Abstract
Chlamydia pneumoniae is an obligate intracellular bacterium which frequently causes airway infection in humans and has been implicated in atherosclerosis. Here we show that infection with C. pneumoniae protects HeLa human epithelioid cells against apoptosis induced by external stimuli. In infected HeLa cells, apoptosis induced by staurosporine and CD95-death-receptor signaling was strongly reduced. Upon treatment with staurosporine, generation of effector caspase activity, processing of caspase-3 and caspase-9 and cytochrome c redistribution were all profoundly inhibited in cells infected with C. pneumoniae. Bacterial protein synthesis during early infection was required for this inhibition. Furthermore, cytochrome c-induced processing and activation of caspases were inhibited in cytosolic extracts from infected cells, suggesting that a C. pneumoniae-dependent antiapoptotic factor was generated in the cytosol upon infection. Infection with C. pneumoniae failed to induce significant NF-κB activation in HeLa cells, indicating that no NF-κB-dependent cellular factors were involved in the protection against apoptosis. These results show that C. pneumoniae is capable of interfering with the host cell's apoptotic apparatus at probably at least two steps in signal transduction and might explain the propensity of these bacteria to cause chronic infections in humans.
All mammalian cells contain an intracellular device which is used to kill the cell upon a specific signal and by a process known as apoptosis (33). Apoptosis is implemented by a specialized signal transduction pathway, and an important function of apoptosis, besides its roles in embryonic development and tissue homeostasis (15, 33), is the defense against environmental stimuli which endanger the organism's integrity; this task involves responses not only to DNA-damaging agents but also to infectious microorganisms (13, 25, 26).
In viral infections apoptosis is very likely used as such a defense mechanism by the host cell. Since the virus depends on the cell to reproduce, death by apoptosis will withdraw the basis for viral replication. Furthermore, the catabolic processes inside a cell dying by apoptosis are likely to degrade viral components, thereby putting an end to the infection. This interpretation is supported by the observation that a number of viruses carry genes whose products can interfere with the cell's apoptosis system and thereby inhibit apoptosis (reviewed in, e.g., reference 22, 32). Some bacterial species also can replicate only inside a host cell. Although they differ from viruses in that they carry their own complete replication machinery, their developmental cycle is adapted in such a way that they can grow only within the host cell and often depend on metabolites provided by the host. The genus Chlamydia encompasses three such species that are pathogenic to humans, Chlamydia trachomatis, Chlamydia psittaci, and Chlamydia pneumoniae, all of which can cause both acute and chronic infections in humans. C. pneumoniae is a common cause of airway infection in humans. The pathogen has also been isolated from arterial walls in humans, and components of the bacteria have frequently been detected in human peripheral blood cells. Although a role for C. pneumoniae in atherosclerosis is still a matter of contention, there is clear evidence that the bacteria can be present within human cells for an extended period of time (2, 10, 29). These data suggest that C. pneumoniae can infect and survive in a variety of cell types in vivo; productive infection of various types of human cells, such as epithelial cells, endothelial cells, smooth muscle cells, and macrophages, has been reproduced in vitro (6, 9).
Several recent studies have addressed the question of whether chlamydial infection interferes with apoptosis. Like viruses, chlamydia depend on host cell factors to replicate, which suggests that the death of an infected cell could impede bacterial replication and thus be favorable to the host. The published results do not, however, unequivocally support this notion. C. psittaci has been found to induce rather than to inhibit apoptosis in infected epithelioid cells and macrophages in vitro (8, 27). C. trachomatis has varyingly been reported to induce apoptosis in vitro (8) and in vivo (during genital infection in mice (28)) and to inhibit experimentally induced apoptosis in vitro (5). The exact reasons for these conflicting findings are unclear (see Discussion).
Although it is largely unclear which signals cause apoptosis in vivo, recent research has greatly enhanced our understanding of the signal transduction in the cell death pathway. At a central position in this pathway, members of the caspase family of cysteine proteases transmit the apoptotic signal and induce the morphological and biochemical changes of apoptosis. Caspases are present in probably all nucleated cells as inactive zymogens (pro-caspases) and become activated upon the signal to apoptosis. Probably two tiers of different caspases are consecutively activated by such a signal, a so-called initiator caspase (probably mainly caspase-8 and−9) and a number of effector caspases (this role has been attributed most often to caspase-3). Effector caspase activity serves to trigger further effector mechanisms and to degrade cellular components, leading to death and disposal of the cell (for a review see references 4 and 30).
Here we investigate how infection with C. pneumoniae affects apoptosis signal transduction in infected human epithelioid cells. Changes in the apoptotic response were assessed in intact cells and in cell extracts. Some of the possible respective contributions of bacteria and the host cell were analyzed.
MATERIALS AND METHODS
Cell lines, chlamydial organisms, and reagents.
The human cervical adenocarcinoma cell line, HeLa 229, and the human laryngeal carcinoma cell line, Hep2, were obtained from the American Type Culture Collection. Cell culturing was done at 37°C with 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum (FCS) and 2 mM l-glutamine. No antibiotics were added. For the propagation of bacteria, cycloheximide (1 μg/ml) was added and culturing was done in the absence of FCS. The mycoplasma-free Chlamydia pneumoniae strain CM-1 (VR-1360) was obtained from the American Type Culture Collection.
For the propagation of chlamydia, Hep2 cells were infected with C. pneumoniae in six-well cell culture plates in a protocol involving centrifugation of bacterial organisms on a host cell monolayer (18). At 72 h of infection, organisms were released from the cells by homogenization and purified on a density gradient as described previously (14, 17). Titers of infection were determined by a serial dilution of preparations in HeLa cells followed by intracellular staining for chlamydial inclusions with a fluorescence-labeled anti-C. pneumoniae-LPS antibody on day 2 (Progen). Harvests were checked for mycoplasmal contamination by PCR, and purified elementary bodies were frozen in aliquots at −70°C for up to 2 months and thawed immediately before infection. Staurosporine, 2,4-dinitrophenol (DNP), and cycloheximide were from Sigma. Anti-CD95 monoclonal antibody (MAb) CH11 was purchased from Upstate Biosciences, and rifampin was from Calbiochem.
Infection of HeLa cells and induction of apoptosis.
HeLa cells were infected with C. pneumoniae as described previously (18). The number of bacteria used was in the range of 1 to 3 inclusion-forming units (IFU) per HeLa cell as specified in the figure legends. HeLa cells were seeded into 6-well (2.5 × 105 cells/well) or 12-well (105 cells/well) plates the day before infection. The next day, medium was replaced with Dulbecco's modified Eagle's medium without FCS, and cells were infected by addition of chlamydia followed by centrifugation for 45 min at 800 × g at 35°C; 10% FCS was added after 3 h. In most experiments, wells were split (1:2) after 24 h. Mock-infected cells were subjected to the same procedure in the absence of chlamydia. Apoptosis was induced by addition of staurosporine (1 μM) or anti-CD95 MAb (100 ng/ml) and DNP (1 mM). Cells were harvested and analyzed as specified in the figure legends.
Assay for nuclear apoptosis.
HeLa cells (2.5 × 105/well) were infected with C. pneumoniae (1 IFU/cell) or mock infected. The next day, cells were split and seeded on glass coverslips in a 12-well plate. Replicate wells were infected with C. pneumoniae (1 IFU/cell) or mock infected. On day 3 of infection, three replicates each were treated with 1 μM staurosporine for 4 h or anti-CD95 and DNP for 5 to 7 h. Cells were then stained with 20 μM Hoechst 33258 (Sigma) for 30 min at 37°C and washed with phosphate-buffered saline (PBS), and nuclear morphology was assessed under a fluorescence microscope. At least 300 nuclei per sample were counted.
Assay for fragmentation of chromosomal DNA.
Infected (1 IFU per cell) or mock-infected HeLa cells (2.5 × 105 cells/well in six-well plates) were split on day 1 and left untreated or were treated with 1 μM staurosporine for 10 h on day 2 of infection. The cells were then harvested by trypsinization, washed with PBS, lysed in detergent-containing buffer (150 mM NaCl–0.5% SDS) supplemented with 500 μg of Proteinase K/ml, and incubated at 37°C for 12 h. Reactions were extracted with phenol-chloroform-isoamylalcohol, and DNA was precipitated by addition of 1 volume of isopropanol. Pellets were washed and redissolved in Tris-EDTA buffer containing RNase A. After incubation at 37°C for 1 h, DNA was run on a 1% agarose gel containing ethidium bromide.
Assay for caspase activity.
HeLa cells were infected (1 IFU per cell) in 12-well plates. To some wells rifampin (10 μg/ml) was added at the time of infection or at different times after infection as indicated in the figure legends. On day 2 of infection, some wells were treated with staurosporine (1 μM) for 4 h or with anti-CD95 and DNP for 6 h. The cells were harvested by trypsinization, washed with PBS, and lysed by incubation in 40 μl of NP-40 lysis buffer (150 mM NaCl, 1% Ipegal CA-630, 50 mM Tris [pH 8.0]) for 15 min on ice. Cell lysates were cleared by centrifugation for 5 min at 15,000 × g at 4°C. Triplicates of 10-μl aliquots of the supernatant were added to 90 μl of DEVD assay buffer (50 mM NaCl, 2 mM MgCl2, 40 mM β-glycerophosphate, 5 mM EGTA, 0,1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate [CHAPS], 100-μg/ml bovine serum albumin, 10 mM HEPES [pH 7.0]) containing 10 μM (final concentration) DEVD–7-amino-4-methyl-coumarin (AMC) fluorimetric substrate. Reactions were incubated for 1 h in 96-well flat-bottomed plates at 37°C. Free AMC was measured at wavelengths of 380 nm (excitation) and 460 nm (emission), and values are presented as arbitrary relative fluorescence units (mean and standard error of the mean for the above-described triplicate reactions).
Western blot analysis.
Infected (1 IFU/cell) or uninfected HeLa cells in 6- or 12-well plates were harvested at various times, washed, and lysed in 40 μl of NP-40 buffer per well as described above. Fifteen-microliter aliquots were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and probed with antibodies specific for human caspase-3, caspase-9, XIAP, and Bcl-2 (all from Pharmingen/Becton Dickinson) or heat shock protein 70 (Stressgen). Proteins were visualized using peroxidase-conjugated secondary antibodies and a chemiluminescence detection system (Roche). In some experiments cells were treated with staurosporine (1 μM) for 4 h prior to analysis, and in some experiments cultures were treated with rifampin (10 μg/ml).
Preparation of cell extracts and caspase activation in extracts.
Infected (1 IFU per cell) or mock-infected HeLa cells were collected on day 3 of infection (cells were infected in six-well plates [between three and nine wells per experiment] and split onto 15-cm-diameter dishes on day 1 of infection). Cells were washed once with PBS and once with cytoplasmic extraction buffer (1 mM Na-EGTA, 1 mM Na-EDTA, 1.5 mM MgCl2, 10 mM KCl, 20 mM HEPES-KOH [pH 7.5]). Cell pellets were resuspended in the same buffer containing a mixture of proteinase inhibitors (Roche) and 1 mM dithiothreitol and incubated for 1 h on ice. The cells were then lysed by repeated passages through a 22-G needle until 80% of cells were disrupted (as judged by eosin staining). After centrifugation at 10,000 × g for 10 min at 4°C, multiple aliquots were frozen at −70°C. The protein concentration of the cell extracts was estimated by measuring the absorption at 280 nm.
Activation of endogenous caspases was induced by addition of 1 mM dATP together with or without 250 μM bovine cytochrome c (Sigma) to the cytosolic extract (500 μg of protein) at a final volume of 40 μl (in cytoplasmic extraction buffer). After incubation at 37°C for 2 or 3 h, triplicates of 10-μl aliquots of the reactions were taken for the measurement of the DEVD-AMC-cleaving activity as described above. For Western blot analysis, 10 to 20 μl of the reactions were used.
Luciferase reporter assays.
HeLa cells (4 × 106) were transfected by electroporation with 16 μg of a plasmid which contained the coding sequence of the firefly luciferase gene under the control of either the β-actin promoter or a triple-NF-κB-binding consensus site in front of the β-interferon minimal promoter (12). The next day, cells were split into 12-well plates and replicates were either mock-infected or infected with C. pneumoniae at 1 IFU/cell. Cells were harvested at 8, 24, or 48 h of infection and analyzed using the luciferase assay system (Promega) and a Lumat 9507 luminometer (Berthold). To some reactions, tumor necrosis factor (TNF) (10 ng/ml) was added either 8 h (for 8-h experiments) or 16 h (for 24- and 48-h experiments) before harvesting.
Intracellular staining for cytochrome c.
HeLa cells were mock infected or infected with C. pneumoniae as described above. On day 1 of infection, wells were split 1:2 onto glass coverslips in 12-well plates. One day later, some wells were treated with staurosporine (1 μM) for 4 or 5 h, fixed with 2% formalin, and stained with anti-cytochrome c MAb (Becton Dickinson) followed by Cy3-labeled anti-mouse antiserum (Jackson), and pictures were taken with a Zeiss laser scanning microscope.
RESULTS
Inhibition of apoptosis by infection with C. pneumoniae.
As a model for the interaction between epithelioid cells and C. pneumoniae, HeLa human epithelioid cells were infected with C. pneumoniae elementary bodies. In human cells, the developmental cycle of C. pneumoniae lasts about 72 h. Large inclusions containing chlamydia developed upon infection in HeLa cells (see Fig. 4, left panel). HeLa cells were infected and monitored for 3 days for apoptotic changes. In parallel experiments, apoptosis was induced in infected or mock-infected HeLa cells by staurosporine, and the effect of chlamydial infection on the progress of apoptosis was analyzed.
FIG. 4.
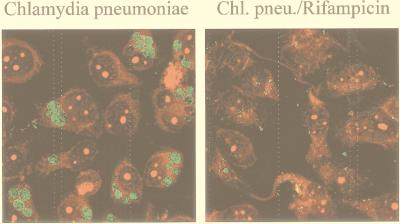
Effect of rifampin on the intracellular growth of C. pneumoniae. HeLa cells were infected with C. pneumoniae at about 1 IFU per cell. To one culture, rifampin (10 μg/ml) was added at the time of infection. Forty-eight hours postinfection, cells were stained with Evans Blue (shown in red) and a fluorescein isothiocyanate-conjugated anti-C. pneumoniae lipopolysaccharide antibody (green), and images were taken by laser scanning microscopy. Note that in the presence of rifampin, bacterial particles can be detected inside the cells, but they fail to develop the large inclusions normally seen.
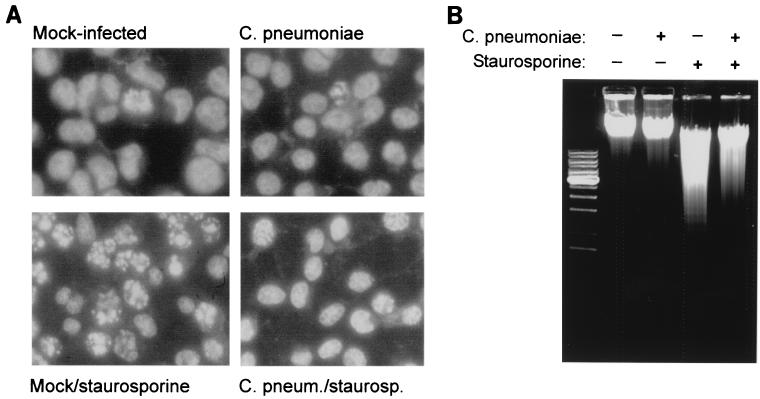
Infection with C. pneumoniae did not induce any morphological changes detectable by light microscopy in HeLa cells up to 72 h postinfection (data not shown). When the cellular DNA was stained to assess nuclear morphology specifically, no nuclear changes indicative of apoptosis were seen in infected HeLa cells (Fig. 1A and Table 1). Apoptosis was next induced by addition of micromolar concentrations of the kinase inhibitor staurosporine. In uninfected HeLa cells, this treatment induced nuclear morphological changes typical of apoptosis in a high percentage of cells over 4 h. When infected cultures were treated with staurosporine, the percentage of cells with apoptotic nuclei was much lower than in uninfected cultures (Fig. 1A and Table 1, experiment 1). Chromosomal DNA was further extracted and analyzed for internucleosomal fragmentation by agarose gel electrophoresis. As shown in Fig. 1B, chlamydial infection did not induce any detectable “laddering” in HeLa cells on its own. Staurosporine treatment of HeLa cells led to the appearance of the DNA degradation pattern typical of apoptosis. The amount of degraded DNA was significantly smaller when infected cells were treated with staurosporine (Fig. 1B). These data show that infection with C. pneumoniae reduces the sensitivity of HeLa cells to staurosporine-induced apoptosis.
FIG. 1.
Infection with C. pneumoniae inhibits staurosporine-induced nuclear apoptosis in HeLa cells. (A) Nuclear morphology. HeLa cells were either left uninfected or infected with C. pneumoniae at about 1.5 IFU per cell. On day 3 of infection, staurosporine (1 μM) was added to one part of the cultures. Four hours later, cells were stained with Hoechst dye and photos of representative areas were taken under a fluorescence microscope. (B) DNA degradation. HeLa cells were either left uninfected or infected with C. pneumoniae at about 1 IFU per cell. On day 2 of infection, cells were either treated with staurosporine (1 μM) for 10 h or left untreated, as indicated. Chromosomal DNA was then extracted and run on a 1% agarose gel containing ethidium bromide.
TABLE 1.
C. pneumoniae infection inhibits the appearance of staurosporine- and CD95- and DNP-induced nuclear apoptosis in HeLa cellsa
| Exp and treatment | % Cells (SD) with apoptotic nuclear morphology
|
|
|---|---|---|
| Mock infected | C. pneumoniae infected | |
| Exp 1 | ||
| None | 0.57 (0.18) | 0.43 (0.38) |
| Staurosporine | 61.23 (7.24) | 17.27 (5.91) |
| Exp 2 | ||
| None | 0.9, 0.3 | 3.0, 2.0 |
| Anti-CD95 and DNP | 40.1, 34.0 | 14.5, 14.9 |
HeLa cells were either mock infected or infected with C. pneumoniae at about 1.5 IFU per cell. For experiment 1, on day 3 of infection, staurosporine (1 μM) was added to one part of the cultures. Four hours later, cells were stained with Hoechst dye and scored for nuclear apoptosis under a fluorescence microscope. Numbers give percentages of apoptotic cells (mean and standard deviation of the mean for three separate wells for each condition; at least 300 nuclei per sample were counted). The data shown are representative of four separate experiments, where apoptosis was induced on either day 2 or day 3 of infection. For experiment 2, on day 2 of infection, anti-CD95 MAb CH11 (100 ng/ml) and DNP (1 mM) were added to duplicate wells. Five hours later, cells were stained with Hoechst dye and nuclear morphology was scored as described above. The numbers give the percentages of apoptotic nuclei for each of the duplicate cultures. This result is representative of two similar experiments.
The generation of caspase activity is reduced in infected cells.
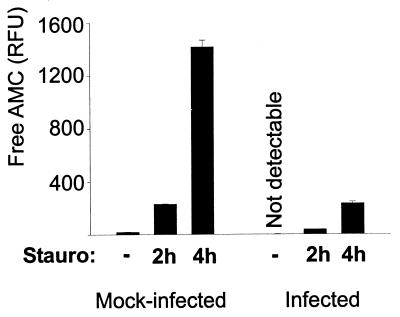
The appearance of the morphological signs of apoptosis is the result of the activation of an intracellular signal transduction pathway. Key components of the apoptotic pathway are members of the caspase protease family. DNA digestion during apoptosis (this is also the case for other morphological features of apoptosis) is the direct result of the appearance of proteolytic activity with specificity for the peptide sequence DEVD and is probably conveyed mainly by caspase-3 (4). To assess the activation of the apoptotic pathway, DEVD-specific (caspase-3-like) proteolytic activity was measured in extracts from HeLa cells. Staurosporine induced strong caspase-3-like activity in HeLa cells. In extracts from HeLa cells infected with C. pneumoniae, the level of detectable activity was much lower than in extracts from mock-infected cells (Fig. 2). The reduction correlated with the amount of elementary bodies used (experiments were performed up to an MOI of about 5; data not shown). Infection with C. pneumoniae therefore appears to inhibit staurosporine-induced apoptosis by blocking either caspase activity or upstream events leading to caspase activation.
FIG. 2.
Infection with C. pneumoniae inhibits the generation of effector caspase activity. HeLa cells were mock infected or infected with C. pneumoniae at about 1 IFU/cell. On day 3 of infection, cells were either treated with staurosporine (Stauro) (1 μM) for 2 or 4 h or left untreated (-), as indicated. Cells were then lysed, and DEVD-cleaving activity was measured in cell extracts. Data are presented as means and standard errors of the means. Each bar represents one well of a 12-well plate. The data are representative of more than five experiments.
Mouse primary embryonic fibroblasts were further analyzed for the apoptotic response to staurosporine. As in HeLa cells, the generated DEVD-cleaving activity was strongly reduced in these cells upon infection with C. pneumoniae (not shown).
Infection with C. pneumoniae inhibits cell death induced by CD95 and DNP.
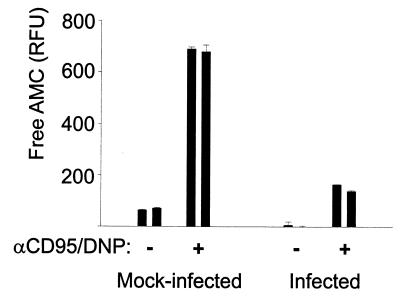
A second apoptotic stimulus was applied which uses a different pathway of apoptosis induction. Apoptosis was induced in HeLa cells by the combination of anti-CD95 stimulation and DNP. At the concentrations used here, DNP does not induce apoptosis by itself but allows the induction of apoptosis by CD95 signaling (20) (HeLa cells are normally not susceptible to CD95-induced apoptosis). Mock-infected and C. pneumoniae-infected HeLa cells were compared in their apoptotic responses to treatment with CD95 and DNP. As shown in Fig. 3 and Table 1 (Exp.2), both the appearance of DEVD-cleaving activity and nuclear fragmentation were reduced in infected cells. The antiapoptotic effect of infection with C. pneumoniae is therefore not restricted to staurosporine treatment.
FIG. 3.
Infection with C. pneumoniae inhibits the generation of DEVD-cleaving activity by treatment with CD95 and DNP in HeLa cells. HeLa cells were either mock infected or infected with C. pneumoniae at about 1 IFU per cell. On day 2 of infection, anti-CD95 MAb CH11 (100 ng/ml) and DNP (1 mM) were added. After 6 h, cells were lysed and DEVD-cleaving activity was measured in extracts. Each bar represents one well of a 12-well plate; activity was measured in triplicate reactions from the lysate of each well, and data are presented as means and standard errors of the means of these values. Similar results were obtained in three separate experiments.
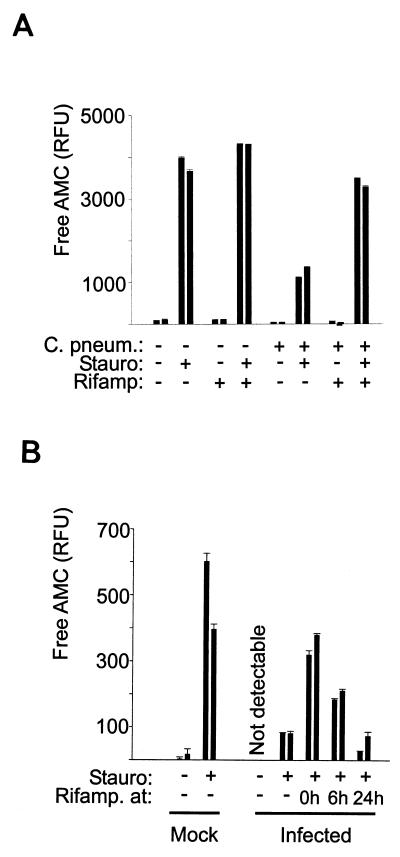
Bacterial protein synthesis is required for the block of caspase activity.
When chlamydial elementary bodies are taken up by a permissive human cell, they commence protein synthesis and start to replicate, forming intracellular inclusions (Fig. 4). In order to distinguish whether the protection against apoptosis requires bacterial metabolism, the effect of the antibiotic rifampin was tested; rifampin acts by blocking bacterial RNA synthesis. In the presence of rifampin, C. pneumoniae was still able to infect HeLa cells but failed to develop the inclusions normally seen (Fig. 4). The appearance of caspase-3-like-activity in response to staurosporine treatment was measured in infected cells which had been exposed to rifampin. As shown in Fig. 5A, rifampin did not affect this response in mock-infected cells. It was, however, able to reverse the protective effect of infection with C. pneumoniae: while in infected HeLa cells the caspase-3-like activity was significantly reduced, the response of infected and rifampin-treated cells was almost the same as the response of uninfected cells. This indicates that bacterial protein synthesis is required for the induction of the antiapoptotic effect following infection with C. pneumoniae. Time course experiments showed that rifampin had to be present during the early phases of infection. When rifampin was added 24 h after infection, its inhibitory effect was not detectable anymore, and this effect was significantly diminished already after 6 h of infection (Fig. 5B). This suggests that early bacterial protein synthesis is required and sufficient for the antiapoptotic effect.
FIG. 5.
Inhibition of bacterial protein synthesis with rifampin reverses the apoptosis-inhibitory effect of C. pneumoniae (C. pneum.). (A) HeLa cells were either infected (about 1 IFU/cell) or mock infected. To some cultures, rifampin (Rifamp) (10 μg/ml) was added at the time of infection. Forty-eight hours postinfection, staurosporine (Stauro) (1 μM) was added to some cultures. After 4 h, cells were lysed, and caspase-3-like activity was measured in the lysates. Data are presented as means and standard errors of the means; each bar represents one well of a 12-well plate. These results are typical of four similar experiments in which apoptotic response was assessed between 48 and 72 h postinfection. (B) Time course of the appearance of antiapoptotic activity. HeLa cells were either mock infected or infected with C. pneumoniae at about 2 IFU per cell. To duplicate cultures, rifampin was added at the indicated times postinfection. At 72 h postinfection, staurosporine (1 μM) was added to some cultures as indicated. Four hours later, cells were extracted and caspase-3-like activity was measured as above. Each bar represents one well of a 12-well plate. These data are representative of four similar experiments.
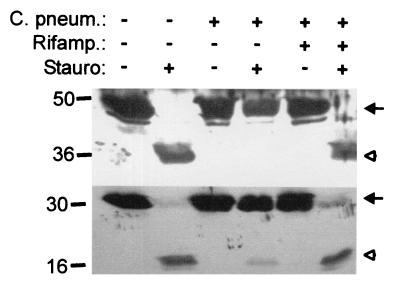
C. pneumoniae infection inhibits apoptosis upstream of caspase activation.
In staurosporine-induced apoptosis, activation of the initiator caspase, caspase-9, precedes the activation of the effector caspase, caspase-3 (36); the trigger for caspase activation is thought to be the release of cytochrome c from mitochondria (1). We next investigated the effect of infection by C. pneumoniae on the activation of caspase-3 and caspase-9. In uninfected HeLa cells, staurosporine treatment led to the processing of both caspase-3 and -9. In cells infected with C. pneumoniae, the extent of processing was strongly reduced for both caspases. When cells were infected and cultured in the presence of rifampin, processing was almost the same as in the case of uninfected cells (Fig. 6). These data suggest that infection with C. pneumoniae exerts its apoptosis-inhibiting effect upstream of caspase activation.
FIG. 6.
Infection with C. pneumoniae inhibits staurosporine-induced apoptosis upstream of caspase activation. HeLa cells were either mock infected or infected with C. pneumoniae (C. pneum.) at about 1 IFU per cell. To one aliquot of cells, rifampin (Rifamp.) (10 μg/ml) was added at the time of infection. At 48 h postinfection, staurosporine (Stauro) (1 μM) was added to one part of the cells. Four hours later, cells were extracted and extracts were analyzed by Western blotting for caspase-9 (top) and caspase-3 (bottom). Arrows indicate procaspases; arrowheads indicate activated forms. This experiment was performed three times with similar results.
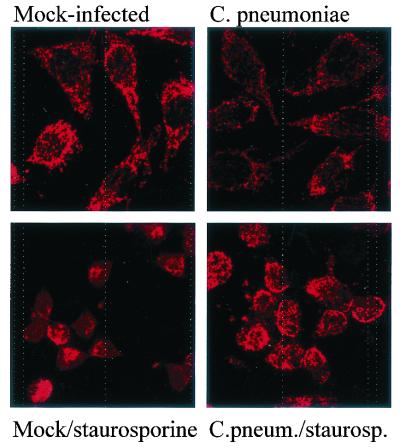
Chlamydial infection reduces the release of cytochrome c from mitochondria.
Mock-infected and C. pneumoniae-infected HeLa cells showed a normal mitochondrial distribution pattern when stained for cytochrome c and analyzed by laser scanning microscopy, indicating that cytochrome c was indeed localized to the mitochondria of the cells (Fig. 7, top panels). When mock-infected HeLa cells had been treated with staurosporine for 5 h, cytochrome c was found to be evenly distributed throughout the cell, including the nucleus, in the majority of the cells (Fig. 7, bottom left). When C. pneumoniae-infected cells were treated with staurosporine, the release was far less complete: in most cells, the staining was still similar to the mitochondrial pattern observed in untreated cells (Fig. 7, bottom right), although the cells had shrunk in size (probably a direct chemical effect of staurosporine). Inhibition of caspases with the peptide inhibitor Z-VAD-fmk did not prevent the redistribution of cytochrome c (data not shown). C. pneumoniae thus exerts an antiapoptotic effect which involves blockade of the release of cytochrome c from mitochondria.
FIG. 7.
Infection with C. pneumoniae inhibits the staurosporine-induced release of cytochrome c from mitochondria. Cells were either mock infected or infected with C. pneumoniae at about 1 IFU/cell. One day later, cells were replated onto glass coverslips. On day 2 of infection, one aliquot each was treated with staurosporine (1 μM) for 5 h, followed by staining for cytochrome c and laser scanning microscopy. The pictures are representative of three similar experiments.
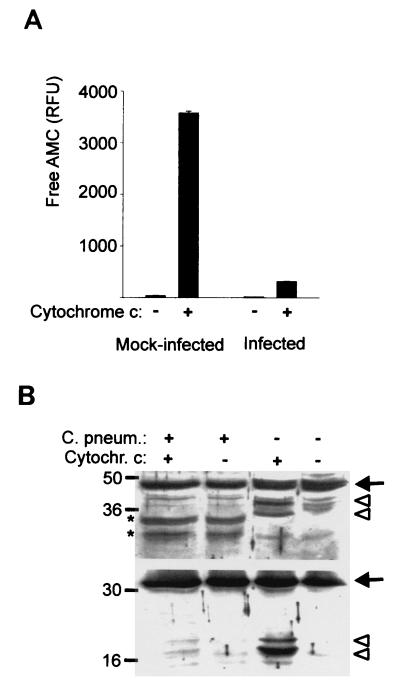
Infection with C. pneumoniae prevents cytochrome c-induced activation of caspases.
Since the prevention of cytochrome c release would be sufficient to explain the observed protection against apoptosis, it came as a surprise to notice that infection with C. pneumoniae also had a pronounced inhibitory effect on effector caspase activation by cytochrome c in a cell-free system. When extracts from mock-infected HeLa cells were incubated in the presence of cytochrome c and dATP, DEVD-cleaving activity could be detected. In extracts from C. pneumoniae-infected cells, the induction of DEVD-cleaving activity was strongly reduced (Fig. 8A). Likewise, cytochrome c treatment led to caspase-3 processing in extracts, which was blocked in extracts from infected cells; caspase-9 processing was also inhibited (Fig. 8B) in these extracts.
FIG. 8.
Infection with C. pneumoniae inhibits caspase activation by cytochrome c in a cell-free system. HeLa cells were either mock infected or infected with C. pneumoniae. Extracts were prepared on day 3 of infection, and aliquots of the extracts were incubated in the presence or absence of cytochrome c as described in Materials and Methods. Aliquots of the reactions were used for measurement of DEVD-cleaving activity (A) or subjected to Western blotting with antibodies specific for human caspase-9 (B, top panel) or caspase-3 (B, bottom panel). Arrows point at the procaspase, and arrowheads indicate the processed forms. All results were obtained in at least three experiments with extracts from separate infections. ∗, these cross-reactive bands were reproducibly seen in extracts from C. pneumoniae-infected cells.
These data suggest that infection with C. pneumoniae changes the composition of the cytosol of infected cells in such a way that the activation of caspases in response to cytochrome c is reduced or prevented. It is at this stage not entirely clear at what step in the apoptotic pathway the activation is inhibited; caspase-9 activation occurs in a large protein complex containing Apaf-1, cytochrome c, caspase-9, and caspase-3 and perhaps further components. It appears likely that a C. pneumoniae-dependent component associates with this complex and affects the processing of caspases.
The antiapoptotic effect is not the result of the induction of NF-κB transcriptional activity.
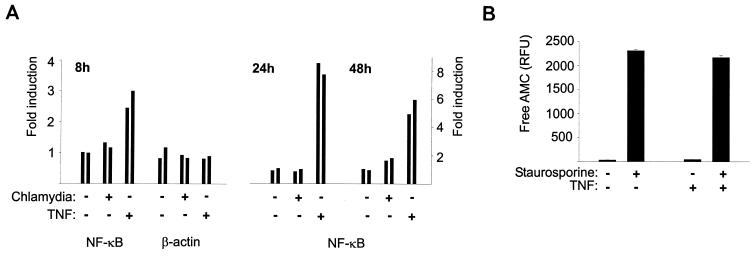
Although chlamydial protein synthesis was required for the antiapoptotic effect of chlamydial infection, this does not exclude the possibility that chlamydial components trigger the generation of a cellular antiapoptotic factor. We therefore investigated a number of cellular factors which were candidates for such a cellular contributing mechanism. In a number of experimental settings it has been shown that activity of the transcription factor family NF-κB affects the sensitivity to apoptotic stimuli. In general, NF-κB activity appears to have a protective effect against apoptosis (34). Infection-induced NF-κB activity has further been found to be induced during infection and to be necessary for survival of endothelial cells infected with the obligate intracellular bacterium Rickettsia rickettsii (3). We therefore investigated whether infection with C. pneumoniae induced NF-κB activity in HeLa cells and whether such activity protected against staurosporine-induced cell death. HeLa cells were transfected with reporter constructs in which expression of the firefly luciferase was under the control of either an NF-κB-regulated promoter or the constitutively active β-actin promoter. Cells were then either left uninfected or infected with C. pneumoniae, and luciferase activity was measured after 8, 24, and 48 h of infection. The detected relative activity was very similar at all three points in time whether cells had been infected or not; in some experiments, there was a slight induction of reporter activity in the range of about 1.3-fold (Fig. 9A). This indicates that chlamydial infection did not significantly affect the activity of NF-κB-dependent promoters. Furthermore, when NF-κB activity was experimentally induced by preculturing the cells with TNF, the cells showed unaltered caspase-3-like activity upon treatment with staurosporine (Fig. 9B). Taken together, these data argue against a participation of NF-κB activity in the antiapoptotic effect of infection with C. pneumoniae.
FIG. 9.
Induction of NF-κB activity does not participate in the inhibition of apoptosis by infection with C. pneumoniae. (A) Lack of induction of NF-κB activity by infection with C. pneumoniae. HeLa cells were transfected by electroporation with reporter constructs for either NF-κB-dependent activity or β-actin promoter activity. The next day, cells were split and either mock infected or infected with C. pneumoniae (about 1 IFU/cell). To some wells, TNF (10 ng/ml) was added as a positive control for NF-κB induction (for 8 or 16 [for the 24- and 48-h values] h). At the indicated time points of infection, cells were lysed and luciferase activity in extracts was measured. Data are presented as relative activity, with the activity in untreated cells set to equal 1. (B) TNF treatment does not prevent staurosporine-induced caspase activation in HeLa cells. HeLa cells were seeded into 12-well plates. To some aliquots, TNF was added for 18 h. Then, some wells were treated with staurosporine (1 μM) for 4 h followed by extraction and detection of DEVD-cleaving activity.
We further investigated cellular proteins which were candidates for inhibitors of apoptosis under these circumstances for a change in expression during infection with C. pneumoniae. Expression of Bcl-2, XIAP, and heat shock protein 70 (hsp70) was not changed by the infection (analysis was done on days 1, 2, and 3; data not shown). Although this is not a comprehensive analysis of the cellular response, these experiments give no indication of a cellular contribution to the antiapoptotic effect of infection with C. pneumoniae.
DISCUSSION
In this study we find that infection with C. pneumoniae protects host cells against apoptosis induced by cytochrome c release. The block of apoptosis lies upstream of caspase activation and extends to apoptosis induced by CD95 engagement. On a molecular level, the results suggest that the induction of apoptosis is blocked at two different steps: both cytochrome c release and cytochrome c-dependent caspase activation are inhibited. Early bacterial protein synthesis is required for this protection.
Like viruses, chlamydia depend on the host cell for reproduction. Although chlamydial protein synthesis is conducted by a bacterial apparatus, replication can occur only inside a mammalian cell and requires cellular components, such as nucleotides. Because of this developmental cycle, chlamydial growth is exquisitely sensitive to death of the host cell. The infecting elementary body develops into a structure called a reticulate body, which is actively replicating but incapable of infection. If the host cell underwent cell death prior to the completion of the cycle, i.e., prior to reorganization into infectious elementary bodies, chlamydial infection would necessarily come to an end. It is thus easily conceivable that the capacity of C. pneumoniae to inhibit apoptosis would be favored and selected in evolution as a bacterial virulence factor.
Previous investigations concerning apoptosis in the interaction between chlamydiae and mammalian host cells have resulted in a number of diverse conclusions. C. psittaci has been shown to induce apoptosis in epithelial cells and macrophages in vitro (8, 27). Interestingly, C. trachomatis was found to induce apoptosis in one in vitro system (8) and during genital infections in mice (28). Another, thorough study of infection with C. trachomatis in vitro, however, described a profound apoptosis-inhibiting activity conferred by bacterial infection and has mapped this activity to the blockade of cytochrome c release (5); this activity is perhaps based on a principle similar to that of one of the activities we find in C. pneumoniae-infected cells. It should be mentioned that one recent study has investigated apoptosis in cultures of human peripheral blood cells infected with C. pneumoniae and has found that the bacterial infection suppressed some forms of apoptosis by inducing the release of interleukin 10 (7). That study, however, was designed by using very low titers of infection to assess only such indirect effects. The basis for these reported differences, i.e., that chlamydia have been found both to induce and to inhibit apoptosis, are unclear. One important uncertainty is whether apoptosis would always serve the host cell or whether it could help to spread the infection, either by releasing bacteria or by inducing uptake of the apoptotic cell by macrophages (which might then themselves become infected and distribute the bacteria). We believe that since there appears to be a strong antiapoptotic mechanism activated by the bacteria, apoptosis is initially used as a defense mechanism by the host. Perhaps apoptosis is induced at later stages by the bacteria (although we were unable to observe it in cell culture with C. pneumoniae) and the conflicting reports reflect largely the different experimental settings and approaches of investigation used. There are further possible explanations; a chlamydial antiapoptotic activity would be necessary only if it were indeed required to inhibit apoptosis occurring during infection. Therefore, host cells are perhaps able somehow to pick up on intracellular infections with chlamydia and to respond to this infection with apoptosis. Assuming this scenario, i.e., that chlamydia can both induce and inhibit apoptosis, it is easily conceivable that chlamydial infection will result in apoptosis in one constellation of cell type and bacterial strain but not in other combinations. Of the three species of chlamydia pathogenic to humans, C. psittaci has the least and C. pneumoniae perhaps the greatest potential to cause chronic infections (an assumption based on the frequent isolation of C. pneumoniae from human blood and arteries). This behavior might be related to their respective capacities to induce and to inhibit apoptosis in infected cells. C. pneumoniae also inhibited apoptosis in mouse embryonic fibroblasts, making it unlikely that the host species (mouse or human) is an important determinant (at least for C. pneumoniae).
The detailed knowledge of the signal transduction in the apoptotic pathway which has become available in recent years allows us to map an antiapoptotic activity within this pathway. The evidence is compelling that one central complex of effector caspases (probably predominantly caspase-3) orchestrates cell death and uptake of the apoptotic cell. Two known upstream branches exist which can lead to the activation of caspase-3, namely, cell death receptors (activating caspase-8) and cytochrome c release (activating caspase-9). Although both branches, dependent on cell type (31), can probably operate independently, there is evidence that death-receptor-induced caspase-8 activation also funnels into cytochrome c release as an effector mechanism (19, 21, 31, 35), which makes cytochrome c release a candidate for one of the central events coordinating apoptosis (reviewed in reference 11).
In this study we found strong evidence for an inhibition of cytochrome c-induced apoptosis by C. pneumoniae. Staurosporine-induced apoptosis requires the release of cytochrome c from mitochondria (1), and this release was inhibited by infection with C. pneumoniae. We further investigated CD95-induced apoptosis and found that this form was also blocked. By preventing the release of cytochrome c, C. pneumoniae infection is likely to inhibit a great number of apoptotic stimuli. The infection does, however, impose an additional block on the apoptosis-inducing action of cytochrome c if and when it reaches the cytosol (as is suggested by the results in a cell-free system). This could be interpreted to mean that cytochrome c-induced apoptosis is indeed a defense mechanism of an infected cell which is on two levels counteracted by C. pneumoniae.
Chlamydial protein synthesis was required for the generation of the C. pneumoniae-dependent antiapoptotic activity; this could mean that a bacterially encoded protein has a direct antiapoptotic effect. The genome of C. pneumoniae encodes proteins with the potential to make up the machinery of a type III secretion system (16), suggesting that the bacteria are able to inject proteins from their inclusion vacuole into the host cell's cytosol. Although no obvious regulators of apoptosis were deduced from the genome, a large number of proteins of unknown function is encoded by C. pneumoniae, which might include such effectors (16). It is also conceivable that a bacterial product induces the expression of a cellular protein which will confer the protection against apoptosis. We were unable to reach a clear distinction between these two possibilities, since an experimental complete abrogation of cellular protein synthesis for the required length of time is not feasible. NF-κB-dependent gene expression, which has been shown to play a role during infection with R. rickettsii, was very likely not involved in the antiapoptotic effect of C. pneumoniae. The cellular inhibitors of apoptosis that were investigated, Bcl-2, XIAP, and Hsp70, were unaltered in expression during infection.
A number of structurally different inhibitors of apoptosis, both of mammalian and of viral origin, are known which block apoptosis at different steps in the pathway. No such inhibitor which would have the profile observed during infection with C. pneumoniae, i.e., inhibition of both cytochrome c release and cytochrome c/Apaf-1-dependent caspase activation, is known. This suggests either the involvement of a novel inhibitor or the concerted action of several members of known classes.
In summary, these results show that infection of permissive human cells with C. pneumoniae confers protection against cytochrome c-dependent apoptotic stimuli. Although some information is available about the infectious biology of these bacteria, the greater part is still completely unknown. We know that C. pneumoniae can replicate in epithelial cells and access the bloodstream upon airway infection in animal models (23, 24) and that the bacteria even can be detected in human atheromatous plaques (for a review see reference 2). Many questions remain, however, such as whether the bacteria replicate actively in artery walls or remain in a quiescent state or whether they are indeed resident in a cell for a long time or rely on release and reinfection of new cells. The data available do, however, suggest that the bacteria can be present in the human body for prolonged periods of time, and inhibition of apoptosis by C. pneumoniae might serve as one mechanism by which the bacteria survive in the host's cells. A therapeutic interference with the antiapoptotic activity of C. pneumoniae could perhaps facilitate elimination of the bacteria and might reduce the risk of atherosclerosis.
ACKNOWLEDGMENT
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Ha 2128/5-1).
REFERENCES
- 1.Bossy-Wetzel E, Newmeyer D D, Green D R. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD- specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell L A, Kuo C C, Grayston J T. Chlamydia pneumoniae and cardiovascular disease. Emerg Infect Dis. 1998;4:571–579. doi: 10.3201/eid0404.980407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clifton D R, Goss R A, Sahni S K, van Antwerp D, Baggs R B, Marder V J, Silverman D J, Sporn L A. NF-kappa B-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc Natl Acad Sci USA. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Earnshaw W C, Martins L M, Kaufmann S H. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 5.Fan T, Lu H, Hu H, Shi L, McClarty G A, Nance D M, Greenberg A H, Zhong G. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaydos C A, Summersgill J T, Sahney N N, Ramirez J A, Quinn T C. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geng Y, Shane R B, Berencsi K, Gonczol E, Zaki M H, Margolis D J, Trinchieri G, Rook A H. Chlamydia pneumoniae inhibits apoptosis in human peripheral blood mononuclear cells through induction of IL-10. J Immunol. 2000;164:5522–5529. doi: 10.4049/jimmunol.164.10.5522. [DOI] [PubMed] [Google Scholar]
- 8.Gibellini D, Panaya R, Rumpianesi F. Induction of apoptosis by Chlamydia psittaci and Chlamydia trachomatis infection in tissue culture cells. Zentbl Bakteriol. 1998;288:35–43. doi: 10.1016/s0934-8840(98)80095-9. [DOI] [PubMed] [Google Scholar]
- 9.Godzik K L, O'Brien E R, Wang S K, Kuo C C. In vitro susceptibility of human vascular wall cells to infection with Chlamydia pneumoniae. J Clin Microbiol. 1995;33:2411–2414. doi: 10.1128/jcm.33.9.2411-2414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grayston J T. Background and current knowledge of Chlamydia pneumoniae and atherosclerosis. J Infect Dis. 2000;181(Suppl. 3):S402–S410. doi: 10.1086/315596. [DOI] [PubMed] [Google Scholar]
- 11.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 12.Hacker H, Mischak H, Hacker G, Eser S, Prenzel N, Ullrich A, Wagner H. Cell type-specific activation of mitogen-activated protein kinases by CpG-DNA controls interleukin-12 release from antigen-presenting cells. EMBO J. 1999;18:6973–6982. doi: 10.1093/emboj/18.24.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haecker G, Vaux D L. Viral, worm and radical implications for apoptosis. Trends Biochem Sci. 1994;19:99–100. doi: 10.1016/0968-0004(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 14.Howard L, Orenstein N S, King N W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974;27:102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson M D, Weil M, Raff M C. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 16.Kalman S, Mitchell W, Marathe R, Lammel C, Fan J, Hyman R W, Olinger L, Grimwood J, Davis R W, Stephens R S. Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat Genet. 1999;21:385–389. doi: 10.1038/7716. [DOI] [PubMed] [Google Scholar]
- 17.Kuo C C, Grayston J T. A sensitive cell line, HL cells, for isolation and propagation of Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990;162:755–758. doi: 10.1093/infdis/162.3.755. [DOI] [PubMed] [Google Scholar]
- 18.Kuo C C, Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976;13:1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Zhu H, Xu C J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 20.Linsinger G, Wilhelm S, Wagner H, Häcker G. Uncouplers of oxidative phosphorylation can enhance a Fas death signal. Mol Cell Biol. 1999;19:3299–3311. doi: 10.1128/mcb.19.5.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 22.Miller L K. Baculovirus interaction with host apoptotic pathways. J Cell Physiol. 1997;173:178–182. doi: 10.1002/(SICI)1097-4652(199711)173:2<178::AID-JCP17>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Moazed T C, Kuo C, Grayston J T, Campbell L A. Murine models of Chlamydia pneumoniae infection and atherosclerosis. J Infect Dis. 1997;175:883–890. doi: 10.1086/513986. [DOI] [PubMed] [Google Scholar]
- 24.Moazed T C, Kuo C, Patton D L, Grayston J T, Campbell L A. Experimental rabbit models of Chlamydia pneumoniae infection. Am J Pathol. 1996;148:667–676. [PMC free article] [PubMed] [Google Scholar]
- 25.Moss J E, Aliprantis A O, Zychlinsky A. The regulation of apoptosis by microbial pathogens. Int Rev Cytol. 1999;187:203–259. doi: 10.1016/s0074-7696(08)62419-5. [DOI] [PubMed] [Google Scholar]
- 26.O'Connor L, Huang D C, O'Reilly L A, Strasser A. Apoptosis and cell division. Curr Opin Cell Biol. 2000;12:257–263. doi: 10.1016/s0955-0674(99)00084-8. [DOI] [PubMed] [Google Scholar]
- 27.Ojcius D M, Souque P, Perfettini J L, Dautry-Varsat A. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J Immunol. 1998;161:4220–4226. [PubMed] [Google Scholar]
- 28.Perfettini J L, Darville T, Gachelin G, Souque P, Huerre M, Dautry-Varsat A, Ojcius D M. Effect of Chlamydia trachomatis infection and subsequent tumor necrosis factor alpha secretion on apoptosis in the murine genital tract. Infect Immun. 2000;68:2237–2244. doi: 10.1128/iai.68.4.2237-2244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saikku P. Chlamydia pneumoniae in atherosclerosis. J Intern Med. 2000;247:391–396. doi: 10.1046/j.1365-2796.2000.00659.x. [DOI] [PubMed] [Google Scholar]
- 30.Salvesen G S, Dixit V M. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 31.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaux D L, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci USA. 1996;93:2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 35.Yin X M, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth K A, Korsmeyer S J. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 36.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]