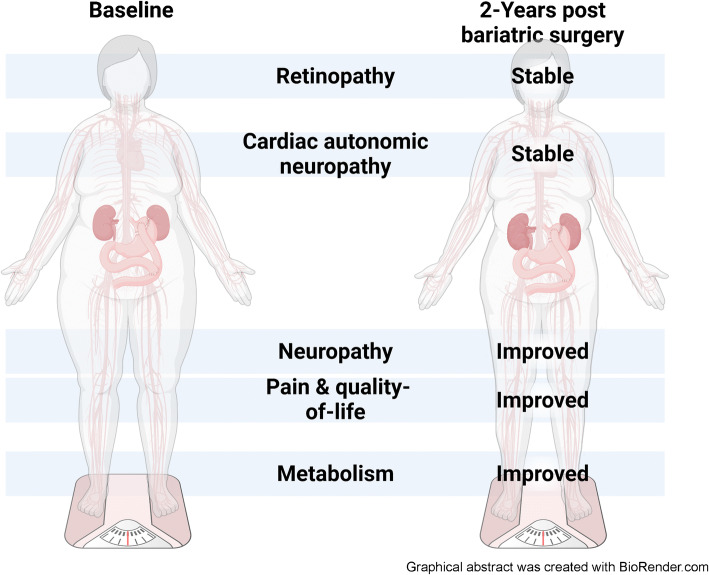
Abstract
Aims/hypothesis
The aim of this study was to determine the effect of bariatric surgery on diabetes complications in individuals with class II/III obesity (BMI > 35 kg/m2).
Methods
We performed a prospective cohort study of participants with obesity who underwent bariatric surgery. At baseline and 2 years following surgery, participants underwent metabolic phenotyping and diabetes complication assessments. The primary outcomes for peripheral neuropathy (PN) were a change in intra-epidermal nerve fibre density (IENFD, units = fibres/mm) at the distal leg and proximal thigh, the primary outcome for cardiovascular autonomic neuropathy (CAN) was a change in the expiration/inspiration (E/I) ratio, and the primary outcome for retinopathy was a change in the mean deviation on frequency doubling technology testing.
Results
Among 127 baseline participants, 79 completed in-person follow-up (age 46.0 ± 11.3 years [mean ± SD], 73.4% female). Participants lost a mean of 31.0 kg (SD 18.4), and all metabolic risk factors improved except for BP and total cholesterol. Following bariatric surgery, one of the primary PN outcomes improved (IENFD proximal thigh, +3.4 ± 7.8, p<0.01), and CAN (E/I ratio −0.01 ± 0.1, p=0.89) and retinopathy (deviation −0.2 ± 3.0, p=0.52) were stable. Linear regression revealed that a greater reduction in fasting glucose was associated with improvements in retinopathy (mean deviation point estimate −0.7, 95% CI −1.3, −0.1).
Conclusions/interpretation
Bariatric surgery may be an effective approach to reverse PN in individuals with obesity. The observed stability of CAN and retinopathy may be an improvement compared with the natural progression of these conditions; however, controlled trials are needed.
Graphical abstract
Keywords: Bariatric surgery, Chronic kidney disease, Diabetes complications, Obesity, Peripheral neuropathy
Introduction
The prevalence of type 2 diabetes is increasing worldwide [1], resulting in substantial morbidity from complications such as peripheral neuropathy (PN), cardiovascular autonomic neuropathy (CAN) and retinopathy [2]. In addition, other individual risk factors for the metabolic syndrome, which is frequently comorbid with type 2 diabetes, such as obesity, hypertension, low HDL-cholesterol and hypertriacylglycerolaemia, are also associated with PN and CAN [3–5]. Conversely, these metabolic factors are not consistently associated with retinopathy [6]. Obesity, particularly central obesity, has emerged as the second leading risk factor for PN after diabetes [3, 7]. Given the independent effects of metabolic risk factors on diabetes complications, interventions that simultaneously target multiple metabolic risk factors are needed.
Bariatric surgery is one intervention that can simultaneously and robustly improve multiple metabolic risk factors compared with other treatments [8]. Recent meta-analyses and systematic reviews have shown that bariatric surgery typically improves diabetes complications [9]. A meta-analysis of four studies comprising 86 participants found that surgical weight loss improved PN [10]. Other studies found that bariatric surgery ameliorated CAN outcomes [11], including a randomised controlled trial that found improvements in both the expiration/inspiration (E/I) ratio and the Valsalva ratio following bariatric surgery [12]. In addition, a meta-analysis of 14 studies comprising 110,300 participants found that the prevalence of retinopathy significantly decreased in surgical patients vs controls [13].
The previous studies that assessed the effect of bariatric surgery on diabetes complications had key limitations, including small sample sizes, limited outcome measures and/or lack of simultaneous assessment of multiple diabetes complications in the same population. Therefore, additional evidence is required to determine whether bariatric surgery can improve outcomes for diabetes complications, and whether it differs in effectiveness for the various complications. Furthermore, it is unknown whether bariatric surgery has differential effects on diabetes complications compared with other interventions, such as medical weight loss. Lastly, more evidence is needed to determine whether changes in specific metabolic risk factors are associated with improvements in diabetes complications.
In this study, we determined the effect of bariatric surgery on diabetes complications in individuals with class II/III obesity (BMI > 35 kg/m2). We also investigated whether changes in individual metabolic risk factors, including anthropometric measurements, were associated with changes in PN, CAN and retinopathy.
Methods
Study population
From April 2015 to May 2018, participants with obesity were enrolled from the University of Michigan bariatric surgery clinic, as previously described [5]. Inclusion criteria were 18 years of age or older and a BMI > 35 kg/m2. Exclusion criteria were use of anticoagulants, BMI > 70 kg/m2, current tobacco, marijuana or nicotine use, active cancer within the last year, a suicide attempt in the last year or multiple suicide attempts, reliance on a wheelchair or scooter, high-dose steroids, a cardiac stent within the last year, a history of open Nissen surgery or oesophagectomy, cirrhosis of the liver and having completed pre-surgical baseline study outcomes more than 6 months prior to surgery. Participants underwent metabolic phenotyping and diabetes complication assessments at baseline (prior to bariatric surgery) and 2 years after bariatric surgery.
Metabolic phenotyping
All participants underwent fasting lipid panel, HbA1c, BP, height, weight and BMI measurements; participants without diabetes also underwent glucose tolerance testing. Anthropometric measurements were obtained by averaging two repeated measurements taken without compressing the subcutaneous adipose tissue at nine separate locations as previously described [7]. Diabetes status was determined using HbA1c and glucose tolerance testing measurements, according to the 2022 ADA Standards of Care [14].
PN outcomes
The primary PN outcomes were intra-epidermal nerve fibre density (IENFD, unit = fibres/mm) measured at the distal leg and proximal thigh, evaluated according to an established protocol [15]. These measures have good diagnostic characteristics for small-fibre PN in individuals with obesity [16]. Secondary PN outcomes included nine nerve conduction study measures of three nerves (peroneal distal motor latency, peroneal amplitude, peroneal F wave index, peroneal CV, sural peak latency, sural amplitude, tibial distal motor latency, tibial amplitude, tibial F wave index), the Michigan Neuropathy Screening Instrument (MNSI) questionnaire, examination and combined index [17], the Utah Early Neuropathy Scale (UENS) [18], quantitative sensory testing (QST) of vibration and cold detection thresholds, vibration perception threshold from neurothesiometer testing [19] and monofilament testing. The nerve conduction studies, QST, neurothesiometer and monofilament testing were completed as previously described [19–21]. We defined clinical PN using the Toronto Consensus Definition of probable neuropathy as determined by one of six neuromuscular specialists (including BCC); this required the presence of at least two neuropathy symptoms, abnormal sensory examination or abnormal reflexes [22].
CAN outcomes
The primary CAN outcome was the E/I ratio, one of five Ewing cardiovascular reflex tests, which are considered the gold standard for autonomic testing [23]. CAN symptoms were assessed using the validated Survey of Autonomic Symptoms (SAS) [24]. Secondary CAN outcomes were heart rate variability (HRV) measurements, which included the resting median heart rate (mHR), low frequency area (LFA), respiratory frequency area (RFA), LFA/RFA ratio, the SD of the normal-to-normal interval (sdNN), the proportion of the number of pairs of successive normal-to-normal intervals that differ by more than 50 ms divided by the total number of normal-to-normal intervals (pNN50), and the root mean square of successive differences of the normal-to-normal interval (rmsSD). We defined clinical CAN using the 5th percentile of E/I ratio values from a control population without obesity, as previously described (E/I ratio <1.09) [5].
Retinopathy outcomes
The primary retinopathy outcome was the mean deviation, and secondary outcomes were the pattern SD and foveal sensitivity, which are sensitive disease markers for retinopathy, and were assessed by frequency doubling technology (FDT) testing using the 24-2 program (Humphrey Matrix 800, Carl Zeiss Meditech, USA) on a Humphrey Matrix, as previously described [25]. We defined clinical retinopathy as a diagnosis of any retinopathy based on a review of non-mydriatic retinal photographs taken by an ophthalmologist (TWG) using a Canon CR-1 Mark II camera.
Chronic kidney disease outcomes
Chronic kidney disease (CKD) was evaluated using the eGFR (ml/min per 1.73 m2), as measured using the 2021 CKD Epidemiology Collaboration equation [26], and the urine albumin to creatinine ratio (mg/g). We defined clinical CKD using the Kidney Disease: Improving Global Outcomes (KDIGO) criteria as eGFR <60 ml/min per 1.73 m2 or albumin to creatinine ratio ≥ 30 mg/g.
Patient-oriented outcomes and other medical comorbidities
The neuropathy-specific quality of life instrument (Neuro-QOL) was used to measure quality of life (QOL), with higher numbers reflecting poorer QOL [27]. Neuro-QOL measures overall neuropathy-specific QOL, overall QOL, the extent that problems with neuropathy impact overall QOL, and QOL specific to pain, reduced sensation, diffuse sensory motor symptoms, activities of daily living, emotional well-being and social well-being [27]. The validated short-form McGill pain questionnaire was used to measure pain using a visual analogue scale (VAS, scale 0–100), a present pain intensity index, and total score summarising 15 descriptors of overall pain and descriptors specific to the sensory and affective dimensions of pain [28]. The Inventory of Depressive Symptomatology Self Report (IDS-SR) was used to assess participant depression [29]. The Impact of Weight on Quality of Life (IWQOL-Lite) questionnaire was used to measure obesity-related QOL [30]. The EuroQOL European Quality of Life 5 Dimensions 3 Level Version (EQ-5D-3L) questionnaire was used to determine participant’s current health state (VAS, scale 0–100) and health status related to mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The Alcohol Use Disorders Identification Test (AUDIT) was used to assess unhealthy alcohol use [31]. Participants also described their physical activity level.
Ethics approval and participant consent
This study was approved by the University of Michigan Institutional Review Board. All study participants provided written informed consent.
Statistical analysis
All study analyses and outcomes were specified beforehand. Primary outcomes included IENFD of the distal leg and proximal thigh for PN, E/I ratio for CAN, and mean deviation by FDT testing for retinopathy. Primary analyses were performed to determine within-participant change in each primary outcome following bariatric surgery. To allow multiple comparisons for the primary outcomes, we used the Bonferroni correction to determine statistical significance. Specifically, for hypothesis testing of the effects of bariatric surgery on within-participant change for each primary outcome, two-tailed p values were calculated, and statistical significance was determined using the Bonferroni-corrected p value threshold of 0.0125. All other analyses were exploratory, and therefore considered hypothesis-generating. As a sensitivity analysis, we assessed the change in each primary outcome stratified by baseline diabetes status.
Descriptive statistics were used to summarise participant demographic information, metabolic phenotyping, and primary and secondary study outcomes at baseline and after 2 years of follow-up. For continuous metabolic risk factors and outcomes, the within-participant change was determined by subtracting baseline values from those collected at follow-up. For categorical variables, the within-participant change was determined as those that improved, were stable or worsened from baseline to follow-up.
We compared the demographic information between participants who were lost to follow-up, those who completed their 2-year follow-up, and those who only completed virtual 2-year follow-up (due to COVID-19) using one-way ANOVA for continuous variables and Pearson’s χ2 tests or Fisher’s Exact tests (as appropriate) for categorical variables. Paired Student t tests (for continuous variables) and Wilcoxon signed-rank tests (for categorical variables) were used to compare within-participant differences at follow-up.
Multivariable linear regression models were fitted to determine the association between changes in primary diabetes complication outcomes and changes in metabolic risk factors. Specifically, we fitted the change in primary outcomes (IENFD of the proximal thigh, IENFD of the distal leg, E/I ratio and mean deviation using FDT) as a function of the change in each metabolic risk factor separately, after adjusting for participant age, sex, baseline BMI and baseline outcome measurements.
Available case analysis was used to manage missing values. For all hypothesis testing, two-tailed p values were calculated to determine statistical significance. All analyses were completed using R statistical software version 4.2.1 (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Austria).
Results
Study participation and missing data
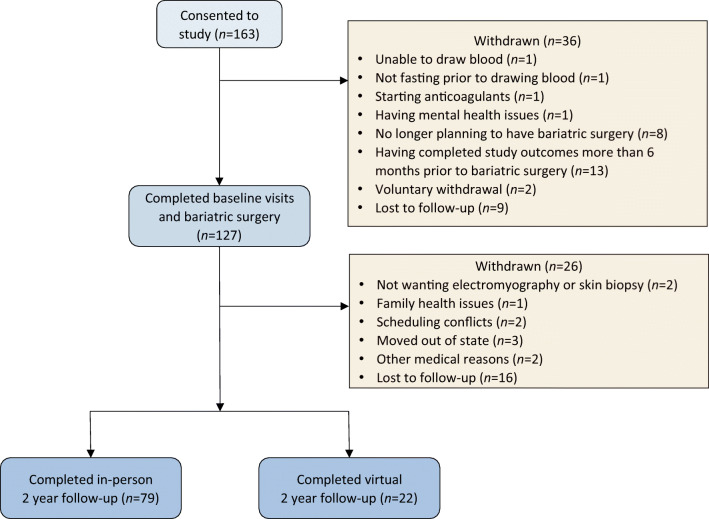
A total of 163 individuals consented to participate in the study, of whom 127 (77.9%) completed all baseline visits and bariatric surgery. Seventy-nine of the 127 participants (62.2%) completed in-person 2-year follow-up visits, and an additional 22 participants completed partial virtual measures due to COVID-19 (total of 79.5% for completion of follow-up). Study participation and loss to follow-up are summarised in Fig. 1. The number of participants with missing metabolic risk factor and outcome information is shown in the relevant tables. After bariatric surgery, many participants were unable to tolerate glucose tolerance testing due to anatomical changes after surgery, and therefore these data were not collected after June 2018. Of the 79 participants who completed in-person follow-up, 71 (89.9%) underwent a sleeve gastrectomy, and 8 (10.1%) underwent gastric bypass surgery.
Fig. 1.
Study participation and loss to follow-up
Demographic characteristics and changes in metabolic risk factors
Participant demographic information is shown in Table 1. The mean age (± SD) for the 79 participants who completed in-person follow-up was 46.0 ± 11.3 years, 73.4% were female, and most were white (79.7%) and non-Hispanic (98.7%). There were no significant demographic differences between those who completed in-person follow-up, completed virtual follow-up, or were lost to follow-up (all p>0.05). All metabolic risk factors significantly improved (all p<0.05) except BP and total cholesterol (Table 2). The number of participants receiving anti-hypertensive medications significantly decreased (27.8% improved, 72.2% stable, 0.0% worsened; p<0.01), whereas the number receiving cholesterol or glucose-lowering medications was stable during follow-up. We also found that the number of participants with diabetes and pre-diabetes decreased during follow-up (54.4% improved, 44.3% stable, 1.3% worsened; p<0.01).
Table 1.
Demographic information for the study participants and those lost to follow-up
| Variable | All participants (N = 127) | Completed in-person follow-up (N = 79) | Completed virtual follow-up (N = 22) | Lost to follow-up (N = 26) | p value |
|---|---|---|---|---|---|
| Age (years) | 44.9 ± 12.8 | 46.0 ± 11.3 | 47.8 ± 14.4 | 40.4 ± 11.9 | 0.10a |
| Female | 101 (79.5) | 58 (73.4) | 18 (81.8) | 22 (84.6) | 0.45a |
| Race | 0.82 | ||||
| Asian | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Black | 20 (15.7) | 12 (15.2) | 5 (22.7) | 3 (11.5) | |
| White | 100 (78.7) | 63 (79.7) | 16 (72.7) | 21 (80.8) | |
| Multiple reported races | 2 (1.6) | 1 (1.3) | 0 (0.0) | 1 (3.8) | |
| Other | 4 (3.1) | 2 (2.5) | 1 (4.5) | 1 (3.8) | |
| Unknown | 1 (0.8) | 1 (1.3) | 0 (0.0) | 0 (0.0) | |
| Ethnicity | 0.61 | ||||
| Hispanic/Latino | 2 (1.6) | 1 (1.3) | 0 (0.0) | 1 (3.8) | |
| Smoking status | 0.95 | ||||
| Ex-smoker | 39 (30.7) | 26 (32.9) | 7 (31.8) | 6 (23.1) | |
| Never smoker | 88 (69.3) | 53 (67.1) | 15 (68.2) | 20 (76.9) | |
| AUDIT alcoholism score | 1.8 ± 1.8 | 1.7 ± 1.9 | 1.6 ± 1.7 | 2.1 ± 1.8 | 0.45a |
| Marital status | 0.77 | ||||
| Divorced | 18 (14.2) | 13 (16.5) | 1 (4.5) | 4 (15.4) | |
| Married | 74 (58.3) | 49 (62.0) | 13 (59.1) | 12 (46.2) | |
| Significant other | 2 (1.6) | 1 (1.3) | 1 (4.5) | 0 (0.0) | |
| Single | 31 (24.4) | 15 (19.0) | 6 (27.3) | 10 (38.5) | |
| Widowed | 2 (1.6) | 1 (1.3) | 1 (4.5) | 0 (0.0) | |
| Education | 0.82 | ||||
| Professional or graduate degree | 20 (15.7) | 10 (12.7) | 6 (27.3) | 4 (15.4) | |
| College degree | 54 (42.5) | 34 (43.0) | 9 (40.9) | 11 (42.3) | |
| Some college or vocational college | 39 (30.7) | 24 (30.4) | 6 (27.3) | 9 (34.6) | |
| High school graduate or GED test | 13 (10.2) | 10 (12.7) | 1 (4.5) | 2 (7.7) | |
| High school or less | 1 (0.8) | 1 (1.3) | 0 (0.0) | 0 (0.0) | |
| Employment status | 0.80 | ||||
| Employed | 86 (67.7) | 51 (64.6) | 15 (68.2) | 20 (76.9) | |
| Retired | 14 (11.0) | 10 (12.7) | 3 (13.6) | 1 (3.8) | |
| Seeking work | 1 (0.8) | 1 (1.3) | 0 (0.0) | 0 (0.0) | |
| Keeping house | 10 (7.9) | 6 (7.6) | 2 (9.1) | 2 (7.7) | |
| Student | 4 (3.1) | 1 (1.3) | 1 (4.5) | 2 (7.7) | |
| Other | 12 (9.4) | 10 (12.7) | 1 (4.5) | 1 (3.8) | |
| Insurance | 0.36 | ||||
| Private insurance | 92 (72.4) | 57 (72.2) | 14 (63.6) | 21 (80.8) | |
| Medicare | 2 (1.6) | 2 (2.5) | 0 (0.0) | 0 (0.0) | |
| Medicaid | 8 (6.3) | 5 (6.3) | 2 (9.1) | 1 (3.8) | |
| Multiple plans | 19 (15.0) | 13 (16.5) | 3 (13.6) | 3 (11.5) | |
| Other | 5 (3.9) | 2 (2.5) | 3 (13.6) | 0 (0.0) | |
| Unknown | 1 (0.8) | 0 (0.0) | 0 (0.0) | 1 (3.8) |
Values are means ± SD or n (%)
Data for the AUDIT score were missing for five participants at in-person follow-up, age was missing for 1 participant of those lost to follow-up
p values were calculated using a χ2 test except where indicated by a superscript a, in which case they represent the result obtained using one-way ANOVA
GED, General Educational Development
Table 2.
Change in metabolic risk factors following bariatric surgery in the 79 patients who completed in-person follow-up
| Variable | Baseline | Two-year follow-up | Change | p value |
|---|---|---|---|---|
| Weight (kg) | 130.4 ± 24.8 | 99.4 ± 21.2 | −31.0 ± 18.4 | <0.01 |
| Height (cm) | 168.0 ± 9.9 | 167.7 ± 10.3 | −0.3 ± 2.2 | 0.21 |
| BMI (kg/m2) | 46.0 ± 6.6 | 35.3 ± 6.4 | −10.8 ± 6.5 | <0.01 |
| NCEP-defined waist circumference (cm) | 131.6 ± 17.2 | 114.3 ± 17.5 | −17.2 ± 14.8 | <0.01 |
| Arm (cm) | 41.5 ± 4.8 | 35.5 ± 4.6 | −6.0 ± 4.2 | <0.01 |
| Forearm (cm) | 30.3 ± 2.6 | 27.3 ± 2.9 | −3.0 ± 2.3 | <0.01 |
| Calf (cm) | 45.8 ± 4.5 | 41.5 ± 4.6 | −4.3 ± 3.0 | <0.01 |
| Mid-thigh (cm) | 64.5 ± 8.4 | 58.3 ± 7.9 | −6.2 ± 6.2 | <0.01 |
| Hips/thigh (cm) | 75.3 ± 9.2 | 66.4 ± 8.7 | −8.9 ± 7.5 | <0.01 |
| Abdomen (cm) | 133.7 ± 17.0 | 113.0 ± 16.4 | −20.6 ± 15.1 | <0.01 |
| Buttocks/hips (cm) | 140.4 ± 15.3 | 121.5 ± 14.0 | −18.6 ± 10.8 | <0.01 |
| High-waist (cm) | 123.4 ± 14.4 | 106.0 ± 14.3 | −17.4 ± 11.0 | <0.01 |
| Systolic BP (mmHg) | 127.9 ± 14.3 | 129.0 ± 16.4 | 1.2 ± 17.3 | 0.55 |
| Diastolic BP (mmHg) | 71.8 ± 11.6 | 73.1 ± 11.2 | 1.3 ± 10.8 | 0.28 |
| Hypertension medication (yes) | 46 (58.2) | 24 (30.4) |
Worsened: 0 (0.0) Stable: 57 (72.2) Improved: 22 (27.8) |
<0.01a |
| Triacylglycerol (mmol/l) | 1.4 ± 0.6 | 1.1 ± 0.6 | −0.3 ± 0.6 | <0.01 |
| HDL-cholesterol (mmol/l) | 1.1 ± 0.3 | 1.6 ± 0.4 | 0.4 ± 0.3) | <0.01 |
| LDL-cholesterol (mmol/l) | 2.6 ± 1.2 | 2.3 ± 0.8 | −0.3 ± 0.9 | 0.01 |
| Cholesterol (mmol/l) | 4.2 ± 1.2 | 4.4 ± 1.0 | 0.2 ± 0.8 | 0.07 |
| Cholesterol medication (yes) | 26 (32.9) | 20 (25.3) |
Worsened: 2 (2.5) Stable: 69 (87.3) Improved: 8 (10.1) |
0.07a |
| Fasting glucose (mmol/l) | 5.7 ± 1.7 | 5.0 ± 1.4 | −0.8 ± 1.0 | <0.01 |
| 2 h glucose (mmol/l) | 6.7 ± 2.2 | 3.4 ± 1.4 | −4.4 ± 2.4 | <0.01 |
| Not collected due to diabetes | 15 | 18 | ||
| Not collected due to tolerability concerns or late follow-upb | 4 | 41 | ||
| HbA1c (%) | 6.0 ± 0.9 | 5.5 ± 0.7 | −0.5 ± 0.6 | <0.01 |
| HbA1c (mmol/l) | 7.0 ± 1.4 | 6.2 ± 1.1 | −0.7 ± 1.0 | <0.01 |
| Diabetes |
Worsened: 1 (1.3) Stable: 35 (44.3) Improved: 43 (54.4) |
<0.01a | ||
| Normoglycaemic | 24 (30.4) | 52 (65.8) | ||
| Pre-diabetes | 32 (40.5) | 21 (26.6) | ||
| Diabetes | 23 (29.1) | 6 (7.6) | ||
| Diabetes medication (yes) | 24 (30.4) | 19 (24.1) |
Worsened: 7 (8.9) Stable: 60 (75.9) Improved: 12 (15.2) |
0.26a |
Values are means ± SD or n (%)
Buttock/hips measurements and LDL-cholesterol measurements were each missing for one participant at follow-up. HbA1c measurements were missing for three participants at baseline. Glucose tolerance testing was not collected for individuals with a previous diabetes diagnosis. In addition, following bariatric surgery, many participants were unable to tolerate glucose tolerance testing due to anatomical changes after surgery, and therefore these data were not collected after June 2018. There were 4 individuals that could not tolerate glucose tolerance testing at baseline, and 41 individuals that could not tolerate it at 2 years. The 2 h glucose measurement was missing for one participant at baseline
p values were calculated using a paired t test except where indicated by a superscript a, in which case they represent the result obtained using Wilcoxon signed-rank test
bLate follow-up after June 2018
Change in PN
One primary PN outcome (IENFD of the proximal thigh) improved during follow-up (+3.4 ± 7.8 fibres/mm, p<0.01) while the other (IENFD of the distal leg) was stable (+0.1 ± 4.1 fibres/mm, p=0.92) (Table 3). Sensitivity analyses revealed that the change in the IENFD of the thigh was significantly improved in those with normoglycaemia (+4.2 ± 6.8, p<0.01) and pre-diabetes (+4.2 ± 7.8, p<0.01), but not diabetes (+0.8 ± 8.1, p=0.65). Two values for secondary PN outcomes, the MNSI questionnaire (−0.8 ± 1.8, p<0.01) and the MNSI combined index (−0.2 ± 0.9, p=0.03) also improved during follow-up. In contrast, three secondary PN outcomes (QST vibration [+1.1 ± 2.4, p<0.01], vibration perception threshold from neurothesiometer testing [+3.4 ± 11.3, p=0.01] and tibial distal motor latency [+0.4 ± 1.0, p<0.01]) worsened during follow-up. All other secondary PN outcomes were stable during follow-up. In addition, we found that the number of participants with clinical PN as defined by the Toronto Consensus Definition for probable neuropathy increased during follow-up (0.0% improved, 89.9% stable, 10.1% worsened; p<0.01).
Table 3.
Diabetes complication and patient-oriented outcome changes following bariatric surgery in the 79 patients who completed in-person follow-up
| Variable | Baseline | Missing | Two-year follow-up | Missing | Change | Missing | p value |
|---|---|---|---|---|---|---|---|
| PN outcomes | |||||||
| IENFD distal leg (fibres/mm) | 8.5 ± 6.9 | 2 | 8.6 ± 6.0 | 3 | 0.1 ± 4.1 | 5 | 0.92 |
| IENFD thigh (fibres/mm) | 15.0 ± 7.9 | 1 | 18.4 ± 7.5 | 1 | 3.4 ± 7.8 | 2 | <0.01 |
| PN (Toronto Consensus Definition of probable neuropathy) | 13 (16.5) | 0 | 21 (27.0) | 0 |
Worsened: 8 (10.1) Stable: 71 (89.9) Improved: 0 (0.0) |
0 | <0.01a |
| Nerve conduction study outcomesb | |||||||
| Peroneal distal motor latency (ms) | 4.7 ± 0.9 | 0 | 4.7 ± 0.9 | 0 | −0.1 ± 0.8 | 0 | 0.50 |
| Peroneal amplitude (μV) | 5.8 ± 2.8 | 0 | 5.3 ± 2.7 | 0 | −0.4 ± 2.3 | 0 | 0.13 |
| Peroneal F wave index (ms) | 49.8 ± 6.3 | 1 | 49.9 ± 5.9 | 0 | −0.03 ± 4.2 | 1 | 0.95 |
| Peroneal CV (m/s) | 45.5 ± 5.6 | 0 | 44.7 ± 6.2 | 0 | −0.7 ± 5.9 | 0 | 0.30 |
| Sural peak latency (ms) | 3.9 ± 0.5 | 1 | 3.8 ± 0.5 | 0 | −0.1 ± 0.5 | 1 | 0.29 |
| Sural amplitude (μV) | 10.9 ± 6.8 | 1 | 11.8 ± 6.8 | 0 | 0.7 ± 5.8 | 1 | 0.32 |
| Tibial distal motor latency (ms) | 4.7 ± 0.9 | 0 | 5.1 ± 0.9 | 0 | 0.4 ± 1.0 | 0 | <0.01 |
| Tibial amplitude (μV) | 8.8 ± 5.5 | 0 | 9.1 ± 5.2 | 0 | 0.4 ± 3.3 | 0 | 0.32 |
| Tibial F wave index (ms) | 51.5 ± 6.1 | 0 | 50.5 ± 6.0 | 1 | −0.8 ± 3.7 | 1 | 0.08 |
| QSTb | |||||||
| QST cold | 10.2 ± 4.2 | 0 | 10.3 ± 3.5 | 0 | 0.2 ± 4.5 | 0 | 0.73 |
| QST vibration | 16.1 ± 4.2 | 0 | 17.2 ± 3.3 | 0 | 1.1 ± 2.4 | 0 | <0.01 |
| MNSI | |||||||
| MNSI questionnaire | 3.1 ± 2.8 | 0 | 2.3 ± 2.5 | 0 | −0.8 ± 1.8 | 0 | <0.01 |
| MNSI exam | 1.0 ± 1.4 | 0 | 1.0 ± 1.4 | 0 | −0.01 ± 1.1 | 0 | 0.96 |
| MNSI index | 1.3 ± 1.1 | 0 | 1.1 ± 0.9 | 0 | −0.2 ± 0.9 | 0 | 0.03 |
| Utah Early Neuropathy Scale (UENS) | 3.2 ± 5.3 | 0 | 3.8 ± 5.9 | 0 | 0.6 ± 3.7 | 0 | 0.08 |
| Neurothesiometer testing | 10.5 ± 15.4 | 0 | 13.9 ± 23.6 | 0 | 3.4 ± 11.3 | 0 | 0.01 |
| Monofilament test |
Worsened: 5 (6.3) Stable: 68 (86.1) Improved: 6 (7.6) |
0.81a | |||||
| Normal | 68 (86.1) | 0 | 71 (89.9) | 0 | 0 | ||
| Reduced | 8 (10.1) | 0 | 4 (5.1) | 0 | 0 | ||
| Absent | 3 (3.8) | 0 | 4 (5.1) | 0 | 0 | ||
| CAN outcomes | 0.89 | ||||||
| E/I ratio | 1.2 ± 0.1 | 0 | 1.2 ± 0.1 | 7 | −0.01 ± 0.1 | 7 | |
| CAN present (E/I ratio <1.09) | 20 (25.3) | 0 | 14 (17.7) | 7 |
Worsened: 5 (6.9) Stable: 58 (80.6) Improved: 9 (12.5) |
7 | 0.30a |
| SAS score | 5.9 ± 5.6 | 1 | 6.2 ± 5.7 | 0 | 0.3 ± 5.3 | 1 | 0.58 |
| RFA | 2.1 ± 3.6 | 0 | 6.0 ± 38.3 | 7 | 4.1 ± 38.5 | 7 | 0.37 |
| LFA | 2.1 ± 2.5 | 0 | 1.5 ± 4.3 | 7 | −0.6 ± 5.0 | 7 | 0.34 |
| LFA/RFA | 242.8 ± 2078.4 | 0 | 289.3 ± 2057.1 | 7 | 28.1 ± 3019.4 | 7 | 0.94 |
| sdNN | 43.4 ± 23.4 | 0 | 49.5 ± 30.5 | 7 | 7.5 ± 30.4 | 7 | 0.04 |
| pNN50 | 9.0 ± 12.0 | 0 | 14.1 ± 16.6 | 7 | 5.7 ± 14.4 | 7 | <0.01 |
| rmsSD | 28.7 ± 21.0 | 0 | 36.4 ± 29.2 | 7 | 9.3 ± 29.3 | 7 | <0.01 |
| Median heart rate | 73.2 ± 9.2 | 0 | 63.8 ± 8.7 | 7 | −9.6 ± 8.7 | 7 | <0.01 |
| Retinopathy outcomes | |||||||
| Mean deviation | −1.2 ± 4.3 | 1 | −1.6 ± 4.2 | 1 | −0.2 ± 3.0 | 2 | 0.52 |
| Retinopathy | 4 (5.1) | 1 | 2 (2.5) | 3 |
Worsened: 1 (1.3) Stable: 71 (94.7) Improved: 3 (4.0) |
4 | 0.50a |
| Pattern SD | 3.2 ± 1.1 | 1 | 3.1 ± 0.9 | 1 | −0.2 ± 0.9 | 2 | 0.09 |
| Foveal sensitivity | 27.2 ± 5.3 | 1 | 27.0 ± 5.0 | 1 | −0.1 ± 4.5 | 2 | 0.91 |
| CKD outcomes | |||||||
| eGFR (ml/min per 1.73 m2) | 98.0 ± 18.5 | 0 | 94.6 ± 19.8 | 1 | −3.4 ± 10.0 | 1 | <0.01 |
| CKD (KDIGO criteria) | 7 (8.9) | 0 | 11 (13.9) | 1 |
Worsened: 5 (6.4) Stable: 72 (92.3) Improved: 1 (1.3) |
1 | 0.13a |
| Microalbumin/creatinine ratio (mg/g) | 0.2 ± 0.3 | 0 | 0.2 ± 0.7 | 0 | 0.05 ± 0.7 | 0 | 0.56 |
| Patient-oriented outcomes | |||||||
| McGill pain scale | |||||||
| McGill VAS | 2.8 ± 2.8 | 0 | 1.8 ± 2.4 | 0 | −1.0 ± 2.6 | 0 | <0.01 |
| McGill present pain intensity index |
Worsened: 8 (10.1) Stable: 55 (69.6) Improved: 16 (20.3) |
<0.01 | |||||
| No pain | 53 (67.1) | 0 | 56 (70.9) | 0 | 0 | ||
| Mild | 11 (13.9) | 0 | 12 (15.2) | 0 | 0 | ||
| Discomforting | 11 (13.9) | 0 | 9 (11.4) | 0 | 0 | ||
| Distressing | 3 (3.8) | 0 | 2 (2.5) | 0 | 0 | ||
| Horrible | 1 (1.3) | 0 | 0 (0.0) | 0 | 0 | ||
| Excruciating | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 | ||
| McGill total | 5.5 ± 6.4 | 0 | 3.5 ± 4.8 | 0 | −2.0 ± 5.5 | 0 | <0.01 |
| McGill sensory | 4.7 ± 5.4 | 0 | 3.0 ± 4.0 | 0 | −1.7 ± 4.4 | <0.01 | |
| McGill affective | 1.1 ± 2.7 | 0 | 0.5 ± 1.1 | 0 | −0.6 ± 2.7 | 0.06 | |
| Neuro-QOL | |||||||
| Neuro-QOL total | 2.9 ± 2.0 | 1 | 2.3 ± 1.5 | 1 | −0.6 ± 1.9 | 2 | <0.01 |
| Neuro-QOL pain | 2.7 ± 2.0 | 0 | 2.2 ± 1.9 | 0 | −0.5 ± 1.9 | 0 | 0.02 |
| Neuro-QOL reduced sensation | 2.0 ± 2.0 | 0 | 1.9 ± 2.2 | 0 | −0.1 ± 1.5 | 0 | 0.49 |
| Neuro-QOL sensory motor | 2.7 ± 2.6 | 0 | 2.1 ± 1.9 | 0 | −0.5 ± 2.4 | 0 | 0.05 |
| Neuro-QOL social/emotional | 2.9 ± 2.8 | 1 | 2.3 ± 1.9 | 1 | −0.7 ± 2.8 | 2 | 0.02 |
| Neuro-QOL activities of daily living | 3.4 ± 2.6 | 1 | 3.2 ± 1.9 | 1 | −0.1 ± 2.7 | 2 | 0.77 |
| Neuro-QOL overall QOL | 2.6 ± 1.0 | 0 | 2.1 ± 0.9 | 0 | −0.5 ± 0.9 | 0 | <0.01 |
| Neuro-QOL foot-specific QOL | 1.5 ± 1.0 | 0 | 1.2 ± 0.6 | 0 | −0.3 ± 1.0 | 0 | <0.01 |
| Impact of Weight on Quality of Life (IWQOL-Lite) | 80.3 ± 24.2 | 5 | 48.7 ± 18.9 | 1 | −31.3 ± 24.1 | 6 | <0.01 |
| EuroQOL EQ-5D-3L | |||||||
| EQ-5D current health status VAS | 66.6 ± 20.8 | 1 | 79.0 ± 14.9 | 0 | 12.4 ± 20.5 | 1 | <0.01 |
| EQ-5D mobility |
Worsened: 4 (5.1) Stable: 59 (74.7) Improved: 16 (20.3) |
0.03a | |||||
| I have no problems walking about | 52 (65.8) | 0 | 64 (81.0) | 0 | 0 | ||
| I have some problems walking about | 27 (34.2) | 0 | 15 (19.0) | 0 | 0 | ||
| I am confined to bed | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 | ||
| EQ-5D self-care |
Worsened: 1 (1.3) Stable: 72 (91.1) Improved: 6 (7.6) |
0.08a | |||||
| I have no problems with self-care | 72 (91.1) | 0 | 77 (97.5) | 0 | 0 | ||
| I have some problems washing or dressing myself | 7 (8.9) | 0 | 2 (2.5) | 0 | 0 | ||
| I am unable to wash or dress myself | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 | ||
| EQ-5D usual activities |
Worsened: 4 (5.1) Stable: 61 (77.2) Improved: 14 (17.7) |
1.00a | |||||
| I have no problems performing my usual activities | 55 (69.6) | 0 | 64 (81.0) | 0 | 0 | ||
| I have some problems performing my usual activities | 23 (29.1) | 0 | 15 (19.0) | 0 | 0 | ||
| I am unable to perform my usual activities | 1 (1.3) | 0 | 0 (0.0) | 0 | 0 | ||
| EQ-5D pain/discomfort |
Worsened: 5 (6.3) Stable: 51 (64.6) Improved: 23 (29.1) |
0.15a | |||||
| I have no pain or discomfort | 32 (40.5) | 0 | 46 (58.2) | 0 | 0 | ||
| I have moderate pain or discomfort | 41 (51.9) | 0 | 31 (39.2) | 0 | 0 | ||
| I have extreme pain or discomfort | 6 (7.6) | 0 | 2 (2.5) | 0 | 0 | ||
| EQ-5D anxiety/depression |
Worsened: 12 (15.2) Stable: 60 (75.9) Improved: 7 (8.9) |
0.78a | |||||
| I am not anxious or depressed | 53 (67.1) | 0 | 47 (59.5) | 0 | 0 | ||
| I am moderately anxious or depressed | 19 (24.1) | 0 | 26 (32.9) | 0 | 0 | ||
| I am extremely anxious or depressed | 7 (8.9) | 0 | 6 (7.6) | 0 | 0 | ||
| Depressive symptomology (IDS-SR) | 18.5 ± 10.9 | 0 | 15.7 ± 13.0 | 1 | −2.9 ± 10.3 | 1 | 0.01 |
| Physical activity level |
Worsened: 16 (20.3) Stable: 31 (39.2) Improved: 32 (40.5) |
0.06a | |||||
| No physical activity | 4 (5.1) | 0 | 1 (1.3) | 0 | 0 | ||
| Only light physical activity in most weeks | 32 (40.5) | 0 | 31 (39.2) | 0 | 0 | ||
| Vigorous physical activity for at least 20 min once or twice per week | 28 (35.4) | 0 | 16 (20.3) | 0 | 0 | ||
| Vigorous physical activity for at least 20 min more than twice per week | 15 (19.0) | 0 | 31 (39.2) | 0 | 0 | ||
p values were calculated using a paired t test except where indicated by a superscript a, in which case they represent the result obtained using Wilcoxon signed-rank test
bSome participants also reported no response for nerve conduction study and QST measurements at baseline (V1) and follow-up (V2). No response was recorded for peroneal distal motor latency (V1: 3, V2: 3), amplitude (V1: 3, V2: 3), CV (V1: 3, V2: 3), and F wave index (V1: 5, V2: 2), sural peak latency (V1: 6, V2: 6), tibial distal motor latency (V1: 2, V2: 2) amplitude (V1: 2, V2: 2), and F wave index (V2: 1), QST cold (V1: 2, V2: 1) and vibration (V2: 1)
Change in CAN
The primary CAN measure (E/I ratio) was stable (−0.01 ± 0.1, p=0.89) during follow-up (Table 3). CAN symptoms (SAS score) were also stable (+0.3 ± 5.3, p=0.58) during follow-up. In contrast, some secondary HRV outcomes, including sdNN (+7.5 ± 30.4, p = 0.04), rmsSD (+9.3 ± 29.3, p<0.01), pNN50 (+5.7 ± 14.4, p<0.01) and mHR (−9.6 ± 8.7, p<0.01) improved during follow-up, while others (RFA, LFA and LFA/RFA) remained stable. Lastly, we found that the number of participants with CAN (E/I ratio <1.09) was also stable during follow-up (12.5% improved, 80.6% stable, 6.9% worsened; p=0.30).
Change in retinopathy
The primary retinopathy outcome, mean deviation, was stable (−0.2 ± 3.0, p=0.52) during follow-up (Table 3). Both of the secondary retinopathy outcomes, pattern SD (−0.2 ± 0.9, p=0.09) and foveal sensitivity (−0.1 ± 4.5, p=0.91), were stable during follow-up, and the number of participants with clinical retinopathy was also stable (4.0% improved, 94.7% stable, 1.3% worsened; p=0.67).
Change in CKD
We found that eGFR significantly worsened (−3.4 ± 10.0 ml/min per 1.73 m2, p<0.01) during follow-up (Table 3). In contrast, the urine albumin to creatinine ratio was stable (+0.05 ± 0.7 mg/g, p=0.56) during follow-up, as was the number of participants meeting the KDIGO criteria for clinical CKD (1.3% improved, 92.3% stable, 6.4% worsened; p=0.13).
Change in patient-oriented outcomes
Multiple patient-oriented outcomes significantly improved 2 years following bariatric surgery. Specifically, we found that pain as assessed using the McGill VAS (−1.0 ± 2.6, p<0.01) and QOL as assessed using the Neuro-QOL instrument (−0.6 ± 1.9, p<0.01) significantly improved after bariatric surgery (Table 3). We also found that overall QOL, QOL related to problems with feet, obesity-specific QOL (IWQOL-Lite), Neuro-QOL sub-scales specific to pain and social/emotional well-being, patient-reported health status (EuroQOL VAS), health status related to mobility (EQ-5D-3L) and depressive symptoms (IDS-SR) significantly improved (p<0.05). In contrast, Neuro-QOL sub-scales specific to activities of daily living, sensory motor and reduced sensation, EQ-5D-3L scales related to self-care, usual activities, pain/discomfort and anxiety/depression, and self-reported physical activity level did not significantly change during follow-up.
Association between metabolic risk factors and diabetes complications
Linear regression models revealed that no changes in metabolic risk factors were associated with changes in the IENFD of the distal leg or thigh or the E/I ratio (Table 4), and only the change in fasting glucose was significantly associated with an improved mean deviation (point estimate −0.7; 95% CI −1.3, −0.1).
Table 4.
Association between changes in metabolic factors and diabetes complications following bariatric surgery in the 79 patients who completed in-person follow-up
| PN | CAN | Retinopathy | ||
|---|---|---|---|---|
| Variable | IENFD distal leg | IENFD thigh | E/I ratio | Mean deviation |
| Weight | −0.03 (−0.1, 0.02) | −0.03 (−0.13, 0.06) | −0.0002 (−0.002, 0.001) | −0.0003 (−0.04, 0.04) |
| Systolic BP | −0.003 (−0.05, 0.05) | −0.05 (−0.14, 0.03) | −0.000007 (−0.001, 0.001) | −0.02 (−0.05, 0.02) |
| Fasting glucose | 0.74 (−0.3, 1.8) | 0.11 (−1.42, 1.65) | 0.01 (−0.02, 0.03) | −0.7 (−1.3, −0.1) |
| HbA1c (mmol/l) | 0.4 (−0.6, 1.3) | −0.3 (−1.9, 1.3) | −0.01 (−0.03, 0.01) | 0.2 (−0.5, 0.9) |
| HbA1c (%) | 0.2 (−1.4, 1.9) | −0.5 (−3.1, 2.0) | 0.002 (−0.04, 0.04) | 0.3 (−1.0, 1.5) |
| HDL-cholesterol | 1.0 (−2.2, 4.1) | 0.7 (−4.4, 5.9) | 0.01 (−0.06, 0.08) | −0.07 (−2.3, 2.2) |
| Triacylglycerol | 0.6 (−0.9, 2.0) | −1.02 (−3.49, 1.45) | −0.02 (−0.05, 0.02) | −0.2 (−1.3, 0.8) |
| NCEP-defined waist circumference | −0.04 (−0.1, 0.02) | −0.03 (−0.13, 0.07) | 0.0006 (−0.0007, 0.002) | −0.01 (−0.05, 0.03) |
| Arm | 0.04 (−0.2, 0.3) | 0.08 (−0.29, 0.46) | −0.002 (−0.01, 0.003) | −0.002 (−0.2, 0.2) |
| Forearm | 0.2 (−0.2, 0.6) | 0.13 (−0.50, 0.77) | −0.002 (−0.01, 0.007) | −0.1, (−0.4, 0.2) |
| Calf | 0.1 (−0.1, 0.4) | 0.006 (−0.48, 0.49) | −0.001 (−0.008, 0.006) | −0.1 (−0.3, 0.1) |
| Mid-thigh | 0.04 (−0.1, 0.2) | −0.01 (−0.25, 0.26) | −0.0002 (−0.003, 0.003) | 0.007 (−0.09, 0.1) |
| Hips/thigh | 0.04 (−0.1, 0.2) | −0.02 (−0.21, 0.17) | −0.0004 (−0.003, 0.002) | −0.4 (−0.1, 0.04) |
| Abdomen | −0.01 (−0.1, 0.05) | −0.03 (−0.13, 0.07) | 0.0006 (−0.001, 0.002) | −0.01 (−0.05, 0.03) |
| Buttocks/hips | 0.01 (−0.1, 0.1) | −0.05 (−0.19, 0.1) | 0.0004 (−0.002, 0.002) | −0.008 (−0.07, 0.06) |
| High-waist | 0.05 (−0.1, 0.1) | 0.03 (−0.07, 0.1) | 0.00001 (−0.001, 0.001) | −0.03 (−0.08, 0.02) |
Values are point estimates (95% CI)
Each row represents a single model adjusting for age, sex, baseline BMI and baseline outcomes
Discussion
In a prospective cohort study of individuals with class II/III obesity, we found that bariatric surgery successfully improved all metabolic risk factors except BP and total cholesterol, including a mean weight loss of over 30 kg 2 years following bariatric surgery. We also found that significantly fewer individuals were taking anti-hypertensive medications following bariatric surgery. Two years after bariatric surgery, we found that one of two primary PN outcomes improved, IENFD in the proximal thigh, whereas the other primary outcome, IENFD in the distal leg, was stable. The primary CAN (E/I ratio) and retinopathy (mean deviation using FDT) outcomes also remained stable, and multiple secondary CAN outcomes improved. Our findings probably indicate an improvement compared with the natural history of worsening PN, CAN and retinopathy over time [32–34]. We found that changes in fasting glucose were associated with improvements in retinopathy, but no other metabolic changes correlated with measurements of diabetes complications. Lastly, we found that bariatric surgery ameliorated multiple patient-oriented outcomes, including QOL, pain and depression.
Two years after bariatric surgery, we found that one of our two primary PN outcomes, two secondary PN outcomes and several PN-related patient-oriented outcomes improved after surgery. As far as we are aware, this is the largest study to assess IENFD before and after bariatric surgery. Our finding of improved PN is consistent with the results of two other small studies, which assessed IENFD and corneal nerve fibre density after bariatric surgery. The first small study (n=11) assessed the change in IENFD at the distal leg 12 months after bariatric surgery, and found an improvement that was not statistically significant [35]. A second small study (n=26) found that bariatric surgery significantly ameliorated corneal nerve fibre density during the 12-month follow-up period [36]. Taken together, our results from the present study, together with those from these small studies [35, 36], indicate that bariatric surgery probably enables regeneration of peripheral nerves, and therefore may be an effective therapy for individuals with obesity. On the other hand, in the present study, the IENFD of the distal leg was stable after bariatric surgery, indicating that reversing damage to the more severely affected distal nerves is more difficult or may require longer follow-up.
Our study also found that the score for the MNSI questionnaire significantly improved, adding to the growing evidence that bariatric surgery improves PN symptoms, as detailed in a recent systematic review, that assessed PN symptoms with the Neuropathy Symptoms Score [10]. On the other hand, the scores for the tibial distal motor latency measure, the QST vibration threshold and vibration perception threshold from neurothesiometer testing worsened, and more participants met the Toronto Consensus Definition for probable PN. One possible explanation for these conflicting results is that bariatric surgery improves small-fibre nerves but does not prevent worsening in large-fibre nerves. Another possibility is that bariatric surgery ameliorates neuropathy in some patients by improving the metabolic profile, but worsens neuropathy in others as a result of nutritional deficiencies, e.g. vitamin B12 deficiency. Further studies are needed to address the reason for the differential effects on outcomes, but it is important to note that the PN-related patient-oriented outcomes all improved after bariatric surgery.
In contrast to previous studies [12, 13], we were surprised to find that primary CAN and retinopathy outcomes were stable after bariatric surgery. One possibility is that, for individuals with long-term metabolic impairment, even a substantial improvement in metabolic risk factors late in the course of disease may not suffice to significantly reverse even mild autonomic and retinal nerve damage. Another possibility is that the effects of bariatric surgery on retinopathy and CAN outcomes take longer than 2 years to manifest. Therefore, future studies with longer-term follow-up are needed. Alternatively, a meta-analysis found that bariatric surgery can result in short-term progression of retinopathy for those already diagnosed with proliferative retinopathy [13]. Thus, stability in retinopathy outcomes may result from initial progression followed by improvement; however, we did not measure retinopathy outcomes throughout the study to assess this possibility. Importantly, given that the natural history of CAN and retinopathy is to worsen over time [33, 34], the observed stability of primary outcomes in this study probably represents an improvement, but controlled studies are needed to provide more definitive evidence.
We also observed improvement in multiple secondary CAN outcomes, including four HRV measures (sdNN, rmsSD, pNN50 and mHR). Notably, this is the third study to observe improvements in sdNN and rmsSD [11, 37], perhaps suggesting that these longer-term HRV measures may be sensitive or early indicators of CAN improvement. As each of these measures is associated with a greater risk of mortality, these outcomes are clinically relevant [38]. Future controlled studies are needed to confirm this important finding, and longer-term studies are required to determine whether HRV improvement eventually reduces the risk of silent myocardial infarction and death. Our results indicate that, for individuals with obesity, bariatric surgery may be an effective approach to reverse peripheral nerve injury and slow the progression of CAN and retinopathy.
While bariatric surgery is one potential intervention to simultaneously improve multiple metabolic risk factors, another comparable intervention is medical weight loss. We previously studied PN and CAN outcomes in 72 participants with obesity 2 years after medical weight loss using a similar study design [21]. We found that medical weight loss (MWL) more modestly improved the metabolic profile compared with bariatric surgery, with 10.3% weight loss for MWL vs 23.8% for bariatric surgery, a reduction in the National Cholesterol Education Program (NCEP)-defined waist circumference of 7.3% for MWL vs 12.5% for bariatric surgery, a reduction in triacylglycerol of 17.7% for MWL vs 19.1% bariatric surgery, increases in HDL-cholesterol of 11.5% for MWL vs 38.6% for bariatric surgery, and a reduction in HbA1c of 5.0% for MWL vs 7.5% for bariatric surgery. MWL stabilised IENFD outcomes, whereas bariatric surgery improved the IENFD of the proximal thigh. Interestingly, both studies found an improvement in PN symptoms, as measured by a reduction in the score for the MNSI questionnaire (21.4% for MWL vs 25.8% for bariatric surgery), although it was more robust after bariatric surgery. Primary CAN outcomes were stable after both interventions, but only bariatric surgery improved multiple secondary HRV outcomes.
Our finding that bariatric surgery improves PN and CAN outcomes to a greater extent than MWL may result from several possibilities. Bariatric surgery improved metabolic abnormalities by greater magnitude and more sustainably, with a weight loss of 31.0 kg for bariatric surgery vs 12.4 kg for MWL, which may directly improve PN and CAN through a dose–response relationship. Alternatively, effects of bariatric surgery that are not related to weight loss may be important. Improved hunger-related behaviours and increased levels of gut satiety hormones have been shown to exert additional pleiotropic effects that have a potential impact on these outcomes. Further, changes that directly influence glucose control, such as increased gastric emptying, modulated bile acids or changes to gut microbiota, may ultimately improve PN and CAN to a greater extent compared with MWL [39]. Regardless of the underlying mechanism, bariatric surgery is a promising disease-modifying therapy for PN, CAN and retinopathy in individuals with obesity.
Simultaneously measuring changes in PN, CAN and retinopathy allowed us to determine whether changes in specific metabolic risk factors exert differential effects on diabetes complications. Improvements in fasting glucose were associated with an improved retinopathy outcome. The relative importance of hyperglycaemia compared with obesity and other metabolic measures for progression of retinopathy is consistent with findings from our previous study [5]. Specifically, in our baseline study in this same population, we found that the prevalence of retinopathy was only higher in obese patients with hyperglycaemia compared with lean control participants, whereas the prevalence of PN and CAN is increased even in obese participants with normoglycaemia [5]. Therefore, the present study adds to the growing body of evidence that, for retinopathy, controlling hyperglycaemia is probably more important than controlling obesity, whereas controlling both hyperglycaemia and obesity may both be necessary for PN and CAN [40]. Given the importance of central obesity measures for PN and CAN in this same population at baseline [5, 7], we were surprised to find that specific changes in measures of central obesity were not differentially associated with either complication. Regression modelling found no relationship between reduced NCEP-defined waist circumference and improved IENFD of the proximal thigh. It is possible that our study lacked statistical power to detect an effect, but another possibility is that the metabolic improvements were secondary to other factors.
Study limitations included a relatively small sample size and lack of a control group. We also had significant loss to follow-up, although, importantly, there were no baseline demographic differences between participants who completed follow-up and those who did not. Prior to the COVID-19 pandemic, our study was on track to retain 85.0% of participants, but nevertheless had a final 79.5% retention rate when including virtual visits. As that we only assessed diabetes complications at baseline and after 2 years of follow-up, the shorter and longer-term effects of bariatric surgery are unknown. In addition, with the present study design, we are unable to make causal claims. Furthermore, our primary assessments of PN included two small-fibre measurements, and the effects of bariatric surgery on large-fibre PN require future study.
In conclusion, we found that, 2 years after bariatric surgery and the resulting substantial improvements in metabolic risk factors, one of two primary measures of PN, plus pain and QOL improved, and CAN and retinopathy were stable. Our study demonstrates that bariatric surgery may be an effective approach to reverse PN in individuals with obesity, either through the direct impact of metabolic improvement, or other beneficial effects of bariatric surgery. Given the natural history of worsening CAN and retinopathy, stability in these complications probably indicates a successful result; however, randomised controlled trials are needed to confirm these findings. For retinopathy, a specific reduction in hyperglycaemia following bariatric surgery is probably required to reverse this complication.
Acknowledgements
Some of the data were presented as an abstract at the 2022 Peripheral Nerve Society Annual Meeting and at the American Diabetes Association 82nd Scientific Sessions.
Authors’ relationships and activities
RP-B receives research support from Novo Nordisk. BCC consults for DynaMed, receives research support from the American Academy of Neurology and performs medical legal consultations including consultations for the Vaccine Injury Compensation Program. The remaining authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
ELR, MB, EC, EV-U, MAE, TWG, RP-B, SP, ELF and BCC were involved in the study design. ELR and MW performed and interpreted the statistical analysis. MB, MAE, TWG, RP-B and SP were involved in interpretation of the data. BCC was involved in interpretation of the statistical analysis. ELR wrote the manuscript and MW, MB, EC, EV-U, MAE, TWG, RP-B, SP and BCC provided critical revisions of the manuscript. BCC is the guarantor of this work. All authors provided final approval of the manuscript.
Abbreviations
- AUDIT
Alcohol Use Disorders Identification Test
- CAN
Cardiovascular autonomic neuropathy
- CKD
Chronic kidney disease
- E/I
Expiration/inspiration
- EQ-5D-3L
European Quality of Life 5 Dimensions 3 Level Version
- FDT
Frequency doubling technology
- HRV
Heart rate variability
- IDS-SR
Inventory of Depressive Symptomatology Self Report
- IENFD
Intra-epidermal nerve fibre density
- IWQOL-Lite
Impact of Weight on Quality of Life questionnaire
- KDIGO
Kidney Disease: Improving Global Outcomes
- LFA
Low frequency area
- mHR
Median heart rate
- MNSI
Michigan Neuropathy Screening Instrument
- MWL
Medical weight loss
- NCEP
National Cholesterol Education Program
- Neuro-QOL
Neuropathy-specific quality of life instrument
- PN
Peripheral neuropathy
- pNN50
Proportion of the number of pairs of successive normal-to-normal intervals that differ by more than 50 ms divided by the total number of normal-to-normal intervals
- QOL
Quality of life
- QST
Quantitative sensory testing
- RFA
Respiratory frequency area
- rmsSD
Root mean square of successive differences of the normal-to-normal interval
- SAS
Survey of Autonomic Symptoms
- sdNN
SD of the normal-to-normal interval
- UENS
Utah Early Neuropathy Scale
- VAS
Visual analogue scale
Funding
The project described was supported by grant number P30DK020572 (MDRC) from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). ELR is supported by the NIH NIDDK (K99DK129785). MAE is supported by the NIH NINDS (5R25NS089450). TWG is supported by the JDRF Center of Excellence at the University of Michigan and the A. Alfred Taubman Medical Research Institute. RP-B is supported by the NIH NIDDK (R01DK107956, U01DK119083, 1U01 DK0945157, R01DK116723) and the JDRF Center of Excellence at the University of Michigan. SP is supported by the NIH NIDDK (P30DK081943, P30DK89503). ELF is supported by the NIH (U01AG057562, U24DK115255, R01DK130913), the Robert E. Nederlander Sr Program for Alzheimer’s Research, the Andrea and Lawrence A. Wolfe Brain Health Initiative Fund, the A. Alfred Taubman Medical Research Institute and the NeuroNetwork for Emerging Therapies. BCC is supported by the NIH NIDDK (R01DK115687).
Data availability
Study datasets are available from the corresponding author on reasonable request.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association Professional Practice Committee 12. Retinopathy, neuropathy, and foot care: standards of medical care in diabetes – 2022. Diabetes Care. 2022;45(Suppl 1):S185–S194. doi: 10.2337/dc22-S012. [DOI] [PubMed] [Google Scholar]
- 3.Callaghan BC, Gao L, Li Y, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol. 2018;5(4):397–405. doi: 10.1002/acn3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams SM, Eleftheriadou A, Alam U, Cuthbertson DJ, Wilding JPH. Cardiac autonomic neuropathy in obesity, the metabolic syndrome and prediabetes: a narrative review. Diabetes Ther. 2019;10(6):1995–2021. doi: 10.1007/s13300-019-00693-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callaghan BC, Reynolds EL, Banerjee M, et al. The prevalence and determinants of cognitive deficits and traditional diabetic complications in the severely obese. Diabetes Care. 2020;43(3):683–690. doi: 10.2337/dc19-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Y, Wang C, Shi K, Yin X. Relation of metabolic syndrome and its components with risk of diabetic retinopathy: a meta-analysis of observational studies. Medicine (Baltimore) 2018;97(38):e12433. doi: 10.1097/MD.0000000000012433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callaghan BC, Reynolds E, Banerjee M, Chant E, Villegas-Umana E, Feldman EL. Central obesity is associated with neuropathy in the severely obese. Mayo Clin Proc. 2020;95(7):1342–1353. doi: 10.1016/j.mayocp.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakobsen GS, Småstuen MC, Sandbu R, et al. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA. 2018;319(3):291. doi: 10.1001/jama.2017.21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien R, Johnson E, Haneuse S, et al. Microvascular outcomes in patients with diabetes after bariatric surgery versus usual care: a matched cohort study. Ann Intern Med. 2018;169(5):300–310. doi: 10.7326/M17-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aghili R, Malek M, Tanha K, Mottaghi A. The effect of bariatric surgery on peripheral polyneuropathy: a systematic review and meta-analysis. Obes Surg. 2019;29(9):3010–3020. doi: 10.1007/s11695-019-04004-1. [DOI] [PubMed] [Google Scholar]
- 11.Casellini CM, Parson HK, Hodges K, et al. Bariatric surgery restores cardiac and sudomotor autonomic C-fiber dysfunction towards normal in obese subjects with type 2 diabetes. PLoS One. 2016;11(5):e0154211. doi: 10.1371/journal.pone.0154211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maser RE, Lenhard MJ, Irgau I, Wynn GM. Impact of surgically induced weight loss on cardiovascular autonomic function: one-year follow-up. Obesity. 2007;15(2):364–369. doi: 10.1038/oby.2007.554. [DOI] [PubMed] [Google Scholar]
- 13.Yu CW, Park LJ, Pinto A, et al. The impact of bariatric surgery on diabetic retinopathy: a systematic review and meta-analysis. Am J Ophthalmol. 2021;225:117–127. doi: 10.1016/j.ajo.2020.12.033. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association Professional Practice Committee 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Suppl 1):S17–S38. doi: 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 15.Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17(7):903–912. doi: 10.1111/j.1468-1331.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- 16.Callaghan BC, Xia R, Reynolds E, et al. Better diagnostic accuracy of neuropathy in obesity: a new challenge for neurologists. Clin Neurophysiol. 2018;129(3):654–662. doi: 10.1016/j.clinph.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herman WH, Pop-Busui R, Braffett BH, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29(7):937–944. doi: 10.1111/j.1464-5491.2012.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singleton JR, Bixby B, Russell JW, et al. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst. 2008;13(3):218–227. doi: 10.1111/j.1529-8027.2008.00180.x. [DOI] [PubMed] [Google Scholar]
- 19.Bril V, Kojic J, Ngo M, Clark K. Comparison of a neurothesiometer and vibration in measuring vibration perception thresholds and relationship to nerve conduction studies. Diabetes Care. 1997;20(9):1360–1362. doi: 10.2337/diacare.20.9.1360. [DOI] [PubMed] [Google Scholar]
- 20.Feng Y, Schlösser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg. 2009;50(3):675–682. doi: 10.1016/j.jvs.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Callaghan BC, Reynolds EL, Banerjee M, et al. Dietary weight loss in people with severe obesity stabilizes neuropathy and improves symptomatology. Obesity (Silver Spring) 2021;29(12):2108–2118. doi: 10.1002/oby.23246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tesfaye S, Boulton AJM, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spallone V, Ziegler D, Freeman R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27(7):639–653. doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 24.Zilliox L, Peltier AC, Wren PA, et al. Assessing autonomic dysfunction in early diabetic neuropathy: the Survey of Autonomic Symptoms. Neurology. 2011;76(12):1099–1105. doi: 10.1212/WNL.0b013e3182120147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joltikov KA, de Castro VM, Davila JR, et al. Multidimensional functional and structural evaluation reveals neuroretinal impairment in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58(6):BIO277–BIO290. doi: 10.1167/iovs.17-21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79(2):268–288. doi: 10.1053/j.ajkd.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Vileikyte L, Peyrot M, Bundy C, et al. The development and validation of a neuropathy- and foot ulcer-specific quality of life instrument. Diabetes Care. 2003;26(9):2549–2555. doi: 10.2337/diacare.26.9.2549. [DOI] [PubMed] [Google Scholar]
- 28.Grafton KV, Foster NE, Wright CC. Test–retest reliability of the Short-Form McGill Pain Questionnaire: assessment of intraclass correlation coefficients and limits of agreement in patients with osteoarthritis. Clin J Pain. 2005;21(1):73–82. doi: 10.1097/00002508-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1986;18(1):65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 30.Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9(2):102–111. doi: 10.1038/oby.2001.13. [DOI] [PubMed] [Google Scholar]
- 31.Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res. 2005;29(5):844–854. doi: 10.1097/01.alc.0000164374.32229.a2. [DOI] [PubMed] [Google Scholar]
- 32.Balducci S, Iacobellis G, Parisi L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20(4):216–223. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Karamitsos DT, Didangelos TP, Athyros VG, Kontopoulos AG. The natural history of recently diagnosed autonomic neuropathy over a period of 2 years. Diabetes Res Clin Pract. 1998;42(1):55–63. doi: 10.1016/s0168-8227(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 34.Meira-Freitas D, Tatham AJ, Lisboa R, et al. Predicting progression of glaucoma from rates of frequency doubling technology perimetry change. Ophthalmology. 2014;121(2):498–507. doi: 10.1016/j.ophtha.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith AG, Graham T, Volckmann E, et al. Bariatric surgery improves peripheral nerve function and intraepidermal nerve fiber density in obese patients without symptomatic neuropathy. Neurology. 2016;86(16 Suppl):1.144. [Google Scholar]
- 36.Azmi S, Ferdousi M, Liu Y, et al. Bariatric surgery leads to an improvement in small nerve fibre damage in subjects with obesity. Int J Obes. 2021;45(3):631–638. doi: 10.1038/s41366-020-00727-9. [DOI] [PubMed] [Google Scholar]
- 37.Jang HN, Moon S, Moon JH et al (2022) 462-P: improvement of cardiovascular autonomic neuropathy after metabolic bariatric surgery in Korean subjects with obesity. Diabetes 71(Suppl 1):462-P. 10.2337/db22-462-P
- 38.Pop-Busui R, Evans GW, Gerstein HC, et al. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. Diabetes Care. 2010;33(7):1578–1584. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornejo-Pareja I, Clemente-Postigo M, Tinahones FJ. Metabolic and endocrine consequences of bariatric surgery. Front Endocrinol. 2019;10:626. doi: 10.3389/fendo.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Study datasets are available from the corresponding author on reasonable request.