Abstract
Objectives
To evaluate the effect of incorporating calcium advice into early pregnancy counseling on calcium intake during pregnancy in the Netherlands.
Methods
A multicenter prospective before-after cohort study was conducted introducing risk-based care including calculating individual pre-eclampsia risk. Part of the intervention was to incorporate calcium advice into routine counseling. We calculated individual daily calcium intake and adequacy of calcium intake (≥1,000 mg/day) at 16, 24 and 34 weeks of pregnancy. We performed a multiple logistic regression adjusting for covariates to identify any differences in the risk of inadequate calcium intake between RC and CAC.
Results
In regular care (RC, 2013–2015, n=2,477) 60% had inadequate calcium intake, compared to 49% during calcium advice care (CAC, 2017–2018, n=774) (aOR 0.75, 95% CI 0.64–0.88). Specific calcium supplements were used by 2% and 29% in RC and CAC, respectively (OR 25.1, 95% CI 17.8–36.0). Determinants of an inadequate calcium intake were lower age (aOR per additional year 0.96, 95% CI: 0.94–0.98), nulliparity (aOR 1.22, 95% CI: 1.03–1.45) and non-Caucasian origin (aOR 1.83, 95% CI 1.09–3.09). In CAC, risk of inadequate intake decreased with increasing predicted pre-eclampsia risk, which was a trend reversal compared to RC.
Conclusions
Incorporating calcium advice into early pregnancy counseling was shown to lead to a decrease in the risk of inadequate calcium intake during pregnancy, but still inadequate intake in half of the women suggesting the need for further study on improving implementation. Awareness of individual increased PE risk had positive effect on calcium intake.
Keywords: calcium, calcium intake, counseling, diet, pre-eclampsia, supplements
Introduction
An adequate calcium intake during pregnancy is of major importance for health of both mother and child [1], [2], [3], [4], [5]. Recommended calcium intake for pregnant women varies between countries from 900 to 1,200 mg/day [6]. In most low-income populations, dietary calcium intakes during pregnancy are alarmingly below these recommendations [7], and even in high-income countries, a substantial proportion of pregnant women fail to meet calcium recommendations by diet [8].
The World Health Organization currently recommends calcium supplementation as part of antenatal care for women with an inadequate dietary calcium intake [9]. Calcium supplement use during pregnancy is low-risk and relatively inexpensive. Advising all pregnant women to use calcium supplements can be expected to cause substantial reductions in the incidence of pre-eclampsia (PE) and related health care costs [10]. However, none of the currently available over-the-counter prenatal vitamin supplements contain a clinically significant amount of calcium. We have recently shown that the majority of Dutch pregnant women do not meet the Dutch Recommended Dietary Allowance (RDA) of 1000 mg/day from the combination of diet and supplement use [11]. Further efforts to optimize calcium intake during pregnancy are therefore desirable.
In 2016, to promote adequate calcium intake, gynecologists and midwives in the southeastern region of the Netherlands started counseling all pregnant women on the importance of an adequate calcium intake and to advise ingesting at least 1,000 mg calcium per day [12]. Other care innovations included calculation of women’s individual PE risk in first trimester and discussing the possibility of prophylactic use of low dose aspirin [13, 14]. In this paper we evaluated whether calcium intakes from both diet and supplement use changed among pregnant women in this area, in comparison with the preceding period. To this aim, we measured calcium intake from both diet and supplement use during pregnancy by means of questionnaires, both before and after the implementation of the calcium advice. In addition to evaluate changes in intake, we aimed to identify determinants of inadequate calcium intake in the pre-intervention period, which could be used in targeted prevention. Lastly, we evaluated whether adequacy of calcium intake in the pre-intervention and intervention period was associated with calculated PE risk.
Materials and methods
Study population
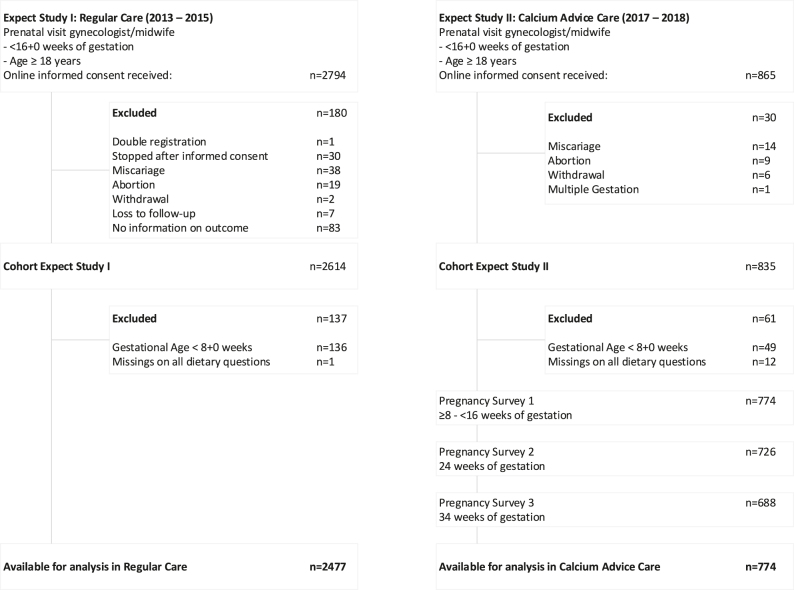
We used data of two prospective cohorts of pregnant women that were previously used for validating and evaluating the impact of first trimester obstetric prediction models [15, 16]. Women enrolled in the validation study (Expect Study I, 2013–2015) received ‘regular care’ (RC), whereas women enrolled in the impact study (Expect Study II, 2017–2018) received ‘calcium advice care’ (CAC), meaning obstetric caregivers agreed to give women the advice to gain an adequate calcium intake. Detailed descriptions of these studies have been published elsewhere. Briefly, women aged 18 years and older and having a singleton pregnancy were recruited at their first prenatal visit (<16 weeks of gestation), in the south eastern region of the Netherlands. Study information and questionnaires were provided in Dutch. Finally, data of 3,251 women were available for analysis; 2,477 women in RC and 774 women in CAC. A flowchart of the study is provided in Figure 1.
Figure 1:
Flowchart of the study population.
Data collection
Data collection was similar for both cohorts. After providing informed consent, women received an online questionnaire (baseline, <16 weeks of gestation) regarding, among other things, diet and supplement use and the healthcare services the women received from their midwife or gynecologist during the first visits. Additional questionnaires were taken in Expect Study II (CAC) at 24 and 34 weeks of gestation. These included questions on health care, delivery and changes in supplement use.
Dietary intake
We based dietary calcium intake on a selection of food products contributing to calcium and vitamin D. We described the selection of these food products extensively elsewhere [11]. In short: the selection procedure resulted in 18 food items, for which both the frequency of use (reference period: last month) and the average daily amount of use were asked: milk and buttermilk; yoghurt and cottage cheese (with or without fruit); yoghurt drinks and other dairy beverages; chocolate milk; custard and pudding; Dutch cheese; non-Dutch cheese and cream cheese; cheese spread; bread spread (sub types: margarine; low-fat margarine; and butter); cooking fat (bake and fry products); and fish (subtypes: fat fish such as salmon, mackerel, eel and white herring; lean fish such as codfish, tilapia, pangasius fish and trout; white fish fillet; smoked or steamed fish; herring; and fish fingers. We included milk, milk products and cheese in the questionnaire as major sources of calcium, bread spread, bake and fry products and fish as major sources of vitamin D. We included the food items in the first questionnaire and these covered an estimated 61.65% of total absolute dietary calcium intake.
Supplements
We included questions regarding supplements which might contain calcium such as prenatal vitamins, general multivitamins and calcium supplements in the baseline questionnaire in RC and all questionnaires during CAC. We requested duration and period of use (start of usage before and during pregnancy, when potentially stopped, current use), brand and any subtype, frequency of use per week, and amount of tablets per day.
We standardized calcium to the elemental form in milligrams, based on the labels. We contacted the manufacturers for clarification when the exact elementary amount of calcium in the supplement was unclear.
Statistical analysis
Baseline characteristics were presented as percentages or means with standard deviations. We imputed missing values in the baseline characteristics of the Expect II cohort regarding level of education (n=3) using the modal value. We calculated individual daily dietary calcium intake by multiplying frequency of consumption by consumed amounts of all assessed food products and combining product intake (grams per day) with calcium content of each product according to the Dutch Food Composition Table of 2010 (NEVO-online 2010) [17] and DNFCS2007-2010 [18]. To account for the incomplete coverage of the food frequency questionnaire we adjusted the estimated calcium intake values (estimated intake * 100/61.65). We used adjusted total calcium intakes in the analyses and presented these in the results. We calculated daily calcium intake from supplement use by combining frequency, amount of supplements and content of specific supplements. In case a participant used a supplement but did not know the exact (subtype) brand, we imputed the modal value. We calculated total calcium intakes in milligrams per day from the combination of diet and supplement use. We compared total calcium intake to the Recommended Dietary Allowance (RDA) of 1,000 mg/day as well as the Estimated Average Requirement (EAR) of 800 mg/day end. The RDA of 1,000 mg calcium per day is considered to be adequate for 97.5% pregnant women [19]. An intake level of 800 mg calcium is expected to satisfy the needs of 50% of all pregnant women [20].
To compare calcium intakes in RC and CAC, we performed a logistic regression analysis without and with adjustment for the following covariates: age (continuous), Body Mass Index (BMI: <20, ≥20–<25, ≥25–<30 and≥30), parity (nulliparous, multiparous), ethnicity (Caucasian, other) and level of education (primary, secondary vocational, secondary general, tertiary university of applied sciences and tertiary university). We compared intake at baseline in RC with intake at 24 weeks of gestation in CAC; we chose not to use intake values at baseline in CAC, since women were usually advised to start calcium supplementation at the beginning of the 2nd trimester (13–14 weeks of gestation), potentially weeks after baseline. Since calcium was not advised during RC, we assumed that the measurements in first trimester (women of 8–16 weeks of gestation) were representative. We undertook additional analyses to evaluate the legitimacy of these choices.
We considered possible determinants of inadequate calcium intake by performing a multiple logistic regression on the RC data, adjusting for the same covariates as previously mentioned. We chose to consider determinants in RC only since, during this period, care providers and pregnant women were still not influenced by the outcomes of the PE risk prediction model [13, 15].
Proportions of women with inadequate calcium intake (<1,000 mg/day) during RC and CAC were plotted using the estimated risk for PE as a continuous variable [13, 15]. A nonparametric local weighted regression (lowess) regression was applied to fit the curves [21]. We evaluated the trend of risk of calcium intake inadequacy by risk of PE for each period by including risk of PE (in %) as a variable in logistic regression. PE-risk for women in RC was calculated after completion of the Expect Study I; women were therefore not specifically alerted to PE risk based on this model during RC.
We calculated mean (± standard deviation, SD) values of total, dietary and supplemental calcium intake, and presented them in milligrams per day for RC and CAC (<16 weeks, 24 weeks and 34 weeks of gestation). We calculated amount and percentages of women using specific calcium supplements during RC and CAC (<16 weeks, 24 weeks and 34 weeks of gestation). All analyses were performed using IBM SPSS Statistics version 23 and SAS version 9.4M7.
Ethical approval
The Medical Ethical Committee of the Maastricht University Medical Centre declared that no ethical approval was necessary for the Expect Study I and II (MEC-13-4-053 and MEC-17-4-057, respectively). All participating women gave informed consent.
Results
Data of 3,251 women were available for analysis; 2,477 women received regular care (RC, Expect I cohort) and 774 women were part of the calcium advice care (CAC) cohort (Expect II). Baseline characteristics are presented in Table 1. The cohorts did not substantially differ with respect to age and BMI; the CAC cohort, however, contained a slightly larger proportion of Caucasian and multiparous women as compared to the RC cohort. Furthermore, the proportion of women with high educational levels was slightly overrepresented in the CAC cohort in comparison to the RC cohort.
Table 1:
Baseline characteristics of the study population (n=3,251).
| Characteristics | Regular care (n=2,477)a | Calcium advice care (n=774)a |
|---|---|---|
| Age, years (mean ± SD) | 30.2 ± 3.9 | 30.7 ± 4.0 |
| Body Mass index before pregnancy, kg/m2 (mean ± SD) | 24.2 ± 4.3 | 24.5 ± 4.4 |
| Ethnicity | ||
|
2,401 (96.9) | 757 (97.8) |
|
76 (3.1) | 17 (2.2) |
| Parity | ||
|
1,265 (51.1) | 384 (49.6) |
|
1,212 (48.9) | 390 (50.4) |
| Level of education | ||
|
9 (0.4) | 4 (0.5) |
|
143 (5.8) | 37 (4.8) |
|
973 (39.3) | 266 (34.4) |
|
944 (38.1) | 325 (42.0) |
|
408 (16.5) | 142 (18.4) |
SD, standard deviation. aPercentages do not always add up to 100% due to rounding.
Total calcium intake
Total mean calcium intake was 949.8 mg/day in RC and 1,070.9 mg/day in CAC (difference, 120.1 mg/day [95% CI 78.7–163.5, p<0.0001). Women in CAC were significantly less likely to have an inadequate calcium intake (<1,000 mg/day) as compared to RC (49 and 60%, respectively; aOR 0.75, 95% CI 0.64–0.88, Table 2). Intakes were <800 mg/day (EAR) in 42% and 36% in RC and CAC, respectively. Results of the logistic regression did not materially change after setting the cut-off to 800 mg/day. Total calcium intake during pregnancy in CAC increased from first to second trimester (mean difference, 49 mg/day [95% CI 33–64]) and remained stable from second to third trimester (mean difference −2 mg/day [95% CI −14 to 10]) (Table 3).
Table 2:
Inadequate calcium intakes (<1,000 m/d) during regular care vs. calcium advice care (n=3,251).
| Total amount per category, n | Amount of inadequate calcium intake, n | Percentage inadequate calcium intake, % | Crude odds ratio [95% CI] | Adjusted odds ratio [95% CI]a | |
|---|---|---|---|---|---|
| Regular care | 2,477 | 1,489 | 60.1 | 1 (ref) | 1 (ref) |
| Calcium advice careb | 726 | 359 | 49.4 | 0.74 [0.63–0.87] | 1.75 [0.64–0.88] |
CI, confidence interval. aCorrected for covariates age, Body Mass Index, parity, ethnicity and level of education. bAt 24 weeks of gestation.
Table 3:
Calcium intake, total and from individual sources.
| Regular care, n=2,477 (mg/d) | Calcium advice care <16 weeks, n=774 (mg/d) | Calcium advice care 24 weeks, n=726 (mg/d) | Calcium advice care 34 weeks, n=688 (mg/d) | |
|---|---|---|---|---|
| Total calcium intakes | ||||
|
| ||||
| Mean ± SD total calcium intake | 949.8 ± 489.7 | 1,006.9 ± 568.2 | 1,070.9 ± 581.8 | 1,065.3 ± 597.3 |
|
| ||||
| Calcium intakes from diet | ||||
|
| ||||
| Mean ± SD calcium intake from diet | 860.8 ± 468.3 | 803.0 ± 494.9 | – | – |
|
| ||||
| Calcium intakes from supplementsa | ||||
|
| ||||
| Mean ± SD calcium intake from prenatal vitamins | 127.1 ± 99.3 | 140.7 ± 97.0 | 135.3 ± 98.1 | 130.5 ± 90.8 |
| Mean ± SD calcium intake from general multivitamins | 128.2 ± 248.8 | 129.1 ± 115.2 | 82.8 ± 92.4 | 78.8 ± 90.7 |
| Mean ± SD calcium intake from calcium supplements | 395.4 ± 296.4 | 596.4 ± 313.1 | 533.5 ± 302.8 | 550.4 ± 315.9 |
SD, standard deviation. aCalculated for users of the specific supplements only.
Calcium intake from individual sources
Mean calcium intake from diet at baseline was 861 mg/day in RC and 803 mg/day in CAC (Table 3). Mean calcium intake from multivitamin supplements (prenatal vitamins and general multivitamins) did not differ much between RC and CAC, also when the analysis was confined to users of such supplements. Multivitamin supplements were taken by 70% of the women in RC at baseline, and in CAC by 72% at baseline, 74% at 24 weeks, and 71% at 34 weeks. Calcium intake from prenatal vitamins did not change much over time, neither in all women or in users only. Calcium intake from general multivitamin supplements showed a decrease during pregnancy. Specific calcium supplements were taken by 2% of the women in RC at baseline, and in CAC by 17% at baseline, 29% at 24 weeks, and 29% at 34 weeks (OR for CAC (at 24 weeks) vs. RC: 25.1 [95%-CI 17.8–36.0]). Mean calcium intake from these supplements in all women was 9 mg/day in RC, and 101, 155, and 158 mg/day at baseline, 24 weeks and 34 weeks in CAC (all differences between RC and CAC being significant at <0.0001 level). Corresponding figures for users only were 396, 596, 534, and 550 mg/day.
Determinants for inadequate calcium intake in RC
Factors significantly associated with an inadequate calcium intake were low age, nulliparity and non-Caucasian ethnicity; medium-low level of education was associated with a decreased risk of an inadequate calcium intake (Table 4). The risk of having an inadequate calcium intake decreased with increasing age (aOR 0.96, 95% CI: 0.94–0.98). Nulliparous women had a higher risk of an inadequate calcium intake (aOR 1.22, 95% CI: 1.03–1.45). Having a secondary general level of education went along with a decreased risk of inadequate calcium intake as compared to tertiary education (aOR 0.79, 95% CI 0.65–0.95). Women with non-Caucasian ethnicities were more likely to have an inadequate calcium intake compared to women with the Caucasian ethnicity (aOR 1.83, 95% CI 1.09–3.09).
Table 4:
The association of inadequate calcium intakes (<1,000 mg/d) during regular care with age, Body Mass Index, parity, ethnicity and level of education, logistic regression (n=2,477).
| Total amount per category, n | Amount of inadequate calcium intake, n | Percentage inadequate calcium intake, % | Crude odds ratio [95% CI] | Adjusted odds ratio [95% CI] | |
|---|---|---|---|---|---|
| Age (per additional year) | – | – | – | 0.95 [0.93–0.97] | 0.96 [0.94–0.98] |
| Body Mass index | |||||
|
331 | 203 | 61.3 | 1.14 [0.89–1.46] | 1.12 [0.87–1.44] |
|
1,333 | 776 | 58.2 | 1 (ref) | 1 (ref) |
|
553 | 345 | 62.4 | 1.19 [0.97–1.46] | 1.21 [0.98–1.49] |
|
260 | 165 | 63.5 | 1.25 [0.95–1.64] | 1.26 [0.95–1.67] |
| Parity | |||||
|
1,265 | 803 | 63.5 | 1.33 [1.13–1.57] | 1.22 [1.03–1.45] |
|
1,212 | 686 | 56.6 | 1 (ref) | 1 (ref) |
| Ethnicity | |||||
|
2,401 | 1,433 | 59.7 | 1 (ref) | 1 (ref) |
|
76 | 56 | 73.7 | 1.89 [1.13–3.17] | 1.83 [1.09–3.09] |
| Level of education | |||||
|
9 | 8 | 88.9 | 4.9 [0.61–39.11] | 4.25 [0.52–34.52] |
|
143 | 91 | 63.6 | 1.07 [0.74–1.54] | 0.93 [0.64–1.35] |
|
973 | 571 | 58.7 | 0.87 [0.72–1.10] | 0.79 [0.65–0.95] |
|
944 | 586 | 62.1 | 1 (ref) | 1 (ref) |
|
408 | 233 | 57.1 | 0.81 [0.64–1.03] | 0.86 [0.68–1.09] |
CI, confidence interval.
Inadequate calcium intake in relation to risk of pre-eclampsia
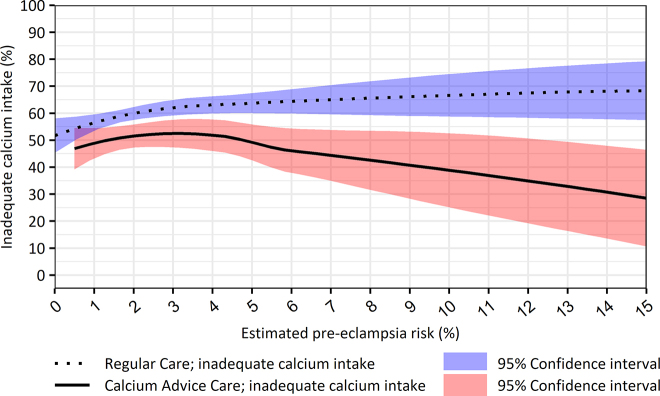
Risk of inadequate calcium intake tended to rise with higher PE risk categories during RC and decrease with higher PE risk categories in CAC (Figure 2). Respective ORs per additional risk percent were 1.04, 95% CI 1.01–1.07) and 0.96 (95% CI 0.93–1.00).
Figure 2:
Estimated pre-eclampsia risks and percentage of inadequate calcium intake (<1000 mg/d) by women receiving regular care or calcium advice care.
Discussion
Main findings
Our results indicate that in CAC vs. RC calcium intake improved, with an absolute decrease of 11% in the probability of having an inadequate intake after the introduction of counselling on calcium. The intake of specific calcium supplements increased from 2 to 29%, while the use of other multivitamin supplements remained comparable to use in the pre-intervention period. We identified low age, nulliparity, and the non-Caucasian ethnicity as risk factors of inadequate calcium intake, while medium-low level of education predicted a decreased risk of an inadequate calcium intake. In CAC, risk of inadequate intake decreased with increasing predicted risk of PE, which was a trend reversal in comparison to RC.
Previous findings
This study is the first to evaluate the effects of active counseling on calcium during early pregnancy on subsequent calcium intake in a Western country. Efforts to increase calcium intake have been taken in low- and middle-income countries (LMIC) [22–25]. Described adherence is high (about 80%), but settings are substantially different in LMIC compared to high income countries (HIC). Moreover, specific calcium supplements were distributed and methodological considerations to be raised concern small sample size, short follow-up period, no calculated dietary calcium intake or dietary intake based on estimated national averages.
Although the effect of the care innovation on mean calcium intake was moderate, literature on effects of other campaigns to improve intakes of other micronutrients during or before pregnancy suggests that very strong effects should not be anticipated. Antenatal micronutrient programmes face challenges in adherence [26, 27]. Even following various public folic acid awareness campaigns, less than 50% of women take folic acid periconceptionally [20]. In a recent adherence study on folic acid and iodine supplementation 38% of the women were found to meet supplementation recommendations [19] In our study, use of specific calcium supplements rose from 2% to 29%. Our data show that over time, the amount of specific calcium supplement users increased to up to 35% in the third of the population that was included latest, supporting the expectation that the uptake of the care innovation will improve even more in time.
In the general population, postmenopausal women, vegetarians and amenorrheic women were found to be at increased risk of inadequate calcium intake [28]. Young age has been demonstrated as risk factor for inadequate micronutrient intake in general and calcium intake was especially low among adolescents and younger women [29], [30], [31]. This is in line with our observations and other findings in the Dutch population. In the Dutch Food Consumption Survey 2007–2010 young women and adolescents were found to have lower mean calcium intakes compared to older women [18]. Maternal education and socio-economic status (SES) have been shown to be positively related to intake of micronutrients and dietary quality in general [30, 32]. We found no clear association for maternal education, although medium-low educational level strikingly went along with a lower probability of an inadequate calcium intake. Our finding of a higher risk of inadequacy of calcium intake in women of non-Caucasian ethnicity appears to be in line with that of a study demonstrating that African-American pregnant women more often had an inadequate calcium intake compared to Caucasian women (OR 3.15, 95% CI: 1.07–9.27). However, the group of women of non-Caucasian ethnicity in our study was very small and had a mix of ethnicities. Nulliparity has not been previously described as risk factor for inadequate calcium intake during pregnancy and needs replication in future studies.
Implications
Since beneficial effects on morbidity and mortality can be expected, costs are low (about 50 euro per pregnancy [10, 33]) and side-effects are uncommon, optimizing calcium intakes during pregnancy seems feasible and can have worldwide impact [1], [2], [3], [4, 10, 33], [34], [35], [36]. This study demonstrates the first results after the incorporating calcium intake advice in early pregnancy counselling for all pregnant women.
Knowledge on risk factors regarding inadequate calcium intake can be useful in targeted strategies. Judging by our results, it might be efficient to concentrate efforts to younger women, nulliparous women, and women of non-Caucasian ethnicity. Furthermore, risk calculation may help in pinpointing groups who have best chances to benefit from improved calcium intake. In our study, calcium advice was not specifically given to women with a high calculated risk of PE, but the results show that adequacy of calcium intake improves with increasing risk estimates, indicating that, in CAC, women took the risk into account in their behaviour. This may be exploited in the further development of calcium intake encouragement strategies.
In order to improve uptake, barriers and facilitators for better counseling and uptake of calcium advice should be identified. This may be achieved by means of qualitative research involving focus group interviews with care professionals and pregnant women with specific characteristics that may influence either delivery of the intervention or uptake.
Strengths and limitations
This study, the first to evaluate the effect of early-pregnancy counseling on calcium on intake of calcium during pregnancy in a Western country, featured a prospective design and cohorts of considerable size that were similar with respect to baseline characteristics. These characteristics are beneficial to validity and precision of the results. Nevertheless, some possible limitations deserve attention. We chose to compare intake at baseline in RC with intake at 24 weeks of gestation in CAC, and therefore not to use intake values at baseline in CAC, since women were usually advised to start calcium supplementation at the beginning of the 2nd trimester (13–14 weeks of gestation), potentially weeks after baseline. Since, in RC, calcium was not routinely discussed, we assumed that intakes in the first trimester (women of 8–16 weeks of gestation) were representative for the remainder of pregnancy. We have several indications supporting the validity of our approach. During CAC, in line with expectation, total calcium intake increased from the first to the second trimester, and then remained stable. The same went for the use of specific calcium supplements. On the other hand, and in line with expectation, during CAC, calcium intake from prenatal vitamins and general multivitamins remained steady through all trimesters of pregnancy, which makes it plausible that such was also the case in RC (although we did not measure calcium intake beyond 13–14 weeks).
The diversity of participating midwifery centers and hospitals, in combination with the broad inclusion criteria, was a prerequisite for obtaining a study population as unselected as possible. However, women of Caucasian ethnicity were still overrepresented, and a higher than average percentage of women were highly educated. Since impaired health literacy is correlated to non-adherence and impaired outcomes, our results may therefore give an overly positive picture of the effects of calcium advice [37, 38].
Another limitation may be that we assumed unchanged dietary habits during pregnancy. Food intake may vary over time. Women may experience nausea in early pregnancy and dietary cravings in the development of their pregnancy, as a result of which dairy product intake may increase [39]. Furthermore, since the advice to improve calcium intake was given during first trimester and supplement intakes increased during second trimester, women who chose to optimize their intake by means of diet may have done so later on. Therefore, we may have underestimated the increase in calcium intake from diet and differences between CAC and RC may have been larger in reality. However, ingestion of the amount of calcium necessary to reach the AI is not easily achieved via diet alone [40] and changing dietary habits has proven to be challenging for many women [41, 42].
Since there is no biochemical assay to display the nutritional calcium status, we had to depend on questionnaires to estimate calcium intake. Our methods for the estimation of calcium intake are identical to our previously published methods during RC [11]. Even though repeated dietary recalls might have been considered as more accurate approaches for food intake assessment, this method would not be achievable in a large cohort. Nevertheless, the FFQ method is widely used for food product and nutrient intake assessment, and although its main strength is in the ranking of individuals according to their intakes of frequently used foods and nutrients it is also considered a feasible tool to gain insight in the percentage of inadequate intake in a large population [43].
Conclusions
Incorporating calcium advice into early pregnancy counseling can lead to a decrease in the risk of inadequate calcium intake during pregnancy. Although the effect that we observed in mean calcium intake was moderate, is was more pronounced among women with an increased risk of PE, who may benefit most from improved calcium intake. Further improvement may result from targeting risk groups for low calcium intake and qualitative studies elucidating barriers and facilitators for better counseling and uptake of calcium advice.
Acknowledgments
We are grateful to all women who participated in the Expect study. We acknowledge participant recruitment by the participating midwifery practices and departments of obstetrics and gynaecology of hospitals in the Province of Limburg. We also acknowledge Nicole Wijckmans, department of Epidemiology, Maastricht University, for selecting the food items for the questionnaire.
Footnotes
Research funding: ZonMw (The Netherlands Organization for Health Research and Development; federal funding) grant numbers 209020007 and 505200098150
Author contributions: LJEM, HCJS, and LJMS contributed to the conception and design of the Expect study I. PM, LJEM, JPMMW, HCJS, and LJMS contributed to the conception and design of the Expect study II. JPMMW, LJEM, PM were responsible for the data collection. MCD was responsible for the selection of food items (FFQ) and assisted in the analysis of dietary intake data. JPMMW and LJMS conducted the statistical analyses, interpreted the data and drafted the manuscript. All authors were involved in interpretation of the outcomes, critically reviewed draft versions and approved the final manuscript.
Competing interests: Authors state no conflict of interest.
Informed consent: Informed consent was obtained from all individuals included in this study.
Ethical approval: The Medical Ethical Committee of the Maastricht University Medical Centre declared that no ethical approval was necessary for the Expect Study I and II (MEC-13-4-053 and MEC-17-4-057, respectively).
References
- 1.Crowther CA, Hiller JE, Pridmore B, Bryce R, Duggan P, Hague WM, et al. Calcium supplementation in nulliparous women for the prevention of pregnancy-induced hypertension, preeclampsia and preterm birth: an Australian randomized trial. FRACOG and the ACT study group. Aust N Z J Obstet Gynaecol. 1999;39:12–8. doi: 10.1111/j.1479-828x.1999.tb03434.x. [DOI] [PubMed] [Google Scholar]
- 2.Bucher HC, Guyatt GH, Cook RJ, Hatala R, Cook DJ, Lang JD, et al. Effect of calcium supplementation on pregnancy-induced hypertension and preeclampsia: a meta-analysis of randomized controlled trials. JAMA. 1996;275:1113–7. doi: 10.1001/jama.1996.03530380055031. [DOI] [PubMed] [Google Scholar]
- 3.Hofmeyr GJ, Manyame S, Medley N, Williams MJ. Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy. Cochrane Database Syst Rev. 2019;9:Cd011192. doi: 10.1002/14651858.CD011192.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/s0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 5.Belizán JM, Villar J, Gonzalez L, Campodonico L, Bergel E. Calcium supplementation to prevent hypertensive disorders of pregnancy. N Engl J Med. 1991;325:1399–405. doi: 10.1056/NEJM199111143252002. [DOI] [PubMed] [Google Scholar]
- 6.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cormick G, Betrán AP, Romero IB, Lombardo CF, Gülmezoglu AM, Ciapponi A, et al. Global inequities in dietary calcium intake during pregnancy: a systematic review and meta-analysis. BJOG. 2019;126:444–56. doi: 10.1111/1471-0528.15512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merialdi M, Mathai M, Ngoc NTN, Purwar M, Campodonico L, Abdel-Aleem H, et al. World health organisation systematic review of the literature and multinational nutritional survey of calcium intake during pregnancy. Fetal Matern Med Rev. 2005;16:97–121. doi: 10.1017/s0965539505001506. [DOI] [Google Scholar]
- 9.World Health Organisation . Guideline calcium supplementation in pregnant women. 2013. http://apps.who.int/iris/bitstream/10665/85120/1/9789241505376_eng.pdf Available from. [PubMed] [Google Scholar]
- 10.Meertens LJE, Scheepers HCJ, Willemse JPMM, Spaanderman MEA, Smits LJM. Should women be advised to use calcium supplements during pregnancy? A decision analysis. Matern Child Nutr. 2018;14 doi: 10.1111/mcn.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willemse J, Meertens LJE, Scheepers HCJ, Achten NMJ, Eussen SJ, van Dongen MC, et al. Calcium intake from diet and supplement use during early pregnancy: the expect study I. Eur J Nutr. 2020;59:167–74. doi: 10.1007/s00394-019-01896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemmens SMP, Röselaers YCM. Limburg obstetric quality system–zorgpaden (aanbevelingen) 2016. https://www.zwangerinlimburg.nl/sites/zwangerinlimburg/files/loqs_zorgpaden_aanbevelingen_vid1.70719.pdf [Google Scholar]
- 13.van Montfort P, Smits LJM, van Dooren IMA, Lemmens SMP, Zelis M, Zwaan IM, et al. Implementing a preeclampsia prediction model in obstetrics: cutoff determination and health care professionals’ adherence. Med Decis Making. 2020;40:81–9. doi: 10.1177/0272989x19889890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Montfort P, Scheepers HCJ, van Dooren IMA, Meertens LJE, Zelis M, Zwaan IM, et al. Low-dose-aspirin usage among women with an increased preeclampsia risk: a prospective cohort study. Acta Obstet Gynecol Scand. 2020;99:875–83. doi: 10.1111/aogs.13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Montfort P, Willemse JP, Dirksen CD, van Dooren IM, Meertens LJ, Spaanderman ME, et al. Implementation and effects of risk-dependent obstetric care in The Netherlands (Expect study II): protocol for an impact study. JMIR Res Protoc. 2018;7:e10066. doi: 10.2196/10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meertens LJE, Scheepers HCJ, van Kuijk SMJ, Aardenburg R, van Dooren IMA, Langenveld J, et al. External validation study of first trimester obstetric prediction models (Expect study I): research protocol and population characteristics. JMIR Res Protoc. 2017;6:e203. doi: 10.2196/resprot.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RIVM . NEVO-online 2010-2.0. Bilthoven: RIVM; 2010. [Google Scholar]
- 18.Rossum van CTM, Fransen HP, Verkaik-Kloosterman J, Buurma-Rethans EJM, Ocké MC. Dutch national food consumption survey 2007–2010. 2011. http://www.rivm.nl/bibliotheek/rapporten/350050006.pdf Available from. [Google Scholar]
- 19.Reynolds AN, Skeaff SA. Maternal adherence with recommendations for folic acid and iodine supplements: a cross-sectional survey. Aust N Z J Obstet Gynaecol. 2018;58:125–7. doi: 10.1111/ajo.12719. [DOI] [PubMed] [Google Scholar]
- 20.Ray JG, Singh G, Burrows RF. Evidence for suboptimal use of periconceptional folic acid supplements globally. BJOG. 2004;111:399–408. doi: 10.1111/j.1471-0528.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- 21.Cleveland WS. LOWESS: a program for smoothing scatterplots by robust locally weighted regression. Am Statistician. 1981;35:54. doi: 10.2307/2683591. [DOI] [Google Scholar]
- 22.Klemm GC, Birhanu Z, Ortolano SE, Kebede Y, Martin SL, Mamo G, et al. Integrating calcium into antenatal iron-folic acid supplementation in Ethiopia: women’s experiences, perceptions of acceptability, and strategies to support calcium supplement adherence. Glob Health Sci Pract. 2020;8:413–30. doi: 10.9745/ghsp-d-20-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thapa K, Sanghvi H, Rawlins B, Karki YB, Regmi K, Aryal S, et al. Coverage, compliance, acceptability and feasibility of a program to prevent pre-eclampsia and eclampsia through calcium supplementation for pregnant women: an operations research study in one district of Nepal. BMC Pregnancy Childbirth. 2016;16:241. doi: 10.1186/s12884-016-1033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin SL, Omotayo MO, Chapleau GM, Stoltzfus RJ, Birhanu Z, Ortolano SE, et al. Adherence partners are an acceptable behaviour change strategy to support calcium and iron-folic acid supplementation among pregnant women in Ethiopia and Kenya. Matern Child Nutr. 2017;13:e12331. doi: 10.1111/mcn.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omotayo MO, Dickin KL, Pelletier DL, Martin SL, Kung’u JK, Stoltzfus RJ. Feasibility of integrating calcium and iron-folate supplementation to prevent preeclampsia and anemia in pregnancy in primary healthcare facilities in Kenya. Matern Child Nutr. 2018;14(1 Suppl):e12437. doi: 10.1111/mcn.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanghvi TG, Harvey PW, WainwrightE Maternal iron-folic acid supplementation programs: evidence of impact and implementation. Food Nutr Bull. 2010;31:S100–7. doi: 10.1177/15648265100312S202. [DOI] [PubMed] [Google Scholar]
- 27.Martin SL, Omotayo MO, Pelto GH, Chapleau GM, Stoltzfus RJ, Dickin KL. Adherence-specific social support enhances adherence to calcium supplementation regimens among pregnant women. J Nutr. 2017;147:688–96. doi: 10.3945/jn.116.242503. [DOI] [PubMed] [Google Scholar]
- 28.National Institutes of Health . Calcium. Fact sheet for health professionals. 2020. https://ods.od.nih.gov/factsheets/calcium-healthprofessional/#h6 Available from. [Google Scholar]
- 29.Drake VJ. Subpopulations at risk for micronutrient inadequacy or deficiency. 2018. https://lpi.oregonstate.edu/mic/micronutrient-inadequacies/subpopulations-at-risk Available from. [Google Scholar]
- 30.Banfield EC, Liu Y, Davis JS, Chang S, Frazier-Wood AC. Poor adherence to US dietary guidelines for children and adolescents in the national health and nutrition examination survey population. J Acad Nutr Diet. 2016;116:21–7. doi: 10.1016/j.jand.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ter Borg S, Verlaan S, Hemsworth J, Mijnarends DM, Schols JM, Luiking YC, et al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr. 2015;113:1195–206. doi: 10.1017/s0007114515000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunst KJ, Wright RO, DiGioia K, Enlow MB, Fernandez H, Wright RJ, et al. Racial/ethnic and sociodemographic factors associated with micronutrient intakes and inadequacies among pregnant women in an urban US population. Publ Health Nutr. 2014;17:1960–70. doi: 10.1017/s1368980013003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zorginstituut Nederland . Medicijnkosten, calcium carbonate. 2016. http://www.medicijnkosten.nl Available from. [Google Scholar]
- 34.Formulary BN. Calcium carbonate. 2016. http://www.medicinescomplete.com/mc/bnf/current/PHP93602-calcium-carbonate.htm?q=calcichew&t=search&ss=text&p=7-_hit Available from. [Google Scholar]
- 35.Hofmeyr GJ, Lawrie TA, Atallah ÁN, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2014;24:CD001059. doi: 10.1002/14651858.CD001059.pub4. [DOI] [PubMed] [Google Scholar]
- 36.von Dadelszen P, Magee LA, Devarakonda RM, Hamilton T, Ainsworth LM, Yin R, et al. The prediction of adverse maternal outcomes in preeclampsia. J Obstet Gynaecol Can. 2004;26:871–9. doi: 10.1016/s1701-2163(16)30137-2. [DOI] [PubMed] [Google Scholar]
- 37.Lupattelli A, Spigset O, Nordeng H. Adherence to medication for chronic disorders during pregnancy: results from a multinational study. Int J Clin Pharm. 2014;36:145–53. doi: 10.1007/s11096-013-9864-y. [DOI] [PubMed] [Google Scholar]
- 38.Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97–107. doi: 10.7326/0003-4819-155-2-201107190-00005. [DOI] [PubMed] [Google Scholar]
- 39.Forbes LE, Graham JE, Berglund C, Bell RC. Dietary change during pregnancy and women’s reasons for change. Nutrients. 2018;10:1032. doi: 10.3390/nu10081032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicklas TA, O’Neil CE, Fulgoni VL. The role of dairy in meeting the recommendations for shortfall nutrients in the American diet. J Am Coll Nutr. 2009;28:73s–81s. doi: 10.1080/07315724.2009.10719807. [DOI] [PubMed] [Google Scholar]
- 41.Wood F, Robling M, Prout H, Kinnersley P, Houston H, Butler C. A question of balance: a qualitative study of mothers’ interpretations of dietary recommendations. Ann Fam Med. 2010;8:51–7. doi: 10.1370/afm.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saba A, Di Natale R. A study on the mediating role of intention in the impact of habit and attitude on meat consumption. Food Qual Prefer. 1999;10:69–77. [Google Scholar]
- 43.Hjartåker A, Andersen LF, Lund E. Comparison of diet measures from a food-frequency questionnaire with measures from repeated 24-hour dietary recalls. The Norwegian Women and Cancer Study. Publ Health Nutr. 2007;10:1094–103. doi: 10.1017/S1368980007702872. [DOI] [PubMed] [Google Scholar]