Abstract
The respiratory tract pathogen Chlamydia pneumoniae has been associated with atherosclerosis. Monocytes are supposed to serve as a vehicle for systemic dissemination of intracellular C. pneumoniae from the lung to the artery vessel wall. We were therefore interested in pathogen-induced cellular events associated with NF-κB, a crucial transcription factor for both inflammatory cytokines and antiapoptotic molecules. In this study we demonstrate by electrophoretic mobility shift assay that C. pneumoniae infection of the human monocytic cell line Mono Mac 6 induces activation of NF-κB over 48 h, with a maximum level at 1 h postinfection. As shown by supershift assay, the activated NF-κB complex consists of the subunits RelA (p65) and NF-κB1 (p50). Apoptotic host cells were not detected during the early stages of the infection when maximal activation of NF-κB was detected. Pretreatment of Mono Mac 6 with the antioxidant and NF-κB inhibitor PDTC (pyrrolidine dithiocarbamate) induced activation of caspase-3 and led to apoptotic cell death. The C. pneumoniae-induced activation of the NF-κB complex was reduced by PDTC, which in parallel resulted in an increased apoptosis, as quantified by annexin V labeling and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling reaction. In the complete absence of activated NF-κB, when Mono Mac 6 cells were pretreated with the more potent NF-κB inhibitors MG-132 and parthenolide a C. pneumoniae-mediated rescue of cells from induced apoptosis could not be achieved. Our results indicate that activation of NF-κB in C. pneumoniae-infected Mono Mac 6 cells is associated with protection of Mono Mac 6 cells against apoptosis and might thereby contribute to systemic spread of the pathogen.
The obligate intracellular human pathogen Chlamydia pneumoniae is a widely distributed agent of usually mild infections of the respiratory tract (14, 15). Based on seroepidemiological studies, C. pneumoniae has also been associated with atherosclerosis (34), the pathological correlate of both coronary and peripheral artery disease. According to the response to injury hypothesis, atherosclerosis can be considered the final stage of a chronic inflammatory process in the artery vessel wall which is characterized by endothelial injury, accumulation of monocytic cells, increased secretion of cytokines and growth factors, foam cell formation, and proliferation of smooth muscle cells (33). In addition to known risk factors, such as smoking, hypercholesterolemia, and hypertension, chronic C. pneumoniae infection has been proposed to induce and maintain inflammatory events within the vessel wall because (i) the agent has been detected in atherosclerotic plaques by culture, PCR, immunocytochemistry, and electronmicroscopy (22, 29), (ii) C. pneumoniae infects in vitro all cell types of the vascular wall, which results in an increased secretion of cytokines and upregulation of cellular receptors and adhesion molecules (17, 28), and (iii) experimental infection of animals leads to progression and aggravation of atherosclerotic lesion development (4).
Chronic vascular C. pneumoniae infection in humans is difficult to prove, and little is known about the underlying molecular mechanisms for persistence. Recently, the modulation of host cell apoptosis has been discussed as a survival strategy of intracellular bacterial pathogens (12). Chlamydia trachomatis has been shown to block host cell apoptosis induced by proapoptotic stimuli during early stages of infection (10), while Chlamydia psittaci induces apoptosis during late stages of infection (27). This points to a well-balanced mechanism which, on the one hand, allows Chlamydia spp. to complete effectively their developmental cycle and on the other hand facilitates the release and spread of infectious elementary bodies. Bacterial factors which determine anti- and proapoptotic activities at different stages of infection are unknown.
In previous investigations it could be demonstrated that C. pneumoniae is able to survive for at least 2 weeks in the human monocytic cell line Mono Mac 6 (17). In addition, growth of bacteria induces production of proinflammatory cytokines and expression of CD14. More recently, PCR studies of Blasi et al. and Boman et al. revealed that human peripheral blood monocytes (PBMCs) contained chlamydial DNA (2, 3). Therefore, monocytes might be the missing link between pulmonary and vascular infection.
The ability to respond to extracellular signals by changes in gene expression via transcription factors is essential for the development and survival of all cells in a living organism (37). Transcription factors of the NF-κB/Rel family are critical for the inducible expression of multiple genes involved both in inflammatory responses and apoptosis. NF-κB dimers, most commonly composed of the RelA (p65) and NF-κB1 (p50) or NF-κB2 (p52) subunits, are sequestered in an inactive cytoplasmatic complex by binding to its inhibitory subunit, IκB. Upon stimulation IκB gets phosphorylated by IκB kinase (IKK). This phosphorylation is followed by ubiquitination and rapid degradation of IκB by a proteasome-dependent pathway and allows translocation of free, active NF-κB complexes into the nucleus, where they bind to specific DNA motifs in the promoter/enhancer regions of target genes and activate transcription (1, 37).
In this paper we demonstrate that C. pneumoniae infection of the human monocytic cell line Mono Mac 6 activates NF-κB and that the level of experimentally modulated NF-κB binding activity corresponds to the extent of apoptosis of the host cells.
MATERIALS AND METHODS
Chlamydial culture and inoculum preparation.
C. pneumoniae strain TW-183 (Washington Research Foundation, Seattle, Wash.) was used throughout the study and was propagated in cycloheximide-treated Hep-2 cells (ATCC CCL-23) according to standard procedures (30). C. pneumoniae cultures were free of mycoplasma contamination, as determined by PCR and 4′,6′-diamidino-2-phenylindole (DAPI) staining (9). Infected monolayers were harvested on day 3 from 6-well plates and were vortexed with glass beads for 2 to 5 min. Cellular debris was removed by centrifugation at 800 × g for 10 min at 4°C. The supernatant was centrifuged at 39,800 × g (Avanti J-25; Beckman) for 1 h at 4°C, and the pellets were resuspended in sucrose-phosphate-glutamate buffer (0.22 M sucrose, 10 mM Na2HPO4, 3.8 mM KH2PO4, 5 mM glutamic acid, pH 7.4). Aliquots containing 5 × 108 inclusion-forming units (IFU) of C. pneumoniae per ml as well as aliquots of control inocula prepared according to the same procedure with uninfected Hep-2 cells (mock) were stored at −70°C until use.
Monocyte cell culture.
The permanent and highly differentiated human monocytic cell line Mono Mac 6, which has been described by Ziegler-Heitbrock in detail (43), was used as a tool to study the interaction of C. pneumoniae with human monocytic cells. Mono Mac 6 cells were purchased from the German Culture Collection (DSMZ 124; Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany) and maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, nonessential amino acids, L-glutamine, insulin, oxalacetate, HEPES, and glucose (43).
Chlamydial infection of Mono Mac 6 cells.
Mono Mac 6 cell infection was performed, with slight modifications, as previously described (17). For nuclear protein extraction, monocytes were seeded for infection at a density of 107 in petri dishes, and diluted stocks of C. pneumoniae were added to obtain a multiplicity of infection (MOI) of 5 IFU per cell. This procedure yielded an infectivity rate of approximately 80% as determined by direct immunofluorescence staining of infected Mono Mac 6 cells using a fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody with specificity to chlamydial lipopolysaccharide (Pathfinder Kallestad, Chaska, Minn.). Infected and mock-infected Mono Mac 6 cells were incubated at 37°C, and cells were harvested for detection of NF-κB and apoptosis at the times indicated below.
Nuclear protein extraction.
Nuclear extracts were prepared as described previously (40). Briefly, Mono Mac 6 cells were washed twice with phosphate-buffered saline (pH 7.4) and were suspended in sucrose buffer (0.32 M sucrose, 3 mM CaCl2, 2 mM MgAc, 100 μM EDTA, 10 mM Tris-HCl, 1 mmol of dithiothreitol [DTT] per liter, 500 μM phenylmethylsulphonyl fluoride [PMSF], and Nonidet P-40 [NP-40]) at a final concentration of 0.5%. Isolated nuclei were resuspended in 0.02 M KCl buffer. Nuclear proteins were extracted by addition of 0.8 M KCl buffer and incubation at 4°C for 20 min. After centrifugation, supernatants with nuclear proteins were transferred into precooled tubes, and protein concentrations were determined by the Bradford assay using a commercially available kit (Bio-Rad, Munich, Germany).
Electrophoretic mobility shift assay (EMSA).
Nuclear protein extracts were incubated with radiolabeled DNA probes in a 20-μl reaction mixture containing 10 mM Tris (pH 7.5), 50 mM NaCl, 1 mM DTT, 3% glycerol, 50 μM MgCl2, and 1 μg of poly(dI-dC) · poly(dI-dC). The DNA probe used in this study included a double-stranded oligonucleotide probe encoding the κB motif of the mouse immunoglobulin kappa light chain enhancer (5′-CTAGTCTCAACAGAGGGGACTTTCCGAGAGGCCAT-3′), which has been endlabeled with 32P. Nucleoprotein complexes were separated by electrophoresis in 4% nondenaturing polyacrylamide gels in Tris glycine buffer. Dried gels were exposed to Kodak-BioMax film at −70°C with intensifying screen. Supershift assays were performed using polyclonal antibodies against the NF-κB proteins NF-κB1 (p50), NF-κB2 (p52), and the Rel proteins RelA (p65), RelB, and c-Rel (Santa Cruz Biotechnology, Santa Cruz, Calif.).
Induction of apoptosis through NF-κB inhibition.
The radical scavenging agent pyrrolidine dithiocarbamate (PDTC) can react with and thereby eliminate reactive oxygen intermediates which are involved in activation of NF-κB (36). In addition, PDTC has been shown as an inductor of apoptosis in monocytes (8). Since NF-κB activation might be involved in protection against apoptosis (5, 19, 38, 41), we studied if C. pneumoniae-infected Mono Mac 6 cells are more resistant to PDTC-induced apoptosis than noninfected Mono Mac 6 cells. Therefore, Mono Mac 6 cells were preincubated for 1 h with 10−4 M PDTC (Sigma Chemicals, Deisenhofen, Germany). One hour after addition of PDTC, cells were infected with C. pneumoniae for 9 h and assessment of apoptosis was performed in infected versus noninfected monocytes, as described below. To investigate the effect of PDTC pretreatment on NF-κB activation in infected Mono Mac 6 cells, cells were harvested and nuclear proteins were extracted for analysis by EMSA. To further characterize the role of NF-κB binding activity for survival of Mono Mac 6 cells, similar experiments were perfomed using the more potent NF-κB inhibitors MG-132 (carbobenzoxylleucinylleucinyl-leucinal-H; Sigma Chemicals) and parthenolide (Sigma Chemicals), which differ in their targets for NF-κB inactivation.
Assessment of apoptosis.
Cells were investigated for signs of apoptosis at 9 h postinfection. Externalization of the membrane phospholipid phosphatidylserine from the inner to the outer leaflet of the plasma membrane occurs when cells enter apoptotic states. Therefore, the Ca2+-dependent phospholipid-binding protein annexin V was used as a probe for identifying cells early in apoptosis (23, 39). Annexin V was used in conjunction with the vital dye propidium iodide to distinguish apoptotic (annexin V positive, propidium iodide negative) from necrotic cells (annexin V positive, propidium iodide positive), because externalization of phosphatidylserine also occurs during necrosis. Therefore, unfixed Mono Mac 6 cells were washed twice with PBS and resuspended in a binding buffer containing 10 mM HEPES–NaOH (pH 7.5), 140 mM NaCl, and 2.5 mM CaCl2. Cells were incubated for 15 min with annexin V-FITC (PharMingen, San Diego, Calif.) and propidium iodide and subsequently were analyzed by flow cytometry with a fluorescence-activated cell sorter (FACStar; Becton Dickinson). Nuclear changes associated with early apoptosis were detected by the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) method using an in situ cell death detection kit (Roche Diagnostics GmbH, Mannheim, Germany). Infected and noninfected Mono Mac 6 cells with and without PDTC pretreatment were washed with PBS, spotted on microscope slides, fixed, and permeabilized. Enzymatic incorporation of fluoresceinated nucleotides was performed according to the instructions of the manufacturer. Cellular fluorescence was evaluated by microscopy (magnification, ×400; Zeiss axiophot 2), and the percentage of TUNEL-positive cells from at least 200 Mono Mac 6 cells was determined by counting fluorescent cells in 10 different fields. Caspase-3 activity in PDTC-treated Mono Mac 6 cells was examined by Western blot analysis. Therefore, equal amounts of cell extract proteins (20 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked in 3% nonfat milk for 10 h and then were incubated with an antibody to caspase-3 (PharMingen, San Diego, Calif.) or poly(ADP-ribose) polymerase (PARP) (PharMingen) for 2 h. After being extensively washed, membranes were incubated with secondary antibody coupled to horseradish peroxidase (Dianova, Hamburg, Germany) for 1 h at room temperature. Signals were visualized with an enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom).
RESULTS
C. pneumoniae infection of Mono Mac 6 induces NF-κB/Rel activation.
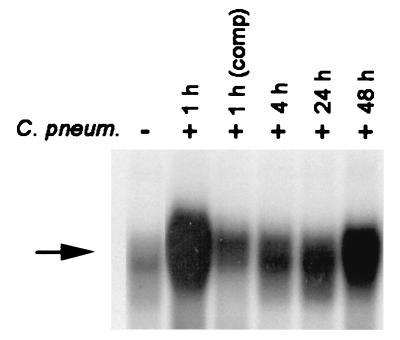
The mature monocytic cell line Mono Mac 6 was used as a model system for studying Chlamydia-host cell interaction. In previous experiments we could demonstrate that C. pneumoniae multiplied within Mono Mac 6 and induced release of proinflammatory cytokines (17). To study the effect of C. pneumoniae on NF-κB binding activity, EMSAs were performed. Mono Mac 6 cells were infected with C. pneumoniae at an MOI of 5 and harvested 1, 4, 24, and 48 h after infection. Nuclear extracts were prepared and incubated with a 32P-endlabeled DNA oligonucleotide containing the recognition site of NF-κB. While little specific NF-κB binding activity was detected in noninfected cells, C. pneumoniae induced NF-κB activity up to 48 h postinfection. Maximal activation of inducible DNA binding activity was detected 1 h postinfection (Fig. 1). The specificity of NF-κB DNA binding induced by C. pneumoniae was confirmed in competition experiments. Incubation with an excess of an unrelated oligonucleotide spanning an activator protein 1 binding site did not antagonize NF-κB binding (data not shown), whereas competition with a 100-fold excess of unlabeled oligonucleotide led to inhibition of binding activity.
FIG. 1.
NF-κB activity in Mono Mac 6 cells. EMSA showing the induction of NF-κB binding activity by C. pneumoniae (C. pneum.). Nuclear extracts were prepared, and equal amounts were reacted with 32P-labeled DNA probe encompassing the κB motif of the mouse kappa light chain enhancer. The arrow indicates the position of the κB-specific DNA binding activity. Data are representative examples of two similar experiments. comp, competition.
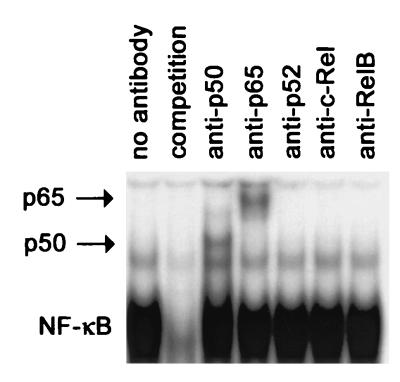
Because NF-κB complexes may constitute a variety of different homo- and heterodimers, the subunit compositions of the C. pneumoniae-induced DNA complex were analyzed by a supershift assay. Antibodies directed against p50, p52, p65, c-Rel, and RelB were added to nuclear extracts of infected Mono Mac 6 cells 1 h postinfection. Anti-p50 and anti-p65 retarded the NF-κB-specific DNA complex (Fig. 2). These data indicate the presence of p50 and p65 in the C. pneumoniae-induced NF-κB complex in Mono Mac 6 cells.
FIG. 2.
Supershift assay identifying the subunit composition of NF-κB complexes in C. pneumoniae-infected Mono Mac 6 cells. Data are representative examples of two similar experiments.
PDTC induces apoptosis in Mono Mac 6 cells by activation of caspase-3.
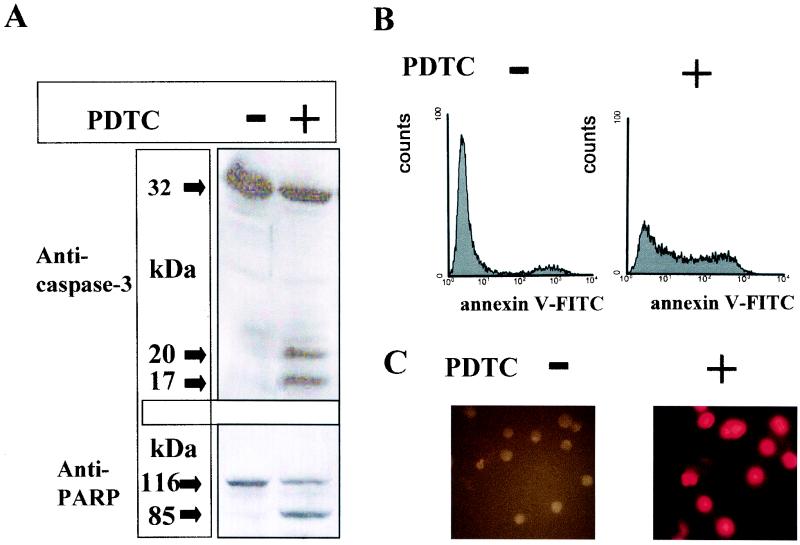
Mono Mac 6 cells were incubated with the NF-κB inhibitor PDTC, which has also been shown to induce apoptotic cell death in the promonocytic cell line U937 (18). To detect plasma membrane damage associated with apoptosis, we used a flow cytometric assay that discriminates between apoptosis and necrosis. Forty percent of PDTC-treated Mono Mac 6 cells were annexin V-positive but did not stain with the vital dye propidium iodide (Fig. 3B and Fig. 4, lane 2). DNA cleavage in PDTC-treated Mono Mac 6 cells was demonstrated by labeling nicked DNA ends using the TUNEL reaction. As shown in Fig. 3C, analysis of labeled cells by fluorescence microscopy revealed a condensed nuclear morphology typical of apoptotic cells in the vast majority of PDTC-treated monocytes. Activation of caspase-3, a key member of the aspartate-specific cysteine protease family, is essential for nuclear condensation and DNA cleavage. As we could demonstrate by Western blot analysis of cell extract proteins, procaspase-3 (p32) was converted into the active subunits p20 and p17 following pretreatment of Mono Mac 6 cells with PDTC (Fig. 3A). PARP becomes activated by DNA damage and has been shown to be a mediator of necrotic cell death by ATP depletion (16). Therefore, cleavage of PARP, which is a target of caspase-3, may be essential for preserving the energy needed to complete the apoptotic cell death program. As demonstrated in Fig. 3A, PDTC treatment of Mono Mac 6 cells leads to degradation of PARP (p116) to a p85 fragment, indicating enzymatic caspase-3 activity.
FIG. 3.
Effect of PDTC on Mono Mac 6 cells. Mono Mac 6 cells were treated with PDTC for 10 h or were left untreated. (A) Western blot analysis using antibodies against caspase-3 (top) or PARP (bottom). The caspase-3 and PARP antibody stainings were developed with a secondary antibody conjugated to horseradish peroxidase followed by visualization using an enhanced chemiluminescence kit as described in Materials and Methods. Data are representative examples of three similar experiments. (B) Flow cytometric analysis of apoptotic cells using annexin V-FITC. Cells were incubated with annexin V-FITC in a buffer containing propidium iodide (PI) and were analyzed by flow cytometry. PI-negative cells were gated and shown as histograms. (C) Detection of apoptosis in Mono Mac 6 cells by TUNEL reaction. The TUNEL reaction was performed as described in Materials and Methods. Fluorescence pictures were taken on a Zeiss axiophot 2 microscope. Data are representative examples of three similar experiments.
FIG. 4.
(A) C. pneumoniae (C. pneu.) reduced PDTC-derived apoptosis in Mono Mac 6 cells, which correlates with NF-κB binding activity. Mono Mac 6 cells were treated with PDTC (10−4 M) for 10 h and/or with C. pneumoniae at an MOI of 5 for 9 h as indicated. Flow cytometric analysis of apoptotic cells using annexin V-FITC is shown. Cells were incubated with annexin V-FITC in a buffer containing propidium iodide (PI) and were analyzed by flow cytometry. PI-negative cells were gated (top). For electromobility shift assay, nuclear extracts were prepared and equal amounts were reacted with 32P-labeled DNA probe encompassing the κB motif of the mouse kappa light chain enhancer. The arrow indicates the position of the κB-specific DNA binding activity (bottom). Data are representative examples of three similar experiments. (B) Relative NF-κB binding activity of Mono Mac 6 cells, which were treated with PDTC (10−4 M) for 10 h and/or C. pneumoniae at an MOI of 5 for 9 h, obtained from densitometric analysis. Scans from EMSAs were analyzed by using the NIH Image software. The density of the NF-κB complex of the untreated cells was set as 1. P values were determined by Student's t test and are indicated by asterisks (n = 3). ∗, P < 0.05; ∗∗, P < 0.005.
C. pneumoniae-infected cells are more resistant to PDTC-induced apoptosis by NF-κB activation.
Infection of Mono Mac 6 cells by C. pneumoniae at an MOI of 5 resulted in an infectivity rate of approximately 80%, a marked activation of NF-κB (Fig. 4A, lane 3) and a low rate of apoptotic cells (9% versus 10% apoptotic cells), which did not differ significantly from that of mock-infected cells, as quantified by annexin V (Fig. 4A, lane 1) and unchanged caspase-3 immunoblot and TUNEL reaction (data not shown). In agreement with a previous study (11), a constitutive low level of NF-κB binding activity was detected in uninfected Mono Mac 6 cells (Fig. 4A, lane 1). Pretreatment of C. pneumoniae-infected Mono Mac 6 cells with the NF-κB inhibitor and apoptosis inductor PDTC decreased NF-κB binding activity, which is accompanied by an increase of apoptotic monocytes (Fig. 4A, lane 4). Obviously, the C. pneumoniae-induced activation of NF-κB in Mono Mac 6 cells was not blocked completely by PDTC, suggesting that the remaining NF-κB binding activity still protects infected monocytes from PDTC-induced cell death.
Survival of C. pneumoniae-infected Mono Mac 6 cells is dependent on NF-κB binding activity.
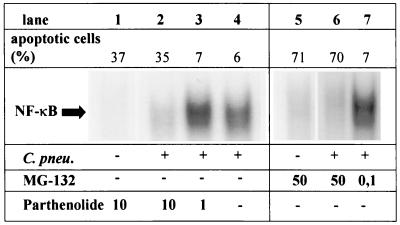
To further characterize the role of NF-κB binding activity for survival of C. pneumoniae-infected monocytes, cells were incubated with different doses of the proteasome inhibitor MG-132, which prevents the proteasome-derived degradation of IκBs, or the IKK inhibitor parthenolide, which targets a component of the IKK complex and prevents the phosphorylation of IκB. When C. pneumoniae-infected Mono Mac 6 cells were treated with doses (10 μM partheonlide or 50 μM MG-132, respectively) that cause a complete suppression of C. pneumoniae-induced NF-κB-activation, a considerable proportion of infected cells underwent apoptotic cell death (Fig. 5, lanes 1 and 5). In contrast, pretreatment of C. pneumoniae-infected monocytes with MG-132 or parthenolide in doses which did not affect the C. pneumoniae-induced NF-κB binding activity resulted in a low rate of apoptotic cells (Fig. 5, lanes 3 and 7) comparable to untreated C. pneumoniae-infected monocytes (Fig. 5, lane 4). These data suggest that suppression of NF-κB nuclear translocation in C. pneumoniae-infected Mono Mac 6 cells by MG-132 or parthenolide is associated with apoptotic cell death.
FIG. 5.
Apoptosis in Mono Mac 6 cells correlates with NF-κB binding activity. Mono Mac 6 cells were treated with MG-132 or parthenolide for 10 h and C. pneumoniae (C. pneu.) at an MOI of 5 for 9 h as indicated. Flow cytometric analysis of apoptotic cells using annexin V-FITC is shown. Cells were incubated with annexin V-FITC in a buffer containing propidium iodide (PI) and were analyzed by flow cytometry. PI-negative cells were gated (top). For electromobility shift assay, nuclear extracts were prepared and equal amounts were reacted with 32P-labeled DNA probe encompassing the κB motif of the mouse kappa light chain enhancer. The arrow indicates the position of the κB-specific DNA binding activity (bottom). Data are representative examples of three similar experiments.
DISCUSSION
Monocytes are present in all stages of atherosclerosis and play a key role in atherosclerotic lesion development. In addition, infected monocytes might be responsible for the systemic spread of C. pneumoniae from the respiratory tract to the artery vessel wall. In this study we demonstrate that infection of the human monocytic cell line MonoMac 6 by C. pneumoniae resulted in a rapid activation of the eukaryotic transcription factor NF-κB, with a maximum at 1 h postinfection. C. pneumoniae-induced activation of DNA binding activity was still detectable at 48 h postinfection. Analysis of the subunit composition of the C. pneumoniae-induced complex confirmed the presence of the p50-p65 heterodimers of NF-κB, of which the p65 subunit (RelA) is thought to be responsible for the strong transcription activating potential of NF-κB (35).
In a previous paper (17) it was demonstrated that growth of C. pneumoniae within the human monocytic cell line Mono Mac 6 induced the production of tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and IL-6 cytokines, which are all regulated by NF-κB. Monocytes/macrophages function as antigen-presenting cells and scavenger cells within the artery vessel wall, but they also may contribute to fibroproliferative processes and chronic inflammatory changes by their capacity to form numerous growth factors and cytokines, in particular platelet-derived growth factor as well as IL-1, IL-6, and TNF-α (32). Therefore, activation of NF-κB in monocytes upon C. pneumoniae infection might also initiate and/or maintain inflammatory events in the atherosclerotic lesion. However, activation of NF-κB upon C. pneumoniae infection of target cells is not restricted to monocytes. Requirement for NF-κB in transcriptional activation of monocyte chemotactic protein 1, plasminogen activator inhibitor 1, and tissue factor by C. pneumoniae has been demonstrated in endothelial cells and smooth muscle cells (6, 20, 24). In C. pneumoniae-infected endothelial cells, activation of different signal transduction pathways, including protein tyrosine phosphorylation, mitogen-activated protein kinase stimulation, and NF-κB activation and/or translocation, was followed by increased mRNA and surface expression of E-selectin, ICAM-1, and VCAM-1, which in turn resulted in enhanced leukocyte-human umbilical vein endothelial cell interaction (21). Therefore, activation of NF-κB seems to be a common response of all important cells of the artery wall upon C. pneumoniae infection, leading to an increased expression of highly relevant genes for inflammation and occlusive lesion development in atherosclerosis.
Recent studies have broadened the role of NF-κB from that of a regulator of immune and inflammatory responses to that of a regulator of apoptosis. Besides its essential importance for development and tissue homeostasis in multicellular organisms, apoptosis allows death and removal of individual, infected cells without damaging the host organism and thereby counteracts the spread of pathogens whose replication is bound to an intracellular niche of a eucaryotic host cell, as is the case for Chlamydia and Rickettsia. There is growing evidence that exploitation of host cell biology by modification of host cell apoptosis constitutes an essential part of the host-pathogen relationship, with important implications for the pathogenesis of infectious diseases, especially of those caused by intracellular bacterial pathogens, including Chlamydia spp. (12, 25, 31, 44).
C. pneumoniae shares with all other members of the genus Chlamydia a unique, complicated, biphasic developmental cycle lasting up to 72 h. Using markers that detect events early in apoptosis, like externalization of phosphatidylserine (Fig. 4A, lanes 1 and 3), DNA fragmentation, and caspase-3 activity (data not shown), we have shown that C. pneumoniae-infected Mono Mac 6 cultures exhibit no evidence of apoptosis during an early time of infection. Therefore, the pathogen could benefit from mechanisms leading to increased host cell resistance to apoptosis in order to efficiently complete the developmental cycle which in turn is required for formation of mature infectious elementary bodies. In this paper, we demonstrate that PDTC-induced apoptosis of Mono Mac 6 cells can be inhibited by C. pneumoniae infection. PDTC-induced apoptosis may be mediated by cytochrome c-dependent mechanisms (7) and by NF-κB (18). Our results are in agreement with data of Geng et al., who reported that C. pneumoniae-infected PBMCs are resistant to apoptosis induced by the photoactivated chemotherapeutic agents 8-methoxypsoralen and hypericin (13). In their paper the resistance to apoptosis observed in PBMCs exposed to C. pneumoniae had been partially attributed to IL-10-induced infection, because depletion of endogenous IL-10 abolished the apoptosis resistance of C. pneumoniae-infected PBMCs. Based on our results, we suggest that activation of NF-κB mediates apoptosis resistance of C. pneumoniae-infected Mono Mac 6 cells, because we could demonstrate that the apoptosis-inducing agent PDTC blocked NF-κB activation in infected cells, probably by its ability to eliminate reactive oxygen intermediates (42). However, total inhibition of C. pneumoniae-induced NF-κB activation by PDTC was not achieved (Fig. 4A and B), resulting in a remaining NF-κB activation which was obviously sufficient to reduce the PDTC-derived apoptosis. Treatment of Mono Mac 6 cells with the proteasome inhibitor MG-132 or the IKK inhibitor parthenolide led to significant apoptosis when used in concentrations which totally inhibit NF-κB binding activity. However, at lower concentrations, when NF-κB binding activity was not affected, similar apoptosis rates to those of unstimulated cells were detected. Therefore, we propose that a constitutive NF-κB binding activity is needed for survival of Mono Mac 6 cells and that C. pneumoniae can overcome PDTC-induced apoptosis via NF-κB activation. This supports findings of Geng and coworkers, because NF-κB probably regulates the transcriptional activity of IL-10 (26). In addition, our results are in agreement with data of Clifton et al. who demonstrated that the obligate intracellular bacteria Rickettsia rickettsii inhibited host cell apoptosis via a mechanism dependent on NF-κB activation, suggesting that NF-κB-mediated increased host cell resistance could be a common survival strategy of obligate intracellular bacteria (5).
Fan et al. were the first to demonstrate that chlamydiae possess antiapoptotic mechanisms, which include blockade of mitochondrial cytochrome c release and caspase activation (10); however, a Chlamydia-induced antiapoptotic factor could not be identified, until now. Members of the family of inhibitor of apoptosis proteins could be promising candidates for potential antiapoptotic factors induced by chlamydiae because they are potent inhibitors of active caspases and are regulated by NF-κB (8).
In conclusion, it was shown that C. pneumoniae infection of Mono Mac 6 cells induces activation of NF-κB and that the NF-κB inhibitor PDTC induces apoptosis in Mono Mac 6 cells, which can be partially inhibited by C. pneumoniae via NF-κB activation. We could also show that activation of NF-κB is required for survival of C. pneumoniae-infected Mono Mac 6 cells. Given that infected monocytes/macrophages are present in the artery vessel wall, C. pneumoniae-induced activation of NF-κB might contribute to the chronic inflammatory events within atherosclerotic lesions as well as to increased host cell resistance to apoptosis, which enables effective replication and may favor systemic dissemination.
ACKNOWLEDGMENT
This study was supported by a grant of the Sonderforschungsbereich SFB 451 to R.M. and A.E.
REFERENCES
- 1.Baeuerle P A, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 2.Blasi F, Boman J, Esposito G, Melissano G, Chiesa R, Cosentini R, Tarsia P, Tshomba Y, Betti M, Alessi M, Morelli N, Allegra L. Chlamydia pneumoniae DNA detection in peripheral blood mononuclear cells is predictive of vascular infection. J Infect Dis. 1999;180:2074–2076. doi: 10.1086/315126. [DOI] [PubMed] [Google Scholar]
- 3.Boman J, Soderberg S, Forsberg J, Birgander L S, Allard A, Persson K, Jidell E, Kumlin U, Juto P, Waldenstrom A, Wadell G. High prevalence of Chlamydia pneumoniae DNA in peripheral blood mononuclear cells in patients with cardiovascular disease and in middle-aged blood donors. J Infect Dis. 1998;178:274–277. doi: 10.1086/517452. [DOI] [PubMed] [Google Scholar]
- 4.Campbell L A, Rosenfeld M, Kuo C C. The role of Chlamydia pneumoniae in atherosclerosis—recent evidence from animal models. Trends Microbiol. 2000;8:255–257. doi: 10.1016/s0966-842x(00)01745-5. [DOI] [PubMed] [Google Scholar]
- 5.Clifton D R, Goss R A, Sahni S K, van Antwerp D, Baggs R B, Marder V J, Silverman D J, Sporn L A. NF-kappa B-dependent inhibition of apoptosis is essential for host cellsurvival during Rickettsia rickettsii infection. Proc Natl Acad Sci USA. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dechend R, Maass M, Gieffers J, Dietz R, Scheidereit C, Leutz A, Gulba D C. Chlamydia pneumoniae infection of vascular smooth muscle and endothelial cells activates NF-kappaB and induces tissue factor and PAI-1 expression: a potential link to accelerated arteriosclerosis. Circulation. 1999;100:1369–1373. doi: 10.1161/01.cir.100.13.1369. [DOI] [PubMed] [Google Scholar]
- 7.Della R F, Cucciolla V, Borriello A, Della P, Manna V C, Galletti P, Zappia V. Pyrrolidine dithiocarbamate induces apoptosis by a cytochrome c-dependent mechanism. Biochem Biophys Res Commun. 2000;268:942–946. doi: 10.1006/bbrc.2000.2161. [DOI] [PubMed] [Google Scholar]
- 8.Erl W, Hansson G K, de Martin R, Draude G, Weber K S, Weber C. Nuclear factor-kappa B regulates induction of apoptosis and inhibitor of apoptosis protein-1 expression in vascular smooth muscle cells. Circ Res. 1999;84:668–677. doi: 10.1161/01.res.84.6.668. [DOI] [PubMed] [Google Scholar]
- 9.Essig A, Heinemann M, Schweitzer R, Simnacher U, Marre R. Decontamination of a Mycoplasma-infected Chlamydia pneumoniae strain by pulmonary passage in SCID mice. Int J Med Microbiol. 2000;290:289–292. doi: 10.1016/S1438-4221(00)80130-7. [DOI] [PubMed] [Google Scholar]
- 10.Fan T, Lu H, Hu H, Shi L, McClarty G A, Nance D M, Greenberg A H, Zhong G. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankenberger M, Pforte A, Sternsdorf T, Passlick B, Baeuerle P A, Ziegler-Heitbrock H W. Constitutive nuclear NF-kappa B in cells of the monocyte lineage. Biochem J. 1994;304:87–94. doi: 10.1042/bj3040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao L Y, Kwaik Y A. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 2000;8:306–313. doi: 10.1016/s0966-842x(00)01784-4. [DOI] [PubMed] [Google Scholar]
- 13.Geng Y, Shane R B, Berencsi K, Gonczol E, Zaki M H, Margolis D J, Trinchieri G, Rook A H. Chlamydia pneumoniae inhibits apoptosis in human peripheral blood mononuclear cells through induction of IL-10. J Immunol. 2000;164:5522–5529. doi: 10.4049/jimmunol.164.10.5522. [DOI] [PubMed] [Google Scholar]
- 14.Grayston J T. Infections caused by Chlamydia pneumoniae strain TWAR. Clin Infect Dis. 1992;15:757–761. doi: 10.1093/clind/15.5.757. [DOI] [PubMed] [Google Scholar]
- 15.Grayston J T, Campbell L A, Kuo C C, Mordhorst C H, Saikku P, Thom D H, Wang S P. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990;161:618–625. doi: 10.1093/infdis/161.4.618. [DOI] [PubMed] [Google Scholar]
- 16.Ha H C, Snyder S H. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci USA. 1999;96:13978–13982. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinemann M, Susa M, Simnacher U, Marre R, Essig A. Growth of Chlamydia pneumoniae induces cytokine production and expression of CD14 in a human monocytic cell line. Infect Immun. 1996;64:4872–4875. doi: 10.1128/iai.64.11.4872-4875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hida A, Kawakami A, Nakashima T, Yamasaki S, Sakai H, Urayama S, Ida H, Nakamura H, Migita K, Kawabe Y, Eguchi K. Nuclear factor-kappaB and caspases co-operatively regulate the activation and apoptosis of human macrophages. Immunology. 2000;99:553–560. doi: 10.1046/j.1365-2567.2000.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iimuro Y, Nishiura T, Hellerbrand C, Behrns K E, Schoonhoven R, Grisham J W, Brenner D A. NF-kappaB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Investig. 1998;101:802–811. doi: 10.1172/JCI483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kol A, Bourcier T, Lichtman A H, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Investig. 1999;103:571–577. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krull M, Klucken A C, Wuppermann F N, Fuhrmann O, Magerl C, Seybold J, Hippenstiel S, Hegemann J H, Jantos C A, Suttorp N. Signal transduction pathways activated in endothelial cells following infection with Chlamydia pneumoniae. J Immunol. 1999;162:4834–4841. [PubMed] [Google Scholar]
- 22.Kuo C C, Shor A, Campbell L A, Fukushi H, Patton D L, Grayston J T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- 23.Martin S J, Reutelingsperger C P, McGahon A J, Rader J A, van Schie R C, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molestina R E, Miller R D, Lentsch A B, Ramirez J A, Summersgill J T. Requirement for NF-κB in transcriptional activation of monocyte chemotactic protein 1 by Chlamydia pneumoniae in human endothelial cells. Infect Immun. 2000;68:4282–4288. doi: 10.1128/iai.68.7.4282-4288.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori N, Prager D. Activation of the interleukin-10 gene in the human T lymphoma line HuT 78: identification and characterization of NF-kappa B binding sites in the regulatory region of the interleukin-10 gene. Eur J Haematol. 1997;59:162–170. doi: 10.1111/j.1600-0609.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 27.Ojcius D M, Souque P, Perfettini J L, Dautry-Varsat A. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J Immunol. 1998;161:4220–4226. [PubMed] [Google Scholar]
- 28.Quinn T C, Gaydos C A. In vitro infection and pathogenesis of Chlamydia pneumoniae in endovascular cells. Am Heart J. 1999;138:S507–S511. doi: 10.1016/s0002-8703(99)70287-5. [DOI] [PubMed] [Google Scholar]
- 29.Ramirez J A. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. The Chlamydia pneumoniae/Atherosclerosis Study Group. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Roblin P M, Dumornay W, Hammerschlag M R. Use of HEp-2 cells for improved isolation and passage of Chlamydia pneumoniae. J Clin Microbiol. 1992;30:1968–1971. doi: 10.1128/jcm.30.8.1968-1971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers H W, Callery M P, Deck B, Unanue E R. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1996;156:679–684. [PubMed] [Google Scholar]
- 32.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 33.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–S420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 34.Saikku P, Leinonen M, Mattila K, Ekman M R, Nieminen M S, Makela P H, Huttunen J K, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz M L, Baeuerle P A. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991;10:3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreck R, Meier B, Mannel D N, Droge W, Baeuerle P A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 38.Ten R M, McKinstry M J, Trushin S A, Asin S, Paya C V. The signal transduction pathway of CD23 (Fc epsilon RIIb) targets I kappa B kinase. J Immunol. 1999;163:3851–3857. [PubMed] [Google Scholar]
- 39.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 40.Wahl C, Liptay S, Adler G, Schmid R M. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Investig. 1998;101:1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S J. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 42.Weber C, Erl W, Pietsch A, Strobel M, Ziegler-Heitbrock H W, Weber P C. Antioxidants inhibit monocyte adhesion by suppressing nuclear factor-kappa B mobilization and induction of vascular cell adhesion molecule-1 in endothelial cells stimulated to generate radicals. Arterioscler Thromb. 1994;14:1665–1673. doi: 10.1161/01.atv.14.10.1665. [DOI] [PubMed] [Google Scholar]
- 43.Ziegler-Heitbrock H W, Thiel E, Futterer A, Herzog V, Wirtz A, Riethmuller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988;41:456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]
- 44.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]