Abstract
Phospholipase A2 (PLA2) is a membrane lytic enzyme that is present in many organisms. Human PLA2 has emerged as a potential biomarker as well as a therapeutic target for several diseases including cancer, cardiovascular diseases, and some inflammatory diseases. The current study focuses on the development of lipo-beads that are very reactive and highly sensitive to PLA2. To develop the best supported lipid bilayer formulation, several lipid combinations were investigated using 10 μm porous silica beads. The reactivity of PLA2 was monitored via the decrease in particle fluorescence because of the release of entrapped fluorescent dye from the particle pores or the disintegration of a fluorescent lipid constituted on the bilayer upon lipid hydrolysis using flow cytometry. The enzyme binding studies indicate that lipo-beads with bulky fluorescent tags in the lipid head group and anionic lipids produce a more pronounced response. The kinetic studies suggest that these lipo-beads are very reactive with PLA2 and can generate a detectable signal in less than 5 min. The enzyme inhibition studies were also conducted with two known PLA2 inhibitors, varespladib and quercetin. We find that quercetin can hydrolyze the supported membrane, and thus inhibition of PLA2 is not observed; however, varespladib has shown significant PLA2 inhibition on lipo-beads. We have demonstrated that our lipo-bead-based approach can detect annexin-3, a known disease biomarker, as low as 10 nM within 5 min after incubation.
Keywords: phospholipase A2, annexin A-3, lipo-beads, supported lipid bilayers, flow cytometry
Graphical Abstract
INTRODUCTION
The human phospholipase A2 (PLA2) enzyme has emerged as a potential diagnostic biomarker for several diseases including breast, colon, pancreatic, lung, and prostate cancer and cardiovascular diseases and as a therapeutic target for pancreatitis, sepsis, atherosclerosis, and several other inflammatory diseases.1–10 Although overexpressed PLA2 in biological fluids and tissues under diseased conditions enables the enzyme to be targeted as a disease marker, under pathogenic conditions, PLA2 is directly or indirectly responsible for the progression of several diseases. PLA2 catalyzes the hydrolysis of phospholipid membranes; thus, the investigation of PLA2–membrane interaction will provide opportunities to develop new methods of disease diagnostics and in vitro analysis in drug development.
There are six main variants of PLA2, and their functions in the human body are different;11 yet, all six variants of PLA2 are capable of hydrolyzing the sn-2 position of the fatty acid chain of phospholipids present in cell membranes and produce arachidonic acids and lysophospholipids.11–13 Besides, the specificity of PLA2 from different sources for a given phospholipid is similar.5 Thus, a common approach that exploits the PLA2–membrane interaction can be utilized to detect any variant of PLA2.
Biological cells can be used as substrates to study the enzyme–membrane interaction; however, the presence of proteins, carbohydrates, and other extracellular components can interfere with PLA2 reactivity, thus hindering the sensitivity and selectivity of the detection. Further, culturing and handling the cells can be tedious and complex, and long-term storage of cells is not convenient. On the other hand, model lipid membrane-based approaches are simple and easy to prepare and handle and thus provide simple and effective ways to investigate specific membrane hydrolysis. Also, PLA2 is more reactive with organized phospholipid bilayers,14 and model membrane systems can be customized with specific lipid compositions, thereby enhancing the enzyme’s response significantly.
The PLA2–lipid membrane interaction has been investigated using model lipid systems comprised of lipid vesicles or supported lipid membranes (SLMs). In lipid vesicle-based studies, colorimetric,3,15 radiometric,16 and steady-state fluorescence techniques17–19 have been implemented to monitor the enzyme–membrane interactions. Furthermore, lipid vesicles have been utilized in PLA2-mediated drug delivery for several cancer types.20,21 Though lipid vesicles are the most commonly used substrates to explore PLA2–membrane interactions3,15–17,19 and may be more suitable for drug-delivery applications, they possess some inherited limitations that are not conducive for disease diagnosis purposes. Lipid vesicles have a short half-life and undefined size and shape; they tend to fuse and are prone to leak the content. In this regard, the use of SLMs is more favorable as the surface of the solid support renders the extra stability to the lipid membranes owing to the membrane–surface interaction.22
Lipid bilayers assembled on microspheres (lipo-beads) are advantageous as they enable the utilization of a sensitive high-throughput method such as flow cytometry.23 The process of forming a lipid bilayer on microspheres is simple and efficient and well characterized. When small unilamellar lipid vesicles (SULVs) mix with microspheres, a single lipid bilayer of about 5 nm thickness will be formed around the microspheres spontaneously because of electrostatic interactions between the vesicles and microspheres. A water layer of about 2 nm trapped between the particle surface and the bilayer helps to retain the fluidity of the lipid bilayer.24,25 The defined size, spherical shape, and bilayer fluidity provide a more cell-like structure, a high surface-to-volume ratio (thus increased reactive surfaces), and ease of handling and storage for an extended period without compromising the reactivity and sensitivity.
A microsphere-based approach has been developed to study the PLA2–lipid bilayer interaction by Chemburu et al. using fluorescence superquenching of silica microspheres derivatized with a fluorescent polyelectrolyte.26 However, the use of a preassembled cationic polyelectrolyte adds an extra synthesis step; an additional reagent (quencher) is needed to detect the enzymatic response, making the approach more complex, and relying on two consecutive binding events can cause an undesirable signal loss. Nevertheless, this work has laid the foundation for a more thorough study to develop more sensitive yet simple fluorescence microsphere-based detection for PLA2. A more detailed kinetic and binding study of PLA2 on lipid monolayer-coated styrene-divinylbenzene beads was reported by Kim and his co-workers.16 In their work, the use of radiolabeled lipids was unconvincing, and the necessity of separating hydrolyzed fatty acids followed by their radiometric analysis was tedious and time-consuming. Further, the hydrophobicity of styrene beads allows only the formation of a lipid monolayer, which is a less accurate representation of the cell membrane. None of the work has studied the use of lipo-beads for PLA2 inhibition, which will be advantageous in analyzing new PLA2 inhibitors as therapeutic drugs as well as disease markers such as annexins that inhibit PLA2.
In this work, we demonstrate the use of fluorescent lipo-beads comprised of various lipid formulations for fast, sensitive, and specific detection of the PLA2–membrane interaction. We utilized two types of fluorescent lipo-beads, one, which has a fluorescent dye encapsulated in porous silica particles using a nonfluorescent lipid coating and another with plain porous silica particles coated with a fluorescent lipid bilayer. In both types, the bilayer will be disintegrated from beads upon reacting with PLA2, resulting in a decrease in the bead fluorescence.27 Most PLA2 inhibition studies have been performed with either lipid micelles or vesicles,3,15,28–31 and to the best of our knowledge, this is the first time that lipo-beads are used for enzyme inhibition studies. We envision that these inhibition studies will initiate the use of lipo-beads in analyzing therapeutic candidates for several diseases. Here, we demonstrate the proof of concept for inhibition studies using two known inhibitors of PLA2, varespladib and quercetin. Then, we explore the feasibility of using lipo-beads to detect annexin A3 (ANXA-3), a potential biomarker for prostate and several other cancer types.32,33
EXPERIMENTAL SECTION
Materials.
1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dipentadecanoyl-sn-glycero-3-phosphocholine (15:0 PC), 1,2-dimyristoyl-sn-glycero-3-phosphorylglycerol (DMPG), and rhodamine phosphoethanolamine (14:0 Liss Rhod PE, RPE) were purchased from Avanti Polar Lipids (Alabaster, AL). Porous silica microspheres of 10 μm diameter with 10 nm pores (Nucleosil 100–10) were purchased from Macherey-Nagel Inc. (Duren, Germany). PLA2 from porcine pancreas, varespladib (LY315920), quercetin dihydrate, phosphate-buffered saline (PBS) (0.01 M phosphate buffer, 0.0027 M KCl, 0.137 M NaCl, pH 7.4 at 25 °C), fluorescein sodium salt, hydrochloric acid (36.5–38%), ammonium hydroxide solution (28–30%), calcium chloride, and chloroform were purchased from Sigma-Aldrich (St. Louis, MO). Hydrogen peroxide (30%) was purchased from VWR (Radnor, PA). Recombinant human annexin A3 (ANXA-3) was purchased from Creative BioMart (Shirley, NY) and Abcam (Cambridge, MA).
Preparation of Fluorescent Lipo-Beads.
The lipo-beads with encapsulated fluorescent dye were prepared by coating a nonfluorescent lipid bilayer on porous silica particles soaked in fluorescein dye, and the lipo-beads with a fluorescent lipid bilayer were prepared by coating the plain porous silica particles with a lipid bilayer comprised of DMPC and a fluorescent derivative of PE (RPE). Additionally, for demonstration purposes, lipo-beads with fluorescein encapsulated and coated with DMPC and RPE were also prepared. Beads with encapsulated fluorescein dye were composed of various nonfluorescent phospholipid bilayers with varying molar composition of lipids (100% DMPC, 100% PC, and 20:80 DMPG/DMPC). For all lipo-bead preparations, first, a known amount of porous silica microspheres were cleaned in a solution of 4% NH4OH and 4% H2O2 (w/v) at 80 °C, followed by a rinse in a solution of 4% H2O2 and 4% HCl (w/v) at 80 °C. SULVs prepared by bath sonication were used to form lipid bilayers on particles, as reported elsewhere.27 Briefly, a lipid solution prepared in chloroform was kept under a stream of N2 to evaporate the solvent and to form a lipid film at the bottom of a glass vial. For lipid mixtures, lipids in chloroform were mixed at the desired molar composition before forming the lipid film. The lipid film was further dried in a vacuum desiccator for an hour and hydrated by mixing with PBS (pH 7.4, 0.01 M). The hydrated lipid was sonicated using a bath sonicator (VWR, Radnor, PA) until the solution became clear, indicating the formation of SULVs. For fluorescein-entrapped microspheres, 100 mg of surface cleaned particles were soaked in 10.0 mL of 1.0 mM fluorescein sodium salt in PBS for 48 h while gently shaking. SLMs were formed by vortexing particles in 500.0 μL of SULV solution (1 mM of total lipid) for 5 min, followed by 20 min shaking using a vortex mixer (VWR, Radnor, PA). The excess lipid and fluorescein dye were removed by rinsing the prepared particles in PBS several times. To prepare lipo-beads coated with fluorescent lipids; 100.0 mg of cleaned particles were used with 500.0 μL of SULV solution (1 mM of total lipid) comprising 100:1 M ratio of DMPC and RPE. The excess lipid was removed as before. Each lipo-bead sample was suspended in 500.0 μL of PBS and stored at 5 °C for extended use. The loss of lipo-bead fluorescence was used to assess the stability of the particles. Thus, the fluorescence intensity of the beads was measured prior to each experiment using a FACSCalibur flow cytometer (BD Bioscience, San Jose, CA). Bead suspensions that were below a predetermined threshold intensity were deemed unstable and discarded.
Confocal Fluorescence Imaging of Lipo-Beads.
To demonstrate the encapsulation of fluorescein and the formation and stability of lipid bilayers on particles, fluorescence imaging was performed using an Olympus Fluoview FV10i confocal laser scanning microscope. Three types of lipo-beads, (a) fluorescein-entrapped porous beads with 100% DMPC lipid bilayer, (b) plain porous beads with a 100:1 (molar ratio) DMPC/RPE lipid bilayer, and (c) fluorescein-entrapped porous beads with 100:1 DMPC/RPE lipid bilayers were imaged after 21 days of preparation. Excitation and emission wavelengths of 473 and 510 nm, respectively, were used for fluorescein, wherein the excitation and emission wavelengths of 559 and 600 nm, respectively, were used for RPE.
Stability Studies of Lipo-Beads.
The stability of DMPC, 100:1 DMPC/RPE, and 20:80 DMPG/DMPC lipo-beads stored at 5 °C was monitored for 3 weeks after preparation. A 10 μL portion from 10 mg/mL stock lipo-beads was diluted to 100 μL with PBS for the fluorescence analysis. All measurements were done in three replicates.
Interaction of PLA2 with Lipid Bilayers on Beads.
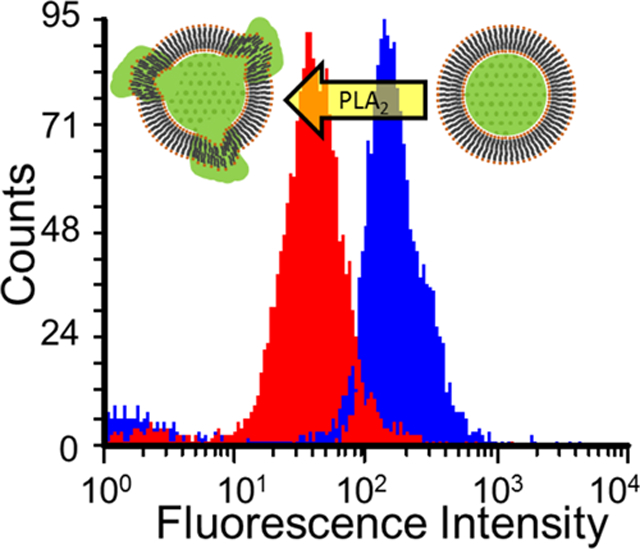
The interaction of PLA2 with the lipid bilayer on beads was explored via the release of fluorescein dye (Figure 1d) and the disintegration of the fluorescent lipid, RPE, (Figure 1e) from the bead surface. The activity of the enzyme was evaluated for four different lipo-bead formulations at two different temperatures, below and above the phase transition temperature (Tm) of the major lipid constituent of the bilayer. In each test, 50.0 μL of 1 mg/mL lipo-beads suspended in PBS was taken into a microcentrifuge tube and incubated with 45.0 μL of 4.1 μM PLA2 and 15.0 μL of 0.01 mM Ca2+ for 30 min. Lipo-beads in enzyme-free PBS with and without 0.01 mM Ca2+ were used as controls, and each test was performed in three replicates. A temperature-controlled digital water bath was used to maintain the desired temperature. The lytic activity of the enzyme was measured via the decrease in the fluorescence intensity of the particles. The fluorescence intensities of the particles before and after reacting with the enzyme were measured at 525 nm for fluorescein-entrapped microspheres and at 585 nm for fluorescent lipid-coated microspheres using a BD FACSCalibur flow cytometer. The percentage decrease was calculated as shown in eq 1
| (1) |
Figure 1.
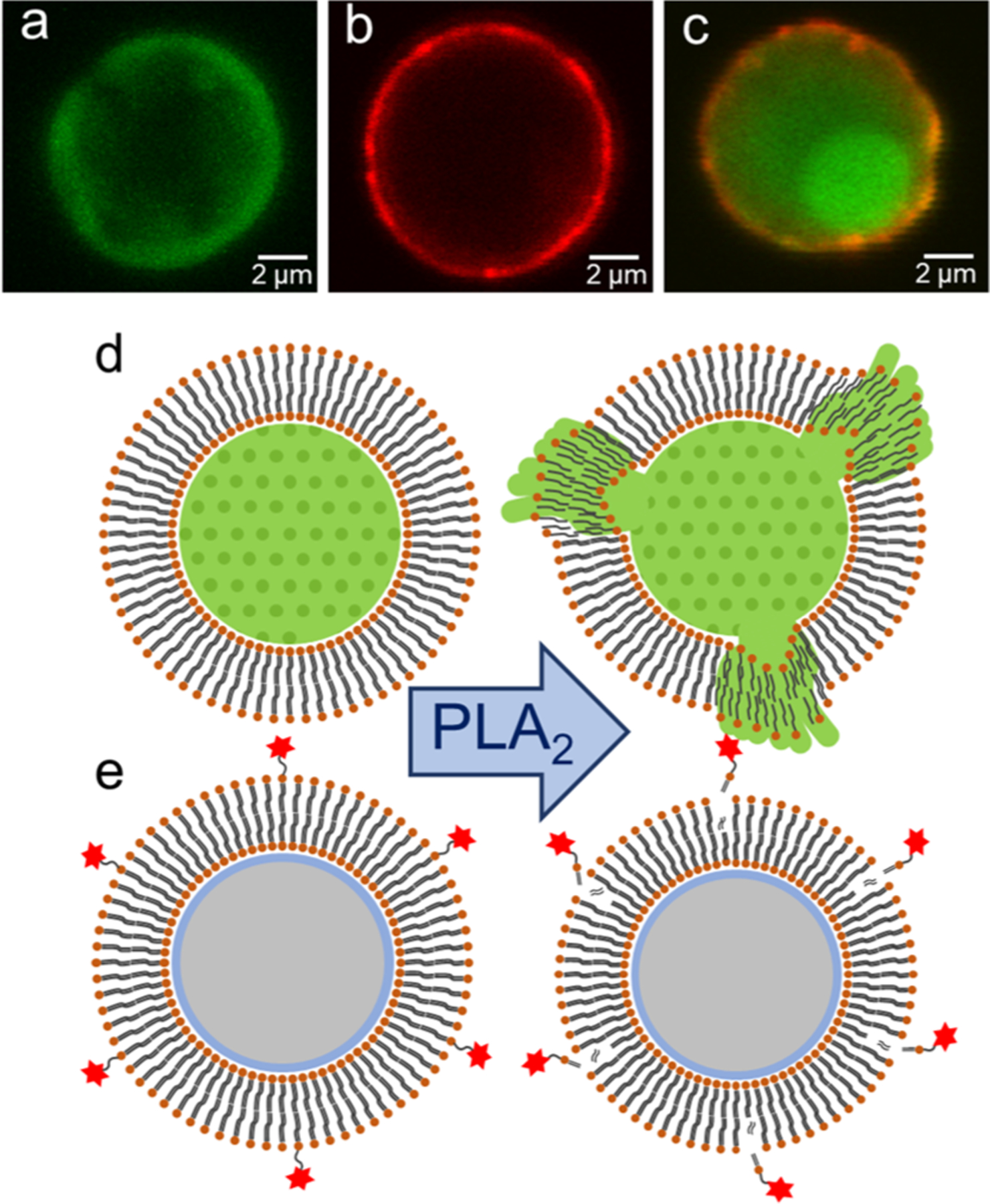
Formation of fluorescent lipo-beads and the two approaches for detecting membrane–PLA2 interaction. Lipo-beads (a) with a nonfluorescent lipid bilayer with encapsulated fluorescein, (b) with a lipid bilayer comprised of fluorescent lipid, RPE, and (c) with a lipid bilayer comprised of RPE and encapsulated fluorescein. The concept of release of (d) fluorescein dye and (e) fluorescent lipid because of the PLA2-mediated membrane hydrolysis.
Here, I0 is the initial fluorescence intensity of the particles and It is the intensity of the particles after reacting with the enzyme. All data collection and analyses were performed using BD CellQuestTM Pro (BD Bioscience, San Jose, CA) and FCS Express 6 (De Novo Software, Glendale, CA) software, respectively.
PLA2 Binding and Kinetic Studies with Lipo-Beads.
Enzyme binding and kinetic assays were performed on different lipo-beads to determine the most reactive lipid composition, optimal PLA2 concentration, and incubation time. During the binding studies, 45.0 μL of PLA2 with various concentrations and 15.0 μL of 0.01 mM Ca2+ for DMPC, PC, and 100:1 DMPC/RPE and 0.3 mM of Ca2+ for 20:80 DMPG/DMPC were mixed with 50.0 μL of 1 mg/mL lipo-beads in microcentrifuge tubes. Lipo-beads incubated in enzyme-free PBS with and without Ca2+ were used as controls, as mentioned previously. All assays were performed at temperatures where the major lipid of the bilayer was in its liquid phase. The fluorescence intensity of the particles in each sample was measured as before. The optimal PLA2 concentration for each lipid composition obtained from the binding studies was used for the respective kinetic experiments. For each kinetic assay, 1 mg/mL suspension of lipo-beads was used with 0.01 mM Ca2+ for DMPC, PC, and 100:1 DMPC/RPE lipo-beads and 0.3 mM Ca2+ for 20:80 DMPG/DMPC lipo-beads. A constant volume of bead sample was withdrawn from the microcentrifuge tube at known time intervals for flow cytometry measurements.
PLA2 Inhibition with Model Inhibitors.
The efficacy of lipo-beads for PLA2 inhibition was evaluated with two model inhibitors, varespladib and quercetin. For varespladib, 35.0 μL of the inhibitor solutions in PBS with various concentrations ranging from 0 to 75.0 μM were mixed first with 50.0 μL of 1.0 μM PLA2 for 30 min at 25 °C. Then, 50.0 μL solution of 1 mg/mL lipo-beads was incubated with the mixture for another 30 min while maintaining the liquid phase of the primary constituent lipid. A calcium concentration of 0.01 mM was maintained for 100% DMPC, 100% PC, and 100:1 DMPC/RPE and 0.3 mM for 20:80 DMPG/DMPC lipo-beads. A positive control comprised of all reagents, but the inhibitor and a negative control comprised of the PBS buffer were also used. For quercetin, 45.0 μL of 4.1 μM PLA2 was preincubated with 45.0 μL of 35.0 μM quercetin before mixing with 50.0 μL of 1 mg/mL 100% DMPC lipo-beads. This mixture was incubated at 30 °C for 30 min before measuring the fluorescence intensity of the particles. The positive control and the negative control were used as before.
Determination of Annexin A-3.
ANXA-3 was determined via PLA2 inhibition using 20:80 DMPG/DMPC and 80:15:5 DMPC/DMPG/RPE lipo-beads. For 20:80 DMPG/DMPC lipo-beads, 10.0 μL of 100.0 nM PLA2 was mixed with 10.0 μL of 10.0 μM Ca2+ and 10.0 μL of 55.0 nM ANXA-3 in PBS. A sample of 20.0 μL of 1 mg/mL 20:80 DMPG/DMPC lipo-beads was added to the preincubated enzyme–ANXA-3 mixture. For 80:15:5 DMPC/DMPG/RPE lipo-beads, 10.0 μL of 20.0 nM PLA2 was preincubated with 10.0 μL of different concentrations of ANXA-3 and 10.0 μL of 10.0 μM Ca2+ before adding to the 20.0 μL of 0.5 mg/mL 80:15:5 DMPC/DMPG/RPE beads. The reaction mixture was incubated at 30 °C for 30 min before measuring the fluorescence intensity of the particles.
The percent inhibition and percent PLA2 activity for two model inhibitors and ANXA-3 were calculated using eqs 2 and 3
| (2) |
| (3) |
where is the decrease in the signal for PLA2 and ID,Inh is the decrease in the signal in the presence of the PLA2–inhibitor mixture. ID was calculated by (see eq 1).
Statistical Analysis.
All experiments were conducted in triplicate with average measurements reported. Statistical analysis (unpaired t-test) was conducted using GraphPad Prism software to report the variations in different measurements at a 95% confidence interval, where it was necessary. Data were considered statistically significant when p-value < 0.05.
RESULTS AND DISCUSSION
Preparation of Fluorescent Lipo-Beads.
PLA2 is more reactive with phospholipid bilayers containing choline, glycerol, and ethanolamine in the lipid-phosphate head group.34,35 Therefore, we utilized several combinations of choline, glycerol, and ethanolamine containing phospholipids as constituents of the supported bilayer. When selecting them, lipids with phase transition temperatures closer to room temperature (around 25 °C) were considered for convenience during the preparation and experimentation. Among the three phospholipids tested, glycerol containing DMPG has a net negative charge at physiological pH, and PLA2 is more reactive with the anionic lipid.3,18,36–38 The silica surface is also negatively charged at pH 7.4;39–41 thus a stable SLM containing 100% DMPG could not be obtained because of strong repulsive electrostatic interactions. Consequently, DMPG was mixed with zwitterionic DMPC to form stable bilayers. Fluorescence spectroscopy-based analysis is simpler and more sensitive than other techniques that have been used to determine the membrane–enzyme interaction in previous studies. Further, a high throughput, fluorescence-based method such as flow cytometry can easily be implemented to monitor the loss of fluorescence in lipo-beads as a consequence of membrane hydrolysis. The presence of a bulky fluorophore in the fluorescently tagged lipid may restrict the enzyme’s access to the membrane. To circumvent this, we used dye-encapsulated microspheres with a nonfluorescent lipid coating (Figure 1a). Yet, to find the efficacy of using lipo-beads with fluorescently tagged lipids, silica microspheres coated with fluorescent lipids were also investigated (Figure 1b). In this study, two choline-containing zwitterionic lipids, DMPC (14:0) or PC (15:0), were used as the primary lipid constituent of the bilayer because most mammalian cell membranes are enriched with phosphatidylcholines.42 Even though the structure of these two lipids differs only by one carbon atom in the fatty acid tail (Figure S1), their phase transition temperatures are 24 and 35 °C. We used 20 mol % DMPG to explore the effect of bilayer charge on PLA2 reactivity. We found that stable lipo-beads with anionic lipids can be formed only when the molar ratio of DMPG is 20% or less under our experimental conditions; thus we kept 20 mol % as the upper limit for the anionic lipid. When the fluorescent tagged lipid RPE is incorporated into the bilayer, we found that 1–5 mol % of RPE would produce lipo-beads with the maximum fluorescence intensity. This can be due to the fluorescence self-quenching at a high fluorophore concentration. During the bead preparation, lipid solutions were maintained above the phase transition temperature of the major lipid, and the prepared lipo-beads were kept at 5 °C during storage. The stability studies conducted for 3 weeks shows that there is no significant loss in particle fluorescence, confirming that the lipid coating on beads is intact even after 3 weeks if kept below the phase transition temperature of the major lipid (Figure S2). Further, these lipo-beads were also stable in artificial urine (Figure S3). Additionally, confocal fluorescence imaging was performed on these beads after 3 weeks from preparation to further confirm the stability and integrity of the bilayers (Figure 1a–c). The obtained images clearly prove that the bilayers are intact and stable even after 3 weeks from preparation. In Figure 1a, fluorescein is retained by the nonfluorescent lipid coating. The sharp fluorescent edge in the sphere (Figure 1b) suggests the existence of a lipid bilayer comprising the fluorescent lipid, RPE, whereas Figure 1c demonstrates the presence of fluorescein in pores and RPE in the coating.
Interaction of PLA2 with Lipo-Beads.
PLA2 requires Ca2+ as a cofactor for the enzymatic hydrolysis of the lipid sn-2 ester bond.11–13,42 In the vesicle-based studies, it has been shown that a high calcium concentration can disrupt vesicles because of membrane fusion.43,44 However, a systematic study on the effect of Ca2+ concentration on solid SLMs has not been reported. To develop a stable, highly sensitive, and reproducible bead-based substrate for the PLA2 assay, the determination of the optimum Ca2+ concentration is essential. From a series of optimizing experiments, we confirmed that a high calcium content could disrupt the supported membrane to some degree (Figure S4), and we limited the maximum concentration to be used with minimum disruption effect to 0.3 mM Ca2+. In Figure 2, the reaction of PLA2 on three different lipo-beads, while keeping the enzyme and calcium concentrations constant, is summarized. Upon PLA2 reaction with lipo-beads, the fluorescence intensity of particles is decreased because of the release of the trapped dye (Figure 1d) or the loss of fluorescent lipid (Figure 1e) as a consequence of bilayer disintegration from the particle surface. Our data suggest that dye-encapsulated DMPC lipo-beads are more reactive (Figure 2a) than the dye-encapsulated PC lipo-beads (Figure 2b). When the incubation temperature is below the phase transition temperature of each lipid, the decrease in the fluorescence intensity is 38 and 33% for DMPC and PC, respectively; however, when the incubation temperature is above the phase transition temperature, PLA2 is more reactive with both lipo-bead types, and the decrease in the fluorescence intensity is now 59 and 47%, respectively. The increased enzymatic activity could be due to the thermal induction or due to the increased fluidity of the membrane, which favors membrane hydrolysis.18,45 Another possible cause is that the lipids in the liquid phase are more structurally disordered than those in the gel phase, and it has been reported that PLA2 is more active on sites with structural defects.46 Our observation with solid-supported bilayers is in good agreement with the PLA2 interaction with lipid vesicles.18,37,45 The difference in activity between PC and DMPC lipo-beads is due to the change in the fatty acid chain lengths as reported by Hoyrup et al. in a lipid vesicle study.47 The statistical analysis of the data suggests that the average fluorescence decreases in the two lipo-bead types are not statistically significant (p = 0.36) when the lipids are in their gel phase; however, the decrease is statistically significant (p = 0.04) when the lipids are in the liquid phase. This observation indicates that the effect of phase change has become more pronounced than the carbon chain length of the fatty acid in the liquid phase. Though the data from lipo-beads with fluorescent lipids (RPE/DMPC) cannot be directly compared with the dye leakage data owing to the two modes of fluorescence loss, the RPE/DMPC beads show greater sensitivity for PLA2. The percent fluorescence decrease in these particles is higher for comparable PLA2 concentrations, and there is no significant difference in responses between the gel and liquid phases (72 and 74%, respectively, Figure 2c). When RPE is present, it is not necessary to hydrolyze the whole bilayer to generate a marked decrease in the fluorescence signal. It has been shown that PLA2 first hydrolyzes the outer monolayer before reaching to inner layer.35 With the bulky group of rhodamine present, we hypothesize that most RPEs are assembled into the outer monolayer, which results in a significant fluorescence decrease upon reacting with the enzyme, a hypothesis worth investigating further. The slow diffusion of the trapped dye can contribute to the low release of the fluorescein in encapsulated particles, resulting in a lower percent decrease in the particle fluorescence. One can anticipate that phase mismatching due to the presence of two lipids might enhance the PLA2 reactivity.48 The phase transition temperatures for DMPC and RPE are 24 and 42 °C, respectively, and by incubating the reaction mixture at 30 °C, only DMPC is in the liquid phase, whereas RPE is in the gel phase. However, the low abundance of RPE (1%) might not generate a phase mismatched bilayer; thus, we conclude the phase mismatch effect is negligible. Even though PLA2 is more reactive with bilayers containing anionic lipids, there was no activity for 20% DMPG at 0.01 mM Ca2+ (Figure S5). However, when the Ca2+ concentration is increased from 0.01 to 0.30 mM, the reactivity of PLA2 was significantly enhanced (Figure 3c). In the presence of an anionic lipid in the bilayer, Ca2+ binds first to the anionic lipid before activating PLA2,49,50 and thus it is necessary to have an extra amount of the divalent cation. Therefore, a higher concentration of Ca2+ was used in tests with DMPG to follow.
Figure 2.
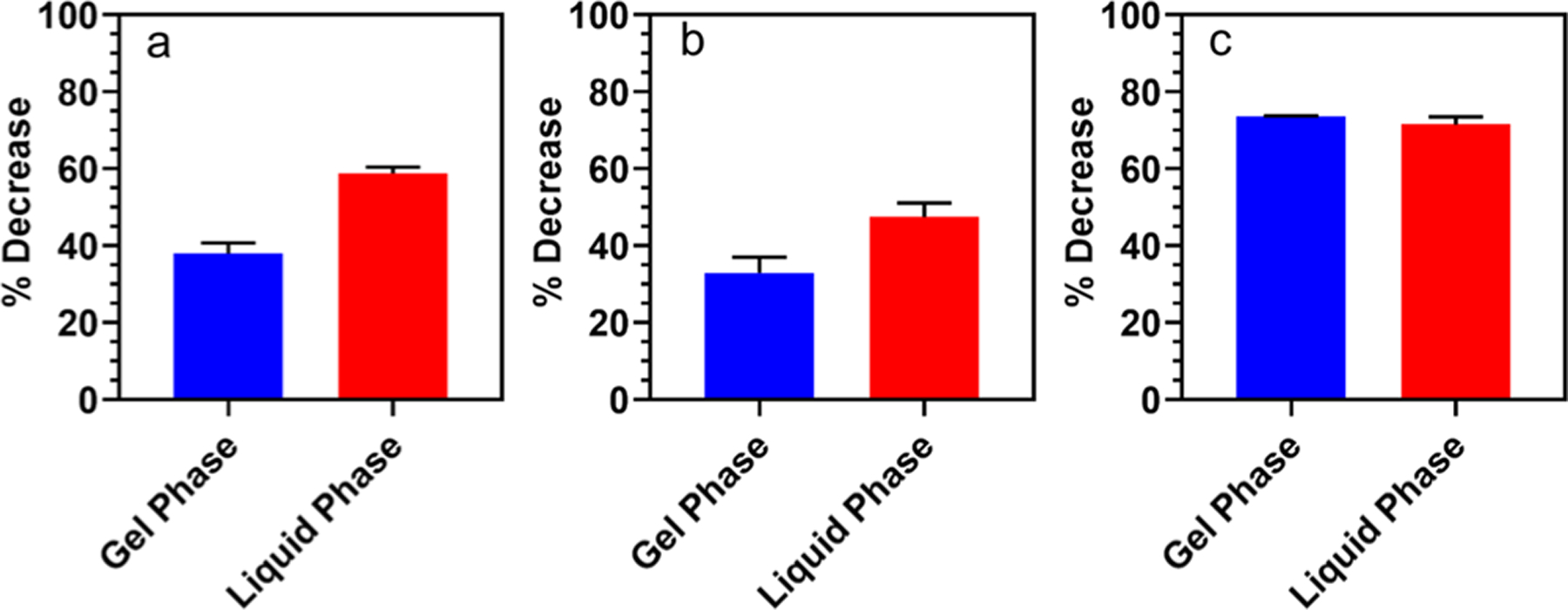
PLA2 activity as the percent decrease of fluorescence intensity for three different lipo-beads in gel and liquid phases of the primary lipid. (a) DMPC at 18 °C (gel phase) and 30 °C (liquid phase), (b) PC (15:0) at 30 °C (gel phase) and 41 °C (liquid phase), and (c) 100:1 DMPC/RPE at 18 °C (gel phase) and 30 °C (liquid phase).
Figure 3.
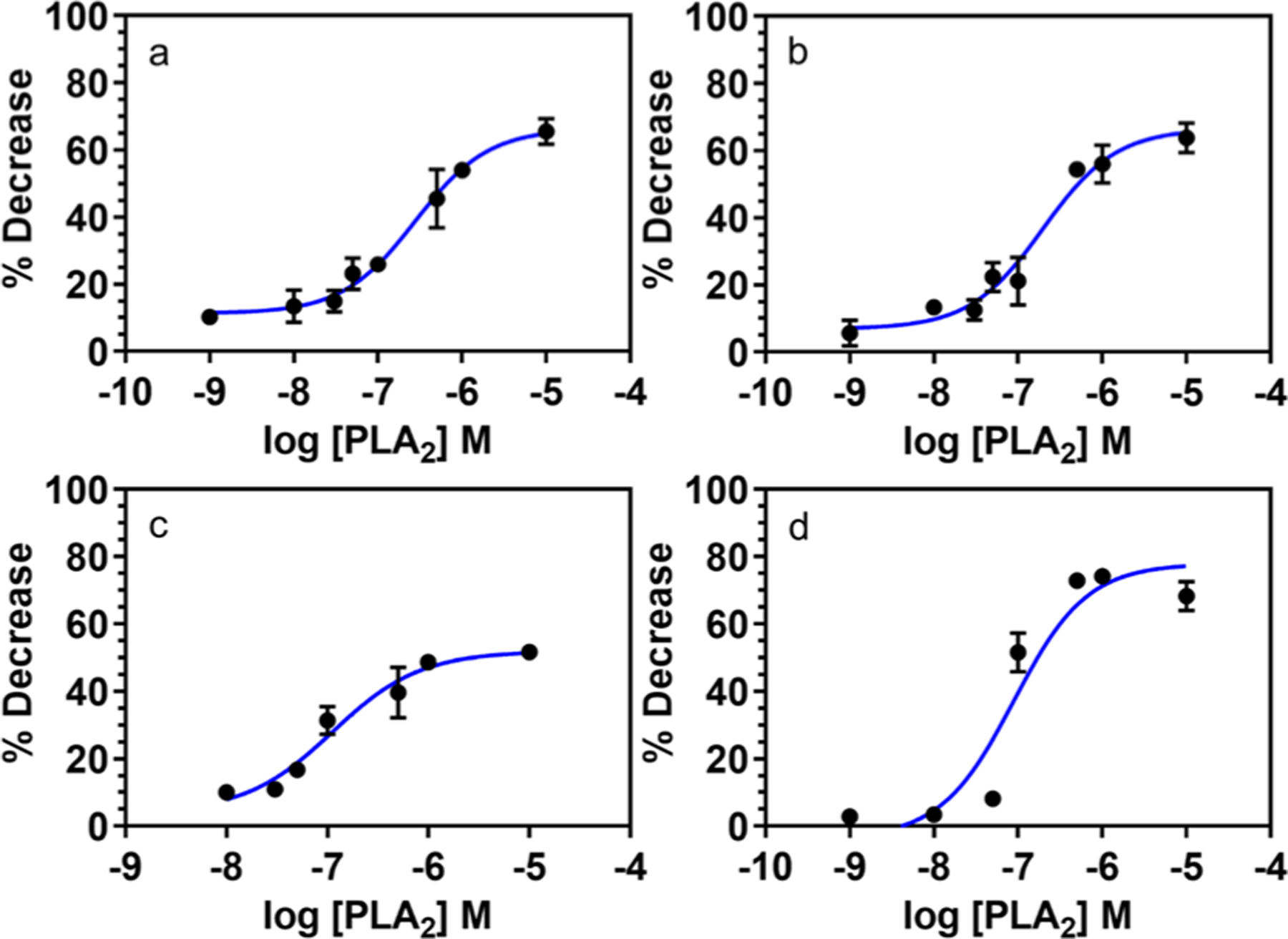
Dose–response curves for PLA2 interaction on different lipo-beads at the liquid phase of the primary constituent lipid. (a) DMPC, (b) PC, (c) 20:80 DMPG/DMPC, and (d) 100:1 DMPC/RPE. The PLA2 activity was measured as the percent decrease of fluorescence intensity of lipo-beads after reacting with PLA2 at different concentrations ranging from 1 nM to 10 μM, except for (c) where the range is from 10 nM to 10 μM.
PLA2 Binding and Kinetic Studies with Lipo-Beads.
EC50 is the 50% effective concentration of the enzyme for the hydrolysis of the phospholipid bilayer, and here it is used to compare the reactivity of the enzyme on different lipo-beads. The EC50 obtained from the enzyme dose–response curves in Figure 3 confirms that PLA2 has enhanced reactivity with lipo-beads containing anionic lipids (Figure 3c). The EC50 for the bilayers containing 20% anionic lipid was 112 nM, whereas for beads with pure zwitterionic lipids, PC and DMPC, the values were 197 and 278 nM, respectively (Figure 3b,a). Figure 3 further suggests that the potency of the enzyme is higher for anionic lipo-beads, though the efficacy is greater for zwitterionic lipo-beads. We anticipate that by further increasing the amount of anionic lipids in the bilayer, more sensitive lipo-beads can be obtained. The EC50 for the lipo-beads with RPE is 89 nM, and its dose response curve suggests that PLA2 is more effective and potent with these lipo-beads than other tested lipo-beads (Figure 3d). As explained earlier, the presence of RPE in the outer layer and less effect from the diffusion-limited release can be the factors for the enhanced reactivity. If we consider a 25% decrease as the minimum detectable response, 100:1 DMPC/RPE lipo-beads have the minimum detection limit of about 48 nM (Figure 3d), whereas 100% DMPC lipo-beads have the minimum detection limit of 92 nM (Figure 3a). The EC50 and PLA2 concentrations needed for a 25% decrease in the fluorescence intensity are summarized in the table of Supporting Information (Table S1).
The kinetic data suggest that PLA2 generates a distinguishable signal in less than 5 min of initiating the reaction (Figure 4). For 100% DMPC and 100% PC lipo-beads, the percent decrease of the mean fluorescence intensity is rapid and it reaches the maximum decrease within 15 min (Figure 4a,b), whereas for the anionic lipo-beads, the maximum decrease reaches within 5 min (Figure 4c), further confirming the enzyme’s enhanced reactivity with negatively charged lipids. On the other hand, lipo-beads with the fluorescent lipid, RPE, reaches the maximum decrease in 10 min (Figure 4d), and the extent of decrease is also greater in this time period, confirming the data in Figure 2c. The kinetic data suggest that both anionic lipo-beads and RPE lipo-beads are fast reactive; yet, RPE lipo-beads are the most sensitive.
Figure 4.
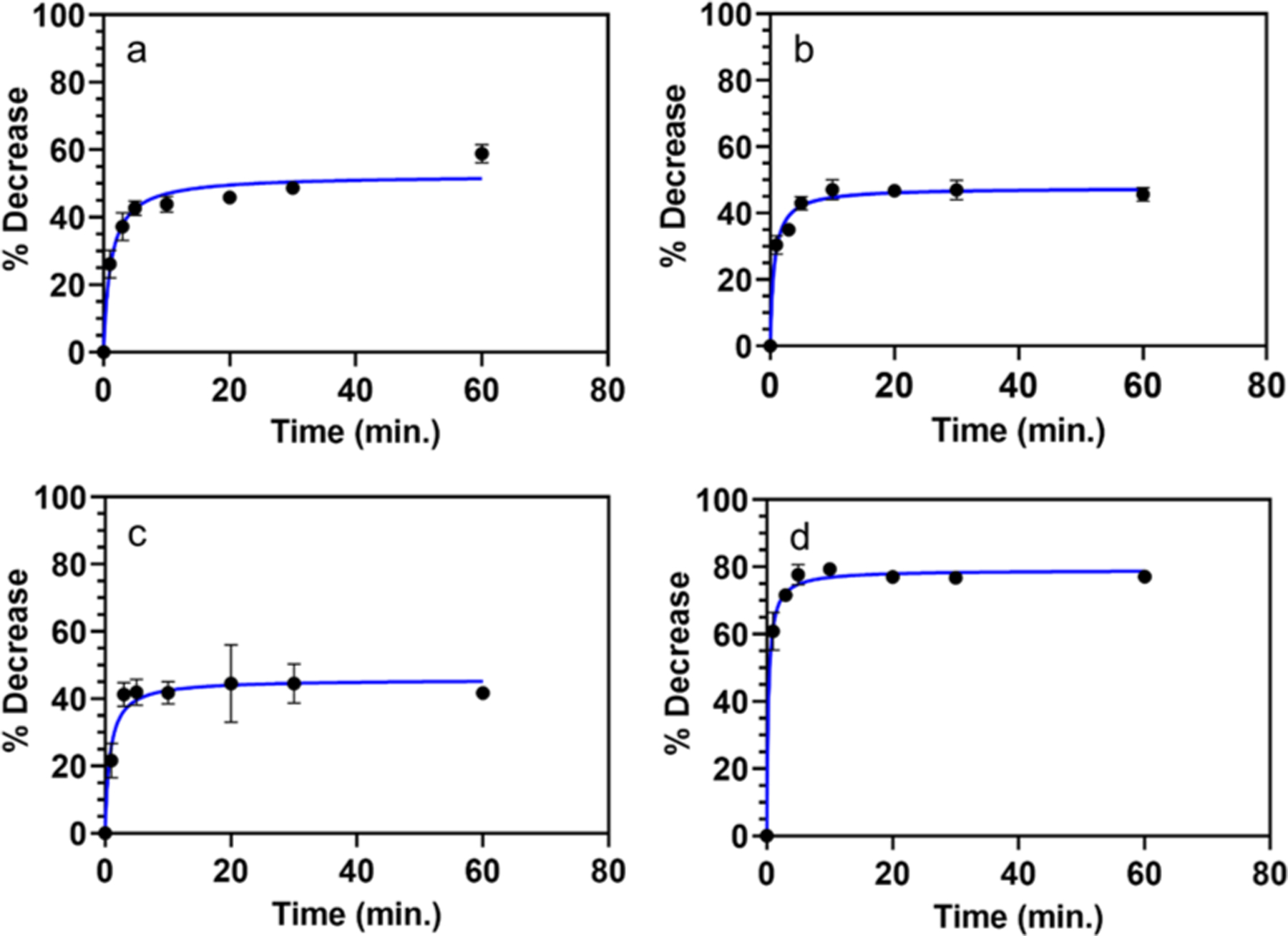
PLA2 kinetic graphs for different lipo-beads at the liquid phase of the primary constituent lipid. (a) DMPC, (b) PC, (c) 20:80 DMPG/DMPC, and (d) 100:1 DMPC/RPE. The kinetic studies were performed by measuring the decrease in the lipo-bead fluorescence over time at a PLA2 concentration.
Inhibition Assays with Model Inhibitors.
Under pathological conditions, PLA2 behaves as a membrane disruptor that triggers several disease conditions, including Alzheimer, multiple sclerosis, and Parkinson disease.51,52 Thus, the development of PLA2 inhibitors as therapeutic agents will be important, and we envision that these lipo-beads can be utilized in in-vitro studies to investigate the efficacy and potency of new PLA2 inhibitory drugs. To demonstrate the proof of concept for utilizing lipo-beads in inhibition studies, known PLA2 inhibitors, varespladib and quercetin, were analyzed. The percent inhibition and the percent PLA2 activity were calculated using eqs 2 and 3 as described before. The varespladib data indicates that the extent of inhibition varies with different lipid substrates (Figure 5). Though DMPC and PC beads show the inhibition (Figure 5a,b), it is not significant as in the anionic DMPG beads (Figure 5c). The statistical analysis shows that there is no significant difference in inhibition in all three lipo-beads (thus no linear correlation) above 5 μM of varespladib (p = >0.05), which indicates that the inhibition is saturated at micromolar concentrations. The lipo-beads comprised of RPE (Figure 5d) show a linear correlation, though the inhibition is less pronounced. As mentioned before, when RPE is present, it is not necessary to hydrolyze the whole bilayer to generate a marked decrease in the fluorescence signal; thus, the direct correlation of enzyme–inhibitor interaction is suggested, whereas in DMPC, PC, and DMPG beads, the direct correlation of enzyme–inhibitor interaction is not observed. Jain et al. have reported that PLA2 has a membrane binding site and a catalytic site, and inhibitors can have three different modes of action. In the first mode, the inhibitor competes with the substrate to bind to the catalytic site of the enzyme, whereas in the second mode, the inhibitor changes the organization of the bilayer, thus affecting the enzyme–substrate binding, and in the third mode, the inhibitor binds directly to the active site or to the interfacial binding site of the enzyme. Therefore, the extent of inhibition depends on the mode of inhibition. Furthermore, the type of lipid substrate can also influence the extent of inhibition.53 Thus, a combined effect from the mode of inhibitory action and the type of lipid substrate is anticipated for the overall PLA2 inhibition on different lipo-beads. The more pronounced inhibition in DMPG beads and the linearity in RPE beads suggest that a combination of DMPG and RPE lipids might produce more sensitive and appropriate lipo-beads. A thorough investigation of the inhibitory effects and mechanisms on different lipo-beads is worth investigating, though it is beyond the scope of the current work. However, our data suggest that these lipo-beads can be utilized to explore the PLA2 inhibition by target molecules, and it is vital to choose the correct type of lipid substrate for PLA2 inhibitory studies.
Figure 5.
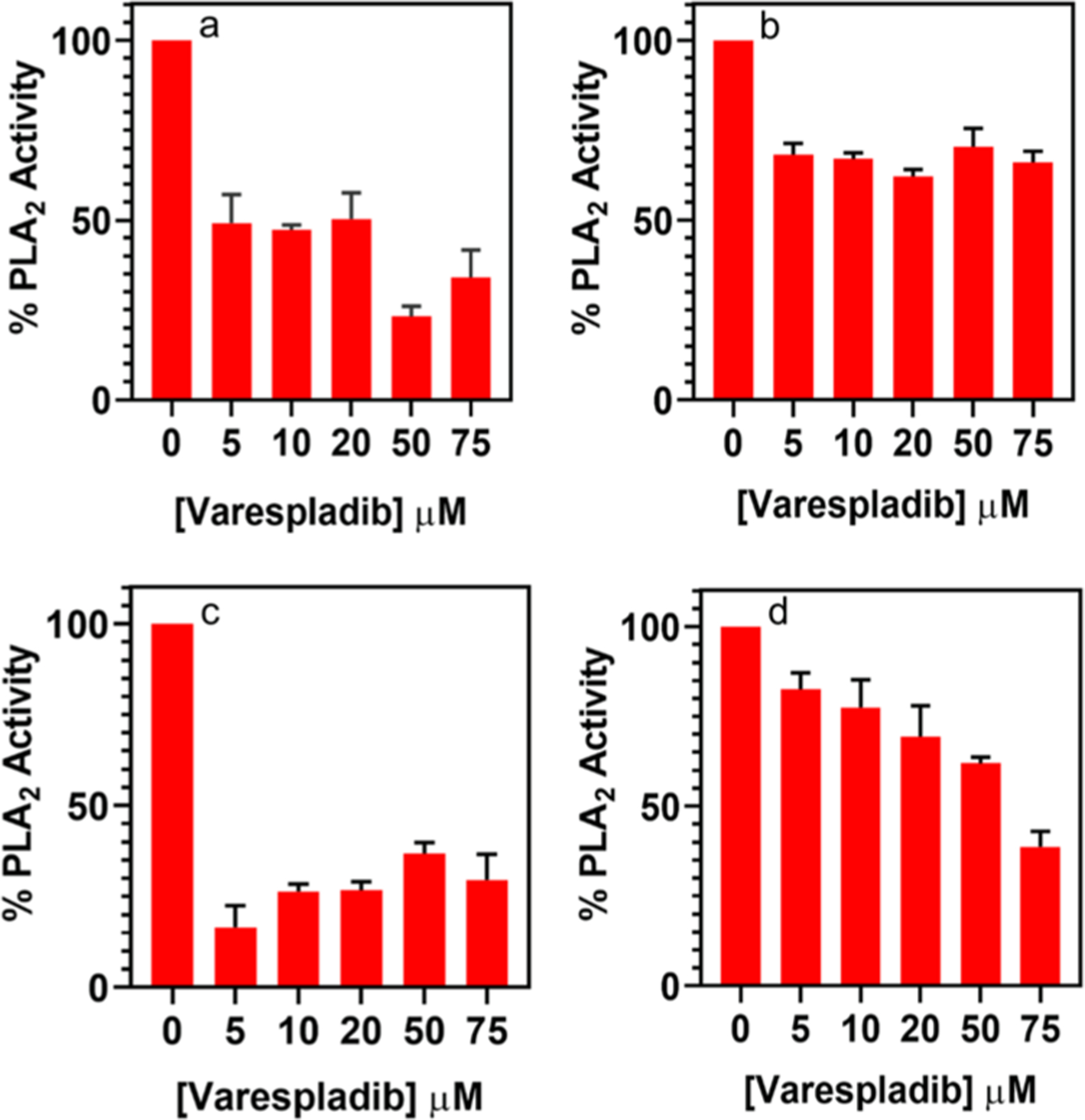
% PLA2 activity in the presence of varespladib for different lipo-beads at the liquid phase of the primary constituent lipid. (a) DMPC, (b) PC, (c) 20:80 DMPG/DMPC, and (d) 100:1 DMPC/RPE. The extent of PLA2 inhibition was converted to the % PLA2 activity using eqs 2 and 3.
The bioflavonoid quercetin, which is normally used for the treatment of inflammation and arteriosclerosis, did not show any inhibition of PLA2 activity when tested with lipo-beads. Instead, it showed a significant disruption of the lipid bilayer (Figure S6). The lytic activity of quercetin could be due to the more structural polarity of quercetin, which helps to interact with the polar head group of a lipid molecule via hydrogen bonding, thus permeating the lipid membrane (see Supporting Information for structures, Figure S1). A previous study of quercetin on 1,2-dipalmitoyl-sn-glycero-3-phosphocholine vesicles has confirmed this type of interaction.54 In another study, quercetin on human erythrocyte membranes showed that quercetin could affect the fluidity of the membrane, thus destabilizing the membrane.55
Determination of Annexin A3.
Annexins have emerged as disease biomarkers for cancer, diabetes, and inflammatory diseases.56–59 They are a family of proteins that binds to phospholipids and are also known to inhibit PLA2 activity.1,31,60 In this work, we performed preliminary studies on annexin detection as another proof of concept for the study using lipo-beads for PLA2 inhibition. Our initial ANXA-3 test with anionic lipo-beads generated a moderate result (Figure S7). The test showed that lipo-beads can be implemented to detect the protein, however, the sensitivity was not high enough for a good detection. Our PLA2 binding data so far have suggested that DMPG and RPE lipo-beads are the most effective in PLA2 detection. Thus, to enhance the sensitivity of lipo-beads further, from a quick inquisitive experiment, we found that lipo-beads with 80:15:5 DMPC/DMPG/RPE would perform better. The dose–response curve for this new lipid formulation gave an EC50 of 15 nM for PLA2 (Figure 6). We chose 20 nM PLA2 for the inhibition assays with varying ANXA-3 concentrations. Figure 7 shows that ANXA-3 as low as 10 nM can easily be detected with 35% PLA2 inhibition. However, to establish the detection limit of our method, a more comprehensive study has to be performed with a wider range of ANXA-3 concentrations. ANXA-3 is currently detected via enzyme-linked immunosorbent assay (ELISA) and has a detection limit of about 0.05 nM (assaypro.com). In comparison to ELISA, the minimum concentration we could detect during this study is 100 times higher. However, our on-going work suggests that the current limit can be lowered further by enhancing the reactivity of lipo-beads by changing the lipid composition and the size and porosity of silica particles. The reported data in Figures 6 and 7 are the average of a minimum of three replicates, and the small standard deviations indicate that the method has good reproducibility. Further, our approach is simple and rapid, and reagents are comparatively inexpensive.
Figure 6.
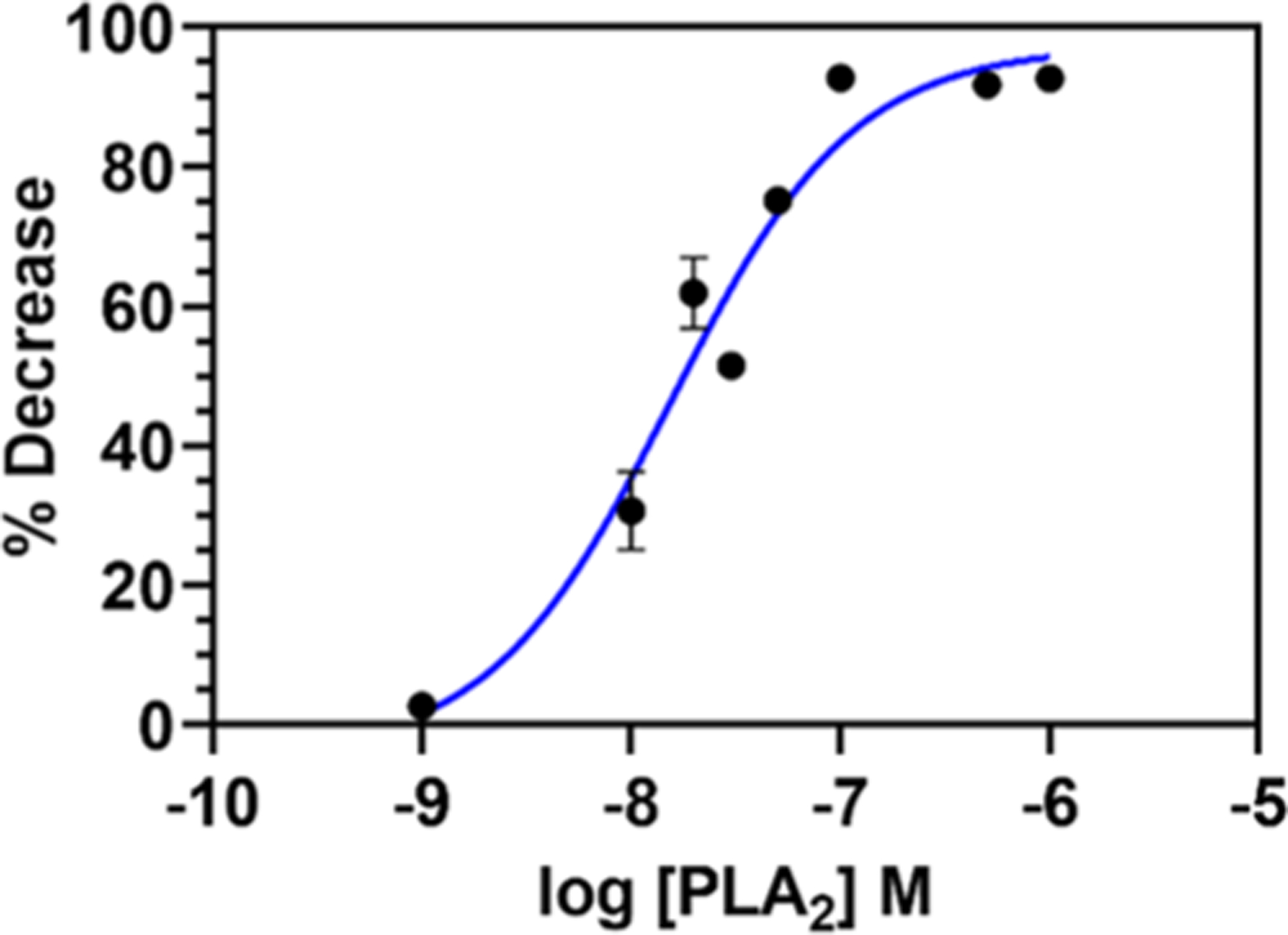
Dose–response curve for PLA2 hydrolysis of the lipid bilayer with 80:15:5 DMPC/DMPG/RPE lipo-beads at 30 °C. The enzyme activity at different concentrations of PLA2, ranging from 1 nM to 1 μM, was calculated as the percent decrease of the fluorescence intensity of lipo-beads using eq 1.
Figure 7.
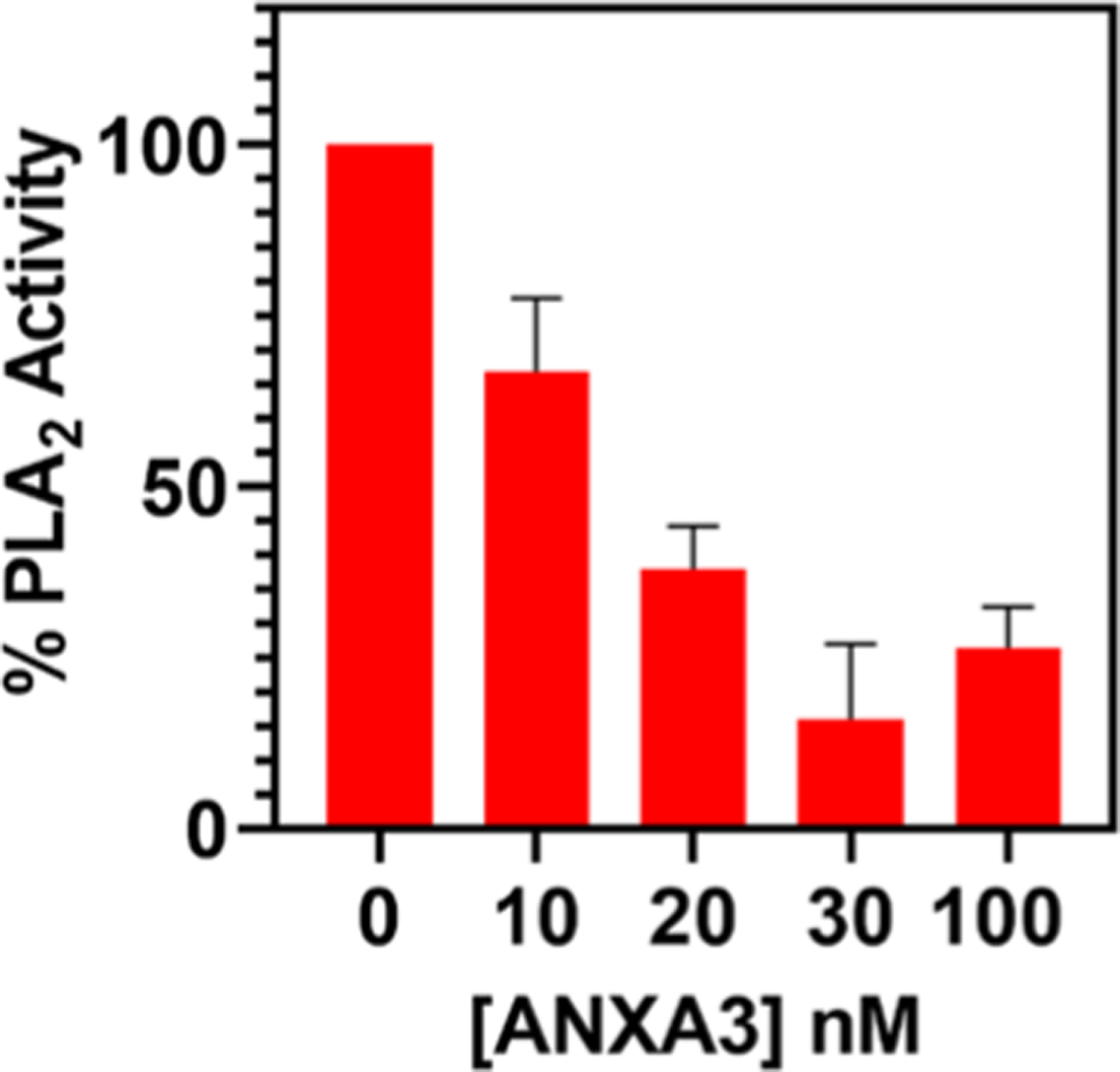
Detection of ANXA-3 via the inhibition of PLA2 activity using lipo-beads comprised of the 80:15:5 DMPC/DMPG/RPE phospholipid bilayer. The PLA2 concentration was maintained at 20 nM while varying the inhibitor ANXA-3 concentration from 10 to 100 nM.
Comparison of the Sensitivity of Lipo-Beads.
The sensitivity of lipo-beads was evaluated by comparing the PLA2 concentrations needed for a 25% decrease of fluorescence intensity of lipo-beads obtained from the dose response curves (Figures 3 and 6). The average % decrease for the most reactive beads at the highest PLA2 concentration we tested in this study was about 75%, and we chose one-third of this maximum to be the criteria for the minimum detectable signal. The data suggest that 85:15:5 DMPC/DMPG/RPE lipo-beads are the most sensitive for PLA2 detection, with a concentration needed for a 25% signal decrease as low as 6.20 nM (see Table S1 in the Supporting Information).
CONCLUSIONS
In this work, we explored several formulations of lipids as bilayers supported on porous silica microspheres to develop a rapid and sensitive particle-based detection method for PLA2–membrane interaction. We have also demonstrated the feasibility of using these lipo-beads for indirect determination of biomarkers that are capable of inhibiting PLA2. We envision that these lipo-beads will be beneficial for disease diagnosis and drug discovery studies. These lipo-beads are very reactive and can generate a detectable signal in less than 5 min. Further, they are stable for several weeks in storage at 5 °C. We have shown that even though PLA2 can hydrolyze most phospholipid membranes, it is essential to have a correct combination of phospholipids for the enhanced reactivity. The current study found that lipo-beads comprised of phosphatidyl ethanolamine tagged with a bulky fluorescent molecule at the hydrophilic head and anionic lipid DMPG provide fast and sensitive detection of PLA2-mediated lipid hydrolysis. Finally, we have demonstrated that ANXA-3, a known disease biomarker, can be detected as low as 10 nM using these lipo-beads. The sensitivity of these lipo-beads can be further improved by optimizing the lipid composition in the supported bilayer. Further, it is our aim to extend the current study to analyze ANXA-3 and other annexins in biological fluids. These sensitive lipo-beads will be useful as a simple, fast, and inexpensive method for the detection of PLA2 and its inhibitors at clinically relevant concentrations.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge funding for an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103451. S.H. would like to acknowledge funds from the office of the Vice President for Research at New Mexico Tech.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsbiomaterials.9b01720.
Chemical structures of RPE, DMPC, DMPG, varespladib, and quercetin; long-term storage stability of different lipo-beads at 5 °C; stability of different lipo-beads after exposure to synthetic urine for 30 min; effect of Ca2+ on the stability of the SLM; PLA2 activity on 20:80 DMPG/DMPC-coated fluorescein-encapsulated beads at 18 °C (gel phase) and 30 °C (liquid phase); effect of quercetin on DMPC lipo-beads; PLA2 inhibition by ANXA3 for 20:80 DMPG/DMPC lipo-beads; and EC50 and PLA2 concentrations needed for a 25% decrease of the fluorescence intensity (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acsbiomaterials.9b01720
The authors declare no competing financial interest.
Contributor Information
Shahriare Hossain, Department of Chemistry, New Mexico Institute of Mining and Technology, Socorro, New Mexico 87801, United States.
Kalika R. Pai, Department of Chemistry, New Mexico Institute of Mining and Technology, Socorro, New Mexico 87801, United States
Menake E. Piyasena, Department of Chemistry, New Mexico Institute of Mining and Technology, Socorro, New Mexico 87801, United States
REFERENCES
- (1).Dong Q; Patel M; Scott KF; Graham GG; Russell PJ; Sved P Oncogenic action of phospholipase A2 in prostate cancer. Cancer Lett. 2006, 240, 9–16. [DOI] [PubMed] [Google Scholar]
- (2).Brglez V; Lambeau G; Petan T Secreted phospholipases A2 in cancer: Diverse mechanisms of action. Biochimie 2014, 107, 114–123. [DOI] [PubMed] [Google Scholar]
- (3).Chapman R; Lin Y; Burnapp M; Bentham A; Hillier D; Zabron A; Khan S; Tyreman M; Stevens MM Multivalent Nanoparticle Networks Enable Point-of-Care Detection of Human Phospholipase-A2 in Serum. ACS Nano 2015, 9, 2565–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Guardiola M; Exeter HJ; Perret C; Folkersen L; van’t Hooft F; Eriksson P; Franco-Cereceda A; Paulsson-Berne G; Palmen J; Li K; Cooper JA; Khaw K-T; Mallat Z; Ninio E; Karabina S-A; Humphries SE; Boekholdt SM; Holmes MV; Talmud PJ PLA2G10 gene variants, sPLA2 activity, and coronary heart disease risk. Circ.: Cardiovasc. Genet 2015, 8, 356–362. [DOI] [PubMed] [Google Scholar]
- (5).Pourhassan H; Clergeaud G; Hansen AE; Østrem RG; Fliedner FP; Melander F; Nielsen OL; O’Sullivan CK; Kjær A; Andresen TL Revisiting the use of sPLA2-sensitive liposomes in cancer therapy. J. Controlled Release 2017, 261, 163–173. [DOI] [PubMed] [Google Scholar]
- (6).Kupert E; Anderson M; Liu Y; Succop P; Levin L; Wang J; Wikenheiser-brokamp K; Chen P; Pinney SM; Macdonald T; Dong Z; Starnes S; Lu T Plasma secretory phospholipase A2-IIa as a potential biomarker for lung cancer in patients with solitary pulmonary nodules. BMC Cancer 2011, 11, 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Yamashita S-I; Yamashita J-I; Ogawa M Overexpression of group II phospholipase A 2 in human breast cancer tissues is closely associated with their malignant potency. Br. J. Cancer 1994, 69, 1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kiyohara H; Egami H; Kurizaki T; Murata K; Ohmachi H; Akagi J; Ohshima S; Yamamoto S; Shibata Y; Ogawa M Possible Relationship between the Expression of Membrane-Associated Phospholipase A2 and the Proliferation of Interstitial Tissue in Human Pancreatic Cancer. International Congress Series; Elsevier, 2003; pp 375–379. [Google Scholar]
- (9).Abe T; Sakamoto K; Kamohara H; Hirano Y -i.; Kuwahara, N.; Ogawa, M. Group II phospholipase A2 is increased in peritoneal and pleural effusions in patients with various types of cancer. Int. J. Cancer 1997, 74, 245–250. [DOI] [PubMed] [Google Scholar]
- (10).Tribler L; Jensen LT; Jørgensen K; Brünner N; Gelb MH; Nielsen HJ; Jensen SS Increased expression and activity of group IIA and X secretory phospholipase A2 in peritumoral versus central colon carcinoma tissue. Anticancer Res. 2007, 27, 3179–3185. [PubMed] [Google Scholar]
- (11).Dennis EA; Cao J; Hsu Y-H; Magrioti V; Kokotos G Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev 2011, 111, 6130–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lambeau G; Gelb MH Biochemistry and Physiology of Mammalian Secreted Phospholipases A2. Annu. Rev. Biochem 2008, 77, 495–520. [DOI] [PubMed] [Google Scholar]
- (13).Burke JE; Dennis EA Phospholipase A2 Biochemistry. Cardiovasc. Drugs Ther 2009, 23, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Berg OG; Gelb MH; Tsai M-D; Jain MK Interfacial Enzymology: The Secreted Phospholipase A2-Paradigm. Chem. Rev 2001, 101, 2613–2654. [DOI] [PubMed] [Google Scholar]
- (15).Aili D; Mager M; Roche D; Stevens MM Hybrid Nanoparticle–Liposome Detection of Phospholipase Activity. Nano Lett 2011, 11, 1401–1405. [DOI] [PubMed] [Google Scholar]
- (16).Kim Y; Lichtenbergova L; Snitko Y; Cho W A Phospholipase A2Kinetic and Binding Assay Using Phospholipid-Coated Hydrophobic Beads. Anal. Biochem 1997, 250, 109–116. [DOI] [PubMed] [Google Scholar]
- (17).Cheng Z; Tsourkas A Monitoring Phospholipase A2 Activity with Gd-encapsulated Phospholipid Liposomes. Sci. Rep 2015, 4, 6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Leidy C; Linderoth L; Andresen TL; Mouritsen OG; Jørgensen K; Peters GH Domain-Induced Activation of Human Phospholipase A2 Type IIA: Local versus Global Lipid Composition. Biophys. J 2006, 90, 3165–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Boyer C; Zasadzinski JA Multiple lipid compartments slow vesicle contents release in lipases and serum. ACS Nano 2007, 1, 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Linderoth L; Fristrup P; Hansen M; Melander F; Madsen R; Andresen TL; Peters GH Mechanistic Study of the sPLA2-Mediated Hydrolysis of a Thio-ester Pro Anticancer Ether Lipid. J. Am. Chem. Soc 2009, 131, 12193–12200. [DOI] [PubMed] [Google Scholar]
- (21).Jensen SS; Andresen TL; Davidsen J; Høyrup P; Shnyder SD; Bibby MC; Gill JH; Jørgensen K Secretory phospholipase A2 as a tumor-specific trigger for targeted delivery of a novel class of liposomal prodrug anticancer etherlipids. Mol. Cancer Ther 2004, 3, 1451. [PubMed] [Google Scholar]
- (22).Castellana ET; Cremer PS Solid supported lipid bilayers: From biophysical studies to sensor design. Surf. Sci. Rep 2006, 61, 429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Fernandez Oropeza N; Zurek NA; Galvan-De La Cruz M; Fabry-Wood A; Fetzer JM; Graves SW; Shreve AP Multiplexed Lipid Bilayers on Silica Microspheres for Analytical Screening Applications. Anal. Chem 2017, 89, 6440–6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Bayerl TM; Bloom M Physical properties of single phospholipid bilayers adsorbed to micro glass beads. A new vesicular model system studied by 2H-nuclear magnetic resonance. Biophys. J 1990, 58, 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Johnson SJ; Bayerl TM; McDermott DC; Adam GW; Rennie AR; Thomas RK; Sackmann E Structure of an adsorbed dimyristoylphosphatidylcholine bilayer measured with specular reflection of neutrons. Biophys. J 1991, 59, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Chemburu S; Ji E; Casana Y; Wu Y; Buranda T; Schanze KS; Lopez GP; Whitten DG Conjugated Polyelectrolyte Supported Bead Based Assays for Phospholipase A2 Activity. J. Phys. Chem. B 2008, 112, 14492–14499. [DOI] [PubMed] [Google Scholar]
- (27).Piyasena ME; Zeineldin R; Fenton K; Buranda T; Lopez GP Biosensors based on release of compounds upon disruption of lipid bilayers supported on porous microspheres. Biointerphases 2008, 3, 38–49. [DOI] [PubMed] [Google Scholar]
- (28).Lewin M; Samuel S; Merkel J; Bickler P Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation. Toxins 2016, 8, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Schevitz RW; Bach NJ; Carlson DG; Chirgadze NY; Clawson DK; Dillard RD; Draheim SE; Hartley LW; Jones ND; Mihelich ED; Olkowski JL; Snyder DW; Sommers C; Wery J-P Structure-based design of the first potent and selective inhibitor of human non-pancreatic secretory phospholipase A2. Nat. Struct. Biol 1995, 2, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Snyder DW; Bach NJ; Dillard RD; Draheim SE; Carlson DG; Fox N; Roehm NW; Armstrong CT; Chang CH; Hartley LW; Johnson LM; Roman CR; Smith AC; Song M; Fleisch JH Pharmacology of LY315920/S-5920,[[3-(Amino-oxoacetyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl]oxy]acetate, a Potent and Selective Secretory Phospholipase A2Inhibitor: A New Class of Anti-Inflammatory Drugs, SPI. J. Pharmacol. Exp. Ther 1999, 288, 1117–1124. [PubMed] [Google Scholar]
- (31).Kim S-W; Ko J; Kim JH; Choi EC; Na DS Differential effects of annexins I, II, III, and V on cytosolic phospholipase A2 activity: specific interaction model. FEBS Lett 2001, 489, 243–248. [DOI] [PubMed] [Google Scholar]
- (32).Schostak M; Schwall GP; Poznanović S; Groebe K; Müller M; Messinger D; Miller K; Krause H; Pelzer A; Horninger W; Klocker H; Hennenlotter J; Feyerabend S; Stenzl A; Schrattenholz A Annexin A3 in urine: a highly specific noninvasive marker for prostate cancer early detection. J. Urol 2009, 181, 343–353. [DOI] [PubMed] [Google Scholar]
- (33).Wu N; Liu S; Guo C; Hou Z; Sun M-Z The role of annexin A3 playing in cancers. Clin. Transl. Oncol 2013, 15, 106–110. [DOI] [PubMed] [Google Scholar]
- (34).Koumanov K; Wolf C; Béreziat G Modulation of human type II secretory phospholipase A2 by sphingomyelin and annexin VI. Biochem. J 1997, 326, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Ghomashchi F; Yu BZ; Berg O; Jain MK; Gelb MH Interfacial catalysis by phospholipase A2: substrate specificity in vesicles. Biochemistry 1991, 30, 7318–7329. [DOI] [PubMed] [Google Scholar]
- (36).Tatulian SA Toward understanding interfacial activation of secretory phospholipase A2 (PLA2): membrane surface properties and membrane-induced structural changes in the enzyme contribute synergistically to PLA2 activation. Biophys. J 2001, 80, 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Østrem RG; Parhamifar L; Pourhassan H; Clergeaud G; Nielsen OL; Kjær A; Hansen AE; Andresen TL Secretory phospholipase A2 responsive liposomes exhibit a potent anti-neoplastic effect in vitro, but induce unforeseen severe toxicity in vivo. J. Controlled Release 2017, 262, 212–221. [DOI] [PubMed] [Google Scholar]
- (38).Diez E; Louis-Flamberg P; Hall RH; Mayer RJ Substrate specificities and properties of human phospholipases A2 in a mixed vesicle model. J. Biol. Chem 1992, 267, 18342–18348. [PubMed] [Google Scholar]
- (39).Behrens SH; Grier DG The charge of glass and silica surfaces. J. Chem. Phys 2001, 115, 6716–6721. [Google Scholar]
- (40).Rimola A; Costa D; Sodupe M; Lambert J-F; Ugliengo P Silica surface features and their role in the adsorption of biomolecules: computational modeling and experiments. Chem. Rev 2013, 113, 4216–4313. [DOI] [PubMed] [Google Scholar]
- (41).Emami FS; Puddu V; Berry RJ; Varshney V; Patwardhan SV; Perry CC; Heinz H Prediction of specific biomolecule adsorption on silica surfaces as a function of pH and particle size. Chem. Mater 2014, 26, 5725–5734. [Google Scholar]
- (42).Leite NB; Aufderhorst-Roberts A; Palma MS; Connell SD; Neto JR; Beales PA PE and PS lipids synergistically enhance membrane poration by a peptide with anticancer properties. Biophys. J 2015, 109, 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Papahadjopoulos D; Nir S; Düzgünes N Molecular mechanisms of calcium-induced membrane fusion. J. Bioenerg. Biomembr 1990, 22, 157–179. [DOI] [PubMed] [Google Scholar]
- (44).Issa ZK; Manke CW; Jena BP; Potoff JJ Ca2+ bridging of apposed phospholipid bilayers. J. Phys. Chem. B 2010, 114, 13249–13254. [DOI] [PubMed] [Google Scholar]
- (45).Ray S; Scott JL; Tatulian SA Effects of lipid phase transition and membrane surface charge on the interfacial activation of phospholipase A2. Biochemistry 2007, 46, 13089–13100. [DOI] [PubMed] [Google Scholar]
- (46).Jørgensen K; Davidsen J; Mouritsen OG Biophysical mechanisms of phospholipase A2 activation and their use in liposome-based drug delivery. FEBS Lett 2002, 531, 23–27. [DOI] [PubMed] [Google Scholar]
- (47).Høyrup P; Mouritsen OG; Jørgensen K Phospholipase A 2 activity towards vesicles of DPPC and DMPC–DSPC containing small amounts of SMPC. Biochim. Biophys. Acta, Biomembr 2001, 1515, 133–143. [DOI] [PubMed] [Google Scholar]
- (48).Jain MK; Yu B-Z; Kozubek A Binding of phospholipase A2 to zwitterionic bilayers is promoted by lateral segregation of anionic amphiphiles. Biochim. Biophys. Acta, Biomembr 1989, 980, 23–32. [DOI] [PubMed] [Google Scholar]
- (49).Melcrová A; Pokorna S; Pullanchery S; Kohagen M; Jurkiewicz P; Hof M; Jungwirth P; Cremer PS; Cwiklik L The complex nature of calcium cation interactions with phospholipid bilayers. Sci. Rep 2016, 6, 38035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Seantier B; Kasemo B Influence of mono-and divalent ions on the formation of supported phospholipid bilayers via vesicle adsorption. Langmuir 2009, 25, 5767–5772. [DOI] [PubMed] [Google Scholar]
- (51).Ong W-Y; Farooqui T; Kokotos G; Farooqui AA Synthetic and Natural Inhibitors of Phospholipases A2: Their Importance for Understanding and Treatment of Neurological Disorders. ACS Chem. Neurosci 2015, 6, 814–831. [DOI] [PubMed] [Google Scholar]
- (52).Sun GY; Xu J; Jensen MD; Simonyi A Phospholipase A2 in the central nervous system implications for neurodegenerative diseases. J. Lipid Res 2004, 45, 205–213. [DOI] [PubMed] [Google Scholar]
- (53).Jain MK; Yuan W; Gelb MH Competitive inhibition of phospholipase A2 in vesicles. Biochemistry 1989, 28, 4135–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Pawlikowska-Pawlęga B; Dziubińska H; Król E; Trębacz K; Jarosz-Wilkołazka A; Paduch R; Gawron A; Gruszecki WI Characteristics of quercetin interactions with liposomal and vacuolar membranes. Biochim. Biophys. Acta, Biomembr 2014, 1838, 254–265. [DOI] [PubMed] [Google Scholar]
- (55).Pawlikowska-Pawlęga B; Gruszecki WI; Misiak LE; Gawron A The study of the quercetin action on human erythrocyte membranes. Biochem. Pharmacol 2003, 66, 605–612. [DOI] [PubMed] [Google Scholar]
- (56).Fatimathas L; Moss SE Annexins as disease modifiers. Histol. Histopathol 2010, 25, 527. [DOI] [PubMed] [Google Scholar]
- (57).Bakar F Annexin Proteins: Novel Promising Targets for Anticancer Drug Development. Unique Aspects of Anti-Cancer Drug Development; IntechOpen, 2017; Vol. 51. [Google Scholar]
- (58).Jeun M; Park S; Kim Y; Choi J; Song SH; Jeong IG; Kim C-S; Lee KH Self-Normalized Detection of ANXA3 from Untreated Urine of Prostate Cancer Patients without Digital Rectal Examination. Adv. Healthcare Mater 2017, 6, 1700449. [DOI] [PubMed] [Google Scholar]
- (59).Zhou T; Li Y; Yang L; Tang T; Zhang L; Shi J Annexin A3 as a prognostic biomarker for breast cancer: a retrospective study. BioMed Res. Int 2017, 2017, 2603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Hofmann A; Raguénès-Nicol C; Favier-Perron B; Mesonero J; Huber R; Russo-Marie F; Lewit-Bentley A The Annexin A3– Membrane Interaction Is Modulated by an N-Terminal Tryptophan. Biochemistry 2000, 39, 7712–7721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


