Abstract
Critical illness polyneuropathies (CIP) and myopathies (CIM) are common complications of critical illness. Several weakness syndromes are summarized under the term intensive care unit-acquired weakness (ICUAW). We propose a classification of different ICUAW forms (CIM, CIP, sepsis-induced, steroid-denervation myopathy) and pathophysiological mechanisms from clinical and animal model data. Triggers include sepsis, mechanical ventilation, muscle unloading, steroid treatment, or denervation. Some ICUAW forms require stringent diagnostic features; CIM is marked by membrane hypoexcitability, severe atrophy, preferential myosin loss, ultrastructural alterations, and inadequate autophagy activation while myopathies in pure sepsis do not reproduce marked myosin loss. Reduced membrane excitability results from depolarization and ion channel dysfunction. Mitochondrial dysfunction contributes to energy-dependent processes. Ubiquitin proteasome and calpain activation trigger muscle proteolysis and atrophy while protein synthesis is impaired. Myosin loss is more pronounced than actin loss in CIM. Protein quality control is altered by inadequate autophagy. Ca2+ dysregulation is present through altered Ca2+ homeostasis. We highlight clinical hallmarks, trigger factors, and potential mechanisms from human studies and animal models that allow separation of risk factors that may trigger distinct mechanisms contributing to weakness. During critical illness, altered inflammatory (cytokines) and metabolic pathways deteriorate muscle function. ICUAW prevention/treatment is limited, e.g., tight glycemic control, delaying nutrition, and early mobilization. Future challenges include identification of primary/secondary events during the time course of critical illness, the interplay between membrane excitability, bioenergetic failure and differential proteolysis, and finding new therapeutic targets by help of tailored animal models.
I. INTRODUCTION
“In adults the disease may attack persons in good health, but who are overworked or ‘run down’ from any cause. Haemorrhage initiates the attack in a few cases. There may be repeated chills; the temperature is high, the pulse rapid, and the respirations are increased. The loss of flesh and strength is very striking.” This description of the symptoms in a condition of acute critical illness, in acute broncho-pneumonic phthisis, by Sir William Osler in his classical textbook on “principles and practice of medicine” (531) is one of the first analytical observations linking an aberrant systemic inflammatory response to a failure of the peripheral nervous system, i.e., muscle wasting and weakness. This condition can either affect the peripheral nerves (critical illness polyneuropathy, CIP), skeletal muscle (critical illness myopathy, CIM) or both (critical illness polyneuromyopathy, CIPNM) (6, 402, 404, 747). These conditions are the primary cause of muscle weakness and paralysis during and following critical illness (402). Weakness appears to be triggered by critical illness and the ICU course regardless of the underlying primary condition (382, 453, 528). The chance of developing muscle weakness correlates with the severity of critical illness, as assessed by several organ dysfunction scores (149, 181, 370, 763), and represents a pathophysiological process that cannot be explained by immobilization alone (181). Mechanical ventilation and muscle immobilization, severe sepsis, and multiple organ dysfunction as well as neuro/myotoxic agents are among the primary risk factors of ICU-acquired neuromyopathy (215, 302, 747). With the advancements in modern intensive care medicine, mortality of sepsis-related ICU conditions has dropped; however, at the cost of a growing incidence of such intensive care unit (ICU)-acquired weaknesses (ICUAW) (64, 332, 622). As sepsis ranks among the top three causes of mortality in some Western countries (185), with incidences of weakness as high as 50–100% in critically ill patients (145, 361, 781), ICUAW is becoming a major problem. Its prevalence not only influences patient prognosis but also bears threats for secondary complications (infection, embolism, etc.), prolongs ICU treatment and rehabilitation, and greatly raises the costs of intensive care medicine worldwide (406, 458). As will be detailed later, the recently introduced term ICUAW (618) shall be considered a syndromal umbrella term and is not synonymous with CIM or CIP “per se” but more pragmatically describes a syndromal complex of weakness in critically ill patients who may be suffering from different pathophysiological entities. For most of these ICUAWs, specific therapies do not yet exist, i.e., for CIP and CIM, because of our patchy knowledge of their underlying pathological mechanisms. However, recent research has identified several interplaying pathways and candidates that might serve as therapy targets. An important issue in dissecting the different entities of ICUAW and their pathophysiological mechanisms, the definition of proper animal models that as closely as possible mimick the phenotype of muscle weakness seen in ICU patients, is crucial. Therefore, in addition to summarizing and discussing the paucity of pathophysiological data, our review will also provide a special emphasis on such animal models and compare the pros and cons involved.
II. HISTORY, TIME COURSE, and CLINICAL FEATURES OF ICU-RELATED WEAKNESS
In the 19th century, patients with life-threatening infections were reported to lose “a lot of flesh and strength” (531). During the next decades, occasional case reports indicated the occurrence of polyneuropathies in coma patients following shock, cardiac arrest, and acute intoxication and in burn patients (65). However, it took another century before this syndrome was described in more detail and was labeled as CIP (67). Almost simultaneously, several authors reported the occurrence of profound flaccid and symmetrical weakness after a severe disease (35, 66, 68, 134, 590, 812). The weakness was accompanied by muscle wasting and often, but not necessarily, by reduction or loss of tendon reflexes but with a relative sparing of the facial muscles. This typically results in grimacing of the facial muscles upon painful limb stimulation but with evoking only limited to absent limb movement. Distal muscles (781, 812) and lower limbs (68, 206, 360, 406, 414) appeared most severely affected. The predominant symptom pointing to the presence of CIP was often weaning failure, as respiratory muscles are also involved (52, 145, 414, 781). Sensory loss, including reduced sensitivity to pain, temperature, and vibration, was variably present (414, 808), but sensory testing in these patients is often unreliable (52, 64, 808, 812) (Table 1). Electrophysiological studies revealed reduced compound motor action potentials and sensory nerve action potentials, variably accompanied by the widespread presence of fibrillation potentials or positive sharp waves. In contrast to the Guillain-Barré syndrome where a demyelination of peripheral nerves results in delayed action potential conduction, nerve conduction velocity was found to be normal or near normal (52, 68, 126, 414, 781, 808, 812). Biopsy and autopsy findings confirmed that the underlying problem was a primary distal axonal degeneration of motor and sensory fibers (44, 67, 68, 193, 812). Later, it was shown that in some patients with electrophysiological evidence of CIP, nerve histology was normal (403), indicating that functional changes may precede structural changes. Consistent with this, rapidly reversible neuropathy was later described (515). Whether this concerns a distinct entity or a different grade of severity is yet unresolved (Table 1). When considering weaning failure, electrophysiological and autopsy studies supported the involvement of the phrenic nerve, diaphragm, as well as the intercostal and other accessory muscles in the process of axonal degeneration and denervation-associated muscle atrophy (450, 611, 781, 808, 812). Recovery from CIP first occurs in the upper and proximal lower limbs. This is then followed by recovery of the respiratory system and finally by the distal lower limbs (812).
Table 1.
Clinical and histological characteristics of CIP and CIM
| Clinical Signs | Spectrum of Histological Findings |
|---|---|
| Critical illness polyneuropathy | |
| Flaccid, symmetrical atrophy, and weakness of the limbs | Nerve:* |
| Distal>proximal | Normal |
| Lower limbs>upper limbs | Mildly reduced myelin fiber density with sporadic axonal degeneration |
| Facial muscles mostly spared | Marked fiber loss with abundant degenerative changes |
| Deep tendon reflexes mostly reduced to absent but may be preserved | Variable fiber regeneration |
| Variable distal sensory loss to pain, temperature, and vibration | Muscle: |
| Weaning failure | Denervation atrophy |
| Critical illness myopathy | |
| Flaccid, symmetrical atrophy and weakness of the limbs and neck flexors | Nerve: |
| Normal | |
| Proximal>distal | Muscle: |
| Fiber atrophy with abnormal variation in size of muscle fibers, angulated fibers, fatty degeneration, fibrosis; mostly affecting both fiber types, may be limited to type II fibers | |
| Facial muscles mostly spared | Focal or diffuse loss of thick myosin filaments |
| Deep tendon reflexes mostly reduced to absent but may be preserved | Scattered to more prominent myonecrosis, vacuolization, and phagocytosis of muscle fibers |
| No sensory loss | |
| Weaning failure | |
No evidence of primary demyelination or inflammatory infiltrates.
In parallel with the description and characterization of CIP, a case of acute quadriplegia developing in a patient treated with corticosteroids and neuromuscular blocking agents was reported (448). Several other authors, by use of muscle biopsies, confirmed the occurrence of an acute myopathy in the intensive care unit, associated with the use of corticosteroids and neuromuscular blocking agents (37, 141, 165, 314, 382). These patients exhibited preferential myosin loss and also demonstrated flaccid weakness and failure to wean from the respirator. Terms used for this syndrome include acute quadriplegic myopathy, acute myopathy of the intensive care, critical care myopathy, acute illness myopathy, and acute myopathy with selective loss of myosin filaments (69). As many critically ill patients, including those with sepsis and multiple organ failure, appeared to have primary muscle involvement not secondary to muscle denervation (125, 403, 812), the term critical illness myopathy was introduced (383, 653, 811). Muscle histology studies (Table 1) further identified other myopathic changes, in addition to preferential myosin loss, which have been variably classified as subtypes of critical illness myopathy (69, 70, 87, 332, 402, 535). In some patients muscle necrosis was present (297, 331, 360, 383, 384, 812), accompanied by variable elevation of creatine kinase levels (69, 150, 382, 383, 384). Finally, in some myopathies, histological changes may be limited to overall atrophy (382, 383), which may be more pronounced in fast type II (IIa and IIx in humans; IIa, IIx, and IIb in rodents) fibers (59, 279) or even be absent in the myopathy with purely functional impairment (402). The generalized flaccid weakness in CIM may be more proximally pronounced (384). Tendon reflexes are generally attenuated. Facial and extraocular muscles are generally spared but may occasionally be involved (100, 382, 529, 643). As in CIP, weaning failure is reported as a dominant symptom (382, 383). CIM itself does not account for changes in sensory nerve function. Table 1 lists clinical symptoms that may differentiate between CIM and CIP.
Accumulating evidence from studies involving detailed electrophysiological testing, including direct muscle stimulation, and muscle histology revealed that both neuropathy and myopathy may co-occur in patients (43, 147, 150, 360, 361). Interestingly, myopathy rather than neuropathy appeared to be the most frequent cause of weakness (43, 125, 297, 370, 383, 403, 413, 577, 583, 614, 764). This led to the concept that CIP and CIM are part of a spectrum of neuromuscular diseases affecting critically ill patients, rather than being separate and distinct disorders (43). The co-occurrence may imply a common pathogenic factor, affecting both the nerves and the muscle in critically ill patients (112, 164, 361, 403). An alternative explanation may be that denervation due to CIP results in increased vulnerability of muscles to several noxious stimuli (70, 145, 382). On the other hand, the diseased muscle might also signal back to affect innervation and the neuromuscular junction. Clinical examination usually cannot differentiate between CIP and CIM as key clinical features are similar and sensory evaluation is typically unreliable (382, 383, 407). Conventional electrophysiological screening including nerve conduction studies and needle electromyography also does not distinguish between the two entities (403). Both in CIP and CIM, reduced compound motor action potentials (CMAPs) are found. Abnormal spontaneous electrical activity presenting as fibrillation potentials or positive sharp waves may be due to denervation in CIP but also may occur in CIM due to muscle sodium channel dysfunction (580, 581) or secondary involvement of nerve endings in primary myopathy (126, 403, 577, 742). Terms such as critical illness polyneuromyopathy or the acronym CRIMYNE subsequently emerged (401, 405, 528). To emphasize the clinical problem of muscle weakness, regardless of its cause, descriptive terminology such as ICU acquired paresis, critical illness neuromuscular abnormalities, acquired neuromuscular disorders, or ICU-acquired (muscle) weakness was launched (145, 618). Although encompassing both entities is useful from a practical point of view, some evidence suggests that for prognostic reasons, it may be relevant to differentiate between CIP, CIM, and other myopathies (381). Early data indicated similar outcome for CIP and CIM (383), but recently it was shown that patients with CIM may recover earlier and better than those with CIP (273). The co-occurrence of CIP with CIM appeared to hamper recovery (370) and was responsible for persisting disabilities (344); however, the majority of patients recover within the first 6–12 mo (190, 504).
Although CIM and CIP are mostly described in association with adult and aged ICU patient populations, they are also a problem in pediatric ICUs (608); however, occurrence of ICU-related weakness in children has only occasionally been reported. From the existing literature it is concluded that clinical and electrophysiological conditions are similar in adults and children (779), but there is still a lack of clinical and animal studies elucidating the significance and nature of weakness in critically ill children.
III. RISK FACTORS and CLINICAL DIAGNOSTIC TOOLS FOR CIP/CIM
A. Risk Factors for the Development of ICU-Acquired Neuromuscular Complications
Several risk factors have been implicated in the development of neuromuscular complications in the ICU (Figure 1A). Several studies attempted to identify independent risk factors by performing multivariable analysis on data obtained during prospective observational studies (44, 100, 147, 149, 231, 499, 763). Additional information on risk factors was based on a number of randomized controlled trials (RCTs) intervening with a particular risk factor and reporting on neuromuscular complications, although in none of these neuromuscular outcome was the primary end point (Table 2).
FIGURE 1.
Risk factors contributing to the development of peripheral nervous system dysfunction seen as muscle weakness in ICU patients and potential differences in disease entities. A: in intensive care unit (ICU) patients, several conditions and risk factors have been associated with the development of muscle weakness pointing to a failure in the peripheral nervous system. Clinically, in the past, the presence of “weakness” has pragmatically been lumped together in the term ICU-acquired weakness (ICUAW), although this term neither discriminates between primary neuropathy or primary myopathy, nor does it account for potentially different mechanisms leading to neuropathy/myopathy. Apart from steroids, denervation, and sepsis, mechanical ventilation and immobilization itself is a prominent risk factor that contributes to the development of ICU-related myopathies. Critical illness myopathy (CIM) seen in critically ill patients is almost exclusively associated with altered muscle excitability, severe atrophy, and a preferential myosin loss, usually seen in critically ill patients who are exposed to several trigger factors at once. ICUAW, on the other hand, is a clinically pragmatic term that can be applied in the absence of any further special electrophysiology or biopsy testing. B: the separation of the distinct disease entities summarized under the term ICUAW as well as unraveling the specific differences of the underlying pathophysiological mechanisms defining those separate disease entities is still a matter of active research, and results from animal models suggest that some of those trigger factors on their own may be able to produce CIM while others may not (e.g., pure sepsis without steroid/denervation and/or mechanical ventilation/immobilization). In ICU patients suffering ICUAW, trigger factors usually combine, and septic ICU patients that are ususally subjected to mechanical ventilation and muscle unloading will present with CIM rather than SIM.
Table 2.
Randomized trials intervening with presumed risk factors and reporting on neuromuscular outcome using electrophysiological or clinical evaluation
| Effect of Intervention on Neuromyopathy |
Other Independent Risk Factors |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference No. | Population | Diagnosis | Intervention | Intervention | Control | P value | Risk factors | Relative risk (95%CI) | P Value |
| 735 | SICU ≥7 days | Electrophysiological diagnosis (SEA) | IIT | 46/181 (25%) | 109/224 (49%) | <0.0001 | Vasopressor support | OR: 1.06 (1.02–1.11) | 0.009 |
| 738 | Duration of ICU stay | OR 1.05 (1.03–1.08) | <0.0001 | ||||||
| RRT | OR 1.9 (1.0–3.8) | NA | |||||||
| Bacteremia | OR 2.3 (1.3–4.1) | NA | |||||||
| 658 | ARDS ≥7days | Pragmatic diagnosis* | Corticosteroids | 26/88 (30%)+ | 21/91 (22%)+ | 0.2 | NA | NA | NA |
| 654 | Septic shock <72 h | Clinical diagnosis | Corticosteroids | 2/234 (1%) | 4/232 (2%) | NS | NA | NA | NA |
| 309 | MICU ≥7 days | Electrophysiological diagnosis (SEA) | IIT | 81/208 (39%) | 107/212 (51%) | 0.02 | Age | OR 0.98 (0.96–0.99) | 0.003 |
| Prolonged NMBA | OR 2.01 (1.10–3.99) | 0.02 | |||||||
| CS (protective) | OR 0.97 (0.94–0.99) | 0.02 | |||||||
| 536 | ARDS ≤48 h | Clinical diagnosis MRC sums score <48 | 48 h of NMBA | 40/112 (36%) | 28/89 (31%) | 0.5 | NA | NA | NA |
| 623 | MV ≤72 h | Clinical diagnosis MRC sum <48 | Early physical and occupational therapy | 15/49 (31%) | 27/55 (49%) | 0.09 | NA | NA | NA |
| 300 | MICU or SICU ≥8 days and random set of short-stayers | Clinical diagnosis MRC sum <48 | Late versus early supplementation of insufficient enteral nutrition in the first week in ICU | 105/305 (34%) | 127/295 (43%) | 0.03 | Age | OR 1.03 (1.01–1.05) | 0.001 |
| APACHE II | OR 1.08 (1.04–1.11) | <0.001 | |||||||
| Sepsis | OR 2.20 (1.30–3.71) | 0.003 | |||||||
| Admission categories (as compared with medical) | 0.03 | ||||||||
| Emergency surgical ICU | OR 0.77 (0.42–1.43) | 0.4 | |||||||
| Elective surgical ICU | OR 2.61 (0.77–8.88) | 0.12 | |||||||
| Cardiac surgery | OR 1.8 (0.80–4.05) | 0.15 | |||||||
| Time to first MRC measurement | OR 1.05 (1.01–1.10) | 0.01 | |||||||
| CS | OR 2.70 (1.73–4.22) | <0.001 | |||||||
| NMBA | OR 2.72 (1.65–4.51) | <0.001 | |||||||
| New infection | OR 2.75 (1.69–4.45) | <0.001 | |||||||
SICU, surgical intensive care unit; MICU, medical intensive care unit; ARDS, acute respiratory distress syndrome; SEA, spontaneous electrical activity; MRC, Medical Research Council; IIT, intensive insulin therapy; MV, mechanical ventilation; OR, odds ratio; CI, confidence interval; RRT, renal replacement therapy; NMBA, neuromuscular blocking agents; CS, corticosteroids; NS, not significant; NA, not available.
Presence of the terms “myopathy,” “myositis,” “neuropathy,” “paralysis,” or “unexplained weakness” in the medical record.
The first 88 patients were retrospectively evaluated.
Based on these data, several key players were suggested. First, the presence and persistence of sepsis, systemic inflammatory response syndrome (SIRS), and multiple organ failure appear to hold a central role. Sepsis was the main suspect in the early days of the discovery of CIP, as it was a common feature in the first case reports (67, 812), and the incidence of CIP reported in patients with sepsis was very high (52, 333). Sepsis, multiple organ failure, and its severity were found to be associated with CIP (166, 403, 415, 416). Sepsis, as such, was identified as an independent risk factor for neuromuscular complications (122, 300) as well as bacteremia (499, 738). The role of the presence and persistence of SIRS and multiple organ failure (44, 147, 149) as well as of certain inflammatory markers (763), however, were confirmed in several studies. As some of these trials specifically focused on septic patients, and sepsis is a major cause of SIRS and organ failure, sepsis was likely to be a crucial factor (146). Indirect evidence for the central role of sepsis is also provided by the independent association of the use of catecholamines (735) as well as central neurological failure (231), which develops earlier during sepsis, with neuromuscular complications. Furthermore, it is confirmed that severity of illness independently predicts the risk of neuromyopathy in critically ill individuals (44, 100, 149). As will be detailed later, animal models have allowed the separation of sepsis and other confounding risk factors, e.g., mechanical ventilation and muscle unloading, which are usually present in combinations in ICU patients. For instance, pure sepsis in animal models has not been able to reproduce hallmarks of the CIM phenotype (see below) when animals were not mechanically ventilated, mechanically unloaded, or subjected to a similar ICU-situation to mimick the situation in patients. However, in septic ICU patients, the CIM phenotype will most likely be seen because critically ill septic patients will almost always also be found subjected to mechanical ventilation, muscle unloading and other contributing factors.
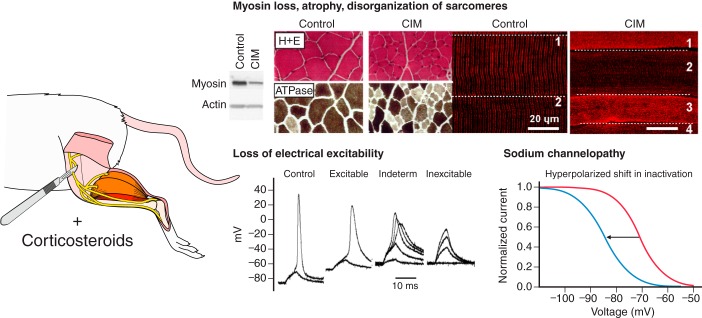
For further discussion of risk factors for ICUAW, it is necessary to define the different syndromes causing weakness. In particular, it is necessary to define what is meant by the term CIM. Many critically ill patients presenting with CIM show several symptoms and alterations on the organ, cellular, and subcellular level of affected muscle, not all of which may be detectable in each patient or at a given stage of investigation: electrical hypoexcitability of muscles; severe atrophy; preferential and significant myosin loss; disorganization of sarcomeres, ultrastructural abnormalities; and impaired autophagy and altered protein turnover.
Whether severe sepsis and sepsis-induced organ failure are already on their own sufficient to create the full-blown pathological manifestations of CIM (in particular the preferential myosin loss) remains unclear. There is no study to date showing preferential or selective loss of myosin secondary to sepsis in the absence of mechanical ventilation and immobilization. In animal models (see sect. XII) where sepsis and immobilization can be separated, sepsis does not trigger preferential loss of myosin pointing towards a differential pathophysiological process in pure sepsis.
Immobilizing patients and mechanically ventilating them during the acute phase of critical illness is suggested to contribute to muscle atrophy and weakness. By itself, the immobilization (pure bed rest) may not be sufficient to explain the degree of muscle atrophy and weakness observed in acutely ill patients (146). “Mechanical silencing” (loss of weight bearing and internal strain caused by activation of contractile proteins) and mechanical ventilation in animal models are sufficient to produce the preferential and significant myosin loss seen in patients (435, 517). Several clinical trials, however, found indicators of the duration of immobilization to be independently related to the occurrence of neuromuscular complications in critical care (147, 735, 781) and the presence of weakness up to 2 yrs after acute respiratory distress syndrome (ARDS) (190). Immobilization, therefore, is likely to contribute to CIM also in patients. This provided the basis for the development of trials to examine the effects of interventions aimed at reducing the duration of immobilization on ICUAW (see sect. XIII). Sedative and analgesic use, by association with time to awakening, can be expected to affect duration of immobilization, but was not found to be independently related with the risk of neuromyopathy in several trials (147, 149, 763).
Hyperglycemia was associated with neuromyopathy in retrospective (51, 306) and prospective studies (100, 147) and was also identified as an independent risk factor (499, 781) (Figure 1A). These findings launched the hypothesis that hyperglycemia might impair the microcirculation in the nerves, contributing to the development of CIP (64). In addition, during critical illness, organs depending on passive uptake of glucose, such as the nervous system, may be subjected to glucose overload resulting in acute neurotoxicity because of the hyperglycemia caused by insulin resistance (308). Hyperglycemia may also contribute to nerve excitability changes or increased generation and deficient scavenging of reactive oxygen species (303, 308). Such observations drove the exploration of restored neuromuscular function by normalizing hyperglycemia in critically ill patients (see sect. XIII).
Myopathy is a well-known adverse effect of glucocorticoid treatment (542). Corticosteroids reduce protein synthesis and increase protein breakdown, resulting in atrophy and weakness. Not surprisingly, after the first case report (448) many followed, suggesting corticosteroids as the culprit of myopathy occurring in critically ill subjects, especially in patients treated with a combination of corticosteroids and neuromuscular blocking agents. Results of prospective trials, however, were equivocal. Some found that corticosteroids were independently associated with neuromyopathy (100, 147, 311), whereas most other trials could not confirm this (44, 51, 149, 231, 499, 654, 658, 735, 763). Several explanations are possible for these divergent findings. First, in addition to the well-known catabolic effects, corticosteroids may improve outcome and shorten duration of organ failure in certain ICU populations, which by itself is an important risk factor for neuromyopathy (21, 22, 473). Although CIP and CIM are characterized by inflammatory infiltrates (150, 193), inflammatory processes are likely involved and corticosteroids could hypothetically downregulate potential detrimental pathways. Corticosteroids also induce hyperglycemia, another risk factor for neuromyopathy. Therefore, clinical effects of corticosteroids may be complex and the impact on the nerves and the muscles could be distinct. Effects may also depend on timing and dosing, as well as on controlling the resulting hyperglycemia (309).
The presumed harmful effects of neuromuscular blocking agents (NMBAs) were extensively reported in many case reports (265). Nondepolarizing agents, often combined with corticosteroids, were considered to be detrimental (165). Pharmacological denervation with neuromuscular blocking agents may increase muscle susceptibility to corticosteroids, as observed in anatomical denervation (466). Despite this well founded hypothesis, again, data from several trials did not consistently show negative effects (44, 149, 150, 190, 306, 499, 735, 763). In three trials, NMBAs were independently associated with neuromuscular complications (231, 300, 309). Type and duration of exposure to NMBAs could possibly explain differential findings. Recent data from an RCT (48 h of NMBAs versus placebo in ARDS patients) indeed did not find an increased incidence of weakness in the intervention group (536). In addition, earlier data may have been confounded by other treatment strategies associated with administration of NMBAs, such as ventilator strategies and sedation regimens no longer used in ICUs (562).
Two trials identified age as an independent predictor of neuromuscular complications in the ICU (300, 309). The role of nutrition has been debated since the first description of CIP. It was hypothesized that malnutrition was a major cause of ICUAW and that the catabolic process could be revoked by adequate parenteral nutrients (67). Some trials evaluating the role of parenteral nutrition, however, could not confirm an association (309, 499, 781). In one trial, parenteral nutrition was an independent risk factor for CIP (231), and in a recent RCT, early parenteral nutrition increased the incidence of ICU-acquired weakness (300). Deleterious effects of refeeding, high amounts of polyunsaturated fats, or metabolic derangements contributing to disturbances in the microcirculation were proposed as mechanisms (752). Autophagy is another pathway that may be involved in neuromuscular effects of nutrition in critically ill individuals. Autophagy is a cellular housekeeping system required for the elimination of damaged organelles and large protein aggregates (122). The process is important for maintaining muscle quality (465). Nutrients, especially amino acids, are powerful suppressors of autophagy (155, 247). Insufficient activation of autophagy was found in muscle biopsies of fed critically ill patients (728), and higher protein delivery in the first week of critical illness was even associated with greater muscle wasting (563). Therefore, muscle quality could be negatively affected by nutrition as shown in a recent RCT (300) (see sect. X).
Several other risk factors were identified in single trials, but current evidence for a role of these factors is limited: low serum albumin (781), hyperosmolality (231), female sex (147), aminoglycosides (499), and renal replacement therapy (738).
B. Aspects of Clinical Evaluation and Diagnostics
There are several potential approaches for diagnosing the problem of intensive care unit acquired weakness as a syndrome (ICUAW). In awake and cooperative patients, a bedside clinical approach can be used. Muscle strength can be quantified using the Medical Research Council (MRC) sum score, a validated ordinal scale which grades muscle weakness in six bilateral muscle groups from upper and lower limbs between 0 (no contraction) and 5 (normal strength) (Table 3). Adding these subscores results in a maximum sum score of 60 (369). ICUAW was defined as MRC sum score for muscle strength to be <48, provided no other cause of weakness could be identified (147). Since then, many other publications have used the MRC sum score to diagnose ICUAW (10, 44, 51, 143, 144, 149, 189, 273, 305, 325, 413, 499, 536, 598, 623, 632) without referring to the precise underlying disease entity (Figure 1B). This clinical approach revealed that weakness affects proximal muscles more than distal muscles (147, 305, 413). The MRC sum score was proposed as a routine and first line screening tool in the ICU for patients at risk for developing ICUAW (268, 402, 622, 661). As reported in various clinical settings [mechanically ventilated patients with acute stroke (267), Guillain-Barré syndrome (369), stimulants (189), post ICU (40, 325)], inter-rater reproducibility was found to be good in a large study of critically ill patients (301), and one smaller series (10), but not in two other small studies (129, 325). Methodological issues such as judging the adequate awakening and cooperation of the patient required to perform the test may be very important.
Table 3.
MRC sum score
| MRC Sum Score Criteria |
|---|
| Evaluation of adequate awakening |
| Open/close your eyes |
| Look at me |
| Open your mouth and put out your tongue |
| Nod your head |
| Raise your eyebrows when I have counted up to 5 |
| Muscle groups evaluated |
| Wrist extension |
| Elbow flexion |
| Shoulder abduction |
| Dorsiflexion of the foot |
| Knee extension |
| Hip flexion |
| Appointed scores |
| 0 no visible/palpable contraction |
| 1 visible/palpable contraction without movement of the limb |
| 2 movement of the limb, but not against gravity |
| 3 movement against gravity |
| 4 movement against gravity and some resistance |
| 5 normal force |
Also, the use of a detailed and stringent protocol adapted for bedridden patients may be crucial (305). MRC sum score <48 is associated with important clinical outcomes (Table 4) and is found to be an independent predictor of prolonged weaning, ICU and hospital stay, as well as ICU, hospital, and 180 day mortality (Table 4). In addition, MRC sum score <48 independently predicted 1 yr mortality (307) and pharyngeal dysfunction (487). The clinical approach is easy and cheap. Drawbacks include the requirement of adequate awakening and cooperation, which limits the number of critically ill patients actually evaluable. Reported figures range from 25% (325) up to 90% (51) during ICU stay. Pain may also interfere with maximal effort. Due to the ordinal character of the scale, subtle changes may not be detected. The MRC sum score only points to weakness but does not provide information on the underlying pathophysiological processes (Figure 1B). It is important to rule out other known causes of weakness, in particular when the clinical picture does not correspond to flaccid and symmetrical weakness, facial muscle sparing, and reduced to absent tendon reflexes. Finally, clinical muscle strength testing does not allow differentiating between a neuropathic and myopathic component of the weakness. Other clinical methods of measuring muscle strength in critically ill patients have been evaluated. These include hand-grip strength measurement using a dynamometer. This proved to be a reliable tool in the ICU with good interobserver reliability (29, 301). Hand grip strength showed good test performance in predicting MRC sum score <48 and was independently associated with mortality in a medical ICU (10). To avoid problems of maximal effort, use of electrical (181) or magnetic (288) muscle stimulation of the adductor pollicis has been described. Data on clinical impact of this kind of measurements are currently lacking. Respiratory muscles are frequently involved in ICUAW. Evaluation of respiratory muscle strength using maximal inspiratory pressure was also suggested as a surrogate marker for ICUAW because of good predictive value for MRC <48 (722) and its association with duration of weaning (143, 722). Similarly, bilateral anterior magnetic stimulation of the phrenic nerves, recording the resulting diaphragmatic contraction using gastric and esophageal balloons, appears feasible and safe, though complex and expensive and, therefore, not routinely applicable in the ICU. Reduced diaphragmatic muscle strength had been shown (299, 387, 760). A clear relationship between respiratory muscle weakness using this technique and worse outcome was recently demonstrated (153, 677). Another way to get around the problem of consciousness and full cooperation is the use of electrophysiological testing, e.g., in combination with direct muscle stimulation (see below).
Table 4.
Studies evaluating clinical outcomes associated with ICUAW determined using the MRC sum score
| Reference No. | Population | Incidence of ICUAW | Timing of Measurement | Univariate Analysis | P Value | Multivariate/Matched Analysis | Relative Risk (95% CI) or Weak Versus Not Weak | P Value |
|---|---|---|---|---|---|---|---|---|
| Prospective studies | ||||||||
| 144,147 | MV ≥7 days | 24/95 (25%) | D7 after awakening | ↑ Duration of MV | <0.001 | Prolonged weaning | HR 2.4 (1.4–4.2) | 0.001 |
| 143,632 | MV ≥7 days | 75/115 (65%) | At awakening | Low MRCb delays successful extubation | 0.001 | Low MRCb delays successful extubation | HR 1.96 (1.27–3.02) | 0.002 |
| ↑ICU mortality | 0.006 | ↑ICU mortality | OR 7.99 (0.99–64.29) | 0.05 | ||||
| ↑Hospital mortality | 0.02 | ↑Hospital mortality | OR 2.02 (1.03–8.03) | 0.048 | ||||
| 499 | ICU stay ≥24 h | 50/474 (11%) | At awakening | ↑ICU mortality | <0.05 | NA | NA | NA |
| ICU stay >10 days | 44/185 (24%) | ↑ICU mortality | <0.05 | |||||
| 10 | MV ≥5 days | 35/136 (26%) | At awakening | ↑Duration of MV | <0.001 | ↓Hospital free days | 41% (12–60%) | 0.007 |
| ↑ICU stay | <0.001 | ↓ICU free days | 54% (67–36%) | 0.001 | ||||
| ↓ICU-free to day 30 | <0.001 | ↑Hospital mortality | OR 7.8 (2.4–25.3) | 0.001 | ||||
| ↑Hospital stay | <0.001 | |||||||
| ↓Hospital-free to day 60 | <0.001 | |||||||
| ↑ICU readmission | 0.01 | |||||||
| ↑Discharged to other location than home | 0.01 | |||||||
| ↑Hospital mortality | <0.001 | |||||||
| 82 | SIRS and MV ≥48 h | 13/39 (33%)a | ICU discharge | ↑Duration of MV | 0.001 | ↑Death before 180 days | NA | 0.009 |
| ↑ICU stay | 0.005 | |||||||
| ↓Functional outcome (Barthel index) at D28 | 0.01 | |||||||
| ↑Death before 180 days | 0.03 | |||||||
| ↑Resources needed (cumulative TISS-28 scores) | 0.008 | |||||||
| 722 | ICU stay ≥7 days | 17/33 (51%) | At awakening | Prolonged weaning | <0.05 | NA | NA | NA |
| ↑ICU stay | 0.001 | |||||||
| ↓MIP | <0.001 | |||||||
| 410c | SICU | 44/95 (46%) | At awakening | ↑ICU stay | 0.002 | ↑ICU stay | IRR 0.98 (0.97–0.98) | <0.001 |
| ↑Hospital stay | 0.001 | ↑Hospital stay | IRR 0.97 (0.97–0.98) | <0.001 | ||||
| ↑Mortality | 0.006 | ↑Hospital mortality | OR 0.95 (0.90–0.99) | 0.04 | ||||
| ↑Duration of MV | 0.01 | |||||||
| 304 | ICU stay ≥8days | 227/415 (55%) | At awakening | ↑Time to alive weaning | <0.001 | ↑Time to alive weaning | 11d (7–22) versus 8d (5–14) | 0.009 |
| ↑Time to alive ICU discharge | <0.001 | ↑Time to alive ICU discharge | 8d (2–14) versus 3d (0–8) | 0.008 | ||||
| ↑ICU mortality | 0.02 | ↑Time to alive hospital discharge | 36d (16–83) versus 23d (13–41) | 0.007 | ||||
| ↑Time to alive hospital discharge | <0.001 | ↓ 6 min WD | 66m (0–207) versus 191m (90–270) | 0.01 | ||||
| ↑Hospital mortality | <0.001 | ↑Total billed hopsitalization costs | 23,277€ (15,370–36,147) versus 17,834€ (12,227–31,306) | 0.04 | ||||
| ↓6 min WD | 0.002 | ↑1 yr mortality | 30.6% versus 17.2% | 0.014 | ||||
| ↑Total billed hopsitalization costs | <0.001 | |||||||
| ↑1 yr mortality | <0.001 | |||||||
| 487 | Long-term ventilated (≥10 days), FEES clinically indicated | 20/30 (67%) | Within 24 h of FEES | Pharangeal dysfunction | Pharangeal dysfunction | |||
| PAS >1 | 0.038 | PAS >1 | 9 (1.3–61.14) | 0.038 | ||||
| VPSR >1 | 0.014 | VPSR >1 | 5.4 (1–28.5) | 0.014 | ||||
| Symptomatic aspiration (retrospective analysis) | 0.003 | Symptomatic aspiration | 9.8 (1.6–60) | 0.009 | ||||
| Retrospective studies | ||||||||
| 51 | ARDS | 27/45 (60%) | At awakening | ↑ICU stay | 0.001 | NA | NA | NA |
| ↑Duration of MV (at 28d) | 0.02 | |||||||
MV, mechanical ventilation; ICUAW, intensive care unit acquired weakness; MRC, medical research council; ICU, intensive care unit; NA, not available; CI, confidence interval; HR, hazard ratio; OR, odds ratio; IRR, incidence rate ratio. All studies used MRC <48 to diagnose ICUAW and to examine relationship with outcome unless explicitly stated:
ankle dorsiflexion not included, ICUAW defined as sum score <35/50;
MRC sum score ≤41;
MRC sum score used as a continuous variable in the predictive models. Additional univariate analysis with MRC cut-off of 48 showed significant association with days on MV, ICU, and hospital stay.
C. Clinical Diagnostic Tools and Potential Contributing Mechanisms
Determining mechanisms contributing to weakness in the acute setting is complicated by a number of factors. One problem is that critically ill patients often cannot cooperate with motor and sensory examination due to sedation or encephalopathy. Because patients cannot cooperate, they are unable to voluntarily recruit motor units during EMG assessment. This prevents use of motor unit morphology and recruitment to distinguish between neuropathy and myopathy. Many studies have used the presence of spontaneous activity on EMG as an indicator of denervation and thus the presence of neuropathy. However, spontaneous activity is also present in CIM, such that this has led to over-diagnosis of neuropathy as the mechanism underlying weakness. To further complicate interpretation of motor unit recruitment during EMG, a recent study in rats has identified reduced motor neuron excitability as a possible contributor to reduced motor unit recruitment (500). Thus, while classic nerve conduction/EMG can be used to serially follow development and resolution of neuropathy and myopathy, it should not be used in isolation to determine mechanisms underlying weakness.
To distinguish between neuropathy and myopathy, more detailed electrophysiological tests, like direct muscle stimulation to measure muscle fiber conduction velocity (12) and velocity recovery cycles of muscle action potentials (801), are needed. In nerve, specialized tests such as strength-duration time constant, threshold electrotonus, current-threshold relationship, and recovery cycles are necessary to identify reduced excitability (800). If nerve and muscle excitability are normal, but sensory and motor amplitudes are reduced, it is likely that axon degeneration is present and the patient has critical illness neuropathy. If small motor units are present that are recruited in a myopathic fashion, it is probably safe to conclude myopathy is present. Muscle biopsy can be used to study atrophy (myofibrillar protein loss equally affecting actin and myosin without change in acto-myosin ratios in pure atrophy) or preferential myosin loss (with significantly decreased myosin-to-actin ratios as in CIM patients and animal models of CIM). Ultrasound might be used to follow muscle atrophy serially. Nerve biopsy is needed to study axon loss. Serum CK can give some estimate of muscle fiber death. By focusing on mechanisms that underlie ICUAW, it may be possible to get a better indication of prognosis. Different mechanisms that contribute to weakness are likely to have a different prognosis for recovery. For example, it appears that loss of electrical excitability in muscle and nerve may be fully reversible and thus have a good prognosis for recovery (515, 583). Other changes such as axon degeneration almost certainly have a poor prognosis. The prognosis of severe myosin loss and severe muscle atrophy are less clear.
Mechanisms underlying weakness may vary with time. Within a few days of onset of critical illness, nerve conductions reveal reduced sensory and motor amplitudes (361, 698, 699). One likely contributor to early reduction in nerve conduction amplitudes is ion channelopathy (515). As available evidence suggests reduction/loss of electrical excitability is fully reversible (515, 583), it is unlikely to be a mechanism underlying chronic weakness. This suggests that there may be an evolution of mechanisms contributing to weakness such that mechanisms underlying acute weakness (initially and within a few days) differ from those contributing to weakness during the ongoing course of critical illness (e.g., within weeks of critical illness). In particular, there may also be phases of various pathophysiological mechanisms contributing to the development of subsequent mechanisms, e.g., membrane dysfunction and depolarization triggering Ca2+ imbalance, metabolic dysregulation which may lead to atrophy and preferential myosin loss (Figure 2). Determining whether mechanisms contributing to weakness evolve over time will require longitudinal use of both electrophysiology and pathology studies and clearly represents a challenge for future clinical and animal studies.
FIGURE 2.

Specific pathophysiological mechanisms in ICUAW may either evolve in time independently or trigger subsequent pathways to present with phases of myopathy during critical illness. Early phases may begin as early as from 24 h of ICU treatment and are characterized by a dysfunction of membrane excitability (hypoexcitability), followed by subcellular putative alterations in Ca2+ homeostasis, bioenergetics, and motor protein function, whereas later phases after several days to 1 wk are marked by hyperproteolysis of myofibrillar proteins (atrophy) and/or preferential myosin loss. Severe loss of sarcomeric proteins may also be responsible for the structural fixation of the disease seen as altered cytoarchitecture and loss of contractile filaments that are only slowly regenerated over months. Note that each mechanism is not limited to a particular phase, and mechanisms may coexist at late stages (i.e., membrane hypoexcitability, myosin loss, impaired autophagy, etc.).
IV. INTRODUCTION TO PATHOPHYSIOLOGICAL MECHANISMS
Multiple mechanisms contribute to development of weakness during critical illness. There seem to be numerous, either independent or interacting, mechanisms that have been identified that contribute to muscular weakness triggered by critical illness (Figure 2) of which some are 1) reduced excitability of muscle and nerve, 2) altered Ca2+ homeostasis, 3) myosin loss following mechanical silencing, 4) atrophy of muscle, 5) death of axons, 6) death of muscle fibers, 7) disturbed anabolism-to-catabolism ratio, 8) bioenergetic failure, and 9) failure of neuromuscular transmission, to name only some that are explained in more detail in the subsequent sections. These will predominantly also focus on the myopathy aspect of critical illness. It is not known whether myopathic mechanisms develop simultaneously or independently, and there may be distinct phases underlying the clinical phenotype of “myopathy” that are inherent to different pathophysiological mechanisms (Figure 2). To develop effective therapy, it may be necessary to prevent multiple contributors to weakness. If all the mechanisms underlying weakness were triggered by the same signal, development of therapy would be straightforward, but if mechanisms underlying weakness were triggered independently by different signals, multiple interventions would be necessary.
V. INFLAMMATORY CYTOKINES IN CRITICAL ILLNESS
A. Cytokine Elevation in Critical Illness
A half-century of research implicates proinflammatory cytokines as potential mediators of critical illness. This concept originally emerged in animal studies designed to characterize individual cytokines. For example, circulating interferon (IFN) levels were markedly increased in mice following endotoxin administration or bacterial infection (663) or in guinea pigs subjected to burn injury (709). In discovering tumor necrosis factor-α (TNF-α), Carswell et al. (106) found that plasma TNF-α levels were increased by administering endotoxin to mice, a finding later extended to rats and rabbits (521). Although these early reports were limited in scope and narrowly focused, they laid the foundation for an explosion of basic science research in the field. It became clear that IFN, TNF-α, members of the interleukin (IL) family, and other cytokine mediators are highly sensitive to experimental conditions that are related to critical illness. A partial listing with representative references includes sepsis (241, 328), mechanical trauma (246), surgery (670, 802), pneumonia (71, 527), drug-induced organ failure (660), thermal injury (484, 743), and peritonitis (36).
Studies of critically ill patients provide more-specific information on cytokine regulation in the clinical setting. An early study by Sullivan et al. (669) tested the hypothesis that plasma cytokine levels might correlate with illness severity or mortality rate in septic children. They found that plasma TNF-α, IL-1, and IL-6 levels were elevated in patients. Cytokine levels correlated with mortality rate; all three cytokines were higher in nonsurvivors than in patients who survived. Damas et al. (140) measured TNF-α, IL-1β, and IL-6 levels in 40 critically ill surgical patients where IL-6 was a robust marker of illness, correlating with both APACHE II score and mortality.
Subsequent refinements in immunoassay technology have enabled broader documentation of cytokine profiles in the clinical setting. Sepsis severity, the evolution of organ failure, and patient death were associated with distinct multicytokine profiles. Hranjec et al. (326) evaluated blood levels of IL-1, -2, -4, -5, -6, -8, -10, and -12, IFN-γ, TNF-α and granulocyte-macrophage colony-stimulating factor (GM-CSF) within 48 h of admission to the ICU in a large patient number. Patients were subclassified based on evidence of infection or trauma. On admission to ICU, specific cytokine profiles were associated with three patient subgroups: patients who lacked infection or trauma (IL-6, -8, -10), infected patients who died (IL-2, -8, -10, GM-CSF), and trauma patients who died (IL-4, -6, -8, TNF-α). Elevation of individual cytokines predicted death in trauma patients (IL-4) and patients with neither trauma nor infection (IL-8) but not in patients with infection. It is interesting to note that in this study, ∼10% of patients in all groups were not mechanically ventilated. However, mechanical ventilation was not separated as an independent variable in the analysis of cytokine profiles. A more targeted study measured circulating cytokines during the first week after admission in 30 septic patients (480). They found that early elevation of IL-8 and monocyte chemoattractant protein (MCP-1) predicted mortality, whereas specific multi-cytokine profiles were associated with either organ failure or shock. Proinflammatory cytokines are suggested to promote muscle atrophy and weakness in critically ill patients (780). Not all cytokines are implicated. Rather, a subset of cytokines has been linked to protein loss or muscle dysfunction in critically ill patients. As outlined in Table 5, these clinical associations were strengthened by mechanistic studies using animal models, isolated tissue preparations, and cell culture systems. Over the past four decades, this vertically integrated approach has generated substantial evidence that cytokines can compromise skeletal muscle performance. The following sections highlight information on three cytokines that are best understood in this context.
Table 5.
Inflammatory mediators associated with critical illness and effects in muscle
| Inflammatory Mediator | Molecular Mass, kDa | Effects in Skeletal Muscle |
|---|---|---|
| TNF-α (cachectin) | 26 (mature secreted form: 17) | Also expressed in type II muscle fibers (550) |
| Depolarization of plasma membrane (712) | ||
| Na+-current inhibition, left shift of Na+ channel activation and inactivation (274) | ||
| Activation of PKC-mediated Na+ channel phosphorylation (148) | ||
| Induces muscle proteolysis and atrophy (148) | ||
| Alters resting Ca2+ levels (decrease in myotubes) (741) | ||
| No change in tetanic Ca2+ amplitudes in muscle fibers (741) | ||
| Decreases Ca2+ transient amplitudes by 50% (in myotubes) (741) | ||
| Depresses tetanic force production in limb and diaphragm muscle (571) | ||
| Activates ubiquitin-proteasome pathway (227,428) | ||
| Increase major histocompatibility complex II surface expression (in combination with IFN-γ) in human muscle cells (359) | ||
| Activation of NF-κB (573) | ||
| Downregulation of MyoD inhibits myogenic differentiation (280) | ||
| Induces insulin resistance in muscle (248) | ||
| IL-1 | 13–17 (precursors: 33) | Produced by activated macrophages; also expressed in muscle fibers in health (619), following exercise (101), and in inflammatory myopathies (26) |
| IL-1α: primarily membrane-bound | Associates with RyR1, blocks SR Ca2+-release, and decreases SR Ca2+ leak in muscle fibers (220) | |
| IL-1β: secreted form | Upregulates iNOS expression (4) | |
| Induces skeletal muscle proteolysis [IL-1α (785), but maybe not IL-1β (228)] | ||
| Stimulates IL-6 production in skeletal muscle cells (IL-1β) (445) | ||
| Stimulates prostaglandin E2 (PGE2) production and proteolysis (250) | ||
| Ubiquitin gene expression ↑ (437) or ⇆ (228) | ||
| Inhibition of protein synthesis (132) | ||
| IL-6 | 23–30 (glycosylation diversity) | Produced by activated macrophages and T-cells, tumor cells, but also produced by muscle fibers (type I > type II) (550) |
| No effect on ubiquitin expression (437) | ||
| Promotes infiltration of myocytes with prostaglandins (780) | ||
| Both pro- and anti-inflammatory actions in critical illness (24) | ||
| Induces skeletal muscle protein breakdown (in rats) (259) | ||
| Reduces insulin-stimulated glucose uptake in muscle (364) | ||
| IL-10 | 18 (unglycosylated in humans) | Anti-inflammatory cytokine (780) |
| Prevents skeletal muscle from IL-6-induced defects in insulin action (364) | ||
| IFN-γ | 20–25 | Produced by activated T-cells and natural killer cells but also in muscle fibers following muscle injury (118) |
| Required for muscle regeneration (118) | ||
| Inhibits protein translation in muscle by stimulating NO synthase activity (223) | ||
| Modulator of TNF-α signaling in muscle (myotubes: downregulation of TNF-R2 and increased NF-κB activity) (710) | ||
| Ubiquitin gene expression ↑ (437) |
B. TNF-α, Muscle Atrophy, and Contractile Dysfunction
TNF-α is the proinflammatory cytokine that has been studied most extensively for effects in skeletal muscle. The protein is synthesized as a 26-kDa parent molecule that is cleaved upon cellular release to form a 17-kDa polypeptide that promotes anti-tumor and immune responses. This molecule was originally named “cachectin” to acknowledge its association with catabolic states of cachexia. Circulating TNF-α concentrations are commonly elevated in critically ill patients (see above) and in animal models of critical illness, e.g., lipopolysaccharide administration (636), peritonitis (507), trauma (539), heart failure (20), and burn injury (799). Increases in TNF-α promote muscle weakness via two general mechanisms: 1) promotion of atrophy and 2) induction of contractile dysfunction.
1. TNF-α promotes muscle atrophy
This was first demonstrated in animal studies where systemic administration of exogenous TNF-α decreased muscle mass and muscle fiber cross-section (83, 226). Initially, TNF-α was thought to be an indirect actor, e.g., via anorexia (485) or glucocorticoid elevation (472), since direct exposure to TNF-α for up to 3 h did not stimulate protein loss in excised muscle preparations (229, 251, 591). However, Li and colleagues (420, 421) later showed a direct catabolic action of TNF-α on differentiated muscle cells. Incubation of cultured myotubes with a clinically relevant TNF-α concentration caused progressive reductions in total protein content, muscle-specific protein levels, and myotube diameter over several days. These changes mimic the response of intact skeletal muscle to critical illness, supporting a potential role for TNF-α. At the cellular level, TNF-α stimulates protein loss via receptor-mediated signaling events that alter muscle gene expression. Skeletal muscle constitutively expresses TNF-α receptor subtype 1 (TNFR1) and subtype 2 (TNFR2). Llovera and associates (438, 439) have shown that TNF-α acts via TNFR1 (and not TNFR2) to stimulate catabolism. In muscle, TNF-α exposure rapidly activates the transcription factor nuclear factor-κB (NF-κB) which is essential for loss of muscle protein (421). The canonical pathway for NF-κB activation is mediated by the I-κB kinase (IKK) complex (546) and is redox sensitive during TNF-α stimulation. NF-κB activation is positively modulated by a transient rise in muscle-derived reactive oxygen species (ROS), which function as second messengers for TNF-α (421), and is negatively modulated by the glutathione cycle (626). TNF-α also rapidly activates the mitogen-activated protein kinases (MAPKs) including p38MAPK, extracellular signal-regulated kinases 1 and 2 (ERK1/2), and c-Jun NH2-terminal kinase (JNK) in differentiated myotubes (418).
In muscle cells, these signaling events stimulate the expression of genes that regulate the ubiquitin-proteasome pathway. TNF-α signaling via NF-κB increases mRNA levels for E2-20k, a ubiquitin carrier protein and murine homolog of human UbcH2 (419). In parallel, TNF/p38MAPK signaling increases the expression of atrogin1/MAFbx, a ubiquitin ligase that mediates muscle atrophy in a variety of catabolic states (418). These changes are associated with TNF-stimulated increases in functional activity of the ubiquitin conjugating pathway. This is the major pathway for regulated degradation of muscle protein via the 26S proteasome. In muscle cells, the rise in ubiquitin conjugating activity is abolished by selective inhibition of p38MAPK (418), NF-κB (421), or E2-20k (419), demonstrating that each of these elements is essential for integrity of the response. Moreover, selective inhibition of NF-κB prevents loss of muscle protein stimulated by TNF (421), identifying NF-κB as a critical regulator of catabolism in response to this cytokine (see also sect. XI).
2. TNF-α and contractile dysfunction
TNF-α also depresses the force of muscle contraction in the absence of atrophy. This was first observed by Wilcox et al. (773) exposing fiber bundles isolated from hamster diaphragm to exogenous TNF-α in vitro. Isometric force normalized to fiber bundle cross-section (“specific force”) was significantly depressed by TNF-α. This was later confirmed in fiber bundles from guinea pig diaphragm (14), mouse diaphragm (423), and mouse flexor digitorum brevis (571). Contractile dysfunction is not simply an artifact of TNF-α exposure in vitro. Li et al. (423) observed a similar decrement in specific force of diaphragm fiber bundles isolated from transgenic mice engineered for cardiac-specific overexpression of TNF-α. In this case, the muscle was exposed to TNF-α in vivo (serum concentration 250–350 pg/ml) prior to excision; exogenous TNF-α was not used. Yet, specific force was approximately half the value of wild-type muscle for twitch and maximum tetanus. The muscle was stimulated directly, eliminating neuromuscular activation as a potential cause of weakness. There was no evidence of muscle atrophy or damage. Body weight, the weights of representative trunk and limb muscles, and the ultrastructure of limb and diaphragm muscle fibers were indistinguishable between transgenic and control animals. Thus chronic exposure to low levels of circulating TNF-α can profoundly weaken muscle fibers without inducing detectable atrophy.
Several studies have addressed the cellular mechanism of TNF-induced dysfunction. Hardin et al. (286) used mice deficient in TNFR1 and TNFR2 to assess the receptor subtype by which TNF-α depresses specific force. One hour following intraperitoneal TNF-α by injection, diaphragm fiber bundles showed marked depression of specific force. TNFR1 deficiency abolished this response, but TNFR2 deficiency did not. Thus, like atrophy, contractile dysfunction is mediated via TNFR1. Studies of post-receptor signaling have largely focused on muscle-derived oxidants as second messengers. Within minutes, TNF-α exposure increases cytosolic oxidant activity (286, 423, 656), which can depress specific force (570). Consistent with this model, preincubating muscle fiber bundles with N-acetylcysteine (NAC), a reduced thiol donor, abolished the effects of exogenous TNF-α (423). NAC pretreatment prevented the rise in cytosolic oxidant activity and preserved specific force at control levels. Similarly, pretreating mice with Trolox, a water-soluble vitemin E analog, abolished the effects of TNF-α injection on cytosolic oxidant activity and specific force (286). These studies reveal that muscle-derived oxidants mediate TNF-α effects on contractile function.
But which are the exact oxidants involved? Skeletal muscle fibers contain multiple intracellular sources of ROS including mitochondria, NADPH oxidase, cyclooxygenase, and xanthine oxidase. Also, muscle fibers constitutively express two enzymatic sources of nitric oxide (NO): the neuronal-type isoform of NO synthase (nNOS) and the endothelial-type isoform (eNOS). Both ROS and NO derivatives are continuously generated by skeletal muscle. Both redox cascades participate in a variety of signal transduction pathways. and in muscle, both cascades appear to be essential for TNF-stimulated dysfunction. Alloati et al. (14) originally reported that TNF-α stimulates NO production by muscle and that pharmacological blockade of constitutive NOS activity prevents the fall in specific force caused by TNF-α. These findings were confirmed by Stasko et al. (656) who identified the nNOS isoform as the molecular source of NO signaling stimulated by TNF-α. In this same study, the investigators also examined ROS involvement. They found no evidence that TNF-α stimulates muscular ROS production. However, selective depletion of ROS (superoxide anions, hydrogen peroxide) in the cytosolic milieu abolished TNF-α effects on specific force without disrupting TNF-α/NO signaling. The authors concluded that TNF-α stimulates NO production by nNOS but that endogenous ROS are obligate comediators of the signal transduction pathway that depresses specific force. The mechanism of NO/ROS interaction was not tested, but it was speculated that peroxynitrite (a reaction product of NO and ROS) might be a downstream mediator required for contractile dysfunction (656). Two lines of evidence identify myofibrillar proteins as the target of TNF-α/NO/ROS signaling. Reid et al. (571) tested TNF-α effects on contractile regulation in intact single murine limb muscle fibers and found that TNF-α depressed specific force of tetanic contractions without altering the magnitude or duration of intracellular calcium transients. They concluded that TNF-α signaling acted on an intracellular target downstream of the calcium transient, i.e., the myofibrillar lattice. This interpretation was confirmed by Hardin et al. (286) in permeabilized muscle fibers isolated from mice injected with either TNF-α or saline. In “skinned” fibers from TNF-treated animals, specific force was depressed at higher calcium concentrations, providing clear evidence of myofibrillar dysfunction. Interestingly, TNF-α effects on the calcium-force relationship closely resemble the effects of direct peroxynitrite exposure (684), consistent with the postulate that peroxynitrite is a downstream mediator of TNF-α signaling.
C. IL-1 and Skeletal Muscle
IL-1 is commonly present at elevated concentrations in the serum of critically ill patients, a biomarker that often correlates with clinical outcome. The cytokine exists as two isoforms. IL-1α is primarily membrane-bound, whereas IL-1β is the secretory form that mediates paracrine and endocrine effects. IL-1 is synthesized both by activated macrophages and by nonimmune cells. These include skeletal muscle fibers which express IL-1β at low levels under basal conditions (619) and at higher levels after exercise (101) and in inflammatory myopathies (26). In critical illness, in particular in the context of sepsis, the release pattern of IL-1 (alongside with other proinflammatory cytokines) shows a distinct time course that is summarized in Figure 3A.
FIGURE 3.
Pro- and anti-inflammatory cytokines and their role in sepsis/critical illness and the systemic inflammation-muscle axis. A: time course of pro- and anti-inflammatory cytokines in the systemic circulation in critical illness as best studied in the scenario of sepsis. During sepsis, pro-inflammatory IL-1 and TNF-α rise early, followed by an increase in IL-1, which is then followed by a rise in anti-inflammatory mediators to initiate the compensatory anti-inflammatory response syndrome (CARS). IL-6 both exerts pro- and anti-inflammatory effects. For example, exercise lacks the pro-inflammatory response and is purely characterized by anti-inflammatory response triggered by almost sole IL-6 release from exercising muscle (“myokine”; note: IL-6 amplitude in both sepsis and exercise scaled to maximum; absolute IL-6 amplitudes are much larger in exercise). Individual time courses may vary depending on the model (e.g., CLP shown in the right panel) and outcome. [Right panel according to data from Chensue et al. (119). Left panel modified from Petersen and Pedersen (545).] B: systemic inflammation-muscle axis during sepsis and critical illness. Activated macrophages in inflamed tissue as well as monocytes and neutrophils in the blood secrete large amounts of pro-inflammatory IL-1 and TNF-α that drive the pro-inflammatory systemic response. These mediators also stimulate myokine production in skeletal muscle, of which IL-6 is the most predominant to initiate the systemic CARS by inhibiting further IL-1/TNF-α release and to stimulate production of IL-10 and IL-1RA, for example.
IL-1 is a potential stimulus for the protein loss and muscle atrophy seen in critical illness. Elevated IL-1 levels clearly promote muscle atrophy in experimental animals. For example, Cooney et al. (132) showed that IL-1α infusion for 6 days decreases muscle weight and protein content of rat gastrocnemius. The pathophysiological relevance is evident in animal models of sepsis where administration of an IL-1 receptor antagonist can preserve muscle mass (131). Studies of underlying mechanisms suggest that IL-1 influences both protein degradation and protein synthesis. Baracos et al. (34) first reported that IL-1, then referred to as leukocytic pyrogen, acts directly on isolated skeletal muscle preparations to increase intralysosomal proteolysis and net protein degradation. This action was augmented at elevated muscle temperatures, a model of fever, and appeared to be mediated by a rise in prostaglandin E2 synthesis. IL-1 did not alter protein synthesis in this preparation. The catabolic actions of IL-1α were demonstrated in vivo by Zamir et al. (795) who found that total and myofibrillar protein breakdown rates were elevated in isolated limb muscles from rats injected with recombinant IL-1α. Consistent with the assertion that IL-1 promotes proteolysis, intravenous administration of this cytokine is reported to increase ubiquitin expression in rodent muscle (437), although ubiquitin upregulation is not always observed (228). IL-1 effects on synthesis pathways also promote net loss of muscle protein. Cooney et al. (132) showed that IL-1α infusion depressed protein synthesis rates in rat gastrocnemius, a muscle composed primarily of fast fibers, whereas soleus muscle (primarily slow fibers) and the heart were unaffected. This finding is consistent with earlier observations that amino acid uptake was depressed in limb muscles of rats treated with IL-1α (794).
Less is known about IL-1 effects on skeletal muscle contraction. However, a recent report on IL-1α effects on calcium release from the sarcoplasmic reticulum (SR) raises intriguing possibilities (220). With the use of permeabilized single fibers, spontaneous colocalization of exogenously applied IL-1α with the RyR1 was found (220). IL-1α exerted reversible effects on SR calcium release and specific force that were strongly dependent on Mg2+ concentration. Long-term IL-1α exposure of intact muscle fibers caused loss of sarcolemmal integrity leading to intracellular deposition of IL-1α. These data suggest a novel mechanism of action by which IL-1 might depress muscle contraction in critically ill patients (see also sect. IX). This hypothesis has not been tested but is consistent with several related observations: 1) specific force of skeletal muscle is depressed by secretory products of lipopolysaccharide-stimulated monocytes (774), which are known to include IL-1; 2) similar to skeletal muscle, IL-1 alters SR calcium release by cardiac myocytes (170); and 3) in the heart, IL-1 depresses the force of myocardial contraction (321). Clearly, the effect of IL-1 on skeletal muscle contraction is a novel topic of potential importance in critical illness.
1. The paradox of IL-6
IL-6 is a pleiotropic cytokine with both pro- and anti-inflammatory properties. It is secreted by various cell types including T cells, macrophages, smooth muscle cells, osteoblasts, and skeletal muscle fibers. Among muscle-derived cytokines, IL-6 has been studied extensively. IL-6 affects skeletal muscle myogenesis, lipid metabolism, glucose uptake, protein synthesis, and protein degradation.
IL-6 is commonly elevated in the serum of critically ill patients (Figure 3A), suggesting a positive association with the loss of muscle that occurs in these individuals. However, the experimental data paint a more nuanced picture: on the one hand, direct IL-6 exposure has not been shown to stimulate muscle proteolysis in vitro. Incubation of rat limb muscles with recombinant IL-6 in vitro does not alter the rate of protein breakdown (230, 259), nor have cell culture studies detected an effect of exogenous IL-6 on the rate of proteolysis in rodent muscle-derived myotubes (179). In contrast, IL-6 appears to promote protein degradation in vivo. High concentrations of circulating IL-6 stimulate muscle proteolysis in rats treated with the recombinant cytokine (259) and in transgenic mice engineered for stable overexpression of IL-6 (718). Conversely, inhibition of circulating IL-6 has been shown to lessen muscle atrophy in an animal model of cancer cachexia (665) and in IL-6 transgenic mice (719). These divergent outcomes simply may be artifacts of experimental design, e.g., IL-6 concentration or the duration of exposure. However, Munoz-Canoves et al. (497) have proposed a more interesting interpretation. They suggest that IL-6 promotes muscle atrophy in the intact animal via indirect effects on insulin-like growth factor I (IGF-I) signaling. Normally, IGF-I promotes muscle growth. The authors reasoned that IL-6 reduces serum levels of IGF-I binding-protein-3 (497) and thereby promotes degradation of serum IGF-I, lessening IGF-I effects on muscle growth. To our knowledge, only one published report has tested the direct effect of IL-6 on contractile function. Janssen et al. (351) incubated diaphragm muscle fiber bundles with recombinant IL-6, then measured specific force and endurance capacity. IL-6 did not alter these parameters.
Apart from a systemic action of IL-6 on muscle, muscle itself has been recognized as an endocrine organ being able to actively secrete several cytokines upon stimulation, e.g., in response to exercise or inflammation. These “myokines,” among which IL-6 seems to be most responsive to physical exercise (545) or electrical stimulation (89), are produced in an ATP- and Ca2+-dependent manner. IL-6 has sometimes been termed “a double-edged sword” exerting both protective and deleterious effects on skeletal muscle (497). On the one hand, IL-6 production is stimulated by TNF-α and IL-1β and may contribute to the proinflammatory cascade in critical illness and sepsis (706); on the other hand, strenuous exercise induces a likewise marked increase in IL-6 plasma levels (up to 100-fold; Ref. 192) without the preceding increase in proinflammatory TNF-α and IL-1 as seen in sepsis (Figure 3A). The fact that the IL-6 peak is followed by rising levels of cytokine inhibitors, i.e., IL-1RA and soluble TNF-α receptors (sTNF-R) has cornered its role for anti-inflammatory effect of exercise (532) but may also be important in initiating the compensatory anti-inflammatory response syndrome (CARS) in sepsis and eventually, during critical illness (533). It is attractive to speculate that systemic IL-6 produced in nonmuscle tissues by TNF-α and IL-1 may contribute to proinflammatory feedback while, once muscle secretes large amounts of IL-6 into the circulation, this would shift the response towards an anti-inflammatory feedback. This hypothesis still awaits experimental clarification, but at least one recent study shows that in mechanically ventilated ICU patients who developed myopathy, the inflammation-induced acute phase response resulted in a marked increase in IL-6 production in skeletal muscle, and also in immobilized muscle (392). Although this increase in inflammation-induced IL-6 myokine production is substantially smaller than in response to strenuous exercise (∼15- vs. 100-fold, respectively; Refs. 192, 392), the results point towards a common denominator in a “muscle-immune system” axis where skeletal muscle feeds back to the proinflammatory response syndrome by tuning the onset of the CARS reaction (Figure 3B). However, since IL-6 also exerts potential detrimental effects on muscle such as muscle wasting, this may even become the more prominent effect in critical illness beyond a putative “tipping point” of IL-6 production such as suggested in Figure 3B.
Apart from IL-6, IL-1 has recently also been shown to be secreted by skeletal muscle in response to inflammasome upregulation (568), and this may be in part responsible for detrimental effects of force production in critical illness (220). However, the role of this “myokine” in regulating systemic immune response on top of the already increased circulating systemic IL-1 is not known.
Figure 3B summarizes the local (muscle) and systemic interaction of cytokines.
VI. BIOMECHANICS, MECHANOSENSATION, and TENSEGRITY IN CRITICAL ILLNESS
In this section, the mechanical activation of skeletal muscle and its pathways that are significantly affected by the ICU condition are discussed in more detail. Mechanical external stimuli may not only influence immediate signaling events, e.g., SR Ca2+ release, via mechanosensitive channels (697) but also influence the shape and growth of all cells via direct effects on protein synthesis and degradation. However, our knowledge of the mechanisms on how mechanical signals, i.e., mechanosensation or “tensegrity” (339–343), are transduced to influence transcriptional regulation and intracellular biochemistry is far from complete. Tensegrity or “tensional integrity,” a term derived from architecture, describes the interaction between isolated components within a system under mechanical compression or tension and resulting structure. It has been introduced to muscle biology to summarize cellular functional and structural responses to mechanical stimuli (574). For example, mechanical signals may induce increased protein synthesis, satellite cell activation, and release of growth factors and may overlap with metabolic, action potential-induced, and oxidative signaling (576). Different major signaling cascades such as PI-3K, MAPKs, Ca2+-calmodulin-calcineurin-NFAT, glycogen synthase kinase (GSK), and AMP activated kinase (AMPK) are downstream effectors of mechanotransduction (86) (Figure 4). Mechanosensing in skeletal muscle involves multiple components and spans from integrins, focal adhesion complexes (FAC), stretch-activated calcium- and sodium-permeable membrane ion channels, caveolin-3 and the caveolin-3-associated nNOS, the sarcodystroglycan complex, intermediate filaments, and a number of mechanosensitive sarcomeric proteins, as well as mechanosignaling via the IGF-I growth factor (76, 86, 346, 390, 391, 641, 652, 686, 687, 754) signaling (Figure 4). In addition, external forces on surface membrane receptors, such as integrins and cadherins, might act at distance and promote coordinated changes in nuclear structures via a hard-wired network including structural actin and specialized nuclear anchoring structures (349, 754). This direct and fast mechanotransduction from the cell surface to the nucleus induces an ion flux through the nuclear membrane and the intranuclear acto-myosin complex may contribute to nuclear prestress and nuclear mechanosensation affecting gene regulation via various mechanisms (754) (Figure 4).
FIGURE 4.
Mechanosignaling in skeletal muscle. Mechanosignaling represents an emerging and dynamic field in biomedical science, but the complexity of the different pathways involved, and how they are interrelated, is not fully resolved. Different pathways involved in mechanosensing and tensegrity in skeletal muscle are briefly summarized, and those reported to be altered in CIM are highlighted in red. However, mechanosignaling has only recently been forwarded as a factor triggering CIM, and the mechanosensing pathways are most probably far more complex than those indicated in red. Multiple signaling pathways influence protein synthesis and degradation in the muscle fiber spanning from the muscle membrane and extracellular matrix to the M-band in the center of the sarcomere. Insulin-like growth factor I (IGF-I) has been suggested to play an important role for the muscle hypertrophy induced by mechanical overload, but a maintained hypertrophy response has been reported in transgenic mice with an IGF-I receptor lacking the ability to bind IGF-I, suggesting multiple other mechanosensitive pathways (651). Calcium- and sodium-permeable stretch-activated channels (SAC) respond to mechanical stimuli and various intracellular signaling cascades. In addition, membrane invaginations, i.e., caveolae, respond to cell stress and stretch-induced signaling, and many different proteins involved in cell signaling bind to caveolins, such as neural nitric oxide synthase (nNOS), G protein subunits, tyrosine kinases, small GTPases, and growth receptors (240, 641). The cytokine leukemia inhibitory factor (LIF) plays an important role in muscle hypertrophy in response to mechanical loading (651). Integrins spanning from the extacellular matrix to the interior of the muscle cell, linked to cytoskeletal actin, directly connect to the nuclei and mitochondria, thus allowing a “hard-wired” and rapid signal propagation to nuclear and mitochondrial DNA (754). The subsarcolemmal dystrophin sarcoglycan complex (DSG) is involved in mechanosensing, and a deficiency in this complex is a feature of muscular dystrophies with defects in the signaling related to mechanical load, resulting in muscle degeneration (793). A number of sarcomeric proteins are involved in mechanosensing, and there is emerging evidence of a very dynamic exchange of multiple sarcomeric proteins to the cytoplasmic pool affecting muscle gene expression in response to mechanical load from the Z-line to the center of the sarcomere in the M-band (76, 234, 390). Multiple major signaling cascades are downstream effectors of mechanosensing, such as PI-3K, MAPKs, calmodulin, calcineurin, glycogen synthase kinase, AMP activated kinase, and AKT/mTOR (86).
The communication between transcription factors and transcriptional modifiers in the sarcomere has recently been reviewed (76). Multiple mechanosensors are embedded in the sarcomere. In the Z-disk, several mechanosensitive elements have been identified with direct transcriptional links to the nucleus, such as MYOZ2 (negative regulator of calcineurin), MLP, and CLOCK, shuttling to the nucleus in response to mechanical strain, and CBFβ (involved in JUNB-mediated gene expression) (Figure 4). In the I-band, different stretch-sensitive muscle ankyrin repeat proteins (MARPs) form complexes with titin and myopallidum/calpain-3 and have been suggested to sense passive stress on the sarcomere (76). At the M-band, the titin kinase interacts with a complex of atrogenes which during mechanical arrest dissociates and translocates to the cytoplasm and nucleus (NBR1, SQSTM1, and MuRFs) (391, 559). These atrogenes are believed to act as transcriptional repressors amplifying the atrophy program affecting both protein synthesis and different degradation pathways (see Ref. 76) (Figure 4).
Different experimental and clinical models have been used to study the effects of immobilization on skeletal muscle structure and function, such as hindlimb unloading, microgravity, bed rest, joint immobilization in plaster (or other types of fixation), denervation, etc. All these models are associated with altered mechanosensing, but effects on protein synthesis and degradation are dependent on type of mechanical load. The mechanical load induced by weight bearing, passive stretch, or shear stress in the sarcomere caused by activation of contractile proteins and the level and type of activation of contractile proteins will involve different mechanosensors targeting specific intracellular signaling pathways with consequences for myofibrillar protein synthesis and degradation. It is accordingly not surprising that the effects of hindlimb unloading, denervation, and immobilization will have different effects on myofibrillar protein expression despite the fact that all are characterized by a decline in muscle mass and force. For instance, strict bed rest for 6 mo results in mild muscle fiber atrophy due to a general loss of contractile proteins that is larger than the loss in muscle fiber size, resulting in decreased single muscle fiber specific force (398). During muscle unloading by hindlimb suspension or microgravity, the loss of muscle mass and function has been reported to be associated with a preferential actin loss (203, 204, 585) and slow-oxidative (type I) to fast-glycolytic (type II) fiber types switching (493). In limb suspension, microgravity, and bed rest models, weight bearing has been removed, but there is still internal strain related to activation of contractile proteins. In immobilization models with joint fixation by, e.g., plaster, weight bearing is typically intact and activation of contractile proteins has not been eliminated, although contractions are mainly isometric. In denervation atrophy, muscles are still passively loaded due to weight bearing or activation of agonists/antagonists. This also applies to spinal cord injury where marked fiber atrophy and slow-to-fast fiber transition occurs with a larger extent in humans compared with rodents (266). In deeply sedated mechanically ventilated ICU patients, with or without neuromuscular blockade, both external (weight bearing or passive movement induced by activation of agonists or antagonists) and internal mechanical load (activation of contractile proteins) have been removed. This complete “mechanical silencing” in ICU patients is, to our knowledge, unique for ICU patients with significant consequences for gene and protein expression and different from the other unloading conditions listed above.
The preferential loss of the molecular motor protein myosin and myosin-associated proteins and maintained expression of thin filament proteins are a hallmark of CIM in ICU patients (396, 397, 399, 400, 519, 662). Neuromuscular blockade, systemic administration of glucocorticosteroids, and sepsis have been forwarded as important factors triggering this type of acquired myopathy, but preferential myosin loss in ICU patients has been reported in the absence of any of these factors (399). Instead, all ICU patients who develop CIM have been exposed to long-term mechanical ventilation and mechanical silencing (396, 519). Furthermore, both experimental and clinical studies have shown that long-term mechanical ventilation and “mechanical silencing” with or without neuromuscular blockade and without both sepsis and systemic steroid administration induce the preferential myosin loss observed in patients with CIM (396, 435, 517, 519). Thus mechanosensation plays an integral role in the complex development of CIM in long-term immobilized and mechanically ventilated ICU patients that distinguishes it from other myopathies under the ICUAW umbrella (Figure 1B).
In pioneering work by Griffiths et al. (269), it was demonstrated that passive mechanical loading 9 h/day for 7 days had a significant positive effect on skeletal muscle in mechanically ventilated ICU patients treated with nondepolarizing neuromuscular blocking agents. In an experimental study using a novel rat ICU model where animals were exposed to neuromuscular blockade and mechanical ventilation for durations varying from <1 day to 14 days, it was confirmed that unilateral loading 12 h/day had a positive effect on skeletal muscle structure and function (574). That is, both the loss of muscle fiber size and specific force at the single muscle fiber level were significantly attenuated in response to passive loading, resulting in a doubling of the functional capacity on the loaded versus the unloaded side (574). The mechanisms underlying the loading effects showed a complex temporal pattern involving oxidative stress, posttranslational protein modifications, transcriptional regulation of myosin synthesis, protein degradation via the E3 ligase MuRF1, and extracellular matrix/cell adhesion proteins (574). Similar effects were observed in both fast- and slow-twitch limb muscles, but the effects of loading were typically more pronounced in slow- versus fast-twitch muscles. In a follow-up clinical study, unilateral ankle joint flexion-extensions 10 h per day for 9 days in sedated, but not pharmacologically paralyzed, neurointensive care patients showed a significant positive effect on single muscle fiber force generation capacity (specific force; Ref. 435). Furthermore, nNOS which is associated with the mechanosensitive caveolin-3 in the muscle membrane was translocated to the cytoplasm in response to the clinical ICU condition, i.e., in a similar fashion as reported in response to hindlimb suspension (687), and was suggested to play a role in the posttranslational deamidation modifications of the myosin motor domain on both the loaded and unloaded side (435). A significant preferential myosin loss, the hallmark of CIM, was observed in response to long-term immobilization and mechanical ventilation in the ICU patients in the absence of neuromuscular blockade, suggesting that the mechanical silencing may play a significantly more important etiological role in triggering CIM than neuromuscular blockers (32, 435). In spite of the significant positive effect of mechanical loading on skeletal muscle structure and function, it did not abolish the negative effects of the ICU condition, suggesting that this mild type of mechanical loading did not impact on all mechanosensitive signaling pathways or that other yet unidentified factors, in addition to mechanosensation, may play an important etiological role. The latter statement is supported by the fact that most CIM patients recover from the muscle paralysis while still mechanically ventilated and immobilized in the ICU. One key difference between passive loading and normal activation of muscle contraction is that during passive loading, muscle action potentials are not occurring. This difference may explain why passive loading does not prevent all the changes triggered by muscle inactivity. In particular, muscle excitation significantly contributes to transcription in a process called excitation-transcription coupling (276, 724).
The significant positive effects of passive loading of skeletal muscle in experimental and clinical CIM intervention studies support the early physical therapy and mobilization of deeply sedated or pharmacologically paralyzed mechanically ventilated ICU patients that has been shown to shorten ventilator- and ICU-days, to reduce health care costs as well as to improve prognosis and quality of life (88, 146, 495, 505, 623).
VII. NEUROMUSCULAR TRANSMISSION IN CRITICAL ILLNESS
Nondepolarizing neuromuscular blocking agents are used to facilitate mechanical ventilation (536). In the 1980s, it was first reported that following prolonged use of neuromuscular blocking agents, some patients developed persistent weakness (529). Subsequent studies identified the mechanism underlying weakness as prolonged neuromuscular blockade (37, 386, 538, 596, 624, 625). Development of prolonged weakness was due to slow clearance of blocking agents or their metabolites and was often associated with renal and/or hepatic failure (37, 386, 624, 625). Because of concerns regarding prolonged weakness, neuromuscular blocking agents are now less frequently used and for as short a period as possible such that prolonged neuromuscular blockade is primarily of historical interest.
At the time of many of the initial reports of prolonged neuromuscular blockade, the role of myopathy as a contributor to development of weakness in critical illness was not yet well recognized. Thus studies generally did not perform muscle biopsies or EMG analysis of motor unit recruitment to look for co-occurrence of myopathy. It was only later proposed that use of neuromuscular blocking agents is a risk factor for the development of ICUAW (141, 255, 256). CIM was likely a contributor to weakness in some patients reported to have weakness due to prolonged neuromuscular blockade (37, 255), but it was not clear whether neuromuscular blockade itself would produce the full-blown picture of CIM. With increased awareness of CIM as a cause of weakness in critically ill patients following the additional use of neuromuscular blocking agents, and more cautious use of neuromuscular blocking agents, most patients with pure motor deficits have been found to have CIM rather than prolonged neuromuscular block as the etiology of weakness, in particular since those patients were always also mechanically ventilated and completely immobilized. Some speculations for neuromuscular blocking agents to trigger myopathy in ICU patients, and in particular CIM, point towards their potential to induce functional denervation and subsequent denervation atrophy during prolonged usage (64, 625).
A. Microcirculation-Induced Denervation
Disturbances of the microcirculation are seen in sepsis and critical illness. Transmigration of immune cells out of blood vessels and cellular infiltration of the tissue may provide the grounds for the specific aberrant effects of inflammatory mediators to both muscle and nerve (149, 193). Additionally, tissue edema, seen in sepsis and critical illness, may also represent a nonspecific compression damage factor to the muscle-nerve compartment, especially since the distal motor axons are located within the muscle fascia and can be prone to compression. In a histochemical and ultrastructural study of skeletal muscle biopsies from several patients with sepsis and multiorgan failure (all being mechanically ventilated), five of which also presented with muscle weakness, ultrastructure showed fiber atrophies and altered capillaries with edema, infiltrates, and segmental necrosis (158). A similar observation was noted in a porcine septic shock model where after 18 and 48 h of septic shock induced by endotoxin injection (no mechanical ventilation or immobilization of animals), endothelial cell damage with endomysial edema and mitochondrial swelling in muscle fibers were detected (293). Endomysial edema increased during the time course of septic shock, and plasma TNF-α levels were significantly increased. However, whether the muscle damage seen was therefore primarily due to edema-induced damage or more directly due to either endotoxin or TNF-α, or both, could not be assessed. Other studies seemed to exclude a role for edema to induce muscle weakness in septic patients. In a clinical study assessing muscle force and edema in 18 septic ICU patients, tissue edema was not associated with skeletal muscle weakness (245). More support to question the direct involvement of tissue edema in the pathophysiological mechanism of ICUAW came from a rat CLP study where EDL muscle microcirculation was observed with intravital microscopy 6–48 h after sepsis induction (549). In septic animals, the number of stopped-flow capillaries was significantly increased but not related to an increase in tissue wet-to-dry weight ratio, indicating that extrinsic compression of capillaries by tissue edema was not a primary cause of interstitial hypoperfusion to induce damage to nerve and muscle. In a recent experimental study on septic nonventilated rats, animals showed marked signs of axonal neuropathy already from day 2 after sepsis induction despite a normal morphology of nerve sections arguing against a major nerve compression by edema (515). Nevertheless, even without compression as the primary cause of tissue hypoperfusion, the latter may contribute to the chronic membrane depolarization of terminal motor axons as concluded from a clinical study on patients with “electrophysiologically proven CIP” who showed reduced nerve superexcitability and increased accommodation to depolarizing and hyperpolarizing currents (800). That the terminal motor axonopathy seen in critical illness-induced weakness also extends further to the neuromuscular transmission was inferred from a clinical study performing nerve conduction studies, electromyography and, in particular, single-fiber electromyography (SFEMG) in several patients with CIP, both before they developed spontaneous muscle electrical activity and during follow-up (621). In particular, the latter technique allows assessing a measure for neuromuscular transmission through quantification of the jitter between “just suprathreshold excitation” and action potential recording, since a jitter <10 μs usually reflects a direct stimulation of fibers (621, 655). Interestingly, patients who developed spontaneous activity in muscles during the study course also had significantly increased jitter time, indicating a delay in neuromuscular transmission.
B. Neuromuscular Transmission in Sepsis and Systemic Inflammation
As sepsis (501, 502, 508, 711) and nonseptic critical illness, i.e., thermal injury and burns (180, 454, 462, 463), have been reported to attenuate the action of nondepolarizing blocking agents, such as d-tubocurarine or rocuronium, a series of studies were performed to determine the underlying mechanisms of this rather unexpected facilitation of neuromuscular transmission in animal models of experimental sepsis, with sepsis as the only confounding condition inducing muscle weakness. Direct exposure of frog sartorius muscle to Escherichia coli endotoxin (10 μg/ml) resulted in elevations of miniature-endplate potential (MEPP) frequencies without changing MEPP amplitudes. These changes were abrogated in Ca2+-free solutions and antagonized by elevating extracellular K+, indicating endotoxin-induced alterations of the presynaptic terminal membrane ion conductances, in particular for Ca2+ (544). In an attempt to better assign neuromuscular transmission changes and neuromuscular blocking action to the stage of sepsis, Narimatsu et al. (502) investigated phrenic nerve-induced twitch-tension responses in hemidiaphragm preparations from rats with early (9 h post CLP) and late (18 h post CLP) acute panperitonitis sepsis. Those represent the bimodal time course of the “hyperdynamic and hypermetabolic” and “hypodynamic and hypometabolic” phases of CLP-induced sepsis in this model (117, 755). Indirect (nerve) and direct (muscle) twitch stimulation produced similar decrements in isometric twitch-tension but much more pronounced in late sepsis (∼65% decrement) compared with early sepsis (∼35% decrement). The dose-response curves of twitch tension for rocuronium, pancuronium, and d-tubocurarine were consistently shifted towards higher concentrations by sepsis with an additionally marked increase from early to late sepsis throughout (502). Since these results were obtained under a low stimulation-frequency regime (0.1 Hz), the NMBA-induced twitch depression predominantly reflects competitive blocking of postjunctional acetylcholine receptors, and it was speculated that hyposensitivity to neuromuscular blockade during sepsis may originate from upregulation of postjunctional nicotinic AChR rather than a decrease in drug-receptor affinity (502), similar as seen in a rat burn injury model (363). In a follow-up study, Niiya et al. (508) focused on elucidating the exact mechanisms underlying changes in neuromuscular transmission during “acute late” sepsis (∼18 h post CLP). This was because results from chronic sepsis either in nonlethal panperitonitis CLP models or intraperitoneal endotoxin-induced sepsis in animals were inconclusive, showing either hyper- or hyposensitivity to d-tubocurarine, or a decrease or no change at all in AChR number (313, 595, 720). Acute late sepsis (∼18 h post CLP) induced a significant increase in endplate potential (EPP; postjunctional potential induced by quantal release of acetylcholine from the motor nerve bouton in response to electric stimulation) amplitudes and EPP quantal content, indicating a facilitation of EPPs and excitability of muscle (508). The authors also measured acetylcholine potentials (AChP; postjunctional potential induced by iontophoretic acetylcholine application), electrotonic potentials (EP; sequential muscle membrane potential changes to direct current applications), spontaneous miniature endplate potentials (MEPP; spontaneous and quantal release of acetylcholine from the motor nerve bouton), and resting membrane potentials (RMP). With the use of this series of techniques, prejunctional effects of sepsis could be dissected from postjunctional ones. Prejunctional effects included an increase in quantal content of EPPs through increased release probability (reflected by an increased MEPP frequency), probably mediated by a sepsis-induced increase in intracellular Ca2+ concentrations to facilitate transmitter release (508). Postjunctionally, acetylcholine-potential analysis revealed a significant decrease in acetylcholine sensitivity during acute late sepsis pointing towards a reduced function/density of AChR and/or increased acetylcholinesterase activity (508). Since previous studies reported an inhibition of the latter in conjunction with inflammation (46, 142), thus expecting an increase in acetylcholine sensitivity, it was concluded that decreased postjunctional nAChR density (720) explained the observed reduced acetylcholine sensitivity by overcoming inhibited acetylcholinesterase activity in acute late sepsis (508). On the other side, also acetylcholine sensitivity in extrajunctional areas might need to be considered because sepsis was shown to induce spread of AChRs along the sarcolemma (461), a condition which would be expected to increase acetylcholine sensitivity rather than decrease it.
The issue of altered AChR expression numbers as well as shifts in expression profiles towards embryonic isoforms (464) in myopathy in critical illness is still puzzling with studies showing upregulation (214, 460, 639), downregulation (508), or no change at all (213, 337), depending on the experimental model of critical illness (464). This is possibly due to the relative contribution of the isolated effects of systemic inflammation and sepsis, immobilization and denervation to the resulting overall AChR expression in each model. For instance, denervation is expected to increase expression of AChR of immature isoforms (788). Immobilization, as a contributing correlate to ICUAW (214), either resulted in increased (214, 461) or unaltered receptor expression (337) in the immobilized limb. Finally, the condition “critical illness” also produced differential outcomes: upregulated (362, 758) or unaltered (455) AChRs in thermal injury animal models, as well as in sepsis animal studies, depending on whether a panperitonitis CLP model [concluded downregulation at the neuromuscular endplate region (508); no change in AChR (595)] or chronic intravenous E. coli infection model (no upregulation of AChRs, Ref. 213) was employed. The difference in the CLP studies may be attributed to the stage of sepsis investigated, but this requires further confirmation studies. It should be noted that none of those animal models involved to induce myopathy in sepsis (“sepsis-induced myopathy”) conducted any kind of mechanical ventilation and/or immobilization and did not assess preferential myosin loss, and although being sometimes termed so in the literature, may not be considered as CIM per se (Figure 1B). Interestingly, to date, there does not seem to be a single study that dissected the effects of sepsis and immobilization in critical illness patients (which strictly means including mechanical ventilation). This could experimentally be overcome in future animal studies combining a pure sepsis animal model with a continuous passive mechanical loading protocol, similar as reported in two clinical studies where passive motion in mechanically ventilated ICU patients without sepsis (435) was able to preserve specific force (435) and prevent fiber atrophy (269).
Taken together, even though presynaptic facilitation of transmission is a common finding in critical illness, the overall loss-of-function aspect of muscle performance seems to be represented by postsynaptic alterations from the neuromuscular junction (this section) to downstream mechanisms (following sections). This ultimately outweighs a facilitated presynaptic component of muscle activation in ICU-related myopathies. Figure 5 summarizes the mechanisms discussed in this chapter.
FIGURE 5.
Alterations at the level of neuromuscular transmission contributing to ICU-acquired weakness. ICUAW is usually a mixed syndrome of either neuropathy- or myopathy-induced weakness during critical illness either due to sepsis (or even sepsis-unrelated conditions, e.g., burns), immobilization, and eventual denervation contributing to alterations to neuromuscular function. Typical effects on either presynaptic (neuropathy) or postsynaptic (myopathy) functions are shown and, where available from literature, dissected into the single confounding conditions where pure immobilization or denervation studies were performed. See text for details. For pure denervation and immobilization, data were also extracted from References 375, 668, and 782.
VIII. MEMBRANE and ION CHANNEL FUNCTION IN CRITICAL ILLNESS
A. Sodium Channels Determine Membrane Excitability in Skeletal Muscle
Voltage-gated sodium channels in the adult skeletal muscle (Nav1.4) determine the membrane excitability of the muscle fiber sarcolemma (599) and are important to initiate the upstroke phase of the action potential upon crossing a certain membrane potential threshold for channel opening during membrane depolarization. This potential threshold is mainly set by the steady-state inactivation of the channels (253, 270). Both changes in steady-state activation and inactivation modulate excitability and action potential properties in skeletal muscle (219) and are thus of special interest in many diseases associated with channelopathies (e.g., Refs. 104, 380). Nav1.4 channels are unevenly distributed over the muscle fiber membrane with highest densities near the neuromuscular junction and dropping towards the ends (15). They are also located in high densities in the t-system to warrant fast and even spread of action potentials from the surface membrane to the triad junctions to trigger excitation-contraction coupling. Detailed information on normal Na+ channel function and membrane excitation is reviewed elsewhere (110, 111). Na+ channels, once opened and having undergone transition into their inactivated state, have to be reactivated by repolarizing the membrane potential well below their activation threshold. This is primarily achieved by K+ efflux through voltage-gated K+ channels. The resting membrane potential in particular is set by the electrochemical diffusion equilibrium of K+, and any changes towards the relative resting permeabilities of other ions (e.g., Na+) over K+ can markedly shift the resting membrane potential and impair action potential generation and spread. Furthermore, skeletal muscle has a severalfold higher Cl− permeability within the tubular membranes over the sarcolemma (168). This larger tubular conductance is required to maintain excitability during bouts of muscle activation where tubular K+ accumulation would otherwise depolarize the tubular potential to either facilitate uncontrolled spontaneous myogenic contractions or lock Na+ channels in an inactivated state, depending on the level of depolarization (173). From this, it is clear that any plasma ionic imbalance caused by critical illness would have greater effects in the t-system over the sarcolemma due to larger concentration gradients present in this diffusion-restricted space.
B. “Sodium Channelopathy” in Muscle in Critical Illness
The first indication that there might be an ion channelopathy in critically ill patients came from studies showing that muscle was electrically unexcitable in the acute phase of severe weakness (577, 583). Subsequently, there have been a number of studies demonstrating reduced muscle excitability that manifests as reduction of muscle fiber conduction velocity, increased relative refractory period, and reduced excitability of fibers in response to direct muscle stimulation (12, 59, 413, 613, 614, 764, 801). Reduction in muscle fiber conduction velocity likely underlies prolongation of compound muscle action potential duration that is seen in patients with CIM (257).
Studies of mechanisms underlying loss of muscle excitability in CIM have been performed in a rat model of the disorder. To simulate CIM in rats, denervation of muscle was combined with corticosteroid treatment (see sect. XII). In steroid-treated, denervated rat muscle, it was found that a combination of depolarization of the resting potential and a hyperpolarized shift in the voltage dependence of sodium channel inactivation resulted in unexcitability of the majority of muscle fibers (196, 580–582). The voltage dependence of sodium channel inactivation was also found to be shifted in the hyperpolarized direction by pure sepsis in the rat (594). These data suggest that increased inactivation of sodium channels is a major contributor to reduced excitability. As the above studies were performed on muscle removed from rats and perfused in Ringer solution, the hyperpolarized shift cannot be accounted for by the presence of a circulating factor acting on the sodium channel. However, there is a study suggesting that circulating factors may contribute to modulation of sodium channel gating. When murine muscle fibers were exposed to serum fractions from septic patients for up to 80 min (1:10 dilutions in standardized external solutions), there was a depolarized shift in the voltage dependence of sodium channel inactivation (218) (Figure 6). Thus, if a circulating factor contributes to altered membrane excitability, its acute effect is opposite to the effect triggered by long-term exposure.
FIGURE 6.
Alterations of ion channels in models of ICU-related myopathies. Graphical summary of documented mechanisms contributing to altered membrane excitability through ion channel dysfunction in ICU-related myopathies. Most research performed points towards reduction in ion current densities, e.g., for Na+, Ca2+, and chloride conductances. For voltage-gated Na+ channels, a hyperpolarizing shift of inactivation curves is induced in chronic sepsis and steroid-denervation models of critical illness, thus reducing availability of Na+ channels for action potential generation at resting potentials, which is even worsened by additional membrane depolarizations. Results shown are taken from References 218, 581, 594, and 696. [Top middle and right panels from Rich and Pinter (580). Copyright John Wiley and Sons. Middle right panel from Friedrich et al. (218). Copyright Springer Science + Business Media.]
One way in which the voltage dependence of sodium channel inactivation might be shifted in the hyperpolarized direction is via expression of different sodium channel isoforms which inactivate at more hyperpolarized potentials. In innervated mature skeletal muscle, only the Nav1.4 sodium channel isoform is expressed. After denervation, steroid treatment, or sepsis, there is upregulation of the Nav1.5 isoform (578, 594, 789). Normally, the Nav1.5 isoform is expressed in embryonic skeletal muscle but is downregulated during development (790). In experiments done in vitro, it has been found that the Nav1.5 isoform activates and inactivates at potentials 15–20 mV more hyperpolarized than Nav1.4 (753, 804). Thus upregulation of Nav1.5 might cause a hyperpolarized shift in inactivation of the total sodium current. There is marked upregulation of Nav1.5 mRNA in steroid-denervated muscle beyond that seen in either denervated or steroid-treated muscle alone (578). However, when μ-conotoxins (GIIIB) were used to selectively block Nav1.4 sodium channels in steroid-denervated muscle, little sodium current remained, and the current that remained was inactivated with similar voltage dependence as the total current (196). These data, as well as more recent protein measurements, indicate that Nav1.4 is the primary sodium channel in both control and steroid-denervated muscle (376). The conotoxin data further suggest that the voltage dependence of both Nav1.4 and Nav1.5 was shifted in the hyperpolarized direction in this rat model of steroid-denervation myopathy (SDM; Figure 1B) that seems to reproduce key symptoms found in CIM muscle (196). Thus, despite in vitro data suggesting that Nav1.5 inactivates at more hyperpolarized potentials than Nav1.4, this may not be the case in vivo. These data suggested that the primary cause of hypoexcitability of steroid-denervated fibers was a hyperpolarized shift in the voltage dependence of inactivation of Nav1.4 (196) (Figure 6).
A recent study of covalent modifications of Nav1.4 found reduced glycosylation (376). However, as previous work found that removal of sialic acid from sodium channels shifts inactivation gating in a depolarizing direction (47, 48, 492), the reduction in glycosylation is likely to be compensatory rather than causative for the hyperpolarized shift in inactivation. One finding that may account for the hyperpolarized shift in inactivation is increased association of sodium channels with proteins of the dystrophin-associated protein complex (376). In particular, there is increased association with nNOS. In mice lacking nNOS, denervation-induced loss of excitability was lessened (376). One way NO might trigger hyperpolarized inactivation is through phosphorylation of Nav1.4; however, global levels of sodium channel phosphorylation were unaltered following denervation and steroid treatment (376). The finding that nNOS is involved in regulation of excitability following denervation adds to earlier findings that implicate NO in the response of skeletal muscle to denervation. Previously, it has been shown that inhibition of nNOS lessens muscle atrophy triggered by denervation (687). Since dramatic muscle atrophy occurs in CIM, this raised the possibility that nNOS signaling plays a role in triggering both electrical hypoexcitability and atrophy of fibers.
In an in vitro TNF-α challenge of isolated muscle fibers, TNF-α induced a reversible dose- and time-dependent inhibition of Na+ currents of up to ∼75% of control currents (275). Steady-state activation and inactivation curves were left-shifted approximately −20 mV towards more negative potentials by TNF-α (Table 5). The time course of current block was almost complete at its maximum potency from 15 min post-exposure for larger concentrations tested (>10 ng/ml) and even earlier for lower concentrations (∼5 min for 2.5 ng/ml) and can be considered an acute effect during sepsis (274), probably also in nonseptic critical illness conditions associated with increase in pro-inflammatory milieu. The lower concentrations for which ∼5% Na+ current (INa) block was observed have been correlated with serum plasma levels found in septic patients with multiple organ failures (548) and lipopolysaccharide (LPS)-induced sepsis in rats (221), although vast variations can be found in the literature with even 100-fold lower concentrations in sepsis (249, 355). Another error variable may stem from the fact that most studies on septic patients do not give further details whether and how patients were subjected to analgo-sedation (i.e., use of fentanyl or related analgo-sedatives to maintain intubation; Ref. 695) and mechanical ventilation and silencing that could further contribute to pro-inflammatory cytokine production. Since interstitial TNF-α concentrations might be substantially higher in LPS-stimulated muscle (known to additionally secrete TNF-α; Ref. 471), a maximum block of INa of ∼75% as assessed by Guillouet et al. (274) might be a realistic scenario. Since the TNF-α effect was complete within minutes, a transcriptional modification like channel numbers or channel isoform, especially a shift towards the embryonic Nav1.5 isoform, seems unlikely in the acute TNF-α challenge model. Moreover, the authors observed a most intriguing effect of blocking PKC-mediated phosphorylation by pretreatment of fibers with chelerythrine (274), a blocker of protein kinase C (761). This suggests a specific PKC-mediated change in Na+ channel gating through phosphorylation, probably between domains III and IV (45) by TNF-α in skeletal muscle, similar to what has been found in neurons (139). Whether TNF-α does so in muscle primarily by either the A2 phospholipase, phospholipase C, or protein kinase A pathways or combination of those, all of which stimulate PKC (593), remains to be determined. Interestingly, the hyperpolarizing shift of Nav1.4 inactivation of about −20 mV is also reproduced in human voltage-gated Nav1.4 channels expressed in human embryonic kidney cells following acute application of lipopolysaccharides at >50 ng/ml (283), pointing towards an acute acquired channelopathy by LPS or TNF-α similar to that seen in critical illness and sepsis (594) (Figure 6).
1. Transferability to patients
Although the muscle membrane hypoexcitability is a hallmark in CIM patients, the presence of the same biophysical changes to Na+ channels as detailed above in animal models also in patients has never been proven to date and is clearly a challenge for future research directions. As detailed later in section XII, those animal models are adequate to reproduce the muscle phenotype of CIM patients; thus a similar Na+ channelopathy in CIM patients is probable. Clearly, in the critically ill patients who succumbed to one of the varieties of the ICU syndrome, many systems will be altered, such as the ionic balance of potassium, sodium and chloride in the plasma, calcium in the plasma, and the osmotic strength of the extracellular fluid bathing the muscle fibers. All of these will have a significant effect on the function of nerve and muscle. However, clinical data on this are extremely scarce. For example, plasma Ca2+ concentration changes have been associated with CIPNM in ICU patients; however, this was true both for hypo- and hypercalcemia (17). Studies on plasma Na+, K+, Cl−, and osmolarity in diagnosed CIM patients seem not to be available and are clearly needed.
C. Membrane Depolarization in Muscle in Critical Illness
A consistent finding in skeletal muscle from animal models and patients with critical illness conditions (sepsis, mechanical ventilation, immobilization, steroid-denervation, etc.) is a substantial membrane depolarization that limits excitability of muscle (138, 178). However, unlike for sepsis or steroid-denervation, for pure mechanical ventilation and/or immobilization, membrane depolarization is much less well documented, both for patients and for animal models, except for one study reporting slight depolarizations (∼5 mV) in a rat limb muscle immobilization model (surgical muscle shortening) (798).
There are plenty potential mechanisms to explain depolarized membrane potentials, e.g., changes in Nernst potentials of respective ion species due to concentration changes (i.e., changes in plasma concentrations of Na+, K+, or Cl−), membrane resistance and conductance changes, as well as energy depletion of ATP stocks that feed electrogenic pumps to maintain negative membrane potentials, i.e., Na+-K+-ATPase. In early studies on skeletal muscles from primates with septic shock, the depolarized membrane potential was associated with increased intracellular sodium chloride content and marked depletion of extracellular content (717). In dogs and rabbits during E. coli-induced bacteremia (338, 635), a marked membrane depolarization of skeletal muscle was also noted. Cellular injury as a result of cell swelling in E. coli-induced sepsis in a rabbit model was used to explain membrane depolarization in muscles before the onset of septic shock (338). The selective increase in Na+ and Cl− concentrations in hindlimb adductor muscle was also confirmed in a dog sepsis model 48 h after infusion of E. coli, without any change to K+ concentrations (635). The increase in Na+ permeability of the membrane was sufficient to explain the observed ∼10 mV depolarization which was also supported by the significant decrease in ATP concentration in those muscles (635). However, another study in rats with subacute E. coli-induced sepsis showed a different scenario: an increased Na+-K+-ATPase activity and unaltered ATP and phosphocreatine levels. The preserved ATP content and increased pump activity were explained by sepsis-induced hyperlactatemia and increased muscle content of lactate, a known stimulator of Na+-K+-ATPase, that even led to decreased intracellular Na+ concentrations (469). Unfortunately, membrane potentials were not recorded in that study. Similar observations were made in a rat CLP model (347). In another mouse LPS-induced sepsis model, Liu et al. (432) observed a marked depolarization in the diaphragm and increased input resistance due to an endotoxin-induced shutdown of sarcolemmal chloride conductance while K+ conductance was unaltered. NO synthase (NOS) inhibitors reversed the observed effects arguing in favor of an NO-mediated alteration of chloride conductance that was also confirmed by the fact that in vitro application of LPS by itself to diaphragm was not able to reproduce the membrane effects seen in the septic mouse, whereas addition of an NO donor was (432). In the CIM model of steroid denervation (SD), a downregulation of Cl− conductance was not seen with SD but only following denervation alone (582).
In several studies, a circulating factor was postulated to be responsible for fast membrane depolarization in the approximately minute range (178, 218). For instance, plasma samples from sheep or pigs in shock were assayed for depolarizing activity when applied to rat diaphragm in vitro (90). The depolarizing effect of a so-called “circulating depolarizing factor” (CDF) was similar as the membrane potentials found in the muscles “in situ” in the original animals in shock the plasma came from (90). Given that sepsis is associated with membrane depolarization, increased Na+ permeability and Na+ content (121, 244, 614), one of the potential mechanisms that contribute to the maintenance of increased [Ca2+]i in septic muscle may be reflected by an increased activity of the Na+/Ca2+ exchanger (NCX) to import Ca2+ for the extrusion of subsarcolemmally elevated Na+. In a rat endotoxemia sepsis model, expression levels for NCX were found increased in the early course of sepsis (4 h) in cardiomyocytes and then declined at later stages (30). However, for skeletal muscle, such recordings are not yet available. The increased Ca2+ uptake as a response to enhanced depolarization is not only a mechanism in pathophysiology of septic myopathy but also visible in exercising muscle where bouts of tetanic stimuli can cause depolarization and Ca2+ uptake (447, 541).
Although much evidence is in favor of the sepsis-induced depolarization hypothesis, isolated inflammatory cytokines, i.e., TNF-α, also produced controversial results. For instance, while Tracey et al. (712) reported a depolarization in single fibers during whole muscle impalements, another study showed a dose-dependent hyperpolarization of membrane potentials by TNF-α in enzymatically isolated peroneus longus fibers (274). However, since those authors used a vigorous collagenase isolation procedure (3 mg/ml collagenase for 2 h at 37°C), their control Em values were already somewhat depolarized (approximately −60 mV) and repolarized by TNF-α, presumably by stimulating the Na+-K+-ATPase (274). Yet, another in vitro study in whole EDL muscles from guinea pigs treated with TNF-α (5 and 10 ng/l for 30 min) showed no effect at all on resting membrane potentials (14).
Although the relationship between sepsis, systemic inflammation, and inflammatory cytokines on the one side and membrane depolarization in skeletal muscle seems well established, it is 1) not yet established that this sepsis-induced myopathy (SIM) is equivalent to CIM since no study yet convincingly has demonstrated the preferential myosin loss in long-term sepsis [on top of the severe atrophy in experimental sepsis affecting both actin and myosin equally seen as a general myofilament release, see below and Williams et al. (776)] and also 2) whether membrane depolarization is indeed present in patients with CIM. Although critically ill patients with clinically diagnosed CIM show reduced muscle membrane excitability, direct in vivo recordings of resting membrane potentials in CIM patients are not available except for one early study that showed substantial resting transmembrane potential depolarization in severely critically ill patients in vivo (138). Unfortunately, details about whether these patients were mechanically ventilated or septic were not reported. This limits our understanding of membrane depolarization in CIM to direct proof from the SD rat animal models of CIM (377, 581) but not yet for mechanical ventilation and immobilization models (517).
D. Membrane Ca2+ Channels in ICU-Related Myopathies
Voltage-gated Ca2+ channels, surprisingly, have only been very modestly studied in conjunction with sepsis and critical illness in muscle. In the SD model of critical illness myopathy, Kraner et al. (377) observed an elevation of RyR1 and CaV1.1 from immunostaining of single fibers that especially were severely atrophic and had disorganized sarcomeres. It was thus concluded that increased Ca2+ release from the SR may contribute to the pathology in CIM. However, SR Ca2+ release was not directly assessed, nor was dihydropyridine receptor (DHPR) function. At least in the heart, studies have pointed towards an impaired SR Ca2+ release through the ryanodine receptor during the hypodynamic phase of sepsis (162, 163), and a decrease in ryanodine receptors was detected in endotoxin shock in the heart (430, 787). L-type Ca2+ currents recorded in single ventricular cardiomyocytes isolated from pigs with early phase hyperdynamic septic shock revealed a significant decrease in Ca2+ current densities (∼25%) with a consistent shift of both activation and inactivation curves ∼5–10 mV towards more negative potentials (659). TNF-α did not induce any further changes to the current properties (659). A similar reduction of L-type Ca2+ currents in ventricular cardiomyocytes from septic guinea pigs 4 h after E. coli LPS injection was found that was, however, unrelated to any changes in steady-state activation or inactivation (810). L-type channel function in skeletal muscle in conjunction with critical illness myopathy has so far only been addressed in one study using the acute serum challenge from CIM patients to normal muscle fibers (cf. sect. XII). Serum fractions from septic and mechanically ventilated critically ill patients acutely reduced Ca2+ current amplitudes when applied to murine normal mouse single fibers without any shifts in steady-state activation and inactivation curves (217). This was interpreted as an acute reduction of the fraction of functional L-type channels by a putative low-molecular-weight factor (218, 290, 318, 759).
In summary, ion channels other than Na+ channels still have only scarcely been addressed but are certainly an interesting field to highlight in future studies involving critical illness myopathies.
1. Are electrically active tissues other than skeletal muscle affected?
One of the major problems triggered by critical illness is multisystem organ failure. The mechanisms underlying organ failure remain poorly understood. The finding that sodium channel dysfunction contributes to failure in force generation in skeletal muscle demonstrates that a novel form of acquired channelopathy may be present during the acute phase of critical illness. If channelopathy affects other electrically active tissues, it could provide a mechanism to account for failure of a number of different organ systems.
It is well established that critical illness can induce neuropathy. The first indication that neuropathy in critically ill patients might not be entirely accounted for by axon degeneration came from a study in which nerve and muscle biopsies were taken in patients with severe critical illness polyneuropathy and myopathy. Nerve biopsy in many patients with electrophysiological evidence of neuropathy was normal (403). This finding suggested there was a functional deficit in nerves of affected patients that did not have a pathological correlate. More recently, it was found that nerve excitability was reduced in patients with critical illness polyneuropathy (800). The presence of a functional deficit in the acute phase of critical illness polyneuropathy raised the possibility of rapid recovery since restoration of excitability could occur more rapidly than regrowth of axons. Rapid improvement has been reported in some patients with critical illness polyneuropathy (515).
In studies of septic rats, peripheral nerves revealed reduced excitability that could not be accounted for by changes in input resistance or resting potential excitability (515). Instead, restoration of normal excitability following brief hyperpolarization of axons suggested that a hyperpolarized shift in the voltage dependence of sodium channel inactivation was the mechanism underlying reduced excitability (515). These data suggest that a sodium channel dysfunction similar to the one in skeletal muscle also occurs in peripheral nerve.
The presence of sodium channelopathy in both muscle and nerve might provide a mechanism to explain why critical illness myopathy and critical illness neuropathy often coexist (43, 44, 64, 147, 361, 403, 413, 528, 614). When patients with co-occurrence of neuropathy and myopathy are studied longitudinally, many evolve into either CIP or CIM (43, 273, 361). This raises the possibility that sodium channelopathy is present at early stages. If reduced excitability of peripheral nerve and muscle is due to sodium channelopathy, the channelopathy would have to affect multiple sodium channel isoforms. In the rat steroid-denervation model of critical illness myopathy, the sodium channel isoforms present in skeletal muscle are Nav1.4 and Nav1.5 (578). In dorsal root axons, Nav 1.6 as well as other sodium channel isoforms are expressed (19, 92).
There is evidence consistent with sodium channelopathy in the heart during sepsis. ECG amplitude is reduced during the acute phase of sepsis (579). One interpretation of this is that there is reduced excitability of myocardium. To directly measure myocardial excitability, a recent study in CLP-induced sepsis in rats measured properties of papillary muscle action potentials with microelectrodes. Although resting potential was not altered, action potential amplitude was reduced and threshold was elevated 24 h after sepsis induction (371). Reduction in the rate of action potential rise suggested the etiology of reduced cardiac excitability was a reduction in sodium current. The in vivo data fit well with an earlier in vitro study in which application of LPS to mouse cardiomyocytes reduced excitability (785). Although the mechanism underlying the reduction in sodium current is not known, these data are consistent with an acquired sodium channelopathy in heart that affects the Nav1.5 Na+ channel isoform. The presence of Na+ channelopathy in heart may contribute to the reduced contractility of heart that occurs during sepsis (481). Partial block of Na+ current in heart with the Na+ channel specific blocker tetrodotoxin mimicked changes in excitability that occurred during sepsis, i.e., reduced excitability and greatly reduced cardiac contractility (371). These data suggest that reduction of Na+ current might contribute to reduction in both cardiac excitability and cardiac contractility during sepsis.
Motoneuron excitability is also significantly reduced within 24 h of sepsis induction in rats, in particular during repetitive firing of action potentials (500). Any reduction in motoneuron excitability will reduce motor unit recruitment and thus contribute to weakness. Motoneurons execute their role in modulating force output by converting the synaptic current delivered to the soma into a firing rate that determines muscle force output. The finding of reduced motoneuron excitability raises the possibility that channelopathy within the central nervous system also contributes to weakness.
To summarize, studies to date in skeletal muscle, peripheral nerve, heart, and spinal cord are all suggestive of some acquired channelopathy that is triggered by critical illness. No studies to date have examined excitability of neurons within the brain. Septic encephalopathy is one of the most common and troubling complications of critical illness (564). Despite a number of studies, the mechanism underlying septic encephalopathy remains a mystery. Presence of channelopathy in multiple tissues raises the possibility that reduced excitability of neurons within the CNS might underlie septic encephalopathy. If this was the case, a single therapy to improve excitability might treat failure of a number of electrically active tissues.
Why might sodium currents be reduced in multiple tissues during sepsis? One possibility is that Na+ currents are especially sensitive to cell sickness/injury. In studies in both skeletal muscle and axons, it appears that damage triggers a hyperpolarized shift in the voltage dependence of the Na+ current activation and inactivation as well as a reduction in maximal current density (72, 195, 494). In injured cells with a depolarized resting potential, a hyperpolarized shift in the voltage dependence of inactivation would result in increased channel inactivation to reduce excitability. Decreased cell excitability might even serve to increase survival of the injured cell by shutting down a Na+ “window” current that is triggered by depolarization of the resting potential (547). The Na+ window current might further depolarize the cell, to cause elevation of intracellular Ca2+ (252, 547) and cell death. One way to prevent this cascade of events is to shut down Na+ current. This has the cost of reducing excitability and, thus, temporarily worsening cell function, but might promote survival. Reduction of Na+ current might serve a similar purpose to the use of barbiturate coma in patients with acute neurologic injury: by decreasing metabolic demand during a period of cell stress, it might increase cell survival to improve long-term outcome (319, 432, 604).
IX. EXCITATION-CONTRACTION COUPLING, Ca2+ HOMEOSTASIS, and MOTOR PROTEINS IN CRITICAL ILLNESS
A. The Normal Electromechanical Signaling Cascade in Muscle
Force output in skeletal muscle is the result of a complex cascade of mechanisms that convey electrical stimulation of the surface and tubular membranes to an intracellular chemical response (rise in myoplasmic Ca2+ concentration) followed by a mechanical contractile response (50, 151, 477). Ca2+ ions are released from the sarcoplasmic reticulum (SR) in response to a tubular action potential (388) in a process called excitation-contraction coupling (EC coupling). Ca2+ ions then quickly diffuse within the myoplasm (41, 151) to bind to troponin C that acts as a Ca2+ switch of the thin filaments of the contractile apparatus (412, 671). This initiates the cross-bridge cycle to recruit weakly or strongly bound cross-bridges, depending on the imposed load (516a). This results in contractile power, either as isometric, isotonic, or a mixed contraction. After the excitation ceases, Ca2+ ions are redistributed into the SR via a sequential buffering to low-affinity buffer proteins (e.g., parvalbumin, etc.) and the SR Ca2+-ATPase pump activity (SERCA) (50, 354, 478). The release machinery at the level of the SR ryanodine receptor (RyR1 in muscle) is a tightly controlled and diversely modulated molecular release gate (169, 281, 558, 813). The mechanical output of the motor proteins is mainly determined by the myosin heavy chain expression which varies from muscle to muscle and is also subject to a high degree of plasticity in health and disease (287). Additional determinants are also the light chain isoforms and their respective phosphorylation (264) that can be affected downstream to many signaling pathways and stimuli, e.g., hormones (422) or weight-bearing function (398). It is apparent that disturbances at any step of the contractile cascade can induce or sustain some of the pathology in all forms of ICU-related myopathies (Figure 1B).
B. Altered Global Muscle Ca2+ Homeostasis in Critical Illness
Early studies in septic rats (bacterial peritonitis; no mechanical ventilation) already pointed towards impaired intracellular Ca2+ regulation: a significant Ca2+ uptake was found in soleus muscle at days 1–3 of sepsis induction that was abolished by the L-type calcium channel blocker diltiazem (57). 45Ca fluxes in septic muscles were ∼25% larger compared with mock treated animals. Diltiazem reduced Ca2+ flux both in septic and control animals, however, to much larger extent in the septic muscle group (57) (Figure 7B). Treatment of normal muscle with the Ca2+ ionophore ionomycin induced a similar increase in Ca2+ flux as seen in the septic rat muscles. The increase in Ca2+ flux was paralleled by an increase in tyrosine release linking increased Ca2+ to a higher proteolysis rate (57, 613). Similar results were obtained after a single intraperitoneal E. coli injection to male rats in soleus muscles 10 h after inoculation (771). Another study using the CLP septic rat model also showed an increased Ca2+ content by 50% in soleus muscle and by 10% in extensor digitorum longus (EDL) muscle when bathed in different Ca2+ solutions for up to 120 min (49). Since 45Ca flux measurements or total Ca2+ content do not reflect free myoplasmic Ca2+ concentrations, Fischer et al. (202) took the next step and measured approximately threefold increased resting fura-2 Ca2+ levels in whole EDL muscles from septic rats 16 h after CLP induction (∼1 μM vs. ∼350 nM in untreated rats) (Figure 7C). Although these control values seem somewhat elevated compared with single fiber Ca2+ determinations (40–100 nM; Refs. 208, 296, 777), they are similar to other studies using fura-2 calcium fluorescence recordings in total muscle (447), probably reflecting some inhomogeneity contributions from dye captured in the intercellular space or contributions from endothelial, vascular smooth muscle cells, or immune cells residing in the muscle tissue. Surprisingly, direct recordings of resting Ca2+ levels in single muscle fibers following septic challenge are still missing. Specifically, most studies on altered Ca2+ homeostasis in sepsis models have been performed in cardiomyocytes because cardiomyopathy is also a complication in sepsis. In rat models of sepsis, either CLP- (807) or LPS-induced (292), a decreased fraction of cardiac shortening was explained by a significant reduction in Ca2+ transient amplitudes 48 h after CLP (807) or 4 h after LPS (292). Reduced SR Ca2+ content of ∼380 nM (LPS) vs. 560 nM (control) was subsequently explained by a significantly compromised SR Ca2+ uptake and increased SR Ca2+ leak in SR vesicles (292) and from largely increased Ca2+ spark frequencies in intact cardiomyocytes (807). Dantrolene, a blocker of the ryanodine receptor, normalized the SR Ca2+ leak in SR cardiac vesicle preparations from LPS-treated rats (292) and significantly reduced elevated resting Ca2+ levels in LPS-treated guinea pig cardiomyocytes (∼230 nM) to control values (∼150 nM) (702). More importantly, Thr-17 phosphorylation of phospholamban was 30% reduced, explaining the compromised SR Ca2+ uptake in septic cardiomyocytes (292). Interestingly, the contractile apparatus properties so far present a diverse picture. In an endotoxemia-induced myocardial dysfunction model in guinea pigs, Ca2+ sensitivity of the myofilaments was unaltered 4 h after LPS induction in chemically skinned cardiomyocytes, although maximum CSA-normalized tension generated was significantly reduced, an effect that was revoked in the presence of protease inhibitors (584). However, another study in endotoxin-treated rabbits showed higher Ca50 values for half-activation of papillary muscle isometric force (1.78 vs. 1.53 μM) already 4 h after a 0.5 mg/kg iv endotoxin treatment, indicating a reduced myofibrillar Ca2+ sensitivity. The latter even worsened after a higher LPS dose (1 mg/kg: Ca50 = 2.08 μM) and with time (2.12 μM after 24 h) but returned to normal after 5 days (692). Treatment of control papillary muscle strips with isoproterenol produced a reduction in myofibrillar Ca2+ sensitivity similar to that seen in LPS-treated preparations, indicating a higher degree of protein kinase A-dependent phosphorylation as a cause for the changes in Ca2+ sensitivity seen in septic hearts (693).
FIGURE 7.
Key findings involved in the reconstruction of the cellular pathology related to Ca2+ homeostasis, EC coupling, and motor protein function in sepsis-related myopathies. A: macrophages (CD68-positive) within muscle tissue in a biopsy from a CIPNM patient. [From De Letter et al. (150). Copyright 2000 Elsevier.] B: Ca2+ flux into soleus muscles from septic (fecal pellet implantation) and sham-operated animals either pretreated or not with diltiazem. [From Bhattacharyya et al. (57).] C: resting fura-2 Ca2+ levels in whole EDL muscles from sham or CLP-septic rats (202). D: depolarization potency of plasma from a sheep in endotoxin shock on rat diaphragm in vitro, compared with direct E. coli LPS depolarizing effect on rat diaphragm (90). E: membrane integrity is compromised differentially in different muscles and sepsis models. Myofiber membrane damage allows passage of extracellular cations and small molecules into the myoplasm (427). F: force recordings in a single skinned fiber bathed in a low Mg2+ (10 μM) environment under control conditions (no IL-1) and in the presence of 25 ng/l IL-1 (α isoform). As long as IL-1 is present, no force is produced. Washing out IL-1 while still in low Mg2+ environment then restores a force transient indicative of IL-1 directly stabilizing the inhibitory Mg2+ site on RyR1. [From Friedrich et al. (220). Reprinted with permission of the American Thoracic Society. Copyright 2015 American Thoracic Society.]
In skeletal muscle, elevated resting cytoplasmic [Ca2+]i also seems to be a major trigger for subsequent downstream proteolysis and metabolic failure events. Strikingly, the vastly increased [Ca2+]i found in whole EDL muscles 16 h after CLP procedure in rats was dantrolene responsive, i.e., dantrolene completely reversed the increased [Ca2+]i to control values (202), suggesting a potential involvement of the SR and the RyR1 in the altered Ca2+ homeostasis during sepsis. The increased [Ca2+]i is furthermore expected to activate calpains for the breakdown of desmin, α-actinin, and other Z-disk anchoring proteins (202, 776). However, the above-mentioned CLP study did not claim to establish a cause-effect relationship for dantrolene to exclusively act on muscle [Ca2+]i, since 1) only one time point was investigated (16 h post CLP or sham) and 2) dantrolene also significantly reduced plasma TNF-α and corticosteroids levels (202). Since TNF-α at 10 nM levels is known to depolarize resting membrane potentials in whole EDL and soleus muscles already after short incubation (712), it can be speculated that the associated increase seen in [Ca2+]i in sepsis 16 h post-CLP may rather be an effect of increased plasma TNF-α because membrane depolarization has been shown to increase the membrane permeability towards Ca2+ (120, 188, 771). Since the enhanced Ca2+ uptake in K+ depolarized skeletal muscle from septic rats was blocked by diltiazem that blocks the dihydropyridine receptor (DHPR) (771), it is likely that the mechanism of this Ca2+ entry in sepsis might be identical to the “excitation-coupled Ca2+ entry” (ECCE) that requires a functional DHPR but is independent of store depletion (33, 334, 446). The upregulation of the Cav1.1-ryanodine receptor complex in a rat model of critical illness myopathy may also point towards this direction (377). Therefore, it is tempting to speculate that membrane depolarization might be a first event that initiates increased membrane Ca2+ permeability and rise in [Ca2+]i followed by activation of Ca2+-dependent proteolysis and, eventually, necrosis.
The above-mentioned studies mostly relate to models of sepsis-induced myopathy; strikingly, for CIM, no study on Ca2+ homeostasis is available, neither for patient muscles nor for animal models.
C. Muscle Ca2+ Stores and Ca2+ Transients in Critical Illness
Another mechanism for the Ca2+ entry in septic muscle may involve a dysregulation of the Ca2+ store with activation of store-operated Ca2+ entry (SOCE). A reduced ryanodine-induced contracture in diaphragm from septic mice (7.5 mg/kg LPS ip) that was reversed by the LPS inhibitor polymyxin B confirmed the involvement of LPS signaling on the sarcoplasmic reticulum regulation (433). This was explained by a similarly reduced SR Ca2+ release by LPS. Whether this is due to a decreased SR content of releasable Ca2+ as a consequence of an increased SR leak similar to cardiac muscle (e.g., Refs. 170, 292) has not yet been addressed. If the SR content was indeed reduced in septic skeletal muscle, SOCE could in principle be activated (254, 806). However, in a steroid-denervation rat model of CIM, ORAI1 (Ca2+ release-activated Ca2+ channel protein 1) expression levels were found ∼20% reduced compared with controls 7 days post-op (377). SR function in skeletal muscle still has only been initially studied in a CLP murine sepsis model by Zink et al. (809). The authors compared “intracellular Ca2+ regulation” in skinned EDL muscle fibers from CLP, sham-operated, and general-anaesthesia-only mice 2–7 days post-procedure, recording caffeine-induced force transients. Relative force transients were decreased in the surgical procedure groups from day two but recovered in the sham group from day three while still declining in the CLP group to a minimum of ∼40% at day three, followed by recovery to control values at day seven. SR reloading conditions were kept identical among groups, though with a slightly overfilled SR compared with the usually only one-third filling of fast-twitch muscles (554). Unfortunately, the study did not provide any mechanistic explanation for the observed force reductions. A link to impaired Ca2+ homeostasis on the SR level was made from conversion of the force responses to “apparent Ca2+ transients,” using the steady-state pCa-force curves. However, this approach is considered qualitative, at best, and has to be interpreted with caution. For example, their calculated Ca2+ transient amplitudes in the general anesthesia group after 10 mM caffeine were close to 2 μM and <1 μM in the CLP group (809). However, direct Ca2+ fluorescence recordings of Ca2+ transients in rat EDL muscles using Mag-fura-2 already gave twitch peak Ca2+ amplitudes of ∼18 μM (41). Therefore, a mechanistic understanding of altered Ca2+ homeostasis in experimental sepsis still awaits experimental clarification. In an isolated in vitro study in C2C12 myotubes, TNF-α treatment (10 ng/ml) decreased electrically evoked Ca2+ transient amplitudes significantly from ∼0.4 to ∼0.2 μM after 48 h (741). Resting Ca2+ levels were also ∼30% reduced. A decreased rate of Ca2+ transient decay by TNF-α, however, suggested that SR Ca2+ pump function might have been compromised with less SR Ca2+ available for release (741). In another in vitro study challenging single isolated flexor digitorum brevis fibers with a rather large dose of TNF-α (500 ng/ml), resting [Ca2+]i and tetanic [Ca2+]i amplitudes were almost identical pre- and 4 h post-treatment (571). A very recent study in mouse EDL skinned fibers challenged with IL-1 (α isoform) revealed a rather unexpected mode of action in skeletal muscle: incubation of skinned fiber bundles with IL-1 at concentrations to those found in septic patients (>10 ng/l) reversibly reduced the SR Ca2+ release induced by relieving the Mg2+ inhibition on the RyR1 (220). Co-immunoprecipitation and immunofluorescence colocalization experiments suggested an association of IL-1α to RyR1 to putatively increase the Mg2+ inhibition on the RyR1. Assessment of the SR leak as the “Ca2+-retention index” (408) indicated a reduction of SR Ca2+ leak by IL-1α (220). In contrast, in cardiac muscle, IL-1 (β isoform) increased SR Ca2+ leak (170). Therefore, in skeletal muscle, this may point towards a novel mechanism where IL-1α associates with the RyR1 to interfere with SR Ca2+ release. Although the store content was found increased (220), the increased blockage of Ca2+ release by IL-1α may be one explanation for weakness in critically ill patients with increased pro-inflammatory response. The question of how IL-1α would enter the membrane barrier from outside was also addressed in the aforementioned study. Prolonged incubation of intact single fibers with IL-1α dose-dependently impaired membrane integrity, and IL-1α was subsequently found deposited within the muscle cells, where it then could associate with RyR1 to impair EC coupling (220) (Figure 7F). In support of this, experimental animal sepsis has been shown to induce membrane damage in myofibers using membrane impermeant dyes. In diaphragm muscle, both CLP and LPS-induced sepsis increased the percentage of myofibers with compromised membrane integrity from <1% (control) to between 15 and 20%. In soleus muscle, LPS failed to compromise membrane integrity but CLP-induced sepsis rose the percentage of damaged fibers to ∼8% (427, 673). Alongside with membrane damage, resting membrane potentials were significantly depolarized in diaphragm muscle fibers (from −79 to −68 mV; Ref. 427) (Figure 7E). Thus sepsis “per se” or the combination of pro-inflammatory cytokines (like TNF-α or IL-1) may compromise membrane integrity with an additional entry pathway for cations and small-molecular-weight factors to depolarize membrane potential, increase resting [Ca2+]i, or exert specific effects like the putative direct binding of IL-1 isoforms to RyR1. The membrane damage mechanism may be unspecific or involve specific signaling pathways, e.g., free radicals that are upregulated in inflammatory myopathies and in response to cytokines (225, 679, 681, 683). Sepsis-induced membrane damage was substantially reduced by NG-monomethyl-l-arginine (l-NMMA), an inhibitor of NOS activity, pointing towards a role of NO-mediated compromise of membrane integrity (427). As long as sufficient myofiber repair mechanisms were still active, such membrane damage would be sufficiently repaired with fibers not necessarily undergoing necrosis (93). However, the damage would be sufficient enough to let Ca2+, other cations and small molecules pass the membrane.
D. Ca2+ Regulation of the Contractile Apparatus in Critical Illness
Apart from the effects of increased [Ca2+]i in septic muscle on motor protein turnover and energy metabolism leading to proteolysis (see sect. XI), it is of interest to highlight regulatory consequences on the motor proteins with respect to Ca2+ activation during EC coupling. Studies in the 1980s already showed an in vitro inhibition of myofibrillar Mg2+-activated ATPase activity in myofibrillar preparations from both rabbit skeletal and dog heart muscles when incubated with E. coli endotoxin (∼0.05–0.3 mg/ml) (537). Already after 2 h, ATPase activity was reduced by 60% in skeletal, and even more pronounced in cardiac myofibrils. Whether those results can be of pathophysiological relevance in the pathogenesis of sepsis-induced dysregulation of myofibrillar activity, however, is unknown. It would require LPS itself to be translocated to the myoplasm of septic muscle to exert this direct effect. A mechanism similar to that found for IL-1 in muscle (220) or in cerebro-microvascular endothelial cells (645) might be possible where endotoxin could enter through local membrane defects, e.g., also induced by NOS activation (427, 673). Probably more important are subsequent inflammatory reactions induced by endotoxin in the tissue, i.e., immune activation of macrophages and release of pro-inflammatory cytokines that provide the grounds for subsequent alterations of contractile performance (692). During endotoxin shock, large amounts of NO, either released by endothelial cells but also by myocytes, seem to be causative for a deregulation of thin filament troponins by raising cGMP and phosphorylation of troponins by cGMP-dependent protein kinases, at least in the heart (75, 629, 673, 693, 791). In a positive-feedback loop, NO released by endothelial and vascular smooth muscle cells stimulates neutrophils in a paracrine fashion to increase their production of cytokines, i.e., TNF-α (739).
In both cardiac and skeletal muscle (diaphragm), a decrease in myofibrillar Ca2+ sensitivity can be routinely detected, either in CLP- or LPS-induced sepsis models (94, 674). This decrease in Ca2+ sensitivity seems to be a direct consequence of production of free radicals since prevention of peroxynitrite formation with superoxide scavengers (e.g., pegylated superoxide dismutase; SOD) or NOS inhibition with l-NMMA not only rescued mitochondrial dysfunction in muscle (61, 95; see sect. X), but also pCa-force curves in diaphragm single fibers from endotoxin-treated rats co-administered with PEG-SOD but not with denatured PEG-SOD (94). The reason for the NO production may be seen in the appropriate cytokine profile during sepsis. In particular, TNF-α and IL-1 are prime candidates that have been shown to result in adjacent activation of NO production. In skeletal muscle, the inducible isoform iNOS is upregulated in response to inflammation or other infectious conditions (74, 394, 609). Upregulation of iNOS by combined incubation of IL-1β and IFN-γ in rat skeletal myoblasts was associated with ERK1/ERK2 activation and was completely abrogated by NF-κB blockade (4). In skeletal muscle biopsies of congestive heart failure patients, a linear correlation between IL-1β content and iNOS expression was detected (4). In C2C12 skeletal muscle myocytes, combinations of TNF-α, IFN-γ, and IL-1α, but not either cytokine alone, were found to substantially increase iNOS activity, and RT-PCR analysis revealed that the iNOS mRNA sequence showed exact homology with macrophage iNOS, indicating stimulation of skeletal muscle NO production via induction of the macrophage-type iNOS gene (778). A 4 h incubation of isolated mouse limb and diaphragm muscle with TNF-α markedly suppressed force production in response to field stimulations up to 150 Hz (571). This force depression was located downstream to Ca2+ signaling since no compromise of tetanic [Ca2+]i by TNF-α (500 ng/ml) was detected (571). A later study by the same group confirmed a depression of field-stimulated myofibrillar force without any changes in Ca2+ sensitivity of the contractile apparatus (286). Using TNFR1-deficient mice treated with TNF-α, the decrease in myofibrillar force was prevented and was similar to wild-type animals treated with vehicles, pointing towards the absolute requirement of TNF-α signaling through its TNFR1 receptor to suppress myofibrillar force, probably indirectly through ROS and RNS production (286). So far, the molecular proteins affected by TNF-α are not yet known (286). Apart from indirect phosphorylation of regulatory proteins by peroxynitrite, a direct interaction of TNF-α with intracellular target proteins similar to as has been suggested for IL-1α (220) may be possible but has not yet been tested.
The literature reviewed above exclusively refers to the association between sepsis, pro-inflammatory mediators, and myopathic changes, indicative of sepsis-induced myopathy. For CIM, surprisingly no published data seem at hand on Ca2+ regulation of the contractile apparatus in CIM patients (i.e., nonseptic). In a preliminary study, sequential vastus lateralis biopsies from 12 critically ill, septic, and mechanically ventilated ICU patients, of which six were clinically diagnosed with CIM, were prepared for pCa-force recordings of skinned fiber bundles. However, although there were some changes in myofibrillar Ca2+ sensitivity in two CIM patients between early (∼2–4 days) and late (7–14 days) biopsies, the baseline sensitivity in early biopsies between CIM and non-CIM patients was similar (Friedrich, unpublished observations). Regarding animal models of CIM, no data on myofibrillar Ca2+ sensitivity are available so far from the steroid-denervation model. In the rat ICU model, myofibrillar Ca2+ sensitivity was consistently found to decrease over the time course of 14 days intensive care management (NMBs, mechanical ventilation and muscle silencing) (517) (see also sect. XII).
E. Muscle Fatigue in Critical Illness
Another important yet rather unresolved symptom of ICU-acquired muscle weakness is “muscle fatigue.” Increased fatigability has been repeatedly documented in patients with exacerbated chronic inflammatory conditions (311, 449), but only very few studies have looked at patients with sepsis and multiorgan failure (181). Interestingly, force decrement after fatiguing tetanic stimulations was not different between patients with sepsis and multiorgan failure and volunteers following 2 wk of immobilization, although the baseline tetanic force was markedly depressed already before the fatigue protocol in the sepsis/MOF group (181). Although it is suggestive that the patients in that study actually suffered from CIM (i.e., were septic, immobilized, and mechanically ventilated), the authors only assessed the muscle weakness and electrophysiology parameters but no muscle biopsies for preferential myosin loss confirmation. Thus, although the authors classify their myopathy as “sepsis-induced myopathy,” patients might in fact have been CIM patients.
Muscle fatigue is a very complex phenomenon that involves multiple cellular correlates, e.g., transmission failure of action potentials, tubular excitation spread failure, voltage sensor dysregulation, SR Ca2+ release, metabolic impairment through ATP depletion or reuptake alterations, or myofibrillar protein alterations (13, 28, 172, 417, 572, 657). In critically ill patients, muscle fatigue is usually only assessed via nerval routes either using voluntary muscle exercise (278) or nerve stimulation (181). In a study on the effect of acute infusion of LPS into healthy volunteers, it was found that there was an acute onset of mild muscle weakness within 3 h (471). Interestingly, there was no deficit in electrically stimulated muscle contractions. The authors concluded that “loss of volition” may be a more important factor than intrinsic dysfunction in acute sepsis-associated human muscle weakness. This finding, together with the recent finding that there may be problems with activation of lower motoneurons in septic patients and septic rats (500), raises the possibility that difficulties with activation of motor units may contribute to weakness and fatigue in critical illness.
Most mechanisms of muscle fatigue in human sepsis had been tracked down to mitochondrial alterations and ATP depletion in cases where muscle biopsies for biochemistry assays were available (211), but other levels had not been looked at. Surprisingly, animal studies on sepsis and muscle fatigue are likewise rare and on animal CIM models, not yet available (but on their way; Larsson, unpublished results). In chronic CLP-induced sepsis, soleus muscles of rats after 7 days of sepsis were stimulated via ventral root single motor units to assess fatigue index which indicated increased fatigability (567). However, mechanisms inherent to impaired nerve transmission were not dissected from fatigue mechanisms within the muscle itself. Another study that also employed CLP-induced sepsis in rats used direct muscular stimulation of isolated diaphragm 16 h post-CLP procedure. Trains of tetanic stimulations (50 Hz for 2 s every 10 s for 5 min) were applied. Times to half decrement of the first peak tetanic tension (T50) as fatigability index revealed an almost 50% shorter T50 indicative of massive fatigue (488). This fatigue was associated with a marked increase in myeloperoxidase activity (MPO) as an index of neutrophil activation in septic muscle tissue. Since in septic patients cAMP stores were found depleted (54), the authors also speculated that phosphodiesterase inhibition would alleviate fatigue patterns. Using the PDE inhibitor olprinone, both MPO activity and fatigue were restored similarly towards control levels, indicating a crucial involvement of activated neutrophils or macrophages within septic tissue to contribute to downstream muscle weakness and increased fatigability, probably through their cytokine release patterns of IL-1 and TNF-α or free radicals (94, 224, 488). In chronic inflammatory conditions of polymyositis and dermatomyositis, muscle weakness in patients was correlated with a strong expression of IL-1α in patient muscle tissues (516). An even more massive expression of several pro-inflammatory cytokines and macrophage infiltration found in muscle biopsies from critically ill patients with probable CIM (patients septic and mechanically ventilated; evidence for CIM from electrophysiology plus immunohistochemistry, but not assessed for preferential myosin loss) (150) (Figure 7A) may already point to a common denominator of such a hypothesis in several forms of myopathy-associated inflammatory conditions. This may also hold true for conditions without primary presence of inflammatory cells in primary inflammatory myopathies, where muscle fibers have been found to intrinsically overexpress IL-1α, TNF-α, IL-2, and IFN-γ (150, 701).
Figure 8 summarizes most of the mechanisms found in septic muscle explained in this section. Again, it has to be kept in mind that results from septic ICU patients cannot assess sepsis as independent variable for CIM development since all those septic patients underwent mechanical ventilation and immobilization simultaneously as part of the ICU treatment. For potential separation of factors, refer to section XII.
FIGURE 8.
Cellular mechanisms that contribute to muscle dysfunction in the phase determined by altered Ca2+ homeostasis, EC coupling, and motor protein function during critical illness. For details, see section IX. CLP, cecal ligation and puncture; LPS, lipopolysaccharides; DHPR, dihydropyridine receptor; SR, sarcoplasmic reticulum; RyR1, ryanodine receptor 1; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; l-NMMA: NG-monomethyl-l-arginine; iNOS, inducible nitric oxide synthase.
X. METABOLIC EFFECTS IN CRITICAL ILLNESS
Critical illness is hallmarked by striking endocrine and metabolic disturbances, the severity of which has been associated with a high risk of morbidity and mortality (730, 732, 784).
Already several decades ago the hypothesis was raised that during sepsis and other types of critical illness, cellular energy metabolism is disturbed in several organs including skeletal muscle. The mitochondria as power plants of the cell are responsible for the majority of the ATP production through oxidative phosphorylation (Figure 9A).
FIGURE 9.
Mechanisms of compromised energy production in muscle during critical illness. A: normal mitochondrial respiratory chain function. Complexes I–V are shown. NAD+ and NADH, nicotinamide dinucleotide, oxidized and reduced form, respectively; FAD and FADH2, flavine adenine dinucleotide, oxidized and reduced form, respectively; ATP, adenosine triphosphate; ADP, adenosine diphosphate; Pi, inorganic phosphate. B: disturbed mitochondrial function in critical illness. A vicious cycle of increased free radical generation resulting from enhanced nitric oxide synthase and from increased escape of electrons from the respiratory chain with formation of superoxide anion radicals reversibly or irreversibly inhibits several mitochondrial respiratory chain enzyme complexes. Consequently, the supply of energy-rich phosphates is compromised.
A. Disturbances in Energy-Rich Phosphates in Critical Illness
The process of oxidative phosphorylation is crucial for adequate production of ATP for energy available in the steady state, but takes some time to respond to alterations in ATP demands. Phosphocreatine (PCr), on the other hand, serves as a rapidly mobilizable short-term storage of high-energy phosphates in skeletal muscle. Several studies on critical illness point towards a disturbance in the levels of these high-energy phosphates in skeletal muscle.
In limb muscle of critically ill patients, especially with sepsis, a low-energy charge potential with reductions in ATP, ADP, and/or PCr and a rise in AMP and free creatine has been described in the presence of high lactate levels and an increased lactate-to-pyruvate ratio (210, 425, 426, 444). In rats with hemorrhagic shock, ATP, ADP, and PCr levels already dropped after 1 h in diaphragm (continuously working muscle) and after 2 h in soleus muscle (more sedentary or resting muscle) (115). These reductions were less severe than those in vital organs as liver and kidney. 31P magnetic resonance spectroscopy studies in rats after CLP sepsis showed a decrease in the PCr/Pi ratio as measure of energy stores after 24 h but not in energy available for immediate use, as reflected in the ATP/Pi ratio (348). The increased Na+-K+-ATPase activity, decreased PCr/ATP, increased PCr breakdown, but unaltered ATP levels and pH in this model were interpreted as increased ATP utilization to help maintain the ionic balance and/or support other metabolic processes, whereas PCr stores are used to buffer the ATP concentration (347, 489). Muscle energy charge was not altered after 1–3 wk of sepsis in another experimental study (475). Interestingly, patients with severe sepsis or septic shock (probably CIM, but not assessed for) who would not survive their illness had lower ATP levels, a smaller total adenine pool, but higher levels of AMP in vastus lateralis muscle biopsies taken within 24 h after ICU admission than patients who would survive (77). Compromised ATP synthesis was described in muscle of septic rats 6 days post CLP (691). Also, burn injury in mice reduced the rate of ATP synthesis, but did not affect high-energy phosphate levels apart from a reduction in PCr (534).
B. Muscle Oxygenation in Critical Illness
Disturbances in cellular energy metabolism and consequent bioenergetic failure in trauma and sepsis in humans were originally ascribed to inadequate tissue perfusion leading to cellular hypoxia and, thus, compromised availability of the final electron acceptor in the respiratory chain (350). This notion was based on the high lactate levels observed in these conditions which are traditionally attributed to anaerobic glycolysis as the result of inadequate oxygen delivery. However, studies on tissue oxygenation yielded ambiguous results. In a rat endotoxemia model, oxygen tension in resting muscle was reduced after 4 h in the presence of a normal microcirculatory perfusion and remained refractory to increasing inspired oxygen (605). A 6-h rat peritonitis model of sepsis-induced myopathy showed reduced oxygen tension in rectus femoris muscle, an increased lactate-to-pyruvate ratio, and reduced levels of ATP and total adenine nucleotides (23). These accompanying abnormalities were still observed when normal tissue oxygenation was maintained. After 36–42 h, no evidence of cellular hypoxia was detected in skeletal muscle or diaphragm in septic rats with peritonitis, based on infusion of a hypoxia marker, although circulating lactate levels increased (324). Patient studies suggested that muscle oxygenation is even increased in fluid-resuscitated, hemodynamically stable sepsis and related to disease severity, unlike in patients with limited infection, cardiogenic shock, or after cardiopulmonary bypass (62, 63, 606). Altogether, these data suggest that cellular hypoxia is not the major driving force of energetic disturbances in critical illness. Importantly, elevated lactate levels in hemodynamically stable patients may well be the consequence of stimulated aerobic glycolysis rather than of tissue hypoxia (350).
C. Intrinsic Mitochondrial Abnormalities in Muscle During Critical Illness: Morphological and Functional Mitochondrial Damage
Recent studies infer a disturbance in oxygen utilization rather than in oxygen delivery, leaving us with an acquired intrinsic derangement in cellular energy metabolism, which has been identified as “cytopathic hypoxia” (198, 640). Mitochondrial ultrastructural damage had been described for hindlimb skeletal muscle of endotoxemic rats already more than 40 years ago with frequent distortion of inner and outer mitochondrial membranes and the presence of large vacuolar areas (620). These alterations were clearly present after 18 h but not yet after 6 or 12 h. In a porcine model of endotoxemia, mitochondrial swelling was observed in skeletal muscle at 18- and 48-h time points (293). A study of serial skeletal muscle biopsies taken after 12 and 24 h and at the time of death showed that bacteremia in baboons led to mitochondrial swelling and even membrane fragmentation with advanced injury (638, 769). Less densely packed cristae were also observed in diaphragm mitochondria of 48 h endotoxemic rats (99). Mitochondria in biopsies of vastus lateralis or intercostal muscles taken after 2–22 days from patients with sepsis-induced multiple organ failure showed no differences in morphology compared with elective surgery patients (210). Early degenerative mitochondrial changes have been observed in skeletal muscle of patients and experimental animal models in conditions of ischemia-reperfusion which may persist for days to weeks after the insult (644, 708).
Evidence for a functional correlate of the mitochondrial morphological damage with an intrinsic dysfunction of the organelles during critical illness continuously increases, although apparently conflicting results have been obtained depending on the species, model, and severity and duration of the insult. Nevertheless, mitochondrial function appears mostly depressed in models of longer duration and greater severity of illness (640). The majority of studies focused on sole sepsis as severe insult which, therefore, will be the focus here. Function of the mitochondrial respiratory chain is most commonly assessed via the activities of its individual enzyme complexes (Figure 9A) or via oxygen consumption studies, with evaluation of state 3 and state 4 mitochondrial respiration as well as the respiratory control ratio or index and the ADP/O ratio.
Very early after an acute insult, mitochondrial function may be enhanced, as illustrated by the increased activities of complex I and citrate synthase in vastus lateralis biopsies taken 2 h (but not at 4 h) after an endotoxin challenge in human healthy volunteers (209). This may be in line with increased cytosolic Ca2+ in sepsis (see previous sections on Ca2+ homeostasis). Elevated cytosolic Ca2+ has been shown to stimulate mitochondrial biogenesis (524). However, such Ca2+-induced stimulation of biogenesis may strongly depend on the level and duration of increased Ca2+ (524). In several (mostly rat) models of sepsis (endotoxin administration, peritonitis induced by CLP, or injection of fecal slurry or live bacteria), no consistent results have been obtained regarding mitochondrial function in limb muscle up to 18–24 h, with either no effect on individual enzyme activities, state 3 and state 4 respiration, respiratory control index, and ADP/O ratio or either marked loss of respiratory control (78, 96, 235, 436, 620). Early partial uncoupling has been described in the diaphragm but was not observed in limb skeletal muscle (61, 436). The time point of 24 h may be a borderline at which mitochondrial functional alterations develop in diaphragm and limb skeletal muscle of endotoxin models, if the administered endotoxin dose, and thus severity of illness, are sufficiently high (96, 97, 552, 672, 716). The decrease in complex I activity in skeletal muscle from rats with fecal peritonitis also depended on the severity of the disease (78). Importantly, mitochondrial functional alterations in vastus lateralis biopsies taken within 24 h after ICU admission were more severe in patients who would succumb to severe sepsis/septic shock than those who would survive (77). Unlike with shorter time windows of illness, mitochondrial function appears uniformly depressed with a prolonged duration of illness beyond 24 h (78, 96–98, 210, 672). There was no uncoupling of the mitochondria beyond this time point (96, 97, 672).
Sepsis appears to most consistently affect the activity of complex I, but also other enzyme complex activities have been found to be reduced (78, 97, 442, 716). The impact of sepsis on mitochondrial function may also differ according to the substrate that is used to stimulate mitochondrial respiration. In that regard, pyruvate- or octanoylcarnitine-dependent respiration, but not succinate-dependent respiration, appeared to be reduced in endotoxin-treated rabbits after 24 h (716). One or two weeks of bacteremia evoked a decrease in pyruvate-dependent respiration, whereas branched-chain keto-acid and ketone body utilization simultaneously increased in rat muscle (475).
Apart from inhibition of electron flow along the mitochondrial respiratory chain or uncoupling of oxidative phosphorylation, disturbances in other metabolic processes may impact on cellular ATP generation. These include altered activities of glycolytic or tricarboxylic acid cycle enzymes, as illustrated by the reduced expression and activity of diaphragmatic phosphofructokinase 36–48 h after endotoxin administration (98). Also, impaired ATP synthase activity and hampered transport of ADP/ATP in and out of mitochondria may compromise cellular ATP generation. The decreased maximal respiration after addition of a chemical uncoupling compound was, however, interpreted as ruling out alterations in ATP synthase activity and transport of ADP or ATP across the inner mitochondrial membrane (97, 672). Nevertheless, the activity and expression of the mitochondrial creatine kinase (MtCK) shuttle appeared dramatically reduced in rat diaphragm 48 h after endotoxin injection (99). In most tissues, the adenine nucleotide transporter system is the most important way by which ATP is transported from the mitochondrial matrix side to the intermembrane space. From there, ATP goes through the outer mitochondrial voltage-dependent anion channel into the cytosol. However, in organs requiring extremely high rates of transmitochondrial ATP transport, a mitochondrial creatine kinase isoform (MtCK) constitutes the major ATP transport system, such as the sarcomeric MtCK in striated muscle. As the MtCK is also an important structural protein that stabilizes mitochondrial membrane architecture by cross-linking of the inner and outer mitochondrial membranes at contact sites, the mitochondrial morphological alterations with less densely packed cristae may be related to the loss of MtCK (99). The cytosolic muscle type creatine kinase (CK-MM) which is responsible for delivery of high-energy phosphates to the myosin ATPase was not affected by sepsis (99).
Several biophysical mechanisms may contribute to mitochondrial dysfunction among which are disruption of mitochondrial membrane integrity, modification of protein side-groups, increased degradation or reduced synthesis of the pathway components, and dissegregation of the multiprotein complexes. Selective depletion of several respiratory chain subunits and other mitochondrial proteins, including multiple cytochromes, has been described in rat diaphragm and/or limb muscle after prolonged sepsis (96, 97, 99, 691), which may be related to decreased gene expression and rate of mitochondrial protein synthesis (98, 592) or increased degradation secondary to side-group modification. It also has been suggested that a reduction in intramitochondrial calcium levels during endotoxemia may contribute to metabolic derangement, specifically through its impact on calcium-dependent intramitochondrial enzymes (358). Interestingly, plasma from patients with sepsis contained increased levels of extracellular mitochondrial DNA and inflammatory cytokines and tended to reduce oxygen consumption by skeletal muscle mitochondria from healthy volunteers (232).
Apart from sepsis, other severe insults have been shown to impair mitochondrial function in skeletal muscle, such as severe burn injury (553). This is illustrated by reduced mitochondrial respiration and activities of individual mitochondrial enzymes after severe burn trauma in children and downregulated expression of several genes involved in glycolysis, tricarboxylic acid cycle, and oxidative phosphorylation in burn-injured mice (135, 136, 534). Furthermore, burn injury elicited early and prolonged activation of apoptosis which was preceded by a disturbance in mitochondrial membrane potential (24, 411, 792) and evoked an early transient rise in the expression of the uncoupling protein UCP3 (803).
Unlike the abundance of data available on mitochondrial function from critically ill septic patients and animal models of sepsis, no studies involving animal models on critical illness to dissect sepsis from mechanical ventilation and immobilization or steroid-denervation have yet been published but are on their way. Profound structural mitochondrial changes were observed early in rats subjected to mechanical ventilation and immobilization in the absence of any other insults (i.e., sepsis). Severe changes developed in the diaphragm already after 6 h of mechanical ventilation and after 18 h in a distal hindlimb muscle (soleus) (Shalah and Larsson, unpublished observations). Likewise, the connection between cytoplasmic and mitochondrial Ca2+ on the one hand and biogenesis on the other hand has not been addressed yet in critical illness or sepsis models. Adequate ICU animal models, such as outlined in section XII, thus represent an important strategy for future studies on mitochondrial dysfunction in CIM.
D. Mitochondrial Dysfunction, Oxidative/Nitrosative Stress, and Impaired Muscle Force During Critical Illness
Endotoxin treatment has been shown to decrease force generation over the entire range of pCa tested, as well as maximal calcium-activated muscle force (Fmax) of skinned myofibers of diaphragm and limb muscle (94, 682). These observations point to altered intrinsic functional properties of the contractile proteins. Interestingly, decreased muscle force coincided with a selective depletion of several proteins and appeared preventable by treatment with a NOS inhibitor or superoxide scavenger (94). Thus increased free radical generation appears to play an important role in the establishment of sepsis-related skeletal muscle dysfunction. Free radicals may impair mitochondrial function, but reciprocally, disturbances in mitochondrial function may also enhance free radical production.
Oxidative stress increases in skeletal muscle early after the onset of sepsis induced by CLP in rats (436). NOS activity rises within a few hours after the insult in this model as well as in endotoxemia and is sustained for several hours or days, especially in diaphragm, but is less unequivocally demonstrated or occurring at a later time point in limb or abdominal skeletal muscle (60, 61, 182, 233, 427, 607). In most of these studies, the rise in total NOS activity is ascribed to the increased expression and activity of the inducible NOS isoform (iNOS), but also increased levels of the eNOS and nNOS isoforms have been reported (182). A study that combined the evaluation of iNOS, muscle histology, and muscle function in rat diaphragm over time detected iNOS protein and activity first in inflammatory cells 6 h after the endotoxemia challenge in the presence of normal histology and muscle force and fatigability (60). At 12 h, histology was still normal and iNOS protein and activity were detected, coinciding with a decreased muscle force but not fatigability. At 24 h, the inflammatory cells had disappeared but iNOS was still found in the myocytes and muscle force remained compromised. By 48 h, iNOS was no longer detectable and muscle force was restored. In acute endotoxemia and subacute peritonitis models, increased iNOS and macrophage staining were associated with sarcolemmal injury in diaphragm and/or limb skeletal muscle which correlated with membrane depolarization (427). Muscle injury and decreases in contractile force were (at least partially) restored or prevented when the rise in iNOS expression was prevented with dexamethasone administration or when iNOS activity was inhibited (60, 61, 94, 182, 233, 427). Later studies demonstrated increased mitochondrial NOS activity in diaphragm and limb skeletal muscle of endotoxemic rats which was ascribed to an inducible (mtiNOS) rather than constitutive isoform (mtcNOS) of NOS, based on the absence of this response in iNOS−/− mice (16, 186, 443).
NO reversibly inhibits the activity of the mitochondrial respiratory chain complex IV by competing with molecular oxygen (Figure 9B). NO can also induce reversible inhibition of complex I by thiol group nitrosylation and has been shown to decrease complex III activity by impairing electron flow at the cytochrome bc1 level. The impairment of the electron transfer chain leads to a decreased mitochondrial membrane potential, leakage of electrons, and formation of more reactive oxygen/nitrogen species, at first instance the superoxide anion radical (O2·−). SODs can convert superoxide to molecular oxygen and hydrogen peroxide which in the presence of transition metal ions can give rise to the extremely reactive hydroxyl radical. Both catalase and glutathione peroxidase are able to detoxify hydrogen peroxide. Importantly, the reaction of NO with superoxide yields peroxynitrite (ONOO−), a potent nitrating agent that irreversibly inhibits the four complexes of the mitochondrial respiratory chain and the ATP synthase (Figure 9B), as well as several other mitochondrial enzymes such as aconitase and the mitochondrial superoxide dismutase (MnSOD), and also oxidizes membrane lipids.
Elevated nitrotyrosine levels as a footprint of ONOO− have been shown in parallel with peak iNOS protein levels in diaphragm of endotoxemic rats (182). Progressive increases in nitration of mitochondrial proteins preceded the decrease in muscle force, which could be reversed by administration of a NOS inhibitor (61). In another study, tyrosine nitrosylation of some mitochondrial proteins preceded the selective depletion of several mitochondrial proteins and compromised mitochondrial function which all could be prevented or attenuated by administration of a NOS inhibitor or superoxide scavenger (96). Selective reductions in some but not all mitochondrial proteins, including components of the electron transfer chain, have been observed simultaneously with disturbed mitochondrial function in other studies but may also be partly related to decreased mRNA expression (97, 98, 99). The rise in nitrotyrosine levels and decrease in muscle force could also be blunted by administration of a NADPH oxidase inhibitor, suggesting involvement of this enzyme as well (684).
Soon after induction of sepsis, mitochondrial production of superoxide radicals and hydrogen peroxide rises, associated with increased (mitochondrial) NOS activity, elevated (intramitochondrial) levels of NO, hydrogen peroxide, and ONOO− and nitration of mitochondrial proteins (16, 61, 436, 506). These responses appeared preventable with NOS inhibition (61). Several studies have implicated increased production of superoxide, hydrogen peroxide, and/or hydroxyl radicals in the decreased muscle contractility of respiratory and limb muscles in endotoxemia, in parallel with markers of oxidative stress-induced macromolecular damage (increased levels of the lipid peroxidation markers malondialdehyde, 8-isoprostane, and of protein carbonyls) (61, 634, 672, 681, 683).
While ROS production is increased, the antioxidant defense mechanisms are compromised in sepsis. A few hours of endotoxemia increased the diaphragmatic activity of the antioxidant enzyme MnSOD but did not affect catalase activity (16). The activities of MnSOD, catalase, and glutathione peroxidase, as well as mitochondrial function, were decreased in limb muscle 12 h after CLP (436). Simultaneously with remarkable inhibition of mitochondrial respiratory chain activity and ATP production, intramitochondrial oxidative stress was observed after 24 h in diaphragm and limb muscle of septic mice with a decreased ratio of oxidized (GSSG) over reduced (GSH) glutathione levels, reduced total glutathione levels, higher activity of the GSH consuming glutathione peroxidase, and lower activity of the GSH regenerating enzyme glutathione reductase (186, 442, 443). These disturbances did not develop in iNOS−/− mice. Also, administration of melatonin, which scavenges reactive oxygen and nitrogen species, counteracted mtNOS induction and respiratory chain failure, and restored the redox status. The degree of the decrease in GSH levels in limb muscle of a fecal peritonitis model was shown to depend on the severity of illness (78). Interestingly, complex I activity correlated inversely with NO production and directly with levels of GSH and ATP. Also, in skeletal muscle of severely burned rats, a remarkable decrease in mitochondrial GSH was observed (456).
E. Mitochondrial Repair Mechanisms in Critical Illness
Persistent mitochondrial damage can be evoked by sustained direct mitochondrial damage and/or impaired or insufficient activation of the mitochondrial repair mechanisms. Damaged organelles in turn can perpetuate further damage, e.g., through production of more ROS. Several repair mechanisms cooperate to remove or compensate for mitochondrial damage. Mitochondria undergo continuous cycles of fusion and fission. Mitochondrial fusion and fission not only determine mitochondrial size and distribution in the cell but are also involved in the repair of mitochondria (116, 700). These processes allow the exchange of damaged mitochondrial components and either dilution of molecular damage over the daughter organelles or bundling of dysfunctional structures by asymmetric fission into a single, irreversibly damaged depolarized organelle, which is fusion-incompetent and is subsequently targeted for removal by mitochondrial autophagy or “mitophagy” (365, 700, 721). Autophagy is a crucial cellular quality control mechanism to clear damaged organelles and potentially toxic protein aggregates (295, 379). Mitochondrial fusion and fission are also important for mitochondrial biogenesis. This pathway generates new mitochondria when needed, such as in response to mitochondrial damage and increased energy demand (282, 615). Under normal physiological conditions, the three processes are nicely balanced. Figure 10 shows a simplified presentation of these processes and their interactions. Compared with mitochondrial damage, much less attention has been paid to the mitochondrial repair mechanisms in critical illness. Actually, interest in these pathways is only recently emerging.
FIGURE 10.
Alterations in the mitochondrial repair mechanisms in muscle during critical illness. Mitochondrial repair mechanisms include three major processes: mitochondrial fusion and fission, removal of damaged mitochondria by autophagy (mitophagy), and generation of new mitochondria via mitochondrial biogenesis. Mitochondrial fusion and fission are important in determining the overall shape of these organelles, have the potential to dilute damage over different organelles, and are also involved in mitochondrial biogenesis and autophagy. Mitofusins and optic atrophy-1 (OPA1) are important in mitochondrial fusion, whereas dynamin-related protein-1 (Drp1) and Fission-1 (Fis1) are required for mitochondrial fission. Asymmetric fission can generate uneven daughter organelles and, thus, can segregate the daughter unit that contains most of the defects. Due to low membrane potential ΔΨm and low OPA1 content, this daughter organelle will have an impaired fusion capacity, hence predisposing the organelle to removal by autophagy. Autophagy is a multiphase process by which portions of cytoplasm and/or organelles are degraded, and this can be either selective or nonselective. In the initiation phase, a phagophore or isolation membrane is formed, which elongates in the elongation phase to surround the material that is to be degraded. This phase ends with the formation of a double-membrane structure, the autophagosome, which in the maturation phase fuses with a lysosome to form an autolysosome. This structure contains all the enzymes that are needed to degrade the sequestered content. Microtubule-associated protein-1 light chain-3 (LC3) plays a central role in the autophagy process. LC3 needs to be converted from its cytosolic precursor form LC3-I to its active mature form LC3-II by lipidation. LC3-II is translocated to the growing autophagosomal membrane where it probably functions as a scaffold protein that supports membrane expansion and is used as an autophagosomal marker. The LC3-II/LC3-I ratio is often used as a marker for autophagosome formation. Mitochondrial biogenesis is controlled by both nuclear and mitochondrial gene expression. Receptor inhibitory protein-140 (RIP-140) is an inhibitor of this process. In contrast, peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) stimulates mitochondrial biogenesis via regulation of the nuclear respiratory factors NRF-1 and NRF-2. The NRFs control mitochondrial transcription factor-A (TFAM), mitochondrial DNA polymerase-γ (Pol-γ), and single-strand binding protein (SSB), which in turn stimulate mtDNA replication and transcription of several mtDNA-encoded respiratory chain complex subunits. The NRFs also regulate other proteins, including several subunits of the respiratory chain complexes that are encoded by the nuclear DNA. The colored signs indicate the reported impact of critical illness on these pathways in different types of skeletal muscle during critical illness (increases indicated by the arrows, equal signs indicate no change).
1. Mitochondrial biogenesis in critical illness
Initially, studies on mitochondrial repair rather focused on mitochondrial biogenesis, mostly in tissues other than skeletal muscle (e.g., liver) where activation of mitochondrial biogenesis preceded restoration of mitochondrial mass and oxidative metabolism and appeared an important pro-survival factor (137, 282, 667). In vastus lateralis muscle biopsies harvested within 1–42 days after ICU admission from patients with sepsis-induced multiple organ failure, most of whom survived their illness, mitochondrial biogenesis may have been partially activated as illustrated by some increased mitochondrial transcription factors, but nevertheless failed to maintain mitochondrial function (212). In another clinical study, an early mitochondrial biogenesis response (within 1–2 days after ICU admission) was observed in muscle from surviving but not from nonsurviving patients with severe sepsis (105). This activation was mainly seen upstream in the respective pathway and was not accompanied by a clear difference in mitochondrial respiratory chain proteins. In a heterogeneous group of critically ill patients, upstream activation of the mitochondrial biogenesis program was observed in postmortem biopsies of rectus abdominis, but not in in vivo biopsies of vastus lateralis taken after 2 wk in ICU, and this was irrespective of future survival status (Figure 10) (727). In muscle taken within 10 days from children with severe burn injury, downregulated mitochondrial biogenesis has been suggested (723). In experimentally denervated gastrocnemius muscle of rats and mice (a model of disuse), strong reductions in mitochondrial content coincided with a remarkable downregulation of several key players of mitochondrial biogenesis several weeks after the insult (5, 750). Several components of the mitochondrial biogenesis pathway appeared downregulated at gene expression level after 24 h of endotoxemia, which was more pronounced in mouse tibialis anterior and soleus muscle than in diaphragm (490). On the other hand, freeze injury of murine gastrocnemius muscle (a model of degeneration and regeneration) suggested that mitochondrial biogenesis plays a role in muscle regeneration because this pathway appeared activated early during muscle regeneration (751).
2. Mitochondrial fusion and fission in critical illness
Data on the impact of critical illness on mitochondrial fusion and fission hardly exist. Both processes need to be appropriately balanced, since both unopposed fusion and unopposed fission are detrimental for cellular function. The only study on mitochondrial fusion and fission in skeletal muscle during critical illness found that key players in these processes were upregulated in postmortem rectus abdominis biopsies, but not in in vivo vastus lateralis biopsies of a heterogeneous group of critically ill patients (Figure 10) (727). There was no relationship with eventual survival, and patients were not investigated to classify for CIM. In a rabbit model of critical illness induced by severe burn injury, key players of mitochondrial fusion and fission in liver and kidney were also not significantly different between survivors and nonsurvivors (277).
3. Autophagy in critical illness
An adequately balanced activation of autophagy appears crucial for maintenance of normal muscle homeostasis. Initial studies on autophagy in conditions of muscle atrophy implicated excessive autophagy in muscle breakdown, in conjunction with the activation of the ubiquitin-proteasome system (42, 451, 756, 805). This conclusion was based on the upregulation of several autophagy-related or -regulating genes in different atrophy models. However, selective genetic inactivation of autophagy in skeletal muscle of mice resulted in a spontaneous induction of atrophy and development of a phenotype of myopathy (465). Myofibers in skeletal muscle of these mice were smaller than in wild-type mice and showed degenerative changes, including vacuolization and central nuclei, damaged mitochondria, and accumulation of aberrant concentric membranous structures. The lipidation of microtubule-associated protein light chain-3 (LC3) with formation of LC3-II from LC3-I, which is required for mature autophagosome formation, was reduced. These abnormalities coincided with the accumulation of p62 protein and aggregates positive for p62 and ubiquitin, which are normally cleared by autophagy. As a functional correlate, muscle force was remarkably reduced. Furthermore, inhibition of autophagy unexpectedly promoted more severe muscle loss instead of protecting against atrophy in several experimental stress models of atrophy, including denervation and fasting (465). Hence, it is clear that autophagy is required to preserve muscle mass, myofiber integrity, and function. This is in line with the crucial role of autophagy in cellular protein quality control, by removal of toxic proteins, aggregates, and dysfunctional organelles that otherwise would amplify the damage. Overall, it remains controversial whether activation of autophagy is actually detrimental in catabolic conditions by contributing to atrophy or whether it is rather beneficial by clearance of damage and promotion of survival (612).
This controversy also holds true for critical illness and associated muscle pathology, in particular. Upregulated gene and/or protein expression of several components of the autophagy-lysosome system and/or accumulation of autophagic vacuoles has been observed in skeletal muscle of experimental models of acute and prolonged critical illness (55, 322, 434, 490, 616, 690). Muscle disuse may be an important factor since multiple (though not all) studies on denervation-induced atrophy (model of chronic muscle disuse) also found such alterations, with one study additionally showing an increase in autophagic flux (356, 525, 526, 715). However, different models of disuse have suggested differential involvement of either the ubiquitin-proteasome system or autophagy in the associated atrophy (58). A human patient study suggested that diaphragm disuse induced by prolonged mechanical ventilation triggers autophagy, based again on the increased expression of several key players in autophagy and an increased number of double-membrane autophagosome vesicles (335). Unlike the diaphragm, quadriceps muscle of these patients showed mostly no changes or even a decrease in these components in response to mechanical ventilation. The development of CIM was not investigated in this study. In contrast, alterations in limb muscle were more pronounced than those in diaphragm of endotoxemic mice (490).
Activation of autophagy suggested by the scarce studies on this pathway in critical illness has been interpreted as a detrimental response which contributes to hypercatabolism and wasting. However, the hypothesis that autophagy could be protective for skeletal muscle and other organs during critical illness had initially been overlooked but warrants consideration in light of the lessons from mice with tissue-selective autophagy (372, 465). In fact, the initial studies did not investigate whether autophagy is sufficiently activated to cope with the severe damage inflicted by critical illness. However, a clinical study that considered consequences of insufficient autophagy confirmed similar changes in skeletal muscle and liver of critically ill patients as observed in autophagy-deficient mice (Figure 10) (154, 728). These included the accumulation of ubiquitin aggregates and other autophagy substrates, such as p62, deformed mitochondria, and aberrant concentric membranous structures. Likewise, an autophagy deficiency phenotype has been observed in skeletal muscle, liver, and kidney of a severe burn injury model of critical illness (155, 277). Signs of insufficient autophagy in muscle were also observed several days after severe insults such as sepsis and denervation (31, 565). Fasting is the strongest physiological activator of autophagy, whereas feeding and insulin inhibit this pathway (247). As in the latter studies patients and animals were artificially and continuously fed, this may indicate that early provision of nutrients during critical illness may impair autophagy activation, thereby reducing damage removal that is required for recovery. This constellation is supported by a study in critically ill rabbits demonstrating that early parenteral nutrition (especially when enriched in amino acids) reduced muscle catabolism at the expense of suppressed autophagy, thus compromising myofiber integrity as well as vital organ function compared with fasting (155). This was confirmed in a recent RCT (300), and this may also explain the delay in recovery observed with early versus late initiation of parenteral nutrition to supplement insufficient enteral nutrition in critically ill patients (107, 109, 300). Indeed, muscle biopsies of the patients showed a suppression of autophagy activation with early compared with late initiation of parenteral nutrition, in the absence of any effect on muscle atrophy markers (300). Importantly, the impact on autophagy was independently associated with a higher risk of clinically relevant muscle weakness. Adequate autophagy activation clearly appeared crucial to confer protection against mitochondrial dysfunction as well as cardiac dysfunction, hepatic injury, and kidney failure in animal models of critical illness as shown by genetic interference or pharmacological activation of autophagy (103, 277, 327). The importance of autophagy for muscle is further underscored by studies in mouse models of muscular dystrophy. Skeletal muscles of Col6a1−/− mice showed impaired activation of autophagy, but myofiber survival could be restored and the dystrophic phenotype could be ameliorated by forced activation of autophagy with genetic, dietary, and pharmacological interventions (271). Potent activation of autophagy also restored the sensitivity of mitochondria to calcium-induced permeability transition pore opening in diaphragm of mdx mice, associated with improved histopathology and force-generating capacity (540).
In summary, a critical balance between autophagy activation and suppression is important for the maintenance of muscle mass, but more importantly, muscle quality. Most studies (human and animal models) show insufficient autophagy activation in critical illness, but the level of suppression of autophagy activation required to contribute to CIM is yet unknown. Also, whether impaired autophagy will be considered as a diagnostic requirement for CIM is a topic for future studies.
F. Glucose Toxicity During Critical Illness
The development of hyperglycemia as a consequence of insulin resistance and increased hepatic glucose production is a common complication of critical illness that has been associated with morbidity and adverse outcome of patients irrespective of the underlying diagnosis that required admission to the ICU (470). In three large randomized clinical studies performed in surgical, medical, and pediatric ICUs of Leuven, insulin infusion to the strict age-adjusted target of normal fasting blood glucose levels during the ICU stay improved survival and reduced morbidity (736, 737, 738, 748). Some studies of other investigators have provided support for the beneficial effects of this intervention, whereas others did not show any benefit or even suggested harm (197, 731, 732). Discussing the controversy surrounding the efficacy and safety of this intervention is beyond the scope of this review, but methodological issues including the quality of glucose control likely played a major role in the different outcomes (734). In fact, the discussion appears not to be about the need for glucose control but rather about which is the optimal level of glycemia to target for.
Maintaining strict normoglycemia with insulin infusion during critical illness in relation to a disturbed cellular energy metabolism was investigated in patients and animal models. The intervention protected the endothelium in patients where lowering of adhesion molecules prevented organ failure and death (393). In critically ill rabbits, glycemic control improved endothelium-mediated relaxation of aortic rings, but had no effect on tissue perfusion and oxygen delivery to different organs, including skeletal muscle (183, 726, 729). Strict glycemic control with insulin protected against severe mitochondrial morphological abnormalities seen in liver from hyperglycemic critically ill patients (725). It also improved the activity of several mitochondrial respiratory chain enzymes. Mitochondrial protection in liver, kidney, and myocardium was attributed to glucose control rather than direct insulin effects, as shown in critically ill rabbits (726, 729). Intriguingly, the intervention had no impact on the mitochondria in skeletal muscle of critically ill patients or rabbits (725, 726). Nevertheless, it attenuated iNOS expression in muscle and prevented excessive NO production (184, 725). The different mechanisms responsible for glucose uptake in these organs were put forward as a potential explanation for the observed organ specificity of the mitochondrial effects. As such, cellular glucose overload may develop in organs with insulin-independent glucose transporters, whereas tissues that rely predominantly on insulin-dependent glucose uptake may be relatively well protected from glucose overload in view of the development of insulin resistance. However, strict glycemic control did improve skeletal muscle mitochondrial function in children with severe burn injury (207). Apart from any impact on the mitochondrial compartment, strict glycemic control with insulin may also affect the muscle by anticatabolic actions in view of the catabolism-promoting property of hyperglycemia and anabolic properties of insulin (205, 261, 262). In critically ill rabbits, increasing the amount of intravenously infused glucose calories was able to dose-dependently attenuate several proteolytic responses but only when hyperglycemia was simultaneously prevented (156). In the same model, prevention of hyperglycemia better preserved autophagy in liver and kidney which correlated with improved mitochondrial function and less organ damage (277). The impact of glycemic control on autophagy in skeletal muscle has not been investigated yet. Several studies support protection of the central and peripheral nervous system during critical illness. Maintenance of normoglycemia during critical illness lowered intracranial pressure and seizures in patients with isolated brain injury and reduced the incidence of critical illness polyneuropathy (309, 735, 736). Normoglycemia also improved neurocognitive development of critically ill children 4 yr after ICU admission (482). Furthermore, strict blood glucose control during critical illness, independently of glucose load, attenuated the neuropathological alterations at the level of neurons, astrocytes, and microglia in vulnerable areas of the brain as shown in human and animal brain specimens (649, 650). The impact of this therapy on peripheral nerves remains to be investigated.
XI. PROTEIN REGULATION IN MUSCLE IN CRITICAL ILLNESS
Critical illness is commonly associated with loss of muscle protein and decrements in skeletal muscle mass (Figure 11A). The resulting muscle atrophy may contribute to weakness, premature fatigue, and glucose intolerance (561) and has been associated with morbidity and mortality in critically ill patients (10, 102, 147). At the cellular level, reductions in myofiber size reflect an imbalance between proteolysis and protein synthesis (Figure 12). This is regulated by complex changes in signaling pathways and gene products that regulate protein breakdown and accretion (353, 575). The influence of critical illness on muscle protein metabolism is outlined below. A central difference to pure atrophy usually seen in bedrest conditions, microgravity, or muscle unloading and sepsis-induced myopathy is that critical illness myopathy is characterized by a preferential myosinolysis that exceeds the mechanism of atrophy (balanced proteolysis of sarcomeric proteins) by far and is responsible for muscle weakness in CIM that is more severe compared with sepsis alone. This is also highlighted in section XII.
FIGURE 11.
Critical illness and skeletal muscle proteolysis. A: primary data from critically ill patients document decreased nitrogen balance and an increased rate of muscle protein breakdown in individuals with sepsis; the rate of muscle protein synthesis was not different between septic and nonseptic individuals. [From Klaude et al. (366).] B: Western blot analyses show higher levels of E3 proteins (MuRF1, MAFbx) and 20S proteasome subunits in muscle biopsies from critically ill patients. [From Constantin et al. (130). Copyright John Wiley and Sons.] C: immunocytochemical staining illustrates greater ubiquitin density in small vs. large muscle fibers of ICU patients, consistent with fiber atrophy via the ubiquitin-proteasome pathway. [From Helliwell et al. (298). Copyright John Wiley and Sons.] D: enzymatic assay data demonstrate greater 20S proteasome activity in muscle of septic individuals. [From Klaude et al. (366).] E: proteasome inhibition lessens degradation of muscle protein in the rat CLP model of sepsis. [From Hobler et al. (315).] F: calpain activity and the rate of protein degradation are elevated in limb muscles of septic rats; proteolysis is partially inhibited by pharmacologic calpain blockade. [From Fareed et al. (191).]
FIGURE 12.
Muscle protein homeostasis in critical illness. Diagram depicts changes to protein metabolism caused by pathophysiological processes in critical illness including sepsis, inflammation, and prolonged exposure to pro-inflammatory cytokines. Upper light grey boxes denote altered regulation of major steps in protein synthesis (left) and degradation (right). Blue boxes identify the downstream effects of altered pathway regulation. PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; AKT, also known as protein kinase B; GSK, glycogen synthase kinase; mTOR, mechanistic target of rapamycin; p70S6K, p70S6 kinase; S6, ribosomal protein S6; elF-4E, eukaryotic translation initiation factor-4E; 4E-BP1, elF-4E binding protein-1; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-κB; FOXO, forkhead box-O; HDAC, histone deacetylase; DNA, deoxyribonucleic acid; RNA, ribonucleic acid; mRNA, messenger RNA; tRNA, transfer RNA; Ca2+, calcium ions; E2, ubiquitin-conjugating enzyme; E3, ubiquitin ligase; U, ubiquitin; ATP, adenosine triphosphate; ADP, adenosine diphosphate.
A. The Ubiquitin-Proteasome Pathway in Critical Illness
The ubiquitin-proteasome pathway is a major mechanism of regulated proteolysis. As reviewed elsewhere (498), structural and regulatory proteins are selectively targeted for degradation by covalent attachment of polyubiquitin chains to lysine residues. This ATP-dependent process requires sequential interaction among three enzyme families: ubiquitin activating enzymes (E1 proteins), ubiquitin conjugating enzymes (E2 proteins), and ubiquitin ligases (E3 protein). E2/E3 protein pairs regulate ubiquitin attachment to protein substrates. The composition of each E2/E3 pair confers substrate specificity, enabling selective tagging for degradation via the 26S-proteasome complex. The 26S complex is composed of a 20S multienzyme core capped at each end by two 19S regulatory subunits. The 19S subunits identify, bind, and unfold ubiquitinated proteins, cleaving and recycling the attached ubiquitin moieties. Peptidases within the 20S core then degrade the unfolded protein to generate small polypeptides.
Critical illness appears to upregulate key components of the ubiquitin-proteasome pathway in skeletal muscle. Constantin et al. (130) found that muscle-specific E3 proteins (MuRF1, MAFbx) and subunits of the proteasome were elevated in vastus lateralis muscle of critically ill patients (assessed by APACHE II scores, no information on mechanical ventilation given). These changes were evident at both the mRNA and protein levels, arguing for their physiological relevance (Figure 11B). On the other hand, another recent study in 63 critically ill ICU patients that demonstrated increased catabolism in vastus lateralis muscle showed the opposite: a downregulation of MuRF1 and atrogin-1 (563). Helliwell et al. (298) found fiber atrophy and reductions in myosin ATPase activity in tibialis anterior muscle biopsies from critically ill patients that were accompanied by an increase in immunocytochemical staining of ubiquitin conjugates (Figure 11C). At the functional level, Klaude et al. (367) have shown that critical illness increases chymotrypsin-like peptidase activity of membrane-associated proteasomes isolated from human leg muscles. The activity of membrane-associated proteasomes was elevated by 30% relative to healthy controls, whereas proteasome activity in the soluble fraction was unaffected by critical illness. A subsequent study by the same group (366) confirmed and extended these findings in patients with sepsis in the ICU. Proteasome activity was elevated by 45–55% in leg muscle biopsies from septic patients (Figure 11D). Interestingly, proteasome activity was also elevated by 30% in serratus anterior, a muscle of the rib cage. In rectus abdominis muscle, a more than twofold increase in chymotrypsin-like activity of the 20S-proteasome has been described (154). Such functional readouts are certainly more informative and preferable over simple transcript or protein content analyses which may not account for the temporary nature of mRNA levels, which can be as short-lived and fall quickly during prolonged muscle proteolysis (409).
Rodent models of sepsis have confirmed observations in humans and provided greater detail on the cellular and molecular mechanisms by which the ubiquitin-proteasome pathway is activated. Tiao et al. (704) first reported that sepsis stimulates nonlysosomal, energy-dependent (i.e., proteasomal) proteolysis in skeletal muscles of rats as a model for sepsis-induced myopathy. The process was shown to affect total and myofibrillar protein levels and to be associated with elevated ubiquitin mRNA. Subsequent work has established that the response is greater in fast- than in slow-twitch muscle (705) and that sepsis-stimulated proteolysis can be blocked using proteasome inhibitors, both in isolated muscle preparations (315) and intact animals (200) (Figure 11E).
Experimental sepsis appears to increase proteasomal activity, at least in part, via endogenous glucocorticoids. Tiao et al. (703) treated septic rats with RU 38486, a glucocorticoid receptor antagonist that inhibited the rise in total and myofibrillar proteolysis and blunted increases in ubiquitin mRNA, free ubiquitin, and ubiquitin conjugates. Conversely, dexamethasone administration to normal rats had the reverse effects. The authors concluded that “…glucocorticoids regulate the energy-ubiquitin-dependent proteolytic pathway in skeletal muscle during sepsis.” Pro-inflammatory cytokines have also been evaluated as downstream mediators. There was no evidence that IL-1β or IL-6 play a significant role in sepsis-associated muscle dysfunction (228, 775). In contrast, Garcia-Martinez and co-workers found that increases in ubiquitin mRNA stimulated by sepsis could be replicated in healthy rats by systemic administration of recombinant TNF-α (227), and that this stimulus increases ubiquitination of skeletal muscle protein (226). This suggested that the elevated TNF-α levels observed in sepsis might be responsible for ubiquitin upregulation. Schakman et al. (616) have evaluated the relative importance of glucocorticoids and TNF-α after systemic endotoxin administration. They found that the glucocorticoid receptor antagonist RU 486 abolished activation of the ubiquitin-proteasome pathway, whereas inhibitors of TNF-α production and NF-κB activation had no effect. Thus, in this LPS sepsis model, glucocorticoid production appears to play a more important role than TNF-α/NF-κB signaling.
Experimental sepsis increases expression of gene products that regulate the ubiquitin-proteasome pathway. This is clearly true for regulatory proteins that control ubiquitin conjugation. In septic rats, Voisin et al. (749) first observed a rise in mRNA for E2-14k, an E2 protein that mediates ubiquitin conjugation via the N-end rule pathway. Potential involvement of E2-14k was reinforced by data from Hobler et al. (316) who observed that E2-14k mRNA was elevated in fast-twitch (but not slow-twitch) muscle of septic rats. Later studies confirmed upregulation of E2-14k in fast-twitch muscles of rats subjected to cecal ligation and puncture (200) or endotoxemia (113). Fischer et al. (201) later found that sepsis also increased mRNA levels for E3α, an E3 ligase that partners with E2-14k. The importance of E3α is reinforced by the finding that elevated rates of ubiquitin conjugation can be suppressed by selectively inhibiting E3α function (648). In aggregate, these findings strongly argue for an involvement of the N-end rule pathway. Other E3 proteins that regulate muscle proteolysis are also upregulated during sepsis. For example, mRNA for MuRF1 and atrogin-1/MAFbx were both elevated in extensor digitorum longus muscles of septic rats (786). Substrates of MuRF1 include actin and the myosin heavy chains. Only MyoD and IF3-f are currently known substrates for atrogin-1 (25). Both are involved in the control of protein synthesis, thus suggesting that atrogin-1 affects protein synthesis rather than proteolysis. Such increases in MuRF1 and atrogin-1 can be inhibited by pretreating septic animals with glucocorticoid receptor antagonists, either RU 38486 (786) or RU 486 (222), identifying these E3 proteins as glucocorticoid-sensitive components of the sepsis response. Downstream events that appear to upregulate atrogin-1/MAFbx include increased signaling via stress-activated protein kinases (123) and FOXO1 (646), complemented by less signaling via PGC-1β (479) and HDAC (9). Alterations in FOXO1 and PGC-1β signaling also appear to stimulate MuRF1 expression (479, 646).
Proteasomal regulation is also altered by experimental sepsis. Voisin et al. (749) demonstrated that mRNA for subunits of the proteasome fluctuate in parallel with muscle proteolysis rates. Proteolytic activity of the proteasome is elevated by experimental sepsis (316), a response that involves both trypsin- and chymotrypsin-like peptidase activities (486).
B. Calpain Regulation in Critical Illness
Calpains comprise a family of calcium-dependent, nonlysosomal proteases that are constitutively expressed in skeletal muscle. Over two decades ago, Bhattacharyya et al. (56) reported that sepsis increases calcium-dependent calpain activity in rat limb muscles. Subsequent rodent studies have generally confirmed this finding (Figure 11F). Greater calpain activity leads to Z-band disintegration and myofibrillar protein breakdown (202, 776), thereby depressing myofibrillar force (674). Calpain inhibitors lessen sepsis effects on muscle, reducing proteolysis (191, 767) and preserving contractile function (675, 685). The mechanism by which sepsis increases calpain activity is less clear. Tissue levels of active calpain protein appear to be elevated (675, 685). This may reflect greater calpain activation by internal calcium stores since sepsis effects are opposed by dantrolene (202). Several studies have detected increases in mRNA for m-calpain, mu-calpain, and p94, a calpain thought to be muscle specific (156, 202, 749, 776). Mu-calpain requires about 10- to 100-fold lower Ca2+ concentrations for its half-maximum activation (in the low micromolar range) compared with m-calpain, with the former, thus, being activated in the range achieved during mild to strenuous exercise and the latter in the range exceeded by normal muscle function (hundreds of micro- to low millimolar range) (39). While muscle can usually fully recover from activation of mu-calpain after strenuous exercise with a period of relative muscle weakness within a few days, global activation of m-calpains will more likely result in irreversible muscle damage and cell death. Finally, apart from activation by Ca2+, the rise in calpain activity may reflect lower activity of calpastatin, the endogenous inhibitor of calpain (767).
The importance of calpain activation in muscle of critically ill patients is largely unstudied. To our knowledge, a recent report by Klaude et al. (368) on eight critically ill patients and seven healthy controls provides first information on this topic. Patients had higher rates of protein degradation and higher activities of proteasomal and lysosomal pathways. Calpain mRNA levels were elevated in muscle but calpain activity was not. These initial data provide very limited information on calpain involvement and illustrate the need for more translational research on this topic.
So far, evidence from experimental sepsis animal models has not been able to point towards a significantly preferential myosin loss, but only a general breakdown of myofibrillar proteins in septic muscle, i.e., similar proportions for actin and myosin (776). This distinguishes the sepsis-induced myopathy from the CIM phenotyope seen in critically ill patients, and sepsis and ICU models in rodents.
C. Caspase Involvement in Critical Illness
The caspase family of cysteine proteases is well recognized as a regulator of apoptosis and immune function. In addition, animal studies suggest that one or more members of the caspase family may contribute to muscle proteolysis in critical illness or sepsis. Caspase-3 has been a primary focus. Du et al. (167) originally observed that recombinant caspase-3 degrades actomyosin (although degradation of acto-myosin by caspase-3 is claimed throughout their work, blots were only probed for actin). They speculated that caspase-3 activation might be an early step in muscle catabolism that promotes proteolysis via the ubiquitin-proteasome pathway. Wei et al. (767) examined this possibility in limb muscles of rats with CLP-induced sepsis. They detected no increases in caspase-3 mRNA, activated caspase-3 protein fragments, or caspase-3 enzyme activity. Nor was the rate of protein degradation altered by Ac-DEVD-CHO, a caspase-3 inhibitor. These investigators concluded that caspase-3 was not involved in the catabolic response to CLP. However, later work showed that CLP increased caspase-3 activity and active caspase-3 protein in rat diaphragm (685). CLP also decreased the specific force of diaphragm muscle fibers; this response was blunted by pretreating animals with zVAD-fmk, a caspase inhibitor. Similarly, endotoxin administration activates caspase-3 and increases procaspase-3 levels in porcine muscle (530). Muscle caspase-3 mRNA levels were elevated in a rat ICU model of critical illness myopathy, but appeared relatively late and were preceded by both the upregulation of the E2 ligases (MuRF1 and atrogin-1) and the preferential myosin loss (434). However, total caspase activity was unaltered in muscles from critically ill patients (368).
Caspase-8 has been identified as a key mediator of diaphragm weakness in endotoxin-treated mice. Supinski et al. (679) originally reported that caspase-8 is activated in C2C12 myotubes exposed to pro-inflammatory cytokines and in diaphragm of endotoxin-treated mice. Endotoxin also activated caspase-3 and depressed specific force of the diaphragm. The latter responses were blocked by pretreating animals with a caspase-8 inhibitor, identifying caspase-8 as an upstream mediator of caspase-3 activation and diaphragm weakness. Subsequent studies by the same group have identified JNK, p38 MAPK, and double-stranded RNA-dependent protein kinase (PKR) as upstream regulators of caspase-8 activation (674, 676, 680). These investigators proposed that pro-inflammatory cytokines, such as TNF-α, stimulate a postreceptor signaling cascade in which PKR is upstream of p38 MAPK, JNK, and other kinases. This cascade triggers cleavage of procaspase-8 to caspase-8. In turn, caspase-8 acts on procaspase-3 to generate caspase-3 which degrades myofibrillar proteins, thereby depressing the specific force of muscle.
Other caspases have also been linked to muscle dysfunction in sepsis or critical illness. Shao et al. (630) have shown that caspase-1 cleaves proteins that regulate glycolysis including aldolase, triose-phosphate isomerase, glyceraldehyde-3-phosphate dehydrogenase, α-enolase, and pyruvate kinase. In a murine model of sepsis, systemic lipopolysaccharide administration activated caspase-1 in diaphragm and inhibited glycolysis in muscle extracts. Glycolytic capacity was preserved in caspase-1-deficient mice, identifying caspase-1 as a mediator of sepsis effects on muscle metabolism. Llano-Diez et al. (434) found that caspase-1 mRNA was increased in a rat model of critical illness myopathy (rats mechanically silenced and ventilated for up to 14 days), a late-phase response observed after 9–14 days. In the same window of time, mRNAs for caspase-4 and caspase-12 were also elevated, suggesting potential roles for these more obscure family members.
D. Cathepsins in Critical Illness
Cathepsins are a family of proteolytic enzymes that have long been recognized for their capacity to degrade proteins and polypeptides (53). In general, these proteases are active in acidic environments, are localized to lysosomes, and are not regulated by calcium availability. Individual cathepsins are categorized as cysteine, serine, or aspartyl proteases based on substrate specificity.
Two members of the cathepsin family, cathepsins B and L, have been evaluated extensively for their contributions to muscle protein loss during sepsis. These studies began three decades ago when Ruff and Secrist (600) first observed that skeletal muscle atrophy caused by experimental sepsis could be prevented by pretreating rats with leupeptin, a cathepsin B inhibitor. Cathepsin B mRNA levels, activity as well as protein degradation rates, were elevated in limb muscles isolated from septic rats (284, 329, 330, 749). The clinical relevance of this finding was reinforced by Helliwell et al. (298) who observed greater immunolabeling for cathepsin B in muscle fibers of critically ill patients. Similarly, Deval et al. (157) reported that cathepsin L mRNA and protein levels were elevated in skeletal muscles of rodents with experimental sepsis. Increased expression of cathepsin L has also been observed in muscles of critically ill patients (130, 154), septic mice (569), and burn-injured rabbits (156). However, the putative roles of cathepsins B and L are highly controversial. In muscles of septic rats, Bhattacharyya et al. (56) were unable to detect elevations in cathepsin B activity or cathepsin L activity, and Ahmad et al. (7) found that cathepsin B protein levels were unaltered. In critically ill patients, Klaude et al. (368) found no changes in the mRNA levels of either cathepsin B or cathepsin L. Interventional studies further challenge the importance of cathepsins. In 1988, Hummel et al. (329) reported that leupeptin, a cathepsin B inhibitor, only partially blunted the rise in protein degradation rate caused by experimental sepsis. They concluded that proteases other than cathepsin B might be involved. Later studies (284) showed that leupeptin inhibited cathepsin B activity in muscles of septic rats but did not alter protein degradation rates. The authors concluded that cathepsin B was not a major regulator of accelerated muscle proteolysis during sepsis. Therefore, there is no current consensus on the role of cathepsins nor are they a compelling target for therapeutic drug development.
E. Muscle Protein Synthesis in Critical Illness
Net loss of muscle protein in critical illness requires that protein is degraded faster than it is synthesized. Preceding sections have outlined mechanisms responsible for increased proteolysis. So, what of synthesis? Relatively few studies have addressed this question directly. Such studies involved small, heterogeneous patient populations and experimental approaches that differed substantially. The results are intriguing, a partial glimpse into a complex problem.
A number of studies have evaluated muscle protein synthesis in critically ill patients. Essen et al. (187) measured the fractional rate of muscle protein synthesis in 15 ICU patients among all of which the fractional synthesis rate for skeletal muscle averaged 1.5%/day (over a 5-fold range individual variation). These values correlated with clinical indices of metabolic status, notably arterial blood Po2 and pH and with illness severity. However, evaluating the distribution of protein synthesis rates among muscle and other tissues, the investigators identified no pattern that was characteristic of critical illness. Gore and Wolfe (261) used isotopic tracer techniques to measure the fractional rates of muscle protein synthesis in six critically ill septic patients and six healthy controls. Under basal conditions, the fractional synthesis rate in muscles of patients was more than twice the value measured in controls. A follow-up study (263) with similar experimental design reached the same conclusion that critical illness significantly increases the fractional synthesis rate of muscle protein. In contrast, Klaude et al. (368) recently compared protein kinetics in leg muscles of 16 ICU patients with sepsis or septic shock to protein kinetics in 8 healthy control subjects using phenylalanine and 3-methylhistidine tracers. Arteriovenous differences were measured across the leg for each tracer. These data were used to compute protein synthesis using both a two-pool model and a three-pool model. Neither model detected any effect of critical illness. However, the variable “sepsis” could not be separated from co-confounding variables of ICU treatment because all the patients studied suffered from multiple organ failure and were analgo-sedated and mechanically ventilated (368).
Of relevance to many ICU patients, Hammarqvist et al. (285) examined the effect of surgery per se on markers of muscle protein synthesis by evaluating ribosomal content in muscle biopsies of 22 patients before and 3 days after elective cholecystectomy. After surgery, they observed decreases in 1) total ribosomal concentration and 2) polyribosomes as a percentage of total ribosomes, suggesting that surgery depresses muscle protein synthesis. Tjader et al. (707) tested this hypothesis by measuring surgery effects on muscle protein fractional synthesis rate. In 28 patients, measurements 90 min before surgery were not different from measurements immediately after surgery. Thus general anesthesia and surgical trauma did not immediately alter muscle protein synthesis. The apparently disparate findings of these two studies may reflect differences between the metabolic states of muscle immediately after surgery versus 3 days later.
Signaling pathways that regulate muscle protein synthesis are sensitive to critical illness. As reviewed by Lang et al. (389), animal studies suggest that signaling via the mammalian target of rapamycin (mTOR) pathway is altered in experimental models of sepsis, interfering with protein translation and depressing protein synthesis. Data from critically ill patients are less clear. On the one hand, Constantin et al. (130) analyzed biopsies from vastus lateralis muscles of 10 patients and 10 age- and sex-matched healthy control subjects. They measured changes in signaling proteins that regulate the initiation of translation. These included v-akt murine thymoma oncogene homolog 1 (i.e., AKT1, PKB), glycogen synthase kinase 3 (GSK3), mTOR, 70-kDa ribosomal protein S6 kinase 1 (p70S6K), and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1). Critical illness affected all of these signaling proteins similarly; in each case, protein phosphorylation was decreased. These findings suggest lower signaling activity in the major pathways that promote protein translation. In contrast, Jespersen et al. (353) used a similar experimental design but obtained very different results analyzing AKT-mTOR-S6K signaling in vastus lateralis biopsies from 12 ICU patients and 12 age- and sex-matched healthy control subjects. In patient muscles, they observed higher phosphorylated-to-total protein ratios for AKT (threonine 308), mTOR (serine 2448), and S6K (threonine 389), whereas phosphorylated/total ratio for 4E-BP1 (threonine 37/46) and GSKβ3 (serine 9) were indistinguishable from controls. It is difficult to reconcile these apparently contradictory data.
In response to critical illness, skeletal muscle appears to upregulate a variety of genes that regulate protein synthesis. Fredriksson et al. (212) analyzed vastus lateralis biopsies from 17 ICU patients and 10 age-matched healthy controls. Using microarray techniques, they detected increased mRNA for 27 translation-related genes in muscles of patients, including proteins of the mitochondrial biogenesis pathway and microRNA regulation. Subsequently, Constantin et al. (130) used PCR techniques to document increased mRNA for key signaling proteins including AKT1, GSK3α, GSK3β, mTOR, p70S6K, and 4E-BP1 in muscles of ICU patients. Less information is available about these molecules at the protein level, but Jespersen et al. (353) observed higher amounts of AKT and marginally higher levels of 4E-BP1 in their patients.
Critical illness also alters gene expression for key structural and functional elements in skeletal muscle. Fredriksson et al. (212) found 1,457 unique genes/identifiers increased in muscles from ICU patients, including genes associated with catabolism, inflammation, and oxidative stress. Decreased expression was observed in 525 genes including genes that regulate mitochondrial biogenesis plus a variety of genes that are skeletal muscle-specific. Importantly, myofibrillar proteins were among the gene products that were downregulated. More recently, myofibrillar downregulation in critical illness was confirmed in muscle biopsies from 208 ICU patients versus 35 healthy controls, the largest clinical study yet completed on this topic (154). mRNA levels were severely reduced for actin and myosin heavy chain I (MyHC-I) and IIa (MyHC-IIa). These findings are consistent with overall reductions in myofiber size that also were observed in these patients. At the protein level, myosin was preferentially depleted by critical illness. The myosin-to-actin ratio was diminished both in limb and trunk muscles of patients relative to control values. Of those patients, >90% were mechanically ventilated and >65% received corticosteroids while between 50 and 75% were septic so that the preferential myosin loss reflects the consequences of critical illness related to the mechanical ventilation and analgo-sedation/steroid therapy and not primarily to sepsis. This molecular imbalance is predicted to alter myofibrillar architecture and cross-bridge stoichiometry, compromising specific force of skeletal muscle.
In summary, the data in the literature related to protein imbalance in critical illness consistently point towards a general increase in protein catabolism and turnover resulting in severe atrophy. So far, no model of pure sepsis as only confounding variable in critical illness, whether in patients or in animal models has convincingly demonstrated preferential myosin loss as in critically ill patients, mechanically ventilated, immobilized, and/or steroid-treated. Thus, while sepsis seems to induce an atrophy-dominated phenotype of muscle weakness, critical illness myopathy in addition alters the quality of the atrophied muscle protein composition towards preferential myosinolysis. More details are given in section XII. Figure 12 summarizes the protein synthesis and degradation mechanisms discussed in this section.
XII. ANIMAL and HUMAN MODELS OF CRITICAL ILLNESS and ICU-RELATED MYOPATHIES
A. The Problem of Choosing the Right Animal Model: Matching Disease Phenotype or Modeling Risk Factors?
When trying to model a clinical endpoint, such as muscle weakness, that can arise from a complex combination of risk factors, one has to either 1) follow a trigger factor-oriented approach of modeling risk factors and their impact on muscle function or 2) define specific pathological alterations found within the syndrome that define a specific disease entity seen in patients. Any trigger factor itself or in combinations capable of reproducing the spectrum of pathological changes is potentially causative for the disease. If the trigger factor produces pathological alterations, but they are distinct from the alterations present in patients, they likely trigger a different disease entity within the syndrome ICUAW (Figure 1B). Potential causes of ICUAW in critically ill patients include, e.g., CIP, CIM, and combinations. Acquired CIM is the most common cause underlying acquired paralysis in critically ill ICU patients, and it has been reported in 25–58% of the general ICU population and in 50–100% of certain subgroups (52, 145, 216, 385, 415). For this reason, we will focus on animal models that have been used to study CIM rather than CIP. As detailed below, there is confusion in the literature about which aspect of CIM is being modeled by any given animal model, e.g., in the case of sepsis models, for which the proof of producing preferential myosin loss is still elusive. In ICU patients suffering from CIM, the heterogeneity introduced by variable timing of disease onset, differences in underlying diseases, age/gender differences, and pharmacological treatments make studies on mechanisms underlying CIM extremely difficult. There is accordingly a strong need for experimental models to explore 1) the relative importance of different trigger factors; 2) the mechanisms underlying muscle specific differences between limb, respiratory, and craniofacial muscles; and 3) the temporal sequence of events leading to the CIM phenotype with severe muscle wasting and preferential loss of myosin in limb muscles. In addition, there is a need for experimental mammalian in vivo models allowing time-resolved analyses from short to long durations in the search for specific intervention strategies targeting CIM, i.e., models allowing detailed studies on early and late effects of the intervention on intracellular signaling, protein synthesis/degradation, and regulation of muscle contraction. In vitro models give important information on specific signaling pathways, but CIM is not caused by an isolated mechanism but is the consequence of a series of changes in a complex biological system, and in vivo models mimicking the ICU condition are accordingly warranted. One might argue that the perfect animal model would combine sepsis and treatment with antibiotics, immobilization and ventilator dependence, corticosteroid treatment, and multiple organ failure as these are characteristics shared by many ICU patients. Clearly, such a model would be expensive and labor-intensive, threatening the feasibility. The key issue for efficient use of animal models of CIM is then which risk factors are relatively inexpensive to model and yet sufficient to trigger CIM, so they can be studied efficiently. A second issue is how well the animal model recreates the pathological phenotype of CIM or just belongs to another form of myopathy under the “ICUAW umbrella” (Figure 1B). Pathological changes present in patients are unique to CIM, suggesting the presence of a unique disease. The phenotype defining CIM in patients should therefore be the starting point of our considerations when comparing the power and limitations of different animal models.
The changes seen in patients with definite CIM that should be ideally reproduced by any animal model of CIM, on top of severe muscle weakness, are as follows:
1) electrical hypoexcitability of the muscle and poor excitation-contraction coupling,
2) severe muscle atrophy (beyond that caused by inactivity alone),
3) preferential and significant myosin loss,
4) eventual sarcomere disorganization,
5) inadequate autophagy activation, and
6) altered protein turnover.
With regard to trigger factor-phenotype relationships, neuromuscular blockade (NMB), corticosteroids, and sepsis have all been suggested to be important etiological factors, but CIM has also been reported in the absence of one or two of these trigger factors in mechanically ventilated ICU patients. However, all ICU patients with CIM have exposure to long-term mechanical ventilation and immobilization in common. Furthermore, the complete “mechanical silencing,” unique for ICU patients, has been shown to play an important role in the development of CIM in experimental and clinical studies (435, 517, 574). An additional factor that needs to be taken into account is the relatively long delay between exposure to trigger factors and the phenotype characterizing CIM, i.e., severe muscle wasting and preferential myosin loss (399, 511, 610). The preferential and significant myosin loss is the result of both a decreased synthesis at the transcriptional level and increased myofibrillar protein degradation (399, 434, 496, 513, 517). Thus early effects via specific signaling pathways on protein synthesis and degradation become manifest relatively late at the myofibrillar protein level, i.e., the preferential myosin loss, due to the slow turnover rate of contractile proteins with myosin having a 1–2% turnover rate per day (647). On the other hand, actin has been reported to have a turnover rate twice as slow as myosin (457). Differences in myosin and actin turnover rates and the preferential targeting of myosin by the E3 ligase MuRF1 for ubiquitination and degradation (127) offer a mechanism underlying the preferential myosin loss in spite of a similar transcriptional downregulation of myosin and actin (513, 517). However, there is reason to believe that the mechanism underlying the preferential myosin loss is more complex, involving other mechanisms such as compromised protection by, e.g., small and large heat shock proteins. There is a need for experimental studies allowing time-resolved analyses with a high temporal resolution to detect early changes in the complex intracellular signaling cascades and effects at the gene/protein levels leading to the CIM phenotype. Apart from the preferential myosin loss, it is important to reproduce the reduced muscle membrane excitability in the animal model, as a hallmark of early manifestation of CIM. In the literature, there have been three principal approaches employed as potential models for CIM: sepsis models; ICU-porcine, -rabbit, and -rat models; and pharmacological steroid-denervation. In the following sections, we present the rationale behind those different models, evaluate their advantages and disadvantages, summarize prime findings and whether the respective model was able to reproduce all phenotypic manifestations of CIM or whether they may point to a different disease entity within the ICUAW syndrome. Finally, some in vitro models are discussed briefly.
B. The Rat Steroid-Denervation Model
The issue of what aspect of muscle immobility triggers CIM in patients is central to the validity of various animal models. There are ways to model different aspects of immobility in patients. Immobility could trigger CIM due to either loss of muscle action potentials or loss of passive muscle movement, or a combination of both. Loss of muscle action potentials can be easily modeled by cutting the sciatic nerve to denervate leg muscles (Figure 13). A rat model of acute CIM using denervation was first developed over 20 yr ago (466, 597). The model involves pairing loss of muscle activity (by denervation) with systemic administration of corticosteroids (Figure 13). The model was established to mimic the situation in patients given systemic neuromuscular blocking agents in conjunction with high-dose corticosteroids to treat asthma and chronic obstructive pulmonary disease (COPD). Those patients develop classical CIM. Denervation does not identically mimic the situation present in immobilized patients as the intact nerve is still present in patients. The rat model using denervation assumes that loss of muscle activity due to denervation has the same effect on muscle as loss of activity due to neuromuscular blockade or immobilization due to sedation. The debate about whether trophic factors from nerve are primarily regulated by activity or by chemical influences is a long-standing one. For an excellent early review on this issue, see McArdle (468). There are several studies which suggest that loss of action potential activity in muscle is the primary factor inducing denervation changes in muscle. In two papers, Lomo and colleagues (440, 441) found that stimulation of denervated muscle prevented the upregulation of extrajunctional AChRs and prevented depolarization of resting potential. More recently, in mutant mice with hyperexcitable muscle due to a mutation in the muscle chloride channel ClC-1, it was found that denervation of muscle did not prevent spontaneous activity. The spontaneous activity was sufficient to prevent both depolarization of the resting potential and development of extrajunctional ACh sensitivity following denervation (757). These studies demonstrated that muscle activity was sufficient to prevent some well described denervation-induced changes. By extension, these data suggest that some properties of muscle are primarily regulated by action potentials rather than chemical trophic influences.
FIGURE 13.
Rat steroid-denervation model of CIM and phenotypic characterization. The steroid-denervation (SD) model involves a combined surgical denervation (partial sciatic nerve removal) and a pharmacological steroid treatment (e.g., dexamethasone 5 mg/kg ip daily). This model produces reduction/loss of electrical excitability in limb muscle and Na+ channelopathy within a few days. In addition, severe atrophy (as seen in reduced fiber cross-sectional areas), preferential myosin loss (as detected in gel electrophoresis), and sarcomere disorganization (as visualized by confocal microscopy) provide most of the phenotypic changes to model CIM as seen in critically ill patients. [Top right panel from Kraner et al. (377). Left panel from Rich et al. (582). Copyright John Wiley and Sons. Bottom middle panel from Rich and Pinter (580). Copyright John Wiley and Sons.]
There are two main differences between the rat model and patients. In the rat model, trophic signaling from the nerve at the endplate is absent, whereas in patients, chemical trophic signaling should still be present. For example, neuregulins are trophic signaling molecules from nerve that modulate muscle acetylcholine receptor expression (586). Loss of neuregulin signaling due to denervation may mediate the terminal Schwann cell response to denervation (586). A second issue is that in patients muscle is unloaded, whereas in the rat leg following denervation by sciatic nerve lesion, there is continued passive movement during gait. In an early study that directly compared unloading to denervation, it was found that unloading triggered atrophy similar to denervation, but did not trigger upregulation of extrajunctional AChRs (440). This suggests that unloading of muscle does not trigger all of the changes triggered by denervation. A recent study in patients examined the effects of mild passive movement on muscle function in immobilized patients and found that, while it improved specific muscle force, it did not improve either atrophy or myosin loss (435). Thus some defects in CIM are likely triggered by unloading, but other defects are triggered by other aspects of critical illness and immobility in the ICU.
While there are differences between the risk factors present in the rat model and patients, the rat steroid denervation model does an excellent job of recreating pathological changes present in patients with CIM. The features recreated by the rat model include (Figure 13):
Thus all the major changes in muscle found in CIM patients are shared by the rat model (it should be noted that inadequate autophagy and metabolic failure have not yet been tested for in this rat steroid-denervation model). The simplest interpretation of these pathological data is that loss of action potentials and steroid treatment cause myopathy in rats, which is very similar to CIM occurring in patients. Major advantages of the model are that it is easy to create and studies are inexpensive. The primary disadvantage of the model is that although it reproduces the phenotypic features of muscle in patients with CIM, the rats are not critically ill and thus lack many of the triggers that are involved in causing CIM in patients. It is important to note that CIM can develop in critically patients and in experimental animal models that have not been exposed to externally applied corticosteroids. Thus, while this model is excellent in studying and treating pathological mechanisms, it should be used with caution for studies aimed at preventing CIM by treating triggers of CIM.
C. Porcine ICU Model
Unfortunately, there are very few experimental models where long-term effects of mechanical ventilation and immobilization on skeletal muscle have been studied in detail. However, experimental porcine models have for many years been used to study the effects of sepsis on organ function (Figure 14) (11, 522) and in the development, treatment, and testing of diagnostic markers of malignant hyperthermia (476). The porcine model was, therefore, forwarded as an ideal candidate for an animal model of CIM, due to the known similarity in metabolism between humans and pigs and extensive experience with this model (511). In this porcine model, the same type of ventilators used in ICU patients can be used in pigs. Previously, experiments with the porcine model were typically for shorter durations, i.e., a day or less. In pilot experiments, it was shown that mechanically ventilated pigs exposed to NMB, systemic administration of corticosteroids, and sepsis for 5 days developed an electrophysiological phenotype resembling previous observations in ICU patients with CIM, i.e., reduced compound muscle action potential (CMAP) amplitudes with maintained motor nerve conduction velocity and intact sensory nerve action potential amplitude and conduction velocity (511, 517) (Figure 14C). This model was subsequently used in a series of studies focusing on the relative importance of different trigger agents such as neuromuscular blockade, sepsis, and steroids in the pathogenesis of CIM, muscle specific differences, as well as the effects of a Ca2+ sensitizer on diaphragm muscle fiber function (1, 2, 32, 511, 517, 520, 566, 686). Specific interest was focused on the early effects of NMB, systemic corticosteroid administration, and sepsis, separately or in combination, on muscle fiber size, regulation of muscle contraction at the single muscle fiber level in limb, respiratory and craniofacial muscles, and electrophysiological properties (1, 2, 32, 517, 520, 686). Exposure to mechanical ventilation and sedation, with or without NMB, did not have a significant effect on limb muscle fiber size or specific force during the 5-day observation period, but the addition of systemic administration of corticosteroids, sepsis, or the combination of corticosteroids and sepsis resulted in a decreased specific force and maintained fiber size (517). The most dramatic decline in specific force was observed in pigs receiving the combination of mechanical ventilation, sedation, NMB, steroids, and sepsis where specific force declined almost 50% in spite of a maintained fiber size. In none of the groups were any significant differences observed between muscle fibers expressing different fast or slow myosin heavy chain isoforms (517). Maximum velocity of unloaded shortening was not affected by any of the different trigger factors in muscle fibers expressing the same myosin isoform, suggesting maintained cross-bridge cycling kinetics in spite of the decreased force generation capacity (517). The CMAP amplitude was reduced in all groups independent of the combination of trigger factors, suggesting that different mechanisms underlie the decline in muscle membrane excitability and force generating capacity in response to the ICU condition (Figure 14C) (517). The sparing of both muscle fiber size and force generation capacity in response to mechanical ventilation with or without NMB were paralleled by a significant upregulation of high- and low-molecular-weight heat shock proteins (HSPs). In animals exposed to sepsis and corticosteroids in addition to mechanical ventilation and NMB, the decline in force generation capacity was accompanied by decreased HSP levels (2, 32). In the diaphragm, 5 days of mechanical ventilation and sedation resulted in a severe decline in muscle fiber force without any structural remodeling at the cellular and motor protein levels. The addition of sepsis, corticosteroids, and NMB, separately or in combination, did not add any significant negative effects, in sharp contrast to observations in limb muscles (520). The unaltered myosin and actin contents in the diaphragm indicate that qualitative changes in contractile proteins by posttranslational modifications may be the dominant mechanisms underlying the ventilator-induced deterioration of diaphragm muscle function. Both muscle fiber size and force generation capacity of the craniofacial masseter muscle were maintained in response to the 5-day exposure to mechanical ventilation, NMB, corticosteroids, and sepsis, in contrast to limb muscles (1). This is consistent with the sparing of craniofacial muscles in ICU patients with CIM. Gene expression analyses revealed highly complex mechanisms underlying this muscle-specific mechanism and HSPs appear to play an important role in this muscle specific difference (1). However, other factors, related to metalloproteinase inhibition, oxidative stress response elements, transcription, and growth factors are also involved in the muscle specific difference between limb and craniofacial muscles (1). The exact mechanism underlying the relative sparing of craniofacial muscles versus limb muscles, which is also reproduced in the porcine animal model, is not known, but intrinsic differences related to differences in embryonic origin and inherent differences in membrane properties and myofibrillar protein expression may have a significant effect on muscle-specific differences also in mechanosensation and responsiveness to mechanical silencing (1, 8).
FIGURE 14.
Rat and porcine ICU animal models of CIM. A: settings of the rodent (top) and porcine (bottom) experimental ICU models. B: single muscle (EDL, soleus) cross-sectional area (CSA) and specific force progressively declined in mechanically ventilated rats with duration of treatment and fell to ∼50% after 2 wk (518). The data show prominent muscle weakness beyond pure atrophy. C: electrical hypoexcitability of porcine tibialis anterior muscle seen as marked drop in CMAP amplitudes 5 days following mechanical ventilation (MV) alone and in combination with NMBAs, corticosteroids (CS), sepsis, or all triggering factors (517). D: preferential myosin loss during mechanical ventilation, documented in Coomassie-stained 12% SDS-PAGE stained gels from rat soleus muscle in a control animal (1) and in a rat mechanically ventilated and immobilized for 10 days (2). The myosin-to-actin ratio was 2.1 in the control and 0.5 after 10 days of complete immobilization. The myosin-to-actin ratio in the diaphragm (3) from a rat mechanically ventilated and immobilized for 10 days was 2.0, i.e., similar to control values in both limb and respiratory muscles. E: electron micrographs from soleus and diaphragm muscles from control rats and rats mechanically ventilated for 10 days. In the soleus muscle, 10 days immobilization and mechanical ventilation (MV + IM) resulted in a disorganization of the A-band in the sarcomere, while an intact A-band is observed in the diaphragm after 10 days of mechanical ventilation, in accordance with the maintained stoichiometric relationship between myosin and actin. In spite of a maintained myosin-to-actin ratio in the diaphragm, a slight general myofibrillar protein loss was observed in response to 10 days of mechanical ventilation. In the diaphragm, mitochondria appeared swollen with disorganized cristae after 10 days of mechanical ventilation (mitochondria are indicated by the arrows). Horizontal bars denote 1 μm.
Both high- and low-molecular-weight HSPs are involved in the protection of proteins in response to different types of stress (1). The high-molecular-weight HSPs include HSPs 60, 70, 90, and 110, and the low-molecular-weight HSPs include HSP 27 and αB-crystallin. HSP 27 and αB-crystallin are translocated from the cytoplasm to the sarcomere where they protect sarcomeric proteins, including myosin, during stress. It was recently shown that low-molecular-weight HSPs also prevent aggregation of the giant sarcomeric protein titin during stress (374). In addition to the protective role of HSPs, they are also involved in different physiological processes affecting muscle size and function. HSP 70 is involved in FOXO signaling, and HSP 27 is a negative regulator of NF-κB in skeletal muscle, and overexpression of these HSPs reduces muscle atrophy during different muscle wasting conditions (160, 627). Thus results from the porcine model indicate there is a loss in muscle function when HSP expression is compromised and that a muscle specific difference in HSP expression is one factor underlying the sparing of craniofacial muscles in critically ill ICU patients.
The 5-day exposure to sepsis in mechanically ventilated pigs is, to our knowledge, significantly longer than in any other porcine sepsis experiment, but still, this duration was too short to induce the preferential myosin loss observed in ICU patients with CIM. This is consistent with clinical observations, i.e., that it typically takes longer than 5 days to develop the CIM phenotype with the preferential myosin loss in mechanically ventilated ICU patients (6). Thus there is a need for an experimental model allowing both short- and long-term studies. In principle, this could be achieved with the porcine model, but it would be associated with significant logistic problems and financial costs. The age of the pigs is an additional problem, i.e., all experiments have so far been performed in piglets weighing ∼25 kg. These animals are in a significant growth phase with an expected approximately fourfold increase in body weight within 6–7 mo, i.e., an anabolic situation significantly different from what is observed in ICU patients with CIM and may more reflect a model of CIM in pediatric patients. Furthermore, the incidence of CIM increases with patients' age, and there are only few reports of CIM in pediatric ICUs (608). However, these disadvantages could, at least in part, be overcome by studying adult mini-pigs who have approximately the same body weight as the piglets.
D. Rodent ICU Model
Experimental rodent ICU models offer a cost-efficient alternative to the porcine ICU model with significant logistic and administrative advantages. Mouse models allow efficient genetic engineering, but there is increasing evidence that the rat is a better model for studies of muscle disease and aging (336, 628). However, long-term mechanical ventilation in small rodents imposes substantial challenges, since commercially available rodent ventilators are unable to maintain life support for longer than a couple of days. Most rodent studies using commercially available ventilators are, therefore, limited to observation periods shorter than 24 h, limiting the interpretative value both by adding a significant confounding factor when studying a failing preparation, and also that it takes significantly longer than 24 h to develop CIM phenotypes.
More than two decades ago, Dworkin and co-workers (174–177) developed an experimental rat model to study blood pressure regulation in a pharmacologically paralyzed rat. A ventilator was designed allowing long-term controlled mechanical ventilation of the rat, and the longest duration one rat has been exposed to NMB and mechanical ventilation is 3 months (174–177). Thus this model offers a unique possibility to conduct time-resolved analyses with a high temporal resolution on the effects of the ICU condition on muscle, muscle specific differences, as well as in the design and evaluation of specific intervention strategies (395). In accordance with clinical and experimental studies using the porcine ICU model, mechanical ventilation and immobilization are required for durations longer than 5 days to induce the CIM phenotype, i.e., muscle wasting and a preferential myosin loss in limb muscles, relative sparing of carniofacial muscles, and severe negative effects on regulation of diaphragm muscle fiber function (8, 133, 517, 520). The CIM phenotype was observed in the rat model in the absence of both systemic corticosteriod hormone administration and sepsis. Importantly, complete “mechanical silencing,” i.e., no external load related to weight bearing and internal load related to activation of contractile proteins, was accordingly forwarded as an important factor underlying the preferential and significant myosin loss, leading to impaired muscle function and persistent paralysis (510–512, 517, 574). Current studies raise the possibility that the complete mechanical silencing in the experimental ICU models and in mechanically ventilated ICU patients is unique for the ICU condition and different from the immobilization during bed rest, joint fixation, hindlimb suspension, microgravity, or peripheral denervation. Detailed time-resolved analyses of global protein synthesis rate, transcriptional regulation of myofibrillar protein synthesis, and activation of major proteolytic degradation pathways in skeletal muscle revealed a unique and complex temporal muscle-specific pattern in response to the ICU intervention. Further studies directly comparing mechanical silencing in the ICU to other manipulations of muscle activity will be necesssary to test this hypothesis. The preferential myosin loss in limb muscles was paralleled by a strong transcriptional downregulation of both actin and myosin, an early activation of the ubiquitin-proteasome degradation pathway preceding the muscle atrophy and the preferential myosin loss. Calpain and lysosomal protein degradation pathways, on the other hand, were preceded by the preferential myosin loss. The MuRF1 and MuRF3 E3 ligases showed a biphasic spatial translocation in response to the mechanical silencing with an early nuclear translocation and were followed by a translocation to the cytoplasm with a distinct perinuclear accumulation after long-term immobilization and mechanical ventilation (517). Mild passive mechanical loading of limb muscles ameliorates both the muscle fiber atrophy and the loss of specific force in the rat ICU model. In the slow-twitch soleus, with a higher protein turnover rate than in fast-twitch muscles, the preferential myosin loss was ameliorated together with lower levels of MuRF1 (574). Thus these insights offer molecular and cellular mechanisms underlying the positive effects of early mobilization in mechanically ventilated and immobilized ICU patients, as well as strongly supporting intense physical therapy in immobilized ICU patients.
The major inspiratory muscle, the diaphragm, showed a different temporal pattern in response to the ICU condition compared with limb muscles. A rapid early decline in maximum diaphragm muscle fiber force, preceding fiber atrophy, was observed in response to mechanical ventilation. Two weeks after controlled mechanical ventilation, residual function of diaphragm muscle to generate force was <15% of control values when combining the effect of both atrophy and loss of specific force at the single muscle fiber level (133). These measurements were performed directly assessing contractile protein function and bypassing EC coupling in permeabilized diaphragm muscle fibers; thus changes in membrane excitability and EC coupling may accordingly add to the effects at the contractile protein level in intact fibers. Furthermore, there was a progressive increase in the number of no force generating fibers with increasing duration of the mechanical ventilation, adding to the 85% reduction in diaphragm muscle function (133). In accordance with observations in limb muscles, there was an early activation of the ubiquitin proteasome, but in contrast to limb muscles, myosin-to-actin ratios were not affected, and transcriptional regulation of myosin isoforms did not show the dramatic and early downregulation as observed in limb muscles. Although the exact underlying mechanisms for the diaphragm weakness are not known, these findings may point towards an altered quality of acto-myosin interactions in the affected diaphragm where also the loading pattern would be changed during ICU management (133, 511, 517, 518). Interestingly, in intercostal muscles, there is a similar decline in myosin-to-actin ratio as in the limb muscle (512). Thus it may be speculated that the passive loading of the diaphragm by the mechanical ventilator (72 times/min in the rat model) had an impact on transcriptional regulation of myofibrillar protein synthesis but not on the activation of protein degradation pathways. However, it cannot be ruled out that intrinsic muscle-specific differences underlie the differences in transcriptional regulation of myofibrillar proteins between diaphragm and limb muscles. While the preferential myosin loss plays an important role for the decreased specific force in limb muscle fibers, other mechanisms are dominating in the diaphragm, e.g., an early onset of oxidative stress, mitochondrial dysfunction, intracellular lipid accumulation, and posttranslational protein modifications (PTMs) (133). Thus qualitative changes in contractile proteins resulting in the severely impaired residual function appear to dominate in diaphragm, while quantitative changes in myosin and myosin-associated proteins play a more important role for the decreased force-generating capacity in limb muscles.
As discussed above, craniofacial muscles are typically spared or less affected than limb muscles in CIM patients. The masticatory masseter muscle was, therefore, studied in the rat ICU model. In contrast to limb muscles, the masseter showed only a mild and delayed decline in myosin-to-actin ratio, smaller reduction in fiber size, and no transcriptional downregulation of myofibrillar protein synthesis. In contrast to both limb muscles and the diaphragm, there was an increase in MuRF1 levels and more sustainable levels of sarcomere protective HSPs and autophagy (8). Thus the better preserved myofibrillar protein expression and muscle fiber size in the masseter muscle compared with limb muscles in response to the ICU condition were the result of muscle-specific differences in both transcriptional regulation of myofibrillar protein synthesis and protein degradation pathways and coupled to a decreased activation of the IGF-I/PI3K/Akt pathway as well as protective effects of HSP and autophagy machineries (8).
The rat ICU model offers the opportunity to investigate effects of different intervention strategies on muscle function. Passive mechanical loading has been discussed above, and the rat experimental ICU model is presently being used in different pharmacological intervention studies. Nutritional status is another important factor in the treatment of critically ill ICU patients, but the role of nutrition in the pathogenesis of CIM remains unknown. In an attempt to study the effects of caloric intake in the pathogenesis of CIM, limb muscles were compared between animals given low versus eucaloric parenteral feeding (523). However, the preferential myosin loss, decline in specific force, and muscle fiber atrophy did not differ between low and eucaloric animals. Furthermore, passive mechanical loading had a sparing effect on muscle weight independent of nutritional status. Thus the mechanical silencing associated with the ICU condition is forwarded as a stronger trigger factor than nutritional status in the pathogenesis of CIM (523).
The major disadvantages of the rat and porcine ICU models are the labor intense experiments with 24 h/day monitoring of heart rate, respiratory function, blood pressure, EEG, body temperature, Pco2, peep, urine output, peripheral perfusion, and Po2. In addition, most of the equipment has to be custom-fabricated, continuously calibrated, and maintained. Despite these disadvantages, the in vivo experimental animal ICU models presented here (porcine and rat) are best at modeling triggers for CIM in patients. These models also do an excellent job at modeling pathological changes such as (Figure 14)
E. Rodent Sepsis Models (CLP and LPS Models)
Rodent models most commonly used in sepsis research are the cecal ligation and puncture (CLP) model and the LPS challenge model. The former is most commonly performed in rats while the latter is preferred in rats or mice. Both models have been extensively used to study muscle wasting associated with sepsis and the underlying mechanisms (317). The various detailed findings associated with CLP- and LPS-induced sepsis are given in the respective sections of this review. This section focuses on distinct differences of both techniques and their strengths and weaknesses, while details on the procedures are given in several reviews elsewhere (317, 588). Although being used in abundance, several lines of critique have recently emerged to the use of CLP and LPS models to adequately reflect conclusions drawn for sepsis in humans (199, 587, 628).
The CLP model has been used for over 30 yr as an animal model of bacterial peritonitis in rodents (601, 772) and has long been considered the “gold standard” in sepsis research (85, 588). It involves suture-ligation below the ileo-cecal valve and a needle puncture, allowing bacterial contamination of the abdominal cavity, followed by peritonitis and septicemia (Figure 15A). The subsequent systemic inflammatory response may then lead to septic shock, multiorgan failure, and death. The severity of sepsis can be modeled by procedure variables such as needle size, length of ligation, and number of punctures (588). However, it is exactly this variability that is often not standardized in experimental sepsis reports and which may complicate comparative results. The model adequately reproduces a hyperdynamic circulatory response and cytokine surge within a few hours (602) and reliably produces a marked increase in myofibrillar protein proteolysis from 6 h post-procedure (200, 291, 317, 776) (Figure 15A). However, myofibrillar protein loss always seems to affect actin and myosin likewise, at least in all studies investigating the time course of proteolysis up to ∼20 h (200, 290, 291, 776). It may be that a significant preferential myosin loss does not develop until longer times post-CLP, but this has not been experimentally proven although CLP in mice has been followed up until day seven, but not for protein contents in skeletal muscle (809). Thus this sepsis model has so far not qualified yet to reproduce all the pathological hallmarks seen in CIM, most importantly, preferential myosin loss, and is thus considered as sepsis-induced myopathy (SIM) under the ICUAW umbrella (Figure 1B). Apart from this specific point, CLP in rodents has been criticized in several further points. First, while the approach is well accepted to mimic the situation of perforated appendicitis or diverticulitis with peritoneal sepsis in patients, this model only applies to a fraction of septic patients, i.e., <50% of septic patients show documented bacteremia (587). Also, CLP animals are often of young age (although considered as adult; use of few weeks old animals may serve as models for human pediatric CIM in future) and without co-morbidities such as usually seen in older patients with peritoneal sepsis. Moreover, the time window of CLP sepsis in rodents is uncomparibly compressed to a few days at most, unlike in patients (199). However, the most prominent argument excluding a simple transfer of the rodent procedure to the human situation of peritoneal sepsis-induced critical illness is the lack of supportive measures in CLP-treated mice or rats, such as mechanical ventilation or complete immobilization shown to produce the CIM pathology (i.e., preferential and significant myosin loss, Ref. 517). Last, probably similar to reasons explained below for the LPS model, differences between the immune system of mice and rats versus humans may preclude simple transfer of conclusions to humans (628). However, it is important to raise general caution against overstating limitations of animal models of human disease. Reasons many animal studies fail to predict clinical success of therapy include poorly designed experiments and small sample size (91, 345).
FIGURE 15.
Rodent animal models of sepsis. Cecum ligation and puncture (CLP) (A) and lipopolysaccharide challenge (LPS) (B) are the most commonly used animal models to mimic various degrees of sepsis from mild to moderate sublethal sepsis and septic shock, depending on details of the techniques. In CLP, the prominent rodent cecum is exposed after median laparatomy, ligated with suture, and punctured with a needle. After gently squeezing the cecum to allow feces to penetrate, the cecum is relocated back into the abdomen and the incision closed. [Photographs from Rittirsch et al. (588). Reprinted by permission from Macmillan Publishers Ltd.] CLP aims to model human peritonitis sepsis. In muscle, myofibrillar protein loss (actin and myosin) is detected from ∼4 h post-CLP. [Data from Williams et al. (776), with permission from The FASEB Journal (www.fasebj.org).] In LPS challenge, lipopolysaccharides from various bacterial sources (mostly E. coli) are injected as single doses either intravenously, intraperitoneally, or subcutaneously. Depending on the doses, the degree of sepsis can be roughly controlled. LPS-induced sepsis results in marked protein loss in skeletal muscle as early as 2 h postinjection. [Modified according to data from Chai et al. (113).]
In the LPS challenge model, endotoxins are injected into animals either subcutaneously, intraperitoneally, or via intravenous routes. As a component of the bacterial wall of gram-negative bacteria, LPS activates the innate immune response via binding to toll-like-4 receptors (TLR-4) with a dose- and administration-dependent response (79). Since continuous or repetitive injections may often result in tolerance, single-dose injections are most commonly employed (317). Depending on the applied dose mild, moderate (sublethal) and severe (lethal) courses of sepsis can be observed (Figure 15B). Sublethal doses (0.5–5 mg/kg body wt) induce a transient increase in pro-inflammatory cytokines and febrile response with recovery of animals within 48 h (317), whereas higher doses produce severe sepsis, multiorgan failure, and endotoxic shock that usually is lethal within 48 h, but there is substantial variation (317). Both moderate and higher doses alter muscle protein metabolism towards an increased myofibrillar proteolysis and decreased protein synthesis in many studies as early as 2 h post-LPS challenge (113) (Figure 15B). A selection of studies and their findings is given in Holecek (317). Although very commonly used as a model mimicking endotoxin burden in septic patients, the LPS model in rodents has been quite recently challenged regarding its transferability to human sepsis (628). In fact, the very transient increase in plasma cytokine levels and rapid clearance of LPS from the systemic circulation is not matching the situation in humans. This may be due to circulating factors in the plasma of mice suppressing the production of pro-inflammatory cytokines following LPS challenge, such as hemopexin (424). This may at least in part explain the vast difference in LPS sensitivity between mice and humans, with mice requiring an LD50 of 1–25 mg/kg body wt, a million times greater dosage than required to induce fever in humans (199, 666). Seok et al. (628) even conclude that “while the genomic responses to different acute inflammatory stresses are highly similar in humans, these responses are not reproduced in current mouse models” (628). Therefore, while the finding of myofibrillar proteolysis in LPS/CLP models might be attractive to transfer to the situation in septic ICU patients, the impact of the sepsis response on muscle in rodent models might be an inadequate correlate of muscle wasting in septic patients. This is because whenever clinical studies in ICU myopathies usually refer to septic ICU patients in the first place with the primary variable of sepsis, those patients are almost always mechanically ventilated and/or immobilized at the same time, at least in the initial phases. Thus, the hallmark of CIM in patients (immobilized and/or mechanically ventilated regardless of whether septic or not) is reflected by a preferential myosin loss that has so far not been confirmed in any pure CLP/LPS model. It should be noted that almost all studies of sepsis in rodents do not include mechanical ventilation and immobilization (exceptions see above). To more accurately mimic CIM in patients, these triggers need to be included.
F. Rabbit Burn Injury Model of Prolonged Critical Illness
A well-validated model of prolonged critical illness is that of catheterized and fluid resuscitated rabbits suffering from hyperinflammation-induced critical illness evoked by third degree burn injury (155). This model displays endocrine and metabolic alterations that are very similar to those in ICU patients suffering from prolonged critical illness (766). Fasted critically ill rabbits lost weight and showed elevated mRNA expression and/or activity of several ubiquitin-proteasome pathway components, calpain-1 and cathepsin-L, as well as a shift towards smaller myofibers in skeletal muscle (155, 156). Intravenous feeding largely counteracted this response, provided hyperglycemia was avoided. Responses in diaphragm and heart were roughly similar. In this model, it was clearly shown that fasting during critical illness also induces activation of autophagy in vital organs and skeletal muscle. Intravenous feeding abolished these responses, with most impact with amino acid-enriched nutrition. Accumulation of p62 and ubiquitinated proteins in muscle and liver, indicative of insufficient autophagy, occurred with parenteral feeding enriched with amino acids and lipids. This was accompanied by fewer autophagosomes, fewer intact mitochondria, suppressed respiratory chain activity, and an increase in markers of organ damage in the liver. In muscle, early parenteral nutrition enriched with amino acids or lipids aggravated vacuolization of myofibers. Hence, just as in human ICU patients, early parenteral nutrition during critical illness in this model was shown to evoke a phenotype of autophagy deficiency in skeletal muscle which in human patients was causally related to ICU-acquired weakness. From these initial studies, the rabbit burn injury model of prolonged critical illness may be a model worth pursuing to further investigate key features of CIM in affected muscles.
G. “Human” Models of ICU-Related Myopathies: The “Circulating Factor Hypothesis”
In a very touching foreword, J. E. Fischer (289) refers to George Clowe's seminal work in 1983 concerning a proteolysis-inducing factor present in plasma from septic patients (124). That study, for the first time, showed that serum from septic patients contained a ∼4-kDa low-molecular-weight protein that was able to induce muscle proteolysis in vitro (124). This proteolysis-inducing factor (PIF) was later suggested to be a degradation product of IL-1β (159). To assess the proteolytic potency of IL-1, its byproducts or other cytokines' ability to induce muscle proteolysis, a series of studies aimed at applying serum, serum fractions, or purified protein factors (cytokines, etc.) to skeletal muscle cells in vitro and studying protein contents and/or cell functions thereafter. Usually, those muscle cells are of animal origin, either freshly isolated fibers, fiber bundles, or myotubes. While some laboratories could not confirm a proteolytic effect induced by IL-1 or TNF-α in vitro (251, 258, 491), TNF-α injection in vivo into rats was effective in accelerating muscle proteolysis, similar to endotoxin treatment, but unlike IL-1 injection which was ineffective (258). Other studies, however, were able to detect a marked stimulation of protein degradation by highly purified human leukocytic pyrogen (IL-1) when applied to isolated rat muscles kept at between 37 and 39°C (34). When the action of purified IL-1 was compared with that of recombinant IL-1 (α or β isoform), recombinant factors failed to reproduce muscle proteolysis in vitro (251). From this finding, Goldberg et al. (251) suggested an additional, still unidentified product of activated monocytes to essentially regulate proteolysis in systemic infections, and which would still be present in IL-1 purifications but not in the recombinant cytokine. Similar as for IL-1, IL-6 was able to induce muscle proteolysis, but only when applied in vivo. In vitro incubation of isolated muscles with IL-6 failed to induce proteolysis (258). Since these studies suggested additional factors present in the plasma during sepsis or systemic infection distinct from the mentioned cytokines, Hasselgren et al. (289) collected plasma from septic rats 16 h after CLP and produced <30-kDa molecular weight fractions to be acutely applied to rat EDL and soleus muscles. Surprisingly, no proteolysis of myofibrillar proteins was detected, further blurring the role of a putative circulating proteolytic factor in sepsis. To look for specific mechanisms in muscle weakness in response to acute challenge with human sepsis serum rather than rodent serum, Friedrich et al. (218) collected serum samples from septic ICU patients with a clinical diagnosis of CIM and applied various molecular weight fractions to single murine muscle fibers (217, 218). Acute serum fraction applications were able to induce a membrane depolarization in time and also reduced caffeine-induced Ca2+-activated force transients. The latter was only seen with small-molecular-weight fractions <10 kDa but was blunted for larger molecular weight fractions. This finding shows that it is important to dissect serum fractions to identify active factors that might be present in a biologically inactivated form in full serum studies. In a later study, van Hees et al. (740) were able to demonstrate a proteolytic effect of plasma from patients with septic shock when applied to C2C12 myotubes.
The in vitro septic serum challenge approach has the advantage that any cell physiology parameter can be tested for using established laboratory techniques in a control versus post-exposure setting similar to as in pharmacological experiments. The use of isolated cells removes many of the uncontrolled variables usually present in in vivo experiments. However, major disadvantages involve the inability to model specific trigger factors inherent to ICUAW, and results are often very inhomogeneous due to the diversity of the underlying patient population, and even the time points samples are retrieved from patients. In conjunction with the small number of patients usually involved, the statistical power remains limited in most studies.
So far, some studies point towards an existing “myotoxic” factor systemically circulating in sepsis and systemic inflammation. Whether this is also true for the controlled setting of CIM, i.e., for pure mechanical ventilation and immobilization, is currently not known. For this, the animal models described in detail above would need to serve as serum source (porcine/rodent ICU model) for subsequent in vitro testing. One hypothesis could be that such a circulating factor was in fact produced by the lungs in the setting of mechanical ventilation. Whatever the source of such a factor, the identity of it has not been resolved yet, even after more than 30 yr of its original proposition.
Table 6 compares the properties, the strengths, and the weaknesses of each model presented in this section.
Table 6.
Comparison of various animal models to study mechanisms in ICU-related myopathies
| Model | Manipulations | Triggers Modeled | Pathological Changes Replicated | Strengths | Weaknesses |
|---|---|---|---|---|---|
| Rat steroid denervation | Lesion to the sciatic nerve, daily administration of corticosteroids | Neuromuscular blockade | Muscle atrophy (377) | Easy to perform | Does not model risk factors such as sepsis, loss of movement |
| Corticosteroid treatment | Preferential loss of myosin (377) | Inexpensive | |||
| Immobilization not modeled | Disorganization of sarcomeres (377) | Models all pathological changes seen in CIM patients | |||
| Electrical hypo/inexcitability (582) | |||||
| Rat ICU | Infusion of neuromuscular blocking agents, mechanical ventilation together with treatments such as LPS and/or corticosteroids | Immobilization | Muscle atrophy (518) | Models atrophy and preferential myosin loss | Labor intensive, difficult to perform |
| Neuromuscular blockade | Preferential loss of myosin (518) | Muscle-specific differences observed in ICU patients with CIM | |||
| Mechanical ventilation | Disorganization of sarcomeres | Suitable for intervention studies | |||
| Sepsis | Similar muscle-specific differences as in ICU patients with CIM | ||||
| Corticosteroid treatment | |||||
| Porcine ICU | Infusion of neuromuscular blocking agents, mechanical ventilation | Immobility | Muscle atrophy (517) | Pigs as good model for human physiology | Labor intensive, difficult to perform |
| Neuromuscular blockade | Preferential loss of myosin (517) | Models all triggers | Expensive | ||
| Mechanical ventilation | Decreased muscle membrane excitability in mechanically ventilated and immobilized animals (independent of the addition of sepsis or systemic steroid administration) (517) | Reproduces atrophy, preferential myosin loss and muscle membrane hypoexcitability | |||
| Corticosteroid treatment | Similar muscle-specific differences as in ICU patients with CIM | Muscle-specific differences observed in ICU patients with CIM | |||
| Sepsis | Suitable for intervention studies | ||||
| Cecum ligation and puncture (CLP) | Median abdominal laparotomy, mobilization of cecum, suture-ligation of cecum and needle puncture, relocation and closure (588) | Various degrees of sepsis (mild, moderate, severe), septic shock, depending on ligation length/level and puncture number | Muscle atrophy | Simple to perform | Animals not immobilized |
| Myofibrillar protein loss (776) | High reproducibility and grading of sepsis severity when sticking to standardized protocols (588) | No mechanical ventilation | |||
| Disorganization of sarcomeres (776) | Systemic response and cytokine surge within 4–6 h postprocedure | Transferability to human problematic | |||
| Decrease in twitch/tetanic force (595) | |||||
| Muscular hypoexcitability (Na+ channel inactivation increased) (594) | |||||
| Mitochondrial dysfunction (543) | |||||
| Lipopolysaccharide challenge (LPS) | Single-dose injections of purified LPS from gram-negative bacteria (e.g., E. coli) sc, ip, or iv | Dose-dependent degrees of sepsis | Muscle atrophy (678) | Simple to perform | Animals not immobilized |
| Mild <0.5 mg/kg body wt | Myofibrillar protein loss (317) | Systemic response and cytokine surge within 4–6 h postprocedure | No mechanical ventilation | ||
| Moderate 0.5–5 mg/kg body wt | Decrease in twitch/tetanic force (678) | Use of genetically modified mice enables study of LPS receptors and innate immune activation | Transferability to humans problematic (628) | ||
| Severe (lethal) >5–10 mg/kg body wt | Muscular hypoexcitability (Na+ channel inactivation increased) (283) | Easy to design studies for drug testing | Discrepancy between LPS sensitivity of mice and humans | ||
| Mitochondrial dysfunction (236) | Systemic activation of the innate immune system | ||||
| Rabbit burn injury model | Third degree burn injury on 15–20% body surface area | Catheterization | Muscle atrophy (156) | Strongly mimics endocrine and metabolic alterations of critically ill patients (183,766) | Labor intensive |
| Parenteral nutrition | Ubiquitin proteasome activation (156,178) | High reproducibility | No mechanical ventilation | ||
| Glucose monitoring/control | Impaired autophagy activation with (parenteral) feeding (156) | ||||
| Hyperinflammation | |||||
| Immobility due to sickness | |||||
| ICU patient in vitro serum challenge model | Serum fractions from ICU patients are applied to skeletal muscle cells (from animals) and acute or long-term effects studied in vitro | Tests for a putatively myotoxic, systemically circulating factor | Muscle atrophy (740) | Easy to perform similar to drug application experiments | Does not model a single trigger factor |
| Preferential myosin loss (740) | Defined in vitro settings of single cells | Very inhomogeneous results profile due to varying patient cohorts | |||
| Decrease in chemically activated force in skinned fibers (218) | Controls (prior to serum application) well established within labs | Only studies with very small sample sizes, patient samples not readily available | |||
| Muscle membrane depolarization (178, 218) | |||||
| Ubiquitin proteasome activation (740) |
XIII. TREATMENT OF ICUAW: DRUG INTERVENTIONS and CLINICAL IMPLICATIONS
A. Intensified Insulin Treatment
Effective strategies to prevent and/or treat ICUAW (CIM, sepsis-induced myopathies) are still scarce. These strategies mainly focused on avoiding or reducing risk factors (303). As neuromuscular ICU complications are associated with sepsis, hyperglycemia, prolonged immobilization, and duration of mechanical ventilation as well as the use of corticosteroids and neuromuscular blocking agents, avoiding these is theoretically of interest (which is probably not feasible for the mechanical ventilation unless external lung assist devices are being considered). Also, strategies that avert the catabolic state associated with critical illness are appealing and could be beneficial. The strongest data currently available concern the treatment of hyperglycemia that inevitably accompanies acute illness. Several RCTs on glycemic control in the ICU were recently performed (197, 557, 736, 738, 748). Two of these, comparing normalization of glycemia using insulin (glycemic target 80–110 mg/dl: intensive insulin therapy, IIT) in critically ill patients, versus tolerating hyperglycemia up to the renal threshold (glycemia tolerated up to 215 mg/dl: conventional insulin therapy, CIT) also evaluated the neuromuscular effects in the subgroup of patients staying in ICU for at least 1 wk. In the 405 long-stay surgical patients, electrophysiological screening revealed that IIT reduced the incidence of critical illness polyneuromyopathy, diagnosed by the presence of abundant spontaneous electrical activity, from 49 to 25% (735). Muscle strength data were not available, but the electrophysiological findings were accompanied by a reduced need for prolonged mechanical ventilation. These results were confirmed in 420 long-stay medical patients (309). Meta-analysis of both trials reported a relative risk for developing neuromuscular complication with IIT of 0.65 (304). Benefits were mainly attributed to glycemic control rather than insulin dose (735). In a post hoc analysis, the beneficial effects were only present in patients in whom actual normalization of glycemia was reached but not in the subgroup of patients reaching intermediate glycemic targets (110–150 mg/dl) (736). Similar findings were made in a retrospective study on the incidence of electrophysiological abnormalities before and after institution of IIT, reflecting daily care practice (306). Insulin resistance is well known to occur in critically ill patients and seems more pronounced in patients with CIM (765). Insufficient GLUT-4 translocation to the sarcolemma may result in decreased glucose supply in patients. Mechanistic studies based on muscle biopsies from insulin trials indicated that despite improving insulin resistance and skeletal muscle GLUT-4 expression (483), IIT basically did not affect myofiber size, myofibrillar protein synthesis capacity, or markers of muscle proteolysis (154). This could indicate that benefits were mainly explained by neuroprotection, although no nerve histology data are available to confirm this. Generalizing treatment of critically ill patients towards reaching normoglycemia was questioned by the NICE SUGAR trial. This multicenter RCT pointed at the risks of broad implementation of IIT in critically ill patients, including higher incidence of hypoglycemia and even increased mortality (197). Various methodological differences between trials may be responsible for the distinct results (734). The discrepancy between results of the large trials has come to the widespread acceptance that hyperglycemia should be avoided (152), but the actual blood glucose target to aim for depends on local expertise and monitoring tools (733).
B. Early Mobilization Strategies
Reducing the duration of immobilization could also conceptually reduce the incidence of ICUAW, since bed rest and mechanical unloading induces a catabolic state, muscle atrophy, and loss of strength (114, 194, 373, 560). A first logical approach to shorten the duration of immobilization is to apply strategies aimed at reducing sedation. During the last decade, multiple beneficial effects of such interventions, including reduced duration of mechanical ventilation and ICU stay as well as less delirium, were described (38, 378, 633). Muscle function, however, was not within the primary or secondary outcomes tested. Shortening duration of immobilization can also be pursued by applying early physiotherapy aiming at mobilizing limbs in the acute phase of illness despite receiving life support treatments. This appears to be safe and feasible in the ICU (27, 429, 495, 551), and a nonrandomized trial suggested beneficial effects on ICU and hospital length of stay (495). One RCT compared 20 min of daily bedside cycling exercise from the fifth day in the ICU in addition to standard physiotherapy with standard physiotherapy alone in 90 patients (88). The trial included patients who were expected to have an ICU stay for another week. The incidence of weakness was not reported in this study, but isometric quadriceps force as well as functional status, measured by the 6-min walking distance at hospital discharge, was significantly higher in the intervention group.
Combining both approaches of minimizing sedation with early mobilization was examined in a second RCT in critically ill patients who were on mechanical ventilation for <72 h (623). Early exercise and mobilization were compared with standard care in 104 patients receiving daily sedative interruption. This trial showed that early exercise and mobilization resulted in better functional outcome at hospital discharge, shorter duration of delirium, and more ventilator-free days. There was no significant reduction in the incidence of ICUAW and, therefore, the intervention may have helped patients to cope with weakness rather than improving muscle strength itself. These are promising findings, but several barriers against the use of early rehabilitation may impair widespread implementation of such practice (410, 551, 796). Some of these, including high severity of illness, may not be modifiable. Others, such as depth of sedation (796) and mental attitude of caregivers (320), can be addressed in training programs. Optimal implementation of rehabilitation programs and resources will require confirmation of beneficial effects, identification of the patient population that benefits, and definition of appropriate intensity of treatment. In a retrospective analysis of 49 patients who failed to wean from mechanical ventilation for at least 14 days, whole body rehabilitation and respiratory muscle training showed to improve strength, weaning outcome, and functional status (459).
Finally, several beneficial effects of rehabilitation strategies following hospital discharge have been reported, although based on limited data (128). It remains unclear to what extent such strategies may promote recovery from ICUAW, as this question has not yet been addressed.
C. Electrical Muscle Stimulation
Some patients may not be able to actively mobilize during the acute phase, and electrical muscle stimulation (EMS) may therefore be another method to preserve muscle mass and function in such a setting. Preliminary data obtained from muscle biopsies of five patients at risk for ICUAW receiving unilateral EMS showed that EMS could improve AMPK activation, glucose utilization, and GLUT-4 translocation (765). The stimulated leg also showed larger cross-sectional area for type 2, but not type 1, fibers. In non-ICU patient populations, EMS has been shown to increase muscle strength and exercise tolerance (637). Preliminary results in small samples of critically ill patients were promising and found that EMS preserved muscle mass (237, 272, 474), reduced muscle catabolism (73), and exerted short-term systemic microcirculatory effects (238). In another eight patients with septic shock however, 7 days of EMS did not preserve muscle volume (555). Several randomized studies examining the effects of EMS on muscle function in the ICU recently emerged. One trial in 140 patients reported a decrease in incidence of ICUAW from 39.3 to 12.5%, accompanied by a shorter time to weaning in the intervention group (598). These data should be interpreted with caution, since significant differences for baseline characteristics, APACHE II, and certain comorbidities were present between groups in the subset of actually evaluated patients (357). Another RCT in COPD patients in the ICU found no incidence of ICUAW in EMS-treated patients. Moreover, quadriceps strength, measured using dynamometry, and 6-min walking distance were increased in the EMS group (3). EMS improved muscle strength and decreased number of days needed to transfer from bed to chair in mechanically ventilated COPD patients (797). No formal assessment for ICUAW was made. Sixteen septic, mechanically ventilated patients were randomized to receive unilateral EMS versus no EMS on the contralateral limbs. Muscle strength was better on the stimulated side (589). In conclusion, data on EMS are promising but remain scarce and, due to several methodological issues, such as small sample size and incomplete outcomes, are prone to bias and should be confirmed in larger trials.
D. Sepsis Treatment
As a key factor, aggressive treatment of sepsis to avoid ICUAW is generally advocated. In addition to the general guidelines for sepsis treatment (152), modulating the inflammatory response as a potential mechanistical pathway may be of special interest with regard to the neuromuscular complications. Overall effects of anti-inflammatory and immune-directed therapies in sepsis appeared disappointing (242, 745), although individualized approaches, including optimal timing of such strategies, may be promising (243, 323). No trial has yet specifically addressed the effects of the immune-modulatory treatments on neuromuscular complications, and this should be considered in future studies.
E. Corticosteroid Management
The use of corticosteroids, although also mentioned as a risk factor, is important to consider, as corticosteroids may reduce duration of organ dysfunction in a subgroup of patients with septic shock (22), which by itself is related to the development of ICUAW. Moreover, when concomitant hyperglycemia is treated, steroids might have beneficial neuromuscular effects in critically ill patients, possibly due to anti-inflammatory properties (309). Several RCTs comparing corticosteroids versus placebo were performed in critically ill patients, but only few reported on neuromuscular complications. The Corticus trial found no effect of corticosteroids on the incidence of polyneuropathy in 499 patients with septic shock (654). However, no diagnostic methods for polyneuropathy were provided, and the figures were unexpectedly low in both groups. Another RCT focused on 180 patients with persisting ARDS and compared treatment with corticosteroids (methylprednisolone) versus placebo (658). There was no difference in the incidence of neuromyopathy between both intervention groups. Methylprednisolone increased the number of ventilator-free and shock-free days during the first 28 days and did not affect mortality. Serious adverse events related to neuropathy or myopathy, however, were reported in nine patients in the steroid group versus none in the control group. In some clinical situations, such as status asthmaticus and systemic vasculitis, corticosteroids are potentially life-saving, and possible side effects should not compromise their prompt institution. In other situations including septic shock (654) or ARDS (658), benefits are less clear and should be weighed against possible side effects, including weakness.
F. Management of Neuromuscular Blockade
Concerning the use of neuromuscular blocking agents, only one RCT studied the effect on ICUAW (536). No difference in incidence of ICUAW was found in patients with early ARDS receiving neuromuscular blocking agents for 48 h versus controls. Neuromuscular blocking agents are still being used, although much less frequently and more selectively to date, for several indications such as urgent intubation, patient-ventilator asynchrony, refractory respiratory failure, intracranial hypertension, and therapeutic hypothermia (265). Despite the lack of equivocal evidence of negative neuromuscular effects, it is generally accepted to limit use of neuromuscular blocking agents (146) and when used, to evaluate depth of neuromuscular blockade applying monitoring techniques (744).
G. Nutritional Strategies
Severe muscle atrophy due to the catabolic state in critically ill patients is generally considered to contribute to muscle weakness. A nutritional deficit rapidly develops during critical illness due to dysfunction of the gastrointestinal tract. This is most troublesome in patients who cannot be fed enterally because of contraindications. Postoperative esophagectomy or pancreato-duodenectomy patients received no enteral feeding in the first 6 days versus enteral feeding through a jejunostomy. No effect on grip strength or respiratory muscle strength was noted, and the fed patients tended to have less rapid recovery of mobility (762). The EPaNIC trial randomized 4,640 patients for early parenteral supplementation (early PN group) versus tolerating the caloric deficit during the first week in ICU (late PN group) (107). Overall, accelerated recovery, shortened duration of mechanical ventilation, and fewer complications were shown for the late PN group compared with the early PN group. Additionally, systematic evaluation of muscle strength using the MRC sum score was performed in patients who stayed in ICU for at least 8 days and were considered to be at high risk for developing ICUAW, as well as in a random sample of short-stay patients (300). The evaluations were performed three times weekly from day eight until ICU discharge or death. The incidence of ICUAW at first evaluation was significantly lower in the late PN group compared with the early PN group. Muscle biopsies from 122 EPaNIC patients showed that this was possibly due to more efficient autophagic qualtity control of myofibers, whereas markers of atrophy were not affected. Another large RCT focused on a specific subgroup of ICU patients, deemed to have contraindications for enteral feeding (161). This trial randomized 1,372 subjects to conventional treatment or early parenteral nutrition and indicated greater muscle wasting in the standard care patients, but no assessment of muscle strength or functional outcome was made (161). The Eden trial studied full enteral versus trophic feeding during the first 6 days in 1,000 acute lung injury patients (503). No strength measurements were provided at ICU or hospital discharge, but at 6 and 12 mo follow-up, physical performance was below the predicted one for a subset of 174 survivors. No difference was found for MRC sum score, handgrip strength, maximal inspiratory pressure, 6-mo walking distance, and quality of life (504). Various supplemental therapies may be of interest. Glutamine concentration in plasma and skeletal muscle are low in critical illness, which independently predicts mortality. This led to the hypothesis that glutamine is a conditionally essential amino acid and that supplementation may not only improve overall outcome but also muscle function (87, 770). A meta-analysis reported beneficial effects of glutamine supplementation in critically ill patients (514), but this was not confirmed in two recent RCTs (18, 312). The latter even showed increased mortality rates in critically ill patients with multiple organ failure (312), and effects on muscle function have not been studied yet. The use of other micronutrients against oxidative stress is also sensible from a pathophysiological point of view. Two recent meta-analyses concluded that antioxidant micronutrients are of potential benefit for critically ill patients (452, 746). Again, effects on muscle weakness were not studied, and the recent large RCT showed no beneficial effects from antioxidants in critically ill patients with multiple organ failure (312). A recent review concluded that there is no evidence for benefit with early nutrition, nor supplemental therapies in critically ill patients (108). Preliminary data from animal experiments showed that mitochondrial emission of ROS is a crucial element in atrophy development and contractile dysfunction of the diaphragm occurring during mechanical ventilation (556) which was prevented by a novel mitochondria-targeted antioxidant.
H. Hormone and Electrolyte Balance
Several other hormones have a key role in the anabolic/catabolic balance of the muscle and could, therefore, possibly be of interest in the prevention of ICUAW. One of the candidates to this effect was growth hormone, since resistance to growth hormone is a characteristic feature of critical illness. Two large randomized trials showed clearly increased mortality rates in patients treated with growth hormone (688). Although the interest in the potential of growth hormone treatment in the recovery phase for selected chronically ill patients is reviving (694), current recommendations are clearly against its use. Low sex hormone concentration in aging may be a key factor in sarcopenia and muscle weakness (642). ICUAW is also associated with hypogonadism in men (631). Testosterone derivatives reduced length of stay and preserved lean body mass in burn patients (352, 783). Data are not supportive for use in trauma (239) or surgery (84) patients, although effects on ICUAW have not formally been addressed. Also, concerns about hepatotoxicity with the use of oxandrolone, an oral synthetic testosterone derivative, have been raised (352). Finally, electrolyte disturbances could affect muscle function (768). The need for correction, rate, and degree to which electrolytes should be corrected depends on multiple factors and generally is not primarily determined by muscle function (768). Also, dedicated weaning protocols might be indicated for weak patients (80) to avoid muscle fatigue (387), although no clinical data are available in this specific group of critically ill patients.
In summary, data on effective strategies to prevent and/or treat muscle weakness in critical illness remain limited, mainly due to lack of well-designed large RCTs. Further research is clearly needed. In particular, strategies to reduce hypoglycemic events during insulin treatment should be pursued, as well as further exploring the potential of early rehabilitation and electrical muscle stimulation and other treatments should be addressed, suggested from targeting pathogenetically involved pathways in this review. Table 7 summarizes current views on strategies with direct, indirect, and potential evidence for therapeutic benefit as well as evidence for harmful outcome and a list of some future challenges is provided at the end of the next chapter.
Table 7.
Therapeutic strategies for ICU-related weakness
| Level of Evidence | Presumed Target | Presumed Target at Cellular/Molecular Level |
|---|---|---|
| Direct evidence for benefit | ||
| Tight glycemic control | Nerve | Improved microcirculation (E) (183, 393, 781) |
| Improved mitochondrial function (E) (735) | ||
| Attenuated loss of nerve fibers (E) (603) | ||
| Withholding parenteral nutrition during first week in ICU | Muscle | Improved autophagic muscle quality control (A) (300) |
| Indirect evidence for benefit | ||
| Sedation sparing protocol | Muscle | Prevention of disuse atrophy caused by decreased protein synthesis, anabolic resistance, and increased protein degradation by the ubiquitin proteasome system (C, D) (81, 260) |
| Early limb mobilization | Muscle | Autophagy (C, D) (294, 689) |
| Potential evidence for harm: limit use unless evidence-based benefit | ||
| Corticosteroids | Muscle | Promoting atrophy by decreased protein synthesis and increased proteolysis by the ubiquitin proteasome and lysosomal system (A, B) (154, 616, 617, 703) |
| Neuromuscular blocking agents | NMJ | Prolonged neuromuscular blockade (A) (265) |
| Muscle | Denervation atrophy (D) | |
| Theoretical benefit | ||
| Careful electrolyte management (Na+, K+, P, Mg2+, Ca2+) | Nerve | Nerve membrane excitability (E) |
| NMJ | Neuromuscular transmission (E) | |
| Muscle | Muscle membrane excitability (E); excitation-contraction coupling (E) | |
| Ventilator weaning protocol | Muscle | Avoiding fatigue (E) (387) |
| Electrical muscle stimulation | Muscle | Prevention of atrophy by suppression of ubiquitin proteasome system and calpain and increased anabolic pathways by induction of growth factors (A, C, D) (73, 171, 467, 664, 765) |
| Autophagy (E) | ||
The level of available evidence is graded as follows: A) human data from critically ill patients; B) experimental data from ICU models; C) human data from noncritically ill or healthy volunteers; D) experimental data from other animal models, including immobilization/denervation; E) hypothesis.
XIV. SUMMARY/OUTLOOK
In this review, we have discussed the different entities of ICU-related weakness as well as potential mechanisms inherent to and differentiating between those entities. One important emphasis of the current consortium is to stress the fact that inconsistencies exist in the literature regarding the classification of weakness in general and myopathies in particular, seen in critically ill patients. For critical illness myopathy to be diagnosed appropriately, several pathological key features shall be present: 1) electrical hypoexcitability of muscles, 2) severe atrophy, 3) preferential and significant myosin loss, 4) disorganization of sarcomeres and ultrastructural abnormalities, and 5) impaired autophagy. These findings may be present between patients in combinations of varying degree. Unless all of those features are confirmed, the definite diagnosis “CIM” does not hold and the more pragmatic term “ICUAW” shall be used. However, it needs further clarification whether the finding of impaired autophagy exclusively applies to CIM patients or also to other subsets of ICUAW. If some of these features are present, e.g., most clinical routine diagnostics will be able to cover features 1, 2, and 4, the term probable CIM may be used, but it is mandatory to perform a focused search for features 3 and 5, in particular because the presence of preferential myosin loss seems, so far, to be the only distinction between CIM and sepsis-induced myopathy, and the presence of impaired autophagy may alter the clinical handling with an initial calorie restriction protocol. Strong symptoms arguing for the presence of CIM in most patients are reduced membrane excitability and preferential myosin loss.
The choice of appropriate animal models mimicking the situation in ICU patients is crucial for current and future studies of missing links in the pathophysiological puzzle and to test for treatment efficiencies. While immobilization is clearly a key risk factor for development of ICUAW and CIM in particular, it is likely that there may also be a contribution of an ongoing inflammatory process. As detailed in our review, the pathophysiological mechanisms found in neuropathies and myopathies in the critically ill are very diverse and affect all facets of neuron and muscle function (Figure 16). One unique complication of critical illness appears to be an acquired channelopathy that affects multiple electrically active tissues and may contribute to myopathy, neuropathy, cardiac failure, and poor recruitment of motor units. However, whether this ion channel dysfunction is a primary pathology or simply a consequence secondary to alterations rendering the ion channel dysfunctional, e.g., electrolyte disturbances, metabolic failure, proteolytic activity in critical illness, etc., is not known and represents a future field of studies where the animal models discussed in this review may become extremely valuable. Other pathological processes include impairments of the neuromuscular transmission and postsynaptic membrane depolarization, so muscle becomes electrically hypo- or inexcitable. Aspects of critical illness related to intracellular Ca2+ homeostasis have only scarcely been investigated in muscle, while not at all in nerve. Evidence points towards substantial alteration in Ca2+ handling mechanisms, at least documented for sepsis-associated weakness. Complete mechanical silencing, i.e., no weight bearing and no internal strain related to activation of contractile proteins, is unique for mechanically ventilated, deeply sedated, or pharmacologically paralyzed ICU patients and has been shown in experimental studies to play a very important role in the pathogenesis of CIM via different mechanosensitive pathways, but other factors have additive/synergistic effects in the development of the CIM phenotype. In addition, the compromised inherent protective mechanisms of sarcomeric proteins and mitochondria by heat shock proteins triggered by systemic corticosteroid hormone administration and sepsis, in combination with mechanical silencing, play an important role for the impaired muscle function in CIM. Thus interventions targeting mechanosenstive pathways and heat shock protein expression offer novel venues for future pharmacological treatments in mechanically ventilated critically ill ICU patients. To date, the only therapeutic strategies with a proven positive outcome include intensified insulin therapy to prevent hyperglycemia and avoiding early parenteral nutrition to allow autophagy activation in muscle. Counteracting immobilization with early mobilization may also help to reduce the severity of myopathy.
FIGURE 16.
Flow chart of proposed mechanisms and their interactions contributing to the development of CIP and CIM. [Extended from Hermans et al. (302).] Summary of pathophysiological mechanisms in ICU-related weakness affecting the nerve/muscle function, demonstrating the various pathways affected and mechanistic explanations for the development of CIP and CIM.
In summary, from a pathophysiological point of view, the dissection of contributing factors to the clinical picture of ICU-related weakness is still a challenging venue for future research. Therefore, appropriate animal models such as the ones presented here with their strengths and weaknesses will become a major focus for critical illness-related research and, hopefully, will become also a reliable tool to more specifically test for therapeutic benefits of new treatment regimes.
A. Future Research Topics (Basic Mechanisms and Possible Therapeutic Interventions)
Future research topics that will certainly represent an advancement in our understaning of the nature of muscle weakness in the critically ill as well as test for novel potential therapeutic interventions include the following.
1. Animal models and human experiments
1) Elucidating the exact time courses of individual phenotypic changes in CIM, e.g., beginning and duration of membrane hypoexcitability, Ca2+ imbalance, metabolic changes, atrophy, preferential myosin loss in serial studies in patients and animal models (Figure 2);
2) Using animal models at ages equivalent to human children as a model for pediatric ICU-acquired weakness;
3) Using animal models with comorbidities (e.g., obese ICU rat model, senescence models, etc.);
4) Extending the porcine ICU model (as model closest to the human) for long-term intensive care treatment (also in adult/old pigs);
5) Exploiting multimodal readout systems (metabolic imaging, metabolomics, multiplex RNA analyses, etc.) to patient samples in serial studies on ICU patients, septic or not, and septic patients with no mechanical ventilation or immobilization to map the pathophysiological mechanisms found in animal models to human models; and
6) Assessing the Na+ channelopathy defined in animal models in ICU patients. In particular, is the hypoexcitability seen in CIM patient muscle also reflected by the same changes in ion channel biophysics as seen in rat SD muscle? Fresh muscle biopsies from CIM patients would be required to be electrophysiologically characterized (voltage/patch clamp).
2. Pathophysiological mechanisms
1) Assessment of complete electrolyte and osmolarity profiles in patients from different ICUAW entities to differentially explain muscle membrane hypoexcitability;
2) Precise involvement of K+, Cl−, and Ca2+ channels in muscle membrane excitability in critical illness and altered Ca2+ homeostasis;
3) More evidence for the direct intracellular action of inflammatory cytokines, e.g., direct binding of IL-1 or TNF-α to regulatory proteins in muscle; and
4) Elucidation of the pathways for altered Ca2+ homeostasis on mitochondrial biogenesis and mitochdonrial activity on ATP outcome in the different animal models of critical illness and criticially ill patients. Is there a myotoxic circulating factor in serum collected from non-septic animal models of ICUAW?
3. Therapeutic concepts to test for
1) Determination of the impact of glycemic control on autophagy in skeletal muscle;
2) Design of strategies for immune modulation in septic patients and animal models to suppress overt systemic inflammatory reaction and to avoid ICUAW;
3) Follow-up the concept of reduced membrane excitability to potentially increase survival of depolarized injured cells (see sect. VIII).
GRANTS
This work was supported by National Institutes of Health Grant NS082354 (to M. Rich) and AR062083 (to M. B. Reid); Research Foundation-Flanders (Fonds voor Wetenschappelijk Onderzoek Vlaanderen), Belgium, Grants G.0399.12 and G.0592.12 (to G. Hermans, I. Vanhorebeek, and G. Van den Berghe); Postdoctoral Fellowship from the Clinical Research Fund (Klinische Onderzoeksfonds, KOF) of the University Hospitals Leuven, Belgium (to G. Hermans); structural research financing via the Methusalem program funded by the Flemish Government (METH08/07 to G. Van den Berghe and METH14/06 to G. Van den Berghe and I. Vanhorebeek), via the University of Leuven; and ERC Advanced Grant AdvG-2012-321670 from the Ideas Program of the EU FP7 (to G. Van den Berghe). The Swedish Medical Research Council (8651), the European Commission (MyoAge, EC Fp7 CT-223756, and COST CM1001), the Swedish Foundation for International Cooperation in Research and Higher Educatiuon (STINT), and Uppsala University Hospital provided support to L. Larsson.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
acknowledgments
Address for reprint requests and other correspondence: O. Friedrich, Institute of Medical Biotechnology, Department of Chemical and Biological Engineering, Friedrich-Alexander-University Erlangen-Nuremberg, Paul-Gordan-Str. 3, 91052 Erlangen, Germany (e-mail: oliver.friedrich@mbt.uni-erlangen.de).
REFERENCES
- 1.Aare S, Ochala J, Norman HS, Radell P, Eriksson LI, Goransson H, Chen YW, Hoffman EP, Larsson L. Mechanisms underlying the sparing of masticatory versus limb muscle function in an experimental critical illness model. Physiol Genomics 43: 1334–1350, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Aare S, Radell P, Eriksson LI, Chen YW, Hoffman EP, Larsson L. The role of sepsis in the development of limb muscle weakness in a porcine intensive care unit model. Physiol Genomics 44: 865–877, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Abdellaoui A, Prefaut C, Gouzi F, Couillard A, Coisy-Quivy M, Hugon G, Molinari N, Lafontaine T, Jonquet O, Laoudj-Chenivesse D, Hayot M. Skeletal muscle effects of electrostimulation after COPD exacerbation: a pilot study. Eur Respir J 38: 781–788, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Adams V, Nehrhoff B, Späte U, Linke A, Schulze PC, Baur A, Gielen S, Hambrecht R, Chuler G. Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappaB activation. An in vitro and in vivo study. Cardiovasc Res 54: 95–104, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Adhihetty PJ, O'Leary MFN, Chabi B, Wicks KL, Hood DA. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol 102: 1143–1151, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Ahlbeck K, Fredriksson K, Rooyackers O, Maback G, Remahl S, Ansved T, Eriksson L, Radell P. Signs of critical illness polyneuropathy and myopathy can be seen early in the ICU course. Acta Anaesthesiol Scand 53: 717–723, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad S, Choudhry MA, Sayeed MM. Ca2+-dependent and Ca2+-independent proteinase contents in the skeletal muscle in septic rats. Shock 5: 247–250, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Akkad H, Corpeno R, Larsson L. Masseter muscle myofibrillar protein synthesis and degradation in an experimental critical illness myopathy model. PLoS One 9: 92622, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alamdari N, Smith IJ, Aversa Z, Hasselgren PO. Sepsis and glucocorticoids upregulate p300 and downregulate HDAC6 expression and activity in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 299: R509–R520, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali NA, O'Brien JM Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, Almoosa K, Hejal R, Wolf KM, Lemeshow S, Connors AF Jr, Marsh CB. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med 178: 261–268, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Allaouchiche B, Duflo F, Debon R, Tournadre JP, Chassard D. Influence of sepsis on minimum alveolar concentration of desflurane in a porcine model. Br J Anaesth 87: 280–283, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Allen DC, Arunachalam R, Mills KR. Critical illness myopathy: further evidence from muscle-fiber excitability studies of an acquired channelopathy. Muscle Nerve 37: 14–22, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Allen DG, Lamg GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Phys Rev 88: 287–332, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Alloati G, Penna C, Mariano F, Camussi G. Role of NO and PAF in the impairment of skeletal muscle contractility induced by TNF-α. Am J Physiol Regul Integr Comp Physiol 279: R2156–R2163, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Almers W, Stanfield PR, Stühmer W. Lateral distribution of sodium and potassium channels in frog skeletal muscle: measurements with a patch-clamp technique. J Physiol 336: 261–284, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez S, Boveris A. Mitochondrial nitric oxide metabolism in rat muscle during endotoxemia. Free Rad Biol Med 37: 1472–1478, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Anastasopoulos D, Kefaliakos A, Michalopoulos A. Is plasma calcium concentration implicated in the development of critical illness polyneuropathy and myopathy? Crit Care 15: R247, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.andrews PJ, Avenell A, Noble DW, Campbell MK, Croal BL, Simpson WG, Vale LD, Battison CG, Jenkinson DJ, Cook JA. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 342: d1542, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Angaut-Petit D, McArdle JJ, Mallart A, Bournaud R, Pincon-Raymond M, Rieger F. Electrophysiological and morphological studies of a motor nerve in “motor endplate disease” of the mouse. Proc R Soc Lond B Biol Sci 215: 117–125, 1982. [DOI] [PubMed] [Google Scholar]
- 20.Anker SD, Rauchaus M. Insights into the pathogenesis of chronic heart failure: immune activation and cachexia. Curr Opin Cardiol 14: 211–216, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De GR, Keh D, Kupfer Y, Oppert M, Meduri GU. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA 301: 2362–2375, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaud P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288: 862–871, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Astiz M, Rackow EC, Weil MH, Schumer W. Early impairment of oxidative metabolism and energy production in severe sepsis. Circ Shock 26: 311–320, 1988. [PubMed] [Google Scholar]
- 24.Astrakas LG, Goljer I, Yasahura S, Padfield KE, Zhang Q, Gopalan S, Mindrinos MN, Dai G, Yu YM, Martyn JAJ, Tompkins RG, Rahme LG, Tzika A. Proton NMR spectroscopy shows lipids accumulate in skeletal muscle in response to burn trauma-induced apoptosis. FASEB J 19: 1431–1440, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Attaix D, Baracos VE. MAFbx/Atrogin-1 expression is a poor index of muscle proteolysis. Curr Opin Clin Nutr Metab Care 13: 223–224, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Authier FJ, Mhiri C, Chazaud B, Christov C, Cherin P, Barlovatz-Meimon G, Gherardi RK. Interleukin-1 expression in inflammatory myopathies: evidence of marked immunoreactivity in sarcoid granulomas and muscle fibres showing ischaemic and regenerative changes. Neuropathol Appl Neurobiol 23: 132–140, 1997. [PubMed] [Google Scholar]
- 27.Bailey P, Thomsen GE, Spuhler VJ, Blair R, Jewkes J, Bezdjian L, Veale K, Rodriquez L, Hopkins RO. Early activity is feasible and safe in respiratory failure patients. Crit Care Med 35: 139–145, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Baker AJ, Languemare MC, Brandes R, Weiner MW. Intracellular tetanic calcium signals are reduced in fatigue of whole skeletal muscle. Am J Physiol Cell Physiol 264: C577–C582, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin CE, Paratz JD, Bersten AD. Muscle strength assessment in critically ill patients with handheld dynamometry: an investigation of reliability, minimal detectable change, and time to peak force generation. J Crit Care 28: 77–86, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Ballard-Croft C, Maass DL, Sikes PJ, Horton JW. Sepsis and burn complicated by sepsis alter cardiac transporter expression. Burns 33: 72–80, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Banduseela VC, Chen YW, Kultima HG, Norman HS, Aare Radell P S, Eriksson LI, Hoffman EP, Larsson L. Impaired autophagy, chaperone expression, and protein synthesis in response to critical illness interventions in porcine skeletal muscle. Physiol Genomics 45: 477–486, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Banduseela VC, Ochala J, Chen YW, Goransson H, Norman H, Radell P, Eriksson LI, Hoffman EP, Larsson L. Gene expression and muscle fiber function in a porcine ICU model. Physiol Genomics 39: 141–159, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Bannister RA, Pessah IN, Beam KG. The skeletal L-type Ca2+ current is a major contributor to excitation-coupled Ca2+ entry. J Gen Physiol 133: 79–91, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baracos V, Rodemann HP, Dinarello CA, Goldberg AL. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med 308: 553–558, 1983. [DOI] [PubMed] [Google Scholar]
- 35.Barat M, Brochet B, Vital C, Mazaux JM, Arne L. Polyneuropathies during prolonged stays in resuscitation. Rev Neurol 143: 823–831, 1987. [PubMed] [Google Scholar]
- 36.Barnett RE, Keskey RC, Rao JM, Billeter AT, Kanaan Z, Cheadle WG. Poor outcome in bacterial peritonitis is associated with dysregulated microRNAs and an increased inflammatory response. Surgery 154: 521–527, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Barohn RJ, Jackson CE, Rogers SJ, Ridings LW, McVey AL. Prolonged paralysis due to nondepolarizing neuromuscular blocking agents and corticosteroids. Muscle Nerve 17: 647–654, 1994. [DOI] [PubMed] [Google Scholar]
- 38.Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 41: 263–306, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Barrett MJ, Goll DE, Thompson VF. Effect of substrate on Ca2+ concentration required for activity of the Ca2+-dependent proteinases, mu- and m-calpain. Life Sci 48: 1659–1669, 1991. [DOI] [PubMed] [Google Scholar]
- 40.Bartlett RH, Roloff DW, Custer JR, Younger JG, Hirschl RB. Extracorporeal life support: the University of Michigan experience. JAMA 283: 904–908, 2000. [PubMed] [Google Scholar]
- 41.Baylor SM, Hollingworth S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibers of mouse muscle. J Physiol 551: 125–138, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bechet D, Tassa A, Taillandier D, Combaret L, Attaix D. Lysosomal proteolysis in skeletal muscle. Int J Biochem Cell Biol 37: 2098–2114, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Bednarik J, Lukas Z, Vondracek P. Critical illness polyneuromyopathy: the electrophysiological components of a complex entity. Intensive Care Med 29: 1505–1514, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Bednarik J, Vondracek P, Dusek L, Moravcova E, Cundrle I. Risk factors for critical illness polyneuromyopathy. J Neurol 252: 343–351, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Bendahhou S, Cummins TR, Potts JF, Tong J, Agnew WS. Serine-1321-independent regulation of the mu1 adult skeletal muscle Na+ channel by protein kinase C. Proc Natl Acad Sci USA 92: 12003–12007, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bengoecchea Gonzalez ME, Perez Casas A, Alvarez Arenal A, Vega Alvarez JA, Tinture Eguren JC. Structure and acetylcholinesterase activity of the neuromuscular junction of rats in a state of septic shock. Rev Clin Esp 167: 359–362, 1982. [PubMed] [Google Scholar]
- 47.Bennett E, Urcan MS, Tinkle SS, Koszowski AG, Levinson SR. Contribution of sialic acid to the voltage dependence of sodium channel gating. A possible electrostatic mechanism. J Gen Physiol 109: 327–343, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennett ES. Isoform-specific effects of sialic acid on voltage-dependent Na+ channel gating: functional sialic acids are localized to the S5–S6 loop of domain I. J Physiol 538: 675–690, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benson DW, Hasselgren PO, Hiyama DT, James JH, Li S, Rigel DF, Fischer JE. Effects of sepsis on calcium uptake and content in skeletal muscle and regulation in vitro by calcium of total and myofibrillar protein breakdown in control and septic muscle: results from a preliminary study. Surgery 106: 87–93, 1989. [PubMed] [Google Scholar]
- 50.Berchtold MW, Brinkmeier H, Müntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Phys Rev 80: 1216–1265, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Bercker S, Weber-Carstens S, Deja M, Grimm C, Wolf S, Behse F, Busch T, Falke KJ, Kaisers U. Critical illness polyneuropathy and myopathy in patients with acute respiratory distress syndrome. Crit Care Med 33: 711–715, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Berek K, Margreiter J, Willeit J, Berek A, Schmutzhard E, Mutz NJ. Polyneuropathies in critically ill patients: a prospective evaluation. Intensive Care Med 22: 849–855, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Bergmann M, Fruton JS. Regarding the general nature of catheptic enzymes. Science 84: 89–90, 1936. [DOI] [PubMed] [Google Scholar]
- 54.Bernardin G, Strosberg AD, Bernard A, Mattei M, Marullo S. β-Adrenergic receptor-dependent and -independent stimulation of adenylate cyclase is impaired during severe sepsis in humans. Intensive Care Med 24: 1315–1322, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Bertsch S, Lang CH, Vary TC. Inhibition of glycogen synthase kinase 3β activity with lithium in vitro attenuates sepsis-induced changes in muscle protein turnover. Shock 35: 266–274, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhattacharyya J, Thompson K, Sayeed MM. Calcium-dependent and calcium-independent protease activities in skeletal muscle during sepsis. Circ Shock 35: 117–122, 1991. [PubMed] [Google Scholar]
- 57.Bhattacharyya J, Thompson KD, Sayeed MM. Skeletal muscle Ca2+ flux and catabolic response during sepsis. Am J Physiol Regul Integr Comp Physiol 265: R487–R493, 1993. [DOI] [PubMed] [Google Scholar]
- 58.Bialek P, Morris C, Parkington J, St andre M, Owens J, Yaworsky P, Seeherman H, Jelinsky SA. Distinct protein degradation profiles are induced by different disuse models of skeletal muscle atrophy. Physiol Genomics 43: 1075–1086, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bierbrauer J, Koch S, Olbricht C, Hamati J, Lodka D, Schneider J, Luther-Schroder A, Kleber C, Faust K, Wiesener S, Spies CD, Spranger J, Spuler S, Fielitz J, Weber-Carstens S. Early type II fiber atrophy in intensive care unit patients with nonexcitable muscle membrane. Crit Care Med 40: 647–650, 2012. [DOI] [PubMed] [Google Scholar]
- 60.Boczkowski J, Lanone S, Ungureanu-Longrois D, Danialou G, Fournier T, Aubier M. Induction of diaphragmatic nitric oxide synthase after endotoxin administration in rats: role on diaphragmatic contractile dysfunction. J Clin Invest 98: 1550–1559, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boczkowski J, Lisdero CL, Lanone S, Samb A, Carreras MC, Boveris A, Aubier M, Poderoso JJ. Endogenous peroxynitrite mediates mitochondrial dysfunction in rat diaphragm during endotoxemia. FASEB J 13: 1637–1646, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Boekstegers P, Weidenhöfer S, Kapsner T, Werdan K. Skeletal muscle partial pressure of oxygen in patients with sepsis. Crit Care Med 22: 640–650, 1994. [DOI] [PubMed] [Google Scholar]
- 63.Boekstegers P, Weidenhöfer S, Pilz G, Werdan K. Peripheral oxygen availability within skeletal muscle in sepsis and septic shock: comparison to limited infection and cardiogenic shock. Infection 19: 317–323, 1991. [DOI] [PubMed] [Google Scholar]
- 64.Bolton CF. Neuromuscular manifestations of critical illness. Muscle Nerve 32: 140–163, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Bolton CF. The discovery of critical illness polyneuropathy: a memoir. Can J Neurol Sci 37: 431–438, 2010. [DOI] [PubMed] [Google Scholar]
- 66.Bolton CF, Brown JD, Sibbald WA. The electrophysiologic investigation of respiratory paralysis in critically ill patients. Neurology 33: 186, 1983. [Google Scholar]
- 67.Bolton CF, Gilbert JJ, Hahn AF, Sibbald WJ. Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatry 47: 1223–1231, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolton CF, Laverty DA, Brown JD, Witt NJ, Hahn AF, Sibbald WJ. Critically ill polyneuropathy: electrophysiological studies and differentiation from Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry 49: 563–573, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bolton CF, Young GB. Critical illness polyneuropathy. Curr Treat Options Neurol 2: 489–498, 2000. [DOI] [PubMed] [Google Scholar]
- 70.Bolton CF, Young GB, Zochodne DW. The neurological complications of sepsis. Ann Neurol 33: 94–100, 1993. [DOI] [PubMed] [Google Scholar]
- 71.Botelho FM, Rangel-Moreno J, Fritz D, Randall TD, Xing Z. Pulmonary expression of oncostatin M (OSM) promotes inducible BALT formation independently of IL-6, despite a role for IL-6 in OSM-driven pulmonary inflammation. J Immunol 191: 1453–1464, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boucher PA, Joos B, Morris CE. Coupled left-shift of Nav channels: modeling the Na+-loading and dysfunctional excitability of damaged axons. J Comput Neurosci 33: 301–319, 2012. [DOI] [PubMed] [Google Scholar]
- 73.Bouletreau P, Patricot MC, Saudin F, Guiraud M, Mathian B. Effects of intermittent electrical stimulations on muscle catabolism in intensive care patients. JPEN J Parenter Enteral Nutr 11: 552–555, 1987. [DOI] [PubMed] [Google Scholar]
- 74.Boveris A, Alvarez S, Navarro A. The role of mitochondrial nitric oxide synthase in inflammation and septic shock. Free Radic Biol Med 33: 1186–1193, 2002. [DOI] [PubMed] [Google Scholar]
- 75.Brady AJB, Poole-Wilson PA, Harding SE, Warren JB. Nitric oxide production within cardiac myocytes reduces their contractility in endotoxemia. Am J Physiol Heart Circ Physiol 263: H1963–H1966, 1992. [DOI] [PubMed] [Google Scholar]
- 76.Braun T, Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol 12: 349–361, 2011. [DOI] [PubMed] [Google Scholar]
- 77.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219–223, 2002. [DOI] [PubMed] [Google Scholar]
- 78.Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwill R, Taylor V, Smolenski RT, Singer M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol 286: R491–R497, 2004. [DOI] [PubMed] [Google Scholar]
- 79.Breuille D, Voisin L, Contrepois M, Arnal M, Rose F, Obled C. A sustained rat model for studying the long-lasting catabolic state of sepsis. Infect Immun 67: 1079–1085, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brochard L, Thille AW. What is the proper approach to liberating the weak from mechanical ventilation? Crit Care Med 37: S410-415, 2009. [DOI] [PubMed] [Google Scholar]
- 81.Brooks NE, Myburgh KH. Skeletal muscle wasting with disuse atrophy is multi-dimensional: the response and interaction of myonuclei, satellite cells and signaling pathways. Front Physiol 5: 99, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brunello AG, Haenggi M, Wigger O, Porta F, Takala J, Jakob SM. Usefulness of a clinical diagnosis of ICU-acquired paresis to predict outcome in patients with SIRS and acute respiratory failure. Intensive Care Med 36: 66–74, 2010. [DOI] [PubMed] [Google Scholar]
- 83.Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J 15: 1753–1765, 1996. [PMC free article] [PubMed] [Google Scholar]
- 84.Bulger EM, Jurkovich GJ, Farver CL, Klotz P, Maier RV. Oxandrolone does not improve outcome of ventilator dependent surgical patients. Ann Surg 240: 472–478, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov 4: 854–865, 2005. [DOI] [PubMed] [Google Scholar]
- 86.Burkholder TJ. Mechanotransduction in skeletal muscle. Front Biosci 12: 174–191, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burnham EL, Moss M, Ziegler TR. Myopathies in critical illness: characterization and nutritional aspects. J Nutr 135: 1818S–1823S, 2005. [DOI] [PubMed] [Google Scholar]
- 88.Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T, Hermans G, Decramer M, Gosselink R. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med 37: 2499–2505, 2009. [DOI] [PubMed] [Google Scholar]
- 89.Bustamante M, Fernandez-Verdejo R, Jaimovich E, Buvinic S. Electrical stimulation induces interleukin-6 in skeletal muscle through extracellular AT, by activating Ca2+ signals and an interleukin-6 autocrine loop. Am J Physiol Endocrinol Metab 306: E869–E882, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Button B, Baker RD, Vertrees RA, Allen SE, Brodwick MS, Kramer GC. Quantitative assessment of a circulating depolarizing factor in shock. Shock 15: 239–244, 2001. [DOI] [PubMed] [Google Scholar]
- 91.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14: 365–376, 2013. [DOI] [PubMed] [Google Scholar]
- 92.Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res 592: 283–297, 1992. [DOI] [PubMed] [Google Scholar]
- 93.Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, Takeshima H, Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem 284: 15894–15902, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Callahan LA, Nethery D, Stofan D, DiMarco A, Supinski GS. Free radical-induced contractile protein dysfunction in endotoxin-induced sepsis. Am J Respir Cell Mol Biol 24: 210–217, 2001. [DOI] [PubMed] [Google Scholar]
- 95.Callahan LA, She ZW, Nosek TM. Superoxide, hydroxyl radical, and hydrogen peroxide effects on single-diaphragm fiber contractile apparatus. J Appl Physiol 90: 45–54, 2001. [DOI] [PubMed] [Google Scholar]
- 96.Callahan LA, Stofan DA, Szweda LI, Nethery DE, Supinski GS. Free radicals alter maximal diaphragmatic mitochondrial oxygen consumption in endotoxin-induced sepsis. Free Radic Biol Med 30: 129–138, 2001. [DOI] [PubMed] [Google Scholar]
- 97.Callahan LA, Supinski GS. Sepsis induces diaphragm electron transport chain dysfunction and protein depletion. Am J Resp Crit Care Med 172: 861–868, 2005. [DOI] [PubMed] [Google Scholar]
- 98.Callahan LA, Supinski GS. Downregulation of diaphragm electron transport chain and glycolytic enzyme gene expression in sepsis. J Appl Physiol 99: 1120–1126, 2005. [DOI] [PubMed] [Google Scholar]
- 99.Callahan LA, Supinski GS. Diaphragm and cardiac mitochondrial creatine kinases are impaired in sepsis. J Appl Physiol 102: 44–53, 2007. [DOI] [PubMed] [Google Scholar]
- 100.Campellone JV, Lacomis D, Kramer DJ, Van Cott AC, Giuliani MJ. Acute myopathy after liver transplantation. Neurology 50: 46–53, 1998. [DOI] [PubMed] [Google Scholar]
- 101.Cannon JG, Fielding RA, Fiatarone MA, Orencole SF, Dinarello CA, Evans WJ. Increased interleukin1 beta in human skeletal muscle after exercise. Am J Physiol Regul Integr Comp Physiol 257: R451–R455, 1989. [DOI] [PubMed] [Google Scholar]
- 102.Caporossi FS, Caporossi C, Borges Dock-Nascimento D, de Aguilar-Nascimento JE. Measurement of the thickness of the adductor pollicis muscle as a predictor of outcome in critically ill patients. Nutr Hosp 27: 490–495, 2012. [DOI] [PubMed] [Google Scholar]
- 103.Carchman EH, Rao J, Loughran PA, Rosengart MR, Zuckerbraun BS. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology 53: 2053–2062, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carle T, Fournier E, Sternberg D, Fontaine B, Tabti N. Cold-induced disruption of Na+ channel slow inactivation underlies paralysis in highly thermosensitive paramyotonia. J Physiol 587: 1705–1714, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carré JE, Orban JC, Re L, Felsmann K, Iffert W, Bauer M, Suliman HB, Piantadosi CA, Mayhew TM, Breen P, Stotz M, Singer M. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med 182: 745–751, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA 72: 3666–3670, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van CS, Ingels C, Meersseman P, Muller J, Vlasselaers D, Debaveye Y, Desmet L, Dubois J, Van AA, Vanderheyden S, Wilmer A, Van den Berghe G. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 365: 506–517, 2011. [DOI] [PubMed] [Google Scholar]
- 108.Casaer MP, Van den Berghe G. Nutrition in the acute phase of critical illness. N Engl J Med 370: 1227–1236, 2014. [DOI] [PubMed] [Google Scholar]
- 109.Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med 187: 247–255, 2013. [DOI] [PubMed] [Google Scholar]
- 110.Catterall WA. Signaling complexes of voltage-gated sodium and calcium channels. Neurosci Lett 486: 107–116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev 57: 397–409, 2005. [DOI] [PubMed] [Google Scholar]
- 112.Chad DA, Lacomis D. Critically ill patients with newly acquired weakness: the clinicopathological spectrum. Ann Neurol 35: 257–259, 1994. [DOI] [PubMed] [Google Scholar]
- 113.Chai J, Wu Y, Sheng ZZ. Role of ubiquitin-proteasome pathway in skeletal muscle wasting in rats with endotoxemia. Crit Care Med 31: 1802–1807, 2003. [DOI] [PubMed] [Google Scholar]
- 114.Chambers MA, Moylan JS, Reid MB. Physical inactivity and muscle weakness in the critically ill. Crit Care Med 37: S337–S346, 2009. [DOI] [PubMed] [Google Scholar]
- 115.Chaudry IH, Sayeed MM, Baue AE. Alterations in high-energy phosphates in hemorrhagic shock as related to tissue and organ function. Surgery 79: 666–668, 1976. [PubMed] [Google Scholar]
- 116.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet 14: R283–R289, 2005. [DOI] [PubMed] [Google Scholar]
- 117.Chen SJ, Wu CC, Yen MH. Alterations of ex vivo vascular reactivity in intraperitoneal sepsis. J Cardiovasc Pharmacol 24: 786–793, 1994. [DOI] [PubMed] [Google Scholar]
- 118.Cheng M, Nguyen MH, Fantuzzi G, Koh TJ. Endogeneous interferon-gamma is required for efficient muscle regeneration. Am J Physiol Cell Physiol 294: C1183–C1191, 2008. [DOI] [PubMed] [Google Scholar]
- 119.Chensue SW, Terebuh PD, Remick DG, Scales WE, Kunkel SL. In vivo biologic and immunohistochemical analysis of interleukin-1 alpha, beta and tumor necrosis factor during experimental endotoxemia. Am J Pathol 138: 395–402, 1991. [PMC free article] [PubMed] [Google Scholar]
- 120.Cherednichenko G, Hurne AM, Fessebden JD, Lee EH, Allen PD, Beam KG, Pessah IN. Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc Natl Acad Sci USA 101: 15793–15798, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chiao JJ, Jones WG 2nd, Shires GT 3rd, Barber AE, Shires GT. Effect of sepsis on intracellular sodium activity, sodium concentration, and water content in thermal injury rat. Circ Shock 38: 42–49, 1992. [PubMed] [Google Scholar]
- 122.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med 368: 651–662, 2013. [DOI] [PubMed] [Google Scholar]
- 123.Clavel S, Siffroi-Fernandez S, Coldefy AS, Boulukos K, Pisani DF, Derijard B. Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol Cell Biol 30: 470–480, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Clowes GH Jr, George BC, Villee CA Jr, Saravis CA. Muscle proteolysis induced by a circulating peptide in patients with sepsis and trauma. N Engl J Med 308: 545–552, 1983. [DOI] [PubMed] [Google Scholar]
- 125.Coakley JH, Nagendran K, Honavar M, Hinds CJ. Preliminary observations on the neuromuscular abnormalities in patients with organ failure and sepsis. Intensive Care Med 19: 323–328, 1993. [DOI] [PubMed] [Google Scholar]
- 126.Coakley JH, Nagendran K, Yarwood GD, Honavar M, Hinds CJ. Patterns of neurophysiological abnormality in prolonged critical illness. Intensive Care Med 24: 801–807, 1998. [DOI] [PubMed] [Google Scholar]
- 127.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol 185: 1083–1095, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Connolly B, Denehy L, Brett S, Elliott D, Hart N. Exercise rehabilitation following hospital discharge in survivors of critical illness: an integrative review. Crit Care 16: 226, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Connolly BA, Jones GD, Curtis AA, Murphy PB, Douiri A, Hopkinson NS, Polkey MI, Moxham J, Hart N. Clinical predictive value of manual muscle strength testing during critical illness: an observational cohort study. Crit Care 17: R229, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Constantin D, McCullough J, Mahajan RP, Greenhaff PL. Novel events in the molecular regulation of muscle mass in critically ill patients. J Physiol 589: 3883–3895, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cooney R, Owens E, Jurasinski C, Gray K, Vannice J, Vary T. Interleukin-1 receptor antagonist prevents sepsis-induced inhibition of protein synthesis. Am J Physiol Endocrinol Metab 267: E636–E641, 1994. [DOI] [PubMed] [Google Scholar]
- 132.Cooney RN, Maish GO 3rd, Gilpin T, Shumate ML, Lang CH, Vary TC. Mechanism of IL-1 induced inhibition of protein synthesis in skeletal muscle. Shock 11: 235–241, 1999. [DOI] [PubMed] [Google Scholar]
- 133.Corpeno R, Dworkin B, Cacciani N, Shalah H, Bergman H, Rvara B, Vitadello M, Gorza L, Gystafson AM, Hedström Y, Peterson J, Feng HZ, Jin JP, Iwamoto H, Yagi N, Artemenko K, Bergquist J, Larsson L. Time-course analysis of mechanical ventilation-induced diaphragm contractile muscle dysfunction. J Physiol 592: 3859–3880, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Couturier JC, Robert D, Monier P. Polynevrites compliquant des sejours prolonges en reanimation. Lyon Medical 252: 247–249, 1984. [Google Scholar]
- 135.Cree MG, Fram RY, Herndon DN, Qian T, Angel C, Greem JM, Mlcak R, Aarsland A, Wolfe RR. Human mitochondrial oxidative capacity is acutely impaired after burn trauma. Am J Surg 196: 234–239, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cree MG, Zwetsloot JJ, Herndon DN, Qian T, Morio B, Fram R, Sanford AP, Aarsland A, Wolfe RR. Insulin sensitivity and mitochondrial function are improved in children with burn injury during a randomized controlled trial of fenofibrate. Ann Surg 245: 214–221, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Crouser ED, Julian MW, Huff JE, Struck J, Cook CH. Carbamoyl phosphate synthase-1: a marker of mitochondrial damage and depletion in the liver during sepsis. Crit Care Med 34: 2439–2446, 2006. [DOI] [PubMed] [Google Scholar]
- 138.Cunningham JN, Carter NW Jr, Rector FC Jr, Seldin DW. Resting transmembrane potential difference of skeletal muscle in nirmal subjects and severely ill patients. J Clin Invest 50: 49–59, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Czeschik JC, Hagenacker T, Schafers M, Busselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett 434: 293–298, 2008. [DOI] [PubMed] [Google Scholar]
- 140.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg 215: 356–362, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Danon MJ, Carpenter S. Myopathy with thick filament (myosin) loss following prolonged paralysis with vecuronium during steroid treatment. Muscle Nerve 14: 1131–1139, 1991. [DOI] [PubMed] [Google Scholar]
- 142.Davis KA, Masella J, Blennerhassett MG. Acetylcholine metabolism in the inflamed rat intestine. Exp Neurol 152: 251–258, 1998. [DOI] [PubMed] [Google Scholar]
- 143.De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, Cerf C, Outin H, Sharshar T. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med 35: 2007–2015, 2007. [DOI] [PubMed] [Google Scholar]
- 144.De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med 30: 1117–1121, 2004. [DOI] [PubMed] [Google Scholar]
- 145.De Jonghe B, Cook D, Sharshar T, Lefaucheur JP, Carlet J, Outin H. Acquired neuromuscular disorders in critically ill patients: a systematic review. Groupe de Reflexion et d'Etude sur les Neuromyopathies en Reanimation. Intensive Care Med 24: 1242–1250, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.De Jonghe B, Lacherade JC, Sharshar T, Outin H. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med 37: S309–S315, 2009. [DOI] [PubMed] [Google Scholar]
- 147.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, Raphael JC, Outin H, Bastuji-Garin S. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 288: 2859–2867, 2002. [DOI] [PubMed] [Google Scholar]
- 148.De Larichaudy J, Zufferli A, Serra F, Isidori AM, Naro F, Dessalle K, Desgeorges M, Piraud M, Cheillan D, Vidal H, Lefai E, Nemoz G. TNF-α- and tumor-induced skeletal muscle atrophy involves sphingolipid metabolism. Skelet Muscle 2: 2, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.De Letter MA, Schmitz PI, Visser LH, Verheul FA, Schellens RL, Op de Coul DA, van der Meche FG. Risk factors for the development of polyneuropathy and myopathy in critically ill patients. Crit Care Med 29: 2281–2286, 2001. [DOI] [PubMed] [Google Scholar]
- 150.De Letter MACJ, van Doorn PA, Savelkoul HFJ, Laman JD, Schmitz PIM, Op de Coul AAW, Visser LH, Kros JM, Teepen JLJM, van der Meche FGA. Critical illness polyneuropathy and myopathy (CIPNM): evidence for local immune activation by cytokine-expression in the muscle tissue. J Neuroimmunol 106: 206–213, 2000. [DOI] [PubMed] [Google Scholar]
- 151.Delbono O, Stefani E. Calcium transients in mammalian skeletal muscle fibers. J Physiol 463: 689–707, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39: 165–228, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, Matecki S, Duguet A, Similowski T, Jaber S. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact–a prospective study. Am J Respir Crit Care Med 188: 213–219, 2013. [DOI] [PubMed] [Google Scholar]
- 154.Derde S, Hermans G, Derese I, Guiza F, Hedstrom Y, Wouters PJ, Bruyninckx F, D'Hoore A, Larsson L, Van den Berghe G, Vanhorebeek I. Muscle atrophy and preferential loss of myosin in prolonged critically ill patients. Crit Care Med 40: 79–89, 2012. [DOI] [PubMed] [Google Scholar]
- 155.Derde S, Vanhorebeek I, Guiza F, Derese I, Gunst J, Fahrenkrog B, Martinet W, Vervenne H, Ververs EJ, Larsson L, Van den Berghe G. Early parenteral nutrition evokes a phenotype of autophagy deficiency in liver and skeletal muscle of critically ill rabbits. Endocrinology 153: 2267–2276, 2012. [DOI] [PubMed] [Google Scholar]
- 156.Derde S, Vanhorebeek I, Ververs EJ, Vanhees I, Darras VM, Van Herck E, Larsson L, Van den Berghe G. Increasing intravenous glucose load in the presence of normoglycemia: effect on outcome and metabolism in critically ill rabbits. Crit Care Med 38: 602–611, 2010. [DOI] [PubMed] [Google Scholar]
- 157.Deval C, Mordier S, Obled C, Bechet D, Combaret L, Attaix D, Ferrara M. Identification of cathepsin L as a differentially expressed message associated with skeletal muscle wasting. Biochem J 15: 143–150, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Diaz NL, Finol HJ, Torres SH, Zambrano CI, Adjounian H. Histochemical and ultrastructural study of skeletal muscle in patients with sepsis and multiorgan failure syndrome (MOFS). Histol Histopathol 13: 121–128, 1998. [DOI] [PubMed] [Google Scholar]
- 159.Dinarello CA, Clowes GH Jr, Gordon AH, Saravis CA, Wolff SM. Cleavage of human interleukin-1: isolation of a peptide fragment from plasma of febrile humans and activated monocytes. J Immunol 133: 1332–1338, 1984. [PubMed] [Google Scholar]
- 160.Dodd S, Hain B, Judge A. Hsp70 prevents disuse muscle atrophy in senescent rats. Biogerontology 10: 605–611, 2009. [DOI] [PubMed] [Google Scholar]
- 161.Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, Davies AR, O'Leary M, Solano T, Peake S. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA 309: 2130–2138, 2013. [DOI] [PubMed] [Google Scholar]
- 162.Dong LW, Ji Y, Wang XQ, Wu LL, Tang CS. Altered ryanodine receptor of rat cardiac sarcoplasmic reticulum and its underlying mechanism during septic shock. Sheng Li Xue Bao 47: 349–356, 1995. [PubMed] [Google Scholar]
- 163.Dong LW, Wu LL, Ji Y, Liu MS. Impairment of the ryanodine-sensitive calcium release channels in the cardiac sarcoplasmic reticulum and its underlying mechanism during the hypodynamic phase of sepsis. Shock 16: 33–39, 2001. [DOI] [PubMed] [Google Scholar]
- 164.Dos Santos CC, Batt J. ICU-acquired weakness: mechanisms of disability. Curr Opin Crit Care 18: 509–517, 2012. [DOI] [PubMed] [Google Scholar]
- 165.Douglass JA, Tuxen DV, Horne M, Scheinkestel CD, Weinmann M, Czarny D, Bowes G. Myopathy in severe asthma. Am Rev Respir Dis 146: 517–519, 1992. [DOI] [PubMed] [Google Scholar]
- 166.Druschky A, Herkert M, Radespiel-Troger M, Druschky K, Hund E, Becker CM, Hilz MJ, Erbguth F, Neundorfer B. Critical illness polyneuropathy: clinical findings and cell culture assay of neurotoxicity assessed by a prospective study. Intensive Care Med 27: 686–693, 2001. [DOI] [PubMed] [Google Scholar]
- 167.Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113: 115–123, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dulhunty AF. Distribution of potassium and chloride permeability over the surface and T-tubule membranes of mammalian skeletal muscle. J Membr Biol 45: 293–310, 1979. [DOI] [PubMed] [Google Scholar]
- 169.Dulhunty AF, Derek RL, Gallant EM, casarotto MG, Pace SM, Curtis S. Activation and inhibition of skeletal RyR channels by a part of the skeletal DHPR II-III loop: effects of DHPR Ser687 and FKBP12. Biophys J 77: 189–203, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Duncan DJ, Yang Z, Hopkins PM, Steele DS, Harrison SM. TNF-alpha and IL-1beta increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium 47: 378–386, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Durigan JL, Peviani SM, Delfino GB, de Souza Jose RJ, Parra T, Salvini TF. Neuromuscular electrical stimulation induces beneficial adaptations in the extracellular matrix of quadriceps muscle after anterior cruciate ligament transection of rats. Am J Phys Med Rehabil 93: 948–961, 2014. [DOI] [PubMed] [Google Scholar]
- 172.Dutka TL, Lamb GD. Transverse tubular system depolarization reduces tetanic force in rat skeletal muscle fibers by impairing action potential repriming. Am J Physiol Cell Physiol 292: C2112–C2121, 2007. [DOI] [PubMed] [Google Scholar]
- 173.Dutka TL, Murphy RM, Stephenson DG, Lamb GD. Chloride conductance in the transverse tubular system of rat skeletal muscle fibres: importance in excitation-contraction coupling and fatigue. J Physiol 586: 875–887, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dworkin BR, Dworkin S. Learning of physiological responses. I. Habituation, sensitization, and classical conditioning. Behav Neurosci 104: 298–319, 1990. [DOI] [PubMed] [Google Scholar]
- 175.Dworkin BR, Dworkin S. Baroreflexes of the rat. III. Open-loop gain and electroencephalographic arousal. Am J Physiol Regul Integr Comp Physiol 286: R597–R605, 2004. [DOI] [PubMed] [Google Scholar]
- 176.Dworkin BR, Dworkin S, Tang X. Carotid and aortic baroreflexes of the rat. I. Open-loop steady-state properties and blood pressure variability. Am J Physiol Regul Integr Comp Physiol 279: R1910–R1921, 2000. [DOI] [PubMed] [Google Scholar]
- 177.Dworkin BR, Tang X, Snyder AJ, Dworkin S. Carotid and aortic baroreflexes of the rat. II. Open-loop frequency response and the blood pressure spectrum. Am J Physiol Regul Integr Comp Physiol 279: R1922–R1933, 2000. [DOI] [PubMed] [Google Scholar]
- 178.Eastridge BJ, Darlington DN, Evans JA, Gann DS. A circulating shock protein depolarizes cells in hemorrhage and sepsis. Ann Surg 219: 298–305, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ebisui C, Tsujinaka T, Morimoto T, Kan K, Iijima S, Yano M, Kominami E, Tanaka K, Monden M. Interleukin-6 induces proteolysis by activating intracellular proteases (cathepsins B and L, proteasome) in C2C12 myotubes. Clin Sci 89: 431–439, 1995. [DOI] [PubMed] [Google Scholar]
- 180.Edwards JP, Hatton PA, Little RA, Pennington RA, Wareham AC. Increased quantal release of acetylcholine at the neuromuscular junction following scald injury in the rat. Muscle Nerve 22: 1660–1666, 1999. [DOI] [PubMed] [Google Scholar]
- 181.Eikermann M, Koch G, Gerwig M, Ochterbeck C, Beiderlinden M, Koeppen S, Neuhäuser M, Peters J. Muscle force and fatigue in patients with sepsis and multiorgan failure. Intensive Care Med 32: 251–259, 2006. [DOI] [PubMed] [Google Scholar]
- 182.El-Dwairi Q, Comtois A, Guo Y, Hussain SN. Endotoxin-induced skeletal muscle contractile dysfunction: contribution of nitric oxide synthases. Am J Physiol Cell Physiol 274: C770–C779, 1998. [DOI] [PubMed] [Google Scholar]
- 183.Ellger B, Debaveye Y, Vanhorebeek I, Langouche L, Giulietti A, Van Etten E, Herijgers P, Mathieu C, Van den Berghe G. Survival benefits of intensive insulin therapy in critical illness: impact of maintaining normoglycemia versus glycemia-independent actions of insulin. Diabetes 55: 1096–1105, 2006. [DOI] [PubMed] [Google Scholar]
- 184.Ellger B, Langouche L, Richir M, Debaveye Y, Vanhorebeek I, Teerlink T, Van Leeuwen PA, Van den Berghe G. Modultaion of regional nitric oxide metabolism: blood glucose control or insulin? Intensive Care Med 34: 1525–1533, 2008. [DOI] [PubMed] [Google Scholar]
- 185.Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski U, John S, Mayer K, Oppert M, Olthoff D, Quintel M, Ragaller M, Rossaint R, Stuber F, Weiler N, Welte T, Bogatsch H, Hartog C, Loeffler M, Reinhart K. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med 33: 606–618, 2007. [DOI] [PubMed] [Google Scholar]
- 186.Escames G, Lopez LC, Tapias V, Utrilla P, Reiter RJ, Hitos AB, Leon J, Rodriguez MI, Acuna-Castroviejo D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J Pineal Res 40: 71–78, 2006. [DOI] [PubMed] [Google Scholar]
- 187.Essen P, McNurlan MA, Gamrin L, Hunter K, Calder G, Garlick PJ, Wernerman J. Tissue protein synthesis rates in critically ill patients. Crit Care Med 26: 92–100, 1998. [DOI] [PubMed] [Google Scholar]
- 188.Everts ME, Clausen T. Effects of thyroid hormones on calcium contents and 45Ca exchange in rat skeletal muscle. Am J Physiol Endocrinol Metab 251: E258–E265, 1986. [DOI] [PubMed] [Google Scholar]
- 189.Fan E, Ciesla ND, Truong AD, Bhoopathi V, Zeger SL, Needham DM. Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Med 36: 1038–1043, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, Himmelfarb CR, Desai SV, Ciesla N, Herridge MS, Pronovost PJ, Needham DM. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med 42: 849–859, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fareed MU, Evenson AR, Wei W, Menconi M, Poylin V, Petkova V, Pignol B, Hasselgren PO. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol 290: R1589–R1597, 2006. [DOI] [PubMed] [Google Scholar]
- 192.Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J 16: 1335–1347, 2002. [DOI] [PubMed] [Google Scholar]
- 193.Fenzi F, Latronico N, Refatti N, Rizzuto N. Enhanced expression of E-selectin on the vascular endothelium of peripheral nerve in critically ill patients with neuromuscular disorders. Acta Neuropathol 106: 75–82, 2003. [DOI] [PubMed] [Google Scholar]
- 194.Ferrando AA, Lane HW, Stuart CA, vis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270: E627–E633, 1996. [DOI] [PubMed] [Google Scholar]
- 195.Filatov GN, Pinter MJ, Rich MM. Role of Ca2+ in injury-induced changes in sodium current in rat skeletal muscle. Am J Physiol Cell Physiol 297: C352–C359, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Filatov GN, Rich MM. Hyperpolarized shifts in the voltage dependence of fast inactivation of Nav1.4 and Nav15 in a rat model of critical illness myopathy. J Physiol 559: 813–820, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360: 1283–1297, 2009. [DOI] [PubMed] [Google Scholar]
- 198.Fink MP. Cytopathic hypoxia: mitochondrial dysfunction as a mechanism contributing to organ dysfunction in sepsis. Crit Care Clin 17: 219–237, 2001. [DOI] [PubMed] [Google Scholar]
- 199.Fink MP. Animal models of sepsis. Virulence 5: 1543-153, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fischer D, Gang G, Pritts T, Hasselgren PO. Sepsis-induced proteolysis is prevented by a proteasome inhibitor in vivo. Biochem Biophys Res Commun 270: 215–221, 2000. [DOI] [PubMed] [Google Scholar]
- 201.Fischer D, Sun X, Gang G, Pritts T, Hasselgren PO. The gene expression of ubiquitin ligase E3alpha is upregulated in skeletal muscle during sepsis in rats: potential role of glucocorticoids. Biochem Biophys Res Commun 267: 504–508, 2000. [DOI] [PubMed] [Google Scholar]
- 202.Fischer DR, Sun X, Williams AB, Gang G, Pritts TA, James JH, Molloy M, Fischer JE, Paul RJ, Hasselgren PO. Dantrolene reduces serum TNFα and corticosterone levels and muscle calcium, calpain gene expression, and protein breakdown in septic rats. Shock 15: 200–207, 2001. [DOI] [PubMed] [Google Scholar]
- 203.Fitts RH, Riley DR, Widrick JJ. Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol 89: 823–839, 2000. [DOI] [PubMed] [Google Scholar]
- 204.Fitts RH, Riley DR, Widrick JJ. Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol 204: 3201–3208, 2001. [DOI] [PubMed] [Google Scholar]
- 205.Flakoll PJ, Hill JO, Abumrad NN. Acute hyperglycemia enhances proteolysis in normal man. Am J Physiol Endocrinol Metab 265: E715–E721, 1993. [DOI] [PubMed] [Google Scholar]
- 206.Fletcher SN, Kennedy DD, Ghosh IR, Misra VP, Kiff K, Coakley JH, Hinds CJ. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med 31: 1012–1016, 2003. [DOI] [PubMed] [Google Scholar]
- 207.Fram RY, Cree MG, Wolfe RR, Mlcak RP, Qian T, Chinkes DL, Herndon DN. Intensive insulin therapy improves insulin sensitivity and mitochondrial function in severely burned children. Crit Care Med 38: 1475–1483, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fraysse B, Desaphy JF, Rolland JF, Pierno S, Liantonio A, Giannuzzi V, Camerino C, Didonna MP, Cocchi D, De Luca A, Conte Camerino D. Fiber type-related changes in rat skeletal muscle calcium homeostasis during aging and restoration by growth hormone. Neurobiol Dis 21: 372–380, 2006. [DOI] [PubMed] [Google Scholar]
- 209.Fredriksson K, Fläring U, Guillet C, Wernerman J, Rooyackers O. Muscle mitochondrial activity increases rapidly after an endotoxin challenge in human volunteers. Acta Anaesthesiol Scand 53: 299–304, 2009. [DOI] [PubMed] [Google Scholar]
- 210.Fredriksson K, Hammarqvist F, Strigard K, Hultenby K, Ljungqvist O, Wernerman J, Rooyackers O. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab 291: E1044–E1050, 2006. [DOI] [PubMed] [Google Scholar]
- 211.Fredriksson K, Rooyackers O. Mitochondrial function in sepsis: respiratory vs. leg muscle. Crit Care Med 35 Suppl: S449–S453, 2007. [DOI] [PubMed] [Google Scholar]
- 212.Fredriksson K, Tjäder I, Keller P, Petrovic N, Ahlman B, Schéele C, Wernerman J, Timmons JA, Rooyackers O. Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PloS One 3: e3686, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Frick CG, Fink H, Gordan ML, Eckel B, Martyn JA, Blobner M. Chronic Escherischia coli infection induces muscle wasting without changing acetylcholine receptor numbers. Intensive Care Med 34: 561–567, 2008. [DOI] [PubMed] [Google Scholar]
- 214.Frick CG, Helming M, Martyn JA, Blobner M, Fink H. Continuous administration of pyridostigmine improves immobilization-induced neuromuscular weakness. Crit Care Med 38: 922–927, 2010. [DOI] [PubMed] [Google Scholar]
- 215.Friedrich O. Critical illness myopathy: what is happening? Curr Opin Clin Nutr Metab Care 9: 403–409, 2006. [DOI] [PubMed] [Google Scholar]
- 216.Friedrich O, Fink RHA, Hund E. Understanding critical illness myopathy: approaching the pathomechanism. J Nutr 135: 1813S–1817S, 2005. [DOI] [PubMed] [Google Scholar]
- 217.Friedrich O, Hund E, von Wegner F. Enhanced muscle shortening and impaired Ca2+ channel function in an acute septic myopathy model. J Neurol 257: 546–555, 2010. [DOI] [PubMed] [Google Scholar]
- 218.Friedrich O, Hund E, Weber C, Hacke W, Fink RH. Critical illness myopathy serum fractions affect membrane excitability and intracellular calcium release in mammalian skeletal muscle. J Neurol 251: 53–65, 2004. [DOI] [PubMed] [Google Scholar]
- 219.Friedrich O, v Wegner F, Wink M, Fink RH. Na+- and K+-channels as molecular targets of the alkaloid ajmaline in skleletal muscle fibres. Br J Pharmacol 151: 63–74, 2007. [DOI] [PubMed] [Google Scholar]
- 220.Friedrich O, Yi B, Edwards JN, Reischl B, Wirth-Hücking A, Buttgereit A, Lang R, Weber C, Polyak F, Liu I, von Wegner F, Cully TR, Lee A, Most P, Völkers M. Interleukin-1α reversibly inhibits skeletal muscle ryanodine receptor: a novel mechanism for critical illness myopathy? Am J Respir Cell Mol Biol 50: 1096–1106, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Frolkis I, Klein Y, Locker C, Nimrod A, Dahan E, Uretzsky G, Shapira I, Sorkine P. Vipera aspis venom reduces lethality and down-regulates tumor necrosis factor-α in a rat model of LPS-induced sepsis. Cytokine 3: 319–324, 2010. [DOI] [PubMed] [Google Scholar]
- 222.Frost RA, Nystrom GJ, Jefferson LS, Lang CH. Hormone, cytokine, and nutritional regulation of sepsis-induced increases in atrogin-1 and MuRF1 in skeletal muscle. Am J Physiol Endocrinol Metab 292: E501–E512, 2007. [DOI] [PubMed] [Google Scholar]
- 223.Frost RA, Nystrom GJ, Lang CH. Endotoxin and interferon-gamma inhibit translation in skeletal muscle cells by stimulating nitric oxide synthase activity. Shock 32: 416–426, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fujimura N, Sumitha Aimono M S, Masuda Y, Shichinohe Y, Narimatsu E, Namiki A. Effect of free radical scavengers on diaphragmatic contractility in septic peritonitis. Am J Respir Crit Care Med 162: 2159–2165, 2000. [DOI] [PubMed] [Google Scholar]
- 225.Garcia YR, May JJ, Green AM, Krolick KA. Acetylcholine receptor-reactive antibody induces nitric oxide production by a rat skeletal muscle cell line: influence of cytokine environment. J Neuroimmunol 120: 103–111, 2001. [DOI] [PubMed] [Google Scholar]
- 226.Garcia-Martinez C, Agell N, Llovera M, Lopez-Soriano FJ, Argiles JM. Tumor necrosis factor-alpha increases the ubiquitinization of rat skeletal muscle proteins. FEBS Lett 323: 211–214, 1993. [DOI] [PubMed] [Google Scholar]
- 227.Garcia-Martinez C, Llovera M, Agell N, Lopez-Soriano FJ, Argiles JM. Ubiquitin gene expression in skeletal muscle is increased by tumour necrosis factor-alpha. Biochem Biophys Res Commun 201: 682–686, 1994. [DOI] [PubMed] [Google Scholar]
- 228.Garcia-Martinez C, Llovera M, Agnell N, Lopez-Soriano FJ, Argiles JM. Ubiquitin gene expression in skeletal muscle is increased during sepsis: involvement of TNF-alpha but not IL-1. Biochem Biophys Res Commun 217: 839–844, 1995. [DOI] [PubMed] [Google Scholar]
- 229.Garcia-Martinez C, Lopez-Soriano FJ, Argiles JM. Acute treatment with tumor necrosis factor-alpha induces changes in protein metabolism in rat skeletal muscle. Mol Cell Biochem 125: 11–18, 1993. [DOI] [PubMed] [Google Scholar]
- 230.Garcia-Martinez C, Lopez-Soriano FJ, Argiles JM. Interleukin-6 does not activate protein breakdown in rat skeletal muscle. Cancer Lett 76: 1–4, 1994. [DOI] [PubMed] [Google Scholar]
- 231.Garnacho-Montero J, Madrazo-Osuna J, Garcia-Garmendia JL, Ortiz-Leyba C, Jimenez-Jimenez FJ, Barrero-Almodovar A, Garnacho-Montero MC, Moyano-Del-Estad MR. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med 27: 1288–1296, 2001. [DOI] [PubMed] [Google Scholar]
- 232.Garrabou G, Moren C, Lopez S, Tobias E, Cardellach F, Miro O, Casademont J. The effects of sepsis on mitochondria. J Infect Dis 205: 392–400, 2012. [DOI] [PubMed] [Google Scholar]
- 233.Gath I, Closs EI, Gödtel-Armbrust U, Schmitt S, Nakane M, Wessler I, Förstermann U. Inducible NO synthase II and neuronal NO synthase I are constitutively expressed in different structures of guinea pig skeletal muscle: implications for contractile function. FASEB J 10: 1614–1620, 1996. [DOI] [PubMed] [Google Scholar]
- 234.Gautel M. Cytoskeletal protein kinases: titin and its relations in mechanosensing. Pflügers Arch 462: 119–134, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Geller ER, Jankauskas S, Kirkpatrick J. Mitochondrial death in sepsis: a failed concept. J Surg Res 40: 514–517, 1986. [DOI] [PubMed] [Google Scholar]
- 236.Gellerich FN, Trumbeckaite S, Opalka JR, Gellerich JF, Chen Y, Neuhof C, Redl H, Werdan K, Zierz S. Mitochondrial dysfunction in sepsis: evidence from bacteraemic baboons and endotoxaemic rabbits. Biosci Rep 22: 99–113, 2002. [DOI] [PubMed] [Google Scholar]
- 237.Gerovasili V, Stefanidis K, Vitzilaios K, Karatzanos E, Politis P, Koroneos A, Chatzimichail A, Routsi C, Roussos C, Nanas S. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care 13: R161, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gerovasili V, Tripodaki E, Karatzanos E, Pitsolis T, Markaki V, Zervakis D, Routsi C, Roussos C, Nanas S. Short-term systemic effect of electrical muscle stimulation in critically ill patients. Chest 136: 1249–1256, 2009. [DOI] [PubMed] [Google Scholar]
- 239.Gervasio JM, Dickerson RN, Swearingen J, Yates ME, Yuen C, Fabian TC, Croce MA, Brown RO. Oxandrolone in trauma patients. Pharmacotherapy 20: 1328–1334, 2000. [DOI] [PubMed] [Google Scholar]
- 240.Gervasio OL, Phillips WD, Cole L, Allen DG. Caveolae respond to cell stretch and contribute to stretch-induced signaling. J Cell Sci 124: 3581–3590, 2011. [DOI] [PubMed] [Google Scholar]
- 241.Giai C, Gonzalez C, Ledo C, Garofalo A, De Genaro MS, Sordelli DO, Gomez MI. Shedding of tumor necrosis factor receptor 1 induced by protein A decreases tumor necrosis factor alpha availability and inflammation during systemic Staphylococcus aureus infection. Infect Immunol 81: 4200–4207, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Giamarellos-Bourboulis EJ. Immunomodulatory therapies for sepsis: unexpected effects with macrolides. Int J Antimicrob Agents 32 Suppl 1: S39–S43, 2008. [DOI] [PubMed] [Google Scholar]
- 243.Giamarellos-Bourboulis EJ. The failure of biologics in sepsis: where do we stand? Int J Antimicrob Agents 42 Suppl: S45–S47, 2013. [DOI] [PubMed] [Google Scholar]
- 244.Gibson WH, Cook JJ, Gatipon G, Moses ME. Effect of endotoxin shock on skeletal muscle cell membrane potential. Surgery 81: 571–577, 1977. [PubMed] [Google Scholar]
- 245.Ginz HF, Bandschapp O, Urwyler A, Girard T, Iaizzo PA. Tissue oedema is not associated with skeletal muscle weakness in septic patients. Acta Anaesthesiol Scand 54: 904, 2010. [DOI] [PubMed] [Google Scholar]
- 246.Glabinski AR, Balasingam V, Tani M, Kunkel SL, Strieter RM, Yong VW, Ransohoff RM. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol 156: 4363–4368, 1996. [PubMed] [Google Scholar]
- 247.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol 221: 3–12, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Görgens SW, Eckardt K, Elsen M, Tennagels N, Eckel J. Chitinase-3-like protein 1 protects skeletal muscle from TNFα-induced inflammation and insulin resistance. Biochem J 459: 479–488, 2014. [DOI] [PubMed] [Google Scholar]
- 249.Gogos C, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 181: 176–180, 2000. [DOI] [PubMed] [Google Scholar]
- 250.Goldberg AL, Baracos V, Rodemann P, Waxman L, Dinarello C. Control of protein degradation in muscle by prostaglandins, Ca2+, and leukocytic pyrogen (interleukin 1). Federation Proc 43: 1301–1306, 1984. [PubMed] [Google Scholar]
- 251.Goldberg AL, Kettelhut IC, Furuno K, Fagen JM, Barascos V. Activation of protein breakdown and prostaglandin E2 production in rat skeletal muscle in fever is signaled by a macrophage product distinct from interleukin-1 or other known monokines. J Clin Invest 81: 1378–1383, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Goldhaber JI, Philipson KD. Cardiac sodium-calcium exchange and efficient excitation-contraction coupling: implications for heart disease. Adv Exp Med Biol 961: 355–364, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gonoi T, Hille B. Gating of Na channels. Inactivation modifiers discriminate among models. J Gen Physiol 89: 253–274, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gonzalez Narvaez AA, Castillo A. Ca2+ store determines gating of store-operated calcium entry in mammalian skeletal muscle. J Muscle Res Cell Motil 28: 105–113, 2007. [DOI] [PubMed] [Google Scholar]
- 255.Gooch JL. AAEM case report #29: prolonged paralysis after neuromuscular blockade. Muscle Nerve 18: 937–942, 1995. [DOI] [PubMed] [Google Scholar]
- 256.Gooch JL, Moore MH, Ryser DK. Prolonged paralysis after neuromuscular junction blockade: case reports and electrodiagnostic findings. Arch Phys Med Rehabil 74: 1007–1011, 1993. [PubMed] [Google Scholar]
- 257.Goodman BP, Harper CM, Boon AJ. Prolonged compound muscle action potential duration in critical illness myopathy. Muscle Nerve 40: 1040–1042, 2009. [DOI] [PubMed] [Google Scholar]
- 258.Goodman MN. Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am J Physiol Endocrinol Metab 260: E727–E730, 1991. [DOI] [PubMed] [Google Scholar]
- 259.Goodman MN. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med 205: 182–185, 1994. [DOI] [PubMed] [Google Scholar]
- 260.Gordon BS, Kelleher AR, Kimball SR. Regulation of muscle protein synthesis and the effects of catabolic states. Int J Biochem Cell Biol 45: 2147–2157, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gore DC, Chinkes DL, Hart DW, Wolf SE, Herndon DN, Sanford AP. Hyperglycemia exacerbates muscle protein catabolism in burn-injured patients. Crit Care Med 30: 2438–2442, 2002. [DOI] [PubMed] [Google Scholar]
- 262.Gore DC, Wolfe RR. Glutamine supplementation fails to affect muscle protein kinetics in critically ill patients. JPEN J Parenter Enteral Nutr 26: 342–349, 2002. [DOI] [PubMed] [Google Scholar]
- 263.Gore DC, Wolfe RR. Metabolic response of muscle to alanine, glutamine, and valine supplementation during severe illness. JPEN J Parenter Enteral Nutr 27: 307–314, 2003. [DOI] [PubMed] [Google Scholar]
- 264.Greenberg MJ, Mealy TR, Jones M, Szczesna-Cordary D, Moore JR. The direct molecular effects of fatigue and myosin regulatory light chain phosphorylation on the actomyosin contractile apparatus. Am J Physiol Regul Integr Comp Physiol 298: R989–R996, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Greenberg SB, Vender J. The use of neuromuscular blocking agents in the ICU: where are we now? Crit Care Med 41: 1332–1344, 2013. [DOI] [PubMed] [Google Scholar]
- 266.Gregory CM, Vandenborne K, Castro MJ, Dudley GA. Human and rat skeletal muscle adaptations to spinal cord injury. Can J Appl Physiol 28: 491–500, 2003. [DOI] [PubMed] [Google Scholar]
- 267.Gregson JM, Leathley MJ, Moore AP, Smith TL, Sharma AK, Watkins CL. Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing 29: 223–228, 2000. [DOI] [PubMed] [Google Scholar]
- 268.Griffiths RD, Hall JB. Intensive care unit-acquired weakness. Crit Care Med 38: 779–787, 2010. [DOI] [PubMed] [Google Scholar]
- 269.Griffiths RD, Palmer TE, Helliwell T, MacLennan P, MacMillan RR. Effect of passive stretching on the wasting of muscle in the critically ill. Nutrition 11: 428–432, 1995. [PubMed] [Google Scholar]
- 270.Groome JR, Lehmann-Horn F, Holzherr BR. Open- and closed-state fast inactivation in sodium channels. Channels 5: 65–78, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Grumati P, Coletto L, Sabatelli P, Cescon M, Angelin A, Bertaggia E, Blaauw B, Urciuolo A, Tiepolo T, Merlini L, Maraldi NM, Bernardi P, Sandri M, Bonaldo P. Autphagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat Med 16: 1313–1320, 2010. [DOI] [PubMed] [Google Scholar]
- 272.Gruther W, Kainberger F, Fialka-Moser V, Paternostro-Sluga T, Quittan M, Spiss C, Crevenna R. Effects of neuromuscular electrical stimulation on muscle layer thickness of knee extensor muscles in intensive care unit patients: a pilot study. J Rehabil Med 42: 593–597, 2010. [DOI] [PubMed] [Google Scholar]
- 273.Guarneri B, Bertolini G, Latronico N. Long-term outcome in patients with critical illness myopathy or neuropathy: the Italian multicentre CRIMYNE study. J Neurol Neurosurg Psychiatry 79: 838–841, 2008. [DOI] [PubMed] [Google Scholar]
- 274.Guillouet M, Gueret G, Rannou F, Giroux-Metges MA, Gioux M, Arvieux C, Pennec JP. TNFα increases resting potential in isolated fibers from rat peroneus longus by a PKC mediated mechanism: involvement in ICU acquired polyneuromyopathy. Cytokine 56: 149–152, 2011. [DOI] [PubMed] [Google Scholar]
- 275.Guillouet M, Gueret G, Rannou F, Giroux-Metges MA, Gioux M, Arvieux C, Pennec JP. Tumor necrosis factor-α downregulates sodium current in skeletal muscle by protein kinase C activation: involvement in critical illness polyneuromyopathy. Am J Physiol Cell Physiol 301: C1057–C1063, 2011. [DOI] [PubMed] [Google Scholar]
- 276.Gundersen K. Excitation-transcription coupling in skeletal muscle: the molecular pathways of exercise. Biol Rev Camp Philos Soc 86: 564–600, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Gunst J, Derese I, Aertgeerts A, Ververs EJ, Wauters A, Van den Berghe G, Vanhorebeek I. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit Care Med 41: 182–194, 2013. [DOI] [PubMed] [Google Scholar]
- 278.Gupta R, Rajani R, Primrose JN, Johnson CD. Body composition, physiological function and psychological changes in patients with predicted severe acute pancreatitis. Pancreatology 1: 58–62, 2001. [DOI] [PubMed] [Google Scholar]
- 279.Gutmann L, Blumenthal D, Gutmann L, Schochet SS. Acute type II myofiber atrophy in critical illness. Neurology 46: 819–821, 1996. [DOI] [PubMed] [Google Scholar]
- 280.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289: 2363–2366, 2000. [DOI] [PubMed] [Google Scholar]
- 281.Haarmann CS, Dulhunty AF, Laver DR. Regulation of skeletal muscle ryanodine receptors by dihydropyridine receptor II-III loop C-region peptides: relief of Mg2+ inhibition. Biochem J 387: 429–436, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Haden DW, Suliman HB, Carraway MS, Welty-Wolf KE, Ali AS, Shitara H, Yonekawa H, Piantadosi CA. Mitochondrial biogenesis restores oxidative metabolism during Staphylococcus aureus sepsis. Am J Respir Crit Care Med 176: 768–777, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Haeseler G, Foadi N, Wiegand E, Ahrens J, Krampfl K, Dengler R, Leuwer M. Endotoxin reduces availability of voltage-gated human skeletal muscle sodium channels at depolarized membrane potentials. Crit Care Med 36: 1239–1247, 2008. [DOI] [PubMed] [Google Scholar]
- 284.Hall-Angeras M, Hasselgren PO, Dimlich RV, Fischer JE. Myofibrillar proteinase, cathepsin B, and protein breakdown rates in skeletal muscle from septic rats. Metabolism 40: 302–306, 1991. [DOI] [PubMed] [Google Scholar]
- 285.Hammarqvist F, Wernerman J, Ali R, von der Decken A, Vinnars E. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Ann Surg 209: 455-61, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Hardin BJ, Campbell KS, Smith JD, Arbogast S, Smith J, Moylan JS, Reid MB. TNF-α acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol 104: 694–699, 2008. [DOI] [PubMed] [Google Scholar]
- 287.Harridge SD. Plasticity of human skeletal muscle: gene expression to in vivo function. Exp Physiol 92: 783–797, 2007. [DOI] [PubMed] [Google Scholar]
- 288.Harris ML, Luo YM, Watson AC, Rafferty GF, Polkey MI, Green M, Moxham J. Adductor pollicis twitch tension assessed by magnetic stimulation of the ulnar nerve. Am J Respir Crit Care Med 162: 240–245, 2000. [DOI] [PubMed] [Google Scholar]
- 289.Hasselgren PO, James JH, Benson DW, Li S, Fischer JE. Is there a circulating proteolysis-inducing factor during sepsis? Arch Surg 125: 510–514, 1990. [DOI] [PubMed] [Google Scholar]
- 290.Hasselgren PO, James JH, Warner BW, Ogle C, Takehara H, Fischer JE. Reduced muscle amino acid uptake in sepsis and the effects in vitro of septic plasma and interleukin-1. Surgery 100: 222–228, 1986. [PubMed] [Google Scholar]
- 291.Hasselgren PO, Talamini M, James H, Fischer JE. Protein metabolism in different types of skeletal muscle during early and late sepsis in rats. Arch Surg 121: 918–923, 1986. [DOI] [PubMed] [Google Scholar]
- 292.Hassoun SM, Marechal X, Montaigne D, Bouazza Y, Decoster B, Lancel S, Neviere R. Prevention of endotoxin-induced sarcoplasmic reticulum calcium leak improves mitochondrial and myocardial dysfunction. Crit Care Med 36: 2590–2596, 2008. [DOI] [PubMed] [Google Scholar]
- 293.Hauptmann S, Klosterhalfen B, Weis J, Mittermayer C, Kirkpatrick CJ. Skeletal muscle oedema and muscle fiber necrosis during septic shock. Observations with a porcine septic shock model. Virchows Arch 424: 653–659, 1994. [DOI] [PubMed] [Google Scholar]
- 294.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481: 511–515, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43: 67–93, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Head SI. Membrane potential, resting calcium and calcium transients in isolated muscle fibers from normal and dystrophic mice. J Physiol 469: 11–19, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Helliwell TR, Coakley JH, Wagenmakers AJ, Griffiths RD, Campbell IT, Green CJ, McClelland P, Bone JM. Necrotizing myopathy in critically-ill patients. J Pathol 164: 307–314, 1991. [DOI] [PubMed] [Google Scholar]
- 298.Helliwell TR, Wilkinson A, Griffiths RD, McClelland P, Palmer TE, Bone JM. Muscle fibre atrophy in critically ill patients is associated with the loss of myosin filaments and the presence of lysosomal enzymes and ubiquitin. Neuropathol Appl Neurobiol 24: 507–517, 1998. [DOI] [PubMed] [Google Scholar]
- 299.Hermans G, Agten A, Testelmans D, Decramer M, Gayan-Ramirez G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care 14: R127, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Hermans G, Casaer MP, Clerckx B, Güiza F, Vanhullebusch T, Derde S, Meerseman P, Derese I, Mesotten D, Wouters PJ, Van Cromphaut S, Debaveye S, Gosselink R, Gunst J, Wilmer A, Van den Berghe G, Vanhorebeek I. Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNic trial. Lancet Resp Med 1: 621–629, 2013. [DOI] [PubMed] [Google Scholar]
- 301.Hermans G, Clerckx B, Vanhullebusch T, Segers J, Vanpee G, Robbeets C, Casaer MP, Wouters P, Gosselink R, Van den Berghe G. Interobserver agreement of Medical Research Council sum score and handgrip strength in the intensive care unit. Muscle Nerve 45: 18–25, 2012. [DOI] [PubMed] [Google Scholar]
- 302.Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G. Clinical review: critical illness polyneuropathy and myopathy. Crit Care 12: 238, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G. Interventions for preventing critical illness polyneuropathy and critical illness myopathy. Cochrane Database Syst Rev 1: CD006832, 2009. [DOI] [PubMed] [Google Scholar]
- 304.Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G. Interventions for preventing critical illness polyneuropathy and critical illness myopathy. Cochrane Database Syst Rev 1: CD006832, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hermans G, Gosselink R. Should we abandon manual muscle strength testing in the ICU? Crit Care 15: 127, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hermans G, Schrooten M, Van Damme P, Berends N, Bouckaert B, De Vooght W, Robberecht W, Van den Berghe G. Benefits of intensive insulin therapy on neuromuscular complications in routine daily critical care practice: a retrospective study. Crit Care 13: R5, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hermans G, Van Mechelen H, Clerckx B, Vanhullebusch T, Mesotten D, Wilmer A, Casaer MP, Meersseman P, Debaveye Y, Van Cromphaut S, Wouters PJ, Gosselink R, Van den Berghe G. Acute outcomes and 1-year mortality of ICU-acquired weakness: a cohort study and propensity matched analysis. Am J Respir Crit Care Med 190: 410–420, 2014. [DOI] [PubMed] [Google Scholar]
- 308.Hermans G, Vanhorebeek I, Derde S, Van den Berghe G. Metabolic aspects of critical illness polyneuromyopathy. Crit Care Med 37: S391–S397, 2009. [DOI] [PubMed] [Google Scholar]
- 309.Hermans G, Wilmer A, Meersseman W, Milants I, Wouters PJ, Bobbaers H, Bruyninckx F, Van den Berghe G. Impact of intensive insulin therapy on neuromuscular complications and ventilator-dependency in MICU. Am J Respir Crit Care Med 175: 480–489, 2007. [DOI] [PubMed] [Google Scholar]
- 311.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS, Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348: 683–693, 2003. [DOI] [PubMed] [Google Scholar]
- 312.Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berger MM, Day AG. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 368: 1489–1497, 2013. [DOI] [PubMed] [Google Scholar]
- 313.Hinohara H, Morita T, Okano N, Kuhimoto F, Goto F. Chronic intraperitoneal endotoxin treatment in rats induces resistance to d-tubocurarine but does not produce up-regulation of acetylcholine receptors. Acta Anaesthesiol Scand 47: 335–341, 2003. [DOI] [PubMed] [Google Scholar]
- 314.Hirano M, Ott BR, Raps EC, Minetti C, Lennihan L, Libbey NP, Bonilla E, Hays AP. Acute quadriplegic myopathy: a complication of treatment with steroids, nondepolarizing blocking agents, or both. Neurology 42: 2082–2087, 1992. [DOI] [PubMed] [Google Scholar]
- 315.Hobler SC, Tiao G, Fischer JE, Monaco J, Hasselgren PO. Sepsis-induced increase in muscle proteolysis is blocked by specific proteasome inhibitors. Am J Physiol Regul Integr Comp Physiol 274: R30–R37, 1998. [DOI] [PubMed] [Google Scholar]
- 316.Hobler SC, Williams A, Fischer D, Wang JJ, Sun X, Fischer JE, Monaco JJ, Hasselgren PO. Activity and expression of the 20S proteasome are increased in skeletal muscle during sepsis. Am J Physiol Regul Integr Comp Physiol 277: R434–R440, 1999. [DOI] [PubMed] [Google Scholar]
- 317.Holecek M. Muscle wasting in animal models of severe illness. Int J Exp Pathol 93: 157–171, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Hollenberg SM, Cunnion RE, Parillo JE. Effect of septic serum on vascular smooth muscle: in vitro studies using rat aortic rings. Crit Care Med 20: 993–998, 1992. [DOI] [PubMed] [Google Scholar]
- 319.Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med 363: 1256–1264, 2010. [DOI] [PubMed] [Google Scholar]
- 320.Hopkins RO, Spuhler VJ, Thomsen GE. Transforming ICU culture to facilitate early mobility. Crit Care Clin 23: 81–96, 2007. [DOI] [PubMed] [Google Scholar]
- 321.Hosenpud JD, Campbell SM, Mendelson DJ. Interleukin-1-induced myocardial depression in an isolated beating heart. J Heart Transplant 8: 460–464, 1989. [PubMed] [Google Scholar]
- 322.Hosokawa S, Koseki H, Nagashima M, Maeyama Y, Yomogida K, Mehr C, Rutledge M, Greenfeld H, Kaneki M, Tompkins RG, Martyn JA, Yasuhara SE. Title efficacy of phosphodiesterase 5 inhibitor on distant burn-induced muscle autophagy, microcirculation, and survival rate. Am J Physiol Endocrinol Metab 304: E922–E933, 2013. [DOI] [PubMed] [Google Scholar]
- 323.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13: 260–268, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Hotchkiss RS, Rust RS, Dence CS, Wasserman TH, Song SK, Hwang DR, Karl IE, Welch MJ. Evaluation of the role of cellular hypoxia in sepsis by the hypoxic marker [18F]fluoromisonidazole. Am J Physiol Regul Integr Comp Physiol 261: R965–R972, 1991. [DOI] [PubMed] [Google Scholar]
- 325.Hough CL, Lieu BK, Caldwell ES. Manual muscle strength testing of critically ill patients: feasibility and interobserver agreement. Crit Care 15: R43, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hranjec T, Swenson BR, Dossett LA, Metzger R, Flohr TR, Popovsky KA, Bonatti HJ, May AK, Sawyer RG. Diagnosis-dependent relationships between cytokine levels and survival in patients admitted for surgical critical care. J Am Coll Surg 210: 833–846, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Hsieh CH, Pai PY, Hsueh HW, Yuan SS, Hsieh YC. Complete induction of autophagy is essential for cardioprotection in sepsis. Ann Surg 253: 1190–1200, 2011. [DOI] [PubMed] [Google Scholar]
- 328.Hsieh PF, Liu YF, Pan YJ, Wu MC, Lin TL, Huang YT, Wang JT. Klebsiella pneumoniae peptidoglycan-associated lipoprotein and murein liporprotein contribute to serum resistance, antiphagocytosis, and proinflammatory cytokine stimulation. J Infect Dis 208: 1580–1589, 2013. [DOI] [PubMed] [Google Scholar]
- 329.Hummel RP 3rd, James JH, Warner BW, Hasselgren PO, Fischer JE. Evidence that cathepsin B contributes to skeletal muscle protein breakdown during sepsis. Arch Surg 123: 221–224, 1988. [DOI] [PubMed] [Google Scholar]
- 330.Hummel RP 3rd, Warner BW, James JH, Hasselgren PO, Fischer JE. Effects of indomethacin and leupeptin on muscle cathepsin B activity and protein degradation during sepsis. J Surg Res 45: 140–144, 1988. [DOI] [PubMed] [Google Scholar]
- 331.Hund E. Myopathy in critically ill patients. Crit Care Med 27: 2544–2547, 1999. [DOI] [PubMed] [Google Scholar]
- 332.Hund E. Neurological complications of sepsis: critical illness polyneuropathy and myopathy. J Neurol 248: 929–934, 2001. [DOI] [PubMed] [Google Scholar]
- 333.Hund E, Grenzwürker H, Böhrer H, Jakob H, Thiele R, Hacke W. Predominant involvement of motor fibres in patients with critical illness polyneuropathy. Br J Anaesth 78: 274–278, 1997. [DOI] [PubMed] [Google Scholar]
- 334.Hurne AM, O'Brien JJ, Wingrove D, Cherednichenko G, Allen PD, Beam KG, Pessah IN. Ryanodine receptor type 1 (RyR1) mutations C4958S and C4961S reveal excitation-coupled calcium entry (ECCE) is independent of sarcoplasmic reticulum store depletion. J Biol Chem 280: 36994–37004, 2005. [DOI] [PubMed] [Google Scholar]
- 335.Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, Danialou G, Matecki S, Jaber S, Petrof BJ, Goldberg P. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med 182: 1377–1386, 2010. [DOI] [PubMed] [Google Scholar]
- 336.Ibebunjo C, Chick JM, Kendall T, Eash JK, Li C, Zhang Y, Vickers C, Wu Z, Clarke BA, Shi J, Cruz J, Fournier B, Brachat S, Gutzwiller S, Ma Q, Markovits J, Broome M, Steinkrauss M, Skuba E, Galarneau JR, Gygi SP, Glass DJ. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cell Biol 33: 194–212, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ibebunjo C, Martyn JA. Fiber atrophy, but not changes in acetylcholine receptor expression, contributes to the muscle dysfunction after immobilization. Crit Care Med 27: 275–285, 1999. [DOI] [PubMed] [Google Scholar]
- 338.Illner HP, Shires GT. Membrane defect and energy status of rabbit skeletal muscle cells in sepsis and septic shock. Arch Surg 116: 1302–1305, 1981. [DOI] [PubMed] [Google Scholar]
- 339.Ingber DE. Control of capillary growth and differentiation by extracellular matrix. Use of a tensegrity (tensional integrity) mechanism for signal processing. Chest 99 Suppl: 34S–40S, 1991. [PubMed] [Google Scholar]
- 340.Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci 104: 613–627, 1993. [DOI] [PubMed] [Google Scholar]
- 341.Ingber DE. Integrins, tensegrity, mechanotransduction. Gravit Space Biol Bull 10: 49–55, 1997. [PubMed] [Google Scholar]
- 342.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 59: 575–599, 1997. [DOI] [PubMed] [Google Scholar]
- 343.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res 91: 877–887, 2002. [DOI] [PubMed] [Google Scholar]
- 344.Intiso D, Amoruso L, Zarrelli M, Pazienza L, Basciani M, Grimaldi G, Iarossi A, Di RF. Long-term functional outcome and health status of patients with critical illness polyneuromyopathy. Acta Neurol Scand 123: 211–219, 2011. [DOI] [PubMed] [Google Scholar]
- 345.Ioannidis JP. Extrapolating from animals to humans. Sci Transl Med 4: 151ps115, 2012. [DOI] [PubMed] [Google Scholar]
- 346.Ito N, Ruegg UT, Kudo A, Miyagoe-Suzuki S, Takeda Y. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat Med 19: 101–106, 2013. [DOI] [PubMed] [Google Scholar]
- 347.Jacobs DO, Kobayashi T, Imaqire J, Grant C, Kesselly B, Wilmore DW. Sepsis alters skeletal muscle energetic and membrane function. Surgery 110: 318–325, 1991. [PubMed] [Google Scholar]
- 348.Jacobs DO, Maris J, Fried R, Settle RG, Rolandelli RR, Koruda MJ, Chance B, Rombeau JL. In vivo phosphorus 31 magnetic resonance spectroscopy of rat hind limb skeletal muscle during sepsis. Arch Surg 123: 1425–1428, 1988. [DOI] [PubMed] [Google Scholar]
- 349.Jain N, Iyer KV, Kumar A, Shivashankar GV. Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proc Natl Acad Sci USA 110: 11349–11354, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable predictor of tissue hypoxia in injury or sepsis. Lancet 354: 505–508, 1999. [DOI] [PubMed] [Google Scholar]
- 351.Janssen SP, Gayan-Ramirez G, Van den Bergh A, Herijgers P, Maes K, Verbeken E, Decramer M. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation 111: 996–1005, 2005. [DOI] [PubMed] [Google Scholar]
- 352.Jeschke MG, Finnerty CC, Suman OE, Kulp G, Mlcak RP, Herndon DN. The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn. Ann Surg 246: 351–360, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Jespersen JG, Nedergaard A, Reitelseder S, Mikkelsen UR, Dideriksen KJ, Agergaard J, Kreiner F, Pott FC, Schjerling P, Kjaer M. Activated protein synthesis and suppressed protein breakdown signaling in skeletal muscle of critically-ill patients. PLoS One 31: e18090, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Johnson JD, Jiang Y, Rall JA. Intracellular EDTA mimics parvalbumin in the promotion of skeletal muscle relaxation. Biophys J 76: 1514–1522, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Joulin O, Petillot P, Labalette M, Lancel S, Neviere R. Cytokine profile of human septic shock serum inducing cardiomyocyte contractile dysfunction. Physiol Res 56: 291–297, 2007. [DOI] [PubMed] [Google Scholar]
- 356.Ju JS, Varadhachary AS, Miller SE, Weihl CC. Quantitation of “autophagic flux” in mature skeletal muscle. Autophagy 6: 929–935, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Karatzanos E, Gerovasili V, Zervakis D, Tripodaki ES, Apostolou K, Vasileiadis I, Papadopoulos E, Mitsiou G, Tsimpouki D, Routsi C, Nanas S. Electrical muscle stimulation: an effective form of exercise and early mobilization to preserve muscle strength in critically ill patients. Crit Care Res Pract 2012: 432752, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Kasden SE, Kirkpatrick JR. Endotoxin and calcium effect on metabolism. Am Surg 56: 707–714, 1990. [PubMed] [Google Scholar]
- 359.Keller CW, Fokken C, Turville SG, Lünemann A, Schmidt J, Münz C, Lünemann JD. TNF-alpha induces macroautophagy and regulates MHC II class expression in human skeletal muscle cells. J Biol Chem 286: 3970–3980, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Kerbaul F, Brousse M, Collart F, Pellissier JF, Planche D, Fernandez C, Gouin F, Guidon C. Combination of histopathological and electromyographic patterns can help to evaluate functional outcome of critical ill patients with neuromuscular weakness syndromes. Crit Care 8: R358–R366, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Khan J, Harrison TB, Rich MM, Moss M. Early development of critical illness myopathy and neuropathy in patients with severe sepsis. Neurology 67: 1421–1425, 2006. [DOI] [PubMed] [Google Scholar]
- 362.Kim C, Fuke N, Martyn JAJ. Burn injury to rat increases nicotinic acetylcholine receptors in the diaphragm. Anesthesiology 68: 401–406, 1988. [DOI] [PubMed] [Google Scholar]
- 363.Kim C, Martyn J, Fuke N. Burn injury to trunk of rat causes denervation-like responses in the gastrocnemius muscle. J Appl Physiol 65: 1745–1751, 1988. [DOI] [PubMed] [Google Scholar]
- 364.Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, Kim JK. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes 53: 1060–1067, 2004. [DOI] [PubMed] [Google Scholar]
- 365.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462: 245–253, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Klaude M, Fredriksson K, Tjäder I, Hammaqvist F, Ahlman B, Rooyackers O, Wernerman J. Proteasome proteolytic activity in skeletal muscle is increased in patients with sepsis. Clin Sci 112: 499–506, 2007. [DOI] [PubMed] [Google Scholar]
- 367.Klaude M, Hammarqvist F, Wemerman J. An assay of microsomal membrane-associated proteasomes demonstrates increased proteolytic activity in skeletal muscle of intensive care unit patients. Clin Nutr 24: 259–265, 2005. [DOI] [PubMed] [Google Scholar]
- 368.Klaude M, Mori M, Tjader I, Gustafsson T, Wernerman J, Rooyackers O. Protein metabolism and gene expression in skeletal muscle of critically ill patients with sepsis. Clin Sci 122: 133–142, 2012. [DOI] [PubMed] [Google Scholar]
- 369.Kleyweg RP, van der Meche FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barre syndrome. Muscle Nerve 14: 1103–1109, 1991. [DOI] [PubMed] [Google Scholar]
- 370.Koch S, Spuler S, Deja M, Bierbrauer J, Dimroth A, Behse F, Spies CD, Wernecke KD, Weber-Carstens S. Critical illness myopathy is frequent: accompanying neuropathy protracts ICU discharge. J Neurol Neurosurg Psychiatry 82: 287–293, 2011. [DOI] [PubMed] [Google Scholar]
- 371.Koesters A, Engisch KL, Rich MM. Decreased cardiac excitability secondary to reduction of sodium current may be a significant contributor to reduced contractility in a rat model of sepsis. Crit Care 18: R54, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169: 425–434, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 297: 1772–1774, 2007. [DOI] [PubMed] [Google Scholar]
- 374.Kotter S, Unger A, Hamdani N, Lang P, Vorgerd M, Nagel-Steger L, Linke WA. Human myocytes are protected from titin aggregation-induced stiffening by small heat shock proteins. J Cell Biol 204: 187–202, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Kouyoumdjian JA. Neuromuscular abnormalities in disues, ageing and cachexia. Arq Neuropsiquiatr 51: 299–306, 1993. [DOI] [PubMed] [Google Scholar]
- 376.Kraner SD, Novak KR, Wang Q, Peng J, Rich MM. Altered sodium channel-protein associations in critical illness myopathy. Skelet Muscle 2: 17, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kraner SD, Wang Q, Novak KR, Cheng D, Cool DR, Peng J, Rich MM. Upregulation of the CaV 1.1-ryanodine receptor complex in a rat model of critical illness myopathy. Am J Physiol Regul Integr Comp Physiol 300: R1384–R1391, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 342: 1471–1477, 2000. [DOI] [PubMed] [Google Scholar]
- 379.Kroemer G, Marin G, Levine B. Autophagy and the integrated stress response. Mol Cell 40: 280–293, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Kuzmenkin A, Jurkat-Rott K, Lehmann-Horn F, Mitrovic N. Impaired slow inactivation due to a polymorphism and substitutions of Ser-906 in the II-III loop of the human Nav1.4 channel. Pflügers Arch 447: 71–77, 2003. [DOI] [PubMed] [Google Scholar]
- 381.Lacomis D. Electrophysiology of neuromuscular disorders in critical illness. Muscle Nerve 47: 452–463, 2013. [DOI] [PubMed] [Google Scholar]
- 382.Lacomis D, Giuliani MJ, Van Gott A, Kramer DJ. Acute myopathy of intensive care: clinical, electromyographic, and pathological aspects. Ann Neurol 40: 645–654, 1996. [DOI] [PubMed] [Google Scholar]
- 383.Lacomis D, Petrella JT, Giuliani MJ. Causes of neuromuscular weakness in the intensive care unit: a study of ninety-two patients. Muscle Nerve 21: 610–617, 1998. [DOI] [PubMed] [Google Scholar]
- 384.Lacomis D, Smith TW, Chad DA. Acute myopathy and neuropathy in status asthmaticus: case report and literature review. Muscle Nerve 16: 84–90, 1993. [DOI] [PubMed] [Google Scholar]
- 385.Lacomis D, Zochodne DW, Bird SJ. Critical illness myopathy. Muscle Nerve 23: 1785–1788, 2000. [DOI] [PubMed] [Google Scholar]
- 386.Lagasse RS, Katz RI, Petersen M, Jacobson MJ, Poppers PJ. Prolonged neuromuscular blockade following vecuronium infusion. J Clin Anesth 2: 269–271, 1990. [DOI] [PubMed] [Google Scholar]
- 387.Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, Tobin MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 167: 120–127, 2003. [DOI] [PubMed] [Google Scholar]
- 388.Lamb GD, Cellini MA, Stephenson DG. Different Ca2+ releasing action of caffeine and depolarisation in skeletal muscle fibers of the rat. J Physiol 531.3: 715–728, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab 293: E453–E459, 2007. [DOI] [PubMed] [Google Scholar]
- 390.Lange S, Ehler E, Gautel M. From A to Z and back? Multicompartment proteins in the sarcomere. Trends Cell Biol 16: 11–18, 2006. [DOI] [PubMed] [Google Scholar]
- 391.Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson LG, Hughes SM, Marchand S, Sejersen T, Richard I, Edstrom L, Ehler E, Udd B, Gautel M. The kinase domain of titin controls muscle gene expression and protein turnover. Science 308: 1599–1603, 2005. [DOI] [PubMed] [Google Scholar]
- 392.Langhans C, Weber-Carstens S, Schmidt F, Hamati J, Kny M, Zhu X, Wollersheim T, Koch S, Krebs M, Schulz H, Lodka D, Saar K, Labeit S, Spies C, Hubner N, Spranger J, Spuler S, Boschmann M, Dittmar G, Butler-Browne G, Mouly V, Fielitz J. Inflammation-induced acute phase response in skeletal muscle and critical illness myopathy. PLoS One 9: e92048, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, Hansen TK, Van den Berghe G. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest 115: 2277–2286, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Lanone S, Mebazza A, Heymes C, Henin D, Poderoso JJ, Panis Y, Zedda C, Billiar T, Payen D, Aubier M, Boczkowski J. Muscular contractile failure in septic patients: role of the inducible nitric oxide synthase pathway. Am J Respir Crit Care Med 162: 2308–2315, 2000. [DOI] [PubMed] [Google Scholar]
- 395.Larsson L. Experimental animal models of muscle wasting in intensive care unit patients. Crit Care Med 35 Suppl: S484-487, 2007. [DOI] [PubMed] [Google Scholar]
- 396.Larsson L. Acute quadriplegic myopathy: an acquired “myosinopathy.” Adv Exp Med Biol 642: 92–98, 2008. [DOI] [PubMed] [Google Scholar]
- 397.Larsson L. Acute Quadriplegic Myopathy, an acquired “myosinopathy.” In: The Sarcomere and Skeletal Muscle Diseases, edited by Laing NG. New York: Springer, 2008, vol. 642, chapt. 8, p. 92–98. [DOI] [PubMed] [Google Scholar]
- 398.Larsson L, Li X, Berg HE, Frontera WR. Effects of removal of weight-bearing function on contractility and myosin isoform composition in single human skeletal muscle cells. Pflügers Arch 432: 320–328, 1996. [DOI] [PubMed] [Google Scholar]
- 399.Larsson L, Li X, Edstrom L, Eriksson LI, Zackrisson H, Argentini C, Schiaffino S. Acute quadriplegia and loss of muscle myosin in patients treated with nondepolarizing neuromuscular blocking agents and corticosteroids: mechanisms at the cellular and molecular levels. Crit Care Med 28: 34–45, 2000. [DOI] [PubMed] [Google Scholar]
- 400.Larsson L, Roland A. Drug induced tetraparesis and loss of myosin. Mild types are probably overlooked. Lakartidningen 93: 2249–2254, 1996. [PubMed] [Google Scholar]
- 401.Latronico N, Bertolini G, Guarneri B, Botteri M, Peli E, andreoletti S, Bera P, Luciani D, Nardella A, Vittorielli E, Simini B, Candiani A. Simplified electrophysiological evaluation of peripheral nerves in critically ill patients: the Italian multi-centre CRIMYNE study. Crit Care 11: R11, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 10: 931–941, 2011. [DOI] [PubMed] [Google Scholar]
- 403.Latronico N, Fenzi F, Recupero D, Guarneri B, Tomelleri G, Tonin P, De Maria G, Antonini L, Rizzuto N, Candiani A. Critical illness myopathy and neuropathy. Lancet 347: 1579–1582, 1996. [DOI] [PubMed] [Google Scholar]
- 404.Latronico N, Guarneri B. Critical illness myopathy and neuropathy. Minerva Anesthesiol 74: 319–323, 2008. [PubMed] [Google Scholar]
- 405.Latronico N, Guarneri B, Alongi S, Bussi G, Candiani A. Acute neuromuscular respiratory failure after ICU discharge. Report of five patients. Intensive Care Med 25: 1302–1306, 1999. [DOI] [PubMed] [Google Scholar]
- 406.Latronico N, Shehu I, Seghelini E. Neuromuscular sequelae of critical illness. Curr Opin Crit Care 11: 381–390, 2005. [DOI] [PubMed] [Google Scholar]
- 407.Latronico N, Tomelleri G, Filosto M. Critical illness myopathy. Curr Opin Rheumatol 24: 616–622, 2012. [DOI] [PubMed] [Google Scholar]
- 408.Launikonis BS, Stephenson DG. Effect of saponin treatment on the sarcoplasmic resticulum of rat, cane toad and crustacean (yabby) skeletal muscle. J Physiol 504.2: 425–437, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin proteasome pathway in normal and disease states. J Nutr 129: 227S–237S, 1999. [DOI] [PubMed] [Google Scholar]
- 410.Lee CM, Fan E. ICU-acquired weakness: what is preventing its rehabilitation in critically ill patients? BMC Med 10: 115, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Lee HY, Kaneki M, andreas J, Tompkins RG, Martyn JA. Novel mitochondria-targeted antioxidant peptide ameliorates burn-induced apoptosis and endoplasmic reticulum stress in the skeletal muscle of mice. Shock 36: 580–585, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Lee RS, Tikunova SB, Kilne KP, Zot HG, Hasbun JE, Minh NV, Swartz DR, Rall JA, Davis JP. Effect of Ca2+ binding properties of troponin C on rate of skeletal muscle force redevelopment. Am J Physiol Cell Physiol 299: C1091–C1099, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Lefaucheur JP, Nordine T, Rodriguez P, Brochard L. Origin of ICU acquired paresis determined by direct muscle stimulation. J Neurol Neurosurg Psychiatry 77: 500–506, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Leijten FS, de Weerd AW. Critical illness polyneuropathy. A review of the literature, definition and pathophysiology. Clin Neurol Neurosurg 96: 10–19, 1994. [DOI] [PubMed] [Google Scholar]
- 415.Leijten FS, de Weerd AW, Poortvliet DC, De Ridder VA, Ulrich C, Harink-De Weerd JE. Critical illness polyneuropathy in multiple organ dysfunction syndrome and weaning from the ventilator. Intensive Care Med 22: 856–861, 1996. [DOI] [PubMed] [Google Scholar]
- 416.Leijten FS, Harinck-de Weerd JE, Poortvliet DC, de Weerd AW. The role of polyneuropathy in motor convalescence after prolonged mechanical ventilation. JAMA 274: 1221–1225, 1995. [PubMed] [Google Scholar]
- 417.Leppik JA, Aughey RJ, Medved I, Fairweather I, Carey MF, McKenna MJ. Prolonged exercise to fatigue in humans impairs skeletal muscle Na+-K+-ATPase activity, sarcoplasmic reticulum Ca2+ regulation, Ca2+ uptake. J Appl Physiol 97: 1414–1423, 2004. [DOI] [PubMed] [Google Scholar]
- 418.Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 19: 362–370, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Li YP, Lecker SH, Chen Y, Waddell ID, Goldberg AL. TNF-alpha increases ubiquitin-conjugating activity in skeletal muscle by upregulating UbcH2/E220k. FASEB J 17: 1048–1057, 2003. [DOI] [PubMed] [Google Scholar]
- 420.Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-κB activation in response to tumor necrosis factor-α. FASEB J 12: 871–880, 1998. [DOI] [PubMed] [Google Scholar]
- 421.Li YP, Reid MB. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am J Physiol Regul Integr Comp Physiol 279: R1165–R1170, 2000. [DOI] [PubMed] [Google Scholar]
- 422.Li X, Hughes SM, Salviati G, Teresi A, Larsson L. Thyroid hormone effects on contractility and myosin composition of soleus muscle and single fibers from young and old rats. J Physiol 494: 555–567, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Li X, Moody MR, Engel D, Walker S, Clubb FJ Jr, Sivasubramanian N, Mann DL, Reid MB. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation 102: 1690–1696, 2000. [DOI] [PubMed] [Google Scholar]
- 424.Liang X, Lin T, Sun G, Beasley-Topliffe L, Cavaillon JM, Warren HS. Hemopexin down-regulates LPS-induced proinflammatory cytokines from macrophages. J Leukoc Biol 86: 229–235, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Liaw KY. Effect of injury, sepsis and parenteral nutrition on high-energy phosphates in human liver and muscle. J Parenter Enteral Nutr 9: 28–33, 1985. [DOI] [PubMed] [Google Scholar]
- 426.Liaw KY, Wei TC, Hsu SC, Lin JK. Effects of severe injury and critical illness on high-energy phosphates in human liver and muscle. J Trauma 25: 628–633, 1985. [DOI] [PubMed] [Google Scholar]
- 427.Lin MC, Ebihara S, El Dwairi Q, Hussain SNA, Yang L, Gottfried SB, Comtois A, Petrof BJ. Diaphragm sarcolemmal injury is induced by sepsis and alleviated by nitric oxide synthase inhibition. Am J Respir Crit Care Med 158: 1656–1663, 1998. [DOI] [PubMed] [Google Scholar]
- 428.Lin SY, Chen WY, Lee FY, Huang CJ, Sheu WH. Activation of ubiquitin-proteasome pathway is involved in skeletal muscle wasting in a rat model with biliary cirrhosis: potential role of TNF-alpha. Am J Physiol Endocrinol Metab 288: E493–E501, 2005. [DOI] [PubMed] [Google Scholar]
- 429.Lipshutz AK, Gropper MA. Acquired neuromuscular weakness and early mobilization in the intensive care unit. Anesthesiology 118: 202–215, 2013. [DOI] [PubMed] [Google Scholar]
- 430.Liu MS, Wu LL. Reduction in the Ca2+-induced Ca2+ release from canine cardiac sarcoplasmic reticulum following endotoxin administration. Biochem Biophys Res Commun 174: 1248–1254, 1991. [DOI] [PubMed] [Google Scholar]
- 431.Liu S, Chen JF. Strategies for therapeutic hypometabothermia. J Exp Stroke Transl Med 5: 31–42, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Liu SH, Lai JL, Lin RH, Lin MJ, Lin-Shiau SY. Involvement of nitric oxide in the in vivo effects of lipopolysaccharides oon the contractile and electrical properties of mouse diaphragm. Naunyn-Schmiedebergs Arch Pharmacol 356: 500–504, 1997. [DOI] [PubMed] [Google Scholar]
- 433.Liu SH, Lai JL, Yang RS, Lin-Shiau SY. Nitric oxide is not involved in the endotoxemia-induced alterations in Ca2+ and ryanodine responses in mouse diaphragms. Naunyn-Schmiedeberg Arch Pharmacol 366: 327–334, 2002. [DOI] [PubMed] [Google Scholar]
- 434.Llano-Diez M, Gustafson AM, Olsson C, Goransson H, Larsson L. Muscle wasting and the temporal gene expression pattern in a novel rat intensive care unit model. BMC Genomics 12: 602, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Llano-Diez M, Renaud G, anderson M, Marrero HG, Cacciani N, Enqquist H, Corpeno R, Artemenko K, Berqquist J, Larsson L. Mechanisms underlying ICU muscle wasting and effects of passive mechanical loading. Crit Care 16: R209, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Llesuy S, Evelson P, Gonzalez-Flecha B, Peralta J, Carreras MC, Poderoso JJ, Boveris A. Oxidative stress in muscle and liver of rats with septic syndrome. Free Rad Biol Med 16: 445–451, 1994. [DOI] [PubMed] [Google Scholar]
- 437.Llovera M, Carbo N, Lopez-Soriano J, Garcia-Martinez C, Busquets S, Alvarez B, Agell N, Costelli P, Lopez-Soriano FJ, Celada A, Argiles JM. Different cytokines modulate ubiquitin gene expression in rat skeletal muscle. Cancer Lett 133: 83–87, 1998. [DOI] [PubMed] [Google Scholar]
- 438.Llovera M, Garcia-Martinez C, Lopez-Soriano J, Agell M, Lopez-Soriano FJ, Garcia I, Argiles JM. Protein turnover in skeletal muscle of tumour-bearing mice overexpressing the soluble TNF receptor-1. Cancer Lett 130: 19–27, 1998. [DOI] [PubMed] [Google Scholar]
- 439.Llovera M, Garcia-Martinez C, Lopez-Soriano J, Carbo N, Agell M, Lopez-Soriano FJ, Argiles JM. Role of TNF receptor 1 in protein turnover during cancer cachexia using gene knockout mice. Mol Cell Endocrionl 142: 183–189, 1998. [DOI] [PubMed] [Google Scholar]
- 440.Lomo T, Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol 221: 493–513, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Lomo T, Westgaard RH. Further studies on the control of ACh sensitivity by muscle activity in the rat. J Physiol 252: 603–626, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Lopez LC, Escames G, Ortiz F, Ros E, Acuna-Castroviejo D. Melatonin restores the mitochondrial production of ATP in septic mice. Neuro Endocrinol Lett 27: 623–630, 2006. [PubMed] [Google Scholar]
- 443.Lopez LC, Escames G, Tapias V, Utrilla P, Leon J, Acuna-Castroviejo D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: its relation with mitochondrial dysfunction and prevention by melatonin. Int J Biochem Cell Biol 38: 267–278, 2006. [DOI] [PubMed] [Google Scholar]
- 444.Loven L, Larsson J, Lennquist S, Liljedahl SO. Hypophosphatemia and muscle phosphate metabolism in severely injured patients. Acta Chir Scand 149: 743–749, 1983. [PubMed] [Google Scholar]
- 445.Luo G, Hershko DD, Robb BW, Wray CJ, Hasselgren PO. IL-1beta stimulates IL-6 production in cultured skeletal muscle cells through activation of MAP kinase signaling pathway and NF-kappaB. Am J Physiol Regul Integr Comp Physiol 284: R1249–R1254, 2003. [DOI] [PubMed] [Google Scholar]
- 446.Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and ORAI1. J Physiol 586: 4815–4824, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Lynch GS, Fary CJ, Williams DA. Quantitative measurement of resting skeletal muscle [Ca2+]i following acute and long-term downhill running exercise in mice. Cell Calcium 22: 373–383, 1997. [DOI] [PubMed] [Google Scholar]
- 448.MacFarlane IA, Rosenthal FD. Severe myopathy after status asthmaticus. Lancet 2: 615, 1977. [DOI] [PubMed] [Google Scholar]
- 449.Mador MJ, Deniz O, Aggarwal A, Kufel TJ. Quadriceps fatigability after single muscle exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 168: 102–108, 2003. [DOI] [PubMed] [Google Scholar]
- 450.Maher J, Rutledge F, Remtulla H, Parkes A, Bernardi L, Bolton CF. Neuromuscular disorders associated with failure to wean from the ventilator. Intensive Care Med 21: 737–743, 1995. [DOI] [PubMed] [Google Scholar]
- 451.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007. [DOI] [PubMed] [Google Scholar]
- 452.Manzanares W, Dhaliwal R, Jiang X, Murch L, Heyland DK. Antioxidant micronutrients in the critically ill: a systematic review and meta-analysis. Crit Care 16: R66, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Maramattom B, Wijdicks EFM, Sundt TM, Cassivi SD. Flaccid quadriplegia due to necrotizing myopathy following lung transplantation. Transplant Proc 36: 2830–2833, 2004. [DOI] [PubMed] [Google Scholar]
- 454.Marathe PH, Dwersteg JF, Pavlin EG, Haschke RH, Heimbach DM, Slattery JT. Effect of thermal injury on the pharmacokinetics and pharmacodynamics of atracurium in humans. Anesthesiology 70: 752–755, 1989. [DOI] [PubMed] [Google Scholar]
- 455.Marathe PH, Haschke RH, Slattery JT, Zucker JR, Pavlin EG. Acetylcholine receptor density and acetylcholine esterase activity in skeletal muscle of rats following thermal injury. Anesthesiology 70: 654–659, 1989. [DOI] [PubMed] [Google Scholar]
- 456.Martensson J, Goodwin CW, Blake R. Mitochondrial glutathione in hypermetabolic rats following burn injury and thyroid hormone administration: evidence of a selective effect on brain glutathione by burn injury. Metabolism 41: 273–277, 1992. [DOI] [PubMed] [Google Scholar]
- 457.Martin AF. Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin-I. J Biol Chem 256: 964–968, 1981. [PubMed] [Google Scholar]
- 458.Martin CM, Hill AD, Burns K, Chen LM. Characteristics and outcomes for critically ill patients with prolonged intensive care unit stays. Crit Care Med 33: 1922–1927, 2005. [DOI] [PubMed] [Google Scholar]
- 459.Martin UJ, Hincapie L, Nimchuk M, Gaughan J, Criner GJ. Impact of whole-body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med 33: 2259–2265, 2005. [DOI] [PubMed] [Google Scholar]
- 460.Martyn JA, Fagerlund MJ, Eriksson LI. Basic principles of neuromuscular transmission. Anaesthesia 64 Suppl 1: 1–9, 2009. [DOI] [PubMed] [Google Scholar]
- 461.Martyn JA, Richtsfeld M. Succinylcholine-induced hyperkalemia in acquired pathologic states. Anesthesiology 104: 158–169, 2006. [DOI] [PubMed] [Google Scholar]
- 462.Martyn JAJ, Szfelbein SK, Ali HH, Matteo RS, Savarese JJ. Increased d-tubocurarine requirements following major thermal injury. Anesthesiology 52: 352–355, 1980. [PubMed] [Google Scholar]
- 463.Martyn JAJ, Tomera JF. Acetylcholine receptor density and acetylcholinesterase enzyme activity in skeletal muscle of rats following thermal injury. Anesthesiology 71: 625–627, 1989. [DOI] [PubMed] [Google Scholar]
- 464.Martyn JA, White DA, Gronert GA, Jaffe RS, Ward JM. Up-and-down regulation of skeletal muscle acetylcholine receptors. Effects on neuromuscular blockers. Anesthesiology 76: 822–843, 1992. [DOI] [PubMed] [Google Scholar]
- 465.Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M. Autophagy is required to maintain muscle mass. Cell Metab 10: 507–515, 2009. [DOI] [PubMed] [Google Scholar]
- 466.Massa R, Carpenter S, Holland P, Karpati G. Loss and renewal of thick myofilaments in glucocorticoid-treated rat soleus after denervation and reinnervation. Muscle Nerve 15: 1290–1298, 1992. [DOI] [PubMed] [Google Scholar]
- 467.Matsumoto A, Fujita N, Arakawa T, Fujino H, Miki A. Influence of electrical stimulation on calpain and ubiquitin-proteasome systems in the denervated and unloaded rat tibialis anterior muscles. Acta Histochem 116: 936–942, 2014. [DOI] [PubMed] [Google Scholar]
- 468.McArdle JJ. Molecular aspects of the trophic influence of nerve on muscle. Prog Neurobiol 21: 135–198, 1983. [DOI] [PubMed] [Google Scholar]
- 469.McCarter FD, Nierman SR, James JH, Wang L, Friedn LA, Fischer JE. Role of skeletal muscle Na+K+ ATPase activity in increased lactate production in sub-acute sepsis. Life Sci 70: 1875–1888, 2002. [DOI] [PubMed] [Google Scholar]
- 470.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin 17: 107–124, 2001. [DOI] [PubMed] [Google Scholar]
- 471.McNicol FJ, Hoyland JA, Cooper RG, Carslon GL. Skeletal muscle contractile properties and proinflammatory cytokine gene expression in human endotoxaemia. Br J Surg 97: 434–442, 2010. [DOI] [PubMed] [Google Scholar]
- 472.Mealy K, van Lanschott JJ, Robinson BG, Rounds J, Wilmore DW. Are the catabolic effects of tumor necrosis factor mediated by glucocorticoids? Arch Surg 125: 42–48, 1990. [DOI] [PubMed] [Google Scholar]
- 473.Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, Tolley EA. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA 280: 159–165, 1998. [DOI] [PubMed] [Google Scholar]
- 474.Meesen RL, Dendale P, Cuypers K, Berger J, Hermans A, Thijs H, Levin O. Neuromuscular electrical stimulation as a possible means to prevent muscle tissue wasting in artificially ventilated and sedated patients in the intensive care unit: a pilot study. Neuromodulation 13: 315–320, 2010. [DOI] [PubMed] [Google Scholar]
- 475.Mela-Riker L, Bartos D, Vlessis AA, Widener L, Muller P, Trunkey DD. Chronic hyperdynamic sepsis in the rat. Characterization of liver and energy metabolism. Circ Shock 36: 83–92, 1992. [PubMed] [Google Scholar]
- 476.Melzer W, Dietze B. Malignant hyperthermia and excitation-contraction coupling. Acta Physiol Scand 171: 367–378, 2001. [DOI] [PubMed] [Google Scholar]
- 477.Melzer W, Herrmann-Frank A, Lüttgau Ch. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibers. Biochim Biophys Acta 1241: 59–116, 1995. [DOI] [PubMed] [Google Scholar]
- 478.Melzer W, Rios E, Schneider MF. A general procedure for determining the rate of calcium release from the sarcoplasmic reticulum in skeletal muscle fibers. Biophys J 51: 849–863, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Menconi MJ, Arany ZP, Alamdari N, Aversa Z, Gonnella P, O'Neal P, Smith IJ, Tizio S, Hasselgren PO. Sepsis and glucocorticoids downregulate the expression of the nuclear cofactor PGC-1beta in skeletal muscle. Am J Physiol Endocrinol Metab 299: E533–E543, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Mera S, Tatulescu D, Cismaru C, Bondor C, Slavcvici A, Zanc V, Carstina D, Oltean M. Multiplex cytokine profiling in patients with sepsis. APMIS 119: 155–163, 2011. [DOI] [PubMed] [Google Scholar]
- 481.Merx MW, Weber C. Sepsis and the heart. Circulation 116: 793–802, 2007. [DOI] [PubMed] [Google Scholar]
- 482.Mesotten D, Gielen M, Sterken C, Claessens K, Hermans G, Vlasselaers D, Lemiere J, Lagae L, Gewillig M, Eyskens B, Vanhorebeek I, Wouters PJ, Van den Berghe G. Neurocognitive development of children 4 years after critical illness and treatment with tight glucose control: a randomized controlled trial. JAMA 308: 1641–1650, 2012. [DOI] [PubMed] [Google Scholar]
- 483.Mesotten D, Swinnen JV, Vanderhoydonc F, Wouters PJ, Van den Berghe G. Contribution of circulating lipids to the improved outcome of critical illness by glycemic control with intensive insulin therapy. J Clin Endocrinol Metab 89: 219–226, 2004. [DOI] [PubMed] [Google Scholar]
- 484.Mester M, Carter EA, Tompkins RG, Gelfand JA, Dinarello CA, Burke JF, Clark BD. Thermal injury induces very early production of interleukin-1 alpha in the rat by mechanisms other than endotoxemia. Surgery 115: 588–596, 1994. [PubMed] [Google Scholar]
- 485.Michie HR, Sherman ML, Spriggs DR, Rounds J, Christie M, Wilmore DW. Chronic TNF infusion causes anorexia but not accelerated nitrogen loss. Ann Surg 209: 19–24, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Minnaard R, Wagenmakers AJ, Combaret L, Attaix D, Drost MR, van Kranenburg GP, Schaart G, Hesselink MK. Ubiquitin-proteasome-dependent proteolytic activity remains elevated after zymosan-induced sepsis in rats while muscle mass recovers. Int J Biochem Cell Biol 37: 2217–2225, 2005. [DOI] [PubMed] [Google Scholar]
- 487.Mirzakhani H, Williams JN, Mello J, Joseph S, Meyer MJ, Waak K, Schmidt U, Kelly E, Eikermann M. Muscle weakness predicts pharyngeal dysfunction and symptomatic aspiration in long-term ventilated patients. Anesthesiology 119: 389–397, 2013. [DOI] [PubMed] [Google Scholar]
- 488.Miyakawa H, Oishi K, Hagiwara S, Kira S, Kitano T, Iwasaka H, Noguchi T. Olprinone improves diaphragmatic contractility and fatigability during abdominal sepsis in a rat model. Acta Anaesthesiol Scand 48: 637–641, 2004. [DOI] [PubMed] [Google Scholar]
- 489.Mizobata Y, Prechek D, Rounds JD, Robinson V, Wilmore DW, Jacobs DO. The duration of infection modifies mitochondrial oxidative capacity in rat skeletal muscle. J Surg Res 59: 165–173, 1995. [DOI] [PubMed] [Google Scholar]
- 490.Mofarrahi M, Sigala I, Guo Y, Godin R, Davis EC, Petrof B, Sandri M, Buruelle Y, Hussain SN. Autophagy and skeletal muscles in sepsis. PLoS One 7: e47265, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Moldawer LL, Swaninger G, Gelin J, Lundholm K. Interleukin 1 (alpha or beta) and tumor necrosis factor-alpha do not regulate protein balance in skeletal muscle. Am J Physiol Cell Physiol 253: C766–C773, 1987. [DOI] [PubMed] [Google Scholar]
- 492.Montpetit ML, Stocker PJ, Schwetz TA, Harper JM, Norring SA, Schaffer L, North SJ, Jang-Lee J, Gilmartin T, Head SR, Haslam SM, Dell A, Marth JD, Bennett ES. Regulated and aberrant glycosylation modulate cardiac electrical signaling. Proc Natl Acad Sci USA 106: 16517–16522, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Moriggi M, Cassano P, Vasso M, Capitanio D, Fania C, Musicco C, Pesce V, Gadaleta MN, Gelfi C. A DIGE approach for the assessment of rat soleus muscle changes during unloading: effect of acetyl-l-carnitine aupplementation. Proteomics 8: 3588–3604, 2008. [DOI] [PubMed] [Google Scholar]
- 494.Morris CE, Boucher PA, Joos B. Left-shifted nav channels in injured bilayer: primary targets for neuroprotective nav antagonists? Front Pharmacol 3: 19, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, Ross A, anderson L, Baker S, Sanchez M, Penley L, Howard A, Dixon L, Leach S, Small R, Hite RD, Haponik E. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med 36: 2238–2243, 2008. [DOI] [PubMed] [Google Scholar]
- 496.Mozaffar T, Haddad F, Zeng M, Zhang LY, Adams GR, Baldwin KM. Molecular and cellular defects of skeletal muscle in an animal model of acute quadriplegic myopathy. Muscle Nerve 35: 55–65, 2007. [DOI] [PubMed] [Google Scholar]
- 497.Munoz-Canoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J 280: 4131–4148, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Murton AJ, Constantin D, Greenhaff PL. The involvement of the ubiquitin proteasome system in human skeletal muscle remodeling and atrophy. Biochim Biophys Acta 1782: 730–743, 2008. [DOI] [PubMed] [Google Scholar]
- 499.Nanas S, Kritikos K, Angelopoulos E, Siafaka A, Tsikriki S, Poriazi M, Kanaloupiti D, Kontogeorgi M, Pratikaki M, Zervakis D, Routsi C, Roussos C. Predisposing factors for critical illness polyneuromyopathy in a multidisciplinary intensive care unit. Acta Neurol Scand 118: 175–181, 2008. [DOI] [PubMed] [Google Scholar]
- 500.Nardelli P, Khan J, Powers R, Cope TC, Rich MM. Reduced motor neuron excitability in a rat model of sepsis. J Neurophysiol 109: 1775–1781, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Narimatsu E, Nakayama Y, Sumita S, Iwasaki H, Fujimura N, Satoh K, Namiki A. Sepsis attenuates the intensity of the neuromuscular blocking effect of d-tubocurarine and the antagonistic actions of neostigmine and edrophonium accompanying depression of muscle contractility of the diaphragm. Acta Anaesthesiol Scand 43: 196–201, 1999. [DOI] [PubMed] [Google Scholar]
- 502.Narimatsu E, Niiya T, Kawamata M, Namiki A. Sepsis stage dependently and differentially attenuates the effects of nondepolarizing neuromuscular blockers on the rat diaphragm in vitro. Anesth Analg 100: 823–829, 2005. [DOI] [PubMed] [Google Scholar]
- 503.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials, Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N, Rock P. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 307: 795–803, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Needham DM, Dinglas VD, Bienvenu OJ, Colantuoni E, Wozniak AW, Rice TW, Hopkins RO. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ 346: f1532, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, Palmer JB, Brower RG, Fan E. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil 91: 536–542, 2010. [DOI] [PubMed] [Google Scholar]
- 506.Nethery D, Callahan LA, Stofan D, Mattera R, Di Marco A, Supinski G. PLA2 dependence of diaphragm mitochondrial formation of reactive oxygen species. J Appl Physiol 89: 72–80, 2000. [DOI] [PubMed] [Google Scholar]
- 507.Newham P, Ross D, Ceuppens P, Das S, Yates JW, Betts C, Reens J, Randall KJ, Knight R, McKay JS. Determination of the safety and efficacy of therapeutic neutralization of tumor necrosis factor-α (TNF-α) using AZD9773, an anti-TNF-α immune Fab, in murine CLP sepsis. Inflamm Res 63: 149–160, 2014. [DOI] [PubMed] [Google Scholar]
- 508.Niiya T, Narimatsu E, Maiki A. Acute late sepsis attenuates effects of a nondepolarizing neuromuscular blocker, rocuronium, by facilitation of endplate potential and enhancement of membrane excitability in vitro. Anesthesiology 105: 968–975, 2006. [DOI] [PubMed] [Google Scholar]
- 510.Nordquist J, Hoglund AS, Norman H, Tang X, Dworkin B, Larsson L. Transcription factors in muscle atrophy caused by blocked neuromuscular transmission and muscle unloading in rats. Mol Med 13: 461–470, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Norman H, Kandala K, Kolluri R, Zackrisson H, Nordquist J, Walther S, Eriksson LI, Larsson L. A porcine model of acute quadriplegic myopathy: a feasibility study. Acta Anaesthesiol Scand 50: 1058–1067, 2006. [DOI] [PubMed] [Google Scholar]
- 512.Norman H, Nordquist J, andersson P, Ansved T, Tang X, Dworkin B, Larsson L. Impact of post-synaptic block of neuromuscular transmission, muscle unloading and mechanical ventilation on skeletal muscle protein and mRNA expression. Pflügers Arch 453: 53–66, 2006. [DOI] [PubMed] [Google Scholar]
- 513.Norman H, Zackrisson H, Hedstrom Y, andersson P, Nordquist J, Eriksson LI, Libelius R, Larsson L. Myofibrillar protein and gene expression in acute quadriplegic myopathy. J Neurol Sci 285: 28–38, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med 30: 2022–2029, 2002. [DOI] [PubMed] [Google Scholar]
- 515.Novak KR, Nardelli P, Cope TC, Filatov G, Glass JD, Khan J, Rich MM. Inactivation of sodium channels underlies reversible neuropathy during critical illness in rats. J Clin Invest 119: 1150–1158, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Nyberg P, Wikman AL, Nennesmo I, Lundberg I. Increased expression of interleukin 1alpha and MHC class I in muscle tissue of patients with chronic, inactive polymyositis and dermatomyositis. J Rheumatol 27: 940–948, 2000. [PubMed] [Google Scholar]
- 516a.Nyitrai M, Rossi R, Adamek N, Pellegrino MA, Bottinelli R, Geeves MA. What limits the velocity of fast-skeletal muscle contraction in mammals? J Mol Biol 355: 432–442, 2006. [DOI] [PubMed] [Google Scholar]
- 517.Ochala J, Ahlbeck K, Radell PJ, Eriksson LI, Larsson L. Factors underlying the early limb muscle weakness in acute quadriplegic myopathy using an experimental ICU porcine model. PLoS One 6: e20876, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Ochala J, Gustafson AM, Diez ML, Renaud G, Li M, Aare S, Qaisar R, Banduseela VC, Hedstrom Y, Tang X, Dworkin B, Ford GC, Nair KS, Perera S, Gautel M, Larsson L. Preferential skeletal muscle myosin loss in response to mechanical silencing in a novel rat intensive care unit model: underlying mechanisms. J Physiol 589: 2007–2026, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Ochala J, Larsson L. Acquired and hereditary sarcomeric protein diseases. In: Muscle: Fundamental Biology and Mechanisms and Disease, edited by Joseph JTS, Hill A, Sweeney HL, Olson EN. London: Academic, 2012, p. 1023–1030. [Google Scholar]
- 520.Ochala J, Renaud G, Llano Diez M, Banduseela VC, Aare S, Ahlbeck K, Radell PJ, Eriksson LI, Larsson L. Diaphragm muscle weakness in an experimental porcine intensive care unit model. PLoS One 6: e20558, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Oettgen HF, Carswell EA, Kassel RL, Fiore N, Williamson B, Hoffmann MK, Haranaka K, Old LJ. Endotoxin-induced tumor necrosis factor. Recent results. Cancer Res 75: 207–212, 1980. [DOI] [PubMed] [Google Scholar]
- 522.Offner PJ, Ogura H, Jordan BS, Pruitt BA Jr, Cioffi WG. Effects of inhaled nitric oxide on right ventricular function in endotoxin shock. J Trauma 39: 179–186, 1995. [DOI] [PubMed] [Google Scholar]
- 523.Ogilvie H, Larsson L. The effect of nutritional status in the pathogenesis of critical illness myopathy (CIM). Biology 3: 368–382, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Ojuka EO, Jones TE, Han DH, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Intermittent increases in cytosolic Ca2+ stimulate mitochondrial biogenesis in muscle cells. Am J Physiol Endocrinol Metab 283: E1040–E1045, 2002. [DOI] [PubMed] [Google Scholar]
- 525.O'Leary MFN, Hood DA. Effect of prior chronic contractile activity on mitochondrial function and apoptotic protein expression in denervated muscle. J Appl Physiol 105: 114–120, 2008. [DOI] [PubMed] [Google Scholar]
- 526.O'Leary MFN, Hood DA. Denervation-induced oxidative stress and autophagy signaling in muscle. Autophagy 5: 230–231, 2009. [DOI] [PubMed] [Google Scholar]
- 527.Opata MM, Ye Z, Hollifield M, Garvy BA. B cell production of tumor necrosis factor in response to Pneumocystis murina infection in mice. Infect Immun 81: 4252–4260, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Op de Coul AA, Verheul GA, Leyten AC, Schellens RL, Teepen JL. Critical illness polyneuromyopathy after artificial respiration. Clin Neurol Neurosurg 96: 261–263, 1991. [DOI] [PubMed] [Google Scholar]
- 529.Op de Coul AA, Lambregts PC, Koeman J, van Puyenbroek MJ, Ter Laak HJ, Gabreels-Festen AA. Neuromuscular complications in patients given Pavulon (pancuronium bromide) during artificial ventilation. Clin Neurol Neurosurg 87: 17–22, 1985. [DOI] [PubMed] [Google Scholar]
- 530.Orellana RA, Suryawan A, Wilson FA, Gazzaneo MC, Fiorotto ML, Nguyen HV, Davis TA. Development aggravates the severity of skeletal muscle catabolism induced by endotoxemia in neonatal pigs. Am J Physiol Regul Integr Comp Physiol 302: R682–R690, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Osler W. The Principles and Practice of Medicine. New York: Appleton, 1892. [Google Scholar]
- 532.Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma interleukin-6 in humans: effect of intensity of exercise. Eur J Appl Physiol 83: 512–515, 2000. [DOI] [PubMed] [Google Scholar]
- 533.Osuchowski MF, Welch K, Siddiqui J, Remich DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol 177: 1967–1974, 2006. [DOI] [PubMed] [Google Scholar]
- 534.Padfield KE, Astrakas LG, Zhang Q, Gopalan S, Dai G, Mindrinos MN, Tompkins RG, Rhame LG, Tzika AA. Burn injury causes mitochondrial dysfunction in skeletal muscle. Proc Natl Acad Sci USA 102: 5368–5373, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Pandit L, Agrawal A. Neuromuscular disorders in critical illness. Clin Neurol Neurosurg 108: 433–439, 2006. [DOI] [PubMed] [Google Scholar]
- 536.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guerin C, Prat G, Morange S, Roch A. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 363: 1107–1116, 2010. [DOI] [PubMed] [Google Scholar]
- 537.Parker CJ, Sardesai VM. The effect of E. coli endotoxin on cardiac and skeletal muscle myofibrillar ATPase. Biochem Med 8: 11–17, 1973. [DOI] [PubMed] [Google Scholar]
- 538.Partridge BL, Abrams JH, Bazemore C, Rubin R. Prolonged neuromuscular blockade after long-term infusion of vecuronium bromide in the intensive care unit. Crit Care Med 18: 1177–1179, 1990. [DOI] [PubMed] [Google Scholar]
- 539.Paterniti I, Impellizzeri D, Di Paola R, Esposito E, Gladman S, Yip P, Priestly JV, Michael-Titus AT, Cuzzocrea S. Docosahexaenoic acid attenuates the early inflammatory response following spinal cord injury in mice: in-vivo and in-vitro studies. J Neuroinflammation 11: 6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Pauly M, Daussin F, Burelle Y, Li T, Godin R, Fauconnier J, Koechlin-Ramonatxo C, Hugon G, Lacampagne A, Coisy-Quivy M, Liang F, Hussain S, Matecki S, Petrof BJ. AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am J Pathol 181: 583–592, 2012. [DOI] [PubMed] [Google Scholar]
- 541.Pedersen TH, de Paoli F, Nielsen OB. Increased excitability of acidified skeletal muscle: role of chloride conductance. J Gen Physiol 125: 237–246, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Pereira RM, Freire de CJ. Glucocorticoid-induced myopathy. Joint Bone Spine 78: 41–44, 2011. [DOI] [PubMed] [Google Scholar]
- 543.Peruchi BB, Petronilho F, Rojas HA, Constantino L, Mina F, Vuolo F, Cardoso MR, Goncalves CL, Rezin GT, Streck EL, Dal-Pizzol F. Skeletal muscle electron transport chain dysfunction after sepsis in rats. J Surg Res 167: e333–e338, 2011. [DOI] [PubMed] [Google Scholar]
- 544.Person RJ. Endotoxin alters spontaneous transmitter release at the frog neuromuscular junction. J Neurosci Res 3: 63–72, 1977. [DOI] [PubMed] [Google Scholar]
- 545.Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol 98: 1154–1162, 2005. [DOI] [PubMed] [Google Scholar]
- 546.Peterson JM, Bakkar N, Guttridge DC. NF-κB signaling in skeletal muscle health and disease. Curr Top Dev Biol 96: 85–119, 2011. [DOI] [PubMed] [Google Scholar]
- 547.Pinet C, Le Grand B, John GW, Coulombe A. Thrombin facilitation of voltage-gated sodium channel activation in human cardiomyocytes: implications for ischemic sodium loading. Circulation 106: 2098–2103, 2002. [DOI] [PubMed] [Google Scholar]
- 548.Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Duport E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest 103: 565–575, 1993. [DOI] [PubMed] [Google Scholar]
- 549.Piper RD, Pitt-Hyde M, Li F, Sibbald WJ, Potter RF. Microcirculatory changes in rat skeletal muscle in sepsis. Am J Respir Crit Care Med 154: 931–937, 1996. [DOI] [PubMed] [Google Scholar]
- 550.Plomguaard P, Penkowa M, Pedersen BK. Fiber type specific expression of TNF-alpha, IL-6 and IL-18 in human skeletal muscle. Exerc Immunol Rev 11: 53–63, 2005. [PubMed] [Google Scholar]
- 551.Pohlman MC, Schweickert WD, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister K, Hall JB, Kress JP. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med 38: 2089–2094, 2010. [DOI] [PubMed] [Google Scholar]
- 552.Porta F, Takala J, Weikert C, Bracht H, Kolarova A, Lauterburg BH, Borotto E, Jakob SM. Effects of prolonged endotoxemia on liver, skeletal muscle and kidney mitochondrial function. Crit Care 10: R118, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Porter C, Herndon DN, Sidossis LS, Borsheim E. The impact of severe burns on skeletal muscle mitochondrial function. Burns 39: 1039–1047, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Posterino GS, Lamb GD. Effect of sarcoplasmic reticulum Ca2+ content on action potential-induced Ca2+ release in rat skeletal muscle fibers. J Physiol 551.1: 219–237, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Poulsen JB, Moller K, Jensen CV, Weisdorf S, Kehlet H, Perner A. Effect of transcutaneous electrical muscle stimulation on muscle volume in patients with septic shock. Crit Care Med 39: 456–461, 2011. [DOI] [PubMed] [Google Scholar]
- 556.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med 39: 1749–1759, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Preiser JC, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chiolero R. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 35: 1738–1748, 2009. [DOI] [PubMed] [Google Scholar]
- 558.Prosser BL, Hernandez-Ochoa EO, Schneider MF. S100A1 and calmodulin regulation of ryanodine receptor in striated muscle. Cell Calcium 50: 323–331, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Puchner EM, Alexandrovich A, Kho AL, Hensen U, Schafer LV, Brandmeier B, Grater F, Grubmuller H, Gaub HE, Gautel M. Mechanoenzymatics of titin kinase. Proc Natl Acad Sci USA 105: 13385–13390, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Puthucheary Z, Harridge S, Hart N. Skeletal muscle dysfunction in critical care: wasting, weakness, and rehabilitation strategies. Crit Care Med 38: S676–S682, 2010. [DOI] [PubMed] [Google Scholar]
- 561.Puthucheary Z, Montgomery H, Moxham J, Harridge S, Hart N. Structure to function: muscle failure in critically ill patients. J Physiol 588: 4641–4648, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Puthucheary Z, Rawal J, Ratnayake G, Harridge S, Montgomery H, Hart N. Neuromuscular blockade and skeletal muscle weakness in critically ill patients: time to rethink the evidence? Am J Respir Crit Care Med 185: 911–917, 2012. [DOI] [PubMed] [Google Scholar]
- 563.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, Velloso C, Seymour J, Agley CC, Selba A, Limb M, Edwards LM, Smith K, Rowlerson A, Rennie MJ, Moxham J, Harridge SD, Hart N, Montgomery HE. Acute muscle wasting in critical illness. JAMA 310: 1591–1600, 2013. [DOI] [PubMed] [Google Scholar]
- 564.Pytel P, Alexander JJ. Pathogenesis of septic encephalopathy. Curr Opin Neurol 22: 283–287, 2009. [DOI] [PubMed] [Google Scholar]
- 565.Quy PN, Kuma A, Piere P, Mizushima N. Proteasome-dependent activation of mammalian target of rapamycin complex 1 (mTOR1) is essential for autophagy suppression and muscle remodeling following denervation. J Biol Chem 288: 1125–1134, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Radell PJ, Remahl S, Nichols DG, Eriksson LI. Effects of prolonged mechanical ventilation and inactivity on piglet diaphragm function. Intensive Care Med 28: 358–364, 2002. [DOI] [PubMed] [Google Scholar]
- 567.Rannou F, Pennec JP, Rossignol B, Morel J, Dorange G, Arvieux C, Gioux M, Giroux-Metges MA. Effects of chronic sepsis on rat motor units: experimental study of critical illness polyneuromyopathy. Exp Neurol 204: 741–747, 2007. [DOI] [PubMed] [Google Scholar]
- 568.Rawat R, Cohen TV, Ampong B, Francia D, Henriques-Pons A, Hoffman EP, Nagaraju K. Inflammasome up-regulation and activation in dysferlin-deficient skeletal muscle. Am J Pathol 176: 2891–2900, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Reed SA, Sandesara PB, Senf SM, Judge AR. Inhibition of FoxO transcriptional activity prevents muscle fiber atrophy during cachexia and induces hypertrophy. FASEB J 26: 987–1000, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Reid MB, Khawli FA, Moody MR. Reactive oxygen species in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol 75: 1081–1087, 1992. [DOI] [PubMed] [Google Scholar]
- 571.Reid MB, Lännergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-α. Involvement of muscle myofilaments. Am J Respir Crit Care Med 166: 479–484, 2002. [DOI] [PubMed] [Google Scholar]
- 572.Reid MB, Stokic DS, Koch SM, Khawli FA, Leis AA. N-acetyl-cysteine inhibits muscle fatigue in humans. J Clin Invest 94: 2468–2474, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Remels AH, Gosker HR, Bakker J, Guttridge DC, Schols AM, Langen RC. Regulation of skeletal muscle oxidative phenotype by classical NF-κB signalling. Biochem Biophys Acta 1832: 1313–1325, 2013. [DOI] [PubMed] [Google Scholar]
- 574.Renaud G, Llano-Diez M, Ravara B, Gorza L, Feng HZ, Jin JP, Cacciani N, Gustafson AM, Ochala J, Corpeno R, Li M, Hedstrom Y, Ford GC, Nair KS, Larsson L. Sparing of muscle mass and function by passive loading in an experimental intensive care unit model. J Physiol 591: 1385–1402, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Rennie MJ. Anabolic resistance in critically ill patients. Crit Care Med 37: S398–S399, 2009. [DOI] [PubMed] [Google Scholar]
- 576.Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol 66: 799–828, 2004. [DOI] [PubMed] [Google Scholar]
- 577.Rich MM, Bird SJ, Raps EC, McCluskey LF, Teener JW. Direct muscle stimulation in acute quadriplegic myopathy. Muscle Nerve 20: 665–673, 1997. [DOI] [PubMed] [Google Scholar]
- 578.Rich MM, Kraner SD, Barchi RL. Altered gene expression in steroid-treated denervated muscle. Neurobiol Dis 6: 515–522, 1999. [DOI] [PubMed] [Google Scholar]
- 579.Rich MM, McGarvey ML, Teener JW, Frame LH. ECG changes during septic shock. Cardiology 97: 187–196, 2002. [DOI] [PubMed] [Google Scholar]
- 580.Rich MM, Pinter MJ. Sodium channel inactivation in an animal model of acute quadriplegic myopathy. Ann Neurol 50: 26–33, 2001. [DOI] [PubMed] [Google Scholar]
- 581.Rich MM, Pinter MJ. Crucial role of sodium channel fast inactivation in muscle fiber inexcitability in a rat model of critical illness myopathy. J Physiol 547: 555–566, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Rich MM, Pinter MJ, Kraner SD, Barchi RL. Loss of electrical excitability in an animal model of acute quadriplegic myopathy. Ann Neurol 43: 171–179, 1998. [DOI] [PubMed] [Google Scholar]
- 583.Rich MM, Teener JW, Raps EC, Schotland DL, Bird SJ. Muscle is electrically inexcitable in acute quadriplegic myopathy. Neurology 46: 731–736, 1996. [DOI] [PubMed] [Google Scholar]
- 584.Rigby SL, Hofmann PA, Zhong J, Adams HR, Rubin LJ. Endotoxemia-induced myocardial dysfunction is not associated with changes in myofilament Ca2+ responsiveness. Am J Physiol Heart Circ Physiol 274: H580–H590, 1998. [DOI] [PubMed] [Google Scholar]
- 585.Riley DA, Bain JL, Thompson JL, Fitts RH, Widrick JJ, Trappe SW, Trappe TA, Costill DL. Decreased thin filament density and length in human atrophic soleus muscle fibers after spaceflight. J Appl Physiol 88: 567–572, 2000. [DOI] [PubMed] [Google Scholar]
- 586.Rimer M. Neuregulins at the neuromuscular synapse: past, present, and future. J Neurosci Res 85: 1827–1833, 2007. [DOI] [PubMed] [Google Scholar]
- 587.Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol 81: 137–143, 2007. [DOI] [PubMed] [Google Scholar]
- 588.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nature Protocols 4: 31–36, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Rodriguez PO, Setten M, Maskin LP, Bonelli I, Vidomlansky SR, Attie S, Frosiani SL, Kozima S, Valentini R. Muscle weakness in septic patients requiring mechanical ventilation: protective effect of transcutaneous neuromuscular electrical stimulation. J Crit Care 27: 319–328, 2012. [DOI] [PubMed] [Google Scholar]
- 590.Roelofs RI, Cerra F, Bielka N, Rosenberg L, Delaney J. Prolonged respiratory insufficiency due to acute motor neuropathy: a new syndrome? Neurology 33: 240, 1983.6681662 [Google Scholar]
- 591.Rofe AM, Conyers RAJ, Bais R, Gamble JR, Vadas MA. The effects of recombinant tumor necrosis factor (cachectin) on metabolism in isolated rat adipocyte, hepatocyte, and muscle preparations. Biochem J 247: 789–792, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Rooyackers OE, Kersten AH, Wagenmakers AJ. Mitochondrial protein content and in vivo synthesis rates in skeletal muscle from critically ill rats. Clin Sci 91: 475–481, 1996. [DOI] [PubMed] [Google Scholar]
- 593.Rossie S. Regulation of voltage-sensitive sodium and calcium channels by phosphorylation. Adv Second Messenger Phosphoprotein Res 33: 23–48, 1999. [DOI] [PubMed] [Google Scholar]
- 594.Rossignol B, Gueret G, Pennec JP, Morel J, Giroux-Metges MA, Talarmin H, Arvieux CC. Effects of chronic sepsis on the voltage-gated sodium channel in isolated rat muscle fibers. Crit Care Med 35: 351–357, 2007. [DOI] [PubMed] [Google Scholar]
- 595.Rossignol B, Gueret G, Pennec JP, Morel J, Rannou F, Giroux-Metges MA, Talarmin H, Gioux M, Arvieux C. Effects of chronic sepsis on contractile properties of fast twitch muscle in an experimental model of critical illness neuromyopathy in the rat. Crit Care Med 36: 1855–1863, 2008. [DOI] [PubMed] [Google Scholar]
- 596.Rossiter A, Souney PF, McGowan S, Carvajal P. Pancuronium-induced prolonged neuromuscular blockade. Crit Care Med 19: 1583–1587, 1991. [DOI] [PubMed] [Google Scholar]
- 597.Rouleau G, Karpati G, Carpenter S, Soza M, Prescott S, Holland P. Glucocorticoid excess induces preferential depletion of myosin in denervated skeletal muscle fibers. Muscle Nerve 10: 428–438, 1987. [DOI] [PubMed] [Google Scholar]
- 598.Routsi C, Gerovasili V, Vasileiadis I, Karatzanos E, Pitsolis T, Tripodaki ES, Markaki V, Zervakis D, Nanas S. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care 14: R74, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Ruff RL. Sodium channel regulation of skeletal muscle membrane excitability. Ann NY Acad Sci 835: 64–76, 1997. [DOI] [PubMed] [Google Scholar]
- 600.Ruff RL, Secrist D. Inhibitors of prostaglandin synthesis or cathepsin B prevent muscle wasting due to sepsis in the rat. J Clin Invest 73: 1483–1486, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Ryan NT, Blackburn GL, Clowes GHA. Differential tissue sensitivity to elevated endogenous insulin levels during experimental peritonitis in rats. Metabolism 23: 1081, 1974. [DOI] [PubMed] [Google Scholar]
- 602.Safranek R, Ishibashi N, Oka Y, Ozaa H, Shirouzu K, Holecek M. Modulation of inflammatory response in sepsis by proteasome inhibition. Int J Exp Pathol 87: 369–372, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Said G, Goulon-Goeau C, Slama G, Tchobroutsky G. Severe early-onset polyneuropathy in insulin-dependent diabetes mellitus. A clinical and pathological study. N Engl J Med 326: 1257–1263, 1992. [DOI] [PubMed] [Google Scholar]
- 604.Sagalyn E, Band RA, Gaieski DF, Abella BS. Therapeutic hypothermia after cardiac arrest in clinical practice: review and compilation of recent experiences. Crit Care Med 37: S223–S226, 2009. [DOI] [PubMed] [Google Scholar]
- 605.Sair M, Etherington PJ, Curzen NP, Winlove CP, Evans TW. Tissue oxygenation and perfusion in endotoxemia. Am J Physiol Heart Circ Physiol 271: H1620–H1625, 1996. [DOI] [PubMed] [Google Scholar]
- 606.Sair M, Etherington PJ, Winlove P, Evans TW. Tissue oxygenation and perfusion in patients with systemic sepsis. Crit Care Med 29: 1343–1349, 2001. [DOI] [PubMed] [Google Scholar]
- 607.Salter RG, Knowles M, Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca2+-dependent and Ca2+-independent nitric oxide synthases. FEBS Lett 291: 145–149, 1991. [DOI] [PubMed] [Google Scholar]
- 608.Salviati L, Laverda AM, Zancan L, Fanin M, Angelini C, Meznaric-Petrusa M. Acute quadriplegic myopathy in a 17-month-old boy. J Child Neurol 15: 63–66, 2000. [DOI] [PubMed] [Google Scholar]
- 609.Sambe A, Ungureanu-Longrois D, Danialou G, Lanone S, Benessiano J, Aubier M, Boczkowski J. Role of nitric oxide on diaphragmatic contractile failure in Escherischia coli endotoxemic rats. Comp Biochem Physiol A 119: 167–175, 1998. [DOI] [PubMed] [Google Scholar]
- 610.Samuelsson J, Zackrisson H, Tokics L, Ljungman P, Lidbrink E, Pircher P, Larsson L. Acute quadriplegic myopathy following autologous peripheral blood stem cell transplantation for breast cancer. Bone Marrow Transplant 23: 835–837, 1999. [DOI] [PubMed] [Google Scholar]
- 611.Sander HW, Saadeh PB, Chandswang N, Greenbaum D, Chokroverty S. Diaphragmatic denervation in intensive care unit patients. Electromyogr Clin Neurophysiol 39: 3–5, 1999. [PubMed] [Google Scholar]
- 612.Sandri M. Involvement of autophagy in muscle atrophy. Am J Physiol Cell Physiol 298: C1291–C1299, 2010. [DOI] [PubMed] [Google Scholar]
- 613.Sayeed MM. Signaling mechanisms of altered cellular responses in trauma, burn and sepsis. Arch Surg 135: 1432–1442, 2000. [DOI] [PubMed] [Google Scholar]
- 614.Sayeed MM. Ion transport in circulatory and/or septic shock. Am J Physiol Regul Integr Comp Physiol 252: R809–R821, 1987. [DOI] [PubMed] [Google Scholar]
- 615.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88: 611–638, 2008. [DOI] [PubMed] [Google Scholar]
- 616.Schakman O, Dehoux M, Bouchuari S, Delaere S, Lause P, Decroly N, Shoelson SE, Thissen JP. Role of IGFI, the TNFα/NFkB pathway, in the induction of muscle atrogenes by acute inflammation. Am J Physiol Endocrinol Metab 303: E729–E739, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Schakman O, Gilson H, Kalista S, Thissen JP. Mechanisms of muscle atrophy induced by glucocorticoids. Horm Res 72 Suppl 1: 36–41, 2009. [DOI] [PubMed] [Google Scholar]
- 618.Schefold JC, Bierbrauer J, Weber-Carstens S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle 1: 147–157, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Schubert W, Kontozis L, Sticker G, Schwan H, Haraldsen G, Jerusalem F. Immunofluorescent evidence for presence of interleukin-1 in normal and diseased human skeletal muscle. Muscle Nerve 11: 890–892, 1988. [DOI] [PubMed] [Google Scholar]
- 620.Schumer W, Erve PR, Obernolte RP. Endotoxemic effect on cardiac and skeletal muscle mitochondria. Surg Gynecol Obstet 133: 433–436, 1971. [PubMed] [Google Scholar]
- 621.Schwarz J, Planck J, Briegel J, Straube A. Single-fiber electromyography, nerve conduction studies, and conventional electromyography in patients with critical-illness polyneuropathy: evidence for a lesion of terminal motor axons. Muscle Nerve 20: 696–701, 1997. [DOI] [PubMed] [Google Scholar]
- 622.Schweickert WD, Hall J. ICU-acquired weakness. Chest 131: 1541–1549, 2007. [DOI] [PubMed] [Google Scholar]
- 623.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister KE, Hall JB, Kress JP. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 373: 1874–1882, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Segredo V, Matthay MA, Sharma ML, Gruenke LD, Caldwell JE, Miller RD. Prolonged neuromuscular blockade after long-term administration of vecuronium in two critically ill patients. Anesthesiology 72: 566–570, 1990. [DOI] [PubMed] [Google Scholar]
- 625.Segredo V, Caldwell JE, Matthay MA, Sharma ML, Gruenke LD, Miller RD. Persistent paralysis in critically ill patients after long-term administration of vecuronium. N Engl J Med 327: 524–528, 1992. [DOI] [PubMed] [Google Scholar]
- 626.Sen CK, Khanna S, Resznick AZ, Roy S, Packer L. Glutathione regulation of tumor necrosis factor-α-induced NF-κB activation in skeletal muscle-derived L6 cells. Biochem Biophys Res Commun 237: 645–649, 1997. [DOI] [PubMed] [Google Scholar]
- 627.Senf SM, Dodd SL, McClung JM, Judge AR. Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J 22: 3836–3845, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DW, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Inflammation, and Host Response to Injury, Large Scale Collaborative Research Program. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Shah AM, Spurgeon HA, Sollott S, Talo A, Lakatta EG. 8-Bromo cGMP reduces the myofilament response to Ca2+ in intact cardiac myocytes. Circ Res 74: 970–978, 1994. [DOI] [PubMed] [Google Scholar]
- 630.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem 282: 36321–36329, 2007. [DOI] [PubMed] [Google Scholar]
- 631.Sharshar T, Bastuji-Garin S, De JB, Stevens RD, Polito A, Maxime V, Rodriguez P, Cerf C, Outin H, Touraine P, Laborde K. Hormonal status and ICU-acquired paresis in critically ill patients. Intensive Care Med 36: 1318–1326, 2010. [DOI] [PubMed] [Google Scholar]
- 632.Sharshar T, Bastuji-Garin S, Stevens RD, Durand MC, Malissin I, Rodriguez P, Cerf C, Outin H, De JB. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med 37: 3047–3053, 2009. [DOI] [PubMed] [Google Scholar]
- 633.Shehabi Y, Bellomo R, Mehta S, Riker R, Takala J. Intensive care sedation: the past, present and the future. Crit Care 17: 322, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Shindoh C, Di Marco A, Nethery D, Supinski G. Effect of PEG-superoxide dismutase on the diaphragmatic response to endotoxin. Am Rev Respir Dis 145: 1350–1354, 1992. [DOI] [PubMed] [Google Scholar]
- 635.Shiono S, Fantini GA, Roberts JP, Chiao J, Shires GT. Assessment of the early cellular membrane response to live Escherichia coli bacteremia. J Surg Res 46: 9–15, 1989. [DOI] [PubMed] [Google Scholar]
- 636.Siegfried A, Berchtold S, Manncke B, Deuschle E, Reber J, Ott T, Weber M, Kalinke U, Hofer MJ, Hatesuer B, Schughart K, Gailus-Durner V, Fuchs H, Hrabe de Angelis M, Weber F, Hornef MW, Autenrieth IB, Bohn E. IFIT2 is an effector protein of type I IFN-mediated amplification of lipopolysaccharide (LPS)-induced TNF-α secretion and LPS-induced endotoxin shock. J Immunol 191: 3913–3921, 2013. [DOI] [PubMed] [Google Scholar]
- 637.Sillen MJ, Speksnijder CM, Eterman RM, Janssen PP, Wagers SS, Wouters EF, Uszko-Lencer NH, Spruit MA. Effects of neuromuscular electrical stimulation of muscles of ambulation in patients with chronic heart failure or COPD: a systematic review of the English-language literature. Chest 136: 44–61, 2009. [DOI] [PubMed] [Google Scholar]
- 638.Simonson SG, Welty-Wolf K, Huang YT, Griebel JA, Caplan MS, Fracica PJ, Piantadosi CA. Altered mitochondrial redox responses in gram negative septic shock in primates. Circ Shock 43: 34–43, 1994. [PubMed] [Google Scholar]
- 639.Simpson LL. The effects of acute and chronic botulinum toxin treatment on receptor number, receptor distribution and tissue sensitivity in rat diaphragm. J Pharmacol Exp Ther 200: 343–351, 1977. [PubMed] [Google Scholar]
- 640.Singer M, Brealey D. Mitochondrial dysfunction in sepsis. Biochem Soc Symp 66: 149–166, 1999. [DOI] [PubMed] [Google Scholar]
- 641.Sinha B, Koster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144: 402–413, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Sipila S, Narici M, Kjaer M, Pollanen E, Atkinson RA, Hansen M, Kovanen V. Sex hormones and skeletal muscle weakness. Biogerontology 14: 231–245, 2013. [DOI] [PubMed] [Google Scholar]
- 643.Sitwell LD, Weinshenker BG, Monpetit V, Reid D. Complete ophthalmoplegia as a complication of acute corticosteroid- and pancuronium-associated myopathy. Neurology 41: 921–922, 1991. [DOI] [PubMed] [Google Scholar]
- 644.Sjöström M, Neglén P, Fridén J, Eklöf B. Human skeletal muscle metabolism and morphology after temporary incomplete ischemia. Eur J Clin Invest 12: 69–79, 1982. [DOI] [PubMed] [Google Scholar]
- 645.Skinner RA, Gibson RM, Rothwell NJ, Pinteaux E, Penny JI. Transport of interleukin-1 across cerebromicrovascular endothelial cells. Br J Pharmacol 156: 1115–1123, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Smith IJ, Alamdari N, O'Neal P, Gonnella P, Aversa Z, Hasselgren PO. Sepsis increases the expression and activity of the transcription factor Forkhead Box O 1 (FOXO1) in skeletal muscle by a glucocorticoid-dependent mechanism. Int J Biochem Cell Biol 42: 701–711, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Smith K, Rennie MJ. The measurement of tissue protein turnover. Baillieres Clin Endocrinol Metab 10: 469–495, 1996. [DOI] [PubMed] [Google Scholar]
- 648.Solomon V, Baracos V, Sarraf P, Goldberg AL. Rates of ubiquitin conjugation increase when muscles atrophy, largely through activation of the N-end rule pathway. Proc Natl Acad Sci USA 95: 12602–12607, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Sonneville R, den Hertog HM, Derde S, Güiza F, Derese I, Van den Berghe G, Vanhorebeek I. Increasing glucose load while maintaining normoglycemia does not evoke neuronal damage in prolonged critically ill rabbits. Clin Nutr 32: 1077–1080, 2013. [DOI] [PubMed] [Google Scholar]
- 650.Sonneville R, den Hertog HM, Güiza F, Gunst J, Derese I, Wouters PJ, Brouland JP, Polito A, Gray F, Chrétien F, Charlier P, Annane D, Sharshar T, Van den Berghe G, Vanhorebeek I. Impact of hyperglycemia on neuropathological alterations during critical illness. J Clin Endocrinol Metab 97: 2113–2123, 2012. [DOI] [PubMed] [Google Scholar]
- 651.Spangenburg EE. Changes in muscle mass with mechanical load: possible cellular mechanisms. Appl Physiol Nutr Metab 34: 328–335, 2009. [DOI] [PubMed] [Google Scholar]
- 652.Spangenburg EE, Brown DA, Johnson MS, Moore RL. Alterations in peroxisome proliferator-activated receptor mRNA expression in skeletal muscle after acute and repeated bouts of exercise. Mol Cell Biochem 332: 225–231, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Spitzer AR, Giancarlo T, Maher L, Awerbuch G, Bowles A. Neuromuscular causes of prolonged ventilator dependency. Muscle Nerve 15: 682–686, 1992. [DOI] [PubMed] [Google Scholar]
- 654.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J. Hydrocortisone therapy for patients with septic shock. N Engl J Med 358: 111–124, 2008. [DOI] [PubMed] [Google Scholar]
- 655.Stålberg E, Ekstedt J, Broman A. Neuromuscular transmission in myasthenia gravis studied with single fibre electromyography. J Neurol Neurosurg Psychiatry 37: 540–547, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Stasko SA, Hardin BJ, Smith JD, Moylan JS, Reid MB. TNF signals via neuronal-type nitric oxide synthase and reactive oxygen species to depress specific force of skeletal muscle. J Appl Physiol 114: 1629–1636, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Steele DS, Duke AM. Metabolic factors contributing to altered Ca2+ regulation in skeletal muscle fatigue. Acta Physiol Scand 179: 39–48, 2003. [DOI] [PubMed] [Google Scholar]
- 658.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354: 1671–1684, 2006. [DOI] [PubMed] [Google Scholar]
- 659.Stengl M, Bartak F, Sykora R, Chvojka J, Benes J, Krouzecky A, Novak I, Sviglerova J, Kuncova J, Matejovic M. Reduced L-type calcium current in ventricular myocytes from pigs with hyperdynamic septic shock. Crit Care Med 38: 579–587, 2010. [DOI] [PubMed] [Google Scholar]
- 660.Steuerwald NM, Foureau DM, Norton HJ, Zhou J, Parsons JC, Chalasani N, Fontana RJ, Watkins PB, Lee WM, Reddy KR, Stolz A, Talwalkar J, Davern T, Saha D, Bell LN, Barnhart H, Gu J, Serrano J, Bonkovsky HL. Profiles of serum cytokines in acute drug-induced liver injury and their prognostic significance. PLoS One 8: e81974, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, De JB, Ali NA, Sharshar T. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med 37: S299–S308, 2009. [DOI] [PubMed] [Google Scholar]
- 662.Stibler H, Edstrom L, Ahlbeck K, Remahl S, Ansved T. Electrophoretic determination of the myosin/actin ratio in the diagnosis of critical illness myopathy. Intensive Care Med 29: 1515–1527, 2003. [DOI] [PubMed] [Google Scholar]
- 663.Stinebring WR, Youngner JS. Patterns of interferon appearance in mice infected with bacteria or bacterial endotoxin. Nature 204: 712, 1964. [DOI] [PubMed] [Google Scholar]
- 664.Strasser EM, Stattner S, Karner J, Klimpfinger M, Freynhofer M, Zaller V, Graf A, Wessner B, Bachl N, Roth E, Quittan M. Neuromuscular electrical stimulation reduces skeletal muscle protein degradation and stimulates insulin-like growth factors in an age- and current-dependent manner: a randomized, controlled clinical trial in major abdominal surgical patients. Ann Surg 249: 738–743, 2009. [DOI] [PubMed] [Google Scholar]
- 665.Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest 89: 1681–1684, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Suffredini AF, Reda D, Banks SM, Tropea M, Agosti JM, Miller R. Effects of recombinant TNF receptor on human inflammatory responses following intravenous endotoxin administration. J Immunol 155: 5038–5045, 1995. [PubMed] [Google Scholar]
- 667.Suliman HB, Carraway MS, Welty-Wolf KE, Whorton AR, Piantadosi CA. Lipopolysaccharide stimulates mitochondrial biogenesis via activation of nuclear respiratory factor-1. J Biol Chem 278: 41510–41518, 2003. [DOI] [PubMed] [Google Scholar]
- 668.Suliman IA, Lindgren JU, Gillberg PG, Diab KM, Adem A. Effect of immobilization on skeletal muscle nicotinic cholinergic receptors in the rat. NeuroReport 8: 2821–2824, 1997. [DOI] [PubMed] [Google Scholar]
- 669.Sullivan JS, Kilpatrick L, Costarino AT Jr, Lee SC, Harris MC. Correlation of plasma cytokine elevations with mortality rate in children with sepsis. J Pediatr 120: 510–515, 1992. [DOI] [PubMed] [Google Scholar]
- 670.Sultan SS. Paravertebral block can attenuate cytokine response when it replaces general anesthesia for cancer breast surgeries. Saudi J Anaesth 7: 373–377, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Sun YB, Brandmeier B, Irving M. Structural changes in troponin in response to Ca2+ and myosin binding to thin filaments during activation of skeletal muscle. Proc Natl Acad Sci USA 103: 17771–17776, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Supinski GS, Callahan LA. Hemin prevents cardiac and diaphragm mitochondrial dysfunction in sepsis. Free Rad Biol Med 40: 127–137, 2006. [DOI] [PubMed] [Google Scholar]
- 673.Supinski GS, Callahan LA. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol 102: 2056–2063, 2007. [DOI] [PubMed] [Google Scholar]
- 674.Supinski GS, Callahan LA. The JNK MAP kinase pathway contributes to the development of endotoxin-induced diaphragm caspase activation. Am J Physiol Regul Integr Comp Physiol 297: R825–R834, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Supinski GS, Callahan LA. Calpain activation contributes to endotoxin-induced diaphragm dysfunction. Am J Respir Cell Mol Biol 42: 80–87, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Supinski GS, Callahan LA. Double-stranded RNA-dependent protein kinase activation modulates endotoxin-induced diaphragm weakness. J Appl Physiol 110: 199–205, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care 17: R120, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Supinski GS, Vanags J, Callahan LA. Effect of proteasome inhibitors on endotoxin-induced diaphragm dysfunction. Am J Physiol Lung Cell Mol Physiol 296: L994–L1001, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Supinski GS, Ji X, Wang W, Callahan LA. The extrinsic caspase pathway modulates endotoxin-induced diaphragm contractile dysfunction. J Appl Physiol 102: 1649–1657, 2007. [DOI] [PubMed] [Google Scholar]
- 680.Supinski GS, Ji XY, Callahan LA. p38 mitogen-activated protein kinase modulates endotoxin-induced diaphragm caspase activation. Am J Respir Cell Mol Biol 43: 121–127, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Supinski GS, Nethery D, DiMarco A. Effect of free radical scavengers on endotoxin-induced respiratory muscle dysfunction. Am Rev Respir Dis 148: 1318–1324, 1993. [DOI] [PubMed] [Google Scholar]
- 682.Supinski GS, Nethery D, Nosek TM, Callahan LA, Stofan D, Di Marco A. Endotoxin administration alters the force vs pCa relationship of skeletal muscle fibers. Am J Physiol Regul Integr Comp Physiol 278: R891–R896, 2000. [DOI] [PubMed] [Google Scholar]
- 683.Supinski GS, Nethery D, Stofan D, DiMarco A. Comparison of the effects of endotoxin on limb, respiratory, and cardiac muscles. J Appl Physiol 81: 1370–1378, 1996. [DOI] [PubMed] [Google Scholar]
- 684.Supinski G, Stofan D, Nethery D, Szweda L, Di Marco A. Apocynin improves diaphragmatic function after endotoxin administration. J Appl Physiol 87: 776–782, 1999. [DOI] [PubMed] [Google Scholar]
- 685.Supinski GS, Wang W, Callahan LA. Caspase and calpain activation both contribute to sepsis-induced diaphragmatic weakness. J Appl Physiol 107: 1389–1396, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Suzuki N, Mizuno H, Warita H, Takeda S, Itoyama Y, Aoki M. Neuronal NOS is dislocated during muscle atrophy in amyotrophic lateral sclerosis. J Neurol Sci 294: 95–101, 2010. [DOI] [PubMed] [Google Scholar]
- 687.Suzuki N, Motohashi N, Uezumi A, Fukada S, Yoshimura T, Itoyama Y, Aoki M, Miyagoe-Suzuki Y, Takeda S. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J Clin Invest 117: 2468–2476, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 341: 785–792, 1999. [DOI] [PubMed] [Google Scholar]
- 689.Tam BT, Siu PM. Autophagic cellular responses to physical exercise in skeletal muscle. Sports Med 44: 625–640, 2014. [DOI] [PubMed] [Google Scholar]
- 690.Tang H, Lee M, Khuong A, Wright E, Shrager JB. Diaphragm muscle atrophy in the mouse after long-term mechanical ventilation. Muscle Nerve 48: 272–278, 2013. [DOI] [PubMed] [Google Scholar]
- 691.Tavakoli H, Mela L. Alterations of mitochondrial metabolism and protein concentrations in subacute septicemia. Infect Immun 38: 536–541, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Tavernier B, Garrique D, Boulle C, Vallet B, Adnet P. Myofilament calcium sensitivity is decreased in skinned cardiac fibers of endotoxin-treated rabbits. Cardiovasc Res 38: 472–479, 1998. [DOI] [PubMed] [Google Scholar]
- 693.Tavernier B, Mebazaa A, Mateo P, Sys S, Ventura-Clapier R, Veksler V. Phosphorylation-dependent alteration in myofilament Ca2+ sensitivity but normal mitochondrial function in septic heart. Am J Respir Crit Care Med 163: 362–367, 2001. [DOI] [PubMed] [Google Scholar]
- 694.Taylor BE, Buchman TG. Is there a role for growth hormone therapy in refractory critical illness? Curr Opin Crit Care 14: 438–444, 2008. [DOI] [PubMed] [Google Scholar]
- 695.Tedders KM, McNorton KN, Edwin SB. Efficacy and safety of analgosedation with fentanyl compared with traditional sedation with propofol. Pharmacotherapy 34: 643–647, 2014. [DOI] [PubMed] [Google Scholar]
- 696.Teener JW, Rich MM. Dysregulation of sodium channel gating in critical illness myopathy. J Muscle Res Cell Motil 27: 291–296, 2006. [DOI] [PubMed] [Google Scholar]
- 697.Teichmann MD, Wegner FV, Fink RH, Chamberlain JS, Launikonis BS, Martinac B, Friedrich O. Inhibitory control over Ca2+ sparks via mechanosensitive channels is disrupted in dystrophin deficient muscle but restored by mini-dystrophin expression. PLoS One 3: e3644, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Tennila A, Salmi T, Pettila V, Roine RO, Varpula T, Takkunen O. Early signs of critical illness polyneuropathy in ICU patients with systemic inflammatory response syndrome or sepsis. Intensive Care Med 26: 1360–1363, 2000. [DOI] [PubMed] [Google Scholar]
- 699.Tepper M, Rakic S, Haas JA, Woittiez AJ. Incidence and onset of critical illness polyneuropathy in patients with septic shock. Neth J Med 56: 211–214, 2000. [DOI] [PubMed] [Google Scholar]
- 700.Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid Redox Signal 12: 503–535, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Tews DS, Goebel HH. Cytokine expression profile in idiopathic inflammatory myopathies. J Neuropathol Exp Neurol 55: 342–347, 1996. [DOI] [PubMed] [Google Scholar]
- 702.Thompson M, Kliewer A, Maass D, Becker L, White DJ, Bryant D, Arteaga G, Horton J, Giroir BP. Increased cardiomyocyte intracellular calcium during endotoxin-induced cardiac dysfunction in guinea pigs. Pediatr Res 47: 669–676, 2000. [DOI] [PubMed] [Google Scholar]
- 703.Tiao G, Fagan J, Roegner V, Lieberman M, Wang JJ, Fischer JE, Hasselgren PO. Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J Clin Invest 97: 339–348, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Tiao G, Fagan JM, Samuels N, James JH, Hudson K, Lieberman M, Fischer JE, Hasselgren PO. Sepsis stimulates nonlysosomal, energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J Clin Invest 94: 2255–2264, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Tiao G, Lieberman M, Fischer JE, Hasselgren PO. Intracellular regulation of protein degradation during sepsis is different in fast- and slow-twitch muscle. Am J Physiol Regul Integr Comp Physiol 272: R849–R856, 1997. [DOI] [PubMed] [Google Scholar]
- 706.Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today 18: 428–432, 1997. [DOI] [PubMed] [Google Scholar]
- 707.Tjader I, Essen P, Garlick PG, McMnurlan MA, Rooyackers O, Wernerman J. Impact of surgical trauma on human skeletal muscle protein synthesis. Clin Sci 107: 601–607, 2004. [DOI] [PubMed] [Google Scholar]
- 708.Tömböl T, Pataki G, Németh A, Hamar J. Ultrastructural changes of the neuromuscular junction in reperfusion injury. Cells Tissues Organs 170: 139–150, 2002. [DOI] [PubMed] [Google Scholar]
- 709.Tokumaru T. The effect of trauma on production of herpes interferon in guinea pigs. Arch Gesamte Virusforsch 21: 61–70, 1967. [DOI] [PubMed] [Google Scholar]
- 710.Tolosa L, Morla M, Iglesias A, Busquets X, Llado J, Olmos G. INF-gamma prevents TNF-alpha-induced apoptosis in C2C12 myotubes through down-regulation of TFN-R2 and increased NF-kappaB activity. Cell Signal 17: 1333–1342, 2005. [DOI] [PubMed] [Google Scholar]
- 711.Tomera JF, Martyn J. Intraperitoneal endotoxin but not protein malnutrition shifts d-tubocurarine dose-repsonse curves in mouse gastrocnemius muscle. J Pharmacol Exp Ther 250: 216–220, 1989. [PubMed] [Google Scholar]
- 712.Tracey KJ, Lowry SF, Beutler B, Cerami A, Albert JD, Shires GT. Cachectin/tumor necrosis factor mediates changes of skeletal muscle plasma membrane potential. J Exp Med 164: 1368–1373, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Trojaborg W. Electrophysiologic techniques in critical illness-associated weakness. J Neurol Sci 242: 83–85, 2006. [DOI] [PubMed] [Google Scholar]
- 714.Trojaborg W, Weimer LH, Hays AP. Electrophysiologic studies in critical illness associated weakness: myopathy or neuropathy–a reappraisal. Clin Neurophysiol 112: 1586–1593, 2001. [DOI] [PubMed] [Google Scholar]
- 715.Trout JJ, Stauber WT, Schottelius BA. Degeneration and regeneration in denervated tonic and phasic skeletal muscle: morphology and acid phosphatase cytochemistry. Virchows Arch B Cell Pathol Incl Mol Pathol 38: 67–76, 1981. [DOI] [PubMed] [Google Scholar]
- 716.Trumbeckaite S, Opalka JR, Neuhof C, Zierz S, Gellerich FN. Different sensitivity of rabbit heart and skeletal muscle to endotoxin-induced impairment of mitochondrial function. Eur J Biochem 268: 1422–1429, 2001. [DOI] [PubMed] [Google Scholar]
- 717.Trunkey DD, Illner H, Wagner Y, Shires GT. The effect of septic shock on skeletal muscle action potentials in the primate. Surgery 85: 638–643, 1979. [PubMed] [Google Scholar]
- 718.Tsujinaka T, Ebisui C, Fujita J, Kishibuchi M, Morimoto T, Ogawa A, Katsume A, Ohsugi Y, Kominami E, Monden M. Muscle undergoes atrophy in association with increase of lysosomal cathepsin activity in interleukin-6 transgenic mouse. Biochem Biophys Res Commun 207: 168–174, 1995. [DOI] [PubMed] [Google Scholar]
- 719.Tsujinaka T, Fujita J, Ebisui C, Yano M, Kominami E, Suzuki K, Tanaka K, Katsume A, Ohsugi Y, Shiozaki H, Monden M. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest 97: 244–249, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Tsukagoshi H, Morita T, Takahashi K, Kunimoto F, Goto F. Cecal ligation and puncture peritonitis model shows decreased nicotinic acetylcholine receptor numbers in rat muscle. Anesthesiology 891: 448–460, 1999. [DOI] [PubMed] [Google Scholar]
- 721.Twig G, Elorza A, Molina AJA, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fusion and fission govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Tzanis G, Vasileiadis I, Zervakis D, Karatzanos E, Dimopoulos S, Pitsolis T, Tripodaki E, Gerovasili V, Routsi C, Nanas S. Maximum inspiratory pressure, a surrogate parameter for the assessment of ICU-acquired weakness. BMC Anesthesiol 11: 14, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Tzika AA, Mintzopoulos D, Mindrinos M, Zhang J, Rahme LG, Tompkins RG. Microarray analysis suggests that burn injury results in mitochondrial dysfunction in human skeletal muscle. Int J Mol Med 24: 387–392, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Valdes JA, Gaggero E, Hidalgo J, Leal N, Jaimovich E, Carrasco MA. NFAT activation by membrane potential follows a calcium pathway distinct from other activity-related transcription factors in skeletal muscle cells. Am J Physiol Cell Physiol 294: C715–C725, 2008. [DOI] [PubMed] [Google Scholar]
- 725.Vanhorebeek I, De Vos R, Mesotten D, Wouters PJ, De Wolf-Peeters C, Van den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet 365: 53–59, 2005. [DOI] [PubMed] [Google Scholar]
- 726.Vanhorebeek I, Ellger B, De Vos R, Boussemaere M, Debaveye Y, Vander Perre S, Rabbani N, Thornalley PJ, Van den Berghe G. Tissue-specific glucose toxicity induces mitochondrial damage in a burn injury model of critical illness. Crit Care Med 37: 1355–1364, 2009. [DOI] [PubMed] [Google Scholar]
- 727.Vanhorebeek I, Gunst J, Derde S, Derese I, Boussemaere M, D'Hoore A, Wouters PJ, Van den Berghe G. Mitochondrial fusion, fission, and biogenesis in prolonged critically ill patients. J Clin Endocrinol Metab 97: E59–E64, 2012. [DOI] [PubMed] [Google Scholar]
- 728.Vanhorebeek I, Gunst J, Derde S, Derese I, Boussemaere M, Guiza F, Martinet W, Timmermans JP, D'Hoore A, Wouters PJ, Van den Berghe G. Insufficient activation of autophagy allows cellular damage to accumulate in critically ill patients. J Clin Endocrinol Metab 96: E633–E645, 2011. [DOI] [PubMed] [Google Scholar]
- 729.Vanhorebeek I, Gunst J, Ellger B, Boussemaere M, Lerut E, Debaveye Y, Rabbani N, Thornalley PJ, Schetz M, Van den Berghe G. Hyperglycemic kidney damage in an animal model of prolonged critical illness. Kidney Int 76: 512–520, 2009. [DOI] [PubMed] [Google Scholar]
- 730.Vanhorebeek I, Langouche L, Van den Berghe G. Endocrine aspects of acute and prolonged critical illness. Nat Clin Pract Endocrinol Metab 2: 20–31, 2006. [DOI] [PubMed] [Google Scholar]
- 731.Vanhorebeek I, Langouche L. Molecular mechanisms behind clinical benefits of intensive insulin therapy during critical illness: glucose versus insulin. Best Pract Res Clin Anaesthesiol 23: 449–459, 2009. [DOI] [PubMed] [Google Scholar]
- 732.Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control with insulin in the ICU: facts and controversies. Chest 132: 268–278, 2007. [DOI] [PubMed] [Google Scholar]
- 733.Van den Berghe G. Intensive insulin therapy in the ICU–reconciling the evidence. Nat Rev Endocrinol 8: 374–378, 2012. [DOI] [PubMed] [Google Scholar]
- 734.Van den Berghe G, Schetz M, Vlasselaers D, Hermans G, Wilmer A, Bouillon R, Mesotten D. Clinical review: intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab 94: 3163–3170, 2009. [DOI] [PubMed] [Google Scholar]
- 735.Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology 64: 1348–1353, 2005. [DOI] [PubMed] [Google Scholar]
- 736.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med 354: 449–461, 2006. [DOI] [PubMed] [Google Scholar]
- 737.Van den Berghe G, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F, Bouillon R, Schetz M. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes 55: 3151–3159, 2006. [DOI] [PubMed] [Google Scholar]
- 738.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med 345: 1359–1367, 2001. [DOI] [PubMed] [Google Scholar]
- 739.Van Dervort AL, Yan L, Madara PJ, Cobb JP, Wesley RA, Corriveau CC, Tropea MM, Danner RL. Nitric oxide regulates endotoxin-induced TNF-alpha production in human neutrophils. J Immunol 152: 4102–4109, 1994. [PubMed] [Google Scholar]
- 740.Van Hees HWH, Schellekens WJM, Linkels M, Leenders F, Zoll J, Donders R, Dekhuijzen PNR, van der Hoeven J, Heunks LMA. Plasma from septic shock patients induces loss of muscle proteins. Crit Care 15: R233, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Van Kann LN, Bakker AJ. Effect of tumor necrosis factor α on electrically induced calcium transients elicited in C2C12 skeletal myotubes. Muscle Nerve 35: 251–253, 2007. [DOI] [PubMed] [Google Scholar]
- 742.Van Mook WN, Hulsewe-Evers RP. Critical illness polyneuropathy. Curr Opin Crit Care 8: 302–310, 2002. [DOI] [PubMed] [Google Scholar]
- 743.Vega GL, Baxter CR. Tumor necrosis factor mediates hypertriglyceridemia during thermal injury in mice genetically susceptible to lipopolysaccharides. J Burn Care Rehabil 12: 4637-4637, 1991. [DOI] [PubMed] [Google Scholar]
- 744.Vender JS, Szokol JW, Murphy GS, Nitsun M. Sedation, analgesia, and neuromuscular blockade in sepsis: an evidence-based review. Crit Care Med 32: S554–S561, 2004. [DOI] [PubMed] [Google Scholar]
- 745.Vincent JL. The rise and fall of drotrecogin alfa (activated). Lancet Infect Dis 12: 649–651, 2012. [DOI] [PubMed] [Google Scholar]
- 746.Visser J, Labadarios D, Blaauw R. Micronutrient supplementation for critically ill adults: a systematic review and meta-analysis. Nutrition 27: 745–758, 2011. [DOI] [PubMed] [Google Scholar]
- 747.Visser LH. Critical illness polyneuropathy and myopathy: clinical features, risk factors and prognosis. Eur J Neurol 13: 1203–1212, 2006. [DOI] [PubMed] [Google Scholar]
- 748.Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet 373: 547–556, 2009. [DOI] [PubMed] [Google Scholar]
- 749.Voisin L, Breuille D, Combaret L, Pouyet C, Taillandier D, Aurousseau E, Obled C, Attaix D. Muscle wasting in a rat model of long-lasting sepsis results from the activation of lysosomal, Ca2+-activated, and ubiquitin-proteasome proteolytic pathways. J Clin Invest 97: 1610–1617, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Wagatsuma A, Kotake N, Mabuchi K, Yamada S. Expression of nuclear-encoded genes involved in mitochondrial biogenesis and dynamics in experimentally denervated muscle. J Physiol Biochem 67: 359–370, 2011. [DOI] [PubMed] [Google Scholar]
- 751.Wagatsuma A, Kotake N, Yamada S. Muscle regeneration occurs to coincide with mitochondrial biogenesis. Mol Cell Biochem 349: 139–147, 2011. [DOI] [PubMed] [Google Scholar]
- 752.Waldhausen E, Mingers B, Lippers P, Keser G. Critical illness polyneuropathy due to parenteral nutrition. Intensive Care Med 23: 922–923, 1997. [PubMed] [Google Scholar]
- 753.Wang DW, George AL Jr, Bennett PB. Comparison of heterologously expressed human cardiac and skeletal muscle sodium channels. Biophys J 70: 238–245, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 10: 75–82, 2009. [DOI] [PubMed] [Google Scholar]
- 755.Wang P, Zhou M, Cioffi WG, Bland KI, Ba ZF, Chaudry IH. Is prostacyclin responsible for producing the hyperdynamic response during early sepsis? Crit Care Med 28: 1534–1539, 2000. [DOI] [PubMed] [Google Scholar]
- 756.Wang X, Blagden C, Fan J, Nowak SJ, Taniuchi I, Littman DR, Burden SJ. Runx1 prevents wasting, myofibrillar disorganization, and autophagy of skeletal muscle. Genes Dev 19: 1715–1722, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Wang X, Li Y, Engisch KL, Nakanishi ST, Dodson SE, Miller GW, Cope TC, Pinter MJ, Rich MM. Activity-dependent presynaptic regulation of quantal size at the mammalian neuromuscular junction in vivo. J Neurosci 25: 343–351, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Ward JM, Martyn JAJ. Burn injury-induced nicotinic acetylcholine receptor changes on muscle membrane. Muscle Nerve 16: 348–354, 1993. [DOI] [PubMed] [Google Scholar]
- 759.Warner BW, Hasselgren PO, James JH, Hummel RP 3rd, Rigel DF, Fischer JE. Reduced amino acid transport in skeletal muscle caused by a circulating factor during endotoxemia. Ann Surg 211: 323–328, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Watson AC, Hughes PD, Louise HM, Hart N, Ware RJ, Wendon J, Green M, Moxham J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med 29: 1325–1331, 2001. [DOI] [PubMed] [Google Scholar]
- 761.Watson CL, Gold MR. Modulation of Na+ current inactivation by stimulation of protein kinase C in cardiac cells. Circ Res 81: 380–386, 1997. [DOI] [PubMed] [Google Scholar]
- 762.Watters JM, Kirkpatrick SM, Norris SB, Shamji FM, Wells GA. Immediate postoperative enteral feeding results in impaired respiratory mechanics and decreased mobility. Ann Surg 226: 369–377, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Weber-Carstens S, Deja M, Koch S, Spranger J, Bubser F, Wernecke KD, Spies CD, Spuler S, Keh D. Risk factors in critical illness myopathy during the early course of critical illness: a prospective observational study. Crit Care 14: R119, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Weber-Carstens S, Koch S, Spuler S, Spies CD, Bubser F, Wernecke KD, Deja M. Nonexcitable muscle membrane predicts intensive care unit-acquired paresis in mechanically ventilated, sedated patients. Crit Care Med 37: 2632–2637, 2009. [DOI] [PubMed] [Google Scholar]
- 765.Weber-Carstens S, Schneider J, Wollersheim T, Assmann A, Bierbrauer J, Marg A, Al Hasani H, Chadt A, Wenzel K, Koch S, Fielitz J, Kleber C, Faust K, Mai K, Spies CD, Luft FC, Boschmann M, Spranger J, Spuler S. Critical illness myopathy and GLUT4: significance of insulin and muscle contraction. Am J Respir Crit Care Med 187: 387–396, 2013. [DOI] [PubMed] [Google Scholar]
- 766.Weekers F, Van Herck E, Coopmans W, Michalaki M, Bowers CY, Veldhuis JD, Van den Berghe G. A novel in vivo rabbit model of hypercatabolic critical illness reveals a biphasic neuroendocrine stress response. Endocrinology 143: 764–774, 2002. [DOI] [PubMed] [Google Scholar]
- 767.Wei W, Fareed MU, Evenson A, Menconi MJ, Yang H, Petkova V, Hasselgren PO. Sepsis stimulates calpain activity in skeletal muscle by decreasing calpastatin activity but does not activate caspase-3. Am J Physiol Regul Integr Comp Physiol 288: R580–R590, 2005. [DOI] [PubMed] [Google Scholar]
- 768.Weiss-Guillet EM, Takala J, Jakob SM. Diagnosis and management of electrolyte emergencies. Best Pract Res Clin Endocrinol Metab 17: 623–651, 2003. [DOI] [PubMed] [Google Scholar]
- 769.Welty-Wolf KE, Simonson SG, Huang YC, Fracica PJ, Patterson JW, Piantadosi CA. Ultrastructural changes in skeletal muscle mitochondria in gram-negative sepsis. Shock 5: 378–384, 1996. [DOI] [PubMed] [Google Scholar]
- 770.Wernerman J. Glutamine supplementation. Ann Intensive Care 1: 25, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 771.Westfall MV, Sayeed MM. Skeletal muscle calcium uptake in bacteremic rats. Am J Physiol Regul Integr Comp Physiol 256: R201–R206, 1989. [DOI] [PubMed] [Google Scholar]
- 772.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock: a review of laboratory models and a proposal. J Surg Res 29: 189–201, 1980. [DOI] [PubMed] [Google Scholar]
- 773.Wilcox P, Miliken C, Bressler B. High-dose tumor necrosis factor alpha produces an impairment of hamster diaphragm contractility. Attenuation with a prostaglandin inhibitor. Am J Respir Crit Care Med 153: 1611–1615, 1996. [DOI] [PubMed] [Google Scholar]
- 774.Wilcox P, Osborne S, Bressler B. Monocyte inflammatory mediators impair in vitro hamster diaphragm contractility. Am Rev Resp Dis 146: 462–466, 1992. [DOI] [PubMed] [Google Scholar]
- 775.Williams A, Wang JJ, Wang L, Sun X, Fischer JE, Hasselgren PO. Sepsis in mice stimulates muscle proteolysis in the absence of IL-6. Am J Physiol Regul Integr Comp Physiol 275: R1983–R1991, 1998. [DOI] [PubMed] [Google Scholar]
- 776.Williams AB, deCourten-Myers GM, Fischer JE, Luo G, Sun X, Hasselgren PO. Sepsis stimulates release of myofilaments in skeletal muscle by a calcium-dependent mechanism. FASEB J 13: 1435–1443, 1999. [DOI] [PubMed] [Google Scholar]
- 777.Williams DA, Head SI, Bakker AJ, Stephenson DG. Resting calcium concentrations in isolated skeletal muscle fibers of dystrophic mice. J Physiol 428: 243–256, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.Williams G, Brown T, Becker L, Prager M, Giroir BP. Cytokine-induced expression of nitric oxide synthase in C2C12 skeletal muscle myocytes. Am J Physiol Regul Integr Comp Physiol 267: R1020–R1025, 1994. [DOI] [PubMed] [Google Scholar]
- 779.Williams S, Horrocks IA, Ouvrier RA, Gillis J, Ryan MM. Critical illness polyneuropathy and myopathy in prediatric intensive care: a review. Pediatr Crit Care Med 8: 18–22, 2007. [DOI] [PubMed] [Google Scholar]
- 780.Winkelmann C. Inactivity and inflammation. Selected cytokines as biologic mediators in muscle dysfunction during critical illness. AACN Clin Issues 15: 74–82, 2004. [DOI] [PubMed] [Google Scholar]
- 781.Witt NJ, Zochodne DW, Bolton CF, Grand'Maison F, Wells G, Young GB, Sibbald WJ. Peripheral nerve function in sepsis and multiple organ failure. Chest 99: 176–184, 1991. [DOI] [PubMed] [Google Scholar]
- 782.Witzemann V, Brenner HR, Sakmann B. Neural factors regulate AChR subunit mRNAs at rat neuromuscular synapses. J Cell Biol 114: 125–141, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Wolf SE, Edelman LS, Kemalyan N, Donison L, Cross J, Underwood M, Spence RJ, Noppenberger D, Palmieri TL, Greenhalgh DG, Lawless M, Voigt D, Edwards P, Warner P, Kagan R, Hatfield S, Jeng J, Crean D, Hunt J, Purdue G, Burris A, Cairns B, Kessler M, Klein RL, Baker R, Yowler C, Tutulo W, Foster K, Caruso D, Hildebrand B, Benjamin W, Villarreal C, Sanford AP, Saffle J. Effects of oxandrolone on outcome measures in the severely burned: a multicenter prospective randomized double-blind trial. J Burn Care Res 27: 131–139, 2006. [DOI] [PubMed] [Google Scholar]
- 784.Wolfe RR, Martini WZ. Changes in intermediary metabolism in severe surgical illness. World J Surg 24: 639–647, 2000. [DOI] [PubMed] [Google Scholar]
- 785.Wondergem R, Graves BM, Li C, Williams DL. Lipopolysaccharide prolongs action potential duration in HL-1 mouse cardiomyocytes. Am J Physiol Cell Physiol 303: C825–C833, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Wray CJ, Mammen JM, Hershko DD, Hasselgren PO. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol 35: 698–705, 2003. [DOI] [PubMed] [Google Scholar]
- 787.Wu LL, Liu MS. Altered ryanodine receptor of canine cardiac sarcoplasmic reticulum and its underlying mechanism in endotoxin shock. J Surg Res 53: 82–90, 1992. [DOI] [PubMed] [Google Scholar]
- 788.Yang B, Jiang JH, Zhou YC, Zhang Y, Li ST. Denervation stage differentially influences reistance to neuromuscular blockers in rat gastrocnemius. J Surg Res 180: 266–273, 2013. [DOI] [PubMed] [Google Scholar]
- 789.Yang JS, Sladky JT, Kallen RG, Barchi RL. TTX-sensitive and TTX-insensitive sodium channel mRNA transcripts are independently regulated in adult skeletal muscle after denervation. Neuron 7: 421–427, 1991. [DOI] [PubMed] [Google Scholar]
- 790.Yang JS, Bennett PB, Makita N, George AL, Barchi RL. Expression of the sodium channel beta 1 subunit in rat skeletal muscle is selectively associated with the tetrodotoxin-sensitive alpha subunit isoform. Neuron 11: 915–922, 1993. [DOI] [PubMed] [Google Scholar]
- 791.Yasuda S, Lew W. Lipopolysaccharide depresses cardiac contractility and β-adrenergic contractile response by decreasing myofilament response to Ca2+ in cardiac myocytes. Circ Res 81: 1011–1020, 1997. [DOI] [PubMed] [Google Scholar]
- 792.Yasuhara S, Perez ME, Kanakubo E, Yasuhara Y, Shin YS, Kaneki M, Fujita T, Martyn JAJ. Skeletal muscle apoptosis after burns is associated with activation of proapoptotic signals. Am J Physiol Endocrinol Metab 279: E1114–E1121, 2000. [DOI] [PubMed] [Google Scholar]
- 793.Yoshida M, Hama H, Ishikawa-Sakurai M, Imamura M, Mizuno Y, Araishi K, Wakabayashi-Takai E, Noguchi S, Sasaoka T, Ozawa E. Biochemical evidence for association of dystrobrevin with the sarcoglycan complex as a basis for understanding sarcoglycanopathy. Hum Mol Genet 9: 1033–1040, 2000. [DOI] [PubMed] [Google Scholar]
- 794.Zamir O, Hasselgren PO, James H, Higashiguchi T, Fischer JE. Effect of tumor necrosis factor or interleukin-1 on muscle amino acid uptake and the role of glucocorticoids. Surg Gynecol Obstet 177: 27–32, 1993. [PubMed] [Google Scholar]
- 795.Zamir O, Hasselgren PO, von Allmen D, Fischer JE. The effect of interleukin-1 alpha and the glucocorticoid receptor blocker RU38486 on total and myofibrillar breakdown in skeletal muscle. J Surg Res 50: 579–583, 1991. [DOI] [PubMed] [Google Scholar]
- 796.Zanni JM, Korupolu R, Fan E, Pradhan P, Janjua K, Palmer JB, Brower RG, Needham DM. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care 25: 254–262, 2010. [DOI] [PubMed] [Google Scholar]
- 797.Zanotti E, Felicetti G, Maini M, Fracchia C. Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest 124: 292–296, 2003. [DOI] [PubMed] [Google Scholar]
- 798.Zemkova H, Teisinger J, Almon RR, Veisada R, Hnik P, Vyskocil F. Immobilization atrophy and membrane properties in rat skeletal muscle fibres. Pflügers Arch 416: 126–129, 1990. [DOI] [PubMed] [Google Scholar]
- 799.Zeng J, Lin X, Fan H, Li C. Hydrogen sulfide attenuates the inflammatory response in a mouse burn injury model. Mol Med Rep 8: 1204–1208, 2013. [DOI] [PubMed] [Google Scholar]
- 800.Z'Graggen WJ, Lin CS, Howard RS, Beale RJ, Bostock H. Nerve excitability changes in critical illness polyneuropathy. Brain 129: 2461–2470, 2006. [DOI] [PubMed] [Google Scholar]
- 801.Z'Graggen WJ, Brander L, Tuchscherer D, Scheidegger O, Takala J, Bostock H. Muscle membrane dysfunction in critical illness myopathy assessed by velocity recovery cycles. Clin Neurophysiol 122: 834–841, 2011. [DOI] [PubMed] [Google Scholar]
- 802.Zhang J, Jiang W, Zuo Z. Pyrrolidine dithiocarbamate attenuates surgery-induced neuroinflammation and cognitive dysfunction possibly via inhibition of nuclear factor κB. Neuroscience S0306-4522: 01057-01059, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 803.Zhang Q, Cao H, Astrakas LG, Mintzopoulos D, Mindrinos MN, Schulz IIIJ, Tompkins RG, Rahme LG, Tzika AA. Uncoupling protein 3 expression and intramyocellular lipid accumulation by NMR following local burn trauma. Int J Mol Med 18: 1223–1229, 2006. [PubMed] [Google Scholar]
- 804.Zhang Y, Hartmann HA, Satin J. Glycosylation influences voltage-dependent gating of cardiac and skeletal muscle sodium channels. J Membr Biol 171: 195–207, 1999. [DOI] [PubMed] [Google Scholar]
- 805.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6: 472–483, 2007. [DOI] [PubMed] [Google Scholar]
- 806.Zhao X, Min CK, Ko JK, Parness J, Kim do H, Weisleder N, Ma J. Increased store-operated Ca2+ entry in skeletal muscle with reduced calsequestrin-1 expression. Biophys J 99: 1556–1564, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Zhu X, Bernecker OY, Haijar RJ, Hellman J, Ichinose F, Valdivia HH, Schmidt U. Increased leakage of sarcoplasmic reticulum Ca2+ contributes to abnormal myocyte Ca2+ handling and shortening in sepsis. Crit Care Med 33: 598–604, 2005. [DOI] [PubMed] [Google Scholar]
- 808.Zifko UA, Zipko HT, Bolton CF. Clinical and electrophysiological findings in critical illness polyneuropathy. J Neurol Sci 159: 186–193, 1998. [DOI] [PubMed] [Google Scholar]
- 809.Zink W, Kaess M, Hofer S, Plachky J, Zausig YA, Sinner B, Weigand MA, Fink RHA, Graf BM. Alterations in intracellular Ca2+-homeostasis of skeletal muscle fibers during sepsis. Crit Care Med 36: 1559–1563, 2008. [DOI] [PubMed] [Google Scholar]
- 810.Zhong J, Hwang TC, Adams HR, Rubin LJ. Reduced L-type calcium current in ventricular myocytes from endotoxemic guinea pigs. Am J Physiol Heart Circ Physiol 273: H2312–H2324, 1997. [DOI] [PubMed] [Google Scholar]
- 811.Zochodne DW, Bolton CF, Thompson RT, Driedger AA, Hahn AF, Gilbert JJ. Myopathy in critical illness. Muscle Nerve 9: 652, 1986. [Google Scholar]
- 812.Zochodne DW, Bolton CF, Wells GA, Gilbert JJ, Hahn AF, Brown JD, Sibbald WA. Critical illness polyneuropathy. A complication of sepsis and multiple organ failure. Brain 110: 819–841, 1987. [DOI] [PubMed] [Google Scholar]
- 813.Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev 49: 1–51, 1997. [PubMed] [Google Scholar]