Abstract
Spinal cord injuries pose grave medical and socioeconomic burdens warranting measures for early diagnosis, triaging, prognostication and therapeutics. Imaging has since long played a pivotal role in this regard, with continuing research and technological advancements opening newer frontiers. One such advanced Magnetic resonance (MR) technique is Diffusion tensor imaging (DTI) which assesses cord microstructure by tracking the movement of water molecules in biological tissues. DTI utilizes the principle of anisotropy exhibited by the normal compact white matter (WM) tracts of the cord, in which direction-dependent water molecular motion is seen along the axonal axis. Disruption of this complex structure in response to injury alters the movement of these molecules, interrupting anisotropy and thereby DTI metrics.
Evaluation of DTI images can be done both by quantitative indices, of which fractional anisotropy (FA) and mean diffusivity (MD) are the most commonly used and by qualitative fiber tracking (tractography) methods in which three-dimensional WM tracts are reconstructed by algorithmic post-processing. Reduced FA is consistently seen at injury sites as a direct consequence of disturbance of anisotropy. Diffusivity values are however more variable with both high and low values recorded across studies. 3D tractography images allow visual assessment of cord integrity, morphology, and orientation. Significant correlation is found between DTI parameters and various spinal injury scores. Furthermore, DTI also helps in accurate lesion mapping and in assessing cord changes distant from injury epicenter providing a holistic evaluation.
From its inception, consistent progress in the understanding and application of DTI has effectuated its clinical utility and impact. Incorporation into day-to-day diagnostics is however still challenging, due to suboptimal image acquisition, difficult post-processing, and lack of standardized protocols & image interpretation guidelines. Further research with technical validation, development of normative and disease data sets, and histological confirmation will help establish this novel technique in routine diagnostics.
Keywords: Spinal cord injury, Diffusion tensor imaging, Tractography, Functional anisotropy
Abbreviations: SCI, Spinal cord injury; DTI, Diffusion tensor imaging; FA, Functional Anisotropy
1. Introduction
Spinal cord injury (SCI) is a crippling condition posing sizeable medical and socioeconomic burdens with a yearly global incidence between 250 000 and 500 000.1 The incidence in India was reported at around 15 new cases per million per year.2 The age distribution is bimodal with higher incidences seen in young adults and the elderly, with males being affected twice as frequently as females.1 Timely and accurate assessment of injury status is therefore critical for management, prognostication, and rehabilitation.
With notable advances in technology, imaging has played a key role in estimating the location, severity, and extent of SCI. Conventional MRI, though routinely performed for assessment and grading of cord injury, shows variable correlation with neurological and functional scores.3,4 The routinely used T1W and T2W sequences are limited in their applications, mainly evaluating macroscopic cord changes caused by edema and hemorrhage.5
Basser et al. first described diffusion tensor imaging (DTI) in 1994,6 and at present it is the only in vivo non-invasive technique that assesses morphological and functional microstructural cord integrity.3 Its basis lies in evaluating the size and direction of motion of water molecules based on their interaction with each other and with the surrounding biological structures. This makes DTI sensitive to early cord changes after injury, as molecular changes precede macroscopic changes, making it suitable for prompt diagnosis, paramount for timely intervention.3,7
Since its advent, DTI mainly had central neurological applications including assessment of several brain pathologies like tumors, trauma, and demyelination. Promising results have led to further research in the field with recent applications extended to include the skeletal muscles, kidneys, and peripheral nervous system, of which spinal cord evaluation has been most promising. Its use in the cord should seem uncomplicated as the central and peripheral nervous systems show similar histo-anatomical makeup with continuing white matter (WM) tracts. However, small cord area, cardiovascular and respiratory motion, CSF pulsation and adjacent bone susceptibility significantly compromise signal-to-noise ratios making acquisition and interpretation of images challenging.8, 9, 10 Higher field strengths, faster imaging techniques, and cardiac pulse gating have all been variably used to mitigate these limitations and improve the overall image quality.9,11
This article attempts to review and evaluate the research done so far on the role of DTI in SCI.
2. Methodology
A systematic search of PubMed was performed using the advanced search keywords ‘((diffusion tensor imaging [Title]) OR (DTI [Title])) AND ((Spinal cord injury [Title]) OR (SCI [Title]))’ in May 2022 which was not limited by date or study type. All the titles and their abstracts were individually screened to include articles that evaluated the role of DTI in SCI including diagnosis, prognosis and therapeutics in humans. The citations from these shortlisted articles were further analyzed to include additional relevant articles.
3. Fundamentals of DTI: a synopsis
3.1. Principle of DTI
Diffusion-weighted imaging (DWI) analyzes tissue microstructure by assessing the Brownian water molecular motion.8,9,12,13 Image acquisition is done by applying two opposing diffusion-sensitizing gradients with the principle that a high signal is obtained only from those water molecules that are ‘restricted’ and are therefore exposed to both these gradients. Moving spins get exposed to only one of these gradients and are shown as signal void. DWI, therefore, provides valuable information on tissue organization.8,9
In isotropic diffusion, molecular movements are equidirectional8,13 as shown by water at body temperature [Apparent diffusion coefficient (ADC) ∼ 3.0 × 10−3 mm2/s].13
Anisotropy with its non-random direction-dependent diffusion forms the core principle of DTI where it is employed for anatomic mapping and quantitative characterization of WM tracts.9,13 This is done by tracking the direction and magnitude of diffusion of water in each voxel.14 In the highly organized WM tracts of cord, diffusion is seen predominantly along the axonal axis, both craniocaudally and caudocranially with perpendicular diffusion limited by cell membranes and myelin sheaths.8
Diffusion coefficient is a measure of the complete sum of diffusion in all directions with the principal diffusion vector oriented along the maximum diffusivity axis. Transverse diffusion constitutes only a small component of this total value.8,9
Image interpretation in DTI can be done by both quantitative anisotropy indices and qualitative fiber tracking techniques (tractography).14
3.2. Anisotropy parameters
Fractional anisotropy (FA) and Mean diffusivity (MD) are the two most commonly used and studied quantitative DTI metrics, with Axial diffusivity (AD) and Radial diffusivity (RD) rounding off the top four.8,12 These indices not only participate in post-processing of fiber tracking but are individually important non-invasive biomarkers on their own.7
FA measures the fraction of total diffusion attributed to anisotropy. Its values range from 0 to 1 representing complete isotropy and perfect anisotropy on both ends of the spectrum.13 Normal cord values range between 0.5 and 0.7.8 Changes in FA parallel variations in primary diffusion axis, however, values are not distinctive to underlying pathology.6
MD estimates water molecular movement in extracellular space, and is an average of the three directional diffusion values [analogous to ADC].8 It depends on membrane permeability and is affected by changes in cellular density and edema,6 for example, MD decreases in neoplastic lesions where the extracellular volumes (ECV) are reduced and increases in necrosis and edema which show increased ECVs.8
AD measures the principal water diffusion axis which within the cord is parallel to the neuronal orientation. RD estimates the component of diffusion occurring perpendicular to principal diffusion axis and is specific for the assessment of myelin integrity.8
3.3. Fiber tracking (tractography)
Tractography algorithms extract information from DTI to reconstruct WM tracts in three dimensions (3D). The two most commonly used algorithms are deterministic and probabilistic, the details of which are not within the purview of this article.8,14,15
Intervoxel connectivity is determined from the direction and alignment of the primary diffusion vector14 which in turn is controlled by fiber diameters, myelination, and the degree of directional congruence of the fibers.16
The fiber orientation data can be color-coded by assigning separate colors for the three orthogonal axes; typically red color for left-right fibers, green for anteroposterior fibers, and blue for superior-inferior fibers,13 Fig. 1, Fig. 2.
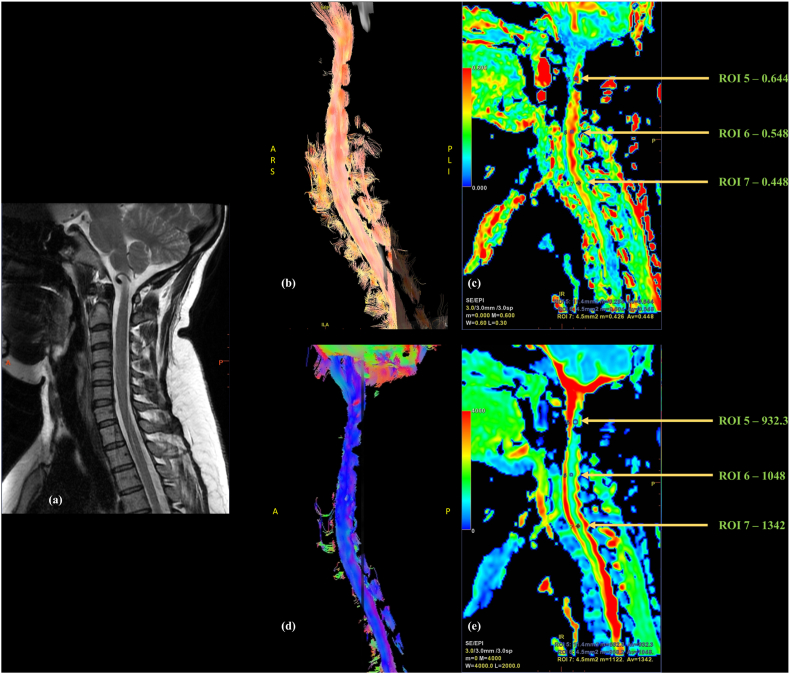
Fig. 1.
27-year-old female with neck pain; Sagittal (a)T2W images of cervical spine with normal appearing cord; (b) Tractography image showing normal orientation and continuity of cord fibers; Colored (c) FA and (e) ADC maps with three region of interests (ROI)s placed at upper (ROI 10), middle (ROI 9) and lower (ROI 8) cervical levels showing values within the normal range (d) Reformatted color FA map with cord fibers in blue indicating superior-inferior fiber direction.
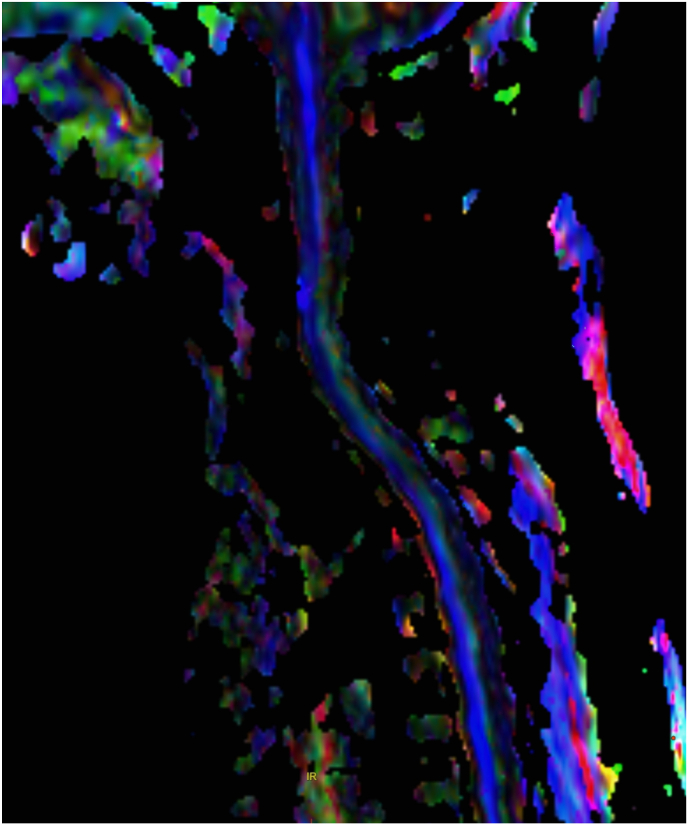
Fig. 2.
Reformatted color FA map of the cervical cord with blue fibers indicating superior-inferior fiber direction.
Tractography datasets provide a qualitative method of visual analysis of cord integrity,5 with presentations ranging from changes in fiber course, altered structural integrity, fiber displacement, and fiber infiltration,8,14 Fig. 3, Fig. 4c. Slow growing processes like tumors usually cause fiber displacement while deformation and interruption are more commonly seen with trauma or ischemia.17
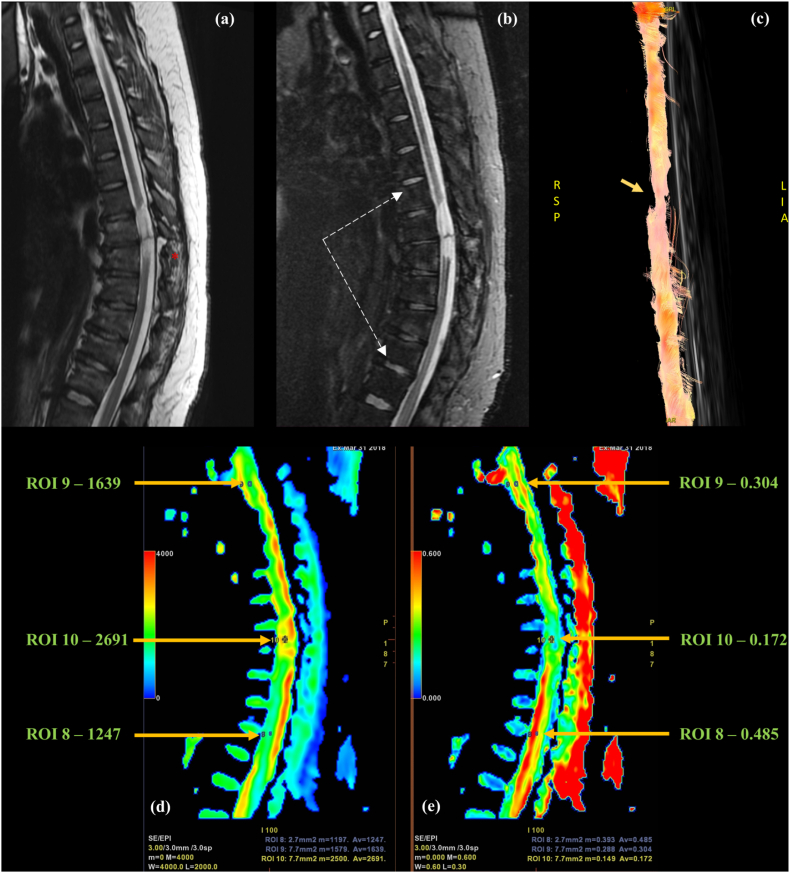
Fig. 3.
24-year-old female with history of road traffic accident; Sagittal (a)T2W and (b)STIR images of dorsal spine showing anterior wedging of D7 to 12 vertebral bodies with variable marrow edema (vertebrae between white dashed arrows in b) with fracture of the posterior elements of D8 (red asterisk in a). Focal intramedullary cord signal alteration at D7-D8 level involving almost the entire cord width; (c) Tractography image showing partial fiber interruption at injury epicenter (yellow arrow). Colored (d) ADC and (e) FA maps with three ROIs placed at injury epicenter (ROI 10), in the rostral cord (ROI 9), and in the caudal cord (ROI 8) showing increased diffusivity and decreased FA at site of injury with abnormal FA values extending for a much longer segment cranially than is apparent on T2W.
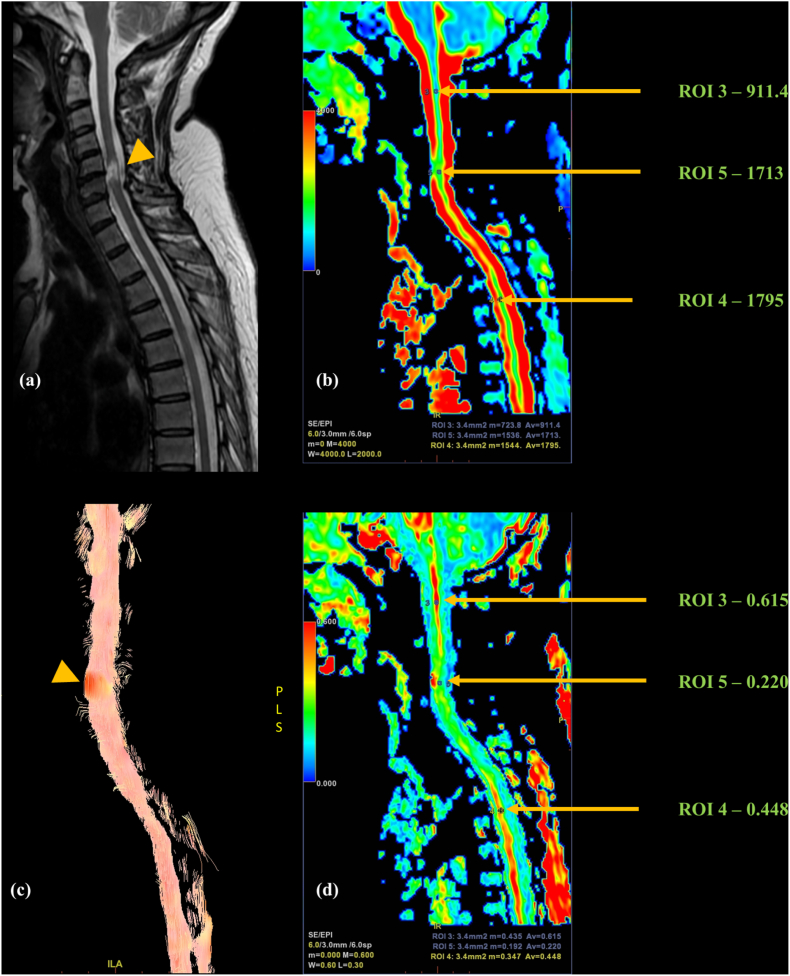
Fig. 4.
Follow up case of a 50-year-old female with post-traumatic quadriparesis; (a)Sagittal T2 image showing short segment myelomalacia at C5–C6 level (yellow arrowhead) involving almost the entire cord width; Colored (b) ADC and (d) FA maps with three ROIs placed at injury epicenter (ROI 5), in the rostral cord (ROI 3) and in the caudal cord (ROI 4) showing increased diffusivity at and below the site of injury; decreased FA involving a much longer cord segment than is apparent on T2; (c) Tractography image showing partial-thickness fiber alteration and disruption with feathering (yellow arrowhead).
In recent times, few experimental quantitative parameters have been obtained from tractography datasets for classifying injuries, including connection rates of fiber tractography (CRFT) and imaginary WM fiber volume (IWMFV). Axial ROIs are placed to include the entire cord cross-section both at injury epicenter, and at a site proximal to injury, to assess the proportion of tracts crossing both points; the higher the proportion, the better the prognosis.5
3.4. DTI variations in normal human spinal cord
DTI in healthy individuals helps in establishing normative controls, allowing cohort matching for better image interpretation. Cervical cord (CC) shows higher signal-to-noise ratios compared to the dorsal region which is likely secondary to the higher T2 values of gray matter, the proportion of which is larger in the former. Topographical diffusivity differences are seen between white and gray matter with significantly higher FA values seen in the anisotropic WM tracts.7 Axial diffusion showed a craniocaudal gradient with higher values within the CC owing to greater proportions of compactly arranged large-diameter axons. Similar changes were seen in FA values.7, 18 ADC values also showed gradation with the highest values in upper cervical segments for dorsal tracts and in the lumbar enlargement for whole cross-section. Mild laterality variations were seen in ADC values.10
DTI indices in normal cord also showed physiological age-related changes, with progressively decreasing CC FA values with age.7 Fig. 1 shows colored FA and ADC maps of the CC of a healthy adult with reconstructed tractography image. Mild craniocaudal gradation is seen in the FA values.
4. Results
The initial database search provided 67 articles which were further filtered by selecting humans under the species section which narrowed the results to 29. 19 articles were further shortlisted after screening the titles and abstracts. Additional citation analysis revealed the remaining 21 papers.
5. DTI in SCI: diagnosis, applications, and literature review
DTI datasets can identify the site and severity of SCI with high sensitivity. Changes may be seen on DTI images earlier than on conventional T2 sequences and sometimes even in cases with normal T2 appearances emphasizing its functional diagnostic potential.6 Figs. 3 and 4 show post-traumatic FA and ADC alterations in the cord extending much beyond the signal abnormality on T2 sequences indicating molecular abnormalities beyond the limits of conventional sequences. Normally maximum diffusion occurs along the tracts with biological barriers limiting perpendicular diffusion. The reverse of this is seen in injury cases that disrupt these barriers.3,8,9,12 Improvements in imaging protocols have allowed DTI applications to be extended to the pediatric population.14 Table 1 reviews the literature evaluating DTI role in SCI highlighting key points.
Table 1.
Summary of literature review evaluating role of DTI IN SCI.
| AUTHORS (YEAR)/STUDY | DEMOGRAPHY | TIMING OF MR POST-INJURY | MR STRENGTH | FINDINGS - CONCLUSIONS | |
|---|---|---|---|---|---|
| 1 | Faro et al 202231https://doi.org/10.1038/s41393-022-00770-5 |
19 cases 36 controls Age: 6–16 years age |
≥6 months | - | Cord areas adjacent to injury sites showed abnormal FA and RD |
| 2 | Krisa et al 202237https://doi.org/10.46292/sci21-00048 |
26 cases 36 controls |
- | 3T | DTI can estimate the severity and location of pediatric SCI, correlating moderately with the CUE-T score. FA shows good correlation with upper extremity muscle strength (UEMS) |
| 3 | Zhu et al 20215https://doi.org/10.1016/j.wneu.2021.01.146 | 20 cases; mean age - 43.9 years | <24 h | 3T Philips | Baseline FA and CRFT can help prognosticate acute Thoracolumbar SCI with higher initial values seen in patients that show good recovery. No significant correlation seen with ADC values. |
| 4 | Zhu et al 202121https://doi.org/10.1097/BRS.0000000000003923 | 24 cases with CSCI | Acute | – | Low FA and WM volumes seen at injury sites with no significant ADC changes. Higher FA values were associated with better AIS grades with good correlation with motor scores |
| 5 | Mossa-Basha et al 202122https://doi.org/10.1259/bjr.20201000 |
15 cases; mean age – 40 ± 20 years 12 controls; mean age – 36 ± 19 years |
≤72 h | 3T Siemens | Changes in segmented DTI metrics at injury sites and at sites remote from injury allow detailed SCI assessment |
| 6 | Singh et al 202040https://doi.org/10.22603/2Fssrr.2020-0048 | 25 cases; mean age approximately – 37 ± 13 years | ≤2 days with 6 months follow-up | - | Progressively increasing FA and decreasing ADC seen in patients showing clinical improvement on follow ups. |
| 7 | Shanmuganathan et al 202026https://doi.org/10.1089/neu.2019.6394 |
20 cases; median age – 53 ± 16.5 years 15 controls; median age - 46 ± 16.5 years |
≤5 days | 1.5T Siemens | Reduced AD values indicate bad prognosis and recovery. AD and MD both correlate significantly with motor scores. |
| 8 | Shabani et al 201938https://doi.org/10.3171/2018.12.FOCUS18595. | 23 cases | At presentation and 1 year follow up | 1.5T GE | High cervical cord FA values can allow stratification and prognostication of cervical SCI |
| 9 | Poplawski et al 201923https://doi.org/10.1089/neu.2018.6092 |
23 cases; mean age - 53 years 45 controls; mean age - 38 years |
≤12hrs | 1.5T Philips | DTI measures immediately rostral to injury sites correlated better with the degree and prognosis of injury compared to parameters at injury sites |
| 10 | Czyz et al 201725https://doi.org/10.4103/EJSS.EJSS_8_17 |
5 cases; median age - 41 years 5 controls |
≤8 h | 1.5T GE | Lower FA values correlate with inferior ASIA scores and neurological outcomes with reverse seen with ADC. ADC could potentially prognosticate SCI with higher initial levels associated with prompter recovery. |
| 11 | Zou et al 201739https://doi.org/10.3760/cma.j.issn.0376-2491.2017.01.005 |
23 cases 15 controls |
Acute - Subacute | - | Changes in RD and FA are most sensitive for SCI prognosis with low FA and high RD indicating poorer prognosis. |
| 12 |
Shanmuganathan et al 201727 https://doi.org/10.1089/neu.2016.4901 |
30 cases; mean age - 53 years 15 volunteers; mean age - 46 years |
≤24 h with 1 year follow-up | 1.5T Siemens | AD can perform as an important biomarker, prognosticating long term outcomes with lower initial values predicting poorer outcomes. |
| 13 | D'souza et al 20173https://doi.org/10.1016/j.injury.2017.02.016 |
20 cases; mean age - 35.95 ± 10.86 years 30 controls; mean age - 35.90 ± 10.13 years |
≤7 days | 3T Siemens | Lower mean FA and higher MD seen at injury sites. Mean FA showed good correlation with clinical-grade |
| 14 | Alizadeh et al 201714https://doi.org/10.1038/sc.2016.121 |
10 cases; age 13.8 ± 3.26 years 10 controls; age 15.13 ± 3.51 years |
> 6 months | 3T Siemens | Changes in DTI and DTT could explain the physiology behind cord injury and repair mechanisms. Decreased FA and tract density seen in SCI patients. |
| 15 | Vedantam et al 201524https://doi.org/10.1016/j.wneu.2013.09.017 | 12 cases; mean age - 54.7 ± 4.0 years | 3.6 ± 0.9 days | 1.5T GE | Increased FA in high cervical region showed positive association with upper limb motor scores and AIS grades. |
| 16 | Mulcahey et al 201332https://doi.org/10.1038/sc.2013.36 |
10 cases; mean age - 13.4 ± 3.9 years 25 controls; mean age - 13.5 ± 4.6 years |
Chronic | 3T Siemens | DTI could help demarcate injury level. Reduced FA and increased diffusivity are found in SCI. |
| 17 |
Koskinen et al 201333 https://doi.org/10.1089/neu.2013.2943 |
28 cases; age 59.9 ± 13.3 years 40 controls; age 40.6 ± 12.2 years |
> 1 year | 3T Siemens | Lower FA and higher ADC seen at injury sites and upper cervical cord with significant correlation between FA and motor scores. |
| 18 | Mulcahey et al 201236https://doi.org/10.1097/BRS.0b013e3182470a08 | 10 CSCI cases | - | 3T Siemens | DTI parameters showed statistically stronger correlations with clinical measures compared to standard MR with FA positively correlating and ADC negatively correlating with ISNCSCI values |
| 19 |
Petersen et al 201210 https://doi.org/10.1089/neu.2011.2027 |
19 cases; mean age - 59.7 years 28 controls; mean age - 58 years |
2 months - 8 years | 3T Philips | DTI parameters help assess SCI severity and can in the future help in assessing response to regeneration therapy. FA values decrease in SCI, correlating with AIS scores and SSEP. |
| 20 | Endo et al 201128https://doi.org/10.1227/NEU.0b013e3182031ce7 |
16 cases; mean age - 65.1 years 11 controls; mean age - 57.8 years |
≤24 h with follow-ups | 1.5T Siemens | Preliminary ADC values predict cavity formation by differentiating cytotoxic from vasogenic edema with lower values associated with higher chances of cord cavitation |
| 21 | Kamble et al 20114https://doi.org/10.4103/0971-3026.85372 |
18 cases; median age - 33.5 years 11 controls; median age - 33 years |
3 to 84 months | 1.5T GE | Mean FA was reduced at sites remote from injury with apparent normal appearances on T2. In future, improvement in FA can indicate axonal regeneration after administration of regeneration inducing therapy. |
| 22 |
Cheran et al 201120 https://doi.org/10.1089/neu.2010.1741 |
25 cases; age - 39.7 ± 13.9 years 11 volunteers; age - 31.5 ± 10.7 years |
1 h to 5 days | 1.5T Siemens | In patients with Nonhemorrhagic contusions, DTI indices correlate well with the severity of injury & ASIA grades |
| 23 | Chang et al 201029https://doi.org/10.1089/neu.2009.1265 |
10 cases; age 47.5 ± 17.7 years 10 controls; age 32.4 ± 5.4 years |
> 1 month | 1.5T GE | Low FA values found in SCI with insignificant ADC differences. FA, imaginary fiber numbers and connection rates correlated with motor scores |
| 24 |
Ellingson et al 200830 DOI 10.3174/ajnr.A1272 |
10 cases; median age - 37 years 13 controls; median age - 25 years |
>4 years | 1.5T GE | Increased diffusion and reduced FA found at injury sites. Remote changes seen in upper cervical cord suggest diffuse involvement of the neuroaxis in injury cases |
| 25 |
Shanmuganathan et al 200819 DOI 10.3174/ajnr.A0916 |
20 cases; mean age - 45.7 ± 17.7 years 8 controls; mean age - 34.2 ± 10.7 years |
2 h - 15 days | 1.5T Siemens | ADC constitutes the most important biomarker of CSCI with the greatest differences in cases with hemorrhagic contusions. Regional differences in cervical cord DTI metrics found in healthy volunteers |
5.1. DTI in acute cases of SCI
Several studies have shown decreased FA in acute cases3,19, 20, 21, 22, 23 which is likely secondary to interruption of the physiological anisotropy. Diverse combinations of demyelination, axonal swelling, and impaired axonal flow contribute to this.24 Complete fiber disruption,24 presence of hemorrhage, infarction, and cyst formation are other causes lowering FA.25
ADC results were more variable with studies showing both increased3,23 and decreased19,20,26,27 values. Lower ADC values are seen in cases developing cytotoxic edema in response to injury, and these correlate with poorer neurological outcomes28 and WM necrosis.26 On the other hand, cases developing vasogenic edema in injury response demonstrate higher ADC values predicting better prognosis and neurological outcomes. Both these case scenarios will be seen as increased signal on T2W sequences and therefore cannot be differentiated.28 Axonal injury and beading due to ischemic and stretching injuries,26 and extrinsic cord compression25 have been postulated as causes of low ADC. Currently, ADC plays more of a biomarker role with higher preliminary values predicting better neurological outcomes and recovery.25,26
5.2. DTI in chronic cases of SCI
Few studies have evaluated the spectrum of DTI alterations in chronic SCI patients. The most consistent finding, as with acute cases, was decreased FA at injury sites compared to the controls,4,10,14,29, 30, 31, 32, 33 Fig. 3, Fig. 4d. In chronic injury cases decreased FA values are linked to reduced fiber numbers and increased extracellular diffusivity secondary to antegrade and retrograde Wallerian degeneration4,10 with variable amounts of axonal loss, atrophy, and demyelination.24
ADC was again, however, more variable with studies reporting increased, decreased, and unchanged values. Most of the studies showed increased ADC at injury sites14,30,32,33 which was explained based on the increased extracellular space with gliosis, loss of neurons, necrosis, and cavitation all contributing variably.25 No significant ADC differences were found by few.29 Fig. 3d depicting a SCI case with subacute – chronic injury duration showing increased MD at injury site. Fig. 4b in a chronic RTA patient showing diffusely increased MD extending caudally from the site of injury.
5.3. Remote cord changes
Changes in DTI parameters are also found in the cord away from the injury epicenter with decrease in FA found to be the most consistent change.4,31,33 ADC results were more heterogeneous and inconsistent.10,30,31,33
DTI parameters rostral to injury epicenter have been shown to better correlate with functional outcomes and recovery as anatomic cord distortion and hemorrhage at injury sites can significantly degrade the image quality.23
5.4. Miscellaneous applications
Accurate demarcation of injury extent is possible by DTI, including its caudal limit which is challenging to discern clinically. Lesion mapping helps to guide therapeutic procedures by targeting appropriate sites for the injection of drugs/stem cells. Furthermore, data on regenerative potential can be sought by characterizing glial scar morphology and estimating axonal dieback.7
The success of several rehabilitation procedures rests upon the presence of intact motor neurons below the injury level which is tough to assess both clinically and electrophysiologically. FA values computed below the injury site can help in this assessment.30
Along with navigation protocols, tractography mapping can assist in surgical planning of safety margins.13
6. Correlation of DTI and tractography with clinical scores
Several classification systems for SCI have been devised over the years to assess and standardize the degree of injury severity, and correlate them with neurological outcomes and prognosis. Injuries are broadly classified into complete and incomplete with the former showing total neurological loss caudal to the injury level. The first classification devised was ‘Frankel grade’ which was a 5-point grading system using both motor & sensory scales. Several other scores were developed over the years with the American Spinal Injury Association Impairment Scale (AIS) also called the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) being the current gold standard.34,35 DTI indices generally showed good correlation with clinical scores.
Significant direct correlation was found between FA and ISNCSCI scores by several authors.10,20,21,24,25,33,36,38,40 Correlation of ADC values with ISNCSCI was more variable with both positive25,27,36 and negative10 results. Frankel grades also showed positive correlation with FA and a reverse correlation with MD.3
Imaginary fiber numbers & connection rates significantly correlated with motor scores.29 Moderate correlation was seen with the Capabilities of the Upper Extremity Test (CUE-T) scores with FA exhibiting a significant association with upper extremity muscle strength (UEMS).37
Changes in DTI parameters on follow-up correlated with recovery with reduced FA and increased RD values predicting poorer outcomes.39,40 Higher baseline FA and CRFT values were associated with better clinical recovery and grade improvement.5
7. Summary of findings
To summarize the findings presented in the aforementioned studies; consistently reduced FA values were found in both acute and chronic spinal cord injury cases indicating direct interruption of the normal axonal anisotropy. These could be due to varying combinations of histopathological processes including axonal degeneration, regeneration, demyelination, remyelination and scarring. ADC values were, however, more variable with ADC playing more of a biomarker role rather than a diagnostic one. Higher initial ADC values were associated with better outcomes indicating component of underlying vasogenic rather than cytotoxic changes and thereby intact cellular morphology. Three-dimensional pictorial fiber tractography images can provide a direct visual interpretation of the axonal/cord injury simplifying interpretation. Early changes in DTI metrics in the presence of normal appearing cord on conventional T2 sequences indicate microstructural changes in response to injury which may be arrested/reversed if timely management is incorporated, potentially improving prognosis. Furthermore, understanding the temporal correlation between mechanisms of injury and healing, and the changes in DTI parameters can help in assessment of response to regeneration therapy and predicting long-term outcomes in rehabilitation cases. The small sample sizes in the studies conducted so far however raise doubts about skewed result interpretation and thereby limit setting up of standard interpretation and diagnostic guidelines. These studies, however, set the ground for further future research confirming or negating the findings known so far.
8. Limitations
This analysis though exhaustive is ridden with a few constraints. Firstly, the article pool was extracted from a single database, Pubmed, potentially overlooking some relevant literature. The sample size is limited as only a handful of studies have been conducted on this topic so far, and these studies further present results on small sample sizes. Third, the imaging parameters used in these studies were not uniform, differing in both the MRI strength and the sequence protocols causing result inhomogeneity. More significant results were obtained on 3 T scanners compared to 1.5 T leading to technical limitations. Differences between vendors and software further add to this ambiguity.8 Machine learning through training data sets and homogenization of acquisition and processing techniques will help improve image reliability.6
Furthermore, modality-based limitations add on to the ambiguity. With advances in technology, though the utility and applications of DTI have widened, it is still fraught with several constraints.9 Small cord cross-sectional areas result in low spatial resolution and signal-to-noise ratios particularly in the dorsal region.7,10 Though partially overcome with newer fast sequences and gating, artifacts caused by respiratory motion, cardiovascular pulsations, and susceptibility, still contribute to significant image degradation.7,10,12,34 Additional functional or anatomic information is often required to supplement the low resolution inherent to DTI13
Extra scan time, lack of normative cohort matched controls and a low number of human studies are other deterrents.12
9. Conclusion and future frontiers
Currently, DTI is the only in vivo non-invasive technique allowing both microstructural and functional cord evaluation. Quantitative and qualitative indices derived thereof have shown significant correlations with injury severity, prognostication, recovery, and therapeutics. By decoding molecular physiology, DTI metrics may in the course of time help differentiate between axonal injury and myelination changes. Monitoring temporal DTI changes with neurophysiological correlation will be the ultimate goal. The purview of DTI though promising is riddled with several technical roadblocks making its use in routine diagnostics expendable. Technique standardization and multicenter studies with data collection will help in validating the utility of this technique, reinforcing the role of DTI as a robust supplement to conventional MR in spinal cord injury protocols.
Credit statement
Dr Geetanjali Nanda: Conceptualization, Methodology, Validation, Writing - Review & Editing, Supervision; Dr Pooja Jain: Conceptualization, Methodology, Investigation, Resources, Writing - Original Draft; Dr Abhishekh Suman: Methodology, Validation, Investigation, Writing - Review & Editing, Visualization; Dr Harsh Mahajan: Conceptualization, Investigation, Resources, Writing - Review & Editing, Supervision.
Funding statement
The authors received no financial support for the research, authorship, and / or publication of this article.
Contributor Information
Geetanjali Nanda, Email: geetutomar@yahoo.co.in.
Pooja Jain, Email: poojajaindoc@gmail.com.
Abhishek Suman, Email: drabhisheksuman@gmail.com.
Harsh Mahajan, Email: hm@mahajanimaging.com.
References
- 1.Spinal cord injury. https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury [Internet]. [cited 2022 May 12]. Available from:
- 2.Jones J.G.A., Cen S.Y., Lebel R.M., Hsieh P.C., Law M. Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. AJNR Am J Neuroradiol. 2013 Feb;34(2):471–478. doi: 10.3174/ajnr.A3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’souza M.M., Choudhary A., Poonia M., Kumar P., Khushu S. Diffusion tensor MR imaging in spinal cord injury. Injury. 2017 Apr;48(4):880–884. doi: 10.1016/j.injury.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Kamble R.B., Venkataramana N.K., Naik A.L., Rao S.V. Diffusion tensor imaging in spinal cord injury. Indian J Radiol Imag. 2011 Jul;21:221–224. doi: 10.4103/0971-3026.85372. 03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu F., Zeng L., Gui S., et al. The role of diffusion tensor imaging and diffusion tensor tractography in the assessment of acute traumatic thoracolumbar spinal cord injury. World Neurosurgery. 2021 Jun;150:e23–30. doi: 10.1016/j.wneu.2021.01.146. [DOI] [PubMed] [Google Scholar]
- 6.Zaninovich O.A., Avila M.J., Kay M., Becker J.L., Hurlbert R.J., Martirosyan N.L. The role of diffusion tensor imaging in the diagnosis, prognosis, and assessment of recovery and treatment of spinal cord injury: a systematic review. Neurosurg Focus. 2019 Mar;46(3):E7. doi: 10.3171/2019.1.FOCUS18591. [DOI] [PubMed] [Google Scholar]
- 7.Vedantam A., Jirjis M., Eckhardt G., et al. Diffusion tensor imaging of the spinal cord: a review. Coluna/Columna. 2013;12(1):64–69. [Google Scholar]
- 8.Noguerol T.M., Barousse R., Amrhein T.J., Royuela-del-Val J., Montesinos P., Luna A. Optimizing diffusion-tensor imaging acquisition for spinal cord assessment: physical basis and technical adjustments. Radiographics. 2020 Mar;40(2):403–427. doi: 10.1148/rg.2020190058. [DOI] [PubMed] [Google Scholar]
- 9.Applications of diffusion tensor imaging and fiber tractography. https://appliedradiology.com/articles/applications-of-diffusion-tensor-imaging-and-fiber-tractography [Internet]. [cited 2022 May 14]. Available from:
- 10.Petersen J.A., Wilm B.J., von Meyenburg J., et al. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma. 2012 May 20;29(8):1556–1566. doi: 10.1089/neu.2011.2027. [DOI] [PubMed] [Google Scholar]
- 11.Li D., Malcolm J., Rindler R., et al. The role of diffusion tensor imaging in spinal pathology: a review. Neurol India. 2017;65(5):982. doi: 10.4103/neuroindia.NI_198_17. [DOI] [PubMed] [Google Scholar]
- 12.Kaushal M., Shabani S., Budde M., Kurpad S. Diffusion tensor imaging in acute spinal cord injury: a review of animal and human studies. J Neurotrauma. 2019 Aug;36(15):2279–2286. doi: 10.1089/neu.2019.6379. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee P., Berman J.I., Chung S.W., Hess C.P., Henry R.G. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. AJNR Am J Neuroradiol. 2008 Apr;29(4):632–641. doi: 10.3174/ajnr.A1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alizadeh M., Intintolo A., Middleton D.M., et al. Reduced FOV diffusion tensor MR imaging and fiber tractography of pediatric cervical spinal cord injury. Spinal Cord. 2017 Mar;55(3):314–320. doi: 10.1038/sc.2016.121. [DOI] [PubMed] [Google Scholar]
- 15.McLachlin S., Leung J., Sivan V., et al. Spatial correspondence of spinal cord white matter tracts using diffusion tensor imaging, fibre tractography, and atlas-based segmentation. Neuroradiology. 2021 Mar;63(3):373–380. doi: 10.1007/s00234-021-02635-9. [DOI] [PubMed] [Google Scholar]
- 16.Facon D., Ozanne A., Fillard P., Lepeintre J.F., Tournoux-Facon C., Ducreux D. vol. 8. 2005. (MR Diffusion Tensor Imaging and Fiber Tracking in Spinal Cord Compression). [PMC free article] [PubMed] [Google Scholar]
- 17.Vargas M.I., Delavelle J., Jlassi H., et al. Clinical applications of diffusion tensor tractography of the spinal cord. Neuroradiology. 2008 Jan;50(1):25–29. doi: 10.1007/s00234-007-0309-y. [DOI] [PubMed] [Google Scholar]
- 18.Ellingson B.M., Ulmer J.L., Kurpad S.N., Schmit B.D. Diffusion tensor MR imaging of the neurologically intact human spinal cord. AJNR Am J Neuroradiol. 2008 Aug;29(7):1279–1284. doi: 10.3174/ajnr.A1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shanmuganathan K., Gullapalli R.P., Zhuo J., Mirvis S.E. Diffusion tensor MR imaging in cervical spine trauma. AJNR Am J Neuroradiol. 2008 Apr;29(4):655–659. doi: 10.3174/ajnr.A0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheran S., Shanmuganathan K., Zhuo J., et al. Correlation of MR diffusion tensor imaging parameters with ASIA motor scores in hemorrhagic and nonhemorrhagic acute spinal cord injury. J Neurotrauma. 2011 Sep;28(9):1881–1892. doi: 10.1089/neu.2010.1741. [DOI] [PubMed] [Google Scholar]
- 21.Zhu F., Liu Y., Zeng L., et al. Evaluating the severity and prognosis of acute traumatic cervical spinal cord injury: a novel classification using diffusion tensor imaging and diffusion tensor tractography. Spine. 2021 May 15;46(10):687–694. doi: 10.1097/BRS.0000000000003923. [DOI] [PubMed] [Google Scholar]
- 22.Mossa-Basha M., Peterson D.J., Hippe D.S., et al. Segmented quantitative diffusion tensor imaging evaluation of acute traumatic cervical spinal cord injury. BJR (Br J Radiol) 2021 Feb 1;94(1118) doi: 10.1259/bjr.20201000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poplawski M.M., Alizadeh M., Oleson C.V., et al. Application of diffusion tensor imaging in forecasting neurological injury and recovery after human cervical spinal cord injury. J Neurotrauma. 2019 Nov 1;36(21):3051–3061. doi: 10.1089/neu.2018.6092. [DOI] [PubMed] [Google Scholar]
- 24.Vedantam A., Eckardt G., Wang M.C., Schmit B.D., Kurpad S.N. Clinical correlates of high cervical fractional anisotropy in acute cervical spinal cord injury. World Neurosurgery. 2015 May;83(5):824–828. doi: 10.1016/j.wneu.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Czyz M., Tykocki T., Szewczyk P., Jarmundowicz W. Application of diffusion tensor imaging in the prognosis of outcome after traumatic. Cervical Spinal Cord Injury. 2017;1(2):4. [Google Scholar]
- 26.Shanmuganathan K., Zhuo J., Bodanapally U.K., et al. Comparison of acute diffusion tensor imaging and conventional magnetic resonance parameters in predicting long-term outcome after blunt cervical spinal cord injury. J Neurotrauma. 2020 Feb 1;37(3):458–465. doi: 10.1089/neu.2019.6394. [DOI] [PubMed] [Google Scholar]
- 27.Shanmuganathan K., Zhuo J., Chen H.H., et al. Diffusion tensor imaging parameter obtained during acute blunt cervical spinal cord injury in predicting long-term outcome. J Neurotrauma. 2017 Nov;34(21):2964–2971. doi: 10.1089/neu.2016.4901. [DOI] [PubMed] [Google Scholar]
- 28.Endo T., Suzuki S., Utsunomiya A., Uenohara H., Tominaga T. Prediction of neurological recovery using apparent diffusion coefficient in cases of incomplete spinal cord injury. Neurosurgery. 2011 Feb 1;68(2):329–336. doi: 10.1227/NEU.0b013e3182031ce7. [DOI] [PubMed] [Google Scholar]
- 29.Chang Y., Jung T.D., Yoo D.S., Hyun J.K. Diffusion tensor imaging and fiber tractography of patients with cervical spinal cord injury. J Neurotrauma. 2010 Nov;27(11):2033–2040. doi: 10.1089/neu.2009.1265. [DOI] [PubMed] [Google Scholar]
- 30.Ellingson B.M., Ulmer J.L., Kurpad S.N., Schmit B.D. Diffusion tensor MR imaging in chronic spinal cord injury. AJNR Am J Neuroradiol. 2008 Nov;29(10):1976–1982. doi: 10.3174/ajnr.A1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faro S.H., Saksena S., Krisa L., et al. DTI of chronic spinal cord injury in children without MRI abnormalities (SCIWOMR) and with pathology on MRI and comparison to severity of motor impairment. Spinal Cord. 2022 May;60(5):457–464. doi: 10.1038/s41393-022-00770-5. [DOI] [PubMed] [Google Scholar]
- 32.Mulcahey M.J., Samdani A.F., Gaughan J.P., et al. Diagnostic accuracy of diffusion tensor imaging for pediatric cervical spinal cord injury. Spinal Cord. 2013 Jul;51(7):532–537. doi: 10.1038/sc.2013.36. [DOI] [PubMed] [Google Scholar]
- 33.Koskinen E., Brander A., Hakulinen U., et al. Assessing the state of chronic spinal cord injury using diffusion tensor imaging. J Neurotrauma. 2013 Sep 15;30(18):1587–1595. doi: 10.1089/neu.2013.2943. [DOI] [PubMed] [Google Scholar]
- 34.Alizadeh A., Dyck S.M., Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019 Mar 22;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts T.T., Leonard G.R., Cepela D.J. Classifications in brief: American spinal injury association (ASIA) impairment Scale. Clin Orthop Relat Res. 2017 May;475(5):1499–1504. doi: 10.1007/s11999-016-5133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulcahey M.J., Samdani A., Gaughan J., et al. Diffusion tensor imaging in pediatric spinal cord injury: preliminary examination of reliability and clinical correlation. Spine. 2012 Jun 1;37(13):E797–E803. doi: 10.1097/BRS.0b013e3182470a08. [DOI] [PubMed] [Google Scholar]
- 37.Krisa L., Middleton D.M., Saksena S., et al. Clinical utility of diffusion tensor imaging as a biomarker to identify microstructural changes in pediatric spinal cord injury. Top Spinal Cord Inj Rehabil. 2022 Apr 12;28(2):1–12. doi: 10.46292/sci21-00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shabani S., Kaushal M., Budde M., Kurpad S.N. Correlation of magnetic resonance diffusion tensor imaging parameters with American Spinal Injury Association score for prognostication and long-term outcomes. Neurosurg Focus. 2019 Mar;46(3):E2. doi: 10.3171/2018.12.FOCUS18595. [DOI] [PubMed] [Google Scholar]
- 39.Zou Z.M., Li J., Cao Q.Y., Lian H.X., He C.N., Wang B. [Clinical value of diffusion tensor imaging parameter value in evaluating the prognosis of spinal cord injury in acute cervical spinal cord injury] Zhonghua Yixue Zazhi. 2017 Jan 3;97(1):17–21. doi: 10.3760/cma.j.issn.0376-2491.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Singh R., Magu S., Baskar A., Rohilla R.K., Kaur K., Kaur S. Correlation of clinical findings in acute spinal injury patients with magnetic resonance including diffusion tensor imaging and fiber tractography. Spine Surg Relat Res. 2020 Oct 27;4(4):305–313. doi: 10.22603/ssrr.2020-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]