieved the acute symptoms, and the question, naturally, was whether it would remain patent and whether there would be long-term complications associated with deep venous insufficiency.
The next slide is an example of the way in which we envisioned the occurrence of deep venous thrombosis and the development of the swollen leg which is so characteristic of this condition. There had to be extensive involvement of the deep venous system so that all the collateral veins were involved before you had the clinical symptomatology of an extensively swollen leg.
The next slide.
This presented clinically in this way initially, the so-called phlegmasia alba dolens or the white leg, also possibly called that because it occurred not uncommonly in the postpartum period during lactation, thus, also called milk leg in women who developed this not uncommonly.
The next slide.
But this could go on to a much more extensive involvement in which there was phlegmasia cerulea dolens, the blue leg, in which there was also compromise of arterial input. And many of these patients would have a significant loss of tissue, as you can see in this example. So it is not a benign condition at all, and there are both the acute symptoms which the authors emphasize with their technique clearly relieving that, but—the next slide—the real question, I think, is about the long-term effects.
And they have a wonderful follow-up of 63 months, therefore giving me an opportunity to focus on that long-term part in my questions.
We believed then, and I believe it still true, that the major problem with deep venous thrombosis following the acute occlusion is the recanalization in which there is dissolution of clot in the middle, but there is organization peripherally, and this involves the valves which sticks them forever to the wall and, thus, it is incompetent and we are left then with a postphlebitic limb.
The final slide.
That postphlebitic limb is the thing that junior faculty members are very familiar with because they are the ones that are asked to see all of these venous problems. And I certainly inherited a number of those in Louisville. A major morbidity to these patients, and a result, we believe, of the recanalization syndrome.
Now the authors say that their study shows that the veins are patent, but I would submit that it is not enough to be patent: they must also have competent valves. And my question, therefore, is directed toward that group of patients in the long-term follow-up. Just as Dr. DePalma asked whether there were long-term postphlebitic problems, how frequently did that occur?
Yes, you showed there was patency, but were there competent valves?
And the second question I have is why do you need a stent unless there is a true venous stenosis, which is uncommon in my experience and from what I have learned from the subsequent literature? Why do you want to put a foreign body in? Even though I recognize that is trying to keep the area of stenosis open, that must be extremely uncommon. Is that not an unnecessary addition to your otherwise fine protocol?
Thank you very much.
Dr. Ali F. Aburahma (Charleston, West Virginia): Thank you, Dr. Aust. Thank you, Dr. DePalma for your comments. In regard to the contraindication or the disaster you see with the lytic therapy, I am sure most of you have seen one or two cases in your practice. Actually, I did see one several years ago. I was telling a good friend of mine about it yesterday. His wife was not part of the study, and, actually, she underwent lytic therapy, kidney transplant, a victim of iliofemoral deep vein thrombosis on the side of the kidney transplant, massive immunological hemorrhage, and death 2 weeks later. So you have to be extremely careful, and that’s why I explained to you we had only 18 patients over 10 years. So I am extremely selective which patient to give lysis.
The dose generally is adjusted with a urokinase or TPA. True, TPA is more delicate, but still you could adjust the dose if you are very careful. And once the fibrinogen level drops below 100 mg, you better stop the lytic therapy, whether TPA, urokinase, or whatever.
Wall stent. The size of the wall stent is determined by the size of the native vein and what you see after you do the lysis. But most if not all of our patients had the size of wall stent vary between 8 to 12 in diameter by 40- to 60-millimeter length.
Filters. We did not do any filter prophylactically on either group; however, two patients who had the PE and the conventional therapy were treated with a filter.
There were seven patients in group 1 with venous ulcerations and two in group 2.
I appreciate Dr. Haller’s constructive comments and his beautiful illustrations. If you look in the manuscript—hopefully, some of you will see it in the near future we refer to a failed vein if the vein is occluded or significant venous reflux was noted in a patient vein.
Stenting, why did we use a stent? Not only just us, but even the venous registry that reported the results of several hundred patients felt that stenting is not needed in certain patients (particularly on the left side).
Footnotes
Presented at the Southern Surgical Association Meeting, December 3–6, 2000, Palm Beach, Florida.
Correspondence: Ali F. AbuRahma, MD, 3100 MacCorkle Ave., SE, Suite 603, Charleston, WV 25304.
E-mail: Ali.aburahma@camcare.com
Accepted for publication December 2000.
References
- 1.Markel A, Manz RA, Bergelin RO, et al. Valvular reflux after deep vein thrombosis: incidence and time occurrence. J Vasc Surg 1992; 15: 377–384. [PubMed] [Google Scholar]
- 2.Sherry S. Thrombolytic therapy for deep venous thrombosis. Semin Intervent Radiol 1985; 4: 331–337. [Google Scholar]
- 3.Akesson H. Venous function assessed during a five-year period after acute iliofemoral venous thrombosis treated with anticoagulation. Eur J Vasc Surg 1990; 4: 43–48. [DOI] [PubMed] [Google Scholar]
- 4.DeWeese JA. Thrombectomy for acute iliofemoral venous thrombosis. J Cardiovasc Surg 1964; 5: 703–712. [PubMed] [Google Scholar]
- 5.Karp RB, Wylie EJ. Recurrent thrombosis after iliofemoral venous thrombectomy. Surg Forum 1966; 17: 147–148. [PubMed] [Google Scholar]
- 6.Lansing AM, Davis WM. Five-year follow-up study of iliofemoral venous thrombectomy. Ann Surg 1968; 168: 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menawat SS, Gloviczki R, Mozes G, et al. Effect of a femoral arteriovenous fistula on lower extremity venous hemodynamics after femorocaval reconstruction. J Vasc Surg 1996; 24: 793–799. [DOI] [PubMed] [Google Scholar]
- 8.Eklof B, Kistner RL. Is there a role for thrombectomy in iliofemoral venous thrombosis? Semin Vasc Surg 1996; 9: 34–45. [PubMed] [Google Scholar]
- 9.Juhan MC, Alimi YS, Barthelemy PJ, et al. Late results of iliofemoral venous thrombectomy. J Vasc Surg 1997; 25: 417–422. [DOI] [PubMed] [Google Scholar]
- 10.Elliot MS, Immelman EJ, Jeffery P. A comparative randomized trial of heparin versus streptokinase in the treatment of acute proximal venous thrombosis: an interim report of a prospective trial. Br J Surg 1979; 66: 838–843. [DOI] [PubMed] [Google Scholar]
- 11.Duckert F, Muller G, Nyman D. Treatment of deep vein thrombosis with streptokinase. Br Med J 1975; 1: 479–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ott P, Eldrup E, Oxholm P, et al. Streptokinase therapy in the routine management of deep venous thrombosis in the lower extremities: a retrospective study of phlebographic results and therapeutic complications. Acta Med Scand 1986; 219: 295–300. [DOI] [PubMed] [Google Scholar]
- 13.Arensen H, Hoiset A, Ly B, et al. Streptokinase or heparin in the treatment of deep vein thrombosis: follow-up results of a prospective study. Acta Med Scand 1982; 211: 65–68. [PubMed] [Google Scholar]
- 14.Semba CP, Dake MD. Iliofemoral deep venous thrombosis: aggressive therapy with catheter-directed thrombolysis. Radiology 1994; 191: 487–494. [DOI] [PubMed] [Google Scholar]
- 15.Comerota AJ. A strategy of aggressive regional therapy for acute iliofemoral venous thrombosis with contemporary venous thrombectomy or catheter-directed thrombolysis. J Vasc Surg 1994; 20: 244–254. [DOI] [PubMed] [Google Scholar]
- 16.Mewissen MW. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology 1999; 211: 39–49. [DOI] [PubMed] [Google Scholar]
- 17.Porter JM, Moneta GL. Reporting standards in venous disease: an update—International Consensus Committee on Chronic Venous Disease. J Vasc Surg 1995; 21: 635–645. [DOI] [PubMed] [Google Scholar]
- 18.Nicolaides AN. Executive Committee of the Ad Hoc Committee, American Venous Forum, 6th Annual Meeting. Classification and grading of chronic venous disease in the lower limbs: a consensus
- 19.Strandness DE Jr. Long-term sequelae of acute venous thrombosis. JAMA 1983; 250: 1289–1292. [PubMed] [Google Scholar]
- 20.Grossman C, McPherson S. Safety and efficacy of catheter-directed thrombolysis for iliofemoral venous thrombosis. AJR Am J Roentgenol 1999; 172: 667–672. [DOI] [PubMed] [Google Scholar]
- 21.Shull KC, Nicolaides AN, Fernandes C, et al. Significance of popliteal reflux in relation to ambulatory venous pressure and ulceration. Arch Surg 1979; 114: 1304–1306. [DOI] [PubMed] [Google Scholar]
- 22.Comerota A, Aldridge SC. Thrombolytic therapy for deep venous thrombosis: a clinical review. Can J Surg 1993; 36: 359–364. [PubMed] [Google Scholar]
- 23.Goldhaber SZ, Buring JE, Lipchick RJ, et al. Pooled analysis of randomized trials of streptokinase and heparin in phlebographically documented acute deep venous thrombosis. Am J Med 1984; 76: 393–397. [DOI] [PubMed] [Google Scholar]
- 24.Hill SL, Martin D, Evans P. Massive vein thrombosis of the extremities. Am J Surg 1989; 158: 131–136. [DOI] [PubMed] [Google Scholar]
- 25.Becker GJ, Rabe FE, Richmond BD, et al. Low-dose fibrinolytic therapy: results and new concepts. Radiology 1993; 148: 663–667. [DOI] [PubMed] [Google Scholar]