Abstract
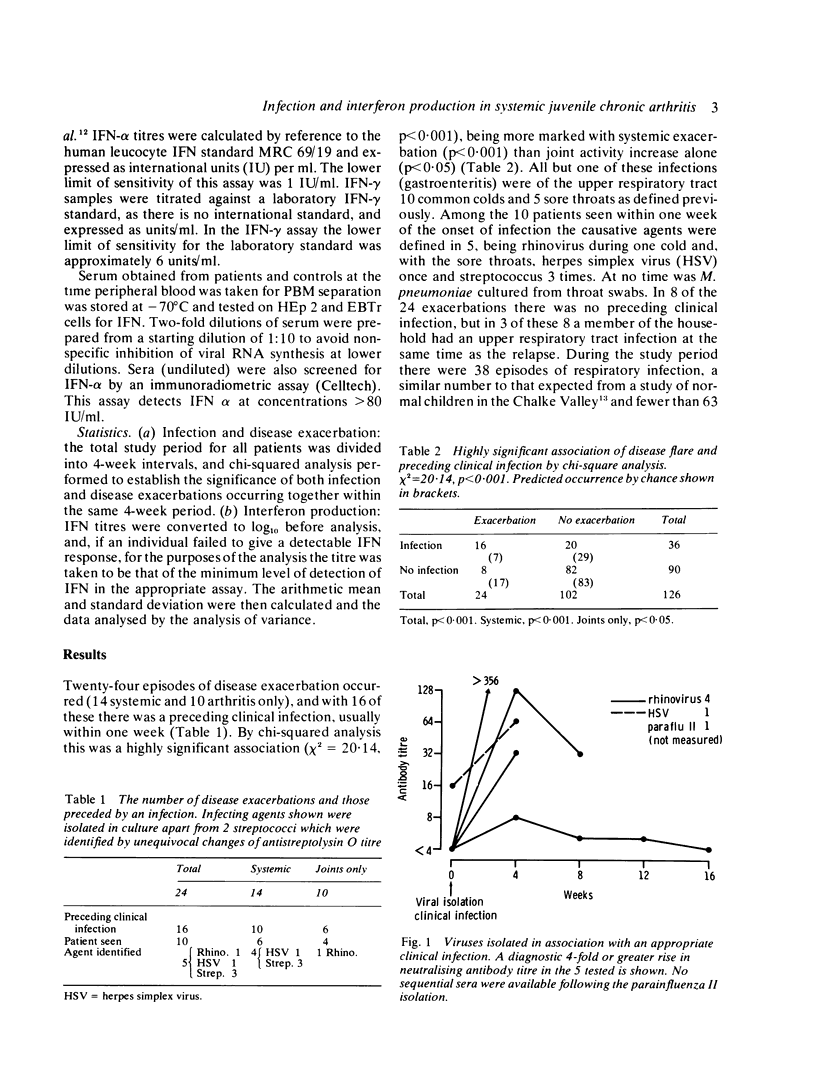
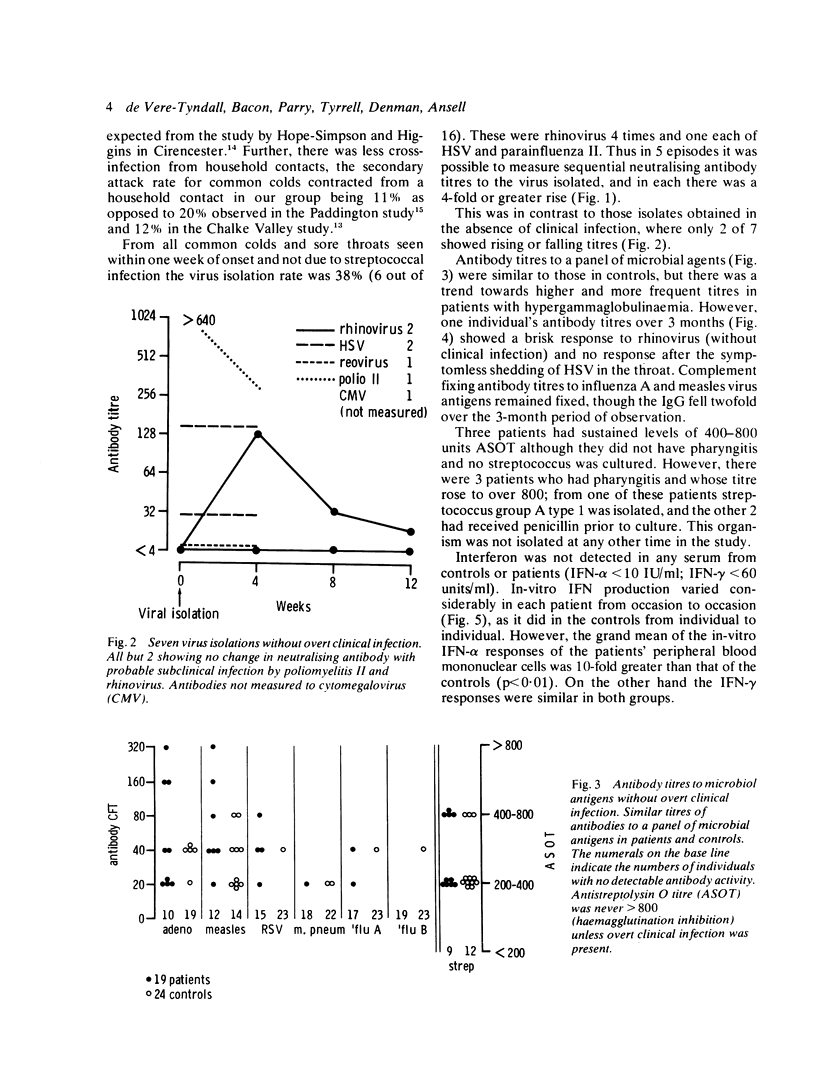
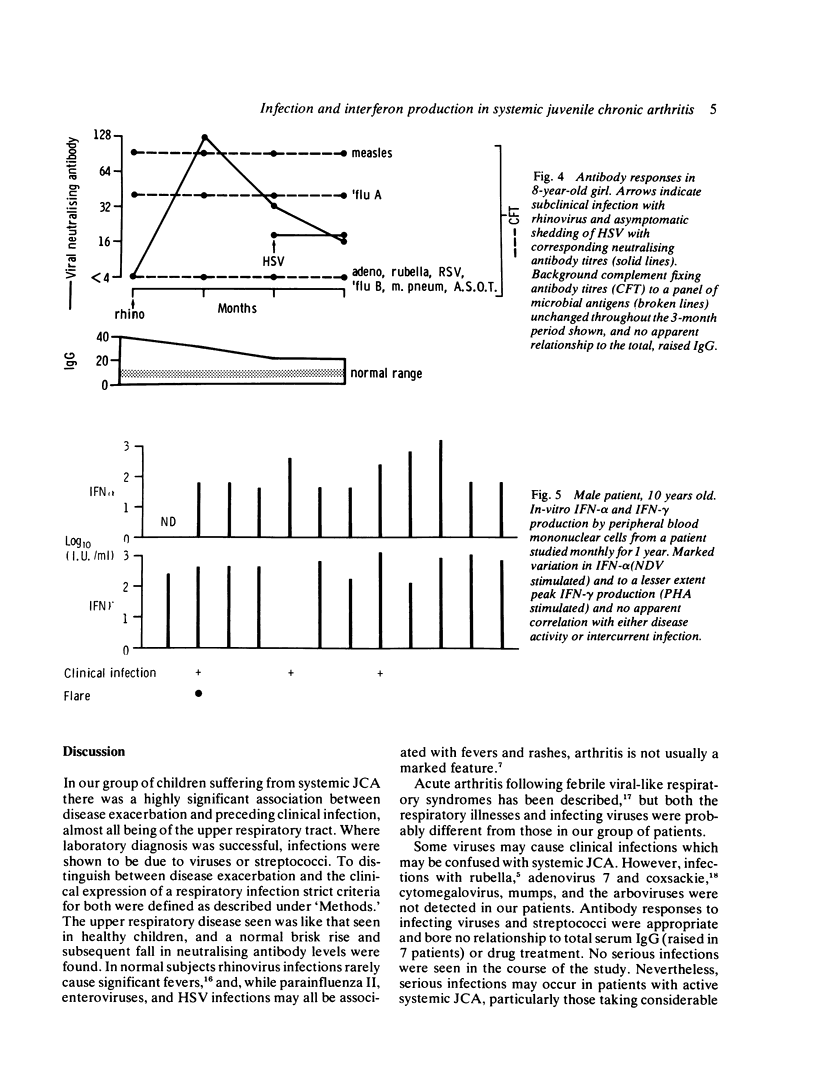
Twenty-four episodes of disease exacerbation in 19 children suffering from systemic juvenile chronic arthritis were studied. Sixteen of these were preceded by an infection (chi 2 = 20.14, p less than 0.001), mostly of the upper respiratory tract. In the 10 cases seen during an infection causative agents were identified in 5 (herpes simplex, rhinovirus, and on 3 occasions streptococcus). The total number of infections was not increased when compared with infection rates predicted by several reported studies. In the absence of clinical infection, specific antibody titres to a panel of microbial antigens were similar to those of a control group but with a trend toward higher titres in patients with hypergammaglobulinaemia. Interferon (IFN) responses were not defective, though sequential in-vitro IFN production from peripheral blood mononuclear cells (PBM) fluctuated considerably in the same patients, occasionally being absent with no obvious clinical correlate. IFN-alpha was induced by stimulation with Newcastle disease virus (NDV), and the mean responses of the patients were significantly greater than those of controls. IFN-gamma production on phytohaemagglutinin (PHA) stimulation was similar in patients and control groups. IFN was not detected in any of the sera from patients or controls.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Schreier M. H., Melchers F. T-cell-dependent B-cell stimulation is H-2 restricted and antigen dependent only at the resting B-cell level. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1612–1616. doi: 10.1073/pnas.77.3.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson Y. J., Winter H. S., Gard S. E., Fischer T. J., Stiehm E. R. Deficiency of immune interferon production by leukocytes of normal newborns. Cell Immunol. 1980 Sep 15;55(1):191–200. doi: 10.1016/0008-8749(80)90150-1. [DOI] [PubMed] [Google Scholar]
- Cassidy J. T., Shillis J. L., Brandon F. B., Sullivan D. B., Brackett R. G. Viral antibody titers to rubella and rubeola in juvenile rheumatoid arthritis. Pediatrics. 1974 Aug;54(2):239–244. [PubMed] [Google Scholar]
- Chantler J. K., Ford D. K., Tingle A. J. Persistent rubella infection and rubella-associated arthritis. Lancet. 1982 Jun 12;1(8285):1323–1325. doi: 10.1016/s0140-6736(82)92398-4. [DOI] [PubMed] [Google Scholar]
- Frick O. L., German D. F., Mills J. Development of allergy in children. I. Association with virus infections. J Allergy Clin Immunol. 1979 Apr;63(4):228–241. doi: 10.1016/0091-6749(79)90106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame R., Armstrong R., Simmons N. A., Mims C. A., Wilton J. M., Laurent R. Isolation of rubella virus from synovial fluid in five cases of seronegative arthritis. Lancet. 1981 Sep 26;2(8248):649–651. doi: 10.1016/s0140-6736(81)90993-4. [DOI] [PubMed] [Google Scholar]
- Hasony H. J., Macnaughton M. R. Prevalence of human coronavirus antibody in the population of southern Iraq. J Med Virol. 1982;9(3):209–216. doi: 10.1002/jmv.1890090308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope-Simpson R. E., Higgins P. G. A respiratory virus study in Great Britain: review and evaluation. Prog Med Virol. 1969;11:354–407. [PubMed] [Google Scholar]
- Isaacs D., Clarke J. R., Tyrrell D. A., Webster A. D., Valman H. B. Deficient production of leucocyte interferon (interferon-alpha) in vitro and in vivo in children with recurrent respiratory tract infections. Lancet. 1981 Oct 31;2(8253):950–952. doi: 10.1016/s0140-6736(81)91153-3. [DOI] [PubMed] [Google Scholar]
- LIDWELL O. M., SOMMERVILLE T. Observations on the incidence and distribution of the common cold in a rural community during 1948 and 1949. J Hyg (Lond) 1951 Dec;49(4):365–381. doi: 10.1017/s0022172400066699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis H. M., Parry J. V., Davies H. A., Parry R. P., Mott A., Dourmashkin R. R., Sanderson P. J., Tyrrell D. A., Valman H. B. A year's experience of the rotavirus syndrome and its association with respiratory illness. Arch Dis Child. 1979 May;54(5):339–346. doi: 10.1136/adc.54.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams M., Finkelstein M. S., Allen P. T., Giron D. J. Assay of chick interferons by the inhibition of viral ribonucleic acid synthesis. Appl Microbiol. 1971 May;21(5):959–961. doi: 10.1128/am.21.5.959-961.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto A. S., Bryan E. R. Microneutralization test for detection of rhinovirus antibodies. Proc Soc Exp Biol Med. 1974 Feb;145(2):690–694. doi: 10.3181/00379727-145-37876. [DOI] [PubMed] [Google Scholar]
- Rahal J. J., Millian S. J., Noriega E. R. Coxsackievirus and adenovirus infection. Association with acute febrile and juvenile rheumatoid arthritis. JAMA. 1976 Jun 7;235(23):2496–2501. doi: 10.1001/jama.235.23.2496. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Iwamoto K., Adam E., Melnick J. L. Measurement of antibodies to herpesvirus types 1 and 2 in human sera. J Immunol. 1970 Mar;104(3):599–606. [PubMed] [Google Scholar]
- TYRRELL D. A. Clinical clues in virus infections. Br Med J. 1963 Feb 23;1(5329):493–496. doi: 10.1136/bmj.1.5329.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingle A. J., Ford D. K., Price G. E., Kettyls D. W. Prolonged arthritis in identical twins after rubella immunization. Ann Intern Med. 1979 Feb;90(2):203–204. doi: 10.7326/0003-4819-90-2-203. [DOI] [PubMed] [Google Scholar]


