Abstract
The hypothalamus, one of the major regulatory centers in the brain, controls various homeostatic processes, and hypothalamic neural stem cells (htNSCs) have been observed to interfere with hypothalamic mechanisms regulating aging. NSCs play a pivotal role in the repair and regeneration of brain cells during neurodegenerative diseases and rejuvenate the brain tissue microenvironment. The hypothalamus was recently observed to be involved in neuroinflammation mediated by cellular senescence. Cellular senescence, or systemic aging, is characterized by a progressive irreversible state of cell cycle arrest that causes physiological dysregulation in the body and it is evident in many neuroinflammatory conditions, including obesity. Upregulation of neuroinflammation and oxidative stress due to senescence has the potential to alter the functioning of NSCs. Various studies have substantiated the chances of obesity inducing accelerated aging. Therefore, it is essential to explore the potential effects of htNSC dysregulation in obesity and underlying pathways to develop strategies to address obesity-induced comorbidities associated with brain aging. This review will summarize hypothalamic neurogenesis associated with obesity and prospective NSC-based regenerative therapy for the treatment of obesity-induced cardiovascular conditions.
Keywords: aging, cardiovascular conditions, hypothalamus, neural stem cells, neuroinflammation, obesity
1. Introduction
Neural stem cells (NSCs) in an adult brain are responsible for neurogenesis and regeneration of brain functions. The two primary NSC reservoirs (neurogenic niches) in an adult mammalian brain are the sub-ventricular zone (SVZ) of the lateral ventricles and the hippocampal dentate gyrus (DG) [1,2,3]. In recent times, a third NSC pool, hypothalamic neural stem cells (htNSCs), were discovered [4,5,6]. The htNSC population is sensitive to variations in nutrient intake and signaling. An increase in neurogenesis in the hypothalamus was observed upon acutely feeding a high fat diet (HFD) [7], whereas a reduced neurogenesis in the hypothalamus was noticed as a result of chronic HFD feeding [8], and ‘inflammation’ was suggested as a major factor in causing such pronounced changes in neurogenesis. Upon htNSCs culturing, we observed a significant increase in htNSCs after eight months in HFD-fed C57BL/6J male adult mice compared to the chow-fed controls (unpublished).
Cellular senescence is an irreversible growth arrest in proliferating cells, which has been implicated in several neurodegenerative diseases [9,10]. During the process of senescence, the NSCs lose their ability to proliferate and generate neurons [11,12,13,14]. Supplementing mono-unsaturated fatty acids, such as oleic acid, in the diet caused lipid droplets to develop in ependymal cells and contributed to a decrease in neurogenesis in SVZ in the Alzheimer’s disease mouse model, 3xTg-AD [15]. Likewise in obesity, SVZ showed an increase in senescent glial cells carrying excessive fat deposits, and genetically ablating these senescent glial cells restored neurogenesis [16]. Thus, modifying the lipid content in the diet can replenish the old neurogenic pool. In this review, we will summarize hypothalamic neurogenesis associated with obesity and aging and explore the possibilities of NSC-based regenerative therapy to treat obesity-induced cardiovascular conditions.
2. HtNSCs and Obesity
NSCs are multipotent and they generate neurons, oligodendrocytes, and glia in the nervous system [17]. Varied levels of neural inflammation are observed in many neurological disorders or neurodegenerative diseases in human beings [18,19]. Their progression involves mediators of inflammation that are synthesized and secreted by various CNS cells, such as astrocytes, microglia, and oligodendrocytes [20]. Both beneficial and detrimental effects are observed in inflammatory conditions, which makes it unclear to specify the exact role of inflammation on NSCs. Certain pathways, after long term activation, cause energy imbalance, abnormal nutrient metabolism, restricted neurogenesis, proliferation, and differentiation of neural stem cells leading to metabolic and cognitive abnormalities. In the hypothalamus, the medio-basal hypothalamus (MBH) and the 3rd ventricle wall are observed to be the NSC niches [8]. Some studies state that mainly adult NSCs are observed in the MBH [7,21]. The MBH is a predominant region for physiological homeostasis of the entire body. Many neural progenitors or specialized ependymal cells that line the 3rd ventricle are observed to be glia-like tanycytes. They send processes to the arcuate nucleus and ventro-medial nucleus of the hypothalamus. Functionally these tanycytes are observed to be glucosensitive, reacting to metabolic stimulation and signal variations caused by feeding and energy balance [4,7,22]. Properties of tanycytes include ATP release, purinergic P2Y1 receptors, ectonucleoside triphosphate diphosphohydrolase 2 (NTPDase2) expression [23], and reacting to the activation of these receptors by the means of intense Ca2+ waves [24]. This is similar to the signaling mechanisms in stem cells. Expression of doublecortin-like [25] proteins, nestin [26,27,28] and vimentin [29,30,31], linked to neural precursor cells are observed in humans and rodent tanycytes. The expression of Sox2 [7,8], a nuclear transcription factor and NSC marker, is found in a few of the tanycytes, especially in the subventricular zone and dentate gyrus. In adult mice, it is mainly expressed in a group of cells in the MBH, particularly within the hypothalamic third-ventricle wall [8]. However, a few studies have shown rare occurrences of proliferating neurogenic progenitors in the human dentate gyrus [32,33]. One of the studies also observed human paralaminar nuclei of the amygdala showing persistence of immature excitatory neurons for decades [34]. Thus, the possibility of observing immature non-proliferative hypothalamic neurons cannot be denied and future studies focusing on confirming their ability to proliferate and differentiate could possibly reveal their normal functionality.
The MBH regulates body weight, feeding, and glucose balance via melanocortin signals based in the arcuate nucleus (ARC), mainly via orexigenic agouti-related peptide (AGRP) neurons and anorexigenic proopiomelanocortin (POMC) neurons [35,36,37,38]. Leptin and insulin, which vary with different fat mass conditions and feeding patterns, affect these two neurons and the process is crucial for body weight homeostasis [36,39,40,41]. The studies also showed decrease in responsiveness to leptin and insulin by these neurons upon chronic feeding of a high-fat diet (HFD), resulting in type-2 diabetes (T2D) and HFD-induced obesity. A 10% loss in POMC neurons was observed in the hypothalamus upon long term HFD feeding [8,21,42]. Neural precursors giving rise to different neurons were observed to have POMC gene expression [43]. Considering these data and mechanisms, there is evidence of dysregulation of neurogenesis in the hypothalamus of obese subjects. Based on many recent studies, neurogenesis has been observed in adult rodents [7,22,44,45] and htNSCs in adult MBH contribute to the regulation of metabolic physiology [8]. Hence, future studies could be focused on developing htNSCs as a treatment regimen for obesity and its related disorders, such as diabetes.
3. HtNSCs and Inflammation
Microglia are brain-resident macrophages that contribute to reduced neurogenesis in aging and play a predominant role in the inflammatory response [46]. Through microglia sorting studies, we observed a significant elevation of activated microglia in the hypothalamus of four-month HFD-fed young adult male mice compared to the chow-fed controls (unpublished). Activated microglia have the potential to release proinflammatory cytokines that can be harmful to NSCs, neurons and other glial cells. Among the complex neural immune reactions in adult NSCs, inflammatory cytokines are observed to majorly affect differentiation, proliferation, migration, and survival [47]. Inhibition of neurogenesis is achieved by pro-inflammatory cytokines whereas an increase in neurogenesis is observed by anti-inflammatory cytokines [48]. The gene expression studies in our lab revealed a significant increase in proinflammatory markers, such as IL1β, MCP1, and TNFα, in the whole hypothalamus of middle-aged, eight-month HFD-fed male mice compared to controls (unpublished). However, an anti-inflammatory cytokine, such as the transforming growth factor-beta (TGFβ), can enhance endothelial cells of adult NSC during aging [49,50]. In addition to these, a chemokine, CCL11, was observed to be increased in aged mice, both in blood and cerebrospinal fluid (CSF), which further caused a decline in neurogenesis leading to cognitive function impairment [51]. Exercise and restriction of calories can cause variations in systemic factors, and hence, act as adult NSC function modulators [52].
Upon over-nutrition, the IκB kinase-β/nuclear transcription factor NF-κB (IKKb/NF-κB) pathway, that plays a crucial role in many physiological processes, gets activated; this can cause SOCS3, a suppressor of cytokine signaling-3 gene upregulation in the hypothalamus, to inhibit insulin and leptin signaling, leading to resistance [53]. Studies have confirmed that, in the neurons of the hypothalamus in mice, SOCS3 knockout leads to an improvement in central leptin signaling and reduced obesity [54,55,56]. Similar effects were observed in central IKKb knockout mice and, in the MBH, SOCS3 overexpression decreased the neural IKKb inhibition effect on obesity reduction [53]. Like SOCS3, protein tyrosine phosphatase 1B (PTP1B) causes inhibition of leptin and insulin signaling and was observed to have a role in the IKKb/NF-kB inflammatory pathway. PTP1B expression in the hypothalamus can be increased by TNF-a by activating the IKKb/NF-kB pathway, mainly by being a transcriptional target [57]. Inhibition of PTP1B in neurons resolved leptin resistance, glucose disorders, and obesity induced by over-nutrition [58,59,60]. It is assumed that neural PTP1B may form a link with metabolic disease pathways and neurodegenerative diseases as it had an effect on genetic mouse models of Alzheimer’s disease [61]. In the forebrain, degeneration of GABAergic interneurons was mediated by an overproduction of the cytokine interleukin-6 in diabetes and obesity, which leads to NF-kB activation and release of neurotoxic inflammatory products [62]. Therefore, alleviating chronic diet-induced neuroinflammation by exploring the pathways associated with the metabolic control function of htNSC and identifying their therapeutic potential is essential.
4. Nrf2, an Important Transcription Factor Affecting NSC Populations in Obesity
Various factors affect NSC populations in obesity, including hormonal factors, transcription factors, inflammatory factors such as cytokines and chemokines, epigenetic changes and chromatin stability, oxidative stress, DNA damage, hyperlipidemia/hyperglycemia, etc. Nuclear factor E2-related factor 2 (Nrf2) is a major transcription factor that regulates basal and induced expression of antioxidant response element genes in response to oxidative stress. Functions of Nrf2 also include stem cell survival, apoptosis, autophagy, mitochondrial biogenesis, and many more, in addition to aging processes [63,64,65,66,67]. Studies in our lab observed an elevated expression of Nrf2 in the hypothalamus of adult obese male mice, along with a significant increase in htNSCs (unpublished). In a previous study, increased oxidation, or reactive oxygen species in adult mouse NSCs, promoted their ability to generate neurons and proliferate [68]. Self-renewal of stem cells was observed to be regulated by Nrf2, along with differentiation initiation with the support of epigenetic factors and transcription regulators [69]. Nrf2 expression and transcriptional activity steadily increased during the induced oluripotent stem cells (iPSC) differentiation process that peaked in later stages [70]. Restoration of age-related loss of hippocampal function was evidenced by transplanting Nrf2-overexpressing young NSCs [71], indicating the critical role of Nrf2 in mediating NSC/neural progenitor cell (NPC)- dependent neurogenesis in aging. Redox homeostasis by Nrf2 critically mediates the differentiation ability of different stem cell types to survive oxidative stress, which could gradually reduce during aging [69]. Thus, obtaining insight into one of the main transcription factors, Nrf2, that can resist oxidative stress, could provide fundamental knowledge about changes in htNSCs during neuroinflammation and lead to development of an associated therapeutic strategy.
5. HtNSCs and Aging
A continuous decline in physiological integrity is observed during aging. Characteristic intertwining factors that contribute to the complex aging process include deregulated nutrient sensing, cellular senescence, epigenetic alterations, genomic instability, loss of proteostasis, mitochondrial dysfunction, telomere attrition, change in intercellular communication, and exhaustion of stem cells [72,73].
It has been observed in various research that the hypothalamus is particularly important in aging [74,75,76,77] but the underlying cellular mechanism is not known in depth. The IκB kinase-β (IKKβ) pro-inflammatory axis in the hypothalamus and its downstream nuclear transcription factor, NF-κB, (IKKβ/NF-κB signaling) is over-stimulated in over-nutrition or aging [77,78,79]. Systemic aging is directed by the hypothalamic IKKβ/NF-κB pathway via inflammatory crosstalk between neurons and microglia by inhibiting gonadotropin-releasing hormone (GnRH) production, and so counteracting inflammation or GnRH therapy could partly regress degenerative signs of aging [77]. Maternal inflammation has been observed to cause reduced ventricular cell proliferation in developing fetal mouse brain [80]. In young mice, a high number of cells co-expressing Sox2 and the polycomb complex protein, Bmi-1, a nuclear protein [81] that is vital for self-renewal of NSCs and hematopoietic stem cells [82], were observed in the third-ventricle wall, whereas the ones in the MBH were found to be sparse. However, a gradual decrease in these cells was observed as age increased, which was initiated in the ventral region of 3rd ventricle wall within the MBH in 11–16-month-old mice and was totally lost in 22-months-and-older ones. Thus, various studies that aim to evaluate the exact time required to intervene in an inflammatory condition/pathway in the brain will provide more understanding upon which to formulate therapeutic clinical strategies for different neural stem cell niches.
Senescent glial cell accumulation is observed in proximity to the lateral ventricles along with excessive fat deposition within them. Upon removal of senescent cells from HFD or obese mice deficient in leptin receptors, neurogenesis being restored and a decline in anxiety-related behavior was observed [16]. Hence from subsequent studies, they concluded that the topmost contributors to obesity-induced anxiety are senescent cells. Therefore, senolytic drugs have opened a novel therapeutic pathway to treat neuropsychiatric disorders.
Alterations in mitochondrial structure and function may cause deleterious effects in adult NSC, which could drive the aging process [83]. Abnormal toxic by-product accumulation, including of reactive oxygen species (ROS), accompanies this event [84]. SOD2, an antioxidant enzyme that is regulated by FoxO3, a transcription factor associated with longevity [85], protects adult NSCs in mice [86]. An increased level of ROS and a decrease in the potential for self-renewal of adult NSCs was observed in mice that were deficient in FoxO1, 3, and 4 [87]. Other dysfunctions of mitochondria that contribute to the aging of NSCs include mitochondrial protein oxidation, variations in mitochondrial membrane composition, and abnormal mitophagy [83,88,89].
During mammalian NSC division, protein segregation is affected by age, mainly by means of diffusion barrier alteration. The stem cells are kept free of damage by the diffusion barrier that facilitates asymmetric segregation of damaged proteins among daughter cells [90]. Like yeast, efficient compartmentalization of cellular damage is achieved in young rodent NSCs and that can protect these proliferative cells. As age advances, this efficiency is reduced, causing aged NSCs to be exposed to excessive cell damage [90].
A mitochondrial function regulator, hypoxia-inducible factor-1α (HIF-1α), is essential for the maintenance of adult NSCs in their hypoxic niches. HIF-1α plays a major part in cell adaptation under hypoxia by inducing transcriptional responses. Thus, for proper adult NSC proliferation and subsequent differentiation, oxygen availability is critically important [91]. An abnormal oxygen-sensing pathway may be responsible for the neurogenic decline in aging [92]. Thus, the use of anti-inflammatory agents along with senolytic and associated htNSC therapy have the potential to strategically counteract diet-induced chronic neuroinflammation and aging. This could possibly pave way to new therapeutic regimens in obesity-induced cardiovascular conditions.
6. Molecular Pathways Associated with NSC Inflammation and Aging
Certain nutrient-sensing mechanisms that can be associated with aging have been considered modifiers of adult NSCs. Adult NSC proliferation and differentiation can be stimulated by insulin-like growth factor 1 (IGF-1) [93], and a reduced IGF-1 level has been associated with cognitive aging [94]. However, lifelong IGF-1 exposure may be the reason for an age-related reduction in adult neurogenesis [95].
An important metabolic regulation coordinator is the mammalian target of rapamycin (mTOR), which has two types, viz., mTORC1 and mTORC2 [96,97]. Regulation of body weight and feeding behavior is primarily controlled by mTOR1 using ghrelin and leptin signaling, in addition to control of gluconeogenesis and adipogenesis peripherally in many tissues [97]. Size, morphology, and neuronal cell numbers are controlled by mTORC2, along with energy balance regulation in the hypothalamus. In POMC neurons in aged mice, an elevation in mTOR activity was observed [98], which can indirectly lead to POMC neuronal soma enlargement and a decline in the projection of neurites to the paraventricular nucleus (PVN), which causes age-dependent obesity [99]. It has been observed that, upon intracerebral injection, rapamycin causes mTOR inhibition which further leads to neurite projection and neuronal excitability in POMC, establishing a decline in body weight and food consumption; hence, age acceleration is achieved by the mTOR pathway. Therefore, to delay aging and improve the lifespan, this pathway can be considered a potential target for therapeutic intervention.
As previously discussed, during aging, a decrease in htNSCs was observed [81]. In addition, mice models with gene silencing mediating Bmi1+ depletion in stem cells showed a significant reduction in cognition, sociality, muscle endurance, coordination, and spatial memory. In other mice models, a decline in lifespan was observed in Sox2+ stem cell-depleted animals. Hence, replenishing new htNSC from a newborn mouse into the MBH of a middle-aged mouse could enhance the lifespan and delay age-associated physiological decline [81]. Exogenous implantation of stem cells into the hypothalamus caused secretion of microRNA-containing exosomes, which delayed physiological deficits in aging. Suppression of NF-kB activation was achieved in neurons due to these microRNAs, and GnRH secretion was also restored [81]. As a result, during aging, htNSC loss might cause systemic physiological changes due to underlying inflammation.
Through Wingless-related integration site (Wnt)-mediated signaling by astrocytes, adult NSC expansion is induced in a paracrine manner [100]. As age increases, Wnt3 expression reduces in astrocytes, which causes further neurogenic decline [101]. Expression of survivin is decreased in adult NSCs due to an age-associated decline in Wnt-mediated signaling in the astrocytes that leads to a quiescent phase in adult NSCs [102]. Release of the Wnt inhibitor, DKK1, from astrocytes is increased in NSC niches during aging, which decreases neurogenesis [103]. Extracellular matrix composition, mechanical properties, and arrangement have a role in adult NSC function, which varies with injury, disease, and aging [104,105].
A high fat mass expression and obesity associated gene (FTO) was observed in adult NSCs [106]. A smaller number of BrdU+ and Ki67+ cells was also observed during FTO loss, showing a decline in adult NSC and reduced proliferating capacity, along with a decline in glial and neuronal differentiation, making adult NSC less multipotent. In addition, in adult mice, FTO loss was observed to decrease adult NSC proliferation and caused inhibition of neuronal differentiation in both SGZ and SVZ regions. Thus, adult NSC activity modulation is achieved by FTO through m6A modification regulation of selective transcripts that can indirectly affect the gene expression [107,108,109].
Alteration of important signaling for neurodevelopment is observed when a change in nutrient or neurotrophic environment is observed. Brain abnormalities and decreased brain weight, along with altered glial and neuronal protein expression is observed in mice that have a paucity of leptin signaling and varied expression of neuronal and glial proteins [110]. Elevated proliferation and decline in neural stem/progenitor cells (NPC) are observed in adult rats with type II diabetes [111] and in rats showing hyperglycemia. NPCs do not respond to growth factors and form neurospheres (NS) that are smaller in size. In addition, a decline in neurogenesis is observed in type I diabetic mice or rats treated with streptozotocin [112]. Along with anorexigenic response signaling, during fetal life, insulin and leptin help in neuronal development and their neurotrophic effects are mediated by the MAPK (ERK/MAPK) pathway that resulted in phosphorylation of ERK1/2 [113]. Significant neuronal differentiation was induced by leptin in differentiation conditions along with elevated early and late neuronal marker expression [114]; whereas the late neuronal marker neuronal nuclei (NeuN) showed no significant increase and a normal elevation in early neuronal markers, such as doublecortin (DCX) and neuron-specific class III β tubulin (Tuj1), were observed upon insulin exposure [114]. According to these studies, it was concluded that maternal diabetes and differential exposure of the fetus to insulin and leptin could result in reduced growth or macrosomia that could have a significant effect on the development of a fetal brain.
7. The Hypothalamus and the Sympathoexcitatory Effect
In various studies of the effects of leptin on the hypothalamus, it has been observed that α-melanocyte-stimulating hormone (α-MSH) or melanotan II (agonist of MC3/4R (MTII)), upon intracerebroventricular (ICV) administration, enhanced sympathetic nerve activity (SNA), however agouti-related protein or MC3/4R broad brain inhibition with ICV SHU9119 blocked leptin’s sympathoexcitatory effect [115,116]. This is based on the understanding that α-MSH and glutamate are two major excitatory signals to the PVN, a cardiogenic center in the hypothalamus (see Figure 1), that can mediate leptin’s sympathoexcitatory effects. POMC neurons synthesize and release α-MSH. These neurons are in arcuate nucleus (ArcN), which projects to various sites in the hypothalamus, including the PVN [117,118,119], and regulates autonomic activity; however, the role of PVN MC3/4 is ambiguous. Glutamatergic signals are received by the PVN from various regions that include the dorsal medial hypothalamus, ventral medial hypothalamus, lateral hypothalamus, and ArcN, wherein elevated SNA is observed due to the action of leptin [120]. A small group of POMC neurons in the ArcN also expresses the glutamate vesicular transporter (VGLUT-2) [121]. PVN glutamate receptors blockade decreases the ArcN’s non-specific chemical stimulation-mediated sympathoexcitatory effects [122,123]. Along with this excitatory signaling, inhibitory neurons, such as neuropeptide Y (NPY) neurons of the ArcN, are projected into the PVN [122,123,124]. NPY neurons are inhibited by leptin in the ArcN [125,126] and PVN neuron firing is inhibited by NPY, which gets stimulated by α-MSH or plasma leptin elevation.
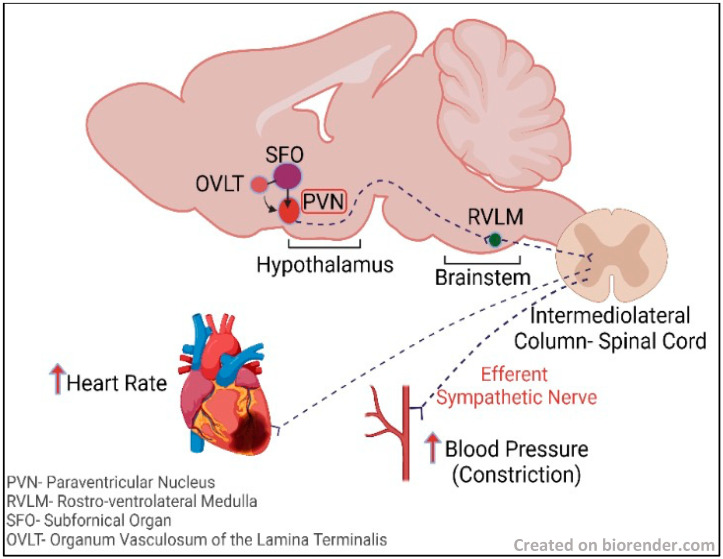
Figure 1.
A simplified pathway showing excitatory circulatory signaling from SFO and OVLT, which are circumventricular organs lacking blood brain barrier, passing to the adjacent PVN in the hypothalamus and RVLM in the brainstem, causing an efferent sympathetic nerve response from the intermediolateral column of the spinal cord to the heart and blood vessels, resulting in increased heart rate and increased blood pressure associated with vasoconstriction.
By various studies it has been identified that elevated SNA is observed in obesity, especially in the kidney and hindlimb, for which a leptin increase and hypothalamic melanocortin activity elevation are predominant activities [127]. In mice and rats, expression of NPY in the ArcN/PVN is reduced by diet-induced obesity or, in the ArcN, by NPY mRNA levels [128,129,130]. In the PVN region, obesity-prone rats that were inbred showed a reduction in agouti-related protein/NPY processes [131]. Tonic NPY inhibition decline is essential for leptin-induced sympathetic outflow driven via PVN MC3/4R. It is inferred from this that obesity plays a role in SNA inhibition and it is due to tonic activity of NPY, which further reveals an elevated α-MSH excitation [132]. htNSCs are predominantly found adjacent to the PVN of the hypothalamus (See Figure 2) lining the 3rd ventricle [133]. Based on these studies, there is a need for detailed investigation into the link between the variation in NSC levels associated with different conditions, such as age, diet etc., and sympathoexcitatory activity.
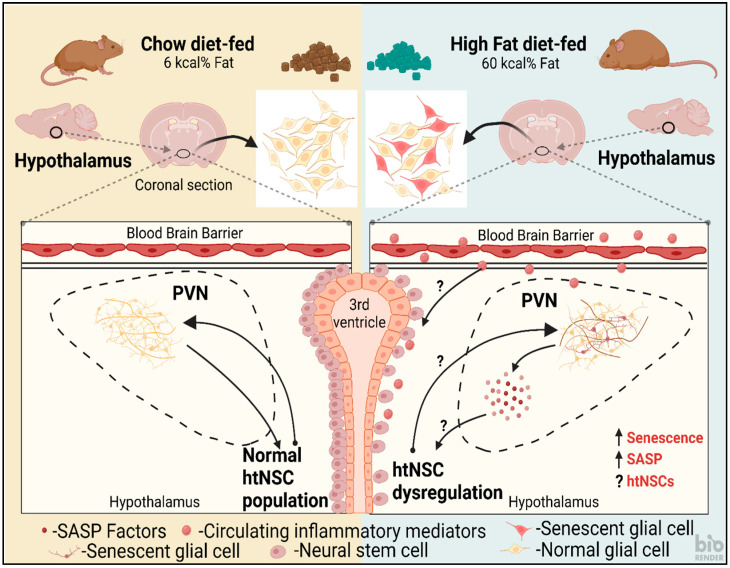
Figure 2.
A schematic illustration of the response to feeding chow (left) and high fat diet (HFD) (right) on hypothalamic paraventricular nucleus (PVN) and hypothalamic neural stem cells (htNSCs) lining the 3rd ventricle in mice. HFD could potentially cause an increase in senescent glial cells within the PVN that can release senescence-associated secretory phenotype (SASP) factors causing a proinflammatory response, further leading to htNSC dysregulation. This could, again, cause a reduction in functional glia and neurons in the PVN. The circulatory inflammatory mediators could also potentially cross the blood brain barrier and cause a direct effect on the htNSCs. Chow-fed control showed normal cell populations.
8. Time-Restricted Feeding and Its Effect on NSCs
Reduced energy consumption without any effect on nutritional value is characteristic of dietary restriction (DR). It can be alternatively described as caloric restriction (CR) and, in a broader way, termed as periodic fasting, short-term starvation, intermittent fasting (IF), and fasting-mimetic diets [134]. In maintaining proper health and physiology, a crucial role is played by the type and amount of diet [135]. Adult stem cells are important for tissue regeneration and homeostasis and these stem cells can differentiate and self-renew into specialized cell types. Dietary changes, environment, and nutrient variation influence the stem cells via function alteration. In various studies, a positive effect was observed in stem cells when calories were restricted, especially an increase in the function of intestine and skeletal muscle stem cells, in addition to an elevated quiescence of hematopoietic stem cells (HSCs). In addition, time-restricted feeding has been shown to protect neuronal stem cells, intestinal stem cells, and HSCs from injury, especially stroke and neurodegenerative diseases in the brain [136,137,138]. HFD impairs neurogenesis and hematopoiesis, and it can create opportunities for tumorigenesis.
Characteristic changes in metabolic pathways in the brain are achieved by IF, mainly by ketogenic amino acid and fatty acid breakdown and an elevation in stress resistance [139,140]. A neuroprotective effect can be achieved via IF by activation of many signaling pathways [141]. IF in rodents has shown an increase in long-term potentiation (LTP) at synapses in the hippocampus and an increase in hippocampal neurogenesis [138] in comparison with animals with a sedentary lifestyle that are fed ad libitum (AL) diet. BrdU-labeled cell number in the dentate gyrus was elevated in the mice that were intermittently fasted [138]. They also used Ki67 as a marker to evaluate cell proliferation by identifying an increase in dentate gyrus Ki67-labeled cells in mice that were fed with an IF diet. Mice subjected to IF for three months showed an elevated level of hippocampal nestin and NeuN (protein markers for precursor/neuronal stem cells), and also PSD95 (a scaffolding protein that is a potent regulator of synaptic strength) compared to AL mice [141], which demonstrated an increase in hippocampal neurogenesis and a strengthening of synaptic connections after IF. The researchers also showed that a pathway essential for neural stem cell maintenance in the mammalian brain [142], the Notch 1 signaling pathway, was shown to become activated mainly by upregulation of full-length Notch 1, Notch intracellular domain (NICD1), and transcription factor HES5 (involved in the formation of neurospheres) after IF.
The stress resistance ability of brain cells is activated by IF by causing various changes in brain metabolic pathways [140]. The changes in metabolic pathways during IF can be injurious to the brain and through activation of the brain-derived neurotrophic factor (BDNF) signaling pathway, a neuroprotective state is achieved. Downstream transcription factor activation that helps in energy balance and neurogenesis is made possible by BDNF, and one such transcription factor is cAMP response element-binding protein (CREB). To differentiate stem cells into matured neurons, collaboration between the Notch signaling pathway and the CREB and BDNF signaling pathways is essential [143,144,145]. An increase in BDNF and p-CREB expression has been seen in IF compared to AL animals [141].
Without leading to malnutrition, CR is a 20–40% reduction in intake of calories. It is known to cause life-span increase, prolonged onset of diseases that are age related, and decrease in the incidence of cancer in different tissues and organisms [146,147,148,149]. The link between CR and longevity is under the influence of the downregulation of major nutrient sensing pathways, including those of insulin or IGF-1, and signaling by mTOR [84,147,150]. Very few studies have been documented on the positive and negative effects of CR on NSCs.
Two-days-a-week fasting or alternate-days fasting (IF) in animals have been shown to decrease clinical symptoms caused by age-related neuronal diseases such as Alzheimer’s disease, and the animals that were fasted also perform better after stroke, which is an acute injury [151]. After three weeks of a three-month period of IF, an elevated NSC proliferation in the rats and mice dentate gyrus was observed [152,153]. An elevated BDNF was associated with these positive effects. However, various studies showed that neuronal survival ability was altered by fasting rather than induction of NSC proliferation. In the dentate gyrus of mice, an increase in neuron and glia numbers was observed within 72 h of feeding a fasting-mimicking diet (FMD), along with a reduced IGF-1/PKA signaling [152,154]. In addition, an increase in mesenchymal stem and progenitor cell number and proliferation were observed on FMD repeated feeding in aged animals, and in aged mice; rebalanced output from HSCs and progenitors were also observed [154,155]. Therefore, time restricting feeding can be a neuroprotective strategy for replenishing lost NSCs in chronic neuroinflammatory conditions.
9. Exosomes from the HtNSCs
HtNSCs have a distinct endocrine function, to release excessive amounts of microRNAs (miRNAs)-containing exosomes [81]. In addtion, they have certain long non-coding RNAs (lncRNAs) that possess the ability to maintain pluripotency and embryonic stem cell neurogenesis [156], self-renewal of cancer stem cells [157], and reprogramming of pluripotent stem cells [158]. LncRNAs may play a unique role in determining the fate of these stem cells in cellular senescence regulation [159]. An abundant lncRNA, Hnscr in the htNSCs of young mice, drastically reduces as the mice age [159]. Hnscr regulates htNSC senescence and mouse aging by binding to YB-1, a multi-functional protein [160] that controls protein translation [161], and also regulates DNA repair [162], protecting it from protein degradation and ubiquitination. YB-1 acts as a repressor of transcription, inhibiting p16INK4A expression in htNSCs [159,163], and hence could be targeted to modulate senescence in htNSC. According to [159], TF2A treatment, isomeric theaflavin monomer, and a black tea derivative [164], improved YB-1 stability, diminished htNSC senescence, and decreased the level of aging related physiological downturn in mice.
By various studies it has been observed that htNSC loss causes systemic aging within a short time and the exosomal miRNAs secreted by these cells (See Figure 3) mediate anti-aging properties [81]. Aging can be correlated with modulation of some gene expressions by certain non-coding RNAs. In aged adult NSC, heterochronic micro-RNA let-7b upregulation is observed. Repression of Hmga2, a high mobility group transcriptional regulator, is observed upon let-7b overexpression, which indirectly potentiates p16lnk4a (an inhibitor of cyclin-dependent kinase and activator of Rb) and p19Arf expression, improving the stability of p53 protein [165]. Therefore, it slows down the progression of cell cycle and induces senescence [166], leading to reduced adult NSC functioning and neurogenesis. However, deficiency of p16INK4a in aged mice diminished this effect [167]. Let-7b initiates differentiation and inhibits proliferation of neural stem cells by targeting Tlx and cyclin D1 in adult NSC and embryonic brains [168]. A higher-to --lower/quiescent shift in NSC proliferative state from fetus to adult is contributed to by Imp1, a different let-7b target, even though it is not expressed in adult NSC [169]. As a result, changes in let-7b may initiate aging in adult NSC. The gene regulation mediated by micro RNAs impacts healthy aging as well as aging associated with neurodegenerative diseases [170]. Administering exosomes derived from NSCs (exo-NSCs) could restore BDNF signaling and memory in HFD mice [171], providing suggestive evidence of the potential therapeutic effect of exo-NSCs on HFD-induced NSC dysregulation in obesity. Hence, further studies on differential expression of certain exosomal non-coding RNAs must be performed to form an understandable association with pathological and healthy aging.
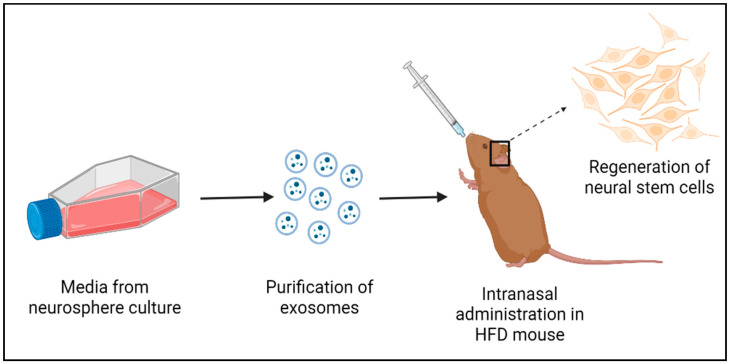
Figure 3.
A simplified protocol for the potential therapeutic effect of exo-NSCs in obesity-induced NSC dysregulation.
10. Challenges Associated with NSCs for Regenerative Medicine and Future Perspectives
Immunological rejection is one of the major difficulties in stem cell therapy. This could be addressed by isolating NSCs from the same subjects that require the therapy to prevent immunological reaction to the newly transplanted stem cells. Administering immunosuppressive drugs could be an additional or alternative option even though it has a lot of side effects. Another challenge is to make certain that the transplanted cells grow enough without causing tumor development and karyotypic instability. According to [172], there is a critical challenge in isolating multipotent NSCs from cell culture for transplantation as the majority of neurospheres in vitro are heterogenic with varying developmental stages and gene expression mainly due to ex vivo culture conditions. Overcoming these challenges and establishing NSC-based therapy for obesity-induced comorbidities, especially cardiovascular conditions, to improve functional outcomes through associated multimodal mechanisms is tremendously foresighted. Based on the difficulty in accessing the brain to collect tissues for processing from live animals, using induced pluripotent stem cell (iPSC) technology is a solution that could produce in vitro NSCs or neurons for transplantation. As iPSCs can be non-invasively obtained from live subjects, and to reduce the risk of immune rejection, reprogramming these cells to NSCs or neurons could provide autologous engraftments [173].
11. Conclusions
HtNSCs could be a potential therapy in obesity-induced cardiovascular diseases. Exosomes derived from htNSCs could be an alternative to or a conjunction with NSC therapy, being a minimally invasive technique to reverse aging and degenerative changes in the CNS. The relationship between htNSC dysregulation and sympathetic nerve response in obesity has never been studied. As brain microglia activation is a predominant indicator of neuroinflammation in hypertension, restoring a normal population of glia and neurons within the cardiogenic centers of the brain cannot be ruled out. Thus, identifying associated htNSC mechanisms and pathways could bring novel insight to therapeutic strategies in obesity-associated hypertension or sympathetic nerve overactivity.
Acknowledgments
We would like to acknowledge Rajasingh Johnson, Department of Bioscience Research, UTHSC for the suggestions made while forming this review.
Author Contributions
B.P. has made substantial contributions to the design and concept of the article including figures, data acquisition and analysis of the quoted unpublished data and interpretation of relevant literature. A.D.A. produced figures and critically revised the literature. M.S. conceptualized and critically revised the article for intellectual content. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created as this is a review, and some data is unavailable due to privacy or ethical restrictions. Data will be published with ethical considerations in future manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by AHA-AIREA Grant #959725 (M.S.), NIH-NHLBI R15 HL14884401 (M.S.), OCASCR Research Grant (M.S.), Research Advisory Committee Grant College of Veterinary Medicine Oklahoma State University PLAKKOT-FY23 (B.P.) and SUBRAMANIAN-FY23 (M.S.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bond A.M., Ming G.L., Song H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell. 2015;17:385–395. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stolp H.B., Molnár Z. Neurogenic niches in the brain: Help and hindrance of the barrier systems. Front. Neurosci. 2015;9:20. doi: 10.3389/fnins.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva-Vargas V., Crouch E.E., Doetsch F. Adult neural stem cells and their niche: A dynamic duo during homeostasis, regeneration, and aging. Curr. Opin. Neurobiol. 2013;23:935–942. doi: 10.1016/j.conb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Bolborea M., Dale N. Hypothalamic tanycytes: Potential roles in the control of feeding and energy balance. Trends Neurosci. 2013;36:91–100. doi: 10.1016/j.tins.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellegrino G., Trubert C., Terrien J., Pifferi F., Leroy D., Loyens A., Migaud M., Baroncini M., Maurage C.-A., Fontaine C., et al. A comparative study of the neural stem cell niche in the adult hypothalamus of human, mouse, rat and gray mouse lemur (Microcebus murinus) J. Comp. Neurol. 2018;526:1419–1443. doi: 10.1002/cne.24376. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y., Tamamaki N., Noda T., Kimura K., Itokazu Y., Matsumoto N., Dezawa M., Ide C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp. Neurol. 2005;192:251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 7.Lee D.A., Bedont J.L., Pak T., Wang H., Song J., Miranda-Angulo A., Takiar V., Charubhumi V., Balordi F., Takebayashi H., et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci. 2012;15:700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J., Tang Y., Cai D. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat. Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campisi J. Cancer, aging and cellular senescence. In Vivo. 2000;14:183–188. [PubMed] [Google Scholar]
- 10.Balasubramanian P., Branen L., Sivasubramanian M.K., Monteiro R., Subramanian M. Aging is associated with glial senescence in the brainstem-implications for age-related sympathetic overactivity. Aging. 2021;13:13460. doi: 10.18632/aging.203111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nada M.B., Slomianka L., Vyssotski A.L., Lipp H.P. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol. Aging. 2010;31:151–161. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Chinta S.J., Woods G., Rane A., DeMaria M., Campisi J., Andersen J.K. Cellular senescence and the aging brain. Exp. Gerontol. 2015;68:3–7. doi: 10.1016/j.exger.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn H.G., Dickinson-Anson H., Gage F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maslov A.Y., Barone T.A., Plunkett R.J., Pruitt S.C. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J. Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton L.K., Dufresne M., Joppé S.E., Petryszyn S., Aumont A., Calon F., Barnabé-Heider F., Furtos A., Parent M., Chaurand P., et al. Aberrant lipid metabolism in the forebrain niche suppresses adult neural stem cell proliferation in an animal model of Alzheimer’s disease. Cell Stem Cell. 2015;17:397–411. doi: 10.1016/j.stem.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Ogrodnik M., Zhu Y.I., Langhi L.G., Tchkonia T., Krüger P., Fielder E., Victorelli S., Ruswhandi R.A., Giorgadze N., Pirtskhalava T., et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 2019;29:1061–1077.e8. doi: 10.1016/j.cmet.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke D.L., Johansson C.B., Wilbertz J., Veress B., Nilsson E., Karlström H., Lendahl U., Frisén J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- 18.Das S., Basu A. Inflammation: A new candidate in modulating adult neurogenesis. J. Neurosci. Res. 2008;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- 19.Lucin K.M., Wyss-Coray T. Immune activation in brain aging and neurodegeneration: Too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block M.L., Hong J.-S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 21.McNay D., Briançon N., Kokoeva M.V., Maratos-Flier E., Flier J.S. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J. Clin. Investig. 2012;122:142–152. doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietrich M.O., Horvath T.L. Fat incites tanycytes to neurogenesis. Nat. Neurosci. 2012;15:651–653. doi: 10.1038/nn.3091. [DOI] [PubMed] [Google Scholar]
- 23.Braun N., Sévigny J., Mishra S.K., Robson S.C., Barth S.W., Gerstberger R., Hammer K., Zimmermann H. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur. J. Neurosci. 2003;17:1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- 24.Frayling C., Britton R., Dale N. ATP-mediated glucosensing by hypothalamic tanycytes. J. Physiol. 2011;589:2275–2286. doi: 10.1113/jphysiol.2010.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saaltink D.-J., Håvik B., Verissimo C.S., Lucassen P., Vreugdenhil E. Doublecortin and doublecortin-like are expressed in overlapping and non-overlapping neuronal cell population: Implications for neurogenesis. J. Comp. Neurol. 2012;520:2805–2823. doi: 10.1002/cne.23144. [DOI] [PubMed] [Google Scholar]
- 26.Baroncini M., Allet C., Leroy D., Beauvillain J.-C., Francke J.-P., Prevot V. Morphological evidence for direct interaction between gonadotrophin-releasing hormone neurones and astroglial cells in the human hypothalamus. J. Neuroendocrinol. 2007;19:691–702. doi: 10.1111/j.1365-2826.2007.01576.x. [DOI] [PubMed] [Google Scholar]
- 27.Barrett P., Ivanova E., Graham E.S., Ross A.W., Wilson D., Plé H., Mercer J.G., Ebling F.J., Schuhler S., DuPré S.M., et al. Photoperiodic regulation of cellular retinoic acid-binding protein 1, GPR50 and nestin in tanycytes of the third ventricle ependymal layer of the Siberian hamster. J. Endocrinol. 2006;191:687–698. doi: 10.1677/joe.1.06929. [DOI] [PubMed] [Google Scholar]
- 28.Wei L.-C., Shi M., Chen L.-W., Cao R., Zhang P., Chan Y. Nestin-containing cells express glial fibrillary acidic protein in the proliferative regions of central nervous system of postnatal developing and adult mice. Dev. Brain Res. 2002;139:9–17. doi: 10.1016/S0165-3806(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 29.Bolborea M., Laran-Chich M.-P., Rasri K., Hildebrandt H., Govitrapong P., Simonneaux V., Pévet P., Steinlechner S., Klosen P. Melatonin controls photoperiodic changes in tanycyte vimentin and neural cell adhesion molecule expression in the Djungarian hamster (Phodopus sungorus) Endocrinology. 2011;152:3871–3883. doi: 10.1210/en.2011-1039. [DOI] [PubMed] [Google Scholar]
- 30.Chauvet N., Prieto M., Alonso G. Tanycytes present in the adult rat mediobasal hypothalamus support the regeneration of monoaminergic axons. Exp. Neurol. 1998;151:1–13. doi: 10.1006/exnr.1998.6784. [DOI] [PubMed] [Google Scholar]
- 31.Sidibe A., Mullier A., Chen P., Baroncini M., Boutin J.A., Delagrange P., Prevot V., Jockers R. Expression of the orphan GPR50 protein in rodent and human dorsomedial hypothalamus, tanycytes and median eminence. J. Pineal Res. 2010;48:263–269. doi: 10.1111/j.1600-079X.2010.00750.x. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez-Buylla A., Cebrian-Silla A., Sorrells S.F., Nascimento M.A., Paredes M.F., Garcia-Verdugo J.M., Yang Z., Huang E.J. Comment on “Impact of neurodegenerative diseases on human adult hippocampal neurogenesis”. Science. 2022;376:eabn8861. doi: 10.1126/science.abn8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terreros-Roncal J., Moreno-Jiménez E.P., Flor-García M., Rodríguez-Moreno C.B., Trinchero M.F., Cafini F., Rábano A., Llorens-Martín M., Rose M.C., Styr B., et al. Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science. 2021;374:1106–1113. doi: 10.1126/science.abl5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorrells S.F., Paredes M.F., Velmeshev D., Herranz-Pérez V., Sandoval K., Mayer S., Chang E.F., Insausti R., Kriegstein A.R., Rubenstein J.L., et al. Immature excitatory neurons develop during adolescence in the human amygdala. Nat. Commun. 2019;10:2748. doi: 10.1038/s41467-019-10765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dietrich M.O., Horvath T.L. Synaptic plasticity of feeding circuits: Hormones and hysteresis. Cell. 2011;146:863–865. doi: 10.1016/j.cell.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Dietrich M.O., Horvath T.L. Hypothalamic control of energy balance: Insights into the role of synaptic plasticity. Trends Neurosci. 2013;36:65–73. doi: 10.1016/j.tins.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Flier J.S. Regulating energy balance: The substrate strikes back. Science. 2006;312:861–864. doi: 10.1126/science.1127971. [DOI] [PubMed] [Google Scholar]
- 38.Niswender K.D., Baskin D., Schwartz M. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol. Metab. 2004;15:362–369. doi: 10.1016/j.tem.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Coll A.P., Farooqi I.S., O’Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Münzberg H., Myers M.G. Molecular and anatomical determinants of central leptin resistance. Nat. Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 41.Olofsson L.E., Unger E.K., Cheung C.C., Xu A.W. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc. Natl. Acad. Sci. USA. 2013;110:E697–E706. doi: 10.1073/pnas.1218284110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thaler J.P., Yi C.-X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O., Zhao X., Sarruf D.A., Izgur V., Maravilla K.R., et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padilla S.L., Carmody J.S., Zeltser L.M. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat. Med. 2010;16:403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kokoeva M.V., Yin H., Flier J.S. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J. Comp. Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- 45.Pierce A.A., Xu A.W. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J. Neurosci. 2010;30:723–730. doi: 10.1523/JNEUROSCI.2479-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fonseca R.S., Mahesula S., Apple D.M., Raghunathan R., Dugan A., Cardona A., O’Connor J., Kokovay E. Neurogenic niche microglia undergo positional remodeling and progressive activation contributing to age-associated reductions in neurogenesis. Stem Cells Dev. 2016;25:542–555. doi: 10.1089/scd.2015.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kokaia Z., Martino G., Schwartz M., Lindvall O. Cross-talk between neural stem cells and immune cells: The key to better brain repair? Nat. Neurosci. 2012;15:1078–1087. doi: 10.1038/nn.3163. [DOI] [PubMed] [Google Scholar]
- 48.Breton J., Mao-Draayer Y. Impact of cytokines on neural stem/progenitor cell fate. J. Neurol. Neurophysiol. 2011;S4:1–12. doi: 10.4172/2155-9562.S4-001. [DOI] [Google Scholar]
- 49.Kandasamy M., Lehner B., Kraus S., Sander P.R., Marschallinger J., Rivera F.J., Trümbach D., Ueberham U., Reitsamer H.A., Strauss O., et al. TGF-beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J. Cell. Mol. Med. 2014;18:1444–1459. doi: 10.1111/jcmm.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wachs F.-P., Winner B., Couillard-Despres S., Schiller T., Aigner R., Winkler J., Bogdahn U., Aigner L. Transforming growth factor-β1 is a negative modulator of adult neurogenesis. J. Neuropathol. Exp. Neurol. 2006;65:358–370. doi: 10.1097/01.jnen.0000218444.53405.f0. [DOI] [PubMed] [Google Scholar]
- 51.Katsimpardi L., Litterman N.K., Schein P.A., Miller C.M., Loffredo F.S., Wojtkiewicz G.R., Chen J.W., Lee R.T., Wagers A.J., Rubin L.L. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouchard J., Villeda S.A. Aging and brain rejuvenation as systemic events. J. Neurochem. 2015;132:5–19. doi: 10.1111/jnc.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kievit P., Howard J.K., Badman M.K., Balthasar N., Coppari R., Mori H., Lee C.E., Elmquist J.K., Yoshimura A., Flier J.S. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4:123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 55.Mori H., Hanada R., Hanada T., Aki D., Mashima R., Nishinakamura H., Torisu T., Chien K.R., Yasukawa H., Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 56.Reed A.S., Unger E.K., Olofsson L.E., Piper M.L., Jr., Myers M.G., Xu A.W. Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes. 2010;59:894–906. doi: 10.2337/db09-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zabolotny J.M., Kim Y.-B., Welsh L.A., Kershaw E.E., Neel B.G., Kahn B.B. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J. Biol. Chem. 2008;283:14230–14241. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banno R., Zimmer D., De Jonghe B.C., Atienza M., Rak K., Yang W., Bence K.K. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J. Clin. Investig. 2010;120:720–734. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bence K., Delibegovic M., Xue B., Gorgun C.Z., Hotamisligil G.S., Neel B.G., Kahn B.B. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat. Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 60.Picardi P.K., Calegari V.C., Prada P.D.O., Moraes J.C., Araújo E., Marcondes M.C.C.G., Ueno M., Carvalheira J.B.C., Velloso L.A., Saad M.J.A. Reduction of hypothalamic protein tyrosine phosphatase improves insulin and leptin resistance in diet-induced obese rats. Endocrinology. 2008;149:3870–3880. doi: 10.1210/en.2007-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liao G., Cheung S., Galeano J., Ji A.X., Qin Q., Bi X. Allopregnanolone treatment delays cholesterol accumulation and reduces autophagic/lysosomal dysfunction and inflammation in Npc1−/− mouse brain. Brain Res. 2009;1270:140–151. doi: 10.1016/j.brainres.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dugan L.L., Ali S.S., Shekhtman G., Roberts A.J., Lucero J., Quick K.L., Behrens M.M. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS ONE. 2009;4:e5518. doi: 10.1371/journal.pone.0005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niture S.K., Jaiswal A.K. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 2012;287:9873–9886. doi: 10.1074/jbc.M111.312694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paek J., Lo J.Y., Narasimhan S.D., Nguyen T.N., Glover-Cutter K., Robida-Stubbs S., Suzuki T., Yamamoto M., Blackwell T.K., Curran S.P. Mitochondrial SKN-1/Nrf mediates a conserved starvation response. Cell Metab. 2012;16:526–537. doi: 10.1016/j.cmet.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murakami S., Motohashi H. Roles of Nrf2 in cell proliferation and differentiation. Free Radic. Biol. Med. 2015;88:168–178. doi: 10.1016/j.freeradbiomed.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 66.Sykiotis G.P., Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balasubramanian P., Asirvatham-Jeyaraj N., Monteiro R., Sivasubramanian M.K., Hall D., Subramanian M. Obesity-induced sympathoexcitation is associated with Nrf2 dysfunction in the rostral ventrolateral medulla. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020;318:R435–R444. doi: 10.1152/ajpregu.00206.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le Belle J.E., Orozco N.M., Paucar A.A., Saxe J.P., Mottahedeh J., Pyle A.D., Wu H., Kornblum H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dodson M., Anandhan A., Zhang D.D., Madhavan L. An NRF2 perspective on stem cells and ageing. Front. Aging. 2021;2:690686. doi: 10.3389/fragi.2021.690686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou G., Meng S., Li Y., Ghebre Y.T., Cooke J.P. Optimal ROS signaling is critical for nuclear reprogramming. Cell Rep. 2016;15:919–925. doi: 10.1016/j.celrep.2016.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ray S., Corenblum M.J., Anandhan A., Reed A., Ortiz F.O., Zhang D.D., Barnes C.A., Madhavan L. A role for Nrf2 expression in defining the aging of hippocampal neural stem cells. Cell Transplant. 2018;27:589–606. doi: 10.1177/0963689718774030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nunan E., Wright C.L., Semola O.A., Subramanian M., Balasubramanian P., Lovern P.C., Fancher I.S., Butcher J.T. Obesity as a premature aging phenotype—Implications for sarcopenic obesity. GeroScience. 2022;44:1393–1405. doi: 10.1007/s11357-022-00567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dacks P.A., Moreno C.L., Kim E.S., Marcellino B.K., Mobbs C.V. Role of the hypothalamus in mediating protective effects of dietary restriction during aging. Front. Neuroendocrinol. 2013;34:95–106. doi: 10.1016/j.yfrne.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sadagurski M., Landeryou T., Cady G., Bartke A., Bernal-Mizrachi E., Miller R.A. Transient early food restriction leads to hypothalamic changes in the long-lived crowded litter female mice. Physiol. Rep. 2015;3:e12379. doi: 10.14814/phy2.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Satoh A., Brace C.S., Rensing N., Cliften P., Wozniak D.F., Herzog E.D., Yamada K.A., Imai S.-I. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang G., Li J., Purkayastha S., Tang Y., Zhang H., Yin Y., Li B., Liu G., Cai D. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature. 2013;497:211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol. Metab. 2013;24:40–47. doi: 10.1016/j.tem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Posey K.A., Clegg D.J., Printz R.L., Byun J., Morton G.J., Vivekanandan-Giri A., Pennathur S., Baskin D.G., Heinecke J.W., Woods S.C., et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol. -Endocrinol. Metab. 2009;296:E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stolp H.B., Turnquist C., Dziegielewska K.M., Saunders N., Anthony D., Molnar Z. Reduced ventricular proliferation in the foetal cortex following maternal inflammation in the mouse. Brain. 2011;134:3236–3248. doi: 10.1093/brain/awr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y., Kim M.S., Jia B., Yan J., Zuniga-Hertz J.P., Han C., Cai D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548:52–57. doi: 10.1038/nature23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molofsky A.V., Pardal R., Iwashita T., Park I.-K., Clarke M.F., Morrison S.J. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stoll E.A., Cheung W., Mikheev A.M., Sweet I.R., Bielas J.H., Zhang J., Rostomily R.C., Horner P.J. Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism. J. Biol. Chem. 2011;286:38592–38601. doi: 10.1074/jbc.M111.252171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finkel T., Deng C.X., Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hagenbuchner J., Ausserlechner M.J. Mitochondria and FOXO3: Breath or die. Front. Physiol. 2013;4:147. doi: 10.3389/fphys.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sakata H., Narasimhan P., Niizuma K., Maier C.M., Wakai T., Chan P.H. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain. 2012;135:3298–3310. doi: 10.1093/brain/aws259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paik J.-H., Ding Z., Narurkar R., Ramkissoon S., Muller F., Kamoun W.S., Chae S.-S., Zheng H., Ying H., Mahoney J., et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gemma C., Vila J., Bachstetter A., Bickford P.C. Oxidative stress and the aging brain: From theory to prevention. Brain Aging. 2007:353–374. [PubMed] [Google Scholar]
- 89.Lionaki E., Markaki M., Palikaras K., Tavernarakis N. Mitochondria, autophagy and age-associated neurodegenerative diseases: New insights into a complex interplay. Biochim. Biophys. Acta (BBA)-Bioenerg. 2015;1847:1412–1423. doi: 10.1016/j.bbabio.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 90.Moore D.L., Pilz G.A., Araúzo-Bravo M.J., Barral Y., Jessberger S. A mechanism for the segregation of age in mammalian neural stem cells. Science. 2015;349:1334–1338. doi: 10.1126/science.aac9868. [DOI] [PubMed] [Google Scholar]
- 91.Mazumdar J., O’Brien W.T., Johnson R., LaManna J., Chavez J.C., Klein P.S., Simon M.C. O2 regulates stem cells through Wnt/β-catenin signalling. Nat. Cell Biol. 2010;12:1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rabie T., Kunze R., Marti H.H. Impaired hypoxic response in senescent mouse brain. Int. J. Dev. Neurosci. 2011;29:655–661. doi: 10.1016/j.ijdevneu.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 93.Aberg M.A., Åberg N.D., Hedbäcker H., Oscarsson J., Eriksson P.S. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sonntag W.E., Ramsey M., Carter C.S. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res. Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 95.Chaker Z., Aïd S., Berry H., Holzenberger M. Suppression of IGF-I signals in neural stem cells enhances neurogenesis and olfactory function during aging. Aging Cell. 2015;14:847–856. doi: 10.1111/acel.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cota D., Proulx K., Blake Smith K.A., Kozma S.C., Thomas G., Woods S.C., Seeley R.J. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 97.de Morentin P.M., Martinez-Sanchez N., Roa J., Ferno J., Nogueiras R., Tena-Sempere M., Dieguez C., Lopez M. Hypothalamic mTOR: The rookie energy sensor. Curr. Mol. Med. 2014;14:3–21. doi: 10.2174/1566524013666131118103706. [DOI] [PubMed] [Google Scholar]
- 98.Yang S.-B., Tien A.-C., Boddupalli G., Xu A.W., Jan Y.N., Jan L.Y. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron. 2012;75:425–436. doi: 10.1016/j.neuron.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mori H., Inoki K., Münzberg H., Opland D., Faouzi M., Villanueva E.C., Ikenoue T., Kwiatkowski D., MacDougald O.A., Myers M.G., et al. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 2009;9:362–374. doi: 10.1016/j.cmet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song H., Stevens C.F., Gage F.H. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 101.Okamoto M., Inoue K., Iwamura H., Terashima K., Soya H., Asashima M., Kuwabara T. Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB J. 2011;25:3570–3582. doi: 10.1096/fj.11-184697. [DOI] [PubMed] [Google Scholar]
- 102.Miranda C.J., Braun L., Jiang Y., Hester M.E., Zhang L., Riolo M., Wang H., Rao M., Altura R.A., Kaspar B.K. Aging brain microenvironment decreases hippocampal neurogenesis through Wnt-mediated survivin signaling. Aging Cell. 2012;11:542–552. doi: 10.1111/j.1474-9726.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seib D.R., Corsini N.S., Ellwanger K., Plaas C., Mateos A., Pitzer C., Niehrs C., Celikel T., Martin-Villalba A. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell. 2013;12:204–214. doi: 10.1016/j.stem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 104.Gattazzo F., Urciuolo A., Bonaldo P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Keung A.J., de Juan-Pardo E.M., Schaffer D.V., Kumar S. Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells. 2011;29:1886–1897. doi: 10.1002/stem.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li L., Zang L., Zhang F., Chen J., Shen H., Shu L., Liang F., Feng C., Chen D., Tao H., et al. Fat mass and obesity-associated (FTO) protein regulates adult neurogenesis. Hum. Mol. Genet. 2017;26:2398–2411. doi: 10.1093/hmg/ddx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haussmann I.U., Bodi Z., Sanchez-Moran E., Mongan N.P., Archer N., Fray R.G., Soller M. m6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540:301–304. doi: 10.1038/nature20577. [DOI] [PubMed] [Google Scholar]
- 108.Nainar S., Marshall P.R., Tyler C.R., Spitale R.C., Bredy T.W. Evolving insights into RNA modifications and their functional diversity in the brain. Nat. Neurosci. 2016;19:1292–1298. doi: 10.1038/nn.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yue Y., Liu J., He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29:1343–1355. doi: 10.1101/gad.262766.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bouret S.G., Simerly R.B. Minireview: Leptin and development of hypothalamic feeding circuits. Endocrinology. 2004;145:2621–2626. doi: 10.1210/en.2004-0231. [DOI] [PubMed] [Google Scholar]
- 111.Lang B.T., Yan Y., Dempsey R.J., Vemuganti R. Impaired neurogenesis in adult type-2 diabetic rats. Brain Res. 2009;1258:25–33. doi: 10.1016/j.brainres.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beauquis J., Saravia F., Coulaud J., Roig P., Dardenne M., Homo-Delarche F., De Nicola A. Prominently decreased hippocampal neurogenesis in a spontaneous model of type 1 diabetes, the nonobese diabetic mouse. Exp. Neurol. 2008;210:359–367. doi: 10.1016/j.expneurol.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 113.Cui H., Cai F., Belsham D.D. Leptin signaling in neurotensin neurons involves STAT, MAP kinases ERK1/2, and p38 through c-Fos and ATF1. FASEB J. 2006;20:2654–2656. doi: 10.1096/fj.06-5989fje. [DOI] [PubMed] [Google Scholar]
- 114.Desai M., Li T., Ross M.G. Fetal hypothalamic neuroprogenitor cell culture: Preferential differentiation paths induced by leptin and insulin. Endocrinology. 2011;152:3192–3201. doi: 10.1210/en.2010-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dunbar J.C., Lu H. Leptin-induced increase in sympathetic nervous and cardiovascular tone is mediated by proopiomelanocortin (POMC) products. Brain Res. Bull. 1999;50:215–221. doi: 10.1016/S0361-9230(99)00197-5. [DOI] [PubMed] [Google Scholar]
- 116.Haynes W.G., Morgan N.A., Djalali A., Sivitz W.I., Mark A.L. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.HYP.33.1.542. [DOI] [PubMed] [Google Scholar]
- 117.Cone R.D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 118.King C., Sarvetnick N. The incidence of type-1 diabetes in NOD mice is modulated by restricted flora not germ-free conditions. PLoS ONE. 2011;6:e17049. doi: 10.1371/journal.pone.0017049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Myers M.G., Münzberg H., Leinninger G.M., Leshan R.L. The geometry of leptin action in the brain: More complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ulrich-Lai Y.M., Jones K.R., Ziegler D.R., Cullinan W.E., Herman J. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: Differential inputs to the anterior versus posterior subregions. J. Comp. Neurol. 2011;519:1301–1319. doi: 10.1002/cne.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mercer A.J., Hentges S.T., Meshul C.K., Low M.J. Unraveling the central proopiomelanocortin neural circuits. Front. Neurosci. 2013;7:19. doi: 10.3389/fnins.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kawabe T., Kawabe K., Sapru H.N. Cardiovascular responses to chemical stimulation of the hypothalamic arcuate nucleus in the rat: Role of the hypothalamic paraventricular nucleus. PLoS ONE. 2012;7:e45180. doi: 10.1371/journal.pone.0045180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kawabe T., Kawabe K., Sapru H.N. Effect of barodenervation on cardiovascular responses elicited from the hypothalamic arcuate nucleus of the rat. PLoS ONE. 2012;7:e53111. doi: 10.1371/journal.pone.0053111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cassaglia P.A., Shi Z., Li B., Reis W.L., Clute-Reinig N.M., Stern J.E., Brooks V.L. Neuropeptide Y acts in the paraventricular nucleus to suppress sympathetic nerve activity and its baroreflex regulation. J. Physiol. 2014;592:1655–1675. doi: 10.1113/jphysiol.2013.268763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jobst E.E., Enriori P.J., Cowley M.A. The electrophysiology of feeding circuits. Trends Endocrinol. Metab. 2004;15:488–499. doi: 10.1016/j.tem.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 126.Varela L., Horvath T.L. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012;13:1079–1086. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hall J.E., da Silva A.A., do Carmo J.M., Dubinion J., Hamza S., Munusamy S., Smith G., Stec D.E. Obesity-induced hypertension: Role of sympathetic nervous system, leptin, and melanocortins. J. Biol. Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kohsaka A., Laposky A.D., Ramsey K.M., Estrada C., Joshu C., Kobayashi Y., Turek F.W., Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 129.La Fleur S.E., Van Rozen A.J., Luijendijk M.C.M., Groeneweg F., Adan R.A.H. A free-choice high-fat high-sugar diet induces changes in arcuate neuropeptide expression that support hyperphagia. Int. J. Obes. 2010;34:537–546. doi: 10.1038/ijo.2009.257. [DOI] [PubMed] [Google Scholar]
- 130.Lin S., Storlien L.H., Huang X.F. Leptin receptor, NPY, POMC mRNA expression in the diet-induced obese mouse brain. Brain Res. 2000;875:89–95. doi: 10.1016/S0006-8993(00)02580-4. [DOI] [PubMed] [Google Scholar]
- 131.Bouret S.G., Gorski J.N., Patterson C.M., Chen S., Levin B.E., Simerly R.B. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab. 2008;7:179–185. doi: 10.1016/j.cmet.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shi Z., Li B., Brooks V.L. Role of the paraventricular nucleus of the hypothalamus in the sympathoexcitatory effects of leptin. Hypertension. 2015;66:1034–1041. doi: 10.1161/HYPERTENSIONAHA.115.06017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Plakkot B., Subramanian M. Evaluation of Hypothalamic Neural Stem Cell Niche–Implications on Obesity-Induced Sympathoexcitation. FASEB J. 2022;36 doi: 10.1096/fasebj.2022.36.S1.R6154. [DOI] [Google Scholar]
- 134.Mattson M.P., Arumugam T.V. Hallmarks of brain aging: Adaptive and pathological modification by metabolic states. Cell Metab. 2018;27:1176–1199. doi: 10.1016/j.cmet.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mana M.D., Kuo E.Y.S., Yilmaz Ö.H. Dietary regulation of adult stem cells. Curr. Stem Cell Rep. 2017;3:1–8. doi: 10.1007/s40778-017-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Arumugam T., Phillips T.M., Cheng A., Morrell C.H., Mattson M.P., Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann. Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Halagappa V.K.M., Guo Z., Pearson M., Matsuoka Y., Cutler R.G., LaFerla F.M., Mattson M.P. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 138.Manzanero S., Erion J.R., Santro T., Steyn F.J., Chen C., Arumugam T.V., Stranahan A.M. Intermittent fasting attenuates increases in neurogenesis after ischemia and reperfusion and improves recovery. J. Cereb. Blood Flow Metab. 2014;34:897–905. doi: 10.1038/jcbfm.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bruce-Keller A.J., Umberger G., McFall R., Mattson M.P. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1999;45:8–15. doi: 10.1002/1531-8249(199901)45:1<8::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 140.Kim J., Kang S.-W., Mallilankaraman K., Baik S.-H., Lim J.C., Balaganapathy P., She D.T., Lok K.-Z., Fann D.Y., Thambiayah U., et al. Transcriptome analysis reveals intermittent fasting-induced genetic changes in ischemic stroke. Hum. Mol. Genet. 2018;27:1497–1513. doi: 10.1093/hmg/ddy057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baik S., Rajeev V., Fann D.Y., Jo D., Arumugam T.V. Intermittent fasting increases adult hippocampal neurogenesis. Brain Behav. 2020;10:e01444. doi: 10.1002/brb3.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hitoshi S., Alexson T., Tropepe V., Donoviel D., Elia A.J., Nye J.S., Conlon R.A., Mak T.W., Bernstein A., van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brai E., Marathe S., Astori S., Ben Fredj N., Perry E., Lamy C., Scotti A., Alberi L. Notch1 regulates hippocampal plasticity through interaction with the reelin pathway, glutamatergic transmission and CREB signaling. Front. Cell. Neurosci. 2015;9:447. doi: 10.3389/fncel.2015.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.He W., Tian X., Yuan B., Chu B., Gao F., Wang H. Rosuvastatin improves neurite extension in cortical neurons through the Notch 1/BDNF pathway. Neurol. Res. 2019;41:658–664. doi: 10.1080/01616412.2019.1610226. [DOI] [PubMed] [Google Scholar]
- 145.Schölzke M.N., Schwaninger M. Transcriptional regulation of neurogenesis: Potential mechanisms in cerebral ischemia. J. Mol. Med. 2007;85:577–588. doi: 10.1007/s00109-007-0196-z. [DOI] [PubMed] [Google Scholar]
- 146.Colman R.J., Beasley T.M., Kemnitz J.W., Johnson S.C., Weindruch R., Anderson R.M. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fontana L., Partridge L., Longo V.D. Extending healthy life span—From yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Longo V.D., Fontana L. Calorie restriction and cancer prevention: Metabolic and molecular mechanisms. Trends Pharmacol. Sci. 2010;31:89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mercken E.M., Carboneau B.A., Krzysik-Walker S.M., de Cabo R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Res. Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Berryman D., Christiansen J.S., Johannsson G., Thorner M.O., Kopchick J.J. Role of the GH/IGF-1 axis in lifespan and healthspan: Lessons from animal models. Growth Horm. IGF Res. 2008;18:455–471. doi: 10.1016/j.ghir.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Longo V.D., Mattson M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee J., Duan W., Long J.M., Ingram D.K., Mattson M.P. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J. Mol. Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- 153.Lee J., Seroogy K.B., Mattson M.P. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem. 2002;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 154.Brandhorst S., Choi I.Y., Wei M., Cheng C.W., Sedrakyan S., Navarrete G., Dubeau L., Yap L.P., Park R., Vinciguerra M., et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cheng C.W., Adams G.B., Perin L., Wei M., Zhou X., Lam B.S., Da Sacco S., Mirisola M., Quinn D.I., Dorff T.B., et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14:810–823. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ng S.Y., Johnson R., Stanton L.W. Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J. 2012;31:522–533. doi: 10.1038/emboj.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang Y., He L., Du Y., Zhu P., Huang G., Luo J., Yan X., Ye B., Li C., Xia P., et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell. 2015;16:413–425. doi: 10.1016/j.stem.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 158.Loewer S., Cabili M.N., Guttman M., Loh Y.H., Thomas K., Park I.H., Garber M., Curran M., Onder T., Agarwal S., et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat. Genet. 2010;42:1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xiao Y.Z., Yang M., Xiao Y., Guo Q., Huang Y., Li C.J., Cai D., Luo X.H. Reducing hypothalamic stem cell senescence protects against aging-associated physiological decline. Cell Metab. 2020;31:534–548.e5. doi: 10.1016/j.cmet.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 160.Bernstein H.-G., Lindquist J.A., Keilhoff G., Dobrowolny H., Brandt S., Steiner J., Bogerts B., Mertens P.R. Differential distribution of Y-box-binding protein 1 and cold shock domain protein A in developing and adult human brain. Brain Struct. Funct. 2015;220:2235–2245. doi: 10.1007/s00429-014-0786-9. [DOI] [PubMed] [Google Scholar]
- 161.En-Nia A., Yilmaz E., Klinge U., Lovett D.H., Stefanidis I., Mertens P.R. Transcription factor YB-1 mediates DNA polymerase α gene expression. J. Biol. Chem. 2005;280:7702–7711. doi: 10.1074/jbc.M413353200. [DOI] [PubMed] [Google Scholar]
- 162.Izumi H., Imamura T., Nagatani G., Ise T., Murakami T., Uramoto H., Torigoe T., Ishiguchi H., Yoshida Y., Nomoto M., et al. Y box-binding protein-1 binds preferentially to single-stranded nucleic acids and exhibits 3′→ 5′ exonuclease activity. Nucleic Acids Res. 2001;29:1200–1207. doi: 10.1093/nar/29.5.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kotake Y., Ozawa Y., Harada M., Kitagawa K., Niida H., Morita Y., Tanaka K., Suda T., Kitagawa M. YB 1 binds to and represses the p16 tumor suppressor gene. Genes Cells. 2013;18:999–1006. doi: 10.1111/gtc.12093. [DOI] [PubMed] [Google Scholar]
- 164.Anandhan A., Tamilselvam K., Radhiga T., Rao S., Essa M.M., Manivasagam T. Theaflavin, a black tea polyphenol, protects nigral dopaminergic neurons against chronic MPTP/probenecid induced Parkinson’s disease. Brain Res. 2012;1433:104–113. doi: 10.1016/j.brainres.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 165.Nishino J., Kim I., Chada K., Morrison S.J. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell. 2008;135:227–239. doi: 10.1016/j.cell.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lowe S.W., Sherr C.J. Tumor suppression by Ink4a–Arf: Progress and puzzles. Curr. Opin. Genet. Dev. 2003;13:77–83. doi: 10.1016/S0959-437X(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 167.Molofsky A.V., Slutsky S.G., Joseph N.M., He S., Pardal R., Krishnamurthy J., Sharpless N.E., Morrison S.J. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhao C., Sun G., Li S., Lang M.-F., Yang S., Li W., Shi Y. MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc. Natl. Acad. Sci. USA. 2010;107:1876–1881. doi: 10.1073/pnas.0908750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nishino J., Kim S., Zhu Y., Zhu H., Morrison S.J. A network of heterochronic genes including Imp1 regulates temporal changes in stem cell properties. Elife. 2013;2:e00924. doi: 10.7554/eLife.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Szafranski K., Abraham K.J., Mekhail K. Non-coding RNA in neural function, disease, and aging. Front. Genet. 2015;6:87. doi: 10.3389/fgene.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Spinelli M., Natale F., Rinaudo M., Leone L., Mezzogori D., Fusco S., Grassi C. Neural Stem Cell-Derived Exosomes Revert HFD-Dependent Memory Impairment via CREB-BDNF Signalling. Int. J. Mol. Sci. 2020;21:8994. doi: 10.3390/ijms21238994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Reekmans K., Praet J., Daans J., Reumers V., Pauwels P., Van Der Linden A., Berneman Z.N., Ponsaerts P. Current challenges for the advancement of neural stem cell biology and transplantation research. Stem Cell Rev. Rep. 2012;8:262–278. doi: 10.1007/s12015-011-9266-2. [DOI] [PubMed] [Google Scholar]
- 173.Bellin M., Marchetto M.C., Gage F.H., Mummery C.L. Induced pluripotent stem cells: The new patient? Nat. Rev. Mol. Cell Biol. 2012;13:713–726. doi: 10.1038/nrm3448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data was created as this is a review, and some data is unavailable due to privacy or ethical restrictions. Data will be published with ethical considerations in future manuscript.