Abstract
Background and Aims
Alterations in body composition are common in inflammatory bowel disease [IBD] and have been associated with differences in patient outcomes. We sought to consolidate knowledge on the impact and importance of body composition in IBD.
Methods
We performed a systematic search of MEDLINE, EMBASE and conference proceedings by combining two key research themes: inflammatory bowel disease and body composition.
Results
Fifty-five studies were included in this review. Thirty-one focused on the impact of IBD on body composition with a total of 2279 patients with a mean age 38.4 years. Of these, 1071 [47%] were male. In total, 1470 [64.5%] patients had Crohn’s disease and 809 [35.5%] had ulcerative colitis. Notably, fat mass and fat-free mass were reduced, and higher rates of sarcopaenia were observed in those with active IBD compared with those in clinical remission and healthy controls. Twenty-four additional studies focused on the impact of derangements in body composition on IBD outcomes. Alterations in body composition in IBD are associated with poorer prognoses including higher rates of surgical intervention, post-operative complications and reduced muscle strength. In addition, higher rates of early treatment failure and primary non-response are seen in patients with myopaenia.
Conclusions
Patients with IBD have alterations in body composition parameters in active disease and clinical remission. The impacts of body composition on disease outcome and therapy are broad and require further investigation. The augmentation of body composition parameters in the clinical setting has the potential to improve IBD outcomes in the future.
Keywords: Body composition, muscle mass, fat mass, inflammatory bowel disease
1. Introduction
Inflammatory bowel disease [IBD] causes significant changes in body composition [BC] including, but not limited to, osteopaenia and osteoporosis, which are well documented.1,2 Other important alterations, such as changes in muscle mass and adiposity along with their consequent effects on IBD outcomes, are less well understood. Furthermore, these changes in BC have not been shown to be associated with body mass index [BMI]. The main drivers of alterations in BC may result from alterations in metabolism due to chronic inflammatory activity, malabsorption or malnutrition.3 In addition, reduced physical activity, because of factors related to their chronic disease, including fatigue, may also contribute to the alterations in BC observed in IBD.4,5
The effects of BC changes are far-reaching and have been associated with complicated Crohn’s disease [CD] course,6 increased post-operative complications7,8 and decreased efficacy of medical therapy.9 Diet and physical activity are important in a patient’s quality of life and have the added benefits of preserving and building on muscle mass.10
BC analysis divides and measures the body’s main tissue compartments of fat [FM] and fat-free mass (lean body mass [LBM]). LBM is anatomically defined as the sum of all non-adipose tissue of the body, whereas the term muscle mass typically refers to the sum of skeletal muscle tissue of the body. LBM appears to play a greater role than the equivalent weight of fat by applying stress to bones and thereby encouraging bone deposition.11–13 LBM deficits may also be associated with demonstrable morbidity, including loss of muscle strength, altered energy metabolism and increased susceptibility to infection.14 Conversely, an increase in FM and obesity has been associated with elevated inflammatory markers and a more severe clinical course in CD patients.15
Skeletal muscle is one of the most plastic human tissues. Although researchers and clinicians from different disciplines describe skeletal muscle mass depletion as either myopaenia or sarcopaenia,16 myopaenia is used to denote clinically significant muscle wasting that is associated either with impaired functional capacity, and/or with increased risk of morbidity or mortality. The term sarcopaenia is typically used to refer to muscle loss associated with ageing and is defined as an appendicular skeletal muscle mass less than two standard deviations below the mean of a young reference group.17
A variety of methods can be employed to measure BC. These include basic [clinical] anthropometry, bioelectrical impedance analysis [BIA], total body water [TBW] measurement [hydrodensitometry], total body potassium measurement, quantitative computed tomography [qCT] scanning, magnetic resonance imaging and dual energy X-ray absorptiometry [DXA]. However, routine clinical assessment of IBD patients using anthropometric techniques such as BMI may provide an inaccurate assessment of BC, because an over-representation of FM may mask deficits in lean mass and lead to under-recognition of malnutrition.18,19 Conversely, measurement of BMI in patients who have a larger burden of lean mass may also result in misclassification of such patients as overweight.
To address these important clinical issues, a systematic review of the literature was undertaken to determine [a] the prevalence of body composition changes in IBD, and [b] the impact/association of body composition changes on disease course and outcomes/treatment response in IBD.
2. Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] guidelines and followed an a priori established protocol.20
2.1. Search strategy
We conducted a comprehensive search of multiple electronic databases from inception to March 2021 including MEDLINE and EMBASE. All potentially relevant studies were identified by combining two key research themes using the Boolean operator ‘AND’. Within each theme, subject headings, title-word and abstract-word search techniques were combined with the Boolean operator ‘OR’. The first theme, Inflammatory Bowel Disease, combined the subject heading Inflammatory Bowel Disease with title-word and abstract-word searches for IBD, Crohn* Disease OR Ulcerative Colitis. The second theme, Body Composition, combined the subject heading Body composition with title- and abstract-word searches for ‘body composition’, ‘muscle’, ‘muscle strength’, and ‘sarcopaenia’ or ‘sarcopenia’.
Additionally, a manual search of conference proceedings of major gastroenterology conferences [Digestive Disease Week, American College of Gastroenterology annual meeting, Advances in Inflammatory Bowel Diseases meeting organized by the Crohn’s and Colitis Foundation of America, and European Crohn’s and Colitis Organization annual meeting] between 2010 and 2021 was conducted to identify additional studies published only in abstract form.
Finally, the reference lists of included studies were screened to identify additional publications of relevance which were not identified through electronic database searches
2.2. Selection criteria
Studies included in this review met the following inclusion criteria: [a] observational and experimental studies involving [b] adult patients with [c] IBD which assessed [d] the alterations in body composition seen in patients with IBD or explored [e] the impact of body composition on outcomes in IBD.
We excluded [a] non-English language papers, [b] studies not available in full-text format, [c] studies published prior to 1992 and [d] all secondary literature.
2.3. Data extraction
Search results were screened for relevance by title and abstract. Full-text review of these shortlisted papers was performed by three authors independently [N.S.D., I.A.B. and D.T.] and the decision for inclusion of each study was made according to the inclusion and exclusion criteria pre-specified above.
Data abstraction was performed by three authors independently [N.S.D., I.A.B. and D.T.] using a standardized form. Data collected fell into the following three categories:
[1] Study characteristics—primary author, year of publication, type of study, definition of active disease or remission
[2] Patient characteristics—age, sex, BMI, disease phenotype, disease duration
[3] Outcomes as defined by:
[a] Effect of IBD on BC as measured by rate of BC alteration [myopaenia, fat-free mass; sarcopaenia, visceral and subcutaneous adiposity in remission and active disease]
[b] Effect of BC on IBD clinical course and prognosis as measured by medical and surgical outcome
2.4. Assessment of study quality
The methodological quality of eligible observational studies was appraised with the Newcastle–Ottawa Scale [NOS]21 while eligible studies following a cross-sectional methodology were assessed according to the AXIS tool.22
Two authors [D.T. and N.S.D.] independently evaluated the included studies by applying these scales. For cohort studies the three domains of selection of cohorts, comparability of cohorts and assessment of outcomes were rated. For case-control studies the three analogous domains of case and control selection, comparability of cases and controls, and ascertainment of exposure were evaluated. Any discrepancies in the risk of bias assessment were resolved by consensus between the two authors through discussion and reference to the original paper.
2.5. Data synthesis
Studies were grouped into two categories according to their primary outcome: [1] the effect of IBD on BC as measured by the rate or prevalence of BC alterations or [2] the effect of BC on IBD clinical course and prognosis as measured by medical or surgical outcomes.
Due to the heterogeneity of study designs among the included citations, a meta-analysis could not be performed.
3. Results
3.1. Search results
Our literature search identified 1134 citations from database searching from 1992 to April 4, 2021. After review of the title and abstracts, a total of 70 citations were selected for full-text review. After excluding studies that did not meet the selection criteria outlined above, a total of 55 articles were eventually included in our review [Figure 1]. Twenty-seven studies followed a cohort study design, a further 27 studies utilized a cross-sectional design and one followed a case-control methodology [Table 1].
Figure 1.
PRISMA flow diagram.
Table 1.
Summary of included studies
| First author | Year | Category | Sample size [n] | Gender [% male] | Age [years] | Study design | Control group | CD [n] | UC [n] | Measure of disease activity |
|---|---|---|---|---|---|---|---|---|---|---|
| Adams23 | 2017 | Effect of IBD on body composition | 90 | 48% | 35 median] | Retrospective cohort | N/A | 76 | 14 | Harvey–Bradshaw Disease Activity Index [HBI] |
| Back24 | 2017 | Effect of IBD on body composition | 141 | UC Group: [64.83%] CD group: [40.74%] | CD: 43.98 [mean] UC: 44.28 [mean] | Cross-sectional | N/A | 54 | 87 | CD: CDAI UC: Mayo Score |
| Bamba25 | 2020 | Impact of body composition on IBD outcomes | 187 | 67% | Male: 19.8 Female 18.9 | Retrospective cohort | N/A | 99 | 88 | CDAI Lichtiger score |
| Bamba26 | 2017 | Impact of body composition on IBD outcomes | 73 | 72.60% | Not provided | Retrospective cohort | N/A | 43 | 29 | N/A |
| Barroso27 | 2018 | Effect of IBD on body composition | 22 | 55% | 47.5 [median] | Cross-sectional | Healthy controls [22] | 18 | 4 | N/A |
| Benjamin28 | 2011 | Effect of IBD on body composition | 123 | 56% | 36.4 [mean] | Cross-sectional | IBD remission | 123 | 0 | N/A |
| Berger29 | 2020 | Impact of body composition on IBD outcomes | 91 | 49.50% | 37 [median] | Retrospective cohort | N/A | 59 | 32 | N/A |
| Boparai30 | 2021 | Impact of body composition on IBD outcomes | 44 | 63.60% | 34.4 [mean] | Retrospective cohort | N/A | 44 | 0 | N/A |
| Bryant31 | 2018 | Impact of body composition on IBD outcomes | 97 | 51% | 31 [median] | Prospective cohort | N/A | 97 | 0 | N/A |
| Bryant1 | 2015 | Effect of IBD on body composition | 137 | 56% | 31 [median] | Cross-sectional | Healthy control | 95 | 42 | CDAI |
| Capristo32 | 1998 | Effect of IBD on body composition | 43 | 53 | 33.8 | Cross-sectional | Healthy controls N = 60 | 43 | 0 | SCDAI |
| Capristo33 | 1998 | Effect of IBD on body composition | 34 | 56 | 36.8 | Cross-sectional | Healthy controls [age- and sex-matched] N = 20 | 18 | 16 | N/A |
| Cravo34 | 2018 | Impact of body composition on IBD outcomes | 71 | 50% | 43 [mean] | Cross-sectional | N/A | 71 | 0 | Harvey–Bradshaw Index |
| Cuoco35 | 2008 | Effect of IBD on body composition | 13 | 69% | 31 [mean] | Cross-sectional | Healthy controls n = 20 | 13 | 0 | N/A |
| Cushing36 | 2018 | Impact of body composition on IBD outcomes | 89 | 64% | 36 [mean] | Retrospective cohort | N/A | 0 | 89 | Endoscopic |
| Ding37 | 2017 | Impact of body composition on IBD outcomes | 106 | 44.30% | 44.84 [median] | Retrospective cohort | N/A | 106 | 0 | N/A |
| Erhayiem6 | 2015 | Impact of body composition on IBD outcomes | 50 | N/A | 49.3 [median] | Cross-sectional | Inflammatory CD vs complicated CD [fistulizing/stricturing] n = 21 | 50 | 0 | N/A |
| Filippi38 | 2006 | Effect of IBD on body composition | 54 | 48% | 39 [mean] | Cross-sectional | Healthy controls N = 25 | 54 | 0 | N/A |
| Fujikawa39 | 2017 | Impact of body composition on IBD outcomes | 69 | 65.20% | 39.8 [mean] | Retrospective cohort | N/A | 0 | 69 | N/A |
| Galata40 | 2020 | Impact of body composition on IBD outcomes | 230 | 43.90% | 37.2 [mean] | Retrospective cohort | N/A | 230 | 0 | N/A |
| Geerling41 | 1998 | Effect of IBD on body composition | 31 | 44% | 40 [mean] | Cross-sectional | Healthy controls N = 32 | 31 | 0 | N/A |
| Gong42 | 2015 | Effect of IBD on body composition | 75 | N/A | 33 [med] | Cross-sectional | N/A | 67 | 8 | CDAI |
| Grillot43 | 2020 | Impact of body composition on IBD outcomes | 88 | 50% | 35.5 [mean] | Retrospective cohort | N/A | 88 | 0 | N/A |
| Holt44 | 2017 | Impact of body composition on IBD outcomes | 68 | 47.10% | 37.6 [mean] | Retrospective cohort | N/A | 63 | 5 | CDAI |
| Holt45 | 2017 | Impact of body composition on IBD outcomes | 44 | 45.50% | 37.8 [mean] | Retrospective cohort | N/A | 44 | 0 | Endoscopic assessment |
| Jahnsen4 | 2003 | Effect of IBD on body composition | 120 | 30% | 47 [mean] | Cross-sectional | Healthy controls n = 60 | 60 | 60 | N/A |
| Katznelson46 | 2003 | Effect of IBD on body composition | 20 | 100% | 45 [mean] | Cross-sectional | Healthy controls n = 20 | 20 | 0 | N/A |
| Labarthe47 | 2020 | Impact of body composition on IBD outcomes | 132 | 43% | 36.9 [median] | Retrospective cohort | N/A | 132 | 0 | Harvey–Bradshaw Index |
| Leslie48 | 2009 | Effect of IBD on body composition | 101 | 42% | 47 [mean] | Prospective cohort | N/A | 56 | 45 | N/A |
| Lim49 | 2020 | Impact of body composition on IBD outcomes | 69 | 60.90% | 43.5 | Cross-sectional | N/A | 69 | 0 | N/A |
| Magro50 | 2018 | Effect of IBD on body composition | 50 | 54% | Not available | Cross-sectional | Healthy controls n = 28 | 50 | 0 | CDAI |
| Mingrone51 | 1996 | Effect of IBD on body composition | 20 | 40 | 29.5 | Cross-sectional | Healthy controls N = 16 | 20 | 0 | CDAI |
| Mingrone52 | 1999 | Effect of IBD on body composition | 18 | 56 | 35.3 | Cross-sectional | Healthy controls [age- and sex-matched] n = 12 | 18 | 0 | N/A |
| Molnar53 | 2017 | Effect of IBD on body composition | 136 | Uncertain | 33.0 mean | Prospective cohort | Healthy age- and gender-matched controls n = 1752 | 136 | 0 | CDAI |
| O’Brien54 | 2018 | Impact of body composition on IBD outcomes | 77 | 60% | 42 [mean] | Retrospective cohort | N/A | 52 | 21 | N/A |
| Pizzoferrato55 | 2019 | Effect of IBD on body composition | 127 | 61% | 41.6 [median] | Cross-sectional | Healthy age- matched controls | 69 | 58 | N/A |
| Rocha56 | 2009 | Effect of IBD on body composition | 102 | 34% | 41.2 [mean] | Cross-sectional | IBD remission N = 74 | 50 | 52 | CDAI |
| Santos57 | 2017 | Effect of IBD on body composition | 26 | 52% | 42 [mean] | Prospective cohort | N/A | 26 | 0 | Harvey–Bradshaw Index [HBI |
| Schneider58 | 2008 | Effect of IBD on body composition | 82 | 48% | 36 [mean] | Cross-sectional | Healthy controls n = 50 | 82 | 0 | N/A |
| Sigurdsson59 | 2020 | Effect of IBD on body composition | 94 | 68% | 21.8 [median] | Prospective cohort | Healthy age-matched controls n = 1294 | 29 | 65 | N/A |
| Steell60 | 2020 | Effect of IBD on body composition | 27 | 44.40% | 23.2 [mean] | Cross-sectional | Healthy controls [age- and sex-matched] n = 27 | 27 | 0 | CDAI |
| Thiberge61 | 2018 | Impact of body composition on IBD outcomes | 149 | 45.60% | 41 [mean] | Retrospective cohort | N/A | 149 | 0 | N/A |
| Tjellesen2 | 2003 | Effect of IBD on body composition | 31 | 42% | 40 [mean] | Cross-sectional | Healthy controls N = 88 | 31 | 0 | N/A |
| Uliveiri62 | 2001 | Effect of IBD on body composition | 43 | 49 | 35.9 [mean] | Prospective cohort | Healthy controls n = 111 | 0 | 43 | N/A |
| Unal63 | 2020 | Effect of IBD on body composition | 344 | 54.10% | 49.4 [mean] | Cross-sectional | N/A | 122 | 222 | Harvey–Bradshaw index and partial Mayo score |
| Urbano64 | 2018 | Impact of body composition on IBD outcomes | 59 | 46.70% | 48.1 [mean] | Cross-sectional | N/A | 0 | 59 | Truelove and Witts criteria |
| Vaisman65 | 2006 | Effect of IBD on body composition | 8 | 50% | 30 [mean] | Cross-sectional | Healthy controls | 0 | 0 | CDAI |
| Valentini66 | 2008 | Effect of IBD on body composition | 47 | 13% | 38 [mean] | Cross-sectional | Healthy controls n = 47 | 30 | 17 | CDAI, UCAI |
| Van Der Sloot67 | 2017 | Impact of body composition on IBD outcomes | 482 | 50% | Not provided | Prospective cohort | N/A | 482 | 0 | N/A |
| Yadav68 | 2017 | Effect of IBD on body composition | 97 | UC Group: [67.9%] CD group: [72.7%] | UC Group: 33.2 [mean] CD group: 41.2 [mean] | Prospective cohort | N/A | 44 | 53 | CDAI and Simple Clinical Colitis Activity Index |
| Zager69 | 2021 | Impact of body composition on IBD outcomes | 121 | 51.20% | 35.98 [mean] | Retrospective cohort | N/A | 121 | 0 | N/A |
| Zalizko70 | 2020 | Impact of body composition on IBD outcomes | 48 | 56% | 36.5 [median] | Prospective cohort | Healthy controls [age- and sex-matched] n = 58 | 23 | 25 | CDAI and Mayo Score |
| Zaltman71 | 2014 | Effect of IBD on body composition | 23 | 0% | 43.9 [mean] | Case control | Healthy controls [age- and sex-matched] n = 23 | 0 | 23 | MAYO |
| Zhang72 | 2017 | Impact of body composition on IBD outcomes | 204 | Not available | Not available | Retrospective cohort | Controls with acute appendicitis | 105 | 99 | Modified Mayo score and Truelove & Witts’ criteria |
| Zhang73 | 2017 | Impact of body composition on IBD outcomes | 114 | 65.80% | 32 [mean] | Retrospective cohort | N/A | 114 | 0 | N/A |
3.2. Assessment of study quality
Assessment of the quality of the included studies is shown in Table 2.
Table 2.
Assessment of study quality
| Author | Study details | Risk of bias assessment | ||||||
|---|---|---|---|---|---|---|---|---|
| Year | Study title | Study design | Risk of bias tool | Selection of cohorts | Comparability of cohorts | Assessment of outcome | Total | |
| Adams23 | 2017 | Sarcopenia is common in overweight patients with inflammatory bowel disease and may predict need for surgery | Retrospective cohort | Newcastle–Ottawa Scale | 3 | 2 | 1 | 6 |
| Back24 | 2017 | Body composition in patients with Crohn’s disease and ulcerative colitis | Cross-sectional | AXIS | N/A | N/A | N/A | 17 |
| Bamba25 | 2020 | Assessment of body composition from CT images at the level of the third lumbar vertebra in inflammatory bowel disease | Retrospective cohort | Newcastle–Ottawa Scale | 4 | 1 | 3 | 8 |
| Bamba26 | 2018 | Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn’s disease | Retrospective cohort | Newcastle–Ottawa Scale | 3 | 2 | 1 | 6 |
| Barroso27 | 2018 | Patients with inflammatory bowel disease have higher abdominal adiposity and less skeletal mass than healthy controls | Cross-sectional | AXIS | N/A | N/A | N/A | 19 |
| Benjamin28 | 2011 | Body composition in Indian patients with Crohn’s disease during active and remission phase | Cross-sectional | AXIS | N/A | N/A | N/A | 16 |
| Berger29 | 2020 | Low skeletal muscle index adjusted for body mass index is an independent risk factor for inflammatory bowel disease surgical complications | Retrospective cohort | Newcastle–Ottawa Scale | 3 | 2 | 3 | 8 |
| Boparai30 | 2021 | Combination of sarcopenia and high visceral fat predict poor outcomes in patients with Crohn’s disease | Retrospective cohort | Newcastle–Ottawa Scale | 3 | 1 | 2 | 6 |
| Bryant31 | 2018 | Visceral adipose tissue is associated with stricturing Crohn’s disease behaviour, fecal calprotectin, and quality of life | Prospective cohort | Newcastle–Ottawa Scale | 3 | 1 | 3 | 7 |
| Bryant1 | 2015 | Low muscle mass and sarcopenia: common and predictive of osteopenia in inflammatory bowel disease | Cross-sectional | AXIS | N/A | N/A | N/A | 20 |
| Capristo32 | 1998 | Effect of disease localization on the anthropometric and metabolic features of Crohn’s disease | Cross-sectional | AXIS | N/A | N/A | N/A | 20 |
| Capristo33 | 1998 | Metabolic features of inflammatory bowel disease in a remission phase of the disease activity | Cross-sectional | AXIS | N/A | N/A | N/A | 18 |
| Cravo34 | 2018 | Lower skeletal muscle attenuation and high visceral fat index are associated with complicated disease in patients with Crohn’s disease: An exploratory study | Cross-sectional | AXIS | N/A | N/A | N/A | 20 |
| Cuoco35 | 2001 | Skeletal muscle wastage in Crohn’s disease: a pathway shared with heart failure? | Cross-sectional | AXIS | N/A | N/A | N/A | 17 |
| Cushing36 | 2018 | Sarcopenia is a novel predictor of the need for rescue therapy in hospitalized ulcerative colitis patients | Retrospective cohort | Newcastle–Ottawa Scale | 3 | 2 | 3 | 8 |
| Daniela37 | 2018 | Visceral fat is increased in individuals with Crohn’s disease: a comparative analysis with healthy controls | Cross Sectional | AXIS | N/A | N/A | N/A | 20 |
| Ding38 | 2017 | The body composition profile is associated with response to anti-TNF therapy in Crohn’s disease and may offer an alternative dosing paradigm | Retrospective cohort | Newcastle–Ottawa Scale | 4 | 1 | 3 | 8 |
| Erhayiem6 | 2015 | Ratio of visceral to subcutaneous fat area is a biomarker of complicated Crohn’s disease | Cross-sectional | AXIS | N/A | N/A | N/A | 17 |
| Fillipi39 | 2006 | Nutritional deficiencies in patients with Crohn’s disease in remission | Cross-sectional | AXIS | N/A | N/A | N/A | 19 |
| Fujikawa40 | 2017 | Impact of sarcopenia on surgical site infection after restorative proctocolectomy for ulcerative colitis | Retrospective cohort | Newcastle–Ottawa Scale | 4 | 2 | 3 | 9 |
| Galata41 | 2020 | Skeletal muscle mass index predicts postoperative complications in intestinal surgery for Crohn’s disease | Retrospective cohort | Newcastle–Ottawa Scale | 4 | 1 | 3 | 8 |
| Geerling42 | 1998 | Comprehensive nutritional status in patients with long-standing Crohn disease currently in remission | Cross-sectional | AXIS | N/A | N/A | N/A | 18 |
| Gong43 | 2015 | Impact of disease activity on resting energy expenditure and body composition in adult Crohn’s disease: a prospective longitudinal assessment | Cross-sectional | AXIS | N/A | N/A | N/A | 18 |
| Grillot44 | 2020 | Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn’s disease | Retrospective cohort | Newcastle–Ottawa Scale | 3 | 2 | 3 | 8 |
| Holt45 | 2017 | Low muscle mass at initiation of anti-TNF therapy for inflammatory bowel disease is associated with early treatment failure: a retrospective analysis | Retrospective cohort | Newcastle–Ottawa Scale | 4 | 2 | 2 | 8 |
| Holt46 | 2017 | Visceral adiposity predicts post-operative Crohn’s disease recurrence | Retrospective cohort | Newcastle–Ottawa Scale | 3 | 1 | 2 | 6 |
| Jahnsen4 | 2003 | Body composition in patients with inflammatory bowel disease: a population-based study | Cross-sectional | AXIS | N/A | N/A | N/A | 20 |
| Katnelzen47 | 2003 | Effects of growth hormone secretion on body composition in patients with Crohn’s disease | Cross-sectional | AXIS | N/A | N/A | N/A | 17 |
| Labarthe48 | 2020 | Magnetic resonance imaging assessment of body composition parameters in Crohn’s disease | Retrospective cohort | Newcastle–Ottawa Scale | 3 | 1 | 2 | 6 |
| Leslie49 | 2009 | Body mass and composition affect bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD cohort study | Prospective cohort | Newcastle–Ottawa Scale | 3 | 2 | 2 | 7 |
| Lim50 | 2020 | The effect of adiposity on anti–tumor necrosis factor-alpha levels and loss of response in Crohn’s disease patients | Cross-sectional | AXIS | N/A | N/A | N/A | 20 |
| Mingrone51 | 1996 | Increased resting lipid oxidation in Crohn’s disease | Cross-sectional | AXIS | N/A | N/A | N/A | 18 |
| Mingrone52 | 1999 | Elevated diet-induced thermogenesis and lipid oxidation rate in Crohn disease | Cross-sectional | AXIS | N/A | N/A | N/A | 19 |
| Molnar53 | 2017 | Body composition assessment of Crohn’s outpatients and comparison with gender- and age-specific multiple matched control pairs | Prospective cohort | Newcastle–Ottawa Scale | 2 | 1 | 2 | 5 |
| O’Brien54 | 2018 | The impact of sarcopenia and myosteatosis on postoperative outcomes in patients with inflammatory bowel disease | Retrospective cohort | Newcastle–Ottawa Scale | 3 | 0 | 2 | 5 |
| Pizzoferrato55 | 2019 | Characterization of sarcopenia in an IBD population attending an Italian gastroenterology tertiary center | Cross-sectional | AXIS | N/A | N/A | N/A | 17 |
| Rocha56 | 2009 | Analysis of fat and muscle mass in patients with inflammatory bowel disease during remission and active phase | Cross-sectional | AXIS | N/A | N/A | N/A | 19 |
| Santos57 | 2017 | Impact of biological therapy on body composition of patients with Crohn’s disease | Prospective cohort | Newcastle–Ottawa Scale | 3 | 0 | 2 | 5 |
| Schneider58 | 2008 | Sarcopenia is prevalent in patients with Crohn’s disease in clinical remission | Cross-sectional | AXIS | N/A | N/A | N/A | 18 |
| Sigurdsson59 | 2020 | Altered body composition profiles in young adults with childhood-onset inflammatory bowel disease | Prospective cohort | Newcastle–Ottawa Scale | 2 | 2 | 2 | 6 |
| Steell60 | 2020 | Muscle deficits with normal bone microarchitecture and geometry in young adults with well-controlled childhood-onset Crohn’s disease | Cross-sectional | AXIS | N/A | N/A | N/A | 20 |
| Thiberge61 | 2018 | Lower subcutaneous or visceral adiposity assessed by abdominal computed tomography could predict adverse outcome in patients with Crohn’s disease | Retrospective Cohort | Newcastle–Ottawa Scale | 4 | 2 | 3 | 9 |
| Tjellesen2 | 2009 | Body composition by dual-energy X-ray absorptiometry in patients with Crohn’s disease | Cross-sectional | AXIS | N/A | N/A | N/A | 17 |
| Uliveiri62 | 2001 | Bone mineral density and body composition in ulcerative colitis: a six-year follow-up | Prospective cohort | Newcastle–Ottawa Scale | 2 | 1 | 3 | 6 |
| Unal63 | 2020 | Malnutrition and sarcopenia are prevalent among inflammatory bowel disease patients with clinical remission | Cross-sectional | AXIS | N/A | N/A | N/A | 20 |
| Urbano64 | 2018 | Associations among body composition, inflammatory profile, and disease extent in ulcerative colitis patients | Cross-sectional | AXIS | N/A | N/A | N/A | 18 |
| Vaisman65 | 2006 | Malabsorption is a major contributor to underweight in Crohn’s disease patients in remission | Cross-sectional | AXIS | N/A | N/A | N/A | 18 |
| Valentini66 | 2008 | Malnutrition and impaired muscle strength in patients with Crohn’s disease and ulcerative colitis in remission | Cross-sectional | AXIS | N/A | N/A | N/A | 19 |
| Van Der Sloot67 | 2017 | Visceral adiposity, genetic susceptibility, and risk of complications among individuals with Crohn’s disease | Prospective cohort | Newcastle–Ottawa Scale | 3 | 2 | 3 | 8 |
| Yadav68 | 2017 | Body composition in Crohn’s disease and ulcerative colitis: correlation with disease severity and duration | Prospective cohort | Newcastle–Ottawa Scale | 3 | 1 | 2 | 6 |
| Zager69 | 2021 | Low psoas muscle area is associated with postoperative complications in Crohn’s disease | Retrospective cohort | Newcastle–Ottawa Scale | 3 | 1 | 2 | 6 |
| Zalizko70 | 2020 | The role of body muscle mass as an indicator of activity in inflammatory bowel disease patients | Prospective cohort | Newcastle–Ottawa Scale | 3 | 0 | 2 | 5 |
| Zaltman71 | 2014 | Lower extremity mobility limitation and impaired muscle function in women with ulcerative colitis | Case control | Newcastle–Ottawa Scale | 2 | 2 | 3 | 7 |
| Zhang72 | 2017 | Prevalence of sarcopenia and its impact on postoperative outcome in patients with Crohn’s disease undergoing bowel resection | Retrospective cohort | Newcastle–Ottawa Scale | 4 | 2 | 2 | 8 |
| Zhang73 | 2017 | Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy | Retrospective cohort | Newcastle–Ottawa Scale | 4 | 1 | 2 | 7 |
Most observational studies in this review [n = 15/28] were deemed at low risk of bias as per the NOS, having received a rating of 7 or more stars out of 9 on the NOS scale. The remaining 12 observational studies were appraised 4–6 stars out of 9 on the NOS scale, indicating a moderate risk of bias. Of the cross-sectional studies included in this review [n = 27], most [20/27] were rated at least 18 out of 20 according to the AXIS tool for the assessment of study quality of cross-sectional studies and were thus deemed to be at low risk of bias. The remainder of the cross-sectional studies included in this review were appraised as 16–17 out of 20 possible points.
3.3. Alterations in BC in IBD
A total of 31 studies investigated the impact of IBD on BC with a total of 2279 patients. Of these, 1071 [47%] patients were male. The mean age for the study cohort was 38.4 years. In total, 1470 [64.5%] patients had CD and 809 [35.5%] had ulcerative colitis [UC]. Activity of disease was noted in 16 studies through use of metrics including the Crohn’s Disease Activity Index [CDAI], Mayo Score and Harvey–Bradshaw Index [HBI].
The techniques of BC analysis used within the studies were heterogeneous. Techniques included DXA, qCT, BIA and anthropometric assessment in addition to functional assessments of muscle strength.
3.3.1. Active disease vs clinical remission
Patients with active IBD have a greater energy expenditure than those in remission,42 resulting in numerous changes to BC. Accordingly, five studies evaluated the impact of active disease vs clinical remission on patient BC.42–46 In these studies, clinical remission was defined as CDAI <150 in the case of CD and as MAYO score <224 or Lichtiger’s Index <1056 in the case of UC.
Four studies compared the impact of active disease on patient BMI in CD.42–56 Each of these analyses identified BMI to be lower in the active disease group in comparison to CD patients in remission. The reduction in mean BMI [kg/m2] between patients with active CD and in patients with CD in remission ranged from 3.9 to 19.8%.42–56
Moreover, additional variables related to LBW including lean body mass index, mid-arm muscle area and thickness of abductor policis muscle were all reduced in patients with active CD relative to those in disease remission.24 Mean lean body mass index was 11.2% lower in patients with active CD, while mid-arm circumference and thickness of abductor policis muscle were 9.4 and 27.5% lower in patients with active CD, respectively.24
In comparison to healthy controls, six studies28,32,33,38,46,52,65 described reductions in FM for patients with CD in clinical remission compared with healthy controls.
Patients with active CD were analysed in three studies,28,51,66 which found reductions in FM probably also associated with malnutrition66 with one study having a lower weight in CD patients compared with controls.51
More recently, magnetic resonance imaging [MRI] has been used to evaluate measurements of BC parameters in CD. This technique uses a single cross-sectional MR image at the L3 level for segmentation of skeletal muscle, visceral fat and subcutaneous adipose tissue areas. BC indices can then be approximated. These include, skeletal muscle index [SMI], which indicates the ratio of the area of skeletal muscle at the L3 level to the patient’s height squared. One study, using this technique, found that sarcopaenia [defined as SMI <38.9 cm2/m2 for females and SMI <54.4 cm2/m2 in males] was more common in patients with active CD compared to those in remission and that SMI and subcutaneous adiposity were lower in patients with active CD.47
3.3.2. Medical therapies
Alterations in BC may result from the use of certain classes of medications in the treatment of IBD. For example, prolonged corticosteroid treatment is known to cause muscle atrophy and lower bone mineral density [BMD].74
Data on the impact of therapy on BC were assessed in five studies.4,25,41,57,75 Steroids were found to decrease BMD in CD patients [n = 120].4 Corticosteroid therapy was found to be associated with lower fat-free mass [FFM]4 and were also observed to negatively impact on SMI.25
More recently, in a prospective study by Santos et al.,57 which evaluated the BC of patients before and after 6 months of exposure to anti-tumour necrosis factor [anti-TNF] therapy, with the exception of phase angle, all BC parameters including BMI, waist circumference, lean mass index and FM index were increased. The gain in FM in these patients is thought to be due to an improvement in the quantity and quality of food intake owing to an improvement in symptoms as well as less catabolism from reduced chronic inflammation and the anti-cachexic effects of TNF-alpha blockade.57 Phase angle, a measure which is obtained using BIA, was the only parameter that was unchanged after anti-TNF therapy.57 Phase angle, which is defined as the arc tangent of the ratio of reactance to electrical resistance, reflects both cell membrane integrity and body cell mass.76 Lower phase angles suggest either cell death or a breakdown in the selective permeability of the cell membrane, while increases in phase angle are associated with improving clinical status.76
Santos et al.’s finding that anti-TNF therapy is associated with increases in BC parameters related to lean and fat mass corroborates an earlier observation by Subramaniam et al.,77 which demonstrated increases in muscle volume and strength at 25 weeks following commencement of anti-TNF therapy in patients with CD.
3.3.3. Disease phenotype
Knowledge of the impact of disease phenotype on BC is limited. CD was associated with increased BMI in one study [n = 75]42 which contrasted with another report identifying lower BMI [n = 8].65 Patients with complicated [fistulating or stricturing] CD were found to have a higher mean fat index as defined by ratio of visceral to subcutaneous fat [0.67 ± 0.29 vs 0.23 ± 0.1, p = 0.001]. Two studies found that CD was associated with a lower FM than UC.4,33 In particular, mean FM in CD patients relative to UC patients, who were in remission, was found to be 12.6 vs 17.1 kg [p < 0.05]56 and 20.8 vs 24.5 kg [p < 0.01].4,33 When undertaking BC assessment according to disease distribution, two studies analysing CD by location concluded that ileal disease was associated with a decreased FM compared to other disease distributions.32,33
More recently, Cravo et al. found that complicated CD phenotypes, including stricturing and penetrating subtypes, were associated with lower mean muscle attenuation, measured using CT cross-sections at the third lumbar vertebra.34 Conversely, this study identified that higher muscle area [MA] was protective against stricturing or penetrating phenotypes (odds ratio [OR] = 0.81 p = 0.002).34 Moreover, a positive association between visceral obesity and complicated disease [OR = 26.1; p = 0.02] was identified.34 This result reinforces the hypothesis that mesenteric fat may promote intestinal inflammation while higher lean mass is protective against more severe disease.34
Alterations in BC seen in patients with UC were examined in two studies.64,72 In the first study, higher body cell mass [BCM], a marker for combined visceral and somatic protein deposits which constitutes all metabolically active tissue in the body, was observed to be protective against more extensive disease64 [OR = 0.92, p < 0.01]. This suggests that lean body mass is associated with reduced susceptibility to more extensive disease activity. In addition, Zhang et al. observed that the sarcopaenia rates were higher and SMI was lower in patients with more severe UC according to Mayo scores.72
3.3.4. Incidence of muscle depletion in IBD
The reported rates of myopaenia in IBD vary due to the heterogeneity of patient populations and modality of measuring the underlying tissue. It is thought that patients in remission also suffer from myopaenia at rates of 50–60%.56,78,79
Furthermore, BC alters during a patient’s phase of disease from remission to active disease, with demonstration of fat mass and muscle mass depletion during an active flare of IBD.56 A study by Rocha et al.56 of 102 patients [50 CD, 52 UC] demonstrated that muscle mass depletion was detected in half of CD and UC patients, with CD patients having lower muscle mass as measured by arm muscle area and triceps skinfold thickness.
Patients with IBD in clinical remission are at greater risk of sarcopaenia.58 A publication by Schneider et al. demonstrated that of 82 patients with CD in clinical remission, 60% were found to be sarcopaenic and 30% osteopaenic, when compared to healthy controls measured by DXA. In total, 91% of sarcopaenic patients were also found to be osteopaenic [T-score < −1.0].58
3.4. Effect of body composition on IBD clinical course
Twenty-four studies dealt predominately with the impact of altered BC upon clinical outcomes in IBD, including treatment response and muscle strength. A total of 2761 patients with IBD [81% CD] were examined across the studies.
3.4.1. Impact of BC changes on muscle strength in IBD patients
Alterations in BC may result in lower muscle mass and subsequently reduced strength.1,41,63,66,71
Seven studies included functional assessments of muscle strength. The method of assessment of strength varied, with hand grip being recorded in six studies1,59,60,63,66,71 while another used hamstring and quadriceps strength.41
In the studies which examined grip strength, patients with IBD were unequivocally found to demonstrate lower maximal isometric grip strengths when compared to healthy, age- and sex-matched controls.1,66,60 The lowest grip strengths were observed in IBD patents with myopaenic and myopaenic-obese BCs.59
Geerling et al. measured hamstring and quadriceps strength in patients with long-standing CD in remission using an isokinetic dynamometer.41 They observed reduced hamstring muscle strength but preserved quadriceps muscle strength in these patients compared with healthy controls. Zaltman et al. extended this functional assessment with use of a sit up test and gait speed test in addition to measuring handgrip strength and quadriceps strength.71 This case control study examined 23 female patents with UC compared with 23 female BMI-matched, healthy controls. They found quadriceps muscle strength was significantly decreased in UC patients compared with controls [−6%]. In addition, UC patients were significantly slower than controls at the sit up test [−32%] and at the gait speed test [−17%]. However, there was no significant difference in hand grip strength between the groups.71
3.4.2. Impact of BC alterations on surgical outcomes
Two studies examined the impact of obesity on post-operative complications by utilizing preoperative CT scans to perform fat and muscle segmentation.7,8 The first, by Connelly et al., measured visceral/intra-abdominal adiposity and subcutaneous adiposity at five axial levels [L1–L5 vertebra] and calculated an average visceral to subcutaneous fat ratio.8 An average was calculated to reduce the likelihood of false values due to patient positioning, distended bowel and body habitus.8 The authors found that an increasing visceral to subcutaneous fat ratio was associated with increased postoperative morbidity (OR = 1.1, 95% confidence interval [CI]: 1.03–1.2, p = 0.03) in those undergoing ileocolectomy.8
A subsequent study by Stidham et al.7 [n = 269] examined the effect of a relative change in fat distribution across spinal levels by defining a parameter which compared the ratio of subcutaneous to visceral fat between the most cephalad [T10] and most caudal [L5] spinal levels. They7 found that a higher subcutaneous to visceral fat ratio in the central region [T10] relative to the mid- to lower abdominal region [L5] was associated with an increased risk of post-operative infectious complications following bowel resection [OR = 2.01, 95% CI 1.20–3.1; p = 0.006].
This study excluded patients with a known abscess or perforation to prevent confounding the outcome assessment. However, the design of the study did not allow for correction for disease activity or severity, which limits the generalizability of the findings.
Moreover, multiple studies have evaluated the impact of sarcopaenia on the need for surgery and as a predictor for post-operative outcomes in IBD.23,26,29,30,39,40,60,73 Five of these studies exclusively evaluated patients with CD,26,30,40,73 one exclusively examined patients with UC39 and two examined cohorts of patients with both UC and CD.23,29 The definition of sarcopaenia utilized in the literature is heterogeneous. While most studies used the SMI,23,26,29,30,40,73 calculated as the cross-sectional area of skeletal muscle at the L3 vertebra relative to patient height squared as a surrogate parameter for sarcopaenia, the SMI cut points used to define sarcopaenia vary from study to study. Most commonly, sarcopaenia was defined according to SMI <55 cm2/m2 for males and SMI <39 cm2/m2 for females.23,29,73 Estimation of Psoas muscle area [PMA], using cross-sectional imaging at the level of the L4 or L5 vertebra, was also used as a surrogate marker to define sarcopaenia in two studies.39,69
In patients with CD, sarcopaenia predicted the need for intestinal resection.26 Specifically, patients with higher SMI were at lower risk of requiring intestinal resection (hazard ratio [HR] = 0.318, p = 0.015).26 However, the indications for intestinal resection among the patients in these studies was not presented.26 Furthermore, the rate at which CD patients with sarcopaenia underwent surgery is particularly striking when compared with those without sarcopaenia [31.6% vs 4%].30 This was thought to be due in part to the observation that more sarcopaenic patients in this study had chronic, continuous disease courses relative to those without sarcopaenia [31.6% vs 16%, p = 0.06].30
In patients with CD, the presence of sarcopaenia was also found to increase the risk of major post-operative complications.40,73 Major complications were defined as a complication which required repeat intervention, intensive care involvement or patient death.73 The odds of these complications was found to be increased by as much as nine-fold by the presence of sarcopaenia [OR = 9.24, p = 0.04].73
Similarly, sarcopaenia was also associated with higher rates of post-operative complications in UC patients. In UC patients who underwent pouch surgery, the odds of suffering a surgical site infection was nearly five times higher than in non-sarcopaenic patients [OR = 4.91, 95% CI: 1.09–23.5, p = 0.03].39
A number of studies also explored the impact of a simultaneous reduction in skeletal muscle mass and increased adiposity on surgical outcomes in IBD patients.23,30 This condition, often referred to as sarcopaenic obesity or myopaenic obesity, is characterized by both low skeletal muscle mass and high levels of adiposity.80 Interestingly, one study identified sarcopaenia to be a predictor of need for surgery only in patients considered overweight according to BMI ≥ 25 and not in non-obese patients.23 Boparai et al.30 stratified patients into three categories: obese, sarcopaenic and both sarcopaenic and obese. In doing so, the authors determined that the cumulative probability of remaining surgery-free was lowest in those with both sarcopaenia and obesity than in those with either or none [38% vs 82% vs 100%, p = 0.01].30
3.4.3. Impact of BC on treatment response
Until recently, there has been a paucity of studies investigating the impact of alterations in BC on treatment response. Studies have assessed the influence of muscle and fat mass on treatment response and failure rates. However, no studies that prospectively examined this relationship were identified.
In a recent retrospective study, Holt et al. assessed the effect of low muscle mass on anti-TNF treatment failure.44 In their study, treatment failure was defined as any post-induction hospital admission or surgery for IBD, escalation of anti-TNF dose or immunosuppressants for clinical loss of response, emergence of a new fistula or CDAI > 150. Patients with skeletal muscle cross-sections [as measured at the third lumbar vertebrae] less than the gender-specific median had a significantly shorter median time to failure [520 vs 1100 days]. Furthermore, Ding et al. went on to demonstrate that myopaenia is associated with primary non-response to anti-TNF therapy in patients with moderate to severe luminal CD [OR = 2.93, 95% CI: 1.28–6.71, p = 0.01].37
With regard to the impact of fat mass on treatment response, Lim et al. demonstrated an inverse relationship between visceral fat area and infliximab trough concentrations in addition to a higher rate of secondary loss of response to adalimumab in patients with increased total adipose area.49 Furthermore, according to Csontos et al.,9 parameters such as FFM index [FFMI] and SMI were found to be inversely correlated with adalimumab trough levels [FFMI: r = −0.494 p = 0.045, SMI r = −0.508, p = 0.038]. However, trough levels did not correlate with fat parameters in this study. Moreover, a study looking into BC parameters and impact on anti-TNF therapy response identified that myopaenia was associated with primary non-response but not secondary loss of response [HR = 4.73, 95% CI: 1.81–12.39].81
In contrast, recent evidence has shown that the efficacy and safety of tofacitinib in patients with UC is not impacted by obesity.82 Patients classified as obese, defined as BMI > 30, do not achieve efficacy endpoints at a lower rate when compared with lower BMI patient groups.82
4. Discussion
Current literature predominantly uses BMI as a marker of nutritional status and a benchmark for research outcomes such as surgery, complications and disease course, with a paucity of data utilizing myopaenia and BC.83,84 Our systematic review serves to highlight the importance of using BC parameters in investigating IBD outcomes. Given the impact of IBD on patients from a young age, more studies with greater clinical data incorporating BC analysis will result in a greater knowledge of the impact of these prevalent changes on IBD therapy and natural history and outcomes.
The major limitation of this review is the quality of data in terms of heterogeneity between study designs and the small sample sizes in some cohorts, which did not allow for quantitative analysis. Furthermore, not all studies assessed the impact of confounders such as diet, exercise, smoking or underlying disease profile. Age represents an important additional confounder that is associated with BC alterations such as reduced skeletal muscle mass and alterations in fat mass and distribution.85 In addition, age is associated with clinical outcomes in IBD including response to therapy86 and complication rates.83 Accordingly, many of the included studies appropriately accounted for the impact of age by using multivariate regression modelling to control for confounding due to age. Results reporting on the impact of BC changes on clinical outcomes in IBD, including need for surgery and postoperative complications, were significant, even after correcting for the effect of age.
With further regard to the limitations of this review, there was significant variability in the techniques utilized for BC analysis in the included studies. These ranged from BIA and skin fold measures to more advanced techniques using CT imaging and software developed specifically for identifying different tissue compartments. Despite these limitations, this review serves to highlight that most patients with IBD experience an alteration in their BC with the most likely being reduction in fat mass, while changes in lean body mass [FFM] independent of BMI are seen in those with active disease. CD has been associated with greater changes in FM and FFM compared with UC. Furthermore, these changes impact greatly upon clinical outcomes, with associations between sarcopaenia and myopaenia as well as higher rates of treatment failure and post-operative morbidity.
4.1. Mechanisms for alteration in BC
Disease activity may contribute to muscle depletion via several mechanisms. Simplistically, malnutrition and inflammation lead to a decrease in muscle cross-sectional area via a catabolic state. Malnutrition according to BMI has been found in 14% of CD patients and 6% of UC patients.56 Furthermore, reduced physical activity because of factors including fatigue and active symptoms may impact upon BC in IBD patients.5
Using albumin as a surrogate marker of disease activity and protein synthesis, elevated whole-body protein synthesis and breakdown in IBD contribute to increased metabolic demands, including energy expenditure, during active gastrointestinal inflammation.49,81 Concurrently, dietary intake is frequently compromised in IBD due to cytokine-induced anorexia and food avoidance due to gastrointestinal symptoms.87 Therefore, metabolic demands of the inflammatory response, including the synthesis of acute phase proteins, are complicated by inadequate dietary intake during gastrointestinal inflammation. In particular, it has been demonstrated that 86.7% of IBD patients with active disease have dietary protein intakes below the recommended 1.2 g/kg while even 36.8% of patients in remission had protein intakes below the recommended 0.8 mg/kg.88 Furthermore, in an animal model, it was shown that disease activity diverts protein synthesis towards albumin production at the expense of muscle protein synthesis.89
BC also comprises fat measurements, and this includes the various components in which fat is stored. It is therefore important to differentiate between subcutaneous and visceral fat. It is thought that mesenteric adipose tissue secretes hormones and molecules which have a role in regulating inflammation.90 Pre-adipocytes have direct phagocytic function and can differentiate into macrophages.91 The roles of fat are being unravelled through molecular studies with a possible difference between UC and CD. A pathway of inflammation [IL-17a] is activated via interaction with cytokines released from pre-adipocytes of IBD patients. It has been shown in IBD patients that higher levels of Substance P [SP] results in upregulation of its respective receptor [NK-1R] along with differential inflammatory cytokine production between UC and CD patients, with lower expression of mRNA and protein level of IL-8 and IL-10 in response to SP treatment of adipocytes. Adipocytes are known to have inflammatory-modulating effects which in an IBD setting may result in phenotypic variation as well as drive therapeutic targets in the future.92
With regard to the impact of adiposity on post-operative outcomes in IBD, two studies included in this review appear to draw conflicting conclusions.7,8 The first, by Connelly et al.,8 determined that increases in the ratio of visceral adiposity [VA] to subcutaneous adiposity [SA] are associated with higher odds of poor 30-day postoperative outcomes in IBD patients undergoing ileocolectomy. This conforms with current understanding that visceral fat is more likely to be pro-inflammatory and is associated with a greater prediction of morbidity than subcutaneous fat.93,94 In contrast, Stidham et al.7 found that a higher subcutaneous [SA] to visceral fat [VA] ratio was associated with an increased risk of post-operative infectious complications following bowel resection. While on the surface these results appear contradictory, there are several important factors which limit their comparability. First, Stidham et al. specifically examined the impact of adiposity on post-operative infections, a narrower outcome than that of postoperative complications, which was used by Connelly et al. In addition, the studies measured adiposity in different ways. While Connelly et al. measured VA and SA at five axial levels [at L1–L5 vertebra] and calculated the mean VA to SA ratio from these measurements, Stidham et al. instead compared the relative change in subcutaneous [SA] to visceral fat [VA] distribution between the most cephalad [T10] and most caudal [L5] spinal levels within the abdominal field. As a result, the findings in Stidham et al. reflect the impact of the distribution of fat between different regions of the abdomen, while those in Connelly et al. reflects the effect of the composition of abdominal fat in the lumbar region, the latter being the more customary approach in the literature.
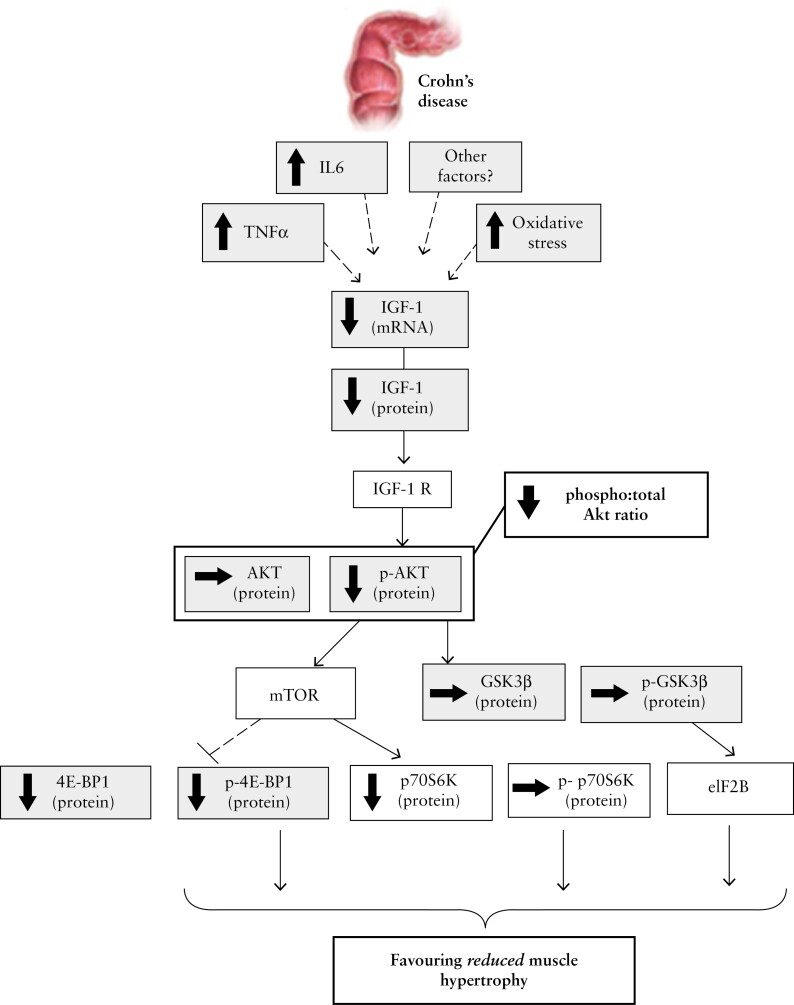
Moreover, it is known that fatigue is an important symptom, which affects IBD patients independent of anaemia. Data show that self-reported fatigue in this setting correlates with muscle depletion.95 Work from van Langenberg et al. demonstrated that subjects had lower serum vitamin D, IGF1 and magnesium, along with higher IL-6 levels, which correlated with muscle fatigue. The same authors also showed that muscle size was 14% lower in patients with CD compared to healthy controls when measured at the quadriceps muscle. They concluded that this could be in part due to impaired activation of muscle protein synthesis pathways with the IGF1-Akt pathway.96 A summary of the signalling targets involved with active CD resulting in reduced muscle hypertrophy is presented in Figure 2 [with permission from Dr van Langenberg].96 Skeletal muscle preservation may also be protective against worsening IBD outcomes as cytokines released by contracting skeletal muscle may generate anti-inflammatory effects and inhibit proinflammatory mediators from visceral fat.10
Figure 2.
Diagram representing the attenuated muscle hypertrophy pathway as reflected by signalling factor levels in CD.96
Inflammation and decreased physical activity are key characteristic features of IBD. Although bed rest alone can result in ~5% loss of LBM in healthy volunteers, the effects of severe stress combined with muscle unloading can be 2- to 3-fold greater.97 The systemic inflammatory response involves stimulation of a number of mediators [mainly cortisol and proinflammatory cytokines], which can directly activate autophagy in skeletal muscle as well as inhibit synthesis of myofibrillar proteins.98 Quality of life significantly decreases with a greater sedentary existence in IBD patients.10 With this perspective, it is important to prevent the loss of muscle and possible gain of visceral fat along with additional pro- and anti-inflammatory properties.
4.2. How to augment body composition favourably?
Altering the BC of IBD patients through physical exercise, medication, nutritional screening and assessment to guide enteral nutrition, including the use of protein-rich nutrition, rehabilitation and close monitoring may be possible as a holistic approach to patient care. Physical activity may alter outcomes in IBD, with resistance exercise shown to prevent and even reverse the progression of sarcopaenia.99 Resistance exercise has been demonstrated to increase muscle cell proliferation, protein synthesis and translational efficiency acutely, while increasing cell numbers as well as the number of myonuclear domains per myofibre chronically. Exercise can also ameliorate the severity of CD and accompanying anorexia while modifying the release of adipokines and ghrelin.100 The effect of lifestyle on the onset of IBD was studied in several studies and physical activity in the pre-illness period was protective against the onset of IBD,101 but this association was stronger for CD than for UC.102
Studies on sedentary patients with inactive or mildly active CD have shown that moderate exercise such as walking programmes or yoga lead to significant improvement in the measures of the quality of life and stress levels.103 Almost 1000 IBD patients [54% with CD and 46% with UC] from the UK completed an online survey which showed that most respondents were undertaking regular exercise, and exercise was found to be beneficial for the symptoms of IBD. However, most of the respondents were requested to stop exercising at some point because of the severity of their symptoms.10
Rehabilitation medicine, with its focus on optimizing function after treatment despite a patient’s symptom burden or impairments, may be beneficial in patients with myopaenia. Indeed, it seems reasonable that optimal physical improvement before treatment [prehabilitation] might result in better outcomes, as seen in oncology patients.104
Appetite regulation and level of physical activity are the major determinants of energy balance and BC changes. It has been suggested that inflammatory mediators play a vital role in appetite regulation. In most patients, systemic inflammation leads to anorexia and fat loss in combination with myopaenia. In others, appetite is sustained, despite activation of systemic inflammation, leading to the association of myopaenia with increased BMI, a condition described as myopaenic obesity. This broad phenotypic variability within the same disease needs careful tailoring of nutritional intervention. Protein intake should be increased to counteract the anabolic resistance associated with inflammation and inactivity.13
Nutritional support or intestinal rehabilitation is integral to supporting IBD patients through acute severe flares of disease. These often require hospitalization and intravenous medical therapy. Along with the potential muscle loss, nutritional deprivation and protein losing enteropathy, patients may require surgery which may be associated with worse, morbidity and mortality. Concomitant enteral nutrition along with infliximab in CD patients has been shown to be more effective than infliximab alone on multivariate analysis.105 In a further study of patients treated with enteral nutrition [EN] >600 kcal/day vs controls, it was found that those given EN [elemental and polymeric diet] were less likely to lose response to infliximab [OR = 0.23, p = 0.0043].106
Of interest, a recent publication demonstrated that in 19 patients treated with infliximab, there was an increase in muscle volume in both legs [reversal of sarcopaenia], which resulted in an increase in muscle strength. Therefore, treatment of the underlying disease alone may halt and indeed reverse sarcopaenia in IBD patients.77
Given the association between BC alterations in IBD such as sarcopaenia and increased adiposity and poor clinical outcomes highlighted here, a key clinical implication of this review is that it reinforces the utility of routine nutritional screening in the care of patients with IBD. The modulation of BC through nutritional intervention may therefore help optimize IBD outcomes, including reducing rates of treatment failure, reducing the need for surgical interventional and lowering the incidence of post-operative complications.
Consistent with current guidelines,107 a practical means of identifying patients who may benefit from review by a registered dietician for consideration of nutritional interventions includes the use of nutritional screening tools [NSTs] in the clinical setting. Multiple NSTs exist, and some have been shown to correlate well with the results from nutritional assessment tools, although at present there is a lack of comprehensive validation to allow recommendation of a single best NST for clinical use.108
Furthermore, the findings of this review highlight the importance of clinically deliverable BC assessment tools in routine clinical practice in the future. Many of the methods of BC assessment utilized in the studies examined in this review are suitable for use in routine clinical practice. For example, basic clinical anthropometry techniques including skin fold assessment and BIA can be readily performed within the clinical setting and enable monitoring of BC over time. In addition, owing to the growing utilization of imaging in IBD management, there is also the potential for more sophisticated methods of BC analysis to become a more routine component of clinical management of IBD in the future. For example, CT or MRI obtained for the purposes of disease assessment and monitoring treatment response109 may be additionally utilized for conducting fat and muscle segmentation to offer a more sensitive means of detecting BC alterations which may impact on IBD management.
5. Conclusion
BC changes occur in IBD in all stages of the disease and are often an occult feature. Myopaenia is a multidimensional condition and imposes a significant impact on the patient’s quality of life and economic burden on healthcare services. With technological advances, reliable diagnostic tools for identifying these changes are possible. IBD care is undergoing a vital shift from a disease-focused management to a more patient–centred approach. Measuring and reporting quality of care drives refinement and an ideal quality improvement framework should be primarily based on the structure, process and outcome of care; hence updated IBD management pathways should take into consideration muscle monitoring as an important metric captured in quality-of-life surveys. To implement an optimal multimodal management of myopaenia in patients with IBD, the following items are required: first, awareness about the impact of muscle deficits; second, expertise in recognising and monitoring relevant deficits; third, knowledge about the relevant treatment options; and, finally, an ability to implement treatment options. The development of such a multimodal package together with the improvement of novel therapeutics promises a new era in the holistic care of IBD.
Acknowledgments
We thank A/Prof. Daniel van Langenberg for permission to reproduce the diagram seen in Figure 2 illustrating attenuating muscle hypertrophy pathways in CD.
Contributor Information
Nik Sheng Ding, Inflammatory Bowel Disease Unit, St Mark’s Hospital, Harrow, UK; Gastroenterology Department, St Vincent’s Hospital, Melbourne, Australia.
Daniel Tassone, Gastroenterology Department, St Vincent’s Hospital, Melbourne, Australia.
Ibrahim Al Bakir, Inflammatory Bowel Disease Unit, St Mark’s Hospital, Harrow, UK.
Kyle Wu, Gastroenterology Department, St Vincent’s Hospital, Melbourne, Australia.
Alexander J Thompson, Gastroenterology Department, St Vincent’s Hospital, Melbourne, Australia.
William R Connell, Gastroenterology Department, St Vincent’s Hospital, Melbourne, Australia.
George Malietzis, Department of Surgery and Cancer, Imperial College, London, UK.
Phillip Lung, Inflammatory Bowel Disease Unit, St Mark’s Hospital, Harrow, UK.
Siddharth Singh, Division of Gastroenterology and Division of Biomedical Informatics, University of California San Diego, La Jolla, California, USA.
Chang-ho Ryan Choi, Inflammatory Bowel Disease Unit, St Mark’s Hospital, Harrow, UK.
Simon Gabe, Inflammatory Bowel Disease Unit, St Mark’s Hospital, Harrow, UK.
John T Jenkins, Department of Surgery and Cancer, Imperial College, London, UK.
Ailsa Hart, Inflammatory Bowel Disease Unit, St Mark’s Hospital, Harrow, UK.
Funding
None.
Conflict of Interest
S.S. is supported by the National Library of Medicine training grant T15LM011271, the American College of Gastroenterology Junior Faculty Development Award, the Crohn’s, and Colitis Foundation of American Career Development Award and by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers K23DK117058 and R03DK129631.
Author Contributions
N.D. (co-first author and corresponding author): study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. D.T.[co-first author]: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. I.A.B.: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. K.W.: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. A.T.: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. W.C.: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. G.M.: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. P.L.: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. S.S.: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. C.R.C.: analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. S.G.: interpretation of data; critical revision of the manuscript for important intellectual content. J.T.J.: interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; study supervision. A.H.: study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content; statistical analysis; study supervision
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Bryant RV, Ooi S, Schultz CG, et al. Low muscle mass and sarcopenia: common and predictive of osteopenia in inflammatory bowel disease. Aliment Pharmacol Ther 2015;41:895–906. [DOI] [PubMed] [Google Scholar]
- 2. Tjellesen L, Nielsen PK, Staun M.. Body composition by dual-energy X-ray absorptiometry in patients with Crohn’s disease. Scand J Gastroenterol 1998;33:956–60. [DOI] [PubMed] [Google Scholar]
- 3. Thangarajah D, Hyde MJ, Konteti VK, Santhakumaran S, Frost G, Fell JM.. Systematic review: body composition in children with inflammatory bowel disease. Aliment Pharmacol Ther 2015;42:142–57. [DOI] [PubMed] [Google Scholar]
- 4. Jahnsen J, Falch JA, Mowinckel P, Aadland E.. Body composition in patients with inflammatory bowel disease: a population-based study. Am J Gastroenterol 2003;98:1556–62. [DOI] [PubMed] [Google Scholar]
- 5. Sousa Guerreiro C, Cravo M, Costa AR, et al. A comprehensive approach to evaluate nutritional status in Crohn’s patients in the era of biologic therapy: a case-control study. Am J Gastroenterol 2007;102:2551–6. [DOI] [PubMed] [Google Scholar]
- 6. Erhayiem B, Dhingsa R, Hawkey CJ, Subramanian V.. Ratio of visceral to subcutaneous fat area is a biomarker of complicated Crohn’s disease. Clin Gastroenterol Hepatol 2011;9:684–7.e1. [DOI] [PubMed] [Google Scholar]
- 7. Stidham RW, Waljee AK, Day NM, et al. Body fat composition assessment using analytic morphomics predicts infectious complications after bowel resection in Crohn’s disease. Inflamm Bowel Dis 2015;21:1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Connelly TM, Juza RM, Sangster W, Sehgal R, Tappouni RF, Messaris E.. Volumetric fat ratio and not body mass index is predictive of ileocolectomy outcomes in Crohn’s disease patients. Dig Surg 2014;31:219–24. [DOI] [PubMed] [Google Scholar]
- 9. Csontos ÁA, Molnár A, Miheller P.. Letter: body surface area and body muscle parameters may influence adalimumab trough levels. Aliment Pharmacol Ther 2015;41:700. [DOI] [PubMed] [Google Scholar]
- 10. Bilski J, Brzozowski B, Mazur-Bialy A, Sliwowski Z, Brzozowski T.. The role of physical exercise in inflammatory bowel disease. Biomed Res Int 2014;2014:429031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE.. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 12. Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol 2014;210:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guadagni M, Biolo G.. Effects of inflammation and/or inactivity on the need for dietary protein. Curr Opin Clin Nutr Metab Care 2009;12:617–22. [DOI] [PubMed] [Google Scholar]
- 14. Carli F, Zavorsky GS.. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care 2005;8:23–32. [DOI] [PubMed] [Google Scholar]
- 15. Walsh MC, Hunter GR, Livingstone MB.. Sarcopenia in premenopausal and postmenopausal women with osteopenia, osteoporosis and normal bone mineral density. Osteoporos Int 2006;17:61–7. [DOI] [PubMed] [Google Scholar]
- 16. Rosenberg IH, editor. Symposium: Sarcopenia: Diagnosis and Mechanisms. Boston, MA: Jean Mayer Human Nutrition Research Center on Aging; Tufts University; 1997. [Google Scholar]
- 17. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–63. [DOI] [PubMed] [Google Scholar]
- 18. Sylvester FA, Leopold S, Lincoln M, Hyams JS, Griffiths AM, Lerer T.. A two-year longitudinal study of persistent lean tissue deficits in children with Crohn’s disease. Clin Gastroenterol Hepatol 2009;7:452–5. [DOI] [PubMed] [Google Scholar]
- 19. Bin CM, Flores C, Alvares-da-Silva MR, Francesconi CF.. Comparison between handgrip strength, subjective global assessment, anthropometry, and biochemical markers in assessing nutritional status of patients with Crohn’s disease in clinical remission. Dig Dis Sci 2010;55:137–44. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. 2000. http://www.ohri.ca/Programs/clinical_epidemiology/oxford.asp.
- 22. Downes MJ, Brennan ML, Williams HC, Dean RS.. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016;6:e011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adams DW, Gurwara S, Silver HJ, et al. Sarcopenia is common in overweight patients with inflammatory bowel disease and may predict need for surgery. Inflamm Bowel Dis 2017;23:1182–6. [DOI] [PubMed] [Google Scholar]
- 24. Back IR, Marcon SS, Gaino NM, Vulcano DSB, Dorna MS, Sassaki LY.. Body composition in patients with Crohn’s disease and ulcerative colitis. Arq Gastroenterol 2017;54:109–14. [DOI] [PubMed] [Google Scholar]
- 25. Bamba S, Inatomi O, Takahashi K, et al. Assessment of body composition from ct images at the level of the third lumbar vertebra in inflammatory bowel disease. Inflamm Bowel Dis 2021;27:1435–42. [DOI] [PubMed] [Google Scholar]
- 26. Bamba S, Sasaki M, Takaoka A, et al. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn’s disease. PLoS One 2017;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barroso T, Conway F, Emel S, et al. Patients with inflammatory bowel disease have higher abdominal adiposity and less skeletal mass than healthy controls. Ann Gastroenterol. 2018;31(5):566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benjamin J, Makharia G, Ahuja V, Joshi YK.. Body composition in Indian patients with Crohn’s disease during active and remission phase. Trop Gastroenterol 2011;32:285–91. [PubMed] [Google Scholar]
- 29. Berger M, Yamada A, Komaki Y, et al. Low skeletal muscle index adjusted for body mass index is an independent risk factor for inflammatory bowel disease surgical complications. Crohns Colitis 360 2020;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boparai G, Kedia S, Kandasamy D, et al. Combination of sarcopenia and high visceral fat predict poor outcomes in patients with Crohn’s disease. Eur J Clin Nutr 2021;75:1491–8. [DOI] [PubMed] [Google Scholar]
- 31. Bryant RV, Schultz CG, Ooi S, et al. Visceral adipose tissue is associated with stricturing crohn’s disease behavior, fecal calprotectin, and quality of life. Inflamm Bowel Dis. 2019;25(3):592–600. [DOI] [PubMed] [Google Scholar]
- 32. Capristo E, Addolorato G, Mingrone G, Greco AV, Gasbarrini G.. Effect of disease localization on the anthropometric and metabolic features of Crohn’s disease. Am J Gastroenterol 1998;93:2411–9. [DOI] [PubMed] [Google Scholar]
- 33. Capristo E, Mingrone G, Addolorato G, Greco AV, Gasbarrini G.. Metabolic features of inflammatory bowel disease in a remission phase of the disease activity. J Intern Med 1998;243:339–47. [DOI] [PubMed] [Google Scholar]
- 34. Cravo ML, Velho S, Torres J, et al. Lower skeletal muscle attenuation and high visceral fat index are associated with complicated disease in patients with Crohn’s disease: an exploratory study. Clin Nutr ESPEN 2017;21:79–85. [DOI] [PubMed] [Google Scholar]
- 35. Cuoco L, Vescovo G, Castaman R, et al. Skeletal muscle wastage in Crohn's disease: a pathway shared with heart failure? Int J Cardiol. 2008;127(2):219–27. [DOI] [PubMed] [Google Scholar]
- 36. Cushing KC, Kordbacheh H, Gee MS, Kambadakone A, Ananthakrishnan AN.. Sarcopenia is a novel predictor of the need for rescue therapy in hospitalized ulcerative colitis patients. J Crohns Colitis. 2018;12(9):1036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ding NS, Malietzis G, Lung PFC, et al. The body composition profile is associated with response to anti-TNF therapy in Crohn’s disease and may offer an alternative dosing paradigm. Aliment Pharmacol Ther 2017;46:883–91. [DOI] [PubMed] [Google Scholar]
- 38. Filippi J, Al-Jaouni R, Wiroth JB, Hébuterne X, Schneider SM.. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm Bowel Dis 2006;12:185–91. [DOI] [PubMed] [Google Scholar]
- 39. Fujikawa H, Araki T, Okita Y, et al. Impact of sarcopenia on surgical site infection after restorative proctocolectomy for ulcerative colitis. Surgery Today 2017;47:92–8. [DOI] [PubMed] [Google Scholar]
- 40. Galata C, Hodapp J, Weiß C, et al. Skeletal muscle mass index predicts postoperative complications in intestinal surgery for Crohn’s disease. J Parenter Enteral Nutr 2020;44:714–21. [DOI] [PubMed] [Google Scholar]
- 41. Geerling BJ, Badart-Smook A, Stockbrügger RW, Brummer RJ.. Comprehensive nutritional status in patients with long-standing Crohn disease currently in remission. Am J Clin Nutr 1998;67:919–26. [DOI] [PubMed] [Google Scholar]
- 42. Gong J, Zuo L, Guo Z, et al. Impact of disease activity on resting energy expenditure and body composition in adult Crohn’s disease: a prospective longitudinal assessment. J Parenter Enteral Nutr 2015;39:713–8. [DOI] [PubMed] [Google Scholar]
- 43. Grillot J, D'Engremont C, Parmentier AL, et al. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn’s disease. Clin Nutr. 2020;39(10):3024–30. [DOI] [PubMed] [Google Scholar]
- 44. Holt DQ, Varma P, Strauss BJG, Rajadurai AS, Moore GT.. Low muscle mass at initiation of anti-TNF therapy for inflammatory bowel disease is associated with early treatment failure: a retrospective analysis. Eur J Clin Nutr 2017;71:773–7. [DOI] [PubMed] [Google Scholar]
- 45. Holt DQ, Moore GT, Strauss BJG, Hamilton AL, De Cruz P, Kamm MA.. Visceral adiposity predicts post-operative Crohn’s disease recurrence. Alimentary Pharmacology & Therapeutics. 2017;45(9):1255–64. [DOI] [PubMed] [Google Scholar]
- 46. Katznelson L, Fairfield WP, Zeizafoun N, et al. Effects of growth hormone secretion on body composition in patients with Crohn’s disease. J Clin Endocrinol Metab 2003;88:5468–72. [DOI] [PubMed] [Google Scholar]
- 47. Labarthe G, Dolores M, Verdalle-Cazes M, et al. Magnetic resonance imaging assessment of body composition parameters in Crohn’s disease. Dig Liver Dis 2020;52:878–84. [DOI] [PubMed] [Google Scholar]
- 48. Leslie WD, Miller N, Rogala L, Bernstein CN.. Body mass and composition affect bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Inflamm Bowel Dis. 2009;15(1):39–46. [DOI] [PubMed] [Google Scholar]
- 49. Lim Z, Welman CJ, Raymond W, Thin L.. The effect of adiposity on anti-tumor necrosis factor-alpha levels and loss of response in Crohn’s disease patients. Clin Transl Gastroenterol 2020;11:e00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Daniela M, Barreto RM, Everton C, et al. Visceral fat is increased in individuals with crohn’s disease: a comparative analysis with healthy controls: P-045. Am J Gastroenterol. 2018;113:S11. [DOI] [PubMed] [Google Scholar]
- 51. Mingrone G, Greco AV, Benedetti G, et al. Increased resting lipid oxidation in Crohn’s disease. Dig Dis Sci 1996;41:72–6. [DOI] [PubMed] [Google Scholar]
- 52. Mingrone G, Capristo E, Greco AV, et al. Elevated diet-induced thermogenesis and lipid oxidation rate in Crohn disease. Am J Clin Nutr 1999;69:325–30. [DOI] [PubMed] [Google Scholar]
- 53. Molnár A, Csontos ÁA, Kovács I, Anton ÁD, Pálfi E, Miheller P.. Body composition assessment of Crohn’s outpatients and comparison with gender- and age-specific multiple matched control pairs. Eur J Clin Nutr. 2017;71(10):1246–50. [DOI] [PubMed] [Google Scholar]
- 54. O’Brien S, Kavanagh RG, Carey BW, Maher MM, O’Connor OJ, Andrews EJ.. The impact of sarcopenia and myosteatosis on postoperative outcomes in patients with inflammatory bowel disease. European Radiology Experimental. 2018;2(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pizzoferrato M, de Sire R, Ingravalle F, et al. Characterization of Sarcopenia in an IBD Population Attending an Italian Gastroenterology Tertiary Center. Nutrients. 2019;11(10):2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rocha R, Santana GO, Almeida N, Lyra AC.. Analysis of fat and muscle mass in patients with inflammatory bowel disease during remission and active phase. Br J Nutr 2009;101:676–9. [DOI] [PubMed] [Google Scholar]
- 57. Santos JCD, Malaguti C, Lucca FA, et al. Impact of biological therapy on body composition of patients with Crohn’s disease. Rev Assoc Med Bras 2017;63:407–13. [DOI] [PubMed] [Google Scholar]
- 58. Schneider SM, Al-Jaouni R, Filippi J, et al. Sarcopenia is prevalent in patients with Crohn’s disease in clinical remission. Inflamm Bowel Dis 2008;14:1562–8. [DOI] [PubMed] [Google Scholar]
- 59. Sigurdsson GV, Schmidt S, Mellström D, et al. Altered body composition profiles in young adults with childhood-onset inflammatory bowel disease. Scand J Gastroenterol 2020;55:169–77. [DOI] [PubMed] [Google Scholar]
- 60. Steell L, Johnston BA, Dewantoro D, et al. Muscle deficits with normal bone microarchitecture and geometry in young adults with well-controlled childhood-onset Crohn’s disease. Eur J Gastroenterol Hepatol 2020;32:1497–506. [DOI] [PubMed] [Google Scholar]
- 61. Thiberge C, Charpentier C, Gillibert A, et al. Lower subcutaneous or visceral adiposity assessed by abdominal computed tomography could predict adverse outcome in patients with crohn’s disease. J Crohns Colitis. 2018;12(12):1429–37. [DOI] [PubMed] [Google Scholar]
- 62. Ulivieri FM, Piodi LP, Taioli E, et al. Bone mineral density and body composition in ulcerative colitis: a six-year follow-up. Osteoporos Int. 2001;12(5):343–8. [DOI] [PubMed] [Google Scholar]
- 63. Ünal NG, Oruç N, Tomey O, Ömer Özütemiz A.. Malnutrition and sarcopenia are prevalent among inflammatory bowel disease patients with clinical remission. Eur J Gastroenterol Hepatol 2021;33:1367–75. [DOI] [PubMed] [Google Scholar]
- 64. Urbano APS, Sassaki LY, Dorna MS, et al. Associations among body composition, inflammatory profile and disease extent in ulcerative colitis patients. Rev Assoc Med Bras 2018;64:133–9. [DOI] [PubMed] [Google Scholar]
- 65. Vaisman N, Dotan I, Halack A, Niv E.. Malabsorption is a major contributor to underweight in Crohn’s disease patients in remission. Nutrition 2006;22:855–9. [DOI] [PubMed] [Google Scholar]
- 66. Valentini L, Schaper L, Buning C, et al. Malnutrition and impaired muscle strength in patients with Crohn’s disease and ulcerative colitis in remission. Nutrition 2008;24:694–702. [DOI] [PubMed] [Google Scholar]
- 67. Van Der Sloot KW, Joshi AD, Bellavance DR, et al. Visceral adiposity, genetic susceptibility, and risk of complications among individuals with crohn’s disease. Inflamm Bowel Dis. 2017;23(1):82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yadav DP, Kedia S, Madhusudhan KS, et al. Body composition in crohn’s disease and ulcerative colitis: correlation with disease severity and duration. Can J Gastroenterol Hepatol. 2017;2017:1215035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zager Y, Khalilieh S, Ganaiem O, et al. Low psoas muscle area is associated with postoperative complications in Crohn’s disease. Int J Colorectal Dis 2021;36:543–50. [DOI] [PubMed] [Google Scholar]
- 70. Zalizko P, Roshofa TH, Meija L, Bodnieks E, Pukitis A.. The role of body muscle mass as an indicator of activity in inflammatory bowel disease patients. Clin Nutr ESPEN. 2020;40:193–200. [DOI] [PubMed] [Google Scholar]
- 71. Zaltman C, Braulio VB, Outeiral R, Nunes T, de Castro CL.. Lower extremity mobility limitation and impaired muscle function in women with ulcerative colitis. J Crohns Colitis 2014;8:529–35. [DOI] [PubMed] [Google Scholar]
- 72. Zhang T, Ding C, Xie T, et al. Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clin Nutr 2017;36:1586–92. [DOI] [PubMed] [Google Scholar]
- 73. Zhang T, Cao L, Cao T, et al. Prevalence of sarcopenia and its impact on postoperative outcome in patients with Crohn’s disease undergoing bowel resection. J Parenter Enteral Nutr 2017;41:592–600. [DOI] [PubMed] [Google Scholar]
- 74. Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol 2001;33:289–94. [DOI] [PubMed] [Google Scholar]
- 75. Mingrone G, Benedetti G, Capristo E, et al. Twenty-four-hour energy balance in Crohn disease patients: metabolic implications of steroid treatment. Am J Clin Nutr 1998;67:118–23. [DOI] [PubMed] [Google Scholar]
- 76. Kumar S, Dutt A, Hemraj S, Bhat S, Manipadybhima B.. Phase angle measurement in healthy human subjects through bio-impedance analysis. Iran J Basic Med Sci 2012;15:1180–4. [PMC free article] [PubMed] [Google Scholar]
- 77. Subramaniam K, Fallon K, Ruut T, et al. Infliximab reverses inflammatory muscle wasting (sarcopenia) in Crohn’s disease. Aliment Pharmacol Ther 2015;41:419–28. [DOI] [PubMed] [Google Scholar]
- 78. Bryant RV, Trott MJ, Bartholomeusz FD, Andrews JM.. Systematic review: body composition in adults with inflammatory bowel disease. Aliment Pharmacol Ther 2013;38:213–25. [DOI] [PubMed] [Google Scholar]
- 79. Bechtold S, Alberer M, Arenz T, et al. Reduced muscle mass and bone size in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2010;16:216–25. [DOI] [PubMed] [Google Scholar]
- 80. Rossi AP, Rubele S, Zamboni M.. Sarcopenic obesity. In: Walrand S, editor. Nutrition and Skeletal Muscle. New York, NY: Academic Press; 2019: 83–92. [Google Scholar]
- 81. Ding NS, Malietzis G, Lung PF, et al. 186 body composition profile: a predictor of therapeutic outcome in patients with moderate to severe Crohn’s disease. Gastroenterology 2016;150:S48–S9. [Google Scholar]
- 82. Farraye FA, Qazi T, Kotze PG, et al. The impact of body mass index on efficacy and safety in the tofacitinib OCTAVE ulcerative colitis clinical programme. Aliment Pharmacol Ther 2021;54:429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Malietzis G, Currie AC, Athanasiou T, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg 2016;103:572–80. [DOI] [PubMed] [Google Scholar]
- 84. Malietzis G, Currie AC, Johns N, et al. Skeletal muscle changes after elective colorectal cancer resection: a longitudinal study. Ann Surg Oncol 2016;23:2539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. St-Onge M-P, Gallagher D.. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition 2010;26:152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. LeBlanc J-F, Wiseman D, Lakatos PL, Bessissow T.. Elderly patients with inflammatory bowel disease: updated review of the therapeutic landscape. World J Gastroenterol 2019;25:4158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rigaud D, Angel LA, Cerf M, et al. Mechanisms of decreased food intake during weight loss in adult Crohn’s disease patients without obvious malabsorption. Am J Clin Nutr 1994;60:775–81. [DOI] [PubMed] [Google Scholar]
- 88. Peters V, Tigchelaar-Feenstra EF, Imhann F, et al. Habitual dietary intake of IBD patients differs from population controls: a case–control study. European Journal of Nutrition 2021;60:345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mackenzie ML, Warren MR, Wykes LJ.. Colitis increases albumin synthesis at the expense of muscle protein synthesis in macronutrient-restricted piglets. J Nutr 2003;133:1875–81. [DOI] [PubMed] [Google Scholar]
- 90. Peyrin-Biroulet L, Chamaillard M, Gonzalez F, et al. Mesenteric fat in Crohn’s disease: a pathogenetic hallmark or an innocent bystander? Gut 2007;56:577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Charrière G, Cousin B, Arnaud E, et al. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem 2003;278:9850–5. [DOI] [PubMed] [Google Scholar]
- 92. Sideri A, Bakirtzi K, Shih DQ, et al. Substance P mediates pro-inflammatory cytokine release form mesenteric adipocytes in inflammatory bowel disease patients. Cell Mol Gastroenterol Hepatol 2015;1:420–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Osborn O, Olefsky JM.. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 2012;18:363–74. [DOI] [PubMed] [Google Scholar]
- 94. Desreumaux P, Ernst O, Geboes K, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology 1999;117:73–81. [DOI] [PubMed] [Google Scholar]
- 95. van den Heuvel TR, Wintjens DS, Jeuring SF, et al. Inflammatory bowel disease, cancer and medication: cancer risk in the Dutch population-based IBDSL cohort. Int J Cancer 2016;139:1270–80. [DOI] [PubMed] [Google Scholar]
- 96. van Langenberg DR, Della Gatta P, Hill B, Zacharewicz E, Gibson PR, Russell AP.. Delving into disability in Crohn’s disease: dysregulation of molecular pathways may explain skeletal muscle loss in Crohn’s disease. J Crohns Colitis 2014;8:626–34. [DOI] [PubMed] [Google Scholar]
- 97. Mawdsley JE, Rampton DS.. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut 2005;54:1481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ.. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- 99. Verdijk LB, Gleeson BG, Jonkers RAM, et al. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci 2009;64:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bilski J, Mazur-Bialy AI, Wierdak M, Brzozowski T.. The impact of physical activity and nutrition on inflammatory bowel disease: the potential role of cross talk between adipose tissue and skeletal muscle. J Physiol Pharmacol 2013;64:143–55. [PubMed] [Google Scholar]
- 101. Sonnenberg A. Occupational distribution of inflammatory bowel disease among German employees. Gut 1990;31:1037–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Persson PG, Leijonmarck CE, Bernell O, Hellers G, Ahlbom A.. Risk indicators for inflammatory bowel disease. Int J Epidemiol 1993;22:268–72. [DOI] [PubMed] [Google Scholar]
- 103. Loudon CP, Corroll V, Butcher J, Rawsthorne P, Bernstein CN.. The effects of physical exercise on patients with Crohn’s disease. Am J Gastroenterol 1999;94:697–703. [DOI] [PubMed] [Google Scholar]
- 104. Silver JK, Baima J.. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil 2013;92:715–27. [DOI] [PubMed] [Google Scholar]
- 105. Hirai F, Ishihara H, Yada S, et al. Effectiveness of concomitant enteral nutrition therapy and infliximab for maintenance treatment of Crohn’s disease in adults. Dig Dis Sci 2013;58:1329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sazuka S, Katsuno T, Nakagawa T, et al. Concomitant use of enteral nutrition therapy is associated with sustained response to infliximab in patients with Crohn’s disease. Eur J Clin Nutr 2012;66:1219–23. [DOI] [PubMed] [Google Scholar]
- 107. Bischoff SC, Escher J, Hébuterne X, et al. ESPEN practical guideline: clinical Nutrition in inflammatory bowel disease. Clin Nutr 2020;39:632–53. [DOI] [PubMed] [Google Scholar]
- 108. Li S, Ney M, Eslamparast T, et al. Systematic review of nutrition screening and assessment in inflammatory bowel disease. World J Gastroenterol 2019;25:3823–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144–64K. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.