Abstract
Iron is indispensable for normal body functions across species because of its critical roles in red blood cell function and many essential proteins and enzymes required for numerous physiological processes. Regulation of iron homeostasis is an intricate process involving multiple modulators at the systemic, cellular, and molecular levels. Interestingly, emerging evidence has demonstrated that many modulators of iron homeostasis contribute to organismal aging and longevity. On the other hand, the age-related dysregulation of iron homeostasis is often associated with multiple age-related pathologies including bone resorption and neurodegenerative diseases such as Alzheimer’s disease. Thus, a thorough understanding on the interconnections between systemic and cellular iron balance and organismal aging may help decipher the etiologies of multiple age-related diseases, which could ultimately lead to developing therapeutic strategies to delay aging and treat various age-related diseases. Here we present the current understanding on the mechanisms of iron homeostasis. We also discuss the impacts of aging on iron homeostatic processes and how dysregulated iron metabolism may affect aging and organismal longevity.
Keywords: Iron, Aging, Longevity, Homeostasis, Human diseases, C. elegans, Drosophila
1. Introduction
Iron is fundamentally important for cell survival, proliferation, and metabolism across multiple organisms. As a key element of many indispensable cellular players and co-factors (e.g., heme groups, iron sulfur clusters), iron is required for oxygen transport and involved in the biosynthesis of collagen, myelin, neurotransmitters, and many components of the mitochondrial electron transport chain (Gardi et al., 2002; T.H. Kim and Wessling-Resnick, 2014; J. Kim and Wessling-Resnick, 2014; Paul et al., 2017; Santiago González et al., 2019). In addition, iron is involved in multiple cellular catabolic and anabolic processes such as DNA synthesis, respiration, and energy metabolism (Abbaspour et al., 2014). Furthermore, iron plays a vital role in the innate immune response to infections, as the first-line host antimicrobial mechanisms often reduce iron availability to pathogens (Johnson and Wessling-Resnick, 2012).
Low concentrations of iron result in restricted erythropoiesis and consequently anemia. By contrast, high concentrations of labile iron are highly toxic to the cell by generating reactive oxygen species (ROS) that may inflict damages on cells and organs (Cabantchik et al., 2005). Thus, iron can act as a double-edged sword, which necessitates tight regulation of its cellular levels and exquisite equilibrium between iron storage and transport (Fig. 1) (Rivella and Crielaard, 2014). Iron uptake is a dynamic process that mainly depends on the absorption from digested food. However, unlike iron uptake, systemic iron excretion occurs at an almost steady basal rate regardless of the physiological concentration of iron (Coffey and Ganz, 2017; Mercadante et al., 2019). Therefore, excess iron can accumulate and result in iron overload. Iron overload could also result from hereditary disorders or pathologies like in the case of hemochromatosis (Piperno et al., 2020), a genetic disease that causes an increased intestinal iron absorption, eventually leading to the iron overload in body tissues and organs and causing tissue damages (Burke et al., 2002). In fact, many genetic variants of iron metabolism modulators have been linked to iron imbalance (Jallow et al., 2020).
Fig. 1. Iron homeostasis is maintained by balancing iron uptake with turnover.

Aging and inflammation can disrupt iron homeostasis by enhancing iron accumulation in different tissues, which may lead to oxidative stress, neurodegeneration, organ damage, as well as cancer. Meanwhile, iron deficiencies are common with old age since dietary iron uptake via intestinal absorption becomes less efficient in the elderly.
Aging has a profound impact on iron homeostasis. In humans, age-related iron accumulation occurs in multiple organs including the liver, kidney, and brain (Cook and Yu, 1998), which has been linked to several age-related pathologies including liver diseases, renal disorders, and Alzheimer’s disease (Anderson and Shah, 2013; Ashraf et al., 2018; Costa et al., 2014; Ward et al., 2014). Conversely, iron deficiency and various forms of anemia are also prominent among older adults as the intestinal dietary iron uptake becomes less efficient at advanced ages (Busti et al., 2014; Grubić Kezele and Ćurko-Cofek, 2020). Notably, there are two major forms of iron deficiency, absolute and functional deficiency. While absolute iron deficiency is a decrease in the total body iron content and is usually due to a decrease in intestinal iron absorption, functional iron deficiency is in general due to iron flux to iron storage sites (McCranor et al., 2013), which leads to iron sequestration in macrophages and renders iron less bioavailable for erythropoeisis (Ganz and Nemeth, 2009). Functional anemia is also known as anemia of inflammation or anemia of chronic disease, and its occurrence mostly results from immune activation (Weiss et al., 2019). Importantly, iron deficiency in general is closely associated with impaired mental and immune functions as well as poor physical performance in the elderly (Beard, 2001; Jáuregui-Lobera, 2014; Penninx et al., 2003). Studies in rodents demonstrated that while restricted iron availability can jeopardize certain physiological processes in the body, iron-enriched diets accelerate aging through increased oxidative stress and inflammation (Arruda et al., 2013). Collectively, a better understanding of the age-related dysregulation of iron homeostasis can help us develop strategies for fine-tuning the process, restoring the balance, and therefore mitigating the associated pathologies. In this review, we discuss our current understanding on the complex interactions between iron homeostasis and aging (Fig. 1).
2. Systemic, cellular, and molecular mechanisms involved in iron homeostasis
Maintaining safe iron levels is critical for sustaining steady iron stores, providing sufficient iron for erythropoiesis, and limiting iron availability to pathogens (Wang and Babitt, 2019). In adult humans, iron enters the body mainly from diet through intestinal dietary iron absorption in two major forms, heme and non-heme iron. Non-heme dietary iron is abundant in plant foods and iron-fortified foods (Monsen, 1988), and it can be in the ferric or more bioavailable ferrous states. Although the chemical nature of non-heme dietary iron is not very well understood, ferric citrate is thought to be the predominant species (Hider, 2002). Existing supplements mainly include ferrous sulfate, ferrous gluconate, ferric citrate, and ferric sulfate (Alleyne et al., 2008; Coates et al., 2010), and the low pH of the digestive system helps keep the non-heme iron in the soluble form. On the other hand, heme iron, which can be ingested from meat, seafood, and poultry, is bound to the porphyrin ring and less affected by diet and other factors, rendering its absorption more efficient (Monsen, 1988).
Iron homeostasis is achieved at both systemic and cellular levels. Cross-talks between the systemic and cellular iron metabolism are key to sensing iron levels, adjusting absorption and recycling accordingly. Below we discuss the underlying mechanisms of iron homeostasis at the systemic, cellular, and molecular levels.
2.1. Systemic iron homeostasis
Systemic iron homeostasis, thoroughly reviewed by Ganz and Nemeth (Ganz and Nemeth, 2012), is maintained by balancing iron supply, utilization, and losses (Wang and Babitt, 2019). Iron is supplied by duodenal enterocytes that absorb dietary iron from the intestinal lumen and by reticuloendothelial macrophages that take iron from aged erythrocytes and supply it to the plasma (Gulec et al., 2014; Knutson and Wessling-Resnick, 2003; Knutson et al., 2005; Sharp and Srai, 2007). Duodenal enterocytes can uptake iron through their apical surface by different mechanisms depending on the type and redox status of iron (Gulec et al., 2014). After uptake, iron can be directly exported into the plasma through the cell’s basolateral surface or stored for a few days (Mackenzie and Garrick, 2005). In humans, around 20 mg of iron are used each day to make 200 billion erythrocytes, which constitutes about 80% of the daily iron use (Girelli et al., 2018; Kautz and Nemeth, 2014). Unused iron can be stored in the liver in the forms of ferritin or hemosiderin (Bonkovsky, 1991). Under normal physiological conditions, iron losses are estimated to be about 1–2 mg per day (Bothwell et al., 1989), and these losses are mainly through shedding of intestinal and skin epithelial cells, menstruation, blood loss, as well as excretion through urine, sweat and stool (Anderson et al., 2009; Green et al., 1968; Hunt et al., 2009; Mercadante et al., 2019; Saito et al., 1964).
Notably, there are no known controlled iron excretion mechanisms in the human body, and thus efficient orchestration of iron absorption is critical for maintaining iron homeostasis. Systemically, this is mainly attained by the hormone hepcidin that is synthesized and secreted by liver hepatocytes (Fig. 2) (Rossi, 2005). Hepcidin is regulated by both the amounts of cellular iron stores and circulating transferrin-bound iron levels (Nemeth and Ganz, 2009). An increase in plasma and tissue iron levels stimulates hepcidin production by activating the BMP-SMAD signaling cascade in hepatocytes (Yu et al., 2008). Hepcidin regulates iron homeostasis by modulating iron release into the circulation (Katsarou and Pantopoulos, 2020). At the cellular level, hepcidin decreases the levels of the iron exporter protein ferroportin (FPN) by directly binding to FPN (Fig. 2), which results in its internalization and subsequent degradation (Pagani et al., 2019). A decrease in intestinal FPN upon the elevated levels of hepcidin reduces iron release from the intestine to the bloodstream and leads to decreased iron absorption (De Domenico et al., 2007). In addition, reduction of FPN levels also occurs in reticuloendothelial macrophages which limits iron efflux from recycling macrophages. Conversely, a decrease in iron levels leads to downregulation of hepcidin production, which then causes upregulated intestinal iron absorption and increased iron release from macrophages (Ganz, 2011, 2005; Ganz and Nemeth, 2012). Interestingly, an increase in interleukin-6 (IL-6) from inflammation stimulates the production of hepcidin and leads to iron dyshomeostasis (Wang and Babitt, 2016). In such cases, iron serum levels decrease while ferritin levels increase (Pagani et al., 2019), a common phenomenon observed during inflammaging and associated anemia (McCranor et al., 2013).
Fig. 2. The regulation of systemic iron homeostasis.

An increase in plasma (circulating) and tissue (stored) iron levels leads to production and secretion of the hormone hepcidin. Hepcidin decreases the levels of ferroportin (FPN) by direct binding, which leads to its internalization and degradation. The reduction of FPN causes a decrease in iron absorption at the intestinal level as well as a decrease in iron efflux by reticuloendothelial macrophages.
2.2. Cellular iron homeostasis
Cellular iron uptake mechanisms vary depending on the cell type and the form of iron. As previously discussed, dietary iron exists in two forms: heme and non-heme. Non-heme iron can be either transferrin-bound (referred to as TBI) or non-transferrin-bound (known as NTBI). Under normal conditions, most of the iron in the circulation is transferrin-bound. However, in cases of iron overload, the potentially toxic NTBI can be found in the circulation at higher levels (Cabantchik et al., 2005; Jacobs et al., 2005). The cellular uptake and metabolism of iron are governed by complex pathways summarized in Fig. 3, and these processes seem to be fairly conserved across eukaryotic species, especially more complex ones (Dlouhy and Outten, 2013; Sherman et al., 2018).
Fig. 3. Important regulators involved in cellular iron homeostasis.

Non-heme iron can enter the cell in two forms: transferrin bound (TFB) and non-transferrin bound (aka NTBI). Ferrous iron or NTBI enters the cell through DMT1. Ferric iron is reduced to ferrous iron by DcytB, whereas ferrous iron is oxidized into ferric iron by the multicopper ferroxidases ceruloplasmin, hephaestin and HephL1. Ferric iron binds to transferrin that in turn binds to the transferrin receptor and gets internalized into the cell via endocytosis. Inside the internalized vesicles, iron is released and transformed into ferrous iron by STEAP3. Ferrous iron will then be exported out of the vesicle and into the cytoplasm by DMT1. Ferrous iron in the cytoplasm constitutes the labile iron pool (LIP), and it can be exported out of the cell by ferroportin, stored in the cell by ferritin, or utilized in different cellular processes. As for heme, it is synthesized in the mitochondria and can be exported to the outside of the cell by FLVCR1. Once in the cytoplasm, heme is cleaved by heme oxygenase (HO1) to release ferrous iron.
2.2.1. Non-transferrin-bound iron uptake
Ferric iron (Fe3+) is reduced to ferrous iron (Fe2+) in order to enter the cell, and this can be achieved by ascorbate or membrane-bound reductases (Cain and Smith, 2021; Ganasen et al., 2018). Ascorbate, a known antioxidant, is a modulator of iron metabolism that can increase levels of cellular iron by increasing cellular ferritin (an iron storage protein) levels (Goralska et al., 1997). Ascorbate cycling across the plasma membrane is associated with ferric citrate iron uptake with this mechanism being more prominent in certain cell types like astrocytes (Lane and Richardson, 2014). As for iron absorption from food, non-heme iron is mainly absorbed by intestinal enterocytes expressing the ferric reductase duodenal cytochrome B (DCYTB), which reduces dietary iron from ferric Fe3+ to ferrous Fe2+ and facilitates its transport through the apical side of the enterocyte cell membrane by the divalent metal transporter DMT1 (Fig. 3) (Ems et al., 2021). In liver hepatocytes, ZIP14 (also known as SLC39A) mediates NTBI absorption by acting as a cellular iron importer (Liuzzi et al., 2006). ZIP8 (also called SLC39A8) is another iron importer that uptakes iron in placental cells (D. Wang et al., 2012; C.-Y. Wang et al., 2012). In addition, NTBI can enter the cell through bulk endocytosis (Sohn et al., 2012). Iron imported into the cell forms the cytoplasmic labile iron pool (LIP), which acts as an intermediate between imported, stored, and utilized iron (Fig. 3). LIP is potentially toxic to the cell due to its redox-active nature, and therefore is maintained at very low levels (for details see Section 2.2.4). Hence, labile iron represents a minor fraction of the total cellular iron content (Kakhlon and Cabantchik, 2002), and in mammalian cells the cellular LIP ranges between 0.2 and 1.5 μM depending on the cell type (Epsztejn et al., 1997).
Iron enters the circulation by being exported through FPN on the basolateral membrane of enterocytes (Fig. 3). FPN, the only known cellular iron export protein, is also responsible for releasing stored iron into the circulation from hepatocytes and macrophages (Donovan et al., 2005; Drakesmith et al., 2015; Zhang et al., 2012).
2.2.2. Transferrin-bound iron uptake
Iron in the circulation can be transported by transferrin that binds iron in the ferric state (Levine and Woods, 1990). After the ferrous iron is exported, multicopper ferroxidases, ceruloplasmin, hephaestin and hephaestin-like protein 1 (HephL1) then oxidize Fe2+ iron into the ferric state and allow it to bind to transferrin (Harris, 2019; Vashchenko and MacGillivray, 2013; Wierzbicka and Gromadzka, 2014). This is thought to facilitate iron release from intestinal enterocytes as well as absorption and release (Chen et al., 2004; Fuqua et al., 2014). Next, the formed TBI complex (ferric iron bound to transferrin) binds to the transferrin receptor TFR1, and TBI-TFR1 complex is then internalized into the cell through the clathrin-dependent receptor-mediated endocytosis (Harding et al., 1983; Muckenthaler et al., 2017). In short, endocytosis of TBI-TFR1 complex is initiated where the vesicle buds from the cell surface, forming a clathrin-coated pit that is separated from the cell membrane with the help of dynamin (Liu et al., 2010; Rosendale et al., 2019). Two populations of early endosomes will form depending on the maturation kinetics, and TBI-TFR1 randomly enters either one of these populations (Killisch et al., 1992; Stoorvogel et al., 1991). This TBI uptake pathway requires multiple trafficking molecules such as Sec15l1 (exocyst complex component Sec15 A), Vps35 (vacuolar protein sorting-associated protein 35), Snx3 (sorting nexin-3) (Chen et al., 2013), as well as the vacuolar-type H+ – ATPase (V- ATPase) assembly factor coiled-coil domain containing 115 (CCDC115) (Sobh et al., 2020). After internalization, TBI-TFR1 eventually fuse with the lysosome, and the acidic pH (4.5–5.5) environment of the lysosome leads to the release of iron from transferrin (Karin and Mintz, 1981), as TFR1 has a higher affinity for the iron-free transferrin at the lower pH (Dautry-Varsat et al., 1983). Afterwards, while transferrin and its receptor are recycled back to the plasma membrane (Harding et al., 1983), six-transmembrane epithelial antigen of the prostate 3 (STEAP3) will reduce ferric Fe3+ to ferrous Fe2+, and then Fe2+ is released from the lysosome into the cytoplasmic LIP via DMT1 (Fig. 3) (Ohgami et al., 2005).
2.2.3. Heme iron uptake
In higher eukaryotes, heme iron is synthesized in the mitochondria or obtained from diet (Chung et al., 2012). Heme is thought to be internalized by receptor-mediated endocytosis (Fig. 3) (Sobh et al., 2020), where heme oxygenases (HO-1 in endoplasmic reticulum or HO-2 inside vesicles) degrade heme and release iron from its porphyrin ring (Yoshida and Migita, 2000). The released iron is then transported into the cytoplasm through DMT1 and incorporated into the LIP (Fig. 3) (West and Oates, 2008). Additionally, in recycling macrophages, the heme importer HRG1 can transport heme from the phagolysosome to the cytoplasm (White et al., 2013). Besides the endocytosis route, heme might be taken by the proton-coupled folate transporter PCFT/HCP1 (encoded by SLC46A1) into the cytoplasm (Fig. 3) (West and Oates, 2008), although it has been argued that the main function of PCFT/HCP1 is to transport folate (Le Blanc et al., 2012). Notably, a genome-wide CRISPR screen in erythroleukemic K562 cells aiming to identify key-players in heme-trafficking revealed a clear role for endocytosis in heme import but did not uncover an explicit cellular heme importer (Sobh et al., 2020). As for the export of heme, feline leukemia virus subgroup C receptor-related protein 1b (FLVCR1b) has been shown to export the mitochondrial heme into the cytoplasm, while the cell membrane FLVCR1 is used to export heme outside of the cell (Fig. 3) (Khan and Quigley, 2013). Nonetheless, how species lacking FLVCR homologs export heme is still unknown.
2.2.4. LIP iron utilization
To keep the low level of intracellular labile iron, the LIP iron can be exported outside of the cell through FPN or stored in the form of ferritin (Fig. 3) (Hynes, 1948). Poly r(C)-binding protein 1 and 2 (PCBP1 and PCBP2) are iron chaperones that are required for iron binding to ferritin (Leidgens et al., 2013). Moreover, the LIP iron can be imported into the mitochondrion through mitoferrin (MTFN1/2) (Paradkar et al., 2009), where it is used for heme biosynthesis, iron-sulfur clusters biosynthesis, tricarboxylic acid (TCA) cycle, or stored in the mitochondrial ferritin (MtFt) (Fig. 3) (Paul et al., 2017; Richardson et al., 2010; Ward and Cloonan, 2019). In addition to its use in the mitochondrion, iron is also widely used as a cofactor by various DNA metabolizing enzymes involved in DNA replication and repair (Puig et al., 2017).
Labile cellular iron is often regarded as a dynamic parameter since it depends on the cell type and the cell’s fluctuating physiological conditions. On the one hand, LIP concentrations may vary within short durations in the same cell to meet the changing catalytic demands (Cabantchik, 2014). On the other hand, it can exist in both ferric and ferrous states with very low concentrations. This renders it difficult to reliably assess the real-time LIP status in living cells (Lv and Shang, 2018; Muir et al., 2019).
2.3. Molecular mechanisms involved in iron homeostasis
Many transcriptional and post-transcriptional mechanisms play important roles in iron homeostasis. For example, hypoxia has been shown to increase the availability of iron in the body through the evolutionarily conserved transcription factors hypoxia inducible factors (HIFs) (Shah and Xie, 2014). HIFs control expression of multiple genes that maintain iron homeostasis, including transferrin, hepcidin, and ferroportin (Peyssonnaux et al., 2008). In the presence of oxygen, HIF-1α is hydroxylated and degraded by the ubiquitin-proteasome degradation. However, when the oxygen level decreases, HIF-1α accumulates and translocates to the nucleus, where it binds to the constitutively expressed HIF-1β subunits and forms a heterodimer to activate the expression of target genes through hypoxia response elements (HREs) (Dengler et al., 2014). Upon activation, the HIF pathway renders iron more available for erythrocyte production by downregulating hepcidin and upregulating transferrin, transferrin receptor, heme oxygenase 1, ferroportin, and ceruloplasmin. Combined, these events enhance intestinal absorption, cellular import, and recycling of iron (Peyssonnaux et al., 2008, 2007; Renassia and Peyssonnaux, 2019). In addition to the transcriptional control, several post-transcriptional mechanisms also contribute to regulating the expression of iron homeostasis genes, including alternative splicing, iron regulatory proteins (IRPs), microRNAs (miRNAs), and proteolytic cleavage.
2.3.1. Alternative splicing
Depending on the splicing sites selection, expression of multi-exon genes can result in distinct proteins that have different sequences and activities (Greenberg and Soreq, 2013). Alternative splicing of several iron-related genes such as ferritin (Jiang et al., 2014), iron-sulfur cluster-containing aconitase 1 (ACO1) (Tejedor et al., 2015), and the most telomeric HLA class I gene HFE (Martins et al., 2011) all result in multiple transcript variants and protein isoforms that have different functions and efficiencies. For example, the HFE protein is expressed in several cell types to compete with transferrin-bound iron for transferrin receptor binding (Bennett et al., 2000), and a HFE splicing variant can regulate cellular transferrin uptake by hepcidin regulation of macrophage iron recycling and by controlling intestinal iron absorption (Laham et al., 2004; Martins et al., 2011). In addition, alternative splicing of SLC11A2 results in two different proteins namely DMT1A and DMT1B. Unlike DMT1A that has an IRE motif at its three prime untranslated region (3’ UTR), DMT1B does not and therefore is irresponsive to iron (Tabuchi et al., 2002). Furthermore, alternative splicing has been shown to contribute to the regulation of ferritin levels in the oriental fruit fly, Bactrocera dorsalis (Jiang et al., 2014).
2.3.2. IRP-IRE pathway
Iron regulatory proteins (IRPs) can bind to the IRE-containing RNA stem-loops in the untranslated regions (UTRs) of target mRNAs, which either negatively affects the expression of the mRNA by inhibiting translation (e.g., FPN1) (Liu et al., 2002) or positively by preventing degradation of the mRNA (e.g., DMT1 and TFR1) (Galy et al., 2008; Lymboussaki et al., 2003). Thus, the binding of iron to IRPs can alter the translation of target mRNAs by disrupting the interaction between IRPs and IRE (dos Santos et al., 2008). Importantly, IRE is present in many genes that are involved in iron uptake, export, transport (e.g., DMT1, TFR1, and ferritin), and utilization (e.g., ACO2 and 5-aminolevulinate synthase (ALAS)) (Muckenthaler et al., 2008; Steinbicker and Muckenthaler, 2013). Notably, IRP-IRE regulation of cellular iron uptake seems to be more prominent for TBI than NTBI (Brissot et al., 2012). Between the two IRPs (IRP1 and IRP2) encoded by the vertebrate genome, IRP2 only responds to iron-related signals and dominates regulation of iron homeostasis in mammals, whereas IRP1 can respond to both iron-dependent and -independent signals (Anderson et al., 2012; Meyron-Holtz et al., 2004; Wallander et al., 2006). Interestingly, IRP1 can also coordinate erythropoietin synthesis with oxygen and iron supply through translationally regulating HIF2a mRNA (Sanchez et al., 2007). Notably, alternative splicing is linked with the IRP-IRE pathway as certain alternatively spliced transcripts of ferroportin that lack the IRE domain can bypass iron deficiency-induced translational repression of its mRNA by IRPs in duodenal and erythroid precursor cells (Zhang et al., 2009). For more details on the post-transcriptional regulation of iron metabolism, readers are encouraged to check other related reviews (Rouault, 2002; Muckenthaler et al., 2008; Kühn, 2015).
2.3.3. microRNAs
miRNAs are single-stranded, noncoding RNA molecules that can interact most commonly with the 3’ UTR region of an mRNA and usually silences the target gene and negatively regulates its expression (O’Brien et al., 2018). Multiple types of miRNAs have been shown to regulate expression of genes involved in iron import, export, storage, and utilization (Davis and Clarke, 2013). For example, miR-122 regulates the expression of hepcidin (Castoldi et al., 2011), while miR-210 (Yoshioka et al., 2012) and miR-148a regulate the expression of transferrin receptor 1 (Babu and Muckenthaler, 2019). Interestingly, miRNAs have also been depicted in the modulation of aging such that upregulation of miR-29 can delay aging by limiting iron delivery to neurons and therefore counteracting the age-associated effects of iron accumulation in these cells (Ripa et al., 2017).
2.3.4. Proteases
In addition to the regulatory mechanisms mentioned above, a role for proteases in the regulation of iron homeostasis was recently revealed. Martipase-2, a serine protease encoded by TMPRSS6, has been shown to be sensitive to the cellular iron levels and negatively regulate the expression of hepcidin (Meynard et al., 2011). In addition, martipase-2 regulates the expression of hemojuvelin, a protein responsible for the increased iron levels in juvenile hemochromatosis (Du et al., 2008; Folgueras et al., 2008; Malyszko, 2009; Ramsay et al., 2009). In fact, different allelic variants of martipase-2 cause altered serum iron and transferrin saturation levels (An et al., 2012; Benyamin et al., 2009; Chambers et al., 2009; Nai et al., 2011; Tanaka et al., 2010), indicating that martipase-2 may act as a global modulator of serum iron concentration and transferrin saturation levels (Wang et al., 2014). Regulation of iron homeostasis by protein degradation has been thoroughly reviewed elsewhere (Thompson and Bruick, 2012; Wang et al., 2014).
3. The impacts of aging on iron homeostasis
As discussed above, we have gained plenty of knowledge about the biological mechanisms underlying iron homeostasis in the past several decades. More recently, emerging evidence suggested that iron homeostasis is interconnected with aging. Older people not only suffer from cellular iron accumulation that can result in multiple pathologies and tissue degeneration, but also are negatively impacted by anemia due to deterrent iron absorption. In fact, anemia in the elderly is considered a significant risk factor for several adverse outcomes including death (den Elzen et al., 2013). Multiple studies have identified unexplained anemia of the elderly (UAE) as one of the most common types of anemia among people 65 years and older (Artz and Thirman, 2011). A dissection of the onset and key players of age-induced dysregulation of iron homeostasis might provide an insight into the etiology of many age-related diseases, thus helping mitigate the effects of iron dyshomeostasis in elderly.
3.1. Age-related decline in systemic iron homeostasis
The most common causes underlying iron-deficiency anemia are gastrointestinal (GI) diseases including ulcers, GI tract malignancies, inflammatory bowel disease, and other pathophysiological conditions that cause bleeding in the GI tract, as these conditions cause iron malabsorption and iron loss (blood loss) (Busti et al., 2014). While the levels of many key modulators of iron homeostasis do not seem to vary steadily with age under normal conditions, certain age-related pathological conditions may alter their levels (Fig. 4). Hepcidin, for instance, does not seem to change with age under normal conditions (Goodnough and Schrier, 2014). However, patients with anemia of inflammation and those at risk for developing Alzheimer’s disease have higher levels of hepcidin, whereas those suffering from iron deficiency anemias have lower plasma hepcidin levels (Chatterjee et al., 2020; den Elzen et al., 2013; Picca et al., 2019). This increase in hepcidin and subsequent sequestration of iron in cells are then associated with the functional iron deficiency and decreased hematopoiesis noted in older adults (Nemeth et al., 2004). In fact, chronic inflammation is known to increase circulating hepcidin levels that lead to a decrease in systemic iron levels and decreased hematopoiesis, which can eventually cause anemia commonly seen in older adults (Cunietti et al., 2004).
Fig. 4. Age-related changes in iron homeostasis.
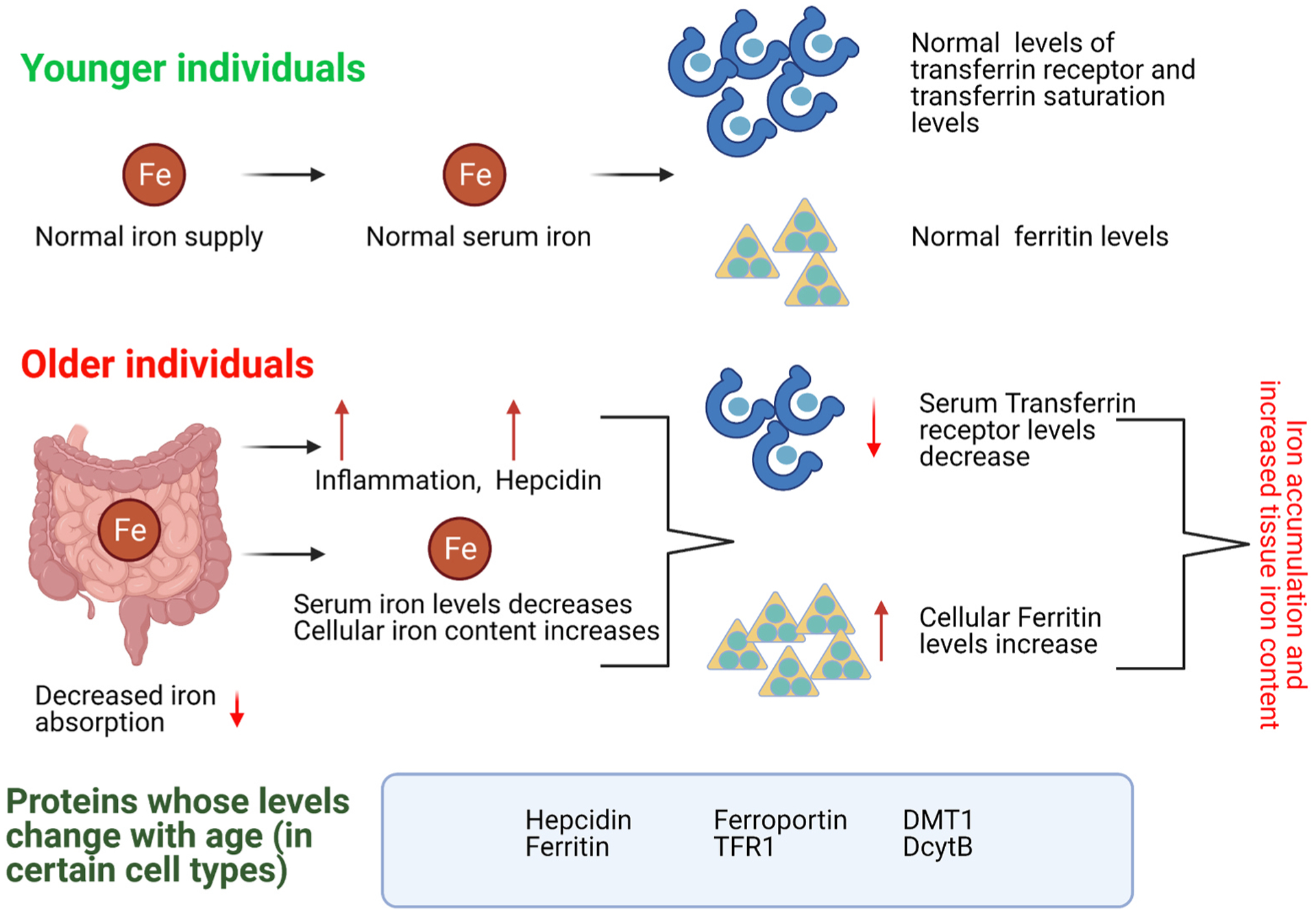
Transferrin receptor levels seem to decrease with age, while ferritin levels increase. Various studies with different animal models also suggest that depending on the organ studied, the levels of certain iron-regulating proteins can vary with age.
Unlike hepcidin whose levels do not usually fluctuate with age under normal conditions, the levels of iron storage protein ferritin increase with age (Fig. 4) (Pusch et al., 1981), and this age-related increase of ferritin is typically induced by aging-driven systemic inflammation, or inflammaging. Although in normal situations iron deficiency leads to decreased ferritin levels, geriatric patients tend to have high ferritin levels even with iron deficiency because of inflammaging, which makes ferritin a potential biomarker for the age-associated inflammation (den Elzen et al., 2013). A 2010 study with 252 geriatric patients showed that 25.4% of the population had anemia while 41% had inflammation (Fairweather-Tait et al., 2014; Lopez-Contreras et al., 2010). Importantly, this study confirmed the link between inflammation markers and ferritin. In addition to anemia, inflammaging is closely associated with many other age-related conditions such as osteoporosis, malignancies, cognitive impairments, atherosclerosis, sarcopenia, and cardiovascular diseases (Ferrucci and Fabbri, 2018; Krabbe et al., 2004; Lencel and Magne, 2011). Thus, a better comprehension of the effect of inflammation on iron homeostasis and vice versa may provide important insights into a better understanding of age-associated diseases.
3.2. Age-related changes in cellular and molecular iron homeostasis
Although heme levels have been clearly shown to decrease with age (Bitar and Weiner, 1983), the change of total iron content with age seems to be less clear. In general, serum iron and soluble transferrin receptor levels decrease with age (Gayar and Deghady, 2015), while ferritin levels increase with age (Cankurtaran et al., 2012) although there are conflicting results from different studies (Fairweather-Tait et al., 2014). In older individuals, iron seems to accumulate in an age-related and tissue/organ-specific manner. For example, the age-associated increase in iron deposition varies between different brain regions and cell types in humans (C.-Y. Wang et al., 2012; D. Wang et al., 2012). In rodent liver and spleen, ferritin levels and ferritin iron saturation increase with age (Bulvik et al., 2012), while old rats have higher iron levels and lower levels of TfR1 and FPN in skeletal muscles (Xu et al., 2012a). Nonetheless, other iron-related factors such as DcytB, DMT1 and FPN1 exhibit the highest levels during the first few weeks in life and then keep decreasing with age, with the lowest levels reported in the oldest rats (Kong et al., 2015). Collectively, aging seems to have complex impacts on the expression levels of distinct systemic, cellular, and molecular factors involved in iron homeostasis.
4. Iron metabolism dysregulation contributes to cellular and organismal aging
The intricate balance of physiological iron levels and proper maintenance of iron homeostasis are key to healthy aging. Iron-induced oxidative stress is linked to age-related pathologies (Smith et al., 1997) and considered as a major contributing factor of aging in humans (Kregel and Zhang, 2007). Intriguingly, suppressing the age-related iron accumulation by limiting iron intake (Polla, 1999) with iron chelators (Bogdan et al., 2016; Kell, 2009; Pouillot et al., 2013) or dietary restriction has been suggested to reduce oxidative stress (Cook and Yu, 1998). Moreover, the rate of iron accumulation is proportional to the rate of aging in Drosophila melanogaster (Massie et al., 1985). Therefore, manipulating iron homeostasis might have direct impacts on aging and longevity.
4.1. Age-associated iron deposition in cellular senescence
Age-related cellular iron deposition can exist in several forms, including labile iron, lipofuscin, and ferritin. As ferritin levels tend to increase with age (Casale et al., 1981; Jung et al., 2008), this could potentially explain the phenomenon of age-related cellular iron sequestration. Very interestingly, senescent cells can have a 10-fold higher concentration of iron compared to younger cells (Killilea et al., 2003), and increased cellular ferritin levels is considered a strong biomarker of cellular aging and senescence (Liu et al., 2019). Furthermore, the increased accumulation of iron is associated with impaired ferritin degradation, altered ferroptosis sensitivity, as well as increased expression of p16, p21 and IL-6 in senescent fibroblasts (Masaldan et al., 2018), all of which are considered as biomarkers of aging (Bernardes de Jesus and Blasco, 2012; González-Gualda et al., 2021).
The iron-dependent ferroptosis process is typically accompanied by iron accumulation and lipid peroxidation (Conrad and Pratt, 2019; Hadian and Stockwell, 2020). Senescent cells often exhibit severely impaired ferritinophagy, a lysosomal degradation process that degrades ferritin and promotes ferroptosis. Thus, the large amount of iron accumulated in senescent cells is primarily trapped in ferritin due to defective ferritinophagy, and cells actually perceive it as iron deficiency (Masaldan et al., 2018). As a result, senescent cells are often resistant to ferroptosis (Masaldan et al., 2018). On the other hand, multiple studies have indicated that cellular free iron is a major driver of both senescence and ferroptosis, and increased ferroptosis might contribute to age-associated neurodegeneration (Cozzi et al., 2019; Do Van et al., 2016; Guiney et al., 2017; Kenny et al., 2019; Morris et al., 2018). It is possible that the amount of LIP iron, but not sequestered iron, might determine the final outcome of ferroptosis in different cell types. Therefore, it is important to decipher whether the mechanisms and initiators of ferroptosis vary among distinct types of cells. A better understanding of the etiology of ferroptosis in aging cells could assist in targeting ferroptosis for various age-related diseases.
Ferritin can accumulate in the cytosol, mitochondria, or form aggregates as dimers, trimers, tetramers and even oligomers (Ashraf et al., 2018). Cytosolic ferritin is degraded by autophagy to release redox-active iron and increase the concentration of LIP (Yu et al., 2003). Notably, ferritin holds large amounts of free iron (theoretically up to 4500 Fe3+ but biologically around 2000 Fe3+ per ferritin molecule), and therefore its degradation can release a large amount of iron (Arosio et al., 2009; Watanabe et al., 2001; Worwood et al., 1976). Excess free irons increase cellular oxidative stress and lead to deteriorating effects on health and longevity (Murillo-Ortiz et al., 2016).
Labile cellular iron can also catalyze the formation of lipofuscin whose accumulation is a hallmark of aging and senescence. As intralysosomal aggregates mainly composed of lipids and oxidized proteins, lipofuscin is resistant to ubiquitin-proteasome degradation (Katz and Robison, 2002). Lipofuscin usually accumulates with age and has cytotoxic effects by the chronic production of oxidants (Höhn et al., 2010). The iron in lipofuscin jeopardizes the stability of the lysosome and triggers apoptosis after lysosome disruption and contents release (Brunk and Terman, 2002). Interestingly, iron chelation has been suggested to alleviate the deteriorating effects lipofuscin on age-related macular degeneration (Ueda et al., 2018).
4.2. Iron metabolism-related genes in animal lifespan regulation
Unlike humans with long lifespan, invertebrate model organisms such as C. elegans and Drosophila feature short lifespan and powerful genetics. As a result, it is more convenient to use these genetic model organisms to investigate the potential impact of iron metabolism-related genes on longevity. In C. elegans, increased dietary iron intake significantly accelerates age-related protein aggregation and negatively affects organismal longevity (Klang et al., 2014). Conversely, reducing overall iron levels using intracellular iron scavengers is sufficient to extend lifespan (Fig. 5A) (Jenkins et al., 2020). Moreover, blocking the iron-dependent ferroptosis can also slow down the aging process and extend lifespan (Jenkins et al., 2020). Furthermore, several core components of the iron-sulfur cluster (ISC) assembly machinery (e.g., ISCU-1, NFS-1) have been recently shown to modulate longevity (Sheng et al., 2021). Interestingly, while ISCU-1 is required for the normal development of larval worms to adults (Fig. 5B), it suppresses longevity during adulthood (Fig. 5C). Thus, the ISC assembly machinery might exhibit antagonistic pleiotropy by being beneficial early in life at the cost of aging.
Fig. 5. Iron metabolism and lifespan regulation in C. elegans.

A, excessive iron accumulation suppresses longevity in C. elegans, where ROS accumulation, ferroptosis, and cellular senescence may act as contributing factors. B, ISCU-1/ISCU, a central mitochondrial protein required for de novo biosynthesis of iron sulfur clusters, is essential for normal development during larval stages but accelerates aging during adulthood. C, suppressing the functions of ISCU-1 by iscu-1 RNAi extends lifespan and promotes stress resistance.
In line with the results of C. elegans, studies in Drosophila have revealed the age-dependent accumulation of iron and holoferritin in addition to altered mitochondrial iron homeostasis and autophagy (Jacomin et al., 2019). In fact, increased dietary iron intake was even considered as an “initiator of senescence” in flies (Massie et al., 1985). On a similar note, green tea can prolong the lifespan of Drosophila through regulating mitoferrin and reducing mitochondrial iron levels (Lopez et al., 2016; Massie et al., 1993). However, improper iron chelation that results in poor liganding of iron species can increase inflammation, demote the fly’s overall health, and decrease its lifespan (Kell, 2009).
Besides C. elegans and Drosophila, studies with several other species have shown that increased cellular iron concentration has negative health impacts that can be potentially ameliorated by iron chelation, including water flea (Dave, 1984), zebrafish (Hamilton et al., 2014; Nakamura et al., 2020), killifish (Kelmer Sacramento et al., 2020; Poeschla and Valenzano, 2020), mouse (Garringer et al., 2016; Song et al., 2014), rat (Bloomer et al., 2008), dog (Bergeron et al., 2004), pig (Gu et al., 2011), and pin monkey (Bergeron et al., 2004, 1999). For example, iron chelation by deferiprone in mouse embryonic fibroblasts with high ferritin levels has been shown to significantly decrease cellular iron content and increase cell viability (Garringer et al., 2016). In addition, iron chelation also decreases oxidative stress during retinal degeneration in mice suffering from iron overload (Song et al., 2014). Although the underlying mechanisms are not thoroughly understood, ferritin, transferrin, and mitochondrial signaling seem to be the key players (Shen, 2020; Shin and Baik, 2017; Whittemore et al., 2019).
4.3. Dysregulated iron homeostasis is a hallmark of human aging
Age-related dysregulation of iron homeostasis contributes to a plethora of human disorders (Fig. 6), including impaired bone homeostasis (Balogh et al., 2018) and osteopenia (Liu et al., 2006), muscle aging (Picca et al., 2019), sarcopenia and skeletal muscle atrophy (DeRuisseau et al., 2013), renal dysfunction (Bloomer et al., 2020), liver disease (Milic et al., 2016), cardiomyopathies (Johnson et al., 1994; Nordestgaard et al., 2010), neurodegenerative diseases such as Alzheimer’s disease (Liu et al., 2018) and Parkinson’s disease (Ayton and Lei, 2014; Rhodes and Ritz, 2008), vision loss (Błasiak et al., 2009), impaired immune system (Dao and Meydani, 2013), and lung damage (Philippot et al., 2014). Alterations in iron homeostasis have been also linked to endocrine system disruptions (Abdulzahra et al., 2011) and gut microbiota imbalance (Yilmaz and Li, 2018). Intriguingly, reduced iron deposition leads to improved motor performance in aging individuals (Kastman et al., 2012), whereas iron supplements negatively affect gut microflora by favoring the pathogenic bacteria and thus potentially leading to gut inflammation (Jaeggi et al., 2015; Zimmermann et al., 2010). Moreover, the serum ferritin level is significantly higher in both women and men with sarcopenia compared to those without sarcopenia (J. Kim et al., 2014; T.H. Kim et al., 2014). Collectively, dysregulated iron homeostasis appears to be intuitively linked to human aging.
Fig. 6. Organ-specific effects of age-related iron accumulation in humans.

Depending on the tissue and organ affected, age-related iron accumulation can lead to various pathologies in humans.
Studies on centenarians have associated healthy aging and increased longevity with increased immune system and systemic anti-inflammatory performance (Balistreri et al., 2012), both of which are modulated by proper iron homeostasis. Intriguingly, a recent GWAS study has linked heme metabolism pathways with human aging (Timmers et al., 2020). It would be very interesting to examine whether the iron homeostasis dysregulation and consequently aging acceleration can be mitigated by reducing iron intake.
5. Conclusions and perspectives
Although the mechanisms and etiologies remain unknown, the negative impacts of age-related iron dyshomeostasis on human health have been well documented (Xu et al., 2012b, 2008). Our knowledge and understanding of iron biology have advanced remarkably during the last couple of decades. However, many key players involved in iron metabolism and homeostasis remain unidentified. For instance, the precise mechanism in cellular heme uptake remains to be identified. In addition, how iron accumulates with age and triggers the associated pathologies are still poorly understood. A better understanding of these questions could help develop diagnostics and therapies for various geriatric pathologies.
Since the age-related increase of ferritin levels appears to underlie the cellular iron accumulation even under conditions of decreased serum iron levels and anemia, mobilizing ferritin iron could potentially mitigate the occurrence of age-associated cellular iron accumulation and its related pathologies. Notably, a recent study has demonstrated that chelating or reducing agents do not mobilize ferritin iron (La et al., 2018). Other studies have investigated the potential overexpression of mitochondrial ferritin to limit the availability of cytosolic active iron by shunting iron to the mitochondria (Nie et al., 2005). The development of real-time iron probes might facilitate studies on ferritin iron mobilization and other aspects of iron homeostasis. Recent advancements in selective fluorescent and genetic iron probes may offer a better monitoring of biological iron in living cells and tissues, which could lead to a better understanding of its involvement in age-related pathologies (Aron et al., 2018; Sahoo and Crisponi, 2019; Weissman et al., 2021).
Taken together, in this review we summarized key players involved in animal iron homeostasis. We also highlighted the link between progressive iron accumulation and age-related pathologies and organ dysfunction. As genetic studies in model organisms suggest that iron accumulation might have a negative impact on aging and longevity, cellular iron homeostasis machineries may present an attractive target to combat aging and age-related diseases.
Acknowledgments
This work was supported by grants from the National Institute on Aging (AG063766, AG028740, AG066654) and the American Cancer Society (RSG-17-171-01-DMC). The authors would like to acknowledge and apologize that not all of the relevant literature have been cited due to space constraints. Model figures were made using BioRender.com.
Footnotes
Declaration of Competing Interest
The authors declare no competing interests.
References
- Abbaspour N, Hurrell R, Kelishadi R, 2014. Review on iron and its importance for human health. J. Res. Med. Sci. J. Isfahan Univ. Med. Sci 19, 164–174. [PMC free article] [PubMed] [Google Scholar]
- Abdulzahra MS, Al-Hakeim HK, Ridha MM, 2011. Study of the effect of iron overload on the function of endocrine glands in male thalassemia patients. Asian J. Transfus. Sci 5, 127–131. 10.4103/0973-6247.83236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleyne M, Horne MK, Miller JL, 2008. Individualized treatment for iron-deficiency anemia in adults. Am. J. Med 121, 943–948. 10.1016/j.amjmed.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An P, Wu Q, Wang H, Guan Y, Mu M, Liao Y, Zhou D, Song P, Wang C, Meng L, Man Q, Li L, Zhang J, Wang F, 2012. TMPRSS6, but not TF, TFR2 or BMP2 variants are associated with increased risk of iron-deficiency anemia. Hum. Mol. Genet 21, 2124–2131. 10.1093/hmg/dds028. [DOI] [PubMed] [Google Scholar]
- Anderson CP, Shen M, Eisenstein RS, Leibold EA, 2012. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim. Biophys. Acta BBA -Mol. Cell Res., Cell Biol. Met 1823, 1468–1483. 10.1016/j.bbamcr.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ER, Shah YM, 2013. Iron homeostasis in the liver. Compr. Physiol 3, 315–330. 10.1002/cphy.c120016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GJ, Frazer DM, McLaren GD, 2009. Iron absorption and metabolism. Curr. Opin. Gastroenterol 25, 129–135. 10.1097/MOG.0b013e32831ef1f7. [DOI] [PubMed] [Google Scholar]
- Aron AT, Reeves AG, Chang CJ, 2018. Activity-based sensing fluorescent probes for iron in biological systems. Curr. Opin. Chem. Biol 43, 113–118. 10.1016/j.cbpa.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arosio P, Ingrassia R, Cavadini P, 2009. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta 1790, 589–599. 10.1016/j.bbagen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Arruda LF, Arruda SF, Campos NA, de Valencia FF, Siqueira EM, de A, 2013. Dietary iron concentration may influence aging process by altering oxidative stress in tissues of adult rats. PloS One 8, e61058. 10.1371/journal.pone.0061058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artz AS, Thirman MJ, 2011. Unexplained anemia predominates despite an intensive evaluation in a racially diverse cohort of older adults from a referral anemia clinic. J. Gerontol. A. Biol. Sci. Med. Sci 66, 925–932. 10.1093/gerona/glr090. [DOI] [PubMed] [Google Scholar]
- Ashraf A, Clark M, So P-W, 2018. The Aging of Iron Man. Front. Aging Neurosci 10, 65. 10.3389/fnagi.2018.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton S, Lei P, 2014. Nigral iron elevation is an invariable feature of Parkinson’s disease and is a sufficient cause of neurodegeneration. BioMed. Res. Int 2014, 581256–581259. 10.1155/2014/581256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu KR, Muckenthaler MU, 2019. miR-148a regulates expression of the transferrin receptor 1 in hepatocellular carcinoma. Sci. Rep 9, 1518. 10.1038/s41598-018-35947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balistreri CR, Candore G, Accardi G, Bova M, Buffa S, Bulati M, Forte GI, Listì F, Martorana A, Palmeri M, Pellicanò M, Vaccarino L, Scola L, Lio D, Colonna-Romano G, 2012. Genetics of longevity. data from the studies on Sicilian centenarians. Immun. Ageing A 9, 8. 10.1186/1742-4933-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh E, Paragh G, Jeney V, 2018. Influence of Iron on Bone Homeostasis. Pharm. Basel Switz 11 10.3390/ph11040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard JL, 2001. Iron biology in immune function, muscle metabolism and neuronal functioning. discussion 580S J. Nutr 131, 568S–579S. 10.1093/jn/131.2.568S. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Lebrón JA, Bjorkman PJ, 2000. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature 403, 46–53. 10.1038/47417. [DOI] [PubMed] [Google Scholar]
- Benyamin B, Ferreira MAR, Willemsen G, Gordon S, Middelberg RPS, McEvoy BP, Hottenga J-J, Henders AK, Campbell MJ, Wallace L, Frazer IH, Heath AC, de Geus EJC, Nyholt DR, Visscher PM, Penninx BW, Boomsma DI, Martin NG, Montgomery GW, Whitfield JB, 2009. Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat. Genet 41, 1173–1175. 10.1038/ng.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron RJ, Wiegand J, Brittenham GM, 1999. HBED: the continuing development of a potential alternative to deferoxamine for iron-chelating therapy. Blood 93, 370–375. [PubMed] [Google Scholar]
- Bergeron RJ, Wiegand J, Weimar WR, Lindstrom TC, Fannin TL, Ratliff-Thompson K, 2004. Comparison of iron chelator efficacy in iron-overloaded beagle dogs and monkeys (Cebus apella). Comp. Med 54, 664–672. [PubMed] [Google Scholar]
- Bernardes de Jesus B, Blasco MA, 2012. Assessing cell and organ senescence biomarkers. Circ. Res 111, 97–109. 10.1161/CIRCRESAHA.111.247866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar M, Weiner M, 1983. Modification of age-induced changes in heme and hemoproteins by testosterone in male rats. Mech. Ageing Dev 23, 285–296. 10.1016/0047-6374(83)90029-5. [DOI] [PubMed] [Google Scholar]
- Błasiak J, Skłodowska A, Ulińska M, Szaflik JP, 2009. Iron and age-related macular degeneration. Klin. Ocz 111, 174–177. [PubMed] [Google Scholar]
- Bloomer SA, Brown KE, Buettner GR, Kregel KC, 2008. Dysregulation of hepatic iron with aging: implications for heat stress-induced oxidative liver injury. Am. J. Physiol. Regul. Integr. Comp. Physiol 294, R1165–R1174. 10.1152/ajpregu.00719.2007. [DOI] [PubMed] [Google Scholar]
- Bloomer SA, Brown KE, Kregel KC, 2020. Renal Iron Accumulation and Oxidative Injury With Aging: Effects of Treatment With an Iron Chelator. J. Gerontol. A. Biol. Sci. Med. Sci 75, 680–684. 10.1093/gerona/glz055. [DOI] [PubMed] [Google Scholar]
- Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y, 2016. Regulators of Iron Homeostasis: New Players in Metabolism, Cell Death, and Disease. Trends Biochem. Sci 41, 274–286. 10.1016/j.tibs.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkovsky HL, 1991. Iron and the liver. Am. J. Med. Sci 301, 32–43. 10.1097/00000441-199101000-00006. [DOI] [PubMed] [Google Scholar]
- Bothwell TH, Baynes RD, MacFarlane BJ, MacPhail AP, 1989. Nutritional iron requirements and food iron absorption. J. Intern. Med 226, 357–365. 10.1111/j.1365-2796.1989.tb01409.x. [DOI] [PubMed] [Google Scholar]
- Brissot P, Ropert M, Le Lan C, Loréal O, 2012. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim. Biophys. Acta 1820, 403–410. 10.1016/j.bbagen.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Brunk UT, Terman A, 2002. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med 33, 611–619. 10.1016/s0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- Bulvik BE, Berenshtein E, Konijn AM, Grinberg L, Vinokur V, Eliashar R, Chevion MM, 2012. Aging is an organ-specific process: changes in homeostasis of iron and redox proteins in the rat. Age Dordr. Neth 34, 693–704. 10.1007/s11357-011-9268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke W, Reyes M, Imperatore G, 2002. Hereditary haemochromatosis: a realistic approach to prevention of iron overload disease in the population. Best. Pract. Res. Clin. Haematol 15, 315–328. [PubMed] [Google Scholar]
- Busti F, Campostrini N, Martinelli N, Girelli D, 2014. Iron deficiency in the elderly population, revisited in the hepcidin era. Front. Pharmacol 5, 83. 10.3389/fphar.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik ZI, 2014. Labile iron in cells and body fluids: physiology, pathology, and pharmacology. Front. Pharmacol 5, 45. 10.3389/fphar.2014.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P, 2005. LPI-labile plasma iron in iron overload. Best. Pract. Res. Clin. Haematol 18, 277–287. 10.1016/j.beha.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Cain TJ, Smith AT, 2021. Ferric iron reductases and their contribution to unicellular ferrous iron uptake. J. Inorg. Biochem 218, 111407 10.1016/j.jinorgbio.2021.111407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cankurtaran M, Yavuz BB, Halil M, Ulger Z, Haznedaroğlu IC, Arıoğul S, 2012. Increased ferritin levels could reflect ongoing aging-associated inflammation and may obscure underlying iron deficiency in the geriatric population. Eur. Geriatr. Med 3, 277–280. 10.1016/j.eurger.2012.06.005. [DOI] [Google Scholar]
- Casale G, Bonora C, Migliavacca A, Zurita IE, de Nicola P, 1981. Serum ferritin and ageing. Age Ageing 10, 119–122. 10.1093/ageing/10.2.119. [DOI] [PubMed] [Google Scholar]
- Castoldi M, Vujic Spasic M, Altamura S, Elmén J, Lindow M, Kiss J, Stolte J, Sparla R, D’Alessandro LA, Klingmüller U, Fleming RE, Longerich T, Gröne HJ, Benes V, Kauppinen S, Hentze MW, Muckenthaler MU, 2011. The liver-specific microRNA miR-122 controls systemic iron homeostasis in mice. J. Clin. Invest 121, 1386–1396. 10.1172/JCI44883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JC, Zhang W, Li Y, Sehmi J, Wass MN, Zabaneh D, Hoggart C, Bayele H, McCarthy MI, Peltonen L, Freimer NB, Srai SK, Maxwell PH, Sternberg MJE, Ruokonen A, Abecasis G, Jarvelin M-R, Scott J, Elliott P, Kooner JS, 2009. Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat. Genet 41, 1170–1172. 10.1038/ng.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P, Mohammadi M, Goozee K, Shah TM, Sohrabi HR, Dias CB, Shen K, Asih PR, Dave P, Pedrini S, Ashton NJ, Hye A, Taddei K, Lovejoy DB, Zetterberg H, Blennow K, Martins RN, 2020. Serum Hepcidin Levels in Cognitively Normal Older Adults with High Neocortical Amyloid-β Load. J. Alzheimers Dis. JAD 76, 291–301. 10.3233/JAD-200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Garcia-Santos D, Ishikawa Y, Seguin A, Li L, Fegan KH, Hildick-Smith GJ, Shah DI, Cooney JD, Chen W, King MJ, Yien YY, Schultz IJ, Anderson H, Dalton AJ, Freedman ML, Kingsley PD, Palis J, Hattangadi SM, Lodish HF, Ward DM, Kaplan J, Maeda T, Ponka P, Paw BH, 2013. Snx3 regulates recycling of the transferrin receptor and iron assimilation. Cell Metab 17, 343–352. 10.1016/j.cmet.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Attieh ZK, Su T, Syed BA, Gao H, Alaeddine RM, Fox TC, Usta J, Naylor CE, Evans RW, McKie AT, Anderson GJ, Vulpe CD, 2004. Hephaestin is a ferroxidase that maintains partial activity in sex-linked anemia mice. Blood 103, 3933–3939. 10.1182/blood-2003-09-3139. [DOI] [PubMed] [Google Scholar]
- Chung J, Chen C, Paw BH, 2012. Heme metabolism and erythropoiesis. Curr. Opin. Hematol 19, 156–162. 10.1097/MOH.0b013e328351c48b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD, 2010. Encyclopedia of Dietary Supplements CRC Press,. [Google Scholar]
- Coffey R, Ganz T, 2017. Iron homeostasis: An anthropocentric perspective. J. Biol. Chem 292, 12727–12734. 10.1074/jbc.R117.781823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M, Pratt DA, 2019. The chemical basis of ferroptosis. Nat. Chem. Biol 15, 1137–1147. 10.1038/s41589-019-0408-1. [DOI] [PubMed] [Google Scholar]
- Cook CI, Yu BP, 1998. Iron accumulation in aging: modulation by dietary restriction. Mech. Ageing Dev 102, 1–13. 10.1016/s0047-6374(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Costa E, Fernandes J, Ribeiro S, Sereno J, Garrido P, Rocha-Pereira P, Coimbra S, Catarino C, Belo L, Bronze-da-Rocha E, Vala H, Alves R, Reis F, Santos-Silva A, 2014. Aging is Associated with Impaired Renal Function, INF-gamma Induced Inflammation and with Alterations in Iron Regulatory Proteins Gene Expression. Aging Dis 5, 356–365. 10.14366/AD.2014.0500356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi A, Orellana DI, Santambrogio P, Rubio A, Cancellieri C, Giannelli S, Ripamonti M, Taverna S, Di Lullo G, Rovida E, Ferrari M, Forni GL, Fiorillo C, Broccoli V, Levi S, 2019. Stem Cell Modeling of Neuroferritinopathy Reveals Iron as a Determinant of Senescence and Ferroptosis during Neuronal Aging. Stem Cell Rep 13, 832–846. 10.1016/j.stemcr.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunietti E, Chiari MM, Monti M, Engaddi I, Berlusconi A, Neri MC, De Luca P, 2004. Distortion of iron status indices by acute inflammation in older hospitalized patients. Arch. Gerontol. Geriatr 39, 35–42. 10.1016/j.archger.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Dao MC, Meydani SN, 2013. Iron biology, immunology, aging, and obesity: four fields connected by the small peptide hormone hepcidin. Adv. Nutr. Bethesda Md 4, 602–617. 10.3945/an.113.004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A, Ciechanover A, Lodish HF, 1983. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc. Natl. Acad. Sci. U. S. A 80, 2258–2262. 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave G, 1984. Effects of waterborne iron on growth, reproduction, survival and haemoglobin in Daphnia magna. Comp. Biochem. Physiol C. 78, 433–438. 10.1016/0742-8413(84)90111-7. [DOI] [PubMed] [Google Scholar]
- Davis M, Clarke S, 2013. Influence of microRNA on the maintenance of human iron metabolism. Nutrients 5, 2611–2628. 10.3390/nu5072611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J, 2007. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol. Biol. Cell 18, 2569–2578. 10.1091/mbc.e07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Elzen WPJ, de Craen AJM, Wiegerinck ET, Westendorp RGJ, Swinkels DW, Gussekloo J, 2013. Plasma hepcidin levels and anemia in old age. The Leiden 85-Plus Study. Haematologica 98, 448–454. 10.3324/haematol.2012.068825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler VL, Galbraith M, Espinosa JM, 2014. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol 49, 1–15. 10.3109/10409238.2013.838205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRuisseau KC, Park Y-M, DeRuisseau LR, Cowley PM, Fazen CH, Doyle RP, 2013. Aging-related changes in the iron status of skeletal muscle. Exp. Gerontol 48, 1294–1302. 10.1016/j.exger.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlouhy AC, Outten CE, 2013. The iron metallome in eukaryotic organisms. Met. Ions Life Sci 12, 241–278. 10.1007/978-94-007-5561-1_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Van B, Gouel F, Jonneaux A, Timmerman K, Geĺe P, Pétrault M, Bastide M, Laloux C, Moreau C, Bordet R, Devos D, Devedjian J-C, 2016. Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol. Dis 94, 169–178. 10.1016/j.nbd.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC, 2005. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1, 191–200. 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- dos Santos CO, Dore LC, Valentine E, Shelat SG, Hardison RC, Ghosh M, Wang W, Eisenstein RS, Costa FF, Weiss MJ, 2008. An iron responsive element-like stem-loop regulates alpha-hemoglobin-stabilizing protein mRNA. J. Biol. Chem 283, 26956–26964. 10.1074/jbc.M802421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakesmith H, Nemeth E, Ganz T, 2015. Ironing out Ferroportin. Cell Metab 22, 777–787. 10.1016/j.cmet.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, Khovananth K, Mudd S, Mann N, Moresco EMY, Beutler E, Beutler B, 2008. The serine protease TMPRSS6 is required to sense iron deficiency. Science 320, 1088–1092. 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ems T, St Lucia K, Huecker MR, 2021. Biochemistry, Iron Absorption. In: StatPearls StatPearls Publishing, Treasure Island (FL). [PubMed] [Google Scholar]
- Epsztejn S, Kakhlon O, Glickstein H, Breuer W, Cabantchik I, 1997. Fluorescence analysis of the labile iron pool of mammalian cells. Anal. Biochem 248, 31–40. 10.1006/abio.1997.2126. [DOI] [PubMed] [Google Scholar]
- Fairweather-Tait SJ, Wawer AA, Gillings R, Jennings A, Myint PK, 2014. Iron status in the elderly. Mech. Ageing Dev 136–137, 22–28. 10.1016/j.mad.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Fabbri E, 2018. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol 15, 505–522. 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgueras AR, de Lara FM, Pendás AM, Garabaya C, Rodríguez F, Astudillo A, Bernal T, Cabanillas R, López-Otín C, Velasco G, 2008. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood 112, 2539–2545. 10.1182/blood-2008-04-149773. [DOI] [PubMed] [Google Scholar]
- Fuqua BK, Lu Y, Darshan D, Frazer DM, Wilkins SJ, Wolkow N, Bell AG, Hsu J, Yu CC, Chen H, Dunaief JL, Anderson GJ, Vulpe CD, 2014. The multicopper ferroxidase hephaestin enhances intestinal iron absorption in mice. PloS One 9, e98792. 10.1371/journal.pone.0098792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy B, Ferring-Appel D, Kaden S, Gröne H-J, Hentze MW, 2008. Iron regulatory proteins are essential for intestinal function and control key iron absorption molecules in the duodenum. Cell Metab 7, 79–85. 10.1016/j.cmet.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Ganasen M, Togashi H, Takeda H, Asakura H, Tosha T, Yamashita K, Hirata K, Nariai Y, Urano T, Yuan X, Hamza I, Mauk AG, Shiro Y, Sugimoto H, Sawai H, 2018. Structural basis for promotion of duodenal iron absorption by enteric ferric reductase with ascorbate. Commun. Biol 1, 120. 10.1038/s42003-018-0121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T, 2011. Hepcidin and iron regulation, 10 years later. Blood 117, 4425–4433. 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T, 2005. Hepcidin–a regulator of intestinal iron absorption and iron recycling by macrophages. Best. Pract. Res. Clin. Haematol 18, 171–182. 10.1016/j.beha.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Ganz T, Nemeth E, 2012. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 1823, 1434–1443. 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T, Nemeth E, 2009. Iron sequestration and anemia of inflammation. Semin. Hematol 46, 387–393. 10.1053/j.seminhematol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardi C, Arezzini B, Fortino V, Comporti M, 2002. Effect of free iron on collagen synthesis, cell proliferation and MMP-2 expression in rat hepatic stellate cells. Biochem. Pharmacol 64, 1139–1145. 10.1016/s0006-2952(02)01257-1. [DOI] [PubMed] [Google Scholar]
- Garringer HJ, Irimia JM, Li W, Goodwin CB, Richine B, Acton A, Chan RJ, Peacock M, Muhoberac BB, Ghetti B, Vidal R, 2016. Effect of Systemic Iron Overload and a Chelation Therapy in a Mouse Model of the Neurodegenerative Disease Hereditary Ferritinopathy. PloS One 11, e0161341. 10.1371/journal.pone.0161341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayar NH, Deghady AA, 2015. Iron status in healthy elderly people: An evaluation of the role of soluble transferrin receptors in elderly - Egypt. J. Obes. Diabetes Endocrinol (n.d.). Retrieved April 28, 2021, from 〈https://www.ejode.eg.net/article.asp?issn=2356-〉. [Google Scholar]
- Girelli D, Ugolini S, Busti F, Marchi G, Castagna A, 2018. Modern iron replacement therapy: clinical and pathophysiological insights. Int. J. Hematol 107, 16–30. 10.1007/s12185-017-2373-3. [DOI] [PubMed] [Google Scholar]
- González-Gualda E, Baker AG, Fruk L, Muñoz-Espín D, 2021. A guide to assessing cellular senescence in vitro and in vivo. FEBS J 288, 56–80. 10.1111/febs.15570. [DOI] [PubMed] [Google Scholar]
- Goodnough LT, Schrier SL, 2014. Evaluation and management of anemia in the elderly. Am. J. Hematol 89, 88–96. 10.1002/ajh.23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goralska M, Harned J, Grimes AM, Fleisher LN, McGahan MC, 1997. Mechanisms by which ascorbic acid increases ferritin levels in cultured lens epithelial cells. Exp. Eye Res 64, 413–421. 10.1006/exer.1996.0227. [DOI] [PubMed] [Google Scholar]
- Green R, Charlton R, Seftel H, Bothwell T, Mayet F, Adams B, Finch C, Layrisse M, 1968. Body iron excretion in man: a collaborative study. Am. J. Med 45, 336–353. 10.1016/0002-9343(68)90069-7. [DOI] [PubMed] [Google Scholar]
- Greenberg DS, Soreq H, 2013. Alternative Splicing. In: Maloy S, Hughes K (Eds.), Brenner’s Encyclopedia of Genetics, Second edition.,. Academic Press, San Diego, pp. 97–98. 10.1016/B978-0-12-374984-0.00043-7. [DOI] [Google Scholar]
- Grubić Kezele T, Ćurko-Cofek B, 2020. Age-Related Changes and Sex-Related Differences in Brain Iron Metabolism. Nutrients 12, 2601. 10.3390/nu12092601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Hua Y, He Y, Wang L, Hu H, Keep RF, Xi G, 2011. Iron accumulation and DNA damage in a pig model of intracerebral hemorrhage. Acta Neurochir. Suppl 111, 123–128. 10.1007/978-3-7091-0693-8_20. [DOI] [PubMed] [Google Scholar]
- Guiney SJ, Adlard PA, Bush AI, Finkelstein DI, Ayton S, 2017. Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochem. Int 104, 34–48. 10.1016/j.neuint.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Gulec S, Anderson GJ, Collins JF, 2014. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Gastrointest. Liver Physiol 307, G397–G409. 10.1152/ajpgi.00348.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadian K, Stockwell BR, 2020. SnapShot: Ferroptosis, 1188–1188.e1 Cell 181. 10.1016/j.cell.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JL, Hatef A, Imran ul-Haq M, Nair N, Unniappan S, Kizhakkedathu JN, 2014. Clinically approved iron chelators influence zebrafish mortality, hatching morphology and cardiac function. PloS One 9, e109880. 10.1371/journal.pone.0109880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C, Heuser J, Stahl P, 1983. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol 97, 329–339. 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ZL, 2019. Chapter 9 - Ceruloplasmin. In: Kerkar N, Roberts EA (Eds.), Clinical and Translational Perspectives on WILSON DISEASE Academic Press, pp. 77–84. 10.1016/B978-0-12-810532-0.00009-4. [DOI] [Google Scholar]
- Hider RC, 2002. Nature of nontransferrin-bound iron. Eur. J. Clin. Invest 32 (Suppl 1), 50–54. 10.1046/j.1365-2362.2002.0320s1050.x. [DOI] [PubMed] [Google Scholar]
- Höhn A, Jung T, Grimm S, Grune T, 2010. Lipofuscin-bound iron is a major intracellular source of oxidants: role in senescent cells. Free Radic. Biol. Med 48, 1100–1108. 10.1016/j.freeradbiomed.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Hunt JR, Zito CA, Johnson LK, 2009. Body iron excretion by healthy men and women. Am. J. Clin. Nutr 89, 1792–1798. 10.3945/ajcn.2009.27439. [DOI] [PubMed] [Google Scholar]
- Hynes M, 1948. Iron Metabolism. J. Clin. Pathol 1, 57–67. 10.1136/jcp.1.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EMG, Hendriks JCM, van Tits BLJH, Evans PJ, Breuer W, Liu DY, Jansen EHJM, Jauhiainen K, Sturm B, Porter JB, Scheiber-Mojdehkar B, von Bonsdorff L, Cabantchik ZI, Hider RC, Swinkels DW, 2005. Results of an international round robin for the quantification of serum non-transferrin-bound iron: Need for defining standardization and a clinically relevant isoform. Anal. Biochem 341, 241–250. 10.1016/j.ab.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Jacomin A-C, Geraki K, Brooks J, Tjendana-Tjhin V, Collingwood JF, Nezis IP, 2019. Impact of Autophagy and Aging on Iron Load and Ferritin in Drosophila Brain. Front. Cell Dev. Biol 7, 142. 10.3389/fcell.2019.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi T, Kortman GAM, Moretti D, Chassard C, Holding P, Dostal A, Boekhorst J, Timmerman HM, Swinkels DW, Tjalsma H, Njenga J, Mwangi A, Kvalsvig J, Lacroix C, Zimmermann MB, 2015. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 64, 731–742. 10.1136/gutjnl-2014-307720. [DOI] [PubMed] [Google Scholar]
- Jallow MW, Cerami C, Clark TG, Prentice AM, Campino S, 2020. Differences in the frequency of genetic variants associated with iron imbalance among global populations. PloS One 15, e0235141. 10.1371/journal.pone.0235141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jáuregui-Lobera I, 2014. Iron deficiency and cognitive functions. Neuropsychiatr. Dis. Treat 10, 2087–2095. 10.2147/NDT.S72491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins NL, James SA, Salim A, Sumardy F, Speed TP, Conrad M, Richardson DR, Bush AI, McColl G, 2020. Changes in ferrous iron and glutathione promote ferroptosis and frailty in aging Caenorhabditis elegans. eLife 9. 10.7554/eLife.56580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X-Z, Cong L, Niu J-Z, Dou W, Wang J-J, 2014. Alternative splicing contributes to the coordinated regulation of ferritin subunit levels in Bactrocera dorsalis (Hendel). Sci. Rep 4, 4806. 10.1038/srep04806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EE, Wessling-Resnick M, 2012. Iron metabolism and the innate immune response to infection. Microbes Infect 14, 207–216. 10.1016/j.micinf.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Fischer JG, Bowman BA, Gunter EW, 1994. Iron nutriture in elderly individuals. FASEB J. Publ. Fed. Am. Soc. Exp. Biol 8, 609–621. 10.1096/fasebj.8.9.8005389. [DOI] [PubMed] [Google Scholar]
- Jung SH, DeRuisseau LR, Kavazis AN, DeRuisseau KC, 2008. Plantaris muscle of aged rats demonstrates iron accumulation and altered expression of iron regulation proteins. Exp. Physiol 93, 407–414. 10.1113/expphysiol.2007.039453. [DOI] [PubMed] [Google Scholar]
- Kakhlon O, Cabantchik ZI, 2002. The labile iron pool: characterization, measurement, and participation in cellular processes(1). Free Radic. Biol. Med 33, 1037–1046. 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- Karin M, Mintz B, 1981. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J. Biol. Chem 256, 3245–3252. [PubMed] [Google Scholar]
- Kastman EK, Willette AA, Coe CL, Bendlin BB, Kosmatka KJ, McLaren DG, Xu G, Canu E, Field AS, Alexander AL, Voytko ML, Beasley TM, Colman RJ, Weindruch RH, Johnson SC, 2012. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J. Neurosci. J. Soc. Neurosci 32, 11897–11904. 10.1523/jneurosci.2553-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsarou A, Pantopoulos K, 2020. Basics and principles of cellular and systemic iron homeostasis. Mol. Asp. Med 75, 100866 10.1016/j.mam.2020.100866. [DOI] [PubMed] [Google Scholar]
- Katz ML, Robison WG, 2002. What is lipofuscin? Defining characteristics and differentiation from other autofluorescent lysosomal storage bodies. Arch. Gerontol. Geriatr 34, 169–184. 10.1016/s0167-4943(02)00005-5. [DOI] [PubMed] [Google Scholar]
- Kautz L, Nemeth E, 2014. Molecular liaisons between erythropoiesis and iron metabolism. Blood 124, 479–482. 10.1182/blood-2014-05-516252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell DB, 2009. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genom 2, 2. 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelmer Sacramento E, Kirkpatrick JM, Mazzetto M, Baumgart M, Bartolome A, Di Sanzo S, Caterino C, Sanguanini M, Papaevgeniou N, Lefaki M, Childs D, Bagnoli S, Terzibasi Tozzini E, Di Fraia D, Romanov N, Sudmant PH, Huber W, Chondrogianni N, Vendruscolo M, Cellerino A, Ori A, 2020. Reduced proteasome activity in the aging brain results in ribosome stoichiometry loss and aggregation. Mol. Syst. Biol 16, e9596 10.15252/msb.20209596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny EM, Fidan E, Yang Q, Anthonymuthu TS, New LA, Meyer EA, Wang H, Kochanek PM, Dixon CE, Kagan VE, Bayir H, 2019. Ferroptosis Contributes to Neuronal Death and Functional Outcome After Traumatic Brain Injury. Crit. Care Med 47, 410–418. 10.1097/CCM.0000000000003555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Quigley JG, 2013. Heme and FLVCR-related transporter families SLC48 and SLC49. Mol. Asp. Med 34, 669–682. 10.1016/j.mam.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killilea DW, Atamna H, Liao C, Ames BN, 2003. Iron accumulation during cellular senescence in human fibroblasts in vitro. Antioxid. Redox Signal 5, 507–516. 10.1089/152308603770310158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killisch I, Steinlein P, Römisch K, Hollinshead R, Beug H, Griffiths G, 1992. Characterization of early and late endocytic compartments of the transferrin cycle. Transferrin receptor antibody blocks erythroid differentiation by trapping the receptor in the early endosome. J. Cell Sci 103 (Pt 1), 211–232. [DOI] [PubMed] [Google Scholar]
- Kim J, Wessling-Resnick M, 2014. Iron and mechanisms of emotional behavior. J. Nutr. Biochem 25, 1101–1107. 10.1016/j.jnutbio.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Hwang H-J, Kim S-H, 2014. Relationship between serum ferritin levels and sarcopenia in Korean females aged 60 years and older using the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV-2, 3), 2008–2009. PloS One 9, e90105. 10.1371/journal.pone.0090105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klang IM, Schilling B, Sorensen DJ, Sahu AK, Kapahi P, Andersen JK, Swoboda P, Killilea DW, Gibson BW, Lithgow GJ, 2014. Iron promotes protein insolubility and aging in C. elegans. Aging 6, 975–991. 10.18632/aging.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson M, Wessling-Resnick M, 2003. Iron metabolism in the reticuloendothelial system. Crit. Rev. Biochem. Mol. Biol 38, 61–88. 10.1080/713609210. [DOI] [PubMed] [Google Scholar]
- Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M, 2005. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc. Natl. Acad. Sci. U. S. A 102, 1324–1328. 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W-N, Wu Q, Shen D, Zhao S-E, Guo P, Duan X-L, Chang Y-Z, 2015. Age-dependent expression of duodenal cytochrome b, divalent metal transporter 1, ferroportin 1, and hephaestin in the duodenum of rats. J. Gastroenterol. Hepatol 30, 513–520. 10.1111/jgh.12830. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H, 2004. Inflammatory mediators in the elderly. Exp. Gerontol 39, 687–699. 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Zhang HJ, 2007. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol 292, R18–R36. 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Kühn LC, 2015. Iron regulatory proteins and their role in controlling iron metabolism. Met. Integr. Biometal Sci 7, 232–243. 10.1039/c4mt00164h. [DOI] [PubMed] [Google Scholar]
- La A, Nguyen T, Tran K, Sauble E, Tu D, Gonzalez A, Kidane TZ, Soriano C, Morgan J, Doan M, Tran K, Wang C-Y, Knutson MD, Linder MC, 2018. Mobilization of iron from ferritin: new steps and details. Met. Integr. Biometal Sci 10, 154–168. 10.1039/c7mt00284j. [DOI] [PubMed] [Google Scholar]
- Laham N, Rotem-Yehudar R, Shechter C, Coligan JE, Ehrlich R, 2004. Transferrin [corrected] receptor association and endosomal localization of soluble HFE are not sufficient for regulation of cellular iron homeostasis. J. Cell. Biochem 91, 1130–1145. 10.1002/jcb.20015. [DOI] [PubMed] [Google Scholar]
- Lane DJR, Richardson DR, 2014. The active role of vitamin C in mammalian iron metabolism: much more than just enhanced iron absorption! Free Radic. Biol. Med 75, 69–83. 10.1016/j.freeradbiomed.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Le Blanc S, Garrick MD, Arredondo M, 2012. Heme carrier protein 1 transports heme and is involved in heme-Fe metabolism. Am. J. Physiol. Cell Physiol 302, C1780–C1785. 10.1152/ajpcell.00080.2012. [DOI] [PubMed] [Google Scholar]
- Leidgens S, Bullough KZ, Shi H, Li F, Shakoury-Elizeh M, Yabe T, Subramanian P, Hsu E, Natarajan N, Nandal A, Stemmler TL, Philpott CC, 2013. Each member of the poly-r(C)-binding protein 1 (PCBP) family exhibits iron chaperone activity toward ferritin. J. Biol. Chem 288, 17791–17802. 10.1074/jbc.M113.460253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencel P, Magne D, 2011. Inflammaging: the driving force in osteoporosis? Med. Hypotheses 76, 317–321. 10.1016/j.mehy.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Levine DS, Woods JW, 1990. Immunolocalization of transferrin and transferrin receptor in mouse small intestinal absorptive cells. J. Histochem. Cytochem. J. Histochem. Soc 38, 851–858. 10.1177/38.6.2186090. [DOI] [PubMed] [Google Scholar]
- Liu AP, Aguet F, Danuser G, Schmid SL, 2010. Local clustering of transferrin receptors promotes clathrin-coated pit initiation. J. Cell Biol 191, 1381–1393. 10.1083/jcb.201008117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Sun Y, Xu G, Snetselaar LG, Ludewig G, Wallace RB, Bao W, 2019. Association between Body Iron Status and Leukocyte Telomere Length, a Biomarker of Biological Aging, in a Nationally Representative Sample of US Adults. J. Acad. Nutr. Diet 119, 617–625. 10.1016/j.jand.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Men P, Kenner GH, Miller SC, 2006. Age-associated iron accumulation in bone: implications for postmenopausal osteoporosis and a new target for prevention and treatment by chelation. Biometals Int. J. Role Met. Ions Biol. Biochem. Med 19, 245–251. 10.1007/s10534-005-6666-2. [DOI] [PubMed] [Google Scholar]
- Liu J-L, Fan Y-G, Yang Z-S, Wang Z-Y, Guo C, 2018. Iron and Alzheimer’s Disease: From Pathogenesis to Therapeutic Implications. Front. Neurosci 12, 632. 10.3389/fnins.2018.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hill P, Haile DJ, 2002. Role of the ferroportin iron-responsive element in iron and nitric oxide dependent gene regulation. Blood Cells Mol. Dis 29, 315–326. 10.1006/bcmd.2002.0572. [DOI] [PubMed] [Google Scholar]
- Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ, 2006. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. U. S. A 103, 13612–13617. 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez TE, Pham HM, Nguyen BV, Tahmasian Y, Ramsden S, Coskun V, Schriner SE, Jafari M, 2016. Green tea polyphenols require the mitochondrial iron transporter, mitoferrin, for lifespan extension in Drosophila melanogaster. Arch. Insect Biochem. Physiol 93, 210–221. 10.1002/arch.21353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Contreras MJ, Zamora-Portero S, Lopez MA, Marin JF, Zamora S, Perez-Llamas F, 2010. Dietary intake and iron status of institutionalized elderly people: relationship with different factors. J. Nutr. Health Aging 14, 816–821. 10.1007/s12603-010-0118-6. [DOI] [PubMed] [Google Scholar]
- Lv H, Shang P, 2018. The significance, trafficking and determination of labile iron in cytosol, mitochondria and lysosomes. Met. Integr. Biometal Sci 10, 899–916. 10.1039/c8mt00048d. [DOI] [PubMed] [Google Scholar]
- Lymboussaki A, Pignatti E, Montosi G, Garuti C, Haile DJ, Pietrangelo A, 2003. The role of the iron responsive element in the control of ferroportin1/IREG1/MTP1 gene expression. J. Hepatol 39, 710–715. 10.1016/s0168-8278(03)00408-2. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Garrick MD, 2005. Iron Imports. II. Iron uptake at the apical membrane in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol 289, G981–G986. 10.1152/ajpgi.00363.2005. [DOI] [PubMed] [Google Scholar]
- Malyszko J, 2009. Hemojuvelin: the hepcidin story continues. Kidney Blood Press. Res 32, 71–76. 10.1159/000208988. [DOI] [PubMed] [Google Scholar]
- Martins R, Silva B, Proença D, Faustino P, 2011. Differential HFE gene expression is regulated by alternative splicing in human tissues. PloS One 6, e17542. 10.1371/journal.pone.0017542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaldan S, Clatworthy SAS, Gamell C, Meggyesy PM, Rigopoulos A-T, Haupt S, Haupt Y, Denoyer D, Adlard PA, Bush AI, Cater MA, 2018. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol 14, 100–115. 10.1016/j.redox.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie HR, Aiello VR, Williams TR, 1993. Inhibition of iron absorption prolongs the life span of Drosophila. Mech. Ageing Dev 67, 227–237. 10.1016/0047-6374(93)90001-8. [DOI] [PubMed] [Google Scholar]
- Massie HR, Aiello VR, Williams TR, 1985. Iron accumulation during development and ageing of Drosophila. Mech. Ageing Dev 29, 215–220. 10.1016/0047-6374(85)90020-x. [DOI] [PubMed] [Google Scholar]
- McCranor BJ, Langdon JM, Prince OD, Femnou LK, Berger AE, Cheadle C, Civin CI, Kim A, Rivera S, Ganz T, Vaulont S, Xue Q-L, Walston JD, Roy CN, 2013. Investigation of the role of interleukin-6 and hepcidin antimicrobial peptide in the development of anemia with age. Haematologica 98, 1633–1640. 10.3324/haematol.2013.087114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadante CJ, Prajapati M, Parmar JH, Conboy HL, Dash ME, Pettiglio MA, Herrera C, Bu JT, Stopa EG, Mendes P, Bartnikas TB, 2019. Gastrointestinal iron excretion and reversal of iron excess in a mouse model of inherited iron excess. Haematologica 104, 678–689. 10.3324/haematol.2018.198382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynard D, Vaja V, Sun CC, Corradini E, Chen S, López-Otín C, Grgurevic L, Hong CC, Stirnberg M, Gütschow M, Vukicevic S, Babitt JL, Lin HY, 2011. Regulation of TMPRSS6 by BMP6 and iron in human cells and mice. Blood 118, 747–756. 10.1182/blood-2011-04-348698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyron-Holtz EG, Ghosh MC, Iwai K, LaVaute T, Brazzolotto X, Berger UV, Land W, Ollivierre-Wilson H, Grinberg A, Love P, Rouault TA, 2004. Genetic ablations of iron regulatory proteins 1 and 2 reveal why iron regulatory protein 2 dominates iron homeostasis. EMBO J 23, 386–395. 10.1038/sj.emboj.7600041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milic S, Mikolasevic I, Orlic L, Devcic E, Starcevic-Cizmarevic N, Stimac D, Kapovic M, Ristic S, 2016. The Role of Iron and Iron Overload in Chronic Liver Disease. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res 22, 2144–2151. 10.12659/msm.896494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsen ER, 1988. Iron nutrition and absorption: dietary factors which impact iron bioavailability. J. Am. Diet. Assoc 88, 786–790. [PubMed] [Google Scholar]
- Morris G, Berk M, Carvalho AF, Maes M, Walker AJ, Puri BK, 2018. Why should neuroscientists worry about iron? The emerging role of ferroptosis in the pathophysiology of neuroprogressive diseases. Behav. Brain Res 341, 154–175. 10.1016/j.bbr.2017.12.036. [DOI] [PubMed] [Google Scholar]
- Muckenthaler MU, Galy B, Hentze MW, 2008. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr 28, 197–213. 10.1146/annurev.nutr.28.061807.155521. [DOI] [PubMed] [Google Scholar]
- Muckenthaler MU, Rivella S, Hentze MW, Galy B, 2017. A Red Carpet for Iron Metabolism. Cell 168, 344–361. 10.1016/j.cell.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir RK, Zhao N, Wei J, Wang Y-H, Moroz A, Huang Y, Chen Y-C, Sriram R, Kurhanewicz J, Ruggero D, Renslo AR, Evans MJ, 2019. Measuring Dynamic Changes in the Labile Iron Pool in Vivo with a Reactivity-Based Probe for Positron Emission Tomography. ACS Cent. Sci 5, 727–736. 10.1021/acscentsci.9b00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo-Ortiz B, Ramírez Emiliano J, Hernández Vázquez WI, Martínez-Garza S, Solorio-Meza S, Albarrán-Tamayo F, Ramos-Rodríguez E, Benítez-Bribiesca L, 2016. Impact of Oxidative Stress in Premature Aging and Iron Overload in Hemodialysis Patients. Oxid. Med. Cell. Longev 2016, 1578235 10.1155/2016/1578235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai A, Pagani A, Silvestri L, Campostrini N, Corbella M, Girelli D, Traglia M, Toniolo D, Camaschella C, 2011. TMPRSS6 rs855791 modulates hepcidin transcription in vitro and serum hepcidin levels in normal individuals. Blood 118, 4459–4462. 10.1182/blood-2011-06-364034. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Saito Y, Gouda T, Imai T, Shimazawa M, Nishimura Y, Hara H, 2020. Therapeutic Effects of Iron Chelation in Atorvastatin-Induced Intracranial Hemorrhage of Zebrafish Larvae. J. Stroke Cerebrovasc. Dis. J. Natl. Stroke Assoc 29, 105215 10.1016/j.jstrokecerebrovasdis.2020.105215. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Ganz T, 2009. The role of hepcidin in iron metabolism. Acta Haematol 122, 78–86. 10.1159/000243791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T, 2004. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest 113, 1271–1276. 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie G, Sheftel AD, Kim SF, Ponka P, 2005. Overexpression of mitochondrial ferritin causes cytosolic iron depletion and changes cellular iron homeostasis. Blood 105, 2161–2167. 10.1182/blood-2004-07-2722. [DOI] [PubMed] [Google Scholar]
- Nordestgaard BG, Adourian AS, Freiberg JJ, Guo Y, Muntendam P, Falk E, 2010. Risk factors for near-term myocardial infarction in apparently healthy men and women. Clin. Chem 56, 559–567. 10.1373/clinchem.2009.139964. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Hayder H, Zayed Y, Peng C, 2018. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol 9, 402. 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE, Fleming MD, 2005. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat. Genet 37, 1264–1269. 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani A, Nai A, Silvestri L, Camaschella C, 2019. Hepcidin and Anemia: A Tight Relationship. Front. Physiol 10, 1294. 10.3389/fphys.2019.01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradkar PN, Zumbrennen KB, Paw BH, Ward DM, Kaplan J, 2009. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol. Cell. Biol 29, 1007–1016. 10.1128/MCB.01685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BT, Manz DH, Torti FM, Torti SV, 2017. Mitochondria and Iron: current questions. Expert Rev. Hematol 10, 65–79. 10.1080/17474086.2016.1268047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BWJH, Guralnik JM, Onder G, Ferrucci L, Wallace RB, Pahor M, 2003. Anemia and decline in physical performance among older persons. Am. J. Med 115, 104–110. 10.1016/s0002-9343(03)00263-8. [DOI] [PubMed] [Google Scholar]
- Peyssonnaux C, Nizet V, Johnson RS, 2008. Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle Georget. Tex 7, 28–32. 10.4161/cc.7.1.5145. [DOI] [PubMed] [Google Scholar]
- Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS, 2007. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J. Clin. Invest 117, 1926–1932. 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot Q, Desĺee G, Adair-Kirk TL, Woods JC, Byers D, Conradi S, Dury S, Perotin JM, Lebargy F, Cassan C, Le Naour R, Holtzman MJ, Pierce RA, 2014. Increased iron sequestration in alveolar macrophages in chronic obstructive pulmonary disease. PloS One 9, e96285. 10.1371/journal.pone.0096285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picca A, Mankowski RT, Kamenov G, Anton SD, Manini TM, Buford TW, Saini SK, Calvani R, Landi F, Bernabei R, Marzetti E, Leeuwenburgh C, 2019. Advanced Age Is Associated with Iron Dyshomeostasis and Mitochondrial DNA Damage in Human Skeletal Muscle. Cells 8. 10.3390/cells8121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno A, Pelucchi S, Mariani R, 2020. Inherited iron overload disorders. Transl. Gastroenterol. Hepatol 5, 25. 10.21037/tgh.2019.11.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeschla M, Valenzano DR, 2020. The turquoise killifish: a genetically tractable model for the study of aging. J. Exp. Biol 223 10.1242/jeb.209296. [DOI] [PubMed] [Google Scholar]
- Polla BS, 1999. Therapy by taking away: the case of iron. Biochem. Pharmacol 57, 1345–1349. 10.1016/s0006-2952(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Pouillot A, Polla A, Polla BS, 2013. Iron and iron chelators: a review on potential effects on skin aging. Curr. Aging Sci 6, 225–231. 10.2174/18746098112059990037. [DOI] [PubMed] [Google Scholar]
- Puig S, Ramos-Alonso L, Romero AM, Martínez-Pastor MT, 2017. The elemental role of iron in DNA synthesis and repair. Met. Integr. Biometal Sci 9, 1483–1500. 10.1039/c7mt00116a. [DOI] [PubMed] [Google Scholar]
- Pusch HJ, Schramm A, Spitz G, Haubitz I, 1981. [Normal range and significance of serum ferritin; variations with age and sex (author’s transl)]. Aktuel-.−. Gerontol 11, 208–211. [PubMed] [Google Scholar]
- Ramsay AJ, Hooper JD, Folgueras AR, Velasco G, López-Otín C, 2009. Matriptase-2 (TMPRSS6): a proteolytic regulator of iron homeostasis. Haematologica 94, 840–849. 10.3324/haematol.2008.001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renassia C, Peyssonnaux C, 2019. New insights into the links between hypoxia and iron homeostasis. Curr. Opin. Hematol 26, 125–130. 10.1097/MOH.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SL, Ritz B, 2008. Genetics of iron regulation and the possible role of iron in Parkinson’s disease. Neurobiol. Dis 32, 183–195. 10.1016/j.nbd.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DR, Lane DJR, Becker EM, Huang ML-H, Whitnall M, Suryo Rahmanto Y, Sheftel AD, Ponka P, 2010. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc. Natl. Acad. Sci. U. S. A 107, 10775–10782. 10.1073/pnas.0912925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripa R, Dolfi L, Terrigno M, Pandolfini L, Savino A, Arcucci V, Groth M, Terzibasi Tozzini E, Baumgart M, Cellerino A, 2017. MicroRNA miR-29 controls a compensatory response to limit neuronal iron accumulation during adult life and aging. BMC Biol 15, 9. 10.1186/s12915-017-0354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivella S, Crielaard BJ, 2014. Disorders of Iron Metabolism: Iron Deficiency and Iron Overload and Anemia of Chronic Diseases. In: McManus LM, Mitchell RN (Eds.), Pathobiology of Human Disease Academic Press, San Diego, pp. 1471–1487. 10.1016/B978-0-12-386456-7.07903-X. [DOI] [Google Scholar]
- Rosendale M, Van TNN, Grillo-Bosch D, Sposini S, Claverie L, Gauthereau I, Claverol S, Choquet D, Sainlos M, Perrais D, 2019. Functional recruitment of dynamin requires multimeric interactions for efficient endocytosis. Nat. Commun 10, 4462. 10.1038/s41467-019-12434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi E, 2005. Hepcidin–the iron regulatory hormone. Clin. Biochem. Rev 26, 47–49. [PMC free article] [PubMed] [Google Scholar]
- Rouault TA, 2002. Post-transcriptional regulation of human iron metabolism by iron regulatory proteins. Blood Cells Mol. Dis 29, 309–314. 10.1006/bcmd.2002.0571. [DOI] [PubMed] [Google Scholar]
- Sahoo SK, Crisponi G, 2019. Recent Advances on Iron(III) Selective Fluorescent Probes with Possible Applications in Bioimaging. Mol. Basel Switz 24 10.3390/molecules24183267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Sargent T, Parker HG, Lawrence JH, 1964. WHOLE-BODY IRON LOSS IN NORMAL MAN MEASURED WITH A GAMMA SPECTROMETER. J. Nucl. Med. Publ. Soc. Nucl. Med 5, 571–580. [PubMed] [Google Scholar]
- Sanchez M, Galy B, Muckenthaler MU, Hentze MW, 2007. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nat. Struct. Mol. Biol 14, 420–426. 10.1038/nsmb1222. [DOI] [PubMed] [Google Scholar]
- Santiago Gonźalez DA, Cheli VT, Wan R, Paez PM, 2019. Iron Metabolism in the Peripheral Nervous System: The Role of DMT1, Ferritin, and Transferrin Receptor in Schwann Cell Maturation and Myelination. J. Neurosci 39, 9940–9953. 10.1523/JNEUROSCI.1409-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah YM, Xie L, 2014. Hypoxia-inducible factors link iron homeostasis and erythropoiesis. Gastroenterology 146, 630–642. 10.1053/j.gastro.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P, Srai S-K, 2007. Molecular mechanisms involved in intestinal iron absorption. World J. Gastroenterol 13, 4716–4724. 10.3748/wjg.v13.i35.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, 2020. An IRON-clad Connection between Aging Organelles. Cell 180, 214–216. 10.1016/j.cell.2019.12.037. [DOI] [PubMed] [Google Scholar]
- Sheng Y, Yang G, Casey K, Curry S, Oliver M, Han SM, Leeuwenburgh C, Xiao R, 2021. A novel role of the mitochondrial iron-sulfur cluster assembly protein ISCU-1/ISCU in longevity and stress response. J. Neurosci 39, 9940–9953. 10.1523/JNEUROSCI.1409-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman HG, Jovanovic C, Stolnik S, Baronian K, Downard AJ, Rawson FJ, 2018. New Perspectives on Iron Uptake in Eukaryotes. Front. Mol. Biosci 5, 97. 10.3389/fmolb.2018.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin C, Baik I, 2017. Transferrin saturation concentrations associated with telomeric ageing: a population-based study. Br. J. Nutr 117, 1693–1701. 10.1017/S0007114517001696. [DOI] [PubMed] [Google Scholar]
- Smith MA, Harris PL, Sayre LM, Perry G, 1997. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. U. S. A 94, 9866–9868. 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobh A, Loguinov A, Zhou J, Jenkitkasemwong S, Zeidan R, El Ahmadie N, Tagmount A, Knutson M, Fraenkel PG, Vulpe CD, 2020. Genetic screens reveal CCDC115 as a modulator of erythroid iron and heme trafficking. Am. J. Hematol 95, 1085–1098. 10.1002/ajh.25899. [DOI] [PubMed] [Google Scholar]
- Sohn Y-S, Ghoti H, Breuer W, Rachmilewitz E, Attar S, Weiss G, Cabantchik ZI, 2012. The role of endocytic pathways in cellular uptake of plasma non-transferrin iron. Haematologica 97, 670–678. 10.3324/haematol.2011.054858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Zhao L, Li Y, Hadziahmetovic M, Song Y, Connelly J, Spino M, Dunaief JL, 2014. The oral iron chelator deferiprone protects against systemic iron overload-induced retinal degeneration in hepcidin knockout mice. Invest. Ophthalmol. Vis. Sci 55, 4525–4532. 10.1167/iovs.14-14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbicker AU, Muckenthaler MU, 2013. Out of balance–systemic iron homeostasis in iron-related disorders. Nutrients 5, 3034–3061. 10.3390/nu5083034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W, Strous GJ, Geuze HJ, Oorschot V, Schwartz AL, 1991. Late endosomes derive from early endosomes by maturation. Cell 65, 417–427. 10.1016/0092-8674(91)90459-c. [DOI] [PubMed] [Google Scholar]
- Tabuchi M, Tanaka N, Nishida-Kitayama J, Ohno H, Kishi F, 2002. Alternative splicing regulates the subcellular localization of divalent metal transporter 1 isoforms. Mol. Biol. Cell 13, 4371–4387. 10.1091/mbc.e02-03-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Roy CN, Yao W, Matteini A, Semba RD, Arking D, Walston JD, Fried LP, Singleton A, Guralnik J, Abecasis GR, Bandinelli S, Longo DL, Ferrucci L, 2010. A genome-wide association analysis of serum iron concentrations. Blood 115, 94–96. 10.1182/blood-2009-07-232496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor JR, Papasaikas P, Valcárcel J, 2015. Genome-wide identification of Fas/CD95 alternative splicing regulators reveals links with iron homeostasis. Mol. Cell 57, 23–38. 10.1016/j.molcel.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Thompson JW, Bruick RK, 2012. Protein degradation and iron homeostasis. Biochim. Biophys. Acta 1823, 1484–1490. 10.1016/j.bbamcr.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers PRHJ, Wilson JF, Joshi PK, Deelen J, 2020. Multivariate genomic scan implicates novel loci and haem metabolism in human ageing. Nat. Commun 11, 3570. 10.1038/s41467-020-17312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Kim HJ, Zhao J, Song Y, Dunaief JL, Sparrow JR, 2018. Iron promotes oxidative cell death caused by bisretinoids of retina. Proc. Natl. Acad. Sci. U. S. A 115, 4963–4968. 10.1073/pnas.1722601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashchenko G, MacGillivray RTA, 2013. Multi-copper oxidases and human iron metabolism. Nutrients 5, 2289–2313. 10.3390/nu5072289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallander ML, Leibold EA, Eisenstein RS, 2006. Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochim. Biophys. Acta 1763, 668–689. 10.1016/j.bbamcr.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Y, Babitt JL, 2019. Liver iron sensing and body iron homeostasis. Blood 133, 18–29. 10.1182/blood-2018-06-815894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Y, Babitt JL, 2016. Hepcidin regulation in the anemia of inflammation. Curr. Opin. Hematol 23, 189–197. 10.1097/MOH.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Y, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, Knutson MD, 2012. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem 287, 34032–34043. 10.1074/jbc.M112.367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Y, Meynard D, Lin HY, 2014. The role of TMPRSS6/matriptase-2 in iron regulation and anemia. Front. Pharmacol 5, 114. 10.3389/fphar.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Li W-B, Wei X-E, Li Y-H, Dai Y-M, 2012. An investigation of age-related iron deposition using susceptibility weighted imaging. PloS One 7, e50706. 10.1371/journal.pone.0050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DM, Cloonan SM, 2019. Mitochondrial Iron in Human Health and Disease. Annu. Rev. Physiol 81, 453–482. 10.1146/annurev-physiol-020518-114742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L, 2014. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13, 1045–1060. 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Yamashita Y, Ohgawara H, Sekiguchi M, Satake N, Orino K, Yamamoto S, 2001. Iron content of rat serum ferritin. J. Vet. Med. Sci 63, 587–589. 10.1292/jvms.63.587. [DOI] [PubMed] [Google Scholar]
- Weiss G, Ganz T, Goodnough LT, 2019. Anemia of inflammation. Blood 133, 40–50. 10.1182/blood-2018-06-856500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman Z, Pinsky M, Donegan RK, Reddi AR, Kornitzer D, 2021. Using genetically encoded heme sensors to probe the mechanisms of heme uptake and homeostasis in Candida albicans. Cell. Microbiol 23, e13282 10.1111/cmi.13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A-R, Oates P-S, 2008. Mechanisms of heme iron absorption: current questions and controversies. World J. Gastroenterol 14, 4101–4110. 10.3748/wjg.14.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, Yuan X, Schmidt PJ, Bresciani E, Samuel TK, Campagna D, Hall C, Bishop K, Calicchio ML, Lapierre A, Ward DM, Liu P, Fleming MD, Hamza I, 2013. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab 17, 261–270. 10.1016/j.cmet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore K, Vera E, Martínez-Nevado E, Sanpera C, Blasco MA, 2019. Telomere shortening rate predicts species life span. Proc. Natl. Acad. Sci. U. S. A 116, 15122–15127. 10.1073/pnas.1902452116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka D, Gromadzka G, 2014. [Ceruloplasmin, hephaestin and zyklopen: the three multicopper oxidases important for human iron metabolism]. Post. Hig. Med. Doswiadczalnej Online 68, 912–924. 10.5604/17322693.1111136. [DOI] [PubMed] [Google Scholar]
- Worwood M, Dawkins S, Wagstaff M, Jacobs A, 1976. The purification and properties of ferritin from human serum. Biochem. J 157, 97–103. 10.1042/bj1570097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Hwang JCY, Lees HA, Wohlgemuth SE, Knutson MD, Judge AR, DupontVersteegden EE, Marzetti E, Leeuwenburgh C, 2012a. Long-term perturbation of muscle iron homeostasis following hindlimb suspension in old rats is associated with high levels of oxidative stress and impaired recovery from atrophy. Exp. Gerontol 47, 100–108. 10.1016/j.exger.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Jia Z, Knutson MD, Leeuwenburgh C, 2012b. Impaired iron status in aging research. Int. J. Mol. Sci 13, 2368–2386. 10.3390/ijms13022368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Knutson MD, Carter CS, Leeuwenburgh C, 2008. Iron accumulation with age, oxidative stress and functional decline. PloS One 3, e2865. 10.1371/journal.pone.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz B, Li H, 2018. Gut Microbiota and Iron: The Crucial Actors in Health and Disease. Pharm. Basel Switz 11 10.3390/ph11040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Migita CT, 2000. Mechanism of heme degradation by heme oxygenase. J. Inorg. Biochem 82, 33–41. 10.1016/s0162-0134(00)00156-2. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y, Kosaka N, Ochiya T, Kato T, 2012. Micromanaging Iron Homeostasis: hypoxia-inducible micro-RNA-210 suppresses iron homeostasis-related proteins. J. Biol. Chem 287, 34110–34119. 10.1074/jbc.M112.356717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT, 2008. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol 4, 33–41. 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Persson HL, Eaton JW, Brunk UT, 2003. Intralysosomal iron: a major determinant of oxidant-induced cell death. Free Radic. Biol. Med 34, 1243–1252. 10.1016/s0891-5849(03)00109-6. [DOI] [PubMed] [Google Scholar]
- Zhang D-L, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA, 2009. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab 9, 461–473. 10.1016/j.cmet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang F, Guo X, An P, Tao Y, Wang F, 2012. Ferroportin1 in hepatocytes and macrophages is required for the efficient mobilization of body iron stores in mice. Hepatol. Baltim. Md 56, 961–971. 10.1002/hep.25746. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Chassard C, Rohner F, N’goran EK, Nindjin C, Dostal A, Utzinger J, Ghattas H, Lacroix C, Hurrell RF, 2010. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d’Ivoire. Am. J. Clin. Nutr 92, 1406–1415. 10.3945/ajcn.110.004564. [DOI] [PubMed] [Google Scholar]


