Abstract
Aims
Cardiotoxicity is associated with doxorubicin (DOX), an effective anticancer drug. Apigenin has cardioprotective properties; it may be employed as a capping and reducing agent in synthesizing gold nanoparticles (AuNPs). This study examined the cardioprotective impact of AuNPs synthesized with apigenin (Api) in DOX-induced cardiotoxicity (DIC).
Main methods
Api-AuNPs were synthesized in a single pot without needing additional reagents for reducing gold ions or stabilizing the NPs. The cytotoxicity of Api-AuNPs on H9c2 heart cells was subsequently determined using the MTT assay. In the animal investigation, 40 male rats were randomly assigned to one of four groups: control, cardiotoxicity (DOX), DOX treated with apigenin (DOX + Api), or DOX treated with Api-AuNPs (DOX + Api-AuNPs). To examine heart function, echocardiography was conducted. Blood samples were obtained to evaluate injury indicators (Lactate dehydrogenase (LDH), creatine kinase MB (CK-MB), Cardiac Troponin I (cTn-I), Alanine transaminase (ALT), and Aspartate transaminase (AST)). The heart was removed under general anesthetic, weighed, and preserved in formalin solution. Six micrometer-thick cardiac tissue sections were stained with hematoxylin, eosin (H&E), and immunohistochemistry to identify cardiomyocyte apoptotic markers (Bax, Bcl-2, and caspase3).
Key findings
Api-AuNPs have an average size of 21.4 ± 11.6 nm and are stable in physiological environments. Api-AuNPs therapy substantially reduced body and heart weight loss compared to the DOX group. Injury indicators were reduced dramatically by Api-AuNPs treatment. Api-AuNPs inhibited myocardial apoptosis via modulating Bax, caspase3, and Bcl-2 and ameliorating tissue damage caused by DOX.
Significance
Api-AuNPs' anti-apoptotic activities provide cardioprotection against DIC. It has the potential to reduce cardiotoxicity and boost myocardial performance.
Keywords: Apigenin, Gold nanoparticles, Doxorubicin, Cardiotoxicity, Apoptosis
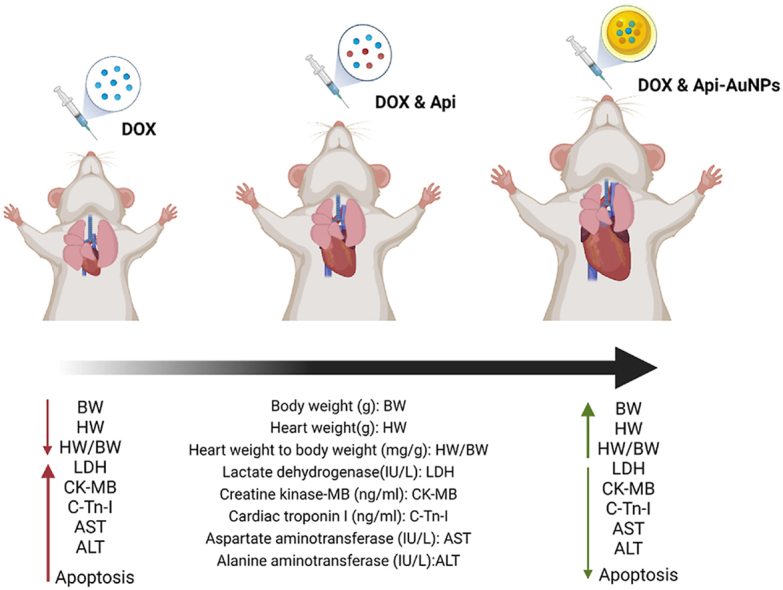
Graphical abstract
Cardioprotective Strategy Against Doxorubicin-induced Cardiotoxicity.
1. Introduction
One of the antitumor agents is DOX. Malignant hematological and solid tumors are widely treated with this drug in adults and children. Cardiotoxicity and other unwanted side effects have been linked to its usage in clinical settings [1,2]. Multiple studies show that DOX causes cardiotoxicity and cardiomyopathy through numerous pathways, including reactive oxygen species (ROS) production and oxidative stress-related damages, apoptotic cell death of cardiomyocytes, intracellular calcium disturbance, and impaired cardiac energy homeostasis [3,4]. Extrinsic and intrinsic apoptosis pathways are both known to be activated by DOX [5]. Therefore, inhibiting apoptosis may help ward off DOX's cardiotoxic effects.
Api is a natural component of plants, fruits, and vegetables. Numerous scientific pieces of research have shown that Api has anti-inflammatory, anti-apoptotic, antioxidant, and anti-carcinogenic characteristics [6,7]. Not only can Api reduce oxidative stress and significantly boost the activity of antioxidant enzymes [8]. It has been observed that Api has potential benefits for cardiovascular illnesses owing to the diminution of pro-apoptotic protein activity, reduction of oxidative stress, and amelioration of mitochondrial damage [9,10]. Therefore, this natural substance is suited for medicinal use.
Among many noble metal nanostructures in recent years, there has been much interest in AuNPs, particularly for biological applications [11,12]. Due to their exceptional features, such as surface plasmon resonance (SPR) that can be tuned, high surface reactivity, biocompatibility, and energy absorbance capacity, are well-suited for diagnostic agents and therapeutic applications [13,14]. The preparation of AuNPs techniques is classified into physical, chemical, and biological processes. A lot of energy is required to produce AuNPs with a physical approach. Also, the quality of the AuNPs is always low [15]. Also, studies have shown the toxicity of chemically prepared AuNPs used in biomedical applications [15,16]. It has been demonstrated that using plant phytochemicals in the biological technique to cap and decrease AuNPs has made this method superior to others. This green synthesis is inexpensive, environmentally friendly, and clinically safe. In prior investigations, plant extracts such as apigenin, gallic acid, and curcumin have all been used [[17], [18], [19]]. Previous research has shown that the action of Api would be augmented by nano-formulations [20].
It has been reported that applying phytochemicals in AuNPs synthesis could increase its antioxidant capacity [21]. AuNPs that phytochemicals have synthesized could decrease DIC [22]. Since Api is poorly soluble in bodily fluids, and we have successfully synthesized the Api-coated AuNPs with high stability in physiological solution, this study aimed to improve the efficacy and bioavailability of Api by capitalizing on its beneficial effects on cardiotoxicity. So, Api-coated AuNPs (Api-AuNPs), highly water-soluble and valuable on DIC, pay particular attention to their protection against cell death especially in cardiotoxicity induced by anti-cancer agents.
2. Materials and methods
2.1. Synthesis of the Api-AuNPs
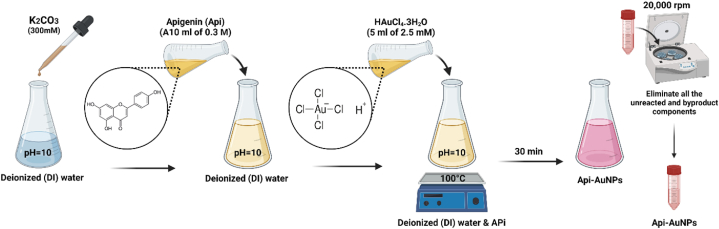
Based on our earlier study, Api-AuNPs were synthesized and characterized [18]. A 10 ml of 0.3 M solutions of Api in deionized (DI) water have been prepared. To increase Api solubility, the pH of the water was adjusted with K2CO3 (300 mM) to a value of 10 before adding the Api to the DI. At the boiling point, 5 ml of HAuCl4.3H2O (2.5 mM) is added to the solution drop by drop. After a while, the appearance of red color is a representation of AuNP synthesis. With centrifugation and decantation at 20,000 rpm, we could eliminate all the unreacted and byproduct components. Therefore, the washing process is performed at least 5 times by centrifugation and supernatant decantation. For further investigations, the final sample was concentrated in less DI water (Fig. 1).
Fig. 1.
A schematic view of the synthesis of Api-AuNPs.
2.2. Characterization of Api-AuNPs
The final gold concentration was determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) analysis after dispersing the last round of sediment in the small volume of (DI) water or other biological solution (Buffer or medium). Physicochemical characterizations, including the efficacy of the washing procedure, the investigation of stability, and the investigation of apigenin coating through FTIR and Raman spectroscopy, were reported in our previous study [17]. Also, the current size, shape, and size distribution of Api-AuNPs were studied using a transmission electron microscope (Zeiss EM 900, Germany), a hydrodynamic diameter (DLS, NANO-flex Particle Sizer Germany), and a double beam UV–visible absorption spectrophotometer (SPEKOL 2000, Analytik Jena, UK).
2.3. In-vitro cytotoxicity evaluation of Api-AuNPs
MTT assay characterized the cytotoxicity of Api-AuNPs on H9c2 heart cells. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM ATCC) with 10% FBS, 100 g/ml streptomycin, and 100 g/ml penicillin G. After adequate growth, 3 × 104 cells/well were placed in three 96-well cell culture plates for the tests. Each well contained 100 μl of cell suspension, and the plates were kept in an incubator at 37 °C and 5% CO2 for 24 h. The cells were separated into control (untreated) and treated (treated with different concentrations of Api-AuNPs). Per well, 100 μL of 2X medium and Api-AuNPs were added for a 24-h incubation period at 37 °C and 5% CO2. The vitality of the cells was then tested after washing them with phosphate-buffered saline (PBS) that was kept sterile. 20 μL of a 5 mg/mL MTT in PBS solution was applied to each well. The purple Formosan crystals were completely dissolved in 100 μL DMSO, and the absorbance was measured at 570 nm using a multimode plate reader after a 4-h incubation.
2.4. Animals and experimental protocols
To create a physiological solution for tail vein injection, stock solutions of gold nanoparticles were sonicated to disperse any aggregation of AuNPs before being diluted with normal saline. By adding NaCl to the sample, AuNPs remained stable. The Experimental Animal Center of Iran University of Medical Sciences supplied 40 adults male Wistar rats weighing 180 and 230 g. Food and water were readily accessible to all animals. The Iran University of Medical Sciences Ethics Committee authorized all experimental procedures and therapies (ethical number: IR.IUMS.REC 1396.32669). This work adhered to the National Institutes of Health's (NIH) recommendations for the care of laboratory animals (NIH Publication No. 85 23, updated 1996). 40 male Wistar rats were randomly divided into four groups: (1) Control group; the animals only received saline as a doxorubicin (DOX) solvent for 12 days (2 mg/kg/day, i.p.), (2) Doxorubicin group (DOX); the animals received a cumulative dose of DOX (12 mg/kg, i.p.) in 6 equal 2 mg/kg doses every 48 h for 12 days (3) DOX + apigenin group (DOX + Api); animals were received a single intravenous (i.v) injection of apigenin (25 mg/kg/day) for 12 days, starting 1 h after the first DOX injection for 12 days and (4) doxorubicin + Api-AuNPs group (DOX + Api-AuNPs): the animals received single i.v dose of Api-AuNPs (20 mg/kg/week) for two weeks. The dosages of DOX and Api were determined in our prior research [23]. The dose of Api-AuNPs was chosen based on Bhattacharya S et al. study [24].
2.5. Blood sampling
On day 12, the animals were weighed and anesthetized deeply with ketamine hydrochloride (80 mg/kg) and Xylazine (8 mg/kg), and blood samples from the left ventricle were taken. The blood samples were then centrifuged at 5000 rpm for 15 min at 4 °C. CK-MB, LDH, cTn-I, ALT, and AST were measured after serum had been isolated and stored at −70 °C.
2.6. Tissue sampling
After collecting blood samples, the hearts were removed by thoracotomy, cleaned with normal saline, and carefully weighed to determine the ratio of heart weight to body weight (HW/BW). Each removed heart was promptly preserved in a 10% formalin solution for 48 h. Embedded in paraffin blocks, sliced using a microtome to a thickness of 6 μm, and stained with hematoxylin and eosin (H&E) and immunohistochemical stains for histological and cardiomyocyte apoptosis evaluations, respectively.
2. 7Measurement of serum parameters
Using particular CK-MB and LDH detection kits (Pars Azmoon, Tehran, Iran) and an auto-analyzer (Roche Hitachi Modular DP Systems; Mannheim, Germany), the serum levels of LDH and CK-MB were calculated (IU/l). An essential and superior marker for myocardial damage is cTnI. cTnI concentrations were determined in ng/ml using a specific kit (Lake Forest, California, USA). ALT and AST-specific kits acquired from Monobind Inc. were used to conduct liver function tests (Lake Forest, California, USA).
2.8. Histopathological evaluation
To examine histological alterations in cardiac tissues, 6 μm slices were deparaffinized, stained with H&E, and evaluated under a light microscope (Olympus BX-50 Olympus Corporation, Tokyo, Japan). Then, digital pictures were taken to assess the histological changes.
2.9. Detection of apoptosis in heart sections
On day 12 post-treatment, cardiac 6 μm slices were stained with polyclonal rabbit anti-Bax, anti-Bcl-2 (ab196495, dilution: 1/100, Abcam), and anti-Caspase-3 primary antibodies, goat antirabbit secondary antibody, and 3,3′-Diaminobenzidine (DAB) chromogen to evaluate apoptotic markers. Under a light microscope, photographs of each stained segment were obtained (BX-51WI Olympus, Tokyo, Japan). The percentage of Bax, Bcl-2, and caspase-3-positive cells in each animal's six random fields and five sections was determined. The assessment was conducted by an examiner who was unaware of the study design.
2.10. Statistical analysis
All data are presented as the mean and standard error of mean (S.E.M). For statistical analysis, GraphPad Prism software (Version 8.3.0) was used. For group comparisons, normally distributed data were analyzed using one-way ANOVA and the Tukey test as a post-doc test. It was deemed statistically significant if P < 0.05.
3. Result
3.1. Characterization of the nanoparticles
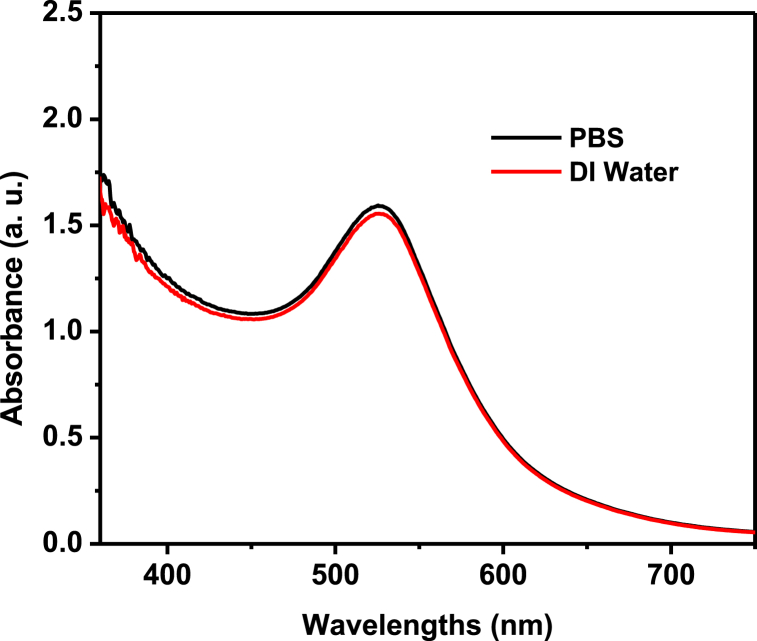
Since the AuNPs may be identified by their distinctive plasmonic peak, a size estimate of AuNPs using UV–Vis spectroscopy has been utilized [25,26], with the information that Haiss et al. have supplied [27]. After final washing, the NP was distributed in PBS or culture medium rather than (DI) water because, as previously shown in our work [17], the Api-AuNPs were very stable. The Api-AuNPs in the PBS medium is exceedingly stable, as seen in Fig. 2.
Fig. 2.
The UV–Vis spectra of Api-AuNPs dispersed in deionized (DI) water and PBS.
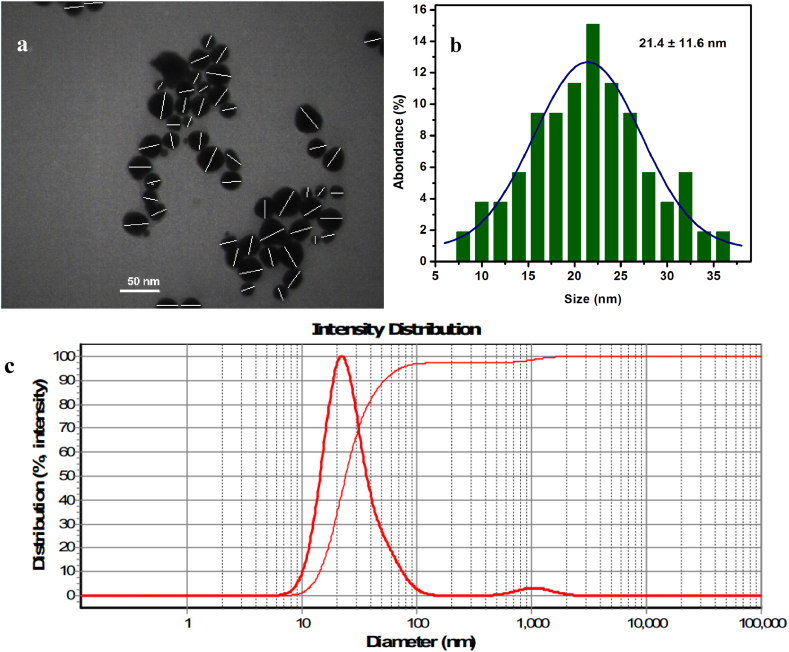
Dynamic Light Scattering (DLS) and transmission electron micrograph (TEM) were used to examine the particle size in more detail. The average size of the NPs was determined by analyzing the TEM experiment's micrograph using digital micrograph software. Our study shows that the nanoparticles' average diameter is 21.4 ± 11.6 nm (Fig. 3 a and b). According to the results from the DLS experiment, the particles' average hydrodynamic diameter is 22.1 nm (Fig. 3 c). Table 1 shows that the supplied findings are all highly correlated.
Fig. 3.
TEM of Api-AuNPs (a) and its size distribution histogram (b). The Hydrodynamic diameter diagram of synthesized Api-AuNPs (c).
Table 1.
Characterization of Api-AuNPs.
| Hydrodynamic diameter | TEM | Zeta potential | |
|---|---|---|---|
| Api-AuNPs | 22.1 nm | 21.4 ± 11.6 nm | −4.3 mV |
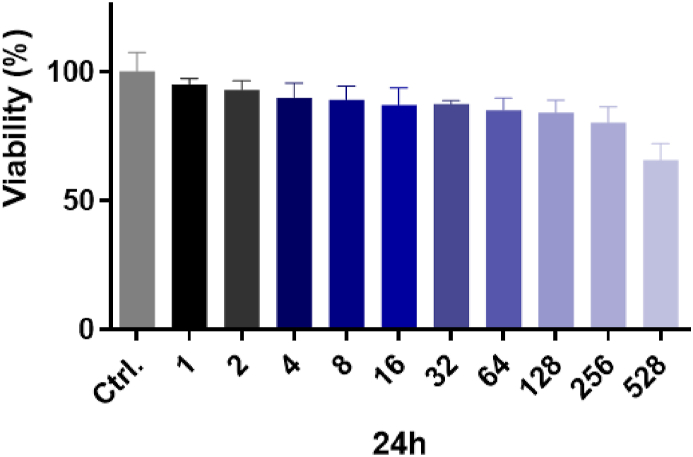
3.2. Cells viability after exposure to Api-AuNPs alone
The MTT test was carried out in H9c2 heart cells for 24 h at 10 concentrations (1–528 ppm). The vitality of H9c2 cells exposed to Api-AuNPs was 91.9% up to 50 ppm, as shown in Fig. 4, indicating no toxicity from such greenly produced AuNPs.
Fig. 4.
The cell viability of H9c2 cells after exposure to apigenin-coated gold nanoparticles (Api-AuNPs) was determined by MTT assay. Cells were treated with different concentrations of Api-AuNPs (1–528 ppm) for 24 h. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Changes in body weight, heart weight, heart weight/body weight
Statistical analysis showed that body weight (BW) was reduced in the DOX group compared with the control and DOX + Api-AuNPs groups (P < 0.001 and P < 0.01, respectively, Table 2). Results show that treatment with Api-AuNPs in DOX-intoxicated rats prevented body weight (BW) and heart weight (HW) reduction compared with DOX and Api + DOX groups (P < 0.001 and P < 0.01). Our results also showed no meaningful changes in heart weight to body weight ratio (HW/BW) between all experimental groups.
Table 2.
Mortality rate and changes in body weight, heart weight, and heart weight/body weight after 12 days of treatment.
| Ctrl | DOX | DOX + Api | DOX + Api-AuNPs | |
|---|---|---|---|---|
| Mortality | 0 | 2 | 1 | 0 |
| BW (g) | 270 ± 5.03 | 203 ± 7.50*** | 218 ± 5.05*** | 254 ± 6.02###$$$ |
| HW (mg) | 1.12 ± 0.08 | 0.62 ± 0.07*** | 0.6 ± 0.08*** | 0.97 ± 0.06##$$ |
| HW/BW (mg/g) | 4.64 ± 0.41 | 3.05 ± 0.67 | 2.75 ± 0.46 | 3.81 ± 0.64 |
Data are presented as Mean ± SEM. BW; body weight (g), HW; heart weight (g), HW/BW; heart weight to body weight (mg/g), Ctrl; Control, DOX; Doxorubicin, Api; Apigenin and Api-AuNPs; Apigenin coated gold nanoparticles.
**P < 0.01 and ***P < 0.001 vs. Ctrl group, ##P < 0.001 and ###P < 0.001 vs. DOX group and $$ P < 0.01 and $$$ P < 0.001 vs. Api + DOX.
3.4. Biochemical Cardiac Injury Markers
Table 3 displays the analysis of cardiac damage enzyme and liver injury indicators (ALT, AST). Serum markers (LDH, CK-MB, and cTn-I) were significantly increased in animals treated with DOX in comparison with control and DOX + Api groups (P < 0.0001). The serum markers of liver injury (ALT, AST) were significantly elevated in DOX treated group in comparison with the control and DOX + Api groups (P < 0.001). Additionally, our findings demonstrated that treatment with Api-AuNPs in the DOX-intoxicated group significantly decreased CK-MB, cTn-I, and LDH, compared to the DOX group (P < 0.001).
Table 3.
Serum levels of LDH, CK-MB, cTn-I, AST, and ALT 12 days after treatment (n = 6).
| Ctrl | DOX | Api + DOX | DOX + Api-AuNPs | |
|---|---|---|---|---|
| LDH (IU/L) | 578.9 ± 39.48 | 1139 ± 78.09**** | 714.4 ± 41.49#### | 733.7 ± 54.08### |
| CK-MB (ng/ml) | 0.255 ± 0.018 | 0.51 ± 0.03**** | 0.32 ± 0.016#### | 0.355 ± 0.019*### |
| cTn-I (ng/ml) | 0.6733 ± 0.044 | 3.475 ± 0.273**** | 1.035 ± 0.069#### | 1.015 ± 0.08512#### |
| AST (IU/L) | 84.14 ± 4.964 | 131.6 ± 10.18** | 99.29 ± 9.426 | 91.86 ± 9.47# |
| ALT (IU/L) | 43.71 ± 3.53 | 70.14 ± 5.535** | 48.00 ± 2.92## | 46.14 ± 4.61## |
Data are presented as Mean ± SEM. LDH; lactate dehydrogenase, CK-MB; Creatine kinase-MB, cTn-I; cardiac troponin I, AST; aspartate aminotransferase, ALT; alanine aminotransferase, DOX; Doxorubicin, Api; Apigenin, and Api-AuNPs; Apigenin coated gold nanoparticles.
**P < 0.01, ***P < 0.001 and ****P < 0.0001, vs. ctrl group and #P < 0.05, ##P < 0.01, ###P < 0.001 and ####P < 0.0001 vs. DOX group.
3.5. Histopathological Changes in Cardiac tissues
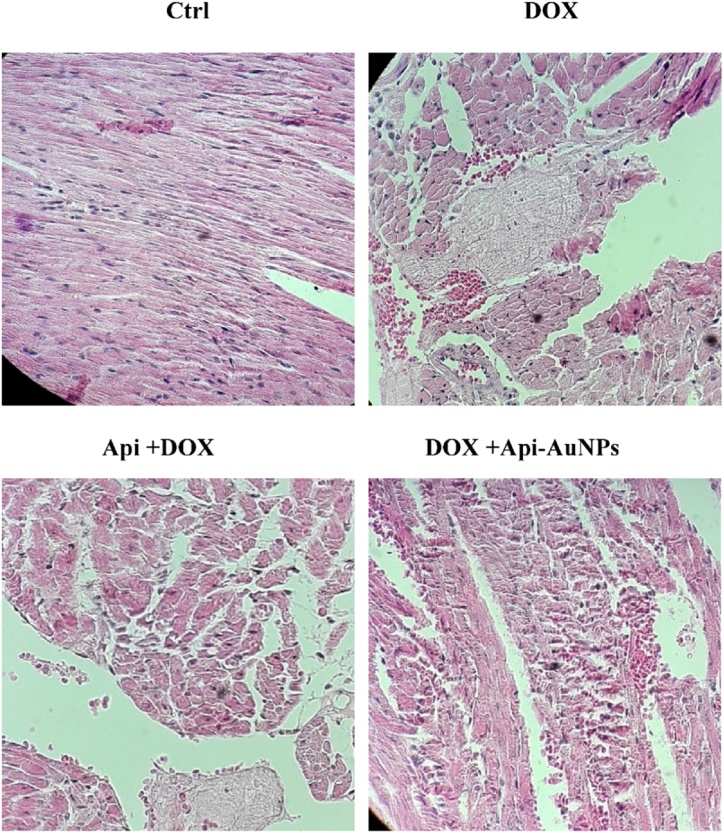
DOX-induced toxicity was assessed by hematoxylin and eosin staining 12 days after treatment. The heart sections of the control group showed regular cell distribution and normal myocardium morphology. However, the cardiac myofibrils in the DOX group showed more intercellular distances and spaces (Fig. 5).
Fig. 5.
Histological changes of myocardial tissue. Heart tissue sections were stained with hematoxylin and eosin (H&E) after 12 days of treatment (n = 5) to detect myocardial changes. × 400 magnifications. DOX; Doxorubicin, Api; Apigenin, Api-AuNPs, Apigenin coated gold nanoparticles. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.6. Effects of apigenin-coated gold nanoparticles on apoptotic markers
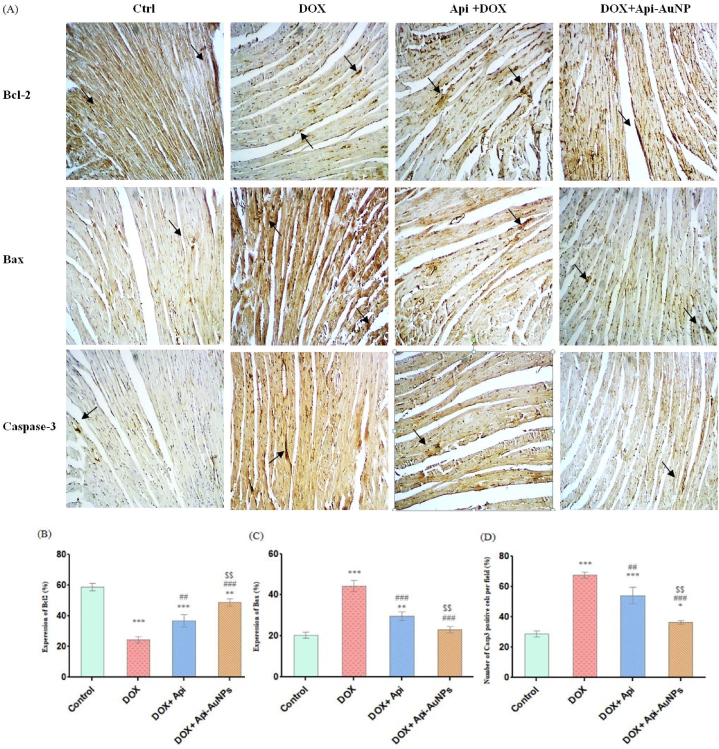
This study investigated apigenin-coated gold nanoparticles' effects on DOX-induced apoptosis. We used immunohistochemical staining to measure Bax, Bcl-2, and Caspase-3 antibodies 12 days after treatment (Fig. 6A). Statistical analysis showed that treatment with Api-AuNPs caused a reduction in the number of Bax-positive cells compared with the DOX group. In addition, the number of Bcl-2 positive cells increased in DOX + Api-AuNPs compared to the DOX group (Fig. 6B–D).
Fig. 6.
Immunohistochemical staining on day 12 after treatment (A, n = 5). Quantification of positive cell numbers of Bcl-2 (B), Bax (C), and (D) Caspase-3. The arrows represent Bcl-2, Bax, and caspase-3 positive cells (brown color, × 400 magnification). DOX; Doxorubicin, Api; Apigenin, Api-AuNPs; Apigenin coated gold nanoparticles. Data are presented as Mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. Ctrl group, ##P < 0.01 and ###P < 0.001 vs. DOX group and $$ P < 0.01 and $$$ P < 0.001 vs. Api + DOX group. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discsussion
As an anthracycline, DOX has been used to treat cancers. However, clinical application is linked to cardiotoxicity [2,28,29]. Previous research has shown that this dosage of DOX causes cardiotoxicity [30,31]. According to pharmacological studies, apigenin has proven anticancer, antibacterial, antioxidant, and anti-inflammatory properties [[32], [33], [34], [35]]. This work injected DOX (2 mg/kg/day for 12 days) into rats to cause cardiotoxicity. Our investigation revealed that DOX administration resulted in cardiotoxicity accompanied by mortality, heart weight, body weight loss, increased cardiac damage markers, cardiomyocyte apoptosis, and histological alterations. In this work, we synthesized AuNPs using Api as reducing or stabilizing agents and demonstrated these particles' cardioprotective properties in a DIC model in male rats.
Using UV–Vis spectroscopy, the average size of the nanoparticles in this experiment is calculated to be 21 nm. According to our earlier research, Api-AuNPs were stable for four weeks in PBS and 24 h in PBS supplemented with 5% FBS daily. It seems that the binding of Api to the surface of the Api-AuNPs is responsible for their stability [17]. DLS and TEM measurements of NPs revealed that Api-AuNPs had a tiny size of around 22.1 nm and 21.4 ± 11.6, respectively.
Api-coated AuNPs were evaluated for their cytotoxicity in vitro using the MTT test. After 24 h, the findings demonstrated that Api-coated AuNPs were non-toxic to H9c2 heart cells, making them appropriate for therapeutic applications. In the animal investigation, DIC resulted in reduced heart and body weight, histological alterations in the heart, elevated LDH, CK-MB, and cTn-I, and myocardial apoptosis. All of these alterations were reversed in DOX-intoxicated rats administered Api-AuNPs with DOX. After 12 days, DOX injection decreased body weight (BW), heart weight (HW), and HW/BW ratio. The HW/BW ratio did not alter after 12 days, comparable to the findings of prior investigations [36,37]. Loss of cardiomyocytes and cytoplasmic vacuolization may cause cardiac weight loss. DOX-induced gastrointestinal toxicity that reduces food intake may also result in a drop in body weight [37,38]. Our results indicated that therapy with Api-AuNPs minimizes heart and body weight loss by reducing cardiomyocyte loss, cytoplasmic vacuolization, and enhancing food intake.
In addition, the induction of cardiotoxicity with DOX considerably elevated the blood levels of LDH, CK-MB, and cTnI. Increased serum enzyme levels indicate heart toxicity and damage [39]. This investigation revealed that intravenous administration of Api-AuNPs significantly decreased serum concentrations of LDH, CK-MB, and cTnI. This research further showed that DIC resulted in morphological changes and cardiomyocyte damage. Api-AuNPs treatment was able to avoid these modifications as much as feasible. Api-AuNPs' protective effects on cardiac tissue, as indicated by hematoxylin and eosin-stained sections and a reduction in myocardial tissue damage, may have prevented the elevation of these markers.
All in all, we examined the impact of Api-AuNPs on DOX-induced myocardial apoptosis. Numerous investigations have shown that apoptosis is crucial to DIC [40]. Previous research demonstrates that DOX decreases Bcl-2, one of the essential regulators of cell death, and increases Bax and Casp3, pro-apoptotic proteins [22,41]. This work also showed that a single intravenous injection of Api-AuNPs increased Bcl-2 expression and reduced Bax and caspase-3 expression. By exerting anti-apoptotic actions, Api-AuNPs prevent cardiac cell death and the loss of functional cardiomyocytes. Consequently, it may reduce heart damage signs and ameliorate DOX-induced weight alterations in treated animals.
5. Conclusion
As a monotherapy or combination therapy, doxorubicin is used in various cancers, and it has been associated with severe cardiac toxicity, making it unsuitable for long-term use. According to the findings of this investigation, Api-AuNPs protect against DOX-induced cardiotoxicity. Api-AuNPs mitigated myocardial injury and apoptosis by enhancing Bcl-2 expression and lowering Bax and Caspase-3 expression. However, more precise mechanistic research is required to corroborate the results with actual signaling pathways. Accumulating shreds of evidence have indicated that Api-AuNPs can exert anticancer activity. Taking the Api-AuNPs into consideration, it can be asked whether these particles enhance the anti-tumor effects of doxorubicin and its cardiovascular benefits. This study may provide a novel therapeutic avenue for doxorubicin in cancer therapy. Investigation of the chronic effects of Api-AuNPs in DOX-induced cardiotoxicity in large animal models with scrutinizing other signaling pathways and functional parameters of the heart would be of value.
Author contribution statement
Zeynab Sharifiaghdam, Seyed Mohammad Amini: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Fereshteh Dalouchi: Performed the experiments; Wrote the paper.
Amir Barzegar Behrooz: Analyzed and interpreted the data; Wrote the paper.
Yaser Azizi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Dr. Yaser Azizi was supported by Iran University of Medical Sciences [96-04-130-32669].
Data availability statement
No data was used for the research described in the article.
Declaration of interest’s statement
The authors declare no conflict of interest.
References
- 1.Zagar T.M., Cardinale D.M., Marks L.B. Breast cancer therapy-associated cardiovascular disease. Nat. Rev. Clin. Oncol. 2016;13(3):172–184. doi: 10.1038/nrclinonc.2015.171. [DOI] [PubMed] [Google Scholar]
- 2.Rochette L., et al. Anthracyclines/trastuzumab: new aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol. Sci. 2015;36(6):326–348. doi: 10.1016/j.tips.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Zhao L., Zhang B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci. Rep. 2017;7(1):1–11. doi: 10.1038/srep44735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo X., et al. Doxorubicin-induced acute changes in cytotoxic aldehydes, antioxidant status and cardiac function in the rat. Biochim. Biophys. Acta, Mol. Basis Dis. 1997;1360(1):45–52. doi: 10.1016/s0925-4439(96)00068-3. [DOI] [PubMed] [Google Scholar]
- 5.Kluza J.m., et al. Mitochondrial proliferation during apoptosis induced by anticancer agents: effects of doxorubicin and mitoxantrone on cancer and cardiac cells. Oncogene. 2004;23(42):7018–7030. doi: 10.1038/sj.onc.1207936. [DOI] [PubMed] [Google Scholar]
- 6.Shukla S., Gupta S. Apigenin: a promising molecule for cancer prevention. Pharmaceut. Res. 2010;27(6):962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du H., et al. Apigenin attenuates acute myocardial infarction of rats via the inhibitions of matrix metalloprotease-9 and inflammatory reactions. Int. J. Clin. Exp. Med. 2015;8(6):8854. [PMC free article] [PubMed] [Google Scholar]
- 8.Jin B.-h., et al. Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress. Eur. J. Pharmacol. 2009;616(1–3):200–205. doi: 10.1016/j.ejphar.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Yang X., et al. Apigenin attenuates myocardial ischemia/reperfusion injury via the inactivation of p38 mitogen-activated protein kinase. Mol. Med. Rep. 2015;12(5):6873–6878. doi: 10.3892/mmr.2015.4293. [DOI] [PubMed] [Google Scholar]
- 10.Patel D., Shukla S., Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise. Int. J. Oncol. 2007;30(1):233–245. [PubMed] [Google Scholar]
- 11.Akbari A., et al. In-vitro investigation of curcumin coated gold nanoparticles effect on human colorectal adenocarcinoma cell line. Nanomed. Res. J. 2022;7(1):66–72. [Google Scholar]
- 12.Ahmadi Kamalabadi M., et al. Folate functionalized gold-coated magnetic nanoparticles effect in combined electroporation and radiation treatment of HPV-positive oropharyngeal cancer. Med. Oncol. 2022;39(12):1–10. doi: 10.1007/s12032-022-01780-2. [DOI] [PubMed] [Google Scholar]
- 13.Amini S.M., Kharrazi S., Jaafari M.R. Radio frequency hyperthermia of cancerous cells with gold nanoclusters: an in vitro investigation. Gold Bull. 2017;50(1):43–50. [Google Scholar]
- 14.Koosha F., et al. Mesoporous silica coated gold nanorods: a multifunctional theranostic platform for radiotherapy and X-ray imaging. J. Porous Mater. 2021;28(6):1961–1968. [Google Scholar]
- 15.Amini S.M., Akbari A. Metal nanoparticles synthesis through natural phenolic acids. IET Nanobiotechnol. 2019;13(8):771–777. doi: 10.1049/iet-nbt.2018.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammadi E., et al. An overview of antimicrobial efficacy of curcumin-silver nanoparticles. Nanomed. Res. J. 2021;6(2):105–111. [Google Scholar]
- 17.Amini S.M., et al. Investigating the in vitro photothermal effect of green synthesized apigenin-coated gold nanoparticle on colorectal carcinoma. IET Nanobiotechnol. 2021;15(3):329–337. doi: 10.1049/nbt2.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neshastehriz A., et al. In-vitro investigation of green synthesized gold nanoparticle's role in combined photodynamic and radiation therapy of cancerous cells. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020;11(4) [Google Scholar]
- 19.Badirzadeh A., et al. Potential therapeutic effects of curcumin coated silver nanoparticle in the treatment of cutaneous leishmaniasis due to Leishmania major in-vitro and in a murine model. J. Drug Deliv. Sci. Technol. 2022;74 [Google Scholar]
- 20.Das S., et al. Efficacy of PLGA-loaded apigenin nanoparticles in Benzo [a] pyrene and ultraviolet-B induced skin cancer of mice: mitochondria mediated apoptotic signalling cascades. Food Chem. Toxicol. 2013;62:670–680. doi: 10.1016/j.fct.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Shaabani E., et al. Curcumin coated gold nanoparticles: synthesis, characterization, cytotoxicity, antioxidant activity and its comparison with citrate coated gold nanoparticles. Nanomed. J. 2017;4(2):115–125. [Google Scholar]
- 22.Sharifiaghdam Z., et al. Curcumin‐coated gold nanoparticles attenuate doxorubicin‐induced cardiotoxicity via regulating apoptosis in a mouse model. Clin. Exp. Pharmacol. Physiol. 2022;49(1):70–83. doi: 10.1111/1440-1681.13579. [DOI] [PubMed] [Google Scholar]
- 23.Zare M.F.R., et al. Apigenin attenuates doxorubicin induced cardiotoxicity via reducing oxidative stress and apoptosis in male rats. Life Sci. 2019;232 doi: 10.1016/j.lfs.2019.116623. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharya S., et al. Apigenin loaded nanoparticle delayed development of hepatocellular carcinoma in rats. Nanomed. Nanotechnol. Biol. Med. 2018;14(6):1905–1917. doi: 10.1016/j.nano.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Fatemi F., et al. Construction of genetically engineered M13K07 helper phage for simultaneous phage display of gold binding peptide 1 and nuclear matrix protein 22 ScFv antibody. Colloids Surf. B Biointerfaces. 2017;159:770–780. doi: 10.1016/j.colsurfb.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 26.Rezaeian A., et al. Plasmonic hyperthermia or radiofrequency electric field hyperthermia of cancerous cells through green-synthesized curcumin-coated gold nanoparticles. Laser Med. Sci. 2021;37(2):1333–1341. doi: 10.1007/s10103-021-03399-7. [DOI] [PubMed] [Google Scholar]
- 27.Haiss W., et al. Determination of size and concentration of gold nanoparticles from UV− Vis spectra. Anal. Chem. 2007;79(11):4215–4221. doi: 10.1021/ac0702084. [DOI] [PubMed] [Google Scholar]
- 28.McGowan J.V., et al. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc. Drugs Ther. 2017;31(1):63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarchi A.A.K., et al. Synthesis and characterisation of liposomal doxorubicin with loaded gold nanoparticles. IET Nanobiotechnol. 2018;12(6):846–849. doi: 10.1049/iet-nbt.2017.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razmaraii N., et al. Crocin treatment prevents doxorubicin-induced cardiotoxicity in rats. Life Sci. 2016;157:145–151. doi: 10.1016/j.lfs.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Razmaraii N., et al. Cardioprotective effect of phenytoin on doxorubicin-induced cardiac toxicity in a rat model. J. Cardiovasc. Pharmacol. 2016;67(3):237–245. doi: 10.1097/FJC.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 32.Lee W.-H., et al. Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013;11(4):338–378. doi: 10.2174/1570159X11311040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trujillo J., et al. Mitochondria as a target in the therapeutic properties of curcumin. Arch. Pharmazie. 2014;347(12):873–884. doi: 10.1002/ardp.201400266. [DOI] [PubMed] [Google Scholar]
- 34.Wang H.-M., et al. PPARγ agonist curcumin reduces the amyloid-β-stimulated inflammatory responses in primary astrocytes. J. Alzheim. Dis. 2010;20(4):1189–1199. doi: 10.3233/JAD-2010-091336. [DOI] [PubMed] [Google Scholar]
- 35.Ganjali S., et al. Investigation of the effects of curcumin on serum cytokines in obese individuals: a randomized controlled trial. Sci. World J. 2014;2014 doi: 10.1155/2014/898361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu J., et al. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in lymphoma nude mice by heme oxygenase-1 induction. Cardiovasc. Toxicol. 2012;12(4):341–349. doi: 10.1007/s12012-012-9178-7. [DOI] [PubMed] [Google Scholar]
- 37.Sahu B.D., et al. Baicalein alleviates doxorubicin-induced cardiotoxicity via suppression of myocardial oxidative stress and apoptosis in mice. Life Sci. 2016;144:8–18. doi: 10.1016/j.lfs.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Carvalho F.S., et al. Doxorubicin‐induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med. Res. Rev. 2014;34(1):106–135. doi: 10.1002/med.21280. [DOI] [PubMed] [Google Scholar]
- 39.Yu W., et al. Apigenin attenuates adriamycin-induced cardiomyocyte apoptosis via the PI3K/AKT/mTOR pathway. Evid. base Compl. Alternative Med. 2017;2017 doi: 10.1155/2017/2590676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo R., et al. Hydrogen sulfide attenuates doxorubicin-induced cardiotoxicity by inhibition of the p38 MAPK pathway in H9c2 cells. Int. J. Mol. Med. 2013;31(3):644–650. doi: 10.3892/ijmm.2013.1246. [DOI] [PubMed] [Google Scholar]
- 41.Rakhshan K., et al. Modulation of apoptosis and oxidative stress with nesfatin-1 in doxorubicin induced cardiotoxicity in male rat. Int. J. Pept. Res. Therapeut. 2022;28(4):1–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.