Abstract
Objective
Childhood vaccination is a cost-effective, essential service to reach a larger population globally. Due to unclear reasons, new emergence and resurgence of vaccine-preventable diseases increase. Thus, the aim of this study is to identify prevalence and determinants of childhood vaccination in Ethiopia.
Design
Community-based cross-sectional study.
Setting
We used data from 2019 Ethiopia Mini Demographic and Health Survey. The survey included all the nine regional states and two city administrations of Ethiopia.
Participants
A weighted sample of 1008 children 12–23 months of age was included in the analysis.
Main outcome measures
A multilevel proportional odds model was fitted to identify determinants of childhood vaccination status. In the final model, variables with a p value of less than 5% and an adjusted OR (AOR) with a 95% CI were reported.
Result
The full childhood vaccination coverage of Ethiopia was 39.09% (95% CI: 36.06%–42.28%). Mothers who attended primary (AOR=2.16; 95% CI: 1.43–3.26), secondary (AOR=2.02; 95% CI: 1.07–3.79) and higher education (AOR=2.67; 95% CI: 1.25–5.71); being in union (AOR=2.21; 95% CI: 1.06–4.58); kept vaccination cards (AOR=26.18; 95% CI: 15.75–43.53); children receiving vitamin A1 (AOR=4.14; 95% CI: 2.9–5.9); living in Afar (AOR=0.14; 95% CI: 0.04–0.45), Somali (AOR=0.19; 95% CI: 0.06–0.60), Gambela (AOR=0.22; 95% CI: 0.06–0.77), Harari (AOR=0.14; 95% CI: 0.04–0.52) and Dire Dawa (AOR=0.23; 95% CI: 0.06–0.79) regions; and rural residents (AOR=0.53; 95% CI: 0.30–0.93) were factors significantly associated with childhood vaccination.
Conclusion
The full childhood vaccination coverage in Ethiopia was low and remained unchanged since 2016. The study identified that both the individual-level and community-level factors affected the vaccination status. Accordingly, public health interventions targeted to these identified factors can increase childhood full vaccination status.
Keywords: Community child health, PREVENTIVE MEDICINE, PUBLIC HEALTH
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study used the most recent nationwide secondary data that show the proportion of the Expanded Program on Immunization (EPI) coverage among children aged 12–23 months in Ethiopia.
We used a multilevel proportional odds model to overcome the hierarchical nature of Demographic and Health Survey data and to identify both individual-level and community-level factors of EPI.
This study did not include some important variables like psychosocial variables of parents, vaccine hesitancy and other related variables, since the study used secondary data of a national survey.
Establishing temporal relationship of causation between childhood vaccination and its determinants was impossible due to the nature of the study design used for the survey.
Recall bias is the most relevant limitation of the study.
Introduction
According to the UNICEF, immunisation is a dynamic constituent of frontline healthcare to access larger populations. Compared with any other health or social activities, immunisation reduces global annual death by a significant proportion.1–4 It saves lives and protects people’s health, improves countries’ productivity and resilience, and helps to ensure a safer and healthier world.5 6
According to the Ethiopian Ministry of Health, a child takes OPV0 (oral polio vaccine) and Bacillus Calmette Guerin (BCG) at birth. At 6 weeks after birth, a child takes OPV1, pentavalent 1 (diphtheria, pertussis and tetanus+hepatits B+Haemophilus influenzae type B), pneumococcal 1 and rotavirus 1. Then, 10 weeks after birth, the child takes OPV2, and pentavalent 2, pneumococcal 2 and rotavirus 2. The child takes OPV3, pentavalent 3, pneumococcal 3 and inactivated polio vaccine (IPV) at 14th week. Other vaccines such as measles-containing vaccine (MCV1) at 9th month and MCV2 at 15th month, and human papilloma virus at 14th year for girls are given. However, this study only considers vaccines between 1 and 2 years. All vaccines are equally important.7 Currently, Ethiopia does not provide rubella, mumps and chickenpox vaccines to immunise children. Depending on the amount of immunity or protection after receiving a dose, a child may need more than one dose for some vaccines.8 Additionally, more than one dose is needed to build high enough immunity to prevent disease and boost immunity that fades over time.9 For vaccines that do not initiate full protection at first introduction, an additional dose is usually initiated.10 However, this does not mean one vaccine is better than the other or more important than another, since it depends on the nature of the disease.
Globally, about 6.6 million children die every year, and half of the deaths would have been prevented by vaccination.11 The global inequality in healthcare distribution forces many low/middle-income countries to live under the bundle of pestilent, cumulative old health problems.12Africa is among the regions severely affected by globally imbalanced immunisation resources distribution. Vaccine-preventable diseases become the big burden in war-affected parts of the world and underdeveloped countries like Ethiopia.13 Despite the existence of highly cost-effective vaccines on global markets, situations such as war, displacement, undervaccination and poor access to vaccine contribute to the prevalence of vaccine-preventable diseases.14 The proportion of full immunisation reflects how resources distribution caused the imbalance in childhood vaccination.1 15
Regardless of advancement and attainment of immunisation, newly emerging vaccine-preventable diseases are still common in Africa.16 There are people deprived of accessing immunisation in some countries, and this becomes the reason for the prevalence of vaccine-preventable diseases as admitted by the WHO and other studies.17–19 In Ethiopia, a study has described that educated (30%) and rich (31%) people more often vaccinate their children.20
Some evidence indicated that global vaccine coverage is decreasing.21 A study summarised by the Demographic Health Surveys (DHS) of sub-Saharan African countries showed that the full vaccination coverage was 59.40% in 2021.22 In Mogadishu, full immunisation was 45.2% and 41.4% in 2020 and 2021, respectively.22–24 In Togo, the full immunisation coverage was 69.3% in Lome district in 2019.25 In Ethiopia, the full immunisation coverage is 76.81% in the Southeast region;26 however, the pooled prevalence among children aged 12–23 months old is 47%.27 The Ethiopia Mini Demographic and Health Survey (EMDHS) 2019 demonstrated that full vaccination coverage was 44%.28 The magnitude of full vaccination increased from 14.3% in 2000 to 44% in 2019.29 From this, we understand that the full vaccination coverage is not adequate.
Many factors support the argument that resource disparity is a reason for low coverage in some countries. A systematic review conducted in sub-Saharan African countries showed that lack of knowledge of immunisation, distance to access point, financial deprivation, lack of partner’s support, and distrust in vaccines and immunisation programmes were the barriers.30 The absence of immunisation card, respondents’ sex, level of education, marital status and organisation of the health system are mentioned in another study.25 A study concluded that many sub-Saharan African countries showed that maternal education, health facility delivery, fathers’ secondary education and above, antenatal care (ANC) visit, postnatal care (PNC) visit, wealth index, media exposure and distance to the health facility affected the vaccination coverage.22 In Ethiopia, from previous four DHS, postnatal check-up, maternal awareness, regional difference (economic, education and lifestyle), educational status, residence and women’s wealth index have influenced vaccination.20 27 31 From another study, ANC visit, higher level of maternal education, good knowledge of immunisation, short distance to health facility and institutional delivery were the determinants of childhood immunisation.32
Different small-scale and large-scale (national level) studies have been done to identify the determinants of childhood vaccination in Ethiopia. However, the majority of studies conducted did not examine the natural ordering of a child’s vaccination status as fully, partially or not vaccinated at all. Hence, we used a multilevel proportional odds model which overcomes the hierarchical nature of the DHS data and considers the natural ordering of the outcome variable. In addition, the full vaccination coverage still remains far behind the National Health Sector Transformation Plan II of Ethiopia (75% by 2024/2025),33 showing the need for further study. Therefore, this study aimed at determining the prevalence of childhood vaccination coverage and its determinants to assist in policy improvements.
Methods
Study design
This study used the 2019 cross-sectional data of the EMDHS. We obtained the dataset from the DHS website (http://dhsprogram.com/data/) after getting permission to access it. The mini-survey was conducted in March 2019–June 2019 for the second time in Ethiopia. The Ethiopian Public Health Institute in collaboration with the Central Statistical Agency and Federal Ministry of Health implemented a nationally representative household survey.
Study setting
The EMDHS included all the nine regions and two city administrations of the country. Ethiopia is the second most populous country in Africa and located in the Horn of Africa.
Participants
The 2019 EMDHS used the sampling frame created for the upcoming Ethiopian population and housing census list of 149 093 enumeration areas (EAs). Participants of the study were selected through a stratified two-stage cluster sampling technique. Initially, they stratified each region in the country into urban and rural areas. Probability proportional to EA size was used to select 305 EAs (93 in urban and 212 in rural areas). In the second stage, a list of households was used as a sampling frame to select 30 households per cluster by equal probability systematic selection technique. Eligible participants for the interview were all women aged 15–49 years and who were the residents of the selected households. Accordingly, 8885 women were interviewed out of 9012 eligible women, giving a response rate of 98.6%. All living children aged 12–23 months at the time of the survey were the source population of the study. The details of the survey methods and procedures are available in the 2019 EMDHS report.29
Eligibility criteria
All children born in the 2 years preceding the survey were included in the analysis. However, children who were not alive at the time of the survey were excluded from this study. Accordingly, data of 1008 children who were born in the last 2 years and surviving were extracted from the 2019 EMDHS dataset for this analysis (figure 1).
Figure 1.
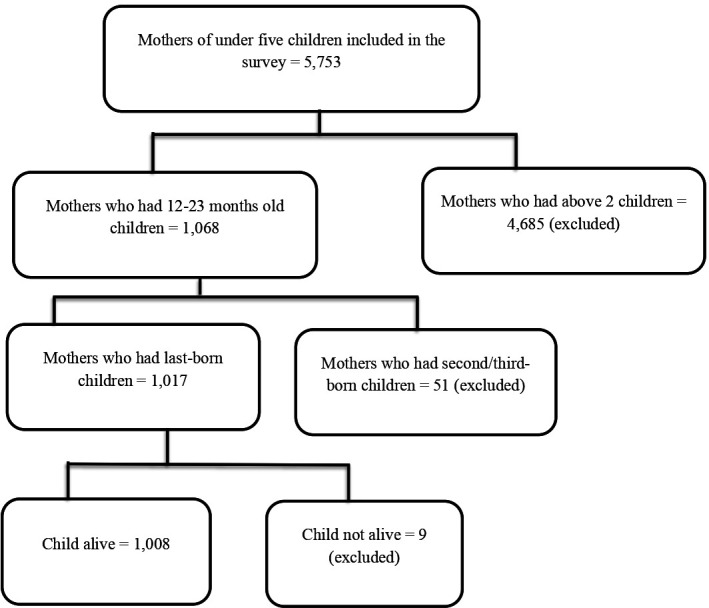
Eligibility assessment for childhood vaccination coverage among children aged 12–23 months in Ethiopia, 2019.
Measurements of the study variables
Dependent variable
Child vaccination status was the outcome variable of the study. We categorised the outcome variable as not vaccinated, partially vaccinated and fully vaccinated.
Fully vaccinated means a child has received one dose of BCG, three doses of pentavalent, three doses of polio, two doses of rotavirus vaccine, three doses of pneumococcal conjugate vaccine and one dose of MCV1.
Partially vaccinated means a child had missed at least one or more of the 13 vaccines/doses.
Not vaccinated means a child has not received any vaccine at all.
The interviewers of the 2019 EMDHS obtained information on vaccination coverage from written vaccination cards (infant immunisation card and other health cards), from mothers’ verbal reports and from health facility records. They request mothers who had a child in the 3 preceding years of the survey to show the infant immunisation card or health card. Subsequently, they copy the dates of each vaccination given in the corresponding questionnaire. Similarly, they ask mothers who did not keep the cards to recall whether the child received that specific vaccination and the number of doses that the child received. In addition, to complement these data (information collected based on the mother’s recall), the field supervisor collects complementary vaccination records from a health facility for a child who visited the health facility and missed vaccination data. Finally, a Health Facility Questionnaire was used to record vaccination information for all children without a vaccination card seen during the mother’s interview; however, we dropped records with the missing data.
Independent variables
In this study, we considered both individual-level and community-level variables. The individual-level variables included are maternal age, maternal educational level, religion, marital status, relationship to household head, sex of the household head, wealth index, number of ANC visits, place of delivery, used PNC, presence of vaccination document, birth order number, preceding birth interval, sex of the child, vitamin A1, total number of children ever born and number of children under 5 years in the household. Region, place of residence, community women education, community poverty and community ANC utilisation are community-level factors of the study. Region is a grouping of the first administrative level of Ethiopia. During the survey period, there were nine regions (Tigray, Amhara, Afar, Oromia, Somali, Benishangul-Gumuz, Southern Nations, Nationalities and People’s Region, Gambela and Harari), and two city administrations (Addis Ababa and Dire Dawa) in the country. Moreover, community women education, community poverty and community ANC utilisation were community-level variables derived by aggregating individual-level factors. They were categorised as ‘low’ or ‘high’ based on the median value, since the EMDHS was not normally distributed.
Information regarding the wealth index is derived from data collected in the Household Questionnaire. The questionnaire includes queries concerning the household’s ownership of several consumer items such as television and car; dwelling characteristics such as flooring material; type of drinking water source; toilet facilities and other characteristics related to wealth status. Each household asset for which information is collected is assigned a weight or factor score generated through principal component analysis. The resulting asset scores are standardised around a standard normal distribution with a mean of 0 and an SD of 1. These standardised scores were then used to create the breakpoints that define wealth quintiles as lowest, second, middle, fourth and highest.34 Here, we further categorised the wealth index into poor (lowest plus poor), average (middle) and rich (rich plus highest).
Data processing and statistical analysis
The data were cleaned, recoded and analysed using STATA/SE version 14.0. We used numbers, mean, percentages or proportions to describe both the individual-level and community-level variables. We used sample weight to manage sampling errors and non-responses. The hierarchical nature of the DHS data (DHS sampling methods) violates the assumption of independence or lack of correlation of the residuals. In the DHS, observations are interdependent as participants nested in the same cluster may behave in the same way and differ from other clusters in terms of the outcome of interest. Consequently, we used a two-level multilevel proportional odds model, as a single-level traditional statistical model might not be adequate to control for the clustering effect. Moreover, we used the multilevel proportional odds model to consider the natural ordering of the response variable (not vaccinated, partially vaccinated and fully vaccinated). Accordingly, we built four consecutive models to identify determinants of child vaccination in Ethiopia. We first fitted the null model or the intercept-only model. Then, model 1 was fitted with the individual-level variables. Similarly, we fitted model 2 (with community-level factors). Finally, model 3 or the mixed-effects model was fitted with both individual-level and community-level factors. The intraclass correlation coefficient (ICC) was used to determine the clustering effect or community variation and it was calculated using the following formula:
ICC =
Where is community-level variance and =3.29 is individual-level variance. On the other hand, we checked proportional change in variance (PCV), median OR (MOR) and deviance by using the following formulas to determine the fitness of the model: PCV=, where is a variance in the null model and is a variance in the consecutive models. MOR=, where indicates the cluster variance. The last model showed the lowest deviance (model 3) and becomes the best-fitted model. Furthermore, we examined the proportional odds assumption that assumes the equal effect of each factor across the outcome categories by score test. We checked variance inflation factor and found 1.60 to assess the presence of multicollinearity. The finding is in the acceptable range. In the bivariable analysis, we kept variables with a p value less than 0.25 for the multivariate multilevel proportional odds analysis. Finally, in the multivariable analysis, we reported variables with a p value less than 5%, adjusted proportional OR and 95% CI.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Result
Sociodemographic characteristics of mothers and children
The current study included 1008 mothers who have children aged 12–23 months. Out of the total respondents, more than half (52.78%) were in the age range of 25–34 years, nearly half (48.91%) have not attended education and almost one-third (32.14%) were Orthodox religion followers. Four out of 10 mothers (39.98%) were from high-income households, and the majority (94.05%) were currently married. Three-fourths of respondents (73.51%) were from rural areas and 12.6% were from Oromia region of the country (table 1).
Table 1.
Sociodemographic characteristics of mothers of children aged 12–23 months in Ethiopia, 2019 (n=1008)
| Variables | Frequency (n) | Percentage |
| Maternal age | ||
| 15–24 | 298 | 29.56 |
| 25–34 | 532 | 52.78 |
| 35–49 | 178 | 17.66 |
| Maternal education | ||
| No education | 493 | 48.91 |
| Primary | 342 | 33.93 |
| Secondary | 99 | 9.82 |
| Higher | 74 | 7.34 |
| Marital status | ||
| Not married | 60 | 5.95 |
| Married | 948 | 94.05 |
| Religion | ||
| Orthodox | 324 | 32.14 |
| Protestant | 177 | 17.56 |
| Muslim | 481 | 47.72 |
| Others | 26 | 2.58 |
| Wealth index | ||
| Poor | 469 | 46.53 |
| Middle | 136 | 13.49 |
| Rich | 403 | 39.98 |
| Region | ||
| Tigray | 93 | 9.23 |
| Amhara | 111 | 11.01 |
| Afar | 99 | 9.82 |
| Oromia | 127 | 12.60 |
| Somali | 85 | 8.43 |
| Benishangul-Gumuz | 83 | 8.23 |
| SNNPR | 116 | 11.51 |
| Gambela | 77 | 7.64 |
| Harari | 73 | 7.24 |
| Addis Ababa | 64 | 6.35 |
| Dire Dawa | 80 | 7.94 |
| Place of residence | ||
| Urban | 267 | 26.49 |
| Rural | 741 | 73.51 |
| Community women education | ||
| Low | 468 | 46.43 |
| High | 540 | 53.57 |
| Community poverty | ||
| Low | 495 | 49.11 |
| High | 513 | 50.89 |
| Community ANC utilisation | ||
| Low | 468 | 46.43 |
| High | 540 | 53.57 |
ANC, antenatal care; SNNPR, Southern Nations, Nationalities and People’s Region.
Healthcare service utilisation related characteristics of mothers
Among the participants, less than half (44.15%) practised home birth, nearly a quarter (24.31%) had no ANC visit and almost one-tenth (13.99%) received PNC service. The majority of mothers (71.73%) kept immunisation document, and 88.1% of the interviewed mothers have less than or equal to two children under 5 years in their households. From the total number of children, half (50.30%) were male, 22.72% were first born and 59.33% received the most recent vitamin A1 (table 2).
Table 2.
Healthcare service utilisation related characteristics of mothers with children aged 12–23 months in Ethiopia, 2019 (n=1008)
| Number of ANC visits | ||
| None | 245 | 24.31 |
| 1–3 times | 313 | 31.05 |
| 4 and above | 450 | 44.64 |
| Place of delivery | ||
| Home | 445 | 44.15 |
| Health facility | 563 | 55.85 |
| PNC service | ||
| No | 836 | 86.01 |
| Yes | 136 | 13.99 |
| Sex of child | ||
| Male | 507 | 50.30 |
| Female | 501 | 49.70 |
| Birth order number | ||
| 1st born | 229 | 22.72 |
| 2nd–3rd | 372 | 36.90 |
| 4th–5th | 204 | 20.24 |
| 6th and above | 203 | 20.14 |
| Presence of vaccination document | ||
| No | 285 | 28.27 |
| Yes | 723 | 71.73 |
| Preceding birth order | ||
| <24 months | 186 | 18.45 |
| 24–48 | 357 | 35.42 |
| 48 and above | 465 | 46.13 |
| Received vitamin A1 | ||
| No | 410 | 40.67 |
| Yes | 598 | 59.33 |
| Total children ever born | ||
| 1–3 | 598 | 59.33 |
| 4–6 | 278 | 27.58 |
| 7 and above | 132 | 13.10 |
| Number of children <5 years in household | ||
| ≤2 | 888 | 88.10 |
| >2 | 120 | 11.90 |
ANC, antenatal care; PNC, postnatal care.
Childhood vaccination status in Ethiopia
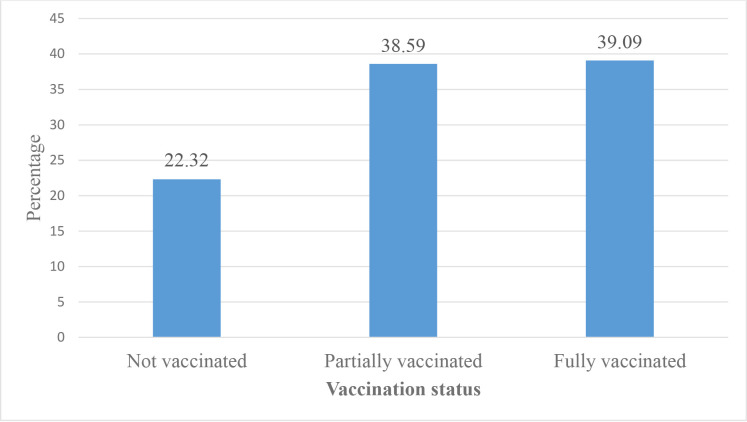
The full vaccination coverage among children aged 12–23 months in Ethiopia was 39.09% (95% CI: 36.06%–42.28%) (figure 2). On the other hand, 2 in 10 children (22.32%) aged 12–23 months have not received any vaccination in 3 years before the 2019 survey period. Among the children aged 12–23 months included in the study, 70.24% received BCG, 57.84% received the third dose of pentavalent, 55.75% received the third dose of polio, 60.62% received the second dose of rotavirus vaccine, 55.16% received the third dose of pneumococcal conjugate vaccine and 57.04% received the first dose of measles vaccination (figure 3).
Figure 2.
Vaccination status of children aged 12–23 months in Ethiopia, 2019.
Figure 3.
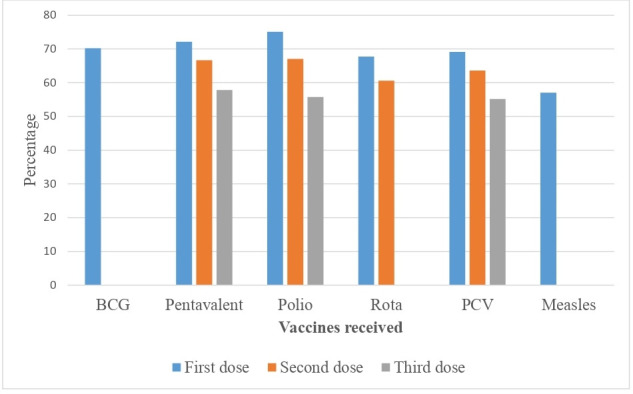
Percentage of children aged 12–23 months who received a particular vaccine in Ethiopia, 2019. PCV, pneumococcal conjugate vaccine.
Factors affecting child vaccination in Ethiopia
The fixed-effects analysis result
The multivariable analysis showed that educational level of mothers, maternal age, marital status, presence of vaccination document, received vitamin A1, region and place of residence were significantly associated with childhood vaccination. Children whose mothers attended primary, secondary and higher education have 2.2 (adjusted OR (AOR)=2.16; 95% CI: 1.43–3.26), 2 (AOR=2.02; 95% CI: 1.07–3.79) and 2.7 (AOR=2.67; 95% CI: 1.25–5.71) times higher partial or full vaccination, respectively, compared with those born to mothers with no education. The odds of partial or full vaccination were 1.8 (AOR=1.79; 95% CI: 1.08–2.99) times higher among children whose mothers are in the age range of 35–49 years compared with 15–19 age group. Mothers who are currently married are 2.2 (AOR=2.21; 95% CI: 1.06–4.58) times more likely to vaccinate their children either partially or fully. Keeping vaccination cards was 26 (AOR=26.18; 95% CI: 15.75–43.53) times more likely associated with partial or full vaccination compared with not keeping vaccination cards. Children who have received vitamin A1 have higher likelihood of partial or full vaccination (AOR=4.14; 95% CI: 2.9–5.9). Children from Afar (AOR=0.14; 95% CI: 0.04–0.45), Somali (AOR=0.19; 95% CI: 0.06–0.60), Gambela (AOR=0.22; 95% CI: 0.06–0.77), Harari (AOR=0.14; 95% CI: 0.04–0.52) and Dire Dawa (AOR=0.23; 95% CI: 0.06–0.79) regions have less partial or full vaccination compared with the children from Tigray region. Children living in rural areas have 47% (AOR=0.53; 95% CI: 0.30–0.93) reduced partial or full vaccination compared with children living in urban areas of the country (table 3).
Table 3.
Multivariate multilevel ordinal logistic regression analysis of individual-level and community-level determinants of child vaccination in Ethiopia, 2019
| Variables | Null model | Model 1 AOR (95% CI) |
Model 2 AOR (95% CI) |
Model 3 AOR (95% CI) |
|
Maternal educational level No education Primary Secondary Higher |
1 1.97 (1.33, 2.93)** 1.80 (0.97, 3.33) 2.85 (1.35, 6.02) |
1 2.16 (1.43, 3.26)*** 2.02 (1.07, 3.79)* 2.67 (1.25, 5.71)* |
||
|
Maternal age 15–24 25–34 35–49 |
1 1.13 (0.78, 1.63) 1.81 (1.09, 3.02)* |
1 1.15 (0.79, 1.66) 1.79 (1.08, 2.99)* |
||
|
Religion Orthodox Protestant Muslim Other |
1 0.46 (0.27, 0.77)** 0.71 (0.47, 1.09) 0.39 (0.14, 1.22) |
1 0.69 (0.36, 1.30) 1.28 (0.73, 2.24) 0.56 (0.18, 1.68) |
||
|
Marital status Not married Married |
1 1.84 (0.89, 3.80) |
1 2.21 (1.06, 4.58)* |
||
|
Immunisation document Available Not available |
1 27.32 (16.44, 45.41)*** |
1 26.18 (15.75, 43.53)*** |
||
|
Received vitamin A1 No Yes |
1 4.32 (3.04, 6.15)*** |
1 4.14 (2.9, 5.9)*** |
||
|
Region Tigray Afar Amhara Oromia Somali Benishangul-Gumuz SNNPR Gambela Harari Addis Ababa Dire Dawa |
1 0.08 (0.03, 0.23)*** 0.59 (0.24, 1.48) 0.21 (0.09, 0.48)*** 0.08 (0.03, 0.22)*** 1.13 (0.37, 3.40) 0.19 (0.08, 0.45)*** 0.38 (0.12, 1.22) 0.43 (0.12, 1.48) 1.27 (0.39, 4.14) 0.78 (0.23, 2.62) |
1 0.14 (0.04, 0.45)** 0.5 (0.22, 1.13) 0.45 (0.18, 1.09) 0.19 (0.06, 0.60)** 0.74 (0.25, 2.22) 0.48 (0.19, 1.17) 0.22 (0.06, 0.77)* 0.14 (0.04, 0.52)** 1.14 (0.37, 3.47) 0.23 (0.06. 0.79)* |
||
|
Place of residence Urban Rural |
1 0.25 (0.14, 0.44)*** |
1 0.53 (0.30, 0.93)* |
||
|
Community women education Low High |
1 1.04 (0.56, 1.94) |
1 0.75 (0.51, 1.10) |
*P<0.05, **p<0.01, ***p<0.001.
AOR, adjusted OR; SNNPR, Southern Nations, Nationalities and People’s Region.
The random-effects analysis result
Table 4 presents the random-effects analysis result of childhood vaccination status in Ethiopia. The appropriateness of application of multilevel analysis and the presence of significant variations of childhood vaccination status between clusters (the clustering effect) were justified by the ICC of the null model (48.19%). The value of ICC decreased from 48.19% in the null model to 14.55% in the final model showing how the effect of clustering changed. In addition, as depicted in the table below, the largest PCV of the final model (81.70%) indicates the goodness of model fitness. The final model (model 3) has the highest capacity than model 1 or model 2 alone to explain the variations in childhood vaccination status. On top of that, model 3 is the best explanatory model to show the variation in the childhood vaccination. The deviance observed in the final model was smaller than that of other models (table 4).
Table 4.
Random-effects analysis and model fit statistics of child vaccination in Ethiopia, 2019
| Parameters | Null model | Model 1 | Model 2 | Model 3 |
| Community-level variance (SE) | 3.06 | 0.76 | 1.21 | 0.56 |
| PCV (%) | Reference | 75.16 | 60.46 | 81.70 |
| ICC (%) | 48.19 | 18.77 | 26.89 | 14.55 |
| MOR | 5.31 | 2.29 | 2.84 | 2.03 |
| Log likelihood | −988.74 | −684.36 | −919.88 | −667.78 |
| Deviance | 1977.48 | 1368.72 | 1839.76 | 1335.56 |
| AIC | 1983.49 | 1418.72 | 1873.77 | 1413.56 |
| BIC | 1998.24 | 1540.70 | 1957.34 | 1603.86 |
AIC, Akaike information criterion; BIC, Bayesian information criterion; ICC, intraclass correlation coefficient; MOR, median OR; PCV, proportional change in variance.
Discussion
In Ethiopia, full vaccination was 39.08%. Compared with other studies in the country, it is less than that of Southeast Ethiopia (76.8%),26 country-level pooled prevalence (47%)27 and in 2016 from another study (67%).35 It is also less than the sub-Saharan African countries vaccination coverage (59.40%):22 East Africa (69.21%),35 Mogadishu (45.2%)23 and Togo (69.3%).25 It is in the same CIs (38.3%) as the 2016 Ethiopia DHS. This indicates that the current prevalence of full vaccination was unchanged since 2016. The reason might be the political instability in the country for the last 5 years. Dropout rate (18.8%) may have also negatively affected childhood vaccination coverage of the country. The inequalities in accessing health services regardless of increased population in those years might be another reason. Lack of maternal education (48.91%) also affected vaccination coverage undesirably; however, it was 67% in 2015.31 It is also better than the sub-Saharan African countries’ vaccination coverage.22 This shows that although there are some improvements, the deviation from standard is big. Only 39.98% of mothers were from higher-income families. The finding is consistent with 36.6% coverage from another study.31
Among the associated factors, maternal education had positive association with higher full or partial vaccination, which is also similar to findings from other studies.22 31 36 The consistency might indicate that education is an independent predictor of full or partial vaccination, which needs focused attention. Increased maternal age puts mothers in the higher level of full immunisation category. Other studies also showed that higher age has association with full immunisation.3 22 This might indicate that maternal maturity is an important factor for higher childhood vaccination coverage in contrast to those who had early marriage. This means childbearing at a younger age and age at first birth need adequate attention. Children whose mothers remained married had higher tendency to be in higher category of full vaccination. From previous studies, there are consistent findings.22 23 This might be an indication that disrupted families might endanger children’s life and so family union should be promoted. Mothers who keep evidence of vaccination tend to be in higher category of vaccination coverage. This is also true for some studies.23 24 31 This means that women who keep their children’s vaccination cards might have indeed great regard for full immunisation while carelessness might start from poor evidence keeping. Children who received vitamin A1 had higher chance of being in the higher vaccination category. The fact that vitamin A1 is scheduled towards the end of most immunisation programmes might raise this association.19 This might be another persisting issue we never eased in previous efforts as per evidence from this study and could be set as an objective to attain other subobjectives. Ethiopia is further classified into city administrations, agrarian and pastoralist regions. Pastoralist regions such as Afar and Somali and semipastoralist regions such as Gambela, Benishangul-Gumuz and Harari had higher tendency of falling into the low category of full immunisation. This is also reported by other studies,31 and confirmed by other studies that compared developed and underdeveloped regions in Ethiopia.31 36 This apparently shows that attention-deprived regions should be the next intervention areas. This problem is relatively similar between the rural and urban place of residence. Other studies also laud that inequalities between rural and urban residence need careful consideration.20 This might be apparently due to access, awareness and availability of resources.
Besides the positive information, this study has also some limitations such as missing data in some records, the disproportion of sample in each region, secondary source data, and time lapse between data collection and analysis. When observable evidence of vaccination is not available, data collectors asked mothers verbally and that may cause some recall and social desirability bias. However, a Health Facility Questionnaire was used to record vaccination information for all children without a vaccination card seen during the mother’s interview.34 Moreover, we are unable to include some relevant variables like psychosocial variables of parents, vaccine hesitancy and other related variables since this study used secondary data of a national survey. The authors followed international protocols of DHS data process and analyses, weighted data, carefully interpreted findings within the period and included complete records.
Conclusion
Although full vaccination increased from Ethiopia DHS 2000 to 2016, the current analysis of EMDHS 2019 results showed that it has remained unchanged. Our analysis identified many factors that might have contributed to the absence of changes. Maternal educational level, maternal age, marital status, presence of vaccination document, receiving vitamin A1, region and place of residence were some of the contributors. Analysing associated factors showed some of the factors are old enough, causing the problem for decades. Even though the political situation in the country might also contributed to the persistency of these factors, it is advisable to work on accessibility, awareness and availability. Maternal education and poverty, especially family and maternal capacity, remained critical and need all efforts such as further government commitment, dedicated international support, and peace and security that can over-ride the problem.
Supplementary Material
Acknowledgments
The authors are grateful to MEASURE DHS, ICF International Rockville, Maryland, USA for allowing them to use the 2019 EMDHS data.
Footnotes
Contributors: SS is the guarantor of the study. SS, GG and SH were equally involved in the conception of the study, interpreted the results, drafted and critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available. The survey datasets used in this analysis are third-party data from the Demographic and Health Survey website (www.dhsprogram.com) and permission to access the data is granted only for registered DHS data user.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study used secondary data from the Demographic and Health Survey files. Initially, the MEASURE DHS team was formally requested to access the datasets by completing the online request form on their website (www.dhsprogram.com). Accordingly, permission to access the data and the letter of authorisation were obtained from ICF international. Therefore, for this study, consent to participate is not applicable. We kept all data confidential, and no effort was made to identify households or individuals. The Ethiopian Health Nutrition and Research Institute (EHNRI) Review Board and the National Research Ethics Review Committee (NRERC) at the Ministry of Science and Technology of Ethiopia approved the EMDHS 2019. The authors also confirm that all methods were carried out in accordance with relevant guidelines and regulations.
References
- 1.WHO vaccine-preventable diseases: monitoring system. 2020 Global Summary 2022;2020:2–3. [Google Scholar]
- 2.Chepkurui V, Amponsah-Dacosta E, Haddison EC, et al. Characterization of national immunization programs in the context of public health emergencies: a case study of 13 countries in the who africa region. Front Public Health 2021;9(September):736532. 10.3389/fpubh.2021.736532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.South Africa: WHO and UNICEF estimates of immunization coverage: 2016 revision. 2018: 1–18. [Google Scholar]
- 4.WHO . Immunization coverage. 2022: 3–7. [Google Scholar]
- 5.Patel MK, Goodson JL, Alexander JP, et al. Progress toward regional measles elimination - worldwide, 2000-2019. MMWR Morb Mortal Wkly Rep 2020;69:1700–5. 10.15585/mmwr.mm6945a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . Counting the impact of vaccines. 2021. [Google Scholar]
- 7.FMOH . Expanded program on immunization. 2022: 1–4. [Google Scholar]
- 8.Klein S. Why some vaccines require more than one dose. 2015: 1–3. [Google Scholar]
- 9.WHO . Why immunizing children matters. 2017: 1–3. [Google Scholar]
- 10.Children’s hospital of Philadelphia . Did you know: some reasons vaccines are given in multiple doses. 2014. [Google Scholar]
- 11.Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci 2014;369:20130433. 10.1098/rstb.2013.0433 Available: 10.1098/rstb.2013.0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues CMC, Plotkin SA. Impact of vaccines; health economic and social perspectives. Front Microbiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghendon Y. Vaccine preventable viral diseases in developing countries. World J Microbiol Biotechnol 1991;7:115–20. 10.1007/BF00328980 [DOI] [PubMed] [Google Scholar]
- 14.Brenzel L, Wolfson LJ, Fox-Rushby J, et al. Vaccine-preventable diseases. In: Jamison DT, Breman JG, Measham AR, eds. Disease Control Priorities in Developing Countries. 2nd edn. edn. Washington (DC): The International Bank for Reconstruction a, [Google Scholar]
- 15.WHO: and UNICEF estimates of immunization coverage: 2019 revision ethiopia. Ethiopia: WHO, 2019: 1–28. [Google Scholar]
- 16.WHO . Immunization WHO regional office for africa. n.d.: 199–207. Available: https://www.afro.who.int/health-topics/immunization [Google Scholar]
- 17.WHO . Organisation mondiale vaccine-preventable diseases and immunization. 2021: 71. [Google Scholar]
- 18.Raslan R, El Sayegh S, Chams S, et al. Re-emerging vaccine-preventable diseases in war-affected peoples of the eastern mediterranean region-an update. Front Public Health 2017;5:283. 10.3389/fpubh.2017.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO . Global immunization news (GIN). 2017. [Google Scholar]
- 20.Shibre G, Zegeye B, Idriss-Wheeler D, et al. Inequalities in measles immunization coverage in ethiopia: a cross-sectional analysis of demographic and health surveys 2000-2016. BMC Infect Dis 2020;20:481. 10.1186/s12879-020-05201-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Figueiredo A, Were F. Local trends in immunisation coverage across africa. Lancet 2019;393:1779–81. 10.1016/S0140-6736(19)30702-0 [DOI] [PubMed] [Google Scholar]
- 22.Fenta SM, Biresaw HB, Fentaw KD, et al. Determinants of full childhood immunization among children aged 12-23 months in sub-saharan africa: a multilevel analysis using demographic and health survey data. Trop Med Health 2021;49:29. 10.1186/s41182-021-00319-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamud Hayir TM, Magan MA, Mohamed LM, et al. Barriers for full immunization coverage among under 5 years children in mogadishu, Somalia. J Family Med Prim Care 2020;9:2664–9. 10.4103/jfmpc.jfmpc_119_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadita ZS, Ayehubizu LM. Full immunization coverage and associated factors among children aged 12-23 months in somali region, eastern ethiopia. PLoS ONE 2021;16:e0260258. 10.1371/journal.pone.0260258 Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zida-Compaore WIC, Ekouevi DK, Gbeasor-Komlanvi FA, et al. Immunization coverage and factors associated with incomplete vaccination in children aged 12 to 59 months in health structures in lomé. BMC Res Notes 2019;12:84. 10.1186/s13104-019-4115-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legesse E, Dechasa W. An assessment of child immunization coverage and its determinants in sinana district, Southeast Ethiopia. BMC Pediatr 2015;15. 10.1186/s12887-015-0345-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nour TY, Farah AM, Ali OM, et al. Immunization coverage in Ethiopia among 12–23 month old children: systematic review and meta-analysis. BMC Public Health 2020;20. 10.1186/s12889-020-09118-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ethiopian Public Health Institute (EPHI) . Ethiopia mini demographic and health survey 2019: final report. Rockville, Maryland, USA: EPHI and ICF, 2021. Available: https://dhsprogram.com/pubs/pdf/FR363/FR363.pdf [Google Scholar]
- 29.Ethiopian Public Health Institute (EPHI) . Mini demographic and health survey 2019: key indicators. Rockville, Maryland, USA: EPHI and ICF, 2019: 35. [Google Scholar]
- 30.Bangura JB, Xiao S, Qiu D, et al. Barriers to childhood immunization in sub-saharan africa: a systematic review. BMC Public Health 2020;20:1108. 10.1186/s12889-020-09169-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakew Y, Bekele A, Biadgilign S. Factors influencing full immunization coverage among 12-23 months of age children in ethiopia: evidence from the national demographic and health survey in 2011. BMC Public Health 2015;15:728. 10.1186/s12889-015-2078-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girmay A, Dadi AF. Full immunization coverage and associated factors among children aged 12-23 months in a hard-to-reach areas of ethiopia. Int J Pediatr 2019;2019:1924941. 10.1155/2019/1924941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ministry of Health-Ethiopia . Health sector transformation plan II (HSTP). 2021.
- 34.Ethiopian Public Health Institute (EPHI) . Ethiopia mini demographic and health survey: key indicators Ethiopia. Rockville, Maryland, USA: EPHI and ICF, 2019. [Google Scholar]
- 35.Gelaye SS, Yenit MK, Baraki AG. Rural vaccination coverage among children aged 12-23 months was higher than the urban counterparts: a comparative cross-sectional study in pawi district, Ethiopia. Pediatric Health Med Ther 2021;12:119–27. 10.2147/PHMT.S299064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wondimu A, Cao Q, Asuman D, et al. Understanding the improvement in full childhood vaccination coverage in Ethiopia using oaxaca-blinder decomposition analysis. Vaccines (Basel) 2020;8:2020;505. 10.3390/vaccines8030505 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The survey datasets used in this analysis are third-party data from the Demographic and Health Survey website (www.dhsprogram.com) and permission to access the data is granted only for registered DHS data user.