Abstract
While people 65 and older represent 16% of the population in the United States, they account for more than 40% of surgical procedures performed each year. Maintaining brain health after anesthesia and surgery is not only important to our patients but is an increasingly important patient safety imperative for the specialty of anesthesiology.
Aging is a complex process that diminishes the reserve of every organ system and often results in a patient that is vulnerable to the stress of surgery. The brain is no exception, and many older patients present with preoperative cognitive impairment that is undiagnosed. As we age, a number of changes occur in the human brain, resulting in a patient that is less resilient to perioperative stress, making older adults more susceptible to the phenotypic expression of perioperative neurocognitive disorders.
This review summarizes the current scientific and clinical understanding of perioperative neurocognitive disorders, and recommends patient-centered, age-focused interventions that can better mitigate risk, prevent harm, and improve outcomes for our patients. Finally, it discusses the emerging topic of sleep and cognitive health, and other future frontiers of scientific inquiry that might inform clinical best practices.
Introduction
Medical systems have grown not only in sophistication but also complexity, and it should not be surprising that harm, from the systems of care intended to improve health, continues to be pervasive. Indeed the World Health Organization has declared that “adverse events due to unsafe care is likely one of the 10 leading causes of death and disability in the world”.1 The specialty of anesthesiology has been a leader in improving patient safety and we have continued to innovate and develop new frontiers in quality and safety.2 In alignment with the progressive complexity of medical systems the specialty has fundamentally changed. Advances in intraoperative monitoring, modern anesthetics, and improved techniques for securing the airway have provided an opportunity for a new era, and our specialty has embraced a more holistic vision of care that is focused on perioperative medicine with embedded processes of care that focus on returning patients to long-term functional, psychosocial, and cognitive health.
In the United States someone turns 65 years old every 8 seconds.3,4 It is estimated that the number of people 65 years and older will double to 95 million by 2060. While this group represents 16% of the population in the United States, it accounts for more than 40% of surgical procedures performed each year.5 In other words, older adults, a cohort of patients vulnerable to preventable harm, undergo a disproportionate percentage of surgical procedures.6,7
Aging is a complex process that diminishes the reserve of every organ system, changes the pharmacokinetics and pharmacodynamics of anesthetics/medications, and produces a pro-inflammatory state, resulting in increased risk of morbidity and mortality associated with surgery and anesthesia.8 Of particular concern to our patients is the risk of postoperative neurocognitive disorders which are common postoperative complications in older adults. A fundamental goal of patient-centric, age-friendly healthcare systems should be to prevent harm to cognitive health across all settings of care. The focus of this review is the key patient safety topic of cognitive health of the older adult during the perioperative period.
From The Patient’s Perspective
To use the term “patient-centric” with integrity we must address the patient’s perspective at the outset. Anecdotes do not define a problem but do start to paint a picture of the perceptions of our patients. In the author’s experience patients do come in with real world perceptions of the effect of surgery and anesthesia on brain health.
Anesthesiologists should be prepared to have discussions with patients and their families regarding brain health concerns and address their perceptions. While many older adults with known cognition issues are quick to express their concerns, most patients aren’t sure which questions to ask regarding perioperative cognitive changes. Older patients should be empowered to ask questions regarding how best to prepare for surgery, what optimization strategies are available before and after surgery, and what role caregivers can have in their recovery. Resources have been created for patients that explain what cognitive changes may occur after surgery with educational guides on maintaining brain health after surgery.9,10 Families are important team members and can provide extra help with prescribed authority to activate a Rapid Response Team when concerned about the condition of the patient. Prompt reporting of symptoms can help reduce the burden of perioperative neurocognitive disorders on overall health.
Perioperative Neurocognitive Disorders
The Nomenclature Consensus Working Group has suggested using the overarching term Perioperative Neurocognitive Disorders (PND) defined as “cognitive impairment or change identified in the preoperative or postoperative period”.11 Perioperative neurocognitive disorders are further characterized in the following domains:
Preoperative cognitive impairment
Postoperative delirium
Delayed neurocognitive recovery - impairment within the first 30-days of surgery
Postoperative neurocognitive disorder - occurring between 30-days and 12-months after surgery.
Postoperative delirium (POD) is the most common surgical complication in older adults and is defined as an acute fluctuating state of confusion, inattention, and level of consciousness that occurs within the first seven days after surgery.11 The reported incidence of POD in the literature has a high degree of variability that is dependent on the study methodology, patient population, and surgical procedure and is generally in the range of 5–65%.12 Further, it is estimated that new or worsening long-term cognitive dysfunction occurs in more than 10% of non-cardiac surgical patients over the age of 60 years, and has been reported in up to 50% of patients, depending on existing comorbidities or type of surgery.13,14
Perioperative neurocognitive disorders are associated with prolonged hospital stays, more days on mechanical ventilation, higher risk of falls, increased healthcare costs, and functional decline. Even after discharge, patients who develop PND are at an increased risk of institutionalization, death, and dementia. Patient health care costs associated with PND can reach $60,000 during the first year after discharge.13,15 The annual national healthcare costs for delirium alone have been estimated at $150 billion.13,15
Given this clear patient safety issue, anesthesiologists have an opportunity to lead in improving safety through perioperative best practices. Recognizing the necessity for quality improvement, the American Geriatrics Society and American College of Surgeons assembled an expert panel on POD to provide a set of evidence-based recommendations for the optimal care of older adults.16,17 In 2015, the American Society of Anesthesiologists launched the Perioperative Brain Health Initiative and published the Best Practices for Postoperative Brain Health,18 promoting attention to this critical issue. Several other evidence-based guidelines have been issued throughout the world.19,20 Despite this call to action, and the fact that simple strategies are projected to reduce the incidence of PND by 40%, these disorders remain a far too frequent event for older adults.16,21
Age as a risk factor for PND
Perioperative neurocognitive disorders most often arise from acute surgical stress in at-risk patients.22 Older patients are at higher risk as age-associated changes take place across multiple physiologic systems and result in diminished function, multiple co-morbidities, and increased vulnerability. Structural and functional alterations that are often present in the aging brain have a spectrum of phenotypic expression; from cognitive “super-agers” who defy normal aging, to a “normal” decline in cognitive ability which allows for a relatively high function and quality of life, to mild cognitive impairment, and finally dementia. Normal aging has its greatest effect on the cognitive domains of processing speed, memory, attention, reasoning, and executive function.
With aging there are widespread structural changes that occur in the brain with atrophy being universal. Atrophy is most prominent in the prefrontal cortex and hippocampus which are responsible for complex thought processes and memory. Atrophy is best explained by a reduction in synaptic density, neuronal size, and synaptic branching.23 As we age there are also reductions in neurogenesis, impairment of the glymphatic system, neurotransmitter imbalances, deterioration of the microcirculation, and dysfunction of the blood-brain barrier. Of particular note is “inflammaging” or a low-level, systemic, chronic inflammatory state that can contribute to diseases of aging and may make the older patient susceptible to an additional inflammatory hit that often occurs with surgery.24,25 There is no unitary theory that fully explains PND but as we age, our brain is less resilient to perioperative stress, making us more susceptible to the phenotypic expression of PND.26,27
The Preoperative Period
Perioperative neurocognitive disorders result from a complex interaction between the patient’s baseline vulnerability, and the physiological stress and inflammation associated with the perioperative period.24,28,29 Perioperative triggers, combined with a decreased physiologic reserve that is often present in the older patient, make older patients more likely to develop PND.26,27 The ability to preoperatively identify and optimize modifiable risk factors presents an opportunity to employ interventions that might decrease the incidence and severity of PND.
Risk Assessment
The framework for risk assessment includes a combination of predisposing factors and precipitating factors (see Figure 1). Predisposing factors include the patient’s baseline medical condition while precipitating factors include factors related to perioperative care and hospitalization. Table 1 identifies predisposing risk as either modifiable or non-modifiable.
Figure 1.
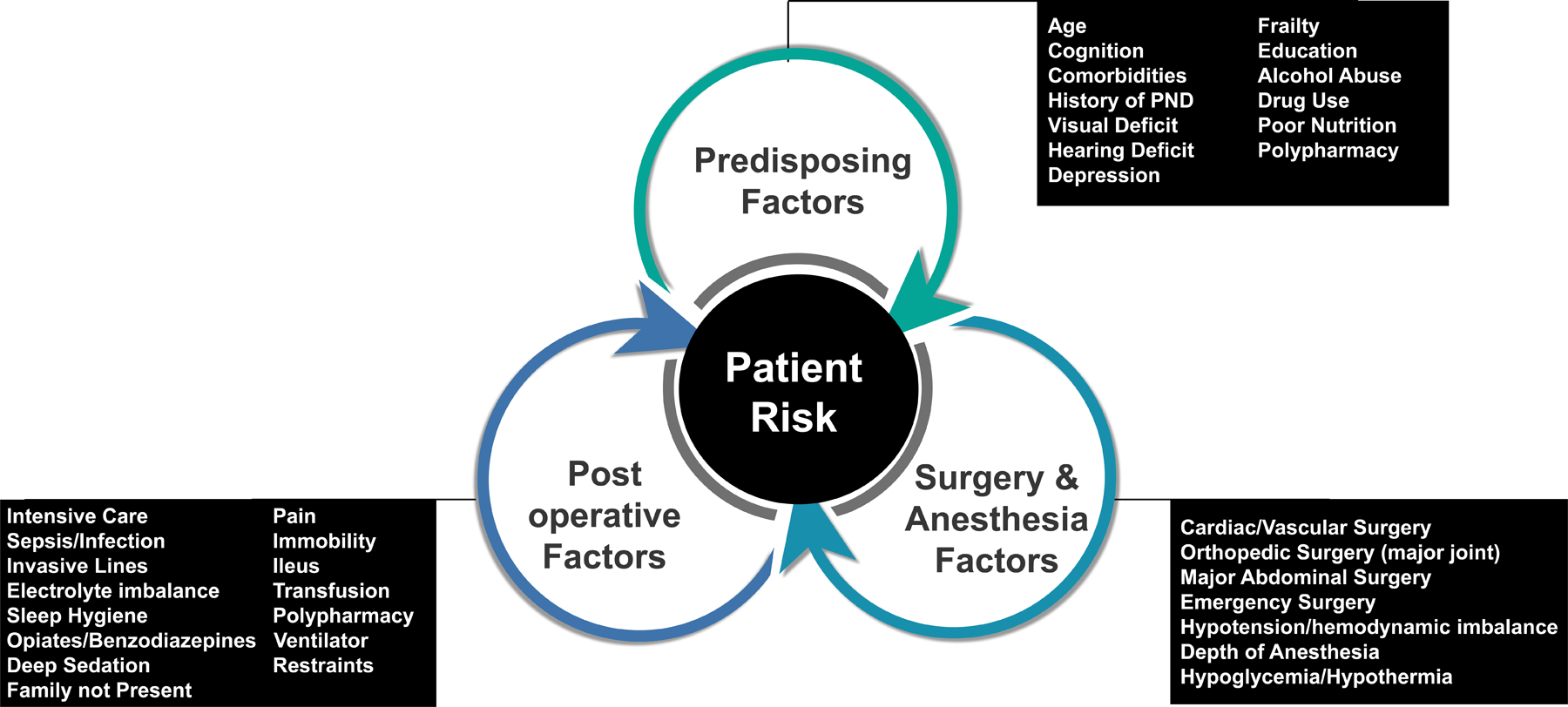
Risk Factors for Perioperative Neurocognitive Disorders. There are three types of factors to consider. 1. Predisposing Factors which encompass the baseline medical condition of the patient. 2. Surgery and Anesthesia Factors which are associated with the type of surgery and anesthetic factors. 3. Postoperative Factors include additional characteristics that occur during the course of hospitalization.
Table 1.
Risk factors for Perioperative Neurocognitive Disorders
| Modifiable Risk Factors | Non-Modifiable Risk Factors |
|---|---|
| Sleep disturbances Frailty Polypharmacy Pain Decreased vision/hearing Poorly controlled diabetes Infection Neuropsychiatric disorders Poor nutrition |
Age > 65 Preoperative cognitive impairment / AD Other Neurologic Disorders Controlled diabetes Severe vascular disease Surgery |
AD = Alzheimer’s Disease
Just as we preoperatively evaluate for cardiovascular risk, identifying risk factors for developing PND allows for implementation of risk-reducing strategies. While several risk factors for PND are non-modifiable (e.g., age) and should be part of the decision matrix for informed consent; other modifiable risk factors (e.g., frailty, polypharmacy, sleep, pain, decreased vision/hearing, diabetes, infection, neuropsychiatric conditions, and poor nutrition) can be optimized perioperatively (Table 1). It is unlikely that one isolated intervention will dramatically change the course of PND, but a comprehensive bundle of interventions based on modifiable risk factors for brain health might positively change the course of this disease.
Although preoperative neurocognitive disorders are relatively common in older adults, they are often undiagnosed and untreated.30,31 It has been reported that up to 24% of patients over 65 years of age have probable cognitive impairment when screened preoperatively.31,32 These patients are more likely to develop PND and have worse outcomes.30–34 In 2012, the American College of Surgeons and the American Geriatrics Society jointly published guidelines recommending routine preoperative neurocognitive assessments in an effort to better determine the risk of PND.30 Yet nearly a decade later, routine preoperative neurocognitive assessments have not been widely implemented.
Cognitive Screening Tools
Various neurocognitive screening tools have been developed to estimate risk and guide clinical decision.35 Screening tools like the Mini-Cog©, the Mini-Mental State Examination (MMSE) or the Montreal Cognitive Assessment (MoCA), are fast and applicable to the preoperative setting.36 For example, the Mini-Cog is a brief test (3–5 minutes) that requires no specialized personnel or technology, has minimal education/cultural/language bias and has shown to be predictive of adverse outcomes including POD.31 The MoCA and the MMSE 2ndedition™ have different examination forms that decrease practice effects in serial administration and provide further information that can be used when developing perioperative care pathways. Other brief cognitive screening tests such as the clock-drawing test and the verbal fluency test are options for very simple and quick assessments.18 Table 2 outlines commonly used screening tools that apply to the preoperative setting.
Table 2.
Examples of preoperative neurocognitive screening tools
| Test | Test time | Notes |
|---|---|---|
| Mini-Cog | 5 min | Fast to administer. High sensitivity and specificity |
| MMSE | 10 min | Widely used. Tests time and spatial orientation, language ability, instant memory, delayed recall, attention, calculation, visual spatial ability, and executive function. Low sensitivity and ceiling effect when this scale is used to detect impairment of a single cognitive domain and mild changes. Possible remote administration. |
| MMSE 2nd Edition | 20 min | Alternative to MMSE. More sensitive to subcortical dementia and to changes associated with aging. No ceiling effect. Different forms that decrease practice effects in serial administration. |
| MoCA | 15 min | High test/retest reliability. Tests cognitive function from visuospatial, executive function, naming, memory, attention, language, abstraction, and orientation. Different forms that decrease practice effects in serial administration. Possible remote administration. |
| NIH Toolbox- cognitive battery | 31 min | Simple and easy to implement. The battery consists of tests to assess: executive function, attention, episodic memory, working memory, language and processing speed. Stability among different age groups, races, genders, and education level. Good reliability and validity. Possible remote administration. |
| Clock-drawing test | < 2 min | Very fast and easy to administer. |
| RUDAS | 5 min | Designed to minimize effect of cultural learning and language diversity |
| Verbal Fluency Test | < 5 min | Very fast to administer. Phonetic test not as sensitive as semantic test. Can be influenced by education |
MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment; RUDAS = Rowland Universal Dementia Assessment Scale; NIH = National Institutes of Health; min = minutes
More comprehensive neurocognitive assessments are sensitive and can track deterioration in several domains of cognition over time, however, they require a high degree of training, and a significant amount of time to perform which are barriers to integration into clinical practice.18 When selecting a test, one must also keep in mind that cognitive screening tools do have limitations, including education, cultural, and language bias.37–39 The international study of post-operative cognitive dysfunction28 and a recent systematic review article40 exploring the methodology of measuring PND, demonstrate the need for broadly testing several memory domains and motor skills through baseline and subsequent timed postoperative neurocognitive assessments.40
A recent consensus statement recommended a practical and pragmatic preoperative assessment, like a brief cognitive test (table 2), for all surgical patients over 65 years of age that helps triage limited resource interventions for the high-risk patient.18 The response to an abnormal preoperative cognitive test should be three-fold: 1) Referral for a comprehensive evaluation that would diagnosis and chronically manage a potential cognitive deficit, 2) The information should be shared with the patient and family as part of the informed consent and shared-decision making process, and 3) Resource-intense targeted interventions should be considered as part of a care pathway specific to this high-risk patient.
Frailty
Frailty, is commonly defined as the decline in physical and cognitive reserve leading to increased vulnerability to stressors, and is associated with adverse postoperative outcomes.41 The presence of frailty either with normal aging or in those with preexisting cognitive impairment, increases the risk for developing PND.42–44 The presence of frailty can affect performance on neurocognitive assessment tests,45,46 and frailty and cognitive screening were recommended by the American College of Surgeons and the American Geriatrics Society in their 2012 jointly published guidelines as a means to risk stratify patients preoperatively.30
Several frailty tests have been developed and described in the literature with the Fried Phenotype (or some derivation) being the most used and studied.47 Screening for frailty and cognitive impairment has become a patient safety priority recognized by the perioperative community as we come to understand that both predict adverse outcomes.48 Given that clinical practices for the perioperative community vary greatly and the frailty assessment may not be implemented in all practices, the American Society of Anesthesiologists has curated a Frailty Toolkit to provide education and resources including various frailty assessment tools such as Frailty Index, Clinical Frailty Scale, and Risk Analysis Index.49
Interventions
Ideally, the decision for surgery should be a “teachable moment” to support lifestyle changes that will improve durable cognitive health for patients. Although not normally in the domain of the pre-operative clinic, ideally patients would be educated on modifiable risk factors for which personal lifestyle changes would improve their long-term cognitive health. The extent to which these interventions would improve short term cognitive resilience to the stress of the perioperative period is speculative. Accordingly, this education intervention would take place in advance of the procedure, with enough time to allow for lifestyle interventions before surgery and continuous postoperative management strategies that involve the family, caregivers, and communities of each patient. This is a fundamental paradigm shift in the way that preoperative clinics currently function, expanding the vision beyond immediate intraoperative risk to a more holistic and integrated approach in the best interest of durable patient health. The guidelines from the World Health Organization regarding “Risk Reduction for Cognitive Decline and Dementia” provide an framework to begin this work.50 They are summarized in Table 3.
Table 3.
A summary of guidelines for risk reduction of cognitive decline and dementia by the World Health Organization.50 The recommendations most plausibly applicable to the preoperative period are listed.
| Strength of Recommendation | |
|---|---|
| 1. Physical activity | Strong |
| 2. Tobacco cessation | Strong |
| 3. Nutritional interventions | Strong |
| 4. Weight management | Conditional |
| 5. Management of hypertension | Conditional |
| 6. Management of diabetes mellitus | Conditional |
| 7. Management of dyslipidemia | Conditional |
| 8. Cognitive interventions | Conditional |
| 9. Interventions for alcohol use disorder | Conditional |
Although there is limited supporting evidence, the World Health Organization’s framework presents key strategies for a prehabilitation program. Increasing resilience through cognitive training, improved nutrition status, reduction of disease burden, strength and mobility training have all been shown to improve postoperative outcomes, including reductions in postoperative complications, POD, and cognitive decline.42,44 There is data regarding prehabilitation programs consisting of interventions targeted to physical health, nutrition, frailty, and anemia leading to a modest decrease in the incidence of delirium.51 Recent evidence offers preliminary support for the use of cognitive prehabilitation to reduce the incidence of POD.52 Incorporating the fundamentals of prehabilitation into comprehensive systems that occur across the continuum of care are even better approaches to mitigate risk (e.g., Hospital Elder Life Program) and will be discussed later in this review.
Avoiding the use of medications such as those listed in the American Geriatrics Society Beers Criteria® list is recommended.53,54 These include benzodiazepines, anticholinergics, antihistamines, antipsychotics and phenothiazines. There are scenarios where the benefit of the above medications may outweigh the risks. e.g., the patient with severe anxiety preoperatively or refractory post-operative nausea and vomiting unresponsive to medications with less risk.
Identifying sleep disturbances, such as obstructive sleep apnea, present the opportunity for interventions such as continuous positive airway pressure therapy to improve the quality of sleep. A final recommendation is the involvement of a physician with expertise in geriatric medicine throughout the perioperative period.
Consent
Informed consent includes a discussion of planned procedures and their risk, benefits and alternatives. While there is some controversy over what is the standard for risk disclosure, it is clear that patients want to know common and serious risks.55 For older surgical patients, POD is the most common complication after surgery. Several expert publications endorse disclosure of the possibility of POD.17,18 However, a recent survey suggests that only about a quarter of anesthesiologists regularly provide information about the risks of delirium.56 To assure that consent for surgery is properly informed, risk of PND should be discussed with older patients and their families.57,58 There has been some controversy regarding whether the etiology of PND defines whether it should be discussed by the anesthesiologist or the surgeon. However, it remains that many complications such as mortality or myocardial infarction are multifactorial, and that each provider should consider these as part of their discussion.
The Intraoperative Period
At face value, intraoperative variables seem like rich targets for interventions to impact the course of PND. Although, a plausible hypothesis, recent data suggests that the type of anesthetic, per se, does not influence PND. A recent single-center cohort study exposed healthy adult volunteers (40 to 80 years old) with no underlying cognitive dysfunction to 2-hours of general anesthesia, without a surgical procedure.59 The primary hypothesis of the study was that time to recovery of cognitive function after general anesthesia increases with age. They did not observe an association between age and recovery to baseline, suggesting that sedative/hypnotics alone do not appear to be associated with an adverse cognitive recovery in healthy adults.59 It should be noted that the investigators studied patients with normal cognition without significant co-morbidities, and the application of these results to patient cohorts with cognitive impairment and frailty is limited.59 Moreover, in two large multicenter randomized trials involving older adults undergoing orthopedic surgery, neuraxial anesthesia was not superior to general anesthesia with respect to the incidence of POD.60,61 In the study by Neuman, et al60 it should be noted that 13.7% of patients in the spinal anesthesia group and 11.7% of patients in the general anesthesia group had pre-existing dementia. The study by Li, et al61 included approximately 40% of patients in each group with pre-existing dementia.
Given the disruptive effect of anesthetics on the brain, some have hypothesized that an anesthetic overdose, might contribute to PND. Specifically, that the use of a processed electroencephalogram (EEG) to minimize the anesthetic dose during surgery might be associated with a lower rate of postoperative neurocognitive decline. Sieber et al,62 randomized patients for hip surgery to receive a spinal anesthetic and either light or deep propofol sedation. They observed that use of light sedation decreased the incidence of post-operative delirium from 40% to 19%. A meta-analysis published in 2018,63 concluded that the use of EEG guided anesthetic depth was associated with a decrease in POD. This was followed by two large randomized clinical trials.64,65 In the ENGAGES trial, EEG-guided hypnotic administration, compared with usual care, did not decrease the incidence of POD. However, subsequently, Evered et al, demonstrated that titrating hypnotic dose to the EEG did indeed reduce the incidence of POD and improved cognitive function at one year. The prevailing hypothesis may be that there is a subset of cognitively frail patients, with preexisting cognitive impairment, who are uniquely susceptible to the adverse effects of a relative hypnotic overdose and might benefit from intraoperative EEG monitoring.66 The use of intraoperative EEG in conjunction with other strategies, such as age-adjusted minimum alveolar concentration are part of the Best Practices for Postoperative Brain Health.18
Several studies have demonstrated an association between intraoperative hypotension and an increased incidence of POD.67–70 There is limited data to support a causal relationship between intraoperative hypotension and POD.71 Maintenance of cerebral perfusion is of utmost importance in vulnerable populations.72 However, the lower limit of cerebral autoregulation varies widely between patients, and absent measuring brain perfusion, it is unknown what the ideal mean arterial pressure is in an individual patient.72 Moreover, many older adults have chronic hypertension which is known to shift the autoregulation curve to the right. The limited data cited above support a recommendation to keep the mean arterial pressure at a minimum of 65 mmHg, although a higher number may be necessary in some patients.
Other recommendations include monitoring of glucose levels and maintenance of normothermia.18 Tight glucose control has been linked to hypoglycemia leading to worse outcomes and an increased rate of POD and is no longer recommended.73,74 Avoiding even mild hypoglicemia74 may be equally as important as avoiding hyperglycemia75 when it comes to preventing PND.
The Postoperative Period
Anesthesiologists often only briefly follow-up with their patients in the immediate postoperative period.18 These conversations mainly focus on pain and antiemetic strategies. New strategies must be adopted in our approach to cognitive recovery, one which engages multimodal tactics, including the return of visual and hearing aids, early mobilization, and resumption of a normal diet. Polysomnographic studies have revealed extreme sleep disruption during hospital stays.76–79 Maintenance of sleep friendly hospital environments (noise reduction, appropriate light), and a decrease of iatrogenic factors such as frequent care-related interruptions was shown to improve outcomes and decrease the incidence of delirium. This is particularly important for the older patient for whom the restorative properties of natural sleep are another key part of their recovery. Importantly, family engagement and social support should be implemented early in the preoperative period and deployed in tandem with the involvement of an interdisciplinary health care team and incorporated in age friendly comprehensive systems of care.
Comprehensive systems of care that implement targeted, structured, multicomponent nonpharmacological approaches are even better approaches to mitigate risk. The Hospital Elder Life Program (HELP™) is an evidence-based intervention that supports this approach.80 One example of the HELP program instituted three universal protocols (orientation, cognitive stimulation and mobilization) as well as targeted protocols dependent on specific risk factors for each patient. In patients over 70 years of age, POD decreased from 19.4% in the control group to 2.6% in the intervention group. Moreover, physical and cognitive function was improved in the intervention group as compared to the control group at 30 days after discharge.81 In a recent study, a novel non-pharmacological, multidisciplinary prevention program was applied to orthopedic, general, or cardiac surgery older patients. Risk factors for delirium and symptoms of delirium were assessed daily with interventions targeted to individual patient needs. Interventions included cognitive, motor, and sensory stimulation, meal companionship, stress relaxation and sleep promotion. Delirium incidence and duration were reduced in the intervention group.82
To paraphrase an old adage “if it isn’t measured it can’t be improved” i.e., if you don’t assess the cognitive state of the patient, the physician has little hope of improving cognitive recovery after anesthesia. For POD, an accepted standard is the Confusion Assessment Method which is simple to use and requires only a few minutes to administer. Often, the first signs of delayed neurocognitive recovery and postoperative neurocognitive disorder are generally clinical signs such as memory impairment and inability to concentrate that may trigger a more sophisticated level of neuropsychometric testing.
A suggested care pathway is outlined in Figure 2. It should be noted that this represents the authors synthesis of current evidence and should be considered a starting point for clinicians to modify to local needs.
Figure 2.
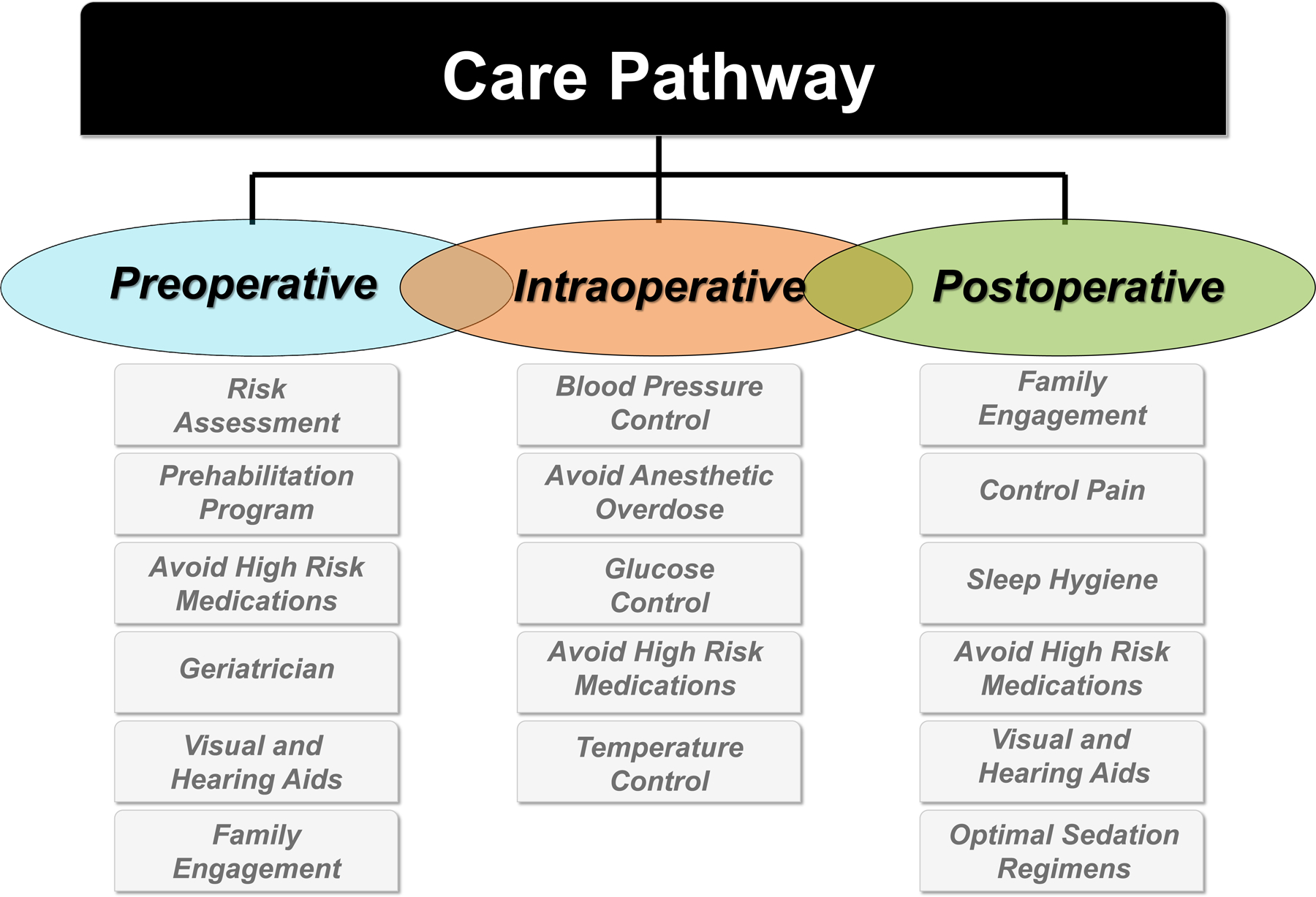
A suggested template to consider when developing a perioperative care pathway for the older adult at risk for Perioperative Neurocognitive Disorders.
Future Directions and Emerging Issues
Much work remains to be accomplished to mature the science that inform best practices regarding PND. Emerging issues that we will highlight include the use of new technologies to define risk and provide decision support, the interaction of sleep and cognition, the cognitive super-ager, and the surgical burden of patients with mild cognitive impairment/Alzheimer’s disease (AD).
While current forms of cognitive screening prior to surgical care are useful for perioperative interventions, new technologies, such as natural language processing and automated speech analysis83–85 as well as gait evaluation technology,86 offer low-cost and pragmatic approaches to risk stratification. One of the advantages of speech analysis is that, by using voice-recognition software, risk prediction can be done virtually.
Current pragmatic and clinically-implementable assessments have moderate accuracy for risk predicition,87,88 and by-in-large utilize hierarchical logistic regression models.89 One technology with promise to advance the identification and treatment of PND is artificial intelligence (AI) and more specifically machine learning (ML). Machine learning is a subset of AI which uses big data and algorithms that imitate the human brain in a way to process large amounts of data to address complex problems with specific applications to define risk, and provide diagnostic and treatment solutions in an efficient manner. Recently, POD prediction models that use ML and AI technologies have been published.90–92 One such model92 used 115 predictive features from electronic health record data with encouraging results as defined by the area under the receiver operating characteristic curve.
Cognitive testing and web-based products that improve long term cognitive health through coaching experiences for the population at large are in development.93 Specific to the perioperative period tools have been developed with the goal of delivering end-to-end digital health platforms that are accessible to the perioperative care team. These tools94–97 introduce in-depth neuropsychological tests in a time efficient manner with minimal barriers to implementation and are reported to be eligible for reimbursement by payors.
Sleep and Cognitive Health
Sleep and its coupling to circadian rhythms are integral to brain health (e.g., memory consolidation, metabolic waste clearance) and other systemic functions.98–101 During normal aging, the duration of total sleep and slow-wave sleep is decreased and is associated with an increase in sleep fragmentation and difficulty of falling asleep.102 The prevalence of sleep disturbances at home, can affect up to 40% of older adults and is associated with impairment of spatial memory, verbal fluency, attention, processing speed, and executive function.101,103 The presence of sleep disturbance before hospital admission is an independent risk factor for PND. Environmental and patient factors in the postoperative hospital setting (pain, light, environmental changes, monitoring systems, constant interruption) can contribute to the impairment of waste clearance, especially for older patients and could contribute to PND.78 Thus, the prevalence of sleep disturbance in the older patient coupled with surgery and hospitalization may result in a nexus of precipitating factors in a patient vulnerable to PND.
The glial-lymphatic (glymphatic) pathway (see Figure 3), a recently theorized cerebral system, facilitates the homeostasis of various brain functions.104–106 Like the peripheral lymphatic system, it clears and empties protein waste products, and is most active during sleep. Further investigation is needed to determine whether perioperative sleep disturbances alter the glymphatic system and if perioperative medications/anesthetics aid or impede glymphatic function.105
Figure 3.
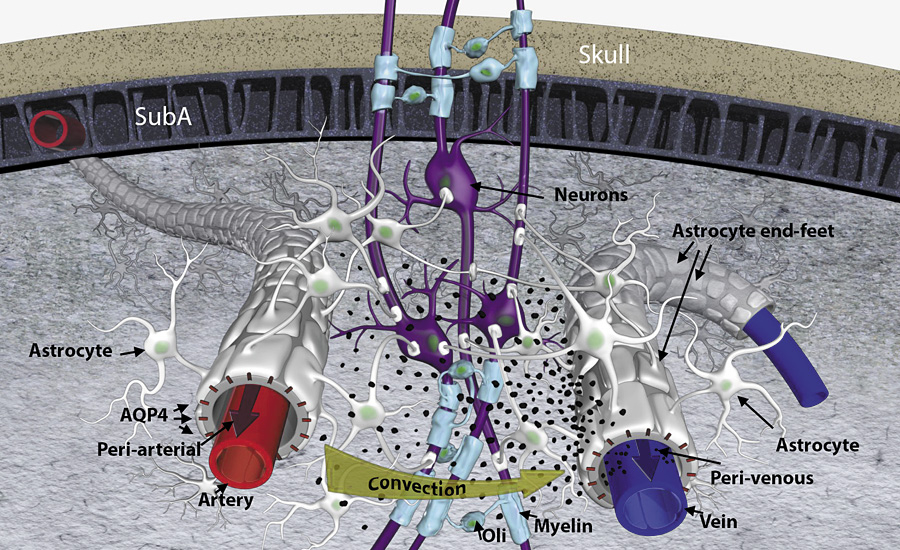
Three-dimensional representation of lymphatic transport and drainage concept highlighting the periarterial and the perivenous space, and the astrocytic end-feet with aquaporin 4 (AQP4) water channels and forming a sheath around the blood vessels. Cerebrospinal fluid is driven by convection through the periarterial space and is propelled across the astroglia end-feet to mix with interstitial fluid and waste products. From there, the waste and excess fluids are driven towards the perivenous space to ultimately be directed towards the lymphatic vessels and general circulation for breakdown and clearance. The black particles represent “waste” particles in the interstitial fluid (e.g., amyloid beta). SubA = subarachnoid space; Oli = oligodendrocyte. Reproduced with permission.106
Anesthesia and surgery alter the circadian rhythm of sleep and delay secretion of melatonin that helps regulate sleep.107 Anesthetic and sedative agents have variable action targets. Those which act by modulating the GABAA receptor converge at the level of the hypothalamus while α2 adrenergic agonists, such as dexmedetomidine, converge on sleep pathways within the brainstem, thus behaving more like natural sleep.108 Several clinical studies reported that the use of dexmedetomidine for sedation in the intensive care unit is associated with reductions in delirium, and improved outcomes when compared to benzodiazepines and/or opioid-based sedation strategies.109–112 Brain waste clearance via the glymphatic system is more efficient during slow-wave sleep,113 which can be mimicked with drugs blocking central adrenergic transmission. Although highly speculative, one hypothesis would suggest that dexmedetomidine’s delirium-preventive effect is related to superior brain waste clearance during comfort sleep when compared to other hypnotic regimens in the intensive care unit setting.105,114 Preclinical studies demonstrated that dexmedetomidine, by blocking norepinephrine release from the locus coeruleus, enhanced glymphatic system transport by 30% relative to other agents.114 Future studies are needed to further elucidate the benefits of dexmedetomidine, among other agents, as good hypnotic/sedative candidates that mirror the restorative functions of sleep.78,115–118
Much can be learned from both ends of a wide spectrum of cognitive function of older patients to identify the complex interplay of factors when older adults present for surgery. On one end, recent studies have shown that there is a subset of patients, the “cognitive super-agers”, who defy the typical age-related decline in cognitive function. Do these patients have enhanced resilience to the stresses of the perioperative period? If so, are there variables that define resilience in these patients that may provide a foundation for interventions in the cohort of patients that are vulnerable to PND? Individuals who have reached extreme age with preserved cognitive health may present an opportunity to investigate factors that promote optimal cognitive function after surgery.119
At the other end of the spectrum, is AD. According to the Alzheimer’s Association over six million Americans live with AD, and that number is projected to grow to 12.7 million people by the year 2050.120 Such numbers are daunting and present a number of perioperative challenges.121 Even more concerning is a likely large number of patients with undiagnosed mild cognitive impairment on the precipice of a steep decline in cognition. Does surgery and anesthesia accelerate cognitive deterioration in this vulnerable cohort of patients? Unfortunately, the delta between what we know, and a mature set of knowledge is substantial.
Population studies have demonstrated a bidirectional association between AD and PND.122–125 Preexisting cognitive impairment is associated with PND,34 and in patients with a high degree of AD-related cortical atrophy, POD is associated with an accelerated rate of long-term cognitive decline.123–126 How perioperative management affects the acceleration of cognitive decline is unknown.127 It has been shown that the apolipoprotein E4 gene was associated with an increased risk for early POD after controlling for known demographic and clinical risk factors.128 Nonetheless, the evidence of the effect of surgery and anesthesia on amyloid deposition is conflicting. Sprung et al, did not find evidence of amyloid deposition after exposure to surgery and anesthesia.129 Klinger et al found that amyloid deposition at six weeks in a surgical cohort was not different from that in AD patients not having surgery, but increased after one year at a rate greater than controls.130 Avidan et al found that the rate of cognitive decline in patients with pre-existing dementia was not affected by the occurrence of a surgical event.131 A recent cohort study also provided some reassurance that the neurocognitive changes identified postoperatively are unlikely to accelerate amyloid or tau related neuropathological processes associated with AD.132
Future studies on these two ends of the patient spectrum (cognitive super-ager and AD) could provide important information on the resilient brain, presenting an opportunity to prioritize perioperative interventions and improve the quality and quantity of life in older patient populations.
Summary
A half a century ago, surgery in older adults was confined to “unequivocally necessary cases”. Today, advances in surgery and anesthesia offer many options meant to improve safety, function, and quality of life. Though not proven causative, it appears that perioperative stresses can jeopardize brain health in vulnerable patients such as the older adult. The associated risks should be evaluated and reviewed within the patient/family-physician partnership in order to develop a perioperative care pathway for those most likely to suffer from PND.
Anesthesiologists, can directly impact this troublesome and growing issue, by coordinating perioperative care, partnering with surgical and other perioperative teams to integrate treatments into a postoperative discharge plan that extends beyond the hospitalization period.133 We must make every effort to safeguard postoperative brain recovery for all our patients.
Funding:
Supported by the National Institutes of Health, National Institute of General Medical Sciences under award K23GM132795 and National Institute of Aging under award R21AG070269 to Dr. Vacas.
Glossary of Terms
- PND
Perioperative Neurocognitive Disorders
- POD
postoperative delirium
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- EEG
electroencephalogram
- HELP
Hospital Elder Life Program
- AD
Alzheimer’s disease
- AI
artificial intelligence
- ML
machine learning
Footnotes
Conflicts of Interest: Stacey Deiner has served as a consultant for Merck and Covidien.
Disclosures:
Daniel J Cole is the current President of the Anesthesia Patient Safety Foundation, is a Past President and current Board Member of the American Society of Anesthesiologists, and Board Member of the American Board of Medical Specialties.
Stacie G. Deiner currently serves on the Board of Directors for the American Board of Anesthesiologists. She has served as an expert witness for legal proceedings
References
- 1.Organization WH. Patient Safety Accessed December 12th 2021, https://www.who.int/news-room/fact-sheets/detail/patient-safety
- 2.Fleisher LA. Brain Health Initiative: A New ASA Patient Safety Initiative. ASA Monitor 2016;80(6):10–11. [Google Scholar]
- 3.Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. United States Census Bureau: The population 65 years and older in the United States. American Community Survey Reports 2018;
- 4.Living AfC. Administration for Community Living 2020 Profile of Older Americans Accessed December 26 2021, https://acl.gov/sites/default/files/Profile%20of%20OA/2020ProfileOlderAmericans_RevisedFinal.pdf
- 5.Centers for Disease Control and Prevention - National Hospital Discharge Survey, 2010 - Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States Accessed February 2020,
- 6.Bureau UC. U.S. Census Bureau Releases 2019 Population Estimates by Demographic Characteristics Accessed April 4, 2021. https://www.census.gov/newsroom/press-releases/2020/65-older-population-grows.html
- 7.Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory Surgery Data From Hospitals and Ambulatory Surgery Centers: United States, 2010. Vol. 102. 2017. National Health Statistics Report February 28, 2017. Accessed April 4, 2021. https://www.cdc.gov/nchs/data/nhsr/nhsr102.pdf [PubMed] [Google Scholar]
- 8.Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Annals of internal medicine Apr 17 2001;134(8):637–43. doi: 10.7326/0003-4819-134-8-200104170-00008 [DOI] [PubMed] [Google Scholar]
- 9.Anesthesiologists ASo. Tools for patients and their families to minimize side effects from anesthesia during surgery Accessed February 10th 2022, 2022. https://www.asahq.org/brainhealthinitiative/toolsforpatients
- 10.Health GCoB. Preserving Your Brain Health During Illness or Surgery: GCBH Recommendations to Prevent and Treat Delirium 2020.
- 11.Evered L, Silbert B, Knopman DS, et al. Recommendations for the Nomenclature of Cognitive Change Associated With Anaesthesia and Surgery-2018. Anesthesia Analgesia Nov 2018;127(5):1189–1195. doi: 10.1213/ane.0000000000003634 [DOI] [PubMed] [Google Scholar]
- 12.Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation Jan 20 2009;119(2):229–36. doi: 10.1161/circulationaha.108.795260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Archives of internal medicine Jan 14 2008;168(1):27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyer CB, Ashton CM, Teasdale TA. Postoperative delirium. A review of 80 primary data-collection studies. Archives of internal medicine Mar 13 1995;155(5):461–5. [DOI] [PubMed] [Google Scholar]
- 15.Leslie DL, Inouye SK. The importance of delirium: economic and societal costs. Journal of the American Geriatrics Society Nov 2011;59 Suppl 2:S241–3. doi: 10.1111/j.1532-5415.2011.03671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohanty S, Rosenthal RA, Russell MM, Neuman MD, Ko CY, Esnaola NF. Optimal perioperative management of the geriatric patient: A best practices guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. J Am Coll Surg May 2016;222(5):930–47. doi: 10.1016/j.jamcollsurg.2015.12.026 [DOI] [PubMed] [Google Scholar]
- 17.Chow WB, Rosenthal RA, Merkow RP, Ko CY, Esnaola NF. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg Oct 2012;215(4):453–66. doi: 10.1016/j.jamcollsurg.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 18.Berger M, Schenning KJ, Brown CHt, et al. Best practices for postoperative brain health: Recommendations from the fifth International Perioperative Neurotoxicity Working Group. Anesthesia and analgesia Dec 2018;127(6):1406–1413. doi: 10.1213/ane.0000000000003841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis D, Searle SD, Tsui A. The Scottish Intercollegiate Guidelines Network: risk reduction and management of delirium. Age Ageing Jul 1 2019;48(4):485–488. doi: 10.1093/ageing/afz036 [DOI] [PubMed] [Google Scholar]
- 20.Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. European journal of anaesthesiology Apr 2017;34(4):192–214. doi: 10.1097/eja.0000000000000594 [DOI] [PubMed] [Google Scholar]
- 21.Peden CJ, Miller TR, Deiner SG, Eckenhoff RG, Fleisher LA. Improving perioperative brain health: an expert consensus review of key actions for the perioperative care team. British journal of anaesthesia Feb 2021;126(2):423–432. doi: 10.1016/j.bja.2020.10.037 [DOI] [PubMed] [Google Scholar]
- 22.Mahanna-Gabrielli E, Schenning KJ, Eriksson LI, et al. State of the clinical science of perioperative brain health: report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. British journal of anaesthesia Oct 2019;123(4):464–478. doi: 10.1016/j.bja.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris JR, Korolchuk VI. Biochemistry and cell biology of ageing. Part II, Clinical science / Harris J. Robin, Korolchuk Viktor I., editors. Subcellular Biochemistry, volume 91. Springer; 2019. [Google Scholar]
- 24.Vacas S, Degos V, Feng X, Maze M. The neuroinflammatory response of postoperative cognitive decline. British medical bulletin 2013;106:161–78. doi: 10.1093/bmb/ldt006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel A, Zhang M, Liao G, et al. A Systematic Review and Meta-Analysis Examining the Impact of Age on Perioperative Inflammatory Biomarkers. Anesthesia and analgesia Dec 28 2021;doi: 10.1213/ane.0000000000005832 [DOI] [PubMed]
- 26.Chen H-L, Lu C-H, Lin H-C, et al. White Matter Damage and Systemic Inflammation in Obstructive Sleep Apnea. Sleep 2015;38(3):361–370. doi: 10.5665/sleep.4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng X, Degos V, Koch LG, et al. Surgery Results in Exaggerated and Persistent Cognitive Decline in a Rat Model of the Metabolic Syndrome. Anesthesiology May 2013;118(5):1098–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet Mar 21 1998;351(9106):857–61. doi:S0140673697073820 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Degos V, Vacas S, Han Z, et al. Depletion of Bone Marrow-derived Macrophages Perturbs the Innate Immune Response to Surgery and Reduces Postoperative Memory Dysfunction. Anesthesiology Mar 2013;118(3):527–36. doi: 10.1097/ALN.0b013e3182834d94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chow WB, Rosenthal RA, Merkow RP, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg Oct 2012;215(4):453–66. doi: 10.1016/j.jamcollsurg.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 31.Culley DJ, Flaherty D, Fahey MC, et al. Poor Performance on a Preoperative Cognitive Screening Test Predicts Postoperative Complications in Older Orthopedic Surgical Patients. Anesthesiology Nov 2017;127(5):765–774. doi: 10.1097/aln.0000000000001859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Culley DJ, Flaherty D, Reddy S, et al. Preoperative Cognitive Stratification of Older Elective Surgical Patients: A Cross-Sectional Study. Anesthesia and analgesia Jul 2016;123(1):186–92. doi: 10.1213/ane.0000000000001277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapoor P, Chen L, Saripella A, et al. Prevalence of preoperative cognitive impairment in older surgical patients.: A systematic review and meta-analysis. J Clin Anesth Nov 5 2021;76:110574. doi: 10.1016/j.jclinane.2021.110574 [DOI] [PubMed] [Google Scholar]
- 34.Silbert B, Evered L, Scott DA, et al. Preexisting cognitive impairment is associated with postoperative cognitive dysfunction after hip joint replacement surgery. Anesthesiology Jun 2015;122(6):1224–34. doi: 10.1097/aln.0000000000000671 [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Huang K, Zhu B, et al. Neuropsychological Tests in Post-operative Cognitive Dysfunction: Methods and Applications. Front Psychol 2021;12:684307. doi: 10.3389/fpsyg.2021.684307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheehan B Assessment scales in dementia. Ther Adv Neurol Disord Nov 2012;5(6):349–58. doi: 10.1177/1756285612455733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gagnon G, Hansen KT, Woolmore-Goodwin S, et al. Correcting the MoCA for education: effect on sensitivity. Can J Neurol Sci Sep 2013;40(5):678–83. doi: 10.1017/s0317167100014918 [DOI] [PubMed] [Google Scholar]
- 38.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society Apr 2005;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 39.Naqvi RM, Haider S, Tomlinson G, Alibhai S. Cognitive assessments in multicultural populations using the Rowland Universal Dementia Assessment Scale: a systematic review and meta-analysis. CMAJ Mar 17 2015;187(5):E169–75. doi: 10.1503/cmaj.140802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borchers F, Spies CD, Feinkohl I, et al. Methodology of measuring postoperative cognitive dysfunction: a systematic review. Br J Anaesth Apr 2 2021;doi: 10.1016/j.bja.2021.01.035 [DOI] [PubMed]
- 41.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med Feb 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Humeidan ML, Reyes JC, Mavarez-Martinez A, et al. Effect of Cognitive Prehabilitation on the Incidence of Postoperative Delirium Among Older Adults Undergoing Major Noncardiac Surgery: The Neurobics Randomized Clinical Trial. JAMA surgery Feb 1 2021;156(2):148–156. doi: 10.1001/jamasurg.2020.4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milder DA, Pillinger NL, Kam PCA. The role of prehabilitation in frail surgical patients: A systematic review. Acta Anaesthesiol Scand Nov 2018;62(10):1356–1366. doi: 10.1111/aas.13239 [DOI] [PubMed] [Google Scholar]
- 44.Whittle J, Wischmeyer PE, Grocott MPW, Miller TE. Surgical Prehabilitation: Nutrition and Exercise. Anesthesiol Clin Dec 2018;36(4):567–580. doi: 10.1016/j.anclin.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 45.Rosado-Artalejo C, Carnicero JA, Losa-Reyna J, et al. Cognitive Performance across 3 Frailty Phenotypes: Toledo Study for Healthy Aging. J Am Med Dir Assoc Sep 1 2017;18(9):785–790. doi: 10.1016/j.jamda.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 46.Canevelli M, Cesari M, Raganato R, et al. Role of frailty in the assessment of cognitive functioning. Mech Ageing Dev Jul 2019;181:42–46. doi: 10.1016/j.mad.2019.111122 [DOI] [PubMed] [Google Scholar]
- 47.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci Mar 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 48.Susano MJ, Grasfield RH, Friese M, et al. Brief Preoperative Screening for Frailty and Cognitive Impairment Predicts Delirium after Spine Surgery. Anesthesiology Dec 1 2020;133(6):1184–1191. doi: 10.1097/ALN.0000000000003523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anesthesia ASfACoG. Frailty for Anesthesiologists. 2021 June 2021.
- 50.Organization WH, ed. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines 2019. Accessed February 2021. https://www.ncbi.nlm.nih.gov/books/NBK542796/ [PubMed]
- 51.Janssen TL, Steyerberg EW, Langenberg JCM, et al. Multimodal prehabilitation to reduce the incidence of delirium and other adverse events in elderly patients undergoing elective major abdominal surgery: An uncontrolled before-and-after study. PloS one 2019;14(6):e0218152. doi: 10.1371/journal.pone.0218152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Humeidan ML, Reyes JC, Mavarez-Martinez A, et al. Effect of Cognitive Prehabilitation on the Incidence of Postoperative Delirium Among Older Adults Undergoing Major Noncardiac Surgery: The Neurobics Randomized Clinical Trial. JAMA Surg Nov 11 2020;doi: 10.1001/jamasurg.2020.4371 [DOI] [PMC free article] [PubMed]
- 53.American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society Apr 2019;67(4):674–694. doi: 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 54.Panel BtAGSBCUE. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society 2015;63(11):2227–2246. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 55.Burkle CM, Pasternak JJ, Armstrong MH, Keegan MT. Patient perspectives on informed consent for anaesthesia and surgery: American attitudes. Acta Anaesthesiol Scand Mar 2013;57(3):342–9. doi: 10.1111/aas.12037 [DOI] [PubMed] [Google Scholar]
- 56.Deiner S, Fleisher LA, Leung JM, Peden C, Miller T, Neuman MD. Adherence to recommended practices for perioperative anesthesia care for older adults among US anesthesiologists: results from the ASA Committee on Geriatric Anesthesia-Perioperative Brain Health Initiative ASA member survey. Perioper Med (Lond) 2020;9:6. doi: 10.1186/s13741-020-0136-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hogan KJ, Bratzke LC, Hogan KL. Informed Consent and Cognitive Dysfunction After Noncardiac Surgery in the Elderly. Anesthesia and analgesia Feb 2018;126(2):629–631. doi: 10.1213/ane.0000000000002689 [DOI] [PubMed] [Google Scholar]
- 58.Hogan KL, Schenning KJ, Hogan KJ. Trouble in Mind: Healthcare Informed Consent, Surgery, Anesthesia, and the Aging Brain. J Leg Med Apr-Jun 2018;38(2):221–270. doi: 10.1080/01947648.2018.1473184 [DOI] [PubMed] [Google Scholar]
- 59.Baxter MG, Mincer JS, Brallier JW, et al. Cognitive Recovery by Decade in Healthy 40- to 80-Year-Old Volunteers After Anesthesia Without Surgery. Anesthesia and analgesia Dec 10 2021;doi: 10.1213/ane.0000000000005824 [DOI] [PMC free article] [PubMed]
- 60.Neuman MD, Feng R, Carson JL, et al. Spinal Anesthesia or General Anesthesia for Hip Surgery in Older Adults. The New England journal of medicine Nov 25 2021;385(22):2025–2035. doi: 10.1056/NEJMoa2113514 [DOI] [PubMed] [Google Scholar]
- 61.Li T, Li J, Yuan L, et al. Effect of Regional vs General Anesthesia on Incidence of Postoperative Delirium in Older Patients Undergoing Hip Fracture Surgery: The RAGA Randomized Trial. Jama 2021;doi: 10.1001/jama.2021.22647 [DOI] [PMC free article] [PubMed]
- 62.Sieber FE, Zakriya KJ, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc 2010;85:18–26. doi: 10.4065/mcp.2009.0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacKenzie KK, Britt-Spells AM, Sands LP, Leung JM. Processed Electroencephalogram Monitoring and Postoperative Delirium: A Systematic Review and Meta-analysis. Anesthesiology Sep 2018;129(3):417–427. doi: 10.1097/aln.0000000000002323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evered LA, Chan MTV, Han R, et al. Anaesthetic depth and delirium after major surgery: a randomised clinical trial. British journal of anaesthesia Nov 2021;127(5):704–712. doi: 10.1016/j.bja.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wildes TS, Mickle AM, Ben Abdallah A, et al. Effect of Electroencephalography-Guided Anesthetic Administration on Postoperative Delirium Among Older Adults Undergoing Major Surgery: The ENGAGES Randomized Clinical Trial. Jama Feb 5 2019;321(5):473–483. doi: 10.1001/jama.2018.22005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vacas S, Hudson AE. Seen and Ignored: Are We Undermining Studies of Brain Health Interventions Before We Start? Anesthesia and analgesia Aug 2020;131(2):464–465. doi: 10.1213/ane.0000000000004367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirsch J, DePalma G, Tsai TT, Sands LP, Leung JM. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non-cardiac surgery. British journal of anaesthesia Sep 2015;115(3):418–26. doi: 10.1093/bja/aeu458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang NY, Hirao A, Sieber F. Association between intraoperative blood pressure and postoperative delirium in elderly hip fracture patients. PloS one 2015;10(4):e0123892. doi: 10.1371/journal.pone.0123892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siepe M, Pfeiffer T, Gieringer A, et al. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery Jul 2011;40(1):200–7. doi: 10.1016/j.ejcts.2010.11.024 [DOI] [PubMed] [Google Scholar]
- 70.Sessler DI, Sigl JC, Kelley SD, et al. Hospital stay and mortality are increased in patients having a `Triple Low’ of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia. Anesthesiology 2012;116doi: 10.1097/ALN.0b013e31825683dc [DOI] [PubMed]
- 71.Brown CHt Neufeld KJ, Tian J, et al. Effect of Targeting Mean Arterial Pressure During Cardiopulmonary Bypass by Monitoring Cerebral Autoregulation on Postsurgical Delirium Among Older Patients: A Nested Randomized Clinical Trial. JAMA Surg Sep 1 2019;154(9):819–826. doi: 10.1001/jamasurg.2019.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vacas S, Cannesson M. Noninvasive Monitoring and Potential for Patient Outcome. Journal of cardiothoracic and vascular anesthesia Aug 2019;33 Suppl 1(Suppl 1):S76–s83. doi: 10.1053/j.jvca.2019.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Finfer S, Liu B, Chittock DR, et al. Hypoglycemia and risk of death in critically ill patients. The New England journal of medicine Sep 20 2012;367(12):1108–18. doi: 10.1056/NEJMoa1204942 [DOI] [PubMed] [Google Scholar]
- 74.Saager L, Duncan AE, Yared JP, et al. Intraoperative tight glucose control using hyperinsulinemic normoglycemia increases delirium after cardiac surgery. Anesthesiology Jun 2015;122(6):1214–23. doi: 10.1097/aln.0000000000000669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ganai S, Lee KF, Merrill A, et al. Adverse outcomes of geriatric patients undergoing abdominal surgery who are at high risk for delirium. Arch Surg Nov 2007;142(11):1072–8. doi: 10.1001/archsurg.142.11.1072 [DOI] [PubMed] [Google Scholar]
- 76.Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed) Apr 6 1985;290(6474):1029–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hilton BA. Quantity and quality of patients’ sleep and sleep-disturbing factors in a respiratory intensive care unit. J Adv Nurs Nov 1976;1(6):453–68. [DOI] [PubMed] [Google Scholar]
- 78.Vacas S, Kurien P, Maze M. Sleep and anesthesia: Common mechanisms of action. Sleep Medicine Clinics 2013;8(1):1–9. doi: 10.1016/j.jsmc.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vacas S, McInrue E, Gropper MA, et al. The Feasibility and Utility of Continuous Sleep Monitoring in Critically Ill Patients Using a Portable Electroencephalography Monitor. Anesthesia and analgesia Jul 2016;123(1):206–12. doi: 10.1213/ane.0000000000001330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: Systematic review and meta-analysis of effectiveness. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry Oct 2018;26(10):1015–1033. doi: 10.1016/j.jagp.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang YY, Yue JR, Xie DM, et al. Effect of the tailored, family-involved Hospital Elder Life Program on postoperative delirium and function in older adults: A randomized clinical trial. JAMA Intern Med Oct 21 2019;doi: 10.1001/jamainternmed.2019.4446 [DOI] [PMC free article] [PubMed]
- 82.Deeken F, Sánchez A, Rapp MA, et al. Outcomes of a Delirium Prevention Program in Older Persons After Elective Surgery: A Stepped-Wedge Cluster Randomized Clinical Trial. JAMA Surg Dec 15 2021:e216370. doi: 10.1001/jamasurg.2021.6370 [DOI] [PMC free article] [PubMed]
- 83.Dickerson LK, Rouhizadeh M, Korotkaya Y, et al. Language impairment in adults with end-stage liver disease: application of natural language processing towards patient-generated health records. NPJ Digit Med 2019;2:106. doi: 10.1038/s41746-019-0179-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yeung A, Iaboni A, Rochon E, et al. Correlating natural language processing and automated speech analysis with clinician assessment to quantify speech-language changes in mild cognitive impairment and Alzheimer’s dementia. Alzheimers Res Ther Jun 4 2021;13(1):109. doi: 10.1186/s13195-021-00848-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ntracha A, Iakovakis D, Hadjidimitriou S, Charisis VS, Tsolaki M, Hadjileontiadis LJ. Detection of Mild Cognitive Impairment Through Natural Language and Touchscreen Typing Processing. Front Digit Health 2020;2:567158. doi: 10.3389/fdgth.2020.567158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu X, Zhao C, Zheng B, et al. Wearable Devices for Gait Analysis in Intelligent Healthcare. Mini Review. Frontiers in Computer Science 2021-May-13 2021;3.doi: 10.3389/fcomp.2021.661676 [DOI]
- 87.Jones RN, Tommet D, Steingrimsson J, et al. Development and internal validation of a predictive model of cognitive decline 36 months following elective surgery. Alzheimers Dement (Amst) 2021;13(1):e12201. doi: 10.1002/dad2.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whitlock EL, Braehler MR, Kaplan JA, et al. Derivation, Validation, Sustained Performance, and Clinical Impact of an Electronic Medical Record-Based Perioperative Delirium Risk Stratification Tool. Anesthesia and analgesia Dec 2020;131(6):1901–1910. doi: 10.1213/ane.0000000000005085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berian JR, Zhou L, Russell MM, et al. Postoperative Delirium as a Target for Surgical Quality Improvement. Ann Surg Jul 2018;268(1):93–99. doi: 10.1097/sla.0000000000002436 [DOI] [PubMed] [Google Scholar]
- 90.Racine AM, Tommet D, D’Aquila ML, et al. Machine Learning to Develop and Internally Validate a Predictive Model for Post-operative Delirium in a Prospective, Observational Clinical Cohort Study of Older Surgical Patients. J Gen Intern Med Feb 2021;36(2):265–273. doi: 10.1007/s11606-020-06238-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu XY, Liu H, Zhao X, et al. Automated machine learning-based model predicts postoperative delirium using readily extractable perioperative collected electronic data. CNS Neurosci Ther Nov 18 2021;doi: 10.1111/cns.13758 [DOI] [PMC free article] [PubMed]
- 92.Bishara A, Chiu C, Whitlock EL, et al. Postoperative delirium prediction using machine learning models and preoperative electronic health record data. BMC Anesthesiol Jan 3 2022;22(1):8. doi: 10.1186/s12871-021-01543-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gray M, Madero EN, Gills JL, et al. Intervention for a Digital, Cognitive, Multi-Domain Alzheimer Risk Velocity Study: Protocol for a Randomized Controlled Trial. JMIR Res Protoc Feb 4 2022;11(2):e31841. doi: 10.2196/31841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology Mar 12 2013;80(11 Suppl 3):S2–6. doi: 10.1212/WNL.0b013e3182872e5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hodes RJ, Insel TR, Landis SC. The NIH toolbox: setting a standard for biomedical research. Neurology Mar 12 2013;80(11 Suppl 3):S1. doi: 10.1212/WNL.0b013e3182872e90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang S, Flores B, Magal R, et al. Diagnostic accuracy of tablet-based software for the detection of concussion. PloS one 2017;12(7):e0179352. doi: 10.1371/journal.pone.0179352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Groppell S, Soto-Ruiz KM, Flores B, et al. A Rapid, Mobile Neurocognitive Screening Test to Aid in Identifying Cognitive Impairment and Dementia (BrainCheck): Cohort Study. JMIR Aging Mar 21 2019;2(1):e12615. doi: 10.2196/12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Misra S, Malow BA. Evaluation of sleep disturbances in older adults. Clin Geriatr Med Feb 2008;24(1):15–26, v. doi:S0749–0690(07)00076–6 [pii] 10.1016/j.cger.2007.08.011 [DOI] [PubMed] [Google Scholar]
- 99.Walker MP. Cognitive consequences of sleep and sleep loss. Sleep medicine Sep 2008;9 Suppl 1:S29–34. doi:S1389–9457(08)70014–5 [pii] 10.1016/S1389-9457(08)70014-5 [DOI] [PubMed] [Google Scholar]
- 100.Eddleston JM, White P, Guthrie E. Survival, morbidity, and quality of life after discharge from intensive care. Critical care medicine Jul 2000;28(7):2293–9. [DOI] [PubMed] [Google Scholar]
- 101.Wang X, Hua D, Tang X, et al. The Role of Perioperative Sleep Disturbance in Postoperative Neurocognitive Disorders. Nat Sci Sleep 2021;13:1395–1410. doi: 10.2147/nss.S320745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moraes W, Piovezan R, Poyares D, Bittencourt LR, Santos-Silva R, Tufik S. Effects of aging on sleep structure throughout adulthood: a population-based study. Sleep medicine Apr 2014;15(4):401–9. doi: 10.1016/j.sleep.2013.11.791 [DOI] [PubMed] [Google Scholar]
- 103.Lu L, Wang SB, Rao W, et al. The Prevalence of Sleep Disturbances and Sleep Quality in Older Chinese Adults: A Comprehensive Meta-Analysis. Behav Sleep Med Nov-Dec 2019;17(6):683–697. doi: 10.1080/15402002.2018.1469492 [DOI] [PubMed] [Google Scholar]
- 104.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med Aug 15 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Benveniste H, Heerdt PM, Fontes M, Rothman DL, Volkow ND. Glymphatic System Function in Relation to Anesthesia and Sleep States. Anesthesia & Analgesia 2019;128(4):747–758. doi: 10.1213/ane.0000000000004069 [DOI] [PubMed] [Google Scholar]
- 106.Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2019;65(2):106–119. doi: 10.1159/000490349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akeju O, Brown EN. Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr Opin Neurobiol Jun 2017;44:178–185. doi: 10.1016/j.conb.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Purdon PL, Pavone KJ, Akeju O, et al. The Ageing Brain: Age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia. British journal of anaesthesia Jul 2015;115 Suppl 1(Suppl 1):i46–i57. doi: 10.1093/bja/aev213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pandharipande P, Ely EW. Sedative and analgesic medications: risk factors for delirium and sleep disturbances in the critically ill. Crit Care Clin Apr 2006;22(2):313–27, vii. doi: 10.1016/j.ccc.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 110.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients - The MENDS randomized controlled trial. Article. JAMA-J Am Med Assoc Dec 2007;298(22):2644–2653. [DOI] [PubMed] [Google Scholar]
- 111.Pandharipande PP, Sanders RD, Girard TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Critical care (London, England) 2010;14(2):R38. doi: 10.1186/cc8916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pisani MA, Murphy TE, Araujo KL, Slattum P, Van Ness PH, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med Jan 2009;37(1):177–83. doi: 10.1097/CCM.0b013e318192fcf9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science (New York, NY) Oct 18 2013;342(6156):373–7. doi: 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Benveniste H, Lee H, Ding F, et al. Anesthesia with Dexmedetomidine and Low-dose Isoflurane Increases Solute Transport via the Glymphatic Pathway in Rat Brain When Compared with High-dose Isoflurane. Anesthesiology Dec 2017;127(6):976–988. doi: 10.1097/aln.0000000000001888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vacas S, Maze M. Can sedation fulfill the physiological role of sleep? Handbook on Burnout and Sleep Deprivation: Risk Factors, Management Strategies and Impact on Performance and Behavior 2015;
- 116.Akeju O, Hobbs LE, Gao L, et al. Dexmedetomidine promotes biomimetic non-rapid eye movement stage 3 sleep in humans: A pilot study. Clin Neurophysiol Jan 2018;129(1):69–78. doi: 10.1016/j.clinph.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Akeju O, Pavone KJ, Westover MB, et al. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology 2014;121(5):978–989. doi: 10.1097/ALN.0000000000000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu J, Vacas S, Feng X, et al. Dexmedetomidine Prevents Cognitive Decline by Enhancing Resolution of High Mobility Group Box 1 Protein-induced Inflammation through a Vagomimetic Action in Mice. Anesthesiology May 2018;128(5):921–931. doi: 10.1097/aln.0000000000002038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kawas CH, Corrada MM. Successful cognitive aging: What the oldest-old can teach us about resistance and resilience. Neurology Aug 25 2020;95(8):329–330. doi: 10.1212/wnl.0000000000010251 [DOI] [PubMed] [Google Scholar]
- 120.Association As. 2021 Alzheimer’s Disease Facts and Figures Accessed Dec 27 2021, 2021. https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf
- 121.Ibinson JW, Cole DJ. Aducanumab, the Novel $56,000 Drug: Perioperative Considerations. ASA Monitor 2022;86(1):1–4. doi: 10.1097/01.ASM.0000805964.38349.d2 [DOI] [Google Scholar]
- 122.Davis DH, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain : a journal of neurology Sep 2012;135(Pt 9):2809–16. doi: 10.1093/brain/aws190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. Lancet Neurol Aug 2015;14(8):823–832. doi: 10.1016/s1474-4422(15)00101-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology 2009;72:1570–1575. doi: 10.1212/WNL.0b013e3181a4129a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Racine AM, Touroutoglou A, Abrantes T, et al. Older Patients with Alzheimer’s Disease-Related Cortical Atrophy Who Develop Post-Operative Delirium May Be at Increased Risk of Long-Term Cognitive Decline After Surgery. Journal of Alzheimer’s disease : JAD 2020;75(1):187–199. doi: 10.3233/jad-190380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weiner MF. Impact of delirium on the course of Alzheimer disease. Arch Neurol Dec 2012;69(12):1639–40. doi: 10.1001/archneurol.2012.2703 [DOI] [PubMed] [Google Scholar]
- 127.Vacas S, Cole DJ, Cannesson M. Cognitive Decline Associated With Anesthesia and Surgery in Older Patients. Jama Aug 2 2021;doi: 10.1001/jama.2021.4773 [DOI] [PMC free article] [PubMed]
- 128.Leung JM, Sands LP, Wang Y, et al. Apolipoprotein E e4 allele increases the risk of early postoperative delirium in older patients undergoing noncardiac surgery. Anesthesiology 2007;107:406–411. doi: 10.1097/01.anes.0000278905.07899.df [DOI] [PubMed] [Google Scholar]
- 129.Sprung J, Warner DO, Knopman DS, et al. Exposure to surgery with general anaesthesia during adult life is not associated with increased brain amyloid deposition in older adults. British journal of anaesthesia May 2020;124(5):594–602. doi: 10.1016/j.bja.2020.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Klinger RY, James OG, Borges-Neto S, et al. 18F-florbetapir Positron Emission Tomography-determined Cerebral β-Amyloid Deposition and Neurocognitive Performance after Cardiac Surgery. Anesthesiology Apr 2018;128(4):728–744. doi: 10.1097/aln.0000000000002103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Avidan MS, Searleman AC, Storandt M, et al. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology 2009;111:964–970. doi: 10.1097/ALN.0b013e3181bc9719 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Berger M, Browndyke JN, Cooter Wright M, et al. Postoperative changes in cognition and cerebrospinal fluid neurodegenerative disease biomarkers. Ann Clin Transl Neurol Feb 1 2022;doi: 10.1002/acn3.51499 [DOI] [PMC free article] [PubMed]
- 133.Mahajan A, Esper SA, Cole DJ, Fleisher LA. Anesthesiologists’ Role in Value-based Perioperative Care and Healthcare Transformation. Anesthesiology Apr 1 2021;134(4):526–540. doi: 10.1097/aln.0000000000003717 [DOI] [PubMed] [Google Scholar]


