Abstract
A novel in vivo expression technology (IVET) was performed to identify Klebsiella pneumoniae CG43 genes that are specifically expressed during infection of BALB/c mice. The IVET employed a UDP glucose pyrophosphorylase (galU)-deficient mutant of K. pneumoniae which is incapable of utilizing galactose and synthesizing capsular polysaccharide, as demonstrated by its low virulence to BALB/c mice and a white nonmucoid colony morphology on MacConkey-galactose agar. By using a functional galU gene as the reporter, an IVE promoter could render the galU mutant virulent while maintaining the white nonmucoid colony phenotype. A total of 20 distinct sequences were obtained through the in vivo selection. Five of them have been identified previously as virulence-associated genes in other pathogens, while another five with characterized functions are involved in regulation and transportation of nutrient uptake, biosynthesis of isoprenoids, and protein folding. No known functions have been attributed to the other 10 sequences. We have also demonstrated that 2 of the 20 IVE genes turn on under iron deprivation, whereas the expression of another five genes was found to be activated in the presence of paraquat, a superoxide generator.
Klebsiella pneumoniae is an important nosocomial pathogen that causes a wide range of infections, including pneumonia, bacteremia, urinary tract infections, and sometimes life-threatening septic shock. As an opportunistic pathogen, it primarily attacks immunocompromised individuals who are hospitalized and/or suffering from severe underlying diseases, such as diabetes mellitus, chronic alcoholism, or pulmonary obstruction (23). Many clinical strains of K. pneumoniae are highly resistant to antibiotics, indicating the relative ineffectiveness of current therapy.
During infections, bacterial pathogens must adapt to various changes in order to persist and proliferate in appropriate locations and to circumvent host defenses. It is reasonable to assume that the expression of many K. pneumoniae genes that participate in pathogenesis could be specifically induced within the host. Ideally, these in vivo-expressed (IVE) genes would serve as useful drug targets and vaccine candidates. Several approaches, including in vivo expression technology (IVET) (11, 15), comparative genomics (2), microarray DNA chips (7), signature-tagged mutagenesis (18, 26), differential display-PCR (1), and differential fluorescence detection (31), have allowed the identification of genes that are essential or specifically activated during infections. Nevertheless, none of these approaches has been applied to K. pneumoniae, primarily due to the limited number of mutants and genetic tools available for the bacterium.
IVE technology (IVET) is a powerful technique that has been used successfully for several important pathogens, including Salmonella enterica serovar Typhimurium (15, 16), Yersinia enterocolitica (33), Staphylococcus aureus (14), Pseudomonas aeruginosa (32), Escherichia coli (12), and Actinobacillus pleuropneumoniae (9). The original IVET, designed to identify promoters that turn on in vivo in S. enterica, used a tandem set of in vivo and in vitro promoterless genes as the reporter (15). There are now several different modifications of the IVET, such as the use of auxotrophic markers and antibiotic resistance genes, and induction of site-specific recombinase as the basis for the selection systems (12, 14, 32). In view of the limited genetic tools available for K. pneumoniae, we have designed a novel IVET selection system for this heavily encapsulated bacterium.
Rationale for the galU-based IVET selection system.
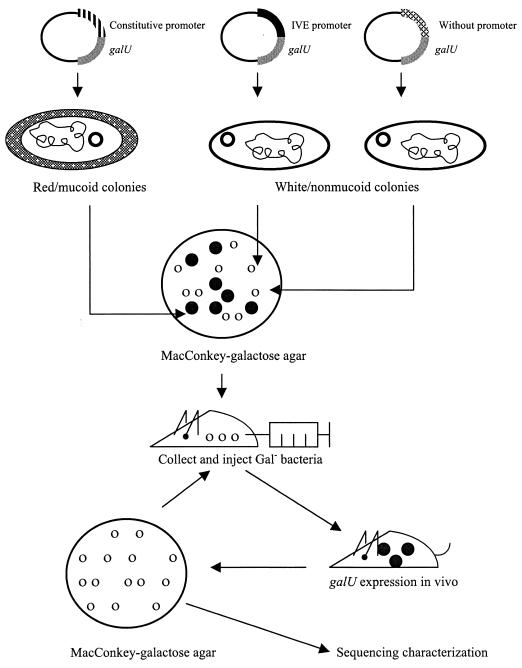
The rationale for the IVET developed for K. pneumoniae is shown in Fig. 1. Instead of using a set of tandem reporter genes, our IVET system incorporates a copy of the promoterless galU gene as a dual-function reporter. The galU gene encodes the enzyme UDP glucose pyrophosphorylase, which regulates the supply of UDP galactose and UDP glucose, two major precursors for the biosynthesis of capsule polysaccharides (CPS) and lipopolysaccharides (LPS) in most enteric bacteria. A GalU− mutant of K. pneumoniae produces defective forms of CPS and LPS and hence loses virulence and the mucoid colony phenotype (5). In addition, the K. pneumoniae GalU− strain is incapable of fermenting galactose, a property that can be readily distinguished by using MacConkey–0.4% galactose agar (5). These unique properties make the galU gene an ideal reporter system for IVE gene selection. The bacterial strains which are able to survive in vivo selection while exhibiting a white nonmucoid colony phenotype on MacConkey-galactose agar would indicate that the DNA fragment upstream of the galU reporter contains an IVE promoter.
FIG. 1.
Overall selection strategy for the galU-based IVET system.
Construction of galU-based reporter gene system.
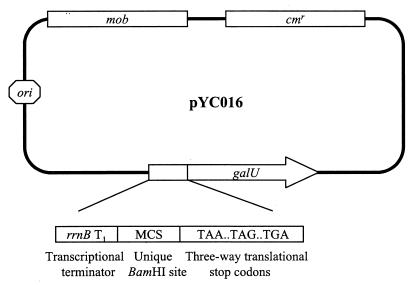
Our IVET selection vector, designated pYC016 (Fig. 2), was constructed by using the mobilizable plasmid pSUP102 (27) as the backbone. Plasmid pYC016 was engineered sequentially in the order described as follows. Initially, a PCR-amplified copy of the Pseudomonas aeruginosa PAO1 galU gene coding sequence was inserted into the unique ClaI site of pSUP102. Subsequently, a nonessential BamHI site in the plasmid was eliminated by end-filling with Klenow fragment of DNA polymerase I, followed by religation. Finally, a cassette from pKK232-8 (Amersham-Pharmacia, Piscataway, N.J.) that contains a transcriptional terminator, multiple cloning sites, and a set of three-way translational stop codons was inserted into a ClaI site of the vector. The main features of pYC016 include (i) a unique BamHI site which is compatible with Sau3AI-digested chromosomal DNA fragments, (ii) use of the galU gene of P. aeruginosa PAO1, with the intention of minimizing the possibility of homologous recombination that could occur between the chromosomal and plasmid-borne galU genes in K. pneumoniae, (iii) the presence of translational stop codons in all three reading frames preceding the ribosome-binding site of the galU gene to ensure transcriptional fusions between inserted DNA fragments and the galU gene, and (iv) a transcriptional terminator, rrnB T1 (4), upstream of the cloned chromosomal fragment to prevent transcription of the galU gene from a fortuitous plasmid promoter.
FIG. 2.
Diagram of K. pneumoniae IVET vector pYC016, showing the locations of the transcriptional terminator (rrnB T1), multiple cloning sites (MCS), three-way translational stop codons, and promoterless galU gene. Only the BamHI site in the multiple cloning site is unique in the vector.
Construction of K. pneumoniae IVET library.
Two isogenic K. pneumoniae strains were used in this study. K. pneumoniae CG43 is a clinical isolate which is highly virulent to laboratory mice, with a 50% lethal dose (LD50) of 10 CFU (22). K. pneumoniae CG43-17 is a GalU− derivative of CG43, generated previously by Tn5 insertion mutagenesis. The virulence of K. pneumoniae CG43-17 was found to be significantly attenuated (LD50, ∼106 CFU) (5). High-molecular-weight chromosomal DNA of K. pneumoniae CG43 was purified and partially digested with Sau3AI. Fragments ranging from 0.5 to 1 kb in size were purified from agarose gels and ligated into the unique BamHI site of pYC016. The ligation mixture was transformed into E. coli S17-1 (27) (hsdR recA pro RP4-2 [Tc::Mu; Km::Tn7]) and selected on Luria-Bertani (LB) agar supplemented with chloramphenicol (30 μg/ml). Approximately 5 × 105 of the transformants, 90% of which contain a plasmid with an insert, were obtained. These plasmids were then mobilized into K. pneumoniae CG43-17 by conjugation. The transconjugants were selected on MacConkey–0.4% galactose plates supplemented with kanamycin (75 μg/ml), chloramphenicol (30 μg/ml), and ampicillin (50 μg/ml). After an 8-h incubation at 37°C, red mucoid colonies were scored as Gal+, whereas white nonmucoid colonies were scored as Gal−. The red-white (Gal+:Gal−) ratio of the transconjugants was about 1:3.
Screening of K. pneumoniae IVE genes.
A pilot study was performed with about 103 CFU of the IVE library. The study demonstrated that after infections in BALB/c mice and subsequent recovery from spleen, the ratio of red to white colonies shifted from the original 1:3 to 60:1, indicating that most of the Gal− clones were eliminated effectively in vivo. To reduce the number of mice used in this study as well as to minimize undesired bacteria of the Gal+ background, approximately 5 × 104 mostly white nonmucoid colonies were collected manually and separated into three batches for the experiments that followed.
Male BALB/c mice (6 to 8 weeks old) with an average weight of 25 g were obtained from the animal center of National Taiwan University and acclimatized in an animal house of our institute for 3 days. Exponential-phase K. pneumoniae was obtained by diluting an overnight broth culture 100-fold into warmed LB broth and shaking at 37°C until the optical density at 600 nm (OD600) reached 0.3 to 0.4.
Typically 104 CFU were used to infect a BALB/c mouse. Prior to infection, bacteria were washed twice and resuspended in 200 μl of 1× phosphate-buffered saline (PBS), and the suspension was then injected intraperitoneally into a BALB/c mouse. The infected mice were sacrificed after 24 to 48 h, and the spleens were dissected, homogenized, serially diluted, and plated on MacConkey-galactose agar supplemented with appropriate antibiotics. The surviving white nonmucoid colonies were then collected for an additional two rounds of in vivo selection. In three independent experiments, each utilizing a different pool of white nonmucoid bacteria and three mice per round of selection, we arbitrarily picked 30 to 60 white nonmucoid colonies from the postselection pool of each infected mouse for plasmid DNA preparation and restriction endonuclease digestion analysis. A total of 20 distinct eletrophoretic patterns were observed, and these IVE clones were subjected to further characterization.
Verification of inducibility of galU fusion constructs in vivo and in vitro.
The plasmid DNA was extracted from the 20 IVE clones and retransformed into K. pneumoniae CG43-17, and the transformants were tested individually for virulence in BALB/c. Approximately 1 × 106 to 5 × 107 CFU could be recovered from 1 g of spleen from the sick mice. The number is comparable to that of the wild-type K. pneumoniae CG43. In contrast, less than 10 CFU of K. pneumoniae CG43-17(pYC016) was observed under these conditions. Moreover, the use of a standard assay method (5) indicated that all these clones did not exhibit detectable UDP glucose pyrophosphorylase activity, confirming that the promoters were indeed turned off under in vitro growth conditions.
A serum susceptibility assay, which correlates LPS quantity in the gram-negative bacteria, was also performed. Less than 1% human serum was sufficient to achieve 50% killing of K. pneumoniae CG43-17, a mutant known to be incapable of synthesizing intact LPS (5). The K. pneumoniae CG43-17 IVE clones exhibited a similar behavior towards 1% human serum and were also killed efficiently. However, the concentration of human serum required to effectively kill the wild-type K. pneumoniae CG43 must exceed 50%. Together, these results indicated that the galU fusion clones were not expressed when grown in enriched medium but could be induced preferentially during infection of BALB/c mice.
Nucleotide sequence determination of IVE genes.
DNA sequence determination was carried out by the PCR-mediated Taq DyeDeoxy Terminator Cycle sequencing kit on an Applied Biosystems model 373A DNA sequencer. The homology search of the GenBank/EMBL and SwissProt databases was performed using the BLAST programs provided by the National Center of Biotechnology Information through the Internet. The result of the sequence analysis is shown in Table 1.
TABLE 1.
K. pneumoniae IVE genes identified in this study
| Predicted function | Accession no. | Homologous gene (accession no.) | % Identity (length of comparable amino acid sequence, no. of residues) | Induction by
|
Predicted protein | |
|---|---|---|---|---|---|---|
| 2′,2′-Dipyridyl | Paraquat | |||||
| Iron acquisition | ||||||
| AJ277397 | E. coli iucA (1073533) | 86a | + | − | Aerobactin biosynthesis | |
| AJ292298 | E. coli fepA (P05825) | 59 (263) | + | − | Ferrienterobactin receptor | |
| Transport/binding proteins | ||||||
| AJ292299 | E. coli ptfA (P24217) | 91 (60) | − | − | Phosphotransferase system | |
| AJ292304 | E. coli uup (P43672) | 91 (40) | − | − | ATP-binding cassette transporter | |
| Regulatory proteins | ||||||
| AJ292310 | E. coli tdcA (P11036) | 78 (175) | − | − | Tdc operon transcriptional activator | |
| AJ292311 | H. influenzae rbsR (P44329) | 38 (94) | − | − | Ribose operon repressor | |
| Amino acid biosynthesis | ||||||
| AJ277396 | E. coli lysA (P455170) | 85 (69) | − | + | Diaminopimelate decarboxylase | |
| Isoprenoid biosynthesis | ||||||
| AJ292312 | E. coli yaeM (P45568) | 71 (115) | − | − | 1-Deoxy-d-xylulose 5-phosphate reductoisomerase | |
| DNA metabolism | ||||||
| AJ292307 | E. coli gyrA (P09097) | 99 (136) | − | − | DNA gyrase subunit A | |
| Protein folding | ||||||
| AJ292309 | S. enterica ppiA (P20753) | 87 (124) | − | − | Peptidylprolyl cis-trans isomerase A | |
| Unknown/hypothetical | ||||||
| AJ292305 | E. coli yjjB (P18389) | 57 (73) | − | − | Protein P-14 | |
| AJ292308 | E. coli ydgH (P76177) | 62 (158) | − | − | Protein YdgH precursor | |
| AJ292300 | E. coli yjjZ (P55914) | 51 (47) | − | − | Hypothetical 8.7-kDa protein | |
| AJ292303 | E. coli yhgI (P46846) | 79 (191) | − | − | Protein YhgI | |
| AJ292306 | E. coli yfjB (P37768) | 90 (43) | − | + | Hypothetical 32.6-kDa protein | |
| AJ292313 | E. coli yfaE (P37910) | 85 (81) | − | − | Hypothetical 9.3-kDa protein | |
| AJ292315 | E. coli yjcC (P32701) | 43 (116) | − | + | Hypothetical 60.8-kDa protein | |
| AJ292301 | KPN_CONTIG 880b | 99a | − | + | Unknown | |
| AJ292302 | KPN_CONTIG 765b | 86a | − | + | Unknown | |
| AJ292314 | Novel sequence | − | − | Unknown | ||
Indicates DNA sequence homology.
Data obtained from the Genomic Sequencing Center, Washington University, St. Louis, Mo.
Among the 20 IVE sequences, 5 have been shown to be genes essential for in vivo growth identified previously in other pathogens, demonstrating the effectiveness of the galU-based selection strategy. iucA and fepA are both involved in iron acquisition, and expression of these genes is known to be activated during infection (3, 8, 11, 17, 20, 31). The ptfA gene encodes a phosphotransfer system for fructose uptake. By using signature-tagged mutagenesis, it has been demonstrated that this gene is crucial for Vibrio cholerae to survive in the host (6). The gene product of rbsR is a repressor responsible for regulating the expression of rbsC, the ribose permease-encoding gene. The expression of rbsR was found to be essential for Brucella melitensis to survive in the host (13). lysA encodes diaminopimelate decarboxylase for lysine biosynthesis (28). It has been shown that in Staphylococcus aureus, lysA is preferentially expressed during infection, presumably due to limited supply of lysine in the host (18).
The other five K. pneumoniae IVE genes found in this study that have been characterized in other organisms include uup (25), an ATP-binding cassette type transporter encoding gene; yaeM (29), which encodes 1-deoxy-d-xylulose 5-phosphate reductoisomerase, which is responsible for terpenoid synthesis; gyrA (19), DNA gyrase subunit A; tdcA (10), the product of which is a transcription activator for the tdc operon, which encodes a system involved in threonine and serine metabolism and transport during anaerobic growth; and ppiA (30), peptidylprolyl cis-trans isomerase A. Among the remaining 10 sequences of unknown function, 7 matched the Escherichia coli hypothetical protein-encoding genes. Two sequences were found in the genome database of K. pneumoniae MGH 78578, established in the Genomic Sequencing Center at Washington University, St. Louis, Mo., and one was a novel sequence.
Inducibility of IVE promoters under iron deprivation and oxidative stress.
On entering the host, bacterial pathogens must circumvent iron deprivation and the attack of reactive oxygen species produced by the immune cells. Therefore, it is reasonable to assume that some of the IVE promoters identified in this study may encode a product that assists the bacteria in countering these stresses. To verify the possibility, the IVE clones were spotted individually on MacConkey-galactose plate containing either 200 μM 2′,2′-dipyridyl, an iron chelator, or 10 μM paraquat, a superoxide generator. The phenotype of the colonies after overnight incubation at 37°C was then examined. Two IVE clones, AJ277397 and AJ292298, representing the promoters of two iron acquisition genes, iucA and fepA, respectively, were found to display the red mucoid colony phenotype in the presence of 2′,2′-dipyridyl that reflects the activation of the promoters under iron deprivation conditions (Fig. 3).
FIG. 3.
Phenotypes of IVE clones on MacConkey-galactose agar containing 10 μM paraquat (A) or 100 μM 2′,2′-dipyridyl (B). The bright red color of the colonies indicates expression of the galU reporter gene.
Paraquat was found to activate five IVE clones, including lysA (AJ277396) and four carrying genes of no known function (AJ292306, AJ292315, AJ292301, and AJ292302). Our result indicates that many genes are likely to be turned on to counter oxidative stress during infection in the host. The number may be more, since it has been shown that E. coli responds to the redox stress imposed by paraquat by activating the synthesis of as many as 80 polypeptides (21). The identification of lysA as a superoxide-inducible gene is intriguing, although we do not have a good explanation for this finding yet.
In summary, we have constructed a novel IVET for identification of virulence-associated genes in gram-negative bacteria. By using this system, we have successfully identified 20 IVE genes in K. pneumoniae. In addition to being used in IVE gene identification, the convenient red-white selection on MacConkey-galactose plates provided by the galU reporter system might be applied in identifying genes specifically expressed under certain growth conditions, such as iron deprivation and oxidative stress. Like many other IVET, the procedure demands that the reporter gene be expressed throughout the course of an infection. Therefore, this strategy may be unable to identify certain IVE genes that are expressed only during a specific stage of infection. However, this drawback can be complemented by screening the IVE library in multiple animal infection models, as demonstrated in Streptococcus pneumoniae with signature-tagged mutagenesis (24).
Acknowledgments
This work was supported in part by the National Science Council of the Republic of China (NSC89-2320-B-009-001 to H.L.P. and 89-2320-B-007-002 to H.Y.C.) and VTY Joint Research Program, Tsou's Foundation (VTY89-p4-28 to H.Y.C.).
We are grateful to J. Vatsyayan for critical reading of the manuscript.
REFERENCES
- 1.Abu Kwaik Y, Pederson L L. The use of differential display-PCR to isolate and characterize a Legionella pneumophila locus induced during the intracellular infection of macrophages. Mol Microbiol. 1996;21:543–556. doi: 10.1111/j.1365-2958.1996.tb02563.x. [DOI] [PubMed] [Google Scholar]
- 2.Arigoni F, Talabot F, Peitsch M, Edgerton M D, Meldrum E, Allet E, Fish R, Jamotte T, Ourchod M L, Loferer H. A genome-based approach for the identification of essential bacterial genes. Nat Biotechnol. 1998;16:851–857. doi: 10.1038/nbt0998-851. [DOI] [PubMed] [Google Scholar]
- 3.Bagg A, Neilands J B. Ferric uptake regulation protein acts as a repressor, employing iron(II) as a co-factor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987;26:5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- 4.Brosius J. Toxicity of an overproduced foreign gene product in Escherichia coli and its use in plasmid vectors for the selection of transcription terminators. Gene. 1984;27:161–172. doi: 10.1016/0378-1119(84)90137-9. [DOI] [PubMed] [Google Scholar]
- 5.Chang H Y, Lee J H, Deng W L, Fu T F, Peng H L. Virulence and outer membrane properties of a galU mutant of Klebsiella pneumoniae CG43. Microb Pathog. 1996;20:255–261. doi: 10.1006/mpat.1996.0024. [DOI] [PubMed] [Google Scholar]
- 6.Chiang S L, Mekalanos J J. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol Microbiol. 1998;27:797–805. doi: 10.1046/j.1365-2958.1998.00726.x. [DOI] [PubMed] [Google Scholar]
- 7.De Saizieuu A, Certa U, Warrington J, Gray C, Keck W, Mous J. Bacterial transcript imaging by hybridization of total RNA to oligonucleotide arrays. Nat Biotechnol. 1998;16:45–50. doi: 10.1038/nbt0198-45. [DOI] [PubMed] [Google Scholar]
- 8.Escolar L, de Lorenzo V, Perez-Martin J. Metalloregulation in vitro of the aerobactin promoter of Escherichia coli by the Fur (ferric uptake regulation) protein. Mol Microbiol. 1997;26:799–808. doi: 10.1046/j.1365-2958.1997.6211987.x. [DOI] [PubMed] [Google Scholar]
- 9.Fuller T E, Shea R J, Thacker B J, Mulks M J. Identification of in vivo induced genes in Actinobacillus pleuropneumoniae. Microb Pathog. 1999;27:311–327. doi: 10.1006/mpat.1999.0309. [DOI] [PubMed] [Google Scholar]
- 10.Ganduri Y L, Sadda S R, Datta M W, Jambukeswaran R K, Datta P. TdcA, a transcriptional activator of the tdcABC operon of Escherichia coli, is a member of the LysR family of proteins. Mol Gen Genet. 1993;240:395–402. doi: 10.1007/BF00280391. [DOI] [PubMed] [Google Scholar]
- 11.Heithoff D M, Conner C P, Hanna P C, Julio S M, Hentschel U, Mahan M J. Bacterial infection as assessed by in vivo gene expression. Proc Natl Acad Sci USA. 1997;94:934–939. doi: 10.1073/pnas.94.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan M A, Isaacson R E. In vivo expression of the beta-glucoside (bgl) operon of Escherichia coli occurs in mouse liver. J Bacteriol. 1998;180:4746–4749. doi: 10.1128/jb.180.17.4746-4749.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lestrate P, Delrue R M, Danese I, Didembourg C, Taminiau B, Mertens P, de Bolle X, Tibor A, Tang C M, Letesson J J. Identification and characterization of in vivo attenuated mutants of Brucella melitensis. Mol Microbiol. 2000;38:543–551. doi: 10.1046/j.1365-2958.2000.02150.x. [DOI] [PubMed] [Google Scholar]
- 14.Lowe A M, Beattie D T, Deresiewicz R L. Identification of novel staphylococcal virulence genes by in vivo expression technology. Mol Microbiol. 1998;27:967–976. doi: 10.1046/j.1365-2958.1998.00741.x. [DOI] [PubMed] [Google Scholar]
- 15.Mahan M J, Slauch J M, Mekalanos J J. Selection of bacterial virulence genes that are specifically induced in host tissues. Science. 1993;259:686–688. doi: 10.1126/science.8430319. [DOI] [PubMed] [Google Scholar]
- 16.Mahan M J, Tobias J W, Slauch J M, Collier P C, Mekalanos J J. Antibiotic-based selection for bacterial genes that are specifically induced during infection of a host. Proc Natl Acad Sci USA. 1995;92:669–673. doi: 10.1073/pnas.92.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez J L, Herrero M, de Lorenzo V. The organization of intercistronic regions of the aerobactin operon of pColV-K30 may account for the differential expression of the iucABCD iutA genes. J Mol Biol. 1994;238:288–293. doi: 10.1006/jmbi.1994.1290. [DOI] [PubMed] [Google Scholar]
- 18.Mei J M, Nourbakhsh F, Ford C W, Holden D W. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol Microbiol. 1997;26:399–407. doi: 10.1046/j.1365-2958.1997.5911966.x. [DOI] [PubMed] [Google Scholar]
- 19.Menzel R, Gellert M. Fusions of the Escherichia coli gyrA and gyrB control regions to the galatokinase gene are inducible by coumermycin treatment. J Bacteriol. 1987;169:1272–1278. doi: 10.1128/jb.169.3.1272-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neilands J B. Mechanism and regulation of synthesis of aerobactin in Escherichia coli K12 (pColV-K30) Can J Microbiol. 1992;38:728–733. doi: 10.1139/m92-119. [DOI] [PubMed] [Google Scholar]
- 21.Nunoshiba T, Hidalgo E, Amabile-Cuevas C F, Demple B. Two-stage control of an oxidative stress regulon: the Escherichia coli SoxR protein triggers redox-inducible expression of the soxS regulatory gene. J Bacteriol. 1992;174:6054–6060. doi: 10.1128/jb.174.19.6054-6060.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng H L, Wang P Y, Chiu C T, Wu C L, Chang H Y. Molecular epidemiology of Klebsiella pneumoniae. Chin J Microbiol Immunol (Taipei) 1991;24:264–271. [PubMed] [Google Scholar]
- 23.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polissi A, Pontiggia A, Feger G, Altieri M, Motti H, Ferrari L, Simon D. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect Immun. 1998;66:5620–5629. doi: 10.1128/iai.66.12.5620-5629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy M, Gowrishankar J. Identification and characterization of ssb and uup mutants with increased frequency of precise excision of transposon Tn10 derivatives: nucleotide sequence of uup in Escherichia coli. J Bacteriol. 1997;179:2892–2899. doi: 10.1128/jb.179.9.2892-2899.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shea J E, Hensel M, Gieeson C, Holden D W. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon R, Connell M O, Labes M, Puhler A. Plasmid vectors for the genetic analysis and manipulation of Rhizobia and other Gram-negative bacteria. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- 28.Stragler P, Danos O, Patte J C. Regulation of diaminopimelate decarboxylase synthesis in Escherichia coli. II. Nucleotide sequence of the lysA gene and its regulatory region. J Mol Biol. 1983;168:321–331. doi: 10.1016/s0022-2836(83)80021-7. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi S, Kuzuyama T, Watanabe H, Seto H. A 1-deoxy-d-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-d-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc Natl Acad Sci USA. 1998;95:9879–9884. doi: 10.1073/pnas.95.17.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran P V, Bannor T A, Doktor S Z, Nichols B P. Chromosomal organization and expression of Escherichia coli pabA. J Bacteriol. 1990;172:397–410. doi: 10.1128/jb.172.1.397-410.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valdivia R H, Falkow S. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol Microbiol. 1996;22:67–78. doi: 10.1046/j.1365-2958.1996.00120.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Mushegian A, Lory S, Jin S. Large-scale isolation of candidate virulence genes of Pseudomonas aeruginosa by in vivo selection. Proc Natl Acad Sci USA. 1996;93:10434–10439. doi: 10.1073/pnas.93.19.10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young G M, Miller V L. Identification of novel chromosomal loci affecting Yersinia enterocolitica pathogenesis. Mol Microbiol. 1997;25:319–328. doi: 10.1046/j.1365-2958.1997.4661829.x. [DOI] [PubMed] [Google Scholar]