Abstract
The bombesin/gastrin-releasing peptide (GRP) family of neuropeptides has been implicated in various in vitro and in vivo models of human malignancies including prostate cancers. It was previously shown that bombesin and/or neurotensin (NT) acts as a survival and migratory factor(s) for androgen-independent prostate cancers. However, a role in the transition from an androgen-dependent to -refractory state has not been addressed. In this study, we investigate the biological effects and signal pathways of bombesin and NT on LNCaP, a prostate cancer cell line which requires androgen for growth. We show that both neurotrophic factors can induce LNCaP growth in the absence of androgen. Concurrent transactivation of reporter genes driven by the prostate-specific antigen promoter or a promoter carrying an androgen-responsive element (ARE) indicate that growth stimulation is accompanied by androgen receptor (AR) activation. Furthermore, neurotrophic factor-induced gene activation was also present in PC3 cells transfected with the AR but not in the parental line which lacks the AR. Given that bombesin does not directly bind to the AR and is known to engage a G-protein-coupled receptor, we investigated downstream signaling events that could possibly interact with the AR pathway. We found that three nonreceptor tyrosine kinases, focal adhesion kinase (FAK), Src, and Etk/BMX play important parts in this process. Etk/Bmx activation requires FAK and Src and is critical for neurotrophic factor-induced growth, as LNCaP cells transfected with a dominant-negative Etk/BMX fail to respond to bombesin. Etk's activation requires FAK, Src, but not phosphatidylinositol 3-kinase. Likewise, bombesin-induced AR activation is inhibited by the dominant-negative mutant of either Src or FAK. Thus, in addition to defining a new G-protein pathway, this report makes the following points regarding prostate cancer. (i) Neurotrophic factors can activate the AR, thus circumventing the normal growth inhibition caused by androgen ablation. (ii) Tyrosine kinases are involved in neurotrophic factor-mediated AR activation and, as such, may serve as targets of future therapeutics, to be used in conjunction with current antihormone and antineuropeptide therapies.
Prostate cancer is the most common noncutaneous cancer in men. The majority of patients die of disseminated disease which is hormone refractory and resistant to conventional therapies (1, 37, 57). Androgen ablation therapy, while initially effective in slowing down the progression of the disease, eventually fails, as androgen-insensitive tumors recur (20, 96).
The antiandrogen therapies usually do not eliminate the expression of the androgen receptor (AR) (87), and at least some forms of androgen insensitivity are thought to be caused by ligand-independent activation of the AR (91). For instance, Culig et al. (30) reported that the AR can be activated in the absence of androgen by growth factors such as keratinocyte growth factor (KGF), insulin-like growth factor 1 (IGF-1), and epidermal growth factor (EGF). Craft et al. (29), Yeh et al. (97) and Wen et al. (93) provide evidence that overexpression of HER2, a growth factor receptor, or its oncogenic variant Neu activates the AR in an androgen-depleted environment. Since all the receptors involved in the above-mentioned cases are tyrosine kinases, which are known initiators of phosphorylation cascades, it was postulated that direct phosphorylation of the AR may be one means to activate the receptor without androgen or to sensitize the receptor toward activation by very low levels of androgen. Indeed, direct phosphorylation of AR by serine/threonine kinases, mitogen-activated protein kinase (MAPK) (29, 97), protein kinase B/AKT (93), protein kinase A (PKA) (60, 76) and protein kinase C (PKC) (33, 45) have been reported. In most of these cases, AR activation, as measured by its ability to transcriptionally activate reporter genes, was also demonstrated. Thus, serine/threonine kinases appear to be mediators of AR activation. The type of protein kinases involved depends on the initiating growth factors and receptors.
In addition to peptide growth factors, neuropeptides such as bombesin and neurotensin (NT) have also been implicated in prostate cancer progression. We and others previously showed that prostate cancer cells often express neuronal markers (2, 3), and some of these cells can be induced to transdifferentiate into neuroendocrine-like cells by interleukin 6 (IL-6) (66, 82), forskolin (12, 27, 28), and androgen withdrawal (19). The association of neuroendocrine cells with prostate cancers has long been recognized (2, 3, 43). Advanced prostate cancers often have increased numbers of neuroendocrine cells, and androgen independence is correlated with elevated levels of neuroendocrine markers in serum (2, 3, 19). Neuroendocrine cells are known to secrete neuropeptides, which are involved in diverse biological processes, including cellular proliferation, transformation, and invasion (74, 94). These neuropeptides, exemplified by bombesin and NT, have been shown to be potent in vitro mitogens (73, 74) and are implicated in a variety of human malignancies in the lung (31, 36, 89, 90), breast (61, 64), and prostate (16, 44, 51, 56). For prostate cancers, it was shown that the receptors for bombesin/gastrin-releasing peptide (GRP) are present in all prostate cancer cell lines examined, including PC3, DU145, and LNCaP (9, 13, 55), and their expression levels are increased in more-advanced tumor specimens compared to less-advanced tumor specimens (55). Bombesin elicits calcium mobilization in PC3 and DU145 cells (9, 38) and enhances the invasive properties of PC3 and LNCaP cells (44). Similarly, NT induces mitogenic responses in PC3 and LNCaP cells (80). The results of these studies suggest that neuropeptides are potential prostate cancer progression factors. The mechanisms by which neuropeptides induce mitogenic and migration responses in prostate cancer cells remain unclear, although the involvement of tyrosine kinases is suggested in some of the reports (74, 75).
In this study, we report that in addition to its mitogenic and chemotactic function for prostate cancer cells, neuropeptides also activate the AR and induce androgen independence. This suggests that neuropeptides and, by extension, neuroendocrine differentiation may play a role in the transition from an androgen-dependent to -independent state. This is particularly relevant, considering that neuroendocrine differentiation of prostate cancer cells can be induced by androgen withdrawal (19) and that androgen ablation therapy is widely used in the treatment of prostate cancers. We also demonstrate in this report that three nonreceptor tyrosine kinases, focal adhesion kinase (FAK), Src, and Etk/BMX are involved in this process. All three tyrosine kinases are known to be engaged in a variety of signal pathways, including mitogenesis, migration, antiapoptosis, and reprogramming of gene expression. They have the potential to activate a number of serine/threonine kinases, which may modify the AR, leading to ligand-independent activation. These tyrosine kinases not only activate but also form a complex with one another. The receptors for bombesin and NT engage G proteins (Gαq or Gα12). Our study thus reveals a cross talk among G-proteins tyrosine kinases and nuclear receptors. In addition to providing insight into the molecular pathways whereby neuropeptides activate the AR, our results suggest that tyrosine kinase inhibitors may be useful in conjunction with androgen ablation in the treatment of prostate cancers.
MATERIALS AND METHODS
Cell culture and reagents.
LNCaP cells (American Type Culture Collection, Rockville, Md.) were maintained in RPMI 1640 medium with 10% fetal bovine serum (FBS). PC3 cells were derived from a poorly differentiated human carcinoma and lack AR expression. PC3(AR)2, a variant line which expresses AR, was established by stable transfection of a wild-type AR gene (40). The control cell line PC3(M) was developed by transfection with an empty vector carrying a hygromycin resistance gene. PC3(AR)2 and PC3(M) were both maintained in 5% charcoal-stripped serum and hygromycin B (100 μg/ml). Bombesin, NT, flutamide, and Flag antibody (M2) were obtained from Sigma. EGF, phorbol myristate acetate (PMA), wortmannin, and pyrazolopyrimidine (PP2) were obtained from Calbiochem-Novabiochem, Ltd. Monoclonal anti-Etk was purified from Etk 13 monoclonal hybridoma culture medium using a protein A/G affinity column (Pierce). The Etk hybridoma was kindly provided by C. H. Tsai (National Taiwan University, Taipei, Taiwan). Anti-T7 antibody was purchased from Novagen (Madison, Wis.). Antibodies to phosphotyrosine (4G10) and to FAK (rabbit polyclonal antibodies) and Src (monoclonal GD11 antibody) were purchased from Upstate Biotechnology Inc. (UBI) (Lake Placid, N.Y.). Phospho-AKT (Ser 473) and AKT antibodies were obtained from Cell Signaling (Beverly, Mass.).
Plasmid constructs.
PSA-Luc (−630/+12) was obtained by PCR-mediated amplification of human genomic DNA using oligonucleotide primers corresponding to the prostate-specific antigen (PSA) gene and ligated with HindIII/XhoI-digested PGL-3 basic vector (Promega, Madison, Wis.). ARE5-luc was constructed by inserting five tandem copies of the androgen-responsive element (ARE) from the androgen-responsive, prostate-specific androgen promoter (5′-TGCAGAACAGCAAGTGCTAGC-3′) upstream of the minimal TATA box into the PGL3 basic vector (Promega). Plasmid pUXLUC (−126/−120) contains two copies of the IL-8 AP-1 binding site from the IL-8 promoter as described previously (48). The T7-tagged wild-type Etk (T7-pcDNA3Etk) or dominant-negative mutant of Etk (T7-EtkDN) as well as the dominant-negative mutant FAK (FAKY397F and FRNK) have been described previously (24, 66, 69) and c-Src (SrcKR) was kindly provided by June Zou (Cancer Center, University of California, Davis) (92)
Transfection and luciferase reporter assays.
LNCaP cells were transiently transfected using Lipofectin reagent (GIBCO/BRL), and PC3(AR)2 and PC3(M) cells were transiently transfected using Fugene 6 from Roche (Indianapolis, Ind.) according to the manufacturer's instructions. Briefly, luciferase reporter construct (250 ng) containing either PSA-Luc or ARE5-Luc was cotransfected with 1 μg of expression vector as indicated or with pcDNA3 empty vector into LNCaP cells in six-well plates for 24 h followed by incubation in charcoal-stripped serum, phenol red-free medium with or without bombesin, NT (100 nM), or R1881 (1 nM) (methyltrienolone; DuPont New England Nuclear) as indicated for 24 h. Luciferase assays were performed on equal amounts of protein (50 μg/sample). Luciferase activities in cell lysates were measured using the Dual Luciferase assay system (Promega). Renilla luciferase expression plasmid, pRL-tk, was used as an internal control for transfection efficiency. The results are presented as fold induction, which is the relative luciferase activity (ratio of reporter luciferases/renilla luciferases) of the treated cells over that of the control cells.
Immunoprecipitation and Western blotting.
LNCaP cells were serum starved for 24 h and then stimulated with 100 nM bombesin or NT for 30 min. Immunoprecipitation and Western blotting were performed as described previously (66). Anti-pY antibody (UBI) was used to detect tyrosine phosphorylation of FAK, Src, and Etk. Total FAK, Src, or Etk detected with anti-FAK, anti-Src, or anti-T7 antibody, respectively, was used as a loading control. Proteins were probed by primary antibody and visualized by using an ECL kit (Pierce, Rockford, Ill.) according to the manufacturer's instructions. For the dose response of Etk phosphorylation, the autoradiograms were scanned using a Gel Doc 1000 scanner (Bio-Rad), and the labeled bands were quantified using Molecular Analyst software program (Bio-Rad).
BrdU labeling proliferation assay.
LNCaP-EtkWT or LNCaP-EtkDN cells (5 × 103) were seeded in 100 μl of charcoal-stripped culture medium containing serum per well in a 96-well, flat-bottom microtiter plate. The next day, the cells were treated with various amounts of bombesin (0.1 to 1,000 nM) and incubated for 4 days at 37°C with 5% CO2. The measurement was performed according to the manufacturer's protocol for the 5-Bromo-2′-deoxyuridine Labeling and Detection Kit III (Roche). Briefly, the cells were incubated with 10 μM bromodeoxyuridine (BrdU) for 2 h at 37°C, and the labeled cells were washed with washing buffer twice and then fixed with 200 μl of precooled ethanol per well for 30 min at −20°C. After fixation, the cells were then incubated with nuclease to partially digest the DNA. Anti-BrdU (monoclonal antibody) conjugated with peroxidase was added to detect incorporated BrdU, and the bound antibody was visualized with the soluble chromogenic peroxidase substrate 2,2′-azinobis(3-ethylbenthiazolinesulfonic acid) (ABTS), which yielded a colorimetric reaction. The plates were measured using an enzyme-linked immunosorbent assay (ELISA) reader.
MTS cell proliferation assay.
The tetrazolium compound (MTS) cell proliferation assay is a quantitative colorimetric assay for mammalian cell survival and proliferation. LNCaP cells (5 × 103) were grown in 100 μl of charcoal-stripped culture medium containing serum per well in a 96-well, flat-bottom microtiter plate. After 24 h, the cells were treated in the absence or presence of 10 μM PP2 or flutamide for 30 min and then treated with bombesin or R1881 for another 48 to 72 h. Then 20 μl of MTS (CellTiter 96 AQueous One Solution Reagent; Promega) was added to each well for 1 to 4 h at 37°C. After incubation, the absorbance was read at a wavelength of 490 nm according to the manufacturer's protocol.
PSA enzyme immunoassay.
The PSA protein was measured in cell culture supernatants from LNCaP cells. LNCaP cells (2 × 104) were grown in 1 ml of 2% charcoal-stripped culture medium containing serum in the presence of bombesin and NT for 72 h. PSA values were expressed in relation to cellular protein levels, which were determined by the method described by Bradford (17).
RESULTS
Bombesin and NT induce androgen-independent growth of LNCaP cells.
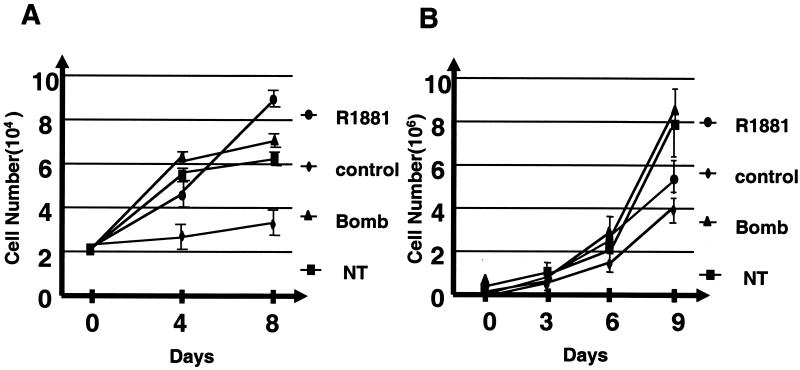
It has been shown that bombesin and NT secreted by neuroendocrine cells exert chemotactic and mitogenic effects on tumor cells in vivo and in vitro (80, 81). These factors are also postulated to play an important role in prostate cancer progression (see introduction). To directly demonstrate their involvement in androgen-independent growth of prostate cancer cells, we measured the effects of NT and bombesin on the growth of the androgen-dependent prostate cancer cell line LNCaP (Fig. 1A). As expected, the growth rate of LNCaP was significantly reduced after androgen depletion. The addition of either NT or bombesin restores the growth of LNCaP cells, with kinetics and extent comparable to those of the synthetic androgen R1881.
FIG. 1.
Effects of bombesin (Bomb) and NT on growth of androgen-dependent prostate cancer cells. Parental LNCaP (A) and CWR22R (B) cells were plated in medium supplemented with 10% charcoal-stripped FBS with 1 nM R1881 or with 50 nM bombesin or NT, and the numbers of cells were counted at the indicated times. These results represent the averages of two independent experiments. Error bars indicate standard errors.
We also tested another androgen-responsive prostate cancer cell line CWR22R, which was derived from a relapsed tumor (83). Although not required, androgen modulates the in vitro growth of CWR22R cells (58, 83). This was reproduced in Fig. 1B, where CWR22R cells were found to grow in the absence of androgen (control) but with an increased rate in the presence of androgen (R1881). NT and bombesin have comparable, if not higher, potencies in stimulating the growth of CWR22R cells. These experiments suggest that NT and bombesin can substitute for androgen as growth factors for androgen-responsive prostate cancer cell lines.
Bombesin and NT activate androgen-dependent promoters.
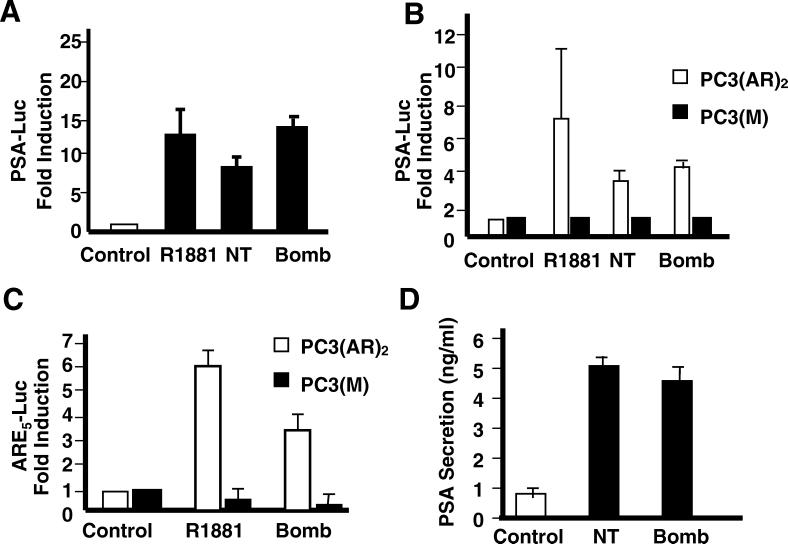
The above results suggest that bombesin and NT can substitute for androgen in stimulating the growth of androgen-responsive prostate cancer cells. There are two possible mechanisms: (i) AR independent (the neurotrophic factors activate their own growth pathways without the participation of the AR) and (ii) AR dependent (the neurotrophic factors activate the AR without the participation of androgen). To distinguish between these two possibilities, we asked whether the AR is activated and whether it is required. Reporter constructs driven by the PSA promoter, known to be a target gene of the activated AR, were used to assess AR activity (78). LNCaP cells were transfected with the PSA (−630/+12) promoter-luciferase reporter plasmid and treated with R1881, NT, or bombesin in charcoal-stripped medium. At the optimal concentration of R1881 (1 nM), PSA luciferase activity was increased about 12-fold (Fig. 2A). In comparison, NT (100 nM) and bombesin (100 nM) increased PSA luciferase activity 8- and 14-fold, respectively. In support of this finding, we also observed that PSA secretion is elevated by NT and bombesin treatment, indicating that the endogenous PSA promoter was also activated (Fig. 2D).
FIG. 2.
Effects of bombesin (Bomb) and NT on androgen-dependent PSA transcription. Stimulation of reporter gene activity in LNCaP and PC3 cells stably expressing AR [PC3(AR)2] or mock-transfected cells [PC3(M)] transfected with the reporter plasmids. (A) LNCaP cells were transiently transfected with the PSA (−630/+12 Luc) plasmid and then treated with either R1881 (1 nM), bombesin (100 nM), or NT (100 nM) for 24 h in medium with 10% charcoal-stripped FBS. (B) PC3(AR)2 and PC3(M) cells were transfected with the PSA (−630/+12 Luc) plasmid. After transfection, cells were treated with either R1881(1 nM), bombesin (100 nM), or NT (100 nM) for 24 h in medium containing 5% charcoal-stripped FBS and 50 μg of hygromycin per ml. (C) PC3(AR)2 and PC3(M) cells were transfected with ARE5-Luc and treated with either R1881 (1 nM), bombesin (100 nM), or NT (100 nM) for 24 h in 5% charcoal-stripped FBS. The results are taken from three independent experiments. The ratio of ARE luciferase to pRL-tk luciferase represents relative luciferase activity. The fold increase indicates the ratio of the normalized luciferase activities between the cells cultured without androgen and with androgen or bombesin. (D) Regulation of PSA secretion in LNCaP cells by bombesin or NT. The cells were incubated in the presence of bombesin or NT for 72 h.
To determine whether this activation requires the AR, we exploit the isogenic PC3 and PC3(AR)2 cell lines. PC3 is a prostate cancer cell line derived from a poorly differentiated human carcinoma which lacks AR expression (34). PC3(AR)2 was derived by transfection of wild-type AR gene and PC3(M) by the vector only (40). If the activation of PSA-Luc by bombesin and NT requires the AR, increased luciferase activity only in PC3(AR)2, but not in PC3 or PC3(M), is expected. The results in Fig. 2B showed that PSA-Luc activity was induced 7.5-fold by R1881 and 3.5- and 4-fold by NT and bombesin, respectively, in PC3(AR)2 cells but not in AR-deficient PC3 cells. These findings suggest that AR is required in the transcriptional process.
To ensure that this activation is via AR binding to the ARE as opposed to other promiscuous enhancer motifs in the PSA promoter, we transiently transfected PC3(AR)2 and PC3(M) cells with a reporter construct, ARE5-Luc that carries only ARE as the enhancer. The luciferase activity was induced 6-fold by R1881 and 3.3-fold by bombesin. These data taken together provide strong evidence that bombesin- and NT-induced responses involve AR and ARE.
Bombesin induced cell growth requires a functional AR.
The induction of PSA transcriptional activity by bombesin appears to be dependent on the AR, as implied by the above experiments. To further test whether bombesin requires the AR for mitogenesis, the antiandrogen flutamide was employed. As shown in Fig. 3, preincubation of LNCaP cells with flutamide blocked bombesin-induced cell growth. Flutamide inhibited androgen-stimulated cell growth as expected. These results lend further support to the notion that bombesin-induced cell growth in LNCaP cells requires a functional AR.
FIG. 3.
Inhibitory effect of flutamide on bombesin-induced cell proliferation. LNCaP cells were preincubated in the absence or presence of flutamide (Flu) for 30 min before the addition of R1881 (1 nM) or bombesin (Bomb) (100 nM) for 72 h under charcoal-stripped serum conditions. Then 20 μl of MTS was added to each well for 2 h at 37°C. After incubation, the absorbance or optical density at a wavelength of 490 nm (OD490nm) was read as described in Materials and Methods.
Tyrosine kinases activated by bombesin and NT in LNCaP.
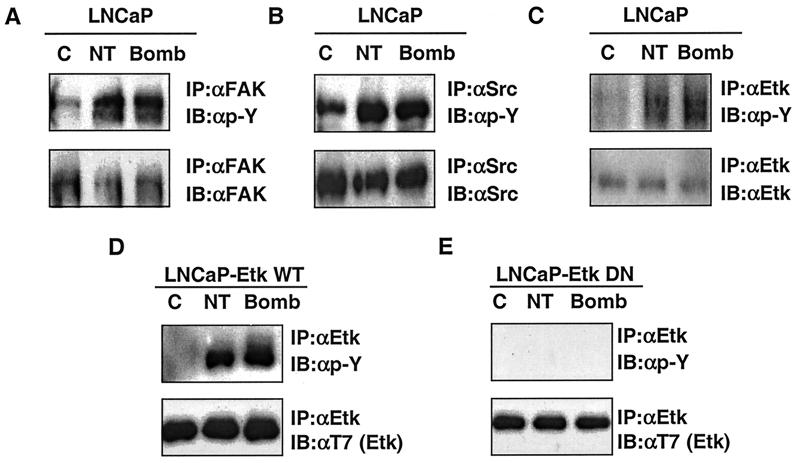
The above finding indicates that AR can be activated by neurotrophic factors. It is not intuitively obvious how neuropeptides, which do not bind nuclear receptor, but rather G-protein-coupled receptor, can function as such. The receptors for mammalian bombesin are GRP-R (gastrin-releasing peptide receptor), NMB-R (neromedullin B receptor), BRS-3 (bombesin receptor subtype 3), and the receptors for NT are NTR1 and NTR2. These receptors are coupled to Gαq and G12α (75). Gαq is known to activate PLCβ, resulting in calcium mobilization and PKC activation. We first tested whether PKC is involved in AR activation. Inhibitors of PKC did not appreciably affect AR activation by bombesin and NT (data not shown). We then turned our attention to other signal pathways. Increasing evidence suggests that G-protein signals engage tyrosine kinases including nonreceptor tyrosine kinases such as Src and Btk (32, 46, 52–54). Furthermore, as discussed earlier, phosphorylation of ARs is shown to be an alternative way of activation other than ligand binding. We then asked whether tyrosine kinases are involved in bombesin- and NT-induced AR activation. We took advantage of our knowledge of the complete tyrosine kinase expression profile of LNCaP cells as determined by an effective reverse transcription-PCR approach developed in our laboratory. Based on this approach, we know there are 21 receptor tyrosine kinases and 11 nonreceptor tyrosine kinases expressed in this cell type (50, 70). The nonreceptor tyrosine kinases are Jak, Tyk2, Src, yes, csk, FAK, Pyk2, Etk, Brk, Abl, and Arg. This information allows us to quickly screen potential tyrosine kinases activated by bombesin and NT, using immunoprecipitation with antibodies to individual kinases followed by Western blot analysis with antiphosphotyrosine antibodies. Among the tyrosine kinases screened, we found that FAK, Src, and Etk/Bmx (Fig. 4A to C, top blots) are prominently activated as reflected by the increased tyrosine phosphorylation on these proteins after neuropeptide treatments. Immunoblotting with antibodies against individual kinases confirm that similar amounts of each protein were loaded in each lane (Fig. 4A to C, bottom blots). The activation of FAK and Src is consistent with previous reports in different cell types (7, 72, 77). Our data confirm and extend these observations to the prostate LNCaP cell line. The finding regarding activation of Etk/Bmx by bombesin and NT is new but is in agreement with the reports that Gαq and Gα12 are activators of the Btk/Tec family of kinases, of which Etk/Bmx is a member (15, 46, 53).
FIG. 4.
Bombesin (Bomb) and NT stimulate the tyrosine phosphorylation of FAK, Src, and Etk compared to control. Antiphosphotyrosine Western blots of immunoprecipitates of FAK (A), Src (B), and Etk kinase (top blots of panels C to E). The protein expression level was confirmed by immunoblotting with antibodies to individual signaling molecules (bottom blots). (D and E) LNCaP cells were transfected with T7-Etk or T7-EtkDN (E42K and K444Q), a dominant-negative mutant, and selected by using 600 μg of G418 per ml. C, control; IP, immunoprecipitation; IB, immunoblotting; αFAK, anti-FAK antibody.
Etk/Bmx is a tyrosine kinase carrying multiple protein-protein interaction modules including a pleckstrin homology (PH) domain, a Src homology 3 (SH3) domain, and an SH2 domain. It was first identified in bone marrow cells by Tamagnone et al. (84); it was identified independently in prostate cancer cells by our group (66, 70). In LNCaP cells, Etk is expressed at a moderate level yet plays important roles in both neuroendocrine differentiation and antiapoptosis processes (66, 95). A cell line (LNCaP-EtkDN), which harbors a dominant-negative ETK KQ, shown to be effective in reversing the Etk-dependent phenotypes of LNCaP, was used in this study (66, 95). This cell line, in contrast to the parental LNCaP cell line (Fig. 4C) and a cell line transfected with wild-type Etk (Fig. 4D), failed to display neuropeptide-induced phosphorylation (Fig. 4E), confirming the kinase-dead nature of the Etk mutant and that enhanced phosphorylation is contributed primarily by autokinase activity.
Roles of Etk and Src tyrosine kinase in bombesin-mediated androgen-independent growth.
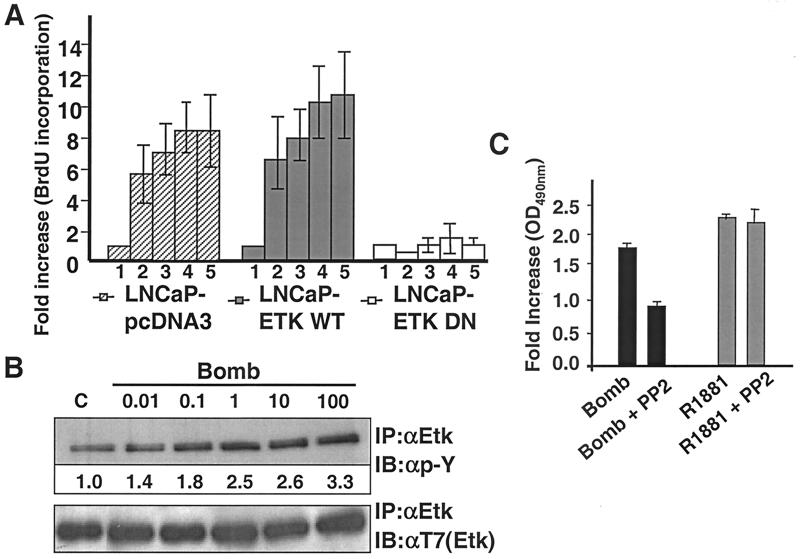
The LNCaP-EtkDN cell line affords us an opportunity to assess directly the involvement of Etk in androgen-independent growth induced by bombesin. LNCaP cell lines transfected with wild-type Etk or with pcDNA vector were used as positive controls. The cell proliferation potential was determined by the measurement of ELISA-based BrdU incorporation (see Materials and Methods). As shown in Fig. 5A, in LNCaP-pcDNA3 and LNCaP-EtkWT cells, 0.1 nM bombesin induced a five- and sixfold increase in proliferation, respectively, over that of untreated samples. A 10-fold increase was observed when the concentration of bombesin was increased to 100 nM. This increase of proliferative capacity is paralleled by an increase of the Etk tyrosine phosphorylation (Fig. 5B). In stark contrast, LNCaP-EtkDN cells were not at all responsive to bombesin-stimulated growth. These data, taken together, suggest that Etk activity is critical in bombesin-induced androgen-independent cell growth. It should be noted that our previous work (95) reproduced in the present study (data not shown) showed that the growth kinetics of LNCaP-EtkDN cells is no different from that of wild-type LNCaP cells in the presence of androgen. These data suggest that Etk tyrosine kinase specifically participates in growth pathway affected by neuropeptides but not by androgen.
FIG. 5.
Dominant-negative Etk (A) and Src (C) inhibits bombesin-mediated cell growth using the BrdU labeling proliferation assay. (A) LNCaP-pcDNA3, LNCaP-EtkWT, and LNCaP-EtkDN cells were incubated in the absence (bar 1) or presence of different concentrations of bombesin as follows: 0.1 nM (bar 2), 1 nM (bar 3), 100 nM (bar 4), and 1 μM (bar 5) in 10% charcoal-stripped FBS for 72 h. At the end of incubation, cells were fixed and stained with BrdU as specified by the manufacturer's protocol and fold increase was measured by ELISA. Each experiment was carried out in triplicate, and the error bars represent standard deviations. For each bar, the fold increase was normalized to the value for the control group. (B) LNCaP cells were not treated (lane C) or treated with different concentrations (nanomolar) of bombesin (Bomb) as indicated for 30 min. Tyrosine phosphorylation of Etk was analyzed by immunoprecipitation using anti-Etk antibody (IP:αEtk) followed by Western blotting (immunoblotting) with anti-pY antibody (IB:αp-Y). Anti-phospho-tyrosine (top blot) (αEtk) (active Etk) and anti-T7 (bottom blot) (αT7) (total Etk) antibodies were used in Western blots (immunoblots [IB]) of Etk immunoprecipitates. Numbers under the bands indicate the fold activation of Etk, as quantitated by video image densitometry. (C) LNCaP cells were preincubated in the absence or presence of PP2 (10 μM) for 30 min before the addition of R1881 (1 nM), or bombesin (Bomb) (100 nM) for 72 h under charcoal-stripped serum conditions. Then 20 μl of MTS was added to each well for 2 h at 37°C. After incubation, the absorbance or optical density at a wavelength of 490 nm (OD490nm) was read.
Taking advantage of the selective inhibitor of Src, PP2, we wish to test the involvement of Src in bombesin-induced cell proliferation of LNCaP cells. The MTS assay was performed on LNCaP cells treated with pyrazolopyrimidine PP2, at a concentration of 10 μM, which selectively inhibits Src family kinases (39, 72). As shown in Fig. 5C, PP2 specifically blocks bombesin-induced LNCaP cell proliferation, but not androgen-induced proliferation (Fig. 5C), confirming the role of Src in bombesin-induced cell proliferation.
Roles of FAK, Src, and Etk in bombesin-induced AR activation.
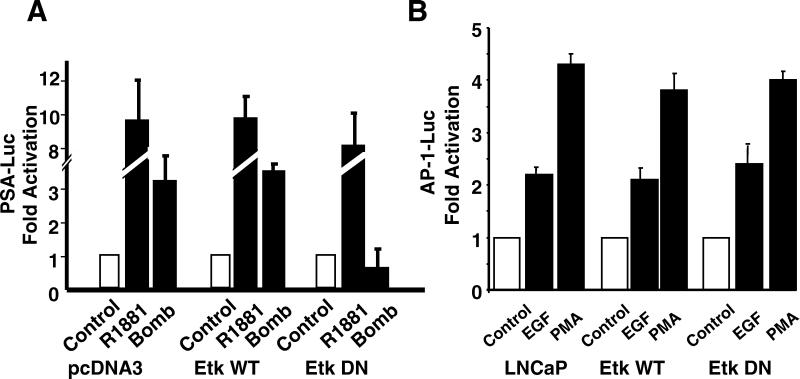
To study whether Etk, FAK, and Src are involved in the activation of the AR by bombesin, bypassing the need for androgen, we used the ARE5-Luc reporter transactivation assay. For AR activation by Etk, the ARE5-Luc reporter was transfected into LNCaP-pcDNA3, LNCaP-EtkWT, or LNCaP-EtkDN cells (Fig. 6A). In LNCaP-EtkWT cells and in LNCaP-pcDNA3 cells, both R1881 and bombesin induce luciferase activity driven by the ARE, whereas in LNCaP-EtkDN cells, the activity is largely reduced in bombesin-treated cells but not in R1881-treated cells. To ensure that the observed unresponsiveness is not due to some peculiarity of LNCaP-EtkDN cells, we tested the abilities of EGF and PMA to activate AP-1 luciferase activity in this cell type (Fig. 6B); AP-1 activity is induced at the same level as those in LNCaP-EtkWT and LNCaP cells.
FIG. 6.
Dominant-negative mutant Etk (EtkDN) blocks bombesin-induced AR pathway but not AP-1 luciferase activity. (A) LNCaP-pcDNA3, LNCaP-EtkWT, or LNCaP-EtkDN cells were cotransfected with pRL-tk vector and PSA-Luc reporter. Cells were then treated with R1881 (1 nM) or bombesin (Bomb) (100 nM) for 24 h in 5% charcoal-stripped FBS. (B) LNCaP, LNCaP-EtkWT, or LNCaP-EtkDN cells were cotransfected with pRL-tk vector and AP-1-Luc reporter (pUXLUC2X(−126/−120). Cells were then treated with EGF (10 ng/ml) or PMA (1 nM) for 24 h. The fold increase represents the ratio of the normalized luciferase activities between the cells cultured without and with EGF or PMA. The results are taken from three independent experiments.
To test the involvement of Src and FAK in AR activation, ARE reporter construct and dominant-negative mutants of FAK or Src, FRNK and SrcKR, respectively, were cotransfected into LNCaP cells (Fig. 7). Bombesin induced ARE luciferase activity in vector-transfected cells but not in cells transfected with the dominant-negative mutants of Src and FAK. These data suggest that FAK and Src, like Etk, are also involved in bombesin-induced AR activation.
FIG. 7.
Dominant-negative mutant of Src (SrcKR) and FAK (FRNK) blocks bombesin-induced AR pathway. LNCaP cells were cotransfected with the PSA-Luc reporter and a pRL-tk reporter plus SrcKR or FRNK or an empty vector and cultured in 5% charcoal-stripped FBS. The ratio of ARE luciferase to pRL-tk luciferase represents relative luciferase activity. The fold increase indicates the ratio of the normalized luciferase activities between the cells cultured without bombesin and with bombesin (Bomb). The results are taken from three independent experiments.
At present, we do not know exactly how this activation is accomplished, although it is unlikely that Etk directly phosphorylates AR on tyrosine residues. More likely, Etk transmits the signals through other serine/threonine kinases or coactivators, which activate the AR (see Discussion). The search for downstream signal pathways is in progress. In the ensuing section, we demonstrate data that addresses the upstream signals from bombesin to the activation of Etk.
Roles of FAK in Etk and Src activation.
Having shown that Etk plays an important role in bombesin-induced androgen-independent growth and AR activation, we were interested in studying how bombesin activates Etk tyrosine kinase. The activation of Etk, like other Btk/Tec family kinases, is thought to require two steps: (i) disruption of the internal folding between the PH domain and the kinase domain by lipids, such as PIP3 (phosphatidylinositol triphosphate) (66), or proteins (47) that have high affinity toward the PH domain; and (ii) phosphorylation of a tyrosine residue by Src-like kinase to activate the catalytic activity (6, 67). We recently reported that the FERM domain of an activated FAK associates tightly with the PH domain of Etk (25). We therefore asked whether FAK, which is activated by bombesin (Fig. 3), is involved in the activation of Etk in LNCaP cells. To this end, hemagglutinin (HA)-tagged wild-type FAK or dominant-negative mutant HA-FAKY397F or HA-FRNK was cotransfected with T7-Etk into LNCaP cells. The phosphorylation of Etk was measured after bombesin treatment. Figure 8 shows that bombesin strongly activates Etk in vector-transfected LNCaP (Fig. 8A, lanes 1 and 2) or in wild-type FAK-transfected LNCaP (Fig. 8A, lanes 4 and 5). FAKY397F has a greatly diminished kinase activity and failed to bind both Src and PI3K (21). Another dominant-negative mutant, FRNK, is a C-terminal variant of FAK, which does not have a kinase domain but retains the focal contact domain (69). Expression of either dominant-negative mutant of FAK (HA-FAKY397F or HA-FRNK) greatly reduced Etk activation by bombesin (Fig. 8A, lanes 6 and 7), suggesting FAK is an activator of Etk. At the same time, HA-FAKY397F also diminishes the activity of Src (Fig. 8B), suggesting that FAK Y397 binding contributes to Src activation. There are several mechanisms by which FAK can activate Etk. FAK can activate Etk directly or indirectly through Src and PI3K. The above mechanisms are not mutually exclusive. We proceeded to investigate the involvement of Src and PI3K in Etk activation.
FIG. 8.
Dominant-negative mutants of FAK, HA-FAKY397F, and HA-FRNK, but not HA-FAKD395A, blocked Etk activation in response to bombesin. (A) Cells were cotransfected with wild-type Etk or T7-Etk with one of the following plasmids: vector, wild-type FAK, HA-FAK, or dominant-negative mutant HA-FAKD395A, HA-FAKY397F, or HA-FRNK. After transfection, LNCaP cells were then treated with 100 nM bombesin (lanes B) or untreated control (lanes C) for 30 min as indicated and subsequently lysed. Tyrosine phosphorylation of Etk was analyzed by immunoprecipitation using anti-Etk antibody (IP:αEtk) followed by Western blotting (immunoblotting) with anti-pY antibody (IB:αp-Y) 4G10 (top blots). The membrane was analyzed further by Western blotting using T7 antibody [IB:αT7(Etk)] (bottom blots). Half of the cell lysates described for Etk were immunoprecipitated with HA antibody [IP:αHA(FAK)] followed by blotting with FAK polyclonal antibody (IB:αFAK) (middle blots). (B) LNCaP cells were transfected with FAKY397F or empty vector, pcDNA3, and the lysates were immunoprecipitated with monoclonal Src antibody (IP:αSrc) and immunoblotted with anti-pY antibody (IB:αp-Y) (top blot) and anti-Src polyclonal antibody (IB:αSrc) (bottom blot).
Role of Src in Etk activation.
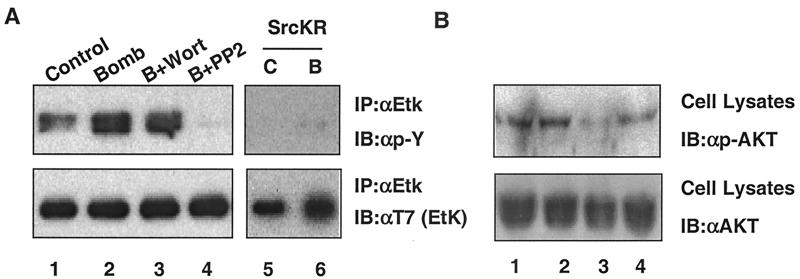
To test the role of Src in Etk activation, we used PP2 or dominant-negative mutant SrcKR. Both experiments yielded convergent results, indicating that Src activity is required for bombesin-induced Etk activation. Figure 9A showed that PP2 treatment or SrcKR transfection significantly diminishes Etk activity (Fig. 9A, compare lanes 4 and 6 to lane 2).
FIG. 9.
Src, but not PI3K, is critical in bombesin-induced Etk activation. (A and B) PP2 blocks the activation of Etk by bombesin (Bomb). LNCaP-EtkWT cells were serum starved for 24 h. The cells were then pretreated with 100 nM wortmannin (Wort), 10 μM PP2, or dimethyl sulfoxide (control or C [lanes 1 and 5]) for 30 min and then treated with bombesin for 30 min as indicated (Bomb or B) (lanes 2 to 4). A dominant-negative Src (c-SrcKR) blocks the activation of Etk by bombesin (lanes 5 and 6). LNCaP cells were cotransfected with Src and Etk dominant-negative mutant (EtkDN and c-SrcKR). At 24 h posttransfection, cells were serum starved for 24 h and treated with bombesin (lane B) for 30 min as indicated. The cell extracts were immunoprecipitated with anti-Etk (IP:αEtk) and then immunoblotted with anti-pY antibody (IB:αp-Y) (top blots) and anti-T7 antibody [IB:αT7 (EtK)] (bottom blots). (B) The cell extracts were immunoblotted with anti-phospho-AKT antibody (IB:αp-AKT) (top blot) and anti-AKT antibody (IB:αAKT) (bottom blot).
Role of PI3K in Etk activation.
We previously showed that Etk, like other Btk/Tec family kinases, is activated by PI3K, presumably via the binding of the Etk PH domain to PIP3, the metabolic product of PI3K. Specifically, we showed that PI3K is required for IL-6-induced activation of Etk (66). Since FAK is known to be an activator of PI3K, we were curious whether PI3K is involved in Etk activation. In contrast to PP2, wortmannin, an inhibitor for PI3K, had little effect on bombesin-induced activation (Fig. 9A, lane 3 versus lane 2). To ensure that wortmannin worked as intended, we included AKT phosphorylation (a known indicator of PI3K activity) as a control. Wortmannin completely abolishes the phosphorylation of AKT, based on the lack of signal in the immunoblot with phospho-AKT antibody (Fig. 9B, lane 3)
To further demonstrate that FAK activation of Etk is through Src, but not PI3K, we used a FAK mutant, FAKD395A, that preserves the Y397 binding context for Src but not PI3K (68). We predicted that this mutant should still activate Etk, and this is indeed the case: FAKD395A only slightly decreased Etk activation (Fig. 8, compare lanes 2 and 3) confirming a major role for Src but not PI3K in Etk activation.
FAK, Src, and Etk form a stable in vivo complex.
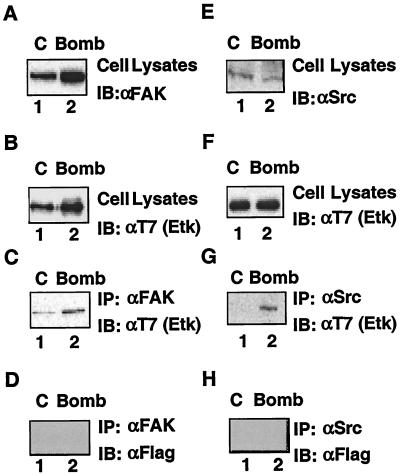
Our data provide evidence that the three nonreceptor kinases FAK, Src, and Etk are all activated upon bombesin treatment of LNCaP cells. Since we know from previous reports for different cell types that FAK interacts with Src (21), Src interacts with Etk (85), and Etk interacts with FAK (25), we were curious to determine whether a complex involving all three components can be found in bombesin-treated LNCaP cells. The results using the LNCaP-EtkWT cells showed that immunoprecipitates of FAK contain Etk, based on Western blot results with T7 (Etk) antibody (Fig. 10C). The formation of this complex, however, does not depend on bombesin treatment, indicating that in LNCaP cells, the Etk and FAK complex has already formed (Fig. 10C, lanes 1 and 2). By contrast, the formation of a complex between Src and Etk depends on bombesin (Fig. 10G, lanes 1 and 2). To further confirm that these associations are specific, we also performed experiments using a nonspecific Flag antibody. The results showed that immunoprecipitates of FAK and Src were not detected after immunoblotting with Flag antibody (Fig. 10D and H), which suggests that the interactions of FAK-Etk and Src-Etk are specific. These findings, together with those of Salazar and Rozengut (77) showing that FAK-Src association depends on treatment with bombesin, suggest that FAK is likely the scaffold that pulls these two components together. Based on the above results, we showed that bombesin induces the formation of a signal complex with three activated tyrosine kinases that has the potential to transmit a phosphorylation cascade that can modify the AR. It is likely the combined action of these phosphorylations that make this pathway particularly active.
FIG. 10.
Etk interacts with FAK and Src in cells. LNCaP-EtkWT cells were either not treated or treated with 100 nM bombesin for 30 min. After 48 h posttransfection, cells were not treated (control [C]) (lanes 1) or treated with 100 nM bombesin (Bomb) (lanes 2). The expression of FAK, Etk, or Src was analyzed by Western blotting (immunoblotting [IB]) using either anti-FAK (αFAK) (A), anti-T7 (Etk) [αT7 (Etk)] (B and F), or anti-Src (αSrc) (E) antibodies, respectively. Half of the cell lysates used above for panels A and D were incubated with anti-FAK antibody and anti-Src antibody, and the immunoprecipitates were then Western blotted (immunoblotted) with anti-T7 (Etk) antibody [IB: αT7 (Etk)] (C and G), respectively, to detect the association of Etk with FAK and Src. The blots from panels C and G were stripped and then Western blotted with anti-Flag antibody (IB: αFLAG) (D and H).
DISCUSSION
There are several significant findings in this study. (i) We establish that neuropeptides such as bombesin and NT are able to substitute for androgen in sustaining the growth of androgen-dependent LNCaP cells, raising the possibility that neuroendocrine cells and their released paracrine factors play important roles in prostate cancer progression. (ii) We show that these neuropeptides are able to activate androgen-dependent promoters and that this process requires a functional AR, implicating their involvement during the transition from an androgen-dependent to -independent state. (iii) We show that bombesin and NT activate a signal complex involving three nonreceptor tyrosine kinases, FAK, Src, and Etk/Bmx, connecting G-protein signaling to that of tyrosine kinases in prostate cancer cells. These kinases are known to be involved in cell motility, transformation, and antiapoptosis, respectively, which may account for some of the properties associated with neuropeptides. (iv) We present evidence that these tyrosine kinases are also involved in the induction of androgen independence, identifying them as potential therapeutic targets. (v) Finally, we demonstrate that Etk/Bmx, can be activated by FAK, possibly through Src, without significant involvement of PI3K, providing new insight into the activation mechanism of Btk/Tec family kinases.
Neuroendocrine cells and prostate cancer progression.
It has long been recognized that neuroendocrine cells are present and intermingled with healthy prostate or prostate cancer epithelial cells (2, 3). Some reports suggest that neuroendocrine cells increase in number during prostate cancer progression (4, 5). It has been proposed that cells may act as a source for paracrine factors that support androgen-independent growth, survival, and migration of the surrounding cancer cells (8, 10). We and others showed that LNCaP can be transdifferentiated by cytokine IL-6 (66) or cyclic AMP agonists (27, 28) into neuroendocrine cells with neuronal morphology. These cells are postmitotic and unable to grow but release neurotrophic factors which potentially can stimulate the growth of the surrounding undiffentiated cancer cells. Among the factors released by neuroendocrine cells, bombesin/GRP has been studied most extensively as an autocrine and paracrine growth factor for many tumor types (9, 42). Bombesin is both a growth factor and migration factor for fibroblasts, lung cancer cells, and prostate cancer cells. In this study, we showed that it may also be a progression factor for androgen independence. Therapeutic modalities based on antagonists of GRP or its receptor have already been developed, and some are currently undergoing clinical trials (79). Our results that bombesin/GRP induces androgen independence via tyrosine kinases suggest that tyrosine kinase inhibitors, which have shown great promise in cancer treatments, may also be used as a combination therapy.
Signal pathways activated by neuropeptides.
Both bombesin and NT bind to the G-protein-coupled receptor (14, 98). The engagement of Gαq to the receptor liberates Gβγ, which activates phospholipase β (PLCβ) (41, 62). PLCβ produces inositol 1,4,5-triphosphate which mobilizes Ca2+ from internal stores and diacylglycerol which in turn, activates PKC (23, 35, 59). A recent study by Buhl et al. showed that Gα12 is also activated by bombesin (18). Gα12 directly associates with Rho-GEF which activates small G-protein Rho (27, 28). Activation of Rho leads to actin polymerization, an important step in cell motility. How and whether these signals generated from G proteins are connected to AR activation is presently unclear. Salazar and Rozengurt (77) showed that in fibroblasts, bombesin-induced actin clustering leads to the activation of FAK tyrosine kinase and a rapid increase in the formation of FAK-Src complexes. This process depends on the integrity of the actin filament network, but not on Ca2+ or PI3K (75). Our results for LNCaP cells are in agreement with this finding, and we propose the following model (Fig. 11) depicting the signals connecting bombesin to the activation of three kinases and eventually to the AR.
FIG. 11.
Summary of the signaling pathway that connects neuropeptides to the AR.
Involvement of nonreceptor tyrosine kinases FAK, Src, and Etk/Bmx.
FAK was originally discovered as a substrate of Src and a kinase activated by integrin clustering (63). FAK, localized in focal adhesion and membrane ruffles, is now thought to play a role in cell motility rather than the formation of focal complexes. FAK is comprised of four domains, the N-terminal FERM domain, a protein-protein interaction domain with homology to band 4.1, the tyrosine kinase domain, and the C-terminal F-actin binding domain. Initial activation of the FAK autokinase is accomplished by actin polymerization or integrin clustering (71), leading to the phosphorylation of Y397, which then serves as the anchor site for either Src or PI3K. The interaction of Y397 with the SH2 domain of Src activates Src kinase activity. The activated Src in turn phosphorylates Y576 and Y577 of FAK, leading to the full activation of FAK (72, 74, 75, 77). This is a case where two kinases act synergistically with each other to reach maximal activity. One of the dominant-negative mutants used in this study, Y397F, is not able to bind Src, and as a result achieves only basal activation levels. The second mutant, FRNK, corresponds to the C-terminal part of FAK which competes with wild-type FAK for localization to focal contacts but lacks kinase activity to transmit a downstream signal. FRNK was shown to be a potent dominant-negative mutant in blocking migration and other phenotypes induced by FAK (69). A third mutant, D395A, was engineered to maintain the binding context around Y397 for Src, but not PI3K. As a result, this mutant is capable of activating only Src, not PI3K (24).
In addition to FAK and Src, our survey of bombesin-activated tyrosine kinases revealed that Etk/Bmx is also activated. Etk/BMX is a tyrosine kinase characterized by having a PH domain at the N terminus and is in the Btk or Tec family of kinases (84, 86, 88). The PH domain, which has a protein-lipid interaction domain as well as a protein-protein interaction domain, regulates Etk activity by a two-step mechanism. The first involves the binding of the PH domain to its lipid-ligand phosphatidylinositol-3,4,5-triphosphate, a product of PI3K, or a protein-ligand such as protein-tyrosine phosphatase D1 (47). This binding presumably opens up the kinase domain, allowing Src-like kinases to phosphorylate tyrosine residue 566, which activates the intrinsic kinase activity of Etk (26, 49). Recently, we showed that the FERM domain of FAK associates with Etk and serves as a ligand to activate Etk (25). The present study extends this observation and demonstrates that FAK, Src, and Etk are engaged in the formation of a complex in bombesin-treated cells. The association between FAK and Etk appears to be preformed, whereas that of Src is induced by neurotrophic factors (Fig. 11). Dominant-negative mutants of FAK and Src inhibitors abolish the Etk activation, while the PI3K inhibitor wortmannin has no effect. This is consistent with the model depicted in Fig. 11 that FAK, Src, and Etk form a complex, with the potential for mutual activations. Within this complex, FAK activates Src (77) and Src activates Etk (85). At the same time, Src is known to activate FAK (21) and FAK has been shown to activate Etk (25). The result is the activation of three tyrosine kinases which each have the potential to transmit phosphorylation signals to the nucleus, resulting in AR activation. Although in this study we focused on the androgen-independent growth aspect of LNCaP cells, it is likely that this complex is also involved in enhanced migration (J. Yang and C. Evans, personal communication).
Role of Etk in androgen independence.
Previous work from this and other labs showed that Etk/Bmx is involved in cellular transformation, antiapoptosis, and differentiation processes of various cell types (66, 85, 95). The present study showed that Etk and Src are required for androgen-independent growth and the activation of the AR pathway. We have not addressed how Etk/Bmx or the FAK-Src-Etk complex channels the signals to the AR. We presume that it is through phosphorylation of the AR by downstream kinases. As discussed earlier, MAPK and AKT have been found to be mediators of androgen-independent activation of the AR. Both kinase pathways are activated by FAK and Src, and AKT was shown to be activated by at least one member of the Tec family of kinases (22). Indeed, recently we have observed that MAPK can be activated by bombesin and that activated MAPK phosphorylated the AR in an in vitro kinase assay (L.-F. Lee and H.-J. Kung, unpublished data). In addition, Etk has the potential to activate a number of other serine kinases, such as PAK (11) and PKC (65), which may also participate in a yet unknown role in AR activation. Alternatively, the target of phosphorylation may be coactivators or corepressors of AR. The association of coactivators or dissociation of corepressors are responsible for AR activation. Phosphorylation of these modulators may change their affinities towards AR, resulting in activation of the AR. Among the known targets of Etk, STAT3, when phosphorylated and activated, was shown to render AR active (26, 85). Experiments to define the downstream pathway leading to AR activation by Etk or the FAK-Src-Etk complex are in progress.
In summary, neurotrophic factors have been implicated in prostate cancer progression (9). Here we show that, in addition to their roles in the stimulation of growth and metastasis of prostate cancer cells, they also induce androgen independence and thus may be involved in the initial transition from an androgen-dependent to -independent state. They do so by activating a signal complex consisting of three nonreceptor tyrosine kinases, FAK, Src, and Etk. This new pathway integrates signals generated by G-protein-coupled receptors, tyrosine kinases, and hormone receptor.
ACKNOWLEDGMENTS
We thank Chris Evan, who kindly provided the PC3(AR)2 and PC3(M) cells, and David L. Boucher for critical reading of the manuscript.
This study was supported in part by a United States Department of Defense postdoctoral fellowship (PC001247 to L.-F.L.) and by grants from the California Prostate Research program, Department of Defense (PC970090), and the National Institutes of Health (CA39207 and CA57179 to H.-J.K.)
REFERENCES
- 1.Abi-Aad A S, Opsomer R J. Prostate cancer–treatment of disseminated disease. Acta Urol Belg. 1996;64:67–76. [PubMed] [Google Scholar]
- 2.Abrahamsson P A. Neuroendocrine cells in tumour growth of the prostate. Endocr Relat Cancer. 1999;6:503–519. doi: 10.1677/erc.0.0060503. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamsson P A. Neuroendocrine differentiation in prostatic carcinoma. Prostate. 1999;39:135–148. doi: 10.1002/(sici)1097-0045(19990501)39:2<135::aid-pros9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Abrahamsson P A, Falkmer S, Falt K, Grimelius L. The course of neuroendocrine differentiation in prostatic carcinomas. An immunohistochemical study testing chromogranin A as an “endocrine marker.”. Pathol Res Pract. 1989;185:373–380. doi: 10.1016/S0344-0338(89)80016-0. [DOI] [PubMed] [Google Scholar]
- 5.Abrahamsson P A, Wadstrom L B, Alumets J, Falkmer S, Grimelius L. Peptide-hormone- and serotonin-immunoreactive tumour cells in carcinoma of the prostate. Pathol Res Pract. 1987;182:298–307. doi: 10.1016/S0344-0338(87)80065-1. [DOI] [PubMed] [Google Scholar]
- 6.Afar D E, Park H, Howell B W, Rawlings D J, Cooper J, Witte O N. Regulation of Btk by Src family tyrosine kinases. Mol Cell Biol. 1996;16:3465–3471. doi: 10.1128/mcb.16.7.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allard P, Beaulieu P, Aprikian A, Chevalier S. Bombesin modulates the association of Src with a nuclear 110-kd protein expressed in dividing prostate cells. J Androl. 2000;21:367–375. [PubMed] [Google Scholar]
- 8.Aprikian A G, Cordon-Cardo C, Fair W R, Zhang Z F, Bazinet M, Hamdy S M, Reuter V E. Neuroendocrine differentiation in metastatic prostatic adenocarcinoma. J Urol. 1994;151:914–919. doi: 10.1016/s0022-5347(17)35121-2. [DOI] [PubMed] [Google Scholar]
- 9.Aprikian A G, Han K, Chevalier S, Bazinet M, Viallet J. Bombesin specifically induces intracellular calcium mobilization via gastrin-releasing peptide receptors in human prostate cancer cells. J Mol Endocrinol. 1996;16:297–306. doi: 10.1677/jme.0.0160297. [DOI] [PubMed] [Google Scholar]
- 10.Aprikian A G, Han K, Guy L, Landry F, Begin L R, Chevalier S. Neuroendocrine differentiation and the bombesin/gastrin-releasing peptide family of neuropeptides in the progression of human prostate cancer. Prostate Suppl. 1998;8:52–61. [PubMed] [Google Scholar]
- 11.Bagheri-Yarmand R, Mandal M, Taludker A H, Wang R A, Vadlamudi R K, Kung H J, Kumar R. Etk/Bmx tyrosine kinase activates PAK-1 and regulates the tumorigenicity of breast cancer cells. J Biol Chem. 2001;276:29403–29409. doi: 10.1074/jbc.M103129200. [DOI] [PubMed] [Google Scholar]
- 12.Bang Y J, Pirnia F, Fang W G, Kang W K, Sartor O, Whitesell L, Ha M J, Tsokos M, Sheahan M D, Nguyen P. Terminal neuroendocrine differentiation of human prostate carcinoma cells in response to increased intracellular cyclic AMP. Proc Natl Acad Sci USA. 1994;91:5330–5334. doi: 10.1073/pnas.91.12.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartholdi M F, Wu J M, Pu H, Troncoso P, Eden P A, Feldman R I. In situ hybridization for gastrin-releasing peptide receptor (GRP receptor) expression in prostatic carcinoma. Int J Cancer. 1998;79:82–90. doi: 10.1002/(sici)1097-0215(19980220)79:1<82::aid-ijc16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Battey J F, Way J M, Corjay M H, Shapira H, Kusano K, Harkins R, Wu J M, Slattery T, Mann E, Feldman R I. Molecular cloning of the bombesin/gastrin-releasing peptide receptor from Swiss 3T3 cells. Proc Natl Acad Sci USA. 1991;88:395–399. doi: 10.1073/pnas.88.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bence K, Ma W, Kozasa T, Huang X Y. Direct stimulation of Bruton's tyrosine kinase by G(q)-protein alpha-subunit. Nature. 1997;389:296–299. doi: 10.1038/38520. [DOI] [PubMed] [Google Scholar]
- 16.Bologna M, Festuccia C, Muzi P, Biordi L, Ciomei M. Bombesin stimulates growth of human prostatic cancer cells in vitro. Cancer. 1989;63:1714–1720. doi: 10.1002/1097-0142(19900501)63:9<1714::aid-cncr2820630912>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 18.Buhl A M, Johnson N L, Dhanasekaran N, Johnson G L. G alpha 12 and G alpha 13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J Biol Chem. 1995;270:24631–24634. doi: 10.1074/jbc.270.42.24631. [DOI] [PubMed] [Google Scholar]
- 19.Burchardt T, Burchardt M, Chen M W, Cao Y, de la Taille A, Shabsigh A, Hayek O, Dorai T, Buttyan R. Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. J Urol. 1999;162:1800–1805. [PubMed] [Google Scholar]
- 20.Carter H B, Isaacs J T. Overview of hormonal therapy for prostate cancer. Prog Clin Biol Res. 1990;350:129–140. [PubMed] [Google Scholar]
- 21.Cary L A, Guan J L. Focal adhesion kinase in integrin-mediated signaling. Front Biosci. 1999;4:D102–D113. doi: 10.2741/cary. [DOI] [PubMed] [Google Scholar]
- 22.Chan T O, Rittenhouse S E, Tsichlis P N. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 23.Charlesworth A, Broad S, Rozengurt E. The bombesin/GRP receptor transfected into Rat-1 fibroblasts couples to phospholipase C activation, tyrosine phosphorylation of p125FAK and paxillin and cell proliferation. Oncogene. 1996;12:1337–1345. [PubMed] [Google Scholar]
- 24.Chen H C, Appeddu P A, Isoda H, Guan J L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 25.Chen R, Kim O, Li M, Xiong X, Guan J L, Kung H J, Chen H, Shimizu Y, Qiu Y. Regulation of the PH-domain-containing tyrosine kinase Etk by focal adhesion kinase through the FERM domain. Nat Cell Biol. 2001;3:439–444. doi: 10.1038/35074500. [DOI] [PubMed] [Google Scholar]
- 26.Chen T, Wang L H, Farrar W L. Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res. 2000;60:2132–2135. [PubMed] [Google Scholar]
- 27.Cox M E, Deeble P D, Bissonette E A, Parsons S J. Activated 3′,5′-cyclic AMP-dependent protein kinase is sufficient to induce neuroendocrine-like differentiation of the LNCaP prostate tumor cell line. J Biol Chem. 2000;275:13812–13818. doi: 10.1074/jbc.275.18.13812. [DOI] [PubMed] [Google Scholar]
- 28.Cox M E, Deeble P D, Lakhani S, Parsons S J. Acquisition of neuroendocrine characteristics by prostate tumor cells is reversible: implications for prostate cancer progression. Cancer Res. 1999;59:3821–3830. [PubMed] [Google Scholar]
- 29.Craft N, Shostak Y, Carey M, Sawyers C L. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 30.Culig Z, Hobisch A, Cronauer M V, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- 31.Cuttitta F, Carney D N, Mulshine J, Moody T W, Fedorko J, Fischler A, Minna J D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature. 1985;316:823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 32.Della R G, van Biesen T, Daaka Y, Luttrell D K, Luttrell L M, Lefkowitz R J. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2, and Src kinase. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 33.de Ruiter P E, Teuwen R, Trapman J, Dijkema R, Brinkmann A O. Synergism between androgens and protein kinase-C on androgen-regulated gene expression. Mol Cell Endocrinol. 1995;110:R1–R6. doi: 10.1016/0303-7207(95)03534-e. [DOI] [PubMed] [Google Scholar]
- 34.Edelstein R A, Carr M C, Caesar R, Young M, Atala A, Freeman M R. Detection of human androgen receptor mRNA expression abnormalities by competitive PCR. DNA Cell Biol. 1994;13:265–273. doi: 10.1089/dna.1994.13.265. [DOI] [PubMed] [Google Scholar]
- 35.Erusalimsky J D, Friedberg I, Rozengurt E. Bombesin, diacylglycerols, and phorbol esters rapidly stimulate the phosphorylation of an Mr = 80,000 protein kinase C substrate in permeabilized 3T3 cells. Effect of guanine nucleotides. J Biol Chem. 1988;263:19188–19194. [PubMed] [Google Scholar]
- 36.Frankel A, Tsao M S, Viallet J. Receptor subtype expression and responsiveness to bombesin in cultured human bronchial epithelial cells. Cancer Res. 1994;54:1613–1616. [PubMed] [Google Scholar]
- 37.Gonzalez M M, Gomez V F, Alvarez C L. Disseminated carcinoma of the prostate: monotherapy or complete androgenic blockade? Arch Esp Urol. 1997;50:1067–1076. . (In Spanish.) [PubMed] [Google Scholar]
- 38.Han K, Viallet J, Chevalier S, Zheng W, Bazinet M, Aprikian A G. Characterization of intracellular calcium mobilization by bombesin-related neuropeptides in PC-3 human prostate cancer cells. Prostate. 1997;31:53–60. doi: 10.1002/(sici)1097-0045(19970401)31:1<53::aid-pros9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 39.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 40.Heisler L E, Evangelou A, Lew A M, Trachtenberg J, Elsholtz H P, Brown T J. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol Cell Endocrinol. 1997;126:59–73. doi: 10.1016/s0303-7207(96)03970-6. [DOI] [PubMed] [Google Scholar]
- 41.Hellmich M R, Battey J F, Northup J K. Selective reconstitution of gastrin-releasing peptide receptor with G alpha q. Proc Natl Acad Sci USA. 1997;94:751–756. doi: 10.1073/pnas.94.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hellmich M R, Ives K L, Udupi V, Soloff M S, Greeley G H J, Christensen B N, Townsend C M J. Multiple protein kinase pathways are involved in gastrin-releasing peptide receptor-regulated secretion. J Biol Chem. 1999;274:23901–23909. doi: 10.1074/jbc.274.34.23901. [DOI] [PubMed] [Google Scholar]
- 43.Hoosein N M. Neuroendocrine and immune mediators in prostate cancer progression. Front Biosci. 1998;3:D1274–D1279. doi: 10.2741/a362. [DOI] [PubMed] [Google Scholar]
- 44.Hoosein N M, Logothetis C J, Chung L W. Differential effects of peptide hormones bombesin, vasoactive intestinal polypeptide and somatostatin analog RC-160 on the invasive capacity of human prostatic carcinoma cells. J Urol. 1993;149:1209–1213. doi: 10.1016/s0022-5347(17)36349-8. [DOI] [PubMed] [Google Scholar]
- 45.Ikonen T, Palvimo J J, Kallio P J, Reinikainen P, Janne O A. Stimulation of androgen-regulated transactivation by modulators of protein phosphorylation. Endocrinology. 1994;135:1359–1366. doi: 10.1210/endo.135.4.7925097. [DOI] [PubMed] [Google Scholar]
- 46.Jiang Y, Ma W, Wan Y, Kozasa T, Hattori S, Huang X Y. The G protein G alpha12 stimulates Bruton's tyrosine kinase and a rasGAP through a conserved PH/BM domain. Nature. 1998;395:808–813. doi: 10.1038/27454. [DOI] [PubMed] [Google Scholar]
- 47.Jui H Y, Tseng R J, Wen X, Fang H I, Huang L M, Chen K Y, Kung H J, Ann D K, Shih H M. Protein-tyrosine phosphatase D1, a potential regulator and effector for Tec family kinases. J Biol Chem. 2000;275:41124–41132. doi: 10.1074/jbc.M007772200. [DOI] [PubMed] [Google Scholar]
- 48.Lee L F, Haskill J S, Mukaida N, Matsushima K, Ting J P. Identification of tumor-specific paclitaxel (Taxol)-responsive regulatory elements in the interleukin-8 promoter. Mol Cell Biol. 1997;17:5097–5105. doi: 10.1128/mcb.17.9.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Wahl M I, Eguinoa A, Stephens L R, Hawkins P T, Witte O N. Phosphatidylinositol 3-kinase-gamma activates Bruton's tyrosine kinase in concert with Src family kinases. Proc Natl Acad Sci USA. 1997;94:13820–13825. doi: 10.1073/pnas.94.25.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin W, Kao H W, Robinson D, Kung H J, Wu C W, Chen H C. Tyrosine kinases and gastric cancer. Oncogene. 2000;19:5680–5689. doi: 10.1038/sj.onc.1203924. [DOI] [PubMed] [Google Scholar]
- 51.Logothetis C, Hoosein N. The inhibition of the paracrine progression of prostate cancer as an approach to early therapy of prostatic carcinoma. J Cell Biochem Suppl. 1992;16H:128–134. doi: 10.1002/jcb.240501229. [DOI] [PubMed] [Google Scholar]
- 52.Ma Y C, Huang J, Ali S, Lowry W, Huang X Y. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 53.Ma Y C, Huang X Y. Identification of the binding site for Gqalpha on its effector Bruton's tyrosine kinase. Proc Natl Acad Sci USA. 1998;95:12197–12201. doi: 10.1073/pnas.95.21.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mao J, Xie W, Yuan H, Simon M I, Mano H, Wu D. Tec/Bmx non-receptor tyrosine kinases are involved in regulation of Rho and serum response factor by Galpha12/13. EMBO J. 1998;17:5638–5646. doi: 10.1093/emboj/17.19.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Markwalder R, Reubi J C. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res. 1999;59:1152–1159. [PubMed] [Google Scholar]
- 56.Milovanovic S R, Radulovic S, Groot K, Schally A V. Inhibition of growth of PC-82 human prostate cancer line xenografts in nude mice by bombesin antagonist RC-3095 or combination of agonist [D-Trp6]-luteinizing hormone-releasing hormone and somatostatin analog RC-160. Prostate. 1992;20:269–280. doi: 10.1002/pros.2990200403. [DOI] [PubMed] [Google Scholar]
- 57.Miron L. The hormonal and chemotherapy of prostatic cancer. Rev Med Chir Soc Med Nat IASI. 1996;100:37–43. . (In Romanian.) [PubMed] [Google Scholar]
- 58.Nagabhushan M, Miller C M, Pretlow T P, Giaconia J M, Edgehouse N L, Schwartz S, Kung H J, de Vere W, Gumerlock P H, Resnick M I, Amini S B, Pretlow T G. CWR22: the first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res. 1996;56:3042–3046. [PubMed] [Google Scholar]
- 59.Nanberg E, Rozengurt E. Temporal relationship between inositol polyphosphate formation and increases in cytosolic Ca2+ in quiescent 3T3 cells stimulated by platelet-derived growth factor, bombesin and vasopressin. EMBO J. 1988;7:2741–2747. doi: 10.1002/j.1460-2075.1988.tb03128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nazareth L V, Weigel N L. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 61.Nelson J, Donnelly M, Walker B, Gray J, Shaw C, Murphy R F. Bombesin stimulates proliferation of human breast cancer cells in culture. Br J Cancer. 1991;63:933–936. doi: 10.1038/bjc.1991.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Offermanns S, Heiler E, Spicher K, Schultz G. Gq and G11 are concurrently activated by bombesin and vasopressin in Swiss 3T3 cells. FEBS Lett. 1994;349:201–204. doi: 10.1016/0014-5793(94)00667-9. [DOI] [PubMed] [Google Scholar]
- 63.Parsons J T, Martin K H, Slack J K, Taylor J M, Weed S A. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- 64.Patel K V, Schrey M P. Activation of inositol phospholipid signaling and Ca2+ efflux in human breast cancer cells by bombesin. Cancer Res. 1990;50:235–239. [PubMed] [Google Scholar]
- 65.Qiu Y, Kung H J. Signaling network of the Btk family kinases. Oncogene. 2000;19:5651–5661. doi: 10.1038/sj.onc.1203958. [DOI] [PubMed] [Google Scholar]
- 66.Qiu Y, Robinson D, Pretlow T G, Kung H-J. Etk/Bmx, a tyrosine kinase with a plekstrin-homology domain, is an effector of phosphatidylinositol 3′-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci USA. 1998;95:3644–3649. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rawlings D J, Scharenberg A M, Park H, Wahl M I, Lin S, Kato R M, Fluckiger A C, Witte O N, Kinet J P. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 68.Reiske H R, Kao S C, Cary L A, Guan J L, Lai J F, Chen H C. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J Biol Chem. 1999;274:12361–12366. doi: 10.1074/jbc.274.18.12361. [DOI] [PubMed] [Google Scholar]
- 69.Richardson A, Parsons T. A mechanism for regulation of the adhesion-associated proteintyrosine kinase pp125FAK. Nature. 1996;380:538–540. doi: 10.1038/380538a0. . (Erratum, 381:810.) [DOI] [PubMed] [Google Scholar]
- 70.Robinson D, He F, Pretlow T, Kung H J. A tyrosine kinase profile of prostate carcinoma. Proc Natl Acad Sci USA. 1996;93:5958–5962. doi: 10.1073/pnas.93.12.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodriguez-Fernandez J L. Why do so many stimuli induce tyrosine phosphorylation of FAK? Bioessays. 1999;21:1069–1075. doi: 10.1002/(SICI)1521-1878(199912)22:1<1069::AID-BIES13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez-Fernandez J L, Rozengurt E. Bombesin, vasopressin, lysophosphatidic acid, and sphingosylphosphorylcholine induce focal adhesion kinase activation in intact Swiss 3T3 cells. J Biol Chem. 1998;273:19321–19328. doi: 10.1074/jbc.273.30.19321. [DOI] [PubMed] [Google Scholar]
- 73.Rozengurt E. Growth factors, cell proliferation and cancer: an overview. Mol Biol Med. 1983;1:169–181. [PubMed] [Google Scholar]
- 74.Rozengurt E. Neuropeptides as cellular growth factors: role of multiple signalling pathways. Eur J Clin Invest. 1991;21:123–134. doi: 10.1111/j.1365-2362.1991.tb01801.x. [DOI] [PubMed] [Google Scholar]
- 75.Rozengurt E. Signal transduction pathways in the mitogenic response to G protein-coupled neuropeptide receptor agonists. J Cell Physiol. 1998;177:507–517. doi: 10.1002/(SICI)1097-4652(199812)177:4<507::AID-JCP2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 76.Sadar M D. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem. 1999;274:7777–7783. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- 77.Salazar E P, Rozengurt E. Bombesin and platelet-derived growth factor induce association of endogenous focal adhesion kinase with Src in intact Swiss 3T3 cells. J Biol Chem. 1999;274:28371–28378. doi: 10.1074/jbc.274.40.28371. [DOI] [PubMed] [Google Scholar]
- 78.Sato N, Gleave M E, Bruchovsky N, Rennie P S, Goldenberg S L, Lange P H, Sullivan L D. Intermittent androgen suppression delays progression to androgen-independent regulation of prostate-specific antigen gene in the LNCaP prostate tumour model. J Steroid Biochem Mol Biol. 1996;58:139–146. doi: 10.1016/0960-0760(96)00018-0. [DOI] [PubMed] [Google Scholar]
- 79.Schally A V, Comaru-Schally A M, Plonowski A, Nagy A, Halmos G, Rekasi Z. Peptide analogs in the therapy of prostate cancer. Prostate. 2000;45:158–166. doi: 10.1002/1097-0045(20001001)45:2<158::aid-pros10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 80.Seethalakshmi L, Mitra S P, Dobner P R, Menon M, Carraway R E. Neurotensin receptor expression in prostate cancer cell line and growth effect of NT at physiological concentrations. Prostate. 1997;31:183–192. doi: 10.1002/(sici)1097-0045(19970515)31:3<183::aid-pros7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 81.Sehgal I, Powers S, Huntley B, Powis G, Pittelkow M, Maihle N J. Neurotensin is an autocrine trophic factor stimulated by androgen withdrawal in human prostate cancer. Proc Natl Acad Sci USA. 1994;91:4673–4677. doi: 10.1073/pnas.91.11.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spiotto M T, Chung T D. STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate. 2000;42:186–195. doi: 10.1002/(sici)1097-0045(20000215)42:3<186::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 83.Sramkoski R M, Pretlow T G, Giaconia J M, Pretlow T P, Schwartz S, Sy M S, Marengo S R, Rhim J S, Zhang D, Jacobberger J W. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim. 1999;35:403–409. doi: 10.1007/s11626-999-0115-4. [DOI] [PubMed] [Google Scholar]
- 84.Tamagnone L, Lahtinen I, Mustonen T, Virtaneva K, Francis F, Muscatelli F, Alitalo R, Smith C I, Larsson C, Alitalo K. BMX, a novel nonreceptor tyrosine kinase gene of the BTK/ITK/TEC/TXK family located in chromosome Xp22.2. Oncogene. 1994;9:3683–3688. [PubMed] [Google Scholar]
- 85.Tsai Y T, Su Y H, Fang S S, Huang T N, Qiu Y, Jou Y S, Shih H M, Kung H J, Chen R H. Etk, a Btk family tyrosine kinase, mediates cellular transformation by linking Src to STAT3 activation. Mol Cell Biol. 2000;20:2043–2054. doi: 10.1128/mcb.20.6.2043-2054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsukada S, Saffran D C, Rawlings D J, Parolini O, Allen R C, Klisak I, Sparkes R S, Kubagawa H, Mohandas T, Quan S. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 87.van der Kwast T H, Schalken J, Ruizeveld D W J, van Vroonhoven C C, Mulder E, Boersma W, Trapman J. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer. 1991;48:189–193. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]
- 88.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–233. doi: 10.1038/361226a0. . (Erratum, 364:362.) [DOI] [PubMed] [Google Scholar]
- 89.Viallet J, Ihde D C. Systemic therapy for small-cell lung cancer: old themes replayed, new ones awaited. J Clin Oncol. 1989;7:985–987. doi: 10.1200/JCO.1989.7.8.985. [DOI] [PubMed] [Google Scholar]
- 90.Viallet J, Sharoni Y, Frucht H, Jensen R T, Minna J D, Sausville E A. Cholera toxin inhibits signal transduction by several mitogens and the in vitro growth of human small-cell lung cancer. J Clin Invest. 1990;86:1904–1912. doi: 10.1172/JCI114923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Voeller H J, Wilding G, Gelmann E P. v-rasH expression confers hormone-independent in vitro growth to LNCaP prostate carcinoma cells. Mol Endocrinol. 1991;5:209–216. doi: 10.1210/mend-5-2-209. [DOI] [PubMed] [Google Scholar]
- 92.Wang B, Zou J X, Ek-Rylander B, Ruoslahti E. R-Ras contains a proline-rich site that binds to SH3 domains and is required for integrin activation by R-Ras. J Biol Chem. 2000;275:5222–5227. doi: 10.1074/jbc.275.7.5222. [DOI] [PubMed] [Google Scholar]
- 93.Wen Y, Hu M C, Makino K, Spohn B, Bartholomeusz G, Yan D H, Hung M C. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- 94.Woll P J. Neuropeptide growth factors and cancer. Br J Cancer. 1991;63:469–475. doi: 10.1038/bjc.1991.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xue L Y, Qiu Y, He J, Kung H J, Oleinick N L. Etk/Bmx, a PH-domain containing tyrosine kinase, protects prostate cancer cells from apoptosis induced by photodynamic therapy or thapsigargin. Oncogene. 1999;18:3391–3398. doi: 10.1038/sj.onc.1202687. [DOI] [PubMed] [Google Scholar]
- 96.Yan G, Fukabori Y, Nikolaropoulos S, Wang F, McKeehan W L. Heparin-binding keratinocyte growth factor is a candidate stromal-to-epithelial-cell andromedin. Mol Endocrinol. 1992;6:2123–2128. doi: 10.1210/mend.6.12.1491693. [DOI] [PubMed] [Google Scholar]
- 97.Yeh S, Lin H K, Kang H Y, Thin T H, Lin M F, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci USA. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zachary I, Rozengurt E. Identification of a receptor for peptides of the bombesin family in Swiss 3T3 cells by affinity cross-linking. J Biol Chem. 1987;262:3947–3950. [PubMed] [Google Scholar]