Abstract
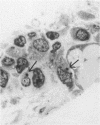
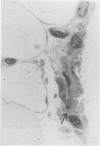
Beige mice carry a gene (bg) which codes for the presence of giant intracellular granules in a variety of cell types. Bone marrow from beige mice was transplanted into irradiated normal mice. Giant granules similar to those seen in beige mouse synovial cells were observed subsequently in the synovial lining cells of marrow recipients, indicating an influx of bone marrow derived cells into the synovial lining. Giant granule bearing cells also appeared in the subintima. On electron microscopy giant granules have been demonstrated only in macrophage-like or type A cells in marrow recipient synovia, despite the occurrence of giant granules in both type A and type B lining cells in donor material (beige). This tends to suggest that only the type A lining cells are derived from bone marrow, as might be expected from their similarity to mononuclear phagocytes elsewhere. However, the possibility remains that type B cells are also derived from bone marrow but have a slower rate of replacement.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barratt M. E., Fell H. B., Coombs R. R., Glauert A. M. The pig synovium, II. Some properties of isolated intimal cells. J Anat. 1977 Feb;123(Pt 1):47–66. [PMC free article] [PubMed] [Google Scholar]
- Coulton L. A., Henderson B., Bitensky L., Chayen J. DNA synthesis in human rheumatoid and nonrheumatoid synovial lining. Ann Rheum Dis. 1980 Jun;39(3):241–247. doi: 10.1136/ard.39.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzner M. A., Lowrie C. T., Jordan H. W. Giant granules in leukocytes of the beige mouse. J Hered. 1967 Nov-Dec;58(6):299–300. doi: 10.1093/oxfordjournals.jhered.a107620. [DOI] [PubMed] [Google Scholar]
- Spector W. G., Lykke A. W. The cellular evolution of inflammatory granulomata. J Pathol Bacteriol. 1966 Jul;92(1):163–167. doi: 10.1002/path.1700920117. [DOI] [PubMed] [Google Scholar]
- Striker G. E., Mannik M., Tung M. Y. Role of marrow-derived monocytes and mesangial cells in removal of immune complexes from renal glomeruli. J Exp Med. 1979 Jan 1;149(1):127–136. doi: 10.1084/jem.149.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Carson D. A., Tavassoli M., Slovin S. F., Speers W. C., Jensen F. B., Vaughan J. H. Evidence for the presence of receptors for C3 and IgG Fc on human synovial cells. Arthritis Rheum. 1980 Jan;23(1):1–9. doi: 10.1002/art.1780230102. [DOI] [PubMed] [Google Scholar]
- Traycoff R. B., Pascual E., Schumacher H. R., Jr Mononuclear cells in human synovial fluid. Identification of lymphoblasts in rheumatoid arthritis. Arthritis Rheum. 1976 Jul-Aug;19(4):743–748. doi: 10.1002/1529-0131(197607/08)19:4<743::aid-art1780190414>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]