Abstract
Host susceptibility to lipopolysaccharide (LPS) is correlated with the levels of circulating tumor necrosis factor alpha (TNF-α) that develop in response to circulating LPS. Mice are resistant, relative to rabbits, to the lethal effects of LPS. This study indicates that mice and rabbits are equally sensitive to the lethal effects of circulating TNF-α but that mice are more resistant than rabbits to the induction of circulating TNF-α by LPS.
The susceptibility of different animal species to the toxicity of lipopolysaccharide (LPS) is highly variable. Although the mechanisms underlying this variability are not well understood, host susceptibility to LPS appears to be correlated with levels of circulating tumor necrosis factor alpha (TNF-α) that develop in response to LPS. For example, humans are exquisitely sensitive to LPS, with the lethal dose of LPS in humans being as low as 1 to 2 μg (18). Injection of 4 ng of Escherichia coli LPS/kg into human volunteers caused a mean peak circulating TNF-α concentration of 240 pg/ml (14). Though not as susceptible to LPS as humans, rabbits are more susceptible to LPS than mice (15). Outbred New Zealand rabbits challenged with a fatal dose of 10 μg of Salmonella enterica serovar Minnesota Re595 LPS/kg developed a mean peak circulating TNF-α concentration of 25.2 ng/ml (13). In contrast, C57BL/6 mice challenged with a higher but nonlethal 50-μg/kg dose of S. enterica serovar Enteritidis LPS developed a peak circulating TNF-α concentration of only 3 ng/ml (17). These data suggest that the TNF-α-inducing potential of LPS is proportionally greater in species with greater sensitivity to LPS. TNF-α is a critical mediator of LPS-induced shock in experimental animals (2, 13), and administration of purified recombinant TNF-α to rats or dogs reproduced much of the vascular instability caused by injection of LPS (20, 21). Resistance to the TNF-α-inducing effects of LPS may therefore explain species-specific responsiveness to LPS.
Alternatively, species resistant to LPS may express a relative resistance to the lethal effects of TNF-α itself. It has even been proposed that the vascular physiology of mice is fundamentally different from that of humans (1). To address these possibilities, we directly compared the LPS-induced levels of TNF-α in serum associated with lethality in mice to those measured in rabbits. The 50% lethal dose (LD50) of S. enterica serovar Typhimurium LPS in BALB/c-AnNCr mice (2 mg/kg) and Dutch belted rabbits (500 μg/kg) have previously been reported (6, 11). To reduce the dose of LPS required for lethality in each species, we induced LPS hypersensitivity in each species by pretreating animals with the superantigen toxic shock syndrome toxin 1 (TSST-1). TSST-1 greatly potentiates LPS-induced serum TNF-α responses in mice (6, 9), and TNF-α was shown to be a required mediator of lethality in mice with superantigen-induced hypersensitivity to LPS (19).
TSST-1 and serovar Typhimurium LPS were prepared as described elsewhere (4, 22) and administered intraperitoneally (i.p.) to BALB/c-AnNCr mice (National Cancer Institute, Frederick, Md.) or intravenously (i.v.) to Dutch belted rabbits (Birchwood Farms, Redwing, Minn.). Serum samples were collected from mice and rabbits, stored at −70°C, and later assayed for cytolytic activity on WEHI clone 13 target cells (7) according to a recently described protocol (6). By convention, 1 U of TNF activity per ml is defined as the concentration of TNF that causes 50% lysis of target cells. The concentration of purified murine recombinant TNF-α (R & D Systems, Minneapolis, Minn.) that typically caused 50% lysis of WEHI clone 13 cells was 1.0 pg/ml. Monoclonal antibodies to rabbit TNF-α (Pharmingen, San Diego, Calif.) neutralized >90% of the cytolytic activity in rabbit serum collected 1 h after injection of LPS (10.0 μg/kg [i.v.]; data not shown). These antibodies were equally effective in neutralizing the cytolytic activity in rabbit serum collected 1 h after the sequential injection of LPS (10.0 μg/kg [i.v.]) and TSST-1 (10 ng/kg [i.v.]), with the dose of TSST-1 given 12 h prior to challenge with LPS (data not shown). The serum cytolytic activity measured in the following experiments was therefore attributed to TNF-α. The lower limit of detection of TNF-α in serum was 20 U/ml. Statistical analyses were performed on log10-transformed scores of measured TNF-α values, and samples containing <20 U of TNF-α/ml were arbitrarily assigned a value of 10 U/ml prior to log transformation. Student's t test for samples with unequal variances was used to determine the significance of differences between independent means. LD50 statistics were calculated as described elsewhere (16).
We have previously published the time course of TNF-α in serum induced by a lethal dose of LPS (400 μg/kg) in BALB/c-AnNCr mice primed for 12 h with 200 μg of TSST-1/kg (6). Peak levels of TNF-α in serum were measured at 1 to 2 h postinjection of LPS in this model, and the maximum enhancement effect of TSST-1 on LPS-induced TNF-α was measured at 2 h postinjection of LPS. At this time point, LPS-induced TNF-α levels in serum were ca. 1,000-fold higher in mice primed with TSST-1 compared to unprimed mice.
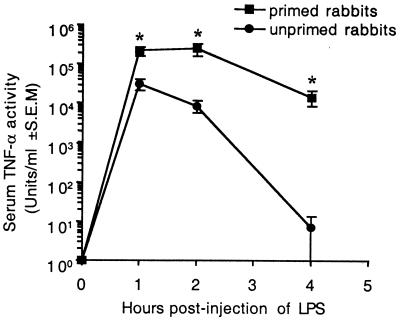
Since the effect of TSST-1 on LPS-induced TNF-α has not been examined in the rabbit, we determined the time course and dose response of LPS-induced TNF-α activity in serum in rabbits primed with TSST-1 for 12 h. When the challenge dose of LPS was held constant at 10 μg/kg, the LD50 for the priming dose of TSST-1 was ca. 10 ng/kg (Table 1). Figure 1 shows the time course of TNF-α in serum that developed in rabbits primed with 10 ng of TSST-1/kg and then challenged with 10 μg of LPS/kg 12 h later. A significant difference between LPS-induced TNF-α levels in serum in unprimed and TSST-1-primed rabbits was measured at each of the time points tested (P ≤ 0.05). As was observed in BALB/c-AnNCr mice (6), peak LPS-induced levels of TNF-α in serum were measured at 1 to 2 h postinjection of LPS. However, in contrast to BALB/c-AnNCr mice, the enhancement effect of TSST-1 on the LPS-induced TNF-α response in serum was greatest at 4 h after injection of LPS in rabbits. At this time point, LPS-induced TNF-α levels in serum were ca. 1,500-fold higher in rabbits primed with TSST-1 compared to unprimed rabbits (Fig. 1). Control rabbits injected with 5 μg/kg (i.v.) of TSST-1 alone, followed by phosphate-buffered saline (PBS) 12 h later, did not develop detectable TNF-α levels in serum.
TABLE 1.
Dose response of LPS-induced lethality and TNF-α activity in serum in rabbits primed with TSST-1
| Expt | Priming dose of TSST-1 (ng/kg)a | Peak TNF-α activity in serum (U/ml ± SEM)b | Magnitude of TNF-α enhancement by TSST-1c | Lethalityd (no. alive/ total no.) |
|---|---|---|---|---|
| 1 | 0.0 | 61,400 ± 20,500 | 3/3 | |
| 1.0 | 191,000 ± 72,200 | 3 | 6/6 | |
| 10.0 | 765,000 ± 289,000 | 12∗ | 3/6 | |
| 2 | 0.0 | 30,720 ± 10,240 | ND | |
| 100.0 | 710,000 ± 332,000 | 23∗ | 0/3 | |
| 500.0 | 4,370,000 ± 8,74,000 | 142∗∗ | ND | |
| 1,000.0 | 7,440,000 ± 3,060,000 | 242∗∗ | ND |
TSST-1 or PBS was injected i.v. 12 h prior to injection of LPS (10 μg/kg [i.v.]).
Mean TNF-α activity in serum collected 2 h after injection of LPS. Control rabbits primed with 5 μg of TSST-1 per kg, followed 12 h later by injection of PBS, did not develop detectable levels of TNF-α.
Statistical analysis was performed on log10-transformed scores of measured TNF-α values. Samples with <20 U of TNF-α per ml were assigned a value of 10 U/ml prior to transformation. Significance was determined by a two-tailed, unpaired t test, assuming unequal sample variances. ∗ = P ≤ 0.05 and ∗∗ = P ≤ 0.01.
ND, not determined.
FIG. 1.
Time course of LPS-induced TNF-α in serum in Dutch belted rabbits primed with TSST-1. Groups of three rabbits were injected with 10.0 ng (i.v.) of TSST-1 (primed)/kg or an equivalent volume of PBS (unprimed). All rabbits were injected with LPS (10 μg/kg [i.v.]) 12 h later. Control rabbits injected with 5 μg of TSST-1 and PBS/kg 12 h later did not develop detectable levels of TNF-α. (∗, P ≤ 0.05).
As shown in Table 1, the peak level of TNF-α in serum induced by LPS in rabbits given the LD50 of TSST-1 and LPS (10 ng of TSST-1/kg plus 10 μg of LPS/kg) was 12-fold higher than that measured in unprimed rabbits treated with LPS alone. Higher priming doses of TSST-1 enhanced peak LPS-induced TNF-α levels in serum by as much as 242-fold.
To determine if lethality was associated with equivalent peak levels of LPS-induced TNF-α activity in serum in mice and rabbits, we directly compared the peak TNF-α activity in serum induced by TSST-1 and LPS in BALB/c-AnNCr mice to that measured in Dutch belted rabbits. Peak TNF-α activity was measured in serum specimens retained from previously conducted time course experiments with BALB/c-AnNCr mice (6) and Dutch belted rabbits (Fig. 1). Serum samples were collected 2 h after injection of LPS from mice (n = 3) that received 200 μg of TSST-1/kg, followed 12 h later by 400 μg of LPS/kg, or from rabbits (n = 3) that received 10 ng of TSST-1/kg, followed 12 h later by 10 μg of LPS/kg. The combined doses of TSST-1 and LPS injected were the minimum doses that consistently caused 100 or 50% fatality rates in mice or rabbits, respectively. Serum samples from each species were tested for TNF-α activity in the same assay. When measured in the bioassay for TNF-α, the specific activity of murine recombinant TNF-α (R & D systems) was 2.56 × 108 U/mg, while that of rabbit TNF-α in conditioned medium (Pharmingen) was 3.72 × 108 U/mg. These specific activities were used to calculate corrected TNF-α scores from measured levels of TNF-α activity in serum. Corrected TNF-α values are expressed as levels of circulating TNF-α protein (in nanograms/milliliter).
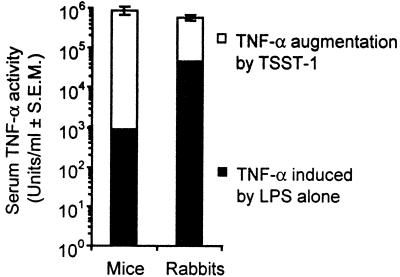
Figure 2 shows the peak levels of circulating TNF-α activity induced by the sequential injection of TSST-1 and LPS into mice and rabbits. The peak levels of TNF-α activity (mean ± the standard error of the mean) in serum measured in mice and rabbits were 874,000 ± 218,000 and 546,000 ± 109,000 U/ml, respectively. The difference between these uncorrected means did not reach statistical significance (P = 0.23). However, when measured TNF-α levels were corrected based on the specific activities of murine and rabbit TNF-α in the bioassay, the mean peak level of circulating TNF-α in mice (3,410 ng/ml) was nearly twice that measured in rabbits (1,470 ng/ml). Although the difference between these corrected means approached statistical significance (P = 0.06), the biological relevance of this difference must be interpreted with caution. If the fatality rates observed for each species were equivalent, then the finding of a greater peak TNF-α response in mice compared to rabbits would lend support to the notion that mice are less sensitive than rabbits to the lethal effects of circulating TNF-α. However, the doses of TSST-1 and LPS injected were the minimum required to cause fatality rates of 100 and 50% in mice and rabbits, respectively. If both the mice and the rabbits had been injected with an LD50 of TSST-1 and LPS, the peak levels of TNF-α measured in the sera of each of the species would probably have been even more equivalent. In view of the observed fatality rates and statistically equivalent peak TNF-α levels in serum in mice and rabbits, we concluded that lethality developed at approximately the same peak circulating level of TNF-α in both species.
FIG. 2.
Direct comparison of LPS-induced TNF-α in serum in mice and rabbits primed with TSST-1. Groups of three BALB/c-AnNCr mice and three Dutch belted rabbits were injected with TSST-1 at doses of 200 μg/kg (i.p.) or 10.0 ng/kg (i.v.), respectively. After 12 h, mice and rabbits were injected with LPS at doses of 400 μg/kg (i.p.) or 10 μg/kg (i.v.), respectively. TNF-α activity in serum was measured at 2 h postinjection of LPS in both species. The levels of TNF-α due to LPS alone were calculated from previous time course studies with the same mice and rabbits. Injection of TSST-1 alone did not induce detectable levels of TNF-α in serum in control animals. The mean peak TNF-α level induced by TSST-1 plus LPS in mice did not significantly differ from that measured in rabbits.
Also shown in Fig. 2 are the fractions of circulating TNF-α attributable to LPS alone, which were determined in our previous time course studies with the same animals. These fractions reveal that LPS is a much more potent inducer of serum TNF-α in rabbits compared to mice. Fortyfold less LPS was given to rabbits compared to mice, but this dose induced ca. fiftyfold more serum TNF-α in rabbits relative to mice. A much greater priming dose of TSST-1 was therefore required to raise peak levels of TNF-α in serum in mice to levels associated with lethality. Finally, since the half-life of circulating TNF-α is short in both mice (t1/2 = 6 min [3]) and rabbits (biphasic t1/2 = 0.5, 11 min [13]), the greater TNF-α-inducing potency of LPS in rabbits probably reflects greater production rather than slower clearance of TNF-α.
Of particular relevance to this study are previous experiments in which purified human TNF-α was administered to mice, rats, or dogs (5, 20, 21). These experiments not only demonstrated that TNF-α was a lethal cytokine but also revealed that mice were relatively resistant to the lethal effects of human TNF-α. Whereas the lethal dose of human TNF-α in mice was in excess of 1 mg/kg, doses of as low as 10 μg of murine TNF-α/kg caused death in mice (5). The resistance of mice to human TNF-α has been attributed to the failure of human TNF-α to engage the murine p75 receptor for TNF (10, 12), and this limited cross-reactivity of TNF-α in vivo has undoubtedly complicated assessments of TNF-α sensitivity across species. By showing that lethality develops in mice and rabbits at comparable circulating levels of homologous TNF-α, our data predict that the lethal dose of homologous TNF-α in rabbits would be close to that measured in mice (ca. 10 μg/kg).
In conclusion, this evidence challenges the notion that the vascular responses of mice to TNF-α or LPS are fundamentally different from species more susceptible to LPS (1). Instead, it favors a theory for murine resistance to LPS that is centered on species differences in the molecules required for potent cytokine induction by LPS. Murine resistance to shock induced by staphylococcal or streptococcal superantigens may likewise be due to a relative impairment in the ability of these toxins to induce cytokine release from murine immune cells. Transgenic mice expressing human CD14 or HLA-DQ6 exhibited significant increases in susceptibility to LPS or staphylococcal enterotoxin B (8, 23), respectively, thereby linking species variability in LPS or superantigen sensitivity to minor species differences in the cellular receptors for these toxins.
Acknowledgments
This work was supported by USPHS research grant AI22159 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.American College of Chest Physicians. Executive summary. From bench to the bedside: the future of sepsis research. National Institute of Allergy and Infectious Disease and National Heart, Lung, and Blood Institute Workshop. Chest. 1997;111:744–753. [PubMed] [Google Scholar]
- 2.Beutler B, Milsark I W, Cerami A C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B A, Milsark I W, Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985;135:3972–3977. [PubMed] [Google Scholar]
- 4.Blomster-Hautamaa D A, Schlievert P M. Purification of toxic shock syndrome toxin-1. Methods Enzymol. 1988;165:37–43. doi: 10.1016/s0076-6879(88)65009-9. [DOI] [PubMed] [Google Scholar]
- 5.Brouckaert P G, Everaerdt B, Libert C, Takahashi N, Fiers W. Species specificity and involvement of other cytokines in endotoxic shock action of recombinant tumour necrosis factor in mice. Agents Actions. 1989;26:196–198. doi: 10.1007/BF02126607. [DOI] [PubMed] [Google Scholar]
- 6.Dinges M M, Schlievert P M. Role of T cells and gamma-interferon during induction of hypersensitivity to lipopolysaccharide by toxic shock syndrome toxin 1 in mice. Infect Immun. 2001;69:1256–1264. doi: 10.1128/IAI.69.3.1256-1264.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 8.Ferrero E, Jiao D, Tsuberi B Z, Tesio L, Rong G W, Haziot A, Goyert S M. Transgenic mice expressing human CD14 are hypersensitive to lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:2380–2384. doi: 10.1073/pnas.90.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henne E, Campbell W H, Carlson E. Toxic shock syndrome toxin 1 enhances synthesis of endotoxin-induced tumor necrosis factor in mice. Infect Immun. 1991;59:2929–2933. doi: 10.1128/iai.59.9.2929-2933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer S M, Aggarwal B B, Eessalu T E, McCabe S M, Ferraiolo B L, Figari I S, Palladino M A., Jr Characterization of the in vitro and in vivo species preference of human and murine tumor necrosis factor-alpha. Cancer Res. 1988;48:920–925. [PubMed] [Google Scholar]
- 11.Lee P K, Deringer J R, Kreiswirth B N, Novick R P, Schlievert P M. Fluid replacement and polymyxin B protection of rabbits challenged subcutaneously with toxic shock syndrome toxins. Infect Immun. 1991;59:879–884. doi: 10.1128/iai.59.3.879-884.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis M, Tartaglia L A, Lee A, Bennett G L, Rice G C, Wong G H, Chen E Y, Goeddel D V. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci USA. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathison J C, Wolfson E, Ulevitch R J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Investig. 1988;81:1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michie H R, Manogue K R, Spriggs D R, Revhaug A, O'Dwyer S, Dinarello C A, Cerami A, Wolff S M, Wilmore D W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988;318:1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- 15.Redl H, Bahrami S, Schlag G, Traber D L. Clinical detection of LPS and animal models of endotoxemia. Immunobiology. 1993;187:330–345. doi: 10.1016/S0171-2985(11)80348-7. [DOI] [PubMed] [Google Scholar]
- 16.Reed L J, Muench H. A simple method for estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 17.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 18.Sauter C, Wolfensberger C. Interferon in human serum after injection of endotoxin. Lancet. 1980;ii:852–853. doi: 10.1016/s0140-6736(80)90189-0. [DOI] [PubMed] [Google Scholar]
- 19.Stiles B G, Campbell Y G, Castle R M, Grove S A. Correlation of temperature and toxicity in murine studies of staphylococcal enterotoxins and toxic shock syndrome toxin 1. Infect Immun. 1999;67:1521–1525. doi: 10.1128/iai.67.3.1521-1525.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tracey K J, Beutler B, Lowry S F, Merryweather J, Wolpe S, Milsark I W, Hariri R J, Fahey T J D, Zentella A, Albert J D, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 21.Tracey K J, Lowry S F, Fahey T J D, Albert J D, Fong Y, Hesse D, Beutler B, Manogue K R, Calvano S, Wei H, et al. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987;164:415–422. [PubMed] [Google Scholar]
- 22.Westphal O, Luderitz O, Blister F. Uber die Extraktion von Bacterien mit Phenol/Wasser. Z Naturforsch. 1952;7b:148–155. [Google Scholar]
- 23.Yeung R S, Penninger J M, Kundig T, Khoo W, Ohashi P S, Kroemer G, Mak T W. Human CD4 and human major histocompatibility complex class II (DQ6) transgenic mice: supersensitivity to superantigen-induced septic shock. Eur J Immunol. 1996;26:1074–1082. doi: 10.1002/eji.1830260518. [DOI] [PubMed] [Google Scholar]