Abstract
Context
Benign prostatic hyperplasia (BPH) associated with lower urinary tract symptoms (LUTS) is diagnosed in up to 80% of men during their lifetime. Several novel ultra-minimally invasive surgical treatments (uMISTs) for BPH/benign prostatic obstruction (BPO) have become available over the past 5 yr.
Objective
To evaluate the perioperative and functional outcomes of recently introduced uMISTs for BPH/BPO, including Urolift, Rezūm, temporary implantable nitinol device, prostatic artery embolization (PAE), and intraprostatic injection.
Evidence acquisition
A systematic literature search was conducted in December 2020 using Medline (via PubMed), Embase (via Ovid), Scopus, and Web of Science (registered on PROSPERO as CRD42021225014). The search strategy used PICO criteria and article selection was conducted in accordance with the PRISMA guidelines. The risk of bias and the quality of the articles included were assessed. A dedicated data extraction form was used to collect the data of interest. Pooled and cumulative analyses were performed to compare perioperative and functional outcomes between study groups. A random-effects model using the DerSimonian and Laird method was used to evaluate heterogeneity. Stata version 15.0 software was used for all statistical analyses.
Evidence synthesis
The initial electronic search identified 3978 papers, of which 48 ultimately met the inclusion criteria and were included in the analysis. Pooled analysis revealed a uMIST benefit in terms of International Prostate Symptom Score (IPSS; −9.81 points, 95% confidence interval [CI] −11.37 to −8.25 at 1 mo; −13.13 points, 95% CI −14.98 to −11.64 at 12 mo), maximum flow rate (from +3.66 ml/s, 95% CI 2.8–4.5 to +4.14 ml/s, 95% CI 0.72–7.56 at 12 mo), and postvoid residual volume (−10.10 ml, 95% CI −27.90 to 7.71 at 12 mo). No negative impact was observed on scores for the International Index of Erectile Function-5, Male Sexual Health Questionnaire-Ejaculatory Dysfunction bother and function scales (overall postintervention change in pooled median score of 1.88, 95% CI 1.34–2.42 at the start of follow-up; and 1.04, 95% CI 0.28–1.8 after 1 yr), or the IPSS-Quality of Life questionnaire.
Conclusions
Novel uMISTs can yield fast and effective relief of LUTS without affecting patient quality of life. Only Rezūm, UroLift, and PAE had a minimal impact on patients’ sexual function with respect to baseline, especially regarding preservation of ejaculation.
Patient summary
We reviewed outcomes for recently introduced ultra-minimally invasive surgical treatments for patients with lower urinary tract symptoms caused by benign prostate enlargement or obstruction. The evidence suggests that these novel techniques are beneficial in terms of controlling symptoms while preserving sexual function.
Take Home Message
Novel ultra-minimally invasive treatments can yield fast and effective relief of lower urinary tract symptoms without affecting a patient’s quality of life.
Keywords: Benign prostatic hyperplasia, Lower urinary tract symptoms, Ultra-minimally invasive, Ejaculation, Micturition
1. Introduction
Benign prostatic hyperplasia (BPH) with lower urinary tract symptoms (LUTS) is the most diagnosed urologic condition among men aged 45–74 yr, with a prevalence ranging from 8% to 80% between the fourth and ninth decades of life [1]. Medical therapy is usually the first treatment choice. However, medication-related side effects and insufficient symptom control resulting in BPH-related adverse events (such as hematuria, recurrent infection, and bladder stones) often lead to pharmacological discontinuation in favor of operative treatment options [2]. Most of these patients are offered surgical treatments, such as the traditional gold-standard transurethral resection of the prostate (TURP), holmium laser enucleation of the prostate (HoLEP) [3], water ablation [4], and simple prostatectomy (either open or minimally invasive [5]). Nevertheless, these techniques carry a non-negligible risk of complications and significantly impact a patient’s sexual function.
In this scenario, several new minimally invasive techniques have been introduced with the aim of providing better symptom relief compared to pharmacological therapy while minimizing the impact on sexual function [6]. Translating the concept from gynecology [7] and orthopedics [8], these new approaches can be broadly defined as ultra-minimally invasive treatments (uMISTs). Among them, steam injection (Rezūm), prostate artery embolization (PAE), intraprostatic injection of fexapotide triflutate (NX-1207) and PRX302, implantation of a prostatic urethral lift (PUL), and the temporary implantable nitinol device (TIND) were introduced in the 2020 European Association of Urology (EAU) guidelines, although their role in the management of BPH remains controversial.
The aim of this review was to summarize and evaluate the perioperative and functional outcomes of these new uMISTs.
2. Evidence acquisition
2.1. Search strategy
After establishing an a priori protocol, a systematic electronic literature search was conducted in December 2020 using the Medline (via PubMed), Embase (via Ovid), Scopus, and Web of Science databases.
The search strategy used the PICO (Patients, Intervention, Comparison, Outcome) [9] criteria: studies among patients with BPH (Patients) undergoing uMIST (Intervention) or MIST (Comparison) to evaluate surgical, micturition, and sexual outcomes (Outcome) were identified.
The following search string was used: “Prostatic Hyperplasia”[MeSH] OR “benign prostatic hyperplasia”[tiab] OR “Lower Urinary Tract Symptoms”[MeSH] OR “lower urinary tract symptoms”[tiab] AND (rezum[tiab] OR vapor/water/steam[tiab] OR “Embolization, Therapeutic”[MeSH] OR PAE[tiab] OR “prostatic urethral lift”[tiab] OR PUL[tiab] OR Urolift[tiab] OR “temporary implantable nitinol device”[tiab] OR TIND[tiab] OR iTIND[tiab] OR Medi-Tate[tiab] OR Meditate[tiab] OR “fexapotide triflutate”[Supplementary Concept] OR “fexapotide triflutate”[tiab] OR “NX-1207”[tiab] OR “PRX302”[Supplementary Concept] OR PRX302[tiab] OR “PRX 302”[tiab]).
2.2. Article selection
The record selection process was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. The study protocol was registered with PROSPERO (registry number CRD42021225014).
Two of the authors (E.C. and S.D.C.) independently reviewed the literature according to the inclusion and exclusion criteria. Disagreements about eligibility were resolved in conjunction with a third reviewer (C.F.) until consensus was reached. We included only prospective studies referring to one of the five uMISTs mentioned in the EAU guidelines [6] and reporting outcomes of interest. Articles not in English, not original investigations (such as editorials, commentaries, abstracts, reviews, and book chapters), studies reporting experimental studies on animals or cadavers, and non–BPH-related surgeries were excluded. As the EAU guidelines do not recommend the use of botulinum toxin A for this indication, this treatment option was not considered and related articles were not eligible. The titles and abstracts were reviewed in accordance with the inclusion criteria. After screening, full-text analysis was performed to confirm whether the selected articles should be included. References from the pooled articles were manually reviewed to identify additional studies of interest. In the case of multiple studies from the same institution and with overlapping study periods, we only included the latest published study. However, studies from the same institution considering different study populations were included in the analysis.
2.3. Risk-of-bias assessment
The risk of bias and the quality of the studies were independently assessed using the standard Cochrane Collaboration risk-of-bias tool for single-arm studies [11], the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool for comparative studies [12], and the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) for randomized controlled trials (RCTs) [13].
2.4. Assessment of study quality
For nonrandomized controlled trials, study quality was assessed using the Newcastle-Ottawa scale (total score: ≤5 = low quality; 6–7 = intermediate quality; 8–9 = high quality) [14]. RCTs were evaluated using the Jadad scale (0 = very poor quality, 5 = rigorous quality) [15]. The level of evidence of each study was assessed according to the Oxford Centre for Evidence-Based Medicine [16].
2.5. Data extraction and analysis
A dedicated data extraction form was used to collect the data of interest. Baseline demographics (age, indwelling catheter, prostate-specific antigen, prostate volume, maximum urinary flow [Qmax], postvoid residual volume [PVR], International Prostate Symptom Score [IPSS], IPSS-Quality of Life [QoL], International Index of Erectile Function [IIEF-5], Male Sexual Health Questionnaire-Ejaculatory Dysfunction [MSHQ-EjD] bother and function, and Sexual Health Inventory for Men [SHIM]), perioperative variables (operative time, number of intraoperative complications, Visual Analog Scale [VAS] score, length of stay, and duration of catheterization), and postoperative complications according to the Clavien-Dindo classification [17] were recorded. Qmax, IPSS, QoL, IIEF-5, PVR, and MSHQ, SHIM, and IIEF-5 scores were collected and analyzed at 1, 3, 6, and 12 mo after the intervention. An indirect comparison among treatments was performed given the absence of studies with a pairwise design.
For continuous variables reported as the median (range), results were converted to the mean ± standard deviation (SD) using a dedicated formula [18]. Data reported as the median and interquartile range (IQR) were excluded because no information regarding the parametric distribution was available. After obtaining the mean ± SD, data were further converted to the mean with 95% confidence interval (CI). The grand mean was used to calculate the overall mean for data for the same variable but split into two groups. For continuous variables, a pooled analysis of the mean (95% CI) was performed using the metan command, whereas for dichotomous values a cumulative analysis of percentages was conducted using the metaprop command. A random-effects model using the DerSimonian and Laird method was used to evaluate the I2 value for heterogeneity. A heterogeneity level above 75% was considered to be high.
Funnel plot analysis was conducted for bias assessment using the metafunnel command.
Stata version 15.0 software (StataCorp LLC, College Station, TX, USA) was used for all statistical analyses.
3. Evidence synthesis
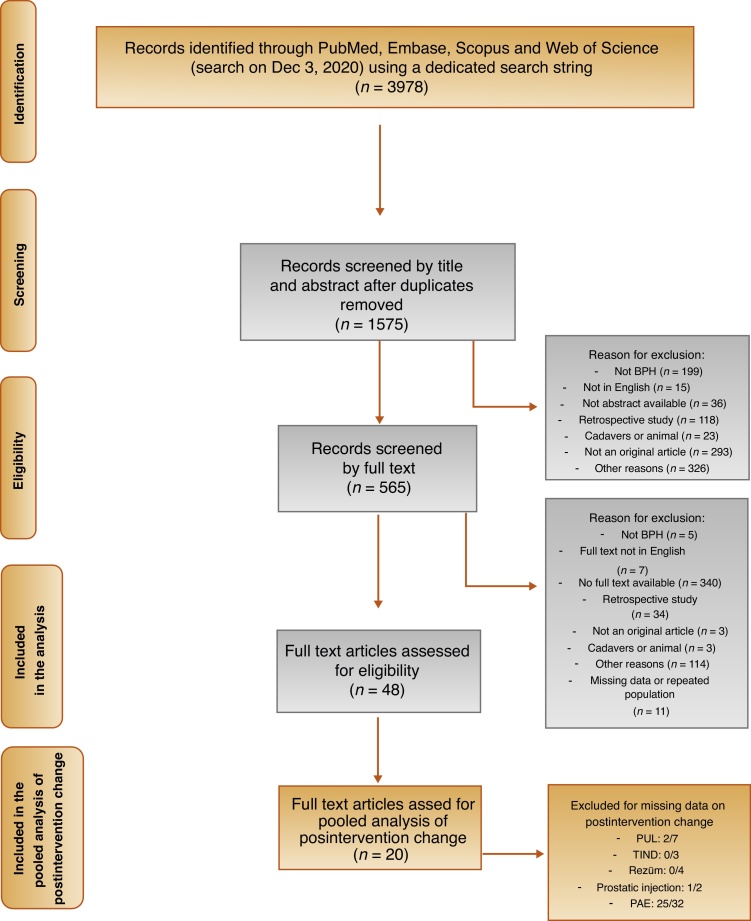
The initial electronic search identified a total of 3978 papers. Of these, 1575 were identified for detailed review, and ultimately 48 studies met the inclusion criteria and were included in the analysis [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66] (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. BPH = benign prostatic hyperplasia; PUL = prostatic urethral lift; TIND = temporary implantable nitinol device; PAE = prostatic artery embolization.
Among these, 35 were single-arm studies [19], [20], [21], [22], [23], [24], [26], [27], [28], [29], [30], [31], [33], [34], [35], [37]–44,46,47,50,51,53,54,[57], [58], [59], [60], [61],66], 12 were RCTs [25], [36], [45], [48], [49], [52], [55], [56], [62], [63], [64], [65], and one was a comparative study [32].
Four studies described Rezūm [53], [54], [55], [56], 32 PAE (including one perfected PAE [PPAE]) [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], two intraprostatic injections [51], [52], seven PUL [60], [61], [62], [63], [64], [65], [66], and three TIND [57], [58], [59]. Only seven studies reported a comparison with TURP, of which five were on PAE [25], [32], [36], [45], [49] and two were on PUL [64], [65].
A total of 2689 patients were evaluated.
3.1. Study quality
The quality of non-RCT studies evaluated with the Newcastle-Ottawa scale ranged from poor to good [19], [20], [21], [22], [23], [24], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [37], [38], [39], [40], [41], [42], [43], [44], [46], [47], [50], [51], [53], [54], [57]–61,66]. All the RCTs were found to be of acceptable quality (≥3 points) [25], [36], [45], [48], [49], [52], [55], [56], [62], [63], [64], [65]. The quality assessment and level of evidence are summarized in Table 1.
Table 1.
Characteristics and methodological assessment of the studies included in the review
| Study | Year | Type of study | uMIS type | Patients (n) |
Outcomes assessed | SQ | LE | |
|---|---|---|---|---|---|---|---|---|
| uMIS | TURP | |||||||
| Pisco et al [19] | 2011 | PSCS | PAE | 15 | – | Clinical success on intent-to-treat basis defined as improvement in IPSS (reduction with score ≤20) and/or Qmax to >7 ml/s after PAE | 5 | 4 |
| Carnevale et al [20] | 2013 | Single-arm PSCS | PAE | 11 | – | Urodynamic investigation, PSA, PV, LUTS, sexual function, QoL | 2 | 4 |
| Pisco et al [21] | 2013 | PSCS | PAE | 89 | – | Safety, morbidity, and short- and intermediate-term results of PAE for BPH after failure of medical treatment | 7 | 4 |
| Pisco et al [22] | 2013 | PSCS | PAE | 255 | – | Improvements in symptoms and QoL | 6 | 4 |
| Antunes et al [23] | 2013 | Single-arm PSCS | PAE | 11 | – | QoL, LUTS, PSA, prostate US and MRI, urodynamic investigation | 4 | 4 |
| Kurbatov et al [24] | 2014 | Single-arm PSCS | PAE | 88 | – | LUTS, QoL, urinary flow, PVR, PSA, sexual function | 5 | 4 |
| Gao et al [25] | 2014 | RCT | PAE | 57 | 57 | Safety and adverse events evaluated from intra- and perioperative data (operative time, fluoroscopy time, radiation dose, changes in hemoglobin and serum sodium within 24 h after PAE, transfusion requirements), postoperative data (hospital stay, catheter requirements), and peri- and postoperative complications | 3 | 1b |
| Bagla et al [26] | 2014 | Single-arm PSCS | PAE | 20 | – | Fluoroscopy time, QoL, LUTS, sexual function, PV, adverse events | 4 | 4 |
| Moreira de Assis et al [27] | 2015 | Single-arm PSCS | PAE | 35 | – | LUTS, urinary flow, urodynamic investigation, PSA, QoL, PV (MRI) | 4 | 4 |
| Li et al [28] | 2015 | PSCS | PAE | 24 | – | Improving symptoms (IPSS total score reduction of ≥25% and score <18 points) after PAE and reduction in QoL of at least 1 point (score ≤3 points), with increase in Qmax by ≥2.5 ml/s and Qmax of ≥7 ml/s | 5 | 4 |
| Lin et al [29] | 2015 | PSCS | PAE | 18 | – | Clinical and morphologic (IPP index and PV) effect of PAE in patients with significant median lobe hyperplasia | 5 | 4 |
| Wang et al [30] | 2015 | PSCS | PAE | 117 | – | Primary endpoints: IPSS reduction of 7 points (or ≥25% of the total score) and increase in Qmax (>3 ml/s) at 24 mo after PAE | 5 | 4 |
| Secondary endpoints: reduction in PV, PVR, and QoL at 24 mo after PAE | ||||||||
| Gabr et al [31] | 2016 | PSCS | PAE | 22 | – | Improvement in LUTS and urinary flow rate, and reduction in PV and serum PSA | 5 | 4 |
| Carnevale et al [32] | 2016 | Prospective comparative study | PAE/PPAE | 15/15 | 15 | Urodynamic investigation, LUTS, PV, QoL, urinary flow, sexual function | 6 | 4 |
| Rampoldi et al [33] | 2017 | PSCS | PAE | 43 | – | Technical feasibility, safety, and efficacy of PAE in patients with BPH-associated BOO managed with IBC and unfit for endoscopic or surgical therapy because of severe comorbidities | 5 | 4 |
| Kløw et al [34] | 2018 | Single-arm PSCS | PAE | 29 | – | PV, LUTS, adverse events | 4 | 4 |
| Franiel et al [35] | 2018 | PSCS | PAE | 30 | – | IPSS, QoL score, IIEF, PSA, Qmax, and PVR assessed before PAE (baseline) and at 1, 3, and 6 mo after PAE | 4 | 4 |
| Abt et al [36] | 2018 | Randomized, open-label noninferiority trial | PAE | 48 | 51 | Urinary flow, PVR, QoL, LUTS, sexual function, adverse events | 3 | 1b |
| Maclean et al [37] | 2018 | PSCS | PAE | 86 | – | Reduction in IPSS of ≥25% | 6 | 4 |
| Singhal et al [38] | 2018 | Single-arm PSCS | PAE | 4 | – | LUTS, QoL, PV, PVR | 2 | 4 |
| Salem et al [39] | 2018 | Single-arm PSCS | PAE | 50 | – | LUTS, QoL, sexual potency, ejaculatory preservation, PVR, urinary flow, adverse events | 5 | 4 |
| Shaker et al [40] | 2016 | Single-arm PSCS | PAE | 28 | – | LUTS, QoL, urinary flow, PV | 4 | 4 |
| Moreira de Assis et al [41] | 2019 | Single-arm PSCS | PAE | 8 | – | LUTS, PSA, urinary flow, PV (MRI), prostate elastography | 4 | 4 |
| Bilhim et al [42] | 2019 | Single-arm, single-blind prospective randomized trial | PAE | 89 | – | LUTS, QoL, sexual function, PV, PSA, urinary flow, PVR | 3 | 1b |
| Lindgren and Bläckberg [43] | 2019 | PSCS | PAE | 37 | – | IPSS reduction of ≥25% or improvement in QoL of 3 points, or freedom from urinary catheter in patients with previous chronic use or CIC, and urinary flow > 10 ml/s | 5 | 4 |
| Malling et al [44] | 2019 | PSCS | PAE | 11 | – | Primary outcome for men with urinary retention: ability to void 6 mo after PAE | 4 | 4 |
| Insausti et al [45] | 2020 | Noninferiority randomized trial | PAE | 23 | 22 | Urinary flow, LUTS, QoL, PV, adverse events | 3 | 1b |
| Cheng et al [46] | 2020 | Prospective single-arm cohort study | PAE | 21 | – | LUTS, urinary flow, PVR, PSA, PV | 4 | 4 |
| Al Rawashdah et al [47] | 2020 | Single-arm PSCS | PAE | 147 | – | LUTS, QoL, urinary flow, PVR, PV, sexual function, ejaculation preservation | 5 | 4 |
| Pisco et al [48] | 2020 | Randomized, single-blind, sham-controlled superiority clinical trial | PAE | 80 | – | LUTS, PSA, urinary flow, PVR, PV, sexual function, fluoroscopy time, procedural time | 3 | 1b |
| Radwan et al [49] | 2020 | RCT | PAE | 20 | 40 | Efficacy and safety of PAE | 2 | 1b |
| Tapping et al [50] | 2020 | Single-arm PSCS | PAE | 50 | – | LUTS, sexual potency, PV (mutiparametric MRI) | 4 | 4 |
| Denmeade et al [51] | 2011 | Phase 1/2 comparative study | PRX302 | 33 | – | LUTS, QoL, PV, PVR, urinary flow | 5 | 4 |
| Elhilali et al [52] | 2013 | RCT | PRX302 | 61 | 31 (PBO) | Primary endpoint: increase in Qmax | 5 | 1a |
| Other endpoints: PV as measured by TRUS, reduction in IPSS | ||||||||
| Dixon et al [53] | 2015 | PSCS | Rezūm | 65 | – | Efficacy and safety | 4 | 4 |
| Dixon et al [54] | 2016 | PSCS | Rezūm | 65 | – | IPSS, QOL instruments (QOL from IPSS, BPHII), and sexual function with IIEF, IIEF-question 9 for ejaculatory function, and MSHQ-EjD (1 center) Uroflowmetry, PVR, and PSA were repeated at 1 wk, and 1, 3, 6, 12, and 24 mo | 5 | 4 |
| McVary et al [55] | 2016 | RCT | Rezūm | 136 | 61 (PBO) | Voiding symptoms (IPSS, QoL, Qmax, incontinence, IIEF-EF score) and ejaculatory function (MSHQ-EjD); effect of median lobe treatment on IPSS and sexual function | 3 | 1b |
| Roehrborn et al [56] | 2017 | Multicenter RCT | Rezūm | 135 | – | LUTS, urinary flow, incontinence rate, sexual function, adverse events | 4 | 1b |
| Porpiglia et al [57] | 2019 | PSCS | TIND | 32 | – | Safe, effective, and well tolerated | 6 | 4 |
| Kadner et al [58] | 2020 | Single-arm, multicenter PSCS | iTIND | 81 | – | LUTS, QoL, urinary flow, PVR, PV, intraoperative complications, sexual function, ejaculatory preservation | 4 | 4 |
| Amparore et al [59] | 2020 | Single-arm, multicenter PSCS | iTIND | 81 | – | Operating room time, postoperative complications, urinary flow, LUTS, PVR, QoL, sexual function, ejaculatory preservation | 4 | 4 |
| Woo et al [60] | 2011 | PSCS | PUL | 19 | – | Safety and feasibility | 5 | 4 |
| Woo et al [61] | 2012 | PSCS | PUL | 64 | – | Effect of PUL procedure on erectile and ejaculatory function | 6 | 4 |
| Cantwell et al [62] | 2014 | Randomized double-blind study | PUL | 66 | – | LUTS, sexual function, ejaculatory preservation, urinary flow, PVR | 4 | 1a |
| McVary et al [63] | 2014 | RCT | PUL | 140 | 66 (PBO) | Improved LUTS and urinary flow rate with preservation of sexual function | 4 | 1b |
| Sønksen et al [64] | 2015 | Multinational prospective, randomized, nonblinded study | PUL | 45 | 35 | LUTS, postoperative recovery, sexual potency, ejaculatory preservation, continence preservation, high-grade complications | 3 | 1b |
| Gratzke et al [65] | 2016 | RCT | PUL | 40 | 40 | Primary endpoint: composite of six validated instruments assessing net health outcome at 1 yr: IPSS, SHIM, MSHQ-EjD, ISI, QoR VAS, and Clavien-Dindo classification of adverse events. | 3 | 1b |
| Rukstalis et al [66] | 2019 | Single-arm PSCS | PUL | 45 | – | LUTS, postprocedural severe complications, QoL, urinary flow, sexual function | 4 | 4 |
uMIST = ultra-minimally invasive treatment; PSCS = prospective study-case series; TURP = transurethral resection of the prostate; SQ = study quality; LE = level of evidence; PAE = prostatic artery embolization; PPAE = perfected PAE; PUL = prostatic urethral lift; PBO = placebo; LUTS = lower urinary tract symptoms; MRI = magnetic resonance imaging; PVR = postvoid residual volume; PV = prostate volume; PSA = prostate-specific antigen; Qmax = maximum urinary flow; CIC = clean intermittent catheterization; QoL = quality of life; IPSS = International Prostate Symptom Score; IIEF = International Index of Erectile Function; IIEF-EF = IIEF-Ejaculatory Function; RCT = randomized controlled trial; IPP = intravesical prostatic protrusion; BPH = benign prostatic hyperplasia; BPHII = BPH Impact Index; BOO = bladder outlet obstruction; IBC = indwelling bladder catheterization; MSHQ-EjD = Male Sexual Health Questionnaire-Ejaculatory Dysfunction; SHIM = Sexual Health Inventory for Men; ISI = Incontinence Severity Index; QoR VAS = Quality of Recovery Visual Analog Scale; TIND = temporary implantable nitinol device; iTIND = second-generation TIND.
3.2. Risk of bias
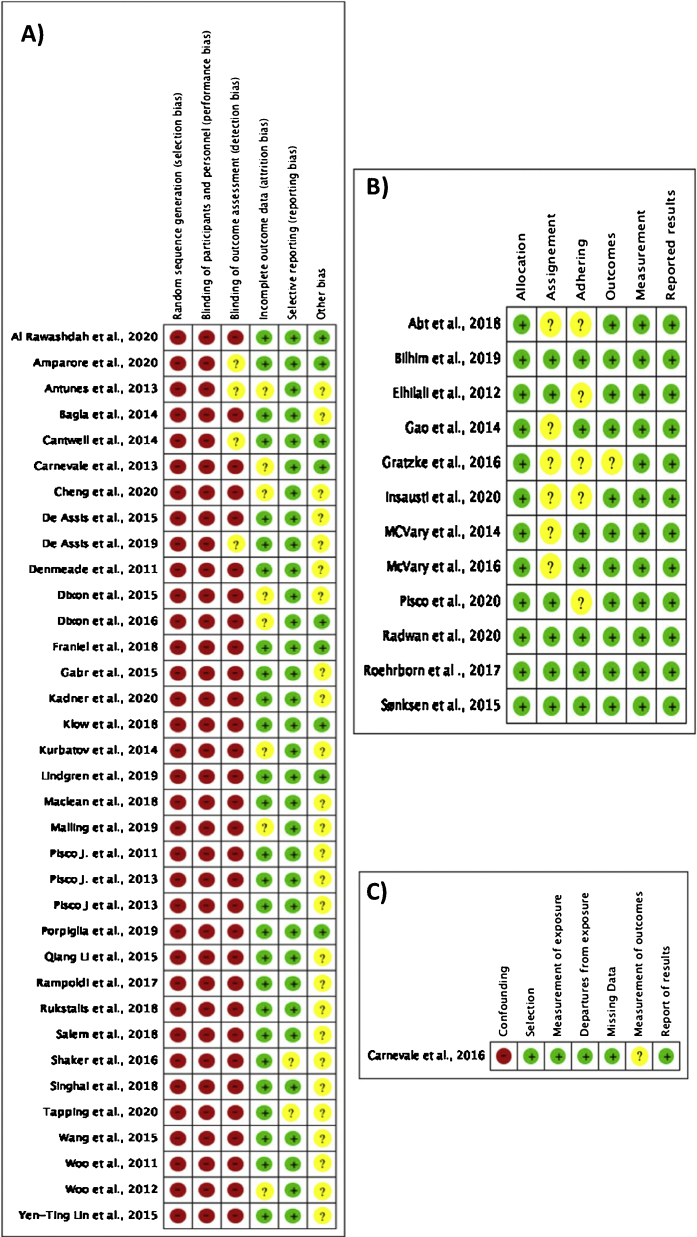
High risk of bias (selection, performance, and detection bias) was observed for all the single-arm studies [19], [20], [21], [22], [23], [24], [26], [27], [28], [29], [30], [31], [33], [34], [35], [37]–44,46,47,50,51,53,54,[57], [58], [59], [60], [61],66] (Fig. 2A), whilst the risk of bias was low for the comparative study [32] and the RCTs [25], [36], [45], [48], [49], [52], [55], [56], [62], [63], [64], [65] (Fig. 2B,C). Funnel plot assessment demonstrated a high risk of bias among the studies, as shown in Supplementary Figure 1.
Fig. 2.
Risk-of-bias assessment for (A) single-arm studies, (B) randomized controlled trials, and (C) the comparative study.
3.3. Baseline characteristics
Baseline data are summarized in Supplementary Table 1. Assessment (Supplementary Fig. 2) revealed that patients in the PAE group were the oldest (68.81 yr, 95% CI 67.79–71.82), were the only ones with indwelling catheters (24%, 95% CI 17–31%), and had the highest prostate volume (88.4 cm3, 95% CI 79.35–97.53). Patients in the PPAE group had both the highest IPSS (24.6 points, 95% CI 22.78–26.42) and the lowest Qmax (5.10 ml/s, 95% CI 3.58–6.62). The TIND group had the lowest IPSS-QoL (3.96 points, 95% CI 3.77–4.15). MSHQ-EjD function and bother scores were only available for Rezūm and UroLift studies. The Rezūm group had a lower MSHQ-EjD function score (7.72 points, 95% CI 7.22–8.23) in comparison to the UroLift group. SHIM scores were only reported in studies assessing the UroLift technique.
3.4. Micturition outcomes
At the start of follow-up, there was a decrease in the pooled IPSS median score with respect to baseline (−9.81 points, 95% CI −11.37 to −8.25) and a further decline by 12 mo (−13.13 points, 95% CI −14.98 to −11.64; Fig. 3A).
Fig. 3.
Forest plots of the pooled postintervention change in micturition outcomes: (A) International Prostate Symptom Score (IPSS), (B) maximum flow rate (QMax), (C) postvoid residual volume (PVR), and (D) IPSS Quality of Life (QoL). CI = confidence interval; PAE = prostatic artery embolization; TIND = temporary implantable nitinol device.
Pooled analysis showed that PAE led to the lowest 1-mo (−6.53 points, 95% CI −7.62 to −5.54) and the highest 12-mo postintervention changes in IPSS (−18.21 points, 95% CI −21.25 to −15.17; Fig. 3A). IPSS data for every time point for all the uMISTs are reported in Supplementary Figure 3.
For Qmax, an overall pooled improvement of 3.66 ml/s (95% CI 2.8–4.5) was recorded at 1 mo of follow-up and remained relatively stable during the study period (4.14 ml/s, 95% CI 0.72–7.56 at 12 mo). The highest postintervention change in Qmax was observed for UroLift at 1 mo (7.80 ml/s, 95% CI 5.78–9.82) and for TIND at 12 mo (7.30 ml/s, 95% CI 6.10–8.50; Fig. 3B and Supplementary Fig. 3).
A significant improvement in PVR was observed at 1 mo (−28.72 ml, 95% CI −52.23 to −5.21) and 6 mo (−23.45 ml, 95% CI −33.48 to −13.45), followed by a slight decrease up to 12 mo (−10.10 ml, 95% CI −27.90 to 7.71; Fig. 3C and Supplementary Fig. 3). The highest postintervention change was found at 12 mo for the TIND technique (−39.51 ml, 95% CI −47.86 to −31.16; Fig. 3C).
3.5. Sexual outcomes
Data for IIEF-5 were available for PAE and PPAE only. The 12-mo IIEF-5 score was higher for the PPAE group than for the PAE group (18.70 vs 15.71; Supplementary Fig. 3). No analysis of postintervention changes in IIEF-5 was possible.
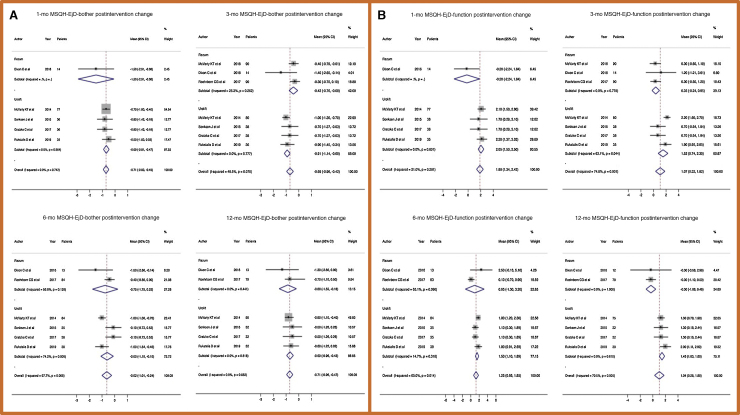
No significant difference in postintervention changes in MSHQ-EjD bother among the treatments was found at 1, 3, 6, and 12 mo (Fig. 4A and Supplementary Fig. 3).
Fig. 4.
Forest plots of the pooled postintervention change in Male Sexual Health Questionnaire-Ejaculatory Dysfunction (MSHQ-EjD) scales for (A) bother and (B) function. CI = confidence interval.
MSHQ-EjD function was not affected by the treatments either at the beginning of follow-up (change in overall pooled median score of 1.88 points, 95% CI 1.34–2.42) or after 1 yr (1.04 points, 95% CI 0.28–1.8). At 12 mo the change in MSHQ-EjD function was lower in the Rezūm group (−0.30 points, 95% CI −1.08 to 0.48; Fig. 4B). Data regarding SHIM scores were only available for UroLift studies (Supplementary Fig. 3).
3.6. QoL outcomes
At 1 mo the pooled change in IPSS-QoL was lowest in the TIND group (−2.74 points, 95% CI −3.17 to −2.32), whereas there was no difference at 3, 6, and 12 mo (Fig. 3D). Overall, the change in QoL at 12 mo was less than −2.5 points (95% CI −2.85 to −2.16).
3.7. Adverse events
Assessment of complications demonstrated an overall rate of 29%, with no difference among the techniques included (Supplementary Fig. 3).
3.8. Interpretation of further study findings
All five uMIST procedures (PAE, UroLift, TIND, Rezūm, and intraprostatic injection) can be performed in the office using intravenous sedation and local anesthesia [67]. Oral sedation has also been described for PUL and Rezūm. Perioperative management is in line with what is expected for a treatment defined as ultra-minimally invasive. Mean operative times for PUL range from 29 to 57 min, while the average treatment times for Rezūm and TIND are 2–6 and 5.8 min, respectively (Supplementary Table 2) [67]. Hospital stays range from 0 to 2 d (Supplementary Table 2), with only one study that reported 6 d for PAE [28].
Rates of postprocedural catheterization for PUL and Rezūm differ, depending on the protocol, with 32–68% of PUL and 55–100% of Rezūm patients still having a catheter in place at discharge [67]. Patients undergoing TIND do not require postoperative catheterization, and thus experience better postprocedural comfort. Our analysis showed that the 1-mo IPSS-QoL (2.08 points, 95% CI 1.87–2.29)] and IPSS-QoL change from baseline (−2.74 points, 95% CI −3.17 to −2.32) were lowest in the TIND group (Fig. 3D).
Compared to other MIST (not uMIST) procedures, TURP [68] and Aquabeam [4] require a significantly longer operative time and a hospital stay for an average of 2.1 d, and a postoperative catheter is always needed.
The number of men requiring surgical intervention vastly exceeds our operative resources, which were further reduced due to pandemic restrictions [69], so uMISTs might represent an attractive and convenient alternative for both patients and physicians.
Focusing on micturition and functional outcomes, TIND devices guarantee a good decrease in IPSS compared to baseline from the first month postoperatively (−11.05 points, 95% CI −12.25 to −9.86), with relatively stable results at 12 mo (−13.63 points, 95% CI −15.09 to −12.16). Similar findings were recorded for PUL and Rezūm. On the contrary, PAE led to a slight improvement in IPSS at the beginning of follow-up (−6.53 points, 95% CI −7.62 to −5.54), with a more significant improvement after 1 yr (−18.21 points, 95% CI −21.25 to −15.17). These differences did not affect the change in Qmax among the different techniques, for which PAE was in line with the other uMISTs. Noteworthy is the UroLift Qmax improvement at 1 mo after intervention (7.80 ml/s, 95% CI 5.78–9.82). PVR was lower in the TIND group at every time point evaluated up to 12 mo of follow-up.
The delayed achievement of a plateau in benefit from PAE can be explained by the different inclusion criteria for patient selection: higher prostate volume might lead to a longer time for the ischemia to induce necrosis in large-volume adenomas. Moreover, the presence of an indwelling catheter could stunt bladder contractility in the first months after the intervention.
Regarding sexual outcomes, a comment should be made with regard to the ideal candidates for these uMISTs. In the past, when first attempts to introduce less invasive treatment options such as transurethral needle ablation and transurethral microwave thermotherapy [70] were made, the target population was often represented by older patients unfit for conventional surgery. Nowadays an ever-younger population is interested in uMISTs since patients prefer to avoid daily consumption of oral medications, which can have considerable sexual side effects (such as erectile and ejaculatory dysfunction). However, these patients do not want to undergo a “standard” surgical procedure requiring general anesthesia and longer recovery, and they prefer to avoid the non-negligible risks of complications, including permanent ejaculatory dysfunction.
In our analysis, data on erectile function in terms of IIEF-5 score were only available for PPAE and PAE groups (mean score at 12 mo: 18.70 vs 15.71). A recently published multicenter Italian Rezūm study showed a statistically significant increase in IIEF-5 score from baseline at 6 mo (20 vs 23.5 [IQR 21–25.5]; p = 0.04), with postoperative scores of 4 and 5 for question 9 of the IIEF-15 questionnaire in 81% and 17.1% of cases, respectively, and a score of 5 for question 10 in 98.5% of cases [71]. These results are in line with the data reported for conventional BPH surgical options.
By contrast, ejaculation dysfunction was widely explored. Our analysis revealed no statistically significant difference for the changes in MSHQ-EjD bother at 1, 3, 6, and 12 mo. In terms of MSHQ-EjD function, substantial stability compared to baseline was recorded. Rezūm seemed to be the most effective treatment at 12 mo, with the lowest score recorded (−0.30 points, 95% CI −1.08 to 0.48).
No case of EjD was recorded in the PUL, TIND, and PAE groups, except for one trial that reported 13.3% loss of ejaculatory volume after PAE [72]. In the Rezūm cohort, EjD ranged from 0% to 4.4%; McVary [55] observed EjD incidence of 2.9% in an RCT, similar to Mollengarden et al [73] (3.1%). More recently, a retrospective review of 62 patients treated in France revealed an even higher anejaculation rate (10.8%) [74]. However, even if Rezūm might carry a higher risk of sexual dysfunction compared to PUL and TIND, this risk is relatively low compared with the rate of retrograde ejaculation reported after TURP (65.4%) [75] and HoLEP (70%) [76].
Cacciamani et al [77] evaluated EjD in a systematic review and pooled analysis of randomized trials and reported that among patients undergoing PUL, MSHQ-EjD bother scores did not reflect significant changes in function score compared to the TURP cohort (−0.1 vs −0.3). The meta-analysis was unable to demonstrate an advantage of PAE over TURP in terms of retrograde ejaculation rates (relative risk 0.73, 95% CI 0.49–1.08; p = 0.11). The physiological mechanisms of ejaculation were recently explored in depth and conventional techniques (TURP or HoLEP) were modified with the aim of avoiding EjD [76].
Acceptable rates of Clavien-Dindo grade III/IV complications have been reported for PAE, PUL, and Rezūm. Data for TIND revealed that 9.9% of cases experienced Clavien-Dindo grade III complications in the first month after the procedure, after which no further complications occurred [78]. For PUL, adverse events were mostly limited to the first 3 mo and consisted of mild to moderate dysuria and hematuria [79]. However, serious related adverse events in the first year included an overnight stay for clot retention related to restarting warfarin therapy and a bladder stone formed from bladder gravel that was detected at baseline. Although rare, TIND patients may experience complications during the implantation period that require treatment abortion [57] or immediate retreatment (2.5–6.2% of cases) [78]. Clavien-Dindo grade I/II adverse events included hematuria, dysuria, urgency, pain, and urinary tract infection, while grade III events included eight episodes (9.9%) of acute urinary retention [59].
Notwithstanding all the above-mentioned benefits of these uMISTs, some obstacles limit their wider adoption, of which equipment limitations represent the first. For example, a specific extra-long bronchoscope lens is necessary for UroLift, and a dedicated computerized radiofrequency steam generator is required for Rezūm.
The second limitation of these new technologies is the cost, which amply exceeds €1000 for just the equipment, besides the requirement to perform the procedures in a dedicated operative setting [80]. In fact, as emerged in a cost-effectiveness analysis, the cheaper MITs were ∼$900 more expensive than the cost of drug therapies over 2 yr. TURP and photovaporization of the prostate provided slightly greater relief of LUTS than MITs at approximately twice the cost over 2 yr [81].
To date, only TIND perfectly meets the uMIST concept [80]: the entire procedure can be performed under local anesthesia or light sedation (without a dedicated setting) with a standard flexible or rigid cystoscope with no extra costs. The procedure is extremely fast and easy to perform, with promising findings for symptom relief up to 3-yr follow-up [59].
Our study is not devoid of limitations. First, the studies included are heterogeneous regarding surgical procedures and study design. In fact, some of these new uMISTs (TIND, Rezūm) lack a comparison with gold-standard options such as TURP; moreover none of the studies directly compared the uMISTs to each other. Although all the studies report similar outcomes, the timings for evaluation differed and complications were not always classified according to the standard Clavien-Dindo system. The paucity of long-term follow-up data did not allow analysis after the first 12 mo postoperatively, despite the promising data from single-center experiences [33], [39], [55], [56], [57], [59], [65], [78]. Lastly, owing to the lack of comparative data with standard TURP, no direct comparison with network meta-analysis was possible among the different uMISTs.
4. Conclusions
Available data suggest that uMISTs might offer effective relief from LUTS in patients with BPH. For Rezūm, UroLift, and PAE, a minimal impact on patients’ sexual function in relation to baseline, especially in terms of preservation of ejaculation, was registered. Longer follow-up and further trials comparing these treatment options with more established techniques are warranted.
Author contributions: Enrico Checcucci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Checcucci.
Acquisition of data: Piana, Granato, Verri, Sica, Meziere, Piscitello, Carbonaro, Zamengo.
Analysis and interpretation of data: Volpi, Carbonara, Veccia, Piramide, De Cillis, Pecoraro.
Drafting of the manuscript: Checcucci, Veccia, Autorino.
Critical revision of the manuscript for important intellectual content: Manfredi, De Luca, Cacciamani, Okhunov, Puliatti, Taratkin, Marenco, Gomez Rivas, Veneziano, Russo.
Statistical analysis: Veccia, Piramide.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia, Fiori, Autorino.
Other: None.
Financial disclosures: Enrico Checcucci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgement
We would like to thanks Dr. Nicoletta Colombi for her assistance in this study.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euros.2021.08.009.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Lim K.B. Epidemiology of clinical benign prostatic hyperplasia. Asian J Urol. 2017;4:148–151. doi: 10.1016/j.ajur.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun Y., Peng B., Lei G.L., Wei Q., Yang L. Study of phosphodiesterase 5 inhibitors and α-adrenoceptor antagonists used alone or in combination for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Minerva Urol Nefrol. 2020;72:13–21. doi: 10.23736/S0393-2249.19.03408-8. [DOI] [PubMed] [Google Scholar]
- 3.Capogrosso P., Fallara G., Pozzi E., Schifano N., Candela L., Costa A., Boeri L., Belladelli F., Cazzaniga W., Scattoni V., Salonia A., Montorsi F. Rates and predictors of postoperative complications after Holmium laser enucleation of the prostate (HoLEP) at a high-volume center. Minerva Urol Nephrol. 2021 doi: 10.23736/S2724-6051.21.04315-9. Epub ahead of print. PMID: 33887894. [DOI] [PubMed] [Google Scholar]
- 4.Fiori C., Checcucci E., Gilling P. All you need to know about “Aquablation” procedure for treatment of benign prostatic obstruction. Minerva Urol Nefrol. 2020;72:152–161. doi: 10.23736/S0393-2249.20.03654-1. [DOI] [PubMed] [Google Scholar]
- 5.Porpiglia F., Checcucci E., Amparore D. Urethral-sparing robot-assisted simple prostatectomy: an innovative technique to preserve ejaculatory function overcoming the limitation of the standard Millin approach. Eur Urol. 2021;80:222–233. doi: 10.1016/j.eururo.2020.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Gravas S., Cornu J.N., Gacci M. European Association of Urology; Arnhem, The Netherlands: 2021. EAU guidelines: management of non-neurogenic male lower urinary tract symptoms.https://uroweb.org/guideline/treatment-of-non-neurogenic-male-luts/ [Google Scholar]
- 7.La Russa M., Liakou C., Burbos N. Ultra-minimally invasive approaches for endometrial cancer treatment: review of the literature. Minerva Med. 2021;112:31–46. doi: 10.23736/S0026-4806.20.07073-1. [DOI] [PubMed] [Google Scholar]
- 8.Rojo-Manaute J.M., Capa-Grasa A., Chana-Rodríguez F. Ultra-minimally invasive ultrasound-guided carpal tunnel release: a randomized clinical trial. J Ultrasound Med. 2016;35:1149–1157. doi: 10.7863/ultra.15.07001. [DOI] [PubMed] [Google Scholar]
- 9.Cumpston M., Li T., Page M.J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;2019 doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Higgins J.P., Altman D.G., Gøtzsche P.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne J.A., Hernán M.A., Reeves B.C. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne J.A.C., Savović J., Page M.J. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O’Connell D et al. The Newcastle Ottawa 1 Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 15.Jadad A.R., Moore R.A., Carroll D. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt G.H., Oxman A.D., Vist G.E. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pisco J.M., Pinheiro L.C., Bilhim T., Duarte M., Mendes J.R., Oliveira A.G. Prostatic arterial embolization to treat benign prostatic hyperplasia. J Vasc Interv Radiol. 2011;22:11–19. doi: 10.1016/j.jvir.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Carnevale F.C., da Motta-Leal-Filho J.M., Antunes A.A. Quality of life and clinical symptom improvement support prostatic artery embolization for patients with acute urinary retention caused by benign prostatic hyperplasia. J Vasc Interv Radiol. 2013;24:535–542. doi: 10.1016/j.jvir.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Pisco J., Campos Pinheiro L., Bilhim T. Prostatic arterial embolization for benign prostatic hyperplasia: short- and intermediate-term results. Radiology. 2013;266:668–677. doi: 10.1148/radiol.12111601. [DOI] [PubMed] [Google Scholar]
- 22.Pisco J.M., Rio Tinto H., Campos Pinheiro L. Embolisation of prostatic arteries as treatment of moderate to severe lower urinary symptoms (LUTS) secondary to benign hyperplasia: results of short- and mid-term follow-up. Eur Radiol. 2013;23:2561–2572. doi: 10.1007/s00330-012-2714-9. [DOI] [PubMed] [Google Scholar]
- 23.Antunes A.A., Carnevale F.C., da Motta Leal Filho J.M. Clinical, laboratorial, and urodynamic findings of prostatic artery embolization for the treatment of urinary retention related to benign prostatic hyperplasia. A prospective single-center pilot study. Cardiovasc Intervent Radiol. 2013;36:978–986. doi: 10.1007/s00270-013-0611-5. [DOI] [PubMed] [Google Scholar]
- 24.Kurbatov D., Russo G.I., Lepetukhin A. Prostatic artery embolization for prostate volume greater than 80 cm3: results from a single-center prospective study. Urology. 2014;84:400–404. doi: 10.1016/j.urology.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 25.Gao Y.A., Huang Y., Zhang R. Benign prostatic hyperplasia: prostatic arterial embolization versus transurethral resection of the prostate—a prospective, randomized, and controlled clinical trial. Radiology. 2014;270:920–928. doi: 10.1148/radiol.13122803. [DOI] [PubMed] [Google Scholar]
- 26.Bagla S., Martin C.P., van Breda A. Early results from a United States trial of prostatic artery embolization in the treatment of benign prostatic hyperplasia. J Vasc Interv Radiol. 2014;25:47–52. doi: 10.1016/j.jvir.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Moreira de Assis A., Mota Moreira A., de Paula Rodrigues V.C. Prostatic artery embolization for treatment of benign prostatic hyperplasia in patients with prostates >90 g: a prospective single-center study. J Vasc Interv Radiol. 2015;26:87–93. doi: 10.1016/j.jvir.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Li Q., Duan F., Wang M.Q., Zhang G.D., Yuan K. Prostatic arterial embolization with small sized particles for the treatment of lower urinary tract symptoms due to large benign prostatic hyperplasia: preliminary results. Chin Med J. 2015;128:2072–2077. doi: 10.4103/0366-6999.161370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Y.T., Amouyal G., Thiounn N. Intra-vesical prostatic protrusion (IPP) can be reduced by prostatic artery embolization. Cardiovasc Intervent Radiol. 2016;39:690–695. doi: 10.1007/s00270-015-1235-8. [DOI] [PubMed] [Google Scholar]
- 30.Wang M.Q., Guo L.P., Zhang G.D. Prostatic arterial embolization for the treatment of lower urinary tract symptoms due to large (>80 mL) benign prostatic hyperplasia: results of midterm follow-up from Chinese population. BMC Urol. 2015;15:33. doi: 10.1186/s12894-015-0026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabr A.H., Gabr M.F., Elmohamady B.N., Ahmed A.F. Prostatic artery embolization: a promising technique in the treatment of high-risk patients with benign prostatic hyperplasia. Urol Int. 2016;97:320–324. doi: 10.1159/000447360. [DOI] [PubMed] [Google Scholar]
- 32.Carnevale F.C., Iscaife A., Yoshinaga E.M., Moreira A.M., Antunes A.A., Srougi M. Transurethral resection of the prostate (TURP) versus original and perfected prostate artery embolization (PAE) due to benign prostatic hyperplasia (BPH): preliminary results of a single center, prospective, urodynamic-controlled analysis. Cardiovasc Intervent Radiol. 2016;39:44–52. doi: 10.1007/s00270-015-1202-4. [DOI] [PubMed] [Google Scholar]
- 33.Rampoldi A., Barbosa F., Secco S. Prostatic artery embolization as an alternative to indwelling bladder catheterization to manage benign prostatic hyperplasia in poor surgical candidates. Cardiovasc Intervent Radiol. 2017;40:530–536. doi: 10.1007/s00270-017-1582-8. [DOI] [PubMed] [Google Scholar]
- 34.Kløw N.E., Grøtta O.J., Bay D. Outcome after prostatic artery embolization in patients with symptomatic benign prostatic hyperplasia. Acta Radiol. 2019;60:1175–1180. doi: 10.1177/0284185118813709. [DOI] [PubMed] [Google Scholar]
- 35.Franiel T., Aschenbach R., Trupp S. Prostatic artery embolization with 250-μm spherical polyzene-coated hydrogel microspheres for lower urinary tract symptoms with follow-up MR imaging. J Vasc Interv Radiol. 2018;29:1127–1137. doi: 10.1016/j.jvir.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Abt D., Hechelhammer L., Müllhaupt G. Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: randomised, open label, non-inferiority trial. BMJ. 2018;361:k2338. doi: 10.1136/bmj.k2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maclean D., Harris M., Drake T. Factors predicting a good symptomatic outcome after prostate artery embolisation (PAE) Cardiovasc Intervent Radiol. 2018;41:1152–1159. doi: 10.1007/s00270-018-1912-5. [DOI] [PubMed] [Google Scholar]
- 38.Singhal S., Sebastian B., Madhurkar R., Uthappa M.C. Prostate artery embolisation: an initial experience from an Indian perspective. Pol J Radiol. 2018;83 doi: 10.5114/pjr.2018.81318. e554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salem R., Hairston J., Hohlastos E. Prostate artery embolization for lower urinary tract symptoms secondary to benign prostatic hyperplasia: results from a prospective FDA-approved investigational device exemption study. Urology. 2018;120:205–210. doi: 10.1016/j.urology.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Shaker M., Abd El Tawab K.A., Abd El Tawab K.H., El-Gharib M. Role of prostatic artery embolization in management of symptomatic benign prostatic hyperplasia. Egyptian J Radiol Nucl Med. 2016;47:839–845. doi: 10.1016/j.ejrnm.2016.04.012. [DOI] [Google Scholar]
- 41.Moreira de Assis A., Mota Moreira A., Carnevale F.C. Effects of prostatic artery embolization on the dynamic component of benign prostate hyperplasia as assessed by ultrasound elastography: a pilot series. Cardiovasc Intervent Radiol. 2019;42:1001–1007. doi: 10.1007/s00270-019-02220-x. [DOI] [PubMed] [Google Scholar]
- 42.Bilhim T., Costa N.V., Torres D., Pisco J., Carmo S., Oliveira A.G. Randomized clinical trial of balloon occlusion versus conventional microcatheter prostatic artery embolization for benign prostatic hyperplasia. J Vasc Interv Radiol. 2019;30:1798–1806. doi: 10.1016/j.jvir.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 43.Lindgren H., Bläckberg M. Introduction of prostate artery embolization (PAE) in Sweden. Scand J Urol. 2019;53:151–155. doi: 10.1080/21681805.2019.1610494. [DOI] [PubMed] [Google Scholar]
- 44.Malling B., Lönn L., Jensen R.J. Prostate artery embolization for lower urinary tract symptoms in men unfit for surgery. Diagnostics. 2019;9:46. doi: 10.3390/diagnostics9020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Insausti I., Sáez de Ocáriz A., Galbete A. Randomized comparison of prostatic artery embolization versus transurethral resection of the prostate for treatment of benign prostatic hyperplasia. J Vasc Interv Radiol. 2020;31:882–890. doi: 10.1016/j.jvir.2019.12.810. [DOI] [PubMed] [Google Scholar]
- 46.Cheng K.C., Wong W.Y., Chan H.C. Prostatic arterial embolisation in men with benign prostatic enlargement and refractory retention considered high-risk surgical candidates. Hong Kong J Radiol. 2020;23:114–121. [Google Scholar]
- 47.Al Rawashdah S.F., Pastore A.L., Velotti G. Sexual and functional outcomes of prostate artery embolisation: a prospective long-term follow-up, large cohort study. Int J Clin Pract. 2020;74:e13454. doi: 10.1111/ijcp.13454. [DOI] [PubMed] [Google Scholar]
- 48.Pisco J.M., Bilhim T., Costa N.V. Randomised clinical trial of prostatic artery embolisation versus a sham procedure for benign prostatic hyperplasia. Eur Urol. 2020;77:354–362. doi: 10.1016/j.eururo.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Radwan A., Farouk A., Higazy A., Samir Y.R., Tawfeek A.M., Gamal M.A. Prostatic artery embolization versus transurethral resection of the prostate in management of benign prostatic hyperplasia. Prostate Int. 2020;8:130–133. doi: 10.1016/j.prnil.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tapping C.R., Little M.W., Macdonald A. The STREAM trial (Prostatic Artery Embolization for the Treatment of Benign Prostatic Hyperplasia) 24-month clinical and radiological outcomes. Cardiovasc Intervent Radiol. 2021;44:436–442. doi: 10.1007/s00270-020-02702-3. [DOI] [PubMed] [Google Scholar]
- 51.Denmeade S.R., Egerdie B., Steinhoff G., Merchant R., Abi-Habib R., Pommerville P. Phase 1 and 2 studies demonstrate the safety and efficacy of intraprostatic injection of PRX302 for the targeted treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. 2011;59:747–754. doi: 10.1016/j.eururo.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elhilali M.M., Pommerville P., Yocum R.C., Merchant R., Roehrborn C.G., Denmeade S.R. Prospective, randomized, double-blind, vehicle controlled, multicenter phase IIb clinical trial of the pore forming protein PRX302 for targeted treatment of symptomatic benign prostatic hyperplasia. J Urol. 2013;189:1421–1426. doi: 10.1016/j.juro.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dixon C., Cedano E.R., Pacik D. Efficacy and safety of Rezūm system water vapor treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urology. 2015;86:1042–1047. doi: 10.1016/j.urology.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 54.Dixon C.M., Cedano E.R., Pacik D. Two-year results after convective radiofrequency water vapor thermal therapy of symptomatic benign prostatic hyperplasia. Res Rep Urol. 2016;8:207–216. doi: 10.2147/rru.s119596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McVary K.T., Gange S.N., Gittelman M.C. Erectile and ejaculatory function preserved with convective water vapor energy treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: randomized controlled study. J Sex Med. 2016;13:924–933. doi: 10.1016/j.jsxm.2016.03.372. [DOI] [PubMed] [Google Scholar]
- 56.Roehrborn C.G., Gange S.N., Gittelman M.C. Convective thermal therapy: durable 2-year results of randomized controlled and prospective crossover studies for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. J Urol. 2017;197:1507–1516. doi: 10.1016/j.juro.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 57.Porpiglia F., Fiori C., Bertolo R. 3-Year follow-up of temporary implantable nitinol device implantation for the treatment of benign prostatic obstruction. BJU Int. 2018;122:106–112. doi: 10.1111/bju.14141. [DOI] [PubMed] [Google Scholar]
- 58.Kadner G., Valerio M., Giannakis I. Second generation of temporary implantable nitinol device (iTind) in men with LUTS: 2 year results of the MT-02-study. World J Urol. 2020;38:3235–3244. doi: 10.1007/s00345-020-03140-z. [DOI] [PubMed] [Google Scholar]
- 59.Amparore D., Fiori C., Valerio M. 3-Year results following treatment with the second generation of the temporary implantable nitinol device in men with LUTS secondary to benign prostatic obstruction. Prostate Cancer Prostat Dis. 2021;24:349–357. doi: 10.1038/s41391-020-00281-5. [DOI] [PubMed] [Google Scholar]
- 60.Woo H.H., Chin P.T., McNicholas T.A. Safety and feasibility of the prostatic urethral lift: a novel, minimally invasive treatment for lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) BJU Int. 2011;108:82–88. doi: 10.1111/j.1464-410X.2011.10342.x. [DOI] [PubMed] [Google Scholar]
- 61.Woo H.H., Bolton D.M., Laborde E. Preservation of sexual function with the prostatic urethral lift: a novel treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Sex Med. 2012;9:568–575. doi: 10.1111/j.1743-6109.2011.02568.x. [DOI] [PubMed] [Google Scholar]
- 62.Cantwell A.L., Bogache W.K., Richardson S.F. Multicentre prospective crossover study of the ‘prostatic urethral lift’ for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. BJU Int. 2014;113:615–622. doi: 10.1111/bju.12540. [DOI] [PubMed] [Google Scholar]
- 63.McVary K.T., Gange S.N., Shore N.D. Treatment of LUTS secondary to BPH while preserving sexual function: randomized controlled study of prostatic urethral lift. J Sex Med. 2014;11:279–287. doi: 10.1111/jsm.12333. [DOI] [PubMed] [Google Scholar]
- 64.Sønksen J., Barber N.J., Speakman M.J. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol. 2015;68:643–652. doi: 10.1016/j.eururo.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 65.Gratzke C., Barber N., Speakman M.J. Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH6 prospective, multicentre, randomized study. BJU Int. 2017;119:767–775. doi: 10.1111/bju.13714. [DOI] [PubMed] [Google Scholar]
- 66.Rukstalis D., Grier D., Stroup S.P. Prostatic urethral lift (PUL) for obstructive median lobes: 12 month results of the MedLift Study. Prostate Cancer Prostat Dis. 2019;22:411–419. doi: 10.1038/s41391-018-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tzeng M., Basourakos S.P., Lewicki P.J., Hu J.C., Lee R.K. New Endoscopic In-office Surgical Therapies for Benign Prostatic Hyperplasia: A Systematic Review. Eur Urol Focus. 2021 doi: 10.1016/j.euf.2021.02.013. Mar 1, S2405-4569(21)00056-0, Epub ahead of print. PMID: 33663982. [DOI] [PubMed] [Google Scholar]
- 68.Tanneru K., Jazayeri S.B., Alam M.U. An indirect comparison of newer minimally invasive treatments for benign prostatic hyperplasia: a network meta-analysis model. J Endourol. 2021;35:409–416. doi: 10.1089/end.2020.0739. [DOI] [PubMed] [Google Scholar]
- 69.Amparore D., Campi R., Checcucci E. Forecasting the future of urology practice: a comprehensive review of the recommendations by international and European associations on priority procedures during the COVID-19 pandemic. Eur Urol Focus. 2020;6:1032–1048. doi: 10.1016/j.euf.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Djavan B., Eckersberger E., Handl M.J., Brandner R., Sadri H., Lepor H. Durability and retreatment rates of minimal invasive treatments of benign prostatic hyperplasia: a cross-analysis of the literature. Can J Urol. 2010;17:5249–5254. [PubMed] [Google Scholar]
- 71.Siena G., Cindolo L., Ferrari G., Maruzzi D., Fasolis G., Condorelli S.V., Varvello F., Visalli F., Rabito S., Toso S., Caroassai S., Mari A., Viola L., Somani B.K., Carini M. Water vapor therapy (Rezūm) for lower urinary tract symptoms related to benign prostatic hyperplasia: early results from the first Italian multicentric study. World J Urol. 2021 doi: 10.1007/s00345-021-03642-4. 1-6 Mar 31. Epub ahead of print. PMID: 33787986; PMCID: PMC8010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lokeshwar S.D., Valancy D., Lima T.F.N., Blachman-Braun R., Ramasamy R. A systematic review of reported ejaculatory dysfunction in clinical trials evaluating minimally invasive treatment modalities for BPH. Curr Urol Rep. 2020;21:54. doi: 10.1007/s11934-020-01012-y. [DOI] [PubMed] [Google Scholar]
- 73.Mollengarden D., Goldberg K., Wong D., Roehrborn C. Convective radiofrequency water vapor thermal therapy for benign prostatic hyperplasia: a single office experience. Prostate Cancer Prostat Dis. 2018;21:379–385. doi: 10.1038/s41391-017-0022-9. [DOI] [PubMed] [Google Scholar]
- 74.Alegorides C., Fourmarier M., Eghazarian C., Lebdai S., Chevrot A., Droupy S. Treatment of benign prostate hyperplasia using the Rezum® water vapor therapy system: results at one year. Prog Urol. 2020;30:624–631. doi: 10.1016/j.purol.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Madersbacher S., Marberger M. Is transurethral resection of the prostate still justified? BJU Int. 1999;83:227–237. doi: 10.1046/j.1464-410x.1999.00908.x. [DOI] [PubMed] [Google Scholar]
- 76.Lebdai S., Chevrot A., Doizi S. Do patients have to choose between ejaculation and miction? A systematic review about ejaculation preservation technics for benign prostatic obstruction surgical treatment. World J Urol. 2019;37:299–308. doi: 10.1007/s00345-018-2368-6. [DOI] [PubMed] [Google Scholar]
- 77.Cacciamani G.E., Cuhna F., Tafuri A. Anterograde ejaculation preservation after endoscopic treatments in patients with bladder outlet obstruction: systematic review and pooled-analysis of randomized clinical trials. Minerva Urol Nefrol. 2019;71:427–434. doi: 10.23736/S0393-2249.19.03588-4. [DOI] [PubMed] [Google Scholar]
- 78.Amparore D., De Cillis S., Volpi G. First- and second-generation temporary implantable nitinol devices as minimally invasive treatments for BPH-related LUTS: systematic review of the literature. Curr Urol Rep. 2019;20:47. doi: 10.1007/s11934-019-0912-6. [DOI] [PubMed] [Google Scholar]
- 79.Roehrborn C.G., Gange S.N., Shore N.D. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. study. J Urol. 2013;190:2161–2167. doi: 10.1016/j.juro.2013.05.116. [DOI] [PubMed] [Google Scholar]
- 80.Elterman D.S., Zorn K.C., Chughtai B., Bhojani N. Is it time to offer true minimally invasive treatments (TMIST) for BPH? A review of office-based therapies and introduction of a new technology category. Can J Urol. 2021;28:10580–10583. [PubMed] [Google Scholar]
- 81.Ulchaker J.C., Martinson M.S. Cost-effectiveness analysis of six therapies for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Clinicoecon Outcomes Res. 2017;10:29–43. doi: 10.2147/CEOR.S148195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.