Abstract
Dendritic cells are powerful antigen-presenting cells that are essential for the priming of T cell responses. In addition to providing T-cell-receptor ligands and co-stimulatory molecules for naive T cell activation and expansion, dendritic cells are thought to also provide signals for the differentiation of CD4+ T cells into effector T cell populations. The mechanisms by which dendritic cells are able to adapt and respond to the great variety of infectious stimuli they are confronted with, and prime an appropriate CD4+ T cell response, are only partly understood. It is known that in the steady-state dendritic cells are highly heterogenous both in phenotype and transcriptional profile, and that this variability is dependent on developmental lineage, maturation stage, and the tissue environment in which dendritic cells are located. Exposure to infectious agents interfaces with this pre-existing heterogeneity by providing ligands for pattern-recognition and toll-like receptors that are variably expressed on different dendritic cell subsets, and elicit production of cytokines and chemokines to support innate cell activation and drive T cell differentiation. Here we review current information on dendritic cell biology, their heterogeneity, and the properties of different dendritic cell subsets. We then consider the signals required for the development of different types of Th immune responses, and the cellular and molecular evidence implicating different subsets of dendritic cells in providing such signals. We outline how dendritic cell subsets tailor their response according to the infectious agent, and how such transcriptional plasticity enables them to drive different types of immune responses.
Keywords: dendritic cells, CD4+ T cells, Th effector responses
Subject terms: Cellular immunity, Conventional dendritic cells, Lymphocyte differentiation, CD4-positive T cells
Introduction
The differentiation of CD4+ Th cells into functionally distinct and context-appropriate effector populations is an important aspect of adaptive immunity, ensuring host protection against a wide range of pathogens that differ greatly in size, structural makeup, and location within tissues and cells. Effector Th cells are typically characterized into Tbet+ IFNγ+ Th1 cells, Gata3+ IL-4+/IL-13+ Th2 cells, RORγt+ IL-17+ Th17 cells, FoxP3+ regulatory T cells (Treg), or BCL6+ IL-21+ T follicular helper cells (Fig. 1). While it is clear that T cells may display intermediate phenotypes and cannot always be easily classified into clearly separated subsets, these Th subsets represent a useful paradigm for the study of Th cell heterogeneity and their development.1 It is known that the differentiation of each Th subset requires a specific set of signals during antigen presentation, and as the primary antigen-presenting cell population dendritic cells (DC) are considered central players in orchestrating CD4+ Th cell responses. Through their ability to surveil their local environment, DC are able to sample self and foreign antigens, and integrate signals from pathogens and bystander cells to convey relevant differentiation signals to naive CD4+ T cells in local lymph nodes (Fig. 2).
Fig. 1.
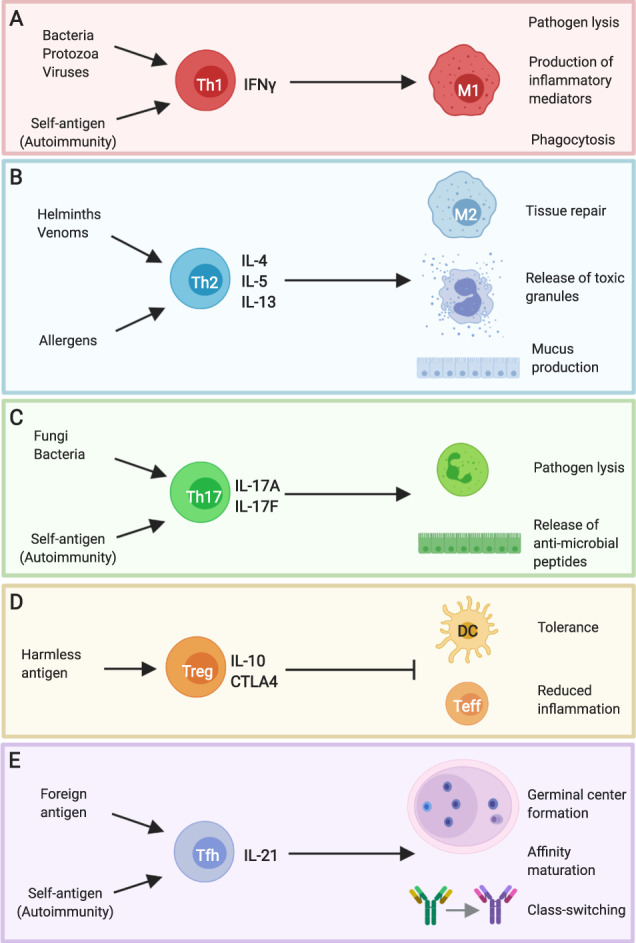
Distinct effector T helper cell phenotypes are associated with different types of pathogens and immune-driven pathologies. a IFNγ+ Th1 cells are associated with bacterial, protozoan, and viral infections, and promote microbicidal activity in macrophages. Similar pathways are invoked in some autoimmune diseases, where Th1 cells are directed against self-antigens. b Helminths and venoms drive IL-4/IL-5/IL-13+ Th2 cells that induce the release of toxic mediators from mast cells and eosinophils as well as promoting epithelial contractility and mucus production. Macrophages adopt a “M2” phenotype under these conditions to support tissue repair. Aberrant Th2 responses are strongly associated with allergic disease. c Extracellular bacterial and fungal infections drive IL-17+ Th17 cells that promote neutrophil activity and the release of anti-microbial peptides from the epithelium. Pathogenic Th17 cells are also associated with autoimmune diseases, such as multiple sclerosis and psoriasis. d Harmless antigens derived from self or foreign sources, such as the food proteins, are associated with the induction of IL-10+ Treg that act on dendritic cells and effector Th cells to regulate their function. e IL-21+ Tfh cells support antibody responses by promoting germinal center formation, affinity maturation, and antibody class switching. Tfh can be specific for foreign antigen, in the context of infection, or self-antigen, in the context of autoimmune disease. This figure was created using BioRender.com
Fig. 2.
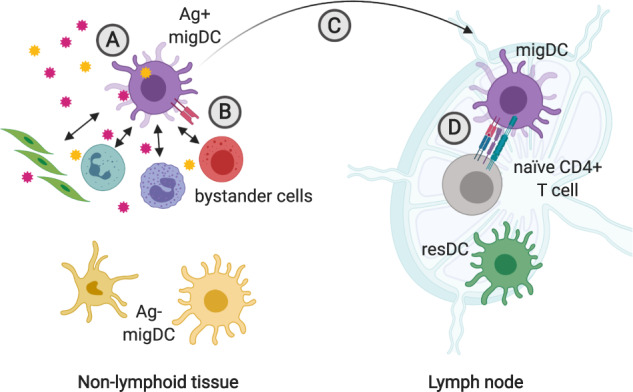
Migratory dendritic cells integrate signals from antigen and bystander cells to inform CD4+ T cell differentiation in local lymph nodes. a Migratory DC (migDC) sample antigen in non-lymphoid tissues. The nature of the antigen (foreign vs. self, immunogenic vs. tolerogenic) determines migDC activation state and expression of CD4+ T cell polarizing factors. b DC communicate with epithelial, stromal, and immune cells in the vicinity. These interactions can inform migDC expression of CD4+ T cell differentiation factors. c Ag+ migDC alter expression of integrins and chemokine receptors to facilitate their migration from the tissue to local lymph nodes. d Ag+ migDC present peptide on MHCII to naive CD4+ T cells expressing a cognate TCR in local lymph nodes. During this interaction, DC provide co-stimulatory signals and instruct CD4+ T cell differentiation through expression of polarizing molecules. This figure was created using BioRender.com
Multiple factors contribute to determine DC expression of Th cell polarizing molecules in vivo, including lineage-specified factors and context-dependent adaptation. The existence of a diverse range of DC subsets that express distinct repertoires of pattern-recognition receptors (PRR) suggests a division of labor and a specialized ability to recognize and respond to different stimuli.2 However, DC are also a highly adaptable population and readily alter their transcriptional profile in a condition-specific manner to support the appropriate Th cell response. Studies mainly carried out in DC cultured in vitro from bone marrow precursors (BM-DC) suggest that DC metabolic state is also affected (recently reviewed in ref. 3). Single-cell (sc)RNASeq data from mice immunized with different inactivated pathogens show that immunization-induced transcriptional changes are highly enriched in a small population of cDC2 that have taken up fluorescent pathogen, suggesting that signals received directly from the pathogen or its immediate environment are important determinants of DC function.4 In this review, we outline the various factors that contribute to DC heterogeneity across tissues, and then discuss how individual DC subsets and their transcriptional plasticity can shape CD4+ T cell responses in vivo.
Dendritic cell subsets across tissues
Conventional DC
Conventional (c)DC are defined by the expression of the transcription factor zBTB46 that differentiates them from other DC populations such as lymphoid-derived plasmacytoid DC (pDC) and monocyte-derived DC (moDC).5,6 cDC populate almost all tissues and are the most abundant DC subtype under steady-state conditions. cDC are a heterogenous population that have been organized into subsets using various criteria depending on the tissue or species under investigation. Aided by high-throughput and high-dimensional techniques, as well as by information on the transcription factors that drive cDC development in mice and humans,7 a unified classification strategy that can be applied across tissues and species has been identified.8,9 Using this strategy cDC can be stratified into one of four subsets based on whether they initially seed lymphoid or non-lymphoid tissues and their developmental requirements.
Migratory and lymphoid-tissue resident cDC populations
cDC can be broadly divided into two populations based on their initial tissue of residence. Lymphoid-tissue-resident (res)DC directly seed lymphoid organs, such as the spleen, lymph nodes, or Peyer’s patches, where they remain as resident sentinels. In contrast, migratory (mig)DC initially seed non-lymphoid tissues, such as the lung, skin, or lamina propria, and migrate to local lymphoid organs either spontaneously under steady-state conditions or upon inflammation-induced activation in a CCR7-dependent manner.10 As such, lymphoid tissues can contain both resDC and tissue-derived migDC. Flow cytometry and histocytometry techniques are generally used to resolve LN resDC and migDC populations by their differential expression of MHCII, CCR7, and CD11c.8,11 MigDC highly express MHCII and CCR7 and have intermediate-to-low expression of CD11c, whereas resDC highly express CD11c and have intermediate expression of MHCII under steady-state conditions.12 Analysis of LN tissue from organ donors suggests that migDC can also be identified by high HLA and CCR7 expression in human samples.13,14 However, it is important to note that the inducibility of MHCII and CCR7 expression on resDC populations during inflammation makes defining resDC and migDC by the sole use of these markers problematic. Unfortunately, stably expressed markers specific for resDC or migDC have yet to be identified. For this reason, the use of photoconvertible mouse models that enable tracking of cell migration are extremely valuable. Several studies have utilized the Kaede or KikGR mouse models to ascertain which LN cells originated from the skin. Cells present in the skin at the time of photoconversion fluoresce Kaede/KikGR-red, differentiating them from all other cells that are Kaede/KikGR-green.15 Following photoconversion migratory Kaede/KikGR-red cells can be detected in the draining LN and consist of migDC, neutrophils, monocytes, and CD4+ T cells, but not resDC.16,17 Similar studies have also highlighted the presence of MHCIIhi CCR7+ migDC expressing CD64, a marker previously thought to be restricted to monocyte and macrophage populations8,18–20 that can also be expressed upon interferon (IFN) exposure (Immgen.com) or by activated cDC.21,22 Given these findings, the use of CD64 as an exclusion marker may not be appropriate for migDC analyses. The use of different markers such as CD88, Ly6C, or MerTK is then required to differentiate cDC from monocyte and macrophage populations in the LN and, more importantly, in non-lymphoid tissues such as the skin and mucosal surfaces. Together with CD26, a marker highly expressed by all cDC populations, the use of CD88 enables a clear distinction between cDC and monocyte/macrophages.8,23
Despite the aforementioned limitations with current surface markers, resDC and migDC sorted from the same lymphoid organ using conventional gating strategies have distinct transcriptional profiles9, which are largely consistent with expression profiles generated using scRNASeq technology.4 Unsurprisingly, many of the differentially expressed genes are associated with cell migration, with transcripts such as Ccr7 and Fscn1 highly expressed by migDC but not resDC (Table 1). Transcripts for MHCII genes including H2-Aa, H2-Ab1, Cd74, and especially H2-DMa and H2-DMab are also expressed at lower levels in migDC compared with resDC, which is consistent with the reduced ability of mature migDC to process additional antigens in LN.4,9 Despite the profound transcriptional shutdown of MHCII genes upon maturation, MHCII protein expression remains high on the surface of migDC to facilitate efficient antigen presentation.4 This example highlights the power of techniques, such as index sorting and CITESeq, that enable the simultaneous assessment of mRNA and protein expression in the same assay.24
Table 1.
Transcriptional signatures and protein markers of described murine DC subsets as determined from published resources4,8,9
| Subset | Transcriptional signature (RNASeq, microarray) | Signature protein markers (flow/spectral cytometry, CyTOF, microscopy) |
|---|---|---|
| Total Lymphoid-resident cDC | Tagap, Tagap1, Hhex, Tmem231, Snord13, Prdx6, Kynu | CD11chi, MHCIIint |
| Total migratory cDC | Fscn1, Ccr7, Cacnb3, Ltc4s, Clu, Epsti1, Ccl17, Il15, Il15ra | MHCIIhi, CCR7+ |
| cDC1 | ||
| All | Xcr1, Cadm1, Clec9a, Naaa, Irf8, Batf3, Id2, Cxcr3, Tlr3, Ifi205, Gcet2 | XCR1+, IRF8+ |
| Migratory | CD103+, CD11b− | |
| Lymphoid-resident | CD8α+, CD11b− | |
| cDC2 | ||
| All | Sirpa, Il2ra, Aldh1a2, Zeb2, Irf4, Sirpb1a, Sirpb1b, Dscam | SIRPα+, IRF4+ |
| Migratory | CD11b+, CD103−a | |
| Lymphoid-resident | CD11b+, CD8α− | |
| LC | Cd207, Fam189a2, Tjp1, Pcdh7, Mtap2 | CD207+, CD326+, XCR1− |
| moDC | Sirpb1c, Ly6c1, C3ar1, Ccr2, Plac8, Ms4a6b, | CD64+, Ly6Chi, CD11c+, CCR2+ |
| pDC | Mctp2, Ly6d, Klk1, Siglech | CD317+, Siglec H+ |
Type-1 and type-2 cDC populations
cDC precursors develop from monocyte-dendritic cell precursors in the bone marrow where they commit to one of two distinct cDC lineages that differ in their developmental requirements.25–27 cDC1 preferentially develop from Ly6C− Siglec H− preDCs25 in a BATF3- and IRF8-dependent manner27–29 and can be identified by their surface expression of XCR130 and, in non-lymphoid tissues, CD103.31,32 cDC2 preferentially develop from Ly6C+ Siglec H− CD115+ preDCs25,27 and are identified by their expression of SIRPα in combination with cDC-specific markers. The transcriptional regulation of cDC2 development has been shown to involve a number of transcription factors including Zeb233 N and Irf4,34–36 which positively regulate the development of all cDC2, and Klf437 and Notch238 Z, which positively regulate the development of various cDC2 subsets. Similar populations of cDC1 and cDC2 precursors have been identified in bone marrow biopsies and peripheral blood samples from healthy volunteers where they can be distinguished on the basis of SIRPα expression.39
preDC seed both lymphoid and peripheral tissues where they undergo clonal expansion.26,40,41 Interestingly, cDC1 and cDC2 localize to different regions within lymphoid and non-lymphoid tissues, a factor that determines their access to antigen and interactions with other immune cell populations (recently reviewed in refs. 42,43). Differential expression of chemokine receptors between cDC1 and cDC2 precursors may partially explain their distinct distribution patterns. Pre-cDC1 highly express CXCR3, whereas pre-cDC2 express CX3CR1.44 Given that the CXCR3 ligands are the IFN-inducible chemokines CXCL9/10/11, preDC1 may be preferentially recruited to sites of IFN-mediated inflammation.44 Further, the relative proportion of each cDC subset differs greatly across tissues.8,13 For example, in mice, cDC1 are rare in skin but are comparatively more abundant in the lung,8 whereas the cDC subset composition is compartmentalized along the intestinal tract.45 These differences in abundance do not always extend to humans, as suggested by the low proportion of cDC1 compared with cDC2 in human lung.13 This variability may suggest a greater impact of environmental and infectious stimuli on human cDC development.
In addition to their divergent developmental pathways, cDC1 and cDC2 have distinct transcriptional profiles, with key transcriptional regulators such as IRF4 and IRF8 among the most differentially expressed genes. Markers used in cDC subset discrimination by flow cytometry, such as XCR1 and SIRPα, are also differentially expressed at the transcript level (Table 1). Interestingly, transcriptional segregation of cDC1 and cDC2 subsets in lymph nodes is much more evident within the resDC compartment.4,9 The relative similarity in transcriptional profiles among migDC subsets suggests that tissue-derived factors and/or cDC migration signals have a significant influence over the migDC transcriptome. Indeed, studies directly comparing the transcriptional and phenotypic profiles of cDC across human tissues have reported marked tissue-specific signatures.13,46
cDC2 subsets
While cDC1 are accepted to be a largely homogenous population, cDC2 are highly heterogenous and have been divided into subpopulations according to various criteria that include the expression of CD11b,47,48 CD301b (MGL2),49 CD206,50 PDL2,51 Esam,38 and Tbet,52 or the developmental reliance on Notch2,38 or Klf4.37 With the exception of CD301b and CD206 that are co-expressed on a subset of cDC2, there is no strong association between each of these classification strategies, highlighting the extent of phenotypic heterogeneity within the cDC2 subset. Tissue-specific adaptation may account for some of this heterogeneity, with a population of Runx/Cbfβ/TGFβR-dependent CD103+ cDC2 unique to intestinal tissues53,54 and CD11b-low SIRPα+ cDC2 enriched in the skin.47,48 A recent single-cell transcriptional study of murine and human cDC2 has provided additional evidence for the existence of distinct cDC2 subsets across tissues.52 These data show that cDC2 can be segregated into Tbet+ and RORγt+ subsets that differ both transcriptionally and epigenetically. However, the study of these populations remains restricted by the need for appropriate reporter mouse strains for the phenotypic identification of the two subsets.
Other DC subsets
In addition to cDC, a number of other DC populations are found in mice and humans. These include moDC, Langerhans cells (LC), and pDC. Circulating Ly6Chi CD11b+ monocytes are actively recruited to sites of inflammation or infection where they can adopt DC-like features, including expression of CD11c and enhanced antigen presentation capacity.55,56 In vitro, GM-CSF and IL-4 promote the differentiation of monocytes into moDC in an IRF4-dependent manner.57,58 In the absence of Irf4, GM-CSF and IL-4-treated monocytes differentiate into macrophages,57 indicating that Irf4 is an important regulator of moDC differentiation. Inflammation and infection are associated with the development of moDC in vivo,59–61 with IFNγ signaling implicated in driving monocyte-to-DC differentiation following T. gondii infection.62 However, the signals involved in the monocyte-to-moDC transition are likely to be context-dependent given that moDC are present in wide range of tissues and inflammatory settings.63
LC are a radio-resistant population that share properties with both DC and macrophages64 and are uniquely positioned in the epidermis where they reside in close contact with surrounding keratinocytes that provide factors required for LC survival and epidermal residence.65,66 Similar to tissue-resident macrophages, LC are continually replenished from a precursor pool67 that is seeded into the epidermis during embryonic development,68,69 but can also be derived from monocytes following injury.70 LC are readily identifiable by cell surface expression of langerin (CD207), E-cadherin, CD326, low CD11b, and, importantly, their lack of XCR1 and CD103 expression, distinguishing them from dermal cDC1.8
Finally, pDC are a distinct DC lineage that can be of lymphoid or myeloid origin71–73 and are identifiable by their expression of IRF7, Siglec H, and high levels of CD317.74–76 Unlike their lymphoid counterparts, myeloid-derived pDC are able to present antigen to T cells,73 but their role in regulating CD4+ T cell responses remains to be determined.
Antigen uptake, transport, and presentation by cDC subsets
Antigen sampling and transport to lymphoid tissues
In their capacity as tissue sentinels DC continually sample antigen from their local environment and process it for presentation to CD4+ Th cells or CD8+ T cells on MHCII or MHCI molecules, respectively.77,78 Exogenous antigen is sampled by phagocytosis,18,79,80 receptor-mediated endocytosis81–83, or micropinocytosis,84,85 depending on the subpopulation and activation status of the DC.81 At barrier sites, such as the epidermis and intestinal epithelium, LC and CX3CR1+ macrophages sample antigen by projecting dendrites through tight junctions into the cornified epithelial layer86,87 or intestinal lumen,88,89 respectively. Dermal cDC2 can also directly access epicutaneously applied antigen through hair follicles.90 Following antigen uptake, human LC transit through the dermis in a CXCR4-dependent manner91 prior to CCR7-dependent migration to the skin-dLNs. LC can also transfer antigen-MHCII complexes directly to cDC within the dermis.92 This transfer likely increases the efficiency at which epidermal-derived antigen reaches the LN as dermal cDC migrate to the LN at a faster rate, and distribute within the LN more widely, than LC.17,47,93 Similarly, sessile intestinal macrophages transfer antigens to migratory cDC via gap junctions for transport to the mediastinal LNs.94
cDC2 are particularly adept at antigen uptake in the skin, making up the majority of antigen-positive (Ag+) cells in skin-dLNs following injection or application of fluorescently labeled particulate antigen.4,95–98 cDC2 also play an important role in the uptake of tumor antigens and their transport to LNs for presentation to T cells.50 Soluble and particulate antigens < 70 kDa do not require active cellular transport to LN and are distributed throughout LN via a conduit network.99,100 It is therefore unsurprising that soluble antigens are taken up more ubiquitously than larger particles. Nevertheless, cDC2 seem to take up higher amount of soluble antigen per cell, and are overrepresented among Ag+ cells in a number of settings,47,101,102 suggesting that cDC2 have an intrinsic capacity for the uptake of exogenous antigens. Further, cDC2 may be optimally positioned for antigen capture within tissues as resDC2 are preferentially located near the subcapsular sinus within LNs, enabling easier access to lymph-borne antigens.98,101
Conversely, cDC1 are located deep within the LN paracortex101 and are efficient at taking up cell-associated antigen and dead cells via receptors such as Clec9A, DEC205, Axl, and TIM3.103 cDC1 preferentially process cell-associated antigen via the cross-presentation pathway for presentation on MHCI to CD8+ T cells, which is essential for antiviral and antitumor immunity.28,103 Indeed, CLEC9a and DEC205 have been successfully targeted with monoclonal antibodies to enhance antigen uptake and cross-presentation in the context of vaccination.104–106 moDC and cDC2 also cross-present antigen under certain conditions, such as in the presence of immune complexes,107–109 suggesting a level of redundancy in this pathway that is particularly apparent in humans.102,110,111
Antigen presentation to naive CD4+ T cells
DC are the primary antigen-presenting cell population in vivo and are essential to elicit antigen-specific activation and expansion of naive CD4+ T cells though the peptide–MHCII interaction with the TCR and co-stimulatory signaling. In line with their superior capacity to take up exogenous antigens, cDC2 are particularly efficient at processing antigen for presentation on MHCII and induce superior CD4+ T cell proliferation compared with cDC1.104,112 Irf4 expression by cDC2 is thought to account for this specialization, with IRF4 linked to enhanced peptide–MHCII formation in cDC.112
In addition to promoting the activation and proliferation of CD4+ T cells, communication between DC and CD4+ T cells during antigen presentation determines Th cell differentiation fate. A number of factors are implicated in regulating the Th cell differentiation program, including the strength and duration of co-stimulatory and peptide-MHCII–TCR interactions.113 For the purpose of this review, we will focus on DC-derived polarization signals, and how DC lineage and condition-specific adaptation regulate CD4+ T cell differentiation in vivo. This is discussed for the five major Th cell phenotypes below and is summarized in Fig. 3.
Fig. 3.
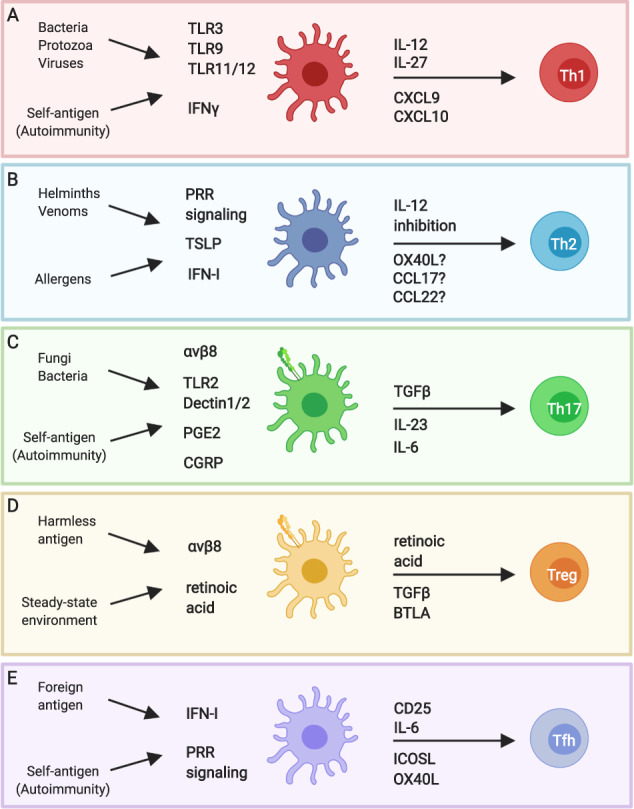
Transcriptional plasticity of dendritic cells determines CD4+ T cell differentiation in vivo. Signals from pathogens, immunogens, and antigens, as well as signals from the local stromal, epithelial, and immune cells influence DC expression of CD4+ T cell polarizing factors in a context-specific manner. This ensures activation of the appropriate effector Th cell phenotype. a IFNγ from bystander cells and signaling through pattern-recognition receptors (PRR), such as TLR3, 9, 11, and 12, induces Il12b upregulation by DC. IFNγ also promotes transcription of the CXCR3 ligands Cxcl9 and Cxcl10. Together with IL-27, DC production of these factors facilitates Th1 differentiation. b Helminth products condition DC to promote Th2 differentiation in vivo, potentially via PRR signaling, and DC integration of IFN-I and TSLP signals produced by bystander cells. The DC-derived factors that specifically promote Th2 differentiation are unclear but may include limiting IL-12 production in addition to a “positive” Th2 signal such as OX40L, CCL17, or CCL22. c Engagement of PRR, such as TLR2 and Dectin-1, promote DC production of IL-6 and IL-23. Fibroblast-derived prostaglandin E2 and neuron-derived calcitonin gene-related peptide also promote IL-23 production by DC. Together with αvβ8-mediated activation of TGFβ, DC production of these factors promotes Th17 differentiation. d At steady state, DC are conditioned into a tolerogenic state by their local tissue environment. Retinoic acid may regulate this state in some tissues/settings. Tolerogenic DC express BTLA and the TGFβ activating integrin αvβ8, and produce retinoic acid. In the absence of immunostimulatory signals, the expression of these factors helps support the generation of Treg. e PRR and IFN-I signaling support IL-6 production by DC. Along with co-stimulatory signaling, IL-6 supports Tfh differentiation. DC also express CD25 to limit IL-2 availability to further support Tfh priming. This figure was created using BioRender.com
DC instruction of CD4+ T cell differentiation
Instruction of Th1 differentiation
Th1 cells are defined by their production of IFNγ and are associated with protective immunity against intracellular pathogens and viruses.114 The Th1 transcriptional program is driven by IFNγ,115,116 IL-12,117 and IL-27,118 which promote the upregulation of Tbet and IL-12Rβ in CD4+ T cells.119 IL-12 signaling reinforces the Th1 program and is essential for optimal Th1 differentiation in both mice and humans.120–122
cDC1 are a major source of IL-12 in vivo.123–125 They constitutively express high levels of Il12b transcripts, with evidence of constitutive IL-12p40 protein production in mice.126,127 As such, cDC1 are commonly associated with Th1 responses and are often portrayed as the principal Th1-inducing cDC subset. Indeed, cDC1 are more efficient than cDC2 at inducing Th1 differentiation in ex vivo coculture systems,128 presumably due to their baseline expression of IL-12. Th1 responses are also significantly compromised in some systemic,129 cutaneous123,130 and intestinal infection models129,131–133 when cDC1 are absent, suggesting that cDC1 are required to support some protective Th1 responses in vivo. However, there is a notable body of literature implicating moDC in driving Th1 responses during infection with Plasmodium,134 T. gondii62, or Salmonella135 as well as in CpG- or CFA-based immunization models.136,137 It is not clear whether moDC can serve as antigen-presenting cells in these settings given their low expression of MHCII and co-stimulatory molecules.18,138 Although inefficient providers of signals 1 and 2, moDC are a good source of signal 3 and can produce high levels of IL-12p40 in vitro137,138 and in vivo (Hilligan et al., submitted manuscript). A cooperation between moDC and cDC has been suggested by several studies that designated moDC as regulators of Th1 differentiation and cDC as inducers of Th1 proliferation.135,136,138,139 Finally, a recent study has demonstrated a role for TNFR2+ cDC2 in driving Th1 responses following intranasal immunization with a mucosal adjuvant.140 Specific targeting of this cDC population reduced recall Th1 responses in the lung and abrogated immunization specific antibody production.140 Together, these data suggest that a number of DC subsets have the capacity to support Th1 differentiation in vivo and the context and location of infection or immunization determines the DC subset requirements.
Given the critical requirement for IL-12 in Th1 immune responses, signals that regulate IL-12 expression in DC likely contribute to potentiating Th1 differentiation. Interestingly, constitutive IL-12p40 production by cDC1 is maintained under germ-free and antigen-free conditions, (Kawabe et al., submitted manuscript) suggesting that IL-12 production is intrinsically coupled to the cDC1 lineage and is not the result of exogenous microbial signals. Homeostatic IL-12 is thought to function as a regulator of Th2 responses126,127 and support the generation of innate-like Tbethi memory-phenotype (MP) CD4+ T cells (Kawabe et al., submitted manuscript) that can produce IFNγ in the absence of TCR signaling and provide early host protection against infection.141
During infection or inflammation, a number of signals induce or enhance IL-12 production by DC. Firstly, signaling through PRR such as TLR3 and TLR9 enhances DC expression of IL-12p40,142–144 and the engagement of multiple PRR synergizes to boost IL-12 production.145 Secondly, CD40:CD40L interactions between DC and T cells promotes transcription of the IL-12p35 subunit in DC,146,147 supporting the production of bioactive IL-12p70. Finally, IFNγ derived from NK or T cells feedbacks on DC to further promote IL-12 production.148–150 IFNγ also promotes cDC and moDC expression of CXCR3 ligands which recruit CXCR3+ pre-Th1 cells to a niche that favors Th1 differentiation.4,151,152 DC–NK cell crosstalk likely facilitates the generation of Th1 cells through this pathway.153 It is possible that the main role of cDC1 in Th1 immunity is their involvement in eliciting early IFNγ from NK154 and CD4+ MP cells141 that then conditions cDC2 and moDC towards a Th1-inducing phenotype.
Instruction of Th2 differentiation
Th2 cells produce IL-4, IL-5, and IL-13155 and are associated with helminth infection, exposure to venoms, and allergic disease.156 IRF4+ cDC2 are necessary for promoting Th2 differentiation in the skin,51 lungs,157 and intestinal tract,132,158 with KLF4-dependent37 and CD301b+ PDL2+ cDC2 subsets49,159 specifically implicated in Th2 models. LC may also support Th2 responses in the skin as LC depletion impairs IL-4 production following epicutaneous application of protein antigen160 or a vitamin D analog that drives an atopic dermatitis-like phenotype in mice.161 In contrast, cDC1 negatively regulate Th2 responses through constitutive IL-12p40 production and their absence can increase the levels of Th2 cytokines in mouse models of helminth infection126,132 and house dust mite allergy.127 The inhibitory effect of cDC1 appears to vary depending on the Th2 model being used, and in some cases it can also be observed for LC,162 which also constitutively express some level of Il12b.9 In this respect it is also important to note that, while lower IL-12 production may result in increased Th2 responses in some models, IL-12-KO mice exposed to Th1 stimuli develop lower Th1 responses without defaulting to Th2.163 Thus, lack of IL-12 may contribute but is not sufficient for Th2 differentiation.
Exposure to helminth products endows cDC2 with the capacity to induce Th2 differentiation, suggesting that DC instruction of Th2 responses is an active process rather than a default one.96,158,164–166 Indeed, there is evidence that the S. mansoni egg antigen (SEA)-derived protein Omega1 inhibits IL-12 production by DC and alters DC morphology to limit contact time with CD4+ T cells,167,168 two factors thought to contribute to Th2 differentiation.169 In an in vitro model, SEA can also trigger Dectin-1 and Dectin-2 receptors on DC, leading to prostaglandin-dependent expression of OX40L and development of Th2 responses.166 In the same study, in vivo experiments in mice also supported a pro-Th2 role of Dectin-2 in the SEA response.
Transcriptomic analysis of helminth- or allergen-conditioned cDC has identified thymic stromal lymphopoietin (TSLP) and IFN-I as upstream regulators of helminth or allergen-induced transcriptional networks in cDC.162,170 These signatures are restricted to Ag+ cDC2, with comparatively little change observed in the transcriptional profile of Ag− cDC2 and cDC1 compared with baseline.4,162 TSLP has long been associated with allergic responses in mice and humans, and is known to be a potent inducer of DC activation accompanied by the upregulation of Tnfsf4 (encoding OX40L), and the expression of the chemokines Ccl17 and Ccl22 to attract CCR4-expressing Th2 cells (reviewed in ref. 171). However, it is important to note that the DC-specific effects of TSLP signaling are complicated by the finding that TSLP also acts directly on T cells to promote the secretion of type-2 cytokines.172,173 A recent OVA allergy model employing Tslprfl/fl animals crossed to cDC-specific (Zbtb46) or CD4+ T cell-specific (Cd4) Cre lines demonstrates that TSLPR signaling in both cell types is important for driving Th2 inflammation.174 IFN-I signaling has been more recently associated with Th2 responses, and interestingly, is only associated with Th2 responses in some settings, such as intradermal injection of N. brasiliensis larvae or house dust mites. In these cases, IFNAR signaling is required to promote robust IL-4 production by Th2 cells in vivo.162,175 The IFN-I requirement for Th2 responses is likely due to a direct effect on DC rather than T cells, as suggested by experiments using immunization with IFNAR-deficient BM-DC.175 IFNAR blockade does not impact cDC2 activation or migration,162 suggesting that other IFN-I driven signals within DC support instruction of Th2 responses. Further investigation of IFN-I driven transcriptional programs in DC may uncover previously unidentified signals involved in Th2 differentiation.
In conclusion, the DC-derived signals required to initiate and reinforce the Th2 differentiation program remain currently unclear. A source of IL-4 appears to be required to support IL-4 production by Th2 cells in vivo,176 a finding that may reflect the recognized role of IL-4 in Th2 differentiation in vitro.177 However, the origin of this early IL-4 production remains undefined, with no evidence suggesting that DC might be the source of such IL-4. While TSLP and IFN-I can condition DC and promote Th2 development, neither is essential except in rare cases. OX40L–OX40 interactions between DC and CD4+ T cells, as well as the DC-derived chemokines CCL17, CCL22, and possibly CCL8, may support Th2 differentiation;178–182 however, it remains to be determined whether these signals are definitive Th2 polarizers or are merely involved in co-stimulation183 and Th2 cell trafficking,184,185 respectively. Future studies may provide new information on this important question.
Instruction of Th17 differentiation
Th17 cells are characterized by production of IL-17 and are required for resolving extracellular bacterial and fungal infections. Th17 cells are also strongly implicated in pathogenesis of many autoimmune diseases, including multiple sclerosis and psoriasis.186 IL-6 and TGFβ are critical signals for Th17 differentiation in vitro,186 whereas IL-1β and IL-23 can support Th17 differentiation in vivo in the presence of IL-6 or TGFβ.187,188
LC have a documented association with cutaneous Th17 responses and accumulate in psoriatic lesions.189 Studies in mice have shown that LC are a significant source of IL-6 and depletion of LC in the context of an epicutaneous C. albicans infection significantly reduces the number of antigen-specific Th17 cells in the dLN.130,190 However, other studies have found no role for LC in driving cutaneous Th17 inflammation.191 Instead, dermal cDC2 have been implicated,192 which is in line with a large body of data showing that IRF4-dependent cDC2 are required to support Th17 differentiation across a number of tissues including the lung36,193 and intestine.38,35,54,194–196 Unlike the cDC2 involved in promoting Th2 immune responses, Th17-promoting cDC2 require Notch2, but not KLF4, for their development.195 Finally, CCR2-dependent moDC have also been associated with promoting Th17 responses in an oropharyngeal candidiasis model. Similar to Th1 models, moDC are thought to cooperate with cDC to facilitate optimal Th17 differentiation.197
DC provide many of the required Th17 differentiation signals in vivo192,193,195,198 and facilitate the activation of latent TGFβ via αvβ8 integrin. Indeed, DC-specific deletion of αvβ8 significantly blunts Th17 responses in the lamina propria199 and limits IL-17-mediated pathology in an experimental autoimmune encephalitis model.200,201 A number of signaling pathways are involved in inducing IL-6 and IL-23 production by cDC, including signaling via TLR2 and Dectin-1, PRR that recognize glucans enriched in fungal cell walls.148,190 TLR2 engagement preferentially induces IL-23 over the related cytokine IL-12,202 suggesting that the distribution of PRR activation directly influences DC expression of Th cell polarization factors. Signaling via other PRR can also promote IL-23 and IL-6 production by DC, including imiquimod engagement of TLR7 in dermal cDC191 and flagellin activation of TLR5 in lamina propria cDC2.196 More recently, interactions between stromal cells, neural networks, and DC have been implicated in eliciting and potentiating Th17 immunity, particularly in the skin. Fibroblast-derived prostaglandin E2 (PGE2) can promote IL-23 production by preactivated human cDC, and skin fibroblasts from psoriasis patients were found to express considerable levels of COX-2, an enzyme involved in prostaglandin synthesis. In turn, cDC-derived TNFα and IL-1β promote fibroblast secretion of PGE2, implicating fibroblast-DC cross-talk in potentiating Th17 inflammation.203 Similarly, calcitonin gene-related peptide (CGRP), a neuropeptide derived from sensory TRPV1+ neurons, promotes IL-23 production by dermal cDC2 following C. albicans infection, or induction of psoriasiform inflammation in mice.204,205 TRPV+ neurons can also trigger CGRP release at sites adjacent to infection, pre-empting cDC responses to help contain infection.206
Instruction of iTreg differentiation
In the periphery, CD4+ T cells can differentiate into inducible regulatory Th cells (iTreg) that limit inflammation and establish immunological tolerance.207 iTreg can be distinguished from the thymus-derived natural Treg, which are not considered in this review, through their expression of the transcription factor Helios, which is expressed by natural Treg but not iTreg.208 TGFβ and IL-2 signaling are important determinants of iTreg differentiation,209–211 with cDC-dependent activation of TGFβ important for optimal iTreg induction.212 In addition, cDC expression of BTLA213 and cDC-derived retinoic acid214,215 support iTreg differentiation in vivo by negatively regulating molecules associated with the differentiation of other effector CD4+ T cell subsets.216,217 Treg-DC cross-talk may also play a role in limiting inflammatory responses as Treg actively deplete CD80, CD86,218,219 and peptide–MHCII complexes from cDC.220
The involvement of DC in instructing iTreg differentiation has been largely studied in the context of the intestine where DC-derived signals are known to play an important role in inducing immunological tolerance to food proteins.221 IRF8- and BATF3-dependent cDC1 are necessary for optimal iTreg induction,129,221 which is in line with their selectively high expression of BTLA,213 Aldh1a2 (encoding retinaldehyde dehydrogenase 2 or RALDH2), an enzyme involved in retinoic acid production, Tgfb2, and Itgb8 (encoding a subunit of αvβ8), an integrin required for TGFβ activation.131,221,222 However, oral tolerance is maintained in animals lacking IRF8-dependent cDC1, suggesting a level of redundancy in intestinal iTreg induction.31,221,223 Experiments in which ovalbumin-specific CD4+ T cells were co-cultured with various mesenteric LN cDC subsets pulsed with ovalbumin peptide showed that CD103+ CD11b+ migDC2 and CD8α+ resDC were also capable of inducing an iTreg differentiation program, albeit to a lesser extent than CD103+ CD11b− migDC1.221
Redundancy in DC subset instruction of iTreg differentiation is further exemplified in the skin where LC and cDC2 also have demonstrable roles in Treg induction. The proximity of epidermal LC to commensal microbes colonizing the surface of the skin has led to the proposal that the main function of LC is to induce tolerant responses. Indeed, in animals where MHCII expression was restricted to LC, epicutaneous immunization failed to induce effector or memory CD4+ T cell responses.224 Further, other studies have shown that depletion of LC reduces Treg numbers and improves effector immune responses in a Leishmania major model.225 LC were also found to mediate the induction of Treg following skin exposure to ionizing radiation.226 In the skin, RALDH2 expression is greatest within the dermal cDC2 population, which were also found to promote iTreg induction ex vivo.214
The signals involved in promoting tolerogenic functions of DC are currently unclear. NFκB signaling may be involved as DC-specific deletion of IKKβ impairs Treg induction, resulting in spontaneous autoimmunity.227 The observation that IKKβ deficiency also reduces the accumulation of tissue-derived cDC in LNs suggests that NFκB signaling may regulate cDC migration rather than a tolerogenic program specifically.227 Instead, adaptation to the tissue microenvironment may be important for initiating a tolerogenic cDC phenotype. TGFβR, retinoic acid, and MyD88 signaling are required for optimal Itgb8 expression by intestinal cDC1 and are sufficient to promote Itgb8 expression by splenic cDC1 ex vivo.222 Further, Aldh1a2 expression is specifically enriched in cDC1 situated in the proximal intestine where iTreg induction is most prominent, indicating strong regionalization of tolerogenic programs within cDC1.45 These data suggest that tolerogenic programming likely occurs in the tissue prior to migration, which may explain the largely concordant nature of transcriptional changes upon homeostatic or immunogenic maturation of cDC1 populations.228
Instruction of Tfh differentiation
Tfh cells are a specialized Th cell subset that localize to B cell areas within secondary lymphoid organs where they support germinal center formation, affinity maturation, and antibody class switching (recently review in ref. 229). The differentiation pathway of Tfh cells is a multistep process that generally requires signals from both DC and B cells.230 IL-6 and inducible co-stimulator (ICOS) signaling initiate the Tfh differentiation program by promoting the expression of Tfh-defining proteins, including BCL6, CXCR5, and IL-21.231–235 An IL-21 autocrine signaling loop further supports their differentiation.235 IFN-I,236 IL-27,237 IL-1β,238 OX40,239 and Notch signaling240 can also support Tfh differentiation in vivo. In contrast, IL-2 is a potent inhibitor of Tfh differentiation241,242 and Tfh-inducing cDC express the IL-2 receptor (CD25) in order to limit the availability of local IL-2.243
DC are thought to play an important role in directing the initial phases of Tfh differentiation, with B cells providing subsequent signals to reinforce the Tfh program. However, a recent study has challenged this notion in the context of Plasmodium infection where cDC were reported to be dispensable and B cells necessary for the generation of antigen-specific Tfh responses.244 Nevertheless, most cDC subsets have the capacity to induce Tfh differentiation in the appropriate conditions (recently reviewed in ref. 245) and cDC2 populations are necessary for regulating Tfh differentiation and humoral responses in a number of settings.98,140,243,246–249 Several mouse models in which cDC2 were specifically depleted report a reduction in Tfh, germinal center B cells, and antibody titers following an immunization or infection protocol.140,246,247,249 In humans, cDC2 isolated from tonsils were identified as the most effective DC subset at inducing Tfh polarization.250 These data suggest that cDC2 support optimal Tfh differentiation in both mice and humans. Interestingly, cDC2 can also have a role in limiting Tfh and antibody responses in the absence of sufficient adjuvanticity. This function is performed by CD301b+ cDC2 and likely serves to prevent the generation of autoantibodies and restrict the generation of antibodies against allergens.251
LC and cDC1 also have the capacity to induce Tfh differentiation, but this only appears to be efficient if antigen is directly targeted to these cell types with Clec9A or Langerin antibodies.252–255 In experiments employing untargeted immunization protocols, LC only partially contributed to Tfh responses,256 and there was no documented defect in Tfh differentiation or antibody responses in animals lacking cDC1.140,246,247,249,256 The role of monocyte-derived cells, including moDC, in Tfh responses is less well defined, with examples of monocytes supporting, inhibiting or having no effect on Tfh numbers or antibody responses depending on the model being assessed. Depletion of monocyte-derived cells had no impact on antibody production in Salmonella infected mice135 or the number of germinal center B cells in papain immunized mice,251 suggesting that cells other than moDC are supporting Tfh responses in these animals. In other models, moDC are shown to negatively regulate Tfh differentiation. Monocyte depletion was associated with increased Tfh differentiation at the expense of Th1 differentiation following Plasmodium infection,134 suggesting that monocyte-derived signals may regulate Th1/Tfh bifurcation.134 There is also evidence that monocytes are required to provide critical Tfh-inducing factors in some settings. MoDC-derived IL-6 boosted Tfh responses in a CpG-B immunization model257 and IL-1β derived from CX3CR1+ patrolling monocytes was required for Tfh differentiation following administration of heat-killed E. coli.238 Together, these studies underscore the context-specific nature of the cell types involved in Tfh differentiation and the extent of cellular redundancy in this pathway.
As mentioned above, a number of redundant signals from cDC, monocytes, and B cells are involved in Tfh differentiation, which makes defining a “Tfh-inducing” profile difficult. One of the most critical factors for Tfh differentiation are early co-stimulatory interactions between DC and naive CD4+ T cells. DC-dependent ICOS signaling is required to induce BCL6 and CXCR5 in CD4+ T cells within the first few days of infection.231 Similarly, cDC expression of CD40,258 OX40L,239,248,259 and Notch ligands240 support Tfh differentiation to varying degrees. The non-canonical NFκB pathway is required for cDC2 expression of ICOSL and OX40L following polyIC stimulation,248 and B-cell expression of ICOSL,260 suggesting that this pathway may facilitate Tfh priming.
In addition to co-stimulation, DC promote Tfh responses through IL-6, with IL-6-KO DC inefficient at promoting IL-21+ Tfh cells.261,262 Early IFN-I is an important inducer of DC-derived IL-6263 and IFNAR deficiency in the DC compartment leads to blunted Tfh responses after VSV infection.262 TRIF-dependent signaling in monocytes has been implicated in endowing these cells with the capacity to induce Tfh differentiation through production of IFN-I and inflammasome activation.238 T cell intrinsic expression of IFNAR and IL-1Rβ was required for optimal Tfh differentiation in this model,238 suggesting that IFN-I supports Tfh responses through actions on DC and T cells. This may explain the IFN-I-dependent enhancement of Tfh cell and germinal center responses in animals co-administered dsRNA with an influenza vaccine.264
Finally, given that co-localization of pre-Tfh with B cells is a requirement for full Tfh differentiation,265 chemokine signaling is an important factor in determining Tfh fate. CXCR5 expression mediated by ICOS signaling occurs within two cell divisions and is necessary for positioning pre-Tfh near B-cell areas.231 In their study, Lonnberg et al. used pseudo-time modeling of scRNASeq data to show Plasmodium-specific Th cells co-express Cxcr3 and Cxcr5 prior to Th1/Tfh bifurcation. This observation coupled with their finding that depletion of monocyte-derived cells favors a Tfh fate over a Th1 fate led them to propose that IFN-dependent moDC production of CXCR3 ligands may act to limit Tfh differentiation by preferentially recruiting antigen-specific CXCR3+ CXCR5+ Th cells to a niche that supports Th1 differentiation.134 This proposal is supported by data demonstrating a requirement for Th cells to locate to areas rich in CXCR3 ligands for optimal Th1 differentiation.151,152 Therefore, chemokine gradients generated by DC and monocytes at the time of CD4+ T cell priming may play a key role in Tfh vs. Th differentiation.
Concluding remarks
Advances in transcriptional profiling and high-dimensional flow cytometry have highlighted the lineage-derived and tissue-imprinted heterogeneity present within the DC compartment,8,13,46,52 as well as the remarkable level of plasticity DC exhibit when responding to different types of stimuli.4 Together, these factors ensure that the DC compartment as a whole has the capacity to sense and respond to a wide variety of pathogens, immunogens, and antigens, and elicit the appropriate CD4+ T cell response.
The tissue environment in which DC capture antigen has a marked impact on their ability to induce particular Th cell differentiation programs. At steady state, tissue-derived signals likely play an important role in programing a tolerogenic vs. immunogenic state in DC,222 as was recently demonstrated in the intestinal tract.45 During infection or inflammatory responses, DC integrate signals derived from the tissue environment as well as the pathogen itself to inform their expression of Th cell polarization factors. Direct interaction with antigen appears to be required for this transcriptional plasticity, as condition-specific transcriptional profiles are only evident among the small proportion of DC that have taken up fluorescent-labeled pathogens.4 This may explain why cDC2, which are superior at taking up exogenous antigen,4,95–98 are pivotal in directing Th cell differentiation in many settings, whereas cDC1, which specialize in the uptake of cell-associated antigen,103 excel at cross-presentation.28
In addition to pathogen-derived signals, DC also sense signals, such as cytokines, derived from surrounding cells responding to the same pathogen. Again, responses to these signals are enriched in Ag+ DC,4 likely due to the relative abundance of the cytokine in the vicinity of the pathogen. However, cytokines derived from bystander cells or DC themselves can have far reaching effects and prime DC at distal sites to anticipate their response, as has been shown with the neuropeptide CGRP promoting IL-23 production by cDC2 at sites adjacent to infection.206 Further, observations of context-specific adaptation of distal DC are now being extended to the epigenetic level in bone marrow precursors.266 These new findings open up the possibility for conditioned responses in DC exceeding the lifespan of tissue-resident cells or the longevity of infection, potentially modulating DC responses to subsequent infections.
Overall, context-dependent adaptation of DC plays a central role in determining Th cell differentiation in vivo. Further study focused on the rare population of DC that take up antigen will likely aid in understanding the specific signals involved in the early regulation of Th cell responses.
Acknowledgements
This work was supported by research grants from the Health Research Council of New Zealand to F.R. and the Malaghan Institute of Medical Research. K.L.H. was supported by a postdoctoral fellowship from the Malaghan Institute of Medical Research, New Zealand.
Author contributions
K.L.H. wrote the manuscript and drafted the figures; F.R. provided edits and comments; and both authors agreed on the final manuscript and figures.
Competing interests
The authors declare no competing interests.
References
- 1.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patente TA, Pelgrom LR, Everts B. Dendritic cells are what they eat: how their metabolism shapes T helper cell polarization. Curr. Opin. Immunol. 2019;58:16–23. doi: 10.1016/j.coi.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Blecher-Gonen R, et al. Single-cell analysis of diverse pathogen responses defines a molecular roadmap for generating antigen-specific immunity. Cell Syst. 2019;8:109–121 e106. doi: 10.1016/j.cels.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Meredith MM, et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J. Exp. Med. 2012;209:1153–1165. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satpathy AT, et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J. Exp. Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilliams M, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilliams M, et al. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity. 2016;45:669–684. doi: 10.1016/j.immuni.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller JC, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohl L, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity. 2012;37:364–376. doi: 10.1016/j.immuni.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J. Exp. Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granot T, et al. Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity. 2017;46:504–515. doi: 10.1016/j.immuni.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haniffa M, et al. Human tissues contain CD141(hi) cross-presenting dendritic cells with functional homology to mouse CD103(+) nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomura M, et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc. Natl Acad. Sci. USA. 2008;105:10871–10876. doi: 10.1073/pnas.0802278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes AJ, et al. Spatiotemporal modeling of the key migratory events during the initiation of adaptive immunity. Front. Immunol. 2019;10:598. doi: 10.3389/fimmu.2019.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomura M, et al. Tracking and quantification of dendritic cell migration and antigen trafficking between the skin and lymph nodes. Sci. Rep. 2014;4:6030. doi: 10.1038/srep06030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamoutounour S, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Langlet C, et al. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J. Immunol. 2012;188:1751–1760. doi: 10.4049/jimmunol.1102744. [DOI] [PubMed] [Google Scholar]
- 20.Tamoutounour S, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 2012;42:3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- 21.Min J, et al. Inflammation induces two types of inflammatory dendritic cells in inflamed lymph nodes. Exp. Mol. Med. 2018;50:e458. doi: 10.1038/emm.2017.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosteels, C. et al. Inflammatory type 2 cDCs acquire features of cDC1s and macrophages to orchestrate immunity to respiratory virus infection. Immunity (2020). In press. 10.1016/j.immuni.2020.04.005. [DOI] [PMC free article] [PubMed]
- 23.Nakano H, et al. Complement receptor C5aR1/CD88 and dipeptidyl peptidase-4/CD26 define distinct hematopoietic lineages of dendritic cells. J. Immunol. 2015;194:3808–3819. doi: 10.4049/jimmunol.1402195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoeckius M, et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods. 2017;14:865–868. doi: 10.1038/nmeth.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlitzer A, et al. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat. Immunol. 2015;16:718–728. doi: 10.1038/ni.3200. [DOI] [PubMed] [Google Scholar]
- 26.Cabeza-Cabrerizo M, et al. Tissue clonality of dendritic cell subsets and emergency DCpoiesis revealed by multicolor fate mapping of DC progenitors. Sci. Immunol. 2019;4:eaaw1941. doi: 10.1126/sciimmunol.aaw1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grajales-Reyes GE, et al. Batf3 maintains autoactivation of Irf8 for commitment of a CD8alpha conventional DC clonogenic progenitor. Nat. Immunol. 2015;16:708–717. doi: 10.1038/ni.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sichien D, et al. IRF8 transcription factor controls survival and function of terminally differentiated conventional and plasmacytoid dendritic cells, respectively. Immunity. 2016;45:626–640. doi: 10.1016/j.immuni.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Bachem A, et al. Expression of XCR1 characterizes the Batf3-dependent lineage of dendritic cells capable of antigen cross-presentation. Front. Immunol. 2012;3:214. doi: 10.3389/fimmu.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edelson BT, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J. Exp. Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginhoux F, et al. The origin and development of nonlymphoid tissue CD103(+) DCs. J. Exp. Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott CL, et al. The transcription factor Zeb2 regulates development of conventional and plasmacytoid DCs by repressing Id2. J. Exp. Med. 2016;213:897–911. doi: 10.1084/jem.20151715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bajana S, Roach K, Turner S, Paul J, Kovats S. IRF4 promotes cutaneous dendritic cell migration to lymph nodes during homeostasis and inflammation. J. Immunol. 2012;189:3368–3377. doi: 10.4049/jimmunol.1102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persson EK, et al. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38:958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Schlitzer A, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tussiwand R, et al. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity. 2015;42:916–928. doi: 10.1016/j.immuni.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis KL, et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–791. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breton G, et al. Human dendritic cells (DCs) are derived from distinct circulating precursors that are precommitted to become CD1c+ or CD141+ DCs. J. Exp. Med. 2016;213:2861–2870. doi: 10.1084/jem.20161135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu K, et al. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 41.Liu K, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat. Rev. Immunol. 2019;19:89–103. doi: 10.1038/s41577-018-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leon B, Lund FE. Compartmentalization of dendritic cell and T-cell interactions in the lymph node: anatomy of T-cell fate decisions. Immunol. Rev. 2019;289:84–100. doi: 10.1111/imr.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cook SJ, et al. Differential chemokine receptor expression and usage by pre-cDC1 and pre-cDC2. Immunol. Cell Biol. 2018;96:1131–1139. doi: 10.1111/imcb.12186. [DOI] [PubMed] [Google Scholar]
- 45.Esterhazy D, et al. Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature. 2019;569:126–130. doi: 10.1038/s41586-019-1125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alcantara-Hernandez M, et al. High-dimensional phenotypic mapping of human dendritic cells reveals interindividual variation and tissue specialization. Immunity. 2017;47:1037–1050 e1036. doi: 10.1016/j.immuni.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ochiai S, et al. CD326(lo)CD103(lo)CD11b(lo) dermal dendritic cells are activated by thymic stromal lymphopoietin during contact sensitization in mice. J. Immunol. 2014;193:2504–2511. doi: 10.4049/jimmunol.1400536. [DOI] [PubMed] [Google Scholar]
- 48.Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol. 2014;14:417–428. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- 49.Kumamoto Y, et al. CD301b(+) dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity. 2013;39:733–743. doi: 10.1016/j.immuni.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binnewies M, et al. Unleashing type-2 dendritic cells to drive protective antitumor CD4(+) T cell immunity. Cell. 2019;177:556–571 e516. doi: 10.1016/j.cell.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao Y, et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. 2013;39:722–732. doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown CC, et al. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell. 2019;179:846–863 e824. doi: 10.1016/j.cell.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bain CC, et al. TGFbetaR signalling controls CD103(+)CD11b(+) dendritic cell development in the intestine. Nat. Commun. 2017;8:620. doi: 10.1038/s41467-017-00658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tenno M, et al. Essential functions of Runx/Cbfβ in gut conventional dendritic cells for priming Rorγt+ T cells. Life Sci. Alliance. 2020;3:e201900441. doi: 10.26508/lsa.201900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 56.Sunderkotter C, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 57.Briseno CG, et al. Distinct transcriptional programs control cross-priming in classical and monocyte-derived dendritic cells. Cell Rep. 2016;15:2462–2474. doi: 10.1016/j.celrep.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Helft J, et al. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+)MHCII(+) macrophages and dendritic cells. Immunity. 2015;42:1197–1211. doi: 10.1016/j.immuni.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 59.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 60.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 61.Plantinga M, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 62.Goldszmid RS, et al. NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. 2012;36:1047–1059. doi: 10.1016/j.immuni.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chow KV, Sutherland RM, Zhan Y, Lew AM. Heterogeneity, functional specialization and differentiation of monocyte-derived dendritic cells. Immunol. Cell Biol. 2017;95:244–251. doi: 10.1038/icb.2016.104. [DOI] [PubMed] [Google Scholar]
- 64.Doebel T, Voisin B, Nagao K. Langerhans cells—the macrophage in dendritic cell clothing. Trends Immunol. 2017;38:817–828. doi: 10.1016/j.it.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohammed J, et al. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-beta. Nat. Immunol. 2016;17:414–421. doi: 10.1038/ni.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Merad M, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoeffel G, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schulz C, et al. A lineage of myeloid cells independent of myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 70.Ferrer IR, et al. A wave of monocytes is recruited to replenish the long-term Langerhans cell network after immune injury. Sci. Immunol. 2019;4:eaax8704. doi: 10.1126/sciimmunol.aax8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cisse B, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nagasawa M, Schmidlin H, Hazekamp MG, Schotte R, Blom B. Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the Ets factor Spi-B. Eur. J. Immunol. 2008;38:2389–2400. doi: 10.1002/eji.200838470. [DOI] [PubMed] [Google Scholar]
- 73.Rodrigues PF, et al. Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells. Nat. Immunol. 2018;19:711–722. doi: 10.1038/s41590-018-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blasius AL, et al. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 75.Zhang J, et al. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–3608. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- 76.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Engleman EG, Benike CJ, Grumet FC, Evans RL. Activation of human T lymphocyte subsets: helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens. J. Immunol. 1981;127:2124–2129. [PubMed] [Google Scholar]
- 78.Doyle C, Strominger JL. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987;330:256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- 79.Reis e Sousa C, Stahl PD, Austyn JM. Phagocytosis of antigens by Langerhans cells in vitro. J. Exp. Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoffmann E, et al. Autonomous phagosomal degradation and antigen presentation in dendritic cells. Proc. Natl Acad. Sci. USA. 2012;109:14556–14561. doi: 10.1073/pnas.1203912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garrett WS, et al. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–334. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 82.Platt CD, et al. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc. Natl Acad. Sci. USA. 2010;107:4287–4292. doi: 10.1073/pnas.0910609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bonifaz LC, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur. J. Immunol. 1997;27:280–288. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 86.Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 2009;206:2937–2946. doi: 10.1084/jem.20091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ouchi T, et al. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J. Exp. Med. 2011;208:2607–2613. doi: 10.1084/jem.20111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niess JH, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 89.Rescigno M, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 90.Tordesillas L, et al. PDL2(+) CD11b(+) dermal dendritic cells capture topical antigen through hair follicles to prime LAP(+) Tregs. Nat. Commun. 2018;9:5238. doi: 10.1038/s41467-018-07716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ouwehand K, et al. CXCL12 is essential for migration of activated Langerhans cells from epidermis to dermis. Eur. J. Immunol. 2008;38:3050–3059. doi: 10.1002/eji.200838384. [DOI] [PubMed] [Google Scholar]
- 92.Yao C, Kaplan DH. Langerhans cells transfer targeted antigen to dermal dendritic cells and acquire major histocompatibility complex II in vivo. J. Investig Dermatol. 2018;138:1665–1668. doi: 10.1016/j.jid.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kissenpfennig A, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 94.Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity. 2014;40:248–261. doi: 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 95.Deckers J, et al. Epicutaneous sensitization to house dust mite allergen requires interferon regulatory factor 4-dependent dermal dendritic cells. J. Allergy Clin. Immunol. 2017;140:1364–1377 e1362. doi: 10.1016/j.jaci.2016.12.970. [DOI] [PubMed] [Google Scholar]
- 96.Connor LM, Tang S-C, Camberis M, Le Gros G, Ronchese F. Helminth-conditioned dendritic cells prime CD4+ T cells to IL-4 production in vivo. J. Immunol. 2014;193:2709–2717. doi: 10.4049/jimmunol.1400374. [DOI] [PubMed] [Google Scholar]
- 97.Bollampalli VP, et al. BCG skin infection triggers IL-1R-MyD88-dependent migration of EpCAMlow CD11bhigh skin dendritic cells to draining lymph node during CD4+ T-cell priming. PLoS Pathog. 2015;11:e1005206. doi: 10.1371/journal.ppat.1005206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gerner MY, Torabi-Parizi P, Germain RN. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity. 2015;42:172–185. doi: 10.1016/j.immuni.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 99.Sixt M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 100.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gerner MY, Casey KA, Kastenmuller W, Germain RN. Dendritic cell and antigen dispersal landscapes regulate T cell immunity. J. Exp. Med. 2017;214:3105–3122. doi: 10.1084/jem.20170335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gilfillan CB, et al. Clec9A(+) dendritic cells are not essential for antitumor CD8(+) T cell responses induced by poly I:C immunotherapy. J. Immunol. 2018;200:2978–2986. doi: 10.4049/jimmunol.1701593. [DOI] [PubMed] [Google Scholar]
- 103.Gutierrez-Martinez E, et al. Cross-presentation of cell-associated antigens by MHC class I in dendritic cell subsets. Front. Immunol. 2015;6:363. doi: 10.3389/fimmu.2015.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 105.Lehmann CHK, et al. DC subset-specific induction of T cell responses upon antigen uptake via Fcgamma receptors in vivo. J. Exp. Med. 2017;214:1509–1528. doi: 10.1084/jem.20160951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Caminschi I, et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112:3264–3273. doi: 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(-) dendritic cells in vivo. J. Exp. Med. 2002;196:817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ballesteros-Tato A, Leon B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat. Immunol. 2010;11:216–224. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baker K, et al. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc. Natl Acad. Sci. USA. 2011;108:9927–9932. doi: 10.1073/pnas.1019037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Le Borgne M, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 111.Segura E, Durand M, Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J. Exp. Med. 2013;210:1035–1047. doi: 10.1084/jem.20121103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lugt BV, et al. Transcriptional programming of dendritic cells for enhanced MHC class II antigen presentation. Nat. Immunol. 2014;15:161–167. doi: 10.1038/ni.2795. [DOI] [PubMed] [Google Scholar]
- 113.van Panhuys N. TCR signal strength alters T-DC activation and interaction times and directs the outcome of differentiation. Front. Immunol. 2016;7:6. doi: 10.3389/fimmu.2016.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015;135:626–635. doi: 10.1016/j.jaci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 115.Afkarian M, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 116.Lighvani AA, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc. Natl Acad. Sci. USA. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu J, et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity. 2012;37:660–673. doi: 10.1016/j.immuni.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 119.Mullen AC, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 120.Hsieh CS, et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 121.Manetti R, et al. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J. Exp. Med. 1994;179:1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heufler C, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur. J. Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 123.Martinez-Lopez M, Iborra S, Conde-Garrosa R, Sancho D. Batf3-dependent CD103+ dendritic cells are major producers of IL-12 that drive local Th1 immunity against Leishmania major infection in mice. Eur. J. Immunol. 2015;45:119–129. doi: 10.1002/eji.201444651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mashayekhi M, et al. CD8alpha(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011;35:249–259. doi: 10.1016/j.immuni.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ruffell B, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Everts B, et al. Migratory CD103+ dendritic cells suppress helminth-driven type 2 immunity through constitutive expression of IL-12. J. Exp. Med. 2015;231:35–51. doi: 10.1084/jem.20150235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Conejero L, et al. Lung CD103+ dendritic cells restrain allergic airway inflammation through IL-12 production. JCI Insight. 2017;2:e90420. doi: 10.1172/jci.insight.90420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maldonado-López R, et al. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arnold IC, et al. BATF3-dependent dendritic cells drive both effector and regulatory T-cell responses in bacterially infected tissues. PLoS Pathog. 2019;15:e1007866. doi: 10.1371/journal.ppat.1007866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Igyarto BZ, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luda KM, et al. IRF8 transcription-factor-dependent classical dendritic cells are essential for intestinal T cell homeostasis. Immunity. 2016;44:860–874. doi: 10.1016/j.immuni.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 132.Demiri, M., Muller-Luda, K., Agace, W. W. & Svensson-Frej, M. Distinct D. C. subsets regulate adaptive Th1 and 2 responses during Trichuris muris infection. Parasite Immunol.39, e12458 (2017). [DOI] [PubMed]
- 133.Muzaki ARBM, et al. Intestinal CD103+CD11b− dendritic cells restrain colitis via IFN-γ-induced anti-inflammatory response in epithelial cells. Mucosal Immunol. 2016;9:336–351. doi: 10.1038/mi.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lonnberg T, et al. Single-cell RNA-seq and computational analysis using temporal mixture modelling resolves Th1/Tfh fate bifurcation in malaria. Sci. Immunol. 2017;2:eaal2192. doi: 10.1126/sciimmunol.aal2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Flores-Langarica A, et al. T-zone localized monocyte-derived dendritic cells promote Th1 priming to Salmonella. Eur. J. Immunol. 2011;41:2654–2665. doi: 10.1002/eji.201141440. [DOI] [PubMed] [Google Scholar]
- 136.De Koker S, et al. Inflammatory monocytes regulate Th1 oriented immunity to CpG adjuvanted protein vaccines through production of IL-12. Sci. Rep. 2017;7:5986. doi: 10.1038/s41598-017-06236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nakano H, et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat. Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chow KV, Lew AM, Sutherland RM, Zhan Y. Monocyte-derived dendritic cells promote Th polarization, whereas conventional dendritic cells promote Th proliferation. J. Immunol. 2016;196:624–636. doi: 10.4049/jimmunol.1501202. [DOI] [PubMed] [Google Scholar]
- 139.Schreiber HA, et al. Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium. J. Exp. Med. 2013;210:2025–2039. doi: 10.1084/jem.20130903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mansouri S, et al. Immature lung TNFR2− conventional DC 2 subpopulation activates moDCs to promote cyclic di-GMP mucosal adjuvant responses in vivo. Mucosal Immunol. 2019;12:277–289. doi: 10.1038/s41385-018-0098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kawabe T, et al. Memory-phenotype CD4+ T cells spontaneously generated under steady-state conditions exert innate TH1-like effector function. Sci. Immunol. 2017;2:eaam9304. doi: 10.1126/sciimmunol.aam9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pompei L, et al. Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. J. Immunol. 2007;178:5192–5199. doi: 10.4049/jimmunol.178.8.5192. [DOI] [PubMed] [Google Scholar]
- 143.Manickasingham SP, Edwards AD, Schulz O, Reis e Sousa C. The ability of murine dendritic cell subsets to direct T helper cell differentiation is dependent on microbial signals. Eur. J. Immunol. 2003;33:101–107. doi: 10.1002/immu.200390001. [DOI] [PubMed] [Google Scholar]
- 144.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat. Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 145.Gautier G, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schulz O, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 147.Cella M, et al. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gerosa F, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J. Exp. Med. 2008;205:1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ma X, et al. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J. Exp. Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sarhan D, et al. Dendritic cell regulation of NK-cell responses involves lymphotoxin-alpha, IL-12, and TGF-beta. Eur. J. Immunol. 2015;45:1783–1793. doi: 10.1002/eji.201444885. [DOI] [PubMed] [Google Scholar]
- 151.Groom JR, et al. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity. 2012;37:1091–1103. doi: 10.1016/j.immuni.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Woodruff MC, et al. Trans-nodal migration of resident dendritic cells into medullary interfollicular regions initiates immunity to influenza vaccine. J. Exp. Med. 2014;211:1611–1621. doi: 10.1084/jem.20132327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martin-Fontecha A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 154.Askenase MH, et al. Bone-marrow-resident NK cells prime monocytes for regulatory function during infection. Immunity. 2015;42:1130–1142. doi: 10.1016/j.immuni.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 156.Fitzsimmons CM, Falcone FH, Dunne DW. Helminth allergens, parasite-specific IgE, and its protective role in human immunity. Front. Immunol. 2014;5:61. doi: 10.3389/fimmu.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Williams JW, et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat. Commun. 2013;4:2990. doi: 10.1038/ncomms3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mayer JU, et al. Different populations of CD11b(+) dendritic cells drive Th2 responses in the small intestine and colon. Nat. Commun. 2017;8:15820. doi: 10.1038/ncomms15820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Murakami R, et al. A unique dermal dendritic cell subset that skews the immune response toward Th2. PLoS ONE. 2013;8:13. doi: 10.1371/journal.pone.0073270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nakajima S, et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J. Allergy Clin. Immunol. 2012;129:1048–1055 e1046. doi: 10.1016/j.jaci.2012.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Elentner A, et al. Langerhans cells are critical in the development of atopic dermatitis-like inflammation and symptoms in mice. J. Cell Mol. Med. 2009;13:2658–2672. doi: 10.1111/j.1582-4934.2009.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Connor LM, et al. Th2 responses are primed by skin dendritic cells with distinct transcriptional profiles. J. Exp. Med. 2017;214:125–142. doi: 10.1084/jem.20160470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jankovic D, et al. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(-/-) setting. Immunity. 2002;16:429–439. doi: 10.1016/s1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 164.MacDonald AS, Straw AD, Bauman B, Pearce EJ. CD8- dendritic cell activation status plays an integral role in influencing Th2 response development. J. Immunol. 2001;167:1982–1988. doi: 10.4049/jimmunol.167.4.1982. [DOI] [PubMed] [Google Scholar]
- 165.de Jong EC, et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse th cell-polarizing signals. J. Immunol. 2002;168:1704–1709. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 166.Kaisar MMM, et al. Dectin-1/2-induced autocrine PGE2 signaling licenses dendritic cells to prime Th2 responses. PLoS Biol. 2018;16:e2005504. doi: 10.1371/journal.pbio.2005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Everts B, et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J. Exp. Med. 2009;206:1673–1680. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Steinfelder S, et al. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J. Exp. Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.van Panhuys N, Klauschen F, Germain RN. T-cell-receptor-dependent signal intensity dominantly controls CD4(+) T cell polarization in vivo. Immunity. 2014;41:63–74. doi: 10.1016/j.immuni.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Janss T, et al. Interferon response factor-3 promotes the pro-Th2 activity of mouse lung CD11b+ conventional dendritic cells in response to house dust mite allergens. Eur. J. Immunol. 2016;46:2614–2628. doi: 10.1002/eji.201646513. [DOI] [PubMed] [Google Scholar]
- 171.Liu YJ. TSLP in epithelial cell and dendritic cell cross talk. Adv. Immunol. 2009;101:1–25. doi: 10.1016/S0065-2776(08)01001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ochiai S, et al. Thymic stromal lymphopoietin drives the development of IL-13(+) Th2 cells. Proc. Natl Acad. Sci. USA. 2018;115:1033–1038. doi: 10.1073/pnas.1714348115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rochman Y, et al. TSLP signaling in CD4(+) T cells programs a pathogenic T helper 2 cell state. Sci. Signal. 2018;11:eaam8858. doi: 10.1126/scisignal.aam8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kabata, H. et al. Targeted deletion of the TSLP receptor reveals cellular mechanisms that promote type 2 airway inflammation. Mucosal Immunol. (2020). 10.1038/s41385-020-0266-x. [DOI] [PMC free article] [PubMed]
- 175.Webb LM, et al. Type I interferon is required for T helper (Th) 2 induction by dendritic cells. EMBO J. 2017;36:2404–2418. doi: 10.15252/embj.201695345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Prout MS, Kyle RL, Ronchese F, Le Gros G. IL-4 is a key requirement for IL-4- and IL-4/IL-13-expressing CD4 Th2 subsets in lung and skin. Front. Immunol. 2018;9:1211. doi: 10.3389/fimmu.2018.01211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J. Exp. Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Burrows KE, et al. OX40 blockade inhibits house dust mite driven allergic lung inflammation in mice and in vitro allergic responses in humans. Eur. J. Immunol. 2014;45:1116–1128. doi: 10.1002/eji.201445163. [DOI] [PubMed] [Google Scholar]
- 179.Hoshino A, et al. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur. J. Immunol. 2003;33:861–869. doi: 10.1002/eji.200323455. [DOI] [PubMed] [Google Scholar]
- 180.Seshasayee D, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J. Clin. Investig. 2007;117:3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chu DK, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J. Allergy Clin. Immunol. 2013;131:187–200 e181. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 182.Ekkens MJ, et al. The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus. J. Immunol. 2003;170:384–393. doi: 10.4049/jimmunol.170.1.384. [DOI] [PubMed] [Google Scholar]
- 183.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu. Rev. Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yoshie O, Matsushima K. CCR4 and its ligands: from bench to bedside. Int Immunol. 2015;27:11–20. doi: 10.1093/intimm/dxu079. [DOI] [PubMed] [Google Scholar]
- 185.Sokol CL, Camire RB, Jones MC, Luster AD. The chemokine receptor CCR8 promotes the migration of dendritic cells into the lymph node parenchyma to initiate the allergic immune response. Immunity. 2018;49:449–463 e446. doi: 10.1016/j.immuni.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 187.Ghoreschi K, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mathers AR, et al. Differential capability of human cutaneous dendritic cell subsets to initiate Th17 responses. J. Immunol. 2009;182:921–933. doi: 10.4049/jimmunol.182.2.921. [DOI] [PubMed] [Google Scholar]
- 190.Kashem SW, et al. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity. 2015;42:356–366. doi: 10.1016/j.immuni.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wohn C, et al. Langerin(neg) conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc. Natl Acad. Sci. USA. 2013;110:10723–10728. doi: 10.1073/pnas.1307569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim TG, et al. Skin-specific CD301b(+) dermal dendritic cells drive IL-17-mediated psoriasis-like immune response in mice. J. Investig. Dermatol. 2018;138:844–853. doi: 10.1016/j.jid.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 193.Linehan JL, et al. Generation of Th17 cells in response to intranasal infection requires TGF-beta1 from dendritic cells and IL-6 from CD301b+ dendritic cells. Proc. Natl Acad. Sci. USA. 2015;112:12782–12787. doi: 10.1073/pnas.1513532112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Welty NE, et al. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. J. Exp. Med. 2013;210:2011–2024. doi: 10.1084/jem.20130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Satpathy AT, et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat. Immunol. 2013;14:937–948. doi: 10.1038/ni.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu H, et al. TLR5 mediates CD172alpha(+) intestinal lamina propria dendritic cell induction of Th17 cells. Sci. Rep. 2016;6:22040. doi: 10.1038/srep22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Trautwein-Weidner K, et al. Antigen-specific Th17 cells are primed by distinct and complementary dendritic cell subsets in oropharyngeal candidiasis. PLoS Pathog. 2015;11:e1005164. doi: 10.1371/journal.ppat.1005164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 199.Steel N, et al. TGFbeta-activation by dendritic cells drives Th17 induction and intestinal contractility and augments the expulsion of the parasite Trichinella spiralis in mice. PLoS Pathog. 2019;15:e1007657. doi: 10.1371/journal.ppat.1007657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Melton AC, et al. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J. Clin. Investig. 2010;120:4436–4444. doi: 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Acharya M, et al. alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J. Clin. Investig. 2010;120:4445–4452. doi: 10.1172/JCI43796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 2001;276:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 203.Schirmer C, Klein C, von Bergen M, Simon JC, Saalbach A. Human fibroblasts support the expansion of IL-17-producing T cells via up-regulation of IL-23 production by dendritic cells. Blood. 2010;116:1715–1725. doi: 10.1182/blood-2010-01-263509. [DOI] [PubMed] [Google Scholar]
- 204.Riol-Blanco L, et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510:157–161. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kashem SakeenW, et al. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity. 2015;43:515–526. doi: 10.1016/j.immuni.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cohen JA, et al. Cutaneous TRPV1(+) neurons trigger protective innate type 17 Anticipatory immunity. Cell. 2019;178:919–932 e914. doi: 10.1016/j.cell.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fantini MC, et al. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 211.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J. Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 212.Paidassi H, et al. Preferential expression of integrin alphavbeta8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology. 2011;141:1813–1820. doi: 10.1053/j.gastro.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jones A, et al. Immunomodulatory functions of BTLA and HVEM govern induction of extrathymic regulatory T cells and tolerance by dendritic cells. Immunity. 2016;45:1066–1077. doi: 10.1016/j.immuni.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Guilliams M, et al. Skin-draining lymph nodes contain dermis-derived CD103(-) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 2010;115:1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 215.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hill JA, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Xiao S, et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ovcinnikovs V, et al. CTLA-4-mediated transendocytosis of costimulatory molecules primarily targets migratory dendritic cells. Sci. Immunol. 2019;4:eaaw0902. doi: 10.1126/sciimmunol.aaw0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gu P, et al. Trogocytosis of CD80 and CD86 by induced regulatory T cells. Cell Mol. Immunol. 2012;9:136–146. doi: 10.1038/cmi.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Akkaya B, et al. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat. Immunol. 2019;20:218–231. doi: 10.1038/s41590-018-0280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Esterhazy D, et al. Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance. Nat. Immunol. 2016;17:545–555. doi: 10.1038/ni.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Boucard-Jourdin M, et al. beta8 Integrin expression and activation of TGF-beta by intestinal dendritic cells are determined by both tissue microenvironment and cell lineage. J. Immunol. 2016;197:1968–1978. doi: 10.4049/jimmunol.1600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Veenbergen S, et al. Colonic tolerance develops in the iliac lymph nodes and can be established independent of CD103(+) dendritic cells. Mucosal Immunol. 2016;9:894–906. doi: 10.1038/mi.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shklovskaya E, et al. Langerhans cells are precommitted to immune tolerance induction. Proc. Natl Acad. Sci. USA. 2011;108:18049–18054. doi: 10.1073/pnas.1110076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kautz-Neu K, et al. Langerhans cells are negative regulators of the anti-Leishmania response. J. Exp. Med. 2011;208:885–891. doi: 10.1084/jem.20102318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Price JG, et al. CDKN1A regulates Langerhans cell survival and promotes Treg cell generation upon exposure to ionizing irradiation. Nat. Immunol. 2015;16:1060–1068. doi: 10.1038/ni.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Baratin M, et al. Homeostatic NF-kappaB signaling in steady-state migratory dendritic cells regulates immune homeostasis and tolerance. Immunity. 2015;42:627–639. doi: 10.1016/j.immuni.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 228.Ardouin L, et al. Broad and largely concordant molecular changes characterize tolerogenic and immunogenic dendritic cell maturation in thymus and periphery. Immunity. 2016;45:305–318. doi: 10.1016/j.immuni.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 229.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50:1132–1148. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Goenka R, et al. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J. Immunol. 2011;187:1091–1095. doi: 10.4049/jimmunol.1100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Choi YS, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Choi YS, et al. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J. Immunol. 2013;190:4014–4026. doi: 10.4049/jimmunol.1202963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hiramatsu Y, et al. c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. J. Leukoc. Biol. 2010;87:703–712. doi: 10.1189/jlb.0909639. [DOI] [PubMed] [Google Scholar]
- 235.Vogelzang A, et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 236.Riteau N, et al. Water-in-oil-only adjuvants selectively promote T follicular helper cell polarization through a type I IFN and IL-6-dependent pathway. J. Immunol. 2016;197:3884–3893. doi: 10.4049/jimmunol.1600883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Batten M, et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J. Exp. Med. 2010;207:2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Barbet G, et al. Sensing microbial viability through bacterial RNA augments T follicular helper cell and antibody responses. Immunity. 2018;48:584–598 e585. doi: 10.1016/j.immuni.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tahiliani V, Hutchinson TE, Abboud G, Croft M, Salek-Ardakani S. OX40 cooperates with ICOS to amplify follicular Th cell development and germinal center reactions during infection. J. Immunol. 2017;198:218–228. doi: 10.4049/jimmunol.1601356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dell’Aringa M, Reinhardt RL. Notch signaling represents an important checkpoint between follicular T-helper and canonical T-helper 2 cell fate. Mucosal Immunol. 2018;11:1079–1091. doi: 10.1038/s41385-018-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ballesteros-Tato A, et al. T follicular helper cell plasticity shapes pathogenic T helper 2 cell-mediated immunity to inhaled house dust mite. Immunity. 2016;44:259–273. doi: 10.1016/j.immuni.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Li J, Lu E, Yi T, Cyster JG. EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature. 2016;533:110–114. doi: 10.1038/nature17947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Arroyo EN, Pepper M. B cells are sufficient to prime the dominant CD4+ Tfh response to Plasmodium infection. J. Exp. Med. 2020;217:e20190849. doi: 10.1084/jem.20190849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Krishnaswamy JK, Alsen S, Yrlid U, Eisenbarth SC, Williams A. Determination of T follicular helper cell fate by dendritic cells. Front. Immunol. 2018;9:2169. doi: 10.3389/fimmu.2018.02169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Krishnaswamy JK, et al. Migratory CD11b(+) conventional dendritic cells induce T follicular helper cell-dependent antibody responses. Sci. Immunol. 2017;2:eaam9169. doi: 10.1126/sciimmunol.aam9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Briseno CG, et al. Notch2-dependent DC2s mediate splenic germinal center responses. Proc. Natl Acad. Sci. USA. 2018;115:10726–10731. doi: 10.1073/pnas.1809925115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Shin C, et al. CD8alpha(-) dendritic cells induce antigen-specific T follicular helper cells generating efficient humoral immune responses. Cell Rep. 2015;11:1929–1940. doi: 10.1016/j.celrep.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 249.Calabro S, et al. Bridging channel dendritic cells induce immunity to transfused red blood cells. J. Exp. Med. 2016;213:887–896. doi: 10.1084/jem.20151720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Durand M, et al. Human lymphoid organ cDC2 and macrophages play complementary roles in T follicular helper responses. J. Exp. Med. 2019;216:1561–1581. doi: 10.1084/jem.20181994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kumamoto Y, Hirai T, Wong PW, Kaplan DH, Iwasaki A. CD301b(+) dendritic cells suppress T follicular helper cells and antibody responses to protein antigens. Elife. 2016;5:e17979. doi: 10.7554/eLife.17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kato Y, et al. Targeting antigen to Clec9A primes follicular Th cell memory responses capable of robust recall. J. Immunol. 2015;195:1006–1014. doi: 10.4049/jimmunol.1500767. [DOI] [PubMed] [Google Scholar]
- 253.Lahoud MH, et al. Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. J. Immunol. 2011;187:842–850. doi: 10.4049/jimmunol.1101176. [DOI] [PubMed] [Google Scholar]
- 254.Bouteau A, et al. DC subsets regulate humoral immune responses by supporting the differentiation of distinct Tfh cells. Front. Immunol. 2019;10:1134. doi: 10.3389/fimmu.2019.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yao C, et al. Skin dendritic cells induce follicular helper T cells and protective humoral immune responses. J. Allergy Clin. Immunol. 2015;136:1387–1397. doi: 10.1016/j.jaci.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Levin C, et al. Critical role for skin-derived migratory DCs and Langerhans cells in TFH and GC responses after intradermal immunization. J. Investig. Dermatol. 2017;137:1905–1913. doi: 10.1016/j.jid.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 257.Chakarov S, Fazilleau N. Monocyte-derived dendritic cells promote T follicular helper cell differentiation. EMBO Mol. Med. 2014;6:590–603. doi: 10.1002/emmm.201403841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Fillatreau S, Gray D. T cell accumulation in B cell follicles is regulated by dendritic cells and is independent of B cell activation. J. Exp. Med. 2003;197:195–206. doi: 10.1084/jem.20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pattarini L, et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J. Exp. Med. 2017;214:1529–1546. doi: 10.1084/jem.20150402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hu H, et al. Noncanonical NF-kappaB regulates inducible costimulator (ICOS) ligand expression and T follicular helper cell development. Proc. Natl Acad. Sci. USA. 2011;108:12827–12832. doi: 10.1073/pnas.1105774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hercor M, et al. Antigen-presenting cell-derived IL-6 restricts the expression of GATA3 and IL-4 by follicular helper T cells. J. Leukoc. Biol. 2017;101:5–14. doi: 10.1189/jlb.1HI1115-511R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.De Giovanni M, et al. Spatiotemporal regulation of type I interferon expression determines the antiviral polarization of CD4+ T cells. Nat. Immunol. 2020;21:321–330. doi: 10.1038/s41590-020-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cucak H, Yrlid U, Reizis B, Kalinke U, Johansson-Lindbom B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity. 2009;31:491–501. doi: 10.1016/j.immuni.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 264.Kulkarni RR, et al. Activation of the RIG-I pathway during influenza vaccination enhances the germinal center reaction, promotes T follicular helper cell induction, and provides a dose-sparing effect and protective immunity. J. Virol. 2014;88:13990–14001. doi: 10.1128/JVI.02273-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Eastman AJ, et al. Epigenetic stabilization of DC and DC precursor classical activation by TNFα contributes to protective T cell polarization. Sci. Adv. 2019;5:eaaw9051. doi: 10.1126/sciadv.aaw9051. [DOI] [PMC free article] [PubMed] [Google Scholar]


