Abstract
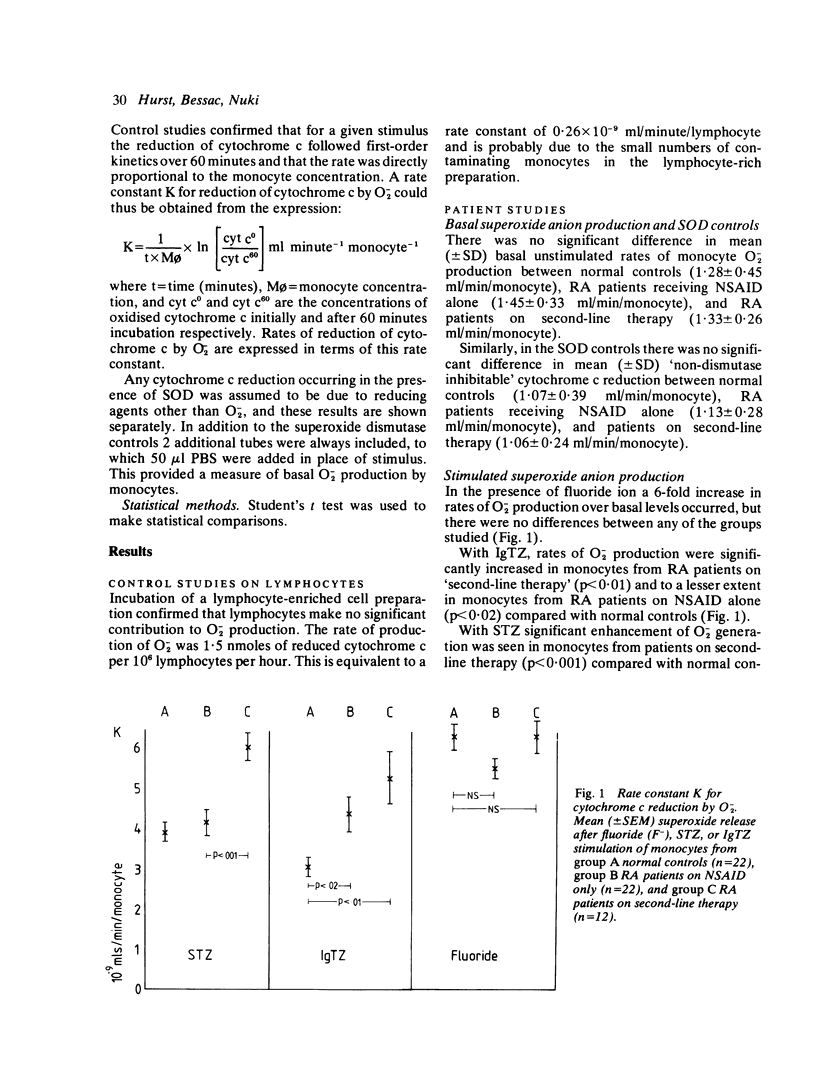
In-vitro studies of superoxide (O-2) anion production by blood monocytes after stimulation with either serum treated zymosan (STZ), IgG treated zymosan (IgGTZ), or fluoride ion (F-) were performed on cells from normal controls (n = 22) and patients with classical or definite rheumatoid arthritis (RA) (n = 35). Twenty-two of the patients were on nonsteroidal anti-inflammatory drugs (NSAID) alone and 13 were on either sodium aurothiomalate, penicillamine, corticosteroids, or a combination. Monocytes from RA patients on 'second-line therapy' showed significantly increased rates of O-2 release in response to STZ compared with normal controls, but no increase was seen in monocytes from patients on NSAID alone. With IgGTZ as the stimulus, rates of O-2 release were increased in monocytes from patients on NSAID alone compared with normal controls (p less than 0.02), but were increased to a greater extent in monocytes from patients on second-line therapy (p less than 0.01). There were no differences in basal unstimulated O-2 production and no differences after stimulation with F-. The enhanced release of O-2 by monocytes from patients on second-line therapy could not be attributed to increased disease activity and may be an effect of therapy.
Full text
PDF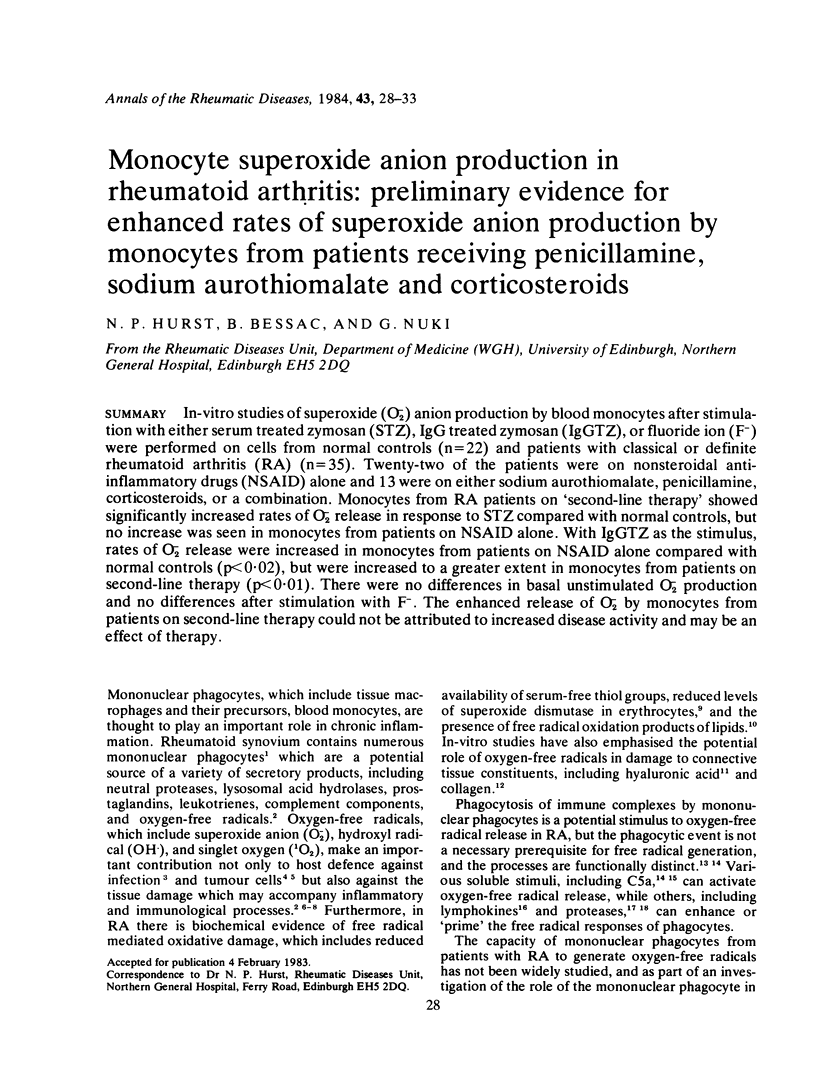



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 1958 REVISION of diagnostic criteria for rheumatoid arthritis. Arthritis Rheum. 1959 Feb;2(1):16–20. doi: 10.1002/1529-0131(195902)2:1<16::aid-art1780020104>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Abramson S., Hoffstein S. T., Weissmann G. Superoxide anion generation by human neutrophils exposed to monosodium urate. Arthritis Rheum. 1982 Feb;25(2):174–180. doi: 10.1002/art.1780250210. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Banford J. C., Brown D. H., Hazelton R. A., McNeil C. J., Sturrock R. D., Smith W. E. Serum copper and erythrocyte superoxide dismutase in rheumatoid arthritis. Ann Rheum Dis. 1982 Oct;41(5):458–462. doi: 10.1136/ard.41.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Kragballe K. Role of oxygen in antibody-dependent cytotoxicity mediated by monocytes and neutrophils. J Clin Invest. 1980 Oct;66(4):676–683. doi: 10.1172/JCI109904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Studies on the mechanism of antibody-dependent polymorphonuclear leukocyte-mediated cytotoxicity. J Immunol. 1977 Oct;119(4):1413–1418. [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M., Karnovsky M. L. Fluoride-mediated activation of the respiratory burst in human neutrophils. A reversible process. J Clin Invest. 1979 Apr;63(4):637–647. doi: 10.1172/JCI109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Inhibition of collagen gelation by action of the superoxide radical. Arthritis Rheum. 1979 Mar;22(3):251–259. doi: 10.1002/art.1780220307. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A. Oxy radicals and connective tissue. J Rheumatol. 1981 Mar-Apr;8(2):185–187. [PubMed] [Google Scholar]
- Hoch S., Schur P. H. Monocyte receptor function in patients with rheumatoid arthritis. Arthritis Rheum. 1981 Oct;24(10):1268–1267. doi: 10.1002/art.1780241006. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Chadwick D. A., Cohn Z. A. Priming of macrophages for enhanced oxidative metabolism by exposure to proteolytic enzymes. J Exp Med. 1981 Jun 1;153(6):1678–1683. doi: 10.1084/jem.153.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Takaku F., Sakamoto S. Evidence that proteases are involved in superoxide production by human polymorphonuclear leukocytes and monocytes. J Clin Invest. 1980 Jan;65(1):74–81. doi: 10.1172/JCI109662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kávai M., Lukács K., Sonkoly I., Páloczi K., Szegedi G. Circulating immune complexes and monocyte Fc function in autoimmune diseases. Ann Rheum Dis. 1979 Feb;38(1):79–83. doi: 10.1136/ard.38.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos H., Blok-Schut B., Kipp B., van Doorn R., Meerhof L. Size distribution, electronic recognition, and counting of human blood monocytes. Blood. 1976 Nov;48(5):743–753. [PubMed] [Google Scholar]
- Lunec J., Halloran S. P., White A. G., Dormandy T. L. Free-radical oxidation (peroxidation) products in serum and synovial fluid in rheumatoid arthritis. J Rheumatol. 1981 Mar-Apr;8(2):233–245. [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McCormick J. R., Harkin M. M., Johnson K. J., Ward P. A. Suppression by superoxide dismutase of immune-complex--induced pulmonary alveolitis and dermal inflammation. Am J Pathol. 1981 Jan;102(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- Munthe E., Kåss E., Jellum E. D-penicillamine-induced increase in intracellular glutathione correlating to clinical response in rheumatoid arthritis. J Rheumatol Suppl. 1981 Jan-Feb;7:14–19. [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Mehta J., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: effect of metabolic inhibitors and antiinflammatory drugs. Arthritis Rheum. 1979 Jul;22(7):755–763. doi: 10.1002/art.1780220711. [DOI] [PubMed] [Google Scholar]
- Walker L., Lowrie D. B. Killing of Mycobacterium microti by immunologically activated macrophages. Nature. 1981 Sep 3;293(5827):69–71. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]
- Waytz P. H., Douglas S. D. Increased antibody-dependent cell-mediated cytotoxicity by monocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1979 May;22(5):490–494. doi: 10.1002/art.1780220508. [DOI] [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- van Furth R. The origin of phagocytic cells in the joint and bone. Scand J Rheumatol Suppl. 1981;40:13–20. doi: 10.3109/03009748109102871. [DOI] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]


