Abstract
Introduction
Dietary supplements are touted for cognitive protection, but supporting evidence is mixed. COSMOS-Mind tested whether daily administration of cocoa extract (containing 500 mg/day flavanols) versus placebo and a commercial multivitamin-mineral versus placebo improved cognition in older women and men.
Methods
COSMOS-Mind, a large randomized two-by-two factorial 3-year trial, assessed cognition by telephone at baseline and annually. The primary outcome was a global cognition composite formed from mean standardized (z) scores (relative to baseline) from individual tests including the Telephone Interview of Cognitive Status, Word List and Story Recall, Oral Trail-Making, Verbal Fluency, Number Span, and Digit Ordering. Using intention-to-treat, the primary endpoint was change in this composite with 3 years of cocoa extract use. The pre-specified secondary endpoint was change in the composite with 3 years of multivitamin-mineral supplementation. Treatment effects were also examined for executive function and memory composite scores, and in pre-specified subgroups at higher risk for cognitive decline.
Results
2262 participants were enrolled (mean age=73y; 60% women; 89% non-Hispanic White), and 92% completed the baseline and at least one annual assessment. Cocoa extract had no effect on global cognition (mean z-score=0.03, 95%CI: −0.02 to 0.08; P=0.28). Daily multivitamin-mineral supplementation, relative to placebo, resulted in a statistically significant benefit on global cognition (mean z=0.07, 95%CI 0.02 to 0.12; P=0.007), and this effect was most pronounced in participants with a history of cardiovascular disease (no history: 0.06, 95%CI 0.01 to 0.11; history: 0.14, 95%CI −0.02 to 0.31; interaction, nominal P=0.01). Multivitamin-mineral benefits were also observed for memory and executive function. The cocoa extract by multivitamin-mineral group interaction was not significant for any of the cognitive composites.
Discussion
Cocoa extract did not benefit cognition. However, COSMOS-Mind provides the first evidence from a large, long-term, pragmatic trial to support the potential efficacy of a multivitamin-mineral to improve cognition in older adults. Additional work is needed to confirm these findings in a more diverse cohort and to identify mechanisms to account for MVM effects.
Keywords: aging, older adults, cognition, cognitive function, cocoa extract, flavanols, multivitamin, clinical trial
1. BACKGROUND
There is an urgent need to identify effective strategies to preserve cognitive function to mitigate the heavy societal burden associated with Alzheimer’s disease (AD) and related dementia, which affect more than 46 million people worldwide.1 No interventions to prevent cognitive decline in asymptomatic older adults have been approved by the FDA, and to date, there is insufficient evidence to support a clinical benefit of any pharmacologic treatment for adults with mild cognitive impairment due to AD.2,3 Identifying a safe, affordable and accessible intervention to protect cognitive function against decline in older adults is a pressing public health priority.4
Cocoa, in its unprocessed form, contains high quantities of catechins and epicatechins, members of a subclass of flavonoids known as flavanols,5,6 and modest amounts of theobromine (an alkaloid of the cacao bean) and caffeine. Dietary consumption of cocoa flavanols may slow cognitive decline through improved cerebral vasodilation,7 blood flow, perfusion and angiogenesis.8,9 Epicatechin, the most common flavanol in cocoa, is rapidly absorbed, readily crosses the blood-brain barrier, can be detected in the brain,10 and may have accumulating physiological effects at higher levels.11,12 Much of the support for cognition-enhancing effects of flavonoids in healthy older adults comes from epidemiological studies,13,14 and a few small clinical trials.15–17 Memory and executive function appear to benefit the most, particularly with higher amounts of cocoa flavanols (500–750 mg/day), according to a recent review.18
Individual micronutrients and minerals target multiple biologic pathways that support normal body and brain function,19 and deficiencies in older adults may increase risk for cognitive decline and dementia.20 Trials of single nutrients such as folic acid with or without other B vitamins,21–24 omega-3 fatty acids,25,26 and vitamin D20,27,28 on cognition have yielded mixed results, which could reflect either no benefit or study design issues that impede cross-study comparisons. Some of these issues relate to the specific micronutrients tested (alone or in combination), the specific cognitive tests administered, the outcome measure (single test vs. composite score), and participant demographics and nutritional status.29,30 Although not without controversy, particularly with respect to potential cognition-enhancing effects of B vitamins,21,22 the conclusion of meta-analytic reviews is that the evidence is insufficient to encourage care providers to recommend use of individual nutrient supplements for brain health.31,32
Longer-term daily intake (>12 months) of a multivitamin-mineral alone or with other dietary supplements to enhance global cognitive function in older adults (≥65 years) has been examined in just one large randomized controlled trial (RCT), which included only male physicians.33 Further study is needed given their widespread use in the general population.34 Here we tested whether daily treatment with cocoa extract (CE) and/or a multivitamin-mineral (MVM) for 3 years protected cognitive function in older adults.
2. METHODS
2.1. Study design
COSMOS-Mind (COcoa Supplement and Multivitamin Outcomes Study of the Mind) was an ancillary study to a large pragmatic, placebo-controlled, 2×2 factorial clinical trial testing the effects of daily supplementation with cocoa extract (CE) and/or a multivitamin-mineral (MVM) on cardiovascular and cancer outcomes.35–37 Given that no additive or synergistic effects were expected with CE and MVM supplementation, the 2×2 factorial design provided an efficient strategy to examine treatment effects of two different agents within a single trial. The CE (Mars Edge, of Mars, Inc.) contained 500 mg of cocoa flavanols, including 80 mg (−)-epicatechins and modest amounts of theobromine (~50 mg/day) and caffeine (~15 mg/day) that likely enhance flavanols’ central and vascular effects.38,39 The 500 mg/day of cocoa flavanols exceeds mean reported intake40 and is consistent with amounts tested in short-term cardiovascular trials.41 The essential nutrients contained in the MVM (Centrum Silver; Pfizer Consumer Healthcare, now Haleon) are listed in Table S6. COSMOS-Mind examined the effects of these supplements on cognitive function, and the details of study design, participant demographics, and baseline characteristics are published.42 The study was conducted in accordance with The Code of Ethics of the World Medical Association, and was approved by the Institutional Review Board of Wake Forest University School of Medicine.
2.2. Participants
Enrollment of two thousand parent trial participants was targeted for COSMOS-Mind. Parent trial eligibility criteria included: 1) no history of myocardial infarction (MI) or stroke (history of other cardiovascular events including transient ischemic attack, congestive heart failure, coronary artery bypass graft, angioplasty, and stent was permitted); 2) no history of cancer within the last 2 years (excluding non-melanoma skin cancer); 3) no serious illness precluding participation; 4) not taking cocoa or vitamin/mineral supplements, or, willing to forego use during the trial; 5) no reported extreme sensitivity to cocoa products or caffeine; 6) successful completion of ≥2-month placebo run-in with ≥75% study pill adherence; and 7) not currently participating in another clinical trial. Additional COSMOS-Mind eligibility criteria included: 1) ≥65 years of age; 2) not taking insulin for diabetes; and 3) able to complete the telephone cognitive assessment (to screen out participants with significant impairment). Participants provided informed consent and received a $15 gift card upon completion of each assessment.
2.3. Randomization and masking
Randomization was controlled by the parent trial using a computer-generated sequential list of random allocations for the 4 treatment combinations.36,37 The random allocation sequence was created using SAS statistical software (version 9.4) and was stratified by sex, age (5-year bins), and recruitment source (Brigham and Women’s Hospital, Women’s Health Initiative). All trial investigators, examiners, and participants were masked to treatment group assignment.
2.4. Procedures
As previously described,42 parent trial participants were recruited through mailings to Women’s Health Initiative Extension Study participants and by Brigham and Women’s Hospital to participants contacted for, but not randomized into, the VITamin D and OmegA-3 TriaL, and through commercial mailing lists and media campaigns. As a result, 35,669 adults began a placebo run-in, of which 21,442 (60%) were ultimately randomized into COSMOS. During the placebo run-in, eligible candidates received an invitation to also participate in COSMOS-Mind. Interested individuals were contacted to 1) provide more information about the ancillary study, 2) test for hearing acuity, and 3) schedule the baseline telephone cognitive assessment. Individuals from traditionally underrepresented groups in research (self-identified as American Indian/Alaska Native, Asian/Pacific Islander, Black/African American, Hispanic; completing 12 years or less of education) were prioritized in the COSMOS-Mind initial contact queue (pre-randomization) to increase their representation in the sample. Demographics and baseline anthropometrics (height, weight) and medical history were provided by participants via questionnaires.
2.5. Outcomes
A standardized telephone cognitive battery was administered at baseline and annually for 3 years to assess general cognitive status, episodic memory and executive function. Tests included the 50-point modified Telephone Interview for Cognitive Status (TICSm with 10-minute short delay word list recall), an additional 40-minute Long Delay Word List Recall,43 immediate and delayed Story Recall (SRI & II),44 Oral Trail-Making Test Part B (OTMT-B, log transformed; Part A was administered for practice only),45 Verbal Fluency by category (VF-C) and letter (VF-L),46 Number Span (NS),47 and Digit Ordering Test (DOT).48 Details about testing procedures, including hearing acuity screening, and administration and scoring are published.42
The primary outcome was a global cognition composite (reported as a z-score based on baseline data from the study cohort) formed from mean standardized (z) scores of pre-specified individual test metrics, with higher scores reflecting better performance. Key secondary outcomes included an episodic memory composite (Long Delay Word List Recall, SRI, SRII) and an executive function composite (OTMT-B, VF-C, VF-L, NST, DOT) that were also formed using mean standardized (z) scores.
Tertiary outcomes included the Cognitive Change Index (CCI),49 a self-report measure of cognitive concerns, and the short 15-item version of the Geriatric Depression Scale (GDS-SF) to permit subgroup analyses for individuals scoring low and high on these scales. Adverse events were recorded by the parent trial, and events reported during a cognitive assessment were relayed to the COSMOS team for tracking and follow-up.
2.6. Statistical analyses
The primary and secondary endpoints have been previously described42 and were pre-specified in the protocol (provided as a supplement). We followed the intention-to-treat approach: all participants were grouped as originally randomly assigned and scores from all cognitive assessments (baseline and Years 1, 2 and 3) for all participants were included in analyses. Scores were used as dependent variables in linear mixed effects models. Time was a categorical factor. No covariates were included in models for the primary comparisons. As specified in the protocol, inference was based on the mean difference of scores obtained across follow-up assessments relative to the baseline score for participants assigned to receive CE versus CE-placebo (primary endpoint), and for participants assigned to receive MVM versus MVM-placebo (key secondary endpoint). That is, for means at Years 0, 1, 2, and 3 represented by μ0, μ1, μ2, and μ3, this can be expressed as (μ1 + μ2 + μ3)/3 − μ0. Separate linear contrasts were used to compare active and placebo arms by CE and by MVM group assignment. For example, the contrast for CE effects is described by [(μCE1 + μCE2 + μCE3)/3 − μCE0] – [(μCE-PL1 + μCE-PL2 + μCD-PL3)/3 − μCE-PL0], where μCE and μCE-PL represent the marginal means for CE and CE-placebo at each time point respectively. This corresponds to an intention-to-treat approach in which all data are included in analyses irrespective of follow-up. We chose to parameterize our primary and secondary endpoints as mean differences from baseline across follow-up to avoid making specific assumptions about the nature of intervention effects (for example, we did not assume that intervention effects accumulated linearly and base comparisons on differences between slopes) and to treat any differences at each of the time points equally. We explored interactions between the CE and MVM treatments and the consistency of CE and MVM treatment effects among subgroups (using forest plots50) based on sex, and baseline age (<70 or ≥70 years), body mass index (BMI; <25, 25–29, ≥30 kg/m2), general cognitive function (TICSm tertiles), subjective cognitive concerns (CCI median split), depressive symptoms (GDS-SF median split), type 2 diabetes, history of hypertension, and history of CVD (based on self-report of transient ischemic attack, congestive heart failure, coronary artery bypass graft, angioplasty, or stent). Subgroups were pre-specified given their possible associations with cognitive and/or cardiovascular response to the interventions.51–53 Multiple imputation by fully conditional specification was used to examine the influence of missing data.54 No adjustments were made for multiple comparisons. The targeted 2,000-person sample was projected to provide >90% power to detect an effect size of 0.10 standard deviations (see Statistical Power supplement for more information). Analyses were performed using SAS v9.4.
3. RESULTS
COSMOS-Mind participants were enrolled from August 2, 2016 to August 17, 2017. 5342 individuals screening for the parent trial were approached for ancillary study enrollment. Phone calls were attempted for 3223 individuals (60%) and of these, 2262 (70%) were enrolled in COSMOS-Mind (Figure 1). Baseline characteristics and baseline cognitive test scores were well balanced across treatment groups (Table 1 and Table S1). Of enrolled participants, 2082 (92%) completed the cognitive assessment at Year 1, 1906 (84%) at Year 2, 1790 (79%) at Year 3, and 1732 (77%) in all 3 years of follow-up. Relative to participants who completed all assessments, those missing at least one assessment were more likely to be from underrepresented racial or ethnic groups, reported less physical activity and chocolate intake and more smoking, tended to have lower education and baseline TICSm scores, and higher prevalence of type 2 diabetes (Table S2). Characteristics of participants with no follow-up data (N=180) are provided in Table S3. Self-reported compliance (pill count) was comparable across treatment groups (% taking ≥75% study pills in Years 1, 2, and 3: 92%, 88%, and 84%).
FIGURE 1.
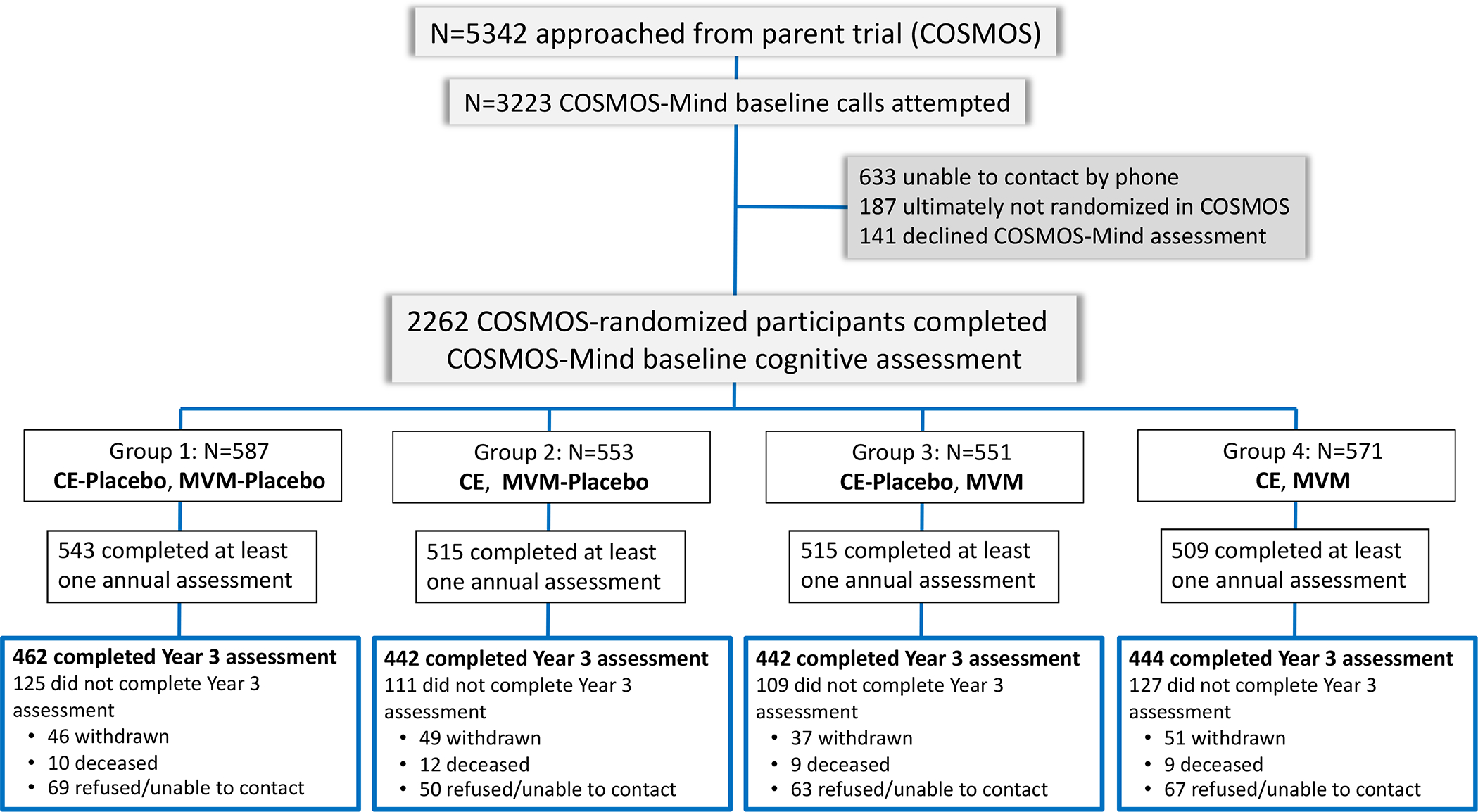
Consort diagram showing flow of participants from first approach through randomization to each of the 4 treatment combinations in the two-by-two factorial design. Abbreviations: CE, cocoa extract; MVM, multivitamin-mineral.
TABLE 1.
Distribution of Baseline Characteristics for COSMOS-Mind Participants by Cocoa Extract (CE) and Multivitamin-Mineral (MVM) Treatment Assignment
| Characteristic a | Overall (N=2262) | CE-Placebo, MVM-Placebo (N=587) | CE, MVM-Placebo (N=553) | CE-Placebo, MVM (N=551) | CE, MVM (N=571) |
|---|---|---|---|---|---|
| Age, mean (SD), years | 72.97 (5.63) | 73.10 (5.72) | 73.21 (5.62) | 72.86 (5.64) | 72.70 (5.52) |
| Sex, N (%) | |||||
| male | 914 (40.4) | 227 (38.7) | 223 (40.3) | 232 (42.1) | 232 (40.6) |
| female | 1348 (59.6) | 360 (61.3) | 330 (59.7) | 319 (57.9) | 339 (59.4) |
| Race and Ethnicity, N (%) | |||||
| American Indian/Alaska Native | 13 (0.6) | 2 (0.3) | 1 (0.2) | 8 (1.5) | 2 (0.4) |
| Asian/Pacific Islander | 39 (1.7) | 8 (1.4) | 12 (2.2) | 10 (1.8) | 9 (1.6) |
| Black/African American | 131 (5.8) | 34 (5.8) | 23 (4.2) | 34 (6.2) | 40 (7.0) |
| Hispanic | 67 (3.0) | 21 (3.6) | 18 (3.3) | 15 (2.7) | 13 (2.3) |
| Multiracial/unknown/not reported | 6 (0.3) | 2 (0.3) | 1 (0.2) | 2 (0.4) | 1 (0.2) |
| Non-Hispanic White | 2006 (88.7) | 520 (88.6) | 498 (90.1) | 482 (87.5) | 506 (88.6) |
| Education, N (%) | |||||
| did not complete high school | 11 (0.5) | 4 (0.7) | 1 (0.2) | 4 (0.7) | 2 (0.4) |
| high school diploma or G.E.D. | 258 (11.4) | 69 (11.8) | 68 (12.3) | 58 (10.5) | 63 (11.0) |
| attended or graduated college | 880 (38.9) | 230 (39.2) | 207 (37.4) | 220 (39.9) | 223 (39.1) |
| post-college | 1113 (49.2) | 284 (48.4) | 277 (50.1) | 269 (48.8) | 283 (49.6) |
| Body Mass Index, mean (SD), kg/m2 | 27.64 (5.16) | 27.48 (5.08) | 27.86 (5.40) | 27.57 (5.15) | 27.65 (5.01) |
| Diabetes, N (%) | 255 (11.3) | 74 (12.6) | 60 (10.9) | 61 (11.1) | 60 (10.5) |
| CVD History, N (%) b | 197 (8.7) | 59 (10.1) | 36 (6.5) | 44 (8.0) | 58 (10.2) |
| Hypertension, N (%) | 1333 (59.2) | 358 (61.3) | 321 (58.3) | 318 (57.7) | 336 (59.3) |
| Medication Use, N (%) | |||||
| antihypertensive | 1013 (54.3) | 327 (56.2) | 292 (53.5) | 296 (54.0) | 314 (55.5) |
| NSAID | 627 (28.0) | 168 (28.9) | 152 (27.7) | 137 (25.3) | 170 (30.0) |
| statin | 976 (43.5) | 262 (45.0) | 240 (43.8) | 224 (41.0) | 250 (44.1) |
| Total Metabolic Equivalent (MET) Hours of Exercise per Week, mean (SD) | 22.41 (23.47) | 21.51 (22.65) | 22.55 (23.83) | 23.57 (25.78) | 22.07 (21.51) |
| Depression, N (%) | 469 (20.9) | 123 (21.2) | 105 (19.1) | 124 (22.8) | 117 (20.6) |
| Smoking Status, N (%) | |||||
| never | 1153 (51.8) | 308 (53.4) | 271 (49.5) | 276 (51.2) | 298 (53.1) |
| past | 1004 (45.1) | 247 (42.8) | 267 (48.7) | 245 (45.5) | 245 (43.7) |
| current | 68 (3.0) | 22 (3.8) | 10 (1.8) | 18 (3.3) | 18 (3.2) |
| Alcohol Intake, N (%) | |||||
| rarely/never | 646 (29.4) | 193 (33.6) | 140 (25.9) | 152 (28.4) | 161 (29.4) |
| monthly | 152 (6.9) | 37 (6.5) | 42 (7.8) | 38 (7.1) | 35 (6.4) |
| weekly | 812 (36.9) | 196 (34.2) | 219 (40.5) | 192 (35.8) | 205 (37.5) |
| daily | 588 (26.8) | 148 (25.8) | 140 (25.9) | 154 (28.7) | 146 (26.7) |
| Baseline Chocolate Intake, N (%) | |||||
| rarely/never | 361 (16.5) | 88 (15.4) | 90 (16.7) | 91 (17.0) | 92 (16.8) |
| monthly | 307 (14.0) | 77 (13.5) | 74 (13.8) | 70 (13.1) | 86 (15.7) |
| weekly | 1274 (58.2) | 337 (59.0) | 319 (59.3) | 312 (58.3) | 306 (55.9) |
| daily | 249 (11.4) | 69 (12.1) | 55 (10.2) | 62 (11.6) | 63 (11.5) |
| Baseline Multivitamin-Mineral Use, N (%) | 956 (42.5) | 242 (41.7) | 232 (42.3) | 238 (43.4) | 244 (42.7) |
| Baseline Cognitive Function, mean (SD) | |||||
| Global Cognition Composite | -0.00 (1.00) | -0.05 (0.99) | 0.02 (1.00) | 0.05 (1.02) | -0.01 (0.99) |
| Executive Function Composite | 0.00 (1.00) | -0.05 (0.99) | -0.03 (0.97) | 0.08 (1.04) | 0.01 (1.00) |
| Episodic Memory Composite | 0.00 (1.00) | -0.03 (1.01) | 0.07 (0.99) | 0.01 (1.00) | -0.04 (1.01) |
| TICSm c | 36.60 (3.89) | 36.45 (3.88) | 36.66 (3.96) | 36.63 (3.84) | 36.68 (3.87) |
| Long Delay Word List Recall d | 3.02 (1.83) | 2.95 (1.78) | 3.05 (1.83) | 3.09 (1.86) | 2.99 (1.85) |
| Story Recall e | |||||
| Immediate | 12.00 (3.85) | 11.91 (3.91) | 12.26 (3.77) | 11.95 (3.79) | 11.89 (3.91) |
| Delayed | 10.77 (3.94) | 10.77 (3.95) | 10.98 (3.88) | 10.75 (3.86) | 10.57 (4.06) |
| Oral Trail-Making Test, median (IQR) f | |||||
| Part A | 9 (8, 11) | 9 (8, 11) | 9 (8, 11) | 9 (8, 11) | 9 (8, 11) |
| Part B | 30 (24, 46) | 30 (23, 44) | 30 (24, 46) | 30 (24, 46) | 31 (24, 48) |
| Verbal Fluency g | |||||
| Category | 35.02 (8.24) | 34.80 (7.88) | 34.89 (8.26) | 35.32 (8.33) | 35.07 (8.51) |
| Letter | 25.54 (8.12) | 25.34 (7.82) | 25.40 (7.67) | 26.01 (8.29) | 25.42 (8.65) |
| Number Span h | |||||
| Forward | 8.19 (2.39) | 8.10 (2.32) | 8.06 (2.43) | 8.39 (2.51) | 8.22 (2.29) |
| Backward | 6.87 (2.32) | 6.85 (2.32) | 6.86 (2.17) | 6.97 (2.53) | 6.80 (2.25) |
| Digit Ordering Test i | 6.27 (2.19) | 6.16 (2.22) | 6.25 (2.08) | 6.39 (2.27) | 6.27 (2.19) |
There were no significant group differences for any baseline characteristic (i.e., p>0.05).
Based on self-report of transient ischemic attack, congestive heart failure, coronary artery bypass graft, angioplasty, or stent.
Telephone Interview for Cognitive Status-modified (TICSm; maximum score=50) is a measure of global cognition and includes short delay word list recall (10-minute delay).
Long Delay Word List Recall (40-minute delay; maximum score=10) measures verbal memory.
Immediate and Delayed Story Recall (SRI & II) measures verbal memory (maximum score per test=25).
Oral Trail-Making Test is a modified version of the original paper and pencil version that measures simple attention (Part A; not included in analyses) and executive function (Part B); times to complete Part B were log transformed prior to analysis.
Verbal Fluency is a measure of language accessibility and includes fluency by category (animals, vegetables) and by letter (F, L).
Number Span measures simple attention and working memory.
Digit Ordering Test is a task of working memory that is similar to but more difficult than Number Span Backward.
Figure 2A shows change in global cognition, relative to baseline, for participants assigned to CE (Figure 1, Groups 2 & 4) versus CE-placebo (Figure 1, Groups 1 & 3). Mean scores in both CE groups increased through the first 2 years (likely due to retest practice effects55) and then plateaued but did not differ significantly from one another. The mean change (CE minus CE-placebo) z-score for global cognition was 0.03 (95%CI: −0.02 to 0.08; P=0.28). In contrast, Figure 2B shows a significant treatment effect of MVM (Figure 1, Groups 3 & 4) versus MVM-placebo (Figure 1, Groups 1 & 2) on global cognition (secondary endpoint), with a mean change (MVM minus MVM-placebo) z-score of 0.07 (95%CI: 0.02 to 0.12; P=0.007). Baseline MVM use was not associated with MVM treatment response (no prior use: 0.062, 95%CI: −0.002 to 0.13; prior use: 0.077, 95%CI: 0.003 to 0.15; interaction, nominal P=0.88).
FIGURE 2.
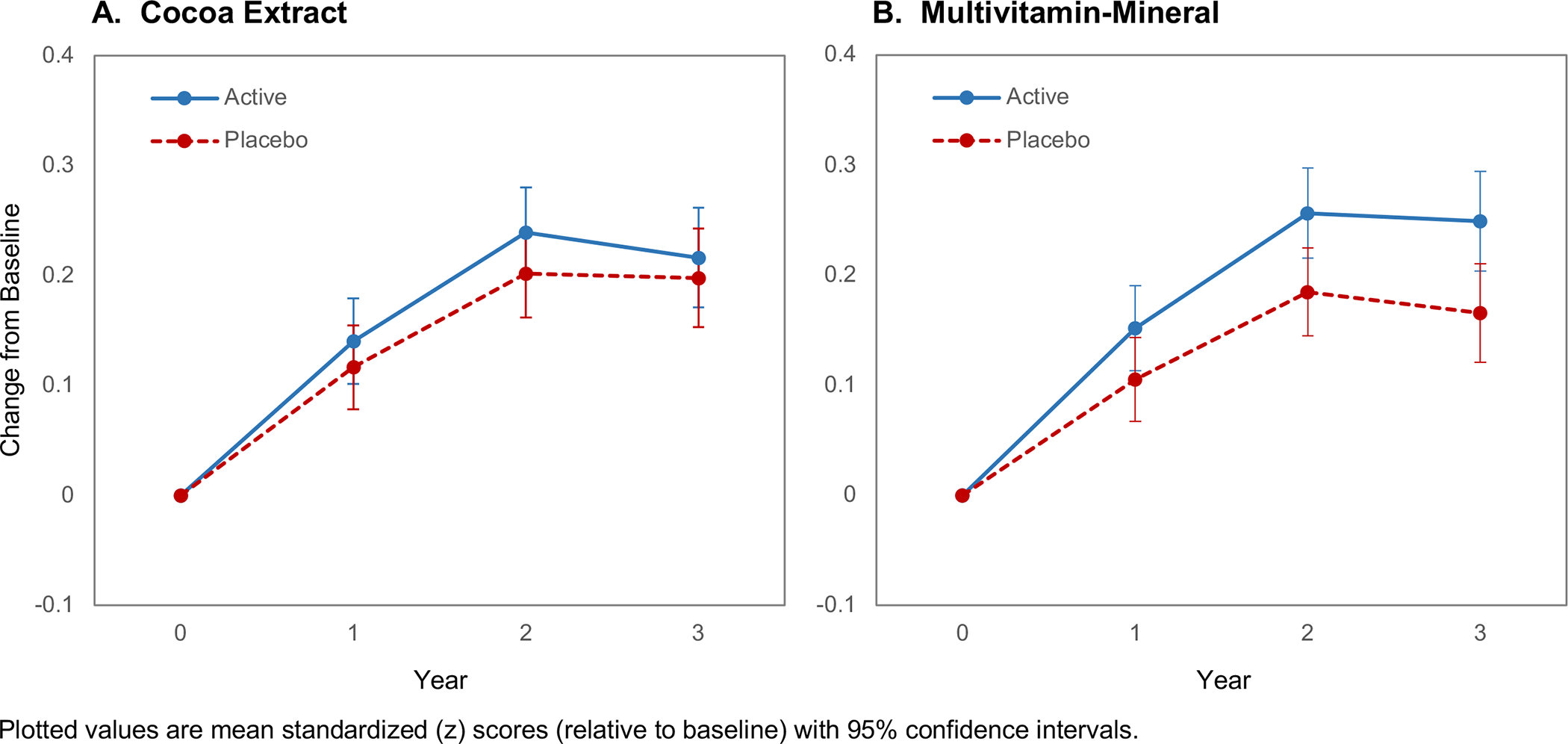
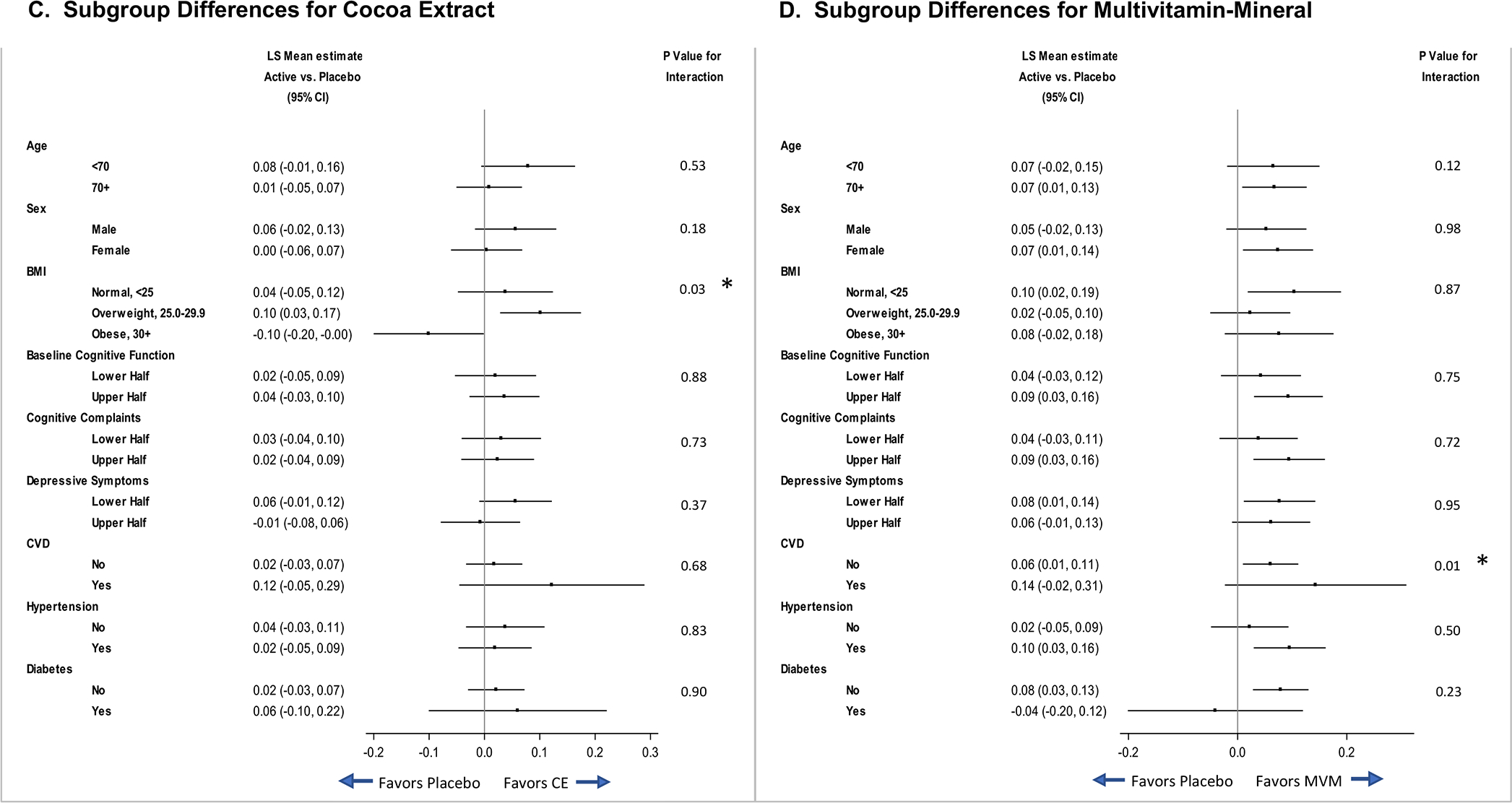
Three-year change in global cognition composite by assignment to (A) daily cocoa extract supplementation, and (B) daily multivitamin-mineral supplementation. Forest plot marginal differences by baseline subgroups for (C) cocoa extract and (D) multivitamin-mineral treatment groups.
In pre-specified subgroup analyses of the primary outcome (global cognition composite), forest plots of CE effects (Figure 2C) suggest that response may vary with baseline BMI. Figure 2D forest plots show a relative benefit of MVM versus MVM-placebo for participants with a CVD history (no history: 0.06, 95%CI: 0.01 to 0.11; history: 0.14, 95%CI: −0.02 to 0.31; interaction, nominal P=0.01). As seen in Figure 3, at baseline, participants without CVD history outperformed those with CVD history on the global cognition composite (mean difference=0.22, 95%CI: 0.08 to 0.37; P=0.003); after Year 1, the MVM-placebo declined while the MVM group showed relative improvement (or protection against decline). There was no evidence of disproportionate loss of Year 3 data by CVD history (Table S4). The CVD history subgroup (compared to subgroup without CVD history) included more men, and tended to be older, have higher BMI, more hypertension, more statin use, more depression, less physical activity, and lower scores on the TICSm. When participants with CVD history were excluded in a sensitivity test from the overall analysis of MVM effects on the global cognition composite, the main MVM finding remained unchanged (mean change z-score=0.06, 95%CI: 0.01 to 0.11; P=0.02). That is, even though the pattern of results differed for subgroups with and without CVD at baseline, 3-years of MVM treatment improved global cognition for all participants, not just those with CVD history.
FIGURE 3.
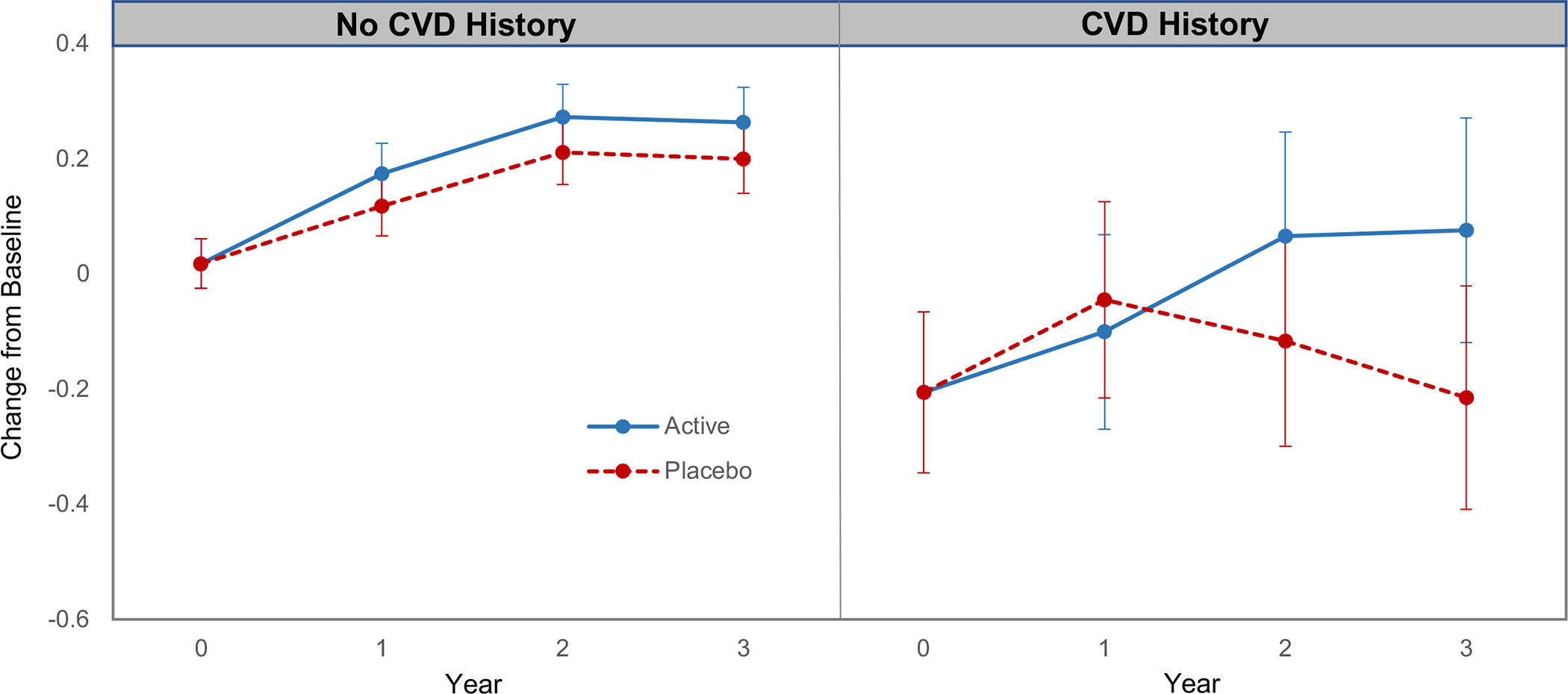
Three-year change in global cognition composite for the active and placebo multivitamin-mineral groups by history of cardiovascular disease, which was based on self-report of transient ischemic attack, congestive heart failure, coronary artery bypass graft, angioplasty, or stent.
Plotted values are mean standardized (z) scores (relative to baseline) with 95% confidence intervals.
CE had no effect on the episodic memory composite (mean change z-score=0.03, 95%CI: −0.04 to 0.09; P=0.40) or on the executive function composite (mean change z-score=0.03, 95%CI: −0.02 to 0.08; P=0.23) (Figure S1). In contrast, but consistent with the positive MVM effect described above, MVM supplementation led to relative improvements both for memory (mean change z-score=0.06, 95%CI: 0.002 to 0.13; P=0.04) and for executive function (mean change z-score=0.06, 95%CI: 0.01 to 0.11; P=0.02) (Figure 4).
FIGURE 4.
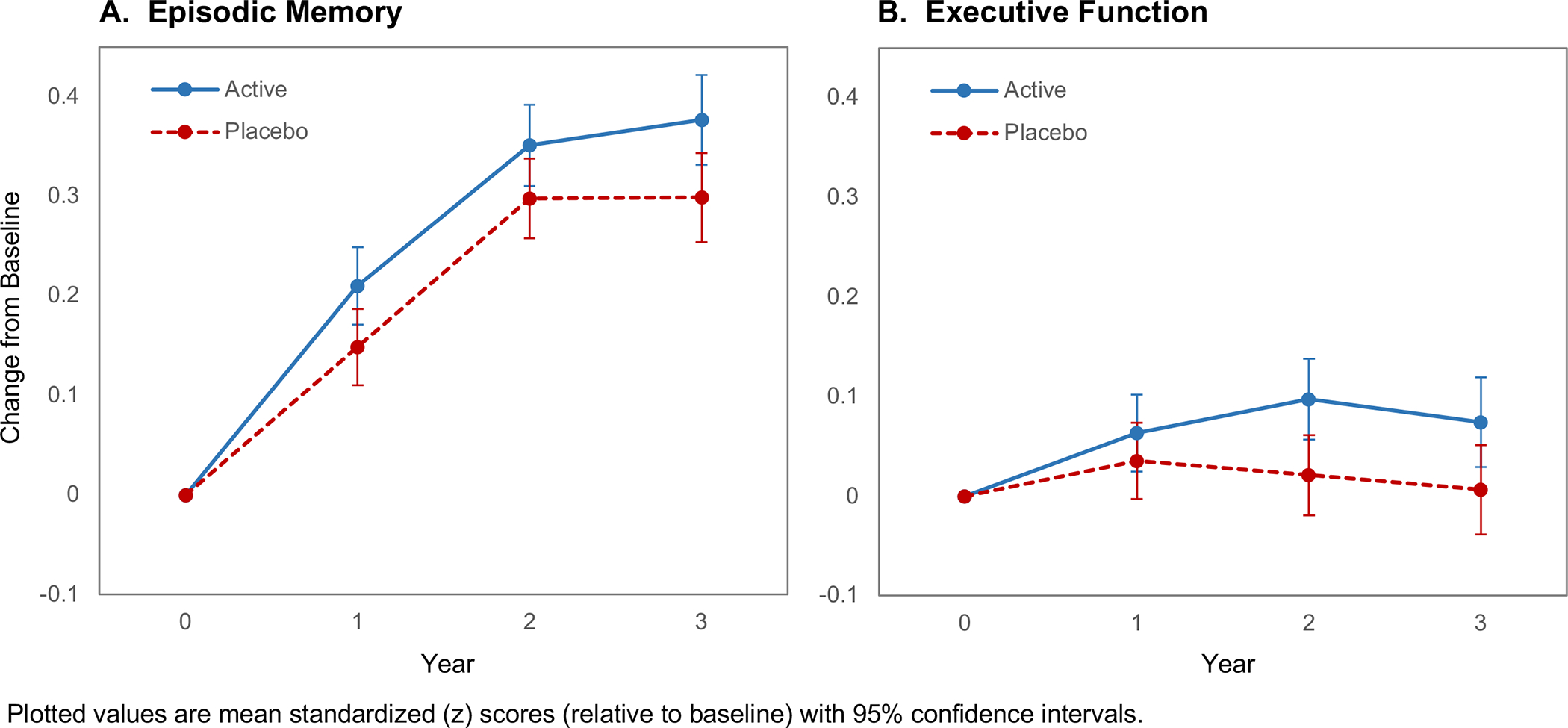
Three-year change in the episodic memory composite (A) and executive function composite (B) for the active and placebo multivitamin-mineral groups.
There was no evidence for an interaction between CE and MVM supplementation for the primary outcome (mean change z-score [95%CI]; CE-placebo/MVM-placebo: 0.15 [0.10 to 0.20]; CE/MVM-placebo: 0.15 [0.11 to 0.20]; CE-placebo/MVM: 0.20 [0.15 to 0.24]; CE/MVM: 0.24 [0.19 to 0.29]; interaction, P=0.40) or for the memory and executive function composites. That is, adding CE did not alter the benefit of MVM on cognition.
Multiple imputation provided no evidence that differential attrition biased the results (Table S5), and there were no safety concerns of supplement use during the trial.
4. DISCUSSION
COSMOS-Mind is the first large-scale, long-term RCT to assess the effects of cocoa extract and a multivitamin-mineral supplement on global cognition in older women and men from the general population. Although our findings did not support a positive effect of CE (primary endpoint), cognition significantly benefited from 3 years of daily MVM use (secondary endpoint). Moreover, the results of pre-planned subgroup analyses indicated that participants with baseline history of CVD may show a more pronounced MVM benefit, suggesting either greater relative improvement or more protection from CVD-related cognitive decline.
Daily CE supplementation for 3 years did not affect cognitive function in our trial. Only a handful of controlled, shorter-term trials have examined the potential benefits of cocoa flavanols on cognition in older adults. While some studies showed cognitive benefits,16,17,56 others did not.8,57–59 In the CoCoA study, a double-blind, 8-week RCT that examined cocoa flavanol effects on cognition, 90 cognitively healthy older adults17 and 90 individuals with amnestic MCI16 received a daily drink containing low (45 mg/day), medium (520 mg/day) or high (993 mg/day) amounts of cocoa flavanols. Performance on the Trail-Making Test, verbal fluency, and the cognitive composite that combined these test scores improved for high- and medium-dose groups relative to the low-dose group, regardless of participant cognitive status. In a smaller (n=37) 3-month RCT comparing 900 mg/day versus 45 mg/day of cocoa flavanols, blood flow increased in the dentate gyrus of the hippocampus, which was associated with a treatment-related improvement on a hippocampal-dependent memory task in adults (50–69 years old).56 In a 12-week RCT of 260, 510, or 770 mg/day of cocoa flavanols in 211 adults (50–75 years old), although there was no effect on the primary endpoint (computer-administered object-recognition task), secondary analyses indicated a dose-dependent improvement on list-learning in adults with a poor quality baseline diet.60 These trials all included short treatment durations showing acute effects of cocoa flavanols on cognition. In COSMOS-Mind, acute cognitive effects of cocoa flavanols (less than 3 months) that were not sustained would have been missed as our assessments were completed only once per year. COSMOS-Mind also differed from these studies as the active supplement included not only cocoa flavanols, but also theobromine and caffeine. Furthermore, it is possible that the COSMOS-Mind cocoa flavanol dose was too low to provide cognitive benefit within the study observation period, particularly in light of previous dose-dependent findings.60
In contrast to the negative CE findings, we found that assignment to 3 years of MVM improved global cognition, episodic memory, and executive function. Previous RCTs of MVM supplements on cognition have reported inconsistent effects. The majority were short in duration (up to 12 months) and relied on individual test scores to assess cognitive effects rather than a composite, which may provide greater statistical power to detect a difference across multiple tests and cognitive domains (if effect sizes are similar across its components).61
The only long-term MVM RCT prior to our study was the 12-year Physician’s Health Study II (PHS II) of older U.S. male physicians that tested whether a daily MVM supplement prevented risk of major CVD62 and cancer.63 A PHS II cognitive substudy of 5,947 healthy, highly educated men (aged ≥65 years) reported no MVM effects on a global cognition composite.33 There are several differences between COSMOS-Mind and PHS II worth highlighting. The COSMOS-Mind and PHS II cohorts differed on key baseline characteristics: PHS II participants were restricted to male physicians who were predominantly White and non-Hispanic, whereas COSMOS-Mind included a relatively more diverse cohort. Although baseline cognitive scores were comparable for participants across studies (i.e., TICSm; VF-C for animals), COSMOS-Mind administered additional tests of executive function (i.e., OTMT-B, VF-L, NS, DOT) and more challenging episodic memory tests (i.e., Long Delay Word List Recall, and a longer version of Story Recall with 55% more components to remember) that may have increased sensitivity to detect effects. In PHS II, the initial cognitive testing began an average of 2.5 years (range: 0.18–5.3 years) after randomization to MVM or placebo, nearly at the point in time when the final follow-up assessment was completed in COSMOS-Mind. Our data suggest that MVM treatment effects increased from baseline in the first 2 years and then remained stable between Years 2 and 3 (Figure 2). As a result, any early cognitive benefits of MVM in PHS II would likely have been missed because of their assessment schedule given the COSMOS-Mind cognitive trajectory showing benefit within 2 years. Also, the specific components of the MVM supplements administered in the two studies differed (Table S6); in COSMOS (parent study), lutein and lycopene were added, amounts of vitamins D and K were 150–300% higher, and amounts of vitamin A and minerals such as iron, magnesium, and copper were lower. Although some reports suggest cognition-protecting benefits of components that were at higher levels in the COSMOS MVM,64–67 no consensus has been reached about the role of specific supplement quantities for brain health.
Our results suggest that MVM cognitive benefits may be more pronounced among older adults with CVD. At baseline, global cognitive function was lower for adults with versus without CVD history, which is consistent with other reports.68 The MVM-treated CVD history subgroup had sustained increases in cognitive function after 2 years (Figure 3), while the placebo-treated CVD history subgroup showed cognitive decline after Year 1. One account for this finding relates to the potential treatment-related improvement in micronutrient levels in CVD-compromised individuals, which could, in turn, have beneficial consequences for brain health. The results of observational studies69 suggest that micronutrient levels are typically lower in patients with CVD versus those without CVD (e.g., heart failure), and may be susceptible to drug interactions.52 In CVD patients, for example, vitamin D deficiency is highly prevalent and predicts disease severity; vitamin K deficiency is linked to coronary artery calcification and increased CVD-related mortality; circulating levels of thiamine, vitamin C and selenium are relatively low (suboptimal, not necessarily deficient);69 and certain medications can reduce vitamin B12 absorption and bioavailability.70 In PHS II, there was no evidence that MVM supplementation affected cardiovascular health status.62 Nonetheless, additional investigation of treatment-related micronutrient status in a more diverse cohort of participants is warranted to confirm COSMOS-Mind findings and to explore mechanisms that might account for the observed benefit.
To estimate clinical significance of our findings, we used COSMOS-Mind data to model treatment-related protection against cognitive aging. At baseline, slope of the global cognition composite scores by participant age (ranging from 64 to 100 years) was −0.045 SD/year (standard error=0.004). The treatment effect of MVM relative to MVM-placebo was 0.083 SDs (95%CI: 0.020 to 0.146) at Year 3. This corresponds to baseline composite scores for individuals who were 1.8 years apart in age. By this albeit imprecise yardstick, 3 years of MVM supplementation appeared to have slowed aging by 1.8 years, or by 60%. Further speculation regarding the clinical significance of our MVM findings is difficult at this time given the relatively short duration of follow-up and the likely impact of practice effects commonly observed in trials with repeat cognitive testing that distort estimates of treatment-related change on metrics such as ‘cognitive age.’ Another trial is needed in a diverse cohort to confirm our findings and further assess the clinical significance of MVM supplementation on cognitive health in older women and men.
COSMOS-Mind had several strengths. The 2×2 factorial design allowed us to efficiently examine the effects of 2 different interventions within a single study, under the assumption of no additive effects when interventions are combined. The pragmatic approach (using mail and telephone only) facilitated recruitment of over 2000 older adults in less than 15 months, minimized participant burden (no travel needed), and provided an opportunity for research participation to individuals who may not have had ready access to an academic institution.
Study limitations included: (1) race and ethnicity of the cohort was not representative of older Americans, which affects generalizability of our results; (2) adherence to study pills and health history (e.g., CVD) were tracked using self-report; (3) inability to assess whether specific components of the COSMOS MVM were responsible for the observed cognitive benefits; (4) data were not collected to permit analyses of biomarkers or potential effect modifiers (e.g., apolipoprotein E genotype) and (5) type 1 error was not controlled across secondary and tertiary analyses in COSMOS-Mind and for outcomes measured in the parent COSMOS trial and its other ancillary studies.
In conclusion, daily intake of cocoa extract for 3 years had no effect on cognition. However, COSMOS-Mind provides the first evidence from a large-scale, long-term, pragmatic RCT to suggest that daily use of a safe, readily accessible, and relatively low-cost MVM supplement has the potential to improve or protect cognitive function for older women and men. An additional trial is needed to confirm these findings in a more representative cohort and to explore potential mechanisms for cognitive benefit. This work may ultimately have important public health implications for standard of care to improve or protect cognitive function in older adults.
Supplementary Material
Highlights.
COSMOS-Mind was a large simple pragmatic randomized clinical trial in older adults conducted by mail and telephone.
The trial used a two-by-two factorial design to assess treatment effects of two different interventions within a single large study.
We found no cognitive benefit of daily cocoa extract administration (containing 500 mg flavanols) for 3 years.
Daily multivitamin-mineral supplementation for 3 years improved global cognition, episodic memory, and executive function in older adults.
The multivitamin-mineral benefit appeared to be greater for adults with cardiovascular disease.
Research in Context.
Systematic review.
The literature was reviewed using PubMed and MEDLINE. Much of the support for potential cognitive benefits of cocoa flavanols in older adults is based on observational studies. Only a few controlled trials have examined the effects of a multivitamin-mineral supplement on cognition for older adults.
Interpretation.
There was no cognitive improvement with daily intake for 3 years of cocoa extract containing 500 mg/day cocoa flavanols. However, we provide the first evidence in a long-term, randomized controlled trial of older women and men that daily use of a safe, readily accessible, and low-cost multivitamin-mineral can improve cognition. This finding could have important public health implications for brain health and resilience against future cognitive decline.
Future directions.
Our results challenge the current status quo regarding the efficacy of multivitamin-mineral supplementation to improve cognitive function and set the stage for new avenues of research to identify mechanisms and alternate approaches involving combination therapy.
ACKNOWLEDGEMENTS
Other Contributions
We thank and acknowledge the contributions of all COSMOS-Mind participants for their time and commitment to the study. We also thank the COSMOS-Mind study team members who provided their expertise to meaningfully connect to participants, keep them engaged, and collect and manage the cognitive data for over 8000 assessments. Wake Forest University School of Medicine (Winston-Salem NC) Team members included Brad Caudle, Debbie Pleasants M.ED., Cheryl Summerville, Alecia Jenkins, Ashley Lentz, Charlene Hunt, Debbie Booth, Deborah Kampman, Dylan Jarrell, Heather Dailey, Leonard Jordan, Marcelle Clavette, Margaret Pierce, Sharnita Duran, Sonya Ashburn, Darrin Harris, Julia Spell, Mary Barr, and Leslie Gordineer. We are especially grateful to the parent trial investigators (Manson, Sesso, and Garnet Anderson, PhD, of the Fred Hutchinson Cancer Research Center, Seattle WA) and the study team at Brigham and Women’s Hospital (Boston, MA) for their partnership from study inception.
Funding/Support
The COSMOS-Mind study and investigators were supported through an award provided by the National Institute on Aging, of the National Institutes of Health (R01AG050657). Drs. Manson and Sesso also received support for the COcoa Supplement and Multivitamin Outcomes Study (COSMOS) through an investigator-initiated grant from Mars Edge, a segment of Mars, Inc. dedicated to nutrition research, which included infrastructure support and the donation of study pills and packaging. Pfizer Consumer Healthcare (now Haleon) provided support through the partial provision of study pills and packaging.
Footnotes
Competing Interests
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health and the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Neither the National Institutes on Health, Mars, nor Pfizer contributed to any aspect of the trial including design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The authors have no competing interests to report.
Access to Data and Data Analysis
Dr. Espeland had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declarations of Interest: None
Data Sharing
The protocol is available upon request. Datasets will be de-identified prior to release for sharing. We will make the data and associated documentation available to users only under a data-sharing agreement that provides for: (a) a commitment to using the data only for COSMOS-approved research purposes and protected identity of any individual participant; (b) a commitment to securing the data using appropriate computer technology; and (c) a commitment to destroying or returning the data after analyses are completed. We will advertise the availability of the study data to other investigators on the study website and on the NIH website following NIH approval.
REFERENCES
- 1.Prince M, Wimo A, Guerchet M, Ali GC, Wu Y, M. P World Alzheimer Report 2015. The global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International;2015. [Google Scholar]
- 2.Cavazzoni P FDA’s Decision to Approve New Treatment for Alzheimer’s Disease. In: Administration USFD, ed2021. [Google Scholar]
- 3.Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIA. Living Long & Well in the 21st Century: Strategic Directions for Research on Aging. 2014.
- 5.Corti R, Flammer AJ, Hollenberg NK, Luscher TF. Cocoa and cardiovascular health. Circulation. 2009;119(10):1433–1441. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Murga L, Tarin JJ, Garcia-Perez MA, Cano A. The impact of chocolate on cardiovascular health. Maturitas. 2011;69(4):312–321. [DOI] [PubMed] [Google Scholar]
- 7.Dorfman LJ, Jarvik ME. Comparative stimulant and diuretic actions of caffeine and theobromine in man. Clinical pharmacology and therapeutics. 1970;11(6):869–872. [DOI] [PubMed] [Google Scholar]
- 8.Sorond FA, Lipsitz LA, Hollenberg NK, Fisher ND. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatric disease and treatment. 2008;4(2):433–440. [PMC free article] [PubMed] [Google Scholar]
- 9.Williams RJ, Spencer JP. Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free radical biology & medicine. 2012;52(1):35–45. [DOI] [PubMed] [Google Scholar]
- 10.Abd El Mohsen MM, Kuhnle G, Rechner AR, et al. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free radical biology & medicine. 2002;33(12):1693–1702. [DOI] [PubMed] [Google Scholar]
- 11.Faria A, Pestana D, Teixeira D, et al. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food & function. 2011;2(1):39–44. [DOI] [PubMed] [Google Scholar]
- 12.Ferruzzi MG, Lobo JK, Janle EM, et al. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: implications for treatment in Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2009;18(1):113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165(12):1364–1371. [DOI] [PubMed] [Google Scholar]
- 14.Moreira A, Diogenes MJ, de Mendonca A, Lunet N, Barros H. Chocolate Consumption is Associated with a Lower Risk of Cognitive Decline. Journal of Alzheimer’s disease : JAD. 2016;53(1):85–93. [DOI] [PubMed] [Google Scholar]
- 15.Socci V, Tempesta D, Desideri G, De Gennaro L, Ferrara M. Enhancing Human Cognition with Cocoa Flavonoids. Front Nutr. 2017;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desideri G, Kwik-Uribe C, Grassi D, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) study. Hypertension. 2012;60(3):794–801. [DOI] [PubMed] [Google Scholar]
- 17.Mastroiacovo D, Kwik-Uribe C, Grassi D, et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the Cocoa, Cognition, and Aging (CoCoA) Study--a randomized controlled trial. The American journal of clinical nutrition. 2015;101(3):538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrera-Reyes PK, de Lara JC, Gonzalez-Soto M, Tejero ME. Effects of Cocoa-Derived Polyphenols on Cognitive Function in Humans. Systematic Review and Analysis of Methodological Aspects. Plant Foods Hum Nutr. 2020;75(1):1–11. [DOI] [PubMed] [Google Scholar]
- 19.Mohajeri MH, Troesch B, Weber P. Inadequate supply of vitamins and DHA in the elderly: implications for brain aging and Alzheimer-type dementia. Nutrition. 2015;31(2):261–275. [DOI] [PubMed] [Google Scholar]
- 20.Littlejohns TJ, Henley WE, Lang IA, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83(10):920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Zhu W, Xing Y, Jia J, Tang Y. B vitamins and prevention of cognitive decline and incident dementia: a systematic review and meta-analysis. Nutrition reviews. 2022;80(4):931–949. [DOI] [PubMed] [Google Scholar]
- 22.Smith AD, Refsum H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu Rev Nutr. 2016;36:211–239. [DOI] [PubMed] [Google Scholar]
- 23.Clarke R, Bennett D, Parish S, et al. Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. The American journal of clinical nutrition. 2014;100(2):657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wald DS, Kasturiratne A, Simmonds M. Effect of folic acid, with or without other B vitamins, on cognitive decline: meta-analysis of randomized trials. The American journal of medicine. 2010;123(6):522–527 e522. [DOI] [PubMed] [Google Scholar]
- 25.Dangour AD, Andreeva VA, Sydenham E, Uauy R. Omega 3 fatty acids and cognitive health in older people. The British journal of nutrition. 2012;107 Suppl 2:S152–158. [DOI] [PubMed] [Google Scholar]
- 26.Mazereeuw G, Lanctot KL, Chau SA, Swardfager W, Herrmann N. Effects of omega-3 fatty acids on cognitive performance: a meta-analysis. Neurobiology of aging. 2012;33(7):1482 e1417–1429. [DOI] [PubMed] [Google Scholar]
- 27.Annweiler C, Llewellyn DJ, Beauchet O. Low serum vitamin D concentrations in Alzheimer’s disease: a systematic review and meta-analysis. Journal of Alzheimer’s disease : JAD. 2013;33(3):659–674. [DOI] [PubMed] [Google Scholar]
- 28.Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Archives of internal medicine. 2010;170(13):1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barberger-Gateau P. Nutrition and brain aging: how can we move ahead?. Eur J Clin Nutr 68, 1245–1249 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Dangour AD, Allen E, Richards M, Whitehouse P, Uauy R. Design considerations in long-term intervention studies for the prevention of cognitive decline or dementia. Nutrition reviews. 2010;68 Suppl 1:S16–21. [DOI] [PubMed] [Google Scholar]
- 31.Butler M, Nelson VA, Davila H, et al. Over-the-Counter Supplement Interventions to Prevent Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer-Type Dementia: A Systematic Review. Annals of internal medicine. 2018;168(1):52–62. [DOI] [PubMed] [Google Scholar]
- 32.Ford AH, Almeida OP. Effect of Vitamin B Supplementation on Cognitive Function in the Elderly: A Systematic Review and Meta-Analysis. Drugs Aging. 2019;36(5):419–434. [DOI] [PubMed] [Google Scholar]
- 33.Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Annals of internal medicine. 2013;159(12):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishra S, Stierman B, Gahche JJ, Potischman N. Dietary suppment use among adults: United States, 2017–2018. Hyattsville, MD: National Center for Health Statistics;2021. [Google Scholar]
- 35.Rist PM, Sesso HD, Johnson LG, et al. Design and baseline characteristics of participants in the COcoa Supplement and Multivitamin Outcomes Study (COSMOS). Contemp Clin Trials. 2022;116:106728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sesso HD, Manson JE, Aragaki AK, et al. Effect of cocoa flavanol supplementation for prevention of cardiovascular disease events: The COSMOS randomized clinical trial. The American journal of clinical nutrition. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sesso HD, Rist PM, Aragaki AK, et al. Multivitamins in the prevention of cancer and cardiovascular disease: The COSMOS randomized clinical trial. The American journal of clinical nutrition. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onatibia-Astibia A, Franco R, Martinez-Pinilla E. Health benefits of methylxanthines in neurodegenerative diseases. Mol Nutr Food Res. 2017;61(6). [DOI] [PubMed] [Google Scholar]
- 39.Sansone R, Ottaviani JI, Rodriguez-Mateos A, et al. Methylxanthines enhance the effects of cocoa flavanols on cardiovascular function: randomized, double-masked controlled studies. The American journal of clinical nutrition. 2017;105(2):352–360. [DOI] [PubMed] [Google Scholar]
- 40.Vogiatzoglou A, Mulligan AA, Luben RN, et al. Assessment of the dietary intake of total flavan-3-ols, monomeric flavan-3-ols, proanthocyanidins and theaflavins in the European Union. The British journal of nutrition. 2014;111(8):1463–1473. [DOI] [PubMed] [Google Scholar]
- 41.Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;4:CD008893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker LD, Rapp SR, Shumaker SA, et al. Design and baseline characteristics of the cocoa supplement and multivitamin outcomes study for the Mind: COSMOS-Mind. Contemp Clin Trials. 2019;83:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welsh KA, Breitner J, Magruder-Habib KM. Detection of dementia in the elderly using the telephone interview for cognitive status. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1993;6:103–110. [Google Scholar]
- 44.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. IntJ Neurosci. 1991;57(3–4):167–178. [DOI] [PubMed] [Google Scholar]
- 45.Ricker JH, Axelrod BN, Houtler BD. Clinical validation of the oral trail making test. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1996;9(1):50–53. [Google Scholar]
- 46.Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City, IA: Univ of Iowa Press; 1976. [Google Scholar]
- 47.Wechsler D Manual for the Wechsler Adult Intelligence Scale-Revised (WAIS-R). New York: Psychological Corporation; 1981. [Google Scholar]
- 48.Hoppe CD, Muller UD, Werheid KD, Thone AD, von Cramon YD. Digit Ordering Test: clinical, psychometric, and experimental evaluation of a verbal working memory test. Clin Neuropsychol. 2000;14(1):38–55. [DOI] [PubMed] [Google Scholar]
- 49.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. 4th ed. New York: Springer; 2010. [Google Scholar]
- 51.Zaninotto P, Batty GD, Allerhand M, Deary IJ. Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English Longitudinal Study of Ageing. J Epidemiol Community Health. 2018;72(8):685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boban M, Bulj N, Kolacevic Zeljkovic M, et al. Nutritional Considerations of Cardiovascular Diseases and Treatments. Nutr Metab Insights. 2019;12:1178638819833705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaramillo Flores ME. Cocoa Flavanols: Natural Agents with Attenuating Effects on Metabolic Syndrome Risk Factors. Nutrients. 2019;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Little RJA, Rubin DB. Statistical analysis with missing data. Wiley; 1987. [Google Scholar]
- 55.Wilson RS, Li Y, Bienias JL, Bennett DA. Cognitive decline in old age: separating retest effects from the effects of growing older. Psychol Aging. 2006;21(4):774–789. [DOI] [PubMed] [Google Scholar]
- 56.Brickman AM, Khan UA, Provenzano FA, et al. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nature neuroscience. 2014;17(12):1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crews WD Jr., Harrison DW, Wright JW. A double-blind, placebo-controlled, randomized trial of the effects of dark chocolate and cocoa on variables associated with neuropsychological functioning and cardiovascular health: clinical findings from a sample of healthy, cognitively intact older adults. The American journal of clinical nutrition. 2008;87(4):872–880. [DOI] [PubMed] [Google Scholar]
- 58.Pase MP, Scholey AB, Pipingas A, et al. Cocoa polyphenols enhance positive mood states but not cognitive performance: a randomized, placebo-controlled trial. Journal of psychopharmacology. 2013;27(5):451–458. [DOI] [PubMed] [Google Scholar]
- 59.Suominen MH, Laaksonen MML, Salmenius-Suominen H, et al. The short-term effect of dark chocolate flavanols on cognition in older adults: A randomized controlled trial (FlaSeCo). Exp Gerontol. 2020;136:110933. [DOI] [PubMed] [Google Scholar]
- 60.Sloan RP, Wall M, Yeung LK, et al. Insights into the role of diet and dietary flavanols in cognitive aging: results of a randomized controlled trial. Sci Rep. 2021;11(1):3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grima NA, Pase MP, Macpherson H, Pipingas A. The effects of multivitamins on cognitive performance: a systematic review and meta-analysis. Journal of Alzheimer’s disease : JAD. 2012;29(3):561–569. [DOI] [PubMed] [Google Scholar]
- 62.Sesso HD, Christen WG, Bubes V, et al. Multivitamins in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA : the journal of the American Medical Association. 2012;308(17):1751–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA : the journal of the American Medical Association. 2012;308(18):1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alisi L, Cao R, De Angelis C, et al. The Relationships Between Vitamin K and Cognition: A Review of Current Evidence. Front Neurol. 2019;10:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crowe-White KM, Phillips TA, Ellis AC. Lycopene and cognitive function. J Nutr Sci. 2019;8:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erdman JW Jr., Smith JW, Kuchan MJ, et al. Lutein and Brain Function. Foods. 2015;4(4):547–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sultan S, Taimuri U, Basnan SA, et al. Low Vitamin D and Its Association with Cognitive Impairment and Dementia. J Aging Res. 2020;2020:6097820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Breteler MM, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. BMJ. 1994;308(6944):1604–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cvetinovic N, Loncar G, Isakovic AM, et al. Micronutrient Depletion in Heart Failure: Common, Clinically Relevant and Treatable. Int J Mol Sci. 2019;20(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohn ES, Kern HJ, Saltzman E, Mitmesser SH, McKay DL. Evidence of Drug-Nutrient Interactions with Chronic Use of Commonly Prescribed Medications: An Update. Pharmaceutics. 2018;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The protocol is available upon request. Datasets will be de-identified prior to release for sharing. We will make the data and associated documentation available to users only under a data-sharing agreement that provides for: (a) a commitment to using the data only for COSMOS-approved research purposes and protected identity of any individual participant; (b) a commitment to securing the data using appropriate computer technology; and (c) a commitment to destroying or returning the data after analyses are completed. We will advertise the availability of the study data to other investigators on the study website and on the NIH website following NIH approval.


