Abstract
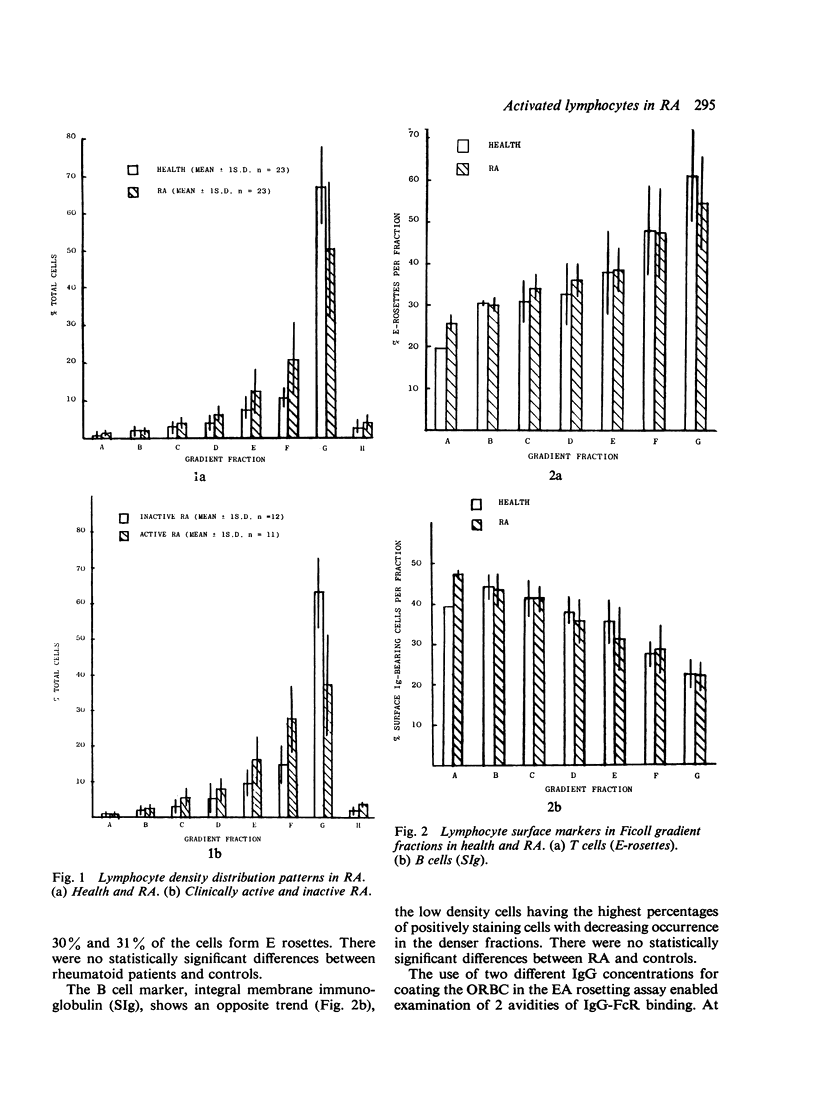
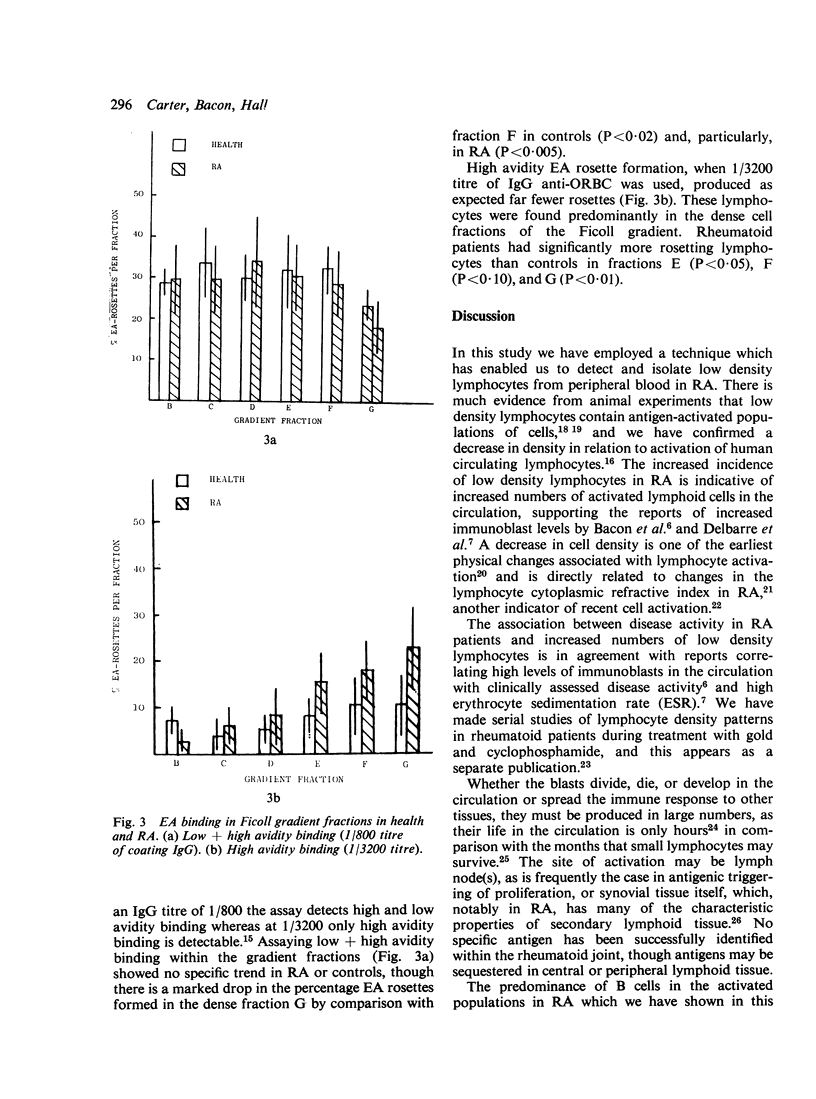
The extent and nature of lymphocyte activation in the circulation in rheumatoid arthritis (RA) was investigated. Peripheral blood lymphocytes (PBL) from RA patients and healthy controls were separated into a number of discrete fractions by density in discontinuous Ficoll density gradients. Low density (activated) lymphocytes were found at significantly higher levels in RA, particularly in patients with clinically active disease. Conversely, patients with clinically inactive RA had normal levels of activated lymphocytes. Lymphocyte populations within the ficoll gradient fractions were detected by E rosettes, staining for surface Ig, and by different avidities of EA binding. The activated population in RA was shown to be relatively depleted of T cells, enriched in surface Ig-bearing lymphocytes, and depleted of lymphocytes with high avidity EA binding. The evidence suggests that many of the activated PBL in RA are B blasts.
Full text
PDF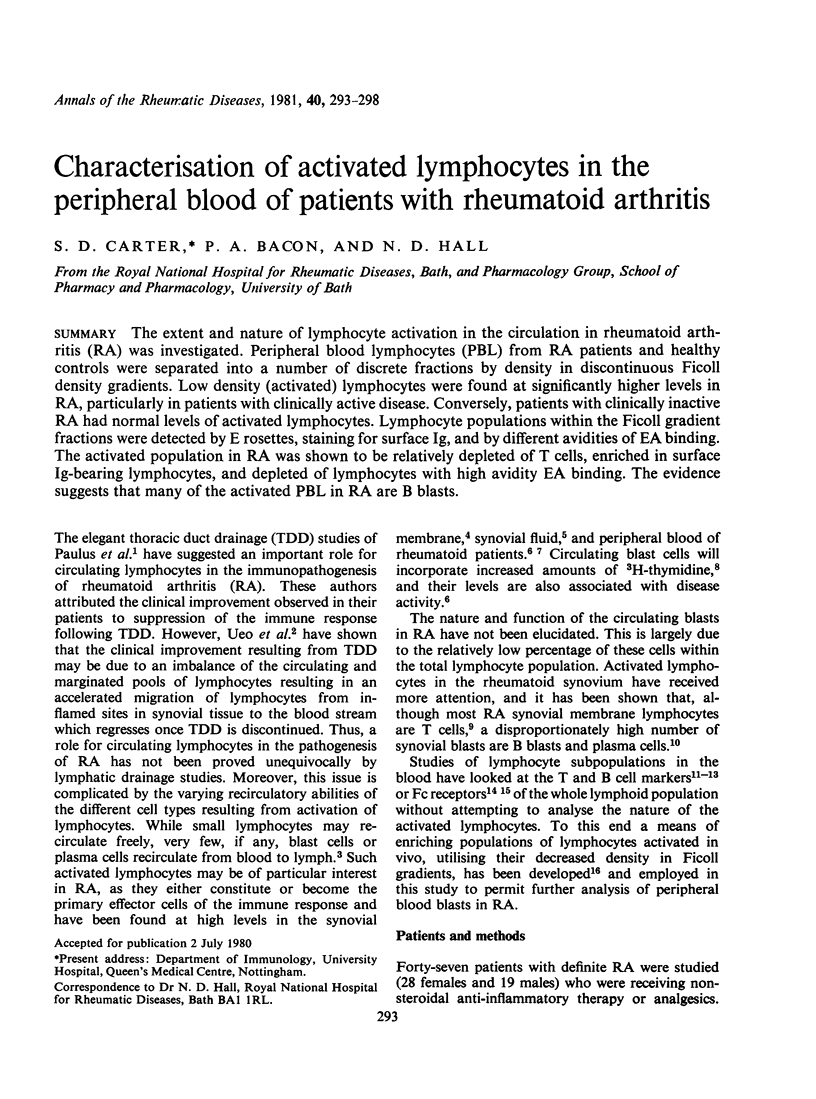



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon P. A., Sewell R. L., Crowther D. Reactive lymphoid cells ('Immunoblasts') in autoimmune and haematological disorders. Clin Exp Immunol. 1975 Feb;19(2):201–208. [PMC free article] [PubMed] [Google Scholar]
- Brenner A. I., Scheinberg M. A., Cathcart E. S. Surface characteristics of synovial fluid and peripheral blood lymphocytes in inflammatory arthritis. Arthritis Rheum. 1975 Jul-Aug;18(4):297–303. doi: 10.1002/art.1780180402. [DOI] [PubMed] [Google Scholar]
- Delbarre F., Le Gô A., Kahan A. Hyperbasophilic immunoblasts in circulating blood in chronic inflammatory rheumatic and collagen diseases. Ann Rheum Dis. 1975 Oct;34(5):422–430. doi: 10.1136/ard.34.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R. M., Miller R. G., Phillips R. A. Homogeneity of antibody-producing cells as analysed by their buoyant density in gradients of Ficoll. Immunology. 1970 Nov;19(5):817–829. [PMC free article] [PubMed] [Google Scholar]
- Hall J. G., Smith M. E. Homing of lymph-borne immunoblasts to the gut. Nature. 1970 Apr 18;226(5242):262–263. doi: 10.1038/226262a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Ziff M. Electron microscopic observations of immunoreactive cells in the rheumatoid synovial membrane. Arthritis Rheum. 1976 Jan-Feb;19(1):1–14. doi: 10.1002/art.1780190101. [DOI] [PubMed] [Google Scholar]
- Jondal M. Surface markers on human B and T lymphocytes. IV. Distribution of surface markers on resting and blast-transformed lymphocytes. Scand J Immunol. 1974;3(6):739–747. doi: 10.1111/j.1365-3083.1974.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Jondal M. Surface markers on human B and T lymphocytes. V. Characterization of the lymphoproliferative response to three different lectins and allogeneic lymphocytes by surface markers. Scand J Immunol. 1974;3(6):749–755. doi: 10.1111/j.1365-3083.1974.tb01310.x. [DOI] [PubMed] [Google Scholar]
- Lobo P. I., Westervelt F. B., Horwitz D. A. Identification of two populations of immunoglobulin-bearing lymphocytes in man. J Immunol. 1975 Jan;114(1 Pt 1):116–119. [PubMed] [Google Scholar]
- Loewi G., Dorling J., Howard A. Mononuclear cells from inflammatory joint effusions: electron-microscopic appearances and immunoglobulin synthesis. J Rheumatol. 1974 Mar;1(1):34–44. [PubMed] [Google Scholar]
- Mellbye O. J., Messner R. P., DeBord J. R., Williams R. C., Jr Immunoglobulin and receptors for C3 on lymphocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1972 Jul-Aug;15(4):371–380. doi: 10.1002/art.1780150408. [DOI] [PubMed] [Google Scholar]
- Parrott D. M., De Sousa M. Thymus-dependent and thymus-independent populations: origin, migratory patterns and lifespan. Clin Exp Immunol. 1971 May;8(5):663–684. [PMC free article] [PubMed] [Google Scholar]
- Paulus H. E., Machleder H. I., Levine S., Yu D. T., MacDonald N. S. Lymphocyte involvement in rheumatoid arthritis. Studies during thoracic duct drainage. Arthritis Rheum. 1977 Jul-Aug;20(6):1249–1262. doi: 10.1002/art.1780200614. [DOI] [PubMed] [Google Scholar]
- Sharpin R. K., Wilson J. D. Increased EA-rosette formation by lymphocytes from patients with rheumatoid arthritis. Clin Exp Immunol. 1977 Aug;29(2):205–212. [PMC free article] [PubMed] [Google Scholar]
- Sharpin R. K., Wilson J. D. Studies on the nature of EA binding by lymphocytes from rheumatoid arthritis patients. Clin Exp Immunol. 1977 Aug;29(2):213–219. [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Haskill J. S., Szenberg A., Legge D. G. Density distribution analysis of lymphocyte populations. Nature. 1967 Dec 23;216(5121):1227–1228. doi: 10.1038/2161227a0. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Machtinger B. G., Fried J., Cohn Z. A. Mouse spleen lymphoblasts generated in vitro. Recovery in high yield and purity after floatation in dense bovine plasma albumin solutions. J Exp Med. 1978 Feb 1;147(2):279–296. doi: 10.1084/jem.147.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surjàn L., Jr, Sebök J. Increase in intranuclear birefringence during chromatin activation reaction. Exp Cell Res. 1973 Mar 30;78(1):241–243. doi: 10.1016/0014-4827(73)90063-3. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., McIntire K. R., Boyse E. A. Immunoglobulin and other surface antigens of cells of the immune system. J Exp Med. 1971 Oct 1;134(4):815–832. doi: 10.1084/jem.134.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueo T., Tanaka S., Tominaga Y., Ogawa H., Sakurami T. The effect of thoracic duct drainage on lymphocyte dynamics and clinical symptoms in patients with rheumatoid arthritis. Arthritis Rheum. 1979 Dec;22(12):1405–1412. doi: 10.1002/art.1780221217. [DOI] [PubMed] [Google Scholar]
- Van Boxel J. A., Paget S. A. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975 Sep 11;293(11):517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Whaley K., Glen A. C., MacSween R. N., Deodhar S., Dick W. C., Nuki G., Williamson J., Buchanan W. W. Immunological responses in Sjogren's syndrome and rheumatoid arthritis. Clin Exp Immunol. 1971 Dec;9(6):721–732. [PMC free article] [PubMed] [Google Scholar]
- Yu D. T., Peter J. B. Cellular immunological aspects of rheumatoid arthritis. Semin Arthritis Rheum. 1974 Fall;4(1):25–52. doi: 10.1016/0049-0172(74)90016-x. [DOI] [PubMed] [Google Scholar]


