Abstract
Periodontitis and inflammatory bowel diseases (IBD) are inflammatory diseases of the gastrointestinal tract that share common features of microbial-induced ecological dysregulation and host immune inflammatory response. The close relationship between periodontitis and IBD is characterized by a higher prevalence of IBD in patients with periodontitis and a higher prevalence and severity of periodontitis in patients with IBD, indicating that periodontitis and IBD are different from the traditional independent diseases and form an “Oral-Gut” axis between the two, which affect each other and thus form a vicious circle. However, the specific mechanisms leading to the association between the two are not fully understood. In this article, we describe the interconnection between periodontitis and IBD in terms of microbial pathogenesis and immune dysregulation, including the ectopic colonization of the gut by pathogenic bacteria associated with periodontitis that promotes inflammation in the gut by activating the host immune response, and the alteration of the oral microbiota due to IBD that affects the periodontal inflammatory response. Among the microbial factors, pathogenic bacteria such as Klebsiella, Porphyromonas gingivalis and Fusobacterium nucleatum may act as the microbial bridge between periodontitis and IBD, while among the immune mechanisms, Th17 cell responses and the secreted pro-inflammatory factors IL-1β, IL-6 and TNF-α play a key role in the development of both diseases. This suggests that in future studies, we can look for targets in the “Oral-Gut” axis to control and intervene in periodontal inflammation by regulating periodontal or intestinal flora through immunological methods.
Keywords: periodontitis, IBD, microbiology, immunity, oral-gut axis
1. Introduction
Periodontitis is one of the most common oral diseases and a major cause of tooth loss in adults. Periodontitis is an infectious disease caused by plaque biofilm that leads to the destruction of tooth-supporting tissues, with clinical symptoms including attachment loss, periodontal pocket formation, and alveolar bone resorption, which, if left untreated, can gradually lead to tooth loosening or even tooth loss (Petersen and Ogawa, 2012; Kinane et al., 2017; Van der Velden, 2017). There is a growing body of research showing that the health of oral tissues interacts with systemic health (Taylor et al., 2000; Genco and Sanz, 2020; Kapila, 2021; Larvin et al., 2022; Teles et al., 2022). Notably, periodontitis is closely associated with several systemic diseases, including atherosclerotic cardiovascular disease (Beck et al., 2001; Van Dyke et al., 2021; Isola et al., 2022), diabetes (Chapple et al., 2013; Genco and Borgnakke, 2020; Nibali et al., 2022), adverse pregnancy outcomes such as miscarriage and preterm delivery (Canakci et al., 2004; Sanz et al., 2013; Miranda-Rius et al., 2021), rheumatoid arthritis(Bartold and Lopez‐Oliva, 2020; Möller et al., 2020; González‐Febles and Sanz, 2021), Alzheimer’s disease (Pizza, 2019; Kamer et al., 2020; Torrealba-García et al., 2021) and inflammatory bowel disease (Cai et al., 2021; Imai et al., 2021; Zhang et al., 2021a; Baima et al., 2022).
The oral cavity is a complex ecosystem in which microorganisms accumulate and multiply, forming dental plaque (Listgarten, 1994; Lourenço et al., 2014). Plaque is the basis for the survival, metabolism and pathogenesis of oral bacterial, and it can serve as an arsenal for bacterial pathogenesis, producing antigens that invade the deep periodontal gingival mucosa and interfere with the host immune defense system, thereby exacerbating the inflammatory response (Murakami et al., 2018; Valm, 2019; Jakubovics et al., 2021). The flora composition and oral ecology of patients with periodontitis becomes more complex, as Socransky et al. suggested that periodontitis is caused by specific pathogens such as “red complex” bacteria (Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia)(Socransky et al., 1998), with Hajishengallis’ in-depth study of its pathogenesis revealing that the etiology of periodontitis is a synergistic effect of a dysregulated microbial community (Hajishengallis and Lamont, 2012), that is, bacteria invade the host tissue and disrupt its immune protective mechanisms, causing local periodontal tissue damage, while facilitating the enrichment of other anaerobic bacteria and increasing the pathogenicity of the entire microbial community, resulting in an inflammatory response.
IBD is a non-specific chronic inflammatory disease of the intestine that has received a lot of attention from scholars because of its high incidence (Windsor and Kaplan, 2019; Agrawal and Jess, 2022; Upadhyay et al., 2022), mainly including ulcerative colitis (UC) and Crohn’s disease (CD). UC mainly damages the colon and rectum, while CD can damage any part of the gastrointestinal tract from the mouth to the anus, with the end of the small intestine and the colon being most commonly affected (Guan, 2019). The main clinical manifestations of IBD are abdominal pain, diarrhea, bloody stools and weight loss (She et al., 2020). Approximately more than 40% of patients will present with extra-intestinal manifestations, such as oral, ocular, cutaneous, hepatobiliary, urinary and neurological lesions may be associated (Jose et al., 2009; Lankarani, 2013; Taleban et al., 2015; Hedin et al., 2018; Marotto et al., 2020). Oral lesions are the most common extraintestinal manifestation in patients with IBD, which can appear as the first symptom and are closely related to disease activity, mainly in the form of recurrent oral aphthae (Sircus et al., 1957), granulomatous inflammation (Dudeney, 1969)and periodontitis (Byrd and Gulati, 2021). In addition, IBD is associated with environmental factors, genetic susceptibility, gut microbiota and host immune response (Ananthakrishnan et al., 2017; Khalili et al., 2018; Sugihara et al., 2019; Amoroso et al., 2020; Agrawal et al., 2022), among which gut microbes are crucial for the development of IBD (Ni et al., 2017; Schirmer et al., 2019). Schreiber et al. found that bacterial diversity in the intestine of IBD patients was reduced, with CD patients having a reduced number of Firmicutes and an increased abundance of Proteobacteria and Bacteroidetes, while UC patients had a relatively mild degree of intestinal microbial dysbiosis, but the exact pathogenesis of IBD is still unclear (Ott, 2004).
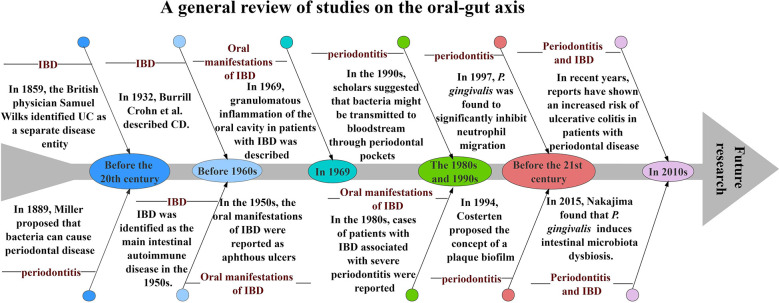
Periodontitis, one of the most common diseases of the oral cavity, has been studied by countless scholars over the years. In 1889, Miller first linked the theory of bacteria to oral disease, clearly implying that bacteria play a role in the late stages of periodontal pathology (LÖE, 1993); in 1994, Socransky et al. classified microorganisms in subgingival plaque into five complexes according to their aggregation and pathogenicity (Socransky et al., 1998). Similar to periodontitis, the signs and symptoms of IBD have been mentioned throughout human history. In 1859, the British physician Samuel Wilks identified UC as a separate disease; in 1932, Burrill Crohn et al. described inflammation of the terminal ileum in an article, a disease that came to be known as CD; in the 1950s, it was noted that symptoms in patients with CD and UC responded to corticosteroids and IBD was identified as a major intestinal autoimmune disease (Malik, 2015). The oral manifestations of IBD were first reported in the 1950s, initially focusing on aphthous ulcers(Sircus et al., 1957); in 1969, Dudeney et al. described granulomatous inflammation of the oral cavity in patients with IBD(Dudeney, 1969); in the 1980s, cases of severe periodontitis in patients with IBD were reported(Engel et al., 1988). Periodontitis may lead to low-grade systemic inflammation and its association with other chronic inflammatory diseases has been studied for many years. In the 1990s, scholars suggested that oral bacteria may spread into the bloodstream through the ulcerated epithelium in periodontal pockets and cause transient bacteremia (Asikainen and Alaluusua, 1993); in 1997, Madianos et al. found that Porphyromonas gingivalis(P. gingivalis) significantly inhibited neutrophil migration induced by stimuli such as Escherichia coli(Madianos et al., 1997); in 2015, Nakajima et al. found that P. gingivalis causes changes in intestinal bacterial composition and induces intestinal microbiota dysbiosis and impaired barrier function(Nakajima et al., 2015); in recent years, scholars have reported a significantly increased risk of ulcerative colitis in patients with periodontal disease(Lin et al., 2018). All these studies and reports provide favorable evidence for the existence of an “oral-gut” axis in periodontitis and IBD ( Figure 1 ).
Figure 1.
A general review of studies on the oral-gut axis.
The oral cavity and the intestine are the starting and ending points of microbial aggregation in the digestive tract, housing unique microbial groups associated with human health and disease (Lynch and Pedersen, 2016), and both share some common flora, including Streptococcus, Bacteroides, and Prevotella (Byrd and Gulati, 2021). Most studies have shown that microorganisms in the oral cavity can be transmitted to the intestine through the digestive tract (Lira-Junior and Figueredo, 2016; Atarashi et al., 2017; Schmidt et al., 2019; Kitamoto et al., 2020a; Byrd and Gulati, 2021). Periodontal pathogenic bacteria can ectopically colonize the gut from the oral cavity, leading to disruption of the intestinal microbiota and inducing an intestinal immune response, thereby exacerbating intestinal inflammation (Atarashi et al., 2017), suggesting a unique association between periodontitis and IBD.
At the same time, harmonious intestinal flora facilitates the control of periodontitis, and the administration of intestinal probiotics with inflammatory regulatory properties is considered one of several new approaches to address bacterial imbalances in periodontitis and prevent bone loss. Several clinical studies have shown that adjuvant oral administration of the intestinal probiotic (Lactobacillus reuteri) after basic periodontal treatment significantly improves periodontal clinical signs (Ikram et al., 2018) and that gavage of the probiotic significantly reduces the number of osteoclasts in the alveolar bone of mice compared with oral inoculation of the probiotic (Gatej et al., 2017). Treatment of IBD is closely related to periodontitis healing and prevention (Newman and Kamada, 2022). Probiotics such as Lactobacillus have beneficial activity for the treatment of gastrointestinal diseases such as IBD, and related longitudinal studies have shown that gavage of probiotics before the formation of periodontitis in mice resulted in a significant increase in β-defensin levels in intestinal and gingival tissues, significantly reduce the levels of inflammatory factors in gingival tissues during periodontitis while modulating intestinal barrier function and improving intestinal inflammation, and alleviate alveolar bone resorption and periodontal membrane destruction (Kobayashi et al., 2017). In addition, altering the progression of chronic systemic inflammatory disease through dietary control may have similarities to improving chronic adult periodontitis (Dawson et al., 2014). Studies have pointed out that certain therapeutic agents of CD, such as steroids, may be able to prevent periodontitis (Chi et al., 2018). Currently, psoralen, vitamin D, and other substances have been shown to achieve the treatment of periodontitis by repairing the barrier of intestinal inflammation damage (Jagelavičienė et al., 2018; Machado et al., 2020; Liu et al., 2021a). Moreover, Zhang et al. proved that with the improvement of periodontitis, IBD was also significantly relieved after exosome treatment (Zhang et al., 2021a). All of the above related longitudinal studies suggest a strong bidirectional association between periodontitis and IBD, with the treatment of one disease having a beneficial effect on the other, which inspires that future studies could use a drug to treat intestinal inflammation by improving the intestinal flora while having a beneficial effect on the prevention and control of periodontitis.
In terms of immune mechanisms, the digestive tract is the main site of communication between the internal and external environment and a key barrier against pathogen invasion, while its defense against pathogen invasion includes both intrinsic and specific immunity, of which the main one is the immune defense involving T lymphocytes. CD4+ T cells are the main cell population mediating various host protective and homeostatic responses and contain many subpopulations (e.g., helper T cells, Th1, Th2, Th17 and regulatory T cells, Treg) (Shale et al., 2013; Jaeger et al., 2021; Hong et al., 2022; Yang et al., 2022), of which two CD4+ T lymphocyte populations synergistically regulate adaptive immunity in the intestinal mucosa: peripherally induced regulatory T cells (pTreg cells) and CD8αα-expressing intraepithelial lymphocytes (CD4IELs) (Shale et al., 2013). However, the exact mechanism of how CD4+ T cells recognize colony antigens and differentiate into CD4IELs remains unknown. According to a recent study by Bousbaine’s team published in Science in 2022, β-hex-specific T cells induced by intestinal commensal antigens can drive differentiation of CD4IELs and regulate intestinal inflammation by expressing interleukin (IL)-10 (Bousbaine et al., 2022). This finding suggests us whether periodontal inflammation can be controlled and intervened by modulating oral or gut microbiota through immune means. Therefore, in this paper, we discuss the association between periodontitis and IBD from both microbial and immune mechanisms, which has important implications for the pathogenesis and treatment of periodontitis and IBD.
2. Correlations between periodontitis and IBD
2.1. Prevalence and comorbidity
Periodontitis as a global public health problem has received wide attention from scholars (Luo et al., 2021; Stødle et al., 2021; Alawaji et al., 2022; Ju et al., 2022; Wu et al., 2022). TaeHyun Kwon et al. found that about 42% of adults in the United States had periodontitis, and 7.8% of them had severe periodontitis (Kwon et al., 2020). In China, the prevalence of periodontitis in adults is as high as 80% to 90%, with chronic periodontitis accounting for 60% to 70% of the prevalence (Meng, 2007). An epidemiological study by Frencken et al. showed that the prevalence of severe periodontitis in the global population remained at about 11.2%, and the prevalence of severe periodontitis increased with age, with a sharp increase in the 30-40 years of age interval. It reaches a peak at the age of 40 years and remains stable thereafter (Frencken et al., 2017).
As an immune-mediated chronic inflammatory disease, the incidence and prevalence of IBD have continued to increase worldwide over time, indicating that it has become a global disease (Ye et al., 2019; Alatab et al., 2020; Kaplan and Windsor, 2020; Zhao et al., 2021). The oral cavity is one of the most vulnerable areas for extraintestinal manifestations of IBD, and numerous studies have found that patients with IBD have a higher risk of periodontitis than those without IBD (Papageorgiou et al., 2017; She et al., 2020; Zhang et al., 2021c; Abrol et al., 2022). In addition, the severity and extent of periodontitis suffered by patients with IBD was found to be greater, with patients having significantly higher mean probing depth, plaque index, calculus index, sulcus bleeding index, and attachment loss than patients with general periodontitis (Zhang et al., 2020; Nijakowski et al., 2021; Domokos et al., 2022). It has also been confirmed that patients with periodontitis have a significantly higher risk of developing IBD than healthy controls without periodontitis (Lin et al., 2018; Kang et al., 2020; Madsen et al., 2022). All of the above studies suggest a close bidirectional association between periodontitis and IBD.
2.2. Microbial associations of periodontitis and IBD
A large number of microorganisms colonize the human oral cavity, including bacteria, fungi, viruses, protozoa, and mycoplasma (Wade, 2013; Mosaddad et al., 2019). Among them, bacterial communities can reach more than 1000 species, including Actinomyces, Bacteroides, Firmicutes, Proteobacteria and Spirochete (Dewhirst et al., 2010). In most cases, bacteria in the oral cavity maintain a relative balance between flora, as well as a dynamic balance between flora and host; however, when the normal flora loses its mutual control or the microorganism and the host lose their balance, it will cause the oral ecological environment to become dysfunctional and the pathogenic bacteria will escape or inhibit the host’s defense function, damaging the host tissue and eventually leading to the development of periodontitis (Darveau, 2010). Mike et al. found a significant increase in oral microbial diversity in patients with periodontitis by 16S rRNA sequencing, with a high concentration of Gram-negative bacteria in the subgingival flora, including the classical “red complex” bacteria (Curtis et al., 2020). Notably, Porphyromonas gingivalis (P. gingivalis), a Gram-negative, specialized anaerobic bacterium, is considered to be the main pathogen involved in the onset and progression of chronic periodontitis (Reyes, 2021), attaching to mucous membranes, periodontal pocket epithelium and other bacterial surfaces by a series of adhesion factors, including bacterial hairs, hemagglutinins and proteases (Lamont and Jenkinson, 2000) and can secrete large amounts of proteases (e.g., gingipains), endotoxins (e.g., lipopolysaccharide, LPS), acid and alkaline phosphatases, indoles, organic acids, and other virulence factors that destroy periodontal tissue by degrading host proteins and evading host immune defenses(Jia et al., 2019a; Xu et al., 2020), leading to clinical manifestations of edema, neutrophil infiltration, and hemorrhage (Travis et al., 1997). In addition, Settem et al. demonstrated that Fusobacterium nucleatum (F. nucleatum) and Tannerella forsythia cause destruction of alveolar bone by synergistically stimulating the immune response in periodontal tissues (Settem et al., 2012), thus demonstrating that the synergistic action of ecologically dysregulated microbial communities plays an important role in the progression of periodontitis. The microbial metabolites are necessary for periodontal pathogenic bacteria to maintain their growth, reproduction and pathogenesis. Compared to healthy subjects, patients with periodontitis had significant differences in the composition of salivary microbial metabolites with higher levels of serine, serotonin, 4-hydroxycinnamic acid, hydrocinnamic acid and isoleucine (Wei et al., 2022). Guan et al. noted that short chain fatty acids (SCFAs) are important pathogenic factors in periodontitis, and butyrate can damage the gingival epithelium by exerting destructive effects on intercellular junctions, leading to cell death (Guan et al., 2020).
The oral cavity and the intestine, as the two ends of the digestive tract, share similar microbial pathogenic mechanisms. When the intestinal microbial balance is disturbed, bacterial metabolites are altered and a large number of virulence factors are released, which can damage host tissues and eventually lead to an inflammatory response (Glassner et al., 2020; Lavelle and Sokol, 2020; Lee and Chang, 2020). The microbiota in the human gut consists mainly of Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria, with Firmicutes (49-76%) and Bacteroidetes (16-23%) predominating (Eckburg et al., 2005; Frank et al., 2007). Host tissues provide a nutrient-rich habitat for the gut flora, while the gut flora can provide short-chain fatty acids and essential vitamins to the host (Mukherjee et al., 2020), and the two complement each other in a harmonious symbiosis, a mutual relationship known as symbiosis; however, when suffering from IBD, the microbiota of the gut is altered, a change known as ecological dysbiosis (Matsuoka and Kanai, 2014). Frank et al. found that, in contrast to periodontitis, the diversity of the intestinal flora is reduced in patients with IBD, with a decrease in the number of Firmicutes and an increase in the number of Proteobacteria (Frank et al., 2007). Changes in the composition of the gut microbiota lead to altered bacterial metabolites, which may play a role in the pathogenesis of IBD. Bile salt hydrolase (BSH), a metabolite of intestinal bacteria (especially the thick-walled phylum), plays a key role in the modification of bile acids, which maintain the integrity of the intestinal epithelial barrier and improve intestinal inflammation through a negative feedback mechanism mediated by the farnesoid X receptor (FXR) (Gadaleta et al., 2011; Ding et al., 2015; Gonzalez et al., 2016). However, when compared to healthy individuals, the number of BSH in Firmicutes was significantly lower in the intestine of IBD patients, which led to a significant reduction in the anti-inflammatory effect of FXR (Ni et al., 2017). Additionally, intestinal microbiota metabolism can produce butyrate, an important energy source for intestinal epithelial cells, and studies have found that the number of butyrate-producing Faecalibacterium prausnitzii in the intestine of IBD patients is significantly reduced, while reduced butyrate levels induce an intestinal inflammatory response (Takaishi et al., 2008). Choline is an essential dietary nutrient for humans, and intestinal microorganisms (Firmicutes) play a key role in the degradation of choline to trimethylamine (TMA), which is further metabolized in the liver to trimethylamine N-oxide (TMAO), the level of which is associated with many adverse host pathologies, including IBD. It has been shown that TMAO levels are significantly elevated in mice fed a choline-rich diet, which leads to increased expression of pro-inflammatory cytokines, and that in colonic epithelial cells, TMAO triggers activation of inflammatory vesicles and production of reactive oxygen species in a dose- and time-dependent manner, thus playing a potential role in the pathogenesis of IBD(Hosseinkhani et al., 2021); in addition, recent studies have found elevated levels of circulating TMAO in patients with stage III-IV periodontitis(Zhou et al., 2021), suggesting a close relationship between intestinal flora metabolites and periodontitis. All of the above studies suggest that alterations in intestinal flora are closely related to the development of IBD.
It was found that microorganisms from the oral cavity can be detected at low levels in stool (Schmidt et al., 2019), suggesting that microorganisms from the oral cavity can spread to the intestine, which also allows pathogenic bacteria to ectopically colonize the intestine and consequently disrupt intestinal homeostasis and abnormally activate the intestinal immune system, thus affecting the development of IBD. Koji et al. suggest that the oral cavity can act as a potential reservoir for intestinal pathogens (Atarashi et al., 2017). For example, when periodontitis develops, Klebsiella and Proteus proliferate in the oral cavity and can migrate to ectopically colonize the lower gastrointestinal tract, thus causing activation of macrophage inflammatory vesicles, which may exacerbate intestinal inflammation (Kitamoto et al., 2020b). Candida, an important pathogenic fungus in the body, is usually found in the oral cavity and intestinal tract of normal people. Generally, small amounts of Candida do not cause disease, but when the body becomes ecologically imbalanced, it can multiply and cause disease. In recent years, numerous studies have found that Candida is closely associated with both periodontitis and IBD. Studies have shown that oral colonization by Candida is strongly associated with the severity of periodontitis. Candidalysin directly induces pro-inflammatory factors and NLRP3 inflammatory vesicles thereby promoting an inflammatory response, and inhibition of Candida albicans by antifungal agents is effective in reducing the severity of periodontitis in women (Ho et al., 2020). Hiengrach et al. found that oral Candida can promote the growth of intestinal bacteria thereby inducing intestinal ecological dysbiosis, which in turn promotes colonic inflammation (Hiengrach et al., 2020); Candida albicans colonize the gut significantly more frequently and with greater severity in patients with CD than in the healthy population (Standaert-Vitse et al., 2009), whereas reducing intestinal fungus reduces the severity of the disease. Furthermore, in the study by Li et al. oral Candida can colonize the gut through the digestive tract and disrupt the intestinal barrier by inducing the release of pro-inflammatory factors, while in the pathogenic mechanism of Candida candidalysin is a key virulence factor in promoting the inflammatory response in the gut (Li et al., 2022b). Oral Candida colonizes the intestine through the digestive tract and thus affects the metabolic and ecological balance of the flora. Another study found that after antibiotic treatment resulting in Candida-dependent oral and intestinal inflammation, SCFA supplementation promoted fungal clearance and restored Th17 and Treg cell SCFA by regulating T cell cytokines, thereby improving the inflammatory response (Bhaskaran et al., 2018). This close link between the oral and intestinal tracts is crucial to our study of the oral-gut axis, and it seems that inhibiting Candida colonization by modulating the intestinal flora as well as microbial metabolites, and thus improving periodontitis through the oral-gut axis may provide us with new therapeutic directions. Furthermore, P. gingivalis and F. nucleatum, as pathogenic bacteria of periodontitis, similarly affect the development of IBD. Lee et al. showed that the number of P. gingivalis in the stool of CD patients was significantly higher, and that P. gingivalis colonization of the intestine led to the loss of intestinal surface epithelium, destruction of crypt structures, and infiltration of inflammatory cells, ultimately exacerbating the manifestation of intestinal inflammation (Lee et al., 2022); Xiao et al. found that the establishment of a mouse periodontitis model by oral administration of P. gingivalis resulted in a significant increase in LPS levels and led to enhanced expression of flavin monooxygenase3 (FMO3) (FMOs promotes the conversion of TMA to TMAO) and plasma TMAO levels, which induced intestinal ecological dysregulation and inflammatory responses (Xiao et al., 2022); Jaclyn et al. found that F. nucleatum is present in the intestinal mucosa of IBD patients and that the invasive potential of F. nucleatum strains is positively correlated with the disease status of the host, thus inferring that F. nucleatum can be used as a potential biomarker for gastrointestinal diseases (Strauss et al., 2011). In addition to the microbes themselves, their metabolites can also enter the intestine via the oral-gut axis, thereby promoting inflammation in the gut. Wang et al. found that periodontitis upregulates the levels of the microbiota metabolite isoleucine (Ile) in saliva, and that Ile translocated through the digestive tract to the intestine and acetylated the NLRP3 protein through its metabolite AcCoA to aggravate colon inflammation in mice (Wang et al., 2022). Microbial metabolites are essential for the regulation of the ecology of the host digestive tract, which in turn suggests that they could be a common therapeutic target for periodontitis and IBD via the oral-gut axis.
Systemic inflammation in patients with IBD also has an impact on the composition of the oral microbiota, and Docktor et al. found an increase in the number of Spirochaetes and Bacteroidetes and a decrease in the number of Firmicutes and Fusobacteria in the oral cavity of patients with IBD(Docktor et al., 2012); Said et al. found a significant increase in Prevotella spp. in the salivary microbiota of patients with IBD by bacterial 16S rRNA sequencing, while Streptococcus spp., which are most abundant in saliva in healthy populations, were significantly reduced(Said et al., 2013). Another study found that IBD causes a disruption of the thick-walled bacterial gate in the patient’s oral cavity, the extent of which is closely related to the severity of the disease, and that this ecological dysregulation subsides over time after successful treatment of IBD (Elmaghrawy et al., 2022). These bacteria enriched in the oral cavity of IBD patients are in turn closely associated with periodontitis, and all of the above studies suggest that IBD causes the development of periodontitis by affecting oral ecological dysregulation.
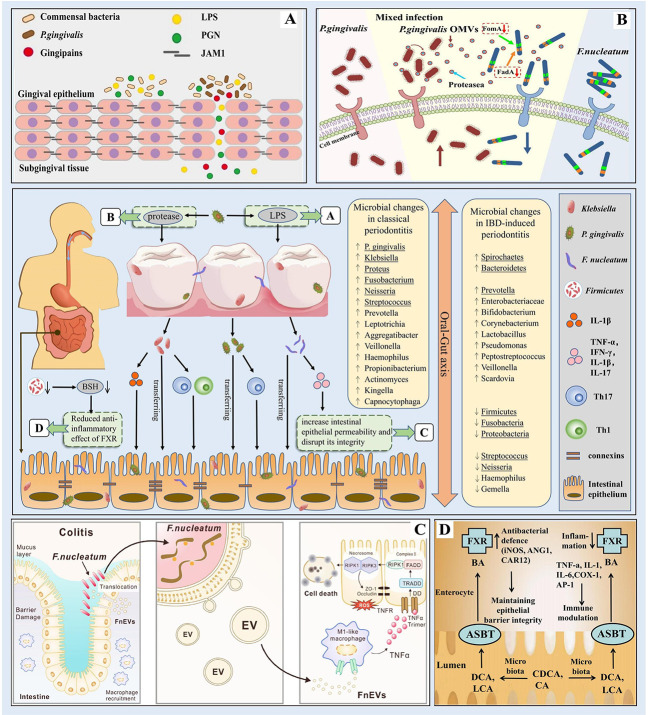
Periodontitis-associated pathogenic bacteria can migrate to the intestine and interfere with intestinal barrier function, thus causing intestinal ecological dysregulation and chronic inflammation, while IBD can cause changes in oral microbial composition and lead to the proliferation of periodontitis-associated pathogenic bacteria, suggesting that oral flora disorders are closely related to intestinal flora abnormalities ( Figure 2 ). This bidirectional association between the oral cavity and the intestine, i.e., the existence of an Oral-Gut axis, is of great significance and also suggests a possible common immunomodulatory mechanism between periodontitis and IBD.
Figure 2.
Microbial correlation between periodontitis and IBD (The bacteria underlined in the diagram are the ones mentioned in our paper). In periodontitis, the oral flora is altered and P. gingivalis evades host immune defense to destroy periodontal tissue by releasing virulence factors such as proteases and lipopolysaccharides; alterations in the composition of the intestinal microbiota leading to changes in bacterial metabolites such as BSH may play an important role in the pathogenesis of IBD; through the oral-gut axis, periodontal pathogenic bacteria such as Klebsiella, P. gingivalis, and F nucleatum can ectopically colonize the intestine and disrupt the intestinal barrier thus leading to intestinal ecological dysregulation and chronic inflammation. (A) P. gingivalis produces virulence factors such as LPS. Source (Takeuchi et al., 2019). (B) P. gingivalis produces virulence factors such as proteases. Source (Zhang et al., 2021d). (C) F nucleatum destroys the intestinal mucosa. Source (Liu et al., 2021b). (D) The role of FXR in IBD. Source (Ding et al., 2015).
2.3. Immune mechanism associations of periodontitis and IBD
Although pathogenic microorganisms can directly destroy host periodontal tissues by releasing virulence factors and metabolites, the host immune response plays an equally important role in the progression of periodontitis (Bosshardt, 2017; Bunte and Beikler, 2019; Pan et al., 2019). The host immune response to microorganisms can be divided into intrinsic immune responses and adaptive immune responses. The intrinsic immune system is the first line of defense against pathogen invasion and consists of different cells (neutrophils, macrophages, etc.) and factors (e.g. complement), among which neutrophils continuously recruit, migrate and infiltrate at the site of inflammation and secrete pro-inflammatory factors such as tumor necrosis factor (TNF)-α, IL-8 etc., but their phagocytic function is significantly reduced (Carneiro et al., 2012; Hajishengallis, 2019; Vitkov et al., 2021); macrophages tend to differentiate towards M1 (pro-inflammatory) in periodontitis and increase the secretion of pro-inflammatory mediators (e.g. interferon-γ, IFN-γ) as well as osteoclast activity (Almubarak et al., 2020; Sun et al., 2021; Yang et al., 2021). Moreover, T cell-mediated adaptive immune responses are crucial in the development of periodontitis, and upon activation through the T cell receptor (TCR), naive CD4+ T cells can differentiate into Th1, Th17, and Treg cells and participate in different types of immune responses (Zhu and Paul, 2010; Campbell et al., 2015; Gonzales, 2015). Philip et al. found that in the P. gingivalis infection-induced periodontitis model, Th1 cell responses were significantly increased and promoted the secretion of the pro-inflammatory factors IFN-γ, IL-1α and IL-1β; activated Th1 cells also highly express receptor activator for nuclear factor-κB ligand (RANKL) and induce osteoclastogenesis, thus causing a widespread inflammatory response and alveolar bone destruction in periodontal tissue (Stashenko et al., 2007). Unlike Th1 cells, Th17 cells mainly secrete the pro-inflammatory factors IL-6, IL-17 and IL-23 to regulate the development of periodontitis, among which IL-17 can promote periodontal connective tissue and alveolar bone destruction by regulating the expression levels of prostaglandin E2 (PGE2), matrix metalloproteinases (MMPs) and RANKL (Cheng et al., 2014; Dutzan and Abusleme, 2019; Huang et al., 2021; Kini et al., 2022). In contrast, Treg cells play a role in reducing the inflammatory response mainly through the secretion of anti-inflammatory factors IL-10, IL-35 and transforming growth factor β (TGF-β) (Alvarez et al., 2018; Zhang et al., 2021b). Zheng et al. found a Th17/Treg imbalance in patients with chronic periodontitis, in which the Th17 ratio was upregulated and the Treg ratio was downregulated, and the expression level of the pro-inflammatory factor IL-17 was significantly increased while the expression of the anti-inflammatory factor IL-10 was decreased, and this imbalance ultimately led to the resorption of alveolar bone (Zheng et al., 2019). Furthermore, recent studies have found that Tregs (CD25/Foxp3 double-positive cells) may lose Foxp3 expression and convert to exFoxP3 Th17 cells in periodontitis, and decreased Tregs activity reduces the synthesis of TGF-β and IL-10, while these exFoxp3 Th17 cells express high levels of RANKL and IL-17; in contrast, when antibiotics were used to reduce the conversion of Treg cells, the Th17/Treg cell imbalance in periodontitis in mice was significantly inhibited and RANKL expression was suppressed, resulting in improved bone resorption (Deng et al., 2021).
In both the innate and adaptive immune responses, macrophages, neutrophils and T cells are important target cells for bacterial invasion of the host, and pathogen molecules can be recognized by surface receptors (e.g., toll-like receptors, TLR) of these cells, initiating intracellular signaling pathways that lead to the release of various inflammatory and chemotactic factors (Kawai and Akira, 2007; Oberg et al., 2011; Fitzgerald and Kagan, 2020), such as macrophage colony-stimulating factor (M-CSF) and RANKL, both of which are key factors in the regulation of osteoclast activity (Cekici et al., 2013). For example, P. gingivalis can activate TLR2 and TLR4 in a variety of cell types, and TLR2 activation can upregulate the expression of M-CSF, thus promoting osteoclast activation (Karlis et al., 2020); meanwhile, bacterial LPS can induce IL-1β production through the TLR pathway to upregulate RANKL, and binding of RANKL to its receptor RANK triggers nuclear factor κB (NF-ĸB) signaling pathway, which induces bone resorption (Cekici et al., 2013; Thummuri et al., 2015; Tsukasaki, 2020). NF-ĸB is a specific transcription factor that plays a major role in the inflammatory response. It is a key transcription factor for intrinsic immune cells (e.g., macrophages) and also plays a role in adaptive immunity by regulating TCR signaling to promote differentiation of Th1 and Th17 cells, and its activation induces the production of pro-inflammatory cytokines such as IL-1, IL-6, IL-12, IL-17 and TNF-α (Liu et al., 2017; Venugopal et al., 2018; Mooney and Sahingur, 2020). In addition, myeloid differentiation factor 88 (MyD88), a key junction molecule in the TLR signaling pathway, binds to the activated TLR/IL-1R (TIR) structural domain, which in turn transmits signals and releases a series of pro-inflammatory factors (Kim et al., 2019; Chen et al., 2020); on this basis, Madeira et al. showed that bacterial LPS can activate the TLR4 pathway through MyD88 signaling to stimulate RANKL expression and induce osteoclast activation; while MyD88 knockout mice reduced alveolar bone resorption by downregulating TNF-α levels (TNF-α increases the expression of M-CSF and RANKL) levels (Madeira et al., 2013). George et al. found that P. gingivalis also activates complement C5a receptor-1 (C5aR-1) and TLR2 in neutrophils and disarms the host protective TLR2-MyD88 pathway via proteasomal degradation of MyD88, while the close association between TLR2 and C5aR activates the phosphatidylinositol 3 kinase (TLR2-Mal-PI3K) pathway, which further prevents phagocytosis by neutrophils, facilitates the invasion of other susceptible bacteria, and stimulates the production of inflammatory cytokines, thereby promoting an inflammatory response (Maekawa et al., 2014). Besides, MMP can also be involved in the process of tissue destruction in periodontitis by degrading the extracellular matrix. Luchian et al. showed that MMP-8 and MMP-9 are the main metalloproteinases participating in periodontal tissue destruction; MMP-8 activation promotes neutrophil migration into the gingival sulcus, leading to extensive destruction of periodontal tissue; while MMP-9 regulates IL-1, IL-6 and IL-8, engaging in the breakdown of proteins in connective tissue and playing a key role in osteoclast-induced bone resorption (Luchian et al., 2022). The high expression of MMP-8 and MMP-9 in chronic periodontitis accurately reflects the patient’s condition and therefore can be used as a biomarker for the diagnosis of periodontitis.
The pathogenesis of IBD is similar to that of periodontitis and is also regulated by a combination of intrinsic and adaptive immunity. Neutrophils and macrophages play an important role in the intrinsic immune response in IBD. Neutrophil activation is followed by the disruption of the intestinal epithelial barrier through the production of high levels of reactive oxygen species (ROS) and the release of proteases, pro-inflammatory cytokines and chemokines, such as IL-8, TNF-α and leukotriene B4 (LTB4)(Wéra et al., 2016; Zhou and Liu, 2017; Kvedaraite, 2021); the number of macrophages in the intestinal mucosa in IBD patients is increased, and the intestinal epithelium damage is aggravated by producing a large number of pro-inflammatory and chemokines, such as TNF-α, IL-1β, IL-12 and IL-23, and enhancing the Th1 and Th17 cell responses (Bain and Schridde, 2018; Na et al., 2019; Pan et al., 2022). CD4+ T cell-mediated adaptive immunity also plays a crucial role in the development of IBD, and Neurath et al. found that CD is characterized by Th1 immune responses, with Th1 increasing the secretion of the pro-inflammatory factors IFN-γ and IL-2, while UC is a Th2-mediated disease that produces excessive amounts of IL-5 and IL-13 (Neurath et al., 2002). In addition to secreting inhibitory cytokines, Treg cells influence the progression of IBD through cytokine deprivation-induced apoptosis and IL-2. CD4+CD25+Foxp3+Treg cells express all three components of the high-affinity IL-2 receptor (IL-2R): CD25, CD122 and CD132, which may compete with responder Foxp3-T cells for IL-2, consume it and inhibit the proliferation of Foxp3-T cells by suppressing the induction of IL-2 mRNA (and mRNA for other effector cytokines) in responder Foxp3-T cells (Shevach, 2009). The constitutive expression of the high-affinity IL-2 receptor containing CD25 allows Treg cells to sustain cytokine uptake in their environment, and Pandiyan et al. showed that in the presence of the pro-apoptotic protein Bim, Treg cells in mouse models of IBD can induce apoptosis of effector CD4+ T cells. The apoptotic pathway triggered is typical of cytokine deprivation, being mediated by inactivation of the anti-apoptotic phosphatidylinositol-3-OH kinase–dependent Akt kinase–Bcl-2 antagonist of cell death pathway and being dependent on the death-promoting protein Bim (Pandiyan et al., 2007); furthermore, IL-2 is not only important for the regulation of Treg cell homeostasis in vivo, but also has a potent inhibitory function. Zhou et al. found that group-3 innate lymphoid cells (ILC3s) in the intestine are the main cellular source of IL-2, and that CD patients showed significant depletion of IL-2 in the small intestine and a significant decrease in Treg levels (Zhou et al., 2019). Clinical studies have demonstrated that low doses of IL-2 can mediate immunomodulation and specifically expand and activate the Treg cell population, thus serving as a potential treatment strategy for patients with IBD (Klatzmann and Abbas, 2015). In recent years, some studies have found that bile acid metabolites, intestinal microbes and TCR signaling intensity act synergistically to influence the balance of Th17/Treg cells, which in turn exacerbates the progression of IBD (Britton et al., 2019; Hang et al., 2019; Yan et al., 2020; Liu et al., 2020b). In particular, bile acids control Th17 cell function by regulating the activity of the characteristic transcription factor RORγt, while a decrease in the abundance of Firmicutes in the intestine of IBD patients leads to a decrease in secondary bile acid levels, which upregulate Th17 differentiation and induce an inflammatory response (Fitzpatrick and Jenabzadeh, 2020); Hemarajata et al. found that intestinal microbes and their bacterial products (e.g., Escherichia coli) can directly act on TLR and other innate immune receptors to mediate the differentiation of Th17 cells and suppress Treg cells, thereby exacerbating intestinal inflammation (Hemarajata and Versalovic, 2012).
Patients with IBD have damage to intestinal tight junction (TJ) barrier, which is manifested by increased permeability of the intestine. NF-κB is involved in regulating TJ barrier function and increasing permeability in animal models of colitis (Yu et al., 2018; Kaminsky et al., 2021; Li et al., 2022a), and studies have found that NF-κB activity is increased in patients with IBD, and inhibition of NF-κB signaling conduction improved the symptoms of colitis in mice (Lin et al., 2019). Additionally, MMP-9 inhibits cell adhesion and wound repair, which is elevated in intestinal tissues, serum and feces of IBD patients and is strongly correlated with disease activity and degree of inflammation (Meijer et al., 2007; Liu et al., 2013; de Bruyn and Ferrante, 2018), while MMP-9 deficiency attenuates intestinal inflammation in mice (Moore et al., 2011). Rana et al. demonstrated that MMP-9 can increase myosin light chain kinase (MLCK) gene and protein expression through NF-κB p65 activation, thereby inducing increased intestinal TJ permeability (Al-Sadi et al., 2021).
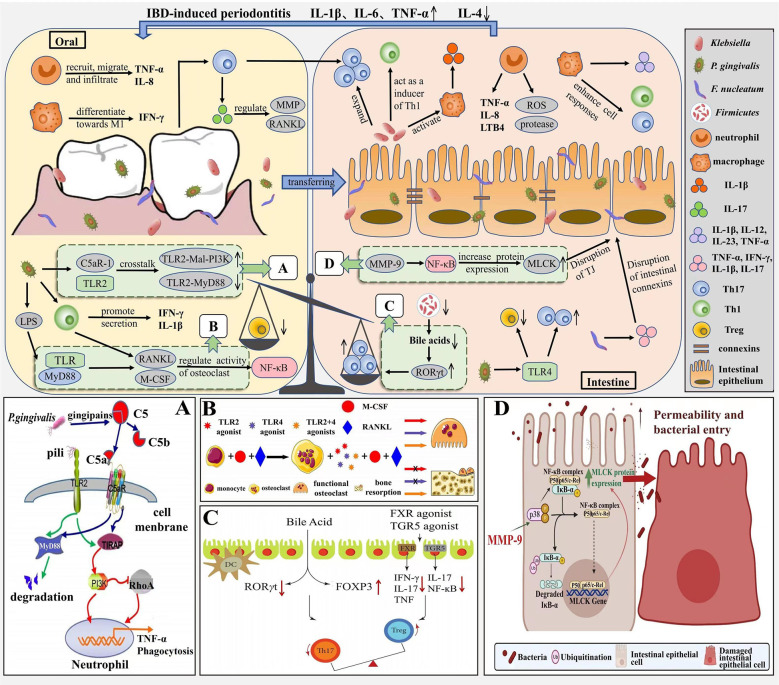
Periodontitis and IBD can be interrelated through immune mechanisms due to the presence of the oral-gut axis ( Figure 3 ). Especially, pro-inflammatory cytokines are associated with the pathogenesis of both periodontitis and IBD. IL-1β is known to influence the progression of periodontitis and IBD, and Kitamoto et al. found that Enterobacteriaceae isolated from the intestine were unable to induce IL-1β secretion, whereas Enterobacteriaceae in the oral cavity (e.g., Klebsiella) could induce intestinal inflammation by activating inflammatory vesicles in macrophages mediating IL-1 signaling (Kitamoto et al., 2020b). Liu et al. found that F. nucleatum, an important causative agent of periodontitis, increases intestinal epithelial permeability, disrupts its integrity by disrupting connexins, promotes the secretion of cytokines TNF-α, IFN-γ, IL-1β, IL-6 and IL-17 and reduces the secretion of anti-inflammatory cytokine IL-10, thereby increasing intestinal inflammation (Liu et al., 2020a). In addition, periodontitis leads to T cells, and pathogenic T cells can migrate from the oral cavity to the intestine, as periodontitis triggers the production of oral Th17 cells, which can migrate to the intestine and expand in response to ectopically colonized Klebsiella, thus promoting worsening intestinal inflammation (Kitamoto et al., 2020b); Koji’s study concluded that Klebsiella, after colonization of the intestine, induces Th1 cell responses by stimulating the innate immune receptor TLR4 and leads to a deficiency of the immunosuppressive factor IL-10, thereby promoting inflammatory responses in the intestine, thus demonstrating that Klebsiella, isolated from the oral microbiota, can act as a strong inducer of intestinal Th1 cells (Atarashi et al., 2017). The Th17/Treg ratio is closely related to the development of IBD, and Lu et al. found that P. gingivalis in periodontitis stimulates CD4+ T cell responses and increases the Th17/Treg ratio in the intestinal lamina propria, upregulates Th17-related transcription factor expression and production of the pro-inflammatory factors IL-17 and IL-6 through the TLR4 pathway, and downregulates the expression of the Treg transcription factor Foxp3 and production of the anti-inflammatory factors TGF-β and IL-10, thereby exacerbating intestinal inflammation (Jia et al., 2020). In oral and intestinal inflammation due to oral Candida infection, the Treg population has a multilayered protective role, one for IL-17A induction in CD4+ T cells and another for immunomodulation to prevent excessive inflammation. In a study by Bhaskaran et al, Foxp3 and IL-17A expression in CD4+ T cells was effectively induced by supplementation with SCFA, thereby increasing the frequency of Foxp3+ Tregs and thus improving the inflammatory response due to Candida infection (Bhaskaran et al., 2018).
Figure 3.
Immune mechanism correlation between periodontitis and IBD. In the pathogenesis of periodontitis, neutrophils are continuously recruited, migrate and infiltrate at the site of inflammation and secrete pro-inflammatory factors such as TNF-α and IL-8, macrophages tend to differentiate towards M1 and secrete IFN-γ. P. gingivalis induces IL-1β production through the TLR pathway, upregulates RANKL and M-CSF expression, and activates the NF-ĸB signaling pathway, thereby inducing bone resorption. The pathogenesis of IBD is similar to periodontitis, with neutrophils and macrophages inducing an inflammatory response through the release of virulence factors, pro-inflammatory mediators, and enhanced Th1 and Th17 cell responses; MMP-9 increases MLCK expression and induces an increase in intestinal TJ permeability through activation of NF-κB p65. Due to the oral-intestinal axis, periodontitis and IBD can be interrelated through immune mechanisms, with periodontal pathogenic bacteria ectopically colonizing the intestine, increasing intestinal Th17 and Th1 cell responses and promoting intestinal inflammation; while Th17/Treg imbalance not only disrupts the oral microbiota and exacerbates bone resorption, but also leads to intestinal ecological dysregulation. (A) Antibactericidal mechanisms of C5a-TLR2 cross-talk induced by P. gingivalis. Source (Jia et al., 2019a). (B) M-CSF and RANKL induce bone resorption. Source (Karlis et al., 2020). (C) The role of bile acid and FXR in Th17/Treg balance. Source (Yan et al., 2020). (D) MMP-9 causes increased intestinal permeability. Source (Al-Sadi et al., 2021).
Meanwhile, the immune response associated with IBD may also contribute to oral inflammation. Dysbiosis of the gut flora not only damages the intestinal barrier, but also disrupts the oral microbiota and exacerbates bone resorption in periodontitis through Th17/Treg imbalance. Yuan et al. found that long-term antibiotic use led to gut ecological dysbiosis, which increased periodontitis-associated pathogens in the oral cavity and decreased oral microbiota probiotics associated with periodontal health, while Th17 cell-associated pro-inflammatory cytokine (IL-17A, IL-6) expression was upregulated and Treg cell-associated cytokine (Foxp3 and IL-10) expression was decreased in periodontal tissues; in contrast, the use of fecal microbiota transplantation (FMT) not only restored the intestinal microbiota of the mice, but even reversed the Th17/Treg imbalance in periodontal tissue and alleviated periodontitis(Yuan et al., 2023). Katarzyna et al. found elevated levels of IL-1β, IL-6 and TNF by measuring salivary inflammatory markers in patients with IBD, with elevated levels of TNF-α and IL-6 being strongly associated with the development of periodontitis (Szczeklik et al., 2012); in the Figueredo team’s study, inflammation scores in gingival tissue were significantly higher in patients with active IBD (including four cytokines, IL-1β, IL-6, IL-21 and sCD40L) (Figueredo et al., 2017); nevertheless, anti-inflammatory factors such as IL-4 decrease with increasing levels of inflammation, and IL-4 levels were found to be significantly lower in the gingival sulcus of IBD patients with periodontitis (de Mello-Neto et al., 2021). This evidence suggests that IBD may be closely associated with the development of periodontitis by decreasing the immune defenses of periodontal tissues.
3. Summary and prospect
At present, there is a growing body of research suggesting a bidirectional association between periodontitis and IBD, with microbial and immune factors combining to influence the development of both diseases ( Table 1 ). Bacteria invade host tissues and cause ecological dysbiosis that damages host tissues and induces host immune responses, leading to inflammation. Periodontitis-associated pathogenic bacteria can ectopically colonize the intestinal tract, thereby exacerbating intestinal inflammation, as evidenced by a higher prevalence of IBD in patients with periodontitis, while the inflammatory response to IBD can alter the composition of the oral microbiota, as evidenced by a higher prevalence and severity of periodontitis in patients with IBD. Among the microbial factors, we highlight the role of Klebsiella, P. gingivalis, F. nucleatum and Candida through the oral-gut axis in both diseases and the modulation of inflammation by microbial metabolites such as short-chain fatty acids; while among the immune mechanisms, the role of cytokines and the Th17/Treg balance contribute to the bidirectional effect between periodontitis and IBD.
Table 1.
Key factors in the oral-intestinal axis (both microbiological and immunological).
| periodontitis | Microbiological factors |
P.gingivalis
F.nucleatum Tannerella forsythia Treponema denticola metabolites (SCFAs) |
| Immune factors | neutrophils macrophages Th17/Treg imbalance TNF-α, IFN-γ, TGF-β IL-1, IL-6, IL-8, IL-12, IL-17, IL-23 PGE2 MMPs M-CSF, RANKL NF-κB |
|
| IBD | Microbiological factors |
Firmicutes
Bacteroidetes Proteobacteria Actinobacteria Faecalibacterium prausnitzii metabolites(BSH, butyrate, TMAO) |
| Immune factors | Neutrophils Macrophages Th17/Treg imbalance TNF-α LTB4 IL-1β, IL-5, IL-8, IL-12, IL-23, IL-13 NF-κB MMP-9 |
|
| Acting simultaneously in both diseases via the oral-gut axis | Microbiological factors |
Klebsiella
Proteus Candida P. gingivalis F. nucleatum metabolites(Ile,TMAO) |
| Immune factors | Th17/Treg imbalance TNF-α IFN-γ IL-1β, IL-6, IL-17 |
In this article, we mentioned that previous research with probiotic supplementation, dietary control and exosomes can treat one disease while benefiting the control of the other. In addition to this, recent studies have found that related drugs exert important anti-inflammatory effects in both the intestinal and oral cavity via the oral-gut axis. In addition to this, recent studies have found that related drugs exert important anti-inflammatory effects in both the intestinal and oral cavity via the oral-gut axis. Berberine has been shown to be widely used in the treatment of gastrointestinal disorders caused by microbial infections. Jia et al. demonstrated that berberine increased butyrate production in the intestinal microbiota, effectively restored the intestinal barrier and significantly inhibited IL-17-related systemic and local immune responses in ovariectomized (OVX)-periodontitis rats, resulting in improved periodontal bone resorption (Jia et al., 2019b); moreover, quercetin supplementation was effective in restoring intestinal microbiota, enhancing butyrate production in the gut and significantly improving intestinal ecological dysbiosis in mice treated with antibiotics (Shi et al., 2020), and Mooney et al. found that oral quercetin helped restore periodontal tissue homeostasis and improved periodontitis by modulating the inflammatory response and oral microbial composition (Mooney et al., 2021). These studies open up new ideas for the future treatment of periodontitis, that is, to prevent and control local inflammation in the oral cavity by modulating the intestinal flora or immune response while improving intestinal inflammation or systemic inflammation with medication.
While numerous studies have demonstrated a close link and bidirectional effects between periodontitis and IBD, there are still some limitations. Periodontitis is an inflammatory disease caused by dental plaque and is usually closely related to oral hygiene, patients with chronic periodontitis are usually accompanied by poor oral hygiene, however, studies have pointed to an inverse correlation between poor oral health and IBD. People with IBD brush their teeth, floss and breath fresheners more frequently, and those with CD have less plaque. excessive oral hygiene may lead to dysbiosis in bacterial colonization, dysregulated innate immune response, and promote inflammatory processes; conversely, poor oral health may help induce immune tolerance and suppress overreactive inflammation, thereby reducing the risk of immune-mediated diseases such as IBD (Yin et al., 2017). In addition, there are some confounding factors such as gender, age and genetics for the study of the association between periodontitis and IBD. Chemokines act in the recruitment of neutrophils and T lymphocytes to the epithelium, which may trigger or promote inflammatory responses, and one study showed enhanced production of chemokines CXCL-8, CXCL-9 and CXCL-10 in oral buccal epithelial cells only in children with CD compared to healthy controls and adults with CD (Damen et al., 2006). Periodontitis and IBD are both chronic inflammatory conditions based on a complex interaction of genetic, environmental, microbial and immune factors, and both share similar mechanisms in terms of bacterial action and specific host responses, but there are also different factors. Smoking, for example, is considered a risk factor for both periodontitis and CD. However, its role in UC is unclear, and some studies have even reported a protective effect of smoking attributed to this condition (Indriolo et al., 2011).
In general, the relationship between periodontitis and IBD is a two-way influence and even forms a vicious circle. In this paper, we elaborate the mechanism of the intrinsic connection between the two diseases through both microbiological and immunological mechanisms, which provides us with directions for the future treatment of periodontitis. In terms of microbiology, we can evaluate the oral and intestinal microenvironment to find the common pathogens or microbial metabolites of both diseases, which can be used as biomarkers for diagnosis and even prevention of both diseases, and through drug treatment, while regulating the intestinal flora, via the oral-gut axis can also play a key role in the treatment of periodontitis; additionally, regulating Th17/Treg balance or inhibiting the related inflammatory signaling pathways through immune means can be a common therapeutic target for both diseases. All these deserve further thought and research.
Author contributions
TZ and WX wrote the manuscript; QW, CJ, and YC sorted literatures; HL, YS and LA revised the review. All authors contributed to the article and approved the submitted version.
Funding Statement
This work was supported by National Natural Science Foundation of China (82201102), General program of Natural Science of Jilin Province (YDZJ202201ZYTS017), Science and Technology Project of Jilin Province Financial Department (JCSZ2021893-21), Science and Technology Project of Jilin Province (20220201121GX), Science and Technology Project of Jilin Province (20200403093SF), Jilin Provincial Development and Reform Commission Project (2021C043-4), Education Science and Technology Research Project of Jilin Province Department (JJKH20221098KJ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abrol N., Compton S. M., Graf D., Parashar P., Heo G., Gibson M. P. (2022). Inflammatory bowel disease and periodontitis: A retrospective chart analysis. Clin. Exp. Dent. Res. 8, 1028–1034. doi: 10.1002/cre2.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal M., Allin K. H., Petralia F., Colombel J.-F., Jess T. (2022). Multiomics to elucidate inflammatory bowel disease risk factors and pathways. Nat. Rev. Gastroenterol. Hepatol. 19, 399–409. doi: 10.1038/s41575-022-00593-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal M., Jess T. (2022). Implications of the changing epidemiology of inflammatory bowel disease in a changing world. United Eur. Gastroenterol. J. 10, 1113–1120. doi: 10.1002/ueg2.12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatab S., Sepanlou S. G., Ikuta K., Vahedi H., Bisignano C., Safiri S., et al. (2020). The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories 1990–2017: a systematic analysis for the global burden of disease study 2017. Nat. Rev. Gastroenterol. Hepatol. 5, 17–30. doi: 10.1016/s2468-1253(19)30333-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alawaji Y. N., Alshammari A., Mostafa N., Carvalho R. M., Aleksejuniene J. (2022). Periodontal disease prevalence, extent, and risk associations in untreated individuals. Clin. Exp. Dental Res. 8, 380–394. doi: 10.1002/cre2.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almubarak A., Tanagala K. K. K., Papapanou P. N., Lalla E., Momen-Heravi F. (2020). Disruption of monocyte and macrophage homeostasis in periodontitis. Front. Immunol. 11. doi: 10.3389/fimmu.2020.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi R., Engers J., Haque M., King S., Al-Omari D., Ma T. Y. (2021). Matrix metalloproteinase-9 (MMP-9) induced disruption of intestinal epithelial tight junction barrier is mediated by NF-κB activation. PloS One 16, e0249544. doi: 10.1371/journal.pone.0249544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez C., Rojas C., Rojas L., Cafferata E. A., Monasterio G., Vernal R. (2018). Regulatory T lymphocytes in periodontitis: A translational view. Mediators Inflammation 2018, 1–10. doi: 10.1155/2018/7806912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso C., Perillo F., Strati F., Fantini M., Caprioli F., Facciotti F. (2020). The role of gut microbiota biomodulators on mucosal immunity and intestinal inflammation. Cells 9, 1234. doi: 10.3390/cells9051234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthakrishnan A. N., Bernstein C. N., Iliopoulos D., Macpherson A., Neurath M. F., Ali R. A. R., et al. (2017). Environmental triggers in IBD: a review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 15, 39–49. doi: 10.1038/nrgastro.2017.136 [DOI] [PubMed] [Google Scholar]
- Asikainen S., Alaluusua S. (1993). Bacteriology of dental infections. Eur. Heart J. 14 (Suppl K), 43–50. doi: 10.1002/ccd.1810300422 [DOI] [PubMed] [Google Scholar]
- Atarashi K., Suda W., Luo C., Kawaguchi T., Motoo I., Narushima S., et al. (2017). Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 358, 359–365. doi: 10.1126/science.aan4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baima G., Muwalla M., Testa G., Mazza F., Bebars A., Perotto S., et al. (2022). Periodontitis prevalence and severity in inflammatory bowel disease: A case–control study. J. Periodontol. 1–10. doi: 10.1002/jper.22-0322 [DOI] [PubMed] [Google Scholar]
- Bain C. C., Schridde A. (2018). Origin, differentiation, and function of intestinal macrophages. Front. Immunol. 9. doi: 10.3389/fimmu.2018.02733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartold P. M., Lopez-Oliva I. (2020). Periodontitis and rheumatoid arthritis: An update 2012-2017. Periodontol. 2000 83, 189–212. doi: 10.1111/prd.12300 [DOI] [PubMed] [Google Scholar]
- Beck J. D., Elter J. R., Heiss G., Couper D., Mauriello S. M., Offenbacher S. (2001). Relationship of periodontal disease to carotid artery intima-media wall thickness. Arterioscler. Thromb. Vasc. Biol. 21, 1816–1822. doi: 10.1161/hq1101.097803 [DOI] [PubMed] [Google Scholar]
- Bhaskaran N., Quigley C., Paw C., Butala S., Schneider E., Pandiyan P. (2018). Role of short chain fatty acids in controlling tregs and immunopathology during mucosal infection. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.01995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshardt D. D. (2017). The periodontal pocket: pathogenesis, histopathology and consequences. Periodontol. 2000 76, 43–50. doi: 10.1111/prd.12153 [DOI] [PubMed] [Google Scholar]
- Bousbaine D., Fisch L. I., London M., Bhagchandani P., Rezende de Castro T. B., Mimee M., et al. (2022). A conserved bacteroidetes antigen induces anti-inflammatory intestinal T lymphocytes. Science 377, 660–666. doi: 10.1126/science.abg5645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton G. J., Contijoch E. J., Mogno I., Vennaro O. H., Llewellyn S. R., Ng R., et al. (2019). Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity 50, 212–224.e4. doi: 10.1016/j.immuni.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunte K., Beikler T. (2019). Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int. J. Mol. Sci. 20, 3394. doi: 10.3390/ijms20143394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd K. M., Gulati A. S. (2021). The “Gum–gut” axis in inflammatory bowel diseases: A hypothesis-driven review of associations and advances. Front. Immunol. 12. doi: 10.3389/fimmu.2021.620124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z., Zhu T., Liu F., Zhuang Z., Zhao L. (2021). Co-Pathogens in periodontitis and inflammatory bowel disease. Front. Med. 8. doi: 10.3389/fmed.2021.723719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L., Millhouse E., Malcolm J., Culshaw S. (2015). T Cells, teeth and tissue destruction - what do T cells do in periodontal disease? Mol. Oral. Microbiol. 31, 445–456. doi: 10.1111/omi.12144 [DOI] [PubMed] [Google Scholar]
- Canakci V., Canakci C. F., Canakci H., Canakci E., Cicek Y., Ingec M., et al. (2004). Periodontal disease as a risk factor for preeclampsia: A case control study. Aust. N Z J. Obstet. Gynaecol. 44 (6), 568–573. doi: 10.1111/j.1479-828X.2004.00323.x [DOI] [PubMed] [Google Scholar]
- Carneiro V. M. A., Bezerra A. C. B., Guimarães M., do C. M., Muniz-Junqueira M. I. (2012). Decreased phagocytic function in neutrophils and monocytes from peripheral blood in periodontal disease. J. Appl. Oral. Sci. 20, 503–509. doi: 10.1590/s1678-77572012000500002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekici A., Kantarci A., Hasturk H., Van Dyke T. E. (2013). Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000 64, 57–80. doi: 10.1111/prd.12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple I. L. C., Genco R., workshop, working (2013). Diabetes and periodontal diseases: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J. Clin. Periodontol. 40, S106–S112. doi: 10.1111/jcpe.12077 [DOI] [PubMed] [Google Scholar]
- Chen L., Zheng L., Chen P., Liang G. (2020). Myeloid differentiation primary response protein 88 (MyD88): The central hub of TLR/IL-1R signaling. J. Med. Chem. 63, 13316–13329. doi: 10.1021/acs.jmedchem.0c00884 [DOI] [PubMed] [Google Scholar]
- Cheng W.-C., Hughes F. J., Taams L. S. (2014). The presence, function and regulation of IL-17 and Th17 cells in periodontitis. J. Clin. Periodontol. 41, 541–549. doi: 10.1111/jcpe.12238 [DOI] [PubMed] [Google Scholar]
- Chi Y.-C., Chen J.-L., Wang L.-H., Chang K., Wu C.-L., Lin S.-Y., et al. (2018). Increased risk of periodontitis among patients with crohn’s disease: a population-based matched-cohort study. Int. J. Colorectal Dis. 33, 1437–1444. doi: 10.1007/s00384-018-3117-4 [DOI] [PubMed] [Google Scholar]
- Curtis M. A., Diaz P. I., Van Dyke T. E. (2020). The role of the microbiota in periodontal disease. Periodontology 2000. 83(1), 14–25. doi: 10.1111/prd.12296 [DOI] [PubMed] [Google Scholar]
- Damen G. M., Hol J., de Ruiter L., Bouquet J., Sinaasappel M., van der Woude J., et al. (2006). Chemokine production by buccal epithelium as a distinctive feature of pediatric crohn disease. J. Pediatr. Gastroenterol. Nutr. 42, 142–149. doi: 10.1097/01.mpg.0000189336.70021.8a [DOI] [PubMed] [Google Scholar]
- Darveau R. P. (2010). Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 8, 481–490. doi: 10.1038/nrmicro2337 [DOI] [PubMed] [Google Scholar]
- Dawson D. R. 3rd, Branch-Mays G., Gonzalez O. A., Ebersole J. L. (2014). Dietary modulation of the inflammatory cascade. Periodontology 2000 64 (1), 161–197. 10.1111/j.1600-0757.2012.00458.x. [DOI] [PubMed] [Google Scholar]
- de Bruyn M., Ferrante M. (2018). Failure of MMP-9 antagonists in IBD: Demonstrating the importance of molecular biology and well-controlled early phase studies. J. Crohns Colitis 12, 1011–1013. doi: 10.1093/ecco-jcc/jjy102 [DOI] [PubMed] [Google Scholar]
- de Mello-Neto J. M., Nunes J. G. R., Tadakamadla S. K., da Silva Figueredo C. M. (2021). Immunological traits of patients with coexistent inflammatory bowel disease and periodontal disease: A systematic review. Int. J. Environ. Res. Public Health 18 (17), 8958. doi: 10.3390/ijerph18178958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Lu C., Zhao Q., Chen K., Ma S., Li Z. (2021). The Th17/Treg cell balance: crosstalk among the immune system, bone and microbes in periodontitis. J. Periodontal Res. 57 (2), 246–255. doi: 10.1111/jre.12958 [DOI] [PubMed] [Google Scholar]
- Dewhirst F. E., Chen T., Izard J., Paster B. J., Tanner A. C. R., Yu W.-H., et al. (2010). The human oral microbiome. J. Bacteriol. 192, 5002–5017. doi: 10.1128/jb.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Yang L., Wang Z., Huang W. (2015). Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 5, 135–144. doi: 10.1016/j.apsb.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docktor M. J., Paster B. J., Abramowicz S., Ingram J., Wang Y. E., Correll M., et al. (2012). Alterations in diversity of the oral microbiome in pediatric inflammatory bowel disease. Inflammation Bowel Dis. 18, 935–942. doi: 10.1002/ibd.21874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domokos Z., Uhrin E., Szabó B., Czumbel M. L., Dembrovszky F., Kerémi B., et al. (2022). Patients with inflammatory bowel disease have a higher chance of developing periodontitis: A systematic review and meta-analysis. Front. Med. 9. doi: 10.3389/fmed.2022.1020126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeney T. P. (1969). Crohn’s disease of the mouth. Proc. R Soc. Med. 62 (12), 1237. doi: 10.1177/003591576906201218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N., Abusleme L. (2019). T Helper 17 cells as pathogenic drivers of periodontitis. Adv. Exp. Med. Biol. 1197, 107–117. doi: 10.1007/978-3-030-28524-1_9 [DOI] [PubMed] [Google Scholar]
- Eckburg P. B., Bik E. M., Bernstein C. N., Purdom E., Dethlefsen L., Sargent M., et al. (2005). Diversity of the human intestinal microbial flora. Science 308, 1635–1638. doi: 10.1126/science.1110591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmaghrawy K., Fleming P., Fitzgerald K., Cooper S., Dominik A., Hussey S., et al. (2022). The oral microbiome in treatment naïve paediatric IBD patients exhibits dysbiosis related to disease severity that resolves following therapy. J. Crohns Colitis jjac155. 1–12. doi: 10.1093/ecco-jcc/jjac155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel L. D., Pasquinelli K. L., Leone S. A., Moncla B. J., Nielson K. D., Rabinovitch P. S. (1988). Abnormal lymphocyte profiles and leukotriene B4 status in a patient with crohn’s disease and severe periodontitis. J. Periodontol. 59, 841–847. doi: 10.1902/jop.1988.59.12.841 [DOI] [PubMed] [Google Scholar]
- Figueredo C. M., Martins A. P., Lira-Junior R., Menegat J. B., Carvalho A. T., Fischer R. G., et al. (2017). Activity of inflammatory bowel disease influences the expression of cytokines in gingival tissue. Cytokine 95, 1–6. doi: 10.1016/j.cyto.2017.01.016 [DOI] [PubMed] [Google Scholar]
- Fitzgerald K. A., Kagan J. C. (2020). Toll-like receptors and the control of immunity. Cell 180, 1044–1066. doi: 10.1016/j.cell.2020.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick L. R., Jenabzadeh P. (2020). IBD and bile acid absorption: Focus on pre-clinical and clinical observations. Front. Physiol. 11. doi: 10.3389/fphys.2020.00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. N., St. Amand A. L., Feldman R. A., Boedeker E. C., Harpaz N., Pace N. R. (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. 104, 13780–13785. doi: 10.1073/pnas.0706625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frencken J. E., Sharma P., Stenhouse L., Green D., Laverty D., Dietrich T. (2017). Global epidemiology of dental caries and severe periodontitis - a comprehensive review. J. Clin. Periodontol. 44, S94–S105. doi: 10.1111/jcpe.12677 [DOI] [PubMed] [Google Scholar]
- Gadaleta R. M., van Erpecum K. J., Oldenburg B., Willemsen E. C. L., Renooij W., Murzilli S., et al. (2011). Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60, 463–472. doi: 10.1136/gut.2010.212159 [DOI] [PubMed] [Google Scholar]
- Gatej S. M., Marino V., Bright R., Fitzsimmons T. R., Gully N., Zilm P., et al. (2017). Probiotic lactobacillus rhamnosus GG prevents alveolar bone loss in a mouse model of experimental periodontitis. J. Clin. Periodontol. 45, 204–212. doi: 10.1111/jcpe.12838 [DOI] [PubMed] [Google Scholar]
- Genco R. J., Borgnakke W. S. (2020). Diabetes as a potential risk for periodontitis: association studies. Periodontol. 2000 83, 40–45. doi: 10.1111/prd.12270 [DOI] [PubMed] [Google Scholar]
- Genco R. J., Sanz M. (2020). Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontol. 2000 83, 7–13. doi: 10.1111/prd.12344 [DOI] [PubMed] [Google Scholar]
- Glassner K. L., Abraham B. P., Quigley E. M. M. (2020). The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 145, 16–27. doi: 10.1016/j.jaci.2019.11.003 [DOI] [PubMed] [Google Scholar]
- Gonzales J. R. (2015). T- and b-cell subsets in periodontitis. Periodontol. 2000 69, 181–200. doi: 10.1111/prd.12090 [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Jiang C., Patterson A. D. (2016). An intestinal microbiota–farnesoid X receptor axis modulates metabolic disease. Gastroenterology 151, 845–859. doi: 10.1053/j.gastro.2016.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Febles J., Sanz M. (2021). Periodontitis and rheumatoid arthritis: What have we learned about their connection and their treatment? Periodontol. 2000 87, 181–203. doi: 10.1111/prd.12385 [DOI] [PubMed] [Google Scholar]
- Guan Q. (2019). A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019, 7247238. doi: 10.1155/2019/7247238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X., Li W., Meng H. (2020). A double-edged sword: Role of butyrate in the oral cavity and the gut. Mol. Oral. Microbiol. 36 (2), 121–131. doi: 10.1111/omi.12322 [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. (2019). New developments in neutrophil biology and periodontitis. Periodontol 2000. 82, 78–92. doi: 10.1111/prd.12313 [DOI] [PubMed] [Google Scholar]
- Hajishengallis G., Lamont R. J. (2012). Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Microbiol. Oral. Immunol. 27, 409–419. doi: 10.1111/j.2041-1014.2012.00663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang S., Paik D., Yao L., Kim E., Trinath J., Lu J., et al. (2019). Bile acid metabolites control TH17 and treg cell differentiation. Nature 576, 143–148. doi: 10.1038/s41586-019-1785-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedin C. R. H., Vavricka S. R., Stagg A. J., Schoepfer A., Raine T., Puig L., et al. (2018). The pathogenesis of extraintestinal manifestations: Implications for IBD research, diagnosis, and therapy. J. Crohns Colitis 13, 541–554. doi: 10.1093/ecco-jcc/jjy191 [DOI] [PubMed] [Google Scholar]
- Hemarajata P., Versalovic J. (2012). Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv. Gastroenterol. 6, 39–51. doi: 10.1177/1756283x12459294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiengrach P., Panpetch W., Worasilchai N., Chindamporn A., Tumwasorn S., Jaroonwitchawan T., et al. (2020). Administration of candida albicans to dextran sulfate solution treated mice causes intestinal dysbiosis, emergence and dissemination of intestinal pseudomonas aeruginosa and lethal sepsis. Shock 53, 189–198. doi: 10.1097/shk.0000000000001339 [DOI] [PubMed] [Google Scholar]
- Ho J., Camilli G., Griffiths J. S., Richardson J. P., Kichik N., Naglik J. R. (2020). Candida albicans and candidalysin in inflammatory disorders and cancer. Immunology 162, 11–16. doi: 10.1111/imm.13255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.-W., Krueger P. D., Osum K. C., Dileepan T., Herman A., Mueller D. L., et al. (2022). Immune tolerance of food is mediated by layers of CD4+ T cell dysfunction. Nature 607, 762–768. doi: 10.1038/s41586-022-04916-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinkhani F., Heinken A., Thiele I., Lindenburg P. W., Harms A. C., Hankemeier T. (2021). The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes 13 (1), 1–22. doi: 10.1080/19490976.2021.1882927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N., Dong H., Luo Y., Shao B. (2021). Th17 cells in periodontitis and its regulation by A20. Front. Immunol. 12. doi: 10.3389/fimmu.2021.742925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikram S., Hassan N., Raffat M. A., Mirza S., Akram Z. (2018). Systematic review and meta-analysis of double-blind, placebo-controlled, randomized clinical trials using probiotics in chronic periodontitis. J. Invest. Clin. Dent. 9, e12338. doi: 10.1111/jicd.12338 [DOI] [PubMed] [Google Scholar]
- Imai J., Ichikawa H., Kitamoto S., Golob J. L., Kaneko M., Nagata J., et al. (2021). A potential pathogenic association between periodontal disease and crohn’s disease. JCI Insight 6, e148543. doi: 10.1172/jci.insight.148543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indriolo A., Greco S., Ravelli P., Fagiuoli S. (2011). What can we learn about biofilm/host interactions from the study of inflammatory bowel disease. J. Clin. Periodontol. 38, 36–43. doi: 10.1111/j.1600-051x.2010.01680.x [DOI] [PubMed] [Google Scholar]
- Isola G., Santonocito S., Distefano A., Polizzi A., Vaccaro M., Raciti G., et al. (2022). Impact of periodontitis on gingival crevicular fluid miRNAs profiles associated with cardiovascular disease risk. J. Periodontal Res. 58(1), 165–174. doi: 10.1111/jre.13078 [DOI] [PubMed] [Google Scholar]
- Jaeger N., Gamini R., Cella M., Schettini J. L., Bugatti M., Zhao S., et al. (2021). Single-cell analyses of crohn’s disease tissues reveal intestinal intraepithelial T cells heterogeneity and altered subset distributions. Nat. Commun. 12, 1921. doi: 10.1038/s41467-021-22164-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagelavičienė E., Vaitkevičienė I., Šilingaitė D., Šinkūnaitė E., Daugėlaitė G. (2018). The relationship between vitamin d and periodontal pathology. Med. (Kaunas). 54, 45. doi: 10.3390/medicina54030045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubovics N. S., Goodman S. D., Mashburn-Warren L., Stafford G. P., Cieplik F. (2021). The dental plaque biofilm matrix. Periodontol. 2000 86, 32–56. doi: 10.1111/prd.12361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L., Han N., Du J., Guo L., Luo Z., Liu Y. (2019. a). Pathogenesis of important virulence factors of porphyromonas gingivalis via toll-like receptors. Front. Cell Infect. Microbiol. 9. doi: 10.3389/fcimb.2019.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X., Jia L., Mo L., Yuan S., Zheng X., He J., et al. (2019. b). Berberine ameliorates periodontal bone loss by regulating gut microbiota. J. Dent. Res. 98, 107–116. doi: 10.1177/0022034518797275 [DOI] [PubMed] [Google Scholar]
- Jia L., Wu R., Han N., Fu J., Luo Z., Guo L., et al. (2020). Porphyromonas gingivalis and lactobacillus rhamnosus GG regulate the Th17/Treg balance in colitis via TLR4 and TLR2. Clin. Transl. Immunol. 9, e1213. doi: 10.1002/cti2.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose F. A., Garnett E. A., Vittinghoff E., Ferry G. D., Winter H. S., Baldassano R. N., et al. (2009). Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflammation Bowel Dis. 15, 63–68. doi: 10.1002/ibd.20604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju X., Harford J., Luzzi L., Jamieson L. M. (2022). Prevalence, extent, and severity of periodontitis among Australian older adults: Comparison of two generations. J. Periodontol. 93, 1387–1400. doi: 10.1002/jper.21-0458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer A. R., Craig R. G., Niederman R., Fortea J., de Leon M. J. (2020). Periodontal disease as a possible cause for alzheimer’s disease. Periodontol. 2000 83, 242–271. doi: 10.1111/prd.12327 [DOI] [PubMed] [Google Scholar]
- Kaminsky L. W., Al-Sadi R., Ma T. Y. (2021). IL-1β and the intestinal epithelial tight junction barrier. Front. Immunol. 12. doi: 10.3389/fimmu.2021.767456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E. A., Chun J., Kim J. H., Han K., Soh H., Park S., et al. (2020). Periodontitis combined with smoking increases risk of the ulcerative colitis: A national cohort study. World J. Gastroenterol. 26, 5661–5672. doi: 10.3748/wjg.v26.i37.5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapila Y. L. (2021). Oral health’s inextricable connection to systemic health: Special populations bring to bear multimodal relationships and factors connecting periodontal disease to systemic diseases and conditions. Periodontol. 2000 87, 11–16. doi: 10.1111/prd.12398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G. G., Windsor J. W. (2020). The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 18, 56–66. doi: 10.1038/s41575-020-00360-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlis G. D., Schöningh E., Jansen I. D. C., Schoenmaker T., Hogervorst J. M. A., van Veen H. A., et al. (2020). Chronic exposure of gingival fibroblasts to TLR2 or TLR4 agonist inhibits osteoclastogenesis but does not affect osteogenesis. Front. Immunol. 11. doi: 10.3389/fimmu.2020.01693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Akira S. (2007). TLR signaling. Semin. Immunol. 19, 24–32. doi: 10.1016/j.smim.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Khalili H., Chan S. S. M., Lochhead P., Ananthakrishnan A. N., Hart A. R., Chan A. T. (2018). The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 15, 525–535. doi: 10.1038/s41575-018-0022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-C., Lee S. E., Kim S. K., Jang H.-D., Hwang I., Jin S., et al. (2019). Toll-like receptor mediated inflammation requires FASN-dependent MYD88 palmitoylation. Nat. Chem. Biol. 15, 907–916. doi: 10.1038/s41589-019-0344-0 [DOI] [PubMed] [Google Scholar]
- Kinane D. F., Stathopoulou P. G., Papapanou P. N. (2017). Periodontal diseases. Nat. Rev. Dis. Primers 3, 17038. doi: 10.1038/nrdp.2017.38 [DOI] [PubMed] [Google Scholar]
- Kini V., Mohanty I., Telang G., Vyas N. (2022). Immunopathogenesis and distinct role of Th17 in periodontitis: A review. J. Oral. Biosci. 64, 193–201. doi: 10.1016/j.job.2022.04.005 [DOI] [PubMed] [Google Scholar]
- Kitamoto S., Nagao-Kitamoto H., Hein R., Schmidt T. M., Kamada N. (2020. a). The bacterial connection between the oral cavity and the gut diseases. J. Dent. Res. 99, 1021–1029. doi: 10.1177/0022034520924633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto S., Nagao-Kitamoto H., Jiao Y., Gillilland M. G., Hayashi A., Imai J., et al. (2020. b). The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell 182, 447–462.e14. doi: 10.1016/j.cell.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Abbas A. K. (2015). The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. nature reviews. Immunology 15, 283–294. doi: 10.1038/nri3823 [DOI] [PubMed] [Google Scholar]
- Kobayashi R., Kobayashi T., Sakai F., Hosoya T., Yamamoto M., Kurita-Ochiai T. (2017). Oral administration of lactobacillus gasseri SBT2055 is effective in preventing porphyromonas gingivalis-accelerated periodontal disease. Sci. Rep. 7 (1), 545. doi: 10.1038/s41598-017-00623-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvedaraite E. (2021). Neutrophil–T cell crosstalk in inflammatory bowel disease. Immunology 164, 657–664. doi: 10.1111/imm.13391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T., Lamster I. B., Levin L. (2020). Current concepts in the management of periodontitis. Int. Dent. J. 71, 462–476. doi: 10.1111/idj.12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont R. J., Jenkinson H. F. (2000). Subgingival colonization by Porphyromonas gingivalis . Oral. Microbiol. Immunol. 15, 341–349. doi: 10.1034/j.1399-302x.2000.150601.x [DOI] [PubMed] [Google Scholar]
- Lankarani K. B. (2013). Oral manifestation in inflammatory bowel disease: A review. World J. Gastroenterol. 19, 8571. doi: 10.3748/wjg.v19.i46.8571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larvin H. 0., Kang J., Aggarwal V. R., Pavitt S., Wu J. (2022). Periodontitis and risk of immune-mediated systemic conditions: A systematic review and meta-analysis. Comm Dent. Oral. Epid. 1–13. doi: 10.1111/cdoe.12812 [DOI] [PubMed] [Google Scholar]
- Lavelle A., Sokol H. (2020). Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17, 223–237. doi: 10.1038/s41575-019-0258-z [DOI] [PubMed] [Google Scholar]
- Lee M., Chang E. B. (2020). Inflammatory bowel diseases (IBD) (Inflammatory bowel diseases and the microbiome: Searching the crime scene for clues). Gastroenterology 160, 524–537. doi: 10.1053/j.gastro.2020.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-C., Liu C.-Y., Lee C.-L., Zhang R.-H., Huang C.-J., Yen T.-L. (2022). The periodontopathic pathogen, porphyromonas gingivalis, involves a gut inflammatory response and exacerbates inflammatory bowel disease. Pathogens 11, 84. doi: 10.3390/pathogens11010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. V., Leonardi I., Putzel G. G., Semon A., Fiers W. D., Kusakabe T., et al. (2022. a). Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature 603, 672–678. doi: 10.1038/s41586-022-04502-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li Q., Xiong B., Chen H., Wang X., Zhang D. (2022. b). Discoidin domain receptor 1(DDR1) promote intestinal barrier disruption in ulcerative colitis through tight junction proteins degradation and epithelium apoptosis. Pharmacol. Res. 183, 106368. doi: 10.1016/j.phrs.2022.106368 [DOI] [PubMed] [Google Scholar]
- Lin C.-Y., Tseng K.-S., Liu J.-M., Chuang H.-C., Lien C.-H., Chen Y.-C., et al. (2018). Increased risk of ulcerative colitis in patients with periodontal disease: A nationwide population-based cohort study. Int. J. Environ. Res. Public Health 15, 2602. doi: 10.3390/ijerph15112602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Wu J., Wang F., Tang F., Sun J., Xu B., et al. (2019). QingBai decoction regulates intestinal permeability of dextran sulphate sodium-induced colitis through the modulation of notch and NF-κB signalling. Cell Prolif. 52, e12547. doi: 10.1111/cpr.12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira-Junior R., Figueredo C. M. (2016). Periodontal and inflammatory bowel diseases: Is there evidence of complex pathogenic interactions? World J. Gastroenterol. 22, 7963. doi: 10.3748/wjg.v22.i35.7963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listgarten M. A. (1994). The structure of dental plaque. Periodontol. 2000 5, 52–65. doi: 10.1111/j.1600-0757.1994.tb00018.x [DOI] [PubMed] [Google Scholar]
- Liu H., Hong X. L., Sun T. T., Huang X. W., Wang J. L., Xiong H. (2020. a). Fusobacterium nucleatum exacerbates colitis by damaging epithelial barriers and inducing aberrant inflammation. J. Dig Dis. 21, 385–398. doi: 10.1111/1751-2980.12909 [DOI] [PubMed] [Google Scholar]
- Liu L., Liang L., Yang C., Zhou Y., Chen Y. (2021. b). Extracellular vesicles of fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microbes 13, 1–20. doi: 10.1080/19490976.2021.1902718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Patel N. R., Walter L., Ingersoll S., Sitaraman S. V., Garg P. (2013). Constitutive expression of MMP9 in intestinal epithelium worsens murine acute colitis and is associated with increased levels of proinflammatory cytokine kc. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G793–G803. doi: 10.1152/ajpgi.00249.2012 [DOI] [PubMed] [Google Scholar]
- Liu Y.-J., Tang B., Wang F.-C., Tang L., Lei Y.-Y., Luo Y., et al. (2020. b). Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 10, 5225–5241. doi: 10.7150/thno.43716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Xu Y., Cui Q., Liu N., Chu F., Cong B., et al. (2021. a). Effect of psoralen on the intestinal barrier and alveolar bone loss in rats with chronic periodontitis. Inflammation 44, 1843–1855. doi: 10.1007/s10753-021-01462-7 [DOI] [PubMed] [Google Scholar]
- Liu T., Zhang L., Joo D., Sun S.-C. (2017). NF-κB signaling in inflammation. Sig Transduct. Target Ther. 2, 17023. doi: 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LÖE H. (1993). Periodontal diseases: a brief historical perspective. Periodontol. 2000 2, 7–12. doi: 10.1111/j.1600-0757.1993.tb00215.x [DOI] [PubMed] [Google Scholar]
- Lourenço T. G. B., Heller D., Silva-Boghossian C. M., Cotton S. L., Paster B. J., Colombo A. P. V. (2014). Microbial signature profiles of periodontally healthy and diseased patients. J. Clin. Periodontol. 41, 1027–1036. doi: 10.1111/jcpe.12302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchian I., Goriuc A., Sandu D., Covasa M. (2022). The role of matrix metalloproteinases (MMP-8, MMP-9, MMP-13) in periodontal and peri-implant pathological processes. Int. J. Mol. Sci. 23, 1806. doi: 10.3390/ijms23031806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Luan H., Wu L., Shi Y., Wang Y., Huang Q., et al. (2021). Secular trends in severe periodontitis incidence, prevalence and disability-adjusted life years in five Asian countries: A comparative study from 1990 to 2017. J. Clin. Periodontol. 48, 627–637. doi: 10.1111/jcpe.13447 [DOI] [PubMed] [Google Scholar]
- Lynch S. V., Pedersen O. (2016). The human intestinal microbiome in health and disease. N Engl. J. Med. 375, 2369–2379. doi: 10.1056/nejmra1600266 [DOI] [PubMed] [Google Scholar]
- Machado V., Lobo S., Proença L., Mendes J. J., Botelho J. (2020). Vitamin d and periodontitis: A systematic review and meta-analysis. Nutrients 12, 2177. doi: 10.3390/nu12082177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira M. F. M., Queiroz-Junior C. M., Cisalpino D., Werneck S. M. C., Kikuchi H., Fujise O., et al. (2013). MyD88 is essential for alveolar bone loss induced byAggregatibacter actinomycetemcomitanslipopolysaccharide in mice. Mol. Oral. Microbiol. 28, 415–424. doi: 10.1111/omi.12034 [DOI] [PubMed] [Google Scholar]
- Madianos P. N., Papapanou P. N., Sandros J. (1997). Porphyromonas gingivalis infection of oral epithelium inhibits neutrophil transepithelial migration. Infect. Immun. 65, 3983–3990. doi: 10.1128/iai.65.10.3983-3990.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen G. R., Bertl K., Pandis N., Stavropoulos A., Burisch J. (2022). The impact of periodontitis on inflammatory bowel disease activity. Inflammation Bowel Dis. 1–9. doi: 10.1093/ibd/izac090 [DOI] [PubMed] [Google Scholar]
- Maekawa T., Krauss J. L., Abe T., Jotwani R., Triantafilou M., Triantafilou K., et al. (2014). Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 15, 768–778. doi: 10.1016/j.chom.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik T. A. (2015). Inflammatory bowel disease: Historical perspective, epidemiology, and risk factors. Surg. Clin. North Am. 95, 1105–110v. doi: 10.1016/j.suc.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Marotto D., Atzeni F., Ardizzone S., Monteleone G., Giorgi V., Sarzi-Puttini P. (2020). Extra-intestinal manifestations of inflammatory bowel diseases. Pharmacol. Res. 161, 105206. doi: 10.1016/j.phrs.2020.105206 [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Kanai T. (2014). The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 37, 47–55. doi: 10.1007/s00281-014-0454-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer M. J. W., Mieremet-Ooms M. A. C., van der Zon A. M., van Duijn W., van Hogezand R. A., Sier C. F. M., et al. (2007). Increased mucosal matrix metalloproteinase-1, -2, -3 and -9 activity in patients with inflammatory bowel disease and the relation with crohn’s disease phenotype. Dig Liver Dis. 39, 733–739. doi: 10.1016/j.dld.2007.05.010 [DOI] [PubMed] [Google Scholar]
- Meng H. (2007). The relationship between periodontitis and diabetes. J. Peking Univ. Health Sci. 39, 18–20. doi: 10.19723/j.issn.1671-167x.2007.01.033 [DOI] [PubMed] [Google Scholar]
- Miranda-Rius J., Brunet-Llobet L., Blanc V., Álvarez G., Moncunill-Mira J., Mashala E. I., et al. (2021). Microbial profile of placentas from Tanzanian mothers with adverse pregnancy outcomes and periodontitis. Oral. Dis. 29(2), 772–785. doi: 10.1111/odi.13962 [DOI] [PubMed] [Google Scholar]
- Möller B., Kollert F., Sculean A., Villiger P. M. (2020). Infectious triggers in periodontitis and the gut in rheumatoid arthritis (RA): A complex story about association and causality. Front. Immunol. 11. doi: 10.3389/fimmu.2020.01108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney E. C., Holden S. E., Xia X.-J., Li Y., Jiang M., Banson C. N., et al. (2021). Quercetin preserves oral cavity health by mitigating inflammation and microbial dysbiosis. Front. Immunol. 12. doi: 10.3389/fimmu.2021.774273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney E. C., Sahingur S. E. (2020). The ubiquitin system and A20: Implications in health and disease. J. Dent. Res. 100, 10–20. doi: 10.1177/0022034520949486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. A., Manthey C. L., Johnson D. L., Bauer A. J. (2011). Matrix metalloproteinase-9 inhibition reduces inflammation and improves motility in murine models of postoperative ileus. Gastroenterology 141, 1283–1292.e4. doi: 10.1053/j.gastro.2011.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosaddad S. A., Tahmasebi E., Yazdanian A., Rezvani M. B., Seifalian A., Yazdanian M., et al. (2019). Oral microbial biofilms: an update. Eur. J. Clin. Microbiol. Infect. Dis. 38, 2005–2019. doi: 10.1007/s10096-019-03641-9 [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Lordan C., Ross R. P., Cotter P. D. (2020). Gut microbes from the phylogenetically diverse genus eubacterium and their various contributions to gut health. Gut Microbes 12, 1802866. doi: 10.1080/19490976.2020.1802866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Mealey B. L., Mariotti A., Chapple I. L. C. (2018). Dental plaque-induced gingival conditions. J. Clin. Periodontol. 45, S17–S27. doi: 10.1111/jcpe.12937 [DOI] [PubMed] [Google Scholar]
- Na Y. R., Stakenborg M., Seok S. H., Matteoli G. (2019). Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 16, 531–543. doi: 10.1038/s41575-019-0172-4 [DOI] [PubMed] [Google Scholar]
- Nakajima M., Arimatsu K., Kato T., Matsuda Y., Minagawa T., Takahashi N., et al. (2015). Oral administration of p. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PloS One 10, e0134234. doi: 10.1371/journal.pone.0134234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F., Finotto S., Glimcher L. H. (2002). The role of Th1/Th2 polarization in mucosal immunity. Nat. Med. 8, 567–573. doi: 10.1038/nm0602-567 [DOI] [PubMed] [Google Scholar]
- Newman K. L., Kamada N. (2022). Pathogenic associations between oral and gastrointestinal diseases. Trends Mol. Med. 28, 1030–1039. doi: 10.1016/j.molmed.2022.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J., Wu G. D., Albenberg L., Tomov V. T. (2017). Gut microbiota and IBD: causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 14, 573–584. doi: 10.1038/nrgastro.2017.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibali L., Gkranias N., Mainas G., Di Pino A. (2022). Periodontitis and implant complications in diabetes. Periodontol. 2000 90, 88–105. doi: 10.1111/prd.12451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijakowski K., Gruszczyński D., Surdacka A. (2021). Oral health status in patients with inflammatory bowel diseases: A systematic review. Int. J. Environ. Res. Public Health 18, 11521. doi: 10.3390/ijerph182111521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg H.-H., Juricke M., Kabelitz D., Wesch D. (2011). Regulation of T cell activation by TLR ligands. Eur. J. Cell Biol. 90, 582–592. doi: 10.1016/j.ejcb.2010.11.012 [DOI] [PubMed] [Google Scholar]
- Ott S. J. (2004). Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 53, 685–693. doi: 10.1136/gut.2003.025403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W., Wang Q., Chen Q. (2019). The cytokine network involved in the host immune response to periodontitis. Int. J. Oral. Sci. 11, 30. doi: 10.1038/s41368-019-0064-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Zhu Q., Pan L.-L., Sun J. (2022). Macrophage immunometabolism in inflammatory bowel diseases: From pathogenesis to therapy. Pharmacol. Ther. 238, 108176. doi: 10.1016/j.pharmthera.2022.108176 [DOI] [PubMed] [Google Scholar]
- Pandiyan P., Zheng L., Ishihara S., Reed J., Lenardo M. J. (2007). CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation–mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 8, 1353–1362. doi: 10.1038/ni1536 [DOI] [PubMed] [Google Scholar]
- Papageorgiou S. N., Hagner M., Nogueira A. V. B., Franke A., Jäger A., Deschner J. (2017). Inflammatory bowel disease and oral health: systematic review and a meta-analysis. J. Clin. Periodontol. 44, 382–393. doi: 10.1111/jcpe.12698 [DOI] [PubMed] [Google Scholar]
- Petersen P. E., Ogawa H. (2012). The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol. 2000 60, 15–39. doi: 10.1111/j.1600-0757.2011.00425.x [DOI] [PubMed] [Google Scholar]
- Pizza M. (2019). Faculty opinions recommendation of porphyromonas gingivalis in alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5 (1), eaau3333. doi: 10.3410/f.734906096.793558207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes L. (2021). Porphyromonas gingivalis. Trends Microbiol. 29, 376–377. doi: 10.1016/j.tim.2021.01.010 [DOI] [PubMed] [Google Scholar]
- Said H. S., Suda W., Nakagome S., Chinen H., Oshima K., Kim S., et al. (2013). Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Res. 21, 15–25. doi: 10.1093/dnares/dst037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz M., Kornman K., workshop*, working (2013). Periodontitis and adverse pregnancy outcomes: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J. Clin. Periodontol. 40, S164–S169. doi: 10.1111/jcpe.12083 [DOI] [PubMed] [Google Scholar]
- Schirmer M., Garner A., Vlamakis H., Xavier R. J. (2019). Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 17, 497–511. doi: 10.1038/s41579-019-0213-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T. S., Hayward M. R., Coelho L. P., Li S. S., Costea P. I., Voigt A. Y., et al. (2019). Extensive transmission of microbes along the gastrointestinal tract. eLife 8, e42693. doi: 10.7554/elife.42693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settem R. P., El-Hassan A. T., Honma K., Stafford G. P., Sharma A. (2012). Fusobacterium nucleatum and tannerella forsythia induce synergistic alveolar bone loss in a mouse periodontitis model. Infect. Immun. 80, 2436–2443. doi: 10.1128/iai.06276-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shale M., Schiering C., Powrie F. (2013). CD4+T-cell subsets in intestinal inflammation. Immunol. Rev. 252, 164–182. doi: 10.1111/imr.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- She Y., Kong X., Ge Y., Liu Z., Chen J., Jiang J., et al. (2020). Periodontitis and inflammatory bowel disease: a meta-analysis. BMC Oral. Health 20, 67. doi: 10.1186/s12903-020-1053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M. (2009). Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30 (5), 636–645. doi: 10.1016/j.immuni.2009.04.010 [DOI] [PubMed] [Google Scholar]
- Shi T., Bian X., Yao Z., Wang Y., Gao W., Guo C. (2020). Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. 11, 8003–8013. doi: 10.1039/d0fo01439g [DOI] [PubMed] [Google Scholar]
- Sircus W., Church R., Kelleher J. (1957). Recurrent aphthous ulceration of the mouth; a study of the natural history, aetiology, and treatment. Q J. Med. 26 (102), 235–249. doi: 10.1056/NEJM195706272562613 [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Haffajee A. D., Cugini M. A., Smith C., Kent R. L. (1998). Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25, 134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x [DOI] [PubMed] [Google Scholar]
- Stødle I. H., Verket A., Høvik H., Sen A., Koldsland O. C. (2021). Prevalence of periodontitis based on the 2017 classification in a Norwegian population: The HUNT study. J. Clin. Periodontol. 48, 1189–1199. doi: 10.1111/jcpe.13507 [DOI] [PubMed] [Google Scholar]
- Standaert-Vitse A., Sendid B., Joossens M., François N., Vandewalle-El Khoury P., Branche J., et al. (2009). Candida albicans colonization and ASCA in familial crohn's disease. Am. J. Gastroenterol. 104, 1745–1753. doi: 10.1038/ajg.2009.225 [DOI] [PubMed] [Google Scholar]
- Stashenko P., Gonçalves R. B., Lipkin B., Ficarelli A., Sasaki H., Campos-Neto A. (2007). Th1 immune response promotes severe bone resorption caused by porphyromonas gingivalis. Am. J. Pathol. 170, 203–213. doi: 10.2353/ajpath.2007.060597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J., Kaplan G. G., Beck P. L., Rioux K., Panaccione R., DeVinney R., et al. (2011). Invasive potential of gut mucosa-derived fusobacterium nucleatum positively correlates with IBD status of the host. Inflammation Bowel Dis. 17, 1971–1978. doi: 10.1002/ibd.21606 [DOI] [PubMed] [Google Scholar]
- Sugihara K., Morhardt T. L., Kamada N. (2019). The role of dietary nutrients in inflammatory bowel disease. Front. Immunol. 9, 3183. doi: 10.3389/fimmu.2018.03183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Gao J., Meng X., Lu X., Zhang L., Chen R. (2021). Polarized macrophages in periodontitis: Characteristics, function, and molecular signaling. Front. Immunol. 12. doi: 10.3389/fimmu.2021.763334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczeklik K., Owczarek D., Pytko-Polończyk J., Kęsek B., Mach T. H. (2012). Proinflammatory cytokines in the saliva of patients with active and non-active crohn’s disease. Pol. Arch. Med. Wewn 122, 200–208. doi: 10.20452/pamw.1256 [DOI] [PubMed] [Google Scholar]
- Takaishi H., Matsuki T., Nakazawa A., Takada T., Kado S., Asahara T., et al. (2008). Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int. J. Med. Microbiol. 298, 463–472. doi: 10.1016/j.ijmm.2007.07.016 [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Sasaki N., Yamaga S., Kuboniwa M., Matsusaki M., Amano A. (2019). Porphyromonas gingivalis induces penetration of lipopolysaccharide and peptidoglycan through the gingival epithelium via degradation of junctional adhesion molecule 1. PloS Pathog. 15, e1008124. doi: 10.1371/journal.ppat.1008124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleban S., Li D., Targan S. R., Ippoliti A., Brant S. R., Cho J. H., et al. (2015). Ocular manifestations in inflammatory bowel disease are associated with other extra-intestinal manifestations, gender, and genes implicated in other immune-related traits. J. Crohns Colitis. 10, 43–49. doi: 10.1093/ecco-jcc/jjv178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. W., Loesche W. J., Terpenning M. S. (2000). Impact of oral diseases on systemic health in the elderly: Diabetes mellitus and aspiration pneumonia. J. Public Health Dent. 60, 313–320. doi: 10.1111/j.1752-7325.2000.tb03341.x [DOI] [PubMed] [Google Scholar]
- Teles F., Collman R. G., Mominkhan D., Wang Y. (2022). Viruses, periodontitis, and comorbidities. Periodontol. 2000 89, 190–206. doi: 10.1111/prd.12435 [DOI] [PubMed] [Google Scholar]
- Thummuri D., Jeengar M. K., Shrivastava S., Nemani H., Ramavat R. N., Chaudhari P., et al. (2015). Thymoquinone prevents RANKL-induced osteoclastogenesis activation and osteolysis in an in vivo model of inflammation by suppressing NF-KB and MAPK signalling. Pharmacol. Res. 99, 63–73. doi: 10.1016/j.phrs.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Torrealba-García D., Garcia-Morales P., Torrealba E., Cejudo J. C., Silvestre-Rangil J. (2021). Is there a relationship between periodontitis and alzheimer’s disease? systematic review and comparative analysis. Alzheimers Dement 17, e051470. doi: 10.1002/alz.051470 [DOI] [Google Scholar]
- Travis J., Pike R., Imamura T., Potempa J. (1997). Porphyromonas gingivalis proteinases as virulence factors in the development of periodontitis. J. Periodontal Res. 32, 120–125. doi: 10.1111/j.1600-0765.1997.tb01392.x [DOI] [PubMed] [Google Scholar]
- Tsukasaki M. (2020). RANKL and osteoimmunology in periodontitis. J. Bone Miner Metab. 39, 82–90. doi: 10.1007/s00774-020-01165-3 [DOI] [PubMed] [Google Scholar]
- Upadhyay K. G., Desai D. C., Ashavaid T. F., Dherai A. J. (2022). Microbiome and metabolome in inflammatory bowel disease. J. Gastroenterol. Hepatol. 38(1), 34–43. doi: 10.1111/jgh.16043 [DOI] [PubMed] [Google Scholar]
- Valm A. M. (2019). The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J. Mol. Biol. 431, 2957–2969. doi: 10.1016/j.jmb.2019.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Velden U. (2017). What exactly distinguishes aggressive from chronic periodontitis: is it mainly a difference in the degree of bacterial invasiveness? Periodontol. 2000 75, 24–44. doi: 10.1111/prd.12202 [DOI] [PubMed] [Google Scholar]
- Van Dyke T. E., Kholy K. E., Ishai A., Takx R. A. P., Mezue K., Abohashem S. M., et al. (2021). Inflammation of the periodontium associates with risk of future cardiovascular events. J. Periodontol. 92, 348–358. doi: 10.1002/jper.19-0441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal P., Koshy T., Lavu V., Ranga Rao S., Ramasamy S., Hariharan S., et al. (2018). Differential expression of microRNAs let-7a, miR-125b, miR-100, and miR-21 and interaction with NF-kB pathway genes in periodontitis pathogenesis. J. Cell Physiol. 233, 5877–5884. doi: 10.1002/jcp.26391 [DOI] [PubMed] [Google Scholar]
- Vitkov L., Muñoz L. E., Schoen J., Knopf J., Schauer C., Minnich B., et al. (2021). Neutrophils orchestrate the periodontal pocket. Front. Immunol. 12. doi: 10.3389/fimmu.2021.788766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade W. G. (2013). The oral microbiome in health and disease. Pharmacol. Res. 69, 137–143. doi: 10.1016/j.phrs.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Wang X., Luo Y., Huang Y., Jin Z., Li Z., Chen J., et al. (2022). Periodontitis-induced oral microbiome alterations provide clues on how periodontitis exacerbates colitis. J. Clin. Periodontol. 1–15. doi: 10.1111/jcpe.13759 [DOI] [PubMed] [Google Scholar]
- Wei Y., Shi M., Nie Y., Wang C., Sun F., Jiang W., et al. (2022). Integrated analysis of the salivary microbiome and metabolome in chronic and aggressive periodontitis: A pilot study. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.959416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wéra O., Lancellotti P., Oury C. (2016). The dual role of neutrophils in inflammatory bowel diseases. J. Clin. Med. 5, 118. doi: 10.3390/jcm5120118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor J. W., Kaplan G. G. (2019). Evolving epidemiology of IBD. Curr. Gastroenterol. Rep. 21 (8), 40. doi: 10.1007/s11894-019-0705-6 [DOI] [PubMed] [Google Scholar]
- Wu L., Zhang S., Zhao L., Ren Z., Hu C. (2022). Global, regional, and national burden of periodontitis from 1990 to 2019: Results from the global burden of disease study 2019. J. Periodontol. 93, 1445–1454. doi: 10.1002/jper.21-0469 [DOI] [PubMed] [Google Scholar]
- Xiao L., Huang L., Zhou X., Zhao D., Wang Y., Min H., et al. (2022). Experimental periodontitis deteriorated atherosclerosis associated with trimethylamine n-oxide metabolism in mice. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.820535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Zhou W., Wang H., Liang S. (2020). Roles of porphyromonas gingivalis and its virulence factors in periodontitis. Adv. Protein Chem. Struct. Biol. 120, 45–84. doi: 10.1016/bs.apcsb.2019.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Luo M., Chen Z., He B. (2020). The function and role of the Th17/Treg cell balance in inflammatory bowel disease. J. Immunol. Res. 2020, 1–8. doi: 10.1155/2020/8813558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Pang X., Li Z., Chen Z., Wang Y. (2021). Immunomodulation in the treatment of periodontitis: Progress and perspectives. Front. Immunol. 12. doi: 10.3389/fimmu.2021.781378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Yu T., Cong Y. (2022). CD4+ T cell metabolism, gut microbiota, and autoimmune diseases: implication in precision medicine of autoimmune diseases. Precis. Clin. Med. 5, pbac018. doi: 10.1093/pcmedi/pbac018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Manne S., Treem W. R., Bennett D. (2019). Prevalence of inflammatory bowel disease in pediatric and adult populations: Recent estimates from Large national databases in the united states 2007–2016. Inflammation Bowel Dis. 26, 619–625. doi: 10.1093/ibd/izz182 [DOI] [PubMed] [Google Scholar]
- Yin W., Ludvigsson J. F., Liu Z., Roosaar A., Axéll T., Ye W. (2017). Inverse association between poor oral health and inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 15, 525–531. doi: 10.1016/j.cgh.2016.06.024 [DOI] [PubMed] [Google Scholar]
- Yu M., Wang Q., Ma Y., Li L., Yu K., Zhang Z., et al. (2018). Aryl hydrocarbon receptor activation modulates intestinal epithelial barrier function by maintaining tight junction integrity. Int. J. Biol. Sci. 14, 69–77. doi: 10.7150/ijbs.22259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Zhou F., Wang H., Xu X., Xu S., Zhang C., et al. (2023). Systemic antibiotics increase microbiota pathogenicity and oral bone loss. Int. J. Oral. Sci. 15, 4. doi: 10.1038/s41368-022-00212-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen J., Fu H., Kuang S., He F., Zhang M., et al. (2021. a). Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int. J. Oral. Sci. 13, 43. doi: 10.1038/s41368-021-00150-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Gao X., Zhou J., Chen S., Zhang J., Zhang Y., et al. (2020). Increased risks of dental caries and periodontal disease in Chinese patients with inflammatory bowel disease. Int. Dental J. 70, 227–236. doi: 10.1111/idj.12542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Guo J., Jia R. (2021. b). Treg: A promising immunotherapeutic target in oral diseases. Front. Immunol. 12. doi: 10.3389/fimmu.2021.667862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Liu S., Zhang S., Li Y., Shi X., Liu D., et al. (2021. d). Porphyromonas gingivalis outer membrane vesicles inhibit the invasion of Fusobacterium nucleatum into oral epithelial cells by downregulating FadA and FomA. J. Periodontol. 93, 515–525. doi: 10.1002/jper.21-0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Qiao D., Chen R., Zhu F., Gong J., Yan F. (2021. c). The association between periodontitis and inflammatory bowel disease: A systematic review and meta-analysis. BioMed. Res. Int. 2021, 1–8. doi: 10.1155/2021/6692420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Gönczi L., Lakatos P. L., Burisch J. (2021). The burden of inflammatory bowel disease in Europe in 2020. J. Crohns Colitis 15, 1573–1587. doi: 10.1093/ecco-jcc/jjab029 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Dong C., Yang J., Jin Y., Zheng W., Zhou Q., et al. (2019). Exosomal microRNA-155-5p from PDLSCs regulated Th17/Treg balance by targeting sirtuin-1 in chronic periodontitis. J. Cell Physiol. 234, 20662–20674. doi: 10.1002/jcp.28671 [DOI] [PubMed] [Google Scholar]
- Zhou J., Chen S., Ren J., Zou H., Liu Y., Chen Y., et al. (2021). Association of enhanced circulating trimethylamine n-oxide with vascular endothelial dysfunction in periodontitis patients. J. Periodontol. 93, 770–779. doi: 10.1002/jper.21-0159 [DOI] [PubMed] [Google Scholar]
- Zhou L., Chu C., Teng F., Bessman N. J., Goc J., Santosa E. K., et al. (2019). Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature 568, 405–409. doi: 10.1038/s41586-019-1082-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G. X., Liu Z. J. (2017). Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J. Dig Dis. 18, 495–503. doi: 10.1111/1751-2980.12540 [DOI] [PubMed] [Google Scholar]
- Zhu J., Paul W. E. (2010). Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 238, 247–262. doi: 10.1111/j.1600-065x.2010.00951.x [DOI] [PMC free article] [PubMed] [Google Scholar]