Abstract
Abstract
Parkinson’s disease (PD) is the second most common neurodegenerative disease but still lacks a preclinical strategy to identify it. The diagnostic value of intestinal mucosal α-synuclein (αSyn) in PD has not drawn a uniform conclusion. The relationship between the alteration of intestinal mucosal αSyn expression and mucosal microbiota is unclear. Nineteen PD patients and twenty-two healthy controls were enrolled in our study from whom were collected, using gastrointestinal endoscopes, duodenal and sigmoid mucosal samples for biopsy. Multiplex immunohistochemistry was performed to detect total, phosphorylate, and oligomer α-synuclein. Next-generation 16S rRNA amplicon sequencing was applied for taxonomic analysis. The results implied that oligomer α-synuclein (OSyn) in sigmoid mucosa of PD patients was transferred from the intestinal epithelial cell membrane to the cytoplasm, acinar lumen, and stroma. Its distribution feature was significantly different between the two groups, especially the ratio of OSyn/αSyn. The microbiota composition in mucosa also differed. The relative abundances of Kiloniellales, Flavobacteriaceae, and CAG56 were lower, while those of Proteobacteria, Gammaproteobacteria, Burkholderiales, Burkholdriaceae, Oxalobacteraceae, Ralstonia, Massilla, and Lactoccus were higher in duodenal mucosa of PD patients. The relative abundances of Thermoactinomycetales and Thermoactinomycetaceae were lower, while those of Prevotellaceae and Bifidobacterium longum were higher in patients’ sigmoid mucosa. Further, the OSyn/αSyn level was positively correlated with the relative abundances of Proteobacteria, Gammaproteobacteria, Burkholderiales, Pseudomonadales, Burkholderiaceae, and Ralstonia in the duodenal mucosa, while it was negatively correlated with the Chao1 index and observed operational taxonomic units of microbiota in sigmoid mucosa. The intestinal mucosal microbiota composition of PD patients altered with the relative abundances of proinflammatory bacteria in the duodenal mucosa increased. The ratio of the OSyn/αSyn level in the sigmoid mucosa indicated a potential diagnostic value for PD, which also correlated with mucosal microbiota diversity and composition.
Key points
• The distribution of OSyn in sigmoid mucosa differed between PD patients and healthy controls.
• Significant alterations in the microbiome were found in PD patients’ gut mucosa.
• OSyn/αSyn level in sigmoid mucosa indicated a potential diagnostic value for PD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00253-023-12410-w.
Keywords: Parkinson’s disease, α-Synuclein, Mucosal microbiota, Intestinal mucosa, Diagnosis
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease (Tysnes et al. 2017). In PD patients, disruption of the α-synuclein (αSyn) structure biases it toward aggregation-prone conformations (Stephens et al. 2019). This accumulation is not limited to the central nervous system but is also found in the enteric nervous system in the postmortem (Ruffmann et al. 2016), making αSyn detection via gastrointestinal biopsies a hot research topic for PD diagnosis. Several studies suggest that αSyn pathology might begin and be detectable in the gastrointestinal tract during the prodromal PD phase before affecting the brain (Hilton et al. 2014; Stokholm et al. 2016). In addition, non-motor symptoms of PD, especially in the gastrointestinal tract, could appear 10 years earlier than motor symptoms, resulting in PD patients with long-term severe constipation (Holmqvist et al. 2014).
Historically, the diagnosis of PD has relied on clinical symptoms and can only be confirmed by postmortem examination of misfolded αSyn in the brain with neuronal loss in the substantia nigra (Dickson et al. 2009), which lacks a preclinical strategy to help identify PD by in vivo biomarkers. Given the close relationship between intestinal αSyn and PD, we hypothesized that the detection of intestinal mucosal αSyn expression would be helpful for the early diagnosis of PD. However, in recent years, αSyn has also been found in healthy people (Coker et al. 2018; Ruffmann et al. 2018), leading to the difficulty of distinguishing PD from normal controls. An assumption is that the “non-PD controls” in which αSyn deposition is detected are also PD patients, but no follow-up study is available to verify and diagnose their disease status. Besides, the previous studies that mainly detected the total and phosphorylate αSyn (PSyn) in the intestinal mucosa showed contradictory conclusions on the reliability of those indicators.
Some recent research reported that the oligomer αSyn (OSyn) was toxic to the nervous system and may relate to the pathogenesis (Ludtmann et al. 2018), while no one has examined its expression in the gastrointestinal mucosa. Intestinal mucosal αSyn might have a potential diagnostic value for PD, but the methodology used in the existing studies cannot make it a gold standard. Hence, one aim of this study is to use multiple fluorescence immunohistochemical technology to simultaneously detect three different αSyn species including the total, phosphorylate, and oligomer types to explore the potential PD biomarkers by intestinal mucosal biopsy. Besides, dysfunction of the microecology-gut-brain axis plays an important role in the pathogenesis of PD. However, previous studies about microecological disorders of PD are mainly based on fecal microbiota, and there is a lack of understanding of the intestinal mucosal microbiota in PD patients. A few studies have reported the characteristics of colonic mucosal microbiota in PD patients, but there are no related reports on the characteristics of the upper gastrointestinal mucosal flora in PD patients. Thus, the other aim of this study is to study the characteristics of duodenal mucosal and sigmoid mucosal microbiota in PD patients.
Materials and methods
Study design
The patients with severe constipation and who were willing to undergo gastrointestinal endoscopy were recruited in our cohort. For the PD group, the volunteers needed to be diagnosed by an experienced neurologist (the stage of Hoehn and Yahr Scale < 5) (Hoehn et al. 1967). We enrolled counterpart-age patients without any other neurodegenerative diseases for the control group. This study was conducted under the ethical principles of the Declaration of Helsinki. The study protocol was approved by Beijing Hospital with the approval number 2019BJYYEC-199-03. Written informed consent was obtained from all subjects before participation. All subjects were informed of the potential risk of bleeding during and after the biopsy.
Collection of biopsy specimens
Biopsy specimens of the duodenal mucosa and sigmoid mucosa were obtained during gastroscopy and colonoscopy. Some of the specimens were preserved in formalin, and 4-μm-thick paraffin sections were prepared for further staining. The other obtained fresh mucosal samples were frozen at − 80 °C within 1 h before subsequent mucosal flora sequencing analysis. Any biopsy that consists entirely or predominantly of abnormal tissue (e.g., carcinoma) was excluded from the final calculation.
Multiplex immunohistochemistry
Multiplex immunohistochemistry (mIHC) was performed according to a sequential multiplexed immunofluorescence protocol (Stack et al. 2014). Briefly, total αSyn (Cat 1B10E9, Proteintech, Chicago, IL, USA), PSyn (Cat MABN826, Merck-Millipore, Boston, MA, USA), and OSyn (Cat ABN2265, Merck-Millipore) were co-stained with corresponding antibodies on the same sample. Then, corresponding secondary antibodies were used in fluorescein isothiocyanate–tyramide signal amplification (FITC-TSA) (PPD520, Panovue, Beijing, China) for total αSyn detection, CY3-TSA (PPD570, Panovue, Beijing, China) for PSyn detection, and CY5-TSA (PPD620, Panovue, Beijing, China) for OSyn detection. Nuclei were highlighted by DAPI (4′,6-diamidino-2-phenylindole) (D9542, Sigma-Aldrich, St Louis, USA). Image J software (Version 1.8.0.112; National Institutes of Health, Bethesda, USA) was applied for location determination and fluorescent intensity evaluation.
Intestinal mucosal bacterial DNA extraction
Bacterial DNA was extracted from the mucosal samples at Novogene Bioinformatics Technology Co. Ltd. (Beijing, China) using the sodium dodecyl sulfate (SDS) method (Natarajan et al. 2016). DNA concentration and purity were monitored on agarose gels. After the concentration was determined, DNA was diluted to 1 ng/μL using sterile water.
16S rRNA gene sequence sequencing
16S rRNA genes of the 16S V4 region were amplified using specific primers (Caporaso et al. 2011) (515F: GTGCCAGCMGCCGCGGTAA; 806R: GGACTACHVGGGTWTCTAAT) with a barcode. The purity and concentration of DNA were detected by 2% agarose gel electrophoresis. Then, the target bands (400–450 bp) were purified and recovered for further experiments. Sequencing libraries were generated using an Illumina TruSeq DNA PCR-Free Library Preparation Kit (Illumina, San Diego, CA, USA) following the manufacturer’s recommendations. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). The library was sequenced on the NovaSeq6000 platform. Sequence data were deposited to the Sequence Read Archive (https://www.ncbi. nlm. nih. gov/ sra/) in the bioproject of PRJNA881840.
16S rRNA gene sequence analysis
(1) Amplicon sequence variants (ASVs): Classify-sklearn algorithm of QIIME2 (Bolyen et al. 2019) used to annotate species for each ASV was used a pre-trained naϊve Bayes classifier. (2) Biodiversity coverage: The biodiversity coverage can directly reflect the rationality of the amount of sequencing data and indirectly reflect the richness of species in the samples. We depicted the rarefaction curves using QIIME2 to show the calculation of species richness and to reflect the biodiversity coverage (Boussarie et al. 2018). When the curve tends to be flat, it shows that the amount of sequencing data is enough, and more data will only produce a small number of new species. (3) Alpha diversity: Alpha diversity was applied in analyzing the complexity of species diversity for a sample through 4 indices including observed operational taxonomic units (OTUs), Chao1, Shannon, and Simpson. We used QIIME2 to achieve this. (4) Beta diversity: Beta diversity analysis was used to evaluate differences of samples in species complexity. Principal coordinate analysis (PCoA) was performed to get principal coordinates and visualize from complex, multidimensional data. Samples with high similarity in community structure tend to gather together, and samples with large community differences will be far away. Analysis of similarities (ANOSIM), calculated by QIIME2, was used to test whether the difference between groups was significantly greater than that within groups. The R statistic, a ratio between within-group and between-group dissimilarities, was between (− 1, 1). R greater than 0 indicated that the difference between groups was significant. R less than 0 indicated that the difference within the group was greater than the difference between groups. (5) Columnar accumulation diagram: A columnar accumulation diagram of the relative abundance of the top 10 species at the phylum and genus levels was used to show the global composition of the bacteria of each group. The plots were drawn by R (version 2.15.3), applying packages Vegan and Reshape. (6) Linear discriminant analysis effect size (LEfSe): LEfSe conducted by LEfSe software (Version 1.0) (Segata et al. 2011) was used to determine which species differed significantly between groups, and the threshold of the linear discriminant analysis (LDA) score was set to 4. (7) Spearman correlation analysis was used to analyze the correlation between mucosal αSyn expression level and alpha diversity and the relative abundance of the top 20 species at phylum, class, order, family, and genus levels of mucosal flora. (8) Functional prediction: Functional prediction was carried out based on KEGG databases (https://www.genome.jp/kegg/). The top 35 functions in abundance were selected to draw a heat map.
Statistical analysis
Statistical analysis was performed by SPSS 26.0 (SPSS Inc., Chicago, IL, USA). The age of our cohort is nonnormal data and is thus being expressed as average (minimum, maximum) deviation using the Wilcoxon test. Counting data were compared using the chi-square test. P value < 0.05 was considered statistically significant in all tests. The mean fluorescence intensity was calculated by Image J (Version 1.8.0.112; National Institutes of Health, Bethesda, USA). The scatterplots and column diagram were exhibited by GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, USA), and the receiver operation characteristic (ROC) curves were depicted by using the web-tool easyROC (http://www.biosoft.hacettepe.edu.tr/easyROC/). The closer the value of the area under curve (AUC) is to 1, the better the diagnostic efficiency is.
Results
Clinical characteristics of enrolled subjects
Nineteen patients and 22 controls were recruited in our cohort to undergo gastrointestinal endoscopy and tissue biopsy. One of the PD patients who had multiple colon polyp forceps provided one biopsy for our study. One of the controls was only acquired from duodenum biopsy due to inadequate preparation for colon cleansing. The basic information of the final enrolled samples in the PD and control groups is shown in Table 1.
Table 1.
Basic information of enrolled subjects
| PD | Control | P valuea | ||
| Number of subjects | 19 | 22 | ||
| Gender | Male | 12 | 16 | 0.511 |
| Female | 7 | 6 | ||
| Ageb | 65 (61, 75) | 64 (56, 68) | 0.129 | |
| Location | Sigmoid | 20 | 21 | 0.570 |
| Duodenum | 10 | 7 | ||
aP values were calculated using the chi-square test (gender and location) and Wilcoxon test (age) to evaluate the characteristics between the two groups. P value > 0.05 was considered no statistical difference
bExhibiting as average (minimum, maximum)
In the present study, duodenal mucosal specimens from 7 healthy controls and 10 PD patients and sigmoid mucosal specimens from 21 healthy controls and 19 PD patients were used to explore the expression of αSyn by mIHC. After the quality control filtration of the amplicon, duodenal mucosal specimens from 7 healthy controls and 7 PD patients and sigmoid mucosal specimens from 18 healthy controls and 17 PD patients were analyzed on the Illumina NovaSeq sequencing platform. The double-end sequencing method of the V4 region of 16S rRNA was performed for the intestinal mucosal microbiota.
The distribution of different types of αSyn in the intestinal mucosa was different between the PD and control subjects
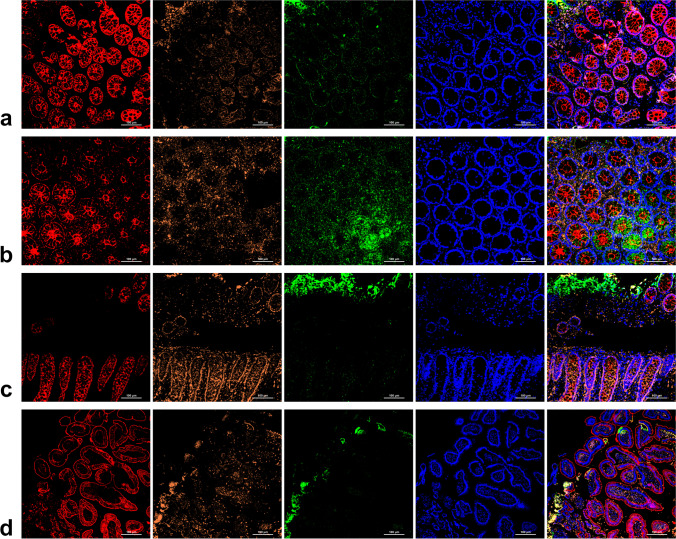
By applying mIHC to detect the three types of αSyn, the landscape of them in intestinal mucosa was depicted (Fig. 1). As observed, in normal controls, αSyn was mainly distributed in the intestinal epithelial cell membrane and a little in the cytoplasm (Fig. 1a). In PD patients, αSyn was distributed in the intestinal epithelial cell membrane and stroma (Fig. 1b–d). The OSyn aggregated from αSyn (Castillo-Carranza et al. 2018) was mainly distributed in the intestinal epithelial cell membrane in normal controls (Fig. 1a), while in PD patients, it was mainly distributed in the stroma and acinar lumen with only a small amount in the intestinal epithelial cell cytoplasm (Fig. 1b–d). As to PSyn, in normal controls, it was also mainly distributed in the intestinal epithelial membrane (Fig. 1a), while in PD patients, PSyn was mainly distributed in the intestinal epithelial membrane and cytoplasm (Fig. 1b–d). To sum up, the distribution of the three types of αSyn in colon mucosae of PD patients differed from the control ones. In particular, OSyn was the most prominent. In the sigmoid mucosa biopsy specimens from PD patients, OSyn was transferred from the intestinal epithelial cell membrane to the cytoplasm, acinar lumen, and stroma. However, the descriptions above were more in accord with that colon tissue rather than in the duodenum samples (Supplemental Fig. S1).
Fig. 1.
The distribution of different types of α-synuclein in the sigmoid mucosa of a normal controls and b–d PD patients; ×200. The scale bars are in the bottom right corner of each picture, indicating 100 μm. Total αSyn staining, red; OSyn staining, orange; PSyn staining, green; DAPI staining, blue; PD: Parkinson’s disease; αSyn: α-synuclein; OSyn: oligomer α-synuclein; PSyn: phosphorylate α-synuclein
The fluorescence intensity ratio of different types of αSyn in colonic mucosa showed a valid diagnosis of PD disease
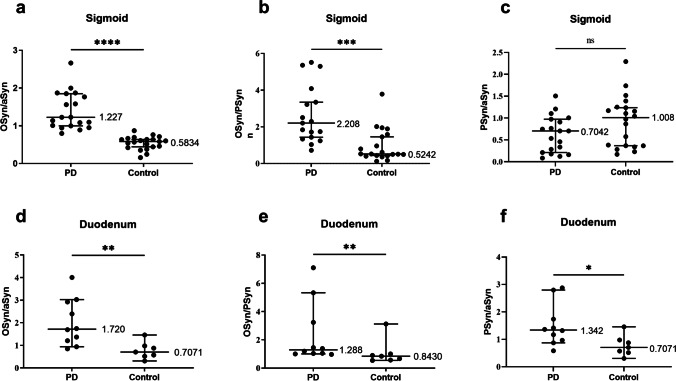
Based on the observations of αSyn distribution, we quantified the mean fluorescence intensity of each channel. Considering the systematic errors between experimental batches, we calculated the ratios between diverse αSyn (Fig. 2). From the data, oligomer α-synuclein/total α-synuclein (OSyn/αSyn) and oligomer α-synuclein/phosphorylate α-synuclein (OSyn/PSyn) of the sigmoid colon demonstrated significant differences between the PD and control groups. Furthermore, we calculated the ROC curve to evaluate the diagnostic efficiency of each ratio (Fig. 3) and the ROC statistics are displayed in Table 2. The ratio of OSyn/αSyn indicates an effective ability to distinguish PD patients and controls, with the cut-off point of 0.799.
Fig. 2.
Comparison of the mean fluorescence intensity ratio of different types of α-synuclein in sigmoid. a OSyn/αSyn in sigmoid mucosa; b OSyn/PSyn in sigmoid mucosa; c PSyn/αSyn in sigmoid mucosa; d OSyn/αSyn in duodenum mucosa; e OSyn/PSyn in duodenum mucosa; f PSyn/αSyn in duodenum mucosa. n = 7–21; ns P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001,****P<0.0001. PD: Parkinson’s disease; OSyn/αSyn: oligomer α-synuclein/total α-synuclein; OSyn/PSyn: oligomer α-synuclein/phosphorylate α-synuclein; PSyn/αSyn: phosphorylate α-synuclein/total α-synuclein
Fig. 3.
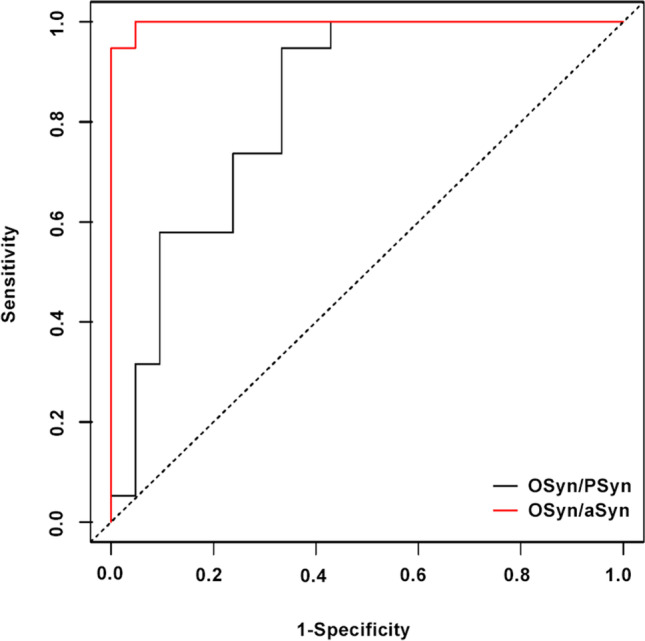
ROC analysis values of the ratio of different αSyn types in PD diagnosis. Black line: OSyn/PSyn; red line: OSyn/αSyn; OSyn/αSyn: oligomer α-synuclein/total α-synuclein; OSyn/PSyn: oligomer α-synuclein/phosphorylate α-synuclein
Table 2.
The related statistics data of receiver operation characteristic (ROC) curves
| Ratio | AUC | SE AUC | Lower limit | Upper limit | P value |
| OSyn/αSyn | 0.99749 | 0.00354 | 0.99055 | 1.00444 | 0 |
| OSyn/PSyn | 0.83208 | 0.06621 | 0.70231 | 0.96185 | 0 |
P value < 0.05 implied the ratios were statistically different between the PD and control groups
AUC area under curve, SE AUC standard error of AUC
The relationship between other factors and the different types of αSyn expression levels in sigmoid mucosa
To validate the diagnostic efficiency, we evaluated other factors that possibly influence the expressions of all types of αSyn. Supplemental Fig. S2 showed that the expression level of different types of αSyn in sigmoid mucosa exhibited no difference between men and women. Our data (Supplemental Fig. S3) did not show the statistical correlations between age and any types of αSyn.
Mucosal microbiome ASVs
An average of 105,232 and 105,918 pyrosequencing reads per sample of duodenal mucosa and sigmoid mucosa were generated after sequencing, respectively. After proper quality control, the number of reads per sample was reduced to 69,114 and 70,791, respectively. The level of biodiversity coverage was assayed through observed species number biodiversity curves (Supplemental Fig. S4), which tended to plateau for each sample, confirming the high accuracy of the performed 16S rRNA profiling analysis.
Comparison of the mucosal microbiota intra- and inter-individual variability among the different groups
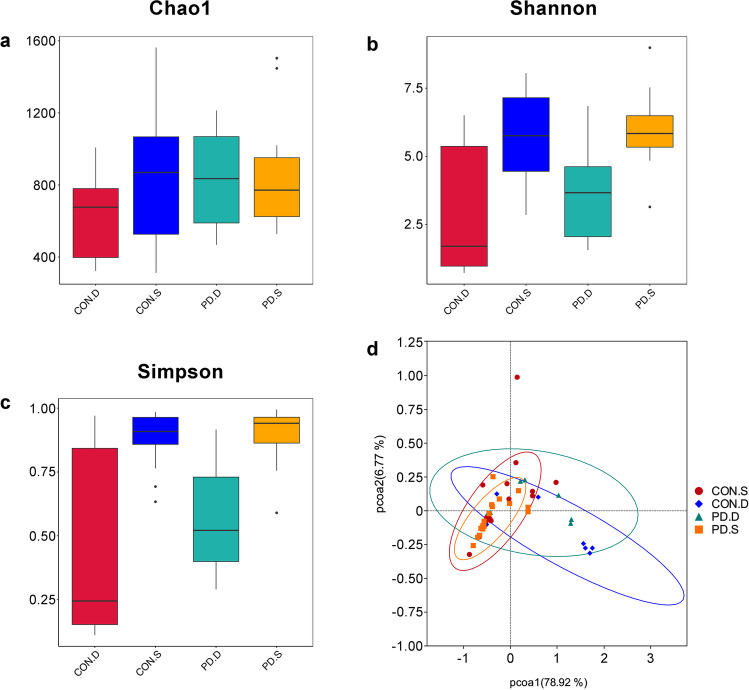
The alpha diversity assessed by three different indexes including Chao1 richness estimator (Fig. 4a), Shannon biodiversity index (Fig. 4b), and Simpson diversity index (Fig. 4c) were used to analyze the complexity of species diversity. Compared to the duodenal mucosa of controls or PD patients, Kruskal–Wallis analysis showed that the microbiotic diversity of sigmoid mucosa was significantly richer according to the Shannon and Simpson diversity indices (P < 0.05). There was no significant difference in the diversity of the duodenal or sigmoid mucosa between the controls and PD patients according to the Chao1, Shannon, and Simpson diversity indices (P > 0.05). The beta diversity among the samples allowed a detailed analysis of the similarities between the gut microbiota composition of the different groups. PCoA (Fig. 4d) revealed that the samples of duodenal mucosa and sigmoid mucosa from PD patients clustered separately (R = 0.30, P = 0.030), while the duodenal or sigmoid mucosal samples of the controls and PD patients appeared to cluster together (R > 0), which was confirmed by ANOSIM.
Fig. 4.
Alpha and beta diversity analysis of duodenal and sigmoid mucosal microbiota. a Chao1 richness estimator, b Shannon biodiversity index, c Simpson diversity index, d unweighted-unifrac principal coordinate analysis (PCA)
Taxonomic analysis of mucosal microbiota composition among different groups
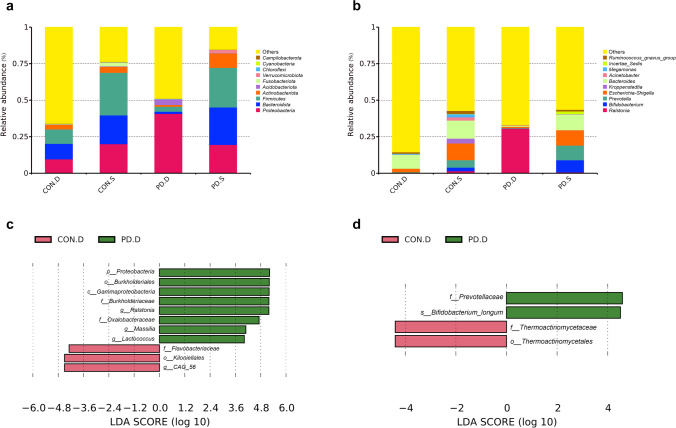
The composition of the top 10 species at the phylum and genus levels was analyzed by the column accumulation diagram of relative abundances of species. At the phylum level (Fig. 5a), the relative abundances of Bacteroidetes and Proteobacteria were the highest in the duodenal mucosa of the controls and PD patients, respectively, while those of Firmicutes in the sigmoid mucosa of the controls and PD patients were the highest. At the genus level (Fig. 5b), Bacteroides and Ralstonia had the highest relative abundance in the duodenal mucosa of the controls and PD patients, respectively, while in the sigmoid mucosa of both the controls and PD patients, Bacteroides had the highest relative abundance.
Fig. 5.
Duodenal and sigmoid mucosal microbiota composition and differential species analysis. Columnar accumulation diagram of relative abundances of the top 10 species at the phylum (a) and genus (b) levels. Linear discriminant analysis effect size of duodenal (c) and sigmoid (d) mucosal microbiota
To identify more precisely, we directly compared the bacterial composition at the phylum, class, order, family, genus, and species levels of the controls and PD patients from the results of the V4 hypervariable region of 16S rRNA gene sequencing, using LEfSe analysis. The results showed that in terms of duodenal mucosal microbiota (Fig. 5c), the relative abundances of Kiloniellales (at order the level), Flavorbacteriaceae (at the family level), and CAG56 (at the genus level) in the control group were higher than those in the PD group. The relative abundances of Proteobacteria (at the phylum level), Gammaproteobacteria (at the class level), Burkholderiales (at the order level), Burkholderiaceae (at the family level), Oxalobacteraceae (at the family level), Ralstonia (at the genus level), Massilla (at the genus level), and Lactococcus (at the genus level) in the normal control group were lower than those in the PD group. In terms of sigmoid mucosal microbiota (Fig. 5d), the relative abundances of Thermoactinomycetales (at the order level) and Thermoactinomycetaceae (at the family level) in the control group were higher than those in the PD group. The relative abundances of Prevotellaceae (at the family level) and Bifidobacterium longum (at the species level) in the control group were lower than those in the PD group.
Correlation analysis between mucosal OSyn/ αSyn expression level and mucosal microbiota composition
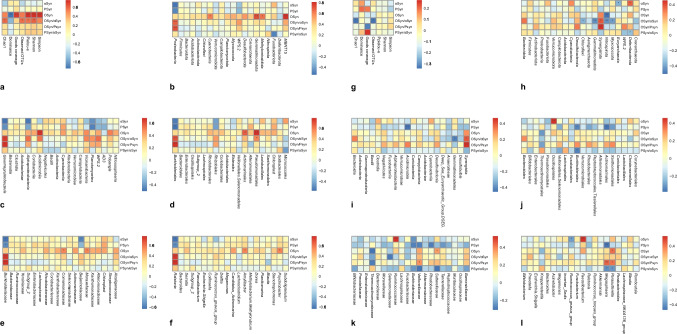
Spearman correlation analysis (Fig. 6a–f) showed that OSyn/ αSyn in duodenal mucosa was positively correlated with the relative abundance of Proteobacteria at the phylum level, Gammaproteobacteria at the class level, Burkholderiales and Pseudomonadales at the order level, Burkholderiaceae at the family level, and Ralstonia at the genus level.
Fig. 6.
Correlation analysis of mucosal αSyn expression with alpha diversity and species abundances of mucosal microbiota. Spearman correlation analysis of duodenal mucosal αSyn expression with alpha diversity (a) and species abundances of duodenal mucosal microbiota at phylum (b), class (c), order (d), family (e), and genus (f) levels. Spearman correlation analysis of sigmoid mucosal αSyn expression with alpha diversity (g) and species abundances of sigmoid mucosal microbiota at phylum (h), class (i), order (j), family (k), and genus (l) levels
OSyn/ αSyn in sigmoid mucosa was negatively correlated with the Chao1 index and observed OTUs of sigmoid mucosa microbiota (Fig. 6g). Besides, OSyn/ αSyn in the sigmoid mucosa was negatively correlated with the relative abundances of Chloroflexi, Gemmatimonadota, Nitrospirota, and Myxococota at the phylum level. In addition, OSyn/ αSyn in the sigmoid mucosa was negatively correlated with the relative abundance of Gemmatimonadetes and positively correlated with the relative abundance of Synergia at the class level (Fig. 6h–l).
Functional prediction
Based on the KEGG method (Fig. 7), pathways correlated with glutathione S-transferase (K00799), methyl acceding chemotaxis protein (K03406), branched-chain amino acid transport system substrate-binding protein (K01999), branched-chain amino acid transport system permease protein (K01997), and branched-chain amino acid transport system ATP-binding protein (K01996) were enriched in the duodenal mucosa of PD patients, while the pathways correlated with putative ABC transport system ATP-binding protein (K02003), phosphoglycolate phosphatase (K01091), and multiple sugar transport system substrate-binding proteins (K02027) were enriched in the sigmoid mucosa of PD patients.
Fig. 7.
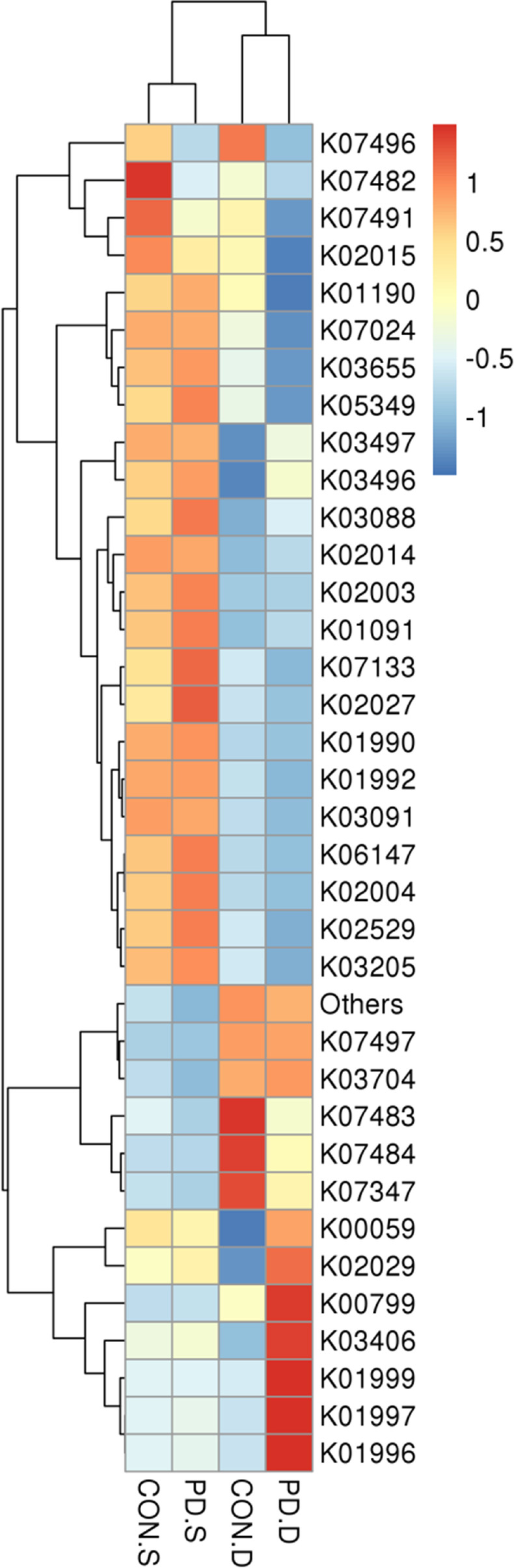
Functional profile prediction among different groups. A heat map of the top 35 functions in abundance based on KEGG database
Discussion
At present, it is believed that the role of the microecology-gut-brain axis in the pathogenesis of PD is as follows: imbalance of intestinal microecology and its metabolites leads to intestinal inflammation and intestinal mucosal barrier impairment. Then, endotoxin and pathogen invasion promote intestinal inflammation, leading to oxidative stress damage and misfolding of αSyn, which activates microglia and further aggravates inflammation. The accumulated αSyn in the gastrointestinal tract is transmitted to the central nervous system through the vagus nerve, resulting in oxidative stress and inflammation. As gastroenterologists, we keep an eye on the gastrointestinal manifestations of PD, comprising dysphagia, delayed gastric emptying, anorectal dysfunction, and especially severe constipation. Hence, our study aimed to analyze the expression of the three different αSyn species by mIHC, including the total, phosphorylate, and oligomer types. In our study, the distribution features of those proteins were different between the PD patients and controls. In addition, the ratios of OSyn/αSyn and OSyn /PSyn in the PD patients were significantly higher than those in the controls. The further ROC analysis of those ratios exhibited that OSyn /αSyn had a potential diagnosis efficiency.
As shown in our study, the total αSyn existed in both groups with different distributions in the intestinal mucosa. Our results indicated that OSyn was transferred from the intestinal epithelial cell membrane to the cytoplasm, acinar lumen, and stroma. OSyn was excreted from the mucous layer, which might partly support the assumption that enteroendocrine cells lining the intestinal tract serve as a reservoir for the central spread of the misfolded αSyn (Chandra et al. 2017) and the transport mediators between cells are exosomes (Guo et al. 2020). Considering that the the OSyn is a pathologic aggregation form of αSyn, the increasing portion of OSyn within the Syn pool also implied the overproduction of OSyn from the gut may be related to the pathological condition, which conformed to the toxic protein accumulation and gut-brain-axis retrograde transport theory (Travagli et al. 2020). Thus, an OSyn/αSyn ratio shows the potential of being a biomarker for PD diagnosis.
Furthermore, we analyzed whether the values and ratios of αSyn species would be influenced by gender or age and no correlation was found. Yang et al. (2020) have shown that the expression of αSyn and OSyn increases normally in the striatum and hippocampus, and Bottner et al. (2012) proposed that the presence of PSyn in the enteric nervous system (ENS) was age-related and might not necessarily be pathological. Taking all the results together, it is possible that the αSyn was formulated in the enteroendocrine cell. The OSyn emerged with aging and retrograde transport to the brain via the vagus nerve. For the healthy group, this process was limited so the accumulation of OSyn only took place in the brain but not in the ENS. For PD patients, this production in the gut might accelerate pathologically, and consequently, the significant increase of OSyn in the colon and brain leads to constipation and mobility disorder.
The dysfunction of the microecology-gut-brain axis plays an important role in the pathogenesis of PD (Tan et al. 2022). Intestinal microecological imbalance increases the risk of PD, which is related to dysbacteriosis, decreased short-chain fatty acids, lipopolysaccharides (LPS), and impaired mucosal barrier function. We found that although the alpha and beta diversity of duodenum and sigmoid mucosal microbiota in PD patients were not significantly different from those in normal individuals, the microflora composition in the duodenal and sigmoid mucosa in PD patients was changed. Specifically, in terms of duodenal mucosal flora, the relative abundances of Kiloniellales, Flavobacteriaceae, and CAG56 in healthy controls were higher than those in PD patients, while those of Proteobacteria, Gammaproteobacteria, Burkholderiales, Burkholderiaceae, Oxalobacteraceae, Ralstonia, Massilla, and Lactococcus in healthy controls were lower than those in PD patients. In terms of sigmoid mucosal flora, the relative abundances of Thermoactinomycetales and Thermoactinomycetaceae in healthy controls were higher than those in PD patients, while those of Prevotellaceae and B. longum were lower than those in PD patients.
Previous studies based on fecal flora show that PD patients have intestinal microecological disorders. Zheng et al. (2021) summarized that the relative abundances of Prevotellaceae, Lachnospiraceae, and Faecalibacterium in the feces of patients with PD decreased, while the relative abundances of Verrucomicrobiaceae, Bifidobacteriaceae, Christensenellaceae, and Ruminococcaceae increased. In addition, the relative abundances of some opportunistic pathogens including Porphyromonas, Prevotella, and Corynebacterium_1 also increased (Zheng et al. 2021). Studies showed that the absolute concentrations of acetate, propionate, and butyrate in fecal samples of PD patients decreased significantly (Unger et al. 2016). Further studies have shown that fecal dysbacteriosis in PD patients is related to the disease course, severity, and clinical manifestations. Specifically, Lactobacillus gasseri and Deferribacterales were positively correlated with the course of PD, while Escherichia, Shigella, Lachnospiraceae, and Clostridium coccoides were negatively correlated with the course of PD. Enterobacteriaceae, Lactobacillaceae, Enterococcus, Escherichia, and Proteus were positively correlated with the severity of PD disease, while Lachnospiraceae, Blautia, Faecalibacterium, and Ruminococcus were negatively correlated with the severity of PD disease. Enterobacteriaceae was positively correlated with the severity of PD motor dysfunction, and Lachnospiraceae was negatively correlated with PD motor dysfunction (Zheng et al. 2021). Further basic study shows that the signal of intestinal microorganisms is necessary for neuroinflammatory response and antibiotic treatment can improve the pathological changes related to PD in mice. Compared to healthy donors, fecal microbiota transplant (FMT) from PD patients enhanced the pathological damage associated with PD in αSyn overexpressed mice (Sampson et al. 2016). Based on the imbalance of the microecology-gut-brain axis in patients with PD, clinical and basic studies have shown that probiotics (Tamtaji et al. 2019; Sun et al. 2021) and FMT (Xue et al. 2020; Zhao et al. 2021) can protect PD, and its mechanism is related to improving intestinal microecological imbalance, strengthening intestinal mucosal barrier, and reducing inflammation.
The intestinal flora contains three biological layers: the inner layer of bacteria is called membrane flora, binding to specific receptors on the surface of intestinal mucosal epithelium and dominated by obligate anaerobic bacteria such as Bifidobacterium and Lactobacillus. The middle layer represents facultative anaerobes dominated by Bacteroides. The outer layer of bacteria is called luminal flora, attached to the surface of the intestinal mucosa and dominated by Escherichia coli, enterococci, and other aerobic or facultative aerobes (Arvans et al. 2005). It can be inferred that the composition of intestinal mucosal flora is different from that of fecal flora, corresponding to the opinion of other scholars (Hou et al. 2022). Keshavarzian et al. (2015) have shown that the relative abundance of Proteobacteria in mucosal flora is higher than that in fecal flora. The relative abundance ratio of Firmicutes to Bacteroides in fecal flora is higher, and the relative abundances of butyrate-producing anti-inflammatory bacteria such as Blautia, Roseburia, and Coprococcus in fecal flora are higher. Besides, the composition of mucosal flora was different in different parts of the gut due to the pH and other environmental factors. Previous studies about dysbacteriosis in PD patients mainly focus on fecal microflora (Qian et al. 2020; Tan et al. 2021). Only sporadic studies have reported sigmoid mucosal flora in PD patients, and there is no report about mucosal flora of the upper digestive tract now. It was found that the change of fecal flora in PD was more prominent than that in sigmoid mucosa. The diversity of sigmoid mucosal microbiota in patients with PD was not different from that in healthy controls, but the composition changed. The relative abundances of Coprobacillaceae, Dorea, and anti-inflammatory bacteria Faecalibacterium in the sigmoid mucosa of PD patients were lower than those of the healthy controls, while the relative abundances of the proinflammatory bacteria including Oxalobacteraceae and Ralstonia were higher than those of the healthy controls (Keshavarzian et al. 2015).
Similar to the previous studies (Nuzum et al. 2020), this research found that the diversity of mucosal flora in PD patients was the same as that in healthy controls, but the species composition changed significantly. At the phylum level, the relative abundance of Proteobacteria in the duodenal mucosa of PD patients increased. Proteobacteria includes lots of pathogens, such as E. coli, Salmonella, Vibrio cholerae, Helicobacter pylori, and so on (Rizzatti et al. 2017). The relative abundance of Gammaproteobacteria in the duodenal mucosa of PD patients increased. The Gammaproteobacteria consists of many important pathogens, such as Salmonella, Yersinia, V. cholerae, and Pseudomonas aeruginosa (Nina Parker 2016). At the order level, Burkholderiales, a species with increased relative abundance in the duodenal mucosa of PD patients, belongs to Betaproteobacteria, which also contains some important pathogenic bacteria, such as Burkholderia and Bordetella (Nina Parker 2016). At the family level, the relative abundances of Burkholderiaceae and Oxalobacteraceae in the duodenal mucosa of PD patients increased. Oxalobacterium belongs to Burkholderiales and was isolated from humans and animals’ rumen or the large intestine (Ning et al. 2022). Its high abundance was found to be associated with a high risk of PD (Ning et al. 2022). At the genus level, the relative abundances of Ralstonia and Massilia in the duodenal mucosa of PD patients increased. Ralstonia is a human opportunistic pathogen leading to septicemia, meningitis, and osteomyelitis (Ryan et al. 2014). Although the frequency of human infection is low, the infections are serious (Ryan et al. 2014). Massilia is also a pathogenic bacterium, which has been isolated from soil, air, immunocompromised patients, and otitis media patients (Park et al. 2013). It can be seen that the relative abundances of many proinflammatory bacteria in the duodenal mucosa of PD patients increased significantly. However, in this study, the change of microbiota in the sigmoid mucosa of PD patients was not as significant as that in the duodenal mucosa.
Our study applied mIHC for the first time to detect three αSyn species in the intestinal mucosa of PD patients, which was mainly used in the cancer research field. Moreover, we collected normal colonic mucosal tissues in this prospective study. Unlike in previous retrospective studies (Aldecoa et al. 2015; Stokholm et al. 2016), in which only the pathological specimens from the PD lesion sites could be collected, the degraded intestinal tissues were hard to be explored. Our results suggested that intestinal biopsy was helpful for the diagnosis of PD, which might provide a novel strategy for the preclinic PD diagnosis. Additionally, unlike some studies (Qian et al. 2020; Tan et al. 2021) mainly focusing on the fecal flora in PD, this study firstly revealed the characteristics of the mucosal flora in different parts of the digestive tract of PD patients, especially the duodenal mucosa and sigmoid mucosa, helping us fully understand the intestinal microecological disorders of PD patients.
Limitations still exist in this study. Firstly, the number of subjects is limited and more volunteers needed to be recruited to further confirm our results. Secondly, in this study, the patients were not separated into different pathogenesis grades as the sample limitation. Thirdly, this study was observational, so interventional studies are needed in the future, to explore the specific mechanisms.
In conclusion, our study found that the distribution features of three types of α-synuclein, especially the oligomer types, were different between PD patients and healthy controls. The OSyn/αSyn expression in sigmoid mucosa had a potential diagnosis efficiency for PD, making it possible to diagnose PD early by intestinal biopsy. The intestinal mucosal microbiota composition of PD patients altered with the relative abundances of proinflammatory bacteria in the duodenal mucosa increased. The OSyn/αSyn expression level was correlated with mucosal microbiota diversity and composition. This study helps to develop in-depth knowledge of the microecology-gut-brain axis in PD patients, but the specific mechanism still needs to be further studied.
Supplementary Information
Figures S1–S4 (PDF 6634 kb)
Author contribution
All of the authors contributed to the study’s conception and design. Experimental design: JS and QL; conduct of the experiment: YW, XX, WL, and QL; data collection and interpretation: YW, DC, KL, JH, and WS; writing of the manuscript: JS, YW, and DC; editing of the manuscript: JS, YW, XX, WL, DC, KL, JH, WS, and QL; funding support: JS and DC. All of the authors read and approved the final version of the manuscript.
Funding
This study was funded by National High Level Hospital Clinical Research Funding (BJ-2021-233), Fundamental Research Funds for the Central Universities (33320211108), and National High Level Hospital Clinical Research Funding (BJ-2021-190).
Data availability
The data underlying this article are available in the article and supplementary material. Specific data underlying this article are available on request from the corresponding author.
Declarations
Ethics statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethical Committee of Beijing Hospital (2019BJYYEC-199-03).
Conflict of interest
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jihua Shi and Yiran Wang contributed equally to this work.
References
- Aldecoa I, Navarro-Otano J, Stefanova N, Sprenger FS, Seppi K, Poewe W, Cuatrecasas M, Valldeoriola F, Gelpi E, Tolosa E. Alpha-synuclein immunoreactivity patterns in the enteric nervous system. Neurosci Lett. 2015;602:145–149. doi: 10.1016/j.neulet.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Arvans DL, Vavricka SR, Ren H, Musch MW, Kang L, Rocha FG, Lucioni A, Turner JR, Alverdy J, Chang EB. Luminal bacterial flora determines physiological expression of intestinal epithelial cytoprotective heat shock proteins 25 and 72. Am J Physiol-Gastr L. 2005;288(4):G696–G704. doi: 10.1152/ajpgi.00206.2004. [DOI] [PubMed] [Google Scholar]
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vázquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottner M, Zorenkov D, Hellwig I, Barrenschee M, Harde J, Fricke T, Deuschl G, Egberts JH, Becker T, Fritscher-Ravens A, Arlt A, Wedel T. Expression pattern and localization of alpha-synuclein in the human enteric nervous system. Neurobiol Dis. 2012;48(3):474–480. doi: 10.1016/j.nbd.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Boussarie G, Bakker J, Wangensteen OS, Mariani S, Bonnin L, Juhel JB, Kiszka JJ, Kulbicki M, Manel S, Robbins WD, Vigliola L, Mouillot D. Environmental DNA illuminates the dark diversity of sharks. Sci Adv. 2018;4(5):eaap9661. doi: 10.1126/sciadv.aap9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. P Natl Acad Sci USA. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Carranza DL, Guerrero-Muñoz MJ, Sengupta U, Gerson JE, Kayed R. α-Synuclein oligomers induce a unique toxic tau strain. Biol Psychiatry. 2018;84(7):499–508. doi: 10.1016/j.biopsych.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra R, Hiniker A, Kuo YM, Nussbaum RL, Liddle RA (2017) α-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight 2(12). 10.1172/jci.insight.92295 [DOI] [PMC free article] [PubMed]
- Coker OO, Dai Z, Nie Y, Zhao G, Cao L, Nakatsu G, Wu WK, Wong SH, Chen Z, Sung JJY, Yu J. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut. 2018;67(6):1024–1032. doi: 10.1136/gutjnl-2017-314281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Leverenz JB, Del Tredici K, Wszolek ZK, Litvan I. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8(12):1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- Guo M, Wang J, Zhao Y, Feng Y, Han S, Dong Q, Cui M, Tieu K. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain. 2020;143(5):1476–1497. doi: 10.1093/brain/awaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, Broughton E, Hagan H, Carroll C. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014;127(2):235–241. doi: 10.1007/s00401-013-1214-6. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, Wang ZY, Roybon L, Melki R, Li JY. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128(6):805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- Hou Y, Dong L, Lu X, Shi H, Xu B, Zhong W, Ma L, Wang S, Yang C, He X, Zhao Y, Wang S. Distinctions between fecal and intestinal mucosal microbiota in subgroups of irritable bowel syndrome. Dig Dis Sci. 2022;67(12):5580–5592. doi: 10.1007/s10620-022-07588-4. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30(10):1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- Ludtmann MHR, Angelova PR, Horrocks MH, Choi ML, Rodrigues M, Baev AY, Berezhnov AV, Yao Z, Little D, Banushi B, Al-Menhali AS, Ranasinghe RT, Whiten DR, Yapom R, Dolt KS, Devine MJ, Gissen P, Kunath T, Jaganjac M, Pavlov EV, Klenerman D, Abramov AY, Gandhi S. α-Synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat Commun. 2018;9(1):2293. doi: 10.1038/s41467-018-04422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan VP, Zhang X, Morono Y, Inagaki F, Wang F. A modified SDS-based DNA extraction method for high quality environmental DNA from seafloor environments. Front Microbiol. 2016;7:986. doi: 10.3389/fmicb.2016.00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nina Parker MS, Anh-Hue Thi T, Lister P, Forster BM. Microbiology. Houston, Texas: OpenStax; 2016. [Google Scholar]
- Ning J, Huang SY, Chen SD, Zhang YR, Huang YY, Yu JT. Investigating casual associations among gut microbiota, metabolites, and neurodegenerative diseases: a mendelian randomization study. J Alzheimers Dis. 2022;87(1):211–222. doi: 10.3233/JAD-215411. [DOI] [PubMed] [Google Scholar]
- Nuzum ND, Loughman A, Szymlek-Gay EA, Hendy A, Teo WP, Macpherson H. Gut microbiota differences between healthy older adults and individuals with Parkinson’s disease: a systematic review. Neurosci Biobehav Rev. 2020;112:227–241. doi: 10.1016/j.neubiorev.2020.02.003. [DOI] [PubMed] [Google Scholar]
- Park MK, Shin HB. Massilia sp. isolated from otitis media. Int J Pediatr Otorhinolaryngol. 2013;77(2):303–305. doi: 10.1016/j.ijporl.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Qian Y, Yang X, Xu S, Huang P, Li B, Du J, He Y, Su B, Xu LM, Wang L, Huang R, Chen S, Xiao Q. Gut metagenomics-derived genes as potential biomarkers of Parkinson’s diseases. Brain. 2020;143(8):2474–2489. doi: 10.1093/brain/awaa201. [DOI] [PubMed] [Google Scholar]
- Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: a common factor in human diseases. Biomed Res Int. 2017;2017:9351507. doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffmann C, Parkkinen L. Gut feelings about α-synuclein in gastrointestinal biopsies: biomarker in the making? Mov Disord. 2016;31(2):193–202. doi: 10.1002/mds.26480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffmann C, Bengoa-Vergniory N, Poggiolini I, Ritchie D, Hu MT, Alegre-Abarrategui J, Parkkinen L. Detection of alpha-synuclein conformational variants from gastro-intestinal biopsy tissue as a potential biomarker for Parkinson’s diseases. Neuropathol Appl Neurobiol. 2018;44(7):722–736. doi: 10.1111/nan.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MP, Adley CC. Ralstonia spp.: emerging global opportunistic pathogens. Eur J Clin Microbiol. 2014;33(3):291–304. doi: 10.1007/s10096-013-1975-9. [DOI] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167(6):1469–1480.e1412. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70(1):46–58. doi: 10.1016/j.ymeth.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Stephens AD, Zacharopoulou M, Kaminski Schierle GS. The cellular environment affects monomeric α-synuclein structure. Trends Biochem Sci. 2019;44(5):453–466. doi: 10.1016/j.tibs.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol. 2016;79(6):940–949. doi: 10.1002/ana.24648. [DOI] [PubMed] [Google Scholar]
- Sun J, Li H, Jin Y, Yu J, Mao S, Su KP, Ling Z, Liu J. Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson’s disease via gut microbiota-GLP-1 pathway. Brain Behav Immun. 2021;91:703–715. doi: 10.1016/j.bbi.2020.10.014. [DOI] [PubMed] [Google Scholar]
- Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F, Borzabadi S, Oryan S, Mafi A, Asemi Z. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(3):1031–1035. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- Tan AH, Chong CW, Lim SY, Yap IKS, Teh CSJ, Loke MF, Song SL, Tan JY, Ang BH, Tan YQ, Kho MT, Bowman J, Mahadeva S, Yong HS, Lang AE. Gut microbial ecosystem in Parkinson disease: new clinicobiological insights from multi-omics. Ann Neurol. 2021;89(3):546–559. doi: 10.1002/ana.25982. [DOI] [PubMed] [Google Scholar]
- Tan AH, Lim SY, Lang AE. The microbiome-gut-brain axis in Parkinson disease - from basic research to the clinic. Nat Rev Neurol. 2022;18(8):476–495. doi: 10.1038/s41582-022-00681-2. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Browning KN, Camilleri M. Parkinson disease and the gut: new insights into pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol. 2020;17(11):673–685. doi: 10.1038/s41575-020-0339-z. [DOI] [PubMed] [Google Scholar]
- Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm. 2017;124(8):901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer KH. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism relat d. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Xue LJ, Yang XZ, Tong Q, Shen P, Ma SJ, Wu SN, Zheng JL, Wang HG. Fecal microbiota transplantation therapy for Parkinson’s disease: a preliminary study. Medicine. 2020;99(35):e22035. doi: 10.1097/MD.0000000000022035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Yu W, Li X, Li X, Yu S. Alpha-synuclein differentially reduces surface expression of N-methyl-D-aspartate receptors in the aging human brain. Neurobiol Aging. 2020;90:24–32. doi: 10.1016/j.neurobiolaging.2020.02.015. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ning J, Bao XQ, Shang M, Ma J, Li G, Zhang D. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome. 2021;9(1):226. doi: 10.1186/s40168-021-01107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SY, Li HX, Xu RC, Miao WT, Dai MY, Ding ST, Liu HD. Potential roles of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res Rev. 2021;69:101347. doi: 10.1016/j.arr.2021.101347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S4 (PDF 6634 kb)
Data Availability Statement
The data underlying this article are available in the article and supplementary material. Specific data underlying this article are available on request from the corresponding author.