Highlights
-
•
ΔR1ρ-fraction extracted from R1ρ measurements at different locking fields provides a new way to quantify microvasculature geometry in the brain.
-
•
ΔR1ρ-fraction values in white matter are significantly correlated with the cognitive status of older adults independent of age.
-
•
This study proposes a new non-invasive approach to characterize microvascular changes in MCI patients compared to healthy controls.
Keywords: MRI, R1ρ dispersion imaging, Microvascular impairment, Cognitive abilities
Abstract
Much previous neuroimaging research in Alzheimer’s disease has focused on the roles of amyloid and tau proteins, but recent studies have implicated microvascular changes in white matter as early indicators of damage related to later dementia. We used MRI to derive novel, non-invasive measurements of R1ρ dispersion using different locking fields to characterize variations of microvascular structure and integrity in brain tissues. We developed a non-invasive 3D R1ρ dispersion imaging technique using different locking fields at 3T. We acquired MR images and cognitive assessments of participants with mild cognitive impairment (MCI) and compared them to age-matched healthy controls in a cross-sectional study. After providing informed consent, 40 adults aged 62 to 82 years (n = 17 MCI) were included in this study. White matter ΔR1ρ-fraction measured by R1ρ dispersion imaging showed a strong correlation with the cognitive status of older adults (βstd = −0.4, p-value < 0.01) independent of age, in contrast to other conventional MRI markers such as T2, R1ρ, and white matter hyperintense lesion volume (WMHs) measured with T2-FLAIR. The correlation of WMHs with cognitive status was no longer significant after adjusting for age and sex in linear regression analysis, and the size of the regression coefficient was substantially decreased (53% lower). This work establishes a new non-invasive method that potentially characterizes impairment of the microvascular structure of white matter in MCI patients compared to healthy controls. The application of this method in longitudinal studies would improve our fundamental understanding of the pathophysiologic changes that accompany abnormal cognitive decline with aging and help identify potential targets for treatment of Alzheimer's disease.
1. Introduction
Alzheimer’s disease (AD) is the most frequent form of dementia in older adults (Prohovnik et al., 2006, Brookmeyer et al., 2007) and research is actively underway to improve diagnosis and develop interventions, both to delay disease onset and to slow the progression of the disease (Bayram et al., 2018). There is increasing recognition that our best chance of maintaining brain function until the end of a life span may be to offer therapies as early as possible or when there is still potential to prevent or delay the onset of cognitive decline (Hane et al., 2017).
Recently, several studies have implicated microvascular changes as early indicators of damage related to later dementia (Nguyen et al., 2021, Meyer et al., 2008, Nation et al., 2019, Hase et al., 2019). For example, Hase et al. showed degenerated capillaries in brain tissues of all dementias measured with standard histopathological methods (Hase et al., 2019). Remarkably, white matter (WM) capillary widths were significantly increased by ∼ 20% across all dementias compared to aging and young controls and dilate further with dementia pathogenesis. These findings suggest that alterations in capillaries in the deep WM may be a compensatory response to improve WM perfusion and integrity during hypoperfused states in aging-related dementias. Therefore, detecting and quantifying altered microvascular changes in the brain may provide a sensitive and specific biomarker for diagnosis and predicting progression toward AD. Over the last two decades, white matter hyperintensities (WMHs) visible on MRI scans, defined as cerebral white matter changes presumably of vascular origin, have been measured using T2-weighted and fluid-attenuated inversion recovery MRI sequences (T2-FLAIR) (Wei et al., 2019, Jimenez-Balado et al., 2022). A recent cross-sectional study showed that different spatial configurations of WMHs are correlated with specific cognitive impairment phenotypes (Jimenez-Balado et al., 2022). Longitudinal data from Dadar et al. showed that WMHs contribute to more severe cognitive deficits in AD and frontotemporal dementia compared to controls, whereas their impact in MCI is not significantly different from controls (Jimenez-Balado et al., 2022, Dadar et al., 2022). However, it also has been demonstrated that WMHs are a common finding in cognitively healthy older adults, with prevalence rates ranging between 39 and 96% (Jimenez-Balado et al., 2022, Prins and Scheltens, 2015). Here, we propose and evaluate a novel, non-invasive MRI measure named R1ρ-weighted dispersion (R1ρD) imaging to directly characterize variations of microvascular structure and density in brain tissues (Adelnia et al., 2021, Spear and Gore, 2014, Spear et al., 2014). We employed the method to assess healthy controls and participants with mild cognitive impairment (MCI).
The purpose of the current study was to determine whether MRI-derived parameters extracted from R1ρD imaging can be used to differentiate these two cohorts. We also sought to quantify the effects of age alone, and how these novel parameters correlate with cognitive and behavioral measures. Specifically, we asked (1) Is there a relationship between neuroimaging measures and age that is not dependent on cognitive status or sex? (2) What are the distinct contributions of age and cognitive impairment to changes in neuroimaging measures in a group of individuals ranging from normal cognition to mild cognitive impairment?
2. Material and methods
2.1. Participants
The Institutional Review Board of Vanderbilt University Medical Center approved the study protocols (IRB #202143). Patients with MCI were clinically diagnosed by and recruited from patients referred to the Behavioral Neurology division in our Department of Neurology as showing signs of early onset cognitive impairment secondary to AD. Diagnosis of MCI is typically made after being seen by this specialty division, review of brain imaging, and ruling out other treatable causes of cognitive change, consistent with the DSM-V criteria for a minor neurocognitive disorder. Most of the patients additionally underwent comprehensive assessment with neuropsychological evaluation, performed by a neuropsychologist. Cognitively normal participants were recruited from family members of cognitively impaired patients and from community advertising. After providing informed consent, all participants underwent a comprehensive cognitive assessment and an MRI scan of the brain. Exclusion criteria aside from the standard contraindications to MRI were a history of other psychiatric and neurological diseases, including multiple sclerosis, brain tumor, seizure disorder, major traumatic brain injury, stroke, infection, or any cranial procedure. One female adult in this study was kicked in the head by a horse at age 14. The study included 23 cognitively normal adults and 17 adults with mild cognitive impairment, with sample characteristics shown in Table 1.
Table 1.
Participant characteristics, cognitive indexes, and MRI biomarkers by diagnosis.
|
Healthy (n = 23) |
MCI (n = 17) |
p-value | |
|---|---|---|---|
| Age (years) | 71.61 ± 4.56 | 74.41 ± 6.42 | 0.05 |
| RBANS | 112.8 ± 12.47 | 84.65 ± 11.43 | <0.0001* |
| MoCA | 26.7 ± 2.82 | 22.53 ± 2.55 | 0.0001* |
| Sex, women (%) | 65% | 41% | ¶0.25 |
| College Education (%) | 13.04% | 17.65% | ¶0.71 |
| WM R2 (Hz) | 16.54 ± 0.56 | 16.51 ± 0.62 | 0.58 |
| WM R1ρ (Hz) | 13.79 ± 0.47 | 13.6 ± 0.47 | 0.16 |
| ªWMHs (mL) | 1.45 ± 0.41 | 1.8 ± 0.43 | 0.01* |
| ßN# WMH | 2.06 ± 0.26 | 2.17 ± 0.22 | 0.26 |
| WM ΔR1ρ F | 0.18 ± 0.01 | 0.19 ± 0.01 | 0.003* |
Differences in age, cognitive indexes, and MRI biomarkers between healthy and patients with mild cognitive impairment (MCI) were defined by the Wilcoxon test. Data are expressed as mean ± standard deviation.
Differences in sex and education were defined by Chi-square p-value.
White matter hyperintensity lesion volume (WMHs) is reported as 1 + log (WMHs).
Number of white matter hyperintensity lesions (N# WMH) is reported as 1 + log (N# WMH).
2.2. Examination of the cognitive abilities
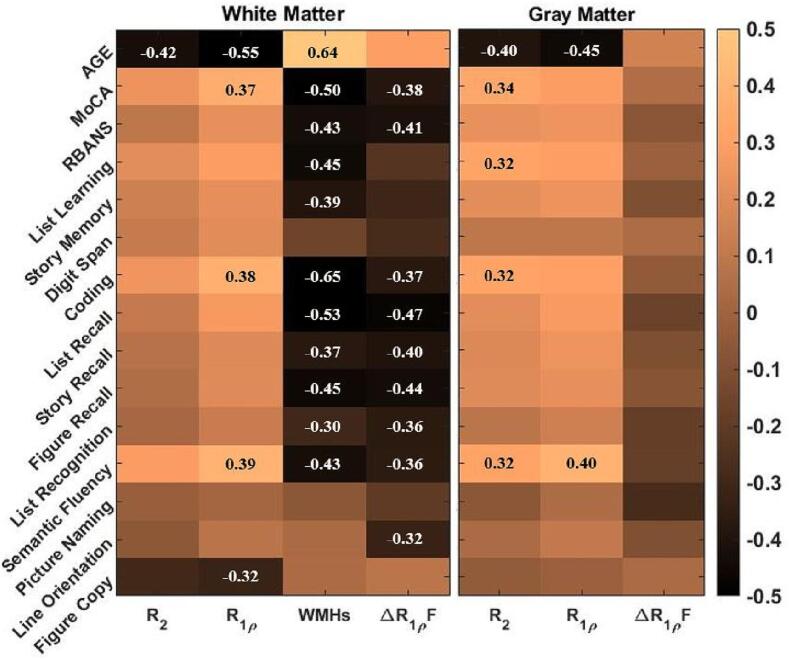
Behavioral tests to evaluate the cognitive abilities of participants included Clinical Dementia Rating Scale (CDR), the Montreal Cognitive Assessment (MoCA), and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). The RBANS consists of 5 Index scores, and 12 sub-indexes include List Recall, Story Recall, Coding, among others (see Fig. 1 and Supplementary Table S1). A trained clinical coordinator (AP) performed cognitive assessments during an in-person visit before the MRI scan following a standard procedure (Randolph et al., 1998). Study participants were classified based on clinical diagnoses into two groups: cognitively healthy persons with CDR of zero and MCI with CDR of 0.5, the latter of which corresponded with the clinical diagnosis previously made by behavioral neurology.
Fig. 1.
Spearman correlation coefficient between the MRI biomarkers and cognitive test indexes. The significant correlations coefficients with p value < 0.05 are added to the relevant quadrants.
2.3. MRI data acquisition
Images were acquired using a 3T Ingenia CX MRI scanner (Philips Healthcare, The Netherlands) equipped with a 32-channel head coil. Initially, the brain was scanned using a 3D dual gradient echo sequence with a FOV of 220 × 180 × 120 mm3 (voxel size 3.4 × 4 × 5 mm3) to acquire a B0 field map, which was then used for image-based field shimming. 3D R1ρ-weighted axial images were obtained using an R1ρ spin-lock preparation (90×-τ/2y-180y-τ/2−y-90×) turbo spin-echo pulse sequence. The acquisition parameters were TR/TE = 3000/10 ms, FOV of 220 × 180 × 120 mm3, voxel size of 1 × 1 × 5 mm3, three locking times; 2, 20, and 48 ms, and two locking field frequency (FSL) of 0 and 300 Hz resulted in 6 dynamics. The scan time for each dynamic was 1 min and 18 sec. The whole brain of each participant was also imaged using conventional multislice T2-FLAIR, 3D-SWI (susceptibility-weighted images), DWI (diffusion-weighted images), and a T1-weighted 3D MPRAGE pulse sequence used for segmentation. All images were reviewed by a board‐certified neuroradiologist (LTD).
2.4. Data analysis
R1ρ (i.e., spin–lattice relaxation rate in the rotating frame) dispersion imaging over weak locking fields reflect the contributions of diffusion of tissue water in intrinsic gradients induced by local inhomogeneities (Adelnia et al., 2021, Spear and Gore, 2016). Such gradients arise from the presence of susceptibility inhomogeneities such as those produced by capillaries and small veins containing paramagnetic deoxyhemoglobin, as well as deoxyhemoglobin-independent factors at microscopic level. Metrics of R1ρ dispersion have the potential to detect abnormal vasculature and specifically may be sensitive to variations in microvascular geometry and density (Spear and Gore, 2014, Zu et al., 2020). Here we choose two different FSLs (0 and 300 Hz) to quantify R1ρ changes. The series of T1ρ-weighted images (T1ρ = 1/R1ρ) acquired from each participant were registered to the T1ρ image collected with FSL = 0 and TSL of 2 ms to correct motion artifacts. R1ρ maps were then generated from a pixel-by-pixel least-squares fit of a monoexponential decay to the signal intensities. The R1ρ value at each FSL was calculated by averaging R1ρ values in white and gray matter regions of interest (ROI) separately. The ΔR1ρ-fraction in each ROI was then calculated by subtracting R1ρ{300 Hz} from R1ρ{0 Hz} and dividing by their average value.
WMHs were quantified using the Lesion Growth Algorithm in the Lesion Segmentation Toolbox of SPM12 with a threshold of 0.1 (Schmidt et al., 2012). The derived lesion probability maps were reviewed and edited in MIPAV by a board‐certified neuroradiologist (LTD). The calculated WMHs and number of lesions were produced based on edited maps using SPM code in MATLAB.
2.5. Statistical analyses
Two‐sample Wilcoxon tests were performed to compare participant characteristics, cognitive indices, and MRI parameters between healthy and MCI groups. Correlations between MRI parameters and cognitive indices were evaluated using the Spearman correlation coefficient for different brain tissues. Linear regression models were used to explore relationships of MRI parameters, including R2 (=R1ρ{0 Hz}), R1ρ (=R1ρ{300 Hz}), ΔR1ρ-fraction with age. The correlations of ΔR1ρ-fraction and WMHs with cognitive status and age were separately and then jointly assessed using linear regression analyses. The interaction between age and cognitive status was tested to understand whether the effect of age on ΔR1ρ-fraction or WMHs is different in healthy or cognitively impaired groups.
The correlation and MRI data analysis were performed in MATLAB (2019b). All regression analyses were performed using R (V3, Austria), and standardized regression coefficients were obtained for multilinear regression analysis. A p-value < 0.05 was considered statistically significant in all analyses.
3. Results
Table 1 compares participant characteristics by clinical diagnosis of cognitive status. Participants of the MCI group showed higher white matter hyperintensity volume and ΔR1ρ-fraction. The cognitive index score of RBANS and MoCA were strongly lower in the MCI group. The sub-index RBANS scores for both groups are reported in Supplementary Table S1. The repeatability of R1ρ dispersion imaging parameters measured on a 26-year-old healthy female are reported in Supplementary Table S2.
The Spearman correlation coefficients between neuroimaging measures and age, cognitive score indexes, and RBANS sub-indexes in white and gray matter are shown in Fig. 1. R2 and R1ρ negatively correlate with age in white and gray matter. ΔR1ρ-fraction of white matter and WMHs illustrated the same behavior in correlation with cognitive index/sub-index scores. Linear regression analysis demonstrated that R2 and R1ρ (= R1ρ{300 Hz}) values of white and gray matter were greater in younger adults, even after adjusting for cognitive status and sex (Table 2). Similar analysis using cognitive index scores, i.e., RBANS and MoCA confirmed that R2 and R1ρ are correlated with aging regardless of the cognition level (Supplementary Table S3). Scatterplots of relationships between age and different MRI parameters in gray and white matter are shown in Supplementary Fig. S1 and S2.
Table 2.
Multiple Linear Regression Models test the association between R2 and R1ρ measured with spin-locking frequency of 300 Hz in white (a) and gray (b) matter with age (Model 1); adjusted by the cognitive status; (Model 2); adjusted by the cognitive status and sex of participants (Model 3).
|
White Matter |
Gray Matter |
|||||||
|---|---|---|---|---|---|---|---|---|
|
R2 |
R1ρ |
R2 |
R1ρ |
|||||
|
β (95 %CI) |
p-value |
β (95 %CI) |
p-value |
β (95 %CI) |
p-value |
β (95% CI) |
p-value | |
| Model 1 | adj_R2 = 0.149 | adj_R2 = 0.264 | adj_R2 = 0.148 | adj_R2 = 0.194 | ||||
| Age | −0.414 | 0.008* | −0.532 | 0.0004* | −0.412 | 0.008* | 0.463 | 0.003* |
| Model 2 | adj_R2 = 0.134 | adj_R2 = 0.249 | adj_R2 = 0.126 | adj_R2 = 0.176 | ||||
| Age | −0.435 | 0.008* | −0.514 | 0.001* | −0.419 | 0.01* | 0.446 | 0.005* |
| §Cognitive status | −0.085 | 0.583 | 0.069 | 0.631 | −0.027 | 0.864 | 0.066 | 0.664 |
| Model 3 | adj_R2 = 0.110 | adj_R2 = 0.228 | adj_R2 = 0.122 | adj_R2 = 0.157 | ||||
| Age | −0.435 | 0.008* | −0.514 | 0.001* | −0.419 | 0.011* | 0.446 | 0.006* |
| §Cognitive status | −0.079 | 0.627 | 0.069 | 0.648 | −0.06 | 0.708 | 0.051 | 0.746 |
| Sex | −0.028 | 0.858 | 0.003 | 0.985 | 0.141 | 0.369 | 0.062 | 0.684 |
An indicator variable used to distinguish healthy control (CDR = 0) from patients with MCI (CDR = 0.5). Similar analysis using cognitive index scores, i.e., RBANS and MoCA are reported in Table SC.
Statistically significant (p-value < 0.05).
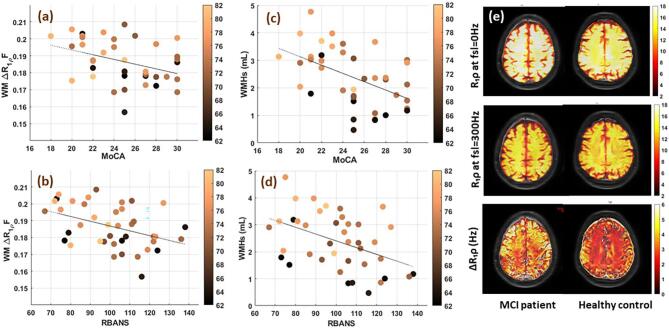
Scatterplots of relationships between age and WMHs/white matter ΔR1ρ-fraction and cognitive indexes of RBANS and MoCA are shown in Fig. 2. To examine the contribution of age and cognitive decline to the changes of WMH/white matter ΔR1ρ-fraction, we performed multilinear regression analysis with both age and cognitive status as independent variables (Table 3). The cognitive status of the individuals shows a stronger correlation with WM ΔR1ρ-fraction (β = −0.474, p-value = 0.002) and does not change (β = −0.424, p-value = 0.006), after adjusting for age. While the correlation of WMH with cognitive status of the participants strongly decreases after accounting for age and is no longer significant, demonstrating that WMH is more age dependent. Similar analysis using RBANS and MoCA, instead of cognitive status confirmed these findings (Supplementary Table S4).
Fig. 2.
Scatterplots of relationships between White Matter ΔR1ρ Fraction and cognitive indexes of (a) MoCA, (b) RBANS and between WMHs and cognitive indexes of (c) MoCA, (d) RBANS. (e) R1ρ maps measured at spin locking field (FSL) of 0 (top), 300 Hz (middle) and ΔR1ρ (=R1ρ{0 Hz} - R1ρ{300 Hz}) maps of 71 years old female healthy control (right), and 72 years female MCI patient (left).
Table 3.
Multiple Linear Regression Models test the association between different MRI biomarkers and the cognitive status of the participants (Model 1); adjusted by age (Model 2); adjusted by age and sex (Model 3).
|
ΔR1ρ-Fraction |
ªWMHs |
|||
|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | |
| Model 1 | adj_R2 = 0.21 | adj_R2 = 0.134 | ||
| §Cognitive status | −0.474 | 0.002* | −0.395 | 0.012* |
| Model 1 | adj_R2 = 0.07 | adj_R2 = 0.357 | ||
| Age | 0.305 | 0.055 | 0.611 | 0.00002* |
| Model 2 | adj_R2 = 0.222 | adj_R2 = 0.404 | ||
| §Cognitive status | −0.424 | 0.006* | −0.256 | 0.052 |
| Age | 0.198 | 0.184 | 0.546 | 0.0001* |
| Model 3 | adj_R2 = 0.209 | adj_R2 = 0.482 | ||
| §Cognitive status | −0.402 | 0.012* | −0.184 | 0.142 |
| Age | 0.198 | 0.188 | 0.546 | <0.0001* |
| Sex | −0.092 | 0.536 | −0.303 | 0.015* |
All regression coefficients are standardized. The interaction term between age and cognitive status was not significant in model 2 (not shown here).
Abbreviations: CI, confidence interval; WMHs, white matter hyperintensity lesion volume; WMHs is ∼ 1 + log (WMH).
An indicator variable used to distinguish healthy control (CDR = 0) from patients with MCI (CDR = 0.5). Similar analysis using cognitive index scores, i.e., RBANS and MoCA are reported in Table S4.
Statistically significant (p-value < 0.05).
4. Discussion
4.1. An aging study
The effect of aging on brain tissue composition has previously been quantified in terms of magnetic resonance relaxation properties of water. It has been shown that T2-relaxation values typically increase with age in multiple brain sites in adults aged from 30 to 66 years old, consistent with an increase in tissue water content (Kumar et al., 2012, MacDonald and Pike, 2021). Only a few regions, such as the putamen and ventral pons, show T2 decreases with age, possibly due to the deposition of iron-bearing proteins in those sites (Kumar et al., 2012). Sex-related differences in T2-relaxation have also been reported in frontal, basal ganglia, thalamic, temporal, occipital, and cerebellar areas (Kumar et al., 2012). Consistent with previous studies, we observed significant age-related reductions in R2 (=1/T2) relaxation rates of WM and GM in our sample of older adults aged from 62 to 82 years across all the individuals studied. R2 is expected to decrease slowly with aging if water content in tissues increases as a consequence of loss of axons or macromolecules. Our results suggest these processes continue even with advancing age in older adults aged from 62 to 82 years in gray and white matter independent of cognitive status.
Our results also showed that R1ρ relaxation rate measured with a locking frequency of 300 Hz in WM and GM decreased with age in older adults. Previously, Haris et al. did not find a signification correlation between age and R1ρ values in white and gray matter measured with a 1.5 T scanner and FSL of 500 Hz, whereas Watts et al. showed that R1ρ values measured with a 3T scanner and FSL of 500 Hz significantly decrease with age in white matter tracts except the WM corticospinal tracts and forceps major, cortical gray matter, putamen, amygdala, and nucleus accumbens (Haris et al., 2011, Watts et al., 2014). It is difficult to compare these results directly because they were measured with different acquisition parameters and static magnetic fields.
The choice of acquisition parameters is particularly important for measurements of R1ρ because it is affected by different relaxation processes whose relative contributions vary with FSL and main magnetic fields. R1ρ values measured at higher magnetic fields (≥3T) are more sensitive to effects of chemical exchange and diffusion amongst susceptibility gradients than at lower fields, while at 3T the contributions of diffusion are more significant at lower locking fields than chemical exchange (Spear and Gore, 2016). Therefore, the differences in R1ρ values between FSL = 300 and 0 reflect diffusion contributions (Adelnia et al., 2021, Spear and Gore, 2014), whereas between FSL 300 and ≥ 500 reflect chemical exchange effects (Spear and Gore, 2016). These dependencies may explain discrepancies between previous R1ρ studies in brain and ours, and emphasize the fact that measurements of R1ρ at a single locking field cannot be interpreted as caused by any single mechanism. The significant interaction between R1ρ measured with FSL 300 Hz in this study and age (Spearman βWM = −0.42; Spearman βGM = −0.45) may reflect the increase of water content in tissue similar to T2-relaxation and a contribution of exchange between water molecules and neurofibrillary tangles (NFTs) and β-amyloid plaques deposited in the brain tissue of older adults. The exchange contribution depends not only on the concentration of macromolecules but also the chemical shifts and exchange rates of the various protons involved, which in turn may depend on pH (Spear and Gore, 2016, Cobb et al., 2011).
In agreement with previous studies, our results showed a significant correlation between WMHs with age in older adults, which remained significant after accounting for sex and cognitive status. Our findings confirm that although WMHs can be associated with various neurological and pathological conditions, they show a stronger correlation with normal brain aging and not, specifically, the early stage of abnormal cognitive decline. On the other hand, WM ΔR1ρ-fraction showed a weak correlation with age, which significantly declined after accounting for cognitive status and sex.
4.2. A cognitive study
Our patients with MCI showed a lower performance on all domains and subsets of the RBANS. These results are consistent with previous reports on patients with AD25 and older adults classified as MCI (Duff et al., 2010). The highest deficits were observed in immediate and delayed memory. The List learning showed the largest difference between healthy controls and patients with MCI within the subset of immediate memory. The List Recall, Story Recall, and Figure Recall show higher contributions to the delayed memory deficit and higher declines in subjects with cognitive impairment. Semantic Fluency and Coding were other clinically relevant subtests strongly associated with cognitive impairment. Similar to previous findings, Picture Naming showed the lowest difference between cognitively impaired patients and healthy controls (p-value > 0.1) (Duff et al., 2008). We also find a significant decline in the Attention memory domain, which was not observed previously in MCI compared to healthy controls (Duff et al., 2010).
Using MRI, it has been shown that WMHs are significantly lower in healthy controls than in subjects with cognitive impairment. For example, Wei et al. showed that the increase of WMHs is associated with age (r = 0.31, p-value = 0.013) and CSF β-amyloid (r = 0.33, p-value = 0.013) separately in a cohort of adults with MCI and healthy controls (Wei et al., 2019). The authors did not verify the contribution of both age and CSF β-amyloid concentration jointly to the changes of WMHs. Though the significant correlation between WMHs and cognitive impairment has been confirmed in several studies, cardiovascular risk factors (King et al., 2013), geriatric syndrome, and age (Saji et al., 2015) are consistently proven to be the strongest predictors of WMH lesion accumulation in community-based studies and not specifically the cognitive status of participants. In line with those studies, we showed that the age of participants is a stronger predictor of higher WMH lesions compared to the clinically assessed levels of cognition. We also found similar results by using the MoCA and RBANS indexes instead of the participant's cognitive status, demonstrating the clinical utility of RBANS/MoCA in detecting cognitive impairment in older adults. Despite this demonstration, the test–retest reliability is poor for cognitive tests such as MoCA, and individuals at the early stage of cognitive impairment with low scores show a better performance in the second assessment. More reliable neuropsychological tests are needed for repeated measurements.
Previous longitudinal data confirmed that primary cardiovascular risk factors such as pulse wave velocity from the aortic arch, systolic blood pressure, hypertension treatment, and congestive heart failure are independent predictors of subsequent WMH lesions in older adults after age (King et al., 2013). In different studies, histopathological findings show that in addition to demyelination, loss of glial cells, axon damage, and spongiosis, WMHs include collagenous thickening and occlusion of venules which are thought to happen because of the insufficient blood supply to the cerebral deep white matter due to vascular pathology (Hase et al., 2019, Challa et al., 2004). Recent post-mortem brain tissue studies showed that microvascular densities decreased by 18% in white matter of patients with AD compared to healthy controls, while the severity of string capillaries or microvessels is higher (Brown, 2010). They also have shown that the capillaries in the WM are marginally but significantly wider in all dementias irrespective of type compared to healthy aging and young controls. Here, we use a novel non-invasive parameter (i.e., ΔR1ρ-fraction measured with R1ρ dispersion imaging) to quantify the proposed microvasculature restructuring measured in post-mortem brain tissue of AD and dementia subjects. Our data showed a significant correlation between WM ΔR1ρ-fraction and cognitive index scores of RBANS/MoCA even after adjusting for age, so the factors that contribute to ΔR1ρ-fraction also affect cognitive status, independent of age, in contrast to WMHs. Theoretical and animal model studies suggest that a larger ΔR1ρ-fraction may arise because of increases in microvascular size and spacing and intrinsic magnetic susceptibility gradients caused by deoxyhemoglobin (Spear and Gore, 2014, Spear et al., 2014). Unlike other parameters, ΔR1ρ-fraction should be independent of changes in tissue water content. We interpret a higher WM ΔR1ρ-fraction in subjects with MCI as reflecting changes in capillary geometry and microvascular restructuring as measured in cognitively impaired post-mortem tissue subjects. This work supports the hypothesis that microvascular deformation in deep white matter differs in adults with abnormal cognitive impairment compared to healthy controls, which can be characterized using ΔR1ρ-fraction values measured over low locking fields.
5. Limitation
We have successfully measured ΔR1ρ changes in a small cohort of older adults, including patients with MCI and healthy controls. Although the results are promising, additional experiments are essential to validate the biological origin of this biomarker in AD and demented brain tissues. This study also needs to be verified in a larger sample size to establish whether ΔR1ρ-fraction is a biomarker of WM disorder in cognitively impaired subjects compared to healthy individuals. Further longitudinal studies are essential to confirm whether this method can capture microvascular structural changes over time with the progression of cognitive impairment that may lead to dementia. Another limitation of this study is the lack of information on attributable risk factors leading to cognitive impairment, namely controlled type 2 diabetes and cardiovascular disease that could be used as covariates in the regression analysis. Additionally, an ROI-based analysis that targets different domains of the brain could clarify the mechanism of cognitive impairment. Such an analysis was not justified in the present study because of the small sample size.
6. Conclusion
In conclusion, the results presented here indicate that WM ΔR1ρ-fraction has a strong correlation with the cognitive level of older adults independent of age in contrast to R2, R1ρ measured at FSL = 300 Hz and WMHs. Our results suggest that changes in microvascular integrity and structure as early indicators of cognitive impairment may be measurable by R1ρ dispersion imaging. Our study also confirms the use of the behavioral RBANS and MoCA tests in clinical studies for the screening of subjects at first visit to quantify human cognitive impairment.
Ethics Statement
Data collection was conducted in accordance with protocols approved by the Institutional Review Board of Vanderbilt University Medical Center. All participants gave written informed consent and were compensated for their participation.
Source of funding
This work was supported by NIH/NIBIB grants R01EB024525 and R01EB024525 Supplement awarded to John C. Gore. Fatemeh Adelnia is funded by a K25 career development award, 1K25AG076864-01, from National Institute on Aging (NIH). Authors have no conflicts to disclose.
CRediT authorship contribution statement
Fatemeh Adelnia: Conceptualization, Methodology, Data curation, Project administration, Formal analysis, Investigation, Supervision, Visualization, Writing - original draft, Writing - review & editing. Larry T. Davis: Formal analysis, Supervision, Visualization, Writing - review & editing. Lealani Mae Acosta: Resources, Supervision, Writing - original draft. Amanda Puckett: Project administration, Data curation, Visualization, Writing - review & editing. Feng Wang: Methodology, Writing - review & editing. Zhongliang Zu: Methodology, Writing - review & editing. Kevin D. Harkins: Formal analysis, Methodology, Writing - review & editing. John C. Gore: Conceptualization, Methodology, Investigation, Resources, Supervision, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the invaluable assistance of Saikat Sengupta, Ph.D. (Vanderbilt University Institute of Imaging Science), for setting up the image-based shimming tool for the 3T MRI scanner; Hakmook Kang, Ph.D. (Department of Biostatistics, Vanderbilt University Medical Center) for consultation on statistical analysis; Muwei Li, Ph.D., and Arabinda Mishra, Ph.D. (Vanderbilt University Institute of Imaging Science) for assistance with SPM12 software. We also thank the subjects and their caregivers for their participation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103366.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Adelnia F., Zu Z., Spear J.T., Wang F., Harkins K.D., Gore J.C. Tissue characterization using R1rho dispersion imaging at low locking fields. Magn. Reson. Imaging. 2021;84:1–11. doi: 10.1016/j.mri.2021.05.006. [DOI] [PubMed] [Google Scholar]
- Bayram E., Caldwell J.Z.K., Banks S.J. Current understanding of magnetic resonance imaging biomarkers and memory in Alzheimer's disease. Alzheimers Dement.. (N Y). 2018;4:395–413. doi: 10.1016/j.trci.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Brown W.R. A review of string vessels or collapsed, empty basement membrane tubes. J. Alzheimers Dis. 2010;21(3):725–739. doi: 10.3233/JAD-2010-100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challa V.R., Thore C.R., Moody D.M., Anstrom J.A., Brown W.R. Increase of white matter string vessels in Alzheimer's disease. J. Alzheimers Dis. 2004;6(4):379–383. doi: 10.3233/jad-2004-6404. discussion 443–379. [DOI] [PubMed] [Google Scholar]
- Cobb J.G., Xie J., Gore J.C. Contributions of chemical exchange to T1rho dispersion in a tissue model. Magn. Reson. Med. 2011;66(6):1563–1571. doi: 10.1002/mrm.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M., Manera A.L., Ducharme S., Collins D.L. White matter hyperintensities are associated with grey matter atrophy and cognitive decline in Alzheimer's disease and frontotemporal dementia. Neurobiol. Aging. 2022;111:54–63. doi: 10.1016/j.neurobiolaging.2021.11.007. [DOI] [PubMed] [Google Scholar]
- Duff K., Humphreys Clark J.D., O'Bryant S.E., Mold J.W., Schiffer R.B., Sutker P.B. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer's disease: sensitivity, specificity, and positive and negative predictive powers. Arch. Clin. Neuropsychol. 2008;23(5):603–612. doi: 10.1016/j.acn.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K., Hobson V.L., Beglinger L.J., O'Bryant S.E. Diagnostic accuracy of the RBANS in mild cognitive impairment: limitations on assessing milder impairments. Arch. Clin. Neuropsychol. 2010;25(5):429–441. doi: 10.1093/arclin/acq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane F.T., Robinson M., Lee B.Y., Bai O., Leonenko Z., Albert M.S. Recent progress in Alzheimer's disease research, Part 3: Diagnosis and treatment. J. Alzheimers Dis. 2017;57(3):645–665. doi: 10.3233/JAD-160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haris M., Singh A., Cai K., et al. T(1rho) MRI in Alzheimer's disease: detection of pathological changes in medial temporal lobe. J. Neuroimaging. 2011;21(2):e86–e90. doi: 10.1111/j.1552-6569.2010.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase Y., Ding R., Harrison G., et al. White matter capillaries in vascular and neurodegenerative dementias. Acta Neuropathol. Commun. 2019;7(1):16. doi: 10.1186/s40478-019-0666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Balado J., Corlier F., Habeck C., Stern Y., Eich T. Effects of white matter hyperintensities distribution and clustering on late-life cognitive impairment. Sci. Rep. 2022;12(1):1955. doi: 10.1038/s41598-022-06019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King K.S., Chen K.X., Hulsey K.M., et al. White matter hyperintensities: use of aortic arch pulse wave velocity to predict volume independent of other cardiovascular risk factors. Radiology. 2013;267(3):709–717. doi: 10.1148/radiol.13121598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Delshad S., Woo M.A., Macey P.M., Harper R.M. Age-related regional brain T2-relaxation changes in healthy adults. J. Magn. Reson. Imaging. 2012;35(2):300–308. doi: 10.1002/jmri.22831. [DOI] [PubMed] [Google Scholar]
- MacDonald M.E., Pike G.B. MRI of healthy brain aging: A review. NMR Biomed. 2021;34(9):e4564. doi: 10.1002/nbm.4564. [DOI] [PubMed] [Google Scholar]
- Meyer E.P., Ulmann-Schuler A., Staufenbiel M., Krucker T. Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2008;105(9):3587–3592. doi: 10.1073/pnas.0709788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation D.A., Sweeney M.D., Montagne A., et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019;25(2):270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen B., Bix G., Yao Y. Basal lamina changes in neurodegenerative disorders. Mol. Neurodegener. 2021;16(1):81. doi: 10.1186/s13024-021-00502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins N.D., Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat. Rev. Neurol. 2015;11(3):157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- Prohovnik I., Perl D.P., Davis K.L., Libow L., Lesser G., Haroutunian V. Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology. 2006;66(1):49–55. doi: 10.1212/01.wnl.0000191298.68045.50. [DOI] [PubMed] [Google Scholar]
- Randolph C., Tierney M.C., Mohr E., Chase T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Saji N., Ogama N., Toba K., Sakurai T. White matter hyperintensities and geriatric syndrome: An important role of arterial stiffness. Geriatr. Gerontol. Int. 2015;15(Suppl 1):17–25. doi: 10.1111/ggi.12673. [DOI] [PubMed] [Google Scholar]
- Schmidt P., Gaser C., Arsic M., et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59(4):3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Spear J.T., Gore J.C. Effects of diffusion in magnetically inhomogeneous media on rotating frame spin-lattice relaxation. J. Magn. Reson. 2014;249:80–87. doi: 10.1016/j.jmr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear J.T., Gore J.C. New insights into rotating frame relaxation at high field. NMR Biomed. 2016;29(9):1258–1273. doi: 10.1002/nbm.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear J.T., Zu Z., Gore J.C. Dispersion of relaxation rates in the rotating frame under the action of spin-locking pulses and diffusion in inhomogeneous magnetic fields. Magn. Reson. Med. 2014;71(5):1906–1911. doi: 10.1002/mrm.24837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts R., Andrews T., Hipko S., Gonyea J.V., Filippi C.G. In vivo whole-brain T1-rho mapping across adulthood: normative values and age dependence. J. Magn. Reson. Imaging. 2014;40(2):376–382. doi: 10.1002/jmri.24358. [DOI] [PubMed] [Google Scholar]
- Wei K., Tran T., Chu K., et al. White matter hypointensities and hyperintensities have equivalent correlations with age and CSF beta-amyloid in the nondemented elderly. Brain Behav. 2019;9(12):e01457. doi: 10.1002/brb3.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Z., Janve V., Gore J.C. Spin-lock imaging of intrinsic susceptibility gradients in tumors. Magn. Reson. Med. 2020;83(5):1587–1595. doi: 10.1002/mrm.28155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.