Abstract
Objective
To determine the harms of ezetimibe in people who need lipid-lowering treatment.
Design
Systematic review and meta-analysis.
Data sources
Randomised controlled trials and cohort studies.
Eligibility criteria for selecting studies
Studies comparing ezetimibe with placebo, standard care, or other lipid-lowering agents in people who need lipid-lowering treatment with a follow-up duration of at least six months (or 24 weeks). The relative effects for potential harms of ezetimibe were pooled by use of random effect pairwise meta-analyses for randomised controlled trials and the evidence from observational studies was narratively summarised. The certainty of evidence was assessed using the Grading of Recommendation Assessment, Development, and Evaluation.
Results
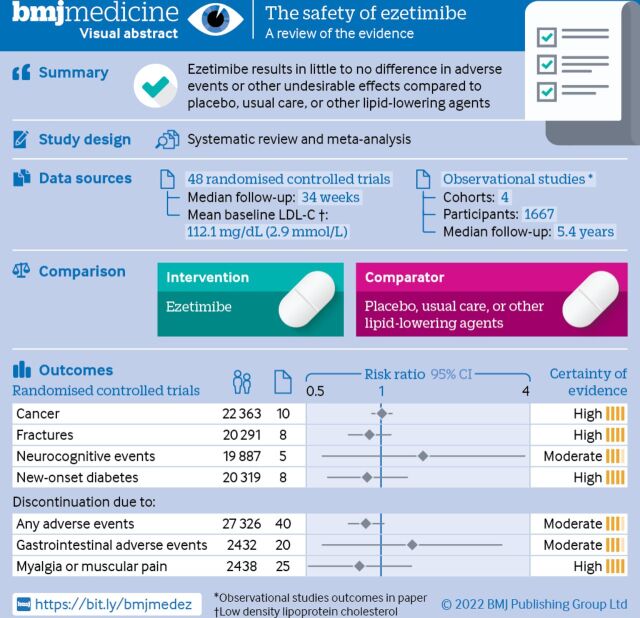
48 randomised controlled trials with 28 444 participants (median follow-up 34 weeks, range 24-312 weeks) and four observational studies with 1667 participants (median follow-up 282 weeks, range 72-400 weeks) were included. The meta-analyses of randomised trials showed moderate to high certainty that ezetimibe was not associated with cancer (relative risk 1.01; 95% confidence interval 0.92 to 1.11), fractures (0.90; 0.74 to 1.10), discontinuation due to any adverse event (0.87; 0.74 to 1.03), gastrointestinal adverse events leading to discontinuation (1.34; 0.58 to 3.08), myalgia or muscular pain leading to discontinuation (0.82; 0.51 to 1.33), neurocognitive events (1.48; 0.58 to 3.81), or new-onset diabetes (0.88; 0.61 to 1.28). The narrative analysis of observational studies provided consistent findings. No credible subgroup effects were identified for the harm outcomes, including shorter versus longer follow-up duration of trials.
Conclusions
Ezetimibe results in little to no difference in adverse events or other undesirable effects compared with placebo, usual care or other lipid-lowering agents.
Review registration
PROSPERO CRD42020187437.
Keywords: Coronary disease, Pharmacology
What is already known on this topic
Ezetimibe lowers low density lipoprotein cholesterol and reduces cardiovascular risks by blocking the gastrointestinal absorption of dietary cholesterol
Although ezetimibe is generally safe, there are concerns about its potential harms including cancer, neurocognitive events, fractures, gastrointestinal adverse events, myalgia, muscular pain, and new-onset diabetes
What this study adds
Adding ezetimibe results in little to no difference in adverse events or other undesirable effects in people who need lipid-lowering treatment
How this study might affect research, practice, or policy
When deciding to add ezetimibe to statins for lipid-lowering treatment, clinicians can have confidence in the evidence that adverse events are rare
Introduction
Cardiovascular disease is one of the leading causes of death and disability worldwide.1–3 Statins are first line cholesterol-lowering drugs for the reduction of cardiovascular risk but can cause adverse effects such as myalgia, muscular pain, and new-onset diabetes.4 5 Ezetimibe, an oral cholesterol-lowering drug taken after statins, which inhibits intestinal cholesterol absorption and decreases biliary cholesterol secretion, lowers low density lipoprotein cholesterol (LDL-C) by 20%.6–9 Clinical trials and systematic reviews have established that ezetimibe can reduce cardiovascular events.10–13 Guidelines from the European Society of Cardiology14 and American Heart Association15 recommend ezetimibe as a second lipid-lowering drug in addition to treatment with statins when LDL-C treatment goals are not met, or as a single drug in case of statin-intolerance. The number of prescriptions of ezetimibe doubled in North America from 2003 to 2006 for the primary and secondary prevention of cardiovascular diseases.16
Although ezetimibe is well tolerated in clinical practice, some studies suggest concerns regarding potential harms such as cancer, neurocognitive events, fractures, gastrointestinal adverse events, myalgia, muscular pain, and new-onset diabetes.4 17–22 The cause and magnitude of adverse events or undesirable effects of ezetimibe remain unclear. Therefore, we conducted a pairwise systematic review and meta-analysis of randomised controlled trials and observational studies to evaluate the safety of ezetimibe in people who need lipid-lowering treatment. This systematic review quantitatively informed the potential harms of ezetimibe for a parallel clinical practice guideline with risk-stratified recommendations for ezetimibe and PCSK9 inhibitors.23 This guideline forms part of a BMJ Rapid Recommendation and is a collaborative effort by the MAGIC Evidence Ecosystem Foundation (https://magicevidence.org) and The BMJ (box 1).24 For the visual abstract of this paper, see figure 1.
Box 1. Linked articles in this BMJ Rapid Recommendations cluster.
-
Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ 2022;377:e069066, doi:10.1136/bmj-2021-069066
Summary of the results from the Rapid Recommendation process
-
Khan SU, Yedlapati SH, Lone AN, et al. Anti-PCSK9 agents and ezetimibe for cardiovascular risk reduction: a systematic review and network meta-analysis. BMJ 2022;377:e069116, doi:10.1136/bmj-2021-069116
Review and network meta-analysis of all available randomised trials that assessed effects of PCSK9 inhibitors and ezetimibe with or without statin therapy for cardiovascular risk reduction
-
Harm reviews
Wang Y, Zhan S, Du H, et al. Safety of ezetimibe in lipid-lowering treatment: systematic review and meta-analysis of randomised controlled trials and cohort studies. BMJ MED 2022;1. doi:10.1136/bmjmed-2022-000134
Li J, Du H, Wang Y, et al. Safety of proprotein convertase subtilisin/kexin 9 inhibitors: a systematic review and meta-analysis. Heart 2022; doi:10.1136/heartjnl-2021-320556
-
MAGICApp (https://app.magicapp.org)
Expanded version of results with multi-layered recommendations, evidence summaries, and decision aids for use on all devices
Figure 1.
Visual abstract
Methods
Study design
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (known as PRISMA) statement and the Meta-analysis of Observational Studies in Epidemiology (known as MOOSE) statement (checklists in online supplemental tables S1 and S2).25 26 We registered the protocol in PROSPERO (CRD42020187437).
bmjmed-2022-000134supp001.pdf (2.2MB, pdf)
Guideline panel and patient involvement
The BMJ Rapid Recommendation panel,24 including clinicians, methodologists, and patients provided critical oversight over the steps of this review. The panel included cardiologists, general practitioners, general internists, endocrinologists, a geriatrician, methodologists, and three patient partners. Patient partners received personal training and individual support in the methods used throughout the guideline development process. The panel assisted in framing the study question, defining the interventions and comparisons, prioritising outcome measures, and proposing subgroup analyses. Three patient partners were members of the guideline panel that contributed to this systematic review and the associated BMJ Rapid Recommendation.
Data sources
We searched Medline, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) from inception to July 2021. We also searched ClinicalTrials.gov for completed unpublished registered trials with results. The search strategy is shown in online supplemental tables S3 and S4.
Paired reviewers (YW and HD) searched the literature and selected studies through screening titles and abstracts. Potentially eligible papers were screened in full text. In case of conflict, a third reviewer (SL) arbitrated disagreement by discussion. We crosschecked the study inclusion with a previously published systematic review.13
We included randomised controlled trials and cohort studies that compared ezetimibe with placebo, standard care, or other lipid-lowering agents with at least six months (or 24 weeks) follow-up duration. We included studies explicitly reporting data for at least one outcome of interest, including cancer (any type), new-onset diabetes mellitus, neurocognitive events, fractures, myalgia or muscular pain leading to discontinuation, discontinuation due to gastrointestinal adverse events, or discontinuation due to any adverse effect. The longest follow-up duration or the largest population study was included when studies reported on the same or overlapping populations. Only studies published in English were included.
Data were collected in a predefined collection form incorporating study characteristics (eg, first author name, year of publication, study design, sample size, follow-up duration, prevention type, the intervention of control, and background treatment); baseline characteristics (eg, age, sex, body mass index, LDL-C, high density lipoprotein cholesterol, and triglycerides); intervention characteristics (eg, drug dose, treatment duration); and safety outcomes (eg, number of events and patients of each outcome) for randomised controlled trials. If a published trial did not report the outcome information, while the corresponding ClinicalTrials.gov reported relevant data, we collected data from the registry report. When the data in publication and ClinicalTrials.gov conflicted, we used the data from the publication. For observational studies, additional data were collected, including prospective or retrospective design, exposure, data source, and methods for comparability (that is, matching or adjusting for confounding variables). Adjusted effect estimates (that is, relative risks or odds ratios) and corresponding 95% confidence intervals were preferred to raw data of adverse events in observational studies. Paired reviewers (YW and HD) performed the data extraction and a third reviewer (SL) judged the discrepancies if any.
Paired reviewers (YW and HD) assessed the risk of bias for randomised controlled trials using the Cochrane Collaboration's risk-of-bias assessment tool27 and that for observational studies with the modified Newcastle-Ottawa quality assessment scale.28 We added one item, which we named "other concerns" because some concerns could not be classified into any of the existing eight items in the scale. A third reviewer (SL) was involved in the discussion if any discrepancy occurred.
Statistical analysis
For the included randomised controlled trials, we pooled relative risks and their 95% confidence intervals using the random effects model for all meta-analyses. Statistical heterogeneity was assessed by χ2 and I2 tests with significance defined by χ2 P<0.1 or I2>50%. We used baseline risks for each outcome based on the pooled event rates of included control groups and calculated absolute effects for each outcome at both five years and two years. As a result of limited data reported and low certainty of evidence, we did not pool outcome data quantitatively in the analysis of observational studies but instead conducted a narrative summary of the included studies.
We analysed three subgroup analyses to explore the potentially hypothetical heterogeneity. Firstly, the follow-up duration (<48 v ≥48 weeks) in particular, potential affects of larger relative effects in studies with longer follow-up duration. Secondly, risk of bias (low v high risk), focusing on larger relative effects in studies with high risk. A high risk of bias is defined if at least two high risk items from the Cochrane Collaboration Risk of Bias Tool are noted. Finally, type of control (placebo or usual care v active agents) with larger relative effects in studies with placebo or usual care.
As recommended by reviewers, we exploratorily performed a meta-regression according to different baseline LDL-C concentrations. When the included number of non-zero-event trials surpassed 10, funnel plots, Begg’s rank correlations, and Egger’s linear regression were applied in evaluating publication bias. We used the fixed effects model to pool the data for each outcome as the sensitivity analysis. All data analyses were done using RStudio (R Pack Version 3.6.1).
To evaluate the certainty of evidence, we used the Grading of Recommendation Assessment, Development, and Evaluation (known as GRADE) framework29 and assessed the credibility of subgroup analyses based on the literature.30
Patient and public involvement
Three patient partners were involved in the design of this research.
Results
Included studies
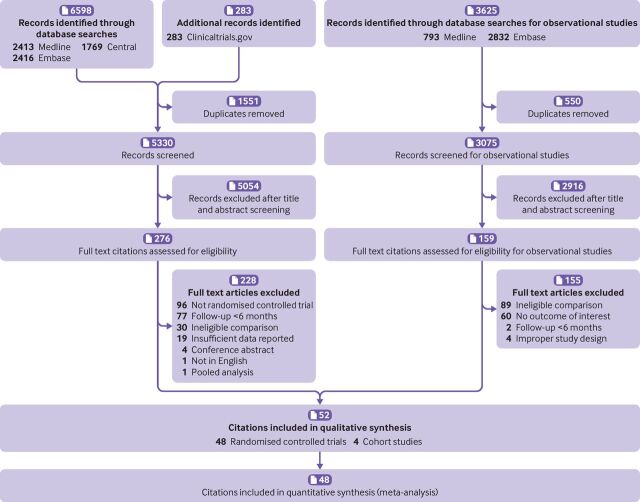
Of 6881 citations of randomised controlled trials, we included 48 with a total of 28 444 participants (figure 2, table 1, and online supplemental tables S6 and S7).11 Follow-up durations ranged from 24 to 312 weeks (median 34 weeks). The mean age of participants was 62.6 years, 71.9% were male, the mean baseline LDL-C was 112.1 mg/dL (2.9 mmol/L), and the mean proportion of individuals using statins at admission was 39.6% (table 1). Of 25 multicentre trials, treatment regimens included ezetimibe in monotherapy (663 participants in 11 trials), ezetimibe plus statin (13 230 participants in 36 trials), and ezetimibe plus fibrate (340 participants in one trial).
Figure 2.
PRISMA flow diagram
Table 1.
Baseline characteristics of included trials
| Characteristics | No (%) | Interquartile range | Range |
| Eligible studies: | |||
| Total No of trials | 48 | — | — |
| Median trial size | 131 | 65-246 | 18-18 144 |
| Median follow-up (weeks) | 34 | 24-52 | 24-312 |
| No of studies funded by pharmaceutical companies | 26 (54.2) | — | — |
| No of studies that were phase 2/3 | 12 (25.0) | — | — |
| No of studies that were not phase 2/3 | 36 (75.0) | — | — |
| Participants: | |||
| Mean age (years) | 62.6 | 57.7-64.0 | 45.9-84.1 |
| Male sex (%) | 71.9 | 51.9-75.3 | 27.6-89.7 |
| Mean LDL-C (mg/dL) at baseline | 112.1 | 109.92-150.47 | 82.05-318.40 |
| Mean proportion (%) of patients receiving statin at baseline | 39.6 | 0-100 | 0-100 |
| Region: | |||
| World | 12 (25.0) | — | — |
| Europe* | 9 (18.8) | — | — |
| Asia† | 20 (41.7) | — | — |
| America | 7 (14.6) | — | — |
| Prevention type: | |||
| Primary prevention | 12 (25.0) | — | — |
| Secondary prevention | 24 (50.0) | — | — |
| Unspecific prevention | 12 (25.0) | — | — |
LDL-C=low density lipoprotain cholesterol (1 mg/dL=0.0259 mmol/L).
*One study conducted in Russia was included in this category.
†One study conducted in Turkey was included in this category.
Of 3625 citations of observational studies, we included four cohort retrospective cohorts with 1667 participants in our narrative summary (figure 2, table 2, and online supplemental table S10).31–34 Follow-up durations ranged from 72 to 400 weeks (that is, 1.38-7.70 years; median 282 weeks (that is, 5.42 years)). The population from two studies were identified from electronic health records.32 34 The mean age of the participants was 59.5 years, 35.6% were male, the mean baseline LDL-C was 191.0 mg/dL (4.9 mmol/L), and 9.7% of participants were using statins at admission. Three studies compared ezetimibe plus statin versus statin alone, and one other compared ezetimibe versus colesevelam.
Table 2.
Characteristics of the included observational studies
| Study | Study design | Data sources | Funding | Location | No of centres | No of participants | Median follow-up duration | Mean LDL-C (mg/dL) at baseline | Prevention type |
| Barkas et al31 | Retrospective cohort study | NR | Not funded | Greece | Single centre | 796 | 6.84 years* | 177.8 | Primary |
| Kim et al32 | Retrospective cohort study | Electronic medical records | Ministry of Health and Welfare, Republic of Korea | Korea | Single centre | 665 | 4 years | NR | NR |
| Kłosiewicz-Latoszek et al33 | Retrospective cohort study | NR | Sanofi | Poland | Single centre | 190 | 7.70 years† | 239.8 | Primary† |
| Rivers et al34 | Retrospective cohort study | Electronic medical records | Sankyo Pharma | US | Single centre | 16 | Phase 1: 305 days; phase 2: 199 days | 166 | NR |
LDL-C=low density lipoprotain cholesterol (1 mg/dL=0.0259 mmol/L); NR=not reported.
*Mean was estimated from median and interquartile range.
†Data in specific subpopulation of interest were not available, so data in overall population were presented.
Risk of bias
We rated the overall risk of bias as low across all 48 included trials; 29 trials raised concerns (online supplemental file 1). We rated 14 (29%) studies as high risk of bias because the number of missing participants was higher than 20% or the analysis was not done by an intention-to-treat protocol. We rated 18 (38%) trials as high risk of bias owing to inadequate masking of participants and personnel, 14 (29%) trials owing to an open label design, and four (8%) trials because the trial design did not have a matching placebo. The overall scores of the Newcastle-Ottawa quality assessment scale were six to seven among the included observational studies, indicating some risks of bias (table 3; online supplemental table S11).
Table 3.
Treatments, outcomes, and risk of bias of included observational studies
| Outcome | Study | Treatment of interest | Control group | Summary of findings | NOS score (0-10) |
| New-onset diabetes | Barkas et al31 | Ezetimibe+statin | Statin | Ezetimibe did not increase the risk of new-onset diabetes (adjusted OR 1.01; 95% CI 0.51 to 1.99). OR was adjusted for the log-transformed baseline fasting glucose levels and follow-up duration, the presence of metabolic syndrome, and family history of diabetes. | 7 |
| Kim et al32 | Simvastatin (20 mg) with ezetimibe (10 or 20 mg) complex | Simvastatin (20 and 40 mg) | Ezetimibe did not increase the risk of new-onset diabetes (adjusted OR via indirect comparison* 1.24; 95% CI 0.65 to 2.39). OR was adjusted for baseline variables, which were not reported explicitly. | 7 | |
| Myalgia or muscular pain leading to discontinuation | Kłosiewicz-Latoszek et al33 | Ezetimibe+statin | Statin | No case of myalgia or muscular pain leading to discontinuation was reported in each group. | 6 |
| Rivers et al34 | Phase 1: ezetimibe; phase 2: ezetimibe+colesevelam | Phase 1: colesevelam; phase 2: ezetimibe+colesevelam | No case of myalgia or muscular pain leading to discontinuation was reported in each group. | 6 | |
| Discontinuation due to any gastrointestinal adverse events | Rivers et al34 | Phase !: ezetimibe; phase 2: ezetimibe+colesevelam | Phase 1: colesevelam; phase 2: ezetimibe+colesevelam | No case of discontinuation due to any gastrointestinal adverse events was reported in each group. | 6 |
| Discontinuation due to any adverse events | Rivers et al34 | Phase 1: ezetimibe; phase 2: ezetimibe+colesevelam | Phase 1: colesevelam; phase 2: ezetimibe+colesevelam | No case of discontinuation due to any adverse events was reported in each group. | 6 |
CI=confidence interval; NOS=Newcastle-Ottawa quality assessment scale; OR=odds ratio.
*The comparison was indirect because the OR and 95% CI of simvastatin and ezetimibe versus imvastatin were calculated from the ORs and 95% CIs of simvastatin versus atorvastatin and of simvastatin and ezetimibe versus atorvastatin.
Meta-analyses of randomised controlled trials
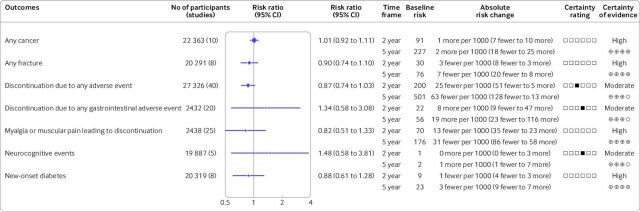
As shown in figure 3, moderate to high certainty evidence indicated that ezetimibe has little to no effect on the risks of cancer (relative risk 1.01; 95% confidence interval 0.92 to 1.11), fractures (0.90; 0.74 to 1.10), discontinuation due to any adverse events (0.87; 0.74 to 1.03), discontinuation due to gastrointestinal adverse events (1.34; 0.58 to 3.08), myalgia or muscular pain leading to discontinuation (0.82; 0.51 to 1.33), neurocognitive events (1.48; 0.58 to 3.81), or new-onset diabetes (0.88; 0.61 to 1.28). We downgraded the certainty of the evidence to moderate for discontinuation due to any adverse events for indirectness because of the composite nature of the outcome. We also downgraded the certainty of evidence of discontinuation due to any gastrointestinal adverse events and neurodegenerative events for imprecision because of wide 95% confidence intervals, which could not support clinical decision making.
Figure 3.
Summary of findings for relative and absolute risks of the safety outcomes of ezetimibe. Hollow squares in the certainty rating column represent six rating domains listed in order from left to right including risk of bias, inconsistency, indirectness, imprecision, publication bias, and other concerns. Black squares means that the certainty was downgraded because of that domain. CI=confidence interval
Subgroups and sensitivity analysis
None of the subgroup analyses identified potential subgroup effects in different trials with different follow-up durations, risk of bias, and type of control (online supplemental table S8 and figures S3–S5). For example, the cancer risk did not show heterogeneity across the subgroups of <48 weeks follow-up (relative risk 0.79; 95% confidence interval 0.21 to 3.01) and ≥48 weeks follow-up (1.01; 0.92 to 1.11) with the interaction P value being 0.72. The meta-regression did not identify any association between baseline LDL-C concentrations and outcomes (online supplemental table S13).
The sensitivity analyses supported the robustness of the pooled results using the fixed effects model (online supplemental figures S7–S19). Neither funnel plots nor Begg’s and Egger’s tests for the outcomes of cancer and discontinuation due to any adverse events did not identify signals of publication bias (online supplemental table S9).
Narrative summary of observational studies
Two retrospective studies31 32 suggested that ezetimibe was not associated with an increased risk of new-onset diabetes (adjusted odds ratio 1.01, 95% confidence interval 0.51 to 1.99; adjusted odds ratio via indirect comparison 1.24, 0.65 to 2.39) during the four to six year follow-up duration (table 3). Two studies reported no instances of myalgia or muscular pain during the follow-up duration.33 34 One study with 16 participants reported no cases of discontinuation due to any adverse events including gastrointestinal effects in a 10 month follow-up duration.34 We consider these findings to be very low certainty evidence due to high risk of bias (new-onset diabetes, myalgia or muscular pain leading to discontinuation, discontinuation due to any gastrointestinal adverse events and discontinuation due to any adverse events), indirectness (new-onset diabetes and discontinuation due to any adverse events) and imprecision (myalgia or muscular pain leading to discontinuation, discontinuation due to any gastrointestinal adverse events and discontinuation due to any adverse events).
Discussion
Main findings
Moderate to high certainty evidence shows that ezetimibe has little to no effect on adverse events (compared with no ezetimibe), including cancer, new-onset diabetes, neurocognitive events, fractures, myalgia or muscular pain leading to discontinuation, or discontinuation due to gastrointestinal adverse events or any adverse events.
Drugs that do not cause adverse events are rare in clinical practice.35 Unlike the pleiotropy of targets for other lipid-lowering drugs, ezetimibe lowers LDL-C concentration by blocking the Niemann-Pick C1 Like 1 (NPC1L1) protein, which inhibits intestinal cholesterol absorption, thus mimicking a low cholesterol diet.36 37 Ezetimibe does not directly interact with the lipid metabolism in the liver and other organs and is biologically safe, except for the potential harms of very low cholesterol intake, which remains open to debate.38
Compared with the previous studies
We identified six previous meta-analyses investigating the safety concerns of ezetimibe.39–44 Results for these studies were consistent with our findings, except that Zhao and colleagues44 significantly linked ezetimibe to increased neurocognitive events in their network meta-analysis (network odds ratio 3.94, 95% confidence interval 1.18 to 13.12).
Neurocognitive safety is one of the most important concerns followed by very low LDL-C concentrations.45 Nevertheless, the findings from our meta-analysis of randomised controlled trials did not show an effect of neurodegenerative events in people treated with ezetimibe, nor did the findings from the observational studies. Our study did not show an effect on cancer, a concern that was raised by the SEAS trial.22 We did not note an association with fractures or gastrointestinal effects, events that could be linked to the limited absorption of lipids in intestines.5 20 21 However, discontinuation due to any gastrointestinal adverse events and neurodegenerative events were downgraded to moderate certainty due to wide 95% confidence intervals. New evidence could change our confidence in these effects.46 47
Strengths and limitations
Our study systematically reviewed all ezetimibe trials and cohort studies from literature and ClinicalTrials.gov and engaged a multidisciplinary panel to contextualise our findings into clinical practice. The GRADE approach based on the absolute effects facilitates the application in clinical practice. In trials, we did not identify credible subgroup effects for any of the harm outcomes regarding different follow-up durations. With the support from observational studies with a median follow-up duration of up to 7.7 years, our study supports the long term safety of the drug. Nevertheless, long term surveillance remains necessary.
The key limitation of this study is that the number of some events (that is, gastrointestinal and neurocognitive events) is rare and therefore findings for these events could be imprecise.Unfortunately, the included observational studies to supplement the trial evidence overall provided very low certainty evidence and were not powered to improve precision for these or other harm outcomes. A large scale, population based study could be helpful in the future. However, such rare events might not alter clinical decision making because of the very low absolute baseline risk. The systematic review did not provide direct evidence for people with characteristics that were not represented by the study population (eg, low LDL-C concentration before treatment). People who might not be represented, therefore, should use when considering the direct evidence.
Conclusion
In this systematic review, moderate to high certainty evidence show that treatment with ezetimibe has little to no effect on adverse events compared with no ezetimibe. Nevertheless, the clinical practice warrants long term surveillance of rare events, especially in unrepresented populations from previous studies.
Footnotes
YW, SZ and HD contributed equally.
Contributors: SL, HT, POV, IR, ND, GB, GG, BA, QH, and SUK conceived the study, YW performed the literature search; YW and JL screened studies for eligibility; YW, SZ, LL, and SL assessed the risk of bias; YW and JL performed data extraction; SL, BA, GG, POV, and NS interpreted the data analysis; SL, BA, GG, QH, ND, and HD assessed the certainty of the evidence; HD wrote the draft of the manuscript; and all other authors revised the manuscript. SL is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was funded by 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (Nos. 19HXFH011, ZYGD18022 and 2020HXF011). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from West China Hospital, Sichuan University for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics approval
Not applicable.
References
- 1. Laslett LJ, Alagona P, Clark BA, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of cardiology. J Am Coll Cardiol 2012;60:S1–49. 10.1016/j.jacc.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 2. GBD 2013 Mortality and Causes of Death Collaborators . Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet 2015;385:117–71. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roth GA, Huffman MD, Moran AE, et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015;132:1667–78. 10.1161/CIRCULATIONAHA.114.008720 [DOI] [PubMed] [Google Scholar]
- 4. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003;289:1681–90. 10.1001/jama.289.13.1681 [DOI] [PubMed] [Google Scholar]
- 5. Wang Z, Li Y, Zhou F, et al. Effects of statins on bone mineral density and fracture risk: a PRISMA-compliant systematic review and meta-analysis. Medicine 2016;95:e3042. 10.1097/MD.0000000000003042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang HH, Portincasa P, Mendez-Sanchez N, et al. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology 2008;134:2101–10. 10.1053/j.gastro.2008.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feingold KR, Anawalt B, Boyce A. Cholesterol Lowering Drugs. Endotext [Internet]: MDText. com, Inc, 2021. [Google Scholar]
- 8. Dujovne CA, Ettinger MP, McNeer JF, et al. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol 2002;90:1092–7. 10.1016/S0002-9149(02)02798-4 [DOI] [PubMed] [Google Scholar]
- 9. Sudhop T, Lütjohann D, Kodal A, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 2002;106:1943–8. 10.1161/01.CIR.0000034044.95911.DC [DOI] [PubMed] [Google Scholar]
- 10. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92. 10.1016/S0140-6736(11)60739-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97. 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 12. Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation 2019;140:992–1003. 10.1161/CIRCULATIONAHA.118.039415 [DOI] [PubMed] [Google Scholar]
- 13. Zhan S, Tang M, Liu F, et al. Ezetimibe for the prevention of cardiovascular disease and all-cause mortality events. Cochrane Database Syst Rev 2018;11:CD012502. 10.1002/14651858.CD012502.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 15. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168–209. 10.1016/j.jacc.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 16. Jackevicius CA, Tu JV, Ross JS, et al. Use of ezetimibe in the United States and Canada. N Engl J Med 2008;358:1819–28. 10.1056/NEJMsa0801461 [DOI] [PubMed] [Google Scholar]
- 17. Navarese EP, Buffon A, Andreotti F, et al. Meta-Analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol 2013;111:1123–30. 10.1016/j.amjcard.2012.12.037 [DOI] [PubMed] [Google Scholar]
- 18. Feingold KR, Grunfeld C. Cholesterol lowering drugs. Endotext [Internet]: MDText. com, Inc, 2018. [Google Scholar]
- 19. Banach M, Rizzo M, Nikolic D, et al. Intensive LDL-cholesterol lowering therapy and neurocognitive function. Pharmacol Ther 2017;170:181–91. 10.1016/j.pharmthera.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 20. Martinez AI, Freeman PR, Moga DC. Statin use and gastrointestinal hemorrhage: a large retrospective cohort study. Am J Cardiovasc Drugs 2019;19:65–74. 10.1007/s40256-018-0301-4 [DOI] [PubMed] [Google Scholar]
- 21. Yamauchi M, Yamaguchi T, Nawata K, et al. Increased low-density lipoprotein cholesterol level is associated with non-vertebral fractures in postmenopausal women. Endocrine 2015;48:279–86. 10.1007/s12020-014-0292-0 [DOI] [PubMed] [Google Scholar]
- 22. Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359:1343–56. 10.1056/NEJMoa0804602 [DOI] [PubMed] [Google Scholar]
- 23. Hao Q, Aertgeerts B, Guyatt G. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: clinical practice guideline with risk stratified recommendations. BMJ 2022;377:e069066. 10.1136/bmj-2021-069066 [DOI] [PubMed] [Google Scholar]
- 24. Siemieniuk RA, Agoritsas T, Macdonald H, et al. Introduction to BMJ rapid recommendations. BMJ 2016;354:i5191. 10.1136/bmj.i5191 [DOI] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stroup DF, Berlin JA, Morton SC, et al. Meta-Analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 27. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wells GA, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford, 2000. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 29. Guyatt GH, Oxman AD, Vist GE, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun X, Briel M, Busse JW, et al. Credibility of claims of subgroup effects in randomised controlled trials: systematic review. BMJ 2012;344:e1553. 10.1136/bmj.e1553 [DOI] [PubMed] [Google Scholar]
- 31. Barkas F, Elisaf M, Liberopoulos E, et al. Statin therapy with or without ezetimibe and the progression to diabetes. J Clin Lipidol 2016;10:306–13. 10.1016/j.jacl.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 32. Kim TM, Kim H, Jeong YJ, et al. The differences in the incidence of diabetes mellitus and prediabetes according to the type of HMG-CoA reductase inhibitors prescribed in Korean patients. Pharmacoepidemiol Drug Saf 2017;26:1156–63. 10.1002/pds.4237 [DOI] [PubMed] [Google Scholar]
- 33. Kłosiewicz-Latoszek L, Cybulska B, Białobrzeska-Paluszkiewicz J, et al. Clinical management of heterozygous familial hypercholesterolemia in a Polish outpatient metabolic clinic: a retrospective observational study. Arch Med Sci 2018;14:962–70. 10.5114/aoms.2017.71855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rivers SM, Kane MP, Busch RS, et al. Colesevelam hydrochloride-ezetimibe combination lipid-lowering therapy in patients with diabetes or metabolic syndrome and a history of statin intolerance. Endocr Pract 2007;13:11–16. 10.4158/EP.13.1.11 [DOI] [PubMed] [Google Scholar]
- 35. Angamo MT, Chalmers L, Curtain CM, et al. Adverse-drug-reaction-related hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Saf 2016;39:847–57. 10.1007/s40264-016-0444-7 [DOI] [PubMed] [Google Scholar]
- 36. Altmann SW, Davis HR, Zhu L-J, L-j Z, et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science 2004;303:1201–4. 10.1126/science.1093131 [DOI] [PubMed] [Google Scholar]
- 37. Temel RE, Tang W, Ma Y, et al. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest 2007;117:1968–78. 10.1172/JCI30060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kosoglou T, Statkevich P, Johnson-Levonas AO, et al. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet 2005;44:467–94. 10.2165/00003088-200544050-00002 [DOI] [PubMed] [Google Scholar]
- 39. Battaggia A, Donzelli A, Font M, et al. Clinical efficacy and safety of ezetimibe on major cardiovascular endpoints: systematic review and meta-analysis of randomized controlled trials. PLoS One 2015;10:e0124587. 10.1371/journal.pone.0124587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Savarese G, De Ferrari GM, Rosano GMC, et al. Safety and efficacy of ezetimibe: a meta-analysis. Int J Cardiol 2015;201:247–52. 10.1016/j.ijcard.2015.08.103 [DOI] [PubMed] [Google Scholar]
- 41. Chaiyasothi T, Nathisuwan S, Dilokthornsakul P, et al. Effects of Non-statin Lipid-Modifying agents on cardiovascular morbidity and mortality among statin-treated patients: a systematic review and network meta-analysis. Front Pharmacol 2019;10:547. 10.3389/fphar.2019.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Davidson MH, Maccubbin D, Stepanavage M, et al. Striated muscle safety of ezetimibe/simvastatin (Vytorin). Am J Cardiol 2006;97:223–8. 10.1016/j.amjcard.2005.08.038 [DOI] [PubMed] [Google Scholar]
- 43. Pandor A, Ara RM, Tumur I, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med 2009;265:568–80. 10.1111/j.1365-2796.2008.02062.x [DOI] [PubMed] [Google Scholar]
- 44. Zhao Z, Du S, Shen S, et al. Comparative efficacy and safety of lipid-lowering agents in patients with hypercholesterolemia: a frequentist network meta-analysis. Medicine 2019;98:e14400. 10.1097/MD.0000000000014400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Olsson AG, Angelin B, Assmann G, et al. Can LDL cholesterol be too low? possible risks of extremely low levels. J Intern Med 2017;281:534–53. 10.1111/joim.12614 [DOI] [PubMed] [Google Scholar]
- 46. Revicki D, Hays RD, Cella D, et al. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 2008;61:102–9. 10.1016/j.jclinepi.2007.03.012 [DOI] [PubMed] [Google Scholar]
- 47. King MT. A point of minimal important difference (mid): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res 2011;11:171–84. 10.1586/erp.11.9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjmed-2022-000134supp001.pdf (2.2MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.