Abstract
Changes in old age that contribute to the complex issue of an increased metabolic cost of walking (mass-specific energy cost per unit distance traveled) in older adults appear to center at least in part on changes in gait biomechanics. However, age-related changes in energy metabolism, neuromuscular function and connective tissue properties also likely contribute to this problem, of which the consequences are poor mobility and increased risk of inactivity-related disease and disability. The U.S. National Institute on Aging convened a workshop in September 2021 with an interdisciplinary group of scientists to address the gaps in research related to the mechanisms and consequences of changes in mobility in old age. The goal of the workshop was to identify promising ways to move the field forward toward improving gait performance, decreasing energy cost, and enhancing mobility for older adults. This report summarizes the workshop and brings multidisciplinary insight into the known and potential causes and consequences of age-related changes in gait biomechanics. We highlight how gait mechanics and energy cost change with aging, the potential neuromuscular mechanisms and role of connective tissue in these changes, and cutting-edge interventions and technologies that may be used to measure and improve gait and mobility in older adults. Key gaps in the literature that warrant targeted research in the future are identified and discussed.
Introduction
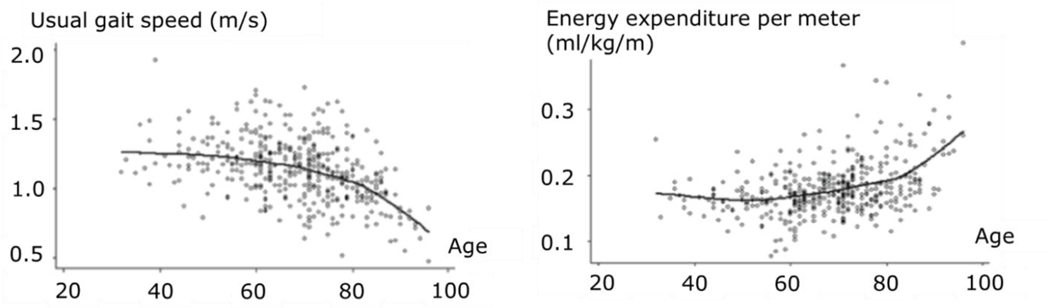
In old age, reduced or limited mobility, defined here as the ability to move freely in the environment, is associated with a loss of independence and an increased risk of morbidity and mortality [1–4]. A hallmark of the age-related decline in mobility is a slowing of gait speed; this can also be accompanied by an increase in the metabolic cost of walking (mass-specific energy cost per unit distance traveled) after the seventh decade of life [5], (Figure 1). Larish et al. [6] reported the first observation that walking requires more energy (or caloric expenditure) in older, compared with younger, adults; this was subsequently demonstrated to be consistent across a range of gait speeds [7]. A higher energy cost of walking is proposed as a risk factor for developing slowed gait speed and reduced mobility in old age [8]. However, the mechanisms for this association between gait speed and energy cost are not yet understood, nor consequently have versatile and effective interventions to mitigate this problem been fully developed. The significant societal, economic, and personal burdens associated with mobility limitations highlight the importance of understanding the mechanisms for increased metabolic cost of walking and slowed walking speed with aging.
Figure 1.
A cross-sectional analysis from the Baltimore Longitudinal Study of Aging shows that walking speed remains relatively constant throughout adulthood until the 7th decade, when usual gait speed begins to decline (left). The curvilinear relationship between Usual gait speed and age is shown with a lowess fit curve. Age-related changes in energy expenditure (right) mirror the changes in walking speed, suggesting an association between usual walking speed and age-related changes in energy use during locomotion. The curvilinear relationship between energy expenditure and age is shown with a lowess fit curve. Figure adapted from Schrack et al., 2012 [5].
The United States National Institute on Aging convened a 2-day workshop titled “Causes and Consequences of Age-Related Changes in Gait Biomechanics: The Metabolic Cost of Walking” in September 2021. Given the holistic approach needed to solve the riddle of reduced mobility with aging, the scientists and clinicians invited to participate in this workshop represented the fields of gerontology, epidemiology, physiology, biochemistry, engineering, physical therapy, neuromechanics, kinesiology, robotics, rehabilitation, evolutionary biology, and biomechanics. The speakers were charged with the task of distilling the state of science in their field into short, focused presentations that identified current knowledge, key knowledge gaps and emerging opportunities that might be applied to the problem of increased energy cost of walking, gait changes, and consequent mobility limitations in aging. Presentations were followed by small group discussions to identify the most important and targetable gaps that should be addressed in order to move the field forward. These small groups reconvened for whole-group synthesis sessions to bring together the new ideas that the small groups had generated. In this way, the workshop identified areas for future research, innovation, and collaboration to advance knowledge of the multifactorial issue of age-related changes in mobility.
This report synthesizes the current state of the field and highlights research gaps to be addressed based on the discussion by the assembled group of experts. The discussion topics were not exhaustive but rather focused on the key areas of existing work, and the assembled experts’ perception of the most critical areas for future focus. The report is structured similarly to the four main sessions of the workshop: i) age-related changes in gait biomechanics; ii) neuromuscular mechanisms for age-related changes in gait; iii) tendon and connective tissues changes with aging; and iv) interventions and assistive technologies to improve mobility in aging. Discussion related to the importance of increasing diversity and inclusivity in this field of research, as well as the context for the problem provided by two keynote talks, are also highlighted. Overall, the consensus from the workshop was that a more complete understanding is clearly needed to identify how an increased metabolic cost of walking develops in old age, and how that might be mitigated to maintain mobility in our aging population. In understanding the changes in gait and energetics with old age, the context in which gait quality degrades (e.g., uneven terrain), the reasons that gait patterns change (e.g., shift to greater use of proximal muscles) and the impact of study activities that may be novel for participants (e.g. treadmill walking) [9] are essential to consider. Finally a suggested aim of future research should be to understand how age-related challenges to mobility develop, and how best to maintain mobility and health throughout the lifespan.
Session 1: Changes in gait biomechanics with aging
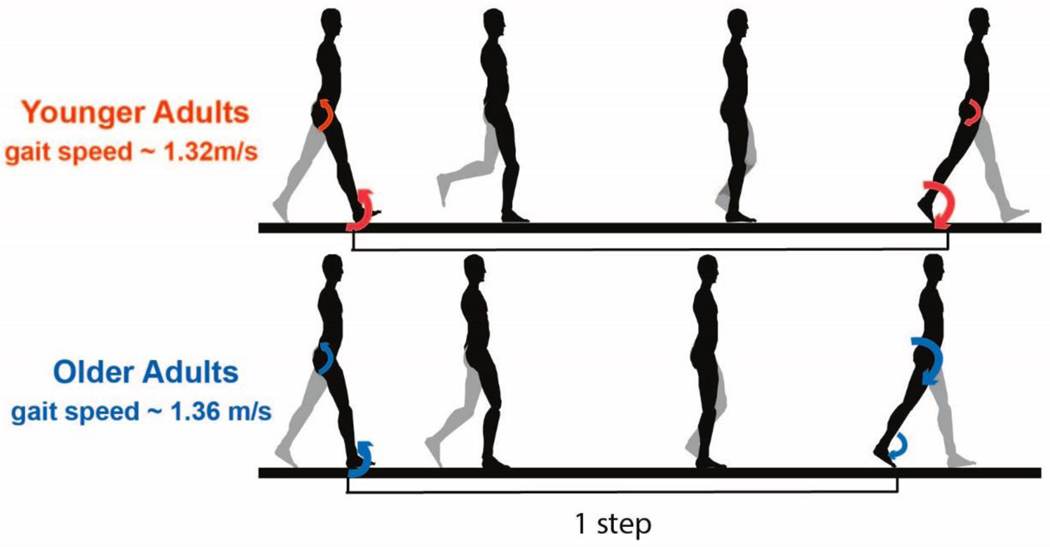
A prevailing concept in the field of gait biomechanics is that there is a “distal-to-proximal shift” in muscle workloads with advancing age such that older adults move away from an “ankle strategy” towards a “hip strategy” for producing propulsion and support during walking [10, 11] (Figure 2). For example, older adults produce less power at the ankle than younger adults at push-off during both level and uphill walking [12, 13]. When gait speeds are matched, older adults also produce more power at the hip than younger adults [10]. Moreover, the greater reliance on hip muscles for walking in older adults was found to be a significant predictor of the greater metabolic cost in older as compared to younger adults [14]. Elevated metabolic cost with a shift from reliance on distal to proximal muscles is consistent with modeling results showing that the plantar flexors function with relatively low energy cost in walking due, in part, to effective storage and release of elastic energy in the Achilles tendon [15]. However, a full understanding of the changes in neuromuscular control responsible for the redistribution of joint mechanical powers in older adults remains elusive [16]. There are limited data for how activation patterns of specific muscle and muscle groups change with age and contribute to the above-mentioned gait changes. For older compared with younger adults, differences including greater levels and prolonged timing of antagonist muscle co-activation have been reported [16–18] and appear to be related to the greater cost of walking in aging [16, 17, 19, 20]. Further knowledge of the nature, causes, and consequences of age-related changes in neuromuscular control is needed to inform interventions targeting problematic alterations in gait with aging. The discussions at the workshop were focused on understanding limitations in our current knowledge of what “aged gait” is, the proposed mechanisms for the observed changes, as well as new scientific approaches and tools available that may be helpful in advancing the science in this area.
Figure 2.
With age, both joint motions (kinematics) and causes of motion (kinetics) differ from those of young adults. Compared with younger adults, older adults, even at speeds matched to the young, walk with shorter strides, cover less distance with each step, have greater double support time and have smaller joint ranges of motion, particularly at the ankle and sometimes the hip. At the end of a step, older adults have greater hip joint moments and powers (large arrows), but smaller ankle moments and powers compared to younger adults (small arrows). Figure based on data from Boyer et al., 2017 [10].
The nature and magnitude of age-related biomechanical adaptations are complicated by factors such as habitual physical activity and self-selected walking speed. Many studies compare gait kinetics and kinematics in younger and older adults, which can be an appropriate study design. However, these cross-sectional studies often find that both groups have a similar preferred walking speed, suggesting that the field is most often examining a highly mobile subset of older adults that may not represent the target population: older adults at risk for or living with walking impairments. Future studies need to include older adults with slowed gait speeds and incident mobility limitations, as these individuals may represent a population of people that often does not make it into laboratories for research studies, but is representative of a large proportion of adults in the community. Indeed, there is merit to comparing low-functioning older adults with high-functioning older adults using a case-control study design.
The mechanisms for altered gait mechanics with age remain unclear. The initial hypothesis has been that reductions in maximal muscle force or power prompt a need to alter gait mechanics in order to avoid approaching the maximal capacity of any one muscle group. However, while healthy, mobile older adults typically produce less positive joint power and work at the ankle, when given biofeedback about their performance they have the capacity to produce similar propulsive power at the ankle as younger adults [21]. Functional demand (i.e., the ratio of external joint kinetics to maximal muscle torque) for both the plantar flexor and knee extensor muscle groups is larger in older compared with younger adults [22, 23], and overall functional demand increases as walking speed increases [24]. Might these differences in functional demand explain greater energy cost and slowed gait speeds in aging? Do these results extend to older adults already experiencing mobility limitations? Estimates vary considerably, however, functional demand generally does not exceed or even approach the maximum capacity for most locomotor muscle groups. In addition, differences in gait mechanics have been reported in mid-life adults (50–64 years) prior to the generally expected onset of age-related declines in muscle function [25, 26]. Notably, several studies report no gait differences between older adults with higher levels of physical activity and, presumably, more “youthful” muscle function than those with low habitual physical activity levels [27, 28]. The concept that muscle power, which involves not only the force produced but also the velocity of the contraction, is a better predictor of future morbidity than torque alone has gained substantial support in recent years [29, 30]. While muscle strength (i.e., maximal voluntary torque) is likely to play some role in the alteration of gait with age, looking beyond muscle strength alone to evaluate the impact of changes in structure, composition, and morphology of muscle and tendon [31]; contraction velocity and power [29]; neural drive to muscle [32]; and interactions among these systems may yield more insight about the mechanisms of age-related changes in gait mechanics.
Tendons work in concert with muscles; therefore, the entire muscle-tendon complex should be considered when exploring the mechanisms for changes in gait biomechanics with aging, and their consequences on the metabolic cost of walking. Advances in ultrasound techniques have yielded new observations of changes in Achilles tendon behavior with age. There is evidence of less independent movement of the subtendons during gait for older adults [33], which may contribute to the lower propulsive power observed at the ankle in older adults [34]. Differences in Achilles tendon moment arm and stiffness may also impact the propulsive power at the ankle and metabolic cost of walking for older adults; however, these are not consistent findings [14, 33]. The Achilles tendon has more capacity for storing and returning elastic energy than do the tendons about the knee and hip, which may help explain, in part, why a “hip strategy” is a less economical way to ambulate [15, 35]. While additional evidence is needed to quantify the role of altered muscle-tendon unit properties on gait mechanics, addressing these changes in plantar flexor muscle tendon unit behavior and propulsive power at the ankle is one potential strategy that could be explored to understand changes in the metabolic cost of walking in the old. Biofeedback or exoskeletons may be useful experimental models for exploring these questions [21, 36]. Without this information, questions will persist as to: the optimal training strategy to improve propulsive power, particularly in those with reduced mobility; when to intervene; and how to maintain these adaptations to decrease the cost of walking and preserve mobility throughout the lifespan. Indeed, a better understanding of causes of changes to gait in aging is needed before effective interventions can be designed [37].
Likewise, strategies intending to reverse age-related changes in gait mechanics must take into consideration the priorities of the individual, along with the effect that targeting a specific biomechanical deficit may have on overall mobility and quality of life in older adults. For example, stroke is a condition that affects almost one million Americans every year [38], the majority of whom are over the age of 65 years. Recovery from stroke often involves physical rehabilitation to regain strength and mobility, and studies have shown that people can improve their walking symmetry post-stroke [39]. However, this increased gait symmetry did not improve the energy cost of walking [40], and may have had a negative impact on balance [41]. Further, although slowed gait speed is considered problematic (as, for example, when crossing a busy street), interventions designed to increase gait speed may also have a negative impact on balance in some people, depending on their strategy for increasing speed [42]. Thus, the goal of a rehabilitation program for a given individual may need to be adjusted to exploit and optimize the residual mobility function by training positive compensatory adaptations, rather than necessarily to restore kinematics or speed to an earlier state. In many situations, personalized solutions may be essential. Thus, the field should focus on establishing methods for determining optimal rehabilitation outcomes for individuals based on their goals, needs, limitations, conditions, and environment.
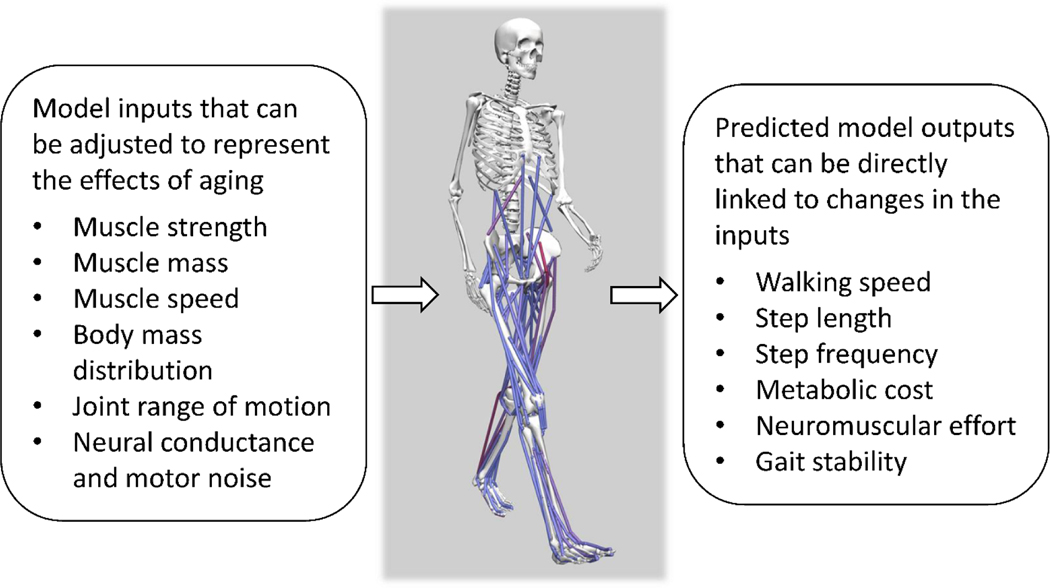
To optimally target and ameliorate age-related changes in gait biomechanics and their effects on the metabolic cost of walking, the specific mechanisms underlying these changes must first be understood. It is often difficult to identify causal relations in biological systems using experimental approaches alone. In the context of understanding age-related gait changes, there are many potential confounding factors, such as muscle weakness, altered neural drive, and decreases in tendon stiffness that may co-vary and interact in complex ways. Computational modeling and simulation (Figure 3) could be integrated with experimental data to better understand the potential impact of each factor on gait in isolation, as well as the nature of their interactions [15, 43]. However, this approach has been applied only sparingly to the questions of muscle-tendon function [33, 44, 45] and gait biomechanics [46–49] older adults. Thus, there is considerable unmet potential in using musculoskeletal modeling and simulation to help identify the biological changes that occur with aging that are most responsible for observed changes in gait biomechanics and the metabolic cost of walking.
Figure 3.
Musculoskeletal modeling is a powerful approach for testing cause-and-effect relations in human movement. In the context of understanding the causes of age-related changes in gait mechanics and energetics, several model inputs (left box) can be adjusted to predict the relative magnitudes of their effects on model outputs (right box). Figure courtesy of B. Umberger.
Human locomotion has been simulated using two major approaches that involve so-called tracking simulations and predictive simulations [50]. Tracking simulations that reproduce experimental data are useful to help understand muscle function [49], and are complementary to experimental analyses based on joint kinematics, kinetics, and electromyography. However, predictive simulations hold considerably more potential for identifying the causes of age-related gait changes. Predictive simulations generate novel movements based on the characteristics of the model [50]. Thus, it is possible with predictive simulations to identify how particular age-related changes in static properties (e.g., muscle strength, joint range of motion) and dynamic properties (e.g., muscle contraction speed, neural conduction speed) of the neuromusculoskeletal system precipitate specific changes in gait performance (e.g., increased metabolic cost of walking, decreased gait speed, impaired dynamic balance), (Figure 3), and thereby provide useful information about which interventions are likely to be most effective at restoring performance [46, 51, 52].
While computational modeling holds considerable promise, challenges remain to maximize its utility as a tool to help solve the broader problem of poor mobility with aging. Key among them is that predictive simulations require the objective or goal of the movement task to be specified, such as weighting metabolic cost against gait stability or movement smoothness [50, 53]. This is an active area of research that must be expanded to include older adults who are, for example, at greater risk of falls and thus may place a greater personal priority on stability than energy cost. Importantly, the field also needs to identify how to best integrate the results of simulation studies with other sources of evidence, such as experimental and epidemiological data, to better understand the primary causes of age-related gait impairments and develop effective, targeted interventions.
In sum, there exist significant knowledge gaps (Table 1) and therefore great potential for future research to advance our understanding of age-related changes in gait and the metabolic energy cost of walking. Multidisciplinary research that integrates experimental studies with mechanistically-focused approaches to capitalize on new technologies is essential to progress in this area. Consideration of study cohort characteristics and their goals will be needed, as will a better understanding of underlying changes before effective interventions can be developed. The next sections examine several factors that have been identified as critical to our understanding of aging, gait, and the metabolic cost of walking.
Table 1.
Knowledge gaps and areas of opportunity for advancing the science investigating the increased energy cost of walking in old age.
| Topic | Knowledge Gap |
|---|---|
| Gait Biomechanics | Magnitude and mechanisms of distal-to-proximal shift in gait patterns |
| Mechanisms of increased metabolic energy cost of walking in older people | |
| At what age and in whom is metabolic energy cost of gait higher? Impact of various moderators including sex, habitual physical activity etc. | |
| Gait alterations and impacts in diverse study groups, including those with slowed self-selected gait speed, incident mobility limitations, etc. |
|
| Mechanisms for greater antagonist muscle activation | |
| Real world measurement of aged gait and impact of environment | |
| Neuromuscular Factors | Effects of fat deposition and distribution |
| Nervous system changes: effects on coordination, synergies, and speed and completeness of activation | |
| Role of weakness and fatigue in gait and metabolic energy cost changes | |
| Tendon & Connective Tissue | Role of muscle tendon complex in gait changes and greater cost of walking? At which joints? |
| What are the changes, mechanisms and impact of age-related alterations in the extracellular matrix in vivo? | |
| Interventions & Sensors | Clinical targets vs. personalized goals? |
| Pros and cons of assistive devices vs. capacity-building interventions | |
| Sensors or devices for detection of early changes that may lead to gait problems | |
| Design and deployment of sensors to track long-term changes, with sufficient data richness and high usability | |
| How to scale to population level? | |
| Study Design | Which problems are common across various sectors of aging population? |
| Effects of age, sex, health status, physical activity level, etc.? | |
| Integration across scales, systems and approaches | |
| Adoption and compliance; cultural, racial and gender needs |
Session 2: Neuromuscular mechanisms for changes in gait biomechanics
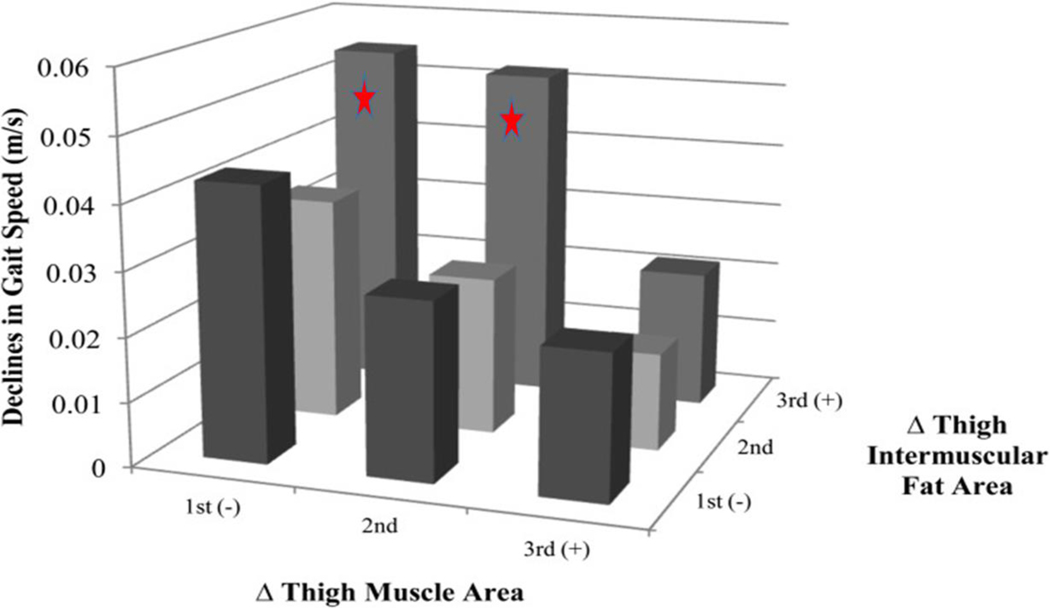
The aging human neuromuscular system undergoes normative changes beyond loss of muscle mass and strength that may also contribute to reduced mobility, although in many cases the specific mechanisms for these changes and their direct impact on gait biomechanics have not yet been fully identified. For example, body mass increases with age up to about age 80 years, due to an increase in fat mass and despite a loss in lean mass in males [54] and females [55]. To fully understand gait changes with aging, particularly as they relate to metabolic energy cost, it is important to clarify why changes in body composition occur over time and how they influence mobility. Importantly, there is little association between low muscle mass and functional decline with age, although muscle strength is a strong predictor of functional decline [56]. Rather, fat accumulation is more strongly associated with age-related mobility impairment than is loss of lean mass [57]. Further, fat distribution may have an even greater impact on mobility than overall fat accumulation. There is an age-related redistribution of fat away from subcutaneous fat and toward internal organs and tissues, including skeletal muscle [58]. Notably, it has been shown that fat within skeletal muscle tissue is the greatest body composition risk factor for slower gait speed and mobility disability [59] (Figure 4). Thus, it is critical to understand how changes in fat distribution may affect mechanical or bioenergetic factors to impact gait and mobility in aging. Likewise, the interaction between or ratio of lean mass to fat mass and its role on strength or power, which is strongly associated with reduced physical function in aging (e.g., [60]), needs more nuanced understanding. The question of causality vs. association must also be addressed in order to understand the mechanistic roles of muscle weakness and fat accumulation and distribution on gait mechanics and the cost of walking in old age.
Figure 4.
Accumulation of intermuscular fat is an important risk factor for slowing gait speed and loss of mobility in aging. Health, Aging and Body Composition Study participants with the greatest decreases in thigh muscle area but increases in thigh intermuscular fat area experienced the greatest declines in gait speed over a 4 year period. 1st, 2nd, and 3rd refer to tertiles. Figure courtesy of B. Nicklas and [59].
The aging nervous system undergoes substantial changes that likely affect the ability of older adults to be active and mobile in their daily lives. The predictive role of low muscle strength and power on functional decline with aging [56] suggests that either reduced muscle mass or an inability to optimally activate muscle could play a role in altered gait mechanics with aging. Documented changes in the nervous system with aging that may affect activation of available muscle include brain atrophy, changes in motor cortex excitability, axonal damage [61], neuromuscular junction damage [61], and muscle denervation [62] leading to a loss of motor units [63]. Central nervous system alterations that may be deleterious to gait such as agonist co-activation and altered muscle synergies also occur with aging [64–66], although the extent to which these are direct effects of aging or indirect effects of inactivity and disease is not clear at present. Regardless, these changes can lower the ability of muscle to produce force and power, and they appear to impact the control of walking and coordination during the various phases of gait [46, 67, 68]. There are emerging opportunities to solidify knowledge relating cognition and changes in cognition with age to the variability, complexity and stability of gait, as well as the implications of these factors for mobility. Research in this area should also expand on work in younger adults that has shown a link between gait variability and the metabolic cost of walking [69]. Degradation of the sensory system also occurs with aging, and can lead to deficits in eyesight, proprioception, vestibular sensing, and cutaneous sensing [70]. Neither the ability of the aging nervous system to adapt to unanticipated challenges or dual tasks during walking, nor the impact of poor sensorimotor function on gait have been resolved. It will also be important to understand how nervous system changes impact skeletal muscle function in its role of supporting locomotion.
An increased metabolic cost of walking with age may have multiple causes, including muscle weakness, slower preferred gait speeds, and altered muscle activation patterns, as discussed above. More directly, greater metabolic cost during gait may also be related to impaired cellular energy metabolism. Intramuscular energy production can be studied noninvasively using phosphorus magnetic resonance spectroscopy. To date, we know that the effects of older age on skeletal muscle oxidative or mitochondrial capacity in vivo vary depending on the muscle group and participant characteristics [71]. For example, a meta-analysis of the literature revealed that the dorsiflexor muscles of older adults have a greater capacity to produce energy (i.e., ATP) by oxidative phosphorylation than do those of younger adults, a result that may be related to a relatively greater duty cycle in this muscle group during the altered gait of older adults. In contrast, the knee extensor muscles were the only muscle group to show a lower oxidative capacity in older compared with younger adults [71], an effect exacerbated in older adults with mobility impairments [72]. Indeed, cross-sectional and more recently longitudinal studies of the quadriceps muscles in the Baltimore Longitudinal Study of Aging have shown that post-exercise oxidative capacity in muscle declines with aging, even when healthy individuals are studied [73, 74]. Independent of age and confounders, oxidative capacity is associated with reduced walking speed, especially in tasks that require endurance, such as the 400m walk. In mediation analysis, this association is explained by lower muscle strength [75–77]. Habitual physical activity level is also a significant moderator of muscle oxidative capacity [71, 72]. The causal nature of the relationship in older adults between a muscle’s daily usage patterns and its ability to produce energy oxidatively remains to be determined.
In addition to evaluating muscle oxidative capacity, it is useful to determine the use of each ATP-producing pathway (i.e., creatine kinase reaction, glycolysis, oxidative phosphorylation) during muscular work. Studies of single muscle groups in vivo suggest that under some conditions, older muscle relies relatively more on oxidative metabolism than younger muscle [78, 79], but this difference is difficult to ascertain during locomotor tasks. Perhaps most relevant to the question of the energy cost of walking, a recent study of knee extensor muscle metabolic economy (torque or work per ATP per unit muscle) indicated similar economy in young and older adults during isometric contractions, but far lower economy in the old during dynamic contractions [80], (Figure 5). This scenario is consistent with a greater metabolic cost of walking with aging, and suggests that intrinsic muscle factors may contribute directly to this problem in older adults. Further, the consequences of lower skeletal muscle economy on muscle fatigue and whole-body fatigability (discussed next), which in turn may exacerbate gait changes in the old, need to be clarified. Given concepts such as the distal-to-proximal shift in gait kinetics with aging, future emphasis should be placed on understanding how energy is produced and used in the hip extensor muscles. Studies that combine measures of in vivo energetics and economy with computational models may prove valuable in identifying specific responses and muscle groups that are contributing most to the greater energy cost of walking in older adults [81].
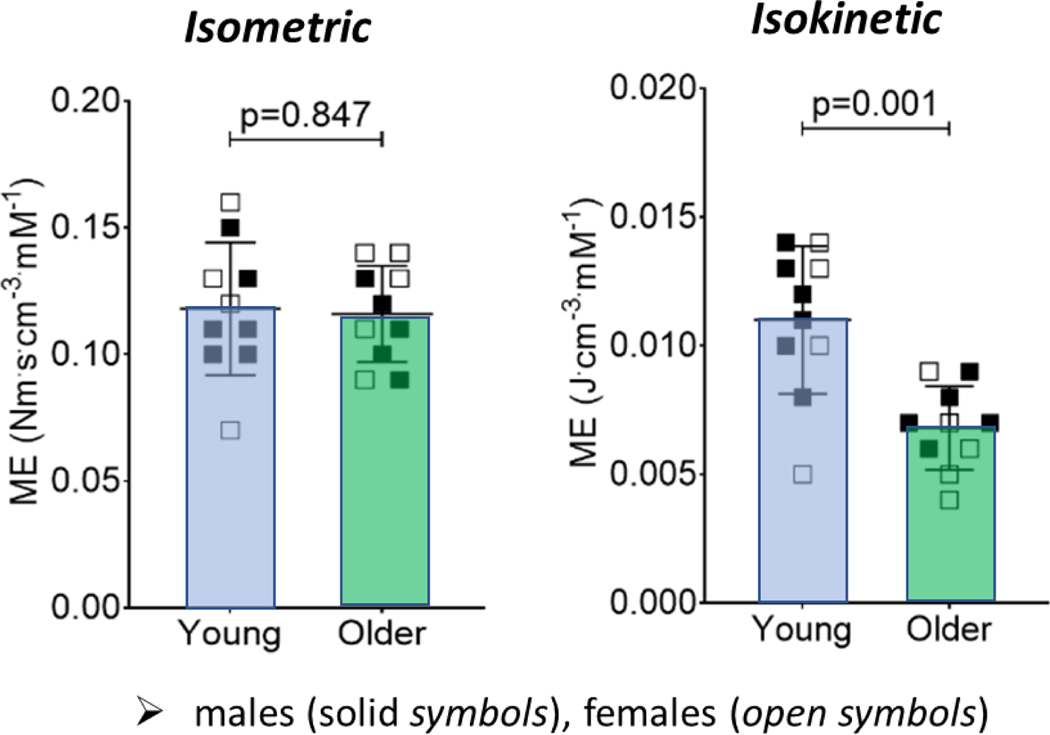
Figure 5.
Knee extensor muscle metabolic economy (size-normalized torque or power produced per mM ATP) is lower in vivo during dynamic, isokinetic (right) but not static, isometric (left), contractions in older compared with younger adults. Dynamic contractions were performed at 120 deg∙s−1. Both conditions involved maximal, single-leg contractions on a magnetic resonance compatible ergometer. The potential impact of lower muscle economy on the increased energy cost of walking in aging remains to be determined. Figure adapted from Fitzgerald et al., 2021 [80].
While muscle fatigue is defined as the transient loss of maximal force- or power-generating capacity of muscle in response to its use [82], the emerging concept of fatigability, or the decline in physical performance in response to a standardized bout of activity [83–85], has important implications for the age-related decline in mobility. It is well known that muscle fatigue is task-specific, meaning that the magnitude and mechanisms of fatigue will vary depending on the intensity, mode and duration of the contraction protocol used to elicit it [82, 86]. For example, for isometric tasks of the lower limb muscles, healthy and mobile older adults are typically less fatigable than young adults but are more fatigable for dynamic contractions, particularly as contraction velocity increases [87–90]. The mechanisms for greater muscle fatigue in older adults during dynamic contractions of single muscle groups appear to include the muscle’s failure to keep pace with the high energy demand of these contractions [91]. In addition to contraction mode and muscle group; sex, age and habitual physical activity are potent moderators of muscle fatigue in aging [92]. The extent to which locomotor muscle fatigue contributes to overall fatigability or the decline in physical performance is unclear at present. Future studies should continue to identify the causes and consequences of skeletal muscle fatigue in all sectors of the aging population, especially as it relates to locomotor tasks, the concept of fatigability, and changes in the metabolic energy cost of walking.
Session 3: Connective tissue function in aging
Connective tissue, including tendons and the extracellular matrix, transmits the forces generated by muscle fibers and acts as a spring during locomotion. As discussed above, despite the key role of connective tissue in locomotion and gait, potential age-related changes in connective tissue function with aging have received relatively little attention to date, particularly in humans. Both the material and mechanical properties of tendon may undergo age-related changes. In adults, tendons are composed predominantly of type I collagen fibers, which provide the tensile, load-bearing properties of the tissue. Other components of tendons include type III and type IV collagens, proteoglycans, and fibroblasts [93]. Animal studies provide the majority of information about changes in material properties of tendons with age, and mainly indicate a decrease in cell density during maturation [94]. At present, there is little information from human studies about changes in cell density, other material properties, or cell function within tendons that occur in old age [95]. Future studies in animals should target the period from maturation to old age, and should account for age-related changes in behaviors such as physical activity. Creative approaches to study the material properties of human tendons should also be explored, including developing new techniques to obtain tissue samples with minimal risk to participants or obtaining tissue during elective surgical procedures. Ultrasound-guided tendon tissue sampling techniques may provide a way to obtain human tendon tissue from the patellar and Achilles tendons [96].
There are known age-related changes in tendon properties. Older adults demonstrate lower Achilles tendon stiffness [97], smaller Young’s modulus, increased hysteresis [98] and greater tendon cross-sectional area than younger adults [99, 100] (Figure 6). However, in the patellar tendon, the results are mixed. One early study reported no differences between older and younger adults in patellar tendon properties [101]. However, more recent data in older males indicates both lower tendon stiffness than younger males and a direct association between patellar tendon stiffness and rate of force development during maximal isometric contractions [102]. The mechanisms for these changes in tendon properties or lack thereof and the role of changes in magnitude and frequency of loading should be explored further. In addition, the potential effects of sex hormones on age-related changes in tendon stiffness and, in turn, the effects of stiffness on the rate of force development are not known but may be highly relevant to the question of aging. The changes in tendon properties may directly impact the energetic cost of locomotion by impacting the elastic energy return but may also increase the metabolic cost of walking by impacting function of the muscle-tendon complex including the muscle fascicle length, rate of force development, and electromechanical delay [31, 93, 103].
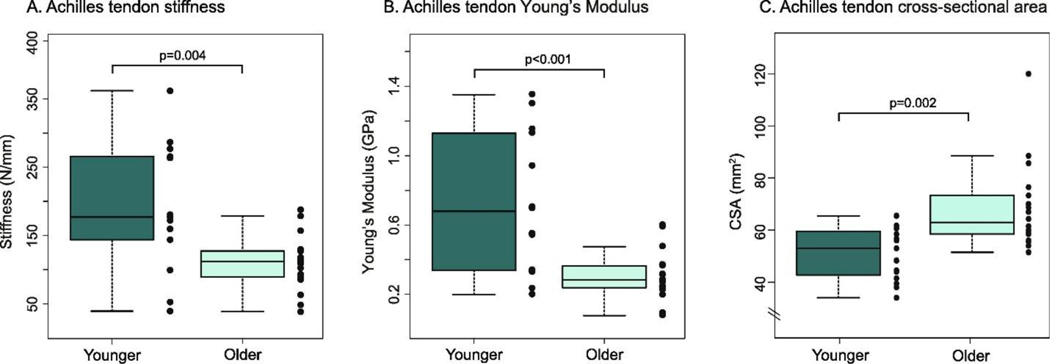
Figure 6.
Achilles tendon stiffness (left) and Young’s Modulus (center) are both lower in older adults with similar physical activity patterns to younger adults. Notably, tendon cross-sectional area is greater in the old (right), which is suggested to be an adaptation to counter reduced material modulus in the tendon. Figure from Lindemann et al., 2020 [99].
Investigators have begun to explore the effects of age-related changes to the mechanical properties of tendon on mobility in older adults. Stenroth et al [104] reported significant associations between 6-minute walk time and Achilles tendon stiffness in older adults. Few studies have examined tendon mechanical properties during mobility tasks in older adults. Overall, research has shown that the mechanical properties of the tendon affect muscle fascicles during the gait cycle [105]. During walking, older adults have altered muscle-tendon complex lengthening during the stance phase, such that increased tendon compliance is accompanied by reduced fascicle lengthening compared to younger adults [106]. Older adults also experience smaller peak tendon stress during push-off in walking compared to younger adults [107]. The implications of age-related changes in tendon mechanical properties for altered self-selected gait speed and metabolic cost of walking in older adults is likely significant but not well understood. Advances in technology that enable measurement of tendon loading during locomotion may support this new line of research [108]. There is some evidence to indicate that tendon properties can be modified through exercise training to optimize mobility in older adults [98, 109]. There is emerging evidence of the molecular mechanisms for mechanotransduction in tendon; understanding age-related changes in these mechanisms will be essential for developing exercise therapies [110–112]. Additional work building on recently-published imaging and genetic engineering approaches in tendon is needed to advance knowledge of the basic mechanisms for tissue property changes with age. Further research is also needed to determine whether changes observed at the tendon translate to changes in the operating length of the sarcomere during static contractions, dynamic contractions, and mobility tasks in vivo.
In addition to the tendon itself, the extracellular matrix (ECM) is an important, although understudied, component of skeletal muscle. The ECM plays a role in force transmission, passive force development, muscle shape changes, gearing, and mechanotransduction [113]. Therefore, a better understanding of how ECM changes in aging constitutes a significant opportunity for improving gait and reducing its energy cost in this population. There are known age-related changes to the ECM of skeletal muscle, including changes to collagen type and cross-linking, collagen content and morphology, fibrosis and stiffening, and a reduced ability to vary gearing (i.e., the ratio of muscle velocity to fiber velocity) [114, 115]. However, it is not known whether or how age-related changes to the ECM may affect mobility. Likewise, changes to collagen and fluid pressure in muscle can affect passive and active force development [116, 117], but future research is needed to understand whether this translates to altered gait in aging. In sum, studies are needed that investigate which specific properties of the ECM, such as its content, morphology, or interactions with fluid, affect skeletal muscle function and consequently gait.
Session 4: Interventions and technologies to measure and improve gait in older adults
A thorough understanding of the age-related changes in biomechanics, and the mechanisms for those changes that lie within the nervous system, skeletal muscle, tendons, and ECM will lead to opportunities for interventions and assistive technologies to allow older adults to be mobile and active in their daily lives. An important consideration when designing interventions to increase mobility in older adults is to identify what the goal(s) should be. That may mean including outcome measures targeting the hypothesized mechanisms, as well as using measures that capture real-world mobility performance. In free-living environments, measures of a person’s mobility in their community can indicate how much someone moves by quantifying metrics such as step counts, speed, locations that a person visited, or activities that a person did. Measures of gait mechanics are less commonly made in the free-living environment, but quantifying mechanics and strategies employed as someone moves through their world, which could be accomplished by using current and future sensor technologies (Figure 7), may yield critical insights to the problem at hand. For example, motion tracking based on wearable sensors such as wearable inertial measurement units (IMUs) can provide direct measurement of body segment and joint movements while out in the community, including locomotion, activities of daily living, and specific activities such as exercise. In addition, shear wave tensiometry has been developed to understand Achilles tendon kinetics during walking, which also can be deployed in a real-world setting [108].
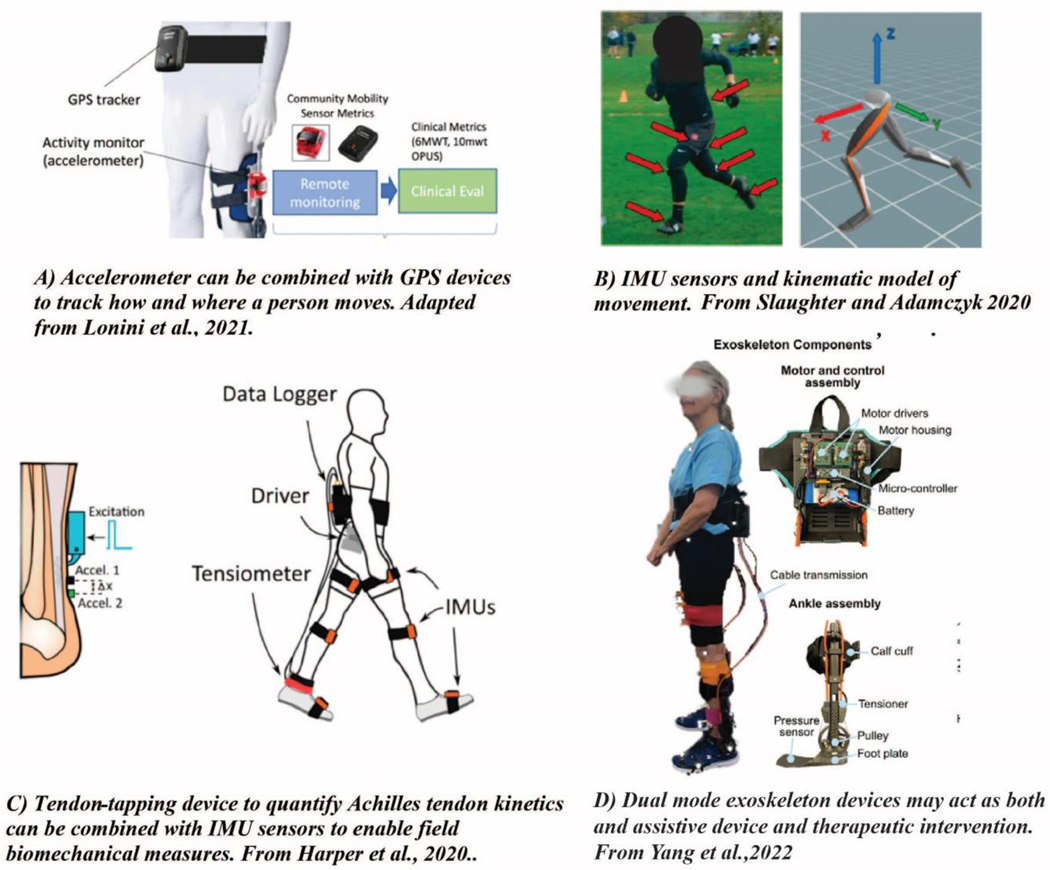
Figure 7.
Measurement of everyday movement can include not only A) how much an individual moves (e.g., step counts, activity intensity, locations) Figure from Lonini et al., 2021 IEEE TNSRE [136], but also B) the pattern of an individual’s movements (e.g., kinematics, muscle coordination) Figure from Slaughter et al., 2020, Sensors [137], and C) the effects of movement on tissue strain patterns Figure from Harper et al., (2020) Sensors [108]. Everyday motions such as walking or chair rising are typically repeated multiple times each day, reducing the barriers to their measurement. In contrast, events such as falls and slips are unique and present a far greater challenge for their identification and study. D) Exoskeleton devices that can switch between assistance and resistance modes may enhance mobility while also acting as a therapeutic intervention to help maintain muscle and tendon function. Figure from Yang et al., 2022 Wearable Technologies.[131]
Researchers will need to consider sensor capabilities, hardiness, size, battery life, data storage, ease of deployment, participant burden, status monitoring, and other factors in their study designs. One study showed that participants can wear IMUs on the feet, wrist, and trunk for four weeks, and that voice recordings can be used by participants to mark loss of balance events, which helps researchers reconstruct foot paths and body motions during a loss of balance event [118]. However, IMUs are not without limitations that need to be carefully considered, including the accuracy and precisions of the selected outcomes. Further advances through sensor fusion to combine kinematic and kinetic measures in the field will make it possible to understand how patterns of motion alter tissue loading in vivo [119]. Sensor technology is rapidly developing to aid in understanding mobility function both within and outside the laboratory, and in a variety of populations. It will be important to establish and maintain collaboration with computer and data scientists for the interpretation of the complex datasets that will be generated by these studies. Integrative team science approaches that combine researchers with topically diverse backgrounds will be particularly useful in this realm.
Sensor technologies will provide a significant opportunity for guiding the development and evaluation of interventions to improve mobility in older adults. For example, a task-oriented motor learning program that takes advantage of active sensor technologies may improve mobility in older adults by improving the skills needed for walking [120]. Different approaches may be needed for those with sensory or vestibular deficits than for those with fatigability or sarcopenia. It will be essential to be able to best match an individual’s deficits and goals with the most efficacious intervention(s) to improve walking and mobility. Finally, attention must be paid to the behavioral issues of perceived needs, motivation, and compliance in order to design effective interventions.
Cognitive impairment is associated with the risk for mobility limitations in older adults [121]; but causality has not been established. Brain aging, including structural and physiological changes, may be a central mechanism affecting both cognition and the neural control of walking. The cognitive domain of executive function may be particularly important, especially for performance of challenging/complex walking tasks [122]. To date, research has shown that exercise training induces neural changes to the central nervous system [123, 124], but a 2018 meta-analysis showed that cognitive training may only improve mobility during challenging walking tasks in older adults [125]. Additional, rigorous studies are needed to show whether exercise training can induce neural changes that directly improve mobility in older adults. To this end, comparative effectiveness randomized controlled trials are needed to assess the ability of cognitive-motor interventions, ideally those that target both cognition and motor function, to reduce mobility disability. As a first step, the feasibility of such trials should be evaluated. Studies will also be needed to examine the mechanisms that explain how cognitive-motor therapies improve motor function in older adults. Quantification of mobility behavior, gait mechanics, muscle-tendon function, brain and muscle activation, energetic cost of walking and detraining effects [126] should all be thoroughly investigated in the context of motor-cognitive therapies.
In addition to interventions designed to modify or improve the innate functional capabilities of older adults, assistive devices may be able to improve gait function in older adults in specific situations, especially if capacity or components of gait cannot be restored with rehabilitation. Traditional assistive devices to improve mobility include canes, crutches, walkers, and rollators [127]. Scientific advances have allowed for the development of exoskeletons as a walking aid to improve gait function and reduce the metabolic cost of walking [128]. The first exoskeleton to break the metabolic cost barrier was an ankle exoskeleton that assisted plantar flexion; it required a power source and had to be used in a laboratory setting, but it showed that an exoskeleton could reduce the metabolic cost of walking [129]. Since then, scientists have continued to develop exoskeletons as walking aids. For example, exoskeletons have been developed that are autonomous, meaning that they are untethered and self-contained when worn by a person [65]. Exoskeletons have also been developed that are passive, meaning that they do not require an input of energy [130]. While these developments are promising, many questions remain before exoskeletons might be widely used in the aging population. First, the best methods of exoskeleton use will need to be established. For instance, it is unclear which lower-limb joint (i.e., hip versus knee versus ankle) is the best target for intervention, and whether this depends on the aspect of mobility that the device is intended to address (e.g., metabolic cost, self-selected walking speed, or balance). The intensity and frequency of use is also uncertain; would it be most effective to use an exoskeleton periodically to train gait and restore mobility, continuously to augment gait in daily life, or by balancing these two approaches? The question of whether “too much” use of an exoskeleton might contribute to atrophy and deconditioning must be addressed and raises the possibility of exoskeletons that can switch between assistance (i.e., to help reduce metabolic cost or increase self-selected walking speed) and resistance (as a therapeutic intervention to help maintain muscle and tendon function) modes [131] (Figure 7). The complexity, cost and regulatory burden of demonstrating clinical efficacy of exoskeletons might limit their utility and scalability to large segments of the population. A simple, passive hip exoskeleton was developed to overcome cost and complexity concerns. It was shown to reduce the cost of walking in older adults in a laboratory setting [132], but its use in a real-world setting is as yet unproven. The possibilities for exoskeletons to improve mobility for older adults are immense, but their use for all older adults, including those with comorbidities, neuromuscular disease, injuries, or cognitive impairment, is uncertain at this time.
Session 5: Considerations for inclusive study design
Moving the field forward towards understanding gait and improving mobility in our aging population will require research that is much more inclusive and diverse than has been common to date. Too frequently, convenience samples from local university communities fail to study people from diverse socioeconomic, racial, ethnic, educational, occupational, and ability backgrounds. Problems generated by a lack of diversity include, for example: uncertainty about how genetic vs. behavioral factors affect the aging process and thus the mechanisms and outcomes related to gait and energetics, or the development of monitoring strategies and interventions that may not be appropriate across all subpopulations.
To increase diversity and inclusion, community partnerships should be forged that are mutually beneficial to community members and researchers. The Johnson County Osteoarthritis Project offers an example of one such successful community partnership [135]. This community-based participatory research project incorporates diversity into its study design in order to engage those individuals at high risk for developing osteoarthritis. Indeed, including stakeholders in pre-study planning, such as diverse patients from the study population of interest, clinicians, caregivers, etc., may help ensure that interventions meet their needs and are implemented in ways that can increase adoption of and compliance with the study goals and design. Despite some successful partnerships in the field of mobility and aging, there remain many unanswered questions about how to facilitate such partnerships, as well as how to interpret results that differ by race or other subgroup. Collaborations with, for example, social scientists, clinical researchers and/or biologists may be important for interpreting data to inform future interventions or advance care for specific populations.
One challenge that the workshop participants thought should be addressed is to identify ways to improve inclusivity in research, such as including all people who are interested in participating in these studies. Stringent inclusion and exclusion criteria are important for creating homogenous research groups in order to tease out differences between groups; however, eligibility criteria may be preventing certain groups of people from being represented in research studies. For example, among adults over 65 years of age, those without disease or disability are often overrepresented in research studies due to their overall good health, relatively good mobility status, and low burden of comorbidities. From a clinical perspective, we can look to the field of geroscience for guidance on addressing variability in responses to exercise intervention programs. Geroscience is the field that aims to understand the basic physiology of aging to explain the increased occurrence of age-related diseases throughout the lifespan [133]. Insights from geroscience and “life course epidemiology” may suggest cellular and molecular targets that allow researchers to identify people at risk for mobility impairments and, further, to identify personalized interventions or novel therapeutics for improving mobility [134].
In addition, clever statistical designs, sharing of data between research groups to create larger datasets, and team science may be important approaches for overcoming the inevitable variability that will be introduced through loosening eligibility criteria to include a more representative sample of the aging population. To that effect, the establishment of a common core set of outcome variables would create unity between research studies and allow for compilation of data and greater comparison of data collected in different laboratories. Further, these measures should be accessible to the majority of researchers, meaning that they can be performed just as easily in small rural laboratories or field sites as in large urban research centers.
Conclusion
The 2021 National Institute on Aging workshop on the causes and consequences of altered gait and increased metabolic energy cost in aging identified key areas where research should be focused in order to move the field forward. To further understand the biomechanics of age-related changes in gait function, research should include multidisciplinary studies of the mechanisms of altered gait as we age. To identify the mechanisms of these alterations, physiologically relevant in vivo, in vitro, and in silico studies of the neural, body composition, bioenergetic, and musculoskeletal factors that support human gait should be conducted. Likewise, tendon and extracellular matrix changes are an understudied area that may provide key information about the mechanisms of age-related changes to mobility. To harness the power of wearable sensors, collaboration with behavioral and data scientists will be important for successful study designs, appropriate interpretation of the data, and overall exploration and management of rich data sets. Exoskeleton technologies are in development that may operate in assistance or resistance modes during locomotion; future studies should investigate how and when to deliver these devices to older adults. Finally, to address all aspects of the problem, inclusive studies of diverse sectors of the population are needed, and creation of common core measurements and data-sharing mechanisms should be explored. Gait is fundamental to mobility, and mobility is essential to optimize health. The study of the causes and consequences of age-related changes in mobility is an untapped opportunity for scientists and clinical gerontologists to improve the mobility, health and quality of life of our aging population.
Highlights.
Altered gait, reduced mobility and an accompanying increase in the energy cost of walking are important problems in our aging population.
Knowledge of the causes, impacts, and potential therapeutic interventions for resolving these challenges to mobility in aging is incomplete at this time.
Effective solutions to this problem will require multi-disciplinary, inclusive and innovative research designed to address the full range of questions within this knowledge gaps.
The purpose of this review is to briefly discuss the current state of knowledge about gait and the energy cost of walking in aging, and highlight potential emerging tools, concepts, and study design approaches that may be useful in addressing current limitations to our understanding of this important societal problem.
Acknowledgements:
The authors thank Lyndon Joseph, PhD in the Division of Geriatrics and Clinical Gerontology at the National Institute on Aging for prioritizing this topic, and organizing and supporting the attendant workshop.
Funding:
National Institute on Aging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooper R, et al. , Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ, 2010. 341(sep09 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper R, et al. , Objective measures of physical capability and subsequent health: a systematic review. Age and Ageing, 2011. 40(1): p. 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guralnik JM, et al. , Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. New England Journal of Medicine, 1995. 332(9): p. 556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Studenski S, et al. , Gait Speed and Survival in Older Adults. JAMA, 2011. 305(1): p. 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrack JA, et al. , The Role of Energetic Cost in the Age-Related Slowing of Gait Speed. Journal of the American Geriatrics Society, 2012. 60(10): p. 1811–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larish DD, Martin PE, and Mungiole M, Characteristic Patterns of Gait in the Healthy Old. Annals of the New York Academy of Sciences, 1988. 515(1 Central Deter): p. 18–32. [DOI] [PubMed] [Google Scholar]
- 7.Martin PE, Rothstein DE, and Larish DD, Effects of age and physical activity status on the speed-aerobic demand relationship of walking. Journal of Applied Physiology, 1992. 73(1): p. 200–206. [DOI] [PubMed] [Google Scholar]
- 8.Schrack JA, et al. , Rising Energetic Cost of Walking Predicts Gait Speed Decline With Aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2016. 71(7): p. 947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das Gupta S, Bobbert MF, and Kistemaker DA, The Metabolic Cost of Walking in healthy young and older adults - A Systematic Review and Meta Analysis. Sci Rep, 2019. 9(1): p. 9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyer KA, et al. , Systematic review and meta-analysis of gait mechanics in young and older adults. Experimental Gerontology, 2017. 95: p. 63–70. [DOI] [PubMed] [Google Scholar]
- 11.DeVita P. and Hortobagyi T, Age causes a redistribution of joint torques and powers during gait. Journal of Applied Physiology, 2000. 88(5): p. 1804–1811. [DOI] [PubMed] [Google Scholar]
- 12.Buddhadev HH and Martin PE, Effects of age and physical activity status on redistribution of joint work during walking. Gait & Posture, 2016. 50: p. 131–136. [DOI] [PubMed] [Google Scholar]
- 13.Franz JR and Kram R, Advanced age and the mechanics of uphill walking: A joint-level, inverse dynamic analysis. Gait & Posture, 2014. 39(1): p. 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delabastita T, et al. , Distal-to-proximal joint mechanics redistribution is a main contributor to reduced walking economy in older adults. Scandinavian Journal of Medicine & Science in Sports, 2021. 31(5): p. 1036–1047. [DOI] [PubMed] [Google Scholar]
- 15.Umberger BR and Rubenson J, Understanding muscle energetics in locomotion: new modeling and experimental approaches. Exerc Sport Sci Rev, 2011. 39(2): p. 59–67. [DOI] [PubMed] [Google Scholar]
- 16.Peterson DS and Martin PE, Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture, 2010. 31(3): p. 355–9. [DOI] [PubMed] [Google Scholar]
- 17.Hortobagyi T, et al. , Association between muscle activation and metabolic cost of walking in young and old adults. J Gerontol A Biol Sci Med Sci, 2011. 66(5): p. 541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hortobagyi T, et al. , Interaction between age and gait velocity in the amplitude and timing of antagonist muscle coactivation. Gait Posture, 2009. 29(4): p. 558–64. [DOI] [PubMed] [Google Scholar]
- 19.Franz JR and Kram R, How does age affect leg muscle activity/coactivity during uphill and downhill walking? Gait Posture, 2013. 37(3): p. 378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortega JD and Farley CT, Effects of aging on mechanical efficiency and muscle activation during level and uphill walking. J Electromyogr Kinesiol, 2015. 25(1): p. 193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browne MG and Franz JR, Ankle power biofeedback attenuates the distal-to-proximal redistribution in older adults. Gait & Posture, 2019. 71: p. 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinoso DH, et al. , Hip, Knee, and Ankle Functional Demand During Habitual and Fast-Pace Walking in Younger and Older Women. J Aging Phys Act, 2019. 27(2): p. 242–251. [DOI] [PubMed] [Google Scholar]
- 23.Samuel D, Rowe P, and Nicol A, The functional demand (FD) placed on the knee and hip of older adults during everyday activities. Arch Gerontol Geriatr, 2013. 57(2): p. 192–7. [DOI] [PubMed] [Google Scholar]
- 24.Hafer JF and Boyer KA, Comparisons of Knee Extensor Functional Demand During Gait by Age, Physical Activity Level, and the Impact of Acute Exercise and Walking Speed. Journal of Applied Biomechanics, 2020. 36(3): p. 163–170. [DOI] [PubMed] [Google Scholar]
- 25.Boyer KA, Andriacchi TP, and Beaupre GS, The role of physical activity in changes in walking mechanics with age. Gait & posture, 2012. 36(1): p. 149–153. [DOI] [PubMed] [Google Scholar]
- 26.Jin L. and Hahn ME, Comparison of lower extremity joint mechanics between healthy active young and middle age people in walking and running gait. Sci Rep, 2019. 9(1): p. 5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krupenevich RL and Miller RH, Habitual endurance running does not mitigate age-related differences in gait kinetics. Exp Gerontol, 2021. 147: p. 111275. [DOI] [PubMed] [Google Scholar]
- 28.Hafer JF, Kent JA, and Boyer KA, Physical activity and age-related biomechanical risk factors for knee osteoarthritis. Gait & Posture, 2019. 70: p. 24–29. [DOI] [PubMed] [Google Scholar]
- 29.Bean JF, et al. , A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci, 2003. 58(8): p. 728–33. [DOI] [PubMed] [Google Scholar]
- 30.Losa-Reyna J, et al. , Impact of Relative Muscle Power on Hospitalization and All-Cause Mortality in Older Adults. J Gerontol A Biol Sci Med Sci, 2022. 77(4): p. 781–789. [DOI] [PubMed] [Google Scholar]
- 31.Krupenevich RL, et al. , Reduced Achilles Tendon Stiffness Disrupts Calf Muscle Neuromechanics in Elderly Gait. Gerontology, 2021. 68: p. 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark DJ, et al. , Does quadriceps neuromuscular activation capability explain walking speed in older men and women? Exp Gerontol, 2014. 55: p. 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franz JR and Thelen DG, Imaging and simulation of Achilles tendon dynamics: Implications for walking performance in the elderly. J Biomech, 2016. 49(9): p. 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasske K. and Franz JR, Aging effects on the Achilles tendon moment arm during walking. Journal of Biomechanics, 2018. 77: p. 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawicki GS, Lewis CL, and Ferris DP, It pays to have a spring in your step. Exercise and sport sciences reviews, 2009. 37(3): p. 130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson RW, et al. , Muscle-tendon mechanics explain unexpected effects of exoskeleton assistance on metabolic rate during walking. Journal of Experimental Biology, 2017. 220(11): p. 2082–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beijersbergen CM, et al. , The biomechanical mechanism of how strength and power training improves walking speed in old adults remains unknown. Ageing research reviews, 2013. 12(2): p. 618–627. [DOI] [PubMed] [Google Scholar]
- 38.Virani SS, et al. , Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation, 2020. 141(9): p. e139–e596. [DOI] [PubMed] [Google Scholar]
- 39.Wist S, Clivaz J, and Sattelmayer M, Muscle strengthening for hemiparesis after stroke: A meta-analysis. Annals of Physical and Rehabilitation Medicine, 2016. 59(2): p. 114–124. [DOI] [PubMed] [Google Scholar]
- 40.Sánchez N. and Finley JM, Individual Differences in Locomotor Function Predict the Capacity to Reduce Asymmetry and Modify the Energetic Cost of Walking Poststroke. Neurorehabilitation and Neural Repair, 2018. 32(8): p. 701–713. [DOI] [PubMed] [Google Scholar]
- 41.Park S, et al. , Using Biofeedback to Reduce Step Length Asymmetry Impairs Dynamic Balance in People Poststroke. Neurorehabil Neural Repair, 2021. 35(8): p. 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Espy DD, et al. , Independent influence of gait speed and step length on stability and fall risk. Gait Posture, 2010. 32(3): p. 378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez JW and Pandy MG, Integrating modelling and experiments to assess dynamic musculoskeletal function in humans. Experimental Physiology, 2006. 91(2): p. 371–382. [DOI] [PubMed] [Google Scholar]
- 44.Hasson CJ and Caldwell GE, Effects of age on mechanical properties of dorsiflexor and plantarflexor muscles. Ann Biomed Eng, 2012. 40(5): p. 1088–101. [DOI] [PubMed] [Google Scholar]
- 45.Thelen DG, Adjustment of muscle mechanics model parameters to simulate dynamic contractions in older adults. J Biomech Eng, 2003. 125(1): p. 70–7. [DOI] [PubMed] [Google Scholar]
- 46.Song S. and Geyer H, Predictive neuromechanical simulations indicate why walking performance declines with ageing. The Journal of Physiology, 2018. 596(7): p. 1199–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pimentel RE, et al. , Muscle metabolic energy costs while modifying propulsive force generation during walking. Computer Methods in Biomechanics and Biomedical Engineering, 2021. 24(14): p. 1552–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hase K, A computer simulation study on the causal relationship between walking and physical malfunctions in older adults. Anthropological Science, 2008. 116(2): p. 95–104. [Google Scholar]
- 49.Lim YP, Lin YC, and Pandy MG, Muscle function during gait is invariant to age when walking speed is controlled. Gait Posture, 2013. 38(2): p. 253–9. [DOI] [PubMed] [Google Scholar]
- 50.Umberger BRM, R.H., Optimal control modeling of human movement, in Handbook of Human Motion, Müller BW, S.I., Editor. 2018: Berlin: Springer. p. 327–348. [Google Scholar]
- 51.Ong CF, et al. , Predicting gait adaptations due to ankle plantarflexor muscle weakness and contracture using physics-based musculoskeletal simulations. PLoS Comput Biol, 2019. 15(10): p. e1006993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson RT, Bianco NA, and Finley JM, Patterns of asymmetry and energy cost generated from predictive simulations of hemiparetic gait. PLoS Comput Biol, 2022. 18(9): p. e1010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen VQ, et al. , Bilevel Optimization for Cost Function Determination in Dynamic Simulation of Human Gait. IEEE Trans Neural Syst Rehabil Eng, 2019. 27(7): p. 1426–1435. [DOI] [PubMed] [Google Scholar]
- 54.Jackson AS, et al. , Longitudinal changes in body composition associated with healthy ageing: men, aged 20–96 years. British Journal of Nutrition, 2012. 107(7): p. 1085–1091. [DOI] [PubMed] [Google Scholar]
- 55.Kamper RS, et al. , Associations between inflammatory markers, body composition, and physical function: the Copenhagen Sarcopenia Study. J Cachexia Sarcopenia Muscle, 2021. 12(6): p. 1641–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaap LA, Koster A, and Visser M, Adiposity, Muscle Mass, and Muscle Strength in Relation to Functional Decline in Older Persons. Epidemiologic Reviews, 2013. 35(1): p. 51–65. [DOI] [PubMed] [Google Scholar]
- 57.Kidde J, et al. , Regional Muscle and Whole-Body Composition Factors Related to Mobility in Older Individuals: A Review. Physiotherapy Canada, 2009. 61(4): p. 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delmonico MJ, et al. , Longitudinal study of muscle strength, quality, and adipose tissue infiltration. The American Journal of Clinical Nutrition, 2009. 90(6): p. 1579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beavers KM, et al. , Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. The American Journal of Clinical Nutrition, 2013. 97(3): p. 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suetta C, et al. , The Copenhagen Sarcopenia Study: lean mass, strength, power, and physical function in a Danish cohort aged 20–93 years. J Cachexia Sarcopenia Muscle, 2019. 10(6): p. 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pratt J, et al. , Plasma neurofilament light levels associate with muscle mass and strength in middle-aged and older adults: findings from GenoFit. J Cachexia Sarcopenia Muscle, 2022. 13(3): p. 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mosole S, et al. , Long-term high-level exercise promotes muscle reinnervation with age. J Neuropathol Exp Neurol, 2014. 73(4): p. 284–94. [DOI] [PubMed] [Google Scholar]
- 63.Hunter SK, Pereira HM, and Keenan KG, The aging neuromuscular system and motor performance. J Appl Physiol (1985), 2016. 121(4): p. 982–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manini TM, Hong SL, and Clark BC, Aging and muscle. Current Opinion in Clinical Nutrition and Metabolic Care, 2013. 16(1): p. 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mooney LM, Rouse EJ, and Herr HM, Autonomous exoskeleton reduces metabolic cost of human walking. Journal of NeuroEngineering and Rehabilitation, 2014. 11(1): p. 151–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piche E, et al. , Metabolic cost and co-contraction during walking at different speeds in young and old adults. Gait & Posture, 2022. 91: p. 111–116. [DOI] [PubMed] [Google Scholar]
- 67.Hafer JF, et al. , Changes in coordination and its variability with an increase in running cadence. Journal of sports sciences, 2016. 34(15): p. 1388–1395. [DOI] [PubMed] [Google Scholar]
- 68.James EG, et al. , Gait coordination impairment is associated with mobility in older adults. Exp Gerontol, 2016. 80: p. 12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Connor SM, Xu HZ, and Kuo AD, Energetic cost of walking with increased step variability. Gait Posture, 2012. 36(1): p. 102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allen D, et al. , Age-Related Vestibular Loss: Current Understanding and Future Research Directions. Front Neurol, 2016. 7: p. 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fitzgerald LF, Christie AD, and Kent JA, Heterogeneous effects of old age on human muscle oxidative capacity in vivo: a systematic review and meta-analysis. Applied Physiology, Nutrition, and Metabolism, 2016. 41(11): p. 1137–1145. [DOI] [PubMed] [Google Scholar]
- 72.Larsen RG, et al. , Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme, 2012. 37(1): p. 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi S, et al. , 31P Magnetic Resonance Spectroscopy Assessment of Muscle Bioenergetics as a Predictor of Gait Speed in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci, 2016. 71(12): p. 1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adelnia F, et al. , Proteomic signatures of in vivo muscle oxidative capacity in healthy adults. Aging Cell, 2020. 19(4): p. e13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tian Q, et al. , Muscle mitochondrial energetics predicts mobility decline in well-functioning older adults: The baltimore longitudinal study of aging. Aging Cell, 2022. 21(2): p. e13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian Q, et al. , Mitochondrial DNA copy number and heteroplasmy load correlate with skeletal muscle oxidative capacity by P31 MR spectroscopy. Aging Cell, 2021. 20(11): p. e13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zane AC, et al. , Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell, 2017. 16(3): p. 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lanza IR, Larsen RG, and Kent-Braun JA, Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. The Journal of physiology, 2007. 583(Pt 3): p. 1093–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Christie AD, et al. , Human skeletal muscle metabolic economy in vivo: effects of contraction intensity, age, and mobility impairment. 2014. p. R1124–R1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fitzgerald LF, et al. , Effects of old age and contraction mode on knee extensor muscle ATP flux and metabolic economy in vivo. The Journal of Physiology, 2021. 599(12): p. 3063–3080. [DOI] [PubMed] [Google Scholar]
- 81.Callahan DM, Umberger BR, and Kent-Braun JA, A computational model of torque generation: neural, contractile, metabolic and musculoskeletal components. PloS one, 2013. 8(2): p. e56013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kent-Braun JA, Fitts RH, and Christie A, Skeletal Muscle Fatigue, in Comprehensive Physiology. 2012, Wiley. p. 997–1044. [DOI] [PubMed] [Google Scholar]
- 83.Eldadah BA, Fatigue and fatigability in older adults. PM R, 2010. 2(5): p. 406–13. [DOI] [PubMed] [Google Scholar]
- 84.Simonsick EM, et al. , Fatigued, but Not Frail: Perceived Fatigability as a Marker of Impending Decline in Mobility-Intact Older Adults. J Am Geriatr Soc, 2016. 64(6): p. 1287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alexander NB, et al. , Bedside-to-Bench conference: research agenda for idiopathic fatigue and aging. J Am Geriatr Soc, 2010. 58(5): p. 967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Enoka RM and Duchateau J, Muscle fatigue: what, why and how it influences muscle function. The Journal of Physiology, 2008. 586(1): p. 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dalton BH, et al. , The age-related slowing of voluntary shortening velocity exacerbates power loss during repeated fast knee extensions. Exp Gerontol, 2012. 47(1): p. 85–92. [DOI] [PubMed] [Google Scholar]
- 88.Paris MT, et al. , Age-related performance fatigability: a comprehensive review of dynamic tasks. J Appl Physiol (1985), 2022. 133(4): p. 850–866. [DOI] [PubMed] [Google Scholar]
- 89.Callahan DM and Kent-Braun JA, Effect of old age on human skeletal muscle force-velocity and fatigue properties. Journal of applied physiology (Bethesda, Md.: 1985), 2011. 111(5): p. 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sundberg CW, et al. , Mechanisms for the age-related increase in fatigability of the knee extensors in old and very old adults. J Appl Physiol (1985), 2018. 125(1): p. 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sundberg CW, et al. , Bioenergetic basis for the increased fatigability with ageing. J Physiol, 2019. 597(19): p. 4943–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Christie A, Snook EM, and Kent-Braun JA, Systematic review and meta-analysis of skeletal muscle fatigue in old age. Medicine and science in sports and exercise, 2011. 43(4): p. 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Svensson RB, et al. , Effect of aging and exercise on the tendon. Journal of Applied Physiology, 2016. 121(6): p. 1237–1246. [DOI] [PubMed] [Google Scholar]
- 94.Stanley RL, et al. , Gap junction protein expression and cellularity: comparison of immature and adult equine digital tendons. Journal of Anatomy, 2007. 211(3): p. 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lui PPY and Wong CM, Biology of Tendon Stem Cells and Tendon in Aging. Frontiers in Genetics, 2020. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Movin T, Tendon tissue sampling. Scandinavian Journal of Medicine & Science in Sports, 2000. 10(6): p. 368–371. [DOI] [PubMed] [Google Scholar]
- 97.Onambele GL, Narici MV, and Maganaris CN, Calf muscle-tendon properties and postural balance in old age. J Appl Physiol (1985), 2006. 100(6): p. 2048–56. [DOI] [PubMed] [Google Scholar]
- 98.Reeves ND, Maganaris CN, and Narici MV, Effect of strength training on human patella tendon mechanical properties of older individuals. The Journal of Physiology, 2003. 548(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lindemann I, et al. , Age-related differences in gastrocnemii muscles and Achilles tendon mechanical properties in vivo. Journal of Biomechanics, 2020. 112: p. 110067–110067. [DOI] [PubMed] [Google Scholar]
- 100.Stenroth L, et al. , Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol (1985), 2012. 113(10): p. 1537–44. [DOI] [PubMed] [Google Scholar]
- 101.Carroll CC, et al. , Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol (1985), 2008. 105(6): p. 1907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quinlan JI, et al. , Muscle and Tendon Contributions to Reduced Rate of Torque Development in Healthy Older Males. The Journals of Gerontology: Series A, 2018. 73(4): p. 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bojsen-Møller J, et al. , Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. Journal of Applied Physiology, 2005. 99(3): p. 986–994. [DOI] [PubMed] [Google Scholar]
- 104.Stenroth L, et al. , Plantarflexor Muscle–Tendon Properties are Associated With Mobility in Healthy Older Adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2015. 70(8): p. 996–1002. [DOI] [PubMed] [Google Scholar]
- 105.Conway KA and Franz JR, Shorter gastrocnemius fascicle lengths in older adults associate with worse capacity to enhance push-off intensity in walking. Gait & Posture, 2020. 77: p. 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mian OS, et al. , Gastrocnemius muscle?tendon behaviour during walking in young and older adults. Acta Physiologica, 2007. 189(1): p. 57–65. [DOI] [PubMed] [Google Scholar]
- 107.Ebrahimi A, et al. , Achilles tendon loading is lower in older adults than young adults across a broad range of walking speeds. Exp Gerontol, 2020. 137: p. 110966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harper SE, et al. , Wearable Tendon Kinetics. Sensors, 2020. 20(17): p. 4805–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quinlan JI, et al. , Muscle and tendon adaptations to moderate load eccentric vs. concentric resistance exercise in young and older males. GeroScience, 2021. 43(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakamichi R, et al. , The mechanosensitive ion channel PIEZO1 is expressed in tendons and regulates physical performance. Sci Transl Med, 2022. 14(647): p. eabj5557. [DOI] [PubMed] [Google Scholar]
- 111.Passini FS, et al. , Shear-stress sensing by PIEZO1 regulates tendon stiffness in rodents and influences jumping performance in humans. Nat Biomed Eng, 2021. 5(12): p. 1457–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang T, et al. , Load-induced regulation of tendon homeostasis by SPARC, a genetic predisposition factor for tendon and ligament injuries. Sci Transl Med, 2021. 13(582). [DOI] [PubMed] [Google Scholar]
- 113.Purslow PP, The Structure and Role of Intramuscular Connective Tissue in Muscle Function. Frontiers in Physiology, 2020. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roberts TJ, et al. , The Multi-Scale, Three-Dimensional Nature of Skeletal Muscle Contraction. Physiology, 2019. 34(6): p. 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sinha U, et al. , Role of the Extracellular Matrix in Loss of Muscle Force With Age and Unloading Using Magnetic Resonance Imaging, Biochemical Analysis, and Computational Models. Frontiers in Physiology, 2020. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sleboda DA and Roberts TJ, Internal fluid pressure influences muscle contractile force. Proc Natl Acad Sci U S A, 2020. 117(3): p. 1772–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sleboda DA and Roberts TJ, Incompressible fluid plays a mechanical role in the development of passive muscle tension. Biol Lett, 2017. 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ojeda LV, et al. , Reconstruction of body motion during self-reported losses of balance in community-dwelling older adults. Medical Engineering & Physics, 2019. 64: p. 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harper SE, et al. , Fusion of Wearable Kinetic and Kinematic Sensors to Estimate Triceps Surae Work during Outdoor Locomotion on Slopes. Sensors (Basel), 2022. 22(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brach JS and VanSwearingen JM, Interventions to Improve Walking in Older Adults. Current Translational Geriatrics and Experimental Gerontology Reports, 2013. 2(4): p. 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Buchman AS, et al. , Cognitive Function Is Associated With the Development of Mobility Impairments in Community-Dwelling Elders. The American Journal of Geriatric Psychiatry, 2011. 19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chatterjee SA, et al. , Interpreting Prefrontal Recruitment During Walking After Stroke: Influence of Individual Differences in Mobility and Cognitive Function. Front Hum Neurosci, 2019. 13: p. 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dishman RK, et al. , Neurobiology of Exercise. Obesity, 2006. 14(3): p. 345–356. [DOI] [PubMed] [Google Scholar]
- 124.Hortobagyi T, et al. , The impact of aerobic and resistance training intensity on markers of neuroplasticity in health and disease. Ageing Res Rev, 2022. 80: p. 101698. [DOI] [PubMed] [Google Scholar]
- 125.Marusic U, Verghese J, and Mahoney JR, Cognitive-Based Interventions to Improve Mobility: A Systematic Review and Meta-analysis. Journal of the American Medical Directors Association, 2018. 19(6): p. 484–491.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hortobagyi T, et al. , Effects of Exercise Dose and Detraining Duration on Mobility at Late Midlife: A Randomized Clinical Trial. Gerontology, 2021. 67(4): p. 403–414. [DOI] [PubMed] [Google Scholar]
- 127.Bertrand K, et al. , Walking Aids for Enabling Activity and Participation. American Journal of Physical Medicine & Rehabilitation, 2017. 96(12): p. 894–903. [DOI] [PubMed] [Google Scholar]
- 128.Sawicki GS, et al. , The exoskeleton expansion: improving walking and running economy. Journal of NeuroEngineering and Rehabilitation, 2020. 17(1): p. 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Malcolm P, et al. , A Simple Exoskeleton That Assists Plantarflexion Can Reduce the Metabolic Cost of Human Walking. PLoS ONE, 2013. 8(2): p. e56137–e56137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Collins SH, Wiggin MB, and Sawicki GS, Reducing the energy cost of human walking using an unpowered exoskeleton. Nature, 2015. 522(7555). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fang YH,K; Franz JR; Lerner ZF, Feasilbility evaluation of a dual-mode ankle exoskeleton to assist and restore community ambualation in older adults Wearable Technologies, 2022. 3(e13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Panizzolo FA, et al. , Reducing the energy cost of walking in older adults using a passive hip flexion device. Journal of NeuroEngineering and Rehabilitation, 2019. 16(1): p. 117–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kennedy BK, et al. , Geroscience: Linking Aging to Chronic Disease. Cell, 2014. 159(4): p. 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ferrucci L, et al. , Age-Related Change in Mobility: Perspectives From Life Course Epidemiology and Geroscience. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2016. 71(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hartman EL, et al. , The Johnston County osteoarthritis project: an illustration of a community-university partnership for population-based research. North Carolina medical journal, 2007. 68(6): p. 459–62. [PubMed] [Google Scholar]
- 136.Lonini L, et al. , Combining Accelerometer and GPS Features to Evaluate Community Mobility in Knee Ankle Foot Orthoses (KAFO) Users. IEEE Trans Neural Syst Rehabil Eng, 2021. 29: p. 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Slaughter PR and Adamczyk PG, Tracking Quantitative Characteristics of Cutting Maneuvers with Wearable Movement Sensors during Competitive Women’s Ultimate Frisbee Games. Sensors (Basel), 2020. 20(22). [DOI] [PMC free article] [PubMed] [Google Scholar]