Abstract
Alzheimer’s Disease (AD) is the cause of around 60–70% of global cases of dementia and approximately 50 million people have been reported to suffer this disease worldwide. The leaves of olive trees (Olea europaea) are the most abundant by-products of the olive grove industry. These by-products have been highlighted due to the wide variety of bioactive compounds such as oleuropein (OLE) and hydroxytyrosol (HT) with demonstrated medicinal properties to fight AD. In particular, the olive leaf (OL), OLE, and HT reduced not only amyloid-β formation but also neurofibrillary tangles formation through amyloid protein precursor processing modulation. Although the isolated olive phytochemicals exerted lower cholinesterase inhibitory activity, OL demonstrated high inhibitory activity in the cholinergic tests evaluated. The mechanisms underlying these protective effects may be associated with decreased neuroinflammation and oxidative stress via NF-κB and Nrf2 modulation, respectively. Despite the limited research, evidence indicates that OL consumption promotes autophagy and restores loss of proteostasis, which was reflected in lower toxic protein aggregation in AD models. Therefore, olive phytochemicals may be a promising tool as an adjuvant in the treatment of AD.
Keywords: olive leaves, bioactive compounds, Alzheimer’s Disease, oleuropein, hydroxytyrosol
1. Introduction
Alzheimer’s Disease (AD) is the cause of around 60–70% of global cases of dementia [1] and approximately 50 million people have been reported to suffer from this disease worldwide [2]. In fact, AD incidence rates double every 5 years from 60 years of age [3] and it is estimated that dementia will affect 81.1 million people worldwide in 2040 [2,4].
The etiopathogenesis of AD is characterized by two histopathological events: the senile plaque aggregation formed by amyloid-β peptides (Aβ) in the central nervous system and the formation of neurofibrillary tangles (NFTs) associated with the accumulation of Tau protein in the hippocampus, neocortical area, and amygdala [4]. These events are associated with an increase in mitochondrial dysfunction, oxidative stress, glucose homeostasis alteration in the brain, neuroinflammation, and disturbances in the proteostatic network, which favor the appearance of senile plaques and NFTs, generating atrophy and neuron death characteristic of AD [5].
AD is a multifactorial disease whose appearance and development are marked by the interaction between genetic predisposition and external factors throughout life [4]. Among the risk factors, aging, gender (higher incidence in women), alcohol and tobacco consumption, obesity, and metabolic disorders such as diabetes mellitus, as well as a low cultural level and family history, have been highlighted [1]. Some of these risk factors, including physical activity, diet, smoking, and alcoholism, could be modified in order to reduce the onset of the disease [4].
Although there is still no pharmacological therapy for its treatment, the preventive and/or therapeutic nutritional interventions against AD have been gaining prominence in recent years [1]. It is known that 35% of dementias could be caused by modifiable risk factors associated with lifestyle, including the type of diet [1]. In particular, the Mediterranean Diet (MD), characterized by a high consumption of legumes, vegetables, fruits, vitamins, and virgin olive oil, and a low consumption of red meat, has been shown to reduce the incidence of AD [1]. MD presents a high contribution of bioactive substances such as phenolic compounds, which have been shown to exert a protective effect in AD [6]. In particular, the intake of phytochemical compounds naturally present in foods, such as oleuropein (OLE), hydroxytyrosol (HT), luteolin (LU), catechin, and curcumin, are related to neuroprotective effects in AD through the modulation of mechanisms such as oxidative stress and neuroinflammation, besides reducing the deposition and toxicity of the misfolded proteins involved [7,8,9,10,11,12].
The leaves of the olive tree (Olea europaea) are the most abundant by-product in the olive grove industry. In Spain, between 1 and 5 tons of waste are generated per hectare in the form of branches and leaves. OLs are long, hard, and lanceolate, and their edges curl up due to desiccation [13]. It is possible to obtain olive leaf extracts enriched in certain compounds after grinding and processing them with an extraction solvent such as methanol, ethanol, or water, or a mixture thereof. In addition, separation or concentration procedures can be applied to enrich extracts in particular molecules [14]. OLs have excellent medicinal properties thanks to the wide variety of bioactive compounds present in them. In this review, the preventive and therapeutic effect of olive compounds against AD were reviewed from the point of view of the molecular mechanisms involved.
2. Phytochemical Characterization of Olive Leaves
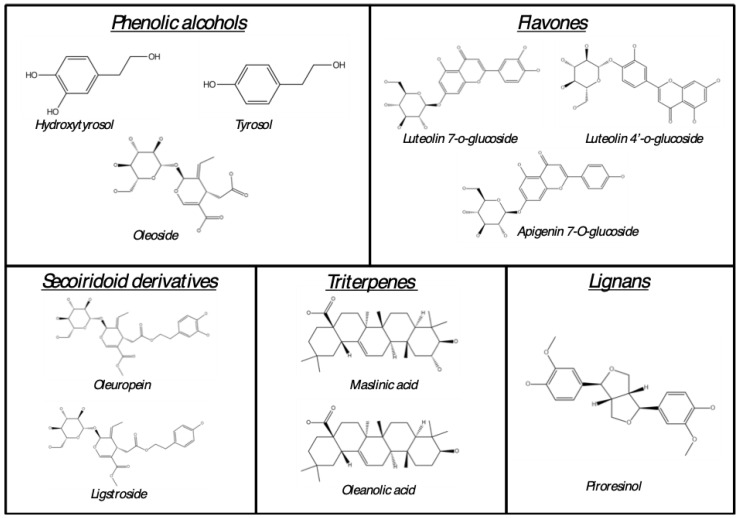
OLs are consumed worldwide as a nutraceutical product due to their numerous and demonstrated health properties. In fact, considerable attention has been given to OLs because of their remarkable content of polyphenols [15]. The most representative compounds of OLs are those illustrated in Figure 1. Table 1 shows most representative compounds present in olive leaves. To date, the best-known phenolic compounds in OLs are secoiridoid derivatives, of which OLE is the most abundant. Additionally, the presence of phenolic alcohols (e.g., HT, tyrosol, and oleoside) and flavones (e.g., LU and luteolin-7-o-glucoside) should be highlighted together with others.
Figure 1.
Most representative phytochemical compounds in OL.
In the same way, other phenolic compounds, such as phenolic acids (e.g., verbascoside), flavanols (e.g., Epicatechin gallate), and flavonols (e.g., kaempferol-7-O-glucoside and rutin), have also been described in OLs (Figure 1). The phytochemical profile of the OL can be affected by several factors, such as the olive tree’s geographical location, cultivars, harvest season, drying temperature of leaves, and the solvents used for extraction. In this regard, Kabbash et al. (2021) demonstrated that olive leaves from Spain presented higher total flavonoid, phenolic, and OLE content compared with olive leaves from Italy and Egypt [16]. Similar results were obtained by Zhang et al. (2022), which showed a higher total flavonoid, OLE, and HT content, among other phenolic compounds, in Spanish olive leaves compared with those harvested in China or Italy [17]. These differences could also be attributed to the use of different cultivars. In this context, Nicoli et al. (2019) found significant differences among 15 different olive tree cultivars from Italy regarding OLE, HT, verbascoside (VB), and flavones (LU and luteolin-7-O-glucoside) content [18]. In the same way, Pasković et al. (2022) reported several differences in OLE, VB, rutin, catechin, phenolic alcohol (HT and tyrosol), and flavone (LU and apigenin derivatives) content in four different olive tree cultivars from Croatia [19]. Furthermore, the harvesting season has been demonstrated to influence the content of most of the phenolic compounds of OLs, even those of the same cultivar, with increases in olives harvested between March and April [16,19]. Additionally, it has been reported that the temperature of leaf drying after pruning can also affect the phenolic content. In this context, olive leaves dried using a freezing protocol (−80 °C) presented higher amounts of phytochemicals compared with those dried using a hot air protocol (105 °C), with the exception of OLE, the content of which increased in hot-air-dried olive leaves [17]. Authors attributed these results to the fact that some molecules, such as flavonoids, easily break down into smaller compounds during dehydration, while the stability of secoiroids, such as OLE, is relatively high. Finally, the method and solvent used for the extraction of olive leaf polyphenols has been reported to dramatically influence the final phenolic content. Most of the evaluated studies in this work used different proportions of methanol [19,20,21,22,23], ethanol [16,17,18], or water [23,24] as solvent to obtain the olive leaf extract. However, only Kontogianni et al. (2013) evaluated the direct influence of the solvent used in OL polyphenol extraction. Independently of the detection method (high performance liquid chromatography or nuclear magnetic resonance spectroscopy), the olive leaves extracted with methanol presented a higher content of OLE, HT, and LU and its derivatives compared with the aqueous OL extract. In fact, LU was not detected in the aqueous extract [23]. For further studies, the phytochemical characterization of OL needs to be standardized to generate a homogeneous phenolic profile through the regulation of the previously mentioned factors.
Table 1.
Phytochemical characterization of olive leaves.
| Phenolic Compound | CAS Number | Molecular Formula | Range of Content | Refs. |
|---|---|---|---|---|
| Phenolic acids | ||||
| Verbascoside | 61276-17-3 | C29H36O15 | 9–465.63 | [18,19,20,22,24,25,26] |
| Gallic acid | 149-91-7 | C7H6O5 | 0.17–140 | [22,24,25] |
| Vanillin | 121-33-5 | C8H8O3 | nd–0.05 | [24,25] |
| Vanillic acid | 121-34-6 | C8H8O4 | 3–274 | [20,22,24] |
| p-coumaric acid | 501-98-4 | C9H8O3 | nd–4.41 | [20,24,25] |
| Ferulic acid | 537-98-4 | C10H10O4 | nd–0.96 | [24,25] |
| Chlorogenic acid | 327-97-9 | C16H18O9 | nd–26 | [17,20,22,24,25] |
| Neochlorogenic acid | 906-33-2 | C16H18O9 | 25 | [22] |
| Caffeic acid | 331-39-5 | C9H8O4 | nd–20.15 | [20,24,25] |
| Plantamajoside | 104777-68-6 | C29H36O16 | nd–1.35 | [17] |
| Rosmarinic acid | 20283-92-5 | C18H16O8 | 0.59 | [24] |
| Phenolic alcohols | ||||
| Hydroxytyrosol | 10597-60-1 | C8H10O3 | 6.68–1092.74 | [17,19,23,25] |
| Hydroxytyrosol 4-O-glucoside | 54695-80-6 | C14H20O8 | nd–11.02 | [17] |
| p-Hydroxybenzoic acid | 99-96-7 | C7H6O3 | 3.63–141 | [20,22,24] |
| 2,3-Dihydroxybenzoic acid | 303-38-8 | C7H6O4 | 2 | [22] |
| 2,5-Dihydroxybenzoic acid | 490-79-9 | C7H6O4 | 1.18 | [24] |
| 3,4-Dihydroxyphenylacetic acid | 102-32-9 | C8H8O4 | 0.62 | [24] |
| Protocatechuic acid | 99-50-3 | C7H6O4 | 17.61–965 | [20,24] |
| Tyrosol | 501-94-0 | C8H10O2 | 3.33–168.12 | [19,22,25] |
| Oleoside | 178600-68-5 | C16H22O11 | 46 | [26] |
| Oleoside 11-methyl ester | 60539-23-3 | C17H24O11 | 23 | [26] |
| Secoiridoid derivatives | ||||
| Oleuropein | 32619-42-4 | C25H32O13 | 2.81–5940 | [16,17,18,19,20,21,22,23,25,26] |
| Secoxyloganin | 58822-47-2 | C17H24O11 | 1.80–138.32 | [17] |
| Ligstroside | 35897-92-8 | C25H32O12 | 17 | [26] |
| Flavanols | ||||
| Catechin | 18829-70-4 | C15H14O6 | nd–77 | [19,22,24] |
| Gallocatechin | 970-73-0 | C15H14O7 | 72 | [22] |
| Epicatechin | 490-46-0 | C15H14O6 | nd–2.17 | [22,24,25] |
| Epicatechin gallate | 1257-08-5 | C22H18O10 | 133 | [22] |
| Epigallocatechin | 970-74-1 | C15H14O7 | 3 | [20] |
| Flavones | ||||
| Luteolin | 491-70-3 | C15H10O6 | nd–266 | [17,18,19,20,22,23,24] |
| Luteolin-7-o-glucoside | 5373-11-5 | C21H20O11 | 30–3978 | [17,18,19,20,22,23,24,25] |
| Luteolin-4′-O-glucoside | 6920-38-3 | C21H20O11 | 3.97–330 | [17,23] |
| Luteolin-3′,7-di-O-glucoside | 52187-80-1 | C27H30O16 | 6.37–31.38 | [17] |
| Diosmetin-7-O-neohesperidoside | 38665-01-9 | C28H32O15 | 0.21–1.27 | [17] |
| Apigenin | 520-36-5 | C15H10O5 | nd–9.38 | [17,19,22,24] |
| Apigenin-7-o-glucoside | 578-74-5 | C21H20O10 | 7.88–214.71 | [17,19,20,22,24,25] |
| Apigenin-7-O-neohesperidoside | 17306-46-6 | C27H30O14 | 18.27–50.40 | [17] |
| Hispidulin | 1447-88-7 | C16H12O | 0.02–0.44 | [17] |
| Flavonols | ||||
| Quercetin | 117-39-5 | C15H10O7 | nd–22 | [17,20,24] |
| Quercetin-3-o-glucoside | 482-35-9 | C21H20O12 | 6.48–31.65 | [17] |
| Quercetin-3-o-galactoside | 482-36-0 | C21H20O12 | 1.08–3.06 | [24,25] |
| Quercetin-4′-O-glucoside | 20229-56-5 | C21H20O12 | nd–1.83 | [17] |
| Rutin | 153-18-4 | C27H30O16 | 1.49–101 | [17,19,20,22,25,26] |
| Kaempferol | 520-18-3 | C15H10O6 | nd–1.18 | [17,20,24,25] |
| Kaempferol-7-O-glucoside | 16290-07-6 | C21H20O11 | 62.19–268.11 | [17] |
| Tiliroside | 20316-62-5 | C30H26O13 | 0.48–1.85 | [17] |
| Flavanonols | ||||
| Taxifolin | 480-18-2 | C15H12O7 | 0.32–27.43 | [17,24] |
| Taxifolin-3-glucoside | 27297-45-6 | C21H22O12 | 0.44–2.89 | [17] |
| Flavanones | ||||
| Eriodictyol | 552-58-9 | C15H12O6 | nd–44.77 | [17,22,24] |
| Hesperidin | 520-26-3 | C28H34O15 | nd–5.72 | [22,24] |
| Coumarins | ||||
| Esculin | 531-75-9 | C15H16O9 | 0.07–2.09 | [17] |
| Coumarin | 91-64-5 | C9H6O2 | 0.40–1.99 | [17] |
| Triterpenes | ||||
| Asiatic acid | 464-92-6 | C30H48O5 | 0.88–2.88 | [17] |
| Oleanonic acid | 17990-42-0 | C30H46O3 | 68.15–226.70 | [17] |
| Maslinic acid | 4373-41-5 | C30H48O4 | 323.55–607.14 | [17] |
| Corosolic acid | 4547-24-4 | C30H48O4 | 91.80–227.40 | [17] |
| Oleanolic acid | 508-02-1 | C30H48O3 | 758.40–1047.67 | [17] |
| Ursolic acid | 77-52-1 | C30H48O3 | 14.64–25.89 | [17] |
| Other compounds | ||||
| Quinic acid | 77-95-2 | C7H12O6 | 605–2519 | [18] |
| Pyrocatechol | 120-80-9 | C6H6O2 | 1.03 | [24] |
| Pinoresinol | 487-36-5 | C20H22O6 | nd–1.56 | [24,25] |
Range of content is expressed as milligrams per 100 g of dry weight. nd: no detectable.
3. Bioaccessibility and Bioavailability of Olive Leaf Polyphenols
A crucial point of drug administration is the capacity of the active principle to be absorbed and passed to the systemic circulation, and to exert its action on the specific sites. In the case of a multicomplex food matrix such as the OL, it is necessary to evaluate the absorption and metabolism of numerous compounds present in it, and to evaluate the role of these compounds in the observed healthy effects. Some studies have investigated the pharmacokinetics of olive leaf phenolics by administering isolated compounds (not explored in this review). However, in this review, the bioaccessibility and bioavailability of individual compounds were examined, but after OL administration.
According with in vitro studies, gastric, intestinal, and colonic digestion significantly reduced the bioaccessibility of numerous compounds naturally present in OLs, such as phenolic acids (e.g., VB, chlorogenic, gallic, and caffeic acid), phenolic alcohols (e.g., HT and tyrosol), secoiridoid derivatives (e.g., OLE), flavones (e.g., luteolin 7-o-glucoside, apigenin 7-o-glucoside), flavanols (e.g., epicatechin), and flavonols (i.e., quercetin-3-o-rutinoside, quercetin-3-o-galactoside, kaempferol, and rutin) [25,26]. However, in vitro digestion also promoted the bioaccessibility and the potential bioavailability of some secoiridoid derivatives related to OLE hydrolysis, such as oleoside and oleoside 11-methyl ester [26].
To date, only three investigations have explored the bioavailability of phenolic compounds from OLs in humans. As can be observed in Table 2, numerous compounds such as secoiridoid derivatives, phenolic alcohols, and flavonoids can be found in plasma or urine samples after OL ingestion. No significant influence of gender on the absorption of OL phenolic compounds was observed in middle-aged people after OL consumption (270 or 400 mg/d). Interestingly, the administration of OLs through liquid glycerol preparation increased the plasma bioavailability of OLE and reduced the time to peak of HT derivatives compared with softgel capsule administration [27].
Table 2.
OL related metabolites found in plasma and urine samples in humans.
| Phenolic Compound | Molecular Formula | Plasma | Urine | Refs. |
|---|---|---|---|---|
| Phenolic alcohols | ||||
| Hydroxytyrosol | C8H10O3 | - | ✓ | [28] |
| Hydroxytyrosol sulfo glucuronide | C14H18O12 | ✓ | ✓ | [27,29] |
| Hydroxytyrosol glucuronide | C14H18O9 | ✓ | ✓ | [27,29] |
| Hydroxytyrosol sulfate | C8H10O6 | ✓ | ✓ | [27,29] |
| Hydroxytyrosol-acetate glucuronide | C16H20O10 | ✓ | ✓ | [29] |
| Tyrosol | C8H10O2 | - | ✓ | [27] |
| Tyrosol glucuronide | C14H18O8 | ✓ | ✓ | [29] |
| Homovanillic alcohol | C9H12O3 | - | ✓ | [28] |
| Homovanillic alcohol sulfate | C9H12O6S | X | ✓ | [29] |
| Homovanillic alcohol glucuronide | C15H20O9 | ✓ | ✓ | [29] |
| Secoiridoid derivatives | ||||
| Oleuropein | C25H32O13 | ✓ | ✓ | [27] |
| Oleuropein aglycone | C19H22O8 | - | ✓ | [28] |
| Oleuropein aglycone dialdehyde | C17H20O6 | - | ✓ | [28] |
| Oleuropein aglycone derivative 1 | C25H32O15 | ✓ | ✓ | [29] |
| Oleuropein aglycone derivative 2 | C25H32O14 | ✓ | ✓ | [29] |
| Oleuropein aglycone glucuronide | C25H30O14 | ✓ | ✓ | [29] |
| Oleuropein sulfate | C19H22O11S | ✓ | ✓ | [29] |
| Elenolic acid | C11H14O6 | X | ✓ | [29] |
| Elenolic acid glucuronide | C17H22O12 | X | ✓ | [29] |
| Flavones | ||||
| Luteolin | C15H10O6 | ✓ | X | [29] |
| Luteolin glucuronide | C21H18O12 | ✓ | X | [29] |
✓: indicates presence of the compound in the sample; X: indicates the absence of the compound in the sample; -: indicates the absence of evidence about the specific metabolite in the sample.
According to the pharmacokinetics, the biological half-life of plasma OLE metabolites (1.33–2.01 h) was shorter than that of HT derivatives (1.73–6.53 h), whereas the excretion peak rate in urine was 8-24 h for both metabolite classes [28,29]. The most abundant compounds found in urine were secoiridoids and HT and its derivatives, probably due to the rapid hydrolysis of OLE in the upper gastrointestinal tract [28,29]. It should be noted that the hydrolysis of OLE is not complete and numerous glucuronidated and sulfated derivatives from OLE can be found in plasma and urine, indicating that OLE is also conjugated by Phase II enzymes [29]. Curiously, a study of pre- and post-menopausal women fed with 250 mg of OL revealed that OL-related metabolites, such as HT glucuronide and sulfate, OLE aglycone glucuronide, and aglycon derivative I, were present in higher concentrations in the plasma from post-menopausal women. The authors attributed these results to potential age-related changes such as alterations in hormonal status and a decrease in gastric emptying [29]. These results are extremely interesting due the existence of a potential increase of bioavailability of phenolic compounds from OL, related, at least in part, to women’s age, opening the door to their potential use in aging and age-related diseases.
4. Toxicological Evaluation of Olive Leaves Bioactive Compounds
Olive leaves have been widely used as therapeutic tools throughout history [30]. In contrast to the classical belief that botanic-related products are completely safe and lack toxicity, these products could cause several side effects due to the fact that most of their chemical content remains uncharacterized. In addition, due to the easy access and low cost of these by-products, as well as the possibility of self-medication without medical advice for many people around the world, the study of OL-related toxicity is necessary. Therefore, in this section, the evidence regarding toxicity related to the intake of olive leaves is discussed.
According to in vitro studies, the co-incubation of different concentrations (51.2, 128, 320, 800, 2000, and 5000 µg/mL) of OL did not reveal pro-mutagenic effect in different Salmonella typhimurium (TA98, TA100, TA1535, and TA1537) and Escherichia coli (WP2 uvrA) strains in the Bacterial Reverse Mutation Test [31]. In the same way, coincubation with rising concentrations of OL (250, 500, 750, 1000, and 1250 µg/mL) did not affect the number of aberrant cells, polyploidy rates, or endoreduplication metaphases in V79 male Chinese hamster lung cells in the Chromosomal Aberration Test [31]. Similarly, treatment with lower OL dosages (20, 40, 60, 70, or 80 µg/mL) was demonstrated not to reduce or even increase viability in different cell lines [32,33].
Acute toxicity of OLs has also been evaluated in in vivo models. In this context, no adverse reactions, toxicity clinical signs, or lethality were observed after 24–48 h of OL administration in Caenorhabditis elegans (0.1, 1, 10, 100, 1000 µg/mL, [C. elegans]), NMRI BR mice (500, 1000, and 2000 mg/kg of body weight [bw]), or Swiss albino mice (2000 mg/kg bw) [31,34,35]. In fact, no genotoxic activity of OL was observed in bone marrow from these NMRI BR mice consuming 500, 1000, or 2000 mg/mL for 48 h [31] or Drosophila melanogaster acutely exposed to 3.75 or 30 µg/mL of OL [36]. Additionally, no embryolethality or embryotoxicity were found after an acute exposure to 100 µg/mL of OL for 24 h in C. elegans Wild-type strain [35]. Regarding sub-chronic toxicity of OL, the intake of 100, 200, 400, or 2000 mg/kg bw of OL daily via gavage for 14 or 28 days did not produce mortality, signs of toxicity, or behavioral and physical alterations in Wistar male and female rats. In addition, necropsy showed no abnormalities in the liver and kidney of treated rats [37]. Similar results were obtained in Wistar rats orally supplemented with 360, 600, or 1000 mg/kg bw of OL daily for three months [31]. Additionally, these authors found no alterations in organ weight (liver, adrenals, kidneys, thyroid/parathyroid, thymus, spleen, brain, heart, epididymides, testes, ovaries, fallopian tubes, and uterus) or pathological lesions in the most representative organs from locomotor, digestive, lymphatic, integumentary, respiratory, cardiovascular, endocrine, excretory, reproductive, and central and peripheral nervous systems [31]. Similarly, the consumption of 250 mg/day of OL in a double-blind, randomized controlled trial for 12 months revealed an absence of side effects in aged women [38]. In accordance with chronic toxicity, only one study evaluated the long-life effect of OL. In this context, lifelong administration of 100 µg/mL did not modify the survival rates in the C. elegans Wild-type strain [35].
Among the in vivo endpoint studies, some research has evaluated the influence of OL treatment in biochemical and hematological parameters. Interestingly, after an acute administration of 2000 mg/kg bw of OL, some hematological (hematocrit, hemoglobin, mean corpuscular volume, red blood cells, and platelets) and biochemical parameters (cholesterol and creatinine levels) were reduced without producing abnormalities in liver or kidneys [37]. It should be noted that the solvent used in this work was a solution made with 51% of ethanol, which could also interfere in hematological and biochemical studies, meaning results may not be attributed to the treatment itself. In fact, when the same solvent was administered for 28 days, the effect on hematological and biochemical parameters disappeared, probably due to an adaptation to alcohol consumption [37]. Similarly, the intake of 360, 600, or 1000 mg/kg bw of OL diluted in distilled water daily for three months did not alter most of the hematological parameters, electrolytes, or renal and hepatic markers studied in rats [31]. According to hepatic markers, no adverse effects were noted related to aspartate and alanine aminotransferase, gamma glutamyl transferase, and alkaline phosphatase levels in middle-aged people who consumed 270 or 400 mg of OL [27]. Similarly, no clinical changes were observed in classical biochemistry, hematological, or electrolytes parameters, or renal- and liver-function-related parameters, in a randomized controlled trial that administered 1000 mg/day of OL for 8 weeks [39].
5. Effects of Olive Leaf Bioactive Compounds in the Molecular Mechanisms Related to AD
In the following section, the scientific evidence regarding the effects of OL bioactive compounds on the main mechanisms involved in the pathogenesis of AD will be discussed. A summary can be found in Table 3.
5.1. Effects on Aβ Aggregation
As mentioned before, an abnormal extracellular accumulation and clearance of the Aβ in the brain is one of the main features of AD, which leads to neuron death and the typical symptoms of dementia [40]. In this regard, several studies have evidenced the protective role of OL and its bioactive molecules.
In vitro experiments indicated that individual compounds from OL were able to reduce both the aggregate size and occurrence of Aβ42 fibrils [40]. In the human neuroblastoma SH-SY5Y cell line, treatment with an OL fraction enriched in triterpenoid compounds (OLE, HT, VB, LU, and quercetin [QU]) reversed the loss of viability induced by the neurotoxic agent Aβ1–42. The lipid profile analysis performed using bioinformatic tools revealed that a significant number of phosphatidylcholines and phosphatidylethanolamines significantly increased, whereas several triacylglycerols decreased in the treated neuroblastoma cells [32]. In the same model, treatment with the aforementioned compounds and commercial preparation of OL exerted a stronger protection against Aβ42-, Cu-Aβ42-, or L-DOPA-Aβ42-induced neurotoxicity, manifesting an increase in cell viability [40]. In accordance with a computational binding affinity test, the neurotoxicity reduction mentioned above may be attributed to the ability of OLE, HT, LU, VB, and QUE, as well as their derivatives, to strongly bind to the hairpin-turn of the Aβ1–40 and Aβ1–42 monomers and the subsequent reduction in Aβ fibrillization [41]. On the other hand, β-secretase site-1 (BACE-1) is involved in the generation of the Aβ aggregation since it participates in the amyloidogenic processing of the amyloid precursor protein (APP). In vitro assays have demonstrated that bioactive compounds present in olive-tree-derived products, both non-flavonoids (e.g., HT, VB) and flavonoids (e.g., RU, QU), have a remarkable inhibitory effect on the BACE-1 enzyme. The commercial olive biophenol extracts (olive leaf extract rich in OLE or HT) also exerted a strong inhibitory activity, the latter being the most powerful. Although the action mechanism of extraction of olive biophenols is not clearly understood, the results showed a synergistic effect of the combination of flavonoid or non-flavonoid compounds in the extracts which are rich in biophenols [42].
Regarding the in vivo evidence, a transgenic strain of C. elegans expressing the human Aβ1–42 peptide in muscle cells was used by Romero-Márquez et al. (2022) for evaluating the anti-Aβ aggregation effect of an olive leaf extract containing 40% of OLE. Results showed a delay in the amyloid-induced paralysis of worms and a reduction in the amount of Aβ deposits stained by Thioflavin T. The RNAi test showed the participation of DAF-16/FOXO, SKN-1/Nrf2, and HSP16.2 pathways in those effects. This extract has been authorized to be used as an ingredient for nutritional supplements in human nutrition, so it could be a very promising approach for AD therapy [35]. Similar results were found for a HT-rich extract from olive fruit [43]. Apart from cells and nematode models, the anti-amyloid effect of olive leaves has also been evaluated in rodents. The AD models 5xFAD and APPswe/PS1dE9 mice overproduce the Aβ peptide and develop progressive cerebral Aβ deposits and learning and memory impairment. Olive leaf extract enriched in OLE mixed with the powdered food was able to reduce the total Aβ deposits in the hippocampus and cortex in both 5xFAD [44] and APPswe/PS1dE9 [40] animals. The soluble Aβ40 levels [44] and the size of Aβ plaques [40], respectively, were also reduced. Furthermore, an increase in the expression of Aβ clearance proteins (P-gp and LRP1) was observed in the treated group. The induction of anti-amyloidogenic protein and enzyme expression (sAPPα and α-secretase) and the reduction in the amyloidogenic protein sAPPβ suggested that the olive leaf extract is able to modulate APP processing [44]. The effect of an olive leaf extract containing 40% OLE on Aβ production was also investigated in male white rabbits, although not in an AD model but in one of cervical myopathy. The increase in Aβ in spinal cord tissue neuron cells after receiving compression treatment was effectively reduced by the treatment [45]. These results could be attributed to the high content of OLE aglycone present in olive leaves, which was also demonstrated to reduce Aβ-induced neurotoxicity through the reduction in Aβ aggregates in rats cerebrally injected with Aβ42 [46]. These results could be explained by results found by Brogi et al. (2020) using molecular docking. In this research, the authors showed that OLE aglycone was able to move deeply within the Aβ fibrils targeting a key motif in Aβ peptide, promoting structural instability and Aβ fibril disaggregation [47].
5.2. Molecules from Olive Leaves and NFTs Formation
Together with Aβ aggregation, AD is characterized by the intracellular accumulation of hyperphosphorylated NFTs. The Tau aggregation has been investigated using the experimental model C. elegans. In this case, a transgenic strain expressing the pro-aggregate human Tau protein in a constitutive pan-neuronal way was used. This strain manifests locomotion defects derived from Tau deposition. Treatment with an OL extract enriched in OLE (40%) improved several locomotive parameters related to Tau neurotoxicity through the modulation of the DAF-16/FOXO, SKN-1/Nrf2, and HSP16.2 pathways. Those transcription factors were also involved in the protective effect of the treatment against the Aβ proteotoxicity [35]. Likewise, in a rabbit model, extracts with the same percentage of OLE decreased the high levels of p-Tau in spinal cord tissue neuron cells after receiving compression treatment in the cervical myelopathy [45]. These results might be attributed to the high content of OLE, OLE aglycone, and HT, which have been shown to prevent Tau fibrillization in vitro [48].
5.3. Actions in Enzymes Related with Neurotransmitters Degradation
The action of specific enzymes on neurotransmitters or certain proteins can lead to the development and progression of neurodegenerative diseases such as AD. The cholinesterases (acetylcholinesterase [AchE] and butyrylcholinesterase [BchE]), histone deacetylase, and tyrosinase are some of the enzymes associated with AD. AChE and BChE hydrolyze choline esters degrade the neurotransmitter acetylcholine. In fact, one of the hypotheses of AD is the cholinergic hypothesis. This system is severely affected in AD, and the over-activation of the mentioned enzymes appears to promote amyloid Aβ fibril formation. The deficit in cerebral cholinergic transmitters ultimately results in memory loss with other cognitive symptoms that are characteristic of AD. In that sense, one enzyme involved is histone deacetylase, which is required for memory formation. Studies have shown the relation between defects in this enzyme and the development of neuropathology and Tau neurofibrillary tangles formation. Likewise, tyrosinase activity is related to processes and sequences involved in the progression of AD [42]. On the other hand, AChE and BChE were effectively inhibited by the aforementioned commercial leaf extracts rich in HT or OLE [42], and by an ethanolic extract of olive leaves from different geographic origins [49]. In addition, different types of olive leaf extracts obtained by supercritical fluid extraction also exerted that activity [50]. The best adsorbent was sea sand, which yielded extracts rich in triterpenes with moderate inhibitory activity of the enzyme [50]. In contrast, HT [42], OLE [42], or maslinic acid [51] alone were not able to inhibit the enzymes, whereas oleanolic acid [52,53,54] and pinoresinol [55] had a very slight inhibitory activity. In the same way, some representative compounds present in OL, such as tyrosol, luteolin 7-O glucoside, and ligstroside, showed a null correlation with both AChE and BchE inhibitory activity [56]. These results suggested that the enzyme inhibitory effect of OL might be caused, once again, by a synergism between different compounds present in the leaves.
5.4. Effects Related to Neuroinflammation
Inflammation is a common condition present in neurodegenerative diseases and is considered another pathological feature of AD. In vitro assays based on the ability of inhibiting the lipoxidase (LOX) demonstrated a modest anti-inflammatory potential of different types of OL extracts obtained by supercritical fluid extraction [50]. Positive results in that sense were also found in cell cultures. Treatment with green olive leaves containing OLE 20% in N1 murine microglia cell culture decreased BSA-AGE-induced NO production [33]. The LPS-induced increase of inflammatory markers was ameliorated by treatment with olive leaf extracts in human THP-1 monocytes [32] and in activated murine macrophages RAW 264.7 [57]. Concentrations of 20 and 40 µg/mL of an olive leaf fraction enriched in triterpenoid compounds reduced IL-6 and IL-1β secretion levels. Furthermore, the highest dose was able to reduce TNF-α levels in the monocyte cell line [32]. RAW 264.7 macrophages were treated with OLE-rich leaf extract in acute (50 µM extract with LPS for 24 h) or chronic exposure (50 µM extract pre-treatment for 24 h followed by LPS). Both acute and chronic treatment decreased NO production and strongly reduced the levels of iNOS and COX-2. In addition, both types also decreased the mRNA expression of IL-1β and IL-6, whereas the acute treatment was able to reduce IL-1βR protein expression and mRNA expression for TGF-β [57].
In the same line, the potential anti-inflammatory activity of olive leaves was also demonstrated in rodents. The 5xFAD mice model accumulated high levels of Aβ along with astrogliosis and microgliosis. Animals treated with an OL extract spiked in refined olive oil and mixed with powdered food showed less astrocyte activation and GFAP levels, together with an ameliorated astrocyte shape, compared with the control group fed only with the vehicle. Microglial activation in both the hippocampus and cortex was reduced, as were the IL-1β levels. In addition, the treatment also decreased NLRP3 in the brain, a finding which is associated with the significant reduction in pro-caspase-1 and pro-caspase-8. Components of the NF-κB, a classical pathway involved in inflammation, were also modulated by the extract. OL consumption significantly reduced the expression of p-IKKβ, an effect that was associated with increased levels of total IκBα and reduced p-IκBα [44]. In addition, the authors studied the receptor for advanced glycated end products (RAGE), which is considered a major source of Aβ entry to the brain and is related with the increase in pro-inflammatory cytokines. The interaction of RAGE with high mobility group box protein 1 (HMGB1) and the upregulation of this last protein in AD are well known. Protein levels of RAGE and HMGB1 were downregulated by the treatment. Overall, the mechanisms involved in the anti-inflammatory activity of olive leaves were the reduction in the NF-κB pathway, which regulates NLRP3 and RAGE/HGMB1 [44]. Likewise, the increase in TNF-α, IL-1β and prostaglandin E2 levels caused by lead (Pb) neurotoxicity were reduced with olive leaf extract in the hippocampus of male Wistar rats. Pb is a well-known neurotoxic agent considered to be a key mediator of inflammation and oxidative-stress-induced neuropathological effects. The oral administration of the extract was able to reduce tissue Pb deposition and prevent the negative effects [58]. However, oral treatment with olive leaf extract to kainic-acid-induced epilepsy Wistar rats did not exert a statistically significant decrease in the pro-inflammatory cytokine TNF-α, although other parameters related to oxidative stress were improved [59]. Additionally, treatment with OL extract containing 40% OLE resulted in a significant decrease in the inflammatory marker CD-68 (biomarker of activated microglia-macrophage) in a model of cervical myopathy in male white rabbits [45]. Inflammatory markers were also reduced in Peripheral Blood Mononuclear Cells (PBMCs) from male human patients treated for 8 weeks with 20 mL of liquid olive leaf extract, which provided 121.8 mg of OLE and 6.4 mg of HT daily. A downregulation of COX-2 and IL-8 gene expression was observed in PBMCs. Furthermore, the authors found a downregulation of the transcription factor jun-B, which is related to macrophage activation, and the Heparin binding EGF-like growth factor, both related to the NF-κB, in the treated group [60].
5.5. Oxidative Stress Attenuation
Oxidative stress (OS) is involved in the occurrence and progression of AD. Aβ plaques and NFTs elevation are associated with increased levels of oxidation products from proteins, lipids, and nucleic acids in the hippocampus and cortex [61]. In this context, OL supplementation was found to reduce DNA damage and protein carbonyls in human PBMCs [62,63,64]. At the brain level, several in vivo studies demonstrated that OL consumption reduced DNA fragmentation, protein carbonyls, lipid peroxidation, and peroxynitrite levels in different murine models of neurodegenerative diseases [58,59,65,66,67,68] or aging [69,70,71]. However, OL supplementation did not alter the urinary markers of oxidative status of healthy young adults, indicating a possible protective role of OL only in redox-homeostasis-impaired conditions such as aging or AD [72]. The antioxidant effects of OL may be explained by the capacity to reduce ROS or NOS content, which leads to the reduction in oxidizing molecules such as DNA, protein, and lipids from different tissues. In this context, some authors have demonstrated the role of OL as a ROS scavenger in vitro and in vivo. Among them, De Cicco et al. (2020) observed that the preincubation with OL was able to reduce the ROS content increase induced by sodium palmitate, a free radical generator, in RAW 264.7 cells [73]. More recently, Romero-Marquez et al. (2022) demonstrated that N2 Wild-type C. elegans strain supplemented with OL presented lower ROS content after an acute exposition to the prooxidant 2,2′-Azobis (2-methylpropionamidine) dihydrochloride [35]. Additionally, OL supplementation has demonstrated protective effects at the neuron level. In this context, a combination of OL with Hibiscus sabdariffa leaves (13:2, w/w) was able to reduce ROS content in human SH-SY5Y neuroblastoma cells damaged by H2O2 [74].
Beyond its free radical scavenger activity, OL supplementation has shown a modulatory activity over some inducible enzymes related to antioxidant response element (ARE). According to the literature, plasma glutathione levels and antioxidant enzyme activity, such as that of glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD), which contribute to the progression of the disease, significantly decreased in early AD [75]. Among the neurodegenerative-like murine model studies, OL supplementation has been found to increase the brain activity of numerous AREs, such as SOD [65,66,67,68,69], CAT [66,67,68,69], GSH-Px [66,69], and glutathione S-transferase (GST) [58], as well as increase glutathione [67,76] brain levels. Notably, the common factor of these AREs is that they are regulated, totally or partially, by the nuclear factor erythroid 2-related factor 2 (Nrf2) [77]. Nrf2 is a transcription factor involved in the protection against OS. Under OS conditions, Nrf2 translocates to the nucleus and promotes the genetic expression of a significant number of AREs [78]. Clinically, the hippocampus from AD patients presents less nuclear Nrf2 compared to healthy controls, although OS markers are higher [79]. This feature indicates that AREs cannot be activated as Nrf2 does not translocate from the cytoplasm into the nucleus in hippocampal neurons in patients with AD [80]. Therefore, Nrf2 activation has been proposed as a novel target in the treatment of AD. Interestingly, some treatments, such as methylene blue, have demonstrated that the reduction in tauopathy, OS, and locomotive and memory impairment induced by NFT formation was mediated by Nrf2 activation in a mouse model of tauopathy [81]. In this context, some authors also described the capacity of OL to modulate Nrf2 in vitro [73] and in vivo [35,82]. Nonetheless, only one work researched the role of OL-induced Nrf2 nuclear translocation to fight AD in vivo. In this context, the authors focused on the role of OL in two different experimental C. elegans AD models. As mentioned in the previous sections, the authors demonstrated that OL treatment was able to reduce the cytotoxic effect of Aβ through the reduction in Aβ plaque aggregation. In the same way, the authors described a reduction in the neurotoxic effect caused by Tau aggregation in OL-treated animals. The authors partially described the mechanism of action of OL using RNAi technology, indicating a key role of SKN-1, a C. elegans ortholog of the human Nrf2, in the progression of Aβ and Tau protein cytotoxicity in C. elegans [35]. Therefore, the increase in Nrf2 translocation, and the subsequent activation of ARE, might be a possible mechanism of action underlying the protective anti-AD effect by olive leaf supplementation.
5.6. Autophagy and Proteostasis Modulation
Scientific evidence indicates that some AD hallmarks such as senile plaque formation are closely related to an alteration in the autophagic pathway and the incapacity to eliminate Aβ1–42 aggregates [83]. Indeed, alterations in autophagic-lysosomal degradation of proteins has been associated with AD, which were correlated with AD progression in both animal models and humans [83]. Recently, it has been demonstrated that the expression of MAPK/p38α protein is upregulated in the brain of APP-PS1 transgenic AD mouse, whereas the knockdown of MAPK in the APP-PS1 mouse stimulates macroautophagy/autophagy, reducing amyloid pathology by increasing autophagic-lysosomal degradation of BACE1 [84]. In the same way, high levels of phosphorylated AKT were associated clinically with the progression of NFT aggregation in AD patients [85]. These features seem to be consistent in a neurodegenerative rodent model induced by Pb. The exposure to Pb induced MAPK/p38 and AKT phosphorylation in the hippocampus of rats. Interestingly, OL supplementation was able to reduce both autophagy markers, which were associated with a reduction in Pb-induced neurotoxicity and an improvement of behavioral and locomotive tests [58].
Although there is limited data available about the modulatory effect of OL on autophagy markers during AD, Leri et al. (2021) investigated the role of an equal mix of OLE aglycone and HT in a cellular model of AD [86]. In this context, the Aβ1−42 oligomers’ exposure to human SH-SY5Y cells increased Beclin1, p62, and S6 expression, as well as the LC3II/I ratio. It seems that Aβ1–42 promotes the expression and activation of autophagy regulation markers, indicating an accumulation of autophagosomes with disrupted degradative activity [87]. In addition, p62 is remarkably involved in AD due to autophagic degradation through the binding of the autophagy marker LC3 [88]. With this background, HT-mix-treated cells presented a significant time-dependent reduction in p62 levels, which was reflected in lower Aβ1–42 oligomers on the surface, suggesting that these aggregates were digested by autophagolysosomes. In the same way, the phosphorylation level of the ribosomal protein S6, a key downstream substrate of TOR, was reduced in cells treated with the HT mix, indicating an involvement of the AMPK pathway in autophagy activation mediated by olive leaf phenols [86].
Similar to autophagy modulation, the role of proteostasis network modulation has been proposed as an intervention for AD management. There is extremely limited information about this topic. As far as it is known, the only work that evaluated the direct role of OL on the proteostasis network component during AD was evaluated in C. elegans. In this research, OL treatment was able to reduce both Aβ and Tau-protein-induced cytotoxicity, whereas an overexpression of HSP-16.2 was reported in a GFP-reporter strain. HSP-16.2 is an important element of protein homeostasis, which involves highly conserved stress responses that prevent protein mismanagement. In C. elegans, HSP-16.2 encodes HSP-16, which directly interacts with Aβ peptide and interferes with oligomerization pathways, leading to reduced formation of toxic species. To confirm the role of this protein in the protective effect shown by OL against AD, the authors used RNAi technology to knock down HSP-16.2 in two different AD-like strains of C. elegans. Interestingly, the authors demonstrated that the protective effect of OL to fight both Aβ and Tau-protein-induced cytotoxicity was mediated by HSP-16.2 overexpression. These results were confirmed by Thioflavin-T staining, which showed lower Aβ accumulation on OL-treated worms, probably due to an increase in Aβ clearance mediated by HSP-16.2 [35]. In conclusion, knowledge about the role of olive leaves in combating AD via autophagy/proteostasis modulation, in order to use it as AD prevention or therapy, is far from being complete. Nonetheless, the limited research available seems to indicate that the protective role of OL might be mediated by an enhancement of autophagy and proteostasis, although more research is needed.
Table 3.
Mechanisms of action of OL in both in vitro and in vivo models.
| Model | Intervention | Effects | Ref. |
|---|---|---|---|
| Anti-Aβ aggregation | |||
| SH-SY5Y cells | Cells exposed to Aβ42 and treated with OL or QU (≥200 µM) | ↓ Aβ42 fibrils aggregation | [40] |
| Cells exposed to Aβ42 and treated with olive phytochemicals: OLE (IC50: 22.9 µM), HT (IC50: 30.4 µg/mL), OL (IC50: 45 µg/mL), OFE (IC50: 95.9 µg/mL), VB (IC50: 22.6 µM), LU (IC50: 36.9 µM) or QU (IC50: 45.9 µM) | ↓ Aβ42 fibrils formation | ||
| SH-SY5Y cells | Cells exposed to Aβ1–42 and treated with OL (40 µg/mL) | ↑ Cells viability | [32] |
| SH-SY5Y cells | Cells pretreated with Olive phytochemicals: OLE, HT, VB, CA, LU, QU, RU or OFE (0–1000 µM) and injured with Aβ42, Aβ42 and Cu or Aβ42 and L-DOPA | ↑ Cells viability | [40] |
| C. elegans | Animals exposed to OL (100 µg/mL) for 72 h | ↓ Aβ-induced toxicity ↓ Aβ deposits |
[35] |
| C. elegans | Animals exposed to OFE (100 µg/mL) for 72 h | ↓ Aβ-induced toxicity ↓ Aβ deposits |
[43] |
| 5xFAD mice | Animals orally supplemented with OL (695 µg/kg/d) for 3 months | ↓ Total Aβ deposits ↓ Soluble Aβ40 levels ↑ Expression of P-gp and LRP1 ↑ sAPPα and α-secretase expression ↓ sAPPβ expression ↑ Memory function |
[44] |
| APPswe/PS1dE9 mice | Animals orally supplemented with OL (50 mg/kg/d) for 16 weeks | ↓ Aβ plaques total number ↓ Aβ plaques size |
[40] |
| New Zealand white rabbits | Animals spinally damaged and orally supplemented with OL (350 mg/kg/d) for 1 week after 14 days of spinal damage | ↓ Aβ presence | [45] |
| Animals spinally damaged and orally supplemented with OL (350 mg/kg/d) for 3 week after 7 days of spinal damage | ↓ Aβ presence | ||
| Anti-tau aggregation | |||
| C. elegans | Animals exposed to OL (100 µg/mL) for 72 h | ↑ Swimming speed and wavelength ↓ Stretching effort |
[35] |
| New Zealand white rabbits | Animals spinally damaged and orally supplemented with OL (350 mg/kg/d) for 1 week after 14 days of spinal damage | ↓ p-Tau presence | [45] |
| Animals spinally damaged and orally supplemented with OL (350 mg/kg/d) for 3 week after 7 days of spinal damage | ↓ p-Tau presence | ||
| Enzyme inhibitory activity | |||
| BACE-1, AChE, BChE, histone and tyrosinase inhibitor screening assay kits | Isolated olive phytochemicals: OFE (IC50: 18 ng), OLE (IC50: 2.7 µM) and HT (IC50: 0.26 µM) | ↓ BACE-1 activity | [42] |
| Isolated olive phytochemicals: OLE (IC50: 101.7 µM), and HT (IC50: 37.6 µM) | ↓ AChE activity | ||
| Isolated olive phytochemicals: OLE (IC50: 84.8 µM) and HT (IC50: 30.6 µM) | ↓ BChE activity | ||
| Isolated olive phytochemicals: HT (IC50: 66.22 µg), OLE (IC50: 1085 µM) | ↓ histone deacetylase activity | ||
| Isolated olive phytochemicals: QU (IC50: 10.73 µM) | ↓ Tyrosinase | ||
| Microtitre assays | OL (500 µg/mL) | ↓ AChE and BChE activity | [49] |
| Colorimetric assays | OL extracts from Cornicabra variety obtained with silicas (IC50: 300.80 to 805.47 µg/mL), aluminum oxide (IC50: 449.50 to 549.49 µg/mL), zeolites (IC50: 391.84 to 418.40 µg/mL) or adsorbates (IC50: 144.43 to 447.64 µg/mL) | ↓ AChE activity | [50] |
| OL extracts from Cornicabra variety obtained with sea sand adsorbate (IC50: 183.82 µg/mL) | ↓ BChE activity | ||
| Anti-inflammation | |||
| Colorimetric assays | OL extracts from Cornicabra variety obtained with silicas (IC50: 83.53 to 548.87 µg/mL), aluminum oxide (IC50: 75.20 to 319.76 µg/mL), zeolites (IC50: 83.27 to 192.65 µg/mL) or adsorbates (IC50: 84.29 to 139.82 µg/mL) | ↓ LOX activity | [50] |
| N1 murine microglia cells | Cells BSA-AGE injured and treated with green OL (EC50: 65 mg/mL) | ↓ NO production | [33] |
| THP-1 cells | Cells injured with LPS and treated with OL (20 or 40 µg/mL) | ↓ IL-1β, IL-6 secretion ↓ TNF-α secretion (only 40 µg/mL) |
[32] |
| RAW 264.7 cells | Cells injured with LPS for 24 h (acute exposure) or 72 h (chronic exposure) and treated with OL (50 µM) | ↓ NO, iNOS and COX-2 levels ↓ IL-1β and IL-6 mRNA expression ↓ IL-1βR protein expression and TGF-β mRNA expression (only in acute exposure) |
[57] |
| 5xFAD mice | Animals orally supplemented with OL (695 µg/kg/d) for 3 months | ↓ Astrocyte and microglial activation ↓ GFAP levels ↓ Astrocyte shape alterations ↓ IL-1β levels ↓ NLRP3 levels ↓ Pro-caspase 1 and 8 levels ↓ RAGE and HMGB1 levels ↓ p-IKKβ and p-IκBα levels ↑ Total IκBα levels |
[44] |
| Wistar rats | Animals neurologically damaged with Pb and orally supplemented with OL (0.1%) for 2 weeks | ↓ TNF-α, IL-1β and PGE2 levels | [58] |
| Wistar rats | Animals neurologically damaged with kainic acid and orally supplemented with OL (300 mg/kg/d) for 4 weeks | =TNF-α | [59] |
| New Zealand white rabbits | Animals spinally damaged and orally supplemented with OL (350 mg/kg/d) for 1 week after 14 days of spinal damage | ↓ CD68 levels | [45] |
| Animals spinally damaged and orally supplemented with OL (350 mg/kg/d) for 3 week after 7 days of spinal damage | ↓ CD68 levels | ||
| Male human patients | Young people orally supplemented with 20 mL of liquid OL daily for 8 weeks | ↓ COX-2, IL-8, JUNB, HBEGF gene expression in PBMCs | [60] |
| Oxidative stress modulation | |||
| SH-SY5Y cells | Cells pretreated with OL (500 µg/mL) and exposed to H2O2 or Cu | ↑ Cell viability | [89] |
| Colorimetric assays | OL extracts from Cornicabra variety obtained with Silicas (IC50: 46.43 to 1789.61 µg/mL), aluminum oxide (IC50: 39.99 to 191.74 µg/mL), zeolites (IC50: 128.49 to 153.55 µg/mL), and adsorbates (IC50: 23.65 to 148.22 µg/mL) |
↓ ABTS | [50] |
| OL extracts from Cornicabra variety obtained with sea sand adsorbate (IC50: 18.27 µg/mL) | ↓ ROS | ||
| OL extracts from Cornicabra variety obtained with sea sand adsorbate (IC50: 1036.86 µg/mL) | ↓ RNS | ||
| SH-SY5Y cells | Cells exposed to a mix (13:2) of OL and Hibiscus sabdariffa L. Flowers (50, 100, 250, 500, 1000 µg/mL) | ↓ ROS content | [90] |
| SH-SY5Y cells | Cells exposed to H2O2 and treated with a mix (13:2) of OL and Hibiscus sabdariffa L. Flowers (0–50 µg/mL) | ↓ ROS content | [74] |
| SH-SY5Y cells | Cells exposed to Aβ1–42 and treated with a mix (75 µM) based on OLE and HT | ↓ ROS content | [86] |
| C. elegans | Animals treated with OL (100 µg/mL) for 72 h and exposed to AAPH | ↓ ROS content |
[35] |
| Wistar rats | Aged animals orally supplemented with OL (50 mg/kg/d) for 6 months | ↑ Midbrain SOD, GPX and CAT activities ↓ Midbrain MDA levels |
[69] |
| Wistar rats | Animals neurologically damaged with Pb and orally supplemented with OL (0.1%) for 2 weeks | ↓ Frontal cortex DNA fragmentation ↑ GST activity |
[58] |
| Wistar rats | Aged animals orally supplemented with OL (500 and 1000 mg/kg/d) in drinking water for 2 months | ↓ Brain MDA, diene conjugate and protein carbonyl (highest dose) | [70] |
| Mongolian gerbils | Animals pretreated with OL (100 mg/kg/d) and subjected to global cerebral ischemia | ↓ Superoxide and nitric oxide production ↓ Lipid peroxidation ↑ SOD activity |
[65] |
| Wistar rats | Animals pretreated with OL (75, 150 and 300 mg/kg/d) for 28 days and neurologically damaged with rotenone | ↓ Midbrain MDA levels ↑ Midbrain SOD, CAT, and GPx levels |
[66] |
| Wistar rats | Animals neurologically damaged with kainic acid and orally supplemented with OL (300 mg/kg) for 4 weeks | ↓ The seizure score ↓ Neuron MDA, nitrite, and nitrate levels ↑ GSH levels |
[59] |
| Autophagy and proteostasis modulation | |||
| SH-SY5Y cells | Cells exposed to Aβ1–42 and treated with a mix (75 µM) based on OLE and HT | ↑ Autophagic flux ↑ p62 levels |
[86] |
| C. elegans | Animals exposed to OL (100 µg/mL) for 72 h | ↑ HSP-16.2::GFP expression | [35] |
Aβ: amyloid beta; AChE: acetylcholine esterase; AGE: advanced glycated end products; BACE-1: prime Aβ producing enzyme β-secretase; BChE: butyrylcholine esterase; BSA: bovine serum albumin; C. elegans: Caenorhabditis elegans; CD68: cluster of differentiation 68; COX-2: cyclooxygenase-2; EC50: half maximal effective concentration; GFAP: glial fibrillary acidic protein; HBEGF: Heparin binding EGF-like growth factor; IC50: half maximal inhibitory concentration; IL: interleukin; HT: hydroxytyrosol; L-DOPA: levodopa; LPS: lipopolysaccharide; LOX: lipoxydase; NO: nitric oxide; OL: olive leaves; OFE: olive fruit extracts; OLE: oleuropein; QU: quercetin; RU: rutin; CA: caffeic acid; LU: luteolin; VB: verbascoside; PBMCs: peripheral blood mononuclear cells; PGE2: prostaglandin E2; RAGE: receptor for advanced glycated end products; TGF-β: transforming growth factor β; TNF-α: tumor necrosis factor α; SOD: superoxide dismutase; CAT: catalase; MDA: Malondialdehyde; GPX: glutathione peroxidase; GST: Glutathione-S transferase; MAPK: p38 mitogen activated protein kinase; TRAP: total radical trapping antioxidant parameter; ROS: reactive oxygen species; RNS: reactive nitrogen species. The symbols ↑ and ↓ mean an increase or decrease of the specific marker, respectively.
6. Trends, Perspectives, and Conclusions
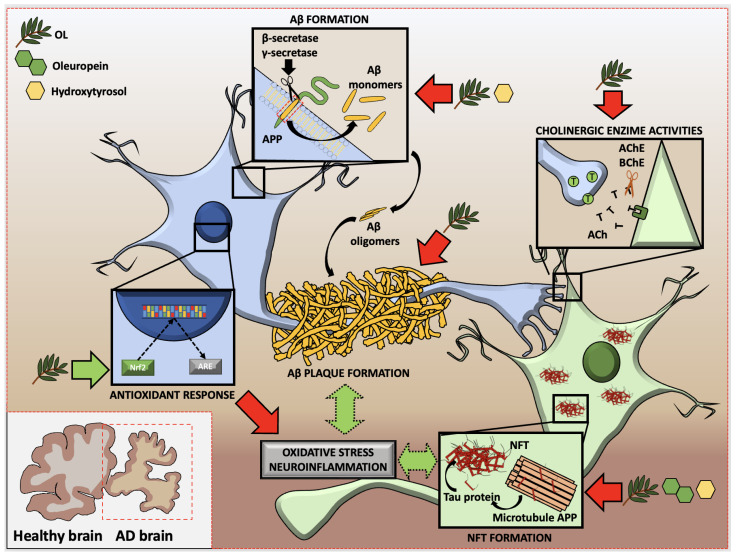
Despite all the efforts, AD continues to remain a challenge, with no effective treatment to combat it, increasing the dependency and, subsequently, the death, of patients. Natural products such as olive leaves and their compounds contribute to the discovery of new anti-AD interventions to combat AD progression. The effectiveness of OL, and of the molecules present in this olive tree by-product, in reducing or even preventing the different processes related to AD, including Aβ and Tau protein production, Aβ fibrogenesis and NFT deposition, inhibition of AD-related enzymes, and oxidative stress and neuroinflammation, have been described (Figure 2). In particular, OL, OLE, and HT reduced both amyloid-β formation and neurofibrillary tangles formation through amyloid protein precursor processing modulation. Although the isolated olive phytochemicals exerted lower cholinesterase inhibitory activity, OL demonstrated high inhibitory activity in the cholinergic tests evaluated. The mechanisms underlying these protective effects might be associated with decreased neuroinflammation and oxidative stress via NF-κB and Nrf2 modulation, respectively.
Figure 2.
Modulatory effect of olive phytochemicals on Alzheimer’s Disease physiopathology. Green arrows represent promotion of the particular process. Red arrows represent reduction in the particular process. Green discontinued arrows represent indirect promotion of the particular process. AChE: acetylcholinesterase; AD: Alzheimer’s disease; APP: amyloid precursor protein; Aβ: amyloid beta; BChE: butyrylcholinesterase; NFT: neurofibrillary tangles; OL: olive leaf.
Furthermore, memory impairment is associated with early AD stage. However, with AD progression, patients tend to develop symptoms of cognitive and behavioral alterations, such as depression, anxiety, disorientation, and paranoia, which affect daily living activities [91]. Some authors have explored the potential therapeutic effect of OL consumption to combat the deleterious effect of neurodegenerative disease induced by chemicals on memory and cognitive function. OL consumption was able to restore the locomotive impairment caused by Pb-induced neurological damage in rats subjected to an open field test [58]. Similarly, in a rat model of Parkinson’s disease induced by rotenone, OL consumption was able to partially restore balance, motor coordination, and muscle strength in a dose-dependent manner [66]. The limited research available that addresses the therapeutic effect of OL in AD-like symptoms failed to find a significant test to measure the behavioral alterations related to AD progression. In this context, Omar et al. (2019) tried to evaluate some behavioral analysis related to anxiety, locomotive function, and orientation in APPswe/PS1dE9 and Wild type mice supplemented or not with OL. As mentioned previously, APPswe/PS1dE9 mice overproduce human Aβ, causing learning and memory impairment. After 5 months of housing, the applied tests failed to show impairments in the studied behavioral parameters in AD or Wild type mice, although Aβ plaque senile deposits were found in AD mice. These results indicate that the test, the age of the mice, or the AD phenotype used were not appropriate for the parameters studied. As mentioned above, behavioral parameters such as anxiety, orientation, and locomotive function are linked to late stages of AD, and it is necessary to produce a relatable late AD stage model with measurable results. In contrast to behavioral tests, Abdallah et al. (2022) found an improvement in memory function using the Morris water maze in OL-treated AD-like 5xFAD mice. Despite the promising results obtained in the Morris water maze test, the authors also failed to measure locomotive impairment related to AD, finding no differences between Wild type and AD mice, treated or not [40]. Although these results suggest that memory impairment related to AD progression might be restored by OL supplementation, more research is necessary to find a correct test to analyze AD behavioral impairment.
Summarizing, despite the fact that direct evidence is still limited, many investigations corroborate the potential use of OL as an adjuvant in AD treatment. Olive leaves have been proven to reduce the toxicity of protein aggregation through the reduction in Aβ formation and aggregation, as well as the reduction in Tau fibrogenesis and deposition. In the same way, the possible mechanism of action underlying the protective effect might be attributed to oxidative stress and neuroinflammation modulation, as well as an increase in toxic protein clearance through the modulation of autophagy and the proteostasis network. Nonetheless, most of the reviewed evidence comes from in vitro studies, indicating that more preclinical and clinical research is needed for a deeper understanding of the molecular mechanisms associated with the observed effects.
Acknowledgments
María D. Navarro-Hortal and José M. Romero-Márquez are FPU fellows with grant reference FPU2017/04358 and FPU2018/05301, respectively, funded by MCIN/AEI/10.13039/501100011033 and FSE “El FSE invierte en tu futuro”. Tamara Forbes-Hernández is supported by a JdC-I post-doctoral contract with grant reference IJC2020-043910-I, funded by NextGenerationEU. Authors are indebted with Monica Glebocki for extensive editing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work has been partially supported by the grants PID2019-106778RB-I00, funded by MCIN/AEI/10.13039/501100011033 FEDER “Una manera de hacer Europa”, and SUSTAINOLIVE (PRIMA H2020-1811).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hill E., Goodwill A.M., Gorelik A., Szoeke C. Diet and Biomarkers of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neurobiol. Aging. 2019;76:45–52. doi: 10.1016/j.neurobiolaging.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X.-X., Tian Y., Wang Z.-T., Ma Y.-H., Tan L., Yu J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021;8:313–321. doi: 10.14283/jpad.2021.15. [DOI] [PubMed] [Google Scholar]
- 3.Erkkinen M.G., Kim M.-O., Geschwind M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018;10:a033118. doi: 10.1101/cshperspect.a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu L.W. Alzheimer’s Disease: Early Diagnosis and Treatment. Hong Kong Med. J. 2012;18:228–237. [PubMed] [Google Scholar]
- 5.Scheltens P., Strooper B.D., Kivipelto M., Holstege H., Chételat G., Teunissen C.E., Cummings J., van der Flier W.M. Alzheimer’s Disease. Lancet. 2021;397:1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrattan A.M., McGuinness B., McKinley M.C., Kee F., Passmore P., Woodside J.V., McEvoy C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019;8:53–65. doi: 10.1007/s13668-019-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendiola-Precoma J., Berumen L.C., Padilla K., Garcia-Alcocer G. Therapies for Prevention and Treatment of Alzheimer’s Disease. Biomed. Res. Int. 2016;2016:2589276. doi: 10.1155/2016/2589276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caruso G., Torrisi S.A., Mogavero M.P., Currenti W., Castellano S., Godos J., Ferri R., Galvano F., Leggio G.M., Grosso G., et al. Polyphenols and Neuroprotection: Therapeutic Implications for Cognitive Decline. Pharmacol. Ther. 2022;232:108013. doi: 10.1016/j.pharmthera.2021.108013. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia N.K., Srivastava A., Katyal N., Jain N., Khan M.A.I., Kundu B., Deep S. Curcumin Binds to the Pre-Fibrillar Aggregates of Cu/Zn Superoxide Dismutase (SOD1) and Alters Its Amyloidogenic Pathway Resulting in Reduced Cytotoxicity. Biochim. Biophys. Acta. 2015;1854:426–436. doi: 10.1016/j.bbapap.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava A., Arya P., Goel S., Kundu B., Mishra P., Fnu A. Gelsolin Amyloidogenesis Is Effectively Modulated by Curcumin and Emetine Conjugated PLGA Nanoparticles. PLoS ONE. 2015;10:e0127011. doi: 10.1371/journal.pone.0127011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arya P., Srivastava A., Vasaikar S.V., Mukherjee G., Mishra P., Kundu B. Selective Interception of Gelsolin Amyloidogenic Stretch Results in Conformationally Distinct Aggregates with Reduced Toxicity. ACS Chem. Neurosci. 2014;5:982–992. doi: 10.1021/cn500002v. [DOI] [PubMed] [Google Scholar]
- 12.Admane N., Srivastava A., Jamal S., Sharma R., Kundu B., Grover A. Molecular Insights into the Critical Role of Gallate Moiety of Green Tea Catechins in Modulating Prion Fibrillation, Cellular Internalization, and Neuronal Toxicity. Int. J. Biol. Macromol. 2022;223:755–765. doi: 10.1016/j.ijbiomac.2022.11.049. [DOI] [PubMed] [Google Scholar]
- 13.Manzanares P., Ruiz E., Ballesteros M., Negro M.J., Gallego F.J., López-Linares J.C., Castro E. Residual Biomass Potential in Olive Tree Cultivation and Olive Oil Industry in Spain: Valorization Proposal in a Biorefinery Context. Span. J. Agric. Res. 2017;15:e0206. doi: 10.5424/sjar/2017153-10868. [DOI] [Google Scholar]
- 14.Papageorgiou C.S., Lymberopoulos S., Bakas P., Zagklis D.P., Sygouni V., Paraskeva C.A. Hydroxytyrosol Enrichment of Olive Leaf Extracts via Membrane Separation Processes. Membranes. 2022;12:1027. doi: 10.3390/membranes12111027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selim S., Albqmi M., Al-Sanea M.M., Alnusaire T.S., Almuhayawi M.S., AbdElgawad H., Al Jaouni S.K., Elkelish A., Hussein S., Warrad M., et al. Valorizing the Usage of Olive Leaves, Bioactive Compounds, Biological Activities, and Food Applications: A Comprehensive Review. Front. Nutr. 2022;9:1008349. doi: 10.3389/fnut.2022.1008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabbash E.M., Ayoub I.M., Gad H.A., Abdel-Shakour Z.T., El-Ahmady S.H. Quality Assessment of Leaf Extracts of 12 Olive Cultivars and Impact of Seasonal Variation Based on UV Spectroscopy and Phytochemcial Content Using Multivariate Analyses. Phytochem. Anal. 2021;32:932–941. doi: 10.1002/pca.3036. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C., Zhang J., Xin X., Zhu S., Niu E., Wu Q., Li T., Liu D. Changes in Phytochemical Profiles and Biological Activity of Olive Leaves Treated by Two Drying Methods. Front. Nutr. 2022;9:854680. doi: 10.3389/fnut.2022.854680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolì F., Negro C., Vergine M., Aprile A., Nutricati E., Sabella E., Miceli A., Luvisi A., De Bellis L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules. 2019;24:1998. doi: 10.3390/molecules24101998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasković I., Lukić I., Žurga P., Majetić Germek V., Brkljača M., Koprivnjak O., Major N., Grozić K., Franić M., Ban D., et al. Temporal Variation of Phenolic and Mineral Composition in Olive Leaves Is Cultivar Dependent. Plants. 2020;9:1099. doi: 10.3390/plants9091099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makowska-Wąs J., Galanty A., Gdula-Argasińska J., Tyszka-Czochara M., Szewczyk A., Nunes R., Carvalho I.S., Michalik M., Paśko P. Identification of Predominant Phytochemical Compounds and Cytotoxic Activity of Wild Olive Leaves (Olea europaea L. Ssp. Sylvestris) Harvested in South Portugal. Chem. Biodivers. 2017;14:e1600331. doi: 10.1002/cbdv.201600331. [DOI] [PubMed] [Google Scholar]
- 21.Mmopele K., Combrinck S., Hamman J., Willers C., Chen W., Viljoen A. Potential Herb-Drug Pharmacokinetic Interactions between African Wild Olive Leaf Extract and Selected Antihypertensive Drugs. Planta Med. 2018;84:886–894. doi: 10.1055/a-0583-0543. [DOI] [PubMed] [Google Scholar]
- 22.Yu M., Gouvinhas I., Rocha J., Barros A.I.R.N.A. Phytochemical and Antioxidant Analysis of Medicinal and Food Plants towards Bioactive Food and Pharmaceutical Resources. Sci. Rep. 2021;11:10041. doi: 10.1038/s41598-021-89437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kontogianni V.G., Charisiadis P., Margianni E., Lamari F.N., Gerothanassis I.P., Tzakos A.G. Olive Leaf Extracts Are a Natural Source of Advanced Glycation End Product Inhibitors. J. Med. Food. 2013;16:817–822. doi: 10.1089/jmf.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarikurkcu C., Locatelli M., Tartaglia A., Ferrone V., Juszczak A.M., Ozer M.S., Tepe B., Tomczyk M. Enzyme and Biological Activities of the Water Extracts from the Plants Aesculus Hippocastanum, Olea europaea and Hypericum Perforatum That Are Used as Folk Remedies in Turkey. Molecules. 2020;25:1202. doi: 10.3390/molecules25051202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martín-Vertedor D., Garrido M., Pariente J.A., Espino J., Delgado-Adámez J. Bioavailability of Bioactive Molecules from Olive Leaf Extracts and Its Functional Value. Phytother. Res. 2016;30:1172–1179. doi: 10.1002/ptr.5625. [DOI] [PubMed] [Google Scholar]
- 26.González E., Gómez-Caravaca A.M., Giménez B., Cebrián R., Maqueda M., Martínez-Férez A., Segura-Carretero A., Robert P. Evolution of the Phenolic Compounds Profile of Olive Leaf Extract Encapsulated by Spray-Drying during in Vitro Gastrointestinal Digestion. Food Chem. 2019;279:40–48. doi: 10.1016/j.foodchem.2018.11.127. [DOI] [PubMed] [Google Scholar]
- 27.de Bock M., Thorstensen E.B., Derraik J.G.B., Henderson H.V., Hofman P.L., Cutfield W.S. Human Absorption and Metabolism of Oleuropein and Hydroxytyrosol Ingested as Olive (Olea europaea L.) Leaf Extract. Mol. Nutr. Food Res. 2013;57:2079–2085. doi: 10.1002/mnfr.201200795. [DOI] [PubMed] [Google Scholar]
- 28.Lockyer S., Corona G., Yaqoob P., Spencer J.P.E., Rowland I. Secoiridoids Delivered as Olive Leaf Extract Induce Acute Improvements in Human Vascular Function and Reduction of an Inflammatory Cytokine: A Randomised, Double-Blind, Placebo-Controlled, Cross-over Trial. Br. J. Nutr. 2015;114:75–83. doi: 10.1017/S0007114515001269. [DOI] [PubMed] [Google Scholar]
- 29.García-Villalba R., Larrosa M., Possemiers S., Tomás-Barberán F.A., Espín J.C. Bioavailability of Phenolics from an Oleuropein-Rich Olive (Olea europaea) Leaf Extract and Its Acute Effect on Plasma Antioxidant Status: Comparison between Pre- and Postmenopausal Women. Eur. J. Nutr. 2014;53:1015–1027. doi: 10.1007/s00394-013-0604-9. [DOI] [PubMed] [Google Scholar]
- 30.Hashmi M.A., Khan A., Hanif M., Farooq U., Perveen S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive) Evid. Based Complement. Alternat. Med. 2015;2015:541591. doi: 10.1155/2015/541591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clewell A.E., Béres E., Vértesi A., Glávits R., Hirka G., Endres J.R., Murbach T.S., Szakonyiné I.P. A Comprehensive Toxicological Safety Assessment of an Extract of Olea europaea L. Leaves (BonoliveTM) Int. J. Toxicol. 2016;35:208–221. doi: 10.1177/1091581815619764. [DOI] [PubMed] [Google Scholar]
- 32.Gallego R., Suárez-Montenegro Z.J., Ibáñez E., Herrero M., Valdés A., Cifuentes A. In Vitro Neuroprotective Potential and Lipidomics Study of Olive Leaves Extracts Enriched in Triterpenoids. Front. Nutr. 2021;8:769218. doi: 10.3389/fnut.2021.769218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandler D., Woldu A., Rahmadi A., Shanmugam K., Steiner N., Wright E., Benavente-García O., Schulz O., Castillo J., Münch G. Effects of Plant-Derived Polyphenols on TNF-Alpha and Nitric Oxide Production Induced by Advanced Glycation Endproducts. Mol. Nutr. Food Res. 2010;54:S141–S150. doi: 10.1002/mnfr.200900504. [DOI] [PubMed] [Google Scholar]
- 34.Misganaw D., Engidawork E., Nedi T. Evaluation of the Anti-Malarial Activity of Crude Extract and Solvent Fractions of the Leaves of Olea europaea (Oleaceae) in Mice. BMC Complement. Altern. Med. 2019;19:171. doi: 10.1186/s12906-019-2567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero-Márquez J.M., Navarro-Hortal M.D., Jiménez-Trigo V., Vera-Ramírez L., Forbes-Hernández T.J., Esteban-Muñoz A., Giampieri F., Bullón P., Battino M., Sánchez-González C., et al. An Oleuropein Rich-Olive (Olea europaea L.) Leaf Extract Reduces β-Amyloid and Tau Proteotoxicity through Regulation of Oxidative- and Heat Shock-Stress Responses in Caenorhabditis Elegans. Food Chem. Toxicol. 2022;162:112914. doi: 10.1016/j.fct.2022.112914. [DOI] [PubMed] [Google Scholar]
- 36.Anter J., Fernández-Bedmar Z., Villatoro-Pulido M., Demyda-Peyras S., Moreno-Millán M., Alonso-Moraga A., Muñoz-Serrano A., Luque de Castro M.D. A Pilot Study on the DNA-Protective, Cytotoxic, and Apoptosis-Inducing Properties of Olive-Leaf Extracts. Mutat. Res. 2011;723:165–170. doi: 10.1016/j.mrgentox.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Guex C.G., Reginato F.Z., Figueredo K.C., da da Silva A.R.H., Pires F.B., da Silva Jesus R., Lhamas C.L., Lopes G.H.H., de Freitas Bauermann L. Safety Assessment of Ethanolic Extract of Olea europaea L. Leaves after Acute and Subacute Administration to Wistar Rats. Regul. Toxicol. Pharmacol. 2018;95:395–399. doi: 10.1016/j.yrtph.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Filip R., Possemiers S., Heyerick A., Pinheiro I., Raszewski G., Davicco M.-J., Coxam V. Twelve-Month Consumption of a Polyphenol Extract from Olive (Olea europaea) in a Double Blind, Randomized Trial Increases Serum Total Osteocalcin Levels and Improves Serum Lipid Profiles in Postmenopausal Women with Osteopenia. J. Nutr. Health Aging. 2015;19:77–86. doi: 10.1007/s12603-014-0480-x. [DOI] [PubMed] [Google Scholar]
- 39.Susalit E., Agus N., Effendi I., Tjandrawinata R.R., Nofiarny D., Perrinjaquet-Moccetti T., Verbruggen M. Olive (Olea europaea) Leaf Extract Effective in Patients with Stage-1 Hypertension: Comparison with Captopril. Phytomedicine. 2011;18:251–258. doi: 10.1016/j.phymed.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Omar S.H., Scott C.J., Hamlin A.S., Obied H.K. Olive Biophenols Reduces Alzheimer’s Pathology in SH-SY5Y Cells and APPswe Mice. Int. J. Mol. Sci. 2018;20:125. doi: 10.3390/ijms20010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang J., Pitsillou E., Man A.Y.L., Madzima S., Bresnehan S.M., Nakai M.E., Hung A., Karagiannis T.C. Utilisation of the OliveNetTM Library to Investigate Phenolic Compounds Using Molecular Modelling Studies in the Context of Alzheimer’s Disease. Comput. Biol. Chem. 2020;87:107271. doi: 10.1016/j.compbiolchem.2020.107271. [DOI] [PubMed] [Google Scholar]
- 42.Omar S.H., Scott C.J., Hamlin A.S., Obied H.K. Biophenols: Enzymes (β-Secretase, Cholinesterases, Histone Deacetylase and Tyrosinase) Inhibitors from Olive (Olea europaea L.) Fitoterapia. 2018;128:118–129. doi: 10.1016/j.fitote.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Romero-Márquez J.M., Navarro-Hortal M.D., Jiménez-Trigo V., Muñoz-Ollero P., Forbes-Hernández T.Y., Esteban-Muñoz A., Giampieri F., Delgado Noya I., Bullón P., Vera-Ramírez L., et al. An Olive-Derived Extract 20% Rich in Hydroxytyrosol Prevents β-Amyloid Aggregation and Oxidative Stress, Two Features of Alzheimer Disease, via SKN-1/NRF2 and HSP-16.2 in Caenorhabditis Elegans. Antioxidants. 2022;11:629. doi: 10.3390/antiox11040629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdallah I.M., Al-Shami K.M., Yang E., Wang J., Guillaume C., Kaddoumi A. Oleuropein-Rich Olive Leaf Extract Attenuates Neuroinflammation in the Alzheimer’s Disease Mouse Model. ACS Chem. Neurosci. 2022;13:1002–1013. doi: 10.1021/acschemneuro.2c00005. [DOI] [PubMed] [Google Scholar]
- 45.Ibrahim S., Adeputra Nasution I.F., Danil M., Sadewo W., Widyawati T., Eyanoer P.C., Ritarwan K., Riawan W., Darmajaya R. Potential Benefit of Olive Leaf Extract in Cervical Spondylotic Myelopathy Model. Ann. Med. Surg. 2022;73:103040. doi: 10.1016/j.amsu.2021.103040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luccarini I., Ed Dami T., Grossi C., Rigacci S., Stefani M., Casamenti F. Oleuropein Aglycone Counteracts Aβ42 Toxicity in the Rat Brain. Neurosci. Lett. 2014;558:67–72. doi: 10.1016/j.neulet.2013.10.062. [DOI] [PubMed] [Google Scholar]
- 47.Brogi S., Sirous H., Calderone V., Chemi G. Amyloid β Fibril Disruption by Oleuropein Aglycone: Long-Time Molecular Dynamics Simulation to Gain Insight into the Mechanism of Action of This Polyphenol from Extra Virgin Olive Oil. Food Funct. 2020;11:8122–8132. doi: 10.1039/D0FO01511C. [DOI] [PubMed] [Google Scholar]
- 48.Daccache A., Lion C., Sibille N., Gerard M., Slomianny C., Lippens G., Cotelle P. Oleuropein and Derivatives from Olives as Tau Aggregation Inhibitors. Neurochem. Int. 2011;58:700–707. doi: 10.1016/j.neuint.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Senol F.S., Ankli A., Reich E., Orhan I.E. HPTLC Fingerprinting and Cholinesterase Inhibitory and Metal-Chelating Capacity of Various Citrus Cultivars and Olea europaea. Food Technol. Biotechnol. 2016;54:275–281. doi: 10.17113/ftb.54.03.16.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suárez Montenegro Z.J., Álvarez-Rivera G., Sánchez-Martínez J.D., Gallego R., Valdés A., Bueno M., Cifuentes A., Ibáñez E. Neuroprotective Effect of Terpenoids Recovered from Olive Oil By-Products. Foods. 2021;10:1507. doi: 10.3390/foods10071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarz S., Loesche A., Lucas S.D., Sommerwerk S., Serbian I., Siewert B., Pianowski E., Csuk R. Converting Maslinic Acid into an Effective Inhibitor of Acylcholinesterases. Eur. J. Med. Chem. 2015;103:438–445. doi: 10.1016/j.ejmech.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Ali M., Muhammad S., Shah M.R., Khan A., Rashid U., Farooq U., Ullah F., Sadiq A., Ayaz M., Ali M., et al. Neurologically Potent Molecules from Crataegus Oxyacantha; Isolation, Anticholinesterase Inhibition, and Molecular Docking. Front. Pharmacol. 2017;8:327. doi: 10.3389/fphar.2017.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mamadalieva N.Z., Youssef F.S., Hussain H., Zengin G., Mollica A., Al Musayeib N.M., Ashour M.L., Westermann B., Wessjohann L.A. Validation of the Antioxidant and Enzyme Inhibitory Potential of Selected Triterpenes Using In Vitro and In Silico Studies, and the Evaluation of Their ADMET Properties. Molecules. 2021;26:6331. doi: 10.3390/molecules26216331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szwajgier D., Baranowska-Wójcik E. Terpenes and Phenylpropanoids as Acetyl- and Butyrylcholinesterase Inhibitors: A Comparative Study. Curr. Alzheimer Res. 2019;16:963–973. doi: 10.2174/1567205016666191010105115. [DOI] [PubMed] [Google Scholar]
- 55.Yu J., Kwon H., Cho E., Jeon J., Kang R.H., Youn K., Jun M., Lee Y.C., Ryu J.H., Kim D.H. The Effects of Pinoresinol on Cholinergic Dysfunction-Induced Memory Impairments and Synaptic Plasticity in Mice. Food Chem. Toxicol. 2019;125:376–382. doi: 10.1016/j.fct.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 56.Gouvinhas I., Garcia J., Granato D., Barros A. Seed Phytochemical Profiling of Three Olive Cultivars, Antioxidant Capacity, Enzymatic Inhibition, and Effects on Human Neuroblastoma Cells (SH-SY5Y) Molecules. 2022;27:5057. doi: 10.3390/molecules27165057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruzzolini J., Chioccioli S., Monaco N., Peppicelli S., Andreucci E., Urciuoli S., Romani A., Luceri C., Tortora K., Calorini L., et al. Oleuropein-Rich Leaf Extract as a Broad Inhibitor of Tumour and Macrophage INOS in an Apc Mutant Rat Model. Antioxidants. 2021;10:1577. doi: 10.3390/antiox10101577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seddik L., Bah T.M., Aoues A., Slimani M., Benderdour M. Elucidation of Mechanisms Underlying the Protective Effects of Olive Leaf Extract against Lead-Induced Neurotoxicity in Wistar Rats. J. Toxicol. Sci. 2011;36:797–809. doi: 10.2131/jts.36.797. [DOI] [PubMed] [Google Scholar]
- 59.Khamse S., Haftcheshmeh S.M., Sadr S.S., Roghani M., Kamalinejad M., Moghaddam P.M., Golchoobian R., Ebrahimi F. The Potential Neuroprotective Roles of Olive Leaf Extract in an Epilepsy Rat Model Induced by Kainic Acid. Res. Pharm. Sci. 2021;16:48–57. doi: 10.4103/1735-5362.305188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boss A., Kao C.H.-J., Murray P.M., Marlow G., Barnett M.P.G., Ferguson L.R. Human Intervention Study to Assess the Effects of Supplementation with Olive Leaf Extract on Peripheral Blood Mononuclear Cell Gene Expression. Int. J. Mol. Sci. 2016;17:2019. doi: 10.3390/ijms17122019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butterfield D.A., Lauderback C.M. Lipid Peroxidation and Protein Oxidation in Alzheimer’s Disease Brain: Potential Causes and Consequences Involving Amyloid Beta-Peptide-Associated Free Radical Oxidative Stress. Free Radic. Biol. Med. 2002;32:1050–1060. doi: 10.1016/S0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 62.Topalović D., Dekanski D., Spremo-Potparević B., Pirković A., Borozan S., Bajić V., Stojanović D., Giampieri F., Gasparrini M., Živković L. Dry Olive Leaf Extract Attenuates DNA Damage Induced by Estradiol and Diethylstilbestrol in Human Peripheral Blood Cells in Vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019;845:402993. doi: 10.1016/j.mrgentox.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Cabarkapa A., Zivković L., Zukovec D., Djelić N., Bajić V., Dekanski D., Spremo-Potparević B. Protective Effect of Dry Olive Leaf Extract in Adrenaline Induced DNA Damage Evaluated Using in Vitro Comet Assay with Human Peripheral Leukocytes. Toxicol. Vitr. 2014;28:451–456. doi: 10.1016/j.tiv.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 64.Türkez H., Toğar B. Olive (Olea europaea L.) Leaf Extract Counteracts Genotoxicity and Oxidative Stress of Permethrin in Human Lymphocytes. J. Toxicol. Sci. 2011;36:531–537. doi: 10.2131/jts.36.531. [DOI] [PubMed] [Google Scholar]
- 65.Dekanski D., Selaković V., Piperski V., Radulović Z., Korenić A., Radenović L. Protective Effect of Olive Leaf Extract on Hippocampal Injury Induced by Transient Global Cerebral Ischemia and Reperfusion in Mongolian Gerbils. Phytomedicine. 2011;18:1137–1143. doi: 10.1016/j.phymed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 66.Sarbishegi M., Charkhat Gorgich E.A., Khajavi O., Komeili G., Salimi S. The Neuroprotective Effects of Hydro-Alcoholic Extract of Olive (Olea europaea L.) Leaf on Rotenone-Induced Parkinson’s Disease in Rat. Metab. Brain Dis. 2018;33:79–88. doi: 10.1007/s11011-017-0131-0. [DOI] [PubMed] [Google Scholar]
- 67.Asghari A.A., Hosseini M., Bafadam S., Rakhshandeh H., Farazandeh M., Mahmoudabady M. Olea europaea L. (Olive) Leaf Extract Ameliorates Learning and Memory Deficits in Streptozotocin-Induced Diabetic Rats. Vicenna J. Phytomed. 2022;12:163–174. doi: 10.22038/AJP.2021.18989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y., Wang S., Cui W., He J., Wang Z., Yang X. Olive Leaf Extract Inhibits Lead Poisoning-Induced Brain Injury. Neural Regen. Res. 2013;8:2021–2029. doi: 10.3969/j.issn.1673-5374.2013.22.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehraein F., Sarbishegi M., Golipoor Z. Different Effects of Olive Leaf Extract on Antioxidant Enzyme Activities in Midbrain and Dopaminergic Neurons of Substantia Nigra in Young and Old Rats. Histol. Histopathol. 2016;31:425–431. doi: 10.14670/HH-11-687. [DOI] [PubMed] [Google Scholar]
- 70.Çoban J., Öztezcan S., Doğru-Abbasoğlu S., Bingül I., Yeşil-Mizrak K., Uysal M. Olive Leaf Extract Decreases Age-Induced Oxidative Stress in Major Organs of Aged Rats. Geriatr. Gerontol. Int. 2014;14:996–1002. doi: 10.1111/ggi.12192. [DOI] [PubMed] [Google Scholar]
- 71.Mikami T., Kim J., Park J., Lee H., Yaicharoen P., Suidasari S., Yokozawa M., Yamauchi K. Olive Leaf Extract Prevents Obesity, Cognitive Decline, and Depression and Improves Exercise Capacity in Mice. Sci. Rep. 2021;11:12495. doi: 10.1038/s41598-021-90589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kendall M., Batterham M., Obied H., Prenzler P.D., Ryan D., Robards K. Zero Effect of Multiple Dosage of Olive Leaf Supplements on Urinary Biomarkers of Oxidative Stress in Healthy Humans. Nutrition. 2009;25:270–280. doi: 10.1016/j.nut.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 73.De Cicco P., Maisto M., Tenore G.C., Ianaro A. Olive Leaf Extract, from Olea europaea L., Reduces Palmitate-Induced Inflammation via Regulation of Murine Macrophages Polarization. Nutrients. 2020;12:3663. doi: 10.3390/nu12123663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chiaino E., Micucci M., Cosconati S., Novellino E., Budriesi R., Chiarini A., Frosini M. Olive Leaves and Hibiscus Flowers Extracts-Based Preparation Protect Brain from Oxidative Stress-Induced Injury. Antioxidants. 2020;9:806. doi: 10.3390/antiox9090806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arslan J., Jamshed H., Qureshi H. Early Detection and Prevention of Alzheimer’s Disease: Role of Oxidative Markers and Natural Antioxidants. Front. Aging Neurosci. 2020;12:231. doi: 10.3389/fnagi.2020.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rabiei Z., Bigdeli M.R., Rasoulian B., Ghassempour A., Mirzajani F. The Neuroprotection Effect of Pretreatment with Olive Leaf Extract on Brain Lipidomics in Rat Stroke Model. Phytomedicine. 2012;19:940–946. doi: 10.1016/j.phymed.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Sajjad N., Wani A., Hassan S., Ali R., Hamid R., Akbar S., Ganai B.A., Bhat E.A. Interplay of Antioxidants in Alzheimer’s Disease. J. Transl. Sci. 2019;5:1–11. doi: 10.15761/JTS.1000313. [DOI] [Google Scholar]
- 78.Osama A., Zhang J., Yao J., Yao X., Fang J. Nrf2: A Dark Horse in Alzheimer’s Disease Treatment. Ageing Res. Rev. 2020;64:101206. doi: 10.1016/j.arr.2020.101206. [DOI] [PubMed] [Google Scholar]
- 79.Ramsey C.P., Glass C.A., Montgomery M.B., Lindl K.A., Ritson G.P., Chia L.A., Hamilton R.L., Chu C.T., Jordan-Sciutto K.L. Expression of Nrf2 in Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2007;66:75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davies D.A., Adlimoghaddam A., Albensi B.C. Role of Nrf2 in Synaptic Plasticity and Memory in Alzheimer’s Disease. Cells. 2021;10:1884. doi: 10.3390/cells10081884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stack C., Jainuddin S., Elipenahli C., Gerges M., Starkova N., Starkov A.A., Jové M., Portero-Otin M., Launay N., Pujol A., et al. Methylene Blue Upregulates Nrf2/ARE Genes and Prevents Tau-Related Neurotoxicity. Hum. Mol. Genet. 2014;23:3716–3732. doi: 10.1093/hmg/ddu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Quraishy S., Othman M.S., Dkhil M.A., Abdel Moneim A.E. Olive (Olea europaea) Leaf Methanolic Extract Prevents HCl/Ethanol-Induced Gastritis in Rats by Attenuating Inflammation and Augmenting Antioxidant Enzyme Activities. Biomed. Pharm. 2017;91:338–349. doi: 10.1016/j.biopha.2017.04.069. [DOI] [PubMed] [Google Scholar]
- 83.Zare-shahabadi A., Masliah E., Johnson G.V.W., Rezaei N. Autophagy in Alzheimer’s Disease. Rev. Neurosci. 2015;26:385–395. doi: 10.1515/revneuro-2014-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schnöder L., Hao W., Qin Y., Liu S., Tomic I., Liu X., Fassbender K., Liu Y. Deficiency of Neuronal P38α MAPK Attenuates Amyloid Pathology in Alzheimer Disease Mouse and Cell Models through Facilitating Lysosomal Degradation of BACE1. J. Biol. Chem. 2016;291:2067–2079. doi: 10.1074/jbc.M115.695916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rickle A., Bogdanovic N., Volkman I., Winblad B., Ravid R., Cowburn R.F. Akt Activity in Alzheimer’s Disease and Other Neurodegenerative Disorders. Neuroreport. 2004;15:955–959. doi: 10.1097/00001756-200404290-00005. [DOI] [PubMed] [Google Scholar]
- 86.Leri M., Bertolini A., Stefani M., Bucciantini M. EVOO Polyphenols Relieve Synergistically Autophagy Dysregulation in a Cellular Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2021;22:7225. doi: 10.3390/ijms22137225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uddin M.S., Stachowiak A., Mamun A.A., Tzvetkov N.T., Takeda S., Atanasov A.G., Bergantin L.B., Abdel-Daim M.M., Stankiewicz A.M. Autophagy and Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Implications. Front. Aging Neurosci. 2018;10:4. doi: 10.3389/fnagi.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma S., Attarwala I.Y., Xie X.-Q. SQSTM1/P62: A Potential Target for Neurodegenerative Disease. ACS Chem. Neurosci. 2019;10:2094–2114. doi: 10.1021/acschemneuro.8b00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Omar S.H., Kerr P.G., Scott C.J., Hamlin A.S., Obied H.K. Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells. Molecules. 2017;22:1858. doi: 10.3390/molecules22111858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chiaino E., Micucci M., Budriesi R., Mattioli L.B., Marzetti C., Corsini M., Frosini M. Hibiscus Flower and Olive Leaf Extracts Activate Apoptosis in SH-SY5Y Cells. Antioxidants. 2021;10:1962. doi: 10.3390/antiox10121962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumar A., Sidhu J., Goyal A., Tsao J.W. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Alzheimer Disease. [Google Scholar]