Abstract
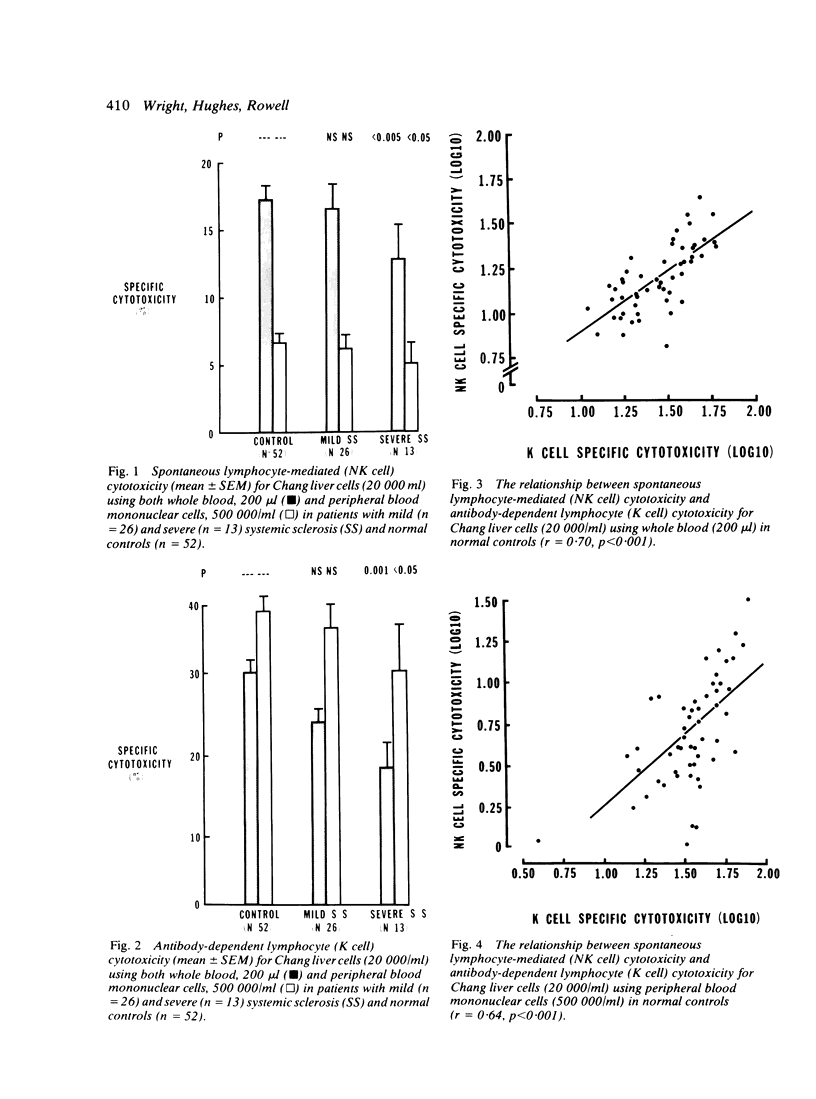
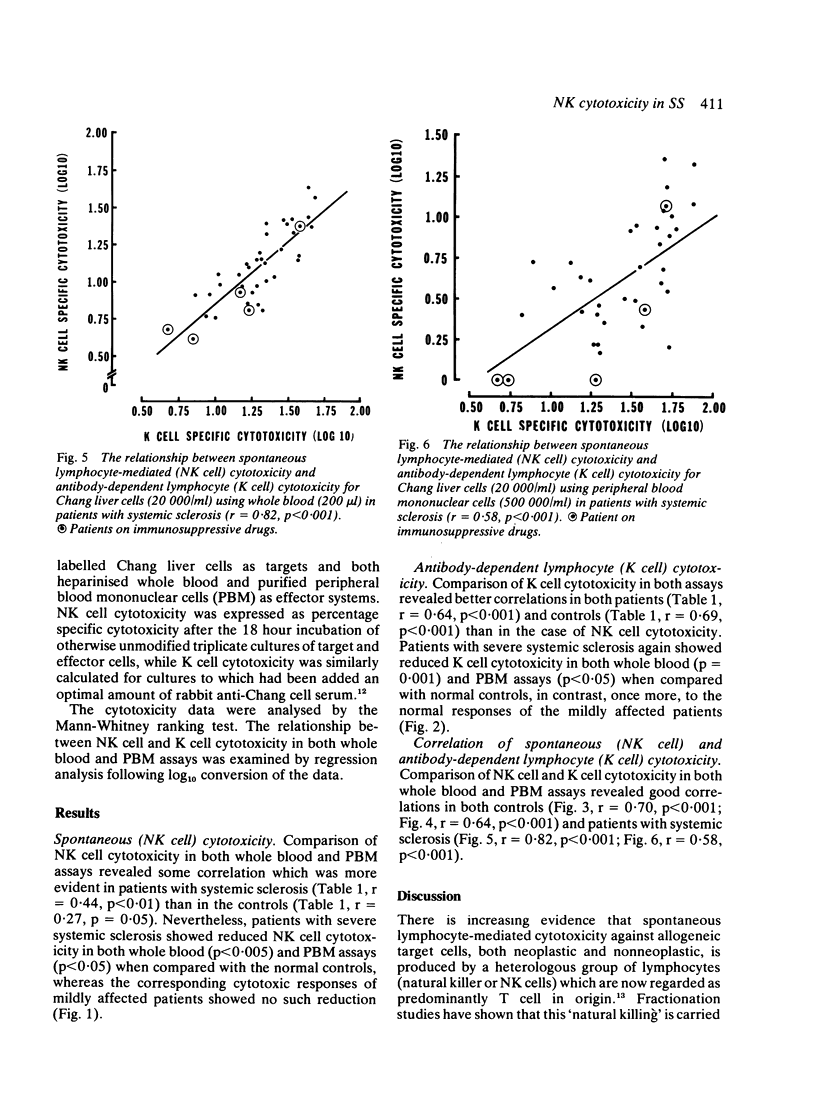
Spontaneous (NK cell) and antibody-dependent (K cell) cytotoxicity were investigated in 39 patients with systemic sclerosis (SS) and compared with that found in 52 normal controls. Cr-labelled Chang liver cells were used as targets in assays utilising both whole blood (WB) and peripheral blood mononuclear cells (PBM) as effectors. Patients with SS, who were severely affected by extensive visceral disease, were found to have significant impairment of both NK (p less than 0.005; p less than 0.05) and K (p less than 0.001; p less than 0.05) cell cytotoxicity by both effector systems, when compared with normal controls. These findings, which seem to be part of a wider defect in cell-mediated immunity, may provide a possible explanation for the described association of malignancy with systemic sclerosis.
Full text
PDF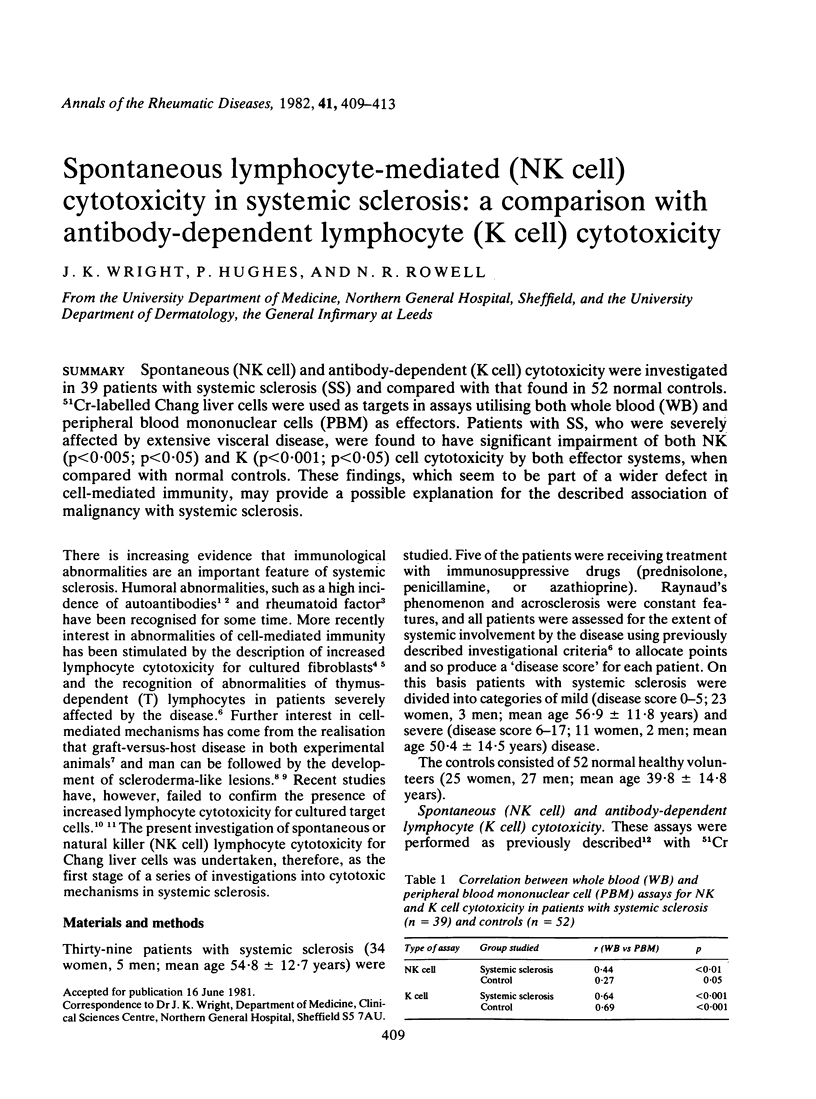


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón-Segovia D., Fishbein E., García-Ortigoza E., Estrada-Parra S. Uracil-specific anti-R.N.A. antibodies in scleroderma. Lancet. 1975 Feb 15;1(7903):363–366. doi: 10.1016/s0140-6736(75)91279-9. [DOI] [PubMed] [Google Scholar]
- BECK J. S., ANDERSON J. R., GRAY K. G., ROWELL N. R. ANTINUCLEAR AND PRECIPITATING AUTOANTIBODIES IN PROGRESSIVE SYSTEMIC SCLEROSIS. Lancet. 1963 Dec 7;2(7319):1188–1190. [PubMed] [Google Scholar]
- Cooper S. M., Friou G. J. Cytotoxicity in progressive systemic sclerosis: no evidence for increased cytotoxicity against fibroblasts of different origin. J Rheumatol. 1979 Jan-Feb;6(1):25–29. [PubMed] [Google Scholar]
- Currie S., Saunders M., Knowles M. Immunological aspects of systemic sclerosis in vitro activity of lymphocytes from patients with the disorder. Br J Dermatol. 1971 May;84(5):400–409. doi: 10.1111/j.1365-2133.1971.tb02523.x. [DOI] [PubMed] [Google Scholar]
- Fenyk J. R., Jr, Smith C. M., Warkentin P. I., Krivit W., Goltz R. W., Neely J. E., Nesbit M. E., Ramsay N. K., Coccia P. F., Kersey J. H. Sclerodermatous graft-versus-host disease limited to an area of measles exanthem. Lancet. 1978 Mar 4;1(8062):472–473. doi: 10.1016/s0140-6736(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Hughes P., Holt S., Rowell N. R., Allonby I. D., Janis K., Dodd J. K. The relationship of defective cell-mediated immunity to visceral disease in systemic sclerosis. Clin Exp Immunol. 1977 May;28(2):233–240. [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Callewaert D. M. Expression of human T-lymphocyte antigens by natural killer cells. J Natl Cancer Inst. 1978 May;60(5):961–964. doi: 10.1093/jnci/60.5.961. [DOI] [PubMed] [Google Scholar]
- Kondo H., Rabin B. S., Rodnan G. P. Cutaneous antigen-stimulating lymphokine production by lymphocytes of patients with progressive systemic sclerosis (scleroderma). J Clin Invest. 1976 Dec;58(6):1388–1394. doi: 10.1172/JCI108594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K., Klein G. O., Kiessling R., Klein G., Roder J. C. Low natural in vivo resistance to syngeneic leukaemias in natural killer-deficient mice. Nature. 1980 Apr 17;284(5757):624–626. doi: 10.1038/284624a0. [DOI] [PubMed] [Google Scholar]
- Lipinski M., Tursz T., Kreis H., Finale Y., Amiel J. L. Dissociation of natural killer cell activity and antibody-dependent cell-mediated cytotoxicity in kidney allograft recipients receiving high-dose immunosuppressive therapy. Transplantation. 1980 Mar;29(3):214–218. doi: 10.1097/00007890-198003000-00010. [DOI] [PubMed] [Google Scholar]
- Masucci G., Poros A., Seeley J. K., Klein E. In vitro generation of K562 killers in human T-lymphocyte subsets. Cell Immunol. 1980 Jul 1;52(2):247–254. doi: 10.1016/0008-8749(80)90346-9. [DOI] [PubMed] [Google Scholar]
- Rothfield N. F., Rodnan G. P. Serum antinuclear antibodies in progressive systemic sclerosis (scleroderma). Arthritis Rheum. 1968 Oct;11(5):607–617. doi: 10.1002/art.1780110502. [DOI] [PubMed] [Google Scholar]
- STASTNY P., STEMBRIDGE V. A., ZIFF M. HOMOLOGOUS DISEASE IN THE ADULT RAT, A MODEL FOR AUTOIMMUNE DISEASE. I. GENERAL FEATURES AND CUTANEOUS LESIONS. J Exp Med. 1963 Oct 1;118:635–648. doi: 10.1084/jem.118.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedmyr E., Jondal M. Cytotoxic effector cells specific for B Cell lines transformed by Epstein-Barr virus are present in patients with infectious mononucleosis. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1622–1626. doi: 10.1073/pnas.72.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge J. E., Meyers K. M., Prieur D. J., Starkey J. R. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980 Apr 17;284(5757):622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- Trayanova T. G., Sura V. V., Svet-Moldavsky G. J. Destruction of human cells in tissue culture by lymphocytes from patients with systemic lupus erythematosus. Lancet. 1966 Feb 26;1(7435):452–454. doi: 10.1016/s0140-6736(66)91456-5. [DOI] [PubMed] [Google Scholar]
- Van Vloten W. A., Scheffer E., Dooren L. J. Localized scleroderma-like lesions after bone marrow transplantation in man. A chronic graft versus host reaction. Br J Dermatol. 1977 Apr;96(4):337–341. doi: 10.1111/j.1365-2133.1977.tb07126.x. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Hughes P., Rowell N. R., Sneddon I. B. Antibody-dependent and phytohaemagglutinin-induced lymphocyte cytotoxicity in systemic sclerosis. Clin Exp Immunol. 1979 Apr;36(1):175–182. [PMC free article] [PubMed] [Google Scholar]


