Abstract
Background
Biologics and Janus kinase (JAK) inhibitors are commonly used to improve ankylosing spondylitis (AS) symptoms if conventional treatments are ineffective or unsuitable. This systematic review aimed to compare the therapeutic effects and safety of JAK inhibitors, tumor necrosis factor-alpha (TNF-α) inhibitors, and interleukin (IL) inhibitors in patients with AS.
Methods
We retrieved literature from various databases including Web of Science, Cochrane, Embase, PubMed, China National Knowledge Infrastructure, Weipu Journal Database, SinoMed, and WanFang Data up to February 1, 2023, and evaluated the quality of the included RCTs using the Cochrane risk-of-bias tool. R 4.1.3, STATA 15.1 were employed for network meta-analyses.
Results
We identified 48 eligible articles including 8,937 patients. Ten articles were rated as “low risk”, 5 as “high risk”, and the others as “some concerns”. In terms of efficacy, IL-17, IL-6, and JAK inhibitors were compared with TNF-α inhibitors in ASAS20 (RR =0.81, 95% CI: 0.66–0.98; RR =0.57, 95% CI: 0.35–0.95; RR =0.77, 95% CI: 0.60–0.99). IL-6 inhibitors were compared with TNF-α inhibitors in ASAS5/6 (RR =0.39, 95% CI: 0.16–0.98). IL-23, JAK inhibitors were compared with TNF-α inhibitors in BASDAI50 (RR =0.35, 95% CI: 0.20–0.60; RR =0.70, 95% CI: 0.49–0.98). IL-17 inhibitors were compared with IL-23 and IL-6 inhibitors in BASFI (MD =−1.05, 95% CI: −1.65–−0.51; MD =−1.46, 95% CI: −2.02–−0.97). In terms of safety, IL-6 inhibitors were compared with JAK, TNF-α inhibitors in AEs (RR =1.38, 95% CI: 1.06–1.88; RR =1.30, 95% CI: 1.01–1.70).
Conclusions
TNF-α inhibitors are significantly superior to both IL and JAK inhibitors, and may be the preferable option to deal with the rapid progression of AS and severe functional limitations. IL-17 inhibitors may better improve the BASDAI50 response compared with JAK, IL-23, and TNF-α inhibitors. The efficacy and safety of IL-6 inhibitors are inferior to other types of drugs, indicating the low efficacy and high risk of IL-6 inhibitors.
Keywords: Ankylosing spondylitis, IL inhibitors, TNF-α inhibitors, JAK inhibitor, Bayesian network meta-analysis
Highlight box.
Key findings
• Tumor necrosis factor-alpha (TNF-α) inhibitors are superior to interleukin (IL) and Janus kinase (JAK) inhibitors and may be the ideal option for treating patients with a rapid progression of ankylosing spondylitis (AS) and severe functional limitations.
What is known and what is new?
• At present, there is no unified experience in drug treatment of AS, and there are still many clinical controversies.
• TNF-α inhibitors are superior to IL inhibitors and JAK inhibitors both in terms of efficacy and safety. IL-17 inhibitors still play an important role in the partial efficacy of AS treatment. IL-6 inhibitors cannot be recommended for the treatment of AS.
What are the implications, and what should change now?
• TNF-α inhibitors can be used as a preferred replacement for conventional drug therapy in AS. This finding is important in guiding the clinical selection of drugs for AS.
Introduction
As a chronic immune system disease, ankylosing spondylitis (AS) mainly influences the spine, paraspinal soft tissue, and peripheral joints (1,2). The incidence of AS varies by region, with a global prevalence of 0.06% to 0.25% (3). The progression of AS results in the fusion of the spinal axis and peripheral joints. This pathological change will affect the normal functions of the human body and may result in different degrees of disability, which undermines the quality of life of patients and places a heavy burden on their families and society.
Patients with advanced AS suffer from irreparable structural damage to the body. The timely application of early interventions can significantly impede the progression of the disease. At present, the commonly used therapeutic drugs to treat AS include non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, biologics, Janus kinase (JAK) inhibitors, etc. Biologics and JAK inhibitors can be used as a replacement for NSAIDs and DMARDs in patients for whom these medications are unsuitable or ineffective, or for those who are sensitive to these treatments. Biologics have been used to treat AS for more than 20 years (4), and the effectiveness of JAK inhibitors in treating AS has recently attracted the attention of clinicians (5). Although the efficacy and safety of the above drugs for the treatment of AS is still under investigation, there is no uniform consensus or experience on the choice of the above drugs, and there are still many clinical disputes. It is a remarkable fact that these drugs tend to be more expensive, placing a severe financial burden on patients. Therefore, it is of great clinical importance to seek more aggressive and effective treatments with fewer side effects.
Some systematic reviews have analyzed randomized controlled trials (RCTs) to compare these drugs. However, some of the studies included in these meta-analyses were not randomized, double-blind trials, or the interventions investigated by some of the included trials coincided with the drugs administered in stable doses in other included trials, resulting in potentially biased meta-analysis results (6,7). In addition, through previous literatures (6,7), we have found that the aforementioned drugs have some efficacy in treating AS. However, direct comparison of the efficacy and safety of interleukin (IL) inhibitors, JAK inhibitors and tumor necrosis factor-alpha (TNF-α) inhibitors in the treatment of AS is rarely reported. Clinicians must select the best drug for treating AS, and thus, it is necessary to evaluate the effectiveness of multiple drugs through network meta-analyses. The present study aims to compare the efficacy and safety of IL, JAK, and TNF-α inhibitors in patients with AS to provide insights into clinical drug use. We present the following article in accordance with the PRISMA-NMA reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-195/rc).
Methods
This study is registered with INPLASY registration No. INPLASY202290117 (URL: https://inplasy.com/inplasy-2022-9-0117/).
Search strategy and eligibility criteria
Two researchers independently searched Web of Science, Cochrane, Embase, PubMed, China National Knowledge Infrastructure (CNKI), Weipu Journal Database, SinoMed, and WanFang Data for randomized controlled trials (RCTs) investigating the effectiveness and safety of TNF-α, JAK, and IL inhibitors in the treatment of AS. Please see Table S1 for details of the literature search strategy.
The inclusion criteria were as follows: (I) The diagnosis of participants followed the 1984 modified New York Criteria for AS (8); (II) “randomized, double-blind, placebo-controlled” trials that compared IL inhibitors or JAK inhibitors or TNF-α inhibitors with a placebo for the treatment of AS were included; (III) the outcome measures were the number of patients satisfying the Assessment of SpondyloArthritis International Society (ASAS) 20 improvement criteria (ASAS 20), ASAS 40 improvement criteria (ASAS 40), at least a 20% improvement in at least five of six ASAS domains (ASAS 5/6), and at least a 50% improvement in the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI50), as well as the Bath Ankylosing Spondylitis Functional Index (BASFI) scores; (IV) all participants took traditional drugs for treating AS at a stable dose before enrollment in an RCT, such as DMARDs, corticosteroids, NSAIDs, etc. The exclusion criteria were as follows: (I) animal experiments; (II) letters, and case reports, meta-analyses, reviews, and conference abstracts; (III) duplicate publication of information from the same research project; (IV) unavailable literature data even after an attempt to contact the author(s).
Data extraction and quality assessment
Two investigators independently extracted the data from the selected literature. Disagreements were settled through discussion to reach a consensus. The results reported in the included studies (including primary trial results and subsequent secondary publications) and their Supplementary Appendix file were used as the primary source of information for our analysis. The extracted information included the year of publication, the first author, interventions, sex ratio, duration of the trials, and results. The primary efficacy endpoint was the number of study subjects selected by ASAS20, and the second efficacy endpoint was the number of patients satisfying the ASAS40 criteria, ASAS5/6 criteria, or BASDAI50. The primary safety endpoint was the number of patients suffering AEs. The number of death among patients during the trial was the secondary safety endpoint.
We evaluated the quality of the included RCTs using the risk of bias tool from the Cochrane Collaboration. The following domains were considered when evaluating the risk of bias: incomplete outcome data, selective reporting, generation of random sequence, allocation concealment, blinding of participants and staff, outcome assessment blinding, and other sources of bias. The included trials were judged as having an unclear, high, or low risk of bias.
Statistical analysis
Based on the Bayesian Hierarchical Model, we used Stata software (version 15.1, StataCorp LLC, USA) and the gemtc package in R software (version 4.1.3, R Development Core Team, New Zealand) to conduct a network meta-analysis. Binary outcomes were synthesized by calculating the relative risk (RR) with a 95% confidence interval (CI) using a random-effects model. A 95% CI crossing 1 indicates no significant differences between the groups and vice versa. Continuous variables were synthesized by calculating the mean difference (MD) with 95% CI using a fix-effects model. Bayesian Hierarchical Models were analyzed by JAGS software (version 4.3.0, Timothy R. Johnson & Kristine M. Kuhn, USA) using Markov chain Monte Carlo (MCMC). The initial value was set by four MCMC chains. The total number of iterations was set to 50,000, and the first 20,000 were used for warmup. The posterior distributions were derived from the prior distributions when MCMC was estimated to reach stable convergence. A Brooks-Gelman-Rubin plot was drawn to assess MCMC convergence, for which the closer all of the values were to 1, the better the convergence, indicating the stability of the model. The node-splitting approach (Figure S1) was used to assess the consistency of the model, with P<0.05 indicating the inconsistency of direct and indirect comparisons, and vice versa. The heterogeneity among the included studies was assessed based on I2, with I2>50% indicating significant heterogeneity and vice versa.
In the network meta-analysis plot, each node represents one intervention, and each edge stands for a direct comparison between two studies that the edge connects. No edge to connect the two studies suggested that an indirect comparison was conducted for them. The thickness of the edge and the number of studies for comparison were directly proportionate. The analysis outcomes were converted into a likelihood or a rating for each condition measured by the surface under the cumulative ranking curve (SUCRA) expressed as a percentage (for example, the best therapy had a value of 100%, while the worst therapy had a value of 0%). The outcome overview was organized using league tables, which rated various treatments based on the frequency of their effects according to corresponding SUCRA values. The risk of bias was assessed using the funnel plot (Figure S2) and Egger’s test.
Results
Study characteristics and risk of bias
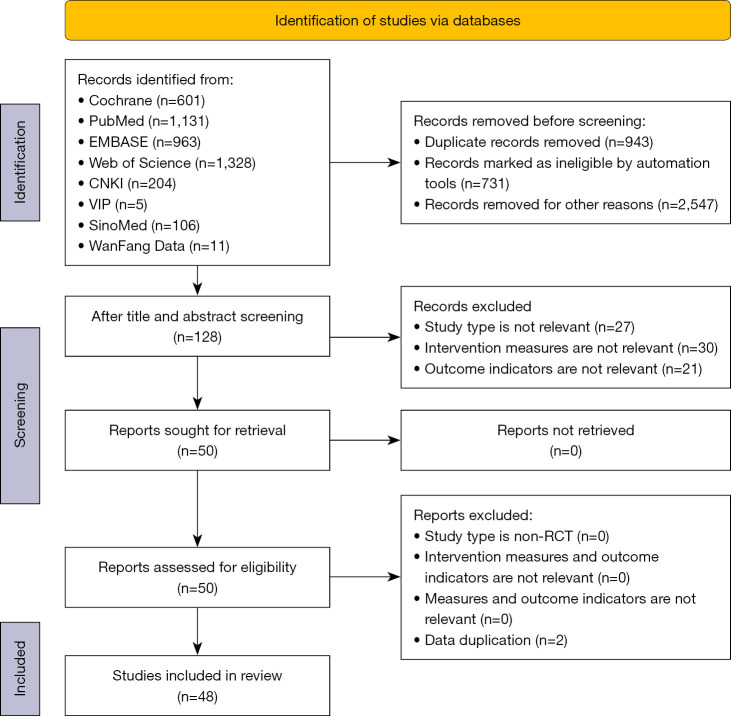
We initially identified a total of 4,349 articles from the databases. Duplicates were identified by manual and automatic screening using the Endnote software (Thomson ResearchSoft, USA). As a result, 943 duplicates were removed, a further 3,278 articles were excluded after screening the titles and abstracts, and 78 documents were removed after screening the full texts. Finally, 48 articles were included for analysis (Figure 1, Table 1). Two of these were republished after the same clinical trial was extended. BASFI was estimated in this network meta-analysis. Among 47 RCTs, 13 investigated IL inhibitors, including nine RCTs on IL-17 inhibitors (Bimekizumab, Ixekizumab, Secukinumab, and Netakimab), with 1,724 participants in the intervention group and 791 in the placebo group; two RCTs on IL-6 inhibitors (Tocilizumab and Sarilumab), with 302 and 101 patients in two groups respectively; and two RCTs on IL-23 inhibitors (Risankizumab and Ustekinumab), with 560 and 260 patients in two groups, respectively. Twenty-nine RCTs investigated TNF-α inhibitors (Certolizumab, Golimumab, Adalimumab, Infliximab, and Etanercept) with 2,442 and 1,558 patients in two groups, respectively. Five RCTs focused on JAK inhibitors (Tofacitinib, Filgotinib, and Upadacitinib) with 651 and 548 patients in the two groups, respectively.
Figure 1.
Study screening flow chart.
Table 1. Baseline characteristics of the eligible literature.
| Author | Year | Country | Intervention | N (M/F) | Duration (weeks) | Outcome |
|---|---|---|---|---|---|---|
| van der Heijde (9) | 2009 | Multiple countries | TNF-α inhibitor | 127 | 24 | BASFI |
| Placebo | 29 | |||||
| van der Heijde (10) | 2020 | Multiple countries | IL17 inhibitor | 243 (207/36) | 12 | ASAS20, ASAS40, ASAS5/6, BASDAI50, BASFI, AEs, mortality |
| Placebo | 60 (49/11) | |||||
| Landewé (11) | 2014 | Multiple countries | TNF-α inhibitor | 121 (88/33) | 12 | ASAS20, ASAS40, ASAS5/6, BASDAI50, BASFI, mortality |
| Placebo | 57 (41/16) | |||||
| Sieper (12) | 2014 | Multiple countries | IL6 inhibitor | 51 (36/15) | 12 | ASAS20, ASAS40, ASAS5/6, AEs, mortality |
| Placebo | 51 (40/11) | |||||
| van der Heijde (13) | 2006 | Multiple countries | TNF-α inhibitor | 208 (157/51) | 12/24 | ASAS20, ASAS40, ASAS5/6, AEs, mortality |
| Placebo | 107 (79/28) | |||||
| Inman (14) | 2008 | Multiple countries | TNF-α inhibitor | 278 (200/78) | 14 | ASAS20, BASDAI50, BASFI, AEs, mortality |
| Placebo | 78 (55/23) | |||||
| van der Heijde (15) | 2005 | Multiple countries | TNF-α inhibitor | 201 (157/44) | 24 | ASAS20, ASAS40, ASAS5/6, BASDAI50, BASFI, AEs, mortality |
| Placebo | 78 (68/10) | |||||
| van der Heijde (16) | 2019 | Multiple countries | JAK inhibitor | 93 (63/30) | 14 | ASAS20, ASAS40, BASDAI50, BASFI, AEs, mortality |
| Placebo | 94 (69/25) | |||||
| van der Heijde (17) | 2017 | Multiple countries | JAK inhibitor | 156 (111/45) | 12 | ASAS20, ASAS40, ASAS5/6, BASDAI50, BASFI, AEs, mortality |
| Placebo | 51 (32/19) | |||||
| van der Heijde (18) | 2018 | Multiple countries | TNF-α inhibitor | 90 (73/17) | 16 | ASAS20, ASAS40, BASDAI50, BASFI, AEs, mortality |
| IL17 inhibitor | 164 (132/32) | |||||
| Placebo | 87 (71/15) | |||||
| van der Heijde (19) | 2018 | Multiple countries | JAK inhibitor | 58 (45/13) | 12 | ASAS20, ASAS40, ASAS5/6, BASFI, AEs, mortality |
| Placebo | 58 (41/17) | |||||
| Baeten (20) | 2015 | Multiple countries | IL17 inhibitor | 394 (269/125) | 16 | ASAS20, ASAS40, ASAS5/6, AEs, mortality |
| Placebo | 196 (141/55) | |||||
| Brandt (21) | 2003 | Multiple countries | TNF-α inhibitor | 14 (10/4) | 6 | ASAS20, BASFI |
| Placebo | 16 (12/4) | |||||
| Tam (22) | 2014 | China | TNF-α inhibitor | 20 (18/2) | 24 | ASAS20, BASFI |
| Placebo | 21 (19/2) | |||||
| Sieper (23) | 2015 | Multiple countries | IL6 inhibitor | 251 (180/71) | 12 | ASAS20, ASAS40, ASAS5/6, BASFI, AEs, mortality |
| Placebo | 50 (38/12) | |||||
| Sieper (24) | 2017 | Multiple countries | IL17 inhibitor | 145 (97/48) | 16 | ASAS20, ASAS40, ASAS5/6 |
| Placebo | 74 (56/18) | |||||
| Deodhar (25) | 2018 | America & Canada | TNF-α inhibitor | 105 (86/19) | 16 | ASAS20, ASAS40, ASAS5/6, BASDAI50, BASFI, AEs, mortality |
| Placebo | 103 (77/26) | |||||
| Pavelka (26) | 2017 | Multiple countries | IL17 inhibitor | 150 (96/54) | 16 | ASAS20, ASAS40, ASAS5/6, AEs, mortality |
| Placebo | 76 (40/36) | |||||
| Pang (27) | 2008 | China | TNF-α inhibitor | 21 | 6 | ASAS20, BASDAI50 |
| Placebo | 19 | |||||
| total | 40 (39/1) | |||||
| Deodhar (28) | 2021 | Multiple countries | JAK inhibitor | 133 (116/17) | 16 | ASAS20, ASAS40, ASAS5/6, BASDAI50, BASFI, AEs, mortality |
| Placebo | 136 (108/28) | |||||
| Hu (29) | 2012 | China | TNF-α inhibitor | 26 (24/2) | 12 | BASFI |
| Placebo | 20 (20/0) | |||||
| Baeten (30) | 2013 | Multiple countries | IL17 inhibitor | 24 (14/10) | 6 | ASAS20, ASAS40, ASAS5/6, AEs |
| Placebo | 6 (5/1) | |||||
| Dougados (31) | 2011 | Multiple countries | TNF-α inhibitor | 39 (37/2) | 12 | ASAS20, ASAS40, ASAS5/6, BASDAI50, BASFI, AEs, mortality |
| Placebo | 43 (39/4) | |||||
| Baeten (32) | 2018 | Multiple countries | IL23 inhibitor | 119 (88/31) | 12 | ASAS20, ASAS40, ASAS5/6, AEs |
| Placebo | 40 (25/15) | |||||
| Bao (33) | 2014 | China | TNF-α inhibitor | 108 (90/18) | 16 | ASAS20, BASFI, AEs, mortality |
| Placebo | 105 (87/18) | |||||
| Maksymowych (34) | 2008 | America & Canada | TNF-α inhibitor | 38 (29/9) | 12 | BASFI, dead |
| Placebo | 44 (36/8) | |||||
| Horneff (35) | 2012 | Germany | TNF-α inhibitor | 17 (10/7) | 12 | ASAS20, ASAS40, BASFI, AEs, mortality |
| Placebo | 15 (7/8) | |||||
| Inman (36) | 2010 | Canada | TNF-α inhibitor | 39 (32/7) | 12 | ASAS20, ASAS40, ASAS5/6, BASDAI50, mortality |
| Placebo | 37 (29/8) | |||||
| Braun (37) | 2002 | Germany | TNF-α inhibitor | 34 (23/11) | 12 | BASDAI50 |
| Placebo | 35 (22/13) | |||||
| Gorman (38) | 2002 | America | TNF-α inhibitor | 20 (13/7) | 16 | ASAS20, BASFI, mortality |
| Placebo | 20 (18/2) | |||||
| Deodhar (39) | 2019 | Multiple countries | IL23 inhibitor | 441 (373/68) | 24 | ASAS20, ASAS40, BASDAI50, BASFI, AEs, mortality |
| Placebo | 220 (181/39) | |||||
| Calin (40) | 2004 | Multiple countries | TNF-α inhibitor | 45 (36/9) | 12 | ASAS20 |
| Placebo | 39 (30/9) | |||||
| Huang (41) | 2020 | China | IL17 inhibitor | 305 (252/53) | 16 | ASAS20, ASAS40, ASAS5/6, AEs, mortality |
| Placebo | 153 (132/21) | |||||
| Davis Jr (42) | 2003 | Multiple countries | TNF-α inhibitor | 138 (105/33) | 24 | ASAS20, BASFI |
| Placebo | 139 (105/34) | |||||
| Erdes (43) | 2020 | Russia & Belarus | IL17 inhibitor | 66 (58/15) | 16 | ASAS20, ASAS40, ASAS5/6, BASFI, AEs, Mortality |
| Placebo | 22 (15/7) | |||||
| Maksymowych (44) | 2010 | Canada | TNF-α inhibitor | 18 (14/4) | 12 | ASAS20, ASAS40 |
| Placebo | 18 (14/4) | |||||
| Huang (45) | 2014 | China | TNF-α inhibitor | 229 (185/44) | 12 | ASAS20, ASAS40, ASAS5/6, BASDAI50, BASFI, AEs, mortality |
| Placebo | 115 (95/20) | |||||
| Kivitz (46) | 2018 | Multiple countries | IL17 inhibitor | 233 (164/69) | 16 | ASAS20, ASAS40, ASAS5/6, AEs, mortality |
| Placebo | 117 (76/41) | |||||
| Deodhar (47) | 2016 | Multiple countries | IL17 inhibitor | 249 (172/77) | 16 | BASFI |
| Placebo | 112 (85/27) | |||||
| Burgos-Vargas (48) | 2022 | Mexico | TNF-α inhibitor | 12 (12/0) | 12 | ASAS20, ASAS40, ASAS5/6, BASFI |
| Placebo | 14 (13/1) | |||||
| van der Heijde (49) | 2022 | Multiple countries | JAK inhibitor | 211 (153/58) | 14 | ASAS20, ASAS40, BASDAI50, AEs |
| Placebo | 209 (158/51) | |||||
| Deng (50) | 2006 | China | TNF-α inhibitor | 26 (26/0) | 6 | ASAS20, BASDAI50, BASFI, ASs |
| Placebo | 26 (24/2) | |||||
| Huang (51) | 2010 | China | TNF-α inhibitor | 74 (63/11) | 6 | ASAS20, ASAS40, ASAS5/6, BASFI, ASs |
| Placebo | 78 (64/14) | |||||
| Huang (52) | 2011 | China | TNF-α inhibitor | 298 | 6 | ASAS20, ASAS5/6, BASDAI50, ASs |
| Placebo | 99 | |||||
| Ma (53) | 2017 | China | TNF-α inhibitor | 13 (12/1) | 14 | ASAS20, ASAS40, BASFI, ASs |
| Placebo | 12 (12/0) | |||||
| Zhang (54) | 2009 | China | TNF-α inhibitor | 43 | 6 | ASAS20, BASDAI50, BASFI |
| Placebo | 43 | |||||
| Zhang (55) | 2009 | China | TNF-α inhibitor | 52 (48/4) | 6 | AEs |
| Placebo | 52 (47/5) | |||||
| Zhang (56) | 2010 | China | TNF-α inhibitor | 115 (99/16) | 6 | ASAS20, ASAS40, ASAS5/6, BASDAI50, BASFI |
| Placebo | 38 (34/4) |
IL, interleukin; TNF-α, tumor-necrosis-factor alpha; JAK, Janus Kinase; ASAS20, 40: Assessment of SpondyloArthritis International Society 20%, 40% improvement; ASAS5/6: ≥20% improvement in 5 out of 6 the Assessment of SpondyloArthritis International Society domains; BASFI: Bath Ankylosing Spondylitis Functional Index; BASDAI50: improvement of at least 50% in the Bath Ankylosing Spondylitis Disease Activity Index; AEs, adverse events.
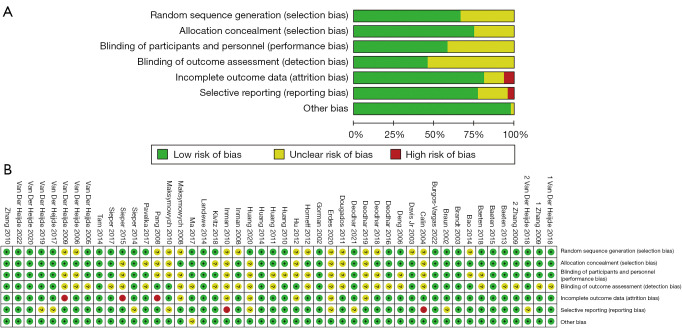
The included trials were published between 2002 and 2022. All trials were of a parallel-group and double-blind design and had a treatment duration of 6 to 24 weeks. The characteristics of the eligible studies are summarized in Table 1. The number of articles that reported on the ASAS20, ASAS40, ASAS5/6, BASDAI50, BASFI, and AEs was 42, 41, 25, 20, 31, and 30, respectively. A total of 26 studies reported on mortality. Among the 48 included articles, 16 referred to “randomization” without describing the methodology (9,13,14,25,27,29,31,33-36,39,40,42-44); allocation concealment was not presented in 12 trials (23,26,27,29,31,36,39-41,43,44,46); blinding of participants and personnel was not adequately described in 21 trials to determine the risk profile (10-12,14-20,22-24,26,28,30,37,38,42,46,47); blinding of outcome assessment was not adequately described in 23 trials to determine the risk profile (10-12,16,17,19-23,27-29,33,37,38,42,44,45,48,49,51,56); the missing outcome data in three trials is likely to be related to the real results (9,23,27); six trials did not mention the reason for the missing data (29,34,36,39,41,43); two trials did not report all prespecified primary outcome measures (36,40); the results of nine trials were not adequately reported to judge the risk profile (12,16,17,19,28,37,41,43,44). Figure 2 depicts the network meta-analysis plot, and the outcomes of the risk of bias assessment are presented in Figure 3.
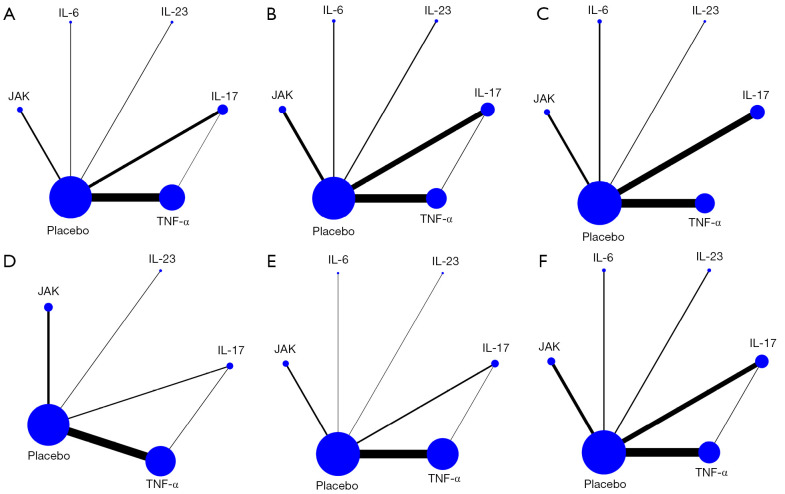
Figure 2.
The network meta-analysis plots. (A) ASAS20; (B) ASAS40; (C) ASAS5/6; (D) BASDAI50; (E) BASFI; (F) AEs. ASAS20, 40: Assessment of SpondyloArthritis International Society 20%, 40% improvement; ASAS5/6: ≥20% improvement in 5 out of 6 the Assessment of SpondyloArthritis International Society domains; BASDAI50: 50% improvement in the Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; AEs, adverse events; IL, interleukin; TNF-α, tumor-necrosis-factor alpha; JAK, Janus Kinase.
Figure 3.
Risk of bias assessment (A) risk of bias graph; (B) risk of bias summary.
The efficacy of IL, TNF-α, and JAK inhibitors for treating AS
Our comparison of the data consistency and inconsistency model (differences in the deviance information criterion less than 5 for both models) illustrated that an indirect comparison among multiple interventions was better than the direct comparison in subsequent meta-analysis. All of the values from the Brooks-Gelman-Rubin plots were close to 1, indicating the stability and reliability of the model (Figure S3).
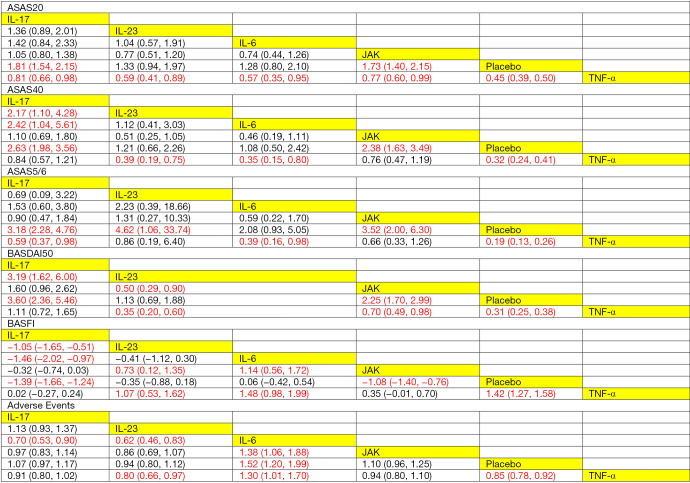
Figure 4 illustrates the league tables to compare the treatment effects of multiple interventions. These league tables showed that the IL-17 and JAK inhibitors were better than the placebo in terms of the ASAS20 response (RR =1.81, 95% CI: 1.54–2.15; RR =1.73, 95% CI: 1.40–2.15, respectively). TNF-α inhibitors were associated with significantly better ASAS20 response, and IL-17, IL-23, IL-6, JAK inhibitors and the placebo were compared with TNF-α inhibitors, respectively (RR =0.81, 95% CI: 0.66–0.98; RR =0.59, 95% CI: 0.41–0.89; RR =0.57, 95% CI: 0.35–0.95; RR =0.77, 95% CI: 0.60–0.99; RR =0.45, 95% CI: 0.39–0.50, respectively). As for the improvement in the ASAS40 response, IL-17 inhibitors were significantly more effective than IL-23 inhibitors, IL-6 inhibitors, and the placebo (RR =2.17, 95% CI: 1.10–4.28; RR =2.42, 95% CI: 1.04–5.61; RR =2.63, 95% CI: 1.98–3.56, respectively). IL-23 inhibitors, IL-6 inhibitors, and the placebo were associated with a significantly lower ASAS40 response compared to the TNF-α inhibitors (RR =0.39, 95% CI: 0.19–0.75; RR =0.35, 95% CI: 0.15–0.80; RR =0.32, 95% CI: 0.24–0.41, respectively). Also, JAK inhibitors were related to a higher ASAS40 response compared to the placebo, and the difference was statistically significant (RR =2.38, 95% CI: 1.63–3.49). Regarding the improvement of the ASAS5/6 responses, IL-17, IL-23, and JAK inhibitors were superior to the placebo, and the differences were statistically significant (RR =3.18, 95% CI: 2.28–4.67; RR =4.62, 95% CI: 1.06–33.74; RR =3.52, 95% CI: 2.00–6.30, respectively). IL-17, IL-6 inhibitors and the placebo was associated with a significantly lower ASAS5/6 response compared to the TNF-α inhibitors (RR =0.59, 95% CI: 0.37–0.98; RR =0.39, 95% CI: 0.16–0.98; RR =0.19, 95% CI: 0.13–0.26). As for the BASDAI50 response, the IL-17 inhibitors were significantly more effective compared to the IL-23 inhibitors and the placebo (RR =3.19, 95% CI: 1.62–6.00; RR =3.60, 95% CI: 2.36–5.46, respectively). The IL-23 inhibitors were associated with significantly lower BASDAI50 response compared to the JAK and TNF-α inhibitors (RR =0.50, 95% CI: 0.29–0.90; RR =0.35, 95% CI: 0.20–0.60). JAK inhibitors were related to a significantly higher BASDAI50 response compared to the placebo (RR=2.25, 95% CI: 1.70–2.99), while IL-23, JAK inhibitors and the placebo was associated with a significantly lower BASDAI50 response compared to TNF-α inhibitors (RR=0.35, 95% CI: 0.20–0.60; RR=0.70, 95% CI: 0.49–0.98; RR=0.31, 95% CI: 0.25–0.38).
Figure 4.
League tables of ASAS20, ASAS40, ASAS5/6, BASDAI50, BASFI, and adverse events. ASAS20, 40: Assessment of SpondyloArthritis International Society 20%, 40% improvement; ASAS5/6: ≥20% improvement in 5 out of 6 the Assessment of SpondyloArthritis International Society domains; BASDAI50: 50% improvement in the Bath Ankylosing Spondylitis Disease Activity Index; BASFI: Bath Ankylosing Spondylitis Functional Index; IL, interleukin; TNF-α, tumor-necrosis-factor alpha; JAK, Janus Kinase.
Regarding BASFI, a better improvement was reported in the IL-17 inhibitor group compared to that in the IL-23 and IL-6 inhibitor groups and the placebo group, and the differences were statistically significant (MD =−1.05, 95% CI: −1.65 to −0.51; MD =−1.46, 95% CI: −2.02 to −0.97; MD =−1.39, 95% CI: −1.66 to −1.24, respectively). IL-23 inhibitors were related to significantly lower BASFI improvement than the JAK and TNF-α inhibitors (MD =0.73, 95% CI: 0.12–1.35; MD= 1.07, 95% CI: 0.53–1.62, respectively). Also, IL-6 inhibitors were associated with significantly lower BASFI improvement of compared with the JAK and TNF-α inhibitors (MD =1.14, 95% CI: 0.56–1.72; MD =1.48, 95% CI: 0.98–1.99). A significantly higher BASFI improvement was observed in the JAK inhibitor group compared to that in the placebo group (MD =−1.08, 95% CI: −1.40 to −0.76), and a significantly lower BASFI improvement was observed in the placebo group compared to the TNF-α inhibitor group (MD =1.42, 95% CI: 1.27–1.58).
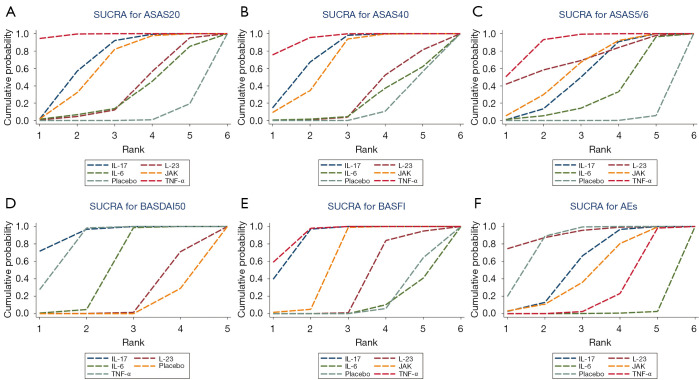
Figure 5A-5E demonstrates that ASAS20 and ASAS40 presented similar SUCRA values. Regarding the value of SUCRA in relation to ASAS20 or ASAS40, multiple treatments are ranked in descending order as follows: TNF-α, IL-17, JAK, IL-23, and IL-6 inhibitors. Concerning ASAS5/6, the treatments are ranked as follows in descending order according to the SUCRA value: TNF-α, IL-23, JAK, IL-17, and IL-6 inhibitors. As for BASDAI50, the treatments are ranked as follows in descending order: IL-17, TNF-α, JAK, and IL23 inhibitors. Regarding BASFI, IL-17 and TNF-α inhibitors had similar SUCRA values, while the remaining treatments are ranked in descending order as follows: JAK inhibitors, IL-23 inhibitors, and IL-6 inhibitors.
Figure 5.
SUCRA. (A) ASAS20; (B) ASAS40; (C) ASAS5/6; (D) BASDAI50; (E) BASFI; (F) AEs. ASAS20, 40: Assessment of SpondyloArthritis International Society 20%, 40% improvement; ASAS5/6: ≥20% improvement in 5 out of 6 the Assessment of SpondyloArthritis International Society domains; BASDAI50: 50% improvement in the Bath Ankylosing Spondylitis Disease Activity Index. SUCRA, surface under the cumulative ranking curve; BASFI, Bath Ankylosing Spondylitis Functional Index; AEs, adverse events; IL, interleukin; TNF-α, tumor-necrosis-factor alpha; JAK, Janus Kinase.
The safety of IL, TNF-α, and JAK inhibitors
The league plots related to the network meta-analysis of AEs associated with the multiple treatments are presented in Figure 4. IL-17, IL-23 inhibitors were related to a significantly lower risk of AEs compared to IL-6 inhibitors (RR =0.70, 95% CI: 0.53–0.90; RR =0.62, 95% CI: 0.46–0.83). IL-23 inhibitors were related to a lower risk of AEs than both the IL-6 and TNF-α inhibitors, with significant differences (RR =0.62, 95% CI: 0.46–0.83; RR =0.80, 95% CI: 0.66–0.97, respectively). IL-6 inhibitors were related to a significantly higher risk of AEs compared to JAK inhibitors, the placebo, and TNF-α inhibitors (RR=1.38, 95% CI: 1.06–1.88; RR=1.52, 95% CI: 1.20–1.99; RR=1.30, 95% CI: 1.01–1.70, respectively). The placebo was related to a lower risk of AEs than the TNF-α inhibitors, with significant differences (RR=0.85, 95% CI: 0.78–0.92).
The SUCRA values of AEs are presented in Figure 5F. IL-23 inhibitors had the lowest SUCRA values. The remaining interventions are ranked in descending order according to their SUCRA values: IL 17 inhibitors, JAK inhibitors, TNF-α inhibitors, and IL6 inhibitors.
Twenty-six articles involving 6,399 patients with AS reported on mortality, with two cases of death in the IL-17 inhibitor group and one case in the placebo group. The two cases in the IL-17 inhibitor group participated in different trials, with one case of death attributed to fatal myocardial infarction after the patient received 75 mg secukinumab and the other case of death due to myocardial infarction unrelated to the treatment. The one case of death in the placebo group was described as suicide. Due to limited data, our study did not investigate the effects of three types of interventions on the risk of mortality.
Results of convergence, inconsistency, publication bias, and heterogeneity analyses
The potential scale reduction factor was limited to 1, reflecting the good convergence of this analysis. Regarding ASAS5/6 and AEs, the funnel plots for the included trials are close to symmetric, and the P values of the Egger’s tests are all greater than 0.05, indicating no significant publication bias. However, for ASAS20, ASAS40, BASDAI50 and BASFI, the P values for the Egger’s test were 0.007, 0.028, 0.004 and 0.000, respectively, indicating publication bias in the included trials. Analysis of heterogeneity showed no significant heterogeneity. The full results of the inconsistency, publication bias, convergence and heterogeneity analyses can be found in Figures S1-S5.
Discussion
At present, the treatment of AS focuses on the relief of inflammation and improvement in body function, aiming to prevent structural damage. The efficacy of IL, TNF-α, and JAK inhibitors has been confirmed by clinical practice. In this study, we collected the latest RCTs investigating IL, TNF-α, and JAK inhibitors and compared the therapeutic effects and safety of these three kinds of treatments directly and indirectly. However, due to the limited data from the included studies, we did not investigate the relationship between the three different types of interventions and AEs (only one of the 48 included studies directly compared IL-17 inhibitors and TNF-α inhibitors in terms of AEs). The included studies mainly compared multiple interventions with the placebo. Figure 4 and Figure 5A-5D show that the three types of treatments, especially TNF-α inhibitors, were associated with better ASAS20, ASAS40, ASAS5/6, and BASDAI50 responses compared to the placebo. TNF-α, JAK, and IL-17 inhibitors performed better than the placebo in terms of the improvement in BASFI but IL-6 inhibitors may be inferior to or not better than the placebo. Also, compared to the placebo, IL-23 inhibitors may be related to a lower risk of AEs.
Through the indirect comparison of various drugs regarding 42 pairs of ASAS20 responses, 31 pairs of ASAS40 responses, and 25 pairs of ASAS5/6 responses, TNF-α inhibitors were significantly superior to IL and JAK inhibitors in improving the ASAS5/6, ASAS20, and ASAS40 responses. No significant differences emerged between the IL-17 and JAK inhibitors in terms of improvement in the ASAS20, ASAS40, and ASAS5/6 responses. In addition, according to the comparison across 20 pairs of BASDAI50, IL-17 inhibitors may provide better BASDAI 50 response improvements than JAK, TNF-α, and IL-23 inhibitors. As for BASFI improvement, no significant difference emerged between the TNF-α and IL-17 inhibitors. This result was based on a comparison of 31 pairs of BASFI results.
Regarding safety, the number of patients experiencing AEs associated with IL-23 inhibitors was significantly smaller than those associated with IL-6, IL-17, JAK, and TNF-α inhibitors. This result was from a comparison of 30 pairs of AEs results. It should be noted that IL-6 inhibitors were inferior to other drugs in terms of the ASAS5/6, ASAS20, ASAS40, BASFI responses, and the risk of AEs, suggesting that IL-6 inhibitors were associated with poor efficacy and high risk. By comparing 26 pairs of mortality data, we found that only two cases of death were reported in the IL-17 inhibitors group; however, one of these cases was caused by a myocardial infarction that was unrelated to IL-17 inhibitors, which deserves more attention in clinical practice.
The special function of TNF-α inhibitors was first revealed by Feldmann and Maini in their study on rheumatoid arthritis (57). Since 1998, various TNF inhibitors have been approved for use, becoming a class of drugs with wide-ranging biological effects. TNF is a class of cytokines produced by activated endotheliocytes, mononuclear macrophages, chondrocytes, and fibrocytes that exerts multiple biological effects. TNF induces synoviocytes to produce collagenase associated with the process of cartilage destruction. Higher levels of TNF in serum have been found in people with AS. In TNF, TNF-α performs its biological function by binding to TNF receptors on the cell surface. TNF-α mediates inflammation and immunomodulatory effects in immunoreactions, including the activation of lymphocytes and the release of other cytokines, prostaglandins, and metalloproteinases. The lesion sites of AS mainly include bone attachment points of the synovium, joint capsule, tendon, and anadesma of the axial joint. The main pathological features are invasive aseptic inflammation of the axial bones including the sacroiliac joint and the surrounding tissues of the joint, eventually progressing to extensive fibrosis and bony ankylosis. Therefore, reducing the activity of TNF-α can significantly increase the efficiency of inflammatory response inhibition and improve patients’ ASAS20, ASAS40, ASAS5/6, and BASDAI50 responses. According to this network analysis, considering the satisfying treatment effects and low risk of AEs associated with TNF-α inhibitors, these drugs may be the best option for people with AS who do not respond well after non-drug therapy or at least 4 weeks of use of more than two types of NSAIDs, which is consistent with the results in the guide (58). However, insufficient evidence has been obtained to suggest significant differences in the effectiveness between TNF-α inhibitors.
Although some meta-analyses of the treatment of AS with biologics had been published before our study was conducted (6,7), they were not based on “randomized, double-blind, placebo-controlled” trials, and interventions in some studies investigated in these meta-analyses overlapped others. Some of our findings through this network meta-analysis echo the conclusions of some previous meta-analyses (6,7). For example, IL-6 inhibitors and IL-23 inhibitors have poor or no therapeutic effects, compared with the placebo, and IL-17 inhibitors present significantly good treatment effects. Previous meta-analyses (6,7) have reported that TNF-α inhibitors were the best option for people with AS who suffer rapid disease progression; however, these analyses found that TNF-α inhibitors were the safest drug and IL-17 inhibitors were irreplaceable for treating AS, which differs from the findings in our study. The distinct differences between our study and previous meta-analyses (6,7) may lie in the types of trials included for analysis, as whether a blinding method was used or not can significantly affect the publication bias of trials. Moreover, our network meta-analysis rejected performing subgroup analyses regarding patients’ age, the course of treatment, and different drugs of the same category, given the inherent drawbacks of network meta-analysis and to avoid the type I error attributed to numerous subgroup analyses. Dougados et al. investigated the effectiveness of etanercept on rheumatic signs in people with advanced AS, and the results of their study are consistent with ours regarding the treatment effects of TNF-α inhibitors based on the measures of ASAS20,40,5/6, BASDAI50, and BASFI (31). It should be noted that there are some contradictions between the results of this study and the included head-to-head comparison studies. This may be partly related to differences in selected populations, drug doses, and with respect to certain molecular and pharmacokinetic characteristics between IL inhibitors. In addition, the results of the subgroup analyses in some traditional meta-analyses did not affect the primary outcomes and there were differences in other outcomes between our study and these traditional meta-analyses (59,60).
This study has some limitations that should be noted and considered. Firstly, the findings regarding the effectiveness and safety of the three types of drugs have to be interpreted with caution because they were based primarily on indirect data, since few “randomized, double-blind” trials have conducted direct comparisons. Additionally, in each RCT that was eligible for this network meta-analysis, differences emerged between subgroups due to the drug type, dosage, and duration of intervention. To confirm that the conclusion of this study can be applied to a more diverse population in clinical practice, the focus should be shifted from RCTs to real-world evidence studies. Finally, publication bias is present in this study, which can be caused by small sample size trials, so conclusions may be affected to some extent.
However, this systematic review analyzed all eligible trials selected from Web of Science, Cochrane, Embase, PubMed, CNKI, Weipu Journal Database, SinoMed, and WanFang Data up to February 1, 2023. The included studies with relatively high quality involved large sample sizes and no significant heterogeneity emerged across most of the included studies. Additionally, considering the high price of biologics and JAK inhibitors targeting AS and the rising cost of research on and development of new drugs, coupled with the deterioration in the global economy, it is particularly important to select more effective drugs for the individually tailored treatment of patients with AS. More effective drugs can also help to relieve the pressure associated with the growth of healthcare spending and reduce the burden on patients.
In the future, triple-blind RCTs with larger sample sizes, adequate allocation concealment, and lower level of loss to follow-up are needed, as are studies based on real-world clinical information. The methodology applied in the present study can be employed for analyzing future RCTs as described above to provide insights for clinicians into the differences between multiple drugs.
Conclusions
TNF-α inhibitors are superior to IL inhibitors and JAK inhibitors and may be the ideal option for treating people with a rapid progression of AS and severe functional limitations. IL-17 inhibitors may better improve the BASDAI50 response compared with JAK, IL-23, and TNF-α inhibitors, which is worthy of attention in clinical practice. No significant difference emerged in improving the BASFI scores between IL-17 and TNF-α inhibitors. IL-6 inhibitors are inferior to other types of drugs in terms of the ASAS20, ASAS40, ASAS5/6, and BASFI responses as well as the risk of AEs, indicating the low efficacy and high risk of IL-6 inhibitors.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors thank all researchers and participants for sharing these data.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Reporting Checklist: The authors have completed the PRISMA-NMA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-195/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-195/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-195/coif). The authors have no conflicts of interest to declare.
(English Language Editor: A. Kassem)
References
- 1.Appel H, Kuhne M, Spiekermann S, et al. Immunohistologic analysis of zygapophyseal joints in patients with ankylosing spondylitis. Arthritis Rheum 2006;54:2845-51. 10.1002/art.22060 [DOI] [PubMed] [Google Scholar]
- 2.Appel H, Kuhne M, Spiekermann S, et al. Immunohistochemical analysis of hip arthritis in ankylosing spondylitis: evaluation of the bone-cartilage interface and subchondral bone marrow. Arthritis Rheum 2006;54:1805-13. 10.1002/art.21907 [DOI] [PubMed] [Google Scholar]
- 3.Stolwijk C, van Onna M, Boonen A, et al. Global Prevalence of Spondyloarthritis: A Systematic Review and Meta-Regression Analysis. Arthritis Care Res (Hoboken) 2016;68:1320-31. 10.1002/acr.22831 [DOI] [PubMed] [Google Scholar]
- 4.Van den Bosch F, Kruithof E, Baeten D, et al. Effects of a loading dose regimen of three infusions of chimeric monoclonal antibody to tumour necrosis factor alpha (infliximab) in spondyloarthropathy: an open pilot study. Ann Rheum Dis 2000;59:428-33. 10.1136/ard.59.6.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adis Editorial . Tofacitinib. Drugs R D 2010;10:271-84. 10.2165/11588080-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Z, Guo J, Li Q, et al. Optimal Biologic Drugs for the Treatment of Ankylosing Spondylitis: Results from a Network Meta-Analysis and Network Metaregression. Biomed Res Int 2022;2022:8316106. 10.1155/2022/8316106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu CL, Yang CH, Chi CC. Drug Survival of Biologics in Treating Ankylosing Spondylitis: A Systematic Review and Meta-analysis of Real-World Evidence. BioDrugs 2020;34:669-79. 10.1007/s40259-020-00442-x [DOI] [PubMed] [Google Scholar]
- 8.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361-8. 10.1002/art.1780270401 [DOI] [PubMed] [Google Scholar]
- 9.van der Heijde DM, Revicki DA, Gooch KL, et al. Physical function, disease activity, and health-related quality-of-life outcomes after 3 years of adalimumab treatment in patients with ankylosing spondylitis. Arthritis Res Ther 2009;11:R124. 10.1186/ar2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Heijde D, Gensler LS, Deodhar A, et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheum Dis 2020;79:595-604. 10.1136/annrheumdis-2020-216980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis 2014;73:39-47. 10.1136/annrheumdis-2013-204231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sieper J, Porter-Brown B, Thompson L, et al. Assessment of short-term symptomatic efficacy of tocilizumab in ankylosing spondylitis: results of randomised, placebo-controlled trials. Ann Rheum Dis 2014;73:95-100. 10.1136/annrheumdis-2013-203559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006;54:2136-46. 10.1002/art.21913 [DOI] [PubMed] [Google Scholar]
- 14.Inman RD, Davis JC, Jr, Heijde Dv, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 2008;58:3402-12. 10.1002/art.23969 [DOI] [PubMed] [Google Scholar]
- 15.van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582-91. 10.1002/art.20852 [DOI] [PubMed] [Google Scholar]
- 16.van der Heijde D, Song IH, Pangan AL, et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet 2019;394:2108-17. 10.1016/S0140-6736(19)32534-6 [DOI] [PubMed] [Google Scholar]
- 17.van der Heijde D, Deodhar A, Wei JC, et al. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis 2017;76:1340-7. 10.1136/annrheumdis-2016-210322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 2018;392:2441-51. 10.1016/S0140-6736(18)31946-9 [DOI] [PubMed] [Google Scholar]
- 19.van der Heijde D, Baraliakos X, Gensler LS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:2378-87. 10.1016/S0140-6736(18)32463-2 [DOI] [PubMed] [Google Scholar]
- 20.Baeten D, Sieper J, Braun J, et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N Engl J Med 2015;373:2534-48. 10.1056/NEJMoa1505066 [DOI] [PubMed] [Google Scholar]
- 21.Brandt J, Khariouzov A, Listing J, et al. Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum 2003;48:1667-75. 10.1002/art.11017 [DOI] [PubMed] [Google Scholar]
- 22.Tam LS, Shang Q, Kun EW, et al. The effects of golimumab on subclinical atherosclerosis and arterial stiffness in ankylosing spondylitis—a randomized, placebo-controlled pilot trial. Rheumatology (Oxford) 2014;53:1065-74. 10.1093/rheumatology/ket469 [DOI] [PubMed] [Google Scholar]
- 23.Sieper J, Braun J, Kay J, et al. Sarilumab for the treatment of ankylosing spondylitis: results of a Phase II, randomised, double-blind, placebo-controlled study (ALIGN). Ann Rheum Dis 2015;74:1051-7. 10.1136/annrheumdis-2013-204963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sieper J, Deodhar A, Marzo-Ortega H, et al. Secukinumab efficacy in anti-TNF-naive and anti-TNF-experienced subjects with active ankylosing spondylitis: results from the MEASURE 2 Study. Ann Rheum Dis 2017;76:571-92. 10.1136/annrheumdis-2016-210023 [DOI] [PubMed] [Google Scholar]
- 25.Deodhar A, Reveille JD, Harrison DD, et al. Safety and Efficacy of Golimumab Administered Intravenously in Adults with Ankylosing Spondylitis: Results through Week 28 of the GO-ALIVE Study. J Rheumatol 2018;45:341-8. 10.3899/jrheum.170487 [DOI] [PubMed] [Google Scholar]
- 26.Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther 2017;19:285. 10.1186/s13075-017-1490-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pang L, Wang L, Suo T, et al. Tumor necrosis factor-alpha blockade leads to decreased peripheral T cell reactivity and increased dendritic cell number in peripheral blood of patients with ankylosing spondylitis. J Rheumatol 2008;35:2220-8. 10.3899/jrheum.080219 [DOI] [PubMed] [Google Scholar]
- 28.Deodhar A, Sliwinska-Stanczyk P, Xu H, et al. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2021;80:1004-13. 10.1136/annrheumdis-2020-219601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Z, Xu M, Li Q, et al. Adalimumab significantly reduces inflammation and serum DKK-1 level but increases fatty deposition in lumbar spine in active ankylosing spondylitis. Int J Rheum Dis 2012;15:358-65. 10.1111/j.1756-185X.2012.01734.x [DOI] [PubMed] [Google Scholar]
- 30.Baeten D, Baraliakos X, Braun J, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 2013;382:1705-13. 10.1016/S0140-6736(13)61134-4 [DOI] [PubMed] [Google Scholar]
- 31.Dougados M, Braun J, Szanto S, et al. Efficacy of etanercept on rheumatic signs and pulmonary function tests in advanced ankylosing spondylitis: results of a randomised double-blind placebo-controlled study (SPINE). Ann Rheum Dis 2011;70:799-804. 10.1136/ard.2010.139261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baeten D, Østergaard M, Wei JC, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis 2018;77:1295-302. 10.1136/annrheumdis-2018-213328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao C, Huang F, Khan MA, et al. Safety and efficacy of golimumab in Chinese patients with active ankylosing spondylitis: 1-year results of a multicentre, randomized, double-blind, placebo-controlled phase III trial. Rheumatology (Oxford) 2014;53:1654-63. 10.1093/rheumatology/keu132 [DOI] [PubMed] [Google Scholar]
- 34.Maksymowych WP, Rahman P, Shojania K, et al. Beneficial effects of adalimumab on biomarkers reflecting structural damage in patients with ankylosing spondylitis. J Rheumatol 2008;35:2030-7. [PubMed] [Google Scholar]
- 35.Horneff G, Fitter S, Foeldvari I, et al. Double-blind, placebo-controlled randomized trial with adalimumab for treatment of juvenile onset ankylosing spondylitis (JoAS): significant short term improvement. Arthritis Res Ther 2012;14:R230. 10.1186/ar4072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inman RD, Maksymowych WP, CANDLE Study Group . A double-blind, placebo-controlled trial of low dose infliximab in ankylosing spondylitis. J Rheumatol 2010;37:1203-10. 10.3899/jrheum.091042 [DOI] [PubMed] [Google Scholar]
- 37.Braun J, Brandt J, Listing J, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 2002;359:1187-93. 10.1016/s0140-6736(02)08215-6 [DOI] [PubMed] [Google Scholar]
- 38.Gorman JD, Sack KE, Davis JC, Jr. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med 2002;346:1349-56. 10.1056/NEJMoa012664 [DOI] [PubMed] [Google Scholar]
- 39.Deodhar A, Gensler LS, Sieper J, et al. Three Multicenter, Randomized, Double-Blind, Placebo-Controlled Studies Evaluating the Efficacy and Safety of Ustekinumab in Axial Spondyloarthritis. Arthritis Rheumatol 2019;71:258-70. 10.1002/art.40728 [DOI] [PubMed] [Google Scholar]
- 40.Calin A, Dijkmans BA, Emery P, et al. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004;63:1594-600. 10.1136/ard.2004.020875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang F, Sun F, Wan WG, et al. Secukinumab provided significant and sustained improvement in the signs and symptoms of ankylosing spondylitis: results from the 52-week, Phase III China-centric study, MEASURE 5. Chin Med J (Engl) 2020;133:2521-31. 10.1097/CM9.0000000000001099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis JC, Jr, Van Der Heijde D, Braun J, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003;48:3230-6. 10.1002/art.11325 [DOI] [PubMed] [Google Scholar]
- 43.Erdes S, Nasonov E, Kunder E, et al. Primary efficacy of netakimab, a novel interleukin-17 inhibitor, in the treatment of active ankylosing spondylitis in adults. Clin Exp Rheumatol 2020;38:27-34. [PubMed] [Google Scholar]
- 44.Maksymowych WP, Salonen D, Inman RD, et al. Low-dose infliximab (3 mg/kg) significantly reduces spinal inflammation on magnetic resonance imaging in patients with ankylosing spondylitis: a randomized placebo-controlled study. J Rheumatol 2010;37:1728-34. 10.3899/jrheum.091043 [DOI] [PubMed] [Google Scholar]
- 45.Huang F, Gu J, Zhu P, et al. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: results of a randomised, controlled trial. Ann Rheum Dis 2014;73:587-94. 10.1136/annrheumdis-2012-202533 [DOI] [PubMed] [Google Scholar]
- 46.Kivitz AJ, Wagner U, Dokoupilova E, et al. Efficacy and Safety of Secukinumab 150 mg with and Without Loading Regimen in Ankylosing Spondylitis: 104-week Results from MEASURE 4 Study. Rheumatol Ther 2018;5:447-62. 10.1007/s40744-018-0123-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deodhar AA, Dougados M, Baeten DL, et al. Effect of Secukinumab on Patient-Reported Outcomes in Patients With Active Ankylosing Spondylitis: A Phase III Randomized Trial (MEASURE 1). Arthritis Rheumatol 2016;68:2901-10. 10.1002/art.39805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burgos-Vargas R, Loyola-Sanchez A, Ramiro S, et al. A randomized, double-blind, placebo-controlled 12-week trial of infliximab in patients with juvenile-onset spondyloarthritis. Arthritis Res Ther 2022;24:187. 10.1186/s13075-022-02877-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Heijde D, Baraliakos X, Sieper J, et al. Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. Ann Rheum Dis 2022;81:1515-23. 10.1136/ard-2022-222608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng XH. Clinical and experimental study on the treatment of ankylosing spondylitis with recombinant human soluble tumor necrosis factor receptor-Fc fusion protein etanercept. Chinese People's Liberation Army Military Medical Training College 2006. Available online: https://cdmd.cnki.com.cn/Article/CDMD-90030-2006086006.htm
- 51.Huang F, Zhang J, Huang JL, et al. A multicenter, randomized, double-blind, placebo-controlled clinical study of etanercept in the treatment of ankylosing spondylitis. Chinese Journal of Internal Medicine 2010;49:741-5. [PubMed] [Google Scholar]
- 52.Huang F, Zhang J, Zheng Y, et al. A multicenter, double-blind, randomized controlled clinical study of etanercept in the treatment of active ankylosing spondylitis. Chinese Journal of Internal Medicine 2011;50:5. [PubMed] [Google Scholar]
- 53.Ma H, Sun F, Zhang YG, et al. Efficacy and safety of golimumab in the treatment of 25 cases of active ankylosing spondylitis. Chinese Journal of Internal Medicine 2017;56:5. [Google Scholar]
- 54.Zhang J, Zhang YM, Zhang JL, et al. Efficacy analysis of etanercept in the treatment of ankylosing spondylitis. Chinese Journal of New Drugs 2009;18:1846-1849+1881.
- 55.Zhang YM, Li XL, Wu HL, et al. Short-term safety analysis of etanercept in the treatment of ankylosing spondylitis. China Drug Application and Monitoring 2009;6:3. [Google Scholar]
- 56.Zhang J. A preliminary study on the evaluation method and efficacy analysis of etanercept in the treatment of ankylosing spondylitis. Chinese People's Liberation Army Military Medical Training College 2010. [Google Scholar]
- 57.Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med 2003;9:1245-50. 10.1038/nm939 [DOI] [PubMed] [Google Scholar]
- 58.Hamilton L, Barkham N, Bhalla A, et al. BSR and BHPR guideline for the treatment of axial spondyloarthritis (including ankylosing spondylitis) with biologics. Rheumatology (Oxford) 2017;56:313-6. 10.1093/rheumatology/kew223 [DOI] [PubMed] [Google Scholar]
- 59.Wang P, Zhang S, Hu B, et al. Efficacy and safety of interleukin-17A inhibitors in patients with ankylosing spondylitis: a systematic review and meta-analysis of randomized controlled trials. Clin Rheumatol 2021;40:3053-65. 10.1007/s10067-020-05545-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yin Y, Wang M, Liu M, et al. Efficacy and safety of IL-17 inhibitors for the treatment of ankylosing spondylitis: a systematic review and meta-analysis. Arthritis Res Ther 2020;22:111. 10.1186/s13075-020-02208-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as