Abstract
Tenecteplase (TNK) is a promising candidate to replace alteplase as the standard of care for acute ischemic stroke (AIS); however, the optimal dosage is still to be investigated. Therefore, we aim to evaluate the safety and efficacy of TNK versus alteplase and to investigate the optimal TNK dosage. A systematic review, pairwise, and network meta-analysis synthesizing randomized controlled trials (RCTs) from WOS, SCOPUS, EMBASE, and PubMed until July 26th, 2022. We used the risk ratio (RR) for dichotomous outcomes presented with the corresponding 95% confidence interval (CI). We registered our protocol in PROSPERO with ID: CRD42022352038. Nine RCTs with a total of 3,707 patients were included. TNK significantly led to complete recanalization (RR: 1.27 with 95% CI [1.02, 1.57], P = 0.03); however, we found no difference regarding early neurological improvement (RR: 1.07 with 95% CI [0.94, 1.21], P = 0.33) and excellent neurological recovery (RR: 1.03 with 95% CI [0.96, 1.10], P = 0.42). Also, TNK was similar to alteplase regarding mortality (RR: 0.99 with 95% CI [0.82, 1.18], P = 0.88), intracranial haemorrhage (RR: 1.00 with 95% CI [0.85, 1.18], P = 0.99), and parenchymal hematoma (RR: 1.13 with 95% CI [0.83, 1.54], P = 0.44). TNK in the dose of 0.25 mg is a viable candidate to displace alteplase as the standard of care in patients with an AIS within 4.5 h of presentation due to its better rate of early neurological recovery and non-inferiority in terms of safety outcomes. However, the evidence regarding TNK’s role in AIS presenting after 4.5 h from symptoms onset, wake-up stroke, and minor stroke/TIA is still lacking, necessitating further double-blinded pragmatic RCTs in this regard.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11239-022-02730-5.
Keywords: Tenecteplase, Alteplase, Stroke, TNK, AIS, Acute ischemic stroke, Systematic review, Meta-analysis, Clinical trials
Highlights
We conducted a systematic review and network meta-analysis to investigate the efficacy and safety of tenecteplase versus alteplase for thrombolysis in patients with acute ischemic stroke and to investigate the most effective tenecteplase dosage.
Given its higher rate of early neurological recovery and non-inferiority in terms of safety outcomes, tenecteplase in the dose of 0.25 mg is a strong candidate to replace alteplase as the standard of care in patients with acute ischemic stroke who present within 4.5 hours of symptoms onset.
Tenecteplase's potential in acute ischemic stroke presenting after 4.5 hours from the onset of symptoms, wakeup stroke, and mild stroke/TIA is still inconclusive.
Introduction
Worldwide, stroke still ranks as the second-leading cause of death and the third-leading cause of composite death and disability (as expressed by disability-adjusted life-years lost—DALYs), amounting to a global health expenditure of over 721 billion US$ (0.66% of the global GDP) [1]. Annually, approximately 700,000 people in the United States experience an acute ischemic stroke (AIS) [2], constituting 85% of stroke cases. There have been greater advances in terms of the management of AIS in comparison with hemorrhagic stroke. Novel treatments such as IV thrombolysis (IVT), and more recently, mechanical thrombectomy (MT) for large vessel occlusion (LVO) have reduced mortality by ten percent compared with the older treatments and improved long-term disability prevention rates after AIS [3, 4].
AIS management guidelines in Europe [5], Canada [6], the United States [7], and the United Kingdom [8], recommend intravenous thrombolysis with the tissue plasminogen activator alteplase (t-PA) within 4.5 h after the onset of stroke and MT within 24 h after onset. Alteplase is the only thrombolytic drug that is FDA-approved for thrombolysis in AIS. Alteplase can lead to a 28% decrease in disability at 90 days and rapid symptom improvement when given within the 4.5 h window period [9]. Despite being promising for a disability-free recovery, implementation of alteplase is restricted due to the narrow time window and adverse effects of alteplase, such as a 6% risk of symptomatic hemorrhage [10]. Moreover, alteplase has demonstrated limited fibrinolytic efficacy; achieving arterial recanalization in fewer than 50% of patients [11]. Also, In patients who achieve recanalization, only 50% recanalize within two hours after drug administration [12]. Furthermore, there has been a rising concern over alteplase’s negative effects on the ischaemic brain, including cytotoxicity and increased permeability of the blood–brain–barrier facilitating cerebral edema [13–16].
In this scenario, a thrombolytic that is safe, easy to administer, and effective can broaden the acceptance of thrombolytic therapy for stroke. Tenecteplase (TNK), a genetically modified variant of alteplase, has been approved by the FDA for thrombolysis in acute myocardial infarction since 2000 after reports from the ASSENT 2 Trial [17]. Multiple clinical trials comparing TNK with alteplase in acute MI have shown that TNK induces faster coronary reperfusion with similar mortality rates [18]. Success in acute MI treatment and animal models for AIS has prompted interest in the replacement of alteplase for TNK therapy in AIS. TNK has several advantages that make it an appealing alternative; it is generally cost-effective, has a high fibrin specificity and longer plasma half-life, enhanced plasminogen Activator Inhibitor-1 (PAI-1) resistance, and can be dispensed as a single bolus; allowing swift treatment without the need for additional equipment such as infusion pumps, making it applicable in the pre-hospital settings [19].
A previous systematic review and network meta-analysis concluded that TNK is at least safe and effective as an alteplase for AIS [20]. However, multiple randomized controlled trials (RCTs) have been recently published with a significantly larger number of participants and conclusions favoring TNK over alteplase [19, 21–23]. Furthermore, in the absence of generalizable results owing to heterogeneous patient population traits, variability in doses administered, and differing clinical endpoints and outcomes evaluated; the relative superiority of TNK over alteplase remains controversial. Therefore, we aim to update the synthesized evidence on the efficacy and safety of TNK versus alteplase for thrombolysis in patients with AIS and to investigate the most effective dosage of TNK.
Methodology
Protocol registration
This systematic review network meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension statement for network meta-analyses [24] and the Cochrane Handbook of Systematic reviews and meta-analysis [25]. The review protocol was published in the International prospective register of systematic reviews (PROSPERO) with ID: CRD42022352038.
Data sources & search strategy
Two reviewers (B.A. and M.A.) independently conducted an electronic systematic search on PubMed (MEDLINE), EMBASE, Web of Science, SCOPUS, and Cochrane Central Register of Controlled Trials (CENTRAL) until July 26th, 2022, without using any search filters. The search strategy for each database is illustrated in (Table S1).
Eligibility criteria
We included RCTs with the following PICO criteria: population (P): adult patients presenting with AIS and undergoing thrombolysis; intervention (I): TNK irrespective of the dose; control (C): alteplase; outcomes (O): efficacy outcomes: early neurological improvement measured by ≥ 4 points reduction in the National Institutes of Health Stroke Scale (NIHSS), excellent neurological recovery (modified Rankin Scale (mRS) 0–1), good neurological recovery (mRS 0–2), and successful reperfusion measured by modified treatment in cerebral ischemia classification or Thrombolysis in Cerebral Infarction (TICI). Furthermore, safety outcomes; all-cause mortality, poor neurological recovery (mRS 4–6), any intracranial hemorrhage (ICH), symptomatic ICH, and any parenchymal hematoma. Conference abstracts, posters, letters to editor, non-randomized trials, single-arm trials, and observational studies were excluded.
Selection process
The selection process was conducted over two steps, first, four reviewers (A.R.S., A.M., E.A., and K.S.) independently screened the titles and abstracts of the retrieved records using Covidence online software [26]. Then they independently screened the full-texts confirming eligibility using the previous eligibility criteria. Disagreements were resolved by discussion or inviting (B.A.) to reach a consensus.
Data extraction
Using a standardized extraction sheet, four reviewers (A.R.S., A.M., E.A., and K.S.) independently extracted the following data from the eligible trials: study characteristics (first author name, year of publication, country, study design, total participants, recruitment duration, intervention dosages, main inclusion criteria, and time window); baseline information (age, sex, number of patients in each arm, onset to infusion time, and stroke risk factors); efficacy outcomes data; and safety outcomes data. Disagreements were resolved through discussion.
Risk of bias and quality assessment
Four reviewers (A.R.S., A.M., E.A., and K.S.) independently investigated the quality of the included trials following The Cochrane Collaboration's tool for assessing the risk of bias (ROB) in randomized trials [27], based on the following domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other potential sources of bias. Disagreements were resolved by discussion. Two reviewers (M.T. and B.A.), guided by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines [28], appraised the quality of the outcome findings. Imprecision, indirectness, inconsistency, publication bias, and risk of bias were considered. Our results about the quality of evidence were rationalized, clarified, and included for each outcome. Any discrepancies were handled through discussion.
Statistical analysis
For the pairwise meta-analysis, we used Revman version 5.4 [29] to pool dichotomous outcomes using risk ratio (RR) along with the corresponding 95% confidence interval (CI). We used the fixed-effect model; however, the random-effect model was used in case of significant heterogeneity. Statistical heterogeneity was evaluated by calculating I2 and conducting a chi-squared test. P-value 0.05 was considered significant, and I2 > 50% indicated substantial heterogeneity, in which case sensitivity analysis was performed by removing one study at a time to determine if there is one study that affects the overall effect estimate.
For network meta-analysis, we performed a network meta-analysis using a frequentist framework [24], pooling dichotomous outcomes using risk ratio (RR) along with the corresponding 95% confidence interval (CI). Analysis was performed using the R-software netmeta and netrank package (R version 4.2.0) and meta-insight software [30–32]. Finally, because we only included less than ten studies in each outcome, we did not conduct funnel plots to reveal publication bias, as advised by Egger et al. [33].
Results
Search results and study selection
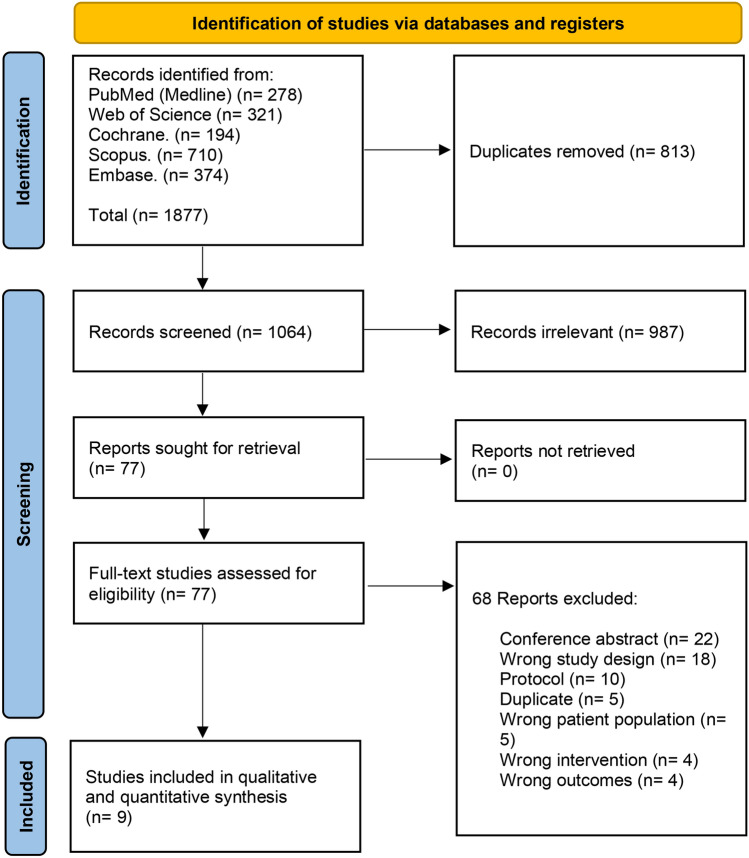
We imported 1877 records after searching databases. Eight hundred and thirteen duplicates were removed using Covidence, leaving 1064 records for the title and abstract screening. We excluded 987 irrelevant records and screened 77 full-text articles, and finally included nine RCTs [19, 21–23, 34–38] (Fig. 1).
Fig. 1.
PRISMA flow chart of the screening process
Characteristics of included studies
Nine RCTs met our inclusion criteria [19, 21–23, 34–38] with a total of 3,707 patients included; of these: 1,967 were allocated to TNK (intervention), and 1,740 were allocated to alteplase (control). Seven trials were multicenter [21–23, 35–38], whereas only two RCTs were single-center trials [19, 34]. The time window was 4.5 h in six trials [19, 21, 22, 34, 35, 37], three hours in two trials [23, 36], and less than six hours in one trial [38]. Table 1 and Table 2 demonstrate the summary and baseline characteristics of the included studies, respectively.
Table 1.
Summary of the included studies
| Study ID | Study Design | Country | Recruitment duration | Total sample size, N | Dosages (mg/kg) | Timing after symptoms onset | |
|---|---|---|---|---|---|---|---|
| Tenecteplase | Alteplase | ||||||
| Bivard et al. 2022 [19] | Phase 2, single center, PROBE | Australia | From June 2019 to November 2021 | N = 104 | (0·25 mg/kg) | (0·9 mg/kg) | 4.5 h |
| Campbell et al. 2018 [35] | Phase 2, multicenter, PROBE | Australia and New Zealand | From March 2015 to October 2017 | N = 202 | (0·25 mg/kg) | (0·9 mg/kg) | 4.5 h |
| Haley et al. 2010 [36] | Phase 2B/3, multicenter, double-blinded, prematurely terminated RCT | USA | From March 2006 to December 2008 | N = 112 |
Group 1 = 0.1 mg/kg Group 2 = 0.25 mg/kg Group 3 = 0.4 mg/kg |
(0.9 mg/kg) | 3 h |
| Huang et al. 2015 [34] | Phase 2, single center, PROBE | Scotland | From January 2012 to September 2013 | N = 96 | (0·25 mg/kg) | (0·9 mg/kg) | 4.5 h |
| Kvistad et al. 2022 [21] | Phase 3, multicenter, PROBE | Norway | From October 2019 to September 2021 | N = 204 |
(0·4 mg/kg) [maximum 40 mg] |
(0·9 mg/kg) | 4.5 h |
| Li et al. 2021 [23] | Phase 2, multicenter, PROBE | China | From May 2018 to February 2020 | N = 236 |
Group 1 = 0.1 mg/kg Group 2 = 0.25 mg/kg Group 3 = 0.32 mg/kg |
(0·9 mg/kg) | 3 h |
| Logallo et al. 2017 [37] | Phase 3, multicenter, PROBE | Norway | From September 2012 to September 2016 | N = 1,100 | (0·4 mg/kg) | (0·9 mg/kg) | 4.5 h |
| Menon et al. 2022 [22] | Phase 3, multicenter, PROBE | Canada | From December 2019 to January 2022 | N = 1,577 | (0·25 mg/kg) | (0·9 mg/kg) | 4.5 h |
| Parsons et al. 2012 [38] | Phase 2B, multicenter, PROBE | Australia | From 2008 to 2011 | N = 75 |
Group 1 = 0.1 mg/kg Group 2 = 0.25 mg/kg |
(0·9 mg/kg) | less than 6 h |
PROBE prospective, randomized, open-label, blinded outcome study, RCT randomized controlled trial, N number, mg milligram, kg kilogram
Table 2.
Baseline characteristics of the included studies
| Study ID | Sample size, n | Age (years) | Male, n(%) | Onset to treatment time, (min) | Baseline NIHSS, mean (SD) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tenecteplase | Alteplase | Tenecteplase | Alteplase | Tenecteplase | Alteplase | Tenecteplase | Alteplase | Tenecteplase | Alteplase | |
| Bivard et al. 2022 [19] | n = 55 | n = 49 | 73.33 ± 18.27 | 71.33 ± 14.51 | 33 (60%) | 30 (61%) | 107.33 ± 67.75 | 96.33 ± 49.64 | 9 ± 6.85 | 10 ± 9.16 |
| Campbell et al. 2018 [35] | n = 101 | n = 101 | 70.4 ± 15.1 | 71.9 ± 13.7 | 58 (57%) | 52 (51%) | 127.6 ± 40.61 | 138 ± 54.15 | 17 ± 7.52 | 17 ± 7.52 |
| Haley et al. 2010 [36] |
TNK 0.1: n = 31 TNK 0.25: n = 31 TNK 0.4: n = 19 |
n = 31 |
TNK 0.1: 67 ± 19 TNK 0.25: 69 ± 15 TNK 0.4: 68 ± 16 |
72 ± 16 |
TNK 0.1: 12 (39%) TNK 0.25: 16 (52%) TNK 0.4: 13 (68%) |
17 (51%) | NA | NA |
TNK 0.1: 8 ± 4.66 TNK 0.25: 10.33 ± 7 TNK 0.4: 10.33 ± 9.6 |
11.6 ± 9.32 |
| Huang et al. 2015 [34] | n = 47 | n = 49 | 71 ± 13 | 71 ± 12 | 30 (64%) | 31 (63%) | 184 ± 44 | 192 ± 45 | 13 ± 6.88 | 11.6 ± 6.11 |
| Kvistad et al. 2022 [21] | n = 100 | n = 104 | 73·2 ± 12·6 | 68·6 ± 15·6 | 45 (45%) | 53 (51%) | 103.16 ± 51.90 | 105 ± 52.62 | 13.4 ± 6.6 | 13.2 ± 6.4 |
| Li et al. 2021 [23] |
TNK 0.1: n = 60 TNK 0.25: n = 57 TNK 0.32: n = 60 |
n = 59 |
TNK 0.1: 62.4 ± 11.1 TNK 0.25: 64.3 ± 12.8 TNK 0.32: 64.8 ± 12.1 |
66.5 ± 12.6 |
TNK 0.1: 48 (80%) TNK 0.25: 42 (73.7%) TNK 0.32: 42 (70%) |
38 (64.4%) |
TNK 0.1: 135 ± 105.6 TNK 0.25: 136 ± 75.3 TNK 0.32:145.33 ± 114.7 |
119.3 ± 128.42 |
TNK 0.1: 7.33 ± 3.80 TNK 0.25: 8.33 ± 5.33 TNK 0.32: 8.5 ± 4.6 |
8.33 ± 5.32 |
| Logallo et al. 2017 [37] | n = 549 | n = 551 | 70.8 ± 14.4 | 71.2 ± 13.2 | 321 (58%) | 339 (62%) | 125.67 ± 75 | 121.6 ± 69.87 | 5.6 ± 5.4 | 5.8 ± 5.2 |
| Menon et al. 2022 [22] | n = 806 | n = 771 | 73.33 ± 14.85 | 72.67 ± 15.6 | 424 (52.6%) | 398 (51.6%) | 135 (69.05%) | 138 ± 69.1 | 10.33 ± 7.43 | 11 ± 8.14 |
| Parsons et al. 2012 [38] |
TNK 0.1: n = 25 TNK 0.25: n = 25 |
n = 25 |
TNK 0.1: 72 ± 6.9 TNK 0.25: 68 ± 9.4 |
70 ± 8.4 |
TNK 0.1: 13 (52%) TNK 0.25: 13 (52%) |
12 (48%) |
TNK 0.1: 3.1 ± 0.9 (h) TNK 0.25: 3.0 ± 0.7 (h) |
2.7 ± 0.8 (h) |
TNK 0.1: 14.5 ± 2.3 TNK 0.25: 14.6 ± 2.3 |
14 ± 2.3 |
| Study ID | Comorbidities, n(%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AF | HTN | DM | Dyslipidaemia | Smoking | ||||||
| Tenecteplase | Alteplase | Tenecteplase | Alteplase | Tenecteplase | Alteplase | Tenecteplase | Alteplase | Tenecteplase | Alteplase | |
| Bivard et al. 2022 [19] | 8 (15%) | 7 (15%) | 30 (55%) | 31 (63%) | 11 (30%) | 17 (35%) | 21 (38%) | 22 (45%) | 8 (15%) | 9 (18%) |
| Campbell et al. 2018 [35] | 27 (27%) | 40 (40%) | 64 (63%) | 63 (62%) | 10 (10%) | 18 (18%) | N/A | N/A | 18 (18%) | 11 (11%) |
| Haley et al. 2010 [36] | NA | NA |
TNK 0.1: 25 (81%) TNK 0.25: 25 (81%) TNK 0.4: 17 (90%) |
22 (71%) |
TNK 0.1: 6 (19%) TNK 0.25: 7 (23%) TNK 0.4: 4 (21%) |
4 (13%) |
TNK 0.1: 16 (52%) TNK 0.25: 15 (48%) TNK 0.4: 8 (42%) |
17 (55%) |
TNK 0.1: 2 (6.5%) TNK 0.25: 7 (23%) TNK 0.4: 0 (0%) |
7 (23%) |
| Huang et al. 2015 [34] | 19 (40%) | 15 (31%) | 20 (43%) | 28 (57%) | 7 (15%) | 7 (14%) | 4 (9%) | 7 (14%) | 13 (28%) | 10 (20%) |
| Kvistad et al. 2022 [21] | 9 (9%) | 8 (8%) | 56 (56%) | 48 (46%) | 17 (17%) | 11 (11%) | 30 (30%) | 33 (32%) | 24 (24%) | 25 (24%) |
| Li et al. 2021 [23] |
TNK 0.1: 8 (13.3%) TNK 0.25: 4 (7.0%) TNK 0.32: 14 (23.3%) |
6 (10.2%) |
TNK 0.1: 43 (71.7%) TNK 0.25: 37 (64.9%) TNK 0.32: 35 (58.3%) |
42 (71.2%) |
TNK 0.1: 14 (23.3%) TNK 0.25: 9 (15.8%) TNK 0.32: 15 (25%) |
11 (18.6%) |
TNK 0.1: 17 (28.3%) TNK 0.25: 13 (22.8%) TNK 0.32: 10 (16.7%) |
11 (18.6%) |
TNK 0.1: 25 (41.7%) TNK 0.25: 25 (43.9%) TNK 0.32: 21 (35%) |
24 (40.7%) |
| Logallo et al. 2017 [37] | 50 (9%) | 69 (13%) | 246 (45%) | 236 (43%) | 72 (13%) | 74 (13%) | 61 (11%) | 65 (12%) | 169 (31%) | 177 (32%) |
| Menon et al. 2022 [22] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Parsons et al. 2012 [38] |
TNK 0.1: 9 (36%) TNK 0.25: 13 (52%) |
6 (24%) |
TNK 0.1: 16 (64%) TNK 0.25: 16 (64%) |
15 (60)% |
TNK 0.1: 8 (32%) TNK 0.25: 6 (24%) |
1 (4%) |
TNK 0.1: 13 (52%) TNK 0.25: 15 (60%) |
9 (36%) |
TNK 0.1: 9 (36%) TNK 0.25: 5 (20%) |
1 (4%) |
NIHSS national institute of health stroke scale, AF atrial fibrillation, HTN hypertension, DM diabetes mellitus, NA not available, n number, SD standard deviation
Risk of bias and quality of evidence
We assessed the quality of the included studies according to the Cochrane risk of bias tool as shown in (Fig. 2). All the included trials had a low risk of random sequence generation bias. All the included studies had a low risk of allocation concealment bias except Haley et al. 2010 [36], which had an unclear risk, while Li et al. 2021 [23] had a high risk of bias. Moreover, all included trials had a high risk of performance bias except for Haley et al. 2010 [36], which had a low risk of performance bias. Furthermore, all included trials had a low risk of detection bias. For the attrition and reporting bias, all our included studies had a low risk of bias. Finally, all the included studies had a low risk of other bias except for Li et al. 2021[23], which had a high risk of bias, and Parsons et al. 2012 [38], which had an unclear risk of bias. Author judgments are furtherly clarified in (Table S2). Finally, the quality of evidence is illustrated in (Table S3).
Fig. 2.
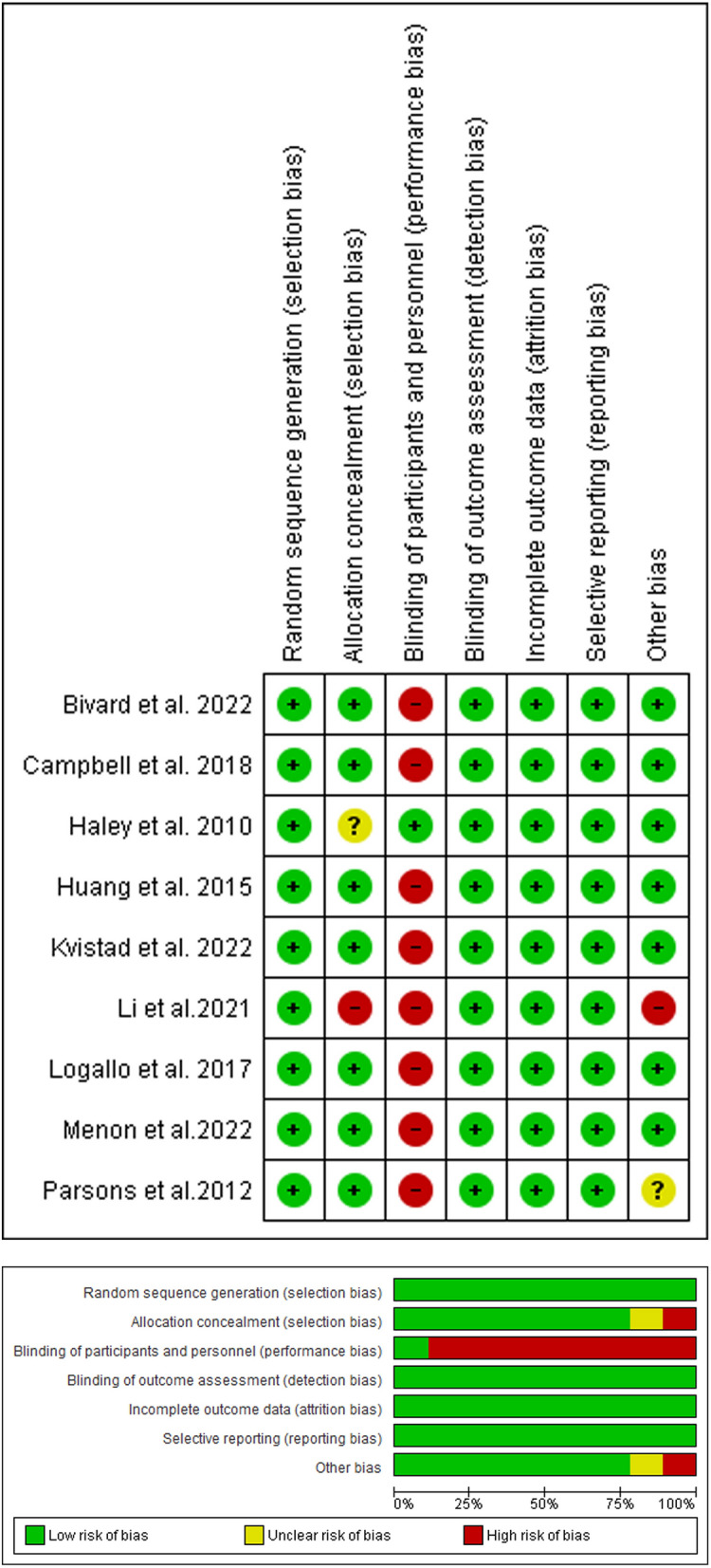
Quality assessment of risk of bias in the studies in the meta-analysis. The upper panel presents a schematic representation of risks (low = red, unclear = yellow, and high = red) for specific types of biases of each of the studies in the review. The lower panel presents risks (low = red, unclear = yellow, and high = red) for the subtypes of biases of the combination of studies included in this review
Efficacy outcomes
Early neurological improvement
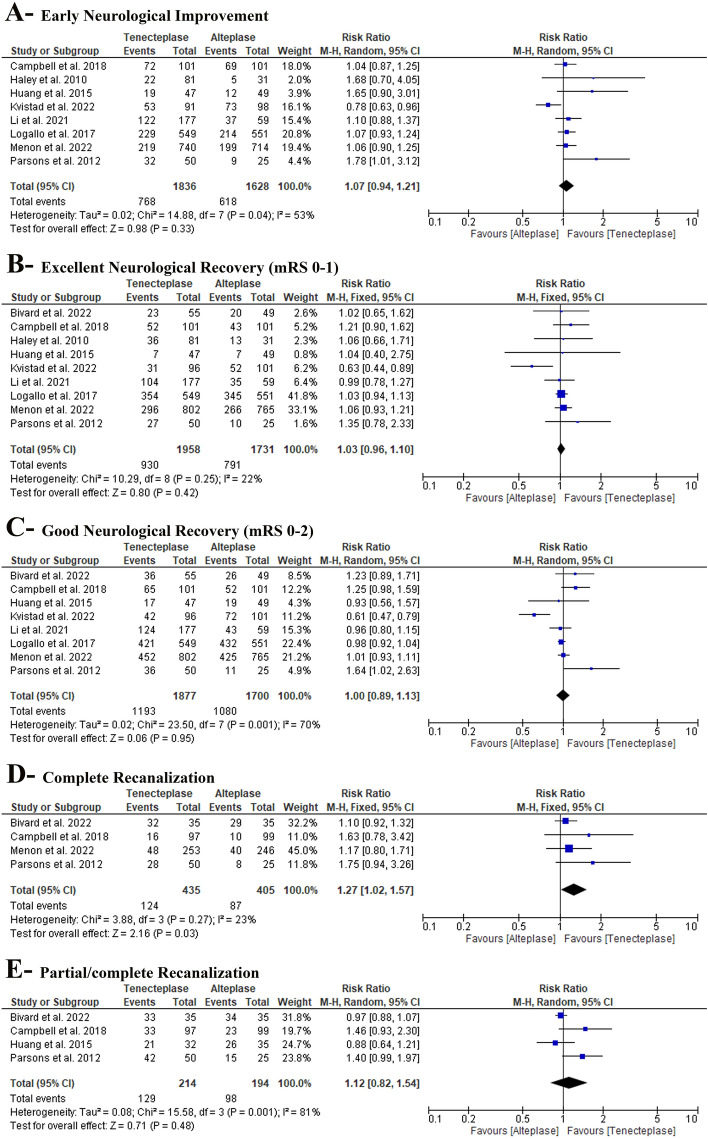
In the pairwise meta-analysis, we found no difference between TNK and alteplase (RR: 1.07 with 95% CI [0.94, 1.21], P = 0.33) (low-quality evidence) (Fig. 3-A, Table S3). Pooled studies were heterogenous (P = 0.04, I2 = 53%). Heterogeneity was best resolved after excluding Kvistad et al. [21] (P = 0.41, I2 = 1%) (Table S4). After excluding Kvistad et al. [21], pooled risk ratio favored TNK (RR: 1.09 with 95% CI [1.01, 1.19], P = 0.04) (Table S4).
Fig. 3.
Forest plot of the efficacy outcomes (A early neurological improvement, B- excellent neurological recovery, C- good neurological recovery, D- complete recanalization, and E- partial/complete recanalization), RR risk ratio, CI confidence interval
In network meta-analysis, all TNK doses showed no statistically significant difference, except TNK-0.25, which showed a statistically significant higher risk for early neurological improvement (RR: 1.24 with 95% CI [1.02, 1.49]) (Table 3, Figures S1-A, S2, S3). No heterogeneity was observed (I2 = 0%).
Table 3.
Ranking table for all our network meta-analyses’ outcomes
| Early neurological improvement | ||||
| TNK 0.4 | ||||
| 0.93 [0.72; 1.19] | Alteplase | |||
| 0.84 [0.54; 1.29] | 0.90 [0.63; 1.29] | TNK 0.32 | ||
| 0.80 [0.56; 1.16] | 0.87 [0.66; 1.14] | 0.96 [0.66; 1.39] | TNK 0.1 | |
| 0.75 [0.55; 1.02] | 0.81 [0.67; 0.98] | 0.90 [0.63; 1.27] | 0.93 [0.72; 1.20] | TNK 0.25 |
| Excellent neurological recovery (mRS 0–1) | ||||
| TNK 0.4 | ||||
| 0.97 [0.70; 1.34] | TNK 0.1 | |||
| 0.90 [0.73; 1.10] | 0.93 [0.71; 1.21] | Alteplase | ||
| 0.86 [0.59; 1.26] | 0.89 [0.62; 1.26] | 0.96 [0.69; 1.33] | TNK 0.32 | |
| 0.79 [0.61; 1.02] | 0.81 [0.63; 1.06] | 0.88 [0.75; 1.03] | 0.92 [0.66; 1.27] | TNK 0.25 |
| Good neurological recovery (mRS 0–2) | ||||
| TNK 0.4 | ||||
| 0.83 [0.55; 1.26] | TNK 0.1 | |||
| 0.82 [0.52; 1.29] | 0.98 [0.66; 1.46] | TNK 0.32 | ||
| 0.81 [0.62; 1.07] | 0.98 [0.72; 1.33] | 0.99 [0.69; 1.43] | Alteplase | |
| 0.71 [0.51; 0.98] | 0.85 [0.63; 1.14] | 0.86 [0.60; 1.24] | 0.87 [0.72; 1.04] | TNK 0.25 |
| Poor neurological recovery (mRS 4–6) | ||||
| TNK 0.4 | ||||
| 0.96 [0.59; 1.58] | TNK 0.1 | |||
| 0.86 [0.68; 1.10] | 0.90 [0.56; 1.46] | Alteplase | ||
| 0.83 [0.43; 1.63] | 0.87 [0.44; 1.72] | 0.97 [0.50; 1.87] | TNK 0.32 | |
| 0.69 [0.45; 1.07] | 0.72 [0.40; 1.30] | 0.80 [0.55; 1.17] | 0.83 [0.39; 1.76] | TNK 0.4 |
| Partial/complete recanalization | ||||
| TNK 0.4 | ||||
| 0.97 [0.58; 1.61] | Alteplase | |||
| 0.84 [0.52; 1.37] | 0.87 [0.67; 1.14] | TNK 0.25 | ||
| Complete recanalization | ||||
| TNK 0.1 | ||||
| 0.64 [0.29; 1.40] | Alteplase | |||
| 0.47 [0.22; 0.99] | 0.73 [0.53; 1.01] | TNK 0.25 | ||
| All-cause mortality at 90 days | ||||
| TNK 0.25 | ||||
| 1.04 [0.45; 2.40] | TNK 0.1 | |||
| 0.99 [0.30; 3.30] | 0.96 [0.29; 3.12] | TNK 0.32 | ||
| 0.87 [0.59; 1.29] | 0.84 [0.38; 1.85] | 0.88 [0.27; 2.80] | Alteplase | |
| 0.66 [0.34; 1.29] | 0.64 [0.24; 1.66] | 0.67 [0.18; 2.41] | 0.76 [0.43; 1.35] | TNK 0.4 |
| Any intracranial hemorrhage | ||||
| TNK 0.25 | ||||
| 0.84 [0.53; 1.34] | Alteplase | |||
| 0.72 [0.29; 1.81] | 0.85 [0.35; 2.10] | TNK 0.1 | ||
| 0.54 [0.17; 1.71] | 0.64 [0.21; 2.01] | 0.75 [0.25; 2.30] | TNK 0.32 | |
| 0.55 [0.28; 1.07] | 0.65 [0.38; 1.11] | 0.76 [0.28; 2.04] | 1.00 [0.29; 3.43] | TNK 0.4 |
| Symptomatic intracranial hemorrhage | ||||
| TNK 0.25 | ||||
| 0.95 [0.60; 1.50] | Alteplase | |||
| 0.99 [0.28; 3.46] | 1.04 [0.31; 3.50] | TNK 0.1 | ||
| 0.93 [0.17; 5.05] | 0.98 [0.19; 5.15] | 0.94 [0.20; 4.32] | TNK 0.32 | |
| 0.57 [0.28; 1.19] | 0.60 [0.33; 1.11] | 0.58 [0.15; 2.19] | 0.62 [0.11; 3.56] | TNK 0.4 |
| Any parenchymal hematoma | ||||
| TNK 0.1 | ||||
| 0.66 [0.11; 4.08] | TNK 0.25 | |||
| 0.53 [0.09; 2.97] | 0.80 [0.39; 1.64] | Alteplase | ||
| 0.07 [0.01; 0.85] | 0.11 [0.02; 0.73] | 0.14 [0.03; 0.79] | TNK 0.4 | |
TNK Tenecteplase, all data are reported in risk ratio (RR) and 95% confidence interval (CI)
Excellent neurological recovery (mRS 0–1).
In pairwise meta-analysis, we found no difference between TNK and alteplase (RR: 1.03 with 95% CI [0.96, 1.10], P = 0.42) (low-quality evidence) (Fig. 3-B, Table S3). Pooled studies were homogenous (P = 0.25, I2 = 22%).
In network meta-analysis, all TNK doses showed no statistically significant difference, compared to alteplase: TNK 0.1 (RR: 0.93 with 95% CI [0.71, 1.21]), TNK 0.25 (RR: 1.14 with 95% CI [0.97, 1.33]), TNK 0.32 (RR: 1.05 with 95% CI [0.75, 1.45]), and TNK 0.4 (RR: 0.9 with 95% CI [0.73, 1.10]) (Table 3, Figures S1-B, S4, S5). No significant heterogeneity was observed (I2 = 12%).
Good neurological recovery (mRS 0–2).
In the pairwise meta-analysis, we found no difference between TNK and alteplase (RR: 1.00 with 95% CI [0.89, 1.13], P = 0.95) (very low-quality evidence) (Fig. 3-C, Table S3). Pooled studies were heterogenous (P = 0.001, I2 = 70%). Heterogeneity was best resolved after excluding Kvistad et al. [21] (P = 0.12, I2 = 41%); however, after excluding Kvistad et al. [21], there was no difference between TNK and alteplase (RR: 1.04 with 95% CI [0.95, 1.13], P = 0.39) (Table S4).
In network meta-analysis, all TNK doses showed no statistically significant difference, compared to alteplase: TNK 0.1 (RR: 0.98 with 95% CI [0.72, 1.33]), TNK 0.25 (RR: 1.15 with 95% CI [0.96, 1.38]), TNK 0.32 (RR: 0.99 with 95% CI [0.69, 1.43]), and TNK 0.4 (RR: 0.81 with 95% CI [0.62, 1.07]) (Table 3, Figures S1-C, S6, S7). No heterogeneity was observed (I2 = 0%).
Complete recanalization
In pairwise meta-analysis, pooled risk ratio favored TNK (RR: 1.27 with 95% CI [1.02, 1.57], P = 0.03) (low-quality evidence) (Fig. 3-D, Table S3). Pooled studies were homogenous (P = 0.27, I2 = 23%).
In network meta-analysis, TNK 0.1, and TNK 0.25 showed no statistically significant difference (RR: 0.64 with 95% CI [0.29, 1.40]), and (RR: 1.37 with 95% CI [0.99, 1.89]), respectively (Table 3, Figures S1-D, S8, S9). No significant heterogeneity was observed (I2 = 24%).
Partial/complete recanalization
In the pairwise meta-analysis, we found no difference between TNK and alteplase (RR: 1.12 with 95% CI [0.82, 1.54], P = 0.48) (very low-quality evidence) (Fig. 3-E, Table S3). Pooled studies were heterogenous (P = 0.001, I2 = 81%). Heterogeneity was not resolved by sensitivity analysis (Table S4).
In network meta-analysis, TNK 0.1, and TNK 0.25 showed no statistically significant difference (RR: 0.97 with 95% CI [0.58, 1.61]), and (RR: 1.15 with 95% CI [0.88, 1.50]), respectively (Table 3, Figures S1-E, S10,S11). No significant heterogeneity was observed (I2 = 32%).
Safety outcomes
Poor neurological recovery (mRS 4–6).
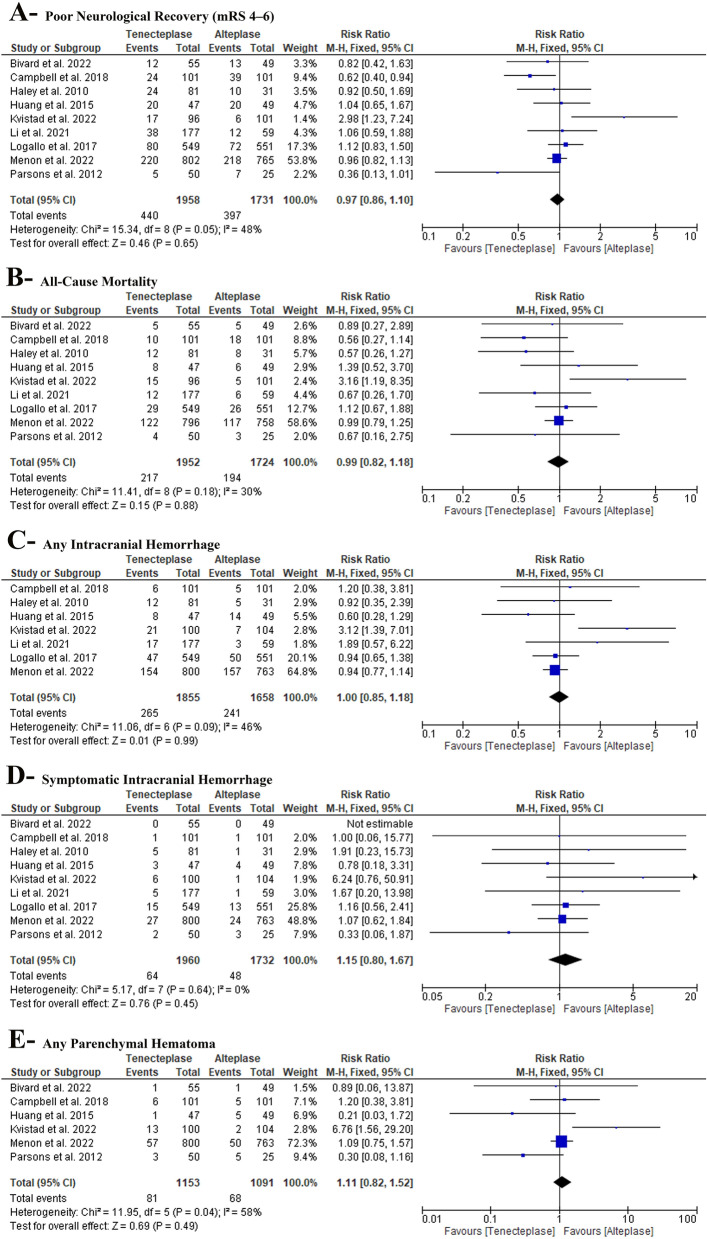
In the pairwise meta-analysis, we found no difference between TNK and alteplase (RR: 0.97 with 95% CI [0.86, 1.10], P = 0.65) (low-quality evidence) (Fig. 4-A, Table S3). Pooled studies were homogenous (P = 0.05, I2 = 48%).
Fig. 4.
F or est plot of the safety outcomes (A- poor neurological improvement, B- all-cause mortality at 90 days, C- any intracranial hemorrhage, D- symptomatic intracranial hemorrhage, and E- any parenchymal hematoma), RR risk ratio, CI confidence interval
In network meta-analysis, all TNK doses showed no statistically significant difference, compared to alteplase: TNK 0.1 (RR: 0.90 with 95% CI [0.56, 1.46]), TNK 0.25 (RR: 0.86 with 95% CI [0.68, 1.10]), TNK 0.32 (RR: 1.04 with 95% CI [0.53, 2.01]), and TNK 0.4 (RR: 1.25 with 95% CI [0.85, 1.82]) (Table 3, Figures S12-A, S13, S14). No heterogeneity was observed (I2 = 0%).
All-cause mortality at 90 days
In the pairwise meta-analysis, we found no difference between TNK and alteplase (RR: 0.99 with 95% CI [0.82, 1.18], P = 0.88) (high-quality evidence) (Fig. 4-B, Table S3). Pooled studies were homogenous (P = 0.18, I2 = 30%).
In network meta-analysis, all TNK doses showed no statistically significant difference, compared to alteplase: TNK 0.1 (RR: 0.84 with 95% CI [0.38, 1.85]), TNK 0.25 (RR: 0.87 with 95% CI [0.59, 1.29]), TNK 0.32 (RR: 0.88 with 95% CI [0.27, 2.80]), and TNK 0.4 (RR: 1.32 with 95% CI [0.74, 2.33]) (Table 3, Figures S12-B, S15, S16). No significant heterogeneity was observed (I2 = 12%).
Any ICH
In the pairwise meta-analysis, we found no difference between TNK and alteplase (RR: 1.00 with 95% CI [0.85, 1.18], P = 0.99) (moderate-quality evidence) (Fig. 4-C, Table S3). Pooled studies were homogenous (P = 0.09, I2 = 46%).
In network meta-analysis, all TNK doses showed no statistically significant difference, compared to alteplase: TNK 0.1 (RR: 1.17 with 95% CI [0.48, 2.88]), TNK 0.25 (RR: 0.84 with 95% CI [0.53, 1.34]), TNK 0.32 (RR: 1.55 with 95% CI [0.50, 4.85]), and TNK 0.4 (RR: 1.55 with 95% CI [0.90, 2.64]) (Table 3, Figures S12-C, S17, S18). No significant heterogeneity was observed (I2 = 12%).
Symptomatic ICH
In the pairwise meta-analysis, we found no difference between TNK and alteplase (RR: 1.15 with 95% CI [0.80, 1.67], P = 0.45) (low-quality evidence) (Fig. 4-D, Table S3). Pooled studies were homogenous (P = 0.64, I2 = 0%).
In network meta-analysis, all TNK doses showed no statistically significant difference, compared to alteplase: TNK 0.1 (RR: 0.96 with 95% CI [0.29, 3.24]), TNK 0.25 (RR: 0.95 with 95% CI [0.60, 1.50]), TNK 0.32 (RR: 1.02 with 95% CI [0.19, 5.38]), and TNK 0.4 (RR: 1.66 with 95% CI [0.90, 3.07]) (Table 3, Figures S12-D, S19, S20). No significant heterogeneity was observed (I2 = 17%).
Any parenchymal hematoma
In the pairwise meta-analysis, we found no difference between TNK and alteplase (RR: 1.13 with 95% CI [0.83, 1.54], P = 0.44) (very low-quality evidence) (Fig. 4-E, Table S3). Pooled studies were heterogenous (P = 0.03, I2 = 59%). Heterogeneity was best resolved after excluding Kvistad et al. [21] (P = 0.27, I2 = 27%); however, after excluding Kvistad et al. [21], there was no difference between TNK and alteplase (RR: 0.95 with 95% CI [0.69, 1.32], P = 0.77) (Table S4).
In network meta-analysis, all other TNK doses showed no statistically significant difference, compared to alteplase, except TNK-0.4, which showed a statistically significant higher risk for hematoma (RR: 7.04 with 95% CI [1.27, 39.08]) (Table 3, Figures S12-E, S21, S22). No significant heterogeneity was observed (I2 = 21%).
Discussion
Our network meta-analysis involving nine RCTs, and 3707 patients is the most comprehensive and recent study to compare the efficacy & safety of various TNK doses with alteplase. Our pairwise meta-analysis showed that TNK was associated with a higher rate of complete recanalization; however, we found no difference between TNK and alteplase regarding early neurological improvement, excellent neurological recovery, good neurological recovery (functional independence), and complete/partial recanalization. Also, safety outcomes, including mortality, ICH, and parenchymal hematoma, were similar between both groups. Moreover, our network meta-analysis showed medium dose (TNK 0.25) to have significantly higher early neurological improvement compared with alteplase. Finally, the high dose (TNK 0.4) showed a significantly higher risk of developing parenchymal hematomas.
Evidence from the previous meta-analysis is in consensus about the non-inferiority of (TNK 0.25) compared with alteplase supporting our findings [39]. The early neurological improvement can be attributed to the pharmacokinetic properties of TNK, having a long half-life, higher fibrin specificity, and more potent clot dissolution, leading to faster vessel recanalization [40]. Moreover, although we found no significant differences between low-tier TNK doses vs. alteplase in other efficacy and safety outcomes, previous studies have extensively shown that (TNK 0.25) is associated with better imaging-based outcomes, partial/complete recanalization, and higher levels of neurological function, with no increased risk of intracerebral bleeding or mortality, compared with alteplase [20, 41–44]. This has important clinical implications because it paves the road for (TNK 0.25) to safely replace alteplase as the standard of care. Moreover, the rapid, single bolus infusion of TNK allows for a give-and-go strategy, whereby giving dosage requires as short as one minute. Therefore, decreasing the door-in-to-door out time. This is important in remote settings with poor resources that lack access to thrombectomy canters and require ambulances for transporting patients to specialized stroke centers. This contrasts with the drip and ship paradigm for multiple boluses and prolonged infusion of alteplase for up to one hour [39].
However, this non-inferiority of TNK over alteplase is still a matter of debate as our analysis revealed that the high dose (TNK 0.4) is significantly associated with developing parenchymal hematomas. Earlier evidence involving this dosage has remained inconsistent and inconclusive due to the small sample sizes and the few investigating RCTs. Huang et al. [41], in their meta-analysis, identified a potential correlation between drug dose and increased risk of ICH hemorrhage; however, they failed to establish plausibility in the results due to the small sample size (19 patients). The adverse effects of TNK 0.4 are speculated to be caused by the relatively longer serum half-life of the drug compared to alteplase delaying the achievement of homeostasis. For alteplase, multiple infusions can be stopped once signs of ICH are detected, yet no evidence shows significant alterations in clinical outcomes [39].
In contrast, recent data from the NOR-TEST [37], with 549 patients enrolled in the high-dose group (TNK 0.4), showed no increased risk of ICH, or mortality after three months [37]. This is inconsistent with Yogendrakumar et al.’s [45] pooled analysis of EXTEND-IA TNK trials showing higher rates of symptomatic ICH with TNK 0.4 and symptomatic ICH and mortality TNK 0.25 [45]. Furthermore, in contrast to NOR TEST [37], NOR TEST 2-A trial [21] failed to demonstrate the non-inferiority of TNK 0.4 to alteplase in moderate or severe ischaemic stroke [21]. In the modified intention-to-treat population, the favorable functional outcome at three months occurred less frequently in patients allocated TNK 0.4 compared with alteplase [21]. Also, the rates of ICH, poor functional outcome, and mortality were higher in the TNK 0.4 group [21]. Kvistad et al. [21] attributed this difference to age imbalance between the two groups, with an average five years higher in the TNK group, patients in the TNK group were more likely to have a disability (mRS score ≥ 1), and more patients in the TNK group were diagnosed with AIS; however, alteplase group had more stroke mimics with a relatively better prognosis [21, 46]. This is supported by the findings of our sensitivity analysis which significantly favored TNK over alteplase regarding early neurological recovery after excluding Kvistad et al. [21]. Therefore, NOR TEST 2-A [21] constitutes an important determinant of our study findings, which is an inherited limitation from the trial itself. Also, the trial was terminated prematurely due to increased harm to patients [21].
Regarding recanalization, despite our pairwise analysis showing significant success with TNK, our network meta-analysis showed no difference. This can be attributed to that different treatment groups are underpowered to show statistical differences. Moreover, Parsons et al. [38] supported the theory that there might be an improved recanalization with increasing TNK doses. Still, there is no evidence regarding recanalization with TNK 0.4; hence, more research is required to prove these dose-related claims, as the recanalization rate is a key indicator for improved outcomes [39].
Intravenous thrombolysis by IV alteplase (0.9 mg/kg, maximum dose 90 mg over 60 min with initial 10% of dose given as bolus over 1 min), on one hand, is the only endorsed systemic reperfusion treatment for patients with AIS according to the 2019 American Heart Association/American Stroke Association (AHA/ASA) Guidelines for emergency management for AIS [7]. AHA/ASA recommended alteplase for selected patients who can be treated within a time window of (< 4.5 h) [7]. Similarly, alteplase administered within 4.5 h of symptom recognition can be beneficial in patients with wake-up stroke, having unclear time onset of stroke (> 4.5 h), or diffusion-weighted magnetic resonance imaging (DW-MRI) lesion smaller than of middle cerebral artery territory with no visible change in fluid-attenuated inversion recovery (FLAIR) [7].
On the other hand, AHA/ASA and European Stroke Organization (ESO) stated that TNK (single IV bolus 0.25 mg/kg maximum 25 mg) may be favored over alteplase in patients without contraindications for IV fibrinolysis and are eligible to undergo mechanical thrombectomy as a bridging therapy [7, 47]. However, the quality of evidence for TNK recommendations remains low, and they recommended further RCTs for a conclusive statement regarding TNK [47]. Accordingly, the addition of the recent findings, especially from the AcT trial [22], can strengthen the evidence about TNK 0.25 to replace alteplase for AIS presenting within 4.5 h [48]. Nonetheless, evidence about TNK’s role in disabling stroke presenting after 4.5 h, wake-up stroke, and minor stroke/TIA is still inconclusive.
Regarding disabling stroke presenting after 4.5 h, ESO guidelines recommend alteplase in patients with AIS presenting after up to nine hours after symptoms start with target mismatch (penumbra: potentially rescuable hypoperfused tissue) on CT perfusion imaging and in whom MT is not planned [47, 48]. In this regard, the TIMELESS trial [49], the ROSE-TNK trial [50], and the ETERNAL trial [51] are currently undergoing to compare TNK 0.25 versus alteplase in AIS presenting beyond 4.5 h along with target mismatch [48].
Similarly, ESO guidelines recommend alteplase for wake-up stroke, provided that the patient fulfills certain imaging criteria [47]. In this regard, the TWIST trial [47] tested TNK 0.25 versus no thrombolysis for patients with wake-up stroke presenting within 4.5 h from awakening; however, it was prematurely terminated and thus underpowered to test the non-inferiority or superiority of TNK over alteplase [48].
Despite that minor stroke’s definition is still controversial with no clear distinguishing between disabling and non-disabling symptoms by currently used scores, such as NIHSS [48], AHA/ASA, and ESO guidelines recommended alteplase for minor stroke with disabling symptoms [7, 47]. Furthermore. AHA/ASA recommended TNK 0.4 for minor stroke based on the NORTEST-1 trial [37], which may not continue after NORTEST-2A [21]. In this regard, the TEMPO-1 trial, a dose escalation trial of TNK in minor stroke/TIA (NIHSS 0–5), found that TNK 0.1 and 0.25 are safe [52]. Currently, the TEMPO-2 trial is comparing TNK 0.25 versus standard of care in patients with minor stroke or TIA who have a confirmed LVO [53].
Notably, all of the included trials were prospective, randomized, open-label, and blinded outcome (PROBE) trials except Haley et al. 2010 [36], which was double-blinded RCT; however, prematurely terminated. Moreover, all the ongoing trials are PROBE trials except the TIMELESS trial, which may provide more subtle results [49, 54]. PROBE trials fail to overcome information bias which may lead to unconvincing outcomes as observed by our GRADE assessment [54]. Hence, future studies should consider the double-blinded design. Furthermore, RCTs that have the potential to reshape management and enhance outcomes for stroke patients are resource-demanding [55], and stroke research funding is considerably lower compared to cancer and heart research [55, 56]. Therefore, the pragmatic design of future RCTs following the AcT trial [22] can decrease the required funding and time needed to register the same factors into variable databases [55]. Finally, using wide inclusion criteria (any AIS patient eligible for thrombolysis), deferred consents, and a simple randomization process are also required in future RCTs, given the time-restricted nature of AIS management [55].
Strengths & Limitations
Our study is the most comprehensive and up-to-date network meta-analysis synthesizing only RCTs constituting the gold standard evidence in this regard. However, our review has a few limitations: first, most of the included RCTs are open-label trials with a high risk of performance bias. Second, the results should be interpreted with caution since the included trials differed in aspects such as advanced imaging for patient selection, presence of large vessel occlusion, the time window for drug administration or endovascular therapy, and variation in patient populations making indirect comparisons less conclusive. Finally, some of our findings show significant heterogeneity, which can limit the generalizability of our results.
Implications for Future Research
Considering the above discussion, rigorous double-blinded, pragmatic RCTs are required to investigate TNK’s potential to replace alteplase as the standard of care in AIS presenting after 4.5 h from symptoms onset, wake-up stroke, and minor stroke/TIA. Furthermore, future RCTs should consider investigating: recanalization time with dose escalation, extending safety outcome measurements to include systematic bleeding events, cerebral infarction in a new vascular area, and vessel re-occlusion [54], other predictors of the stroke care pathway, such as door to groin time, and efficacy of TNK as a bridging therapy before MT. Moreover, all of the completed and ongoing trials are from high-income regions with Caucasian ethnicity predominance; thus, RCTs in low- and middle-income regions and different ethnicities are still required [54]. Finally, cost benefits and drug administration techniques of TNK versus alteplase can also be assessed as considerations in this area that would be valuable for universal access to stroke care in low and middle-income countries.
Conclusion
TNK in the dose of 0.25 mg is a promising candidate to replace alteplase as the standard of care in patients with AIS presenting within 4.5 h of symptom onset, given its higher rate of early neurological recovery and non-inferiority in terms of safety outcomes. However, the evidence regarding TNK’s potential in AIS presenting after 4.5 h from symptoms onset, wake-up stroke, and minor stroke/TIA is still lacking, which accordingly warrants conducting further double-blinded, large-scale, and pragmatic RCTs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
ARS conceived the idea. BA and MT designed the research workflow. BA and MT searched the databases. AM, ARS, EA, and SK screened the retrieved records, extracted relevant data, assessed the quality of evidence, and BA resolved the conflicts. MT and AKA performed the analysis. MT, UA, and BA wrote the final manuscript. BA supervised the project. All authors have read and agreed to the final version of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). We received no funding for this study.
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicale.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohamed Abuelazm, Email: dr.mabuelazm@gmail.com.
Amith Reddy Seri, Email: seriamithreddy@gmail.com.
References
- 1.Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17:18–29. doi: 10.1177/17474930211065917. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 3.Carandang R, Seshadri S, Beiser A, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. J Am Med Assoc. 2006;296:2939–2946. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- 4.Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97:S6–S16. doi: 10.1212/WNL.0000000000012781. [DOI] [PubMed] [Google Scholar]
- 5.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) – European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on mechanical thrombectomy in acute ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE) Eur Stroke J. 2019;4:6–12. doi: 10.1177/2396987319832140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulanger JM, Lindsay MP, Gubitz G, et al (2018) Canadian stroke best practice recommendations for acute stroke management: prehospital, emergency department, and acute inpatient stroke care, 6th Edition, Update 2018. Int J Stroke 13:949–984. 10.1177/1747493018786616 [DOI] [PubMed]
- 7.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. J Vasc Surg. 2018;67:1934. doi: 10.1016/j.jvs.2018.04.007. [DOI] [Google Scholar]
- 8.(2022) Overview | Stroke and transient ischaemic attack in over 16s: diagnosis and initial management | Guidance | NICE. Nice [PubMed]
- 9.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue Plasminogen Activator for Acute Ischemic Stroke (NINDS Study) N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 10.Miller DJ, Simpson JR, Silver B, Silver B. Safety of thrombolysis in acute ischemic stroke: a review of complications, risk factors, and newer technologies. Neurohospitalist. 2011;1:138–147. doi: 10.1177/1941875211408731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: Real-world experience and a call for action. Stroke. 2010;41:2254–2258. doi: 10.1161/STROKEAHA.110.592535. [DOI] [PubMed] [Google Scholar]
- 12.Yeo LLL, Paliwal P, Teoh HL, et al. Timing of recanalization after intravenous thrombolysis and functional outcomes after acute ischemic stroke. JAMA Neurol. 2013;70:353–358. doi: 10.1001/2013.jamaneurol.547. [DOI] [PubMed] [Google Scholar]
- 13.Kaur J, Zhao Z, Klein GM, et al. The neurotoxicity of tissue plasminogen activator? J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- 14.Nicole O, Docagne F, Ali C, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001 doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 15.Parcq J, Bertrand T, Montagne A, et al. Unveiling an exceptional zymogen: The single-chain form of tPA is a selective activator of NMDA receptor-dependent signaling and neurotoxicity. Cell Death Differ. 2012;19:1983–1991. doi: 10.1038/cdd.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci. 2009;32:48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 17.F. Vv de W, J. A, D. A, , et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354:716–722. doi: 10.1016/S0140-6736(99)07403-6. [DOI] [PubMed] [Google Scholar]
- 18.Melandri G, Vagnarelli F, Calabrese D, et al. Review of tenecteplase (TNKase) in the treatment of acute myocardial infarction. Vasc Health Risk Manag. 2009;5:249–256. doi: 10.2147/vhrm.s3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bivard A, Zhao H, Churilov L, et al. Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne Mobile Stroke Unit (TASTE-A): a phase 2, randomised, open-label trial. Lancet Neurol. 2022;21:520–527. doi: 10.1016/S1474-4422(22)00171-5. [DOI] [PubMed] [Google Scholar]
- 20.Kheiri B, Osman M, Abdalla A, et al. Tenecteplase versus alteplase for management of acute ischemic stroke: a pairwise and network meta-analysis of randomized clinical trials. J Thromb Thrombolysis. 2018;46:440–450. doi: 10.1007/s11239-018-1721-3. [DOI] [PubMed] [Google Scholar]
- 21.Kvistad CE, Næss H, Helleberg BH, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol. 2022;21:511–519. doi: 10.1016/S1474-4422(22)00124-7. [DOI] [PubMed] [Google Scholar]
- 22.Menon BK, Buck BH, Singh N, et al. Articles Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada ( AcT ): a pragmatic, multicentre. Lancet. 2022 doi: 10.1016/S0140-6736(22)01054-6. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Pan Y, Wang Z, et al. Safety and efficacy of tenecteplase versus alteplase in patients with acute ischaemic stroke (TRACE): a multicentre, randomised, open label, blinded-endpoint (PROBE) controlled phase II study. Stroke Vasc Neurol. 2022;7:47–53. doi: 10.1136/svn-2021-000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Cochrane Handb Syst Rev Interv. 2019 doi: 10.1002/9781119536604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Innovation VH Covidence systematic review software
- 27.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011 doi: 10.1136/BMJ.D5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt GH, Oxman AD, Vist GE, et al. Rating Quality of Evidence and Strength of Recommendations: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ Br Med J. 2008;336:924. doi: 10.1136/BMJ.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.RevMan | Cochrane Training. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman. Accessed 3 Aug 2021
- 30.Owen RK, Bradbury N, Xin Y, et al. MetaInsight: An interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res Synth Methods. 2019;10:569–581. doi: 10.1002/jrsm.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team (2021) R Core Team 2021 R: A language and environment for statistical computing. R foundation for statistical computing. https://www.R-project.org/. R Found Stat Comput 2:2019
- 32.Rücker G, Schwarzer G, Krahn U, König J (2016) netmeta: Network meta-analysis using frequentist methods. R package version 0.9–8.
- 33.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/BMJ.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang X, Cheripelli BK, Lloyd SM, et al. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): A phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol. 2015;14:368–376. doi: 10.1016/S1474-4422(15)70017-7. [DOI] [PubMed] [Google Scholar]
- 35.Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N Engl J Med. 2018;378:1573–1582. doi: 10.1056/nejmoa1716405. [DOI] [PubMed] [Google Scholar]
- 36.Haley EC, Thompson JLP, Grotta JC, et al. Phase IIB/III trial of tenecteplase in acute ischemic stroke: Results of a prematurely terminated randomized clinical trial. Stroke. 2010;41:707–711. doi: 10.1161/STROKEAHA.109.572040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Logallo N, Novotny V, Assmus J, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017;16:781–788. doi: 10.1016/S1474-4422(17)30253-3. [DOI] [PubMed] [Google Scholar]
- 38.Parsons MW, Spratt N, Bivard A, et al. Tenecteplase versus Alteplase for Acute Ischemic Stroke (TAAIS) trial: a randomized trial using advanced CT selection. Stroke. 2012;43:1099–1107. doi: 10.1161/str.43.suppl_1.a57. [DOI] [PubMed] [Google Scholar]
- 39.Ba M, Sj L. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke. Stroke. 2019;50:2156–2162. doi: 10.1161/STROKEAHA.119.025080. [DOI] [PubMed] [Google Scholar]
- 40.Frühwald T, Gärtner U, Stöckmann N, et al. In vitro examination of the thrombolytic efficacy of tenecteplase and therapeutic ultrasound compared to rt-PA. BMC Neurol. 2019 doi: 10.1186/s12883-019-1404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang X, MacIsaac R, Thompson JLP, et al. Tenecteplase versus alteplase in stroke thrombolysis: An individual patient data meta-analysis of randomized controlled trials. Int J Stroke. 2016;11:534–543. doi: 10.1177/1747493016641112. [DOI] [PubMed] [Google Scholar]
- 42.Zang Y, Hou J, Wang LY. Therapeutic effect of tenecteplase on treatment of cerebral arterial thrombosis: a meta-analysis. Eur Rev Med Pharmacol Sci. 2016;20:4369–4379. [PubMed] [Google Scholar]
- 43.Bivard A, Huang X, Levi CR, et al. Tenecteplase in ischemic stroke offers improved recanalization. Neurology. 2017;89:62–67. doi: 10.1212/WNL.0000000000004062. [DOI] [PubMed] [Google Scholar]
- 44.Coutts SB, Dubuc V, Mandzia J, et al. Tenecteplase-tissue-type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke. 2015;46:769–774. doi: 10.1161/STROKEAHA.114.008504. [DOI] [PubMed] [Google Scholar]
- 45.Yogendrakumar V, Churilov L, Mitchell PJ, et al. Safety and efficacy of tenecteplase in older patients with large vessel occlusion. Neurology. 2022;98:e1292–e1301. doi: 10.1212/WNL.0000000000013302. [DOI] [PubMed] [Google Scholar]
- 46.Kvistad CE, Novotny V, Næss H, et al. Safety and predictors of stroke mimics in The Norwegian Tenecteplase Stroke Trial (NOR-TEST) Int J Stroke. 2019;14:508–516. doi: 10.1177/1747493018790015. [DOI] [PubMed] [Google Scholar]
- 47.Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021 doi: 10.1177/2396987321989865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahmood A, Muir KW. Tenecteplase or alteplase: what is the thrombolytic agent of the future? Curr Treat Options Neurol. 2022;24:503–513. doi: 10.1007/s11940-022-00733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.NCT03785678 (2020) Tenecteplase in stroke patients between 4 and 24 hours (TIMELESS). In: ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/record/NCT03785678. Accessed 21 Oct 2022
- 50.NCT04752631 (2021) MRI-guided thrOmbolysis for Stroke bEyond Time Window by TNK. In: https://clinicaltrials.gov/show/NCT04752631. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02235143/full. Accessed 22 Oct 2022
- 51.NCT04454788 (2020) Extending the Time Window for Tenecteplase by Effective Reperfusion in Patients With Large Vessel Occlusion. In: https://clinicaltrials.gov/show/NCT04454788. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02134080/full. Accessed 21 Oct 2022
- 52.…, Goyal M, Patil S, et al (2015) Tenecteplase–tissue-type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke [DOI] [PubMed]
- 53.NCT02398656 (2015) A Randomized Controlled Trial of TNK-tPA Versus Standard of Care for Minor Ischemic Stroke With Proven Occlusion (TEMPO-2). In: https://clinicaltrials.gov/ct2/show/NCT02398656. https://clinicaltrials.gov/ct2/show/NCT02398656. Accessed 22 Oct 2022
- 54.Li G, Wang C, Wang S, et al. Tenecteplase in ischemic stroke: challenge and opportunity. Neuropsychiatr Dis Treat. 2022;18:1013–1026. doi: 10.2147/NDT.S360967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandset EC, Tsivgoulis G. Tenecteplase for acute ischaemic stroke. Lancet. 2022;400:138–139. doi: 10.1016/S0140-6736(22)01107-2. [DOI] [PubMed] [Google Scholar]
- 56.Pendlebury ST. Worldwide under-funding of stroke research. Int J Stroke. 2007;2:80–84. doi: 10.1111/j.1747-4949.2007.00126.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.