Abstract
The Drosophila Dscam1 gene encodes a vast number of cell recognition molecules through alternative splicing. These exhibit isoform-specific homophilic binding and regulate self-avoidance, the tendency of neurites from the same cell to repel one another. Genetic experiments indicate that different cells must express different isoforms. How this is achieved is not known, as the expression of alternative exons in vivo has not been shown. Here, we modified the endogenous Dscam1 locus to generate splicing reporters for all variants of exon 4. We demonstrate that splicing does not occur in a cell-type specific fashion, that cells identified by their unique locations express different exon 4 variants in different animals, and that splicing in identified neurons can change over time. Probabilistic expression is compatible with a widespread role in neural circuit assembly through self-avoidance and is incompatible with models in which specific isoforms of Dscam1 mediate recognition between processes of different cells.
Introduction
Neural circuits are assembled through interactions between neurites, both axons and dendrites, of vast numbers of neurons. This assembly relies upon many different receptors and ligands mediating repulsive and adhesive interactions between neurites. Recent studies have highlighted the importance of repulsive interactions between neurites of the same cell for patterning neural circuits. This process, first described in the leech and termed self-avoidance (Kramer and Stent, 1985), contributes to circuit assembly in both vertebrates and invertebrates (Hattori et al., 2007; Hughes et al., 2007; Lefebvre et al., 2012; Matthews et al., 2007; Millard et al., 2010; Soba et al., 2007; Wang et al., 2002a). Self-avoidance relies on neurites acquiring a cell surface identity specific to each neuron, different from other neurons they encounter during development (Schmucker and Chen, 2009; Zipursky and Grueber, 2013; Zipursky and Sanes, 2010).
Self-avoidance is understood best in Drosophila where a large family of immunoglobulin (Ig) containing proteins encoded by the Dscam1 locus mediates this process (Hughes et al., 2007; Matthews et al., 2007; Soba et al., 2007; Wang et al., 2002a; Zhan et al., 2004). Dscam1 proteins exhibit isoform-specific homophilic binding, both in vitro and in vivo (Wojtowicz et al., 2004; Wojtowicz et al., 2007; Wu et al., 2012). Upon contact between neurites of the same cell, homophilic binding of Dscam1 triggers repulsion (Hughes et al., 2007; Matthews et al., 2007; Soba et al., 2007; Wu et al., 2012). The importance of Dscam1 for self-avoidance has been demonstrated in axons, dendrites, and postsynaptic elements at multiple contact synapses (Hattori et al., 2007; Hughes et al., 2007; Matthews et al., 2007; Millard et al., 2010; Soba et al., 2007; Wang et al., 2002a; Zhan et al., 2004). Genetic studies indicate that thousands of isoforms are necessary for self-avoidance and neurons must express different Dscam1 isoforms from their neighbors for normal patterning (Hughes et al., 2007; Matthews et al., 2007; Soba et al., 2007). As neurons often encounter the neurites of many different cells, particularly in dense neuropil in the developing central nervous system (CNS), robust mechanisms must exist to ensure that neurons that encounter each other during development express different isoforms.
Dscam1 isoforms are generated through alternative splicing (Schmucker et al., 2000). Each extracellular domain shares the same overall domain structure, but differs in amino acid sequence in one or more of three variable Ig domains, designated Ig2, Ig3, and Ig7. These are encoded by clusters of alternative exons with 12 variants of exon 4 (encoding half of Ig2), 48 variants of exon 6 (encoding half of Ig3), and 33 variants of exon 9 (encoding Ig7 in its entirety) (Figure 1A). Each combination of three variable domains determines the unique binding specificity of each isoform. Indeed, some 18,000 of the 19,008 potential extracellular domains exhibit strong isoform-specific homophilic binding (Wojtowicz et al., 2004; Wojtowicz et al., 2007). The ability of neurites to discriminate between self and non-self depends critically on the pattern of alternative splicing of Dscam1 in each neuron.
Figure 1. The Design of Reporters for Splicing of Alternative Exon 4 Variants.
(A) Schematic representation of the Dscam1 genomic locus. Color-coded exons are alternatively spliced in a mutually exclusive manner, such that one variant each from exon 4, exon 6, exon 9, and exon 17 clusters are included in the mature mRNA. Exons 4, 6, and 9 correspond to three variable Ig domains in the extracellular domain that determine the binding specificity of the isoform and encode 12, 48, and 33 variants, respectively. Thus, this gene has the potential to generate up to 19,008 distinct extracellular domains.
(B) Splicing reporter design. The exon 4.5 reporter is shown as an example. All variants of exon 4, except exon 4.5, were mutated by a single base pair insertion. A transmembrane domain (TM), “self-cleaving” 2A peptide, and Gal4 followed by a stop codon and polyA site were fused in frame to exon 5. Splicing of exon 4.5 results in the translation of Gal4, which drives the expression of GFP markers under the control of UAS elements. Splicing of any other alternative exon 4 results in a frame shift, generating a stop codon in exon 5. Reporters were generated for all 12 alternative variants. A positive control with all wild-type exon 4 variants and a negative control with all exon 4 variants mutated were also generated. For detailed experimental strategy of knock-in generation, see Extended Experimental Procedures and Figure S1.
Although genetic studies indicate that neurons with overlapping dendrites and axons must express different isoforms, the expression of Dscam1 isoforms in vivo has not been described. Microarray studies on cDNAs prepared by RT-PCR from a small number of single R7 and R3/R4 photoreceptor neurons, isolated by FACS, indicated that a single neuron expresses multiple variants of exon 9 (Neves et al., 2004). Although it remains unclear whether photoreceptor neurons require Dscam1 for circuit assembly, using the same molecular approach we later reported a similar mode of expression in a small number of mushroom body (MB) neurons, which require Dscam1 for self-avoidance (Zhan et al., 2004). These findings indicate that photoreceptor and MB neurons express different combinations of Dscam1 isoforms conferring unique cell identities to them.
It has not been demonstrated how the expression of a unique combination of Dscam1 isoforms in each neuron is determined. Neurons may express Dscam1 isoforms in a highly regulated or deterministic fashion reflecting their cell type, their unique location (e.g. their dorsoventral or anteroposterior location), developmental history (e.g. their birthdate or lineage) or some combination of such developmental determinants. Alternatively, the pattern of isoform expression may not be regulated in a deterministic fashion, but rather may result from lack of regulation. A probabilistic choice of isoforms, in theory, provides a robust and efficient means by which neurons acquire unique identities (Forbes et al., 2011; Hattori et al., 2009). Furthermore, different strategies may be employed in different systems. Distinguishing between these possibilities has been hampered by the difficulties in assessing the expression of alternative isoforms at the level of identified neurons within the developing nervous system.
Here we report that Dscam1 splicing is probabilistic through the analysis of alternative exon 4 expression using splicing reporters. Live imaging revealed that the alternative variants spliced in a given cell also change over time. A similar mode of splicing was observed in cells requiring Dscam1 for self-avoidance in axons, dendrites, and postsynaptic elements, and more generally throughout the nervous system. These findings support the notion that the ability of neurites to discriminate between self and non-self relies on a probabilistic mechanism to endow developing neurons with unique cell surface identities. As specific cell types were not found to reproducibly splice the same alternative exon 4, and thus they do not express isoforms with the same binding specificity, it is unlikely that neurons use Dscam1 to specify interactions between different neuronal cell types. Conversely, widespread probabilistic splicing supports a role for Dscam1 diversity in self-recognition throughout the developing nervous system.
Results
Modification of the Endogenous Locus to Detect Expression of Each Alternative Variant of Exon 4
We devised a splicing reporter system to visualize the expression of single alternative variants of exon 4 from the endogenous Dscam1 locus in individual cells in vivo. Reporter constructs were knocked into the endogenous locus by homologous recombination of exons 3–5 with a replaceable cassette, followed by phiC31 recombination mediated cassette exchange (RMCE; see Figure S1 and Extended Experimental Procedures). The detection system was designed to monitor expression in fixed preparations and in live animals at different time points, and to identify cells expressing different alternative exons by their morphology, co-expression of specific markers, or both.
We generated reporters for each of the 12 alternative variants of exon 4. Each variant is referred to as exon 4.X, where X indicates the position of the alternative variant within the cluster from 5’ to 3’ (i.e. exon 4.5 is the fifth alternative exon downstream from exon 3). In each reporter, only a single alternative variant was in-frame with a downstream indicator, the transcriptional activator GAL4. The remaining 11 alternative variants all included a frame-shift mutation that rendered the indicator out-of-frame (Figure 1B). Thus, GAL4 was expressed only in neurons that spliced the single remaining wild type alternative variant. These neurons were visualized by expression of either membrane-bound or nuclear GFP under the control of tandemly arranged UAS elements. Positive and negative control alleles were also generated, in which all variants were in frame (Dscam1positive-Gal4) or out of frame (Dscam1negative-Gal4). As the reporter knock-in mutations inactivate Dscam1, all experiments were done in a heterozygous background. No morphological or developmental defects were observed in this background in any of the systems we analyzed. As expected, positive controls were expressed widely, and only very few weakly stained scattered cells were observed in the negative control. By contrast, each of the 12 single exon reporters was expressed in a salt-and-pepper pattern within the developing nervous system. Here, we describe the expression patterns of all 12 variants of alternative exon 4 in developing MB neurons in the central brain, dendritic arborization (da) neurons in the periphery, and visual system neurons.
MB Neurons Express Each Variant of Alternative Exon 4 at a Characteristic Level and Frequency
A role for Dscam1 in self-avoidance was first described in the MB (Zhan et al., 2004). MB is a bilobed structure in the central brain, with each lobe containing one of two sister branches of intrinsic MB neurons (i.e. Kenyon cells) (Figure 2A). Each MB contains about 2,500 Kenyon cells. These cells send axons within a common nerve called the peduncle. Axons bifurcate at the base of the peduncle, and the branches segregate and extend into separate lobes with high fidelity. In the absence of Dscam1, as assessed in single mutant cells, sister branches often fail to segregate, and instead, the two branches extend within the same lobe. Here, we argued that axons from different neurons express different Dscam1 isoforms, such that at the common branch point branches discriminate between self and non-self. This “self-recognition” would lead to homophilic repulsion and branch segregation (Hattori et al., 2007; Wang et al., 2002a; Zhan et al., 2004).
Figure 2. Alternative Variants of Exon 4 Are Expressed at Different Frequencies in the MB.
Membrane-bound GFP tagged with a V5 epitope (white) was used as the readout of the splicing reporters in the MB lobes (blue) and their intrinsic neurons, Kenyon cells (blue), of mid-pupal brains (65 hr after puparium formation). MB lobes and Kenyon cells were visualized by anti-Fasciclin II and anti-Dachshund, respectively.
(A) Schematic of the MB. Kenyon cell bodies form a cluster in the posterior part of the brain. Each Kenyon cell sends an axon through the peduncle (P). Each axon bifurcates and the branches extend into two different lobes. This segregation of the sister branches requires repulsion induced by homophilic binding of the Dscam1 isoforms. Before entering the peduncle, Kenyon cells also form a dendritic field in a structure called the calyx (C).
(B and C) The negative control allele resulted in a few weakly stained Kenyon cells (B2), whereas the positive control shows strong expression in the MB lobes (C1) and Kenyon cells (C2).
(D-G) The frequency of cells expressing the splicing reporters for exons 4.1, 4.2, 4.9, and 4.12 in the MB and Kenyon cells varied, although the number of cells stained was similar between duplicates of the same reporter. The remaining eight reporters also exhibited these characteristics (see Figure S2). Two independent samples are shown for the MB and the Kenyon cells. Scale bars: 40 µm, B1 – G1; 30 µm, B2 – G2.
We assessed expression of all 12 variants of exon 4 during MB development in pupae. Dscam1positive-Gal4 was expressed strongly in both lobes of the MB (Figure 2C1). This resulted from the reporter expression in the Kenyon cells and possibly other neurons sending process into the lobes (i.e. MB extrinsic neurons). Most, if not all, Kenyon cells expressed Dscam1positive-Gal4 (Figure 2C2), consistent with previous data indicating Dscam1 expression in these cells (Zhan et al., 2004). By contrast, only a couple of weakly stained cells were observed in the negative control (i.e., Dscam1negative-Gal4) (Figures 2B1 and 2B2). The source of this background expression is not known.
Each of the 12 exon 4 variants was expressed in a subset of cells (Figures 2D–2G and Figures S2A–S2J). For a given alternative variant, the expression level and number of cells expressing the variant were qualitatively similar between animals. By contrast, the expression frequency for different alternative variants was different (Figures 2D–2G and Figures S2A–S2J). For example, the frequency of exon 4.2 splicing was consistently lower than any other alternative variant we analyzed (Figures 2E1 and 2E2). In contrast, exon 4.12 was expressed in many more cells at much higher levels (Figures 2G1 and 2G2). Furthermore, although the frequency of expression of a given variant was similar between animals, there was no obvious similarity in the distribution of cells in different animals. The overall bias in splicing of the alternative variants is consistent with our previous expression data obtained on populations of Kenyon cells isolated by FACS (Zhan et al., 2004). For example, microarray and cDNA sequencing results both indicated that exons 4.2 and 4.12 represent the two extremes of the frequency spectrum with exon 4.2 expressing the least and exon 4.12 the most. The convergence of these data argues that the splicing trap method accurately reflects splicing in vivo.
Splicing in Single Class IV da Neurons Is Probabilistic
To assess rigorously whether Dscam1 alternative splicing is probabilistic, we analyzed expression in class IV da neurons. The dendrites of these neurons require Dscam1 for self-avoidance. In wild type, each class IV da neuron forms highly branched dendrites that do not overlap (Grueber et al., 2002). By contrast, in the absence of Dscam1, these dendrites frequently cross one another, form clumps, and as a consequence disrupt coverage of the receptive field (Hughes et al., 2007; Matthews et al., 2007; Soba et al., 2007).
Each class IV da neuron can be identified reliably from animal to animal based on its unique position, morphology, and marker expression. There is a dorsal (ddaC), lateral (v’ada), and ventral (vdaB) class IV neuron in each abdominal hemisegment A1 through A7 (Grueber et al., 2002; Han et al., 2012) (Figure 3A). Thus, this system allows us to score the alternative splicing of exon 4 in 42 different uniquely identified neurons in each animal, and to compare expression between these cells and between the same cell in different animals.
Figure 3. Splicing of Exon 4 in Class IV da Neurons Is Probabilistic.
(A) Schematic indicating cell body locations of the three class IV da neurons (blue) in a right abdominal hemisegment. Cell bodies of other classes (I – III) of da neurons are also indicated (orange). This organization is repeated in abdominal segments A1 - A7, allowing identification of 42 class IV neurons in each animal.
(B) Schematic representation of expression of exon 4 reporter in class IV da neurons in A1-A7. Three class IV neurons (blue ellipse) in each hemisegment correspond to the three neurons in (A). The expression of GFP-tagged histone H2A in the nucleus under the control of UAS element is represented as a white circle. Grey ellipses represent neurons in which staining could not be quantified. This animal corresponds to the first column in (J).
(C) A class IV da neuron with nuclear GFP expression in white (above) and no expression (below). Red dashed circle indicates the location of the nucleus. Scale bar, 5 µm.
(D–J) Expression patterns of the control and splicing reporters for exons 4.1, 4.2, 4.7, 4.9, and 4.12. Each row represents a cell defined by its unique position: Left and right; dorsal (D), lateral (L), and ventral (V); and abdominal segments A1-A7. Each column represents expression in one animal. Red indicates neurons that do not express the reporter (OFF), blue indicates neurons that express the reporter (ON), and grey indicates neurons that could not be scored. For other splicing reporters, see Figure S3.
(K) Boxplots showing different frequencies of expression of the splicing reporters. Y-axis represents percentage of ON neurons per animal. Boxes indicate the first and third quartiles, whiskers denote 1.5 times the interquartile range, and outliers are shown as circles. Numbers in parentheses indicate the numbers of animals analyzed for each alternative exon variant. On average ∼39 neurons could be scored from each animal. The statistical significance of the differences in expression between different alternative variants is given in Figure S3H. *See Extended Experimental Procedures.
We first set out to address whether the choice of alternative exon 4 variant is always the same for a class IV neuron in the same location in different animals. GFP-tagged histone H2A was used to detect the expression of each splicing reporter, and class IV da neurons were identified by the expression of tandem dimer Tomato under the control of a genomic element from the pickpocket locus (Han et al., 2011) (Figures 3B and 3C). Each neuron in abdominal segments A1 through A7 was identified, and its nuclear GFP intensity was quantified to determine the expression (i.e., simply ON or OFF). GFP intensity in Dscam1negative-Gal4 was used to determine the threshold between ON and OFF. Briefly, the histogram was fit to a Gamma probability distribution function to determine a threshold value; 312/314 of the control cells were below this value (for details, see Extended Experimental Procedures). With this threshold, the positive control was expressed in all neurons scored except one (i.e., 1/299), confirming Dscam1 expression at this stage (Figure 3E). By contrast, in every animal, only a subset of the 42 class IV da neurons expressed each alternative exon 4 variant (Figures 3F–3I and Figures S3A–S3G). Moreover, the set of neurons that spliced a given variant was different between individual animals, and no cell expressed the same variant across all animals, with the exception of 3 cells that expressed exon 4.2 in all 8 animals analyzed (Figures 3F–3J and Figures S3A–S3G). Given that exon 4.2 is expressed in ∼80% of class IV neurons, we would expect on average 7 cells to express this alternative exon in all animals by chance. Thus, the splicing choice is not deterministic.
To determine whether the probability of splicing a specific variant is the same for each class IV da neuron (i.e., whether there is a splicing bias for cells in certain locations), we performed statistical simulations. For a given alternative exon 4 variant, the spatial splicing patterns were shuffled to generate a set of randomized patterns. The pairwise Pearson correlation coefficient (PCC) was then calculated for every pair within the set, and the average score was determined. This process was repeated a thousand times to generate a distribution of the average PCC of the randomized sets and fit to a normal distribution (Figure S4A). The differences between the distribution of average PCCs from the simulations and the experimental data for each exon 4 variant were not statistically significant (p>0.025; two-tailed test) (Figure 4 and Figures S4B).
Figure 4. Expression of Exon 4 Variants Is Randomly Distributed Among the 42 Class IV da Neurons.
Statistical test results for exons 4.1, 4.2, 4.9, and 4.12, indicating no significant difference between the average Pearson correlation coefficients of experimental data (red line) and one thousand trials of randomized patterns (shown as histograms in blue) (p>0.025; two-tailed test). This result was similar for each of the remaining eight variants (see Figure S4). Thus, although the frequency of expression of different exons varies, the spatial expression pattern is not biased towards any cell and is random. For methods, see text and Figure S4A.
These data are consistent with the notion that the splicing probability of each exon 4 variant is equivalent among class IV da neurons and that the spatial pattern of splicing within the class of neurons is the result of a random process. Thus, the spatial location of a neuron does not contribute to the splicing choice. In a separate series of experiments, we assessed the splicing of alternative variants of exon 4 in ventral class III (vdaD) and class IV neurons, which share overlapping receptive fields. There was no correlation in splicing with each cell expressing alternative variants independent of the other cell (Figure S3I). Thus, we conclude that the selection of the specific exon 4 variant to include within a transcript is made probabilistically on a cell-by-cell basis, such that each alternative variant displays varying frequencies of expression (i.e. between ∼6–80% for class IV da neurons) (Figure 3K).
The Frequency of Inclusion of Exon 4 Variants Is Different Between Cell Types
Cell-type specific differences were observed in the frequency of exon 4 variant splicing in class IV da neurons and in Kenyon cells. While the likelihood that a given alternative variant was expressed in class IV da neurons was consistent from animal to animal, there were significant differences in the percentage of cells expressing different alternatives (Figure 3K and Figure S3H). This distribution was strikingly different from those observed in the Kenyon cells. For instance, exon 4.2 was expressed in ∼78% of the class IV da neurons, yet it was only rarely expressed in Kenyon cells (Figures 2E2, 3G, and 3K). By contrast, exon 4.1 was expressed in only ∼6% of the class IV da neurons, while it was expressed in many more Kenyon cells than exon 4.2 (Figures 2D2, 3F, and 3K). These data demonstrate that the probability of splicing a given alternative exon 4 varies between cell types that require Dscam1 for self-avoidance. This may reflect differences in splicing factors expressed in cells or the level of their expression, as suggested previously by Chess and colleagues (Neves et al., 2004).
The Splicing Choice of Alternative Exon 4 Variant Changes Over Time
We sought to address whether a set of alternatively spliced transcripts is generated early in da neuron development and maintained, or whether different transcripts are expressed at different times. To this end, we analyzed splicing of exon 4 variants in the same set of dorsal class IV da neurons ∼48 hours apart, imaged in late second and wandering third instar larval stages. Splicing was visualized in reporters for exons 4.6 and 4.10, using GFP-tagged histone-H2A. These were chosen arbitrarily among the variants that exhibited intermediate levels of expression. We assessed whether any neuron switched exon 4.6 or 4.10 expression from OFF to ON or ON to OFF during this time window. Dscam1negative-Gal4 animals were used to determine the threshold between ON and OFF (Figure 5A). With the positive control, we confirmed that Dscam1 was expressed in all class IV neurons at these developmental stages (Figure 5B). This is consistent with a previous study indicating that these neurons continue to arborize during this time period (Grueber et al., 2003).
Figure 5. Splicing of Exon 4 in Class IV da Neurons is Dynamic.
(A–D) Expression of exon 4.6 and 4.10 (and controls) was assessed in the dorsal 14 class IV da neurons of second instar larvae, and the expression in these same cells was determined 48 hours later. Thresholds for expression were determined by fitting a Gamma probability density function to the values from negative control animals (for details, see Extended Experimental Procedures). Neurons whose expression changed between the two time points are indicated with asterisks. Color codes are as in Figure 3. If the expression in neurons could not be quantified at either of the two stages, they were not included in the analysis and are indicated as grey boxes. Cells are indicated by their positions in each row, and each column represents the cells scored in each animal.
(E) Example of a neuron switching exon 4.6 expression from OFF to ON. White, GFP-tagged H2A driven by exon 4.6 reporter; red dashed circles, locations of nuclei; and blue, class IV specific marker. Signals were saturated in this panel post-acquisition for viewing purposes. Scale bar, 2µm.
(F) Summary table of the number of neurons that switched expression from OFF to ON (left columns) and from ON to OFF (right columns). * p<0.05, Pearson’s chi-squared test.
The pattern of exon 4.6 and 4.10 changed over time. Seventy-five neurons were identified that initially lacked exon 4.6 expression, and of these, 11 neurons switched to ON (Figures 5C, 5E, and 5F). Conversely, seventeen neurons were identified that initially expressed exon 4.6, and of these, 3 neurons switched to OFF (Figures 5C and 5F). Thus, one in every 6 to 7 neurons (∼15%) changed splicing of exon 4.6. OFF to ON changes were also observed with exon 4.10, although less frequently; ON to OFF changes were not statistically significant (Figures 5D and 5F). For both exons 4.6 and 4.10, the number of ON to OFF cells may be an underestimate, as GFP tagged H2A and Gal4 proteins could persist well after the reporter mRNA. Based on the analysis of these two variants, we estimate that the splicing of each variant changes in ∼10% of the cells (i.e. from ON to OFF and from OFF to ON). By extrapolating these data to all 12 alternative variants of exon 4, many class IV da neurons are likely to change the exon 4 variants spliced during this 48h period. As the positive control indicates that some variant of exon 4 is expressed in all neurons throughout the imaging period, our results demonstrate that the choice of alternative exon, and thus the isoforms expressed in a cell, can change over time.
The Pattern of Dscam1 Splicing in the Visual System Is Consistent with a Broad Role in Self-recognition in the CNS
In the previous sections, we demonstrated that Dscam1 is spliced in a probabilistic fashion in neurons utilizing Dscam1 for axon and dendritic self-avoidance. In addition to these functions, Dscam1 regulates synaptic organization at tetrad synapses in the visual system through self-avoidance (Millard et al., 2010). Tetrad synapses are multiple contact synapses between photoreceptor axons and the dendrites of target cells called lamina neurons. Each tetrad contains a single presynaptic release site and an invariant pair of postsynaptic elements, one from an L1 lamina neuron and the other from L2 (Figures 6A - 6A”). Each L1 and L2 neuron contributes postsynaptic elements to multiple tetrads along the same photoreceptor cell axon, and each photoreceptor neuron is presynaptic to only one L1 and to only one L2 neuron. Two additional dendrites from other cells complete the tetrad. While L1/L1 and L2/L2 pairs are not seen at wild type tetrads, in the absence of both Dscam1 and Dscam2 (a paralog expressing two isoforms), many L1/L1 and L2/L2 pairs were observed. We argued that L1 and L2 express different isoforms of Dscam1 and Dscam2. This would prevent pairing of postsynaptic elements from the same cell through homophilic repulsion, and, in this way, this mechanism contributes to the appropriate pairing of L1 and L2. We sought to determine whether L1 and L2 express different Dscam1 isoforms, and, if they do, how this is regulated.
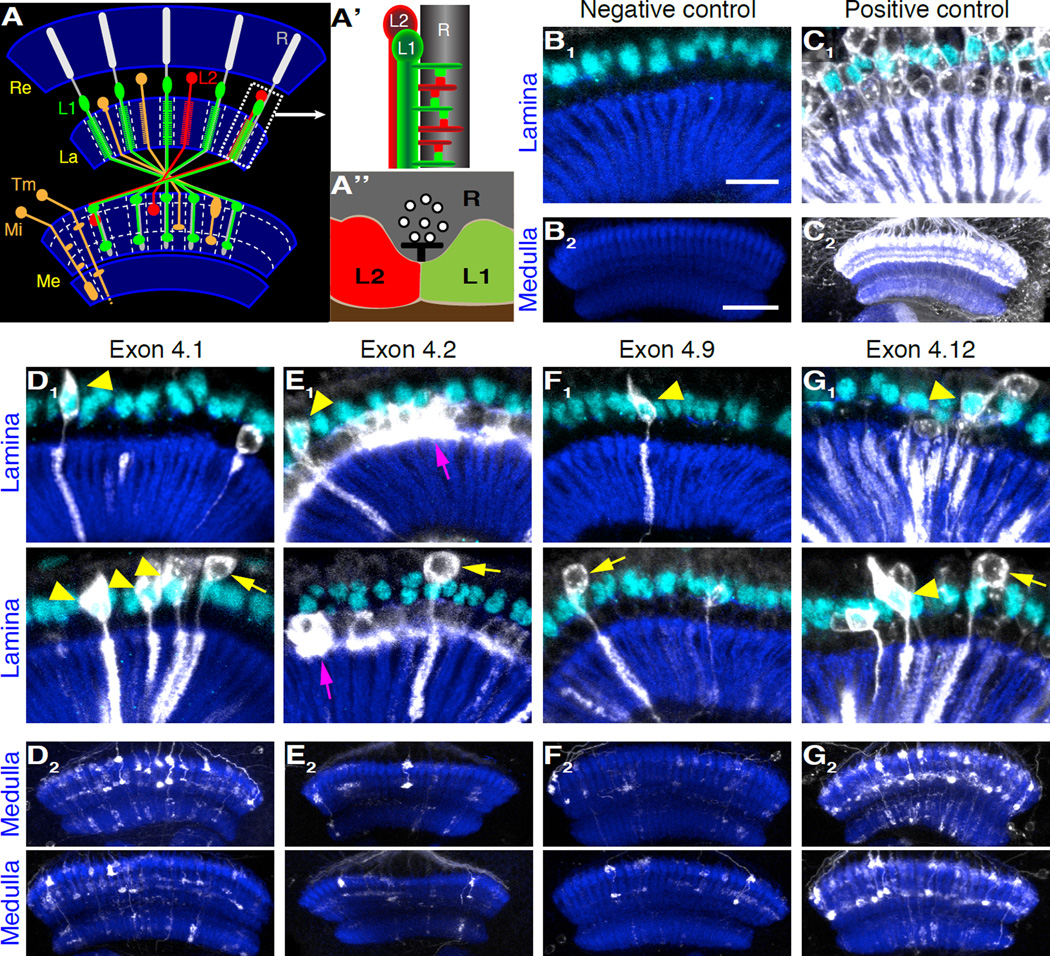
Figure 6. Scattered Neurons of Specific Cell Types Express Alternative Variants of Exon 4 in the Visual System.
(A-A”) Schematic representation of the Drosophila visual system indicating the relationship between the retina (Re), lamina (La), and medulla (Me) (A). The photoreceptors (R), a few classes of lamina monopolar neurons including the L1 and L2 neurons, and two medulla neurons (medulla intrinsic neuron (Mi) and transmedullary neuron (Tm)) are shown. This is only a small subset of the greater than 60 cell types innervating these structures. All neuronal subtypes shown are repeated in the medulla. As such, if specific alternative versions of exon 4 were expressed in each cell of any of these cell types, or others of similar periodicity, highly regular columnar structures and uniform layers would be seen (see Figure S5A). Such patterns were not observed. Importantly, L1 and L2 require Dscam1 for normal patterning of tetrad synapses via self-avoidance (see text). Each tetrad synapse comprises four postsynaptic elements (one L1, one L2, and two other variable cell types (not shown)) abutting a presynaptic site on a photoreceptor (A”). L1/L1 or L2/L2 pairs are prevented through self-avoidance. A pair of L1 and L2 makes multiple tetrads along a photoreceptor axon (A’).
(B and C) Expression of the negative and positive controls visualized by V5-tagged membrane bound GFP (white) in the lamina and the medulla. Neuropile structures of the lamina and the medulla were visualized by staining against N-cadherin (dark blue). The negative control does not show any expression in the lamina or the medulla, while the positive control shows strong expression in both. In the lamina, repeated columnar structures can be seen with the positive control, and most, if not all, L1 neurons are labeled. L1 nuclei are identified by specific expression of Seven-up (cyan). Similarly, layers are seen in the medulla, in large part reflecting prominent terminals of lamina monopolar neurons.
(D–G) Expression of splicing reporters for exons 4.1, 4.2, 4.9, and 4.12. Two examples for the lamina and medulla are shown. Both L1 (yellow arrowheads) and L2 neurons (yellow arrows), identified by their morphology and the expression of seven-up in L1, were observed with all the splicing reporters tested, but only a subset of each class of neurons expressed a particular alternative variant. Cell-type specific expression of exon 4.2 was observed in the proximal satellite glia (magenta arrow), but few neuronal projections expressing exon 4.2 were found. As no phenotype was observed in flies lacking exon 4.2 (data not shown), the significance of the glial expression is not clear. Color code as in (B) and (C). 5 µm z-stack projections in the lamina, 10 µm projections in the medulla. Scale bars: 15 µm, lamina (B1-G1); 30 µm, medulla (B2-G2). For other alternative variants, see Figure S5.
Most, if not all, lamina neurons express Dscam1positive-Gal4 as tetrad synapses are forming within the developing lamina during mid-pupal development, and none expressed Dscam1negative-Gal4 (Figures 6B and 6C). These findings are consistent with protein expression studies indicating that Dscam1 proteins are expressed on the dendrites of L1 and L2 neurons during this developmental time period (Millard et al., 2010). Although the morphologies of L1 and L2 neurons are very similar, these neurons can be distinguished from one another by the selective expression of the transcription factor Seven-up in L1 (Figure 6A) (Claude Desplan, personal communication). All the individual splicing reporters were expressed in subsets of L1 and L2 neurons scattered throughout the lamina with no apparent pattern (Figures 6D1-6G1 and Figures S5B1-S5I1; yellow arrowheads and arrows). Occasionally, these neurons were in the same cartridge, as one would expect from a probabilistic splicing. Presumably, these differ from one another through the expression of additional alternative versions of exon 4 (as in class IV da neurons (see Discussion)), through differential expression of alternative versions from the two other clusters of alternative exons encoding variable recognition domains, or through both mechanisms. Thus, these data are consistent with the notion that probabilistic splicing provides unique cell identities to L1 and L2 neurons thus preventing inappropriate pairing of postsynaptic elements from the same cell.
As multiple contact synapses are common throughout the fly visual system, we sought to assess whether isoform splicing occurs in a probabilistic fashion more generally in different classes of visual system neurons. Each of the 12 exon 4 reporters was expressed in a salt and pepper pattern throughout the visual system during periods of synapse formation (Figures 6D–6G and Figures S5B–S5I). Given the repetitive structure of the visual system (e.g. columns in the lamina and medulla where axons of different cell types branch or terminate in discrete layers; Figure 6A), if cell-type specific splicing were a common feature of Dscam1 expression, then we would anticipate observing regular patterns of processes in a columnar or layered arrangement (Figure S5A). This was not observed, suggesting that splicing is determined neither by cell type nor spatial location in the visual system. Thus, like L1 and L2, it seems likely that the vast majority of visual system cells express alternative variants of exon 4 in a probabilistic fashion. As multiple contact synapses are common throughout the fly visual system, this would be consistent with Dscam1 playing a broad role in regulating their synaptic composition through self-recognition.
Discussion
As differences in Dscam1 expression between neurons is essential for self-avoidance, how isoform expression is regulated is a critical issue in neural circuit assembly in Drosophila. The observation that single photoreceptor neurons express multiple isoforms of Dscam1 and that they express different combinations of them led Chess and co-workers to propose that Dscam1 provided neurons with a unique identity largely through a stochastic process (Neves et al., 2004). These findings were published just prior to our report that Dscam1 isoforms exhibit isoform-specific homophilic binding and followed earlier work from Lee and colleagues describing MB neuron branch segregation defects in Dscam1 mutants (Wang et al., 2002a; Wojtowicz et al., 2004; Wojtowicz et al., 2007). Together these three observations, and our findings that MB neurons also express multiple isoforms and different sets of them (Zhan et al., 2004), led us to propose that sister branches of MB neurons utilize homophilic recognition as a means of discriminating between sister branches and the branches of other neurons. Extensive genetic and biochemical analyses provide a strong case for Dscam1 as a critical determinant of self-avoidance in this and other contexts in the developing fly peripheral and central nervous systems (Hattori et al., 2008; Hughes et al., 2007; Matthews et al., 2007; Soba et al., 2007). Ironically, while the nature of Dscam1 expression lies at the heart of self-avoidance, this aspect of Dscam1 biology has remained poorly understood, in large part due to considerable technical challenges.
Here we took a genetic approach to visualize Dscam1 isoform expression. By monitoring exon 4 splicing as a surrogate for isoform expression in neurons, we assessed splicing in vivo broadly throughout the developing nervous system and in specific cell types, assessed splicing in single identified cells between different animals, and followed splicing in the same cell at different times during development. We demonstrated that splicing is probabilistic in class IV da neurons where it is required for dendritic self-avoidance. The patterns of splicing in both the MB and L1/L2 neurons, where Dscam1 is required for axon branch self-avoidance and appropriate pairing at multiple contact synapses, respectively, are also consistent with a probabilistic mode of splicing.
The analysis of exon 4 splicing in class IV da neurons revealed that, on average each neuron expresses multiple exon 4 variants. The sum of the average expression probability of all the alternative variants of exon 4 in the class IV da neurons is 393 +/− 38 % (Figure 3K), arguing the splicing of about four variants in a single neuron. If the splicing mechanism were probabilistic, not only at the level of single neurons but also at the level of each round of mRNA processing, it might be expected that the most abundantly spliced variant (i.e. exon 4.2) would be expressed in all neurons, albeit at varying levels, given enough rounds of transcription. Thus, the scattered splicing pattern within a neuronal population may reflect a splicing mechanism in which the same variant is included in multiple mRNAs while others are excluded (e.g. through the assembly of stable splicing complex associated with chromatin). Alternatively, this pattern of expression may result from low copy numbers of total Dscam1 mRNAs in each class IV da neuron.
The expression of multiple isoforms in each neuron is a key to robust self-avoidance. Previous studies using RT-PCR analysis on single MB neurons also indicated that each neuron expresses multiple variants of exon 9 (Zhan et al., 2004). Monte Carlo simulations and mathematical modeling suggest that expression of multiple isoforms in a neuron through probabilistic splicing can provide a robust mechanism to endow each neuron with a unique cell surface identity (Forbes et al., 2011; Hattori et al., 2009). Indeed, this robustness is supported by the observation that the differential Dscam1 expression in L1 and L2 neurons arises from probabilistic splicing in these neurons. The large number of isoforms encoded by Dscam1 is likely to be sufficient to offset the reduced diversity caused by biased exon usage. In addition, dynamic splicing further minimizes the risk that neighboring neurons share the same Dscam1 isoforms for an extended period.
Recent studies suggest that a similar mechanism for self-avoidance has evolved in vertebrates. In the mouse retina and cerebellum, self-avoidance is mediated by a large family of isoform-specific homophilic binding proteins encoded by the clustered protocadherin γ locus (Lefebvre et al., 2012). RT-PCR analyses showed that Purkinje cells express different isoforms, and this is consistent with probabilistic expression of multiple protocadherin γ isoforms in each neuron (Kaneko et al., 2006). Here, regulation appears to be at the level of alternative promoter choice rather than alternative splicing (Tasic et al., 2002; Wang et al., 2002b). Thus, probabilistic expression may have evolved as a common strategy, albeit via different molecular mechanisms, by which neurons acquire unique self-identities.
Although it is clear that Dscam1 plays a prominent role in regulating self-avoidance in multiple contexts, the extraordinary selectivity of homophilic binding and the vast number of different isoforms seems particularly well-suited to mediating recognition between different neurons, for instance between pre and postsynaptic partners. Indeed, Sanes and colleagues have demonstrated that two chick Dscam paralogs (N.B. these genes do not encode multiple isoforms), each with mutually exclusive binding specificities, are expressed in different pairs of synaptic partners in the inner plexiform layers, a structure analogous to the medulla in the fly visual system (Yamagata and Sanes, 2008). Furthermore, gain and loss of function studies support a role for them in matching synaptic partners. If this were the case for Dscam1 isoforms, we would anticipate reproducible cell-type specific patterns of expression of exon 4 variants as homophilic binding requires precise matching of all three variable domains (i.e. encoded by variable exons 4, 6, and 9). No reproducible patterns of exon 4 expression were observed in any region of the visual system or elsewhere in the developing postembryonic brain. Thus, cell-type specific expression, if it occurs at all, is rare. Thus, it seems unlikely that Dscam1 isoforms selectively regulate matching synaptic partners. Rather, our data provide compelling evidence that a probabilistic mechanism endows cells with unique individual identities throughout the nervous system and is consistent with a widespread role for Dscam1 diversity in patterning neural circuits by preventing inappropriate interactions between axons, dendrites, and postsynaptic elements of the same cell.
Experimental Procedures
Additional information on the experimental methods used here can be found in the Extended Experimental Procedures available online.
The strategy used to generate knock-ins of the splicing reporters is indicated in Figure S1 and is based on ends-out homologous recombination followed by phiC31 recombination mediated cassette exchange (RMCE) (Bateman et al., 2006; Gong and Golic, 2003) (see Extended Experimental Procedures). Transgenic strains used in this study as well as the immunohistochemistry protocol for pupal brains are described in Extended Experimental Procedures. The immunohistochemistry protocol for da neurons has been described previously by Grueber et al (Grueber et al., 2002). Class IV da neurons were identified using the expression of tandem dimer Tomato (tdTom) expressed specifically in these neurons by sequences from the pickpocket gene (Han et al., 2012). Nuclear GFP intensity in class IV da neurons was quantified in single confocal planes. Nuclei of class IV da neurons were identified using the exclusion of tdTom signal from the nuclei. Live imaging was done using a custom built 2-photon microscope. The threshold between ON and OFF was determined using the GFP values obtained from nuclei of negative control animals. All images were analyzed using Fiji, and statistics were done in R.
Supplementary Material
Highlights.
Alternative splicing of Dscam1 mRNA was visualized in vivo using splicing reporters
Alternative splicing of Dscam1 mRNA is probabilistic at the level of single neurons
Reproducible splicing patterns between animals were not observed
Expression studies suggest Dscam1 plays a widespread role in self-avoidance
ACKNOWLEDGEMENTS
We thank Yasushi Hiromi, Yuh Nung Jan, Aljoscha Nern, Barret Pfeiffer, and Gerald Rubin for reagents, members of our laboratory for discussion, and Edward De Robertis, Wesley Grueber, Daisuke Hattori, Kelsey Martin, and Alex Plocik for their comments on the manuscript. We also thank Orkun Akin and Josh Trachtenberg for their help with the 2-photon microscope, Jinfei Ni for his early work in generating the reporter constructs, Wei Wu for her initial assistance in assessment of the transgenic flies, and GenetiVision and Genetic Services Inc. for their injection services. This work was supported by a fellowship from the Nakajima Foundation to S.K.M., Canadian Institutes of Health Research (CIHR) Fellowship and UCLA Computational Bioscience Initiative Fellowship to K.X.Z., and N.I.H grants to B.R.G (5R01GM067842) and S.L.Z (5R01DC006485). S.L.Z. is an investigator of Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR INFORMATION
The authors declare no competing financial interests.
REFERENCES
- Bateman JR, Lee AM, Wu CT. Site-specific transformation of Drosophila via phiC31 integrase-mediated cassette exchange. Genetics. 2006;173:769–777. doi: 10.1534/genetics.106.056945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EM, Hunt JJ, Goodhill GJ. The combinatorics of neurite self-avoidance. Neural computation. 2011;23:2746–2769. doi: 10.1162/NECO_a_00186. [DOI] [PubMed] [Google Scholar]
- Gong WJ, Golic KG. Ends-out, or replacement, gene targeting in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2556–2561. doi: 10.1073/pnas.0535280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Ye B, Moore AW, Jan LY, Jan YN. Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Current biology : CB. 2003;13:618–626. doi: 10.1016/s0960-9822(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Han C, Jan LY, Jan YN. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9673–9678. doi: 10.1073/pnas.1106386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Wang D, Soba P, Zhu S, Lin X, Jan LY, Jan YN. Integrins regulate repulsion-mediated dendritic patterning of drosophila sensory neurons by restricting dendrites in a 2D space. Neuron. 2012;73:64–78. doi: 10.1016/j.neuron.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori D, Chen Y, Matthews BJ, Salwinski L, Sabatti C, Grueber WB, Zipursky SL. Robust discrimination between self and non-self neurites requires thousands of Dscam1 isoforms. Nature. 2009;461:644–648. doi: 10.1038/nature08431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori D, Demir E, Kim HW, Viragh E, Zipursky SL, Dickson BJ. Dscam diversity is essential for neuronal wiring and self-recognition. Nature. 2007;449:223–227. doi: 10.1038/nature06099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori D, Millard SS, Wojtowicz WM, Zipursky SL. Dscam-mediated cell recognition regulates neural circuit formation. Annual review of cell and developmental biology. 2008;24:597–620. doi: 10.1146/annurev.cellbio.24.110707.175250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, Bortnick R, Tsubouchi A, Baumer P, Kondo M, Uemura T, Schmucker D. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–427. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko R, Kato H, Kawamura Y, Esumi S, Hirayama T, Hirabayashi T, Yagi T. Allelic gene regulation of Pcdh-alpha and Pcdh-gamma clusters involving both monoallelic and biallelic expression in single Purkinje cells. The Journal of biological chemistry. 2006;281:30551–30560. doi: 10.1074/jbc.M605677200. [DOI] [PubMed] [Google Scholar]
- Kramer AP, Stent GS. Developmental arborization of sensory neurons in the leech Haementeria ghilianii. II. Experimentally induced variations in the branching pattern. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1985;5:768–775. doi: 10.1523/JNEUROSCI.05-03-00768.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre JL, Kostadinov D, Chen WV, Maniatis T, Sanes JR. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature. 2012;488:517–521. doi: 10.1038/nature11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, Grueber WB. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Millard SS, Lu Z, Zipursky SL, Meinertzhagen IA. Drosophila dscam proteins regulate postsynaptic specificity at multiple-contact synapses. Neuron. 2010;67:761–768. doi: 10.1016/j.neuron.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Zucker J, Daly M, Chess A. Stochastic yet biased expression of multiple Dscam splice variants by individual cells. Nature genetics. 2004;36:240–246. doi: 10.1038/ng1299. [DOI] [PubMed] [Google Scholar]
- Schmucker D, Chen B. Dscam and DSCAM: complex genes in simple animals, complex animals yet simple genes. Genes & development. 2009;23:147–156. doi: 10.1101/gad.1752909. [DOI] [PubMed] [Google Scholar]
- Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, Dixon JE, Zipursky SL. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101:671–684. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- Soba P, Zhu S, Emoto K, Younger S, Yang SJ, Yu HH, Lee T, Jan LY, Jan YN. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron. 2007;54:403–416. doi: 10.1016/j.neuron.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B, Nabholz CE, Baldwin KK, Kim Y, Rueckert EH, Ribich SA, Cramer P, Wu Q, Axel R, Maniatis T. Promoter choice determines splice site selection in protocadherin alpha and gamma pre-mRNA splicing. Molecular cell. 2002;10:21–33. doi: 10.1016/s1097-2765(02)00578-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Zugates CT, Liang IH, Lee CH, Lee T. Drosophila Dscam is required for divergent segregation of sister branches and suppresses ectopic bifurcation of axons. Neuron. 2002a;33:559–571. doi: 10.1016/s0896-6273(02)00570-6. [DOI] [PubMed] [Google Scholar]
- Wang X, Su H, Bradley A. Molecular mechanisms governing Pcdh-gamma gene expression: evidence for a multiple promoter and cis-alternative splicing model. Genes & development. 2002b;16:1890–1905. doi: 10.1101/gad.1004802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, Clemens JC. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell. 2004;118:619–633. doi: 10.1016/j.cell.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz WM, Wu W, Andre I, Qian B, Baker D, Zipursky SL. A vast repertoire of Dscam binding specificities arises from modular interactions of variable Ig domains. Cell. 2007;130:1134–1145. doi: 10.1016/j.cell.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Ahlsen G, Baker D, Shapiro L, Zipursky SL. Complementary chimeric isoforms reveal Dscam1 binding specificity in vivo. Neuron. 2012;74:261–268. doi: 10.1016/j.neuron.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Zhan XL, Clemens JC, Neves G, Hattori D, Flanagan JJ, Hummel T, Vasconcelos ML, Chess A, Zipursky SL. Analysis of Dscam diversity in regulating axon guidance in Drosophila mushroom bodies. Neuron. 2004;43:673–686. doi: 10.1016/j.neuron.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Grueber WB. The molecular basis of self-avoidance. Annual review of neuroscience. 2013;36:547–568. doi: 10.1146/annurev-neuro-062111-150414. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Sanes JR. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell. 2010;143:343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.