Abstract
Background
When advanced, heart failure (HF) with preserved ejection fraction (HFpEF) is readily apparent. However, diagnosis of earlier disease may be challenging, as exertional dyspnea is not specific for HF, and biomarkers and hemodynamic indicators of volume overload may be absent at rest.
Methods and Results
Patients with exertional dyspnea and EF>50% were referred for hemodynamic catheterization. Those with no significant coronary disease, normal BNP, and normal resting hemodynamics (mean pulmonary artery (PA) pressure<25 mmHg & PA wedge (PCWP) pressure <15 mmHg; n=55) underwent exercise study. The exercise PCWP was used to classify patients as having HFpEF (PCWP≥25 mmHg; n=32) or non-cardiac dyspnea (NCD, PCWP<25 mmHg; n=23). At rest, HFpEF patients displayed higher resting PA pressures and PCWP, though all values fell within normal limits. Exercise-induced elevation in PCWP in HFpEF was confirmed by greater increases in left ventricular end-diastolic pressure, and was associated with blunted increases in heart rate, systemic vasodilation and cardiac output. Exercise-induced pulmonary hypertension was present in 88% of HFpEF patients and was related principally to elevated PCWP, as pulmonary vascular resistances dropped similarly in both groups. Exercise PCWP and PASP were highly correlated. An exercise PASP≥45mmHg identified HFpEF with 96% sensitivity and 95% specificity.
Conclusions
Euvolemic patients with exertional dyspnea, normal BNP and normal cardiac filling pressures at rest may have markedly abnormal hemodynamic responses during exercise, suggesting that chronic symptoms are related to heart failure. Earlier and more accurate diagnosis using exercise hemodynamics may allow better targeting of interventions to treat and prevent HFpEF progression.
Keywords: heart failure, exercise, hemodynamics, diastole, diagnosis
INTRODUCTION
Approximately half of heart failure (HF) patients have preserved ejection fraction (HFpEF)1–3. The natural history of HFpEF is not comprehensively defined, as most previous studies have focused on progression of disease after an index event, typically hospitalization for acutely decompensated HF with volume overload1–4. Many patients with decompensated incident HFpEF relate a prior history of chronic exertional dyspnea or exercise intolerance4, yet this earlier phase of HFpEF remains poorly characterized. Symptoms of exertional dyspnea and intolerance are highly sensitive for HF5, but they are also nonspecific and widely prevalent, particularly in the elderly where a number of conditions other than HF may cause or contribute to impaired functional capacity6–9.
Diagnosis of HFpEF based upon current guidelines requires objective evidence of elevated filling pressures, either at catheterization, by echocardiography, or brain natriuretic peptide (BNP) assays10. The potential for over-diagnosis of HFpEF, particularly when relying on surrogate markers of elevated filling pressures, has been appropriately emphasized by a number of investigators6–11. However, less is known regarding the potential for under-diagnosis of HFpEF in patients with lifestyle limiting symptoms, but no clinical evidence of hypervolemia.
We hypothesized that patients with HFpEF may present in a milder or “early” phase of disease characterized by exertional symptoms in the absence of volume overload. This group may fail to meet current diagnostic criteria based upon resting hemodynamics alone, developing hemodynamic derangements characteristic of HF only during the stress of exercise. To test this hypothesis, we studied consecutive patients referred to the cardiac catheterization laboratory for diagnostic evaluation of unexplained exertional dyspnea who had normal BNP levels and normal resting hemodynamics. In such patients, we examined the hemodynamic responses to exercise to determine if a subset of these patients displayed hemodynamic evidence of HF during exercise.
METHODS
Study Population
We retrospectively examined consecutive patients referred to the Mayo Clinic cardiac catheterization laboratory between 8/05 and 8/09 for right heart catheterization (with or without left heart catheterization) for the clinical assessment of exertional dyspnea and/or fatigue. The study population included 55 consecutive patients with normal EF (≥50%) and normal resting hemodynamics (defined below). Patients with elevated BNP or NT-proBNP (>200 pg/ml or >220 pg/ml10), significant coronary artery disease (stenosis≥50%), valvular heart disease (any stenosis, >mild regurgitation), hypertrophic or infiltrative cardiomyopathy, constrictive pericarditis, exercise-induced pulmonary hypertension due to vascular disease (mean exercise pulmonary artery pressure>30mmHg with pulmonary capillary wedge<15mmHg12) or radiographic pulmonary congestion were excluded. The study was approved by the Mayo Clinic Institutional Review Board. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Catheterization Protocol
Patients were studied on chronic medications in the fasted state after minimal sedation in the supine position. Standard right heart catheterization was performed through a 7–9 Fr sheath via the internal jugular or femoral vein. Left heart catheterization was performed via the radial or femoral artery (6 Fr). High fidelity right and left-sided pressure measurements were obtained in most patients using 2 Fr micromanometer-tipped catheters (Millar Instruments, Houston, TX) advanced through the lumen of the corresponding fluid-filled catheter. Mean micromanometer pressures were calibrated to mean fluid-filled pressures at the beginning and throughout each case. Transducers were zeroed at mid-axilla, measured by calipers in each patient.
Pressures in the right atrium (RA), pulmonary artery (PA), pulmonary capillary wedge (PCWP), and left ventricle at end-diastole (LVEDP) were measured at end-expiration and represent the mean of ≥3 beats. Because intrathoracic pressure swings are enhanced with the increased work of breathing during exercise, exercise pressures are also reported as the mean of inspiration and expiration. PCWP was verified based upon characteristic waveforms, appearance on fluoroscopy and oxygen saturation. LVEDP was determined following the atrial systolic deflection, prior to the onset of isovolumic contraction. Systolic blood pressure (BP) was measured in the LV, or by arm cuff if left heart cath was not performed. Mean brachial BP was obtained from an oscillometric arm cuff sphygmomanometer.
Cardiac output was determined by the Fick method using directly measured saturations and oxygen consumption (MedGraphics, St. Paul, MN) or by thermodilution, and indexed to body surface area (cardiac index, CI). Pulmonary vascular resistance index (PVRI=[mean PA-PCWP]/CI) and total systemic vascular resistance index (SVRI=[mean arterial BP/CI]) were determined. Baseline oximetry run was performed to rule out left to right shunting.
Exercise Protocol
After assessment of resting hemodynamics, patients exercised via supine cycle ergometry or out-stretched arm-adduction lifting 4 lb weights (if femoral access had been obtained). Patients performing leg exercise cycled at 60 rpm starting at 20 Watts (W) workload, increasing by 10W increments in 3 minutes stages to maximum tolerated levels. For arm exercise, repetition frequency was gradually increased to subjective fatigue. PCWP was determined at baseline, prior to exercise with passive leg elevation (if applicable), after 1.5 minutes at low-level (20W) exercise, at peak and at 1 minute recovery (legs still elevated for ergometry). Peak exercise CI was determined by the Fick method (direct oxygen consumption measurements) or thermodilution. Exercise cardiac output was not determined in the majority of patients (11/13) performing arm exercise.
Case Definitions
Prior studies in normal controls have shown that peak PCWP and LVEDP during supine exercise are <20–23mmHg13, 14 and <25mmHg15, 16, respectively. For this analysis, patients with peak exercise PCWP≥25 mmHg were classified as HFpEF and those with values<25mmHg were classified as non-cardiac dyspnea (NCD). Exercise-induced pulmonary hypertension was defined as mean PA>30mmHg12.
Statistical Analysis
Results are reported as mean±SD. Between-group differences were compared by ANOVA or 2. Bivariate (Pearson coefficient) was used to examine correlations between exercise measures. Multivariate linear regression was performed to adjust for group differences in baseline characteristics (age and BMI), heart rate, blood pressure, exercise protocol, peak exercise workload and cardiac output measurement method. Regression analyses assumed that distributions were Gaussian, relationships between dependent and independent variables were linear, and that errors were homoscedastic. These assumptions were tested by visual inspection of the distributions and of the standardized residual plots. Logistic regression with receiver operating characteristic analysis was used to identify clinical testing results associated with invasively-confirmed HFpEF.
RESULTS
Subject Characteristics
The sample population consisted of predominantly middle-aged to elderly hypertensive women with NYHA Class II symptoms (Table 1). HFpEF patients were older and had higher body mass than NCD. Gender, race, medical comorbidities, symptoms, labs and medication use were similar.
Table 1.
Baseline Characteristics
| NCD (n=23) | HFpEF (n=32) | p | |
|---|---|---|---|
| Age (years) | 47±17 | 65±13 | <0.001 |
| Gender (% female) | 65 | 72 | 0.6 |
| Race (% white) | 100 | 91 | 0.3 |
| Body Mass Index (kg/m2) | 27.3±5.5 | 32.0±5.9 | 0.004 |
| Obesity (%) | 40 | 56 | 0.2 |
| Hypertension (%) | 57 | 72 | 0.2 |
| Diabetes (%) | 22 | 16 | 0.6 |
| Atrial fibrillation (%) | 9 | 6 | 0.7 |
| NYHA class II/III | 20/3 | 27/5 | 0.8 |
| GFR (ml/min) | 95±36 | 86±31 | 0.3 |
| BNP (pg/ml) | 49±54 | 71±49 | 0.3 |
| NT-ProBNP (pg/ml) | 38±22 (n=4) | 104±62 (n=7) | 0.07 |
| Hemoglobin (gm/dL) | 13.2±1.5 | 13.6±1.2 | 0.3 |
| β–Blockers (%) | 35 | 44 | 0.5 |
| ACEI or ARB (%) | 30 | 38 | 0.6 |
| Diuretic (%) | 35 | 53 | 0.18 |
Clinical Evaluation prior to Catheterization
HFpEF patients were more likely to display cardiomegaly on chest film, with trends toward more left ventricular hypertrophy and left atrial enlargement (Table 2). Mean tissue-Doppler medial E’ velocities were lower and E/e’ ratios higher in HFpEF compared with NCD, but there was substantial overlap, and only 9% of HFpEF patients displayed elevated E/e’. Echo-estimated pulmonary artery systolic pressures and EF were similar in HFpEF and NCD. Applying contemporary diagnostic guidelines10, 34% of HFpEF and 24% of NCD patients would have been diagnosed as having HFpEF prior to catheterization (p=0.4).
Table 2.
Clinical Evaluation Prior to Hemodynamic Assessment
| NCD (n=23) | HFpEF(n=32) | p | |
|---|---|---|---|
| Radiographic | |||
| Cardiomegaly (%) | 4 | 25 | 0.04 |
| Echocardiographic | |||
| LV Ejection Fraction (%) | 61±6 | 62±7 | 0.4 |
| LV mass (gm/m2) | 84±22 | 92±20 | 0.16 |
| LV Hypertrophy (%) | 17 | 34 | 0.06 |
| LA enlargement (%) | 38 | 65 | 0.06 |
| E (cm/s) | 80±20 | 80±20 | 0.8 |
| A (cm/s) | 60±30 | 80±30 | 0.08 |
| E/A | 1.3±0.5 | 1.1±0.5 | 0.10 |
| Medial E’ (cm/s) | 10±3 | 9±3 | 0.02 |
| E/E’ | 8±3 | 10±3 | 0.04 |
| E/E’>15 (%) | 5 | 9 | 0.5 |
| Estimated PASP (mmHg) | 31±6 | 33±8 | 0.4 |
| PASP>35 mmHg (%) | 28 | 26 | 0.9 |
| ESC HFpEF Dx (%) | 24 | 34 | 0.4 |
Resting Hemodynamics
Resting heart rate, blood pressure and LVEDP were similar in NCD and HFpEF (Table 3). PA pressures and PCWP were higher in HFpEF at rest, though all values fell within normal limits. Pulmonary and systemic vascular resistances were higher in HFpEF compared with NCD, while resting CI was lower.
Table 3.
Resting Hemodynamics
| NCD (n=23) | HFpEF (n=32) | p | |
|---|---|---|---|
| Heart Rate (bpm) | 72±12 | 70±9 | 0.5 |
| Arterial Systolic Pressure (mmHg) | 131±19 | 137±23 | 0.3 |
| Arterial Mean Pressure (mmHg) | 88±12 | 94±14 | 0.4 |
| RA Pressure (mmHg) | 4±2 | 5±2 | 0.04 |
| PA Systolic Pressure (mmHg) | 24±6 | 31±7 | 0.0003 |
| PA Mean Pressure (mmHg) | 15±4 | 19±4 | 0.001 |
| End-Expiration PCWP (mmHg) | 9±3 | 11±2 | 0.002 |
| Average PCWP (mmHg) | 9±3 | 11±2 | 0.003 |
| LV End Diastolic Pressure (mmHg) | 12±3 | 13±2 | 0.13 |
| Cardiac Index (L/min/m2) | 3.2±0.8 | 2.8±0.6 | 0.04 |
| Pulmonary Vascular Resistance Index(WU*m2) | 2.1±1.0 | 3.2±1.5 | 0.006 |
| Systemic Vascular Resistance Index (DSC*m2) | 2300±700 | 2800±600 | 0.02 |
Exercise Hemodynamics
The majority of patients (76%) performed leg exercise (Table 4). Increases in heart rate and cardiac output were lower with arm exercise compared with leg ergometry, though changes in BP and filling pressures were similar (Supplementary table). Importantly, all within- and between-group comparisons of hemodynamic responses with exercise were similar when examining leg or arm exercise responses separately (not shown). Exercise RA pressure was measured in 17/55 patients (11/23 NCD; 6/32 HFpEF), and exercise LVEDP was measured in 25/55 (7/23 NCD; 18/32 HFpEF).
Table 4.
Exercise Hemodynamics
| NCD (n=23) | HFpEF (n=32) | p | |
|---|---|---|---|
| Arm/Leg Exercise | 3/20 | 10/22 | 0.11 |
| Peak Leg Ergometry Workload (Watts) | 64±36 | 47±19 | 0.06 |
| Heart Rate (bpm) | 122±24 | 104±21 | 0.004 |
| Arterial Systolic Pressure (mmHg) | 153±26 | 182±34 | 0.002 |
| Arterial Mean Pressure (mmHg) | 101±15 | 125±20 | 0.0001 |
| RA Pressure (mmHg; n=11/6) | 6±3 | 14±4 | 0.0004 |
| PA Systolic Pressure (mmHg) | 35±7 | 59±11 | <0.00001 |
| PA Mean Pressure (mmHg) | 23±5 | 43±7 | <0.00001 |
| End-Expiration PCWP (mmHg) | 13±5 | 32±6 | <0.00001 |
| Average PCWP (mmHg) | 11±5 | 28±7 | <0.00001 |
| LV End Diastolic Pressure (mmHg) | 14±4 | 34±6 | <0.00001 |
| Cardiac Index (L/min/m2) | 6.7±1.4 | 4.9±1.0 | <0.0001 |
| PVRI (WU*m2) | 1.9±0.9 | 2.4±1.2 | 0.17 |
| Exercise Induced Pulmonary HTN (%) | - | 88 | - |
| SVRI (DSC*m2) | 1300±400 | 1900±400 | 0.0007 |
p<0.0001 for all paired changes (within groups) compared with rest
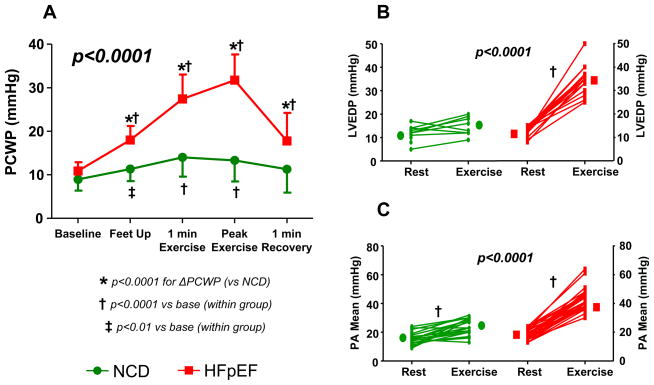
The changes in PCWP by group throughout exercise study are shown in Figure 1A. Passive elevation of the legs (prior to cycle exercise) was associated with an increase in PCWP in both groups, but the increase was much more prominent in HFpEF compared with NCD (+7±3 vs +2±3mmHg, p<0.0001; Figure 1A). By case definition, the increase in PCWP during peak exercise was greater in HFpEF than NCD (Figure 1A, Table 4). Intriguingly, most of the change in PCWP occurred within the first 1.5 minutes of exercise, at low-level (20W) workload (+5±3mmHg in NCD, 89% of peak; +16±6mmHg in HFpEF, 80% of peak; p<0.0001). Higher left heart filling pressures were confirmed by greater exercise increases in LVEDP in HFpEF (Figure 1B, Table 4). Each of these differences remained significant after adjusting for age and BMI. In the immediate recovery phase (1 minute post exercise), PCWP returned to baseline leg up (leg exercise) or supine rest (arm) values in both groups (Figure 1A). PCWP averaged over the respiratory cycle tended to be lower compared with end-expiration values (Table 4), but this difference was not significant (p=0.2) and the two values were highly correlated (R2=0.89, p<0.0001). PCWP averaged over the respiratory cycle was≥20mmHg in all HFpEF patients and 18mmHg in 22/23 NCD patients.
Figure 1.
[A] Pulmonary capillary wedge pressure (PCWP) increased to a greater extent in HFpEF (red) compared with non-cardiac dyspnea (NCD, green) with leg elevation and during exercise. PCWP returned to baseline almost immediately in recovery. [B] Left ventricular end-diastolic (LVEDP) and [C] mean pulmonary artery (PA) pressures also rose with exercise more dramatically in HFpEF (p values for exercise change between groups).
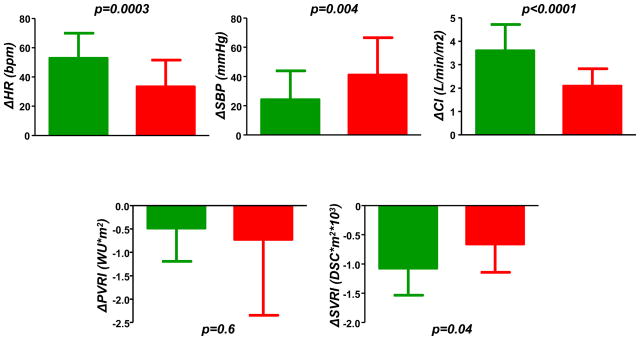
Similar to PCWP, increases in PA pressures were markedly greater in HFpEF (Table 4, Figure 1C), with 88% of patients meeting criteria for exercise-induced PH12. This was related exclusively to pulmonary venous hypertension, as PVRI dropped similarly in HFpEF and NCD (Figure 2). Exercise-induced augmentation in heart rate and cardiac index were blunted in HFpEF compared with NCD, while HFpEF patients displayed a greater hypertensive response and less systemic vasodilation (Figure 2). Exercise changes and peak exercise PCWP, LVEDP and PA pressures all remained significantly higher in HFpEF after adjusting for age, BMI, heart rate, blood pressure, exercise type, peak workload and method of cardiac output determination (all p 0.0002), while cardiac output response remained lower (p=0.02). Heart rate responses were no longer different in HFpEF and NCD after adjusting for age, although prior studies have confirmed chronotropic incompetence in HFpEF even compared to age matched controls17–20.
Figure 2.
Increases in heart rate (ΔHR), cardiac index (ΔCI) and systemic arterial vasodilation (ΔSVRI) with exercise were impaired in HFpEF (red) compared with non-cardiac dyspnea (NCD, green). Systolic blood pressure (ΔSBP) increased to greater extent in HFpEF, while pulmonary vasodilation (ΔPVRI) was similar.
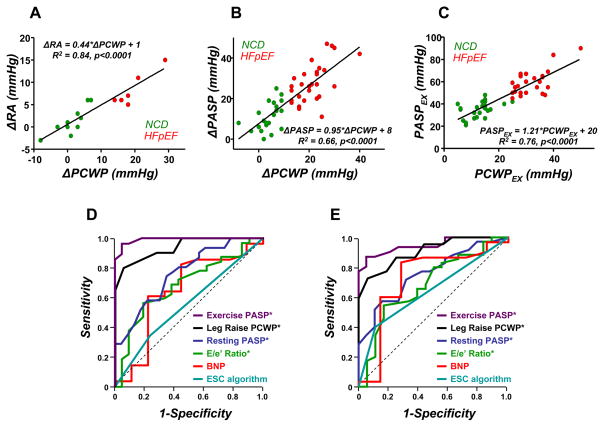
Exercise change in PCWP ( PCWP) was highly correlated with change in RA pressure (Figure 3A), with an intercept near zero and slope of 0.44 (R2=0.84, p<0.00001, Figure 3A). Peak exercise PCWP and PASP were also highly correlated, as were exercise changes in each pressure (Figure 3B-C). Receiver operating characteristic (ROC) analysis showed that none of the clinical, radiographic or echocardiographic parameters identified in Table 2 adequately identified catheterization-verified HFpEF (all ROC areas under the curve, AUC<0.70, Figure 3D). In contrast, leg elevation PCWP and exercise PASP was highly predictive of HFpEF, with AUC 0.94 and 0.99, respectively. An exercise PASP≥45mmHg identified HFpEF with 96% sensitivity and 95% specificity.
Figure 3.
[A,B] Exercise changes in PCWP (ΔPCWP) were highly correlated with the corresponding changes in right atrial pressure (ΔRA) and pulmonary artery systolic pressure (ΔPASP). [C] Peak exercise PCWP (PCWPEX) and PASP (PASPEX) were strongly associated. [D] Clinical measures (BNP, E/e’) and diagnostic algorithms (ESC) did not robustly distinguish HFpEF from NCD. In contrast, PCWP with leg raise and exercise PASP showed excellent discrimination between HFpEF and NCD. Panel [E] shows receiver-operating characteristic curves using PCWP>15mmHg to define HFpEF. See text for details.
Sensitivity Analysis
Because the partition values for exercise PCWP which define “abnormality” are not well-established, we repeated analyses defining HFpEF by exercise PCWP>15mmHg, >18mmHg and >20mmHg. Each of these analyses produced similar results, with all differences in resting and exercise hemodynamic responses depicted in Figures 1-2 and Tables 3–4 persisting (not shown). Four patients would be reclassified as having HFpEF using the most liberal partition value (15mmHg). ROC analysis using PCWP>15mmHg to define HFpEF shows similar results as PCWP≥25mmHg (Figure 3), where leg-elevation PCWP and exercise PASP are most predictive of HFpEF.
DISCUSSION
This study examined hemodynamic responses to exercise, measured invasively in consecutive patients with preserved EF referred for catheterization to evaluate the cause of exertional dyspnea. Of 55 patients with normal BNP and normal resting pressures, 58% displayed an abnormal increase in left heart filling pressures with exercise consistent with HFpEF. This was coupled with additional hemodynamic derangements characteristic of heart failure, including secondary pulmonary hypertension and impairments in heart rate, vasodilation, and cardiac output reserve. Noninvasive diagnostic testing including radiography, BNP and echocardiography did not distinguish HFpEF from non-cardiac dyspnea. Invasively-measured exercise pulmonary artery pressures were highly correlated with left heart pressures, suggesting the potential for use of Doppler estimated exercise pulmonary artery pressures in noninvasive screening. These findings provide support for the hypothesis that an earlier or milder stage of HFpEF characterized by normal resting but abnormal exercise hemodynamics exists. These data also suggest the utility of hemodynamic exercise testing to identify this population of patients with less advanced HFpEF. Further study is warranted to describe the prevalence and natural history of this early stage of disease, determine sensitive and specific but simple approaches to diagnosis, and explore whether interventions in this population may prevent progression to more advanced stages of HFpEF.
Clinical Diagnosis of HFpEF
Exertional dyspnea and fatigue are hallmark symptoms of HF5, 10, 21, but they are also reported in a variety of non-cardiac conditions, including obesity, pulmonary disease, anemia, deconditioning, and pulmonary vascular disease6, 8, 11, 22, 23. Symptoms of exercise intolerance may even be regarded as inevitable byproducts of “normal aging”, particularly if there is no clear evidence for LV dysfunction or pulmonary disease. While population-based studies have reported that approximately half of HF patients in the community have preserved EF1–3, it has been questioned whether many of these patients truly suffer from HF7, 9. Plausible alternative sources of dyspnea may be present, such as spirometric abnormalities or obesity6, though cardiac and pulmonary disease may coexist within a given patient24, and obesity is a commonly-observed comorbidity and risk factor in HFpEF1–3, 23.
A recent study found that while patients with a clinical diagnosis of HFpEF had severe subjective and objective exercise intolerance, mean NT-proBNP levels were similar to healthy controls—leading the authors to question the diagnosis of HF11. However, natriuretic peptide levels more accurately reflect wall stress rather than filling pressures, and both wall stress and BNP levels are known to be lower in HFpEF compared to HF with reduced EF25. Echo-Doppler/tissue-Doppler measures such as E/e’ have been shown to serve as reasonable noninvasive measures of LV filling pressures and diastolic dysfunction26. Indeed, the E/e’ ratio serves as a key decision point in a recent diagnostic algorithm proposed for HFpEF10. Similarly, Tschope and colleagues found that NT-proBNP levels accurately identify the presence of diastolic dysfunction in patients with normal EF27. However, in each of these studies, measurements were performed only at rest, and if filling pressures (and hence wall stress) are only intermittently elevated (e.g. during exercise), it follows that these noninvasive markers of congestion may appear normal or near-normal when measured in the clinic.
This study indicates that currently utilized diagnostic criteria may fail to accurately distinguish euvolemic patients with less advanced HFpEF from those with non-cardiac dyspnea. Identification of HFpEF should not rely upon a “diagnosis of exclusion”, as recently suggested9, but should rather be based upon positive identification of objective measurable criteria. Our data suggests that exercise hemodynamics may provide such criteria. Exercise testing is not likely to be needed in congested patients where the diagnosis of HFpEF is obvious, but it may play an important role in identifying patients with intermediate probability—serving as an extension rather than a replacement to current guidelines10. This will be important for both clinical care and to more accurately identify patients to enroll in future clinical trials. Indeed, one of the concerns raised regarding the negative results in prior trials in HFpEF has been that some of the subjects enrolled may not have truly had HF9, 23.
“Early” HFpEF
The ACC/AHA HF staging system complements the NYHA functional class designation by emphasizing the progressive nature of HF from risk (Stage A and B), to overt symptoms (Stage C), to pre-terminal (Stage D) disease21. The current data suggest that HFpEF patients may present with overt (Stage C) but primarily exertional (NYHA class II-III) symptoms without clinical volume overload at rest. This group is not well-characterized, as prior studies have focussed largely on patients hospitalized for HFpEF1–4. Our patients predominantly complained of NYHA class II symptoms and had not been formally diagnosed with HF. Intriguingly, commonly observed phenotypic markers of HFpEF, such as diastolic dysfunction, left atrial enlargement, LV hypertrophy and atrial fibrillation were present, but were not as severely “abnormal” as in previous studies examining more advanced HFpEF28–30. The patients in the current study also displayed a number of impairments in cardiovascular reserve function with exercise that are increasingly recognized in patients with advanced HFpEF17–20, 31–33. Collectively these observations are consistent with the notion that this represents an “early” form of disease. The natural history of early HFpEF remains unclear; but we speculate that it may represent an important group in which interventions may be targeted with higher yield to prevent or delay the transition to advanced HFpEF, where changes in the material properties of the ventricle and vasculature may be irreversible.
Mechanisms of Exercise-Induced Pulmonary Venous Hypertension
Diastolic dysfunction is considered a cornerstone in the pathophysiology of HFpEF28, 30, 34, and the observed increases in PCWP with exertion in the current study are likely related in large part to abnormalities in diastolic reserve. Kitzman and colleagues first showed that normal exercise increases in LV preload volume are blunted in HFpEF, despite marked increases in PCWP31. Similar to the current study, Kitzman et al. also studied compensated outpatients and found that resting PCWP was not elevated—despite marked elevations during stress. This emphasizes that congestion may only be an intermittent phenomenon in HFpEF. Other groups have recently corroborated these findings, showing exaggerated increases in LV diastolic pressures during handgrip exercise34. In the current study, passive elevation of the legs prior to cycle ergometry (which increases venous return16) was associated with a larger increase in PCWP in HFpEF compared to NCD. This is consistent with an inability to utilize the Frank-Starling mechanism: the increase in venous return with passive leg raise could not be accommodated without raising filling pressures. Disparities in PCWP became even more pronounced during low-level and peak exercise. Pressures promptly returned towards baseline within 1 minute of recovery, emphasizing that these patients were not hypervolemic per se, but that increases in venous return acutely raised ventricular and atrial pressures which were transmitted to the pulmonary venous bed during exercise.
Increases in blood pressure were greater and reductions in vascular resistance lower in HFpEF, and because high afterload impairs diastolic function35, these disparities in vasorelaxation may have contributed to the observed increases in PCWP in HFpEF. Because LV relaxation kinetics and end-diastolic pressure-volume relationships were not measured, we cannot discern to what extent changes in diastolic relaxation, compliance or extrinsic forces might have contributed. Dauterman and colleagues showed that approximately 40% of measured LV diastolic pressure is related to forces external to the left ventricle, mediated by right heart-left heart interaction and pericardial restraint36. We found that RA pressure rose in tandem with PCWP during exercise in HFpEF and NCD, and intriguingly the slope of this relationship was remarkably close to the 40% external contribution reported by Dauterman et al36 and others37.
Pulmonary Hypertension and HFpEF
It has increasingly become appreciated that pulmonary hypertension (PH) is a common finding in HFpEF38, 39. Pulmonary pressures increase with aging and vascular stiffening40, processes strongly implicated in the pathogenesis of HFpEF23, 35. Tolle and colleagues recently reported that ~50% of patients referred for invasive exercise hemodynamic testing display exercise-induced PH due to pulmonary venous hypertension12. Elderly patients with PH are more likely to display elevated PCWP39, and 83% of HFpEF patients have PH38. The presence of PH predicts increased mortality in HFpEF, and elevated PASP has been shown to be a more powerful predictor of HFpEF than other noninvasive measures of diastolic dysfunction38. The current results, coupled with these previous studies, reinforce the notion that PH noted at rest or with exercise may be more of an indication of elevated left heart pressures than pulmonary vascular disease in many patients, particularly among the elderly39.
The current results suggest a potential role for exercise PASP to noninvasively screen for HFpEF in patients with exertional dyspnea. In this study, commonly used radiographic, laboratory and echocardiographic variables did not distinguish HFpEF from NCD (all AUC<0.70). Resting PASP at catheterization was slightly more robust (AUC 0.75), but exercise PASP showed superior discriminative ability (AUC 0.99, p<0.0001). Because exercise PH may be also be due to pulmonary vascular disease, invasive study may still be required, but if echo-Doppler derived PASP is validated during exercise, it may serve as a useful screen in patients with exertional dyspnea of uncertain etiology.
Study Limitations
The study population included patients referred for cardiac catheterization and was not randomly selected, thus there is referral bias that may limit generalizability of the findings. There may also be greater inaccuracy in the assessment of subjective findings such as NYHA class or volume status because of the retrospective nature of the study. The type of exercise performed (arm or leg), the method of cardiac output assessment (Fick or thermodilution) and the measurement of RA and LV pressures with exercise were not uniform in all patients, being based upon catheterization indications, operator preference and vascular access. However, this was a retrospective study, and the intent was simply to examine patients who displayed an abnormal response (exercise PCWP≥25mmHg), regardless of the type of exercise stressor. Importantly, stratified analysis comparing only patients who performed leg or arm exercise separately showed similar differences within and between groups for all hemodynamic responses. As in community-based studies28, the HFpEF group was older-aged and had higher body mass, but PA and PCWP differences with exercise persisted after adjusting age and BMI. As this was supine testing, these results may not apply to upright exercise. Objective exercise effort was not quantified, but prior studies have shown that maximal increases in PA and PCWP occur early on during submaximal supine exercise15, and our data similarly showed that 80–90% of the peak increase in PCWP was apparent during the first stage. Indeed, the observation that abnormalities are detectable at low workload is notable and enhances feasibility for application in laboratories not equipped for time-consuming exercise studies. The study population excluded patients with PH due to pulmonary vascular disease and HFpEF patients with congestion apparent at rest, and the results may not apply to these populations. It is not established what a “normal” exercise PCWP in the supine position is, or whether this differs with age, gender or body size12–16.
Contractile function, which has been shown to be mildly impaired at rest and with exercise in HFpEF18, 41, was not assessed in this study and may have contributed to the observed differences in cardiac output reserve. We chose a more rigid partition value (≥25mmHg at end-expiration, corresponding to ≥20mmHg averaged throughout respiration) to identify HFpEF, but it is very possible that many of the patients with less dramatic increases in PCWP also have or will develop HFpEF. Importantly, each of the hemodynamic differences observed between groups persisted in sensitivity analyses examining different exercise PCWP partition values to define HF. Finally, while all patients developed dyspnea during testing, the onset or severity of dyspnea was not determined or correlated with PCWP elevation, and factors other than pulmonary venous hypertension, such as ergoreflex activation or ventilation abnormalities may also contribute to dyspnea in this population18, 42.
Conclusions
Symptoms of dyspnea and fatigue with exertion are common in practice and may be due to a wide variety of cardiac and non-cardiac diseases. Despite clinical euvolemia, normal natriuretic peptide levels, echocardiography and normal resting filling pressures, patients may display a number of hemodynamic changes characteristic of HFpEF during exercise stress. Invasive exercise hemodynamic testing may enhance diagnosis of HFpEF in this expanding population of patients with exertional dyspnea of unknown etiology. Future research is required to better define and phenotype the clinical behavior, optimal treatment strategies for and natural history of this “early” form of HFpEF, and future trials may examine whether progression to more “advanced” HFpEF can be delayed or prevented through interventions targeted to this group of patients.
Supplementary Material
Acknowledgments
Sources of Funding
BAB was supported by a grant from the Mayo Clinic Center for Translational Science Activities and the NIH (UL RR024150) and the Marie Ingalls Career Development Award in Cardiovascular Research. MMR is supported by NIH HL 63281, PO1 HL 76611 and UO 1 HL 84907.
Footnotes
Disclosures
None.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 3.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. Jama. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 4.Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, Kalman J, Phillips RA, Steingart R, Brown EJ, Jr, Berkowitz R, Moskowitz R, Soni A, Mancini D, Bijou R, Sehhat K, Varshneya N, Kukin M, Katz SD, Sleeper LA, Le Jemtel TH. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol. 2004;43:1432–1438. doi: 10.1016/j.jacc.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 5.Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ. Assessing diagnosis in heart failure: which features are any use? Qjm. 1997;90:335–339. doi: 10.1093/qjmed/90.5.335. [DOI] [PubMed] [Google Scholar]
- 6.Caruana L, Petrie MC, Davie AP, McMurray JJ. Do patients with suspected heart failure and preserved left ventricular systolic function suffer from "diastolic heart failure" or from misdiagnosis? A prospective descriptive study. BMJ (Clinical research ed) 2000;321:215–218. doi: 10.1136/bmj.321.7255.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee P, Banerjee T, Khand A, Clark AL, Cleland JG. Diastolic heart failure: neglected or misdiagnosed? J Am Coll Cardiol. 2002;39:138–141. doi: 10.1016/s0735-1097(01)01704-1. [DOI] [PubMed] [Google Scholar]
- 8.Azevedo A, Bettencourt P, Pimenta J, Frioes F, Abreu-Lima C, Hense HW, Barros H. Clinical syndrome suggestive of heart failure is frequently attributable to non-cardiac disorders--population-based study. Eur J Heart Fail. 2007;9:391–396. doi: 10.1016/j.ejheart.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Cleland JG, Taylor J, Tendera M. Prognosis in heart failure with a normal ejection fraction. N Engl J Med. 2007;357:829–830. doi: 10.1056/NEJMc076179. [DOI] [PubMed] [Google Scholar]
- 10.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 11.Ingle L, Cleland JG, Clark AL. Perception of symptoms is out of proportion to cardiac pathology in patients with "diastolic heart failure". Heart. 2008;94:748–753. doi: 10.1136/hrt.2007.131144. [DOI] [PubMed] [Google Scholar]
- 12.Tolle JJ, Waxman AB, Van Horn TL, Pappagianopoulos PP, Systrom DM. Exercise-induced pulmonary arterial hypertension. Circulation. 2008;118:2183–2189. doi: 10.1161/CIRCULATIONAHA.108.787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thadani U, Parker JO. Hemodynamics at rest and during supine and sitting bicycle exercise in normal subjects. Am J Cardiol. 1978;41:52–59. doi: 10.1016/0002-9149(78)90131-5. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida A, Kadota K, Kambara H, Tamaki S, Suzuki Y, Kawai C, Tamaki N, Torizuka K. Left ventricular responses to supine bicycle exercise assessed by radionuclide angiocardiography and a Swan-Ganz catheter. Jpn Circ J. 1985;49:661–671. doi: 10.1253/jcj.49.661. [DOI] [PubMed] [Google Scholar]
- 15.Parker JO, Thadani U. Cardiac performance at rest and during exercise in normal subjects. Bulletin europeen de physiopathologie respiratoire. 1979;15:935–949. [PubMed] [Google Scholar]
- 16.McCallister BD, Yipintsoi T, Hallermann FJ, Wallace RB, Frye RL. Left ventricular performance during mild supine leg exercise in coronary artery disease. Circulation. 1968;37:922–931. doi: 10.1161/01.cir.37.6.922. [DOI] [PubMed] [Google Scholar]
- 17.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 18.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global Cardiovascular Reserve Dysfunction in Heart Failure with Preserved Ejection Fraction. J Am Coll Card. 2010 doi: 10.1016/j.jacc.2010.03.077. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 22.Thomas MD, Fox KF, Wood DA, Gibbs JS, Coats AJ, Henein MY, Poole-Wilson PA, Sutton GC. Echocardiographic features and brain natriuretic peptides in patients presenting with heart failure and preserved systolic function. Heart. 2006;92:603–608. doi: 10.1136/hrt.2005.063768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53:905–918. doi: 10.1016/j.jacc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Rutten FH, Cramer MJ, Grobbee DE, Sachs AP, Kirkels JH, Lammers JW, Hoes AW. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J. 2005;26:1887–1894. doi: 10.1093/eurheartj/ehi291. [DOI] [PubMed] [Google Scholar]
- 25.Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006;47:742–748. doi: 10.1016/j.jacc.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschope C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 27.Tschope C, Kasner M, Westermann D, Gaub R, Poller WC, Schultheiss HP. The role of NT-proBNP in the diagnostics of isolated diastolic dysfunction: correlation with echocardiographic and invasive measurements. Eur Heart J. 2005;26:2277–2284. doi: 10.1093/eurheartj/ehi406. [DOI] [PubMed] [Google Scholar]
- 28.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 30.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 31.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 32.Ennezat PV, Lefetz Y, Marechaux S, Six-Carpentier M, Deklunder G, Montaigne D, Bauchart JJ, Mounier-Vehier C, Jude B, Neviere R, Bauters C, Asseman P, de Groote P, Lejemtel TH. Left ventricular abnormal response during dynamic exercise in patients with heart failure and preserved left ventricular ejection fraction at rest. J Card Fail. 2008;14:475–480. doi: 10.1016/j.cardfail.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M, Sanderson JE. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36–46. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 34.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschope C. Role of Left Ventricular Stiffness in Heart Failure With Normal Ejection Fraction. Circulation. 2008;117:2051–60. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 35.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart failure clinics. 2008;4:23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dauterman K, Pak PH, Maughan WL, Nussbacher A, Arie S, Liu CP, Kass DA. Contribution of external forces to left ventricular diastolic pressure. Implications for the clinical use of the Starling law. Ann Intern Med. 1995;122:737–742. doi: 10.7326/0003-4819-122-10-199505150-00001. [DOI] [PubMed] [Google Scholar]
- 37.Tebbe U, Scholz KH, Kreuzer H, Neuhaus KL. Changes in left ventricular diastolic function during exercise in patients with coronary artery disease. Eur Heart J. 1987;8 (Suppl G):21–28. doi: 10.1093/eurheartj/8.suppl_g.21. [DOI] [PubMed] [Google Scholar]
- 38.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shapiro BP, McGoon MD, Redfield MM. Unexplained pulmonary hypertension in elderly patients. Chest. 2007;131:94–100. doi: 10.1378/chest.06-1571. [DOI] [PubMed] [Google Scholar]
- 40.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-Associated Increases in Pulmonary Artery Systolic Pressure in the General Population. Circulation. 2009;119:2663–70. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark AL, Poole-Wilson PA, Coats AJ. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol. 1996;28:1092–1102. doi: 10.1016/S0735-1097(96)00323-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.