Abstract
The neural regulation of emotional perception, learning, and memory is essential for normal behavioral and cognitive functioning. Many of the symptoms displayed by individuals with schizophrenia may arise from fundamental disturbances in the ability to accurately process emotionally salient sensory information. The neurotransmitter dopamine (DA) and its ability to modulate neural regions involved in emotional learning, perception, and memory formation has received considerable research attention as a potential final common pathway to account for the aberrant emotional regulation and psychosis present in the schizophrenic syndrome. Evidence from both human neuroimaging studies and animal-based research using neurodevelopmental, behavioral, and electrophysiological techniques have implicated the mesocorticolimbic DA circuit as a crucial system for the encoding and expression of emotionally salient learning and memory formation. While many theories have examined the cortical-subcortical interactions between prefrontal cortical regions and subcortical DA substrates, many questions remain as to how DA may control emotional perception and learning and how disturbances linked to DA abnormalities may underlie the disturbed emotional processing in schizophrenia. Beyond the mesolimbic DA system, increasing evidence points to the amygdala-prefrontal cortical circuit as an important processor of emotionally salient information and how neurodevelopmental perturbances within this circuitry may lead to dysregulation of DAergic modulation of emotional processing and learning along this cortical-subcortical emotional processing circuit.
Keywords: dopamine, prefrontal cortex, amygdala, sensitization, emotional learning, emotion, ventral tegmental area, memory
Introduction
Schizophrenia is a unique human disorder comprising a complex array of neurophysiological, neurochemical, and psychological disturbances. Adding to this complexity is the existence of both “positive” symptoms, such as psychotic ideation and sensory hallucinations, and “negative” symptoms, such as apathy, anhedonia, and social isolation.1,2 At the core of many of these manifestations of schizophrenia lies a fundamental disturbance in emotional processing, perception, and regulation. Indeed, our seemingly effortless ability to make sense of the constant sensory information entering our brains depends upon our capacity to place this information in its appropriate emotional context. However, in addition to performing adaptive emotional contextualization, our brains need to form learned associations between sensory cues in our environments and their associated emotional meanings. These conditioned associations form memories upon which our future behavioral responses and cognitive patterns are determined. When these processes go awry, sensory stimuli within our environments may trigger maladaptive emotional or motivational responses eventually leading to psychotic ideation and/or delusions based upon these faulty conditioned associations and memories. Patients suffering with schizophrenia often assign inappropriate emotional significance (either abnormally potentiated or severely blunted) to sensory stimuli in their environments that healthy individuals would be able to appropriately perceive and emotionally contextualize. When the brain does not properly process the emotional meaning of sensory stimuli, it becomes inherently difficult to perform adaptive motivated behaviors in response to those stimuli, in present or future contexts.
Given the interrelationship between emotional learning, memory, and motivation, is there a common neurobiological system which may account for the deficits in these psychological processes underlying the bewildering array of schizophrenia-related symptoms? Considerable evidence from both the animal and human neuroscience literature implicates disturbances in the functional balance between dopaminergic (DAergic) signaling pathways, subcortical brain regions (such as the hippocampus [HC], amygdala, thalamus, striatum, and midbrain), and cortical structures (such as the medial and lateral prefrontal regions and cingulate cortex). In this review, research examining the role of the catecholamine neurotransmitter DA in the processing of emotion, motivation, and sensory processing will be considered. In this context, I will focus primarily on 4 interconnected brain regions that have been consistently implicated in the processing of emotional sensory information, motivational control, and emotional associative learning: the prefrontal cortex (PFC), basolateral amygdala (BLA), ventral tegmental area (VTA), and the nucleus accumbens (NAcc). I will compare and contrast evidence from recent theories and research discoveries suggesting that the underlying emotional processing disturbances observed in schizophrenia may be related to a dysregulation of DA systems within these interconnected cortical-subcortical neural circuits. In addition, the role of this neural circuitry in the processing and encoding of emotional information and how disturbances in DA regulation of these emotional learning circuits may underlie the aberrant emotional and motivational processing present within the schizophrenic syndrome are also discussed.
The Role of DA in Emotional Processing, Learning, and Motivation: Anatomical and Functional Considerations
The neurotransmitter DA has received perhaps the greatest amount of basic research attention in the context of emotional and motivational regulation.3 At the pharmacological level, DA receptors are broadly classified into 2 families: the D1 like (comprising D1 and D5 subtypes) and D2 like (comprising D2, D3, and D4 subtypes).4 While DA receptors are found throughout the nervous system, they demonstrate high concentrations within the brain's limbic regions, basal ganglia, and frontal cortical areas, all of which are involved importantly in emotional and motivational regulation. At the systems level, the A10 DAergic neurons located within the midbrain VTA and their associated efferent targets in the NAcc (the mesolimbic pathway) and the PFC (the mesocortical pathway) have been the focus for decades of research, revealing critical roles for both these DAergic systems in the processing and regulation of emotion and motivation.3,5–7 The mesolimbic pathway, in particular, has been implicated as a critical neural system for the processing of motivationally salient information, including both drugs (opiates, nicotine, cocaine, alcohol, cannabinoids, and amphetamine) and naturally appetitive stimuli.5,7–11 In terms of emotional processing and perception, functional neuroimaging studies in human subjects have consistently demonstrated that the amygdala, PFC, VTA, and striatal regions (including the NAcc) are activated by emotionally salient sensory cues, including both “rewarding” and “aversive” emotional events.12–18
Whereas early theories about the role of DA transmission in emotional processing proposed that DA represented a neurochemical transducer of reward or “pleasure,” continuing evidence in both human and animal research implicates DA transmission in the processing of the rewarding and aversive stimulus properties of various drugs and other emotionally salient stimuli.6,10 For example, DAergic midbrain neurons increase firing in response to aversive stimuli, such as tail pinch or other stressors,6 and increase responding in response to primary, conditioned, or secondary rewards19 and novel or unpredicted stimuli.20,21 The general response patterns of DAergic neurons to salient stimuli, regardless of valence, has suggested that DA transmission may encode the emotional or motivational “salience” of primary or conditioned stimuli.22,23 Thus, rather than being involved exclusively in either the rewarding or aversive emotional encoding of specific stimulus information, the role of DA transmission in the context of emotional processing, learning, and memory appears far more ubiquitous than previously thought. Furthermore, this more global role for DA transmission in modulating emotional processing would suggest that fundamental disturbances in DA transmission, particularly within neuronal emotional learning and memory circuits, could have profound effects on affective regulation, possibly leading to global distortions in emotional processing and perception. As will be discussed presently, recent evidence from basic animal research and human neuroimaging studies is pointing to this conclusion.
DA Modulation of Emotional Processing, Learning, and Plasticity: Implications for Schizophrenia
The DA hypothesis of schizophrenia posits that excessive DA transmission, primarily within forebrain regions, is responsible for the psychotic symptoms of schizophrenia.24–26 This conclusion is based on several compelling pieces of evidence. First, clinical evidence has consistently demonstrated the efficacy of DA D2 receptor–blocking drugs in the treatment of schizophrenia-related psychosis.24–26 Second, excessive DA transmission in human schizophrenics is suggested by neuroimaging studies which have found that patients with schizophrenia display greater amphetamine-induced potentiation of striatal synaptic DA levels.27,28 Finally, reports from both human and animal research demonstrate that addictive, psychostimulant drugs, such as amphetamine, which strongly potentiate DA transmission, can induce psychotic states that are virtually indistinguishable from those observed in patients with schizophrenia.29,30 Furthermore, these drugs can worsen or precipitate psychotic episodes in these patients.31,32 Nevertheless, there are several pieces of evidence that are inconsistent with the DA hypothesis of schizophrenia. For example, little direct evidence suggests a primary pathology in DA neurotransmission in schizophrenia, or for pathological alterations in the levels of DA or DA receptors that can be confirmed without the potential confound of long-term medication artifacts.33 Despite this, the efficacy of neuroleptic medications in the treatment of schizophrenia-related psychosis is tightly linked to their ability to block DA D2 receptors.24
As previously discussed, DA transmission has been demonstrated to strongly modulate emotional and motivational processing within the mesolimbic pathway. However, beyond this system, various studies focusing specifically on the amygdala-prefrontal cortical circuit have demonstrated that the PFC and amygdala regions are involved importantly in emotional processing and regulation for a wide array of emotionally and motivationally salient events.34–40 For example, both regions show strong activation following the presentation of emotionally salient stimuli such as visual representations of emotional expressions in human faces or disturbing imagery.36,41,42 Patients with schizophrenia show attenuated activation in the amygdala-cortical circuit following emotional stimulus presentations, suggesting that deficits in emotional perception and processing observed in these patients may be related to a disturbance in this emotional processing circuit.41,42 Within the frontal cortex, abnormal neuronal activity is associated with psychotic symptoms in patients with schizophrenia43,44 that appears to be related to disturbed DAergic transmission.45,46 In nonhuman primates, the PFC is involved in the generation of hallucinatory-like behaviors,47 and in patients with schizophrenia, functional magnetic resonance imaging studies report that frontal cortical regions are activated during auditory hallucinations48–50 and during the occurrence of positive schizophrenia-related symptoms.43
Beyond the level of functional neuroimaging, more detailed electrophysiological studies have demonstrated the role of single neurons within both the PFC and amygdala in the active encoding and expression of learned emotional conditioned associations. For example, when an emotionally salient event such as an electric foot shock is paired with a sensory cue, such as a tone, light, or olfactory stimulus51–54 neurons in either of these regions, will increase their firing rates specifically in response to the associatively conditioned cue. The ability of single neuron within the PFC and amygdala to encode emotional associative learning is dependent upon signaling through DA receptors,51,53 and emotional processing within PFC neurons is strongly modulated by cannabinoid receptor signaling system,34,52 a neurotransmitter system that functionally interacts with DA receptor substrates.34 In addition to a role in encoding emotional memory associations, subpopulations of neurons within the rodent PFC also signal the extinction or “unlearning” of emotionally salient learned associations and can regulate the activity and associative encoding of amygdalar neurons during emotional learning tasks.55–59 Together, this evidence demonstrates a fundamental role for the amygdala-prefrontal cortical circuit in the processing of emotional information. Given the structural and functional abnormalities observed in patients with schizophrenia, how disturbances in DA signaling might lead to aberrant emotional processing? As will now be discussed, increasing evidence shows important implications for functional interactions between cortical and subcortical emotional processing centers and suggests that disturbed DA transmission within this circuitry may underlie these symptom patterns in schizophrenia.
DA Modulation of Cortical and Subcortical Functional Interactions: Implications for Emotional Processing
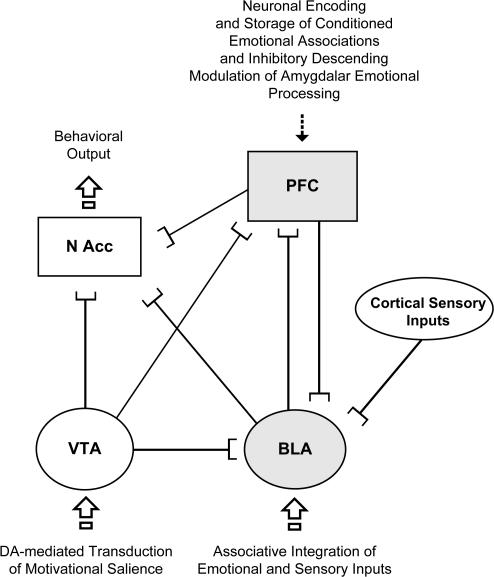
A functional role for DA neurotransmission in emotional processing extends to interconnected cortical and subcortical neural regions. Thus, the PFC, amygdala, VTA, and NAcc all share critical functional connections, which are neither fully understood nor completely characterized at the anatomical, neurochemical, and functional levels. In figure 1, a simplified schematic is presented demonstrating some of the known functional connections between these neural regions and their hypothesized roles in emotional processing, learning, and memory encoding. While a detailed review of these connections is beyond the scope of this review, some of these connections have important implication for DAergic regulation of cortical-subcortical emotional regulatory processes. For example, in addition to the well-established DAergic VTA projection to the NAcc, DAergic VTA efferents target also the amygdala, NAcc, and PFC.60 The amygdala sends excitatory, presumably excitatory glutamatergic, inputs to the NAcc and PFC,38 and the PFC sends important descending, excitatory inputs to the NAcc,61 VTA,62 and various amygdalar subnuclei.38,53,54 In addition to these 4 regions, the HC and various thalamic nuclei functionally interact with the cortex, VTA, and amygdala, and these connections are modulated by DA transmission.61 While neither the HC nor the thalamus are implicated directly in emotional processing, the HC is critical for the transmission of contextual learning elements, and the thalamus serves as a primary relay center for the organization of incoming sensory input. Although the complexity of these functional interactions appears overwhelming, several important implications for the processing of emotional information in schizophrenia are emerging.
Fig. 1.
A Simplified Schematic Showing Some of the Known Anatomical and Functional Connections Between the Prefrontal Cortex (PFC), Ventral Tegmental Area (VTA), Basolateral Amygdala (BLA), and Nucleus Accumbens (NAcc). Neurons within the BLA may serve as a primary integration center for emotional and/or motivational signals with incoming cortical sensory. However, PFC neuronal subpopulations also encode emotionally salient conditioned associations, and this process requires active BLA inputs.51,52 In addition, descending inhibitory influences from PFC to the BLA can strongly modulate both the acquisition of emotional learning in the amygdala and the extinction of learned conditioned associations. It is presently not known whether learned emotional associations and memories are first integrated within the amygdala and then transferred to higher cortical regions for long-term storage or if both regions may simultaneously encode these associations. Converging outputs from BLA, VTA, and PFC may integrate the behavioral output or motivated response selections mediated by the NAcc.
For example, West and Grace61 have proposed a functional model wherein converging glutamatergic inputs from the HC and BLA onto spiny projection NAcc neurons regulate PFC throughput to limbic/motor NAcc output pathways. In the context of schizophrenia, it is proposed that dysregulated gating of cortical inputs by hippocampal inputs may result in hypoactivity in accumbal-pallidal circuits, possibly contributing to the “negative” symptom profile of schizophrenia, whereas disturbances in amygdala-mediated inputs to the NAcc lead to exaggerated influence of amygdalar inputs to the NAcc, resulting in aberrant emotional processing and conceivably leading to the disturbed emotional processing underlying the “positive” symptoms of schizophrenia. Given the critical role for the BLA in the encoding and signaling of emotionally salient information, one possibility is that converging inputs from the PFC and BLA may serve as an integration mechanism for emotionally salient sensory information encoded in BLA amygdalar neurons.53,54 Such a mechanism may interact with motivational drive from the mesolimbic DA pathway originating in the VTA which would conceivably implicate the NAcc as a nexus point for the integration of motivational signals from the VTA DA system and emotional information from the amygdala. Nevertheless, further evidence is required in order to fully characterize the role of the NAcc in the processing and/or integration of emotionally salient information. Thus, it is presently not known if neurons in the NAcc, which anatomically serve as a nexus point between amygdala, VTA, and PFC inputs, actually serve to encode emotionally salient associative encoding or rather act as a nodal motivational/motor output action integrator during behavioral responding. In other words, do NAcc neurons subserve the acquisition and/or encoding or simply the expression of emotional learning and memory? One possibility is that the NAcc serves as a behavioral response/output generator based upon converging emotional, associative, contextual, and motivational inputs from the BLA, PFC, HC, and VTA, respectively (figure 1). Future studies are required to examine these questions more specifically.
Beyond the NAcc-PFC circuitry, recent evidence points increasingly to a crucial role for neuronal subpopulations within the PFC and amygdala as active encoders during the acquisition, expression, and extinction phases of both emotional learning and memory.51–58 Thus, the functional and anatomical connections between the amygdala, particularly the BLA, and the PFC are increasingly recognized as a critical cortical-subcortical emotional processing circuit, and one that appears disturbed in schizophrenic patients.41–43 Animal research has found that ascending inputs from the BLA to the PFC are necessary for the transmission of emotionally salient learned associations to individual neurons within the medial PFC.34,51,52 In particular, specific subpopulations of neurons within the PFC that receive monosynaptic inputs from the BLA can actively encode and express emotional conditioned associations, an effect that is blocked by inactivation of the BLA prior to the learning taking place.51,52 Neurons within the PFC are particularly interesting in this regard because they are also critical for transmitting extinction information while the animal “unlearns” a previously conditioned emotional association.55–57 Future studies are needed to fully characterize the neuronal populations that may be differentially involved in these emotional learning phenomena.
In addition to the BLA>PFC pathway, PFC neurons send important descending inputs to the amygdala and can control emotional learning and neuronal activity of single neurons within the amygdala during emotional associative learning processing. For example, stimulation of PFC inputs to the amygdala can block the ability of amygdala neurons to encode emotional learning.54 This has important implications for prefrontal cortical pathology in the schizophrenic syndrome; a loss of PFC inhibition on emotional processing mechanisms within the amygdala would likely render the individual incapable of accurately encoding the emotional salience of incoming sensory information from the sensory cortex or motivational signals arising form DAergic input from the VTA. Together, this evidence points to critical cortical-subcortical functional interactions between the amygdala and PFC during the encoding and expression of emotional learning and memory formation. As will be described presently, DA inputs from the VTA to both these regions seem to modulate these emotional processing circuits and regulate cellular and behavioral emotional learning and memory plasticity.
DA Regulation of Emotional Learning Plasticity in the Amygdala-Prefrontal Cortical Circuit
Considerable evidence from both the human neuroimaging and animal research literature now points to a critical role for emotional processing circuits within the amygdala-prefrontal cortical circuitry in the underlying pathology of schizophrenia. Given the importance of DA transmission in the underlying pathology of schizophrenia, it is perhaps not surprising that DA receptor signaling is crucial for emotional learning plasticity and memory encoding within this circuit. For example, DA transmission is required for encoding aversive conditioned associations both in the amygdala and PFC; systemic or local blockade of DA receptor activation in these brain regions prevents the acquisition and expression of learned emotional associations at the level of the single neuron.51–53 Blockade of DA receptors within the amygdala impairs the recall of emotionally salient conditioned fear associations63 and prevents the formation of associative plasticity in the form of long-term potentiation within the lateral amygdala.64 Within the medial PFC, blockade of the DA D4 receptor subtype prevents the expression and acquisition of fear-related behaviors and emotional associative learning both behaviorally and in single neurons,51,65 demonstrating the critical role of DA receptor transmission for the processing of emotionally salient information within this circuit.
In terms of disturbed DA signaling within neural emotional processing circuitry in schizophrenia, perturbations in the laminar distribution of DA D2 receptors have been reported in the cortex of postmortem human schizophrenics.66 While there is a wealth of evidence implicating disturbances in amygdala activity during the processing of emotionally salient sensory information in schizoprenia,67–69 there is less direct evidence for disturbances in amygdalar DA transmission as an underlying cause of the aberrant emotional processing. Reynolds70 found evidence of increased DA levels specifically within the left amygdala of schizophrenic patients; however, as with many postmortem tissue analyses, the potential confounding effects of medication cannot be ruled out. Future studies are required to more fully characterize potential disturbances in amygdalar DA transmission in the context of schizophrenia and emotional processing, particularly given the increasing evidence implicating the importance of amygdalar DA signaling in the regulation of emotional associative learning and memory.
One consistent finding in the literature is the demonstration of perturbed DA transmission within the thalamus, a subcortical structure with intermediate levels of DA D2 receptors.71 Using raclopride-C11 positron emission tomography imaging, Talvik et al72 reported lowered levels of DA D2 receptors in the thalamic region of patients with schizophrenia, suggesting an increase in presynaptic DA release in these patients. Although there is no direct evidence that the thalamus serves as a primary site for emotional information processing, the well-known role of the thalamus in sensory gating and integration may suggest that DAergic disturbances within this region may be involved importantly in the disturbed sensory gating present in patients with schizophrenia, particularly via functional interactions with the HC, VTA, and PFC. For example, Floresco and Grace73 reported that the mediodorsal thalamus exerts complex gating control of HC and VTA input to the PFC and can both facilitate excitatory HC input and inhibit VTA-mediated inhibition of individual PFC neurons, further implicating thalamic inputs to the PFC in the integration of emotional, motivational, and contextually salient information. Future research will undoubtedly shed light on how thalamic interactions with neural regions involved in emotional processing, learning, and memory may modulate these psychological processes, particularly in the context of disturbances in DAergic transmission within this circuitry.
Neuroplasticity of DA Transmission and the Modulation of Motivational and Emotional Processing: Implications for Schizophrenia
Recent research endeavors, particularly in the fields of behavioral neuropharmacology and electrophysiology, have examined the plasticity of DA transmission both at the systems and single-neuron levels of analysis. Indeed, DA pathways demonstrate considerable plasticity particularly following repeated administration of psychostimulant drugs that potentiate DA release and receptor activation within the mesocorticolimbic system. Typically, such studies measure the locomotor activity of rodents throughout the course of psychostimulant drug exposure and find that with repeated, intermittent administration of DA-stimulating drugs, the animal begins to display a hyperlocomotor response in the presence of the drug.65–67 Other indications of psychotic-like behaviors induced by psychostimulants, such as amphetamine, include perseverative stereotypical behavior patterns, which also display sensitization. In an extensive review of this evidence, Lieberman et al33 proposed that a neurodevelopmental process of DAergic sensitization induced by a combination of predisposing genetic variables and intermittent life experiences, such as severely stressful life events, may underlie the eventual manifestation of schizophrenia by inducing pathological sensitization of DAergic signaling pathways, which in turn leads to remittent episodes of psychosis. Several pieces of compelling evidence suggest that plastic alterations in DA transmission may underlie the disturbed motivational and emotional processing observed in of DA-related disorders, including addiction and schizophrenia. As previously noted, high doses of psychostimulants induce psychotic symptoms, including hallucinations and disturbed cognitive processes, which mimic schizophrenia in previously healthy individuals.29,74 In addition, psychostimulants exacerbate psychotic symptoms in schizophrenic patients,32,75 an effect that can be blocked with DA receptor antagonist pretreatment.30
If a pathological sensitization of DA transmission underlies the psychotic manifestations of schizophrenia, can such a process account for the disturbances in motivational and emotional processing observed in schizophrenia, and if so, which neural circuit(s) may be primarily affected by such DAergic abnormalities? The overwhelming majority of DA sensitization research has focused on behavioral correlates of drug sensitization, such as hyperlocomotion. This focus primarily has been upon the mesolimbic system in the context of addiction. Nevertheless, to date, there is no compelling evidence that psychomotor correlates of psychostimulant administration are functionally related to the modulation of emotion and motivation. If plasticity within the mesocorticolimbic DAergic system as a result of dysregulated DAergic tone were responsible for emotional and motivational disturbances in schizophrenia, it would be reasonable to expect changes in emotional and motivational processing as a result of a sensitized DA system. Indeed, given the important functional interactions between the mesocorticolimbic DA system with the amygdala and frontal cortex summarized in figure 1, “sensitized” or otherwise dysregulated DAergic input to this amygdala-prefrontal cortical emotional learning circuit may underlie the distorted emotional processing, learning, and memory encoding observed in schizophrenia. In figure 2, I present a hypothetical schematic wherein disturbed DAergic transmission from the VTA to the BLA>PFC circuit could potentially distort emotional processing, learning, and memory encoding. For example, DA receptor activation potentiates amygdala neuron output and excitability,64,76 an effect that may be expected to amplify emotional signaling within the BLA or along its output pathways and which could also disturb the balance between incoming sensory information to the amygdala and the motivational salience being attributed to such information due to amplified DAergic salience signals arising from the VTA. To the extent that DA signaling may represent a “reinforcement” signal, these aberrant DA signals arriving at the BLA simultaneously may maladaptively reinforce distorted associations between sensory inputs and amplified emotional/motivational salience. In addition, increased DAergic input to the PFC may serve to inhibit neuronal subpopulations within the PFC that normally provide inhibitory influences upon amygdalar emotional processing neurons54–56 (figure 2). Furthermore, increased activation of BLA output neurons to the PFC caused by hyper-DAergic VTA inputs may amplify emotional associative learning and memory encoding within PFC neurons through the BLA>PFC circuit. If PFC neurons encode long-term storage of these conditioned emotional associations, these aberrant learned associations and memories would become increasingly and persistently embedded within this emotional memory circuit.
Fig. 2.
In This Hypothetical Model of Aberrant Emotional Processing and Memory Encoding in Schizophrenia, Dopaminergic (DAergic) Inputs to the BLA>PFC Circuit Are Dysregulated, Leading to Abnormal Amplification and Distortion of Emotional and Sensory Information. These conditioned associations may then be reinforced by incoming ventral tegmental area (VTA) DA inputs and then transferred to emotional memory encoding neuronal subpopulations in the PFC. Behavioral output determined by integrative mechanisms in the nucleus accumbens (NAcc) would then reflect this pathological emotional processing and motivational output coming from VTA, BLA, and/or PFC inputs leading to aberrant behaviors and inappropriate responses to incoming emotional information.
In terms of motivational processing, certainly chronic exposure to various psychotomimetic drugs known to activate the DAergic system can induce qualitative alterations in how the DAergic system regulates drug reward information.77,78 For example, chronic opiate or nicotine exposure is sufficient to induce a switch to DA-dependent neural motivational system during the addiction process, whereas in previously drug-naive animals, DA transmission is not required for mediating the rewarding, “hedonic” properties of these drugs.79,80 Robinson, Berridge, and colleagues77–79 have proposed an “incentive salience” sensitization model which proposes that DA transmission signals the incentive or motivational “salience” of appetitive cues, such as drugs of abuse, and that this process occurs gradually over time with continued and intermittent exposure to psychostimulant drugs. While this model does not directly address the implications of DAergic plasticity in the context of emotional and motivational learning and processing in schizophrenia, it provides an interesting framework wherein plastic alterations in DA signaling over time may similarly lead to aberrant attribution of emotional or motivational salience to stimuli in the environment which under normal circumstances would be ignored and/or placed within its appropriate emotional or motivational context.
As suggested at the outset of this review, one feature of schizophrenia is the consistent attribution of inappropriate emotional and motivational salience to sensory stimuli in the environment, either real or imagined. Schizophrenics often cannot ignore sensory signals (such as auditory or olfactory hallucinations) possibly because such stimuli are inappropriately tagged with emotional salience. Indeed, drug addicts exhibit similar patterns of disinhibited control of emotional reactivity when they encounter conditioned cues within their environments that trigger drug-experience associations and memories. However, is there evidence from a functional neuroanatomical perspective to suggest that sensitization along the mesocorticolimbic system may indeed cause disturbances in emotional or motivational processing? One conceptual difficulty arising from the extant animal drug sensitization models is that no consistent demonstration of an “emotional” component to DA-related sensitization phenomena has been elucidated. Thus, the presence of potentiated locomotor activation following intermittent administration of DA agonist drugs says little about the underlying emotional and/or motivational processes that may or may not be influenced by chronic psychostimulant drug exposure. Indeed, little evidence suggests that a sensitized DA transmission within the mesolimbic circuit specifically underlies alterations in emotional or motivational salience attribution. Thus, it is presently unknown if a sensitized mesolimbic DA system could be responsible for the emotional processing and perception disturbances observed in schizophrenia. Nevertheless, increasing evidence suggests that potentiated DA neurotransmission in substrates beyond the NAcc may be related to disturbances in emotional learning and processing, particularly within the mesocortical pathway, as will be discussed presently.
To examine how neurodevelopmental disturbances may lead to neuropathological alterations related to schizophrenia, several animal models have been developed using pre- or postnatal chemical lesions or chemotoxic exposure protocols in an attempt to induce neuroanatomical, neurochemical, and/or behavioral symptoms characteristic of those observed in human schizophrenia patients. Several of these models have demonstrated DA sensitization–like phenotypes with corresponding behavioral indices of disturbed DAergic processing primarily within the VTA>PFC pathway along with corresponding behavioral abnormalities consistent with sensory and behavioral disturbances observed in schizophrenia. For example, Lipska et al81 demonstrated that neonatal lesions of the ventral HC induced profound alterations in behavioral responsiveness to amphetamine and stress. Further studies linked these effects to disturbed neuronal activity within the VTA>PFC pathway such that neurons within the PFC demonstrated markedly potentiated neuronal responsiveness to VTA inputs.82 Rajakumar et al83 reported that neonatal injections of a p75 antibody into the developing PFC of rats induced a hyper-DAergic phenotype in adult rats characterized by sensory processing deficits and potentiated locomotor and stereotypical responses to systemic amphetamine administration relative to controls. These effects were correlated with specific disruptions in PFC neuronal development related to disturbed neurotrophin activity during this developmental window. Lavin et al84 reported that prenatal exposure to mitotoxin methylazooxymethanol acetate in rats lead to an adult phenotype characterized by hyperresponsiveness of PFC neurons to stimulatory inputs from the VTA concurrent with decreased neuronal responsiveness to DA administration. All these neurodevelopmental animal models of schizophrenia have reported behavioral abnormalities in response to stimulation of DA substrates; effects that were correlated with disturbed neuronal signaling between subcortical (VTA) inputs to cortical target neurons. Amphetamine-induced sensitization of DA systems has been reported to disturb emotional processing and learning within the amygdala.85 In addition, amphetamine sensitization of hallucinatory behaviors is dependent upon the PFC because PFC lesions block the ability of DA sensitization to potentiate hallucinatory-like behaviors in primates.47 Interestingly, these same PFC lesions enhanced locomotor sensitization in these animals, suggesting dissociation between DA-mediated locomotor sensitization and the psychotic symptoms of schizophrenia.47
Fletcher et al86 have demonstrated that amphetamine sensitization can induce attentional deficits in a 5-choice serial reaction time test in rodents and that this effect can be reversed by D1 receptor activation within the PFC. Together, this evidence points to an important role for DAergic modulation of the amygdala-cortical emotional learning circuit and suggests that subcortical disturbances in DA transmission can profoundly influence emotional processing and learning within this emotional learning circuit. While further studies are required to better understand how disturbed DA transmission may specifically modulate emotional processing within the amygdala or PFC, the corresponding evidence from human neuroimaging studies that have reported disturbances in amygdalar and PFC regions87–89 during the processing of emotionally salient information in schizophrenic patients point to an important role for this circuit in the context of cortical-subcortical DAergic signaling abnormalities in schizophrenia.
Summary: DA as a Final Common Pathway for Emotional Processing Disturbances in Schizophrenia
Human and animal research has demonstrated a fascinating and multivariate role for DA transmission in the processing and encoding of emotionally salient information, both at the anatomical and signal transduction levels of analysis. Undoubtedly, DA represents a critical neural receptor substrate in the clinical treatment of psychosis. However, whether the DA system represents a final common pathway in either the etiological or symptomatic phases of schizophrenic pathology is a contentious issue for a variety of reasons. First, the heterogeneous nature of schizophrenic psychopathology makes it exceptionally difficult to pinpoint a specific role for DA transmission that could account for both the positive and negative symptom spectra. However, this conceptual difficulty is largely determined by how we envision the role of DA transmission in the context of emotional and/or motivational processes. For example, given the increasing evidence implicating DA transmission in both appetitive and aversive emotional stimuli, it is conceivable that dysregulated DA transmission acting on emotional processing circuits that are responsible for positive and negative affective valences could ultimately be responsible for disturbances in inappropriate “reinforcement” of maladaptive conditioned emotional associations or for the flat affect and lack of motivational drive characteristic of the negative symptoms. This could be conceptually consistent with the notion that DA transmission, rather than serving as a specific emotional valence signal, serves to “tag” the emotional salience of incoming sensory information, regardless of valence. In addition, psychosis-like phenomena can be induced by drugs that do not directly interact with DA receptors. For example, phencyclidine, which interacts with glutamatergic signaling pathways, is well established as a psychotomimetic agent and, similarly to DA activating drugs, can exacerbate psychotic relapse90,91 Further, anticholinergics and/or drugs that interact with the cannabinoid CB1 receptor system, both induce schizophrenia-like psychotic symptoms.34,92 Nevertheless, all these neurotransmitter systems functionally interact with DAergic substrates, particularly during the generation of psychosis-like symptoms.34,93 Thus, a critical challenge for neuropharmacologists and behavioral neuroscientists is elucidating the relative roles of these disparate neurochemical pathways and their functional interactions with DA systems, which still represent the most critical clinical target of all effective antipsychotic treatments.24 Understanding how the neurochemical pathways responsible for emotional processing interact with one another at both the molecular and neuroanatomical levels will hopefully lead to the development of pharmacotherapeutic compounds that can target multiple receptor substrates and/or modulate functional interactions between these emotional learning and memory encoding circuits. Indeed, identifying pharmacological targets that can modulate or normalize aberrant emotional processing and encoding may have tremendous treatment potential for patients with schizophrenia.
Nevertheless, the question remains: do disturbances in DAergic regulation of cortical-subcortical emotional processing circuits represent a final common pathway for the spectrum of symptoms observed in schizophrenia? The potential answer to this question will largely depend upon our criteria for a final common pathway. In this sense, such a system would likely display “plasticity” in the sense that the psychotic and other manifestations of schizophrenia are not static but emerge, regress, and reemerge in a labile manner. Certainly, as the evidence discussed above has suggested, DAergic modulation of emotional and motivational processing meets this plasticity criteria. Second, such a system would likely be vulnerable to neurodevelopmental insults that could in turn lead to the later manifestation of schizophrenia, consistent with the typical onset of schizophrenic psychopathology in late adolescence and early adulthood. As noted previously, the DAergic system is highly vulnerable to both pre- and perinatal disturbances that can lead to adult-onset phenotypes similar to schizophrenic symptoms. Third, disturbances in such a system would likely be capable of inducing both positive- and negative-like manifestations of schizophrenia. Given the emerging conceptualization of DAergic signaling as an emotional salience signal irrespective of valence, it is conceivable that disturbances in DAergic regulation of cortical-subcortical emotional learning circuits may underlie aberrant reinforcement of incoming sensory information, leading to distorted emotional learning and memory formation. This process may ultimately result in the inability to perceive, respond adaptively, or understand the emotional significance of either the internal or external sensory environment. The ongoing convergence of neuroscience research in both the animal and human domains will hopefully elucidate more clearly the significance of cortical-subcortical interactions in the context of DAergic regulation of emotional processing as the search for a final common neurobiological pathway for schizophrenia-related pathology continues.
References
- 1.Williamson PC. Mind, Brain, and Schizophrenia. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 2.Berman I. Differential relationships between positive and negative symptoms and neuropsychological deficits in schizophrenia. Schizophr Res. 1997;25:1–10. doi: 10.1016/S0920-9964(96)00098-9. [DOI] [PubMed] [Google Scholar]
- 3.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 4.Missale C. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 5.Wise RA. Interactions between medial prefrontal cortex and meso-limbic components of brain reward circuitry. Prog Brain Res. 2000;126:255–262. doi: 10.1016/S0079-6123(00)26018-4. [DOI] [PubMed] [Google Scholar]
- 6.Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 7.Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 8.Wise RA. Dopamine and food reward: back to the elements. Am J Physiol Regul Integr Comp Physiol. 2004;286:R13. doi: 10.1152/ajpregu.00590.2003. [DOI] [PubMed] [Google Scholar]
- 9.Laviolette SR. Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behav Brain Res. 2002;129:17–29. doi: 10.1016/s0166-4328(01)00327-8. [DOI] [PubMed] [Google Scholar]
- 10.Laviolette SR. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- 11.Grace AA. The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 1995;37:111–129. doi: 10.1016/0376-8716(94)01066-t. [DOI] [PubMed] [Google Scholar]
- 12.Phelps EA. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Adolphs R. Cortical systems for the recognition of emotion in facial expressions. J Neurosci. 1996;16:7678–7687. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ljungberg T. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- 15.Becerra L. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- 16.Volkow ND. Drug addiction: the neurobiology of behavior gone awry. Nat Rev Neurosci. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein RZ. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang GJ, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- 19.Hyland BI. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- 20.Miller JD. Mesencephalic dopaminergic unit activity in the behaviorally conditioned rat. Life Sci. 1981;29:110–121. doi: 10.1016/0024-3205(81)90231-9. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen K. Single unit response of noradrenergic, serotonergic and dopaminergic neurons in freely moving cats to simple sensory stimuli. Brain Res. 1986;369:336–340. doi: 10.1016/0006-8993(86)90546-9. [DOI] [PubMed] [Google Scholar]
- 22.Le Moal M. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 23.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 24.Kapur S. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse. 1987;1:133–152. doi: 10.1002/syn.890010203. [DOI] [PubMed] [Google Scholar]
- 26.Snyder SH. The dopamine hypothesis of schizophrenia: focus on the dopamine receptor. Am J Psychiatry. 1976;133:197–202. doi: 10.1176/ajp.133.2.197. [DOI] [PubMed] [Google Scholar]
- 27.Breier A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laruelle M, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angrist BM. The phenomenology of experimentally induced amphetamine psychosis-preliminary observation. Biol Psychiatry. 1970;2:95–107. [PubMed] [Google Scholar]
- 30.Angrist BM. Amphetamine psychosis: behavioral and biochemical aspects. J Psychiatr Res. 1974;11:13–23. doi: 10.1016/0022-3956(74)90064-8. [DOI] [PubMed] [Google Scholar]
- 31.Angrist B. Responses to apomorphine, amphetamine, and neuroleptics in schizophrenic subjects. Psychopharmacology. 1980;67:31–88. doi: 10.1007/BF00427592. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman JA. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology. 1987;91:415–433. doi: 10.1007/BF00216006. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman JA. Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology. 1997;17:205–229. doi: 10.1016/S0893-133X(97)00045-6. [DOI] [PubMed] [Google Scholar]
- 34.Laviolette SR. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci. 2006;63:1597–1613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams LM, et al. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 2001;14:1070–1079. doi: 10.1006/nimg.2001.0904. [DOI] [PubMed] [Google Scholar]
- 36.Ledoux JM. Emotion: clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- 37.Baxter MG. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 38.Aggleton JP. The Amygdala: A Functional Analysis. 2nd ed. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 39.Feenstra MG. Dopamine and noradrenaline release in the prefrontal cortex in relation to unconditioned and conditioned stress and reward. Prog Brain Res. 2000;126:133–163. doi: 10.1016/S0079-6123(00)26012-3. [DOI] [PubMed] [Google Scholar]
- 40.Feenstra MG. Rapid sampling of extracellular dopamine in the rat prefrontal cortex during food consumption, handling and exposure to novelty. Brain Res. 1996;274:17–24. doi: 10.1016/s0006-8993(96)00945-6. [DOI] [PubMed] [Google Scholar]
- 41.Williams LM, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry. 2004;161:480–489. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- 42.Paradiso S, et al. Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. Am J Psychiatry. 2003;160:1775–1783. doi: 10.1176/appi.ajp.160.10.1775. [DOI] [PubMed] [Google Scholar]
- 43.Sabri O. Correlation of positive symptoms exclusively to hyperfusion or hypoperfusion of cerebral cortex in never-treated schizophrenics. Lancet. 1997;349:1735–1739. doi: 10.1016/S0140-6736(96)08380-8. [DOI] [PubMed] [Google Scholar]
- 44.Silbersweig DA, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- 45.Dolan RF. Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature. 1995;378:180–182. doi: 10.1038/378180a0. [DOI] [PubMed] [Google Scholar]
- 46.Egan MF, et al. Effect of COMT Val108-158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castner SA. Amphetamine sensitization of hallucinatory-like behaviors is dependent on prefrontal cortex in nonhuman primates. Biol Psychiatry. 2003;54:105–110. doi: 10.1016/s0006-3223(03)00292-0. [DOI] [PubMed] [Google Scholar]
- 48.Lennox BR. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res. 2000;100:13–20. doi: 10.1016/s0925-4927(00)00068-8. [DOI] [PubMed] [Google Scholar]
- 49.McGuire PK. Increased blood flow in Brocas area during auditory hallucinations in schizophrenia. Lancet. 1993;342:703–706. doi: 10.1016/0140-6736(93)91707-s. [DOI] [PubMed] [Google Scholar]
- 50.Shergill SS. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- 51.Laviolette SR. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning through burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laviolette SR. Cannabinoids dramatically potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala. J Neurosci. 2006;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenkranz JA. Dopamine-mediated modulation of odor-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- 54.Rosenkranz JA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milad MR. Neurons in the medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 56.Milad MR. Electrical stimulation of the medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 57.Garcia R. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- 58.Rosenkranz JA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pezze MA. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Simon H. Efferents and afferents of the ventral tegmental-A10 region studied after local injection of [3H]leucine and horseradish peroxidase. Brain Res. 1991;178:17–40. doi: 10.1016/0006-8993(79)90085-4. [DOI] [PubMed] [Google Scholar]
- 61.West AR. Frontal subcortical circuits in psychiatric and neurological disorders. In: Lichter DG, editor. New York: Guilford Publications; 2001. 372–400. [Google Scholar]
- 62.Sesack SR. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens and on dopamine neurons of the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- 63.Nader K. The dopaminergic modulation of fear: quinpirole impairs the recall of emotional memories in rats. Behav Neurosci. 1999;113:152–165. doi: 10.1037//0735-7044.113.1.152. [DOI] [PubMed] [Google Scholar]
- 64.Bissiere S. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- 65.Shah AA. Selective antagonism of medial prefrontal D4 receptors decreases fear-related behaviors in rats. Eur J Neurosci. 2004;19:3392–3397. doi: 10.1111/j.0953-816X.2004.03447.x. [DOI] [PubMed] [Google Scholar]
- 66.Goldsmith SK. Disrupted pattern of D2 dopamine receptors in the temporal lobe in schizophrenia. A postmortem study. Arch Gen Psychiatry. 1997;54:649–658. doi: 10.1001/archpsyc.1997.01830190077008. [DOI] [PubMed] [Google Scholar]
- 67.Morrison R. Deficits in facial affect recognition and schizophrenia. Schizophr Bull. 1998;14:67–83. doi: 10.1093/schbul/14.1.67. [DOI] [PubMed] [Google Scholar]
- 68.Edwards J. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr Res. 2001;48:235–253. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- 69.Mandal MK. Facial expressions of emotions and schizophrenia: a review. Schizophr Bull. 1998;24:399–412. doi: 10.1093/oxfordjournals.schbul.a033335. [DOI] [PubMed] [Google Scholar]
- 70.Reynolds GP. Increased concentrations and lateral asymmetry of amygdala dopamine in schizophrenia. Nature. 1983;305:527–529. doi: 10.1038/305527a0. [DOI] [PubMed] [Google Scholar]
- 71.Talvick M. Decreased thalamic D2/D3 receptor binding in drug-naive patients with schizophrenia: a PET study with [11C]FLB 457. Int J Neuropsychopharmacol. 2003;6:361–370. doi: 10.1017/S1461145703003699. [DOI] [PubMed] [Google Scholar]
- 72.Talvik M, et al. Dopamine D2 receptor binding in drug-naive patients with schizophrenia examined with raclopride-C11 and positron emission tomography. Psychiatry Res. 2006;148:165–173. doi: 10.1016/j.pscychresns.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Floresco SB. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. J Neurosci. 2003;23:3930–3943. doi: 10.1523/JNEUROSCI.23-09-03930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Griffith JD. Paranoid episodes induced by drug. JAMA. 1968;205:39. [Google Scholar]
- 75.Janowsky DS. Methylphenidate, dextroamphetamine and levamphetamine. Effects on schizophrenia symptoms. Arch Gen Psychiatry. 1976;33:304–308. doi: 10.1001/archpsyc.1976.01770030024003. [DOI] [PubMed] [Google Scholar]
- 76.Kroner S. Dopamine modulates excitability of basolateral amygdala neurons in vitro. J Neurophysiol. 2005;93:1598–1610. doi: 10.1152/jn.00843.2004. [DOI] [PubMed] [Google Scholar]
- 77.Berridge KC. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 78.Robinson TE. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 79.Robinson TE. The psychology and neurobiology of addiciton: an incentive-sensitization view. Addiction. 2000;95(suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 80.Laviolette SR. Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci. 2004;7:160–169. doi: 10.1038/nn1182. [DOI] [PubMed] [Google Scholar]
- 81.Lipska BK. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 82.O'donnell P. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- 83.Rajakumar N. Altered neurotrophin receptor function in the developing prefrontal cortex leads to adult-onset dopaminergic hyperresponsivity and impaired prepulse inhibition of acoustic startle. Biol Psychiatry. 2004;55:797–803. doi: 10.1016/j.biopsych.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 84.Lavin A. Prenatal disruption of neocortical development alters prefrontal cortical neuron responses to dopamine in adult rats. Neuropsychopharmacology. 2005;30:1426–1435. doi: 10.1038/sj.npp.1300696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suzuki T. Enhancement of delayed release of dopamine in the amygdala induced by conditioned fear stress in methamphetamine-sensitized rats. Eur J Pharmacol. 2002;435:59–65. doi: 10.1016/s0014-2999(01)01563-1. [DOI] [PubMed] [Google Scholar]
- 86.Fletcher PJ. A sensitizing regimen of amphetamine impairs visual attention in the 5-choice serial reaction time test: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Neuropsychopharmacology. 2006;32:1122–1132. doi: 10.1038/sj.npp.1301221. [DOI] [PubMed] [Google Scholar]
- 87.Paradiso S, et al. Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. Am J Psychiatry. 2003;160:1775–1783. doi: 10.1176/appi.ajp.160.10.1775. [DOI] [PubMed] [Google Scholar]
- 88.Fahim C, et al. Brain activity during emotionally negative pictures in schizophrenia with and without flat affect: an fMRI study. Psychiatry Res. 2005;140:1–15. doi: 10.1016/j.pscychresns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi H. The role of extrastriatal dopamine D2 receptors in schizophrenia. Biol Psychiatry. 2006;59:919–928. doi: 10.1016/j.biopsych.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 90.Javitt DC. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 91.Luby ED. Model psychoses and schizophrenia. Am J Psychiatry. 1962;119:61–67. doi: 10.1176/ajp.119.1.61. [DOI] [PubMed] [Google Scholar]
- 92.Yeomans JS. Role of tegmental cholinergic neurons in dopaminergic activation, antimuscarinic psychosis and schizophrenia. Neuropsychopharmacology. 1995;12:3–16. doi: 10.1038/sj.npp.1380235. [DOI] [PubMed] [Google Scholar]
- 93.Jentsch JD. Dysregulation of mesoprefrontal dopamine neurons induced by acute and repeated phencyclidine administration in the nonhuman primate: implications for schizophrenia. Adv Pharmacol. 1998;42:810–824. doi: 10.1016/s1054-3589(08)60870-4. [DOI] [PubMed] [Google Scholar]