Abstract
Objectives
The aims of the study were to assess the management of low-density lipoprotein cholesterol (LDL-C) and the goal achievement, as well as to investigate the association between baseline LDL-C level, lipid-lowering treatment (LLT), and stroke recurrence in patients with ischaemic stroke or transient ischaemic attack (TIA).
Design
Our study was a post hoc analysis of the Third China National Stroke Registry (CNSR-III).
Setting
We derived data from the CNSR-III - a nationwide clinical registry of ischaemic stroke and TIA based on 201 participating hospitals in mainland China.
Participants
15,166 patients were included in this study with demographic characteristics, etiology, imaging, and biological markers from August 2015 to March 2018.
Primary and secondary outcome measures
The primary outcome was a new stroke, LDL-C goal (LDL-C<1.8mmol/L and LDL-C<1.4mmol/L, respectively) achievement rates, and LLT compliance within 3, 6, and 12 months. The secondary outcomes included major adverse cardiovascular events (MACE) and all caused death at 3 and 12 months.
Results
Among the 15,166 patients, over 90% of patients received LLT during hospitalization and 2 weeks after discharge; the LLT compliance was 84.5% at 3 months, 75.6% at 6 months, and 64.8% at 12 months. At 12 months, LDL-C goal achievement rate for 1.8mmol/L and 1.4mmol/L was 35.4% and 17.6%, respectively. LLT at discharge was associated with reduced risk of ischemic stroke recurrence (HR=0.69, 95% CI: 0.48-0.99, p=0.04) at 3 months. The rate of LDL-C reduction from baseline to 3-month follow-up was not associated with a reduced risk of stroke recurrence or major adverse cardiovascular events (MACE) at 12 months. Patients with baseline LDL-C ≤1.4mmol/L had a numerically lower risk of stroke, ischemic stroke and MACE at both 3 months and 12 months.
Conclusions
The LDL-C goal achievement rate has increased mildly in the stroke and TIA population in mainland China. Lowered baseline LDL-C level was significantly associated with a decreased short- and long-term risk of ischemic stroke among stroke and TIA patients. LDL-C<1.4mmol/L might be a safe standard for this population.
Keywords: Neurology, Lipid disorders, Stroke medicine, Stroke
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This hospital-based study analysed the low-density lipoprotein cholesterol levels and lipid-lowering therapy in patients with ischaemic stroke (IS)/transient ischaemic attack (TIA) in the general population of mainland China.
The study included the largest sample of patients with IS/TIA and recorded detailed prognostic characteristics.
The design of the cohort study did not allow for further detailed analysis of lipid-lowering medication use, such as dose change and duration.
Some undetected confounding factors, including residual risk, were not able to be assessed in this study.
Introduction
Low-density lipoprotein cholesterol (LDL-C) has been well established as an independent risk factor for ischaemic stroke (IS).1 Intensive lipid-lowering treatment (LLT) has been proven to reduce cardiovascular event recurrence in patients with IS/transient ischaemic attack (TIA). The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study2 showed that intensive atorvastatin treatment for 5 years reduced the risk of stroke recurrence up to 16% (HR 0.84, 95% CI 0.71 to 0.99; p<0.03) in IS/TIA. Recently, Treat Stroke to Target (TST) Study3 also demonstrated that patients with IS/TIA who had a target LDL-C level of less than 70 mg/dL (1.8 mmol/L) had a lower risk of subsequent cardiovascular events than those who had a target range of 90–110 mg/dL (HR 0.78, 95% CI 0.61 to 0.98; p=0.04). Therefore, European Stroke Organisation and American Stroke Association both updated the IS/TIA second prevention guideline with a recommendation of LDL-C target goal to be less than 70 mg/dL (1.8 mmol/L).4 5
However, there are still clinical questions not thoroughly investigated. First, SPARCL and TST trials are randomised controlled trials conducted mainly in the Caucasian population,2 6 whereas studies focusing on the Asian population on lipid management in patients who had a stroke are limited. Since there are more patients with intracranial artery stenosis (ICAS)7 8 and cerebral small vessel disease in Asia,9 10 especially in east Asia, the applicability of the conclusions of these two trials to Asian people should be discreet. Second, there are inconsistencies and conflicts about whether the reduced LDL-C level, especially during the acute or subacute phase, could increase the risk of intracranial haemorrhage (ICH). In the SPARCL Study, subgroup analysis indicated that atorvastatin treatment might increase the risk of ICH, which led to a big concern for statin usage during the acute phase of IS/TIA.11 In contrast, the TST Study showed that the incidence of ICH did not differ significantly between the lower-target and higher-target groups.3 Third, with emerging evidence from non-stain therapies such as IMPROVE-IT,12 FOURIER13 and ODYSSEY,14 a lower LDL-C target of less than 1.4 mmol/L or even 1.0 mmol/L has been recommended for adoption as international guidelines. However, the benefits of a lower LDL-C target lower than 1.8 mmol/L have not been investigated.
The Third China National Stroke Registry (CNSR-III) is one of the world’s most extensive IS/TIA cohort studies and it includes comprehensive medical histories, centralised the Trial of Org 10172 in Acute Stroke Treatment classification judication and follow-up outcomes. We aimed to collect data from CNSR-III to investigate China’s current lipid management practices and the associations between LDL-C level, LLT and stroke recurrence in patients with IS or TIA.
Methods
Study design and participants
This study was based on the CNSR-III database. The CNSR-III is a nationwide clinical registry of IS or TIA based on aetiology, imaging and biological markers in China from August 2015 to March 2018.15 Two hundred one participating hospitals were selected in China, and 15 166 patients were eligible and had complete information at baseline. The total 15 166 patients were included in the analysis. Among all the clinical centres included in CNSR-III, 169 centres voluntarily participated in the prespecified blood biomarker substudy, with all the patients at these centres participating in the biomarker substudy. Such patients provided a separate written informed consent form that included their consent for blood sample collection and further study of biomarkers.
To be eligible for this second analysis research, patients had to meet the following criteria: (1) age 18 years or older; (2) hospitalised with a primary diagnosis of acute IS or TIA; (3) direct hospital admission from a physician’s clinic or an emergency department; and (4) informed consent provided by the patient or legally authorised representative. Patients with ICH, subarachnoid haemorrhage or undetermined stroke were not included in this study.
Data collection and management
Patient information, including demographics, risk factors, comorbidities, medications, selected laboratory tests and hospital-level characteristics, was collected systematically during hospitalisation and at discharge by trained research coordinators at each participating hospital. National Institutes of Health Stroke Scale (NIHSS) score at admission, and IS recurrence, composite vascular event, and modified Rankin Scale at 3 months and 1 year after stroke onset were also collected.
Venous blood samples were collected from fasting patients within 24 hours from admission. Serum specimens were extracted, aliquoted and transported through the cold chain to the central laboratory in Beijing Tiantan Hospital and stored at −80°C. LDL-C measurements were centrally and blindly assayed by enzymatic method on the Cobas 8000 analyser c702 module (Roche Diagnostics, Mannheim, Germany).
Follow-up and clinical outcome evaluations
Patients were followed up through face-to-face interviews at 3 months and by telephone interviews at 6 and 12 months by trained research coordinators who followed a standardised interview protocol. Information collected at each follow-up included cardiovascular and cerebrovascular events, all causes of death and medication use. Vascular events were confirmed with the treating hospital, and death was either confirmed based on a death certificate issued by the attended hospital or the local civil registry.
The primary outcome was a new stroke (defined as a new neurological deficit lasting more than 24 hours or rehospitalisation with a diagnosis of IS, ICH or subarachnoid haemorrhage), LDL-C goal (LDL-C <1.4 mmol/L and LDL-C <1.8 mmol/L, respectively) achievement rates and LLT compliance in China within 3, 6 and 12 months. The secondary outcomes included major adverse cardiovascular events (MACE) (including stroke, myocardial infarction or vascular death) and all-cause death at 3 months and 12 months.
All reported efficacy and safety outcomes were verified by a central independent adjudication committee blinded to study treatment assignments and baseline LDL-C level.
Patients were categorised into four groups according to the baseline LDL-C levels and LLT during hospitalisation and after discharge: LDL-C ≤1.4 mmol/L, 1.4 mmol/L<LDL-C≤1.8 mmol/L, 1.8 mmol/L<LDL≤2.6 mmol/L, LDL >2.6 mmol/L.
LLT compliance was defined as the continuation of LLT medication from discharge to 3, 6 or 12 months after the onset of symptoms. Patients assigned to LLT at discharge but later discontinuing LLT at any follow-up point within 3, 6 or 12 months were considered ‘non-persistent’. Patients were considered persistent if they discontinued one medication but replaced it with another statin medication that they continued taking through 3, 6 or 12 months after enrolment.
Statistical analysis
Baseline variables were presented as median with the interquartile range (IQR) for continuous variables and percentages for categorical variables.
To analyse the association of baseline LDL-C levels and outcomes, we only included those subjects who provided 3-month or 12-month biosample. Univariate and multivariate Cox proportional hazard regression models were used. The model included the following covariates: age, sex, education, current smoking, heavy drinking, medical history, stroke severity on the NIHSS, history of stroke, history of diabetes and history of hypertension. Adjusted hazard ratios (HR) with 95% confidence intervals (CI) were calculated. The dose–response relationship curves were also presented.
To analyse the effect of discharge LLT on outcomes, we excluded subjects who reached the endpoint (stroke recurrence or MACE, death and loss to follow-up) during hospitalisation. We performed a univariate model and multivariate analysis by adjusting for age, sex, education, current smoking, heavy drinking, medical history, stroke severity on the NIHSS, history of stroke, history of diabetes and history of hypertension.
In addition, to analyse the association of 3-month LDL-C change with stroke recurrence and MACE within 12 months, we excluded subjects who reached the endpoint (stroke recurrence or MACE, death and loss to follow-up) within 3 months.
All statistical analyses in the study were performed by SAS V.9.4 software. All statistical analyses adopted a two-sided test which was performed at a 5% significance level.
Patient and public involvement
This registry study was designed and conducted without patient and public involvement. Our results will be disseminated to the public through publication in this journal.
Results
Characteristics of study participants
From August 2015 to March 2018, a total of 15 166 patients with acute stroke and TIA were recruited to the CNSR-III and entered our final analysis. The average age of patients was 62.2±11.3 years, 31.7% of patients were women, 14 146 (93.3%) had an index event of stroke and 1020 (6.7%) had a TIA.15
Baseline LDL-C levels
There were 10 738 patients in LDL-C analysis set: 1407 (13.1%), 1636 (15.2%), 3655 (34.0%) and 4040 (37.6%) patients with the baseline LDL-C ≤1.4 mmol/L, 1.4–1.8 mmol/L, 1.8–2.6 mmol/L, and ≥2.6 mmol/L, respectively (table 1).
Table 1.
Baseline characteristics for the LDL-C analysis set
| Variables | LDL ≤1.4 mmol/L N=1407 |
1.4<LDL≤1.8 mmol/L N=1636 |
1.8<LDL≤2.6 mmol/L N=3655 |
LDL >2.6 mmol/L N=4040 |
P value |
| Women, n (%) | 378 (26.9) | 439 (26.8) | 1057 (28.9) | 1517 (37.6) | <0.001 |
| Mean age, years (SD) | 60.8±11.9 | 62.4±11.3 | 62.2±11.3 | 62.8±11.1 | <0.001 |
| Ethnicity (non-Han), n (%) | 30 (2.1) | 49 (3.0) | 122 (3.3) | 104 (2.6) | 0.07 |
| Current smoker, n (%) | 435 (30.9) | 525 (32.1) | 1239 (33.9) | 1198 (29.7) | <0.001 |
| Heavy drinker, n (%)* | 185 (13.2) | 210 (12.8) | 545 (14.9) | 589 (14.6) | 0.12 |
| Triglycerides (IQR) | 1.3 (0.9–1.9) | 1.3 (1.0–1.8) | 1.3 (1.0–1.8) | 1.5 (1.1–2.0) | <0.001 |
| TC, mmol/L | 2.7 (2.4–3.1) | 3.2 (3.0–3.5) | 3.8 (3.5–4.1) | 4.9 (4.5–5.5) | <0.001 |
| HDL-C, mmol/L | 0.8 (0.7–1.0) | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 1.0 (0.8–1.2) | <0.001 |
| LDL-C, mmol/L | 1.2 (1.0–1.3) | 1.6 (1.5–1.7) | 2.2 (2.0–2.4) | 3.2 (2.9–3.8) | <0.001 |
| BMI | 24.4 (22.5–26.4) | 24.5 (22.7–26.6) | 24.4 (22.5–26.4) | 24.5 (22.7–26.7) | 0.06 |
| Systolic pressure, mm Hg | 145.0 (132.5–160.0) | 146.5 (133.0–161.0) | 148.5 (135.0–163.5) | 150.0 (136.0–166.5) | <0.001 |
| Medical history, n (%) | |||||
| Ischaemic stroke | 369 (26.2) | 429 (26.2) | 715 (19.6) | 748 (18.5) | <0.001 |
| TIA | 44 (3.1) | 46 (2.8) | 115 (3.6) | 102 (2.5) | 0.38 |
| Coronary heart diseases | 147 (10.5) | 193 (11.8) | 366 (10.0) | 449 (11.1) | 0.20 |
| Atrial fibrillation | 93 (6.6) | 124 (7.6) | 272 (7.4) | 257 (6.4) | 0.19 |
| Hypertension | 897 (63.8) | 1045 (63.9) | 2295 (62.8) | 2516 (62.3) | 0.62 |
| Diabetes mellitus | 386 (27.4) | 394 (24.1) | 824 (22.5) | 960 (23.8) | 0.004 |
| Hypercholesterolaemia | 119 (8.5) | 120 (7.3) | 302 (8.3) | 341 (8.4) | 0.56 |
| NIHSS at admission, median (IQR) | 3.0 (1.0–6.0) | 3.0 (1.0–6.0) | 3.0 (1.0–6.0) | 3.0 (1.0–6.0) | <0.001 |
| NIHSS 0–3 | 743 (52.8) | 914 (55.9) | 1974 (54.0) | 2073 (51.3) | 0.009 |
| NIHSS ≥4 | 664 (47.2) | 722 (44.1) | 1681 (46.0) | 1967 (48.7) | |
| mRS (IQR) | 0 (0–1.0) | 0 (0–1.0) | 0 (0–1.0) | 0 (0–0) | <0.001 |
| Stroke subtype, n (%) | |||||
| LAA | 303 (21.5) | 390 (23.8) | 933 (25.5) | 1092 (27.0) | 0.0095 |
| CE | 81 (5.8) | 96 (5.9) | 251 (6.9) | 256 (6.3) | |
| SAO | 312 (22.2) | 359 (21.9) | 740 (20.3) | 819 (20.3) | |
| Other | 21 (1.5) | 16 (1.0) | 38 (1.0) | 47 (1.2) | |
| Unknown | 690 (49.0) | 775 (47.4) | 1693 (46.3) | 1826 (45.2) | |
| Prestroke antiplatelet therapy, n (%) | 1357 (97.4) | 1569 (97.1) | 3504 (96.7) | 3894 (97.0) | 0.57 |
| Prestroke LLT, n (%) | 1359 (97.6) | 1558 (96.4) | 3498 (96.5) | 3897 (97.1) | 0.15 |
| Statin, n (%) | 1355 (97.3) | 1556 (96.3) | 3491 (96.3) | 3887 (96.9) | 0.27 |
BMI, body mass index; CE, cardiogenic embolism; HDL-C, high-density lipoprotein cholesterol; LAA, large artery atherosclerosis; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SAO, small artery occlusion; TC, total cholesterol; TIA, transient ischaemic attack.
Patients in the lower baseline LDL-C level group (≤1.4 mmol/L) were more likely to be younger (p<0.0001) and had a greater prevalence of cardiovascular risk factors (previous stroke, hypertension, hypercholesterolaemia, diabetes mellitus and history of stroke) (p<0.0001) and lower levels of triglycerides, total cholesterol and high-density lipoprotein (p<0.0001). About 97% of the patients had a history of antiplatelet and LLT, and the rates showed no difference among the four baseline LDL-C groups.
Association between baseline LDL-C levels and outcomes at 3 months and 12 months
There were 656 (6.1%) new stroke occurrences at 3 months and 1037 (9.7%) at 12 months (table 2).
Table 2.
Association between baseline LDL-C levels and outcomes at 3 months and 12 months
| Total | Events (n%) | HR (95% CI) unadjusted | P value | HR (95% CI) adjusted | P value | |
| 3 months | ||||||
| Stroke recurrence | ||||||
| LDL ≤1.4 mmol/L | 1407 | 69 (4.9) | 0.72 (0.55 to 0.94) | 0.01 | 0.74 (0.57 to 0.97) | 0.03 |
| 1.4<LDL≤1.8 mmol/L | 1636 | 95 (5.8) | 0.85 (0.68 to 1.08) | 0.18 | 0.89 (0.70 to 1.12) | 0.32 |
| 1.8<LDL≤2.6 mmol/L | 3655 | 219 (6.0) | 0.88 (0.74 to 1.05) | 0.17 | 0.91 (0.76 to 1.08) | 0.28 |
| LDL >2.6 mmol/L | 4040 | 273 (6.8) | Reference | – | Reference | – |
| Ischaemic stroke | ||||||
| LDL ≤1.4 mmol/L | 1407 | 65 (4.6) | 0.72 (0.55 to 0.95) | 0.02 | 0.74 (0.56 to 0.98) | 0.03 |
| 1.4<LDL≤1.8 mmol/L | 1636 | 88 (5.4) | 0.84 (0.66 to 1.07) | 0.16 | 0.87 (0.68 to 1.11) | 0.27 |
| 1.8<LDL≤2.6 mmol/L | 3655 | 201 (5.5) | 0.86 (0.72 to 1.04) | 0.11 | 0.89 (0.74 to 1.07) | 0.22 |
| LDL >2.6 mmol/L | 4040 | 257 (6.4) | Reference | – | Reference | – |
| Haemorrhagic stroke | ||||||
| LDL ≤1.4 mmol/L | 1407 | 4 (0.3) | 0.52 (0.18 to 1.51) | 0.23 | 0.55 (0.19 to 1.61) | 0.28 |
| 1.4<LDL≤1.8 mmol/L | 1636 | 9 (0.6) | 1.01 (0.46 to 2.19) | 0.98 | 1.03 (0.47 to 2.26) | 0.93 |
| 1.8<LDL≤2.6 mmol/L | 3655 | 20 (0.6) | 1.00 (0.55 to 1.84) | 0.99 | 0.93 (0.50 to 1.73) | 0.82 |
| LDL >2.6 mmol/L | 4040 | 22 (0.5) | Reference | – | Reference | – |
| MACE | ||||||
| LDL ≤1.4 mmol/L | 1407 | 71 (5.1) | 0.72 (0.56 to 0.94) | 0.01 | 0.75 (0.57 to 0.97) | 0.03 |
| 1.4<LDL≤1.8 mmol/L | 1636 | 100 (6.1) | 0.88 (0.70 to 1.10) | 0.27 | 0.91 (0.72 to 1.15) | 0.42 |
| 1.8<LDL≤2.6 mmol/L | 3655 | 231 (6.3) | 0.91 (0.77 to 1.08) | 0.29 | 0.93 (0.78 to 1.11) | 0.43 |
| LDL >2.6 mmol/L | 4040 | 279 (6.9) | Reference | – | Reference | – |
| 12 months | ||||||
| Stroke recurrence | ||||||
| LDL ≤1.4 mmol/L | 1407 | 114 (8.1) | 0.76 (0.62 to 0.93) | 0.009 | 0.77 (0.62 to 0.95) | 0.01 |
| 1.4<LDL≤1.8 mmol/L | 1636 | 158 (9.7) | 0.91 (0.76 to 1.08) | 0.30 | 0.92 (0.76 to 1.10) | 0.36 |
| 1.8<LDL≤2.6 mmol/L | 3655 | 339 (9.7) | 0.87 (0.76 to 1.01) | 0.06 | 0.89 (0.77 to 1.03) | 0.12 |
| LDL >2.6 mmol/L | 4040 | 426 (10.5) | Reference | – | Reference | – |
| Ischaemic stroke | ||||||
| LDL ≤1.4 mmol/L | 1407 | 102 (7.6) | 0.72 (0.58 to 0.90) | 0.004 | 0.73 (0.59 to 0.91) | 0.005 |
| 1.4<LDL≤1.8 mmol/L | 1636 | 145 (8.9) | 0.89 (0.73 to 1.07) | 0.22 | 0.90 (0.74 to 1.09) | 0.27 |
| 1.8<LDL≤2.6 mmol/L | 3655 | 304 (8.3) | 0.83 (0.72 to 0.97) | 0.02 | 0.86 (0.74 to 1.00) | 0.04 |
| LDL >2.6 mmol/L | 4040 | 400 (9.9) | Reference | – | Reference | – |
| Haemorrhagic stroke | ||||||
| LDL ≤1.4 mmol/L | 1407 | 12 (0.9) | 0.96 (0.50 to 1.84) | 0.89 | 0.97 (0.50 to 1.88) | 0.93 |
| 1.4<LDL≤1.8 mmol/L | 1636 | 15 (0.9) | 1.03 (0.56 to 1.87) | 0.94 | 1.02 (0.56 to 1.88) | 0.95 |
| 1.8<LDL≤2.6 mmol/L | 3655 | 37 (1.0) | 1.13 (0.72 to 1.80) | 0.59 | 1.10 (0.69 to 1.75) | 0.69 |
| LDL >2.6 mmol/L | 4040 | 36 (0.9) | Reference | – | Reference | – |
| MACE | ||||||
| LDL≤1.4 mmol/L | 1407 | 119 (8.5) | 0.76 (0.62 to 0.93) | 0.008 | 0.77 (0.62 to 0.94) | 0.01 |
| 1.4<LDL≤1.8 mmol/L | 1636 | 170 (10.4) | 0.94 (0.79 to 1.12) | 0.47 | 0.94 (0.79 to 1.13) | 0.50 |
| 1.8<LDL≤2.6 mmol/L | 3655 | 363 (9.9) | 0.90 (0.78 to 1.03) | 0.13 | 0.91 (0.79 to 1.05) | 0.20 |
| LDL >2.6 mmol/L | 4040 | 444 (11.0) | Reference | – | Reference | – |
LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular events.
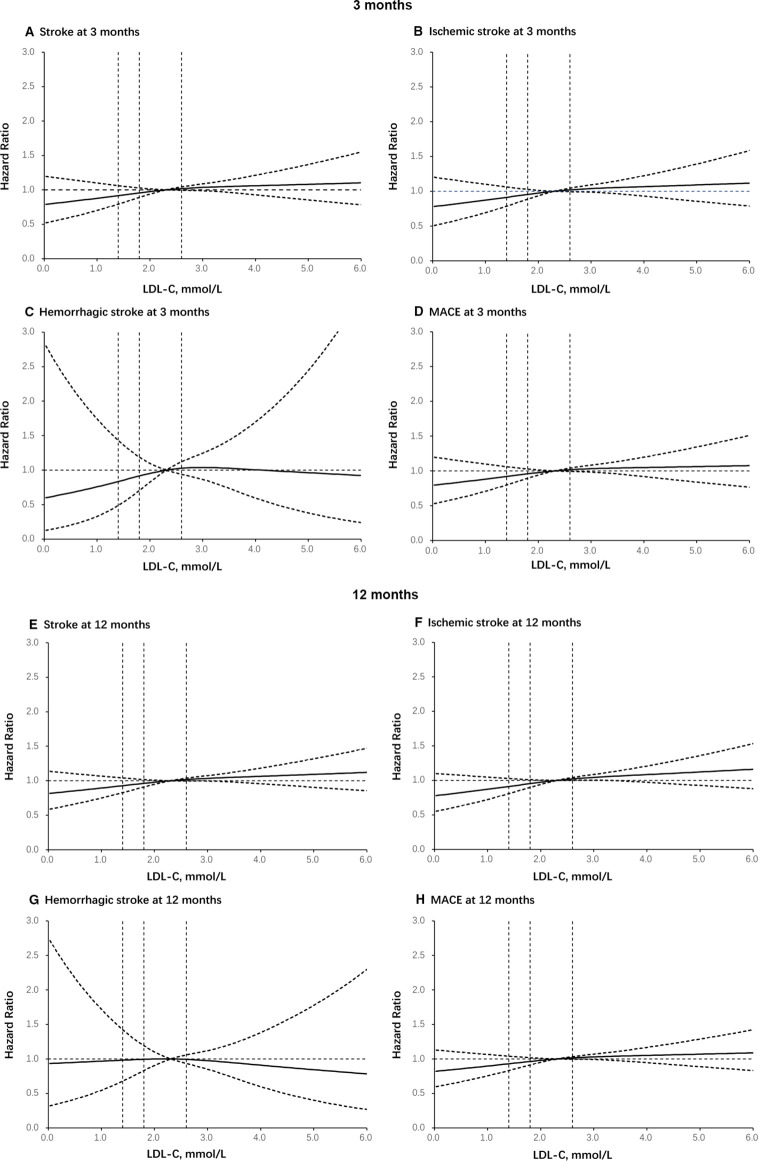
Compared with patients with other LDL-C level subgroups, the patients with LDL-C ≤1.4 mmol/L had a numerically lower risk of stroke (HR=0.742, 95% CI: 0.568 to 0.970, p=0.0291), IS (HR=0.741, 95% CI: 0.562 to 0.976, p=0.0329) and MACE (HR=0.746, 95% CI: 0.573 to 0.972, p=0.0297) at 3 months. Similar results were found for the outcome of stroke (HR=0.767, 95% CI: 0.622 to 0.946, p=0.0131), IS (HR=0.731, 95% CI: 0.587 to 0.911, p=0.0052) and MACE (HR=0.766, 95% CI: 0.624 to 0.940, p=0.0106) at 12 months after the initial event. Lower baseline LDL-C level was not associated with an increased risk of haemorrhagic stroke at either 3 months or 12 months (table 2). Using a Cox regression model with restricted cubic splines, a strong association was also found between baseline LDL-C level and risk of stroke, IS, haemorrhagic stroke and MACE (figure 1).
Figure 1.
Dose–response relationship curves. Adjusted OR of events and MACE at 3 months and 12 months according to LDL-C at baseline in patients: (A) stroke at 3 months; (B) ischaemic stroke at 3 months; (C) haemorrhagic stroke at 3 months; (D) MACE at 3 months; (E) stroke at 12 months; (F) ischaemic stroke at 12 months; (G) haemorrhagic stroke at 12 months; (H) MACE at 12 months. The full line indicates the adjusted HR and the dashed lines the 95% CI bands. Reference is LDL-C >2.6 mmol/L. Data were fitted using a logistic regression model of restricted cubic spline with three knots (the 5th, 50th, 90th percentiles) for LDL-C level, adjusting for potential covariates. LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular events.
Lipid-lowering management, LLT compliance, and association of discharge LLT and outcomes
LLT management and compliance of the included patients during hospitalisation, at discharge, 3 months, 6 months and 12 months after the initial event were shown in table 3.
Table 3.
Lipid-lowering treatment (LLT) and the compliance of patients in CNSR-III
| Hospitalisation | Discharge | 3 months | 6 months | 12 months | |
| Non-LLT | 547 (3.6) | 1300 (8.6) | 2590 (17.4) | 3007 (22.4) | 3754 (26.0) |
| LLT | 14 506 (96.4) | 13 831 (91.4) | 12 271 (82.6) | 11 726 (77.6) | 10 682 (74.0) |
| Compliance | |||||
| Non-persistent | / | / | 2147 (15.5) | 3382 (24.5) | 4863 (35.2) |
| Persistent | / | / | 11 684 (84.5) | 10 449 (75.6) | 8968 (64.8) |
CNSR-III, Third China National Stroke Registry.
Over 90% of patients received LLT during hospitalisation and for 2 weeks after discharge. The LLT compliance was 84.5% at 3 months, 75.6% at 6 months and 64.8% at 12 months. The drug regimens of LLT for the patients in CNSR-III at 3 months, 6 months and 12 months were shown in table 4.
Table 4.
Lipid-lowering treatment of the included patients in CNSR-III at 3-month, 6-month, 12-month follow-up (n=15 166)
| Treatment | Patients with statins, N (%) | ||||
| Hospitalisation | Discharge | 3 months | 6 months | 12 months | |
| Atorvastatin | 10 527 (69.4) | 9851 (65.0) | 8656 (57.1) | 8228 (54.3) | 7470 (49.3) |
| <40 mg | 7442 (70.7) | 8770 (89.0) | 8284 (95.7) | 7963 (96.8) | 7269 (97.4) |
| ≥40 mg | 3083 (29.3) | 1081 (11.0) | 372 (4.3) | 265 (3.2) | 198 (2.7) |
| Rosuvastatin | 3546 (23.4) | 3395 (22.4) | 2903 (19.1) | 2779 (18.3) | 2489 (16.4) |
| <20 mg | 2876 (81.2) | 2983 (87.9) | 2650 (91.4) | 2536 (91.3) | 2313 (93.0) |
| ≥20 mg | 668 (18.9) | 412 (12.1) | 250 (8.6) | 242 (8.7) | 176 (7.1) |
| Simvastatin | 272 (1.8) | 239 (1.6) | 390 (2.6) | 411 (2.7) | 444 (2.9) |
| Pravastatin | 166 (1.1) | 165 (1.1) | 137 (0.9) | 128 (0.8) | 100 (0.7) |
| Lovastatin | 25 (0.2) | 24 (0.2) | 33 (0.2) | 33 (0.2) | 30 (0.2) |
| Fluvastatin | 54 (0.4) | 53 (0.4) | 52 (0.3) | 43 (0.3) | 47 (0.3) |
| Pravastatin | 61 (0.4) | 78 (0.5) | 70 (0.5) | 64 (0.4) | 61 (0.4) |
CNSR-III, Third China National Stroke Registry.
Compared with the non-discharge LLT group, LLT at discharge was associated with reduced risk of IS (HR=0.65, 95% CI: 0.45 to 0.94, p=0.02) and stroke recurrence (HR=0.69, 95% CI: 0.48 to 0.99, p=0.04) at 3 months (table 5).
Table 5.
The association of discharge lipid-lowering therapy (LLT) and outcomes
| Total | Events (n%) | HR (95% CI) unadjusted |
P value | HR (95% CI) adjusted |
P value | |
| 3 months | ||||||
| Stroke recurrence | ||||||
| Discharge LLT | 13 248 | 269 (2.0) | 0.68 (0.48 to 0.96) | 0.03 | 0.69 (0.48 to 0.99) | 0.04 |
| Non-discharge LLT | 1181 | 35 (3.0) | Reference | Reference | ||
| Ischaemic stroke | ||||||
| Discharge LLT | 13 263 | 245 (1.9) | 0.68 (0.47 to 0.98) | 0.04 | 0.65 (0.45 to 0.94) | 0.02 |
| Non-discharge LLT | 1188 | 32 (2.7) | Reference | Reference | ||
| Haemorrhagic stroke | ||||||
| Discharge LLT | 13 740 | 31 (0.2) | 0.71 (0.25 to 2.01) | 0.52 | 1.19 (0.36 to 3.98) | 0.78 |
| Non-discharge LLT | 1266 | 4 (0.3) | Reference | Reference | ||
| MACE | ||||||
| Discharge LLT | 13 248 | 299 (2.3) | 0.71 (0.51 to 1.003) | 0.052 | 0.74 (0.52 to 1.04) | 0.08 |
| Non-discharge LLT | 1181 | 37 (3.1) | Reference | Reference | ||
| 12 months | ||||||
| Stroke recurrence | ||||||
| Discharge LLT | 13 248 | 758 (5.7) | 0.88 (0.7 to 1.12) | 0.30 | 0.89 (0.7 to 1.14) | 0.36 |
| Non-discharge LLT | 1181 | 75 (6.4) | Reference | Reference | ||
| Ischaemic stroke | ||||||
| Discharge LLT | 13 263 | 683 (5.2) | 0.87 (0.68 to 1.11) | 0.26 | 0.86 (0.67 to 1.10) | 0.23 |
| Non-discharge LLT | 1188 | 69 (5.8) | Reference | Reference | ||
| Haemorrhagic stroke | ||||||
| Discharge LLT | 13 740 | 86 (0.6) | 0.97 (0.47 to 2.00) | 0.94 | 1.23 (0.56 to 2.69) | 0.60 |
| Non-discharge LLT | 1266 | 8 (0.6) | Reference | Reference | ||
| MACE | ||||||
| Discharge LLT | 13 248 | 838 (6.3) | 0.94 (0.75 to 1.19) | 0.60 | 0.96 (0.76 to 1.21) | 0.72 |
| Non-discharge LLT | 1181 | 78 (6.6) | Reference | Reference |
Patients who reached the endpoint (stroke recurrence or MACE, death and loss to follow-up) during hospitalisation were excluded.
MACE, major adverse cardiovascular events.
LDL-C goal achievement and the association of LDL-C changes (from baseline to 3 months) with outcomes at 12 months
The overall blood lipid levels at baseline and at 3-month and 12-month follow-up were shown in table 6. LDL-C goal of 1.8 mmol/L was achieved by 28.3% of patients at baseline, 46.7% at 3 months and 35.4% at 12 months; LDL-C goal of 1.4 mmol/L was achieved by 13.1% of patients at baseline, 25.6% at 3 months and 17.6% at 12 months.
Table 6.
Blood lipid level of the included patients at baseline, 3 months and 1 year in CNSR-III
| Lipids, mmol/L | Baseline N=10 738 |
3 months N=6034 |
1 year N=4899 |
| Median triglycerides (IQR), mmol/L | 1.37 (1.03–1.87) | 1.32 (0.98–1.81) | 1.46 (1.04–2.16) |
| Total cholesterol, mmol/L | 3.97 (3.31–4.72) | 3.74 (3.13–4.54) | 3.92 (3.25–4.76) |
| HDL-C, mmol/L | 0.93 (0.78–1.12) | 1.02 (0.86–1.21) | 0.99 (0.79–1.2) |
| LDL-C, mmol/L | 2.31 (1.73–2.97) | 1.87 (1.39–2.55) | 2.14 (1.57–2.87) |
| LDL <1.4 mmol/L, n (%) | 1407 (13.1) | 1547 (25.6) | 862 (17.6) |
| 1.4<LDL≤1.8 mmol/L, n (%) | 1636 (15.2) | 1272 (21.1) | 872 (17.8) |
| 1.8<LDL≤2.6 mmol/L, n (%) | 3655 (34.0) | 1785 (29.6) | 1533 (31.3) |
| LDL >2.6 mmol/L, n (%) | 4040 (37.6) | 1430 (23.7) | 1632 (33.3) |
CNSR-III, Third China National Stroke Registry; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
We did not find any significant association between the LDL-C reduction rate from baseline to 3-month follow-up and the risk of stroke and MACE at 12 months (table 7).
Table 7.
Association of LDL-C changes (from baseline to 3 months) with outcomes at 12 months
| Percentage of LDL level decrease (compared with baseline) | Total | Events (n%) | HR (95% CI) unadjusted | P value | HR (95% CI) adjusted | P value |
| 12 months | ||||||
| Stroke recurrence* | ||||||
| <30%, n (%) | 3526 | 137 (3.9) | 1.48 (0.92 to 2.39) | 0.11 | 1.42 (0.87 to 2.30) | 0.16 |
| 30%–50%, n (%) | 1146 | 45 (3.9) | 1.50 (0.88 to 2.56) | 0.14 | 1.44 (0.84 to 2.47) | 0.19 |
| >50%, n (%) | 718 | 19 (2.7) | Reference | Reference | ||
| MACE† | ||||||
| <30%, n (%) | 3526 | 149 (4.2) | 1.46 (0.92 to 2.20) | 0.11 | 1.39 (0.88 to 2.21) | 0.16 |
| 30%–50%, n (%) | 1146 | 47 (4.1) | 1.41 (0.84 to 2.36) | 0.19 | 1.36 (0.81 to 2.28) | 0.24 |
| >50%, n (%) | 718 | 21 (2.9) | Reference | Reference |
*Patients with stroke recurrence, death and loss to follow-up within 3 months were excluded.
†Patients with MACE, death and loss to follow-up within 3 months were excluded.
LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular events.
Discussion
This national hospital-based study described the current LDL-C level and LLT of patients with IS/TIA in the real world. We described the LLT management and LDL-C goal achievement. We also found that a lowered baseline LDL-C level was associated with a decreased risk of new IS and MACE at both 3 months and 12 months after the initial event, without an increased risk of ICH. In addition, LLT at discharge was associated with a reduced risk of cardiovascular events at 3 and 12 months. Given the large sample size of LDL-C levels of patients with IS/TIA and comprehensive prognostic characteristics recorded, these findings may have important clinical implications.
First, LDL-C of 1.4 mmol/L might be a reasonable target for the high-risk population. Our study indicated that the LDL ≤1.4 mmol/L group, with the highest risk factors, had the lowest stroke and MACE rates at 3 and 12 months. The paradox of high risk of stroke with low LDL-C level could be due to the previous intensive LLT and rigid LDL-C control. It is consistent with the previous study that fixed-dose statin regimens are less effective than targeting LDL-C levels of 1.8 or 1.4 mmol/L when pretreatment LDL-C levels exceed 4 mmol/L16; and the target of 1.4 mmol/L recently advocated in particularly high-risk patients is most effective when pretreatment LDL-C exceeds 3 mmol/L.16 In addition, 2019 European Society of Cardiology/European Atherosclerosis Society Guidelines for the management of dyslipidaemias set the most aggressive target of less than 1.4 mmol/L and a reduction of more than 50% in LDL-C.17
Second, our findings suggested the safety of the LDL-C ≤1.4 mmol/L at least in Chinese population, because this level was not associated with an increased risk of haemorrhagic stroke. Studies of LDL-C and ICH have reported conflicting results. In a 20-year epidemiological study, an excess risk of haemorrhagic stroke was observed in patients with uncontrolled hypertension and LDL-C <70 mg/dL (1.8 mmol/L).18 However, in a subgroup analysis of FOURIER trial,19 among patients with prior stroke, the risk of haemorrhagic stroke did not increase, even when the median LDL-C decreased from 2.4 mmol/L at randomisation to 0.8 (0.5–1.2) mmol/L at 48 weeks in the evolocumab group. All stroke and IS rates were reduced, and the rate of haemorrhagic stroke was not significantly changed. Meanwhile, in a systematic review and meta-analysis, the higher level of LDL-C tended to be associated with a lower risk of haemorrhagic stroke.20 Thus, our study indicated the efficacy and safety of the baseline LDL-C of <1.4 mmol/L in patients with IS/TIA, providing evidence for the first and second prevention strategies.
Third, we described the epidemiological characteristics of Chinese patients with IS/TIA in relation to their LDL-C levels and LLT. Compared with the study conducted in 2013,21 our study indicated some progress in blood lipid management in mainland China. Notably, about 97% of patients had LLT medication history prior to the entry into our study. Also, compared with the LLT rate of 79.6% in 2013, over 90% of patients in our cohort received LLT during hospitalisation and at discharge; the LLT compliance was 84.5% at 3 months, 75.6% at 6 months and 64.8% at 12 months. In addition, LDL-C goal achievement for 1.8 mmol/L had improved mildly, from 27.4% to 35.4%, and LDL-C goal achievement for 1.4 mmol/L was 17.6% at 12 months. The less than perfect LLT compliance and LDL-C control rate might be due to statin intolerance in Asian people, including statin-associated myopathy and haemorrhagic stroke.22 23 An earlier meta-analysis indicated that statins increase the risk of haemorrhagic stroke in a medication dose-dependent and type of index brain vascular injury-dependent manner, while proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors do not increase haemorrhagic stroke risk.24 Thus, statins, rather than low level of LDL-C, might closely relate to the risk of haemorrhagic stroke. Accordingly, PCSK9 inhibitors might be a more promising lipid-lowering medication class in patients with an elevated risk of haemorrhagic stroke. In addition, our analysis revealed a significant association between LLT at discharge and 3-month outcomes, indicating the importance of early LLT implementation.
Fourth, we did not observe the correlation between the 3-month LDL-C decrease amplitude and 12-month outcomes. To analyse the association of 3-month LDL-C change with 12-month outcomes, we excluded subjects who reached the endpoint within 3 months, which led to a reduction of our sample size and a loss of a considerable number of target events, for most stroke recurrences occurred within 3 months.25 Another critical factor was that we could not adjust some risk factors in the model, such as interleukin-6 level or the evidence of relevant ICAS, which were independent risk factors of the residual risk. Although substantially reduced by secondary prevention treatment, there was still 8.3% residual risk of 12-month recurrent stroke even in patients with persistent adherence to guideline-based secondary stroke prevention.26
Our study has several limitations. First, only LLT medication use at the follow-up time points was recorded, whereas additional details of use during the whole study, such as continuous use, intermittent use and the dose changes, were not subjected to specific analysis. Thus, lipid-lowering agent use at 3 months and 12 months provided only a partial picture of the course of medication during the study. Second, statin use before admission was not recorded in the study which may confound the results. Furthermore, details of medication use, such as class, dose, duration and adherence to lipid-lowering agents, did not enter the regression model. Third, there could be some undetected confounding factors in addition to those regarded as the residual risk. Fourth, the use of dual antiplatelet therapy may reduce the risk of a 3-month recurrence of stroke for more than half of the patients presented with an initial NIHSS score of ≤3. Fifth, the study was conducted exclusively on Chinese patients. The finding in this study needs to be further validated in studies with a larger sample size and non-Asian populations.
Conclusions
The LDL-C goal achievement has increased mildly in the population who had a stroke and with TIA in mainland China, and its further improvement is still an essential task for secondary prevention of stroke. The lowered baseline LDL-C level was significantly associated with a decreased short-and long-term risk of IS among patients who had a stroke and with TIA. LDL-C <1.4 mmol/L could be a safe standard for this population.
Supplementary Material
Acknowledgments
We thank Dr Feng Sheng for his important intellectual contributions to the article. We thank all participating hospitals, their physicians and nurses. We appreciate all the patients who took part in the CNSR-III.
Footnotes
Collaborators: The complete list of CNSR-III members and sites: Beijing Tiantan Hospital Capital Medical University, Beijing, China: Yongjun Wang; Aerospace Central Hospital, Beijing, China: Jilai Li; Beijing Qinghua Changgeng Hospital, Beijing, China: Jian Wu; Beijing Longfu Hospital, Beijing, China: Mei Zhang; Beijing Shijitan Hospital Capital Medical University, Beijing, China: Maolin He; Beijing Hospital, Beijing, China: Tao Gong; The Hospital of Shunyi District Beijing, Beijing, China: Quping Ouyang; Fuxing Hospital Capital Medical University, Beijing, China: Guang Huang; Beijing Haidian Hospital, Beijing, China: Fengchun Yu; Civil Aviation General Hospital, Beijing, China: Chenlong Wang; Chinese People’s Liberation Army 263 Hospital, Beijing, China: Jinli Zhang; Beijing Ditan Hospital Capital Medical University, Beijing, China: Wenqing Wu; Affiliated Hospital of the Chinese People’s Armed Police Force Logistics College, Tianjin, China: Yi Wang; Yaoyu Yu; Tianjin People’s Hospital, Tianjin, China: Meiyun Zhang; Tianjin Huanhu Hospital, Tianjin, China: Zhongping An; The Third Hospital of Hebei Medical University, Shijiazhuang, China: Junyan Liu; Shijiazhuang First Hospital, Shijiazhuang, China: Wanying Shi; Tangshan Gongren Hospital, Tangshan, China: Baoquan Lu; Luannan County Hospital, Tangshan, China: Lijun Geng; Kailuan General Hospital, Tangshan, China: Shujuan Wang; Yutian County Hospital, Tangshan, China: Xu Zhang; Zunhua People’s Hospital, Tangshan, China: Ruifang Liu; The No. 2 Hospital of Baoding, Baoding, China: Fengli Zhao; Handan City First Hospital, Handan, China: Jie Lin; Handan Central Hospital, Handan, China: Xinping Liu; Wu’an First People’s Hospital, Handan, China: Xuebing Sun; Shexian Hospital, Handan, China: Tianyuan Li; Affiliated Hospital of Hebei University of Engineering, Handan, China: Youming Wang; Hengshui People’s Hospital, Hengshui, China: Xinxia He; Hengshui Fifth People Hospital, Hengshui, China: Weiqiang Yuan; Cangzhou Hospital of Integrated TCM-WM, Hebei, Cangzhou, China: Ronghua Dou; Cangzhou People’s Hospital, Cangzhou, China: Lihai Liu, Yanling Wang; Cangzhou Central Hospital, Cangzhou, China: Junling Zhang; Chengde Central Hospital, Chengde, China: Haisong Du; Xingtai Third Hospital, Xingtai, China: Yuqing Wei; Weixian People’s Hospital, Xingtai, China: Cunrui Wang; First Hospital of Zhangjiakou, Zhangjiakou, China: Limin Wang; The First Affiliated Hospital of Hebei North University, Zhangjiakou, China: Yu’an Zou; Shanxi Cardiovascular Hospital, Taiyuan, China: Xiaofei Chen; Shanxi Provincial People’s Hospital, Taiyuan, China: Fengyun Hu; Yangquan Coalmine Group General Hospital, Yangquan, China: Jinfeng Liu; Changzhi People’s Hospital, Changzhi, China: Lili Zhao; The Second People’s Hospital of Jinzhong, Jinzhong, China: Fanping He; The Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China: Xingchen Wang; Shandong Police General Hospital, Jinan, China: Qingwei Zhao; Jinan Central Hospital, Jinan, China: Xiaohong Li; Leling People’s Hospital, Leling, China: Jun Zhao; Liaocheng People’s Hospital, Liaocheng, China: Zhangyong Xia; Dong’a County People’s Hospital, Liaocheng, China: Hongjin Li; Penglai Traditional Chinese Medicine Hospital, Penglai, China: Mingzong Yan; Penglai People’s Hospital, Penglai, China: Guiru Zhang; Yantaishan Hospital of Yantai City, Yantai, China: Hui Liang; Taian City Central Hospital, Taian, China: Yunlin Liu; Zhengzhou Yihe Hospital affiliated to Henan University, Zhengzhou, China: Jun Xu; Zhengzhou Central Hospital, Zhengzhou, China: Runqing Wang; Gongyi City People’s Hospital, Gongyi, China: Yuhui Han; Qixian People’s Hospital, Hebi, China: Xianghong Meng, Mingzhen Li; Jiyuan People’s Hospital, Jiyuan, China: Ting Wang; Kaifeng Central Hospital, Kaifeng, China: Xinsheng Han; Yucheng County People’s Hospital, Luoyang, China: Hongtian Zhang; Luoyang Central Hospital, Luoyang, China: Congmin Ma; Pingdingshan First People’s Hospital, Pingdingshan, China: Wenjun Xue; Ruzhou First People’s Hospital, Ruzhou, China: Chun Wang; Shangqiu First People’s Hospital, Shangqiu, China: Yan Fang; Changge Municipal People’s Hospital, Xuchang, China: Gexia Liu; Dalian Central Hospital, Dalian, China: Jianfeng Wang; Affiliated Zhongshan Hospital of Dalian University, Dalian, China: Qiang Ma; Dalian Friendship Hospital, Dalian, China: Xiaohong Li; Wenxu Zheng; Xinhua Hospital Affiliated to Dalian University, Dalian, China: Haitao Chi; The Fourth Affiliated Hospital of China Medical University, Shenyang, China: Lianbo Gao; First People’s Hospital of Shenyang, Shenyang, China: Jin Zhou; People’s Liberation Army Shenyang Military Region General Hospital, Shenyang, China: Huisheng Chen; Shengjing Hospital of China Medical University, Shenyang, China: Juan Feng; Anshan Central Hospital, Anshan, China: Hongbo Xiao; Third People Hospital of Liaoyang, Liaoyang, China: Lijun Xiao; Jilin University First Hospital, Changchun, China: Yi Yang; The First Affiliated Hospital of Harbin Medical University, Harbin, China: Guozhong Li; The Second Affiliated Hospital of Harbin Medical University, Harbin, China: Yulan Zhu, Lihua Wang; Hongqi Hospital Affiliated to Mudanjiang Medical College, Mudanjiang, China: Yindong Yang; Qiqihar City Rongjian Stroke Prevention and Treatment Institute, Qiqihar, China: Xuerong Qiu; Daqing Oilfield General Hospital, Daqing, China: Xuhai Gong; Wuhan First Hospital, Wuhan, China: Guohua Chen; Hubei Third People’s Hospital, Wuhan, China: Xiaoxiang Peng; The Central Hospital of Enshi Autonomous Prefecture, Wuhan, China: Qunhui Liu; Zhongxiang Hospital of Renmin Hospital of Wuhan University, Jingmen, China: Shiping Gong; Xiangyang Central Hospital, Xiangyang, China: Hongbin Zhou; Zhangzhou First People’s Hospital, Chenzhou, China: Haipeng Li; The First Affiliated Hospital of Nanhua University, Hengyang, China: Yong You; Xiangtan Central Hospital, Xiangtan, China: Jinsheng Li; Nanjing Drum Tower Hospital, Nanjing, China: Yun Xu; The Second Chinese Medicine Hospital of Jiangsu, Nanjing, China: Lei Sheng; The Second Affiliated Hospital of Suzhou University, Suzhou, China: Heqing Zhao; The Second Affiliated Hospital of Lianyungang, Lianyungang, China: Aixia Zhuang; Affiliated Hospital of Nantong University, Nantong, China: Kaifu Ke; The First Affiliated Hospital of Suzhou University, Nantong, China: Qi Fang; The First People’s Hospital of Nantong, Nantong, China: Zhengxie Dong; The Affiliated Hospital of Xuzhou Medical College, Xuzhou, China: Guiyun Cui, Deqin Geng; The Second Affiliated Hospital of Xuzhou Medical College, Xuzhou, China: Liangqun Rong; Yixing People’s Hospital, Yixing, China: Junfeng Shi; Affiliated Hospital of Jiangsu University, Zhenjiang, China: Ming Yu; Subei People’s Hospital of Jiangsu, Yangzhou, China: Jun Xu; Zhejiang Provincial People’s Hospital, Hangzhou, China: Yu Geng; The First Affiliated Hospital of Zhejiang University, Hangzhou, China: Benyan Luo; Lishui Center Hospital, Lishui, China: Xueli Cai; Shaoxing Central Hospital, Shaoxing, China: Jun Zhou; Yiwu Hospital Affiliated to Wenzhou Medical University, Yiwu, China: Yi Wu; Zhoushan Hospital, Zhoushan, China: Weiguo Tang; Taizhou First People’s Hospital, Taizhou, China: Zhimin Wang; The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China: Yangmei Chen; Third Affiliated Hospital of the Third Military Medical University of the Chinese People’s Liberation Army, Chongqing, China: Yanjiang Wang; Affiliated Hospital of the Third Military Medical University, Chongqing, China: Kangning Chen; Qinghai Provincial People’s Hospital, Qinghai, China: Shizheng Wu; Huainan Chaoyang Hospital, Huainan, China: Wenguang Bu; Huangshan People’s Hospital, Huangshan, China: Xiaohua Cheng; The Third Affiliated Hospital of Zhongshan University, Guangzhou, China: Zhengqi Lu; The First Affiliated Hospital of Jinan University, Guangzhou, China: An’ding Xu; Southern Hospital of Southern Medical University, Guangzhou, China: Jia Yin; The University of Hong Kong, Shenzhen Hospital, Shenzhen, China: Jifu Cai; Shenzhen People’s Hospital, Shenzhen, China: Yi Guo; Shenzhen Hospital of Peking University, Shenzhen, China: Jun Wu; The People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China: Lvli Li; Wuzhou People’s Hospital, Wuzhou, China: Li Pan; Fujian Provincial Hospital, Fuzhou, China: Yinzhou Wang; The First Affiliated Hospital of Fujian Medical University, Fuzhou, China: Ning Wang; The Second Hospital of Xiamen, Xiamen, China: Jianping Niu; Xiamen Haicang Hospital, Xiamen, China: Qing Li; The First Affiliated Hospital of Medical College, Shihezi University, Shihezi, China: Hong Wang; Xinjiang Uygur Autonomous Region People’s Hospital, Urumqi, China: Hongyan Li; Xinjiang Production and Construction Corps Hospital, Urumqi, China: Xiaoying Zhang; Kunming Yan’an Hospital, Kunming, China: Liping Zhan; Wuyuan County People’s Hospital, Bayannaoer, China: Yongming Chen; Baotou Central Hospital, Baotou, China: Baojun Wang; First Affiliated Hospital of Baotou Medical University, Baotou, China: Li’e Wu; Chifeng Municipal Hospital, Chifeng, China: Li Liu; Affiliated Hospital of Chifeng University, Chifeng, China: Yanru Zhao; Ordos Center Hospital, Ordos, China: Yingchun Wu; Inner Mongolia People’s Hospital, Hohhot, China: Runxiu Zhu; Ningxia Medical University General Hospital, Yinchuan, China: Yanhui Du; The Third People’s Hospital of Ningxia, Yinchuan, China: Yongxia Wen; Xi’an Central Hospital, Xi’an, China: Ye Tian; Xi’an First Hospital, Xi’an, China: Songdi Wu; Yan’an University Affiliated Hospital, Yan’an, China: Yongcai Qu; First People’s Hospital Affiliated to Shanghai Jiaotong University, Shanghai, China: Yuncheng Wu; The Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China: Jianren Liu; Huashan Hospital Affiliated to Fudan University, Shanghai, China: Qiang Dong; Shanghai Pudong New Area People’s Hospital, Shanghai, China: Qingke Bai; The Sixth People’s Hospital Affiliated to Shanghai Jiaotong University, Shanghai, China: Yuwu Zhao; The Eighth People’s Hospital of Shanghai Province, Shanghai, China: Xu Chen; The Third People’s Hospital of Hainan Province, Sanya, China: Chaoming He; Shenzhen Second People’s Hospital, Shenzhen, China: Lijie Ren; Lishui People’s Hospital, Lishui, China: Weiwen Qiu; Sanmenxia Central Hospital, Sanmenxia, China: Shufang Yao; Yantai Yuhuangding Hospital, Yantai, China: Xuwen Sun; The Second Xiangya Hospital of Central South University, Changsha, China: Hainan Zhang; Taiyuan Central Hospital, Taiyuan, China: Weirong Li; Zhumadian Central Hospital, Zhumadian, China: Ligong Gao; Qingyuan People’s Hospital, Qingyuan, China: Xianglin Chen; The First Hospital of Beijing Fangshan District, Beijing, China: Jianhua Li; Affiliated Hospital of North China University of Technology, Tangshan, China: Qiuyan Shi; Tangshan People’s Hospital, Tangshan, China: Yan Wang; Zhongli County People’s Hospital, Zhengzhou, China: Mingzhi Zhao; First Affiliated Hospital of Zhongshan University, Guangzhou, China: Jinsheng Zeng; Kaifeng Second People’s Hospital, Kaifeng, China: Liping Wang; People’s Liberation Army No. 309 Hospital, Beijing, China: Wei Wang; Naval General Hospital, Beijing, China: Feng Qiu; Beijing Huairou Hospital of University of Chinese Academy of Sciences, Beijing, China: Zhaochen Li; Affiliated Hospital of Chengde Medical College, Chengde, China: Liang Zhao; Yingyang City People’s Hospital, Xingyang, China: Tianbao Chen; Zhoukou Central Hospital, Zhoukou, China: Lei Xia; Changzi People’s Hospital, Changzhi, China: SuYun Yang; Qinyang City People’s Hospital, Qinyang, China: Yazhou Han; Lianyungang Municipal Oriental Hospital, Lianyungang, China: Liyan Liu; Qingyuan County People’s Hospital, Lishui, China: Xinxiao Wu; Xunxian People’s Hospital, Hebi, China: Beihai Jiang; The People’s Hospital of Hebi, Hebi, China: Lizhong Li; Longquan People’s Hospital, Longquan, China: Weidong Lou; Hospital of Jianshui, Jianshui, China: Xiaoqian Shen; Affiliated Nanhua Hospital, University of South China, Hengyang, China: Ping Zhang; Jingning County People’s Hospital, Lishui, China: Weiming Lan; Jinzhong City First People’s Hospital, Jinzhong, China: Aihu Zheng; Shanxi Qixian People’s Hospital, Jinzhong, China: Qifu Bai; Jiangsu Jiangbei People’s Hospital, Nanjing, China: Lifang Luan; The Second Affiliated Hospital of Nanhua University, Hengyang, China: Lin Chen; Fenxi Mining Bureau Hospital, Jinzhong, China: Liqing Yan; Hejian City People’s Hospital, Hejian, China: Yanxia Wang; Wenzhou City Third People’s Hospital, Wenzhou, China: Xuerong Huang; Huangdao District People’s Hospital, Qingdao, China: Xiangting Chai; Anyang County People’s Hospital, Jiyuan, China: Yanshu Liu; Nanyang Second People’s Hospital, Nanyang, China: Liangjun You; Jiyuan City Hospital of Traditional Chinese Medicine, Jiyuan, China: Hongqin Yang; The Second Affiliated Hospital of Shanxi Medical University, Taiyuan, China: Dongfang Li; The Second Hospital of Hebei Medical University, Shijiazhuang, China: Huijuan Wang; Hengshui City Yinzhou District Hospital, Hengshui, China: Linying Gui; The No. 4 People’ Hospital of Hengshui, Hengshui, China: Aisheng Wu, Jianling Zhang; Huimin County People’s Hospital, Binzhou, China: Dengling Wang; The Western Hospital of Shandong Provincial Hospital, Jinan, China: Qinghua Zhang; The Fifth Affiliated Hospital of Zhengzhou University, Zhengzhou, China: Yunhong He; Qingdao City, Hai Ci Hospital, Qingdao, China: Ruiyou Guo; the Affiliated Hospital of Qingdao University, Qingdao, China: Jijun Teng; The Zhengzhou First People’s Hospital, Zhengzhou, China: Ping Lou.
Contributors: YX and XM conceived and designed the study. XM and YJW served as scientific advisors. XM, ZL, HL and YJW critically reviewed the study proposal. XM, XZ, LL and YLW collected and assembled the data. MW and YP did statistical analyses. YX and WC interpreted the data. YX drafted the manuscript and did the language editing. XM is responsible for the overall content as the guarantor. All the authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding: This work was supported by the National Key R&D Program of China (no. 2018YFC1312903), National Natural Science Foundation of China (no. 81870905, 82071295, 81801139) and Beijing Hospitals Authority Innovation Studio of Young Staff Funding Support (code: 202113).
Competing interests: None declared.
Patient and public involvement: This registry study was designed and conducted without patient and public involvement. Our results will be disseminated to the public through publication in this journal.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
the CNSR-III Investigators:
Yongjun Wang, Jilai Li, Jian Wu, Mei Zhang, Maolin He, Tao Gong, Quping Ouyang, Guang Huang, Fengchun Yu, Chenlong Wang, Jinli Zhang, Wenqing Wu, Yi Wang, Yaoyu Yu, Meiyun Zhang, Zhongping An, Junyan Liu, Wanying Shi, Baoquan Lu, Lijun Geng, Shujuan Wang, Xu Zhang, Ruifang Liu, Fengli Zhao, Jie Lin, Xinping Liu, Xuebing Sun, Tianyuan Li, Youming Wang, Xinxia He, Weiqiang Yuan, Ronghua Dou, Lihai Liu, Yanling Wang, Junling Zhang, Haisong Du, Yuqing Wei, Cunrui Wang, Limin Wang, Yu’an Zou, Xiaofei Chen, Fengyun Hu, Jinfeng Liu, Lili Zhao, Fanping He, Xingchen Wang, Qingwei Zhao, Xiaohong Li, Jun Zhao, Zhangyong Xia, Hongjin Li, Mingzong Yan, Guiru Zhang, Hui Liang, Yunlin Liu, Jun Xu, Runqing Wang, Yuhui Han, Xianghong Meng, Ting Wang, Xinsheng Han, Hongtian Zhang, Congmin Ma, Wenjun Xue, Chun Wang, Yan Fang, Gexia Liu, Jianfeng Wang, Qiang Ma, Xiaohong Li, Wenxu Zheng, Haitao Chi, Lianbo Gao, Jin Zhou, Huisheng Chen, Juan Feng, Hongbo Xiao, Lijun Xiao, Yi Yang, Guozhong Li, Yulan Zhu, Lihua Wang, Yindong Yang, Xuerong Qiu, Xuhai Gong, Guohua Chen, Xiaoxiang Peng, Qunhui Liu, Shiping Gong, Hongbin Zhou, Haipeng Li, Yong You, Jinsheng Lin, Yun Xu, Lei Sheng, Heqing Zhao, Aixia Zhuang, Kaifu Ke, Qi Fang, Zhengxie Dong, Guiyun Cui, Deqin Geng, Liangqun Rong, Junfeng Shi, Ming Yu, Jun Xu, Yu Geng, Benyan Luo, Xueli Cai, Jun Zhou, Yi Wu, Weiguo Tang, Zhimin Wang, Yangmei Chen, Yanjiang Wang, Kangning Chen, Shizheng Wu, Wenguang Bu, Xiaohua Cheng, Zhengqi Lu, An’ding Xu, Jia Yin, Jifu Cai, Yi Guo, Jun Wu, Lvli Li, Yinzhou Wang Li Pan, Ning Wang, Jianping Niu, Qing Li, Hong Wang, Hongyan Li, Xiaoying Zhang, Liping Zhan, Yongming Chen, Baojun Wang, Li’e Wu, Yanru Zhao Li Liu, Yingchun Wu, Runxiu Zhu, Yanhui Du, Yongxia Wen, Ye Tian, Songdi Wu, Yongcai Qu, Yuncheng Wu, Jianren Liu, Qiang Dong, Qingke Bai, Yuwu Zhao, Xu Chen, Chaoming He, Lijie Ren, Weiwen Qiu, Shufang Yao, Xuwen Sun, Hainan Zhang, Weirong Li, Ligong Gao, Xianglin Chen, Jianhua Li, Qiuyan Shi, Yan Wang, Mingzhi Zhao, Jinsheng Zeng, Liping Wang, Wei Wang, Feng Qiu, Zhaochen Li, Liang Zhao, Tianbao Chen, Lei Xia, SuYun Yang, Yazhou Han, Liyan Liu, Xinxiao Wu, Beihai Jiang, Lizhong Li, Weidong Lou, Xiaoqian Shen, Ping Zhang, Weiming Lan, Aihu Zheng, Qifu Bai, Lifang Luan, Liqing Yan, Yanxia Wang, Xuerong Huang, Xiangting Chai, Yanshu Liu, Liangjun You, Hongqin Yang, Dongfang Li, Huijuan Wang, Linying Gui, Aisheng Wu, Dengling Wang, Qinghua Zhang, Yunhong He, Ruiyou Guo, Jijun Teng, and Ping Lou
Data availability statement
Data are available upon reasonable request. The datasets used in this study are not publicly available, but these can be provided on reasonable request after the approval.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the ethics committee at Beijing Tiantan Hospital (KY2019-109-01). The study protocol of the CNSR-III was approved by the ethics committee at Beijing Tiantan Hospital (IRB approval number: KY2015-001-01) and all participating centres. Every participant provided written informed consent before participation.
References
- 1. Wang Y-J, Li Z-X, Gu H-Q, et al. China stroke statistics 2019: a report from the National center for healthcare quality management in neurological diseases, China national clinical research center for neurological diseases, the Chinese stroke association, National center for chronic and non-communicable disease control and prevention, Chinese center for disease control and prevention and Institute for global neuroscience and stroke collaborations. Stroke Vasc Neurol 2020;5:211–39. 10.1136/svn-2020-000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–59. 10.1056/NEJMoa061894 [DOI] [PubMed] [Google Scholar]
- 3. Amarenco P, Kim JS, Labreuche J, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med 2020;382:9. 10.1056/NEJMoa1910355 [DOI] [PubMed] [Google Scholar]
- 4. Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: a European stroke organisation guideline. Eur Stroke J 2019;4:198–223. 10.1177/2396987319841187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/american stroke association. Stroke 2021;52:e364–467. 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 6. Amarenco P, Kim JS, Labreuche J, et al. Treat stroke to target trial design: first trial comparing two LDL targets in patients with atherothrombotic strokes. Eur Stroke J 2019;4:271–80. 10.1177/2396987319838100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet 2014;383:984–98. 10.1016/S0140-6736(13)61088-0 [DOI] [PubMed] [Google Scholar]
- 8. Wong LKS. Global burden of intracranial atherosclerosis. Int J Stroke 2006;1:158–9. 10.1111/j.1747-4949.2006.00045.x [DOI] [PubMed] [Google Scholar]
- 9. Mok V, Srikanth V, Xiong Y, et al. Race-ethnicity and cerebral small vessel disease -- comparison between Chinese and white populations. Int J Stroke 2014;9 Suppl A100:36–42. 10.1111/ijs.12270 [DOI] [PubMed] [Google Scholar]
- 10. Wolma J, Nederkoorn PJ, Goossens A, et al. Ethnicity a risk factor? The relation between ethnicity and large- and small-vessel disease in white people, black people, and Asians within a hospital-based population. Eur J Neurol 2009;16:522–7. 10.1111/j.1468-1331.2009.02530.x [DOI] [PubMed] [Google Scholar]
- 11. Collins R, Armitage J, Parish S, et al. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004;363:757–67. 10.1016/S0140-6736(04)15690-0 [DOI] [PubMed] [Google Scholar]
- 12. Oyama K, Giugliano RP, Blazing MA, et al. Baseline low-density lipoprotein cholesterol and clinical outcomes of combining ezetimibe with statin therapy in Improve-IT. J Am Coll Cardiol 2021;78:1499–507. 10.1016/j.jacc.2021.08.011 [DOI] [PubMed] [Google Scholar]
- 13. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22. 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 14. McKenney JM, Koren MJ, Kereiakes DJ, et al. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol 2012;59:2344–53. 10.1016/j.jacc.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 15. Wang Y, Jing J, Meng X, et al. The third China national stroke registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke Vasc Neurol 2019;4:158–64. 10.1136/svn-2019-000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soran H, Adam S, Durrington PN. Optimising treatment of hyperlipidaemia: quantitative evaluation of UK, USA and european guidelines taking account of both LDL cholesterol levels and cardiovascular disease risk. Atherosclerosis 2018;278:135–42. 10.1016/j.atherosclerosis.2018.08.040 [DOI] [PubMed] [Google Scholar]
- 17. Authors/Task Force Members, ESC Committee for Practice Guidelines (CPG), ESC National Cardiac Societies . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis 2019;290:140–205. 10.1016/j.atherosclerosis.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 18. Zhang X, Liu J, Wang M, et al. Twenty-year epidemiologic study on LDL-C levels in relation to the risks of atherosclerotic event, hemorrhagic stroke, and cancer death among young and middle-aged population in china. J Clin Lipidol 2018;12:1179–89. 10.1016/j.jacl.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 19. Giugliano RP, Pedersen TR, Saver JL, et al. Stroke prevention with the PCSK9 (proprotein convertase subtilisin-kexin type 9) inhibitor evolocumab added to statin in high-risk patients with stable atherosclerosis. Stroke 2020;51:1546–54. 10.1161/STROKEAHA.119.027759 [DOI] [PubMed] [Google Scholar]
- 20. Wang X, Dong Y, Qi X, et al. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta-analysis. Stroke 2013;44:1833–9. 10.1161/STROKEAHA.113.001326 [DOI] [PubMed] [Google Scholar]
- 21. Wang C-J, Wang Y-L, Li Z-X, et al. The management of LDL cholesterol and predictors of goal achievement in stroke patients in China: a cross-sectional study. CNS Neurosci Ther 2016;22:577–83. 10.1111/cns.12543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tomlinson B, Chan P, Liu Z-M. Statin responses in Chinese patients. J Atheroscler Thromb 2018;25:199–202. 10.5551/jat.40204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tomlinson B, Chan P, Liu Z-M. Statin intolerance-an Asian perspective. J Atheroscler Thromb 2020;27:485–8. 10.5551/jat.50435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sanz-Cuesta BE, Saver JL. Lipid-Lowering therapy and hemorrhagic stroke risk: comparative meta-analysis of statins and PCSK9 inhibitors. Stroke 2021;52:3142–50. 10.1161/STROKEAHA.121.034576 [DOI] [PubMed] [Google Scholar]
- 25. Coull AJ, Lovett JK, Rothwell PM, et al. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326. 10.1136/bmj.37991.635266.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan Y, Li Z, Li J, et al. Residual risk and its risk factors for ischemic stroke with adherence to guideline-based secondary stroke prevention. J Stroke 2021;23:51–60. 10.5853/jos.2020.03391 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The datasets used in this study are not publicly available, but these can be provided on reasonable request after the approval.